-
核技术的广泛应用,会产生大量含有铀、钚等核素的放射性的废物。其具有毒性大,衰变时间长等特点,如不妥善处理将会对人类和生态造成长久的危害[1—4]。放射性废物中的铀在水相环境下一般以U(Ⅳ)和U(Ⅵ)的方式存在,四价铀具有很强的还原性,易被氧化为六价铀以
$ {\mathrm{U}\mathrm{O}}_{2}^{2+} $ 的形式存在于水相环境中,若处于地下水环境中,还会与地下水中的杂质离子反应,形成多种铀酰化合物[5-6]。如何安全、有效的处理这些放射性核素是目前急需解决的问题,常用的U(Ⅵ)处理方法有离子交换法、吸附法、化学沉淀法、电化学处理法、膜分离法等[7—13]。这些方法普遍存在投入高、消耗大等不足。材料吸附法作为一种简单易操作的方法被广泛应用于去除溶液中的U(Ⅵ)。粘土岩矿物作为一种常用的吸附材料,具有不透水,自封闭性良好、吸附能力强等优点,因此受到广泛的关注。3-氨基丙基三乙氧基硅烷(3-APTES)能够增加粘土岩表面的吸附位点,增强粘土岩的吸附性能。因此,3-APTES已经作为一种常用的改性附载材料被应用到很多领域中。
本实验利用3-氨基丙基三乙氧基硅烷对粘土岩进行改性,测试其吸附性能并探究其吸附U(Ⅵ)的反应机理,为缓冲回填材料提供更好的选择,为其在地质处置中的应用提供基础理论依据。
-
JJ-2恒温磁力搅拌器、DZTW恒温电热套、DHG-9020-2控温红外烘箱、UPT-Ⅱ-10T纯水机、FA2004A 电子分析天平、DZS-708L 电子pH计、L500高速离心机、UV-3150紫外分光光度计、Axios X射线荧光光谱仪、Spectrum One红外光谱仪、UItra 55场发射扫描电子显微。
-
3-氨基丙基三乙氧基硅烷(3-APTES)、无水乙醇、硝酸、盐酸、氢氧化钠、氯化钾、氯化钠、碳酸钠、氯化镁、硝酸钠、硫酸钠、碳酸氢钠、偶氮胂Ⅲ、双氧水、标准溶液U(Ⅵ)(pH=5,浓度为200 mg·L−1)。
-
取4 g粘土岩矿物,经破碎、研磨并过200目筛后,放入干燥箱中80 ℃烘烤至完全干燥,向干燥后的粘土岩中加入200 mL的去离子水、2 mL 1 mol·L−1的盐酸和2 mL 2 mol·L−1的硝酸,在室温下使用恒温磁力搅拌器以1000 r·min−1的转速搅拌6 h,对粘土岩进行酸性活化。将活化后粘土岩放入三颈烧瓶中,加入200 mL无水乙醇,三颈烧瓶保持氮气循环的条件下,放置在恒温电热套中恒温加热2 h,使其充分完成水解、聚合后再逐滴加入2 mL的3-APTES并充分震荡,继续恒温加热4 h。反应完成后的产物放入恒温真空抽滤箱中,在80 ℃的真空条件下进行恒温干燥,每3 h用无水乙醇进行冲洗1次,重复操作6—8次,待产物完全干燥后即可得到3-APTES改性粘土岩.
-
称取0.04 g 3-APTES改性粘土岩到离心管中,加入2 mL 的200 µg·mL−1 的U(Ⅵ)标液并定容至8 mL。将离心管放置到恒温震荡箱中进行振荡,按照设定的接触时间分别取出离心管,放入高速离心机中以3000 r·min−1的转速离心20 min。离心后3-APTES改性粘土岩聚集在离心管底部,移取1 mL上清液于10 mL的容量瓶,先后加入0.5 mL的0.5 mol·L−1盐酸和2 mL质量浓度为0.1%的偶氮胂Ⅲ显色剂,摇匀后使用紫外分光光度计测量其浓度并记录数据。本实验通过吸附分配系数(Kd值)来表征吸附性能[14—15]:
式中,A0—U(Ⅵ)的初始浓度,μg·mL−1;At—平衡后水相中U(Ⅵ)的浓度,μg·mL−1;V —反应介质的总体积,mL;M—加入3-APTES改性粘土岩的质量,g。
-
粘土岩及3-APTES改性粘土岩的XRF分析结果如表1所示。由表1可知,粘土岩及3-APTES改性粘土岩化学组成复杂,Si、Ca、Al元素所占比重较大,其中SiO2的含量在粘土岩及改性粘土岩中超过50.00%,还含有少量的Fe、K、Mg、Na等元素,且都以稳定氧化物的形式存在于3-APTES改性粘土岩中。
-
改性前后的粘土岩红外光谱见图1。由图1可知,3415.31 cm−1处为Si—OH键伸缩振动峰,2917.77 cm−1处为C—H键的伸缩振动峰(反称),1644.98 cm−1处为H2O—OH键弯曲振动峰,改性前后土样这3个峰完全一样,说明进行附载改性后,这部分结构并未发生改变。1454.06 cm−1处为C—O—H键伸缩振动峰,改性后该峰基本消失,说明粘土岩中的C—O—H键被3-APRES中的—NH2破坏。1031.73 cm−1处为Si—O键面内伸缩振动峰,改性后该峰的面积明显增大,说明Si—O键数量增加,这与3-APTES的化学成分相符。873.59 cm−1处的C—H键弯曲振动消失。在470.54 cm−1处的Si—O—Al键弯曲振动峰面积增加,说明该键的数量增加,这是由于3-APTES中的部分Si—O键与粘土岩中的Al原子组合成新的Si—O—Al键。由此可知3-APTES改性粘土岩的改性过程主要发生在C—O—H键、Si—O键和Si—O—Al键处。其主要组分未发生变化,仍为伊利石等粘土矿物。
-
3-APTES改性粘土岩的扫面电镜结果如图2所示。由图2可以看出,3-APTES改性粘土岩没有固定的表面结构,多为呈不规则多边型的薄片状晶体,形态各异没有规律,且空间结构分布分散,各晶体间孔隙大,空位多。这样的结构使得3-APTES改性粘土岩拥有更大的比表面积、更多的吸附位点,即其具有更强的表面吸附能力。
-
室温下,称取0.04 g 3-APTES改性粘土岩到离心管中,加入2 mL 的200 µg·mL−1 的U(Ⅵ)标液并定容至8 mL。在pH 5 的条件下,考察吸附时间对改性粘土岩对U(Ⅵ)的影响,结果如图3所示。由图3可知, 3-APTES改性粘土岩对U(Ⅵ)的吸附量随反应时间的增加而增加,吸附速率随时间的增加而不断减小。120 min时吸附量达到最大,吸附达到平衡。
-
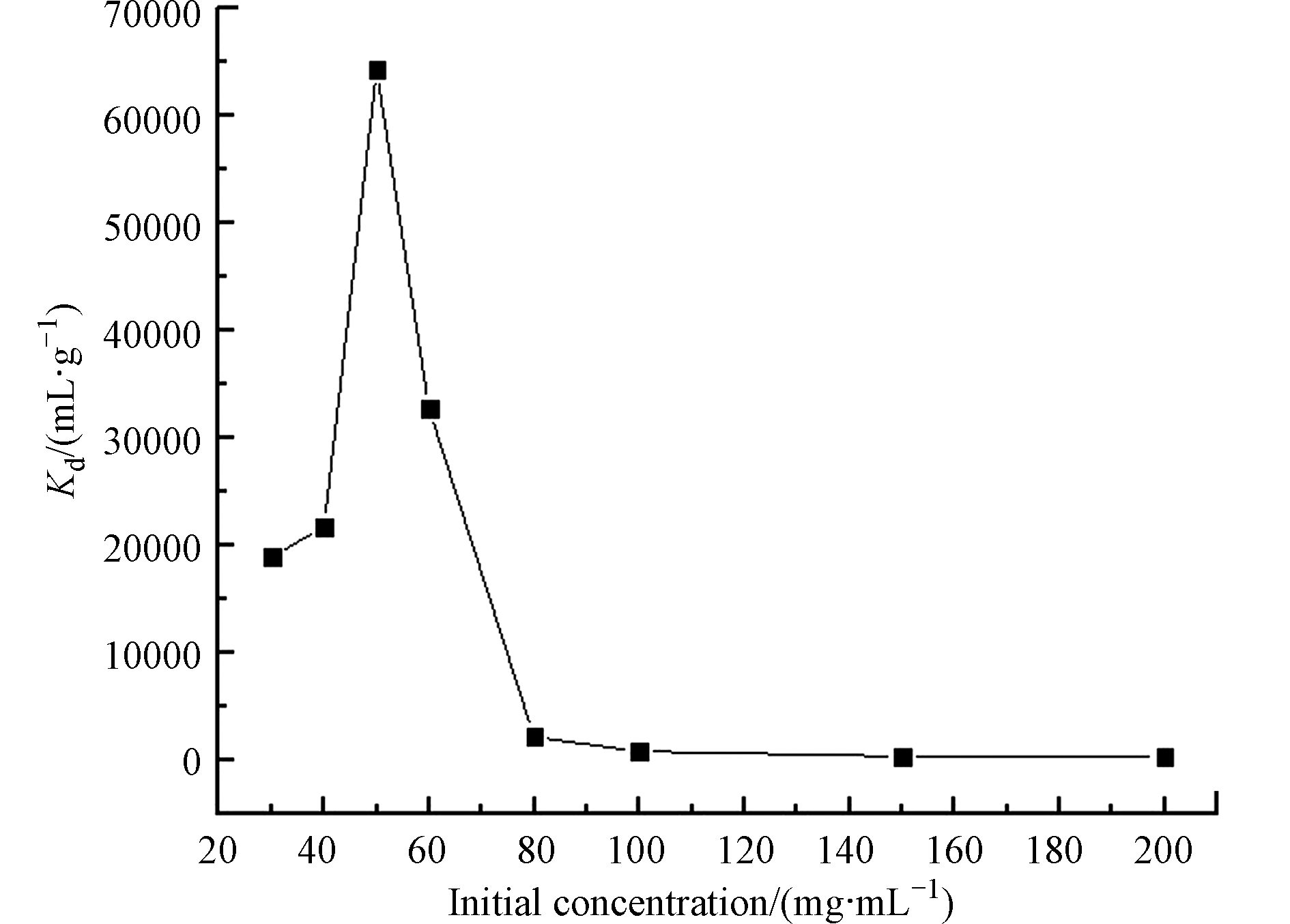
室温下,称取0.04 g 3-APTES改性粘土岩到离心管中,加入2 mL 的不同初始浓度的U(Ⅵ)标液并定容至8 mL。在pH 5 的条件下,吸附120 min,考察不同浓度对改性粘土岩对U(Ⅵ)的影响,结果如4所示。由图4可知,3-APTES改性粘土岩对U(Ⅵ)的吸附性能随着U(Ⅵ)初始浓度的增大先增强后减弱,最后趋于稳定。分析认为是由于3-APTES改性粘土岩含有大量的氨基、羟基、碳碳双键等有机活性基团,能与溶液中的U(Ⅵ)以化学键的形式结合,同时3-APTES改性粘土岩不规则的形状及松散多孔的表面结构,可为对U(Ⅵ)的吸附提供足够的吸附位点,使分配系数增大;当U(Ⅵ)初始浓度为50 μg·mL−1时,3-APTES改性粘土岩中的吸附点位已基本被U(Ⅵ)所占据,没有多余的位点用于继续吸附溶液中过量的U(Ⅵ),U(Ⅵ)就会与溶液中的其他离子结合,以铀酰化合物的形式游离存在,以致溶液中的U(Ⅵ)浓度降低,而被吸附的U(Ⅵ)的量保持一定,导致分配系数呈下降趋势,直至Kd值趋于一定。50 μg·mL−1为3-APTES改性粘土岩吸附U(Ⅵ)的最佳浓度。
-
室温下,称取一定量的3-APTES改性粘土岩到离心管中,加入2 mL 的50 μg·mL−1的U(Ⅵ)标液并定容至8 mL。在pH 5 的条件下,吸附120 min,考察不同固液比对改性粘土岩对U(Ⅵ)的影响,结果如图5所示。由图5可知,随着固液比的增大分配系数Kd值先增大后减小。这是因为当3-APTES改性粘土岩投入量较少时,3-APTES改性粘土岩表面的吸附位点不足以将U(Ⅵ)完全吸附,U(Ⅵ)以铀酰离子的形式游离存在于溶液中;随着投入量的增加,吸附位点和配位点增多,故分配系数增大;当岩样用量达到一定值后,吸附达到饱和,继续增加投入量U(Ⅵ)将不再被吸附,单位面积上的吸附量减少,Kd值下降。1∶200 为此过程的最佳固液比。此时,分配系数最高,最佳吸附量达9.969 mg·g−1。同等条件下未改性粘土岩的最佳吸附量为2.352 mg·g−1,改性粘土岩吸附能力显著增加。
-
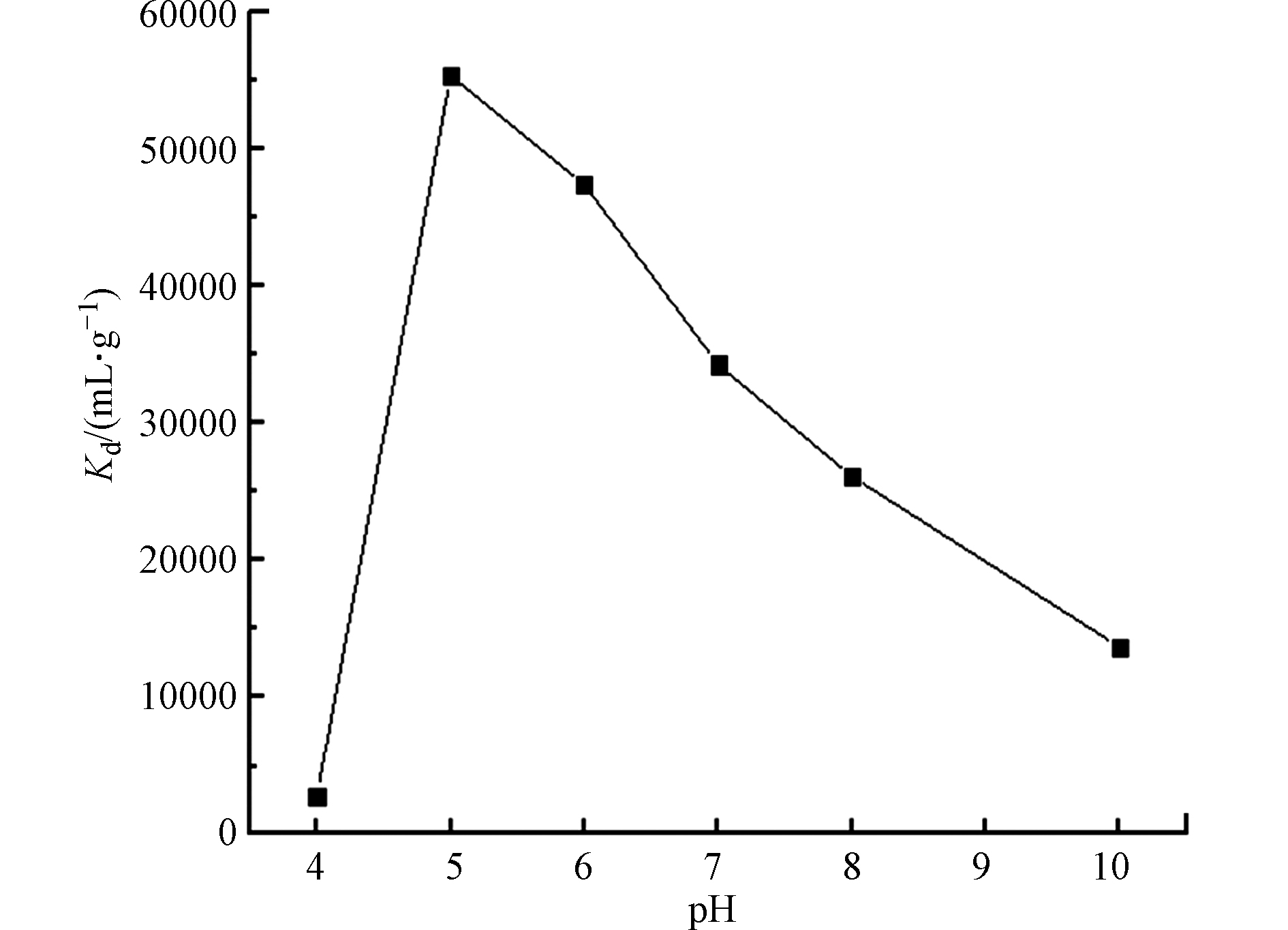
室温下,称取0.04 g 3-APTES改性粘土岩到离心管中,加入2 mL 的50 μg·mL−1的U(Ⅵ)标液并定容至8 mL。吸附120 min,考察不同pH对改性粘土岩对U(Ⅵ)的影响,结果如图6所示。由图6可知,当pH值小于5时,3-APTES改性粘土岩对U(Ⅵ)的吸附分配系数随pH的增大而增大;当pH值等于5时,Kd值达到最大,为最佳吸附pH 值;当pH值大于5时,分配系数随pH的增大而减小。这是由于酸性条件下,U(Ⅵ)在溶液中以游离的
$ {\mathrm{U}\mathrm{O}}_{2}^{2+} $ 形式存在,U(Ⅵ)的吸附主要为$ {\mathrm{U}\mathrm{O}}_{2}^{2+} $ 与土样表面的有机活性官能团成键结合,而溶液中含有的量H+,会与3-APTES改性粘土岩上—NH2和—OH结合,与$ {\mathrm{U}\mathrm{O}}_{2}^{2+} $ 竞争吸附空位,明显抑制对了U(Ⅵ)的吸附作用;随着pH值的增大,UO2CO3与溶液中的OH−络合生成$ {{\mathrm{U}\mathrm{O}}_{2}\left({\mathrm{C}\mathrm{O}}_{3}\right)}_{2}^{2-} $ ,吸附效果下降。随着OH−的持续增加,$ {{\mathrm{U}\mathrm{O}}_{2}\left({\mathrm{C}\mathrm{O}}_{3}\right)}_{2}^{2-} $ 会与过量的OH−反应生成UO2(CO3)34−,其极难被3-APTES改性粘土岩吸附,从而抑制对U(Ⅵ)的吸附。同时过量的OH−还会与土样中的有机活性基团配位,占据大量的吸附配位点,U(Ⅵ)游离存在与溶液中,从而使分配系数Kd值降低。 -
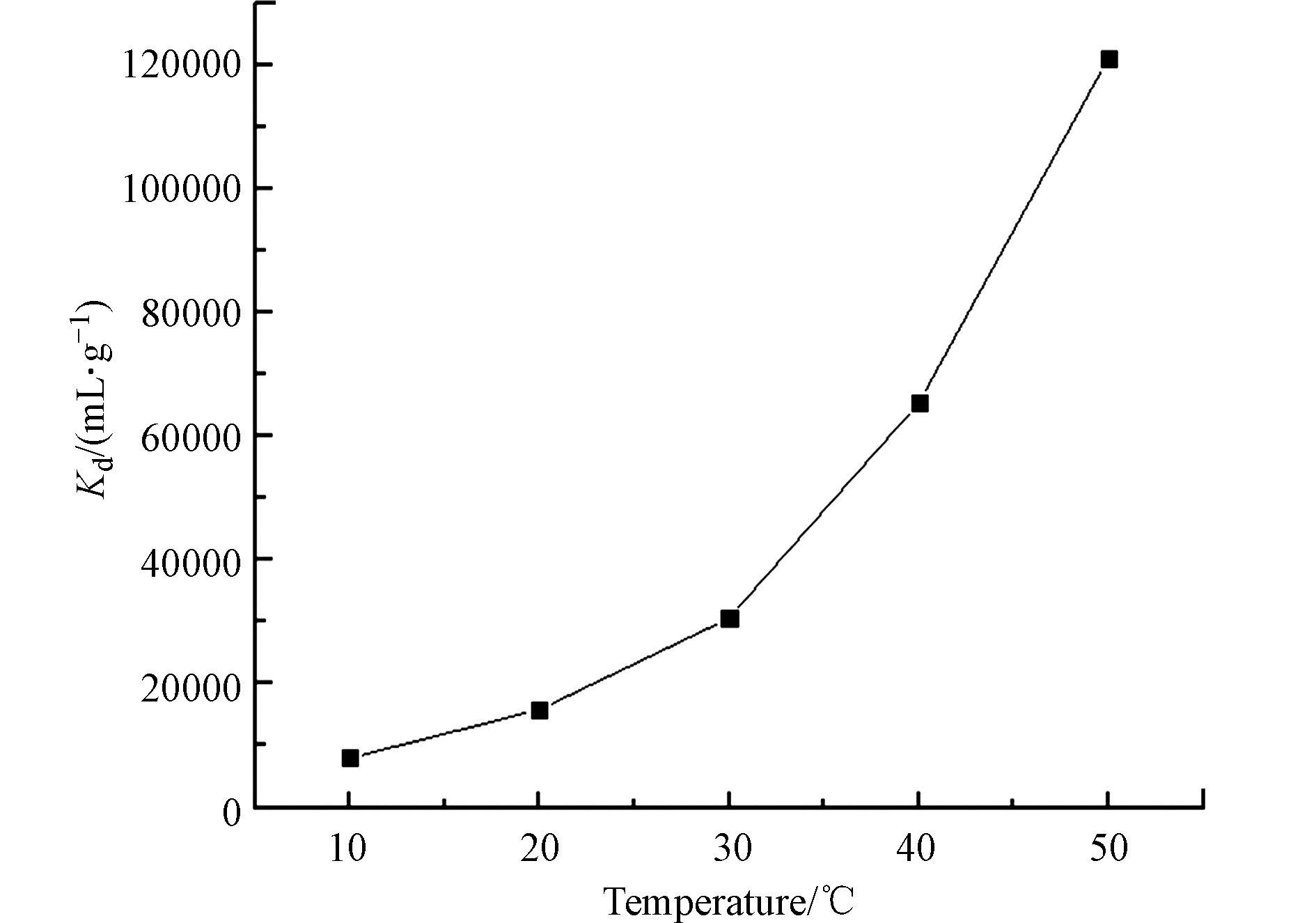
称取0.04 g 3-APTES改性粘土岩到离心管中,加入2 mL 的50 μg·mL−1的U(Ⅵ)标液并定容至8 mL。在pH 5的条件下,吸附120 min,考察不同温度对改性粘土岩对U(Ⅵ)的影响,结果如图7所示。由图7可知,分配系数受温度影响较大,随着温度的增高吸收分配系数急剧增加,吸附反应速率加快,说明此过程为吸热反应,升温增大3-APTES改性粘土岩对U(Ⅵ)的吸附量。由图7可知,温度20 ℃时分配系数较高,已达15800 mL·g−1,吸附效果良好,考虑实验条件,吸附可在常温下进行。
-
称取0.04 g 3-APTES改性粘土岩到离心管中,加入2 mL 的50 μg·mL−1的U(Ⅵ)标液并定容至8 mL。在pH 5的条件下,吸附120 min,考察不同离子对改性粘土岩对U(Ⅵ)的影响,结果如图8所示。由图8可知,Na+对吸附效果基本无影响,K+、Mg2+对吸附的抑制作用较弱,Ca2+抑制效果最显著。这是由于带正电荷的离子会在库仑力的作用下附着在3-APTES改性粘土岩的表面,使其表面可用于吸附的位点减少,从而抑制3-APTES改性粘土岩对U(Ⅵ)的吸附;同时Ca2+还会与溶液中的阴离子形成络合物,堵塞3-APTES改性粘土岩表面的吸附孔位,大大降低了吸附效果。阴离子中
${\rm{NO}}_3^{-} $ 、${\rm{SO}}_4^{2-} $ 对吸附行为基本没有影响,${\rm{HCO}}_3^{-} $ 、${\rm{CO}}_3^{2-} $ 的抑制效果明显,这是由于${\rm{CO}}_3^{2-} $ 水解会产生OH−,其能与UO2CO3配位生成$ {{\mathrm{U}\mathrm{O}}_{2}\left({\mathrm{C}\mathrm{O}}_{3}\right)}_{2}^{2-} $ 等,同时${\rm{CO}}_3^{2-} $ 还能与U(Ⅵ)络合,形成多种难以被吸附的化学物质,从而导致U(Ⅵ)的吸附量下降;而${\rm{HCO}}_3^{-} $ 能电离产生${\rm{CO}}_3^{2-} $ ,其抑制吸附的机理与${\rm{CO}}_3^{2-} $ 相同,但是${\rm{HCO}}_3^{-} $ 的水解能力较弱,产生的OH−较少,所以其对吸附的抑制效果弱于${\rm{CO}}_3^{2-} $ 。 -
本文以3-APTES改性粘土岩作为研究对象,通过XRF、FT-IR和SEM和等仪器对3-APTES改性粘土岩的化学成分及结构进行表征,以静态吸附的方式,探究了在不同条件下其对U(Ⅵ)的吸附影响规律。得出以下结论:
(1)3-APTES改性粘土岩化学组成复杂,Si、Ca、Al元素所占比重较大,其中SiO2含量最大;其表面空洞多,结构松散,利于吸附;3-APTES改性粘土岩的改性过程主要发生在C—O—H键、Si—O键和Si—O—Al键处。
(2) 3-APTES改性粘土岩吸附水相中U(Ⅵ)的平衡时间为120 min,最佳固液比为1:200,最佳U(Ⅵ)初始浓度为50 μg·mL−1。3-APTES改性粘土岩对U(Ⅵ)吸附能力随pH的增大先增强后减弱,吸附的最佳pH值为5;Ca2+、
${\rm{CO}}_3^{2-} $ 和${\rm{HCO}}_3^{-} $ 对吸附进程的抑制作用明显;K+、Na+、${\rm{NO}}_3^{-} $ 、${\rm{SO}}_4^{2-} $ 对U(Ⅵ)的吸附作用影响较弱。
3-氨基丙基三乙氧基硅烷(3-APTES)改性粘土岩的制备及其对铀U(Ⅵ)的吸附
Preparation of 3-APTES modified clay rock and its adsorption for U(Ⅵ)
-
摘要: 本文用3-氨基丙基三乙氧基硅烷(3-APTES)改性粘土岩,并通过X射线荧光光谱分析(XRF)、红外光谱仪(FT-IR)和扫描电子显微镜(SEM)对其进行了表征。结果显示,3-APTES改性粘土岩没有固定的表面结构,多为呈不规则多边型的薄片状晶体。以3-APTES改性粘土岩为吸附介质,探讨了反应时间、初始浓度、水相pH值、固液比、实验温度和离子种类等对该材料吸附U(Ⅵ)的影响。实验结果表明,pH为5、U(Ⅵ)初始浓度为50 µg·mL−1、固液比为1∶200时,经过120 min 3-APTES改性粘土岩对U(Ⅵ)的吸附达到平衡,吸附效果最佳。升温有助于提高其吸附性能;溶液中Ca2+、
${\rm{HCO}}_3^{-} $ 、${\rm{CO}}_3^{2-} $ 等3种离子极大的抑制了3-APTES改性粘土岩的吸附性能。-
关键词:
- 3-氨基丙基三乙氧基硅烷(3-APTES) /
- 改性 /
- 粘土岩 /
- 吸附 /
- U(Ⅵ)
Abstract: 3-aminopropyltriethoxysilane (3-APTES) modified clay rock was characterized by X-ray fluorescence spectrometry (XRF), infrared spectrometer (FT-IR) and scanning electron microscope (SEM). The characterization results showed that 3-APTES modified clay rock had no fixed surface structure and was mostly lamellar crystal with irregular polygonal shape. Using 3-APTES modified clay rock as the adsorption medium, the effect of reaction time, initial concentration, aqueous phase pH value, solid-liquid ratio, experimental temperature and ionic species on absorption of U(Ⅵ) were explored. The experimental results showed that when the pH was 5, the initial concentration of U(Ⅵ) was 50 µg·mL−1, and the solid-liquid ratio was 1∶200, the adsorption of U(Ⅵ) on the 3-APTES modified clay rock reached equilibrium after 120 minutes. The adsorption effect was the best. Warming helps to improve its adsorption performance; Ca2+,${\rm{HCO}}_3^{-} $ and${\rm{CO}}_3^{2-} $ ions in the solution greatly inhibited the adsorption performance of 3-APTES modified clay rock.-
Key words:
- 3-APTES /
- modification /
- clay rock /
- adsorption /
- U(Ⅵ)
-

-
表 1 粘土岩、 3-APTES改性粘土岩主要成分(%)
Table 1. Main components of claystone、3-APTES modified claystone(%)
成分Composition 岩样1
Claystone1岩样2
Claystone2岩样平均值
Average value改性岩样1
Modified claystone 1改性岩样2
Modified claystone 2改性岩平均值
Average valueSiO2 52.92 49.08 51.00 49.67 52.88 51.28 CaO 21.84 18.53 20.19 21.68 17.69 19.69 Al2O3 14.71 15.20 14.96 14.86 15.23 15.04 Fe2O3 5.82 5.93 5.88 5.66 5.98 5.82 K2O 3.09 2.56 2.83 2.70 2.88 2.79 MgO 2.75 2.88 2.82 2.82 2.68 2.75 Na2O 0.87 0.95 0.91 0.87 0.94 0.90 TiO2 0.63 0.68 0.66 0.63 0.67 0.65 -
[1] 曹存存, 吕俊文, 夏良树, 等. 土壤胶体对渗滤液中铀(Ⅵ)迁移影响的研究进展 [J]. 核化学与放射化学, 2012, 34(1): 1-7. CAO C C, LU J W, XIA L S, et al. Impact of colloids on migration of U(Ⅵ) from uranium waste rock leachate [J]. Journal of Nuclear Radiochemistry, 2012, 34(1): 1-7(in Chinese).
[2] 冯孝杰, 祁芳芳, 秦冰, 等. 几种阴离子对土壤中铀的浸取的影响 [J]. 核技术, 2013, 36(9): 42-46. FENG X J, QI F F, QIN B, et al. Influence of several anions on uranium desorption in U-contaminated soil [J]. Nuclear Techniques, 2013, 36(9): 42-46(in Chinese).
[3] 朱莉, 王津, 刘娟, 等. 铀尾矿库中铀, 钍及部分金属的模拟淋浸实验初探 [J]. 环境化学, 2013, 32(4): 678-685. doi: 10.7524/j.issn.0254-6108.2013.04.021 ZHU L, WANG J, LIU J, et al. Preliminary study on uranium, thorium and some other metals leached from uranium tailings under simulated natural environmental conditions [J]. Environmental Chemistry, 2013, 32(4): 678-685(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.04.021
[4] 方俊, 黄炜飞, 解小凡, 等. 榕树气生根对铀吸附性能的初步研究 [J]. 环境化学, 2016, 35(3): 555-561. doi: 10.7524/j.issn.0254-6108.2016.03.2015081101 FANG J, HUANG W F, XIE X F, et al. Sorption of uranium (Ⅵ) from aqueous solution by biomass of aerial root of Ficus microcarpa [J]. Environmental Chemistry, 2016, 35(3): 555-561(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.03.2015081101
[5] 王彦惠, 成建峰, 赵玉婷, 等. Fe3O4@SiO2-NH2磁性复合纳米材料对铀(Ⅵ)的吸附性能 [J]. 环境化学, 2019, 38(9): 2149-2158. doi: 10.7524/j.issn.0254-6108.2019011003 WANG Y H, CHENG J F, ZHAO Y T, et al. Adsorption of Fe3O4@SiO2-NH2 magnetic composite nanomaterials on Uranium (Ⅵ) [J]. Environmental Chemistry, 2019, 38(9): 2149-2158(in Chinese). doi: 10.7524/j.issn.0254-6108.2019011003
[6] YU G, ZHANG M G, CHENG X M, et al. Carbon nanotube versus graphene in modifying the electrical and optical properties of organic nonlinear optical material [J]. Applied Nanoscience, 2020, 10(6): 1893-1901. doi: 10.1007/s13204-020-01435-6 [7] 黄永锋, 许紫洋. 放射性废物处置研究进展 [J]. 化工设计通讯, 2017, 43(2): 105-122. doi: 10.3969/j.issn.1003-6490.2017.02.084 HUANG Y F, XU Z Y. Research progress of radioactive waste disposal [J]. Chemical Engineering Design Communications, 2017, 43(2): 105-122(in Chinese). doi: 10.3969/j.issn.1003-6490.2017.02.084
[8] ALI AYOUB, WILFRIED PFINGSTEN, LUCA PODOFILLINI, et al. Uncertainty and sensitivity analysis of the chemistry of cesium sorption in deep geological repositories [J]. Applied Geochemistry, 2020, 117(104607): 1-12. [9] 潘多强. U(Ⅵ)、Th(Ⅳ)和Eu(Ⅲ)在花岗岩组分矿物及膨润土上的吸附行为研究[D]. 兰州: 兰州大学, 2014. PANG D Q. Sorption of U(Ⅵ), Th(Ⅳ) and Eu(Ⅲ) on mineralogical compoenents of granite and benton [D]. Lanzhou: Lanzhou University, 2014(in Chinese).
[10] ZEYNEP M Ş, SELÇUK Ş, HALIL İ U, et al. Insight from adsorption properties of Xylidyl Blue embedded hydrogel for effective removal of uranyl: Experimental and theoretical approaches [J]. Polymer Testing, 2020, 88: 106566. doi: 10.1016/j.polymertesting.2020.106566 [11] 罗辉, 王驹, 谢敬礼, 等. 一种高放废物地质处置深钻孔布置方法[P]. CN110322982A, 2019-10-11. LUO H, WANG J, XIE J L, et al. A layout method of deep boreholes for geological disposal of high level radioactive waste[P]. Beijing: CN110322982A, 2019-10-11(in Chinese).
[12] 孙东阳, 赵帅维, 李洪辉. 高放废物深地质处置缓冲材料中(239)Pu核素迁移计算 [J]. 环境工程, 2018(36): 573-579. SUN D Y, ZHAO S W, LI H H. Conculation of nuclide migration model of 239 Pu in buffer material in deep geology disposal [J]. Environmental Engineering, 2018(36): 573-579(in Chinese).
[13] 马立平, 韩永国. 核废物地质处置缓冲/回填材料研究综述 [J]. 四川建筑, 2014, 34(2): 92-94. doi: 10.3969/j.issn.1007-8983.2014.02.037 MA L P, HAN Y G. Review of buffer / backfill materials for geological disposal of nuclear waste [J]. Sichuan Architecture, 2014, 34(2): 92-94(in Chinese). doi: 10.3969/j.issn.1007-8983.2014.02.037
[14] 杜浪, 李玉香, 马雪, 等. 偶氮胂Ⅲ分光光度法测定微量铀 [J]. 冶金分析, 2015, 35(1): 68-71. DU L, LI Y X, MA X, et al. Determination of micro uranium by arsenazo Ⅲ spectrophtometry [J]. Metallurgical Analysis, 2015, 35(1): 68-71(in Chinese).
[15] 杜作勇, 王彦惠, 李东瑞, 等. 膨润土对U(Ⅵ)的吸附机理研究 [J]. 核技术, 2019, 42(2): 22-29. DU Z Y, WANG Y H, LI D R, et al. Adsorption mechanism of U(Ⅵ) by bentonite [J]. Nuclear Techniques, 2019, 42(2): 22-29(in Chinese).
-








 下载:
下载: