-
早在1998年,Havers等[1]对德国农村和城市采集的颗粒物分析表明,大气颗粒物(particulate matter,PM)中超过10%的物质属于腐殖酸和富里酸这类大分子,样品通过离子交换树脂分离后采用傅里叶红外光谱(fourier transform infrared spectroscopy, FTIR)、核磁共振(nuclear magnetic resonance, NMR)等对其结构进行了表征,认为其结构和土壤、水生系统中的腐殖酸和富里酸相似,但分子量更低并富含脂肪族和碳水化合物,称为类腐殖酸(humic-like substances,HULIS)。HULIS是一类弱极性、水溶性、相对分子量较高(一般为200—500 Da,也有的分子量比200低)的有机混合物[2-3],由多个共轭结构和含有羧基、羟基和羰基等极性官能团的脂肪族侧链的芳香结构组成[4-6],但与陆/水生腐殖质相比,HULIS的表面活性更高、酸性更小、分子量和芳香性更低[2]。HULIS普遍存在于雾、云、积雪和PM中[7-10],是水溶性有机物的重要组成部分[11]。
大气中HULIS来源广泛,包括生物质燃烧[12-13]、机动车尾气排放[14]、海上迁移[15-16]和二次反应[17-18],其中生物质燃烧[19-21]和二次反应(气相和液相)是最主要的两个来源[22]。在模拟生物质燃烧排放的木质素化合物(3,5-二羟基苯甲酸)与羟基自由基(·OH)在云中的反应时,发现产物与腐殖酸和黄腐酸的UV-vis光谱基本一致,表明前体物在大气液相中通过自由基聚合为大分子量的HULIS[23];本课题组开展四乙基酚大气液相氧化实验,也测定出产物中含有HULIS,导致吸光性增强[24];Lin等[21]研究表明,受生物质燃烧影响的区域大气HULIS浓度明显升高。Limbeck等[25]认为目前已经确定的HULIS来源—生物质燃烧并不能完全解释欧洲HULIS水平,他提出了在硫酸气溶胶催化下异戊二烯或萜类通过非均相低聚反应形成HULIS;汽车或船舶机油内燃几乎不产生一次源HULIS,但排放的芳香烃(如甲苯、二甲苯等)和硝酸盐可通过二次反应而形成HULIS,因此冬季夜间硝酸盐与有机物液相氧化是HULIS形成的一个重要来源[26]。Iinuma等[27]研究了在酸性粒子存在下,α-蒎烯的臭氧化形成SOA,并经测定结果显示,颗粒相有机物增加了40%,且形成了具有HULIS性质的化合物。研究表明[28-29],硫酸和有机化合物的低聚反应形成高分子量的有机硫酸盐和有机硫酸酯(一类重要的HULIS)。最近有研究[13]提出废物焚烧排放大量的多环芳烃(polycyclic aromatic hydrocarbons, PAHs),也是形成HULIS的来源之一。
HULIS在大气过程中起着重要作用,例如形成云凝结核(cloud condensation nuclei,CCN)、通过吸湿生长增加单个粒子的反照率[30-32],及吸收紫外线从而引发辐射转移和光化学反应等[33]。近年来, 受污染的空气中某些物质通过细胞诱导产生活性氧化物种(reactive oxygen species,ROS),包括H2O2、O2-•、·OH和RO·,从而导致肺炎、哮喘等疾病率升高的研究引起了广泛关注[34]。很多研究[35-36]围绕HULIS或者HULIS-过渡金属螯合产生ROS,从而导致健康影响风险的作用机制开展。
尽管大气颗粒物中HULIS的研究比较多,但关于其特性、在大气转化中的作用以及对人体健康的影响机制等还存在很多不确定性,因此研究大气中的HULIS具有重要的意义。Zheng等[11]综述了HULIS的测定方法,重点关注了外场观测大气颗粒物中HULIS浓度时空变化及来源。本文总结了HULIS的分离和提取方法、测定方法、细胞毒性机制、吸光特性、光敏性等,重点关注了气溶胶致毒机理、ROS的测定方法和HULIS的毒理性,从而为读者全面了解大气PM中HULIS的特性提供参考。
-
气溶胶中HULIS分离提取方法包括固相萃取法(solid phase extraction,SPE)[37]、毛细管电泳法(capillary electrophoresis,CE)[7]、离子交换色谱法(ion exchange chromatography,IEC)[4]、反相高效液相色谱法(reversed-phase liquid chromatography,RPLC)[38]、分子排阻色谱法(size exclusion chromatography,SEC)[39]等。这些方法原理不同,则提取出的HULIS的化学性质和结构(如相对分子质量、极性和酸度等)稍有差异。此外,HULIS的分离和提取方法、测定方法的选择与研究目的(包括吸湿增长性、表面张力、吸光性等)有关,不同的研究目的关注测定的HULIS物种不同。
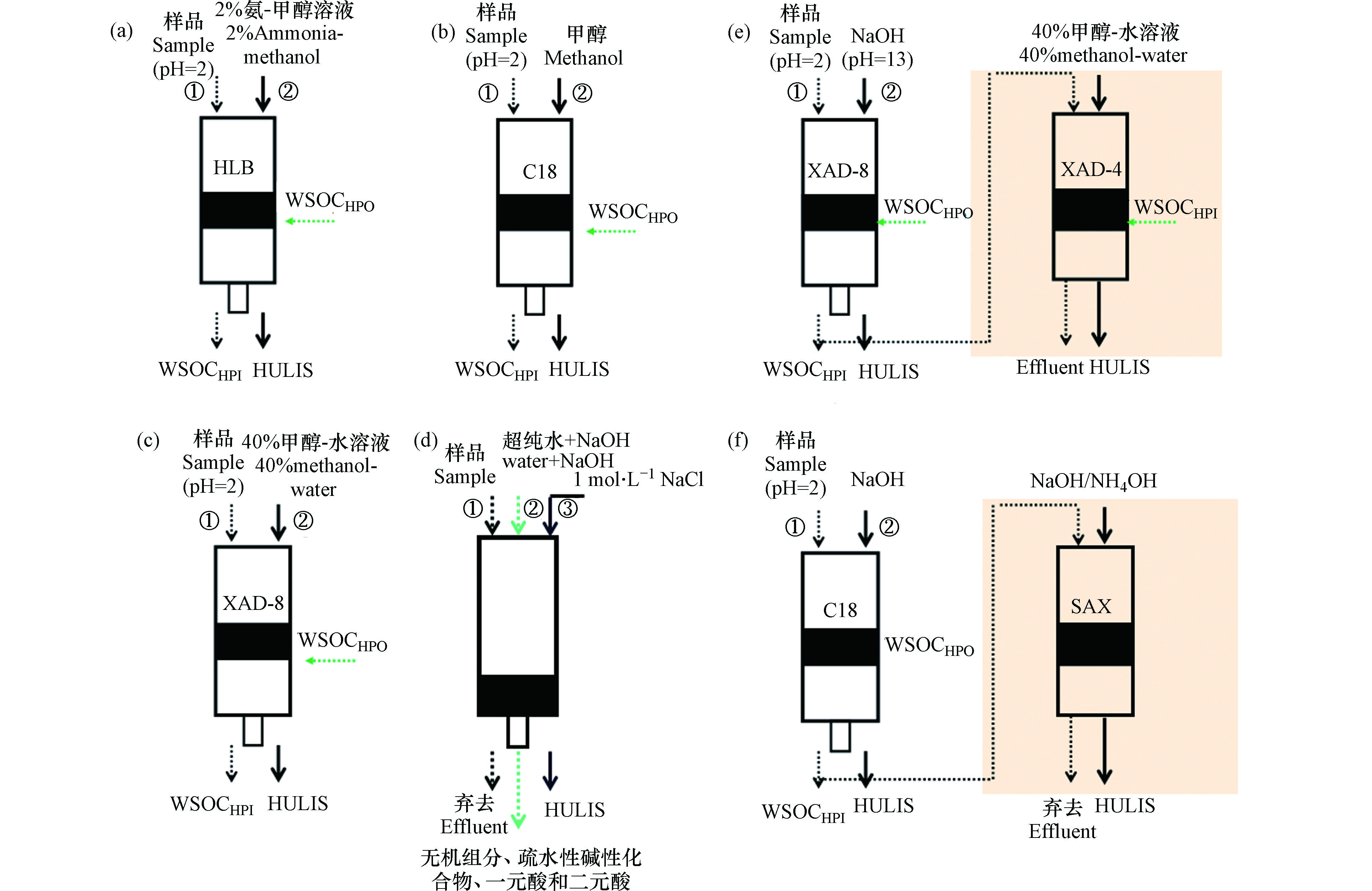
上述方法中,SPE法由于具有操作简便、选择性强等优点,是目前应用最广泛的的方法。常用的SPE柱包括C18(高度疏水性、非极性)[40]、HLB(亲水亲脂平衡、反相吸附剂)[18]、XAD(非离子大孔树脂)[41]、DEAE(弱碱性阴离子交换树脂)[1]和SAX(强碱性阴离子交换树脂)[42]等5种,约占大气PM中HULIS提取和定量分析的80%[43]。前3种利用其疏水性分离HULIS,而DEAE和SAX利用其多酸性结构进行分离。总的提取步骤:提取液(通常为水溶液)调节pH值后通过SPE柱截留水溶性有机物中疏水性有机物(用WSOCHPO表示)到吸附柱上,流出的为无机水溶性组分和亲水性有机物(用WSOCHPI表示),然后用甲醇、碱性的甲醇(例如2%氨-甲醇溶液)或NaOH淋洗出吸附柱上的部分WSOCHPO, 即为HULIS。为了提高提取效率,也有采样两种不同SPE柱串联提取的方法。WSOCHPI通常为低分子量(小于3或4个碳原子)的脂肪酸或羰基化合物、有机胺和糖类;WSOCHPO通常为高分子量(长链)的脂肪酸或羰基化合物、芳香酸、苯酚、有机硝酸盐、环酸和富里酸[44]。日本名古屋大学课题组[45] 最近开展了进一步将HULIS通过碱性甲醇和甲醇两种溶液分步提取出HULIS-n(中性)和HUILS-a(酸性)的研究。
HULIS含有羧基、羟基、芳香基等官能团,当样品中其他化合物含有这些官能团,就会影响提取的HULIS的物质组分和浓度。HULIS的提取效果受SPE柱类型、样品成分、提取条件和淋洗液组分的影响。图1概括了4种不同SPE柱提取HULIS的步骤,不同SPE吸附柱结构和适用范围见表1。
C-18柱:样品需酸化(pH=2),HULIS和弱极性、低分子量的有机化合物保留在柱子上[10],无机盐和强极性的高分子量化合物如糖类等被有效分离出去。该方法提取的HULIS可能包括低极性的有机化合物(如芳香酸、苯酚)、大于4个碳原子的一元酸、二元酸,硝基PAHs和苯醌等[46]。
HLB柱:填料为亲水性N-乙烯基吡咯烷酮和亲脂二乙烯基苯,样品需酸化(pH=2),HLB柱可以截留更多的大分子有机物,例如芳香环和极性官能团的化合物(如—COOH,—C=O)也会被截留(导致HULIS浓度偏高),但含有一个或多个羟基的化合物,如愈创木酚、左旋葡聚糖、苯酚等截留率很低。Lin等[18]用HLB法测试大气中存在的有机化合物的保留率,这些物质包括分子量小且具挥发性的有机酸(如乙酸、草酸等)、非挥发性大分子化合物(糖类)以及芳香族化合物,其中苯甲酸和邻苯二甲酸的保留率最高,这归因于苯环和—COOH官能团对吸附剂的亲和作用。
XAD(或DAX)柱:非离子型大孔树脂,常用XAD-8和XAD-4提取HULIS[41]。XAD-8保留组分主要为疏水结构的酸性化合物组成(如芳香酸、酚类、有机硝酸盐和长链脂肪酸),但仅芳香酸才可被后续NaOH溶液洗脱出来,即分离出HULIS主要为芳香酸,小部分的弱吸附有机物(如辛二酸、3,5-二羟基苯甲酸和邻苯二甲酸)被一起洗脱废弃掉导致提取的HULIS浓度偏低[47]。为了得到组分比较全面的HULIS,有研究[47]采用XAD-8/XAD-4两步法联合提取HULIS,即通过XAD-8的淋洗液再次通过XAD-4柱子,分离出小分子亲水性HULIS(例如糖类、胺类和相对分子量小的脂肪族一元酸和二元酸)。
DEAE柱:不需要进行pH调节,但需要高离子强度试剂例如NaCl溶液作为洗脱剂,仅3,5-二羟基苯甲酸干扰测定[43],因此DEAE法对大气中HULIS的分离具有更高的选择性,更有利于紫外吸收强、高分子量、高芳香度的化合物的提取。
-
HULIS测定方法很多,包括光谱法(UV-vis,荧光,NMR,FTIR等)和非光谱法(HPLC-MS-MS、HPLC、GC-MS等)[48],目前应用于大气中HULIS测定的主要是光谱法、蒸发光散射(evaporative light scattering detector,ELSD)HPLC法。还有用有机碳分析仪(TOC)测定得到HULIS-C浓度后再乘以转换因子的换算法。无论用那种方法分析,都需要标准品进行定量,目前常用苏万尼河黄腐酸(SRFA,国际腐殖物质协会)和Pahokee泥腐殖酸(PPHA)[42]。值得说明的是,实际大气中HULIS比标准SRFA的分子量低、氧化性更低且含更多的脂肪族化合物[3]。
-
UV-vis法和荧光法[49-50]是依据分离出的HULIS的吸光度和发射荧光特性与HULIS的标准物质(如SRFA)的比较而间接得到HULIS的浓度。在UV-vis测定中[1],重点关注λ=250、285、300、350、375 nm处的吸光度变化, 采用350 nm和450 nm吸光度比值(E350/E450=5.9)可以作为小分子HULIS的指示;也可以通过对比HULIS样品与标准浓度的SRFA在波长250 nm、285 nm处吸光度得到样品的HULIS浓度,借助280 nm处的摩尔吸收系数ε(mol-1· L· cm-1),通过M=534+1.33ε还可计算出HULIS的平均分子量[51]。荧光法通过特征激发波长(λexc)和发射波长(λem)范围: λexc=330—350 nm, λem=420—480 nm (富里酸);λexc=250—260 nm, λem=380—480 nm (HULIS)来表征HULIS特性和强度[52]。荧光强度与物质的吸光性、与金属离子配位的程度、分子量和分子构象等有关,低分子量的HULIS显示高的荧光强度[2]。这两种光谱法都无法获得HULIS的化学结构信息,且没有明显的峰值吸收和发射波长,定量不够精确。1H-NMR[53-54]和FTIR可以获得HULIS的化学结构以及官能团的信息;1H-NMR分析表明,H原子优先存在多聚糖(约40%)和脂肪糖(约50%)中,只有小部分(11.5%)存在芳香烃中;Samburova等[40]采用优化的1H-NMR分析了大气PM1中HULIS的官能团,发现二元酸、丙烯酸、苯酚、脂肪族等官能团浓度为9×10-11—6×10-8 mol·m-3,占总HULIS的14%, 冬天由于木材燃烧HULIS中芳香基浓度高于夏天。
光谱方法简单快捷,但无法从分子水平得到HULIS中化合物的准确组分,适合光学特性等定性分析,不适用于准确定量分析。
-
ELSD-HPLC法是近年来提出的一种定量检测方法[55],相比于其它方法,测定的HULIS浓度更准确,因为其响应与光学性质无关(发色团的类型和数量)。ELSD本身可以作为一些最常用的基于极性的分离方法(例如SPE)的补充步骤,分离过程中未分离的疏水的低分子物质在实际检测之前,可以通过ELSD中包含的热探测器部分(即蒸发管)蒸发[56],避免了溶剂的干扰和因温度变化引起的基线漂移,即使用梯度洗脱不会产生基线漂移,检测基线平稳。该方法特别适用于像HULIS这样未知结构的化合物的检测。Lin等[18]采用蔗糖, NAFA和SRFA的3种标准物质,用ELSD检测其响应与浓度的关系,发现尽管3种物质的结构不同,但响应面积与浓度的对应关系(都取对数作图)完全一致,说明ELSD是一种可靠且适合于未知结构化合物检测的准万能的质量检测器。其检出限为7 ng·μL-1,不确定度为10%,因此只要被测组分的挥发性低于流动相即可检测,但ELSD的检测效率随被测组分的挥发性增强而显著降低。Emmenegger等[55]首次提出采用ELSD-HPLC法测定大气PM中的HULIS浓度,通过C18柱固相萃取HULIS组分后用该方法测定了瑞士苏黎世城郊PM1中HULIS日平均浓度为1.1 μg·m-3。为了解HULIS中化合物的详细结构,很多研究[48]采用LC-MS/MS对水溶性、高分子量的HULIS中的官能团(脂肪族、芳香化合物、二元酸、有机硫化合物、碳水化合物)进行定量分析。
由于用的是单一标准物质进行比较而测定的,因此得到的HULIS浓度并不是准确的,只是与腐殖质相当,而且测定的HULIS仅体现出与腐殖质酸性、极性和结构特征相似[57],不能代表整体HULIS水平,所以仍需探索更好、更具代表性的标准物质。HPLC-ESI-MS测定发现HULIS的分子量分布,最高不超过4000 Da,大气气溶胶中HULIS的分子量一般不超过400 Da;Kiss等[58]也采用质谱的方法估算出大气PM中HULIS的平均分子量为215—345 Da。
-
TOC法测定的是HULIS-C的浓度,需乘以转换因子(1.81[18]—2.5[59])来估计HULIS浓度,这种方法非常适合大气野外观测HULIS浓度的测定,测定结果与OC和EC等碳质组分可以直接比较而用于气溶胶的源解析。其中OC的检出限为0.11 μgC·m-3,EC的检出限为0.05 μgC·m-3,OC的精密度为4%,EC为7%。转换因子受采样地点、采样时间和季节等的影响,城市是1.81[18],对于农村是1.93[5]。研究表明[60],大气颗粒物种HULIS-C约占WSOC的15%—60%,但有时也高达70%以上。本课题组[61]测定了常州夏季和冬季PM2.5中HULIS的昼夜吸光特性,冬季HULIS-C/WSOC比值为50%左右[62]。表2汇总了部分国内外城市和农村、海洋、森林等测定的HULIS方法和浓度。由表可知,PM2.5中HULIS浓度变化范围较广(超过两个数量级),从0.08 μg·m-3到11.8 μg·m-3不等,但在大多数情况下,HULIS浓度在1—7 μg·m-3之间。总体来说,采用HLB和C18两种柱子提取分离的方法多。青藏高原HULIS-C/WSOC比值高。另外,生物质燃烧PM中的HULIS-C约占总WSOC的60%[21],高于煤燃烧HULIS-C/WSOC占比(45.5%)[63]。当然,HULIS-C/ WSOC占比还受季节、昼夜的变化。Lin等[64]对珠江三角洲区域农村地区采集的PM2.5颗粒中HULIS浓度为5.9—18.1 μg·m-3;Kanpur的Indo-Gangetic平原冬季大气观测数据表明[65],白天HULIS-OM(17.3—38.0 µg·m-3)占PM10中总OM的20%—30%,夜晚HULIS-OM浓度(19.8—40.6 µg·m-3)稍高但在总OM中占比较低,HULIS-C/WSOC白天和夜间分别为0.37和0.45。
-
HULIS是一类弱极性的混合有机物,目前关于其分子和元素组成等化学特性的研究还较少。分子组成测定通常是按照1.1中提取后,采用N,O-双(三甲基硅基)三氟乙酰胺(bis(trimethylsilyl)trifluoroacetamide,BSTFA)烷基化,再用GC-MS测定羧基、羟基化合物。Ma[74]等采用HLB柱固相萃取后,仅仅用GC-MS对中国香港郊区夏季大气PM2.5中HULIS中的11种芳香酸(羟基苯甲酸、邻(-间-对)苯二甲、苯三甲酸、香草酸、丁香酸)、2种硝基苯酚(硝基邻苯二酚、硝基苯酚)和3种脂肪酸(己二酸、庚二酸、壬二酸)共16种含羧基和羟基的化合物进行了鉴定和定量,但这不能代表整体HULIS分子量水平。Chen等[45]采用FTIR和高分辨四级时间飞行质谱仪表征了PM2.5中中性和酸性HULIS(HULIS-n和HULIS-a)的化学结构和分子量分布,发现HULIS-n富含脂肪结构和C9-C18的羟基化合物,而HULIS-a含有大量的低分子量羧酸和醇(C4-C10)。Stone等[48]LC-MS-MS基于不同结构的HULIS保留时间不同测定了PM2.5中HULIS的脂肪类、羧酸类、糖类、有机硫酸酯等的含量。Lin等[64]采用正、负电喷雾离子(ESI)耦合高分辨质谱(ESI-UHRMS)研究了珠江三角洲农村地区丰收季节PM2.5中HULIS的元素组成,即CHN、CHON、CHO、CHOS、CHONS等元素组成的物种及元素比(O/C、N/C),检测出有机硫酸酯、硝氧有机硫酸酯即为硫酸根与VOCs光氧化反应产物,这个研究也说明HULIS中含有大量的羰基化合物和硝基化合物,即包含大量孤对电子而容易与重金属元素配合,对大气化学和金属的氧化还原反应产生影响,进而对健康有害。因此,生物质燃烧气溶胶的WSOC中检测到含金属的有机化合物[75]。也有采用X射线光电子能谱(XPS)测定HULIS的元素的报道[54]。
-
PM2.5对人体健康有负面影响,不仅会引起呼吸系统疾病[76-77],而且对神经系统、心血管系统、内分泌系统等皆有不良影响。大气颗粒物中产生ROS的物质主要为有机物和重金属离子。毒性研究表明,重金属离子、有机物(例如HULIS)产生ROS通过激酶级联、转录因子和炎症介质的释放,引起体内细胞大分子(DNA、脂类)破坏[78]。在细胞中,O2被还原为超氧化物(O2-•),然后O2-•在超氧化物歧化酶(SOD)的作用下歧化为O2和H2O2,H2O2在谷胱甘肽过氧化物酶和过氧化氢酶作用下维持在较低浓度。在生理浓度下,活性氧化物种(ROS)包括H2O2、O2-•、·OH和RO·,是介导细胞生长、迁移和分化的信号分子,而ROS积累过度引起机体氧化应激效应,可能引起细胞死亡与凋亡并导致机体受损。研究[79-80]发现,吸入污染颗粒后,部分供电子有机物产生稳态ROS,从而引发佐剂效应(adjuvant effect)[81],导致肺或者心脏的而使器官受损[82]。H2O2和有机过氧化物是大气PM中最重要的ROS[83]。颗粒物的致毒机理还不明晰,但颗粒物中某些反应产生的ROS浓度过高,随后氧化反应引发机体损失是大家公认的一种可能的机制,因此颗粒物对人体健康的影响可以用氧化潜势(oxidative potential, OP)来表征。汽车尾气、生物质燃烧、香烟烟雾、烹饪烟雾等产生的PM中的重金属浓度越高,ROS浓度越高,对人体衰老和致癌影响越大[80]。一项柴油机颗粒物特性研究[84]表明其在细胞中ROS浓度高是导致癌症等疾病的重要原因,与柴油车产生更多的9,10-菲醌或者1,4-萘醌等PAHs有关。
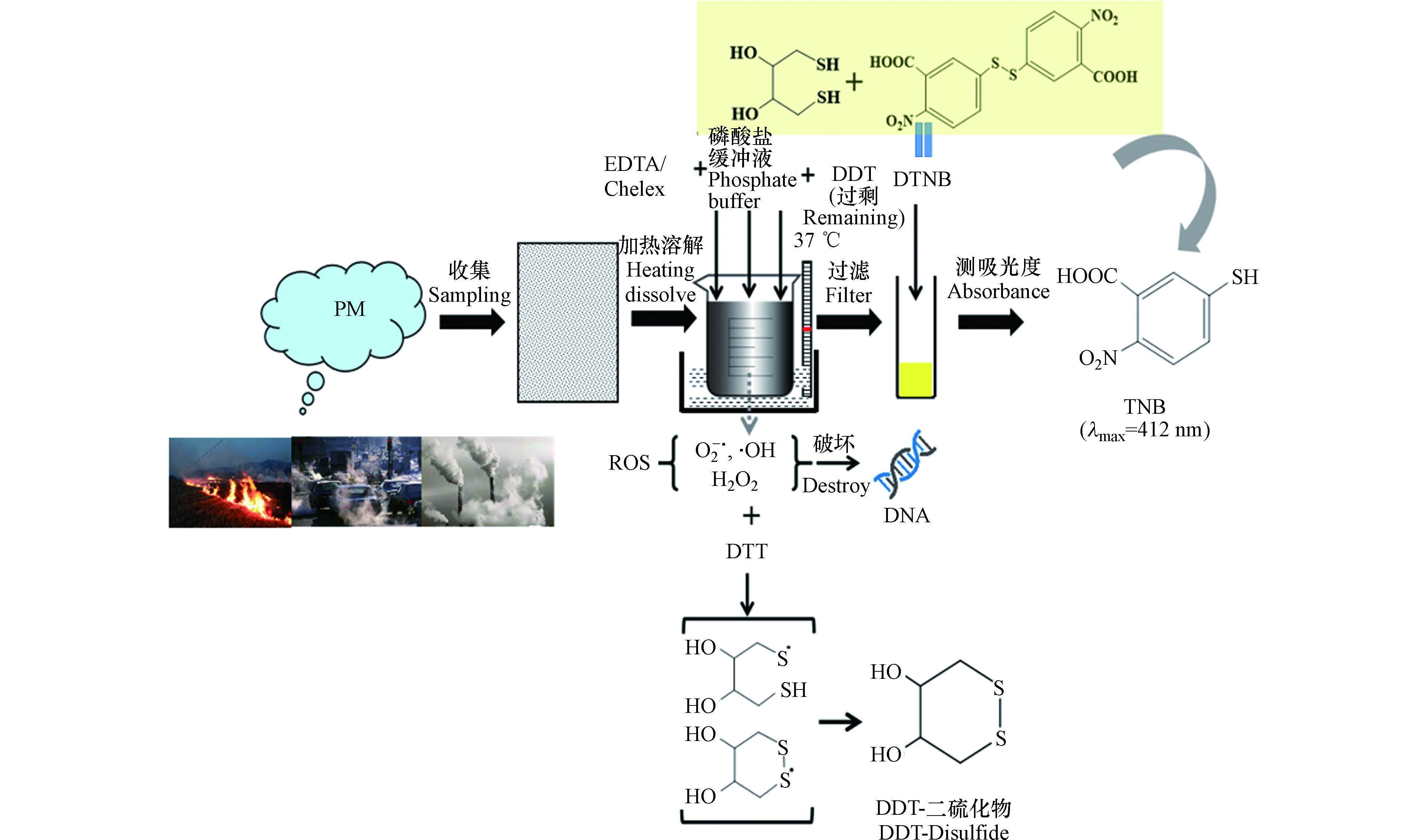
由于PM-ROS浓度低、寿命短,很难直接测定其准确浓度,因此常通过生物或者化学中的逆氧化消耗法测定。常用的方法包括[85]:二硫苏糖醇(dithiothreitol,DTT)法[86]、电子自旋共振(electron spin-resonance spectroscopy,EPR)法、二氯荧光素(2',7'-dichlorofluorescein,DCFH)荧光法[87-88]、无细胞抗坏血酸法[89-90]和模拟肺液法(surrogate lung fluid,SLF)[91], 其中DTT是一种用于评价颗粒的氧化潜能和毒性的定量方法,因其操作简单、实验设备低廉而被广泛用来评价大气颗粒物的OP[86]。DTT测定ROS的基本原理见图2 [92]。
PM中的一些氧化还原性化合物(例如苯醌、萘醌等PAHs)能有效催化DTT(电子供体)电子转移到溶解O2产生O2-•(DTT+2O2→DTT-二硫化物+2O2-•)[93],O2-•进一步形成H2O2等ROS,体系中剩余的DTT与5,5'-二硫代双(2-硝基苯甲酸)(DTNB)反应生成2-硝基-5-硫代苯甲酸(TNB),412 nm处TNB有特征吸收峰,因此定时向其中加入DTNB测溶液吸光度,即可得到剩余DTT浓度,然后计算出DTT的消耗速率[94](单位时间单位重量PM或者采样体积下DTT消耗量,以DTTm或DTTv表示, 单位为pmol·min-1μg-1或nmol·min-1m-3),该值被认为与人类暴露的健康影响直接相关。DTTm或DTTv值越高,说明ROS产生速率越高,PM2.5对健康的影响越大。很多研究[95-96]通过关注DTT消耗速率与PM2.5中的OC、重金属、PAHs的关系建立PM2.5中组分对健康的影响贡献。研究表明[97],颗粒物粒径越小,细胞组织越容易渗透,导致ROS浓度越高;颗粒物老化程度越高,氧化潜能越大。
DCFH通常用于在线实时观测PM中ROS浓度,此方法通常联合采用PILS[88]或者喷雾洗涤器[86]在线收集PM2.5,DCFH测定中ROS的浓度,检测线可以达到nmol级。结果表明ROS的范围为81.1—668.1 nmol·L-1或1.3—19.1 nmol·m-3,取决于PILS中气流的停留时间(停留时间越长,成核等气粒转化越彻底)[88];采用DCFH测定美国佐治亚州亚特兰大的城市和农村PM中的ROS浓度分别为0.26 nmol ·m-3H2O2和0.14 nmol ·m-3H2O2。无细胞抗坏血酸测定法定量PM的氧化潜能(OPAA),即PM氧化产生ROS能力,对于大气颗粒物,这个值通常在0.2—2.0 nmol·min-1·m-3 [89]。这种方法可以建立PM的化学特性和毒理性之间的联系,发现PM中Cu和苯醌对OPAA贡献比较大。
-
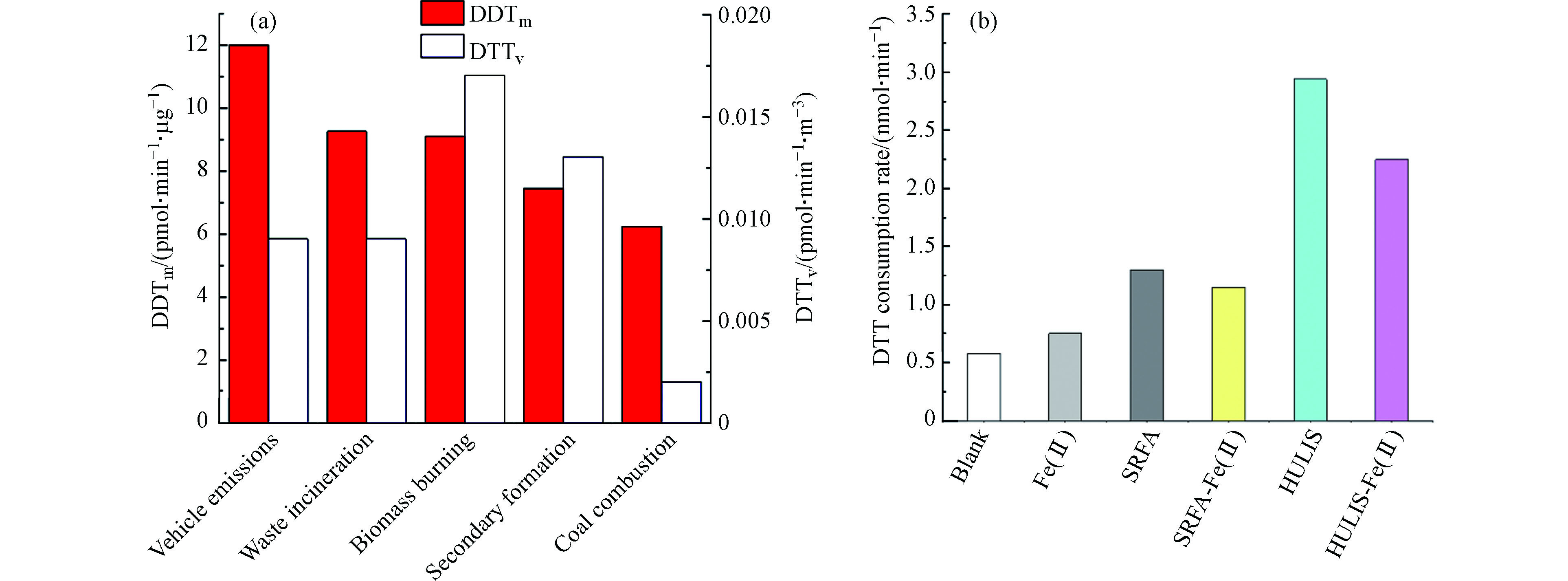
HULIS作为大气PM的重要组成部分,其毒理性近年才引起重视。前人的研究[13]发现HULIS因其含有可逆的氧化还原位点,作为电子载体催化细胞中(用DTT代替细胞中的抗坏血酸等)ROS的形成[79],以醌类化合物为例,与DTT的反应通过先形成半醌化合物(semi-Q),然后电子转移O2形成O2-• ,见方程式(1—3)。Lin等[36]2011年首次研究了PM中HULIS催化的ROS产生并用DTT测定ROS,研究表明弱极性的HULIS占所有WSOC的毒性贡献的80%,DTT消耗速率与HULIS浓度正相关;随后Lin和Yu[86]采用无细胞DTT法测定,通过使用强的鳌合剂(二乙基三胺五乙酸,DTPA)来抑制金属的干扰从而得到HULIS介导的ROS,并研究了不同初始浓度DTT对测定HULIS和HULIS-Cu(Mn)产生的ROS的影响,发现高的初始DTT浓度测定出的ROS浓度更高,说明ROS测定方法还需进一步研究。Verma等[98]的研究表明PM中的弱极性有机物(例如HULIS属于疏水)对PM的ROS起决定作用。之后研究了与PM2.5中ROS相关的组分特性,表明高氧化性OA和生物质燃烧OA(弱极性部分便即为HULIS)对ROS产生的贡献最大[99],见图4(a)。Gonzalez等[100]测定出美国Fresno生物质燃烧气溶胶(含57%HULIS)中含有大量的·OH。DTT活性在不同季节有显著变化[101],取决于HULIS的来源不同。冬季DTT活性最高,原因是冬季生物质燃烧是HULIS的主要来源,夏季则因二次有机气溶胶的形成也表现出较高的DTT活性。
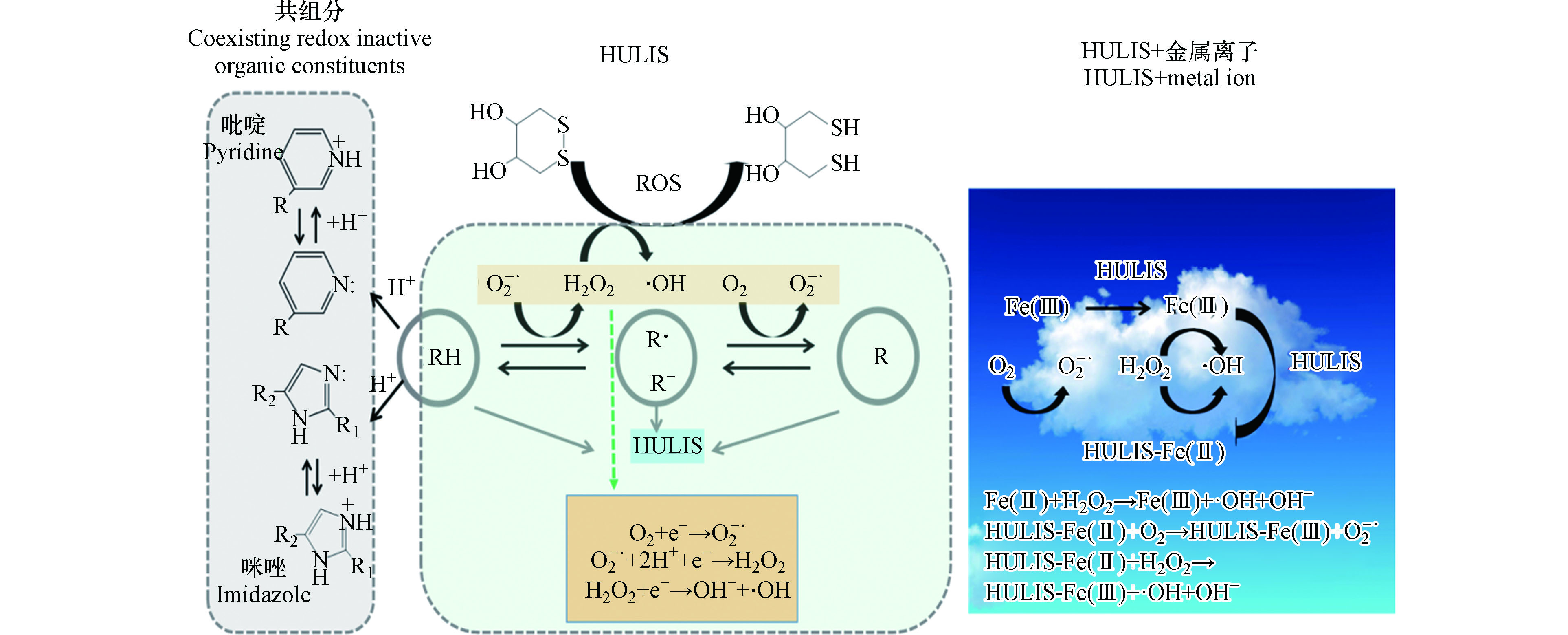
尽管上述研究都证实HULIS确实可产生ROS,但其机理还不是很清晰。PM中HULIS与重金属都能产生ROS,但机理显著不同,当两者同时存在时,还存在耦合或者抵消效应。笔者概括了单独HULIS、金属和其它共存有机物存在时,HULIS催生ROS的机理,见图3。图3以苯醌(RH)为例,描述通过基于苯醌自由基(R•和R-)参与的氧化还原反应产生O2-•,再通过H+和e-添加,形成H2O2、·OH等ROS。
HULIS中含有大量的酸,强大的可电离位点(又称为配体,主要为苯酚基、羧基等)。醌类作为一种HULIS,能在细胞内催化生成ROS,然而,醌的大气浓度通常非常低(通常小于2000 pg·m-3)[92],使得难以解释其对环境PM的DTT活性的主要贡献。为此,HULIS催化形成ROS的能力还受共存组分的影响,比如生物质燃烧产生的一些VOCs经过进一步氧化形成具有氧化还原活性的产物,这些产物随HULIS提取到溶液中从而消耗DTT。还有因为硫酸盐、硝酸盐和臭氧与HULISws成正相关,因此HULIS可能由较低DTT活性的SOA组成,比如有机硫酸盐、有机硝酸盐。例如Li等[84]比较臭氧老化的和新鲜的柴油废气颗粒的DTT活性,发现两者都有较高的DTT活性,但臭氧老化后DTT活性更高,因为柴油废气产生的氮氧化物被氧化形成硝酸盐,而硝酸盐又进一步与废气中的VOCs反应生成有机硝酸盐,从而提高了DTT活性。McWhinney等[102]提出在有氧化还原活性的有机气溶胶中,除了测量到的醌类化合物,还有更多的化合物推动了PM的DTT活性。生物质燃烧产生的含氮有机物中的—NO2基团可以通过促进电子转移,从而促进ROS的形成。同时生物质燃烧产生的生物碱也可促进ROS形成。Dou等[35]用DTT法测定吡啶、咪唑及其烷基衍生物的氧化还原活性,发现这些物质虽然本身具有较小的氧化还原活性,但却能增强醌类化合物和HULIS催化生成ROS,且其增强作用与在溶液里的含量和溶液碱度成正比。来自香港一项PM2.5中HULIS的研究[103]也表明生物质燃烧产生的HULIS的DTT活性最高,而二次反应形成的HULIS的DTT最低。图3以吡啶、咪唑等供电子化合物为共组分,描述其通过提供e-促进ROS的形成的机理。
大气中重金属离子可以直接通过电子转移产生ROS[78],以大气PM中最常见的Fe2+为例,Fe2++O2→Fe3++ O2-•;Fe2+O2-•+2H+→Fe3++H2O2。HULIS的存在可以使反应变得复杂,进而改变颗粒物的物理化学特性。当HULIS和金属同时存在时,HULIS可以与氧化性的金属离子产生稳定的配合物[104],随后经历光化学反应形成金属有机酸例如Fe-草酸。例如HULIS作为强大的螯合剂可通过促进金属离子(以大气中最常见的Fe(Ⅲ)为例,雾水中浓度大概200 μmol·L-1[105])在云滴中的溶解而提高其浓度,并在酸性、黑暗条件下HULIS将Fe(Ⅲ)还原为Fe(Ⅱ),随后Fe(Ⅱ)发生芬顿反应形成·OH,为此大气夜间HULIS的存在导致OH浓度升高。很多研究表明[106],Fenton中加入HULIS(例如苯醌、有机酸)等,可以加速Fe3+向Fe2+的转化,从而加快夜间Fenton降解大气液相中有机物的速度,这些都表明HULIS可以促进ROS的形成。
大气中HULIS的存在,也可以与氧化-还原活性的金属离子(Fe2+、Mn2+、Cu2+等)配位,例如HULIS与Fe螯合后,进一步与PM中的O2、H2O2反应,导致•OH、O2-•等浓度升高,因此HULIS与Fe(Ⅱ)螯合可以加速O2转化为O2-•和H2O2分解为•OH,体系中•OH浓度升高[100]。HULIS与金属离子的反应可以使金属迁移到水、土壤和大气中,反过来,也会增加HULIS进入大气液相的可能性,促进大气液相氧化反应。总之,金属离子与HULIS络合是评估金属毒性、生物可用度和迁移转化时需考虑的重要因素。HULIS与金属反应形成ROS而引起的细胞死亡率与几个因素密切相关,如HULIS的金属结合能力[107]、络合时间[107]、pH值[108]和Fenton反应[109]等。HULIS与金属的络合能力主要来源于羧基和酚基,通过去质子化而与金属离子结合,结合能力随pH的降低而减小,因为氢离子会竞争阳离子与HULIS结合。
很多研究[104]采用SRFA、苯甲酸、水杨酸等开展HULIS健康影响实验,但更多的是采用无细胞DTT测定ROS的方法。研究表明,HULIS的DTTm或DTTv活性与其来源有很大关系,见图4(a),生物质来源的DTT活性高,而煤燃烧的DTT活性低。为了更好的理解HULIS的健康影响,需要进一步通过基于细胞分析方法获得产生ROS的信息。然而,目前有关于HULIS和过渡金属通过细胞基质和组织配合后产生ROS的研究还很少。2019年,Lu等[107]首次通过无细胞DTT法和生物鉴定法(3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐,MTT)对比实际雾霾和非雾霾天气PM2.5中HULIS和HULIS-Fe(Ⅱ)络合物的氧化潜能(ROS),为实际大气HULIS的健康影响提供直接证据。结果表明,HULIS的ROS值比Fe(Ⅱ)产生的ROS高,见图4(b)。HULIS与Fe(Ⅱ)络合后机体的细胞死亡率增加,霾天ROS产生量更大,对健康的影响更大等。然而,大气PM中水溶性过渡态金属借助还原反应产生ROS,由于其浓度远高于PM中苯醌等氧化-还原性有机物,因此不能忽略金属离子单独存在时对ROS的贡献。
-
HULIS在辐射转移和光化学过程中有着重要贡献,在紫外线范围内有很强的吸收能力,266 nm处吸收与棕色碳(BrC)相当[110],在可见光区的吸收几乎可以忽略不计。PM中HULIS对吸收系数的贡献占所有碳质组分的32%—81%[111],Lee等[111]估算了韩国首尔PM2.5中HULIS和EC的季节性吸收系数范围为0.09—11.64 mol·L-1·m-1和0.11—3.04 mol·L-1·m-1,说明HULIS对PM的辐射强迫贡献显著。众多研究[112-113]通过对比气溶胶、雾或云中分离的HULIS与腐殖酸(或黄腐酸)的UV-vis光谱,证实了其相似性。HULIS的UV-vis基本没有特征吸收波长,但250—300 nm波长范围内,HULIS和黄腐酸的肩部清晰,这通常归因于含有C=C和C=O双键的基团中的π-π*电子跃迁。HULIS的吸光特性与分子结构有很大关系。总体来说,HULIS吸光度随波长(200—400 nm)的增加而递减,随芳香度增大而增加。具体的,270—280 nm之间的吸光主要归因于多烯、苯甲酸、2—3环PAHs、苯胺衍生物等的π-π*电子跃迁[20]。
HULIS的吸光性与平均分子量和芳香性有关[114]。254 nm和280 nm的吸光度(SUVA254和SUVA280)、365 nm的质量吸收效率(MAE365, m2·gC-1)[73]以及250和365 nm的吸光度比值(E250/E365)等,被广泛地应用于表征HULIS的化学特性和来源分析。E250/E365低、MAE365高,HULIS的芳香度和分子量越高[114]。生物质燃烧产生的HULIS芳香度高,而二次光化学反应的芳香度低。研究表明[63],生物质燃烧产生的HULIS的MAE365值是煤烟的约1.5—3倍。HULIS的吸光性还受燃料类型、燃烧条件、季节等的影响。Fan等[63]研究生物质和化石燃料燃烧产生烟的PM2.5中HULIS的特性,分别测定了其SUVA254和E250/E365,煤烟的E250/E365远高于生物质; Huo等[115]在研究3种秸秆的含水率和堆叠方式对其HULIS吸光能力的影响时,发现3种秸秆的MAE365随含水率的增加而减小,而E250/E365值相反;季节性差异表现为,HULIS的E250/E365夏季高于冬季[116],MAE365值冬季高于夏季[73],原因是夏季更多生物源VOCs光化学反应形成脂肪族化合物导致E250/E365升高,吸光性下降。大气颗粒物HULIS的吸光性与颗粒的粒径也有很大关系,越小的颗粒包含的吸光性发色团越多,导致MAE365升高[114];同样的,大气颗粒的吸湿性影响含水率和化学组分,从而对HULIS的吸光性也有一定影响[111]。
-
很多光敏反应可以生成HULIS[117]。Tsui等[118]通过实验发现了咪唑-2-甲酸(IC)的光敏反应可促进SOA的形成,在紫外光照下,含有IC的气溶胶与异戊二烯、柠檬烯、α-烯、β-烯和甲苯反应有明显的SOA形成。苯酚和三线态的硝基萘(光敏剂)反应生成HULIS,暗示燃烧产生硝基芳香烃和苯酚,两者发生光敏化反应形成HULIS物质,具有一定的激发-发射(EEM)荧光特性[119]。同时HULIS本身也是一种光敏剂[118]。HULIS可以吸收太阳光产生电子激发态从而导致·OH等的形成,即HULIS参与大气反应可以通过吸光后的激发三重态(3HULIS*)直接光解大气中的VOC形成SOA,也可以还原O2产生1O2、HOx等活性自由基,通过自由基氧化液相中的VOCs形成SOA。Wentworth等[60]采用没食子酸(GA)作为PM中常见的一种HULIS物质,与FeCl3进行非均相光敏化反应产生SOA。也有研究表明,HULIS参与的光化学反应生成不吸光的物质后,也会产生光漂白(photobleaching)效应。
HULIS参与的光化学反应途径主要包括(见式4—10)。HULIS吸收太阳光或紫外光形成激发态(1HULIS*, 3HULIS*)后直接参与反应,或激发态与O2反应生成·OH、O2−•、1O2等自由基,从而氧化降解其它物质。HULIS作光敏剂时可以通过催化S(Ⅳ)(通常为SO2)转化为S(Ⅵ)而加速大气中的硫酸盐的形成,因此生物质燃烧区域(HULIS的重要来源)HULIS对硫酸气溶胶的形成有重大贡献[120]。
-
本文综述了大气PM中HULIS的分离和提取方法,并详细分析了SPE分离HULIS时不同分离柱对提取的HULIS的化学组分的差异性,对比了各自的优缺点和适用性,总结了国内外不同采样点大气HULIS、HULIS-C的浓度差异。借助SPE分离和后续测定方法,开展大气PM中HULIS浓度、季节性差异和来源的研究已经比较多,由于HULIS成分的复杂性,造成不同文献中的数据没有直接的可比性,且提取的HULIS溶液后续分析主要针对其光谱性,很难准确定量具体的HULIS分子组成。因此,应尽快完善HULIS的分离提取方法和定量分析方法。HULIS含有可逆的氧化还原位点,参与氧化还原反应生成ROS,而ROS在人体内过度积累会导致细胞死亡、机体损伤等健康问题,因此研究HULIS的氧化潜能和毒理性有重要意义。DTT作为一种胞外表征HULIS氧化能力的方法被广泛应用,但想要科学准确地了解HULIS吸入人体后的反应机制和影响程度,还需要进行胞内(cell)实验,直接获得基于细胞分析方法获得单独HULIS或与金属螯合产生ROS的信息。建议今后可以重点开展3方面的研究:(1)HULIS、金属等通过细胞基质和组织配合后产生ROS的测定及与机体中毒特性相关的研究;(2)HULIS的化学结构、分子组分等的定量分析,建立物质结构与毒性之间的作用机制和关系;(3)研发在线高时间分辨质谱仪等先进设备进行大气外场观测,直接测定大气HULIS化学组分浓度,更好地揭示HULIS的形成机制、理化性质以及对人类健康和环境的影响。
大气颗粒物中类腐殖质的测定、理化特性及健康影响
Determination, physical and chemical characterization and health risk of HULIS in atmospheric particulate matter
-
摘要: 类腐殖质(humic-like substance,HULIS)是一类水溶性、相对分子量高的有机混合物,常见于雾滴、云滴、积雪和大气颗粒物(PM)中。本文主要综述了大气PM中HULIS的提取和分析方法、毒理性、吸光特性和光敏性,重点探讨了PM中有机物和过渡金属(尤其是Fe离子)产生活性氧化性物种(reactive oxygen species, ROS)从而破坏DNA的致毒机理,进一步提出HULIS(含有可逆的氧化还原位点)单独或与过渡金属螯合成有机-金属配体形成ROS的机理。最后对未来大气HULIS的研究方向进行了展望,指出今后应该加强大气HULIS、金属等通过细胞内催生ROS的测定及细胞毒性相关的研究,并关注HULIS的化学结构、分子组分等的定量分析,更好地揭示物质结构与细胞毒性之间的作用机制和关系, 以期为大气HULIS的健康影响提供支持。Abstract: Humic-like substances are a kind of water-soluble, high molecular weight organic mixtures, which usually exist in fog droplets, cloud droplets, snow and atmospheric particulate matters (PM). This paper mainly summarize extraction and isolation, analytical methods, toxicological property, absorbing-light characteristic and photosensitivity, with emphasis on exploring toxic mechanism on DNA damage via generation of reactive oxygen species (ROS) from organic matter and transition metals (especially iron), and further put forward ROS formation mechanism via individual HULIS or HULIS chelating with metal to form organic-metal complexes. At last, the research direction of ambient particle HULIS (reversible redox-active sites) in the future is prospected. It was pointed out that we should highlight intracellular ROS determination and cytotoxicity from atmospheric HULIS and metals, with more attention on quantitation on chemical structure, molecular composition. On the Basis of above study, we can better obtain driving mechanism and correlation between matter structure and cytotoxicity, and try to provide insights into the health risks caused by atmospheric HULIS.
-

-
表 1 不同SPE吸附柱结构和适用范围
Table 1. Different SPE adsorption column structure and application range
SPE柱
SPE
column填充成分及特点
Filling composition
and characteristics分离原理
Separation principle干扰物组分
Chaff interferent适用范围
Range of
applicationC-18 C18烷基链,非极性,
高疏水性利用亲、疏水性
分离、酸化长链有机羧酸、芳香酸、芳香
醇等极性有机物,硝基
PAHs、苯醌等非极性到中等极
性化合物分离HLB N-乙烯基吡咯烷酮和
亲脂二乙烯基苯利用亲、疏水性
分离、酸化芳香酸、短链羧酸、羟基酸等
具有
芳香结构和极性官能
团的化合物极性化合物分离 XAD-8 聚合甲基丙烯酸酯,
非离子型、大孔
径、弱极性利用化合物极性分离、
反冲洗脱处理长链高分子量二元酸、芳香酸 高共轭、酸性、疏
水性物质分离DEAE 二乙氨乙基纤维素,
弱阴离子交换柱利用化合物酸性分离、不
需要酸化、需要高离子
强度试剂作为洗脱剂3,5-二甲氧基苯甲酸 高分子量、高芳香
性化合物分离SAX 强阴离子交换树脂 串联在C18 柱之后使用 表 2 大气PM中HULIS测定方法及浓度
Table 2. Determination method and concentration of HULIS in atmospheric PM
采样地点
Site样品
Sample type采样时间
Sampling period分离方法
Isolation
methodHULIS-C
/(µg·m-3)HULIS/
(µg·m-3)HULIS-
C/WSOC测定方法
Quantification
method中国
城市北京 PM2.5 2012.6—2013.4 HLB 7.5 — 59.5% TOC[66] 上海 PM2.5 2011.12—2012.11 HLB 2.69 — 62.8% TOC[67] 常州 PM2.5 2017.1—2017.2 HLB 4.18 — 51.4% TOC[62] 兰州 PM2.5 2012.12 HLB — 7.24 84.7% ELSD[68] 2013.6—2013.7 — 2.15 80.2% 西安 PM2.5 2008.7—2009.8 HLB 3.1 — 34.5% 热光法[69] 广州 PM2.5 2009.1—2009.12 HLB 4.83 — 48.0% ELSD[12] 中国
农村广州 TSP 2006.7 HLB — 4.3 37.0% 重力法[54] 2007.1 — 7.8 44.0% 香港 PM2.5 2007.11 HLB — 11.8 60.0% ELSD[18] 外国
城市瑞士 PM10 2002.8—2002.9 SEC — 1.32 30.0% UV-vis[50] 法国 PM10 2007.11—2008.2 DEAE 2.28 — 38.6% TOC[20] 日本 TSP 2011.7—2011.9 HLB 1.75 — 51.0% TOC[45] 2011.12—2012.2 1.50 — 30.0% 外国
农村印度 PM10 2015.12—2016.1 HLB 15.3 — 45.1% TOC[70] 葡萄牙 PM2.5 2002.7—2003.7 XAD-8+XAD-4 1.97 — 51.6% TOC[71] 瑞士 PM1 2005.12—2006.1 C18 — 1.10 — ELSD[55] 韩国 PM2.5 2015.6—2015.7 HLB 1.3 — 50.0% TOC[72] 2015.12—2016.1 1.9 / 60.0% 海洋 中国广州 PM2.5 2007.7—2008.8 HLB — 7.1 — ELSD[21] 葡萄牙 PM2.5 2002.9—2004.5 C18+
SAX0.08 — 21.0% TOC[10] 高原 西藏 TSP 2015.8—2015.9 HLB 0.22 — 59.0% TOC[73] 森林 中国广州 TSP 2006.7 HLB — 5.7 40.5% 重力法[54] 法国 PM2.5 2002.9—2004.5 C18+
SAX0.35 — 23.0% TOC[10] 德国 PM2.5 2002.9—2004.5 C18+
SAX0.37 — 14.0% TOC[10] -
[1] HAVERS N, BURBA P, LAMBERT J, et al. Spectroscopic characterization of humic-like substances in airborne particulate matter [J]. Journal of Atmospheric Chemistry, 1998, 29(1): 45-54. doi: 10.1023/A:1005875225800 [2] GRABER E R, RUDICH Y. Atmospheric HULIS: how humic-like are they? A comprehensive and critical review [J]. Atmospheric Chemistry and Physics, 2006, 6(3): 729-753. doi: 10.5194/acp-6-729-2006 [3] KRISTENSEN T B, DU L, NGUYEN Q T, et al. Chemical properties of HULIS from three different environments [J]. Journal of Atmospheric Chemistry, 2015, 72(1): 65-80. doi: 10.1007/s10874-015-9302-8 [4] DECESARI S, FACCHINI M C, FUZZI S, et al. Characterization of water-soluble organic compounds in atmospheric aerosol: A new approach [J]. Journal of Geophysical Research:Atmospheres, 2000, 105(D1): 1481-1489. doi: 10.1029/1999JD900950 [5] KISS G, VARGA B, GALAMBOS I, et al. Characterization of water-soluble organic matter isolated from atmospheric fine aerosol [J]. Journal of Geophysical Research:Atmospheres, 2002, 107(D21): 1-8. [6] WIN M S, TIAN Z, ZHAO H, et al. Atmospheric HULIS and its ability to mediate the reactive oxygen species (ROS): A review [J]. Journal of Environmental Sciences, 2018, 71: 13-31. doi: 10.1016/j.jes.2017.12.004 [7] KRIVACSY Z, KISS G, VARGA B, et al. Study of humic-like substances in fog and interstitial aerosol by size-exclusion chromatography and capillary electrophoresis [J]. Atmospheric Environment, 2000, 34(25): 4273-4281. doi: 10.1016/S1352-2310(00)00211-9 [8] FACCHINI M C, DECESARI S, MIRCEA M, et al. Surface tension of atmospheric wet aerosol and cloud/fog droplets in relation to their organic carbon content and chemical composition [J]. Atmospheric Environment, 2000, 34(28): 4853-4857. doi: 10.1016/S1352-2310(00)00237-5 [9] VOISIN D, JAFFREZO J L, HOUDIER S, et al. Carbonaceous species and humic like substances (HULIS) in Arctic snowpack during OASIS field campaign in Barrow [J]. Journal of Geophysical Research:Atmospheres, 2012, 117(D0): 19. [10] FECZKO T, PUXBAUM H, KASPER-GIEBL A, et al. Determination of water and alkaline extractable atmospheric humic-like substances with the TU Vienna HULIS analyzer in samples from six background sites in Europe [J]. Journal of Geophysical Research, 2007, 112(D23): 10. [11] ZHENG G J, HE K B, DUAN F K, et al. Measurement of humic-like substances in aerosols: a review [J]. Environmental Pollution, 2013, 181: 301-314. doi: 10.1016/j.envpol.2013.05.055 [12] KUANG B Y, LIN P, HUANG X H H, et al. Sources of humic-like substances in the Pearl River Delta, China: positive matrix factorization analysis of PM2.5 major components and source markers [J]. Atmospheric Chemistry and Physics, 2015, 15(4): 1995-2008. doi: 10.5194/acp-15-1995-2015 [13] MA Y Q, CHENG Y B, QIU X H, et al. Sources and oxidative potential of water-soluble humic-like substances (HULISWS) in fine particulate matter (PM2.5) in Beijing [J]. Atmospheric Chemistry and Physics, 2018, 18(8): 5607-5617. doi: 10.5194/acp-18-5607-2018 [14] HADDAD I E, MARCHAND N, DRON, J, et al. Comprehensive primary particulate organic characterization of vehicular exhaust emissions in France [J]. Atmospheric Environment, 2009, 43(39): 6190-6198. doi: 10.1016/j.atmosenv.2009.09.001 [15] CALACE N, PETRONIO B M, CINI R, et al. Humic marine matter and insoluble materials in Antarctic snow [J]. International Journal of Environmental Analytical Chemistry, 2001, 79(4): 331-348. doi: 10.1080/03067310108044393 [16] CAVALLI F, FACCHINI M C, DECESARI S, et al. Advances in characterization of size-resolved organic matter in marine aerosol over the North Atlantic [J]. Journal of Geophysical Research, 2004, 109(D24): 215. [17] SALMA I, MESZAROS T, MAENHAUT W. Mass size distribution of carbon in atmospheric humic-like substances and water soluble organic carbon for an urban environment [J]. Journal of Aerosol Science, 2013, 56: 53-60. doi: 10.1016/j.jaerosci.2012.06.006 [18] LIN P, HUANG X F, HE L Y, et al. Abundance and size distribution of HULIS in ambient aerosols at a rural site in South China [J]. Journal of Aerosol Science, 2010, 41(1): 74-87. doi: 10.1016/j.jaerosci.2009.09.001 [19] MAYOL-BRACERO O L, GUYON P, GRAHAM B, et al. Water-soluble organic compounds in biomass burning aerosols over Amazonia 2. apportionment of the chemical composition and importance of the polyacidic fraction [J]. Journal of Geophysical Research, 2002, 107(D20): 8091. doi: 10.1029/2001JD000522 [20] BADUEL C, VOISIN D, JAFFREZO J L, et al. Seasonal variations of concentrations and optical properties of water soluble HULIS collected in urban environments [J]. Atmospheric Chemistry and Physics, 2010, 10(9): 4085-4095. doi: 10.5194/acp-10-4085-2010 [21] LIN P, ENGLING G, YU J Z. Humic-like substances in fresh emissions of rice straw burning and in ambient aerosols in the Pearl River Delta Region, China [J]. Atmospheric Chemistry and Physics, 2010, 10(14): 6487-6500. doi: 10.5194/acp-10-6487-2010 [22] ALTIERI K E, SEITZINGER S P, CARLTON A G, et al. Oligomers formed through in-cloud methylglyoxal reactions: chemical composition, properties, and mechanisms investigated by ultra-high resolution FT-ICR mass spectrometry [J]. Atmospheric Environment, 2008, 42(7): 1476-1490. doi: 10.1016/j.atmosenv.2007.11.015 [23] HOFFER A, KISS G, BLAZSO M, et al. Chemical characterization of humic-like substances (HULIS) formed from a lignin-type precursor in model cloud water [J]. Geophysical Research Letters, 2004, 31(L6): 115. [24] YE Z L, QU Z X, MA S S, et al. A comprehensive investigation of aqueous-phase photochemical oxidation of 4-ethylphenol [J]. Science of the Total Environment, 2019, 685: 976-985. doi: 10.1016/j.scitotenv.2019.06.276 [25] LIMBECK A, KULMALA M, PUXBAUM H, et al. Secondary organic aerosol formation in the atmosphere via heterogeneous reaction of gaseous isoprene on acidic particles [J]. Geophysical Research Letters, 2003, 30(19): 1-4. [26] ZHAO M F, QIAO T, LI Y L, et al. Temporal variations and source apportionment of Hulis-C in PM2.5 in urban Shanghai [J]. the Science of the Total Environment, 2016, 571(16): 18-26. [27] IINUMA Y, MULLER C, BOGE O, et al. The formation of organic sulfate esters in the limonene ozonolysis secondary organic aerosol (SOA) under acidic conditions [J]. Atmospheric Environment, 2007, 41(27): 5571-5583. doi: 10.1016/j.atmosenv.2007.03.007 [28] IINUMA Y, MULLER C, BERNDT T, et al. Evidence for the existence of organosulfates from β-pinene ozonolysis in ambient secondary organic aerosol [J]. Environmental Science and Technology, 2007, 41(19): 6678-6683. doi: 10.1021/es070938t [29] SURRATT J D, GOMEZ-GONZALEZ Y, CHAN A W H, et al. Organosulfate formation in biogenic secondary organic aerosol [J]. the Journal of Physical Chemistry A, 2008, 112(36): 8345-8378. doi: 10.1021/jp802310p [30] GYSEL M, WEINGARTNER E, NYEKI S, et al. Hygroscopic properties of water-soluble matter and humic-like organics in atmospheric fine aerosol [J]. Atmospheric Chemistry and Physics, 2004, 4(1): 35-50. doi: 10.5194/acp-4-35-2004 [31] DINAR E, TARANIUK I, GRABER E R, et al. Cloud condensation nuclei properties of model and atmospheric HULIS [J]. Atmospheric Chemistry and Physics, 2006, 6(66): 2465-2482. [32] WANG B, KNOPF D A. Heterogeneous ice nucleation on particles composed of humic-like substances impacted by O3 [J]. Journal of Geophysical Research, 2011, 116(D3): 205. [33] HOFFER A, GELENCSER A, GUYON P, et al. Optical properties of humic-like substances (HULIS) in biomass-burning aerosols [J]. Atmospheric Chemistry and Physics, 2006, 6(68): 3563-3570. [34] DELFINO R J, STAIMER N, TJOA T, et al. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel [J]. Journal of Exposure Science and Environmental Epidemiology, 2013, 23(5): 466-473. doi: 10.1038/jes.2013.25 [35] DOU J, LIN P, KUANG B Y, et al. Reactive oxygen species production mediated by humic-like substances in atmospheric aerosols: enhancement effects by pyridine, imidazole, and their derivatives [J]. Environmental Science and Technology, 2015, 49(11): 6457-6465. doi: 10.1021/es5059378 [36] LIN P, YU J Z. Generation of reactive oxygen species mediated by humic-like substances in atmospheric aerosols [J]. Environmental Science and Technology, 2011, 45(24): 10362-10368. doi: 10.1021/es2028229 [37] VARGA B, KISS G, GANSZKY I, et al. Isolation of water-soluble organic matter from atmospheric aerosol [J]. Talanta, 2001, 55(3): 561-572. doi: 10.1016/S0039-9140(01)00446-5 [38] GORA R, HUTTA M. Reversed-phase liquid chromatographic characterization and analysis of air particulates humic (-like) substances in presence of pollens [J]. Journal of Chromatography A, 2005, 1084(1/2): 39-45. [39] ANDRACCHIO A, CAVICCHI C, TONELLI D, et al. A new approach for the fractionation of water-soluble organic carbon in atmospheric aerosols and cloud drops [J]. Atmospheric environment, 2002, 36(32): 5097-5107. doi: 10.1016/S1352-2310(02)00238-8 [40] SAMBUROVA V, DIDENKO T, KUNENKOV E, et al. Functional group analysis of high-molecular weight compounds in the water-soluble fraction of organic aerosols [J]. Atmospheric Environment, 2007, 41(22): 4703-4710. doi: 10.1016/j.atmosenv.2007.03.033 [41] SULLIVAN A P, WEBER R J. Chemical characterization of the ambient organic aerosol soluble in water: 1. isolation of hydrophobic and hydrophilic fractions with a XAD-8 resin [J]. Journal of Geophysical Research, 2006, 111(D5): 314. [42] BADUEL C, VOISIN D, JAFFREZO J L. Comparison of analytical methods for humic-like substances (HULIS) measurements in atmospheric particles [J]. Atmospheric Chemistry and Physics, 2009, 9(175): 5949-5962. [43] FAN X J, SONG J Z, PENG P A. Comparison of isolation and quantification methods to measure humic-like substances (HULIS) in atmospheric particles [J]. Atmospheric Environment, 2012, 60: 366-374. doi: 10.1016/j.atmosenv.2012.06.063 [44] MIYAZAKI Y, KONDO Y, SHIRAIWA M, et al. Chemical characterization of water-soluble organic carbon aerosols at a rural site in the Pearl River Delta, China, in the summer of 2006 [J]. Journal of Geophysical Research, 2009, 114(D14): 208. [45] CHEN Q C, IKEMORI F, HIGO H, et al. Chemical structural characteristics of HULIS and other fractionated organic matter in urban aerosols: results from mass spectral and FT-IR analysis [J]. Environmental Science and Technology, 2016, 50(4): 1721-1730. doi: 10.1021/acs.est.5b05277 [46] LIMBECK A, HANDLER M, NEUBERGER B, et al. Carbon-specific analysis of humic-like substances in atmospheric aerosol and precipitation samples [J]. Analytical Chemistry, 2005, 77(22): 7288-7293. doi: 10.1021/ac050953l [47] DUARTE R M B O, DUARTE A C. Application of non-ionic solid sorbents (XAD resins) for the isolation and fractionation of water-soluble organic compounds from atmospheric aerosols [J]. Journal of Atmospheric Chemistry, 2005, 51(1): 79-93. doi: 10.1007/s10874-005-8091-x [48] STONE E A, HEDMAN C J, SHEESLEY R J, et al. Investigating the chemical nature of humic-like substances (HULIS) in North American atmospheric aerosols by liquid chromatography tandem mass spectrometry [J]. Atmospheric Environment, 2009, 43(27): 4205-4213. doi: 10.1016/j.atmosenv.2009.05.030 [49] ZAPPOLI S, ANDRACCHIO A, FUZZI S, et al. Inorganic, organic and macromolecular components of fine aerosol in different areas of Europe in relation to their water solubility [J]. Atmospheric Environment, 1999, 33(17): 2733-2743. doi: 10.1016/S1352-2310(98)00362-8 [50] SAMBUROVA V, ZENOBI R, KALBERER M. Characterization of high molecular weight compounds in urban atmospheric particles [J]. Atmospheric Environment and Physics, 2005, 5(53): 2163-2170. [51] PAVLOVIC J, HOPKE P K. Chemical nature and molecular weight distribution of the water-soluble fine and ultrafine PM fractions collected in a rural environment [J]. Atmospheric Environment, 2012, 59: 264-271. doi: 10.1016/j.atmosenv.2012.04.053 [52] SANTOS P S M, OTERO M, FILIPE O M S, et al. Comparison between DAX-8 and C-18 solid phase extraction of rainwater dissolved organic matter [J]. Talanta, 2010, 83(2): 505-512. doi: 10.1016/j.talanta.2010.09.050 [53] CHANG J L, THOMPSON J E. Characterization of colored products formed during irradiation of aqueous solutions containing H2O2 and phenolic compounds [J]. Atmospheric Environment, 2010, 44(4): 541-551. doi: 10.1016/j.atmosenv.2009.10.042 [54] SONG J Z, HE L L, PENG P A, et al. Chemical and isotopic composition of humic-like substances (HULIS) in ambient aerosols in Guangzhou, South China [J]. Aerosol Science and Technology, 2012, 46(5): 533-546. doi: 10.1080/02786826.2011.645956 [55] EMMENEGGER C, REINHARDT A, HUEGLIN C, et al. Evaporative light scattering: a novel detection method for the quantitative analysis of humic-like substances in aerosols [J]. Environmental Science and Technology, 2007, 41(7): 2473-2478. doi: 10.1021/es061095t [56] YOUNG C S, DOLAN J W. Success with evaporative light-scattering detection [J]. LC-GC Europr, 2003, 16(3): 132-137. [57] 项萍, 谭吉华, 马永亮, 等. 大气颗粒物中类腐殖酸的研究进展 [J]. 环境化学, 2015, 34(3): 401-409. doi: 10.7524/j.issn.0254-6108.2015.03.2014071902 XIANG P, TAN J H, MA Y L, et al. Research progress of humic-like substances (HULIS) in atmospheric particles [J]. Environmental Chemistry, 2015, 34(3): 401-409(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.03.2014071902
[58] KISS G, TOMBACZ E, VARGA B, et al. Estimation of the average molecular weight of humic-like substances isolated from fine atmospheric aerosol [J]. Atmospheric Environment, 2003, 37(27): 3783-3794. doi: 10.1016/S1352-2310(03)00468-0 [59] POLIDORI A, TURPIN B J, DAVIDSON C I, et al. Organic PM2.5: Fractionation by polarity, FTIR spectroscopy, and OM/OC ratio for the pittsburgh aerosol [J]. Aerosol Science and Technology, 2008, 42(3): 233-246. doi: 10.1080/02786820801958767 [60] WENTWORTH G R, AL-ABADLEH H A. DRIFTS studies on the photosensitized transformation of gallic acid by iron(III) chloride as a model for HULIS in atmospheric aerosols [J]. Physical Chemistry Chemical Physics, 2011, 13(14): 6507-6516. doi: 10.1039/c0cp01953d [61] YE Z l, LI Q, MA S S, et al. Summertime day-night differences of PM2.5 components (inorganic Ions, OC, EC, WSOC, WSON, HULIS, and PAHs) in Changzhou, China [J]. Atmosphere, 2017, 8(10): 189. [62] 顾远, 李清, 黄雯倩, 等. 常州市冬季PM2.5中类腐殖质昼夜特征分析 [J]. 环境科学, 2019, 40(3): 1091-1100. GU Y, LI Q, HUANG W Q, et al. Day-night characteristics of humic-like substances in PM2.5 during winter in Changzhou] [J]. Environmental Science, 2019, 40(3): 1091-1100(in Chinese).
[63] FAN X J, WEI S Y, ZHU, M B, et al. Comprehensive characterization of humic-like substances in smoke PM2.5 emitted from the combustion of biomass materials and fossil fuels [J]. Atmospheric Chemistry and Physics, 2016, 16(20): 13321-13340. doi: 10.5194/acp-16-13321-2016 [64] LIN P, RINCON A G, KALBERER M, et al. Elemental composition of HULIS in the Pearl River Delta Region, China: results inferred from positive and negative electrospray high resolution mass spectrometric data [J]. Environmental Science and Technology, 2012, 46(14): 7454-7462. doi: 10.1021/es300285d [65] KUMAR V, RAJPUT P, GOEL A. Atmospheric abundance of HULIS during wintertime in Indo-Gangetic Plain: impact of biomass burning emissions [J]. Journal of Atmospheric Chemistry, 2018, 75(4): 385-398. doi: 10.1007/s10874-018-9381-4 [66] LI X, HAN J Z, HOPKE P K, et al. Quantifying primary and secondary humic-like substances in urban aerosol based on emission source characterization and a source-oriented air quality model [J]. Atmospheric Chemistry and Physics, 2019, 19(4): 2327-2341. doi: 10.5194/acp-19-2327-2019 [67] 黄众思, 修光利, 蔡婧, 等. 大气PM2.5中水溶性有机碳和类腐殖质碳的季节变化特征 [J]. 环境科学学报, 2013, 33(10): 2664-2670. HUANG Z S, XIU G L, CAI J, et al. Seasonal characterization of water-soluble organic carbon and humic-like substance carbon in atmospheric PM2.5 [J]. Acta Scientiae Circumstantiae, 2013, 33(10): 2664-2670(in Chinese).
[68] TAN J H, XIANG P, ZHOU X M, et al. Chemical characterization of humic-like substances (HULIS) in PM2.5 in Lanzhou, China [J]. the Science of the Total Environment, 2016, 573: 1481-1490. doi: 10.1016/j.scitotenv.2016.08.025 [69] 倪海燕, 韩永明, 曹军骥. 西安水溶性类腐殖质气溶胶(HULIS)的污染特征及其来源[C]. 十一届全国气溶胶会议暨第十届海峡两岸气溶胶技术研讨会, 2013: 98. NI H Y, HAN Y M, CAO J J. Pollution characteristics and sources of water soluble humic-like substances aerosol (HULIS) in Xi 'an[C]. 11st National Aerosol Conference and 10th Cross-strait Workshop for Aerosol Science and Technology, 2013: 98(in Chinese).
[70] KUMAR V, GOEL A, RAJPUT P. Compositional and surface characterization of HULIS by UV-Vis, FTIR, NMR and XPS: wintertime study in Northern India [J]. Atmospheric Environment, 2017, 164: 468-475. doi: 10.1016/j.atmosenv.2017.06.008 [71] DUARTE R M B O, SANTOS E B H, PIO C A, et al. Comparison of structural features of water-soluble organic matter from atmospheric aerosols with those of aquatic humic substances [J]. Atmospheric Environment, 2007, 41(37): 8100-8113. doi: 10.1016/j.atmosenv.2007.06.034 [72] PARK S, SON S C, LEE S. Characterization, sources, and light absorption of fine organic aerosols during summer and winter at an urban site [J]. Atmospheric Research, 2018, 213: 370-380. doi: 10.1016/j.atmosres.2018.06.017 [73] WU G M, WAN X, GAO S P, et al. Humic-like substances (HULIS) in aerosols of central Tibetan Plateau (Nam Co, 4730 m asl): abundance, light absorption properties, and sources [J]. Environmental Science and Technology, 2018, 52(13): 7203-7211. doi: 10.1021/acs.est.8b01251 [74] MA Y Q, CHENG Y B, GAO G, et al. Speciation of carboxylic components in humic-like substances (HULIS) and source apportionment of HULIS in ambient fine aerosols (PM2.5) collected in Hong Kong [J]. Environmental Science and Pollution Research, 2020, 27: 23172-23180. doi: 10.1007/s11356-020-08915-w [75] CHANG-GRAHAM A L, PROFETA L T M, JOHNSON T J, et al. Case study of water-soluble metal containing organic constituents of biomass burning aerosol [J]. Environmental Science and Technology, 2011, 45: 1257-1263. doi: 10.1021/es103010j [76] SINT T, DONOHUE J F, GHIO A J. Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease [J]. Inhalation Toxicology, 2008, 20(1): 25-29. doi: 10.1080/08958370701758759 [77] FAJERSZTAJN L, VERAS M, BARROZO L V, et al. Air pollution: A potentially modifiable risk factor for lung cancer [J]. Nature Reviews. Cancer, 2013, 13(9): 674-678. doi: 10.1038/nrc3572 [78] GHIO A J, CARRAWAY M S, MADDEN M C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems [J]. Journal of Toxicology and Environmental Health Part B Critical Reviews, 2012, 15(1): 1-21. doi: 10.1080/10937404.2012.632359 [79] LI N, HAO M Q, PHALEN R F, et al. Particulate air pollutants and asthma A paradigm for the role of oxidative stress in PM-induced adverse health effects [J]. Clinical Immunology (Orlando), 2003, 109(3): 250-265. doi: 10.1016/j.clim.2003.08.006 [80] SEE S W, WANG Y H, BALASUBRAMANIAN R. Contrasting reactive oxygen species and transition metal concentrations in combustion aerosols [J]. Environmental Research, 2007, 103(3): 317-324. doi: 10.1016/j.envres.2006.08.012 [81] LI N, WANG M Y, BRAMBLE L A, et al. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential [J]. Environmental Health Perspectives, 2009, 117(7): 1116-1123. doi: 10.1289/ehp.0800319 [82] GURGUEIRA S A, LAWRENCE J, COULL B, et al. Rapid Increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation [J]. Environmental Health Perspectives, 2002, 110(8): 749-755. doi: 10.1289/ehp.02110749 [83] ZIEMANN P J. Evidence for low-volatility diacyl peroxides as a nucleating agent and major component of aerosol formed from reactions of O3 with cyclohexene and homologous compounds [J]. Journal of Physical Chemistry A, 2002, 106(17): 4390-4402. doi: 10.1021/jp012925m [84] LI Q F, WYATT A, KAMENS R M. Oxidant generation and toxicity enhancement of aged-diesel exhaust [J]. Atmospheric Environment, 2009, 43(5): 1037-1042. doi: 10.1016/j.atmosenv.2008.11.018 [85] YU H R, WEI J L, CHENG Y L, et al. Synergistic and antagonistic interactions among the particulate matter components in generating reactive oxygen species based on the dithiothreitol assay [J]. Environmental Science and Technology, 2018, 52(4): 2261-2270. doi: 10.1021/acs.est.7b04261 [86] LIN M F, YU J Z. Dithiothreitol (DTT) concentration effect and its implications on the applicability of DTT assay to evaluate the oxidative potential of atmospheric aerosol samples [J]. Environmental Pollution, 2019, 251: 938-944. doi: 10.1016/j.envpol.2019.05.074 [87] KING L E, WEBER R J. Development and testing of an online method to measure ambient fine particulate reactive oxygen species (ROS) based on the 2, 7-dichlorofluorescin (DCFH) assay [J]. Atmospheric Measurement Techniques, 2013, 6(7): 1647-1658. doi: 10.5194/amt-6-1647-2013 [88] VENKATACHARI P, HOPKE P K. Development and laboratory testing of an automated monitor for the measurement of atmospheric particle-bound reactive oxygen species (ROS) [J]. Aerosol Science and Technology, 2008, 42(8): 629-635. doi: 10.1080/02786820802227345 [89] PIETROGRANDE M C, BERTOLI I, MANARINI F, et al. Ascorbate assay as a measure of oxidative potential for ambient particles: Evidence for the importance of cell-free surrogate lung fluid composition [J]. Atmospheric Environment, 2019, 211: 103-112. doi: 10.1016/j.atmosenv.2019.05.012 [90] DISTEFANO E, EIGUREN-FERNANDEZ A, DELFINO R J, et al. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles [J]. Inhalation Toxicology, 2009, 21(9): 731-738. doi: 10.1080/08958370802491433 [91] VIDRIO E, PHUAH C H, DILLNER A M, et al. Generation of hydroxyl radicals from ambient fine particles in a surrogate lung fluid solution [J]. Environmental Science and Technology, 2009, 43(3): 922-927. doi: 10.1021/es801653u [92] CHARRIER J G, ANASTASIO C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals [J]. Atmospheric Chemistry and Physics, 2012, 12(19): 9321-9333. doi: 10.5194/acp-12-9321-2012 [93] LI N, SIOUTAS C, CHO A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage [J]. Environmental Health Perspectives, 2003, 111(4): 455-460. doi: 10.1289/ehp.6000 [94] 张曼曼, 李慧蓉, 杨闻达, 等. 基于DTT法测量广州市区PM2.5的氧化潜势 [J]. 中国环境科学, 2019, 39(6): 2258-2266. doi: 10.3969/j.issn.1000-6923.2019.06.003 ZHANG M M, LI H R, YANG W D, et al. Measurement based on DTT method of the PM2.5 oxidative potential in Guangzhou urban area [J]. China Environment Science, 2019, 39(6): 2258-2266(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.06.003
[95] LIU W J, XU Y S, LIU W X, et al. Oxidative potential of ambient PM2.5 in the coastal cities of the Bohai Sea, northern China: seasonal variation and source apportionment [J]. Environmental Pollution, 2018, 236: 514-528. doi: 10.1016/j.envpol.2018.01.116 [96] HEO J, ANTKIEWICZ D S, SHAFER M M, et al. Assessing the role of chemical components in cellular responses to atmospheric particle matter (PM) through chemical fractionation of PM extracts [J]. Analytical and Bioanalytical Chemistry, 2015, 407(20): 5953-5963. doi: 10.1007/s00216-015-8749-4 [97] SAFFARI A, DAHER N, SHAFER M M, et al. Global perspective on the oxidative potential of airborne particulate matter: a synthesis of research findings [J]. Environmental Science and Technology, 2014, 48(13): 7576-7583. doi: 10.1021/es500937x [98] VERMA V, RICO-MARTINEZ R, KOTRA N, et al. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols [J]. Environmental Science and Technology, 2012, 46(20): 11384-11392. doi: 10.1021/es302484r [99] VERMA V, FANG T, XU L, et al. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5 [J]. Environmental Science and Technology, 2015, 49(7): 4646-4656. doi: 10.1021/es505577w [100] GONZALEZ D H, CALA C K, PENG Q Y, et al. HULIS enhancement of hydroxyl radical formation from Fe(II): kinetics of fulvic acid-Fe(II) complexes in the presence of lung antioxidants [J]. Environmental Science and Technology, 2017, 51(13): 7676-7685. doi: 10.1021/acs.est.7b01299 [101] VERMA V, FANG T, GUO H, et al. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: spatiotemporal trends and source apportionment [J]. Atmospheric Chemistry and Physics, 2014, 14(23): 12915-12930. doi: 10.5194/acp-14-12915-2014 [102] MCWHINNEY R D, ZHOU S, ABBATT J P D. Naphthalene SOA: redox activity and naphthoquinone gas–particle partitioning [J]. Atmospheric Chemistry and Physics, 2013, 13(19): 9731-9744. doi: 10.5194/acp-13-9731-2013 [103] MA Y Q, CHENG Y B, QIU X H, et al. Optical properties, source apportionment and redox activity of humic-like substances (HULIS) in airborne fine particulates in Hong Kong [J]. Environmental Pollution, 2019, 255: 113087. doi: 10.1016/j.envpol.2019.113087 [104] KOSTIC I, ANDJELKOVIC T, NIKOLIC R, et al. Copper(II) and lead(II) complexation by humic acid and humic-like ligands [J]. Journal of the Serbian Chemical Society, 2011, 76(9): 1325-1336. doi: 10.2298/JSC110310115K [105] ARAKAKI T, SAITO K, OKADA K, et al. Contribution of fulvic acid to the photochemical formation of Fe(II) in acidic Suwannee River fulvic acid solutions [J]. Chemosphere, 2010, 78(8): 1023-1027. doi: 10.1016/j.chemosphere.2009.11.035 [106] MOONSHINE M, RUDICH Y, KATSMAN S, et al. Atmospheric HULIS enhance pollutant degradation by promoting the dark Fenton reaction [J]. Geophysical Research Letters, 2008, 35(20): 807. [107] LU S L, WIN M S, ZENG J Y, et al. A characterization of HULIS-C and the oxidative potential of HULIS and HULIS-Fe(II) mixture in PM2.5 during hazy and non-hazy days in Shanghai [J]. Atmospheric Environment, 2019, 219(117058): 1-8. [108] ZHOU P, YAN H, GU B H. Competitive complexation of metal ions with humic substances [J]. Chemosphere, 2005, 58(10): 1327-1337. doi: 10.1016/j.chemosphere.2004.10.017 [109] AL-ABADLEH H A. Review of the bulk and surface chemistry of iron in atmospherically relevant systems containing humic-like substances [J]. RSC Advances, 2015, 5(57): 45785-45811. doi: 10.1039/C5RA03132J [110] UTRY N, AJTAI T, FILEP A, et al. Mass specific optical absorption coefficient of HULIS aerosol measured by a four-wavelength photoacoustic spectrometer at NIR, VIS and UV wavelengths [J]. Atmospheric Environment, 2013, 69: 321-324. doi: 10.1016/j.atmosenv.2013.01.003 [111] LEE J, JUNG C, KIM Y. Estimation of optical properties for HULIS aerosols at Anmyeon island, Korea [J]. Atmosphere, 2017, 8(7): 120-138. [112] KISS G, VARGA B, GELENCSER A, et al. Characterisation of polar organic compounds in fog water [J]. Atmospheric Environment, 2001, 35(12): 2193-2200. doi: 10.1016/S1352-2310(00)00473-8 [113] DUARTE R M B O, PIO C A, DUARTE A C. Spectroscopic study of the water-soluble organic matter isolated from atmospheric aerosols collected under different atmospheric conditions [J]. Analytica Chimica Acta, 2005, 530(1): 7-14. doi: 10.1016/j.aca.2004.08.049 [114] VOLIOTIS A, PROKES R, LAMMEL G, et al. New insights on humic-like substances associated with wintertime urban aerosols from central and southern Europe: Size-resolved chemical characterization and optical properties [J]. Atmospheric Environment, 2017, 166: 286-299. doi: 10.1016/j.atmosenv.2017.07.024 [115] HUO Y Q, LI M, JIANG M H, et al. Light absorption properties of HULIS in primary particulate matter produced by crop straw combustion under different moisture contents and stacking modes [J]. Atmospheric Environment, 2018, 191: 490-499. doi: 10.1016/j.atmosenv.2018.08.038 [116] FAN X J, SONG J Z, PENG P A. Temporal variations of the abundance and optical properties of water soluble humic-like substances (HULIS) in PM2.5 at Guangzhou, China [J]. Atmospheric Research, 2016, 172/173: 8-15. doi: 10.1016/j.atmosres.2015.12.024 [117] VIONE D, MAURINO V, MINERO C. Photosensitised humic-like substances (HULIS) formation processes of atmospheric significance: a review [J]. Environmental Science and Pollution Research International, 2014, 21(20): 11614-11622. doi: 10.1007/s11356-013-2319-0 [118] TSUI W G, MCNEILL V F. Modeling secondary organic aerosol production from photosensitized humic-like substances (HULIS) [J]. Environmental Science and Technology Letters, 2018, 5(5): 255-259. doi: 10.1021/acs.estlett.8b00101 [119] LAURENTIIS E D, SUR B, PAZZI M, et al. Phenol transformation and dimerisation, photosensitised by the triplet state of 1-nitronaphthalene: A possible pathway to humic-like substances (HULIS) in atmospheric waters [J]. Atmospheric Environment, 2013, 70: 318-327. doi: 10.1016/j.atmosenv.2013.01.014 [120] WANG X, GEMAYEL R, HAYECK N, et al. Atmospheric photosensitization: a new pathway for sulfate formation [J]. Environmental Science and Technology, 2020, 54(6): 3114-3120. doi: 10.1021/acs.est.9b06347 -



 下载:
下载: