-
近年来婴儿性早熟、雄鱼雌化病案频发,内分泌干扰物(EDCs)已成为全球广泛关注的一类重要的新兴污染物[1]。双酚A(BPA)作为一种典型的EDCs被广泛用于制造塑料制品[2],并且自2000年以来其消费量增长了10倍,达到每年300万吨,用存量每年人均增加0.8 kg[3]。由于塑料制品高温下的析出和工业废水的排放等原因导致BPA在环境中被不断检出[4]。有研究表明,我国沿海地区的32个取样点中均检测到BPA,冬季、夏季平均浓度分别为449.2、186.3 ng·L−1,由其引发的生态风险占总风险系数的71%以上[5]。由于传统的污水处理工艺对BPA的去除效果有限,尾水中残留的BPA易通过食物网和环境介质再次进入人和动物体内,可引发内分泌紊乱、生殖障碍、幼体变异等病变[6],寻求高效深度处理技术对人类的健康生存有着重大的意义。
近年来,高级氧化法(AOPs)凭借其反应速度快、降解能力强、对污染物没有选择性等独特优势脱颖而出[7],其中过硫酸盐氧化技术得到了广泛关注和研究。过硫酸盐(PDS, S2O82−)在热、碱、过渡金属、紫外光等外界能量的激活下,可以产生高氧化还原电位(2.4—3.1 V)[8] 和较长半衰期(4 s)的硫酸根自由基(
${\rm{SO}}_4^{-}\cdot $ )[9],对难降解有机物有很好的去除效果。在各类活化方式中,Fe2+/S2O82−体系无毒、低能耗、高效,受到了广泛关注[10]。相比于其它传统的AOP技术(如芬顿、光催化等),Fe2+/S2O82−的优势还体现在其在地下水和土壤原位化学修复(ISCO)领域具有更广阔应用前景[11-14]。过硫酸盐较过氧化氢稳定性更好,更适于场地修复过程试剂的运送及储存,且Fe2+/S2O82−体系无需额外能量投入,对水质透光性也无特殊要求。Dong等[15]研究发现,Fe2+/S2O82−体系对碘仿有较好的去除效果,当Fe2+/PDS的物质的量比为1∶5、PDS初始浓度为15 μmol·L−1、pH值为3.0,碘仿去除率最佳可达83%;Ren等[16]考察了Fe2+/PDS体系中pH值、水中无机阴离子等因素对磺胺嘧啶降解效率的影响,发现去除率高达到90%,${\rm{SO}}_4^{-}\cdot $ 为主要活性物质。目前,利用Fe2+/S2O82−体系来降解BPA鲜有报道,尤其是围绕Fe2+/S2O82−体系中关键性影响因素,如溶解氧、投加比及优化策略等方面的研究比较有限。本文拟采用Fe2+/S2O82−技术降解BPA,通过单因素变量法考察了Fe2+和PDS投加剂量及比例对BPA降解效率及动力学的影响;探讨了初始pH对体系效率的影响机制;分析了Fe2+的投加方式和PDS的投加方式对BPA降解动力学的影响及潜在的优化方式;确定了Fe2+/PDS体系对BPA降解起主要作用的活性物质;分析了BPA在体系中的矿化效率和降解路径。
-
双酚A(BPA,纯度≥99%)、PDS(K2S2O8,纯度≥99%)、叔丁醇(C4H10O,分析纯)、磷酸(H3PO4,色谱级)、5,5-二甲基-1-吡咯啉-N-氧化物(DMPO,分析纯)都购自于Sigma-Aldrich公司;硫酸亚铁(FeSO4·7H2O)、氢氧化钠(NaOH)、硫酸(H2SO4)均为分析纯,购自于上海国药集团化学试剂有限公司;甲醇(CH4O,色谱级)购于TEDIA公司。实验超纯水由超纯水机(Smart-DUV,上海和泰仪器有限公司,18 MΩ·cm)制得。
-
所有实验反应体系溶液总体积均为100 mL。先配置100 mL浓度为0.02 mmol·L−1的BPA反应液,向烧杯中加入一定量的PDS储备液,使用磁力搅拌器混合,搅拌速度恒定为500 r·min−1。使用1.0 mol·L−1 的H2SO4或NaOH调节体系至所需的初始pH值,同时加入一定量的亚铁储备液引发反应(投加PDS和Fe2+对溶液总体积的影响忽略不计),按照预设的取样时间准确取1.0 mL的样品于液相小瓶中,取样前在液相小瓶中加入0.1 mL的甲醇作为淬灭剂进行淬灭反应。
-
BPA浓度采用高效液相色谱(Dionex Ultimate 3000)测定,具体条件为:C18反相色谱柱(Thermo,5 μm×250 mm×4.6 mm),流动相采用0.1%磷酸水溶液和甲醇(V/V=30/70)的混合液,流速为1.0 mL·min−1,进样量为50 μL,色谱柱温度为35 °C,紫外检测波长为225 nm。
通过具有X波段场扫描的Bruker EMX-10/12设备检测电子自旋共振(ESR)信号。其参数设置为:中心场= 3480 G,扫描宽度= 200 G,微频率= 9.736 GHz,功率= 19.92 mW。DMPO用作捕获剂。
BPA的降解产物利用高效液相色谱和质谱联用(LCMS)进行分析(LTQ Orbitrap XL, Thermo Fisher),配有电喷雾离子源和静电场轨道离子阱质量分析器。用安捷伦Polaris 3 C18-A 色谱柱(5 μm particle size, 250 × 3.0 mm)进行产物分离,甲醇和水梯度洗脱,流速为0.5 mL·min−1。BPA及产物均用负模式检测。
-
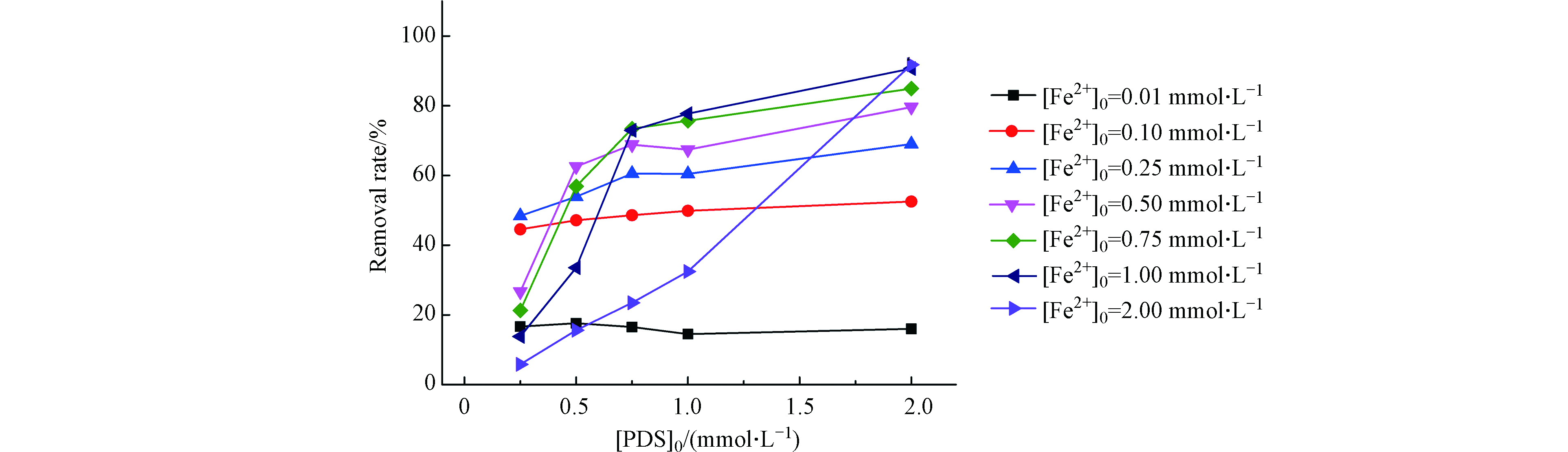
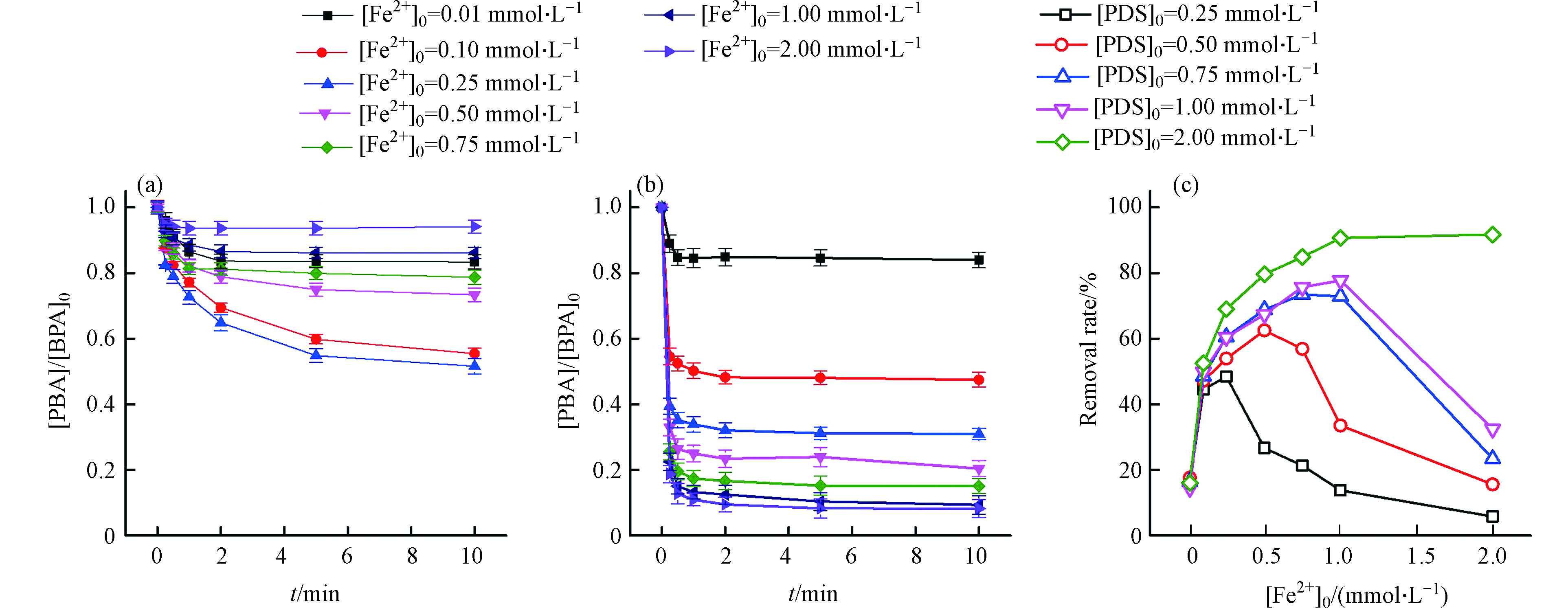
分别在不同PDS浓度水平下(0.25、0.50、0.75、1.00、2.00 mmol·L−1)考察了Fe2+的剂量对BPA降解的影响,以探寻PDS和Fe2+的相对浓度对体系效能的影响,结果如图1所示,图1a和1b分别为PDS浓度在0.25 mmol·L−1和2.00 mmol·L−1水平下BPA随着Fe2+浓度变化的降解动力学情况。
当PDS浓度较低时(图1a),随着Fe2+的初始浓度从0.01 mmol·L−1增到0.25 mmol·L−1,BPA降解率从16.65%增加到48.4%,然而Fe2+初始浓度继续增加到2.00 mmol·L−1,BPA降解率反降低到5.82%,BPA的总体去除率随Fe2+浓度升高呈现先促进后抑制的趋势。Fe2+可以活化PDS生成大量的
${\rm{SO}}_4^{-}\cdot $ 或·OH(反应式1—2)[17],所以适量增加Fe2+浓度可加速PDS的活化从而促进BPA的降解(PDS对BPA的直接氧化作用几乎检测不到);随着Fe2+浓度进一步增加,过量的Fe2+会与${\rm{SO}}_4^{-}\cdot $ 、·OH反应生成Fe3+(反应式3—4),与BPA形成自由基竞争抑制其降解[18],且高浓度的Fe2+导致自由基生成速率过快,自由基之间的自我淬灭效应也影响其利用效率(反应式5—6)[17]。当PDS浓度较高时(图1b),BPA降解速率随Fe2+浓度增加而增加,在Fe2+浓度为2.00 mmol· L−1时BPA有最大去除率91.77%。这说明铁浓度对Fe2+/S2O82−体系的影响与PDS的浓度水平也密切相关。图1c更清晰地展示了BPA的去除率随Fe2+浓度的变化趋势,最佳Fe2+浓度值随着PDS浓度水平的增高而逐渐增大,当PDS浓度分别为0.25、0.50、0.75、1.00、2.00 mmol·L−1时,Fe2+分别在浓度为0.25、0.50、0.75、1.00、2.00 mmol·L−1时取得最高的BPA降解效率。从图1c中可以较明显地看出,当在PDS足量的情况下,Fe2+与PDS的物质的量比约为1∶1时,BPA的降解效果最好。该现象与Tan 等[18]研究Fe2+/PDS体系降解敌草隆过程相似,提高Fe2+浓度可以提高${\rm{SO}}_4^{-}\cdot $ 生成速率,加快敌草隆的降解效率,然而随着Fe2+含量的进一步增加,敌草隆的降解速率没有增加,当Fe2+与PDS的物质的量比为1:1时敌草隆的降解效果最好。此外,BPA的降解动力学总体呈现两阶段特征,与均相Fenton体系类似,BPA在初期(< 2 min)呈现快速减少,之后进入缓慢降解阶段甚至停滞期,且这种两阶段的降解动力学在高PDS和Fe2+浓度条件下更为明显。这是因为反应刚开始时Fe2+活化PDS产生大量自由基快速降解BPA(第一阶段),而这个过程瞬间产生高浓度的SO4·−或·OH,自由基彼此发生淬灭反应以及大量的自由基使Fe2+过快的氧化,导致反应后期体系中缺少Fe2+,因此BPA不能有效地去除(第二阶段)。
-
考察了PDS的浓度对Fe2+活化PDS降解BPA的影响。图2显示了不同Fe2+投加浓度下,BPA的10 min去除率随着PDS浓度的变化情况。
当Fe2+浓度为0.01 mmol·L−1时,增加PDS浓度对BPA去除效果几乎没有影响,这是由于Fe2+浓度过低成为了反应(1)的限制性因素;当Fe2+浓度为0.10、0.25、0.50、0.75、1.00 mmol·L−1时,BPA的去除率随着PDS浓度增大而增大,但在PDS浓度较低时BPA去除率增加显著,浓度较高时BPA去除率增加缓慢,实验结果与王鸿斌等[10]研究亚铁活化过硫酸盐降解水中双氯芬酸钠一致。这也是因为过高浓度的PDS也会与SO4·−、·OH反应,减弱自由基的利用效率(反应式7—8)。当Fe2+浓度为2.00 mmol·L−1时,BPA去除率在PDS浓度较高时增加更快。结果表明,BPA的降解效率受PDS的浓度影响较大,在Fe2+浓度一定条件下,BPA去除率随着PDS浓度的增加而增加,因为Fe2+活化PDS时,PDS投加量越多,可以产生更多的自由基。然而Fe2+浓度在0.10 mmol·L−1到1.00 mmol·L−1时,过高的PDS浓度不能显著提高BPA的去除率,主要是因为Fe2+在活化PDS的过程中快速转化成了Fe3+ (反应式1、3、4),尽管PDS浓度增加,生成的Fe3+无法有效活化PDS产生自由基。而且,如上所述,自由基彼此发生淬灭反应减弱了自由基的利用效率(反应式5—6)。这也说明了,增加PDS浓度只有在Fe2+浓度充足的情况下才能对污染物降解起到明显的促进作用。当PDS的投加量一定时,与PDS浓度相同的Fe2+活化PDS时,BPA去除率最高,该现象与上述研究结果一致。
-
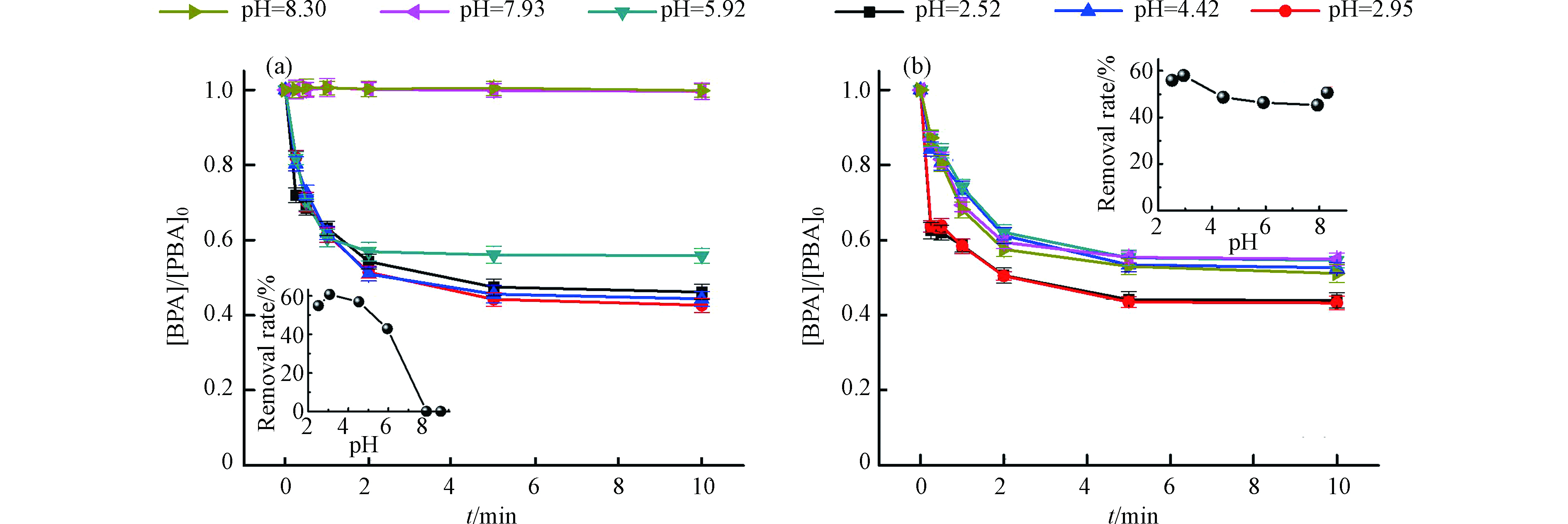
由于溶液pH值可以影响体系中Fe(Ⅱ)、Fe(Ⅲ)的形态分布,继而影响PDS的活化效率。本研究首先考察了初始pH值在2.5—8.5范围内对Fe2+活化PDS体系降解BPA的影响。如图3a所示,在Fe2+/PDS体系中,初始pH值为2.52、2.95、4.42、5.92、7.93和8.30时,反应10 min后,BPA的降解率分别为54%、57%、56%、44%、1%、1%。当溶液初始pH < 5时,BPA的降解效率较高,且在pH < 5范围内初始pH的变化对BPA降解率影响较弱。而当初始pH = 5.92时,BPA的降解率下降到44%;初始pH > 7后,BPA几乎不发生降解。其原因可能有两方面:(1)中性偏碱性条件下,溶液中氢氧根离子逐渐变多,与体系中的Fe2+发生络合,限制了Fe2+对PDS的活化作用;(2)Fe2+在碱性条件下更易被溶解氧氧化成为Fe(Ⅲ)(FeOH2+、Fe2(OH)24+、Fe(OH)2+、Fe(OH)3等)[19],从而导致体系中二价铁减少。根据Fe(OH)2的Ksp值(8.0 × 10−16)[20]计算可得,实验条件下([Fe2+]0 = 0.5 mmol·L−1)当pH ≈ 8左右时Fe2+开始沉淀,pH ≈ 8.9时Fe2+几乎完全转变成Fe(OH)2。而在pH为3—4左右时,Fe2+几乎没生成Fe(OH)2,仍保持离子形态的Fe2+能够高效活化PDS。过低的pH值导致BPA的降解略微降低,可能是由于调pH使用的稀硫酸,HSO4−在强酸性条件下(HSO4−的pKa为1.9)对羟基自由基有一定的淬灭作用(kOH = 1 × 106 L·mol−1·s−1)[21]。然而,实际实验中还发现,在pH值为7.93和8.30条件下,尽管根据理论计算Fe2+并没有完全生成Fe(OH)2,但BPA几乎不发生降解。这也说明pH对体系效率的影响不仅和上述原因(1)相关,还可能与原因(2)有关,即Fe(Ⅱ)在水中溶解氧存在的条件下向Fe(Ⅲ)转化,进而生成Fe(OH)3,而Fe(OH)3的Ksp值非常低(4.0 × 10−38)[20],在pH ≈ 3.2左右就沉淀完全,如果体系中的二价铁转化为Fe(Ⅲ)会严重影响体系中有效的铁含量。在实验中也发现,当pH越高时,体系中的深黄色悬浊物越多,说明体系中Fe(Ⅱ)向Fe(Ⅲ)的转化程度越高,Fe(OH)3沉淀越多,对体系活化PDS越不利。
为进一步验证pH对Fe2+/PDS体系的影响机制,对Fe2+/PDS体系中通入氮气,降低体系的溶解氧考察BPA的降解情况(图3b)。Fe2+/PDS体系中通入氮气后,初始pH < 6时,通入氮气的Fe2+/PDS体系中BPA的降解率与未通入氮气的Fe2+/PDS体系中BPA的降解率几乎相同。当初始pH > 6时,BPA的降解率从未通入氮气的1%提高到了54%。这说明当初始pH > 6时Fe2+/PDS体系污染物降解效率骤降主要由于溶解氧的存在加速了二价铁氧化,使体系中Fe2+的有效浓度大幅降低,导致BPA几乎不发生降解。
综上,酸性pH值对Fe2+/PDS体系降解BPA更有利,pH 3左右具有最高的BPA降解效率,中性及弱碱性条件下由于Fe2+与OH–的络合及体系中溶解氧加速Fe(Ⅱ)向Fe(Ⅲ)转化使得BPA的降解受限。
-
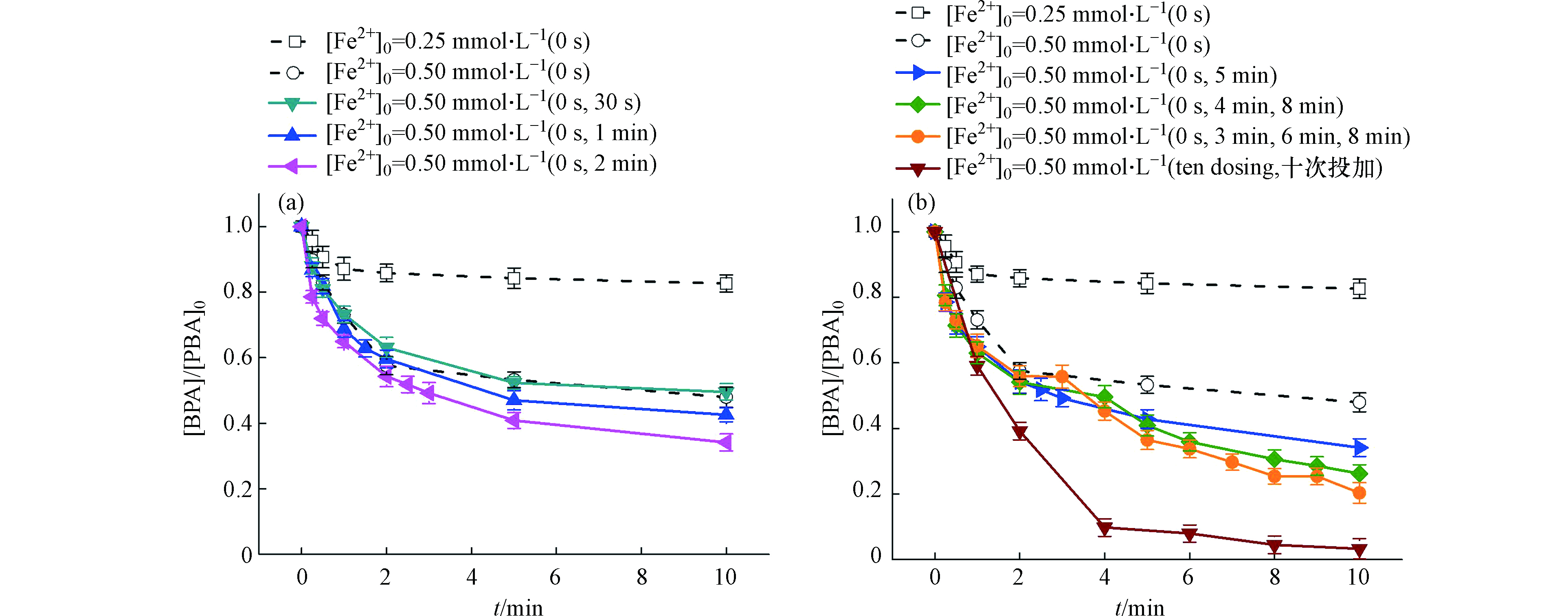
上述实验发现BPA的降解动力学呈现两个阶段,迅速降解阶段与平缓降解阶段。为分析BPA缓慢降解阶段的主要原因,进行Fe2+的分批投加实验,结果如图4所示。图4a为将Fe2+均分为两批次投加的实验,第一次均在反应开始时投加Fe2+,第二次分别在反应开始后的30 s、1 min与2 min时投加Fe2+,两次投加总和为0.5 mmol·L−1 (每次投加0.25 mmol·L−1的Fe2+)。与一次性直接投加0.5 mmol·L−1 Fe2+相比,反应开始后的30 s再次投加Fe2+时BPA降解率几乎不变,反应开始后的1 min再次投加Fe2+时BPA降解率提高了5%,反应开始后的2 min再次投加Fe2+时BPA降解率提高了13%。这说明适当延迟Fe2+的二次投加时间有助于更充分利用体系产生的自由基。但是分两次投加Fe2+总体来说对BPA的降解效率提高不显著,又进一步考察了多次投加Fe2+对BPA降解的影响。从图4b中可见,总量为0.5 mmol·L−1的Fe2+分批投加次数越多,BPA的降解越彻底,当分十次投加(每次投加0.05 mmol·L−1的Fe2+)时BPA去除率可达97%。这说明了Fe2+少量多次投加可以显著提高自由基利用率,也证明了Fe2+的自由基淬灭作用(反应式3—4)是影响体系自由基利用效率的关键性因素。这与Tan等[22]研究Fe2+活化过一硫酸盐体系降解染料过程中发现的规律一致,他们发现分三批投加Fe2+显著提高了体系染料的去除率。
-
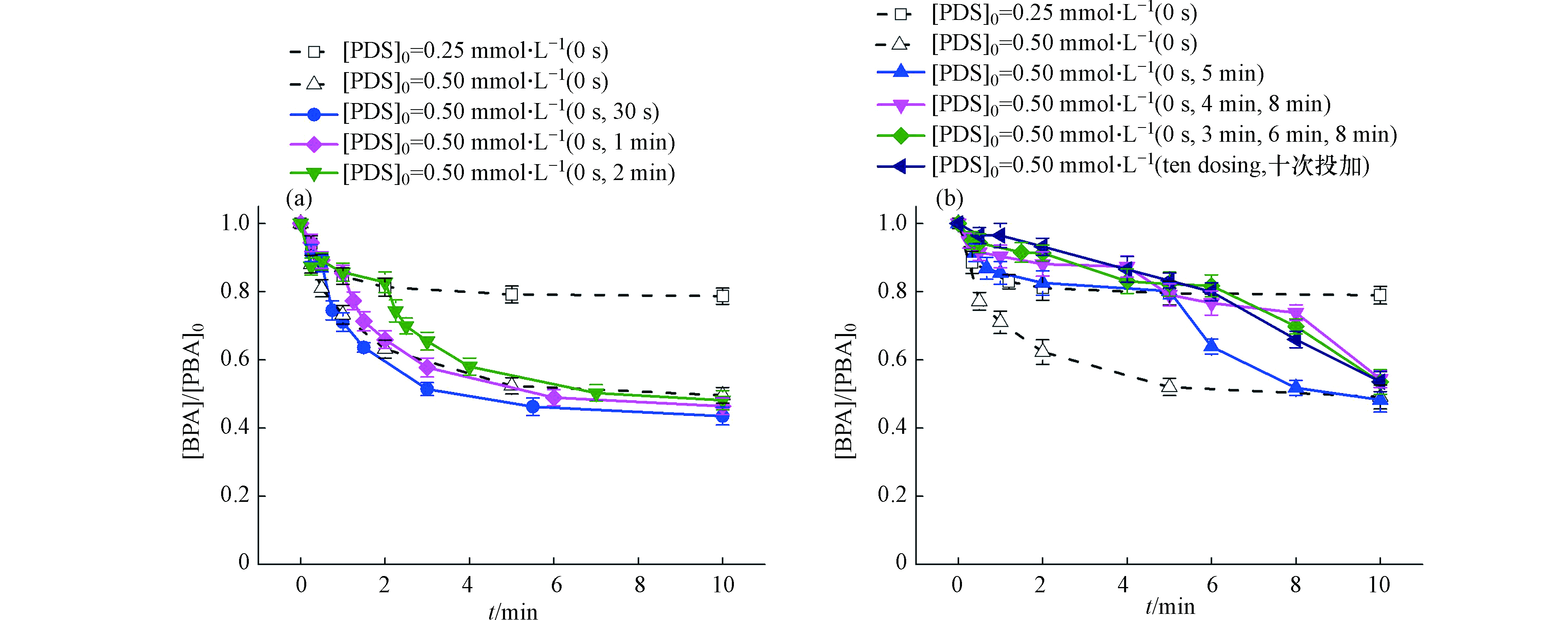
有研究显示在Fenton反应中采用分步投加H2O2的形式也可以有效提高污染物的去除效率[23],故进一步考察了Fe2+/PDS体系中PDS的投加方式对BPA降解的影响,结果如图5所示。图5a为将PDS平均分两份投加,投加总浓度为0.5 mmol·L−1。第一次在反应起始(0 s)投加0.25 mmol·L−1的PDS,第二次分别在反应开始后的30 s、1 min、2 min时投加另外0.25 mmol·L−1的PDS。实验结果表明,分批次投加PDS时,第一次投加PDS后BPA的变化趋势与一次性投加0.25 mmol·L−1PDS中BPA的降解动力学完全一致,而在第二次加入PDS后,BPA的降解速率有所提升,因为二次投加的PDS补充了体系中缺少的S2O82−,使得活化反应继续进行,持续产生
${\rm{SO}}_4^{-}\cdot $ 。反应10 min后,在两次投加的时间间隔为30 s、1 min、2 min的体系中,BPA的降解率分别达到了58%、54%、52%,均略高于一次性投加0.5 mmol·L−1 PDS体系中BPA的降解率,并且投加间隔时间为30 s体系中BPA降解率最高。根据2.2中的研究结果,PDS浓度越高体系会产生更多的活性氧,但是没有被污染物及时利用的活性氧会自我淬灭(反应式5—6),也可能和过量的PDS发生反应(反应式7—8),也会影响活性氧的利用效率,适量控制PDS的供给量有助于控制活性氧的产生速率,但两次投加的间隔越久,体系中Fe2+被消耗的越多,即使补充了PDS,也可能无法有效产生自由基。又进一步考察了多次投加PDS对BPA降解的影响。从图5b可以看出,均分两次投加PDS(0 s和5 min投加),前5 min内BPA的降解速率与投加0.25 mmol·L−1 PDS一致,5 min之后BPA降解速率迅速增加,最终与投加0.5 mmol·L−1 PDS达到了一致,降解程度与一次性投加0.5 mmol·L−1 PDS一致。增加PDS的投加次数(3次、4次、10次),BPA前期降解率显著减慢,因为前期PDS投加剂量越小,产生的自由基量少,不利于BPA降解。分多次投加PDS反应10 min 后BPA降解率趋于一致,但均略小于一次性投加0.5 mmol·L−1PDS时BPA的降解程度。结果表明,少量多次投加PDS对BPA的降解率影响不大。
-
为了探究Fe2+/PDS体系中产生的活性物质,采用甲醇(MeOH)和叔丁醇(TBA)作为自由基淬灭剂。羟基自由基与甲醇的反应速率常数(9.7×108 L·mol−1·s−1)和与叔丁醇的反应速率常数((3.8—7.6)×108 L·mol−1·s−1)在相同的量级上,硫酸根自由基与甲醇的反应速率常数((0.2—2.5)×107 L·mol−1·s−1)比与叔丁醇的反应速率常数((4—9.1)×105 L·mol−1·s−1)大得多[24],所以甲醇既是硫酸根自由基也是羟基自由基的淬灭剂,叔丁醇则对羟基自由基有淬灭作用。实验结果如图6a所示,当没有加任何淬灭剂的时候,BPA降解率为52%,当分别加入1 mmol·L−1的TBA与1 mmol·L−1的MeOH时,10 min反应后BPA降解率分别降低了9%和13%,说明了TBA和MeOH均对BPA降解有抑制作用,且甲醇的抑制作用更明显;当分别加入5 mmol·L−1的TBA、5 mmol·L−1的MeOH 时,10 min反应后BPA降解率分别降低了13%和20%,说明TBA和MeOH对BPA降解的抑制作用随着其浓度的升高而升高。实验结果进一步表明,Fe2+活化PDS过程中既产生了硫酸根自由基也产生了羟基自由基,并都对BPA降解起到了一定作用。硫酸根自由基主要由反应(1)产生,羟基自由基由硫酸根自由基部分水解形成,如反应(2)。
通过电子自旋共振(ESR)捕获技术进一步确定Fe2+/PDS体系中的活性物质。在Fe2+/PDS体系加入70 mmol·L−1的DMPO,得到如图6b所示谱图。根据谱图特征峰1∶2∶2∶1的比例和超精细耦合常数(αN=αHβ=14.9)确定为DMPO-OH[25],由
${\rm{SO}}_4^{-}\cdot $ 部分水解形成的·OH与DMPO反应形成,与抑制剂实验结果一致。谱图中没有看到硫酸根自由基的特征峰,其原因可能为${\rm{SO}}_4^{-}\cdot $ 可以与水发生反应转换生成·OH。 -
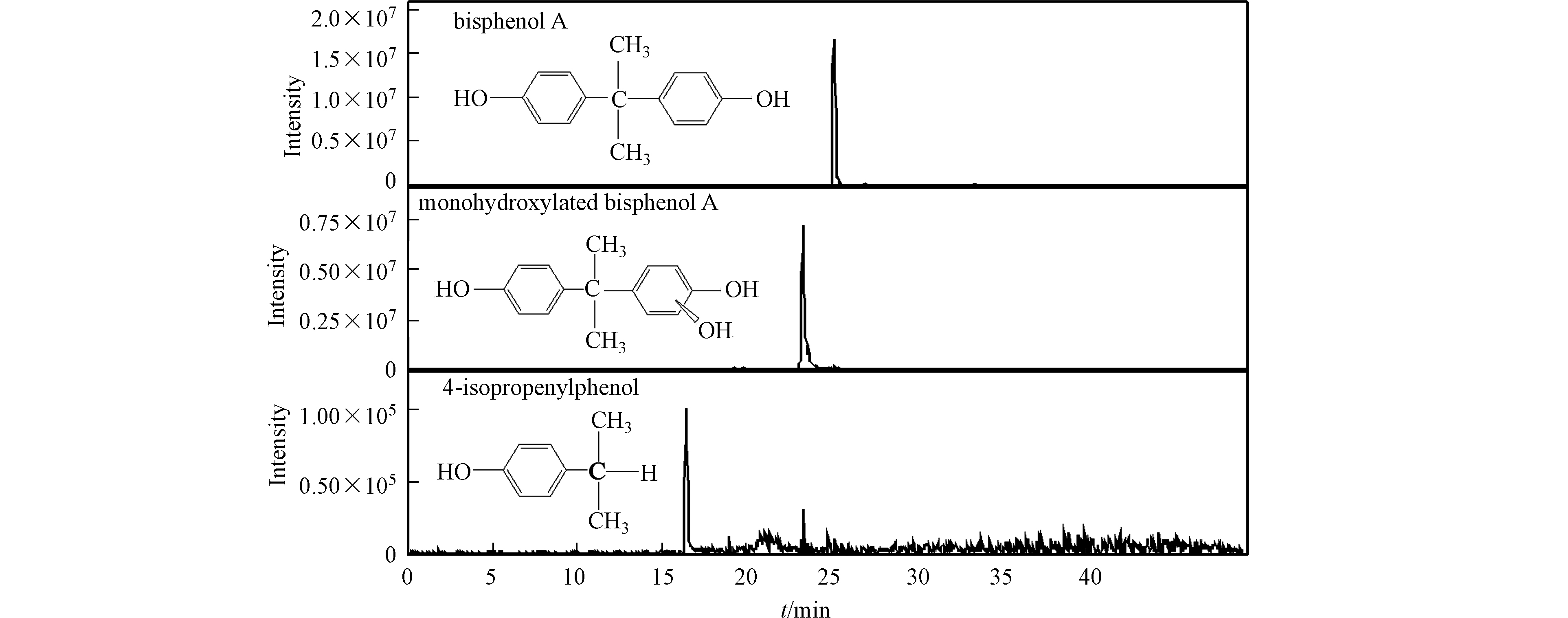
为提高BPA降解产物分析的准确性,将BPA的初始浓度提高至0.05 mmol·L−1进行分析,初始pH为3.0,Fe2+和PDS的初始浓度均为2.0 mmol·L−1。由于反应进行的较快,对反应15 s后的样品进行液质检测,以分析BPA的初期反应产物。从质谱的提取离子色谱图(EIC)(图7)中可见,在BPA的出峰位置(24.9 min)前有两个主要的初期降解产物,分别为23.5 min位置的羟基化双酚A([M-1]– = 243)和4-异丙基苯酚([M-1]– = 135)。其中,羟基化产物为·OH自由基主导的高级氧化体系的典型反应路径[26-27],由·OH攻击苯环产生。此外,SO4·−攻击苯环也可形成羟基化产物(反应式9—10)[28]。另外,BPA分子结构中的异丙基和苯环连接的C—C键由于具有较高的前沿电子密度易受到·OH和SO4·−的攻击[29],生成4-异丙基苯酚。由此推测,BPA的降解路径主要包括自由基对苯环的攻击和对异丙基和苯环连接的C—C键的攻击。
-
(1) Fe2+浓度的增加对BPA去除起先促进后抑制的作用;最佳Fe2+浓度值随着PDS浓度水平的增高而逐渐增大,在PDS充足的情况下当Fe2+与PDS的物质的量比约为1∶1时,BPA的降解效果最好。增加PDS浓度促进BPA的降解,当铁浓度一定时,PDS在低浓度范围内增加对BPA降解影响更明显。
(2) 初始pH值为2.5—8.5时,BPA在酸性条件下降解更快且在pH = 2.95时降解率最高;在中性偏碱性条件下,Fe2+因为与体系中的HO−发生络合、被水中溶解氧氧化成Fe(Ⅲ)而浓度下降抑制了BPA降解。
(3) 分批投加Fe2+的方式较分批投加PDS更能充分利用Fe2+/PDS体系产生的活性氧自由基,使BPA降解更彻底。
(4) 通过抑制剂和ESR分析验证了Fe2+/PDS体系中降解BPA的主要活性物质是硫酸根自由基和羟基自由基。
(5) 通过产物分析发现Fe2+/PDS体系对BPA的降解途径主要有自由基攻击苯环和自由基攻击异丙基和苯环连接的C—C键两方面。
Fe2+/S2O82-体系对双酚A的降解性能及优化
The performance and optimization of Fe2+/S2O82- process for bisphenol A degradation
-
摘要: 研究了Fe2+活化过硫酸盐(PDS, S2O82-)高级氧化技术对水中内分泌干扰物双酚A(BPA)的降解性能及优化方式,考察了Fe2+剂量、PDS剂量、初始pH、Fe2+和PDS投加方式对BPA降解的影响,确定了Fe2+/PDS体系中起主要作用的活性物质,分析了BPA的降解路径。结果表明,Fe2+剂量的增加对BPA的降解呈现先促进后抑制的作用,当Fe2+与PDS物质的量比为1∶1时BPA去除效果最好;BPA去除率随着PDS浓度的增加而增加。初始pH值为2.5—8.5时,BPA在酸性条件下能有效降解,且在pH = 2.95时降解率最高,主要原因是Fe2+与OH–的络合及溶解氧在中性及碱性条件下加速了二价铁的氧化。通过研究Fe2+和PDS分批投加对BPA降解的影响发现,均分两次投加Fe2+时适当延迟Fe2+的二次投加时间可促进BPA的降解,少量多次投加Fe2+可以显著提高自由基的利用率,而改变PDS的投加方式对BPA降解影响不大。通过分析甲醇及叔丁醇对BPA降解的抑制作用,结合电子自旋共振(ESR)确定体系中主要活性物质是硫酸根自由基和羟基自由基。利用液质联用技术对BPA的初期降解产物进行分析发现,BPA的降解主要为自由基攻击苯环和攻击异丙基和苯环连接的C—C键两种路径。Abstract: The advanced oxidation technology of persulfate (PDS) activation by Fe2+ for the degradation of endocrine disruptor bisphenol A (BPA) in water was investigated in this study. The effects of Fe2+ dosage, PDS dosage, initial pH, the modes of dosing Fe2+ or PDS on BPA degradation were examined systematically. The degradation pathways of BPA in Fe2+/PDS process was also examined. The reactive oxygen species (ROS) in Fe2+/PDS system were analyzed. Results showed that the increase of Fe2+ dosage could promote BPA degradation but overhigh concentration of Fe2+ could inhibit BPA degradation, and the best molar ratio of Fe2+ to PDS was found to be 1∶1. The removal rate of BPA increased with the increase of PDS dosage. When the initial pH was between 2.5—8.5, BPA could be degraded efficiently at acidic conditions than that at neutral or alkaline conditions, and the degradation rate was the highest at pH=2.95. The adverse effects of neutral or alkaline conditions were found mainly due to the enhanced oxidation of Fe2+ by dissolved oxygen. By evaluating the dosing modes of both Fe2+ and PDS, it was found that, when adding Fe2+ in two batches, moderate delay of Fe2+ addition was beneficial to the degradation of BPA. Adding Fe2+ in multiple batches could significantly improve the utilization of ROS. However, altering the dosing modes of PDS hardly had any effect on BPA degradation. Based on quenching experiments using methanol and tert-butanol and electron spin resonance (ESR) analysis, the main active substances were determined as sulfate radicals and hydroxyl radicals. Based on the analysis of primary degradation intermediates of BPA using LCMS, both hydroxylation of the benzene ring and attack on the connecting carbon were found to be the main reaction pathways.
-
Key words:
- bisphenol A /
- ferrous ion /
- persulfate /
- sulfate radical /
- advanced oxidation
-

-
图 4 (a)二次均分投加总量为0.5 mmol·L−1的Fe2+对BPA降解的影响;(b)多次均分投加总量为0.5 mmol·L−1的Fe2+对BPA降解的影响([BPA]0 = 0.02 mmol·L−1, [PDS]0 = 0.5 mmol·L−1, pH0 = 3.0)
Figure 4. (a) Effect of dosing 0.5 mmol·L−1 Fe2+ in two splits on the degradation of BPA;(b) Effect of dosing 0.5 mmol·L−1 Fe2+ in multiple splits on the degradation of BPA
图 5 (a)二次均分投加总量为0.5 mmol·L-1的PDS对BPA降解的影响;(b)多次均分投加总量为0.5 mmol·L-1的PDS对BPA降解的影响([BPA]0 = 0.02 mmol·L-1, [Fe2+]0 = 0.5 mmol·L-1, pH0 = 3.0)
Figure 5. (a) Effect of dosing 0.5 mmol·L-1 PDS in two splits on the degradation of BPA;(b) Effect of dosing 0.5 mmol·L-1 PDS in multiple splits on the degradation of BPA
-
[1] WANG R, MA X, LIU T, et al. Degradation aspects of endocrine disrupting chemicals: A review on photocatalytic processes and photocatalysts [J]. Applied Catalysis a-General, 2020, 597: 117547. doi: 10.1016/j.apcata.2020.117547 [2] 宋作栋, 仇雁翎, 张华, 等. 水体中双酚类物质的赋存现状及研究进展 [J]. 环境化学, 2020, 39(6): 1496-1503. doi: 10.7524/j.issn.0254-6108.2019081413 SONG Z D, QIU Y L, ZHANG H, et al. The occurrence and research progress of bisphenol analogues in aquatic environment [J]. Environmental Chemistry, 2020, 39(6): 1496-1503(in Chinese). doi: 10.7524/j.issn.0254-6108.2019081413
[3] JIANG D, CHEN W Q, ZENG X, et al. Dynamic stocks and flows analysis of Bisphenol A (BPA) in China: 2000—2014 [J]. Environmental Science & Technology, 2018, 52(6): 3706-3715. [4] 赵荧, 李媛, 陈永柏. 双酚A和酞酸酯对鱼类内分泌干扰效应及繁殖毒性研究 [J]. 水生态学杂志, 2017, 38(6): 1-10. ZHAO Y, LI Y, CHEN Y Y. Research progress of endocrine-disrupting effects and reproductive toxicity of Bisphenol A and phthalic acid esters on fish [J]. Journal of Water Ecology, 2017, 38(6): 1-10(in Chinese).
[5] LU J, ZHANG C, WU J, et al. Seasonal distribution, risks, and sources of endocrine disrupting chemicals in coastal waters: Will these emerging contaminants pose potential risks in marine environment at continental-scale? [J]. Chemosphere, 2020, 247: 125907. doi: 10.1016/j.chemosphere.2020.125907 [6] 熊美昱, 夏雨琪, 彭程. 典型类雌激素的降解方法及其影响因素研究进展 [J]. 环境化学, 2020, 39(3): 610-623. doi: 10.7524/j.issn.0254-6108.2019101303 XIONG M Y, XIA Y Q, PENG C. Degradation methods and factors of typical estrogen like substances [J]. Environmental Chemistry, 2020, 39(3): 610-623(in Chinese). doi: 10.7524/j.issn.0254-6108.2019101303
[7] HU L, WANG P, SHEN T. The application of microwaves in sulfate radical-based advanced oxidation processes for environmental remediation: A review [J]. Science of the Total Environment, 2020, 722: 137831. doi: 10.1016/j.scitotenv.2020.137831 [8] HOU X, ZHANG G, HUANG X, et al. Persulfate activation induced by ascorbic acid for efficient organic pollutants oxidation [J]. Chemical Engineering Journal, 2020: 382;122355. [9] 邓靖, 冯善方, 马晓雁, 等. 均相活化过硫酸氢盐高级氧化技术研究进展[J]. 水处理技术, 2015, 41(4): 13-19. DENG J, FENG S F, MA X Y, et al. Reach development in advanced oxidation processes based on homogeneous activation of peroxymonosulfate[J]. Water Treatment Technology, 2015, 41(4): 13-19(in Chinese).
[10] 王鸿斌, 王群, 刘义青, 等. 亚铁活化过硫酸盐降解水中双氯芬酸钠 [J]. 环境化学, 2020, 39(4): 869-875. doi: 10.7524/j.issn.0254-6108.2019040806 WANG H B, WANG Q, LIU Y Q, et al. Degradation of diclofenac by ferrous activated persulfate [J]. Environmental Chemistry, 2020, 39(4): 869-875(in Chinese). doi: 10.7524/j.issn.0254-6108.2019040806
[11] TSITONAKI A, PETRI B, CRIMI M, et al. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review [J]. Critical Reviews in Environmental Science and Technology, 2010, 40(1): 55-91. doi: 10.1080/10643380802039303 [12] 吴昊, 孙丽娜, 王辉, 等. 活化过硫酸钠原位修复石油类污染土壤研究进展 [J]. 环境化学, 2015, 34(11): 2085-2095. WU H, SUN L N, WANG H, et al. Persulfate In-situ remediation of petroleum hydrocarbon contaminated soil [J]. Environmental Chemistry, 2015, 34(11): 2085-2095(in Chinese).
[13] 张易旻, 陈铮铮, 陈昆柏, 等. 氯代有机物污染土壤高级化学氧化修复技术研究进展 [J]. 环境化学, 2019, 38(3): 480-493. doi: 10.7524/j.issn.0254-6108.2018050203 ZHANG Y M, CHEN Z Z, CHEN K B, et al. Remediation of chlorohydrocarbon contaminated soil by advanced oxidation technologies: A review [J]. Environmental Chemistry, 2019, 38(3): 480-493(in Chinese). doi: 10.7524/j.issn.0254-6108.2018050203
[14] 谷得明, 郭昌胜, 冯启言, 等. 基于硫酸根自由基的高级氧化技术及其在环境治理中的应用 [J]. 环境化学, 2018, 37(11): 2489-2508. doi: 10.7524/j.issn.0254-6108.2018012102 GU D M, GUO C S, FENG Q Y, et al. Sulfate radical-based advanced oxidation processes and its application in environmental remediation [J]. Environmental Chemistry, 2018, 37(11): 2489-2508(in Chinese). doi: 10.7524/j.issn.0254-6108.2018012102
[15] DONG Z J, WANG F, SONG X L, et al. Fe (II)-activated persulfate oxidation effectively degrades iodoform in water: Influential factors and kinetics analysis [J]. Arabian Journal of Chemistry, 2020, 13(4): 5009-5017. doi: 10.1016/j.arabjc.2020.01.023 [16] REN H Y, ZHANG H M, JIA Q Z, et al. Oxidation of sulfamerazine with Fe2+/Persulfate system: Effects of inorganic anions and degradation mechanism based on independent reaction [J]. Fresenius Environmental Bulletin, 2020, 29(2): 1096-1103. [17] WANG S, WU J, LU X, et al. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways [J]. Chemical Engineering Journal, 2019, 358: 1091-1100. doi: 10.1016/j.cej.2018.09.145 [18] TAN C, GAO N, CHU W, et al. Degradation of diuron by persulfate activated with ferrous ion [J]. Separation and Purification Technology, 2012, 95: 44-48. doi: 10.1016/j.seppur.2012.04.012 [19] RAO Y F, QU L, YANG H, et al. Degradation of carbamazepine by Fe(II)-activated persulfate process [J]. Journal of Hazardous Materials, 2014, 268: 23-32. doi: 10.1016/j.jhazmat.2014.01.010 [20] 贾之慎. 无机分析化学[M]. 2版. 杭州: 高等教育出版社, 2015: 409. JIA Z S. Inorganic and Analytical Chemistry[M]. Second Edition. Hangzhou: Higher Education Press, 2015: 409(in Chinese).
[21] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (OH/O) in aqueous-solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [22] TAN C, DONG Y, SHI L, et al. Degradation of orange II in ferrous activated peroxymonosulfate system: Efficiency, situ EPR spin trapping and degradation pathway study [J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 83: 74-81. doi: 10.1016/j.jtice.2017.11.014 [23] CHU W, CHAN K H, KWAN C Y, et al. Degradation of atrazine by modified stepwise-Fenton's processes [J]. Chemosphere, 2007, 67(4): 755-761. doi: 10.1016/j.chemosphere.2006.10.039 [24] LI Y, BAGHI R, FILIP J, et al. Activation of peroxydisulfate by ferrite materials for phenol degradation [J]. Acs Sustainable Chemistry & Engineering, 2019, 7(9): 8099-8108. [25] YAN S, SHI Y, TAO Y, et al. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4/Fe2O3 heterojunction [J]. Chemical Engineering Journal, 2019, 359: 933-943. doi: 10.1016/j.cej.2018.11.093 [26] XU L J, CHU W, LEE P H, et al. The mechanism study of efficient degradation of hydrophobic nonylphenol in solution by a chemical-free technology of sonophotolysis [J]. Journal of Hazardous Materials, 2016, 308: 386-393. doi: 10.1016/j.jhazmat.2016.01.075 [27] XU L J, CHU W, GRAHAM N. A systematic study of the degradation of dimethyl phthalate using a high-frequency ultrasonic process [J]. Ultrasonics Sonochemistry, 2013, 20(3): 892-899. doi: 10.1016/j.ultsonch.2012.11.005 [28] SHARMA J, MISHRA I M, DIONYSIOU D D, et al. Oxidative removal of bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway [J]. Chemical Engineering Journal, 2015, 276: 193-204. doi: 10.1016/j.cej.2015.04.021 [29] DU J, BAO J, LIU Y, et al. Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A [J]. Journal of Hazardous Materials, 2016, 320: 150-159. doi: 10.1016/j.jhazmat.2016.08.021 -



 下载:
下载: