-
近年来,随着脱硫技术的发展和推广,大气中NO2已逐渐取代SO2,成为大气中主要的酸性污染气体[1]。近来研究显示,硝酸盐成为了我国华北地区夏季灰霾污染的主要诱因[2]。因此通过研究硝酸盐的形成机制及其来源,对了解区域空气污染的成因和制定空气污染的对策有重要意义。人为源(如化石燃料燃烧)和自然源(闪电和土壤微生物活动)释放到大气中的NOx(NO+NO2)主要以NO为主,NO在大气中被臭氧(O3)和过氧自由基(RO2·)氧化成NO2。在白天,NO2被光解产物羟基自由基(·OH)氧化为HNO3;在晚上,NO2被臭氧进一步氧化成三氧化氮(NO3),然后NO2与NO3化合生成五氧化二氮(N2O5)。N2O5在颗粒表面水解和NO3+VOCs是夜间HNO3生成的主要途径。由于从NO到HNO3途径中,参与的氧化剂的氧同位素不同(例如δ18O-O3的值为90‰—120‰[3],大气降雨中δ18O-H2O的值为−36‰—−4‰(http://isohis.iaea.org),大气中羟基自由基的δ18O-OH的值为−60‰—−30‰[4-5]),因此不同路径形成的δ18O-HNO3也不同。例如,NO2+OH(40‰—60‰)路径形成的δ18O-HNO3值远低于N2O5+H2O和NO3+VOCs(80‰—110‰)路径[1,4-6]。因此δ18O-NO3−值被广泛用于定性和定量分析大气气溶胶和雨水中NO3−的形成路径[1,4-7]。
另外,由于不同排放源释放的NOx的δ15N-NOx值不同,例如煤燃烧释放的δ15N-NOx值(13.5‰±4.9‰)[8-9]明显高于汽车尾气(−4.3‰±4.5‰)[10-12]、闪电固氮(−0.5‰—+1.4‰)[13]和土壤微生物(−28.9‰±8.2‰)[10,14-15]释放的δ15N-NOx值,且大气中NOx的最终产物是NO3−,所以δ15N-NO3−值在早期研究中主要用于定性描述大气气溶胶和雨水中NO3−的来源[7,16]。基于热力学分馏理论,Walters等[6]量化了从NOx氧化成HNO3过程中的氮同位素分馏系数。例如,NO2+OH路径形成的δ15N-HNO3值稍低于δ15N-NO2值(−3‰—0‰),但是在300 K的条件下,N2O5+H2O路径形成的δ15N-HNO3值比δ15N-NO2值高25.5‰,相反NO3+VOCs路径形成的δ15N-HNO3值比δ15N-NO2值低18.0‰。基于不同路径形成HNO3所引起的氮同位素分馏、不同来源的δ15N-NOx值和贝叶斯同位素混合模型,δ15N-NO3−已被用于定量评估大气硝酸盐来源的相对贡献[17-19]。因此,联合δ18O-NO3−值和δ15N-NO3−值,可以评估大气硝酸盐的来源。
基于硝酸盐浓度估算大气降雨和气溶胶中硝酸盐沉降通量季节变化[7,16,20]和基于δ15N-NO3−和δ18O-NO3−值探讨大气硝酸盐来源和形成机制季节变化[1,4-5]的研究较多,但是还未有研究报道大气NOy(NOx + HNO3 + NO3−)干沉降的昼夜变化及其双同位素(δ15N-NO3−和δ18O-NO3−值)的昼夜分布特征。另外,南昌位于我国中东部地区,近年来空气污染加剧,且出现硝酸盐和硫酸盐交替主导空气污染[4]。本研究通过对南昌夏季昼夜NOy干沉降样本中硝酸盐氮浓度和氮氧同位素的测定,来探讨NOy干沉降昼夜的变化特征、主要来源以及形成机制。
-
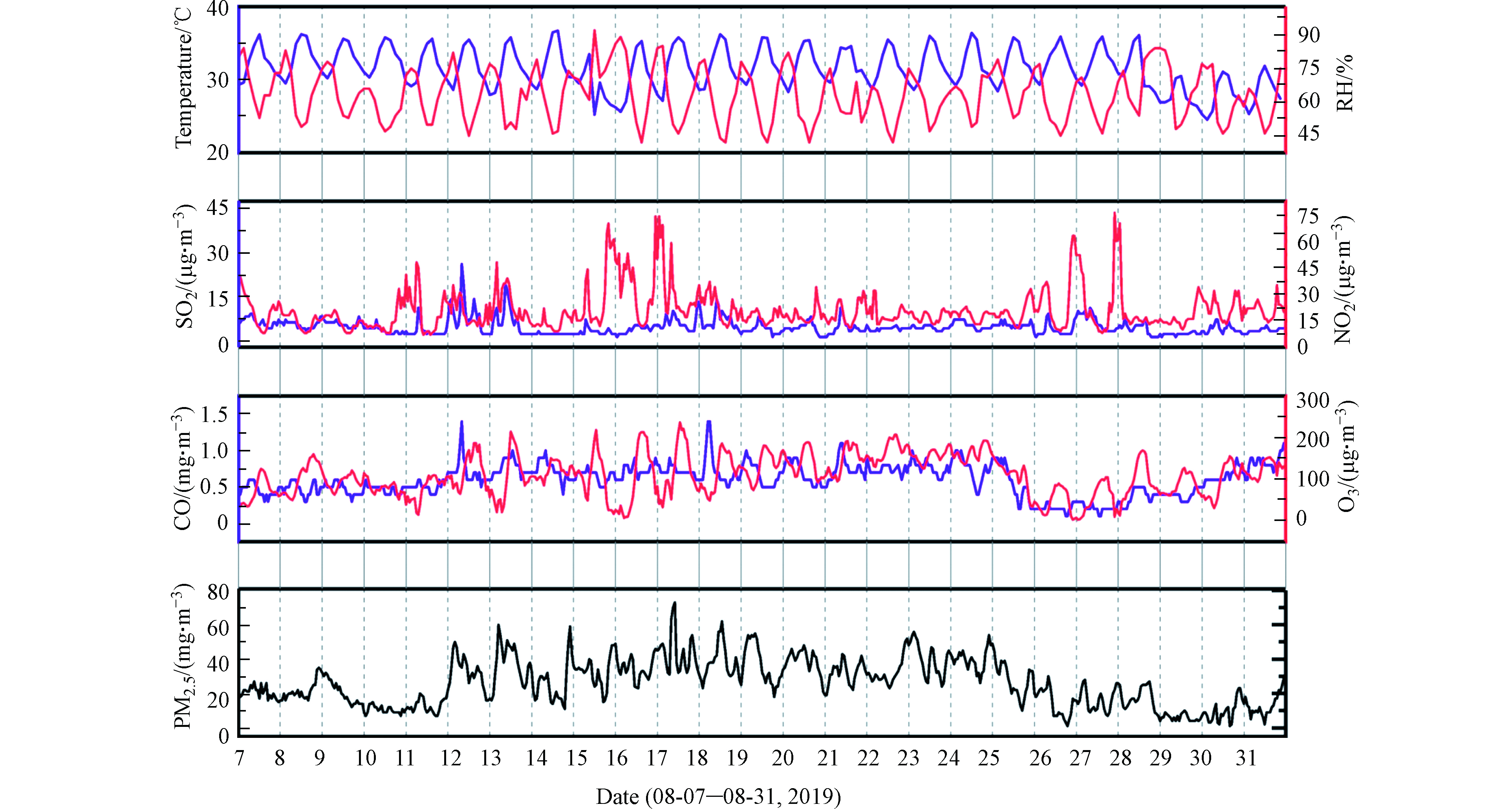
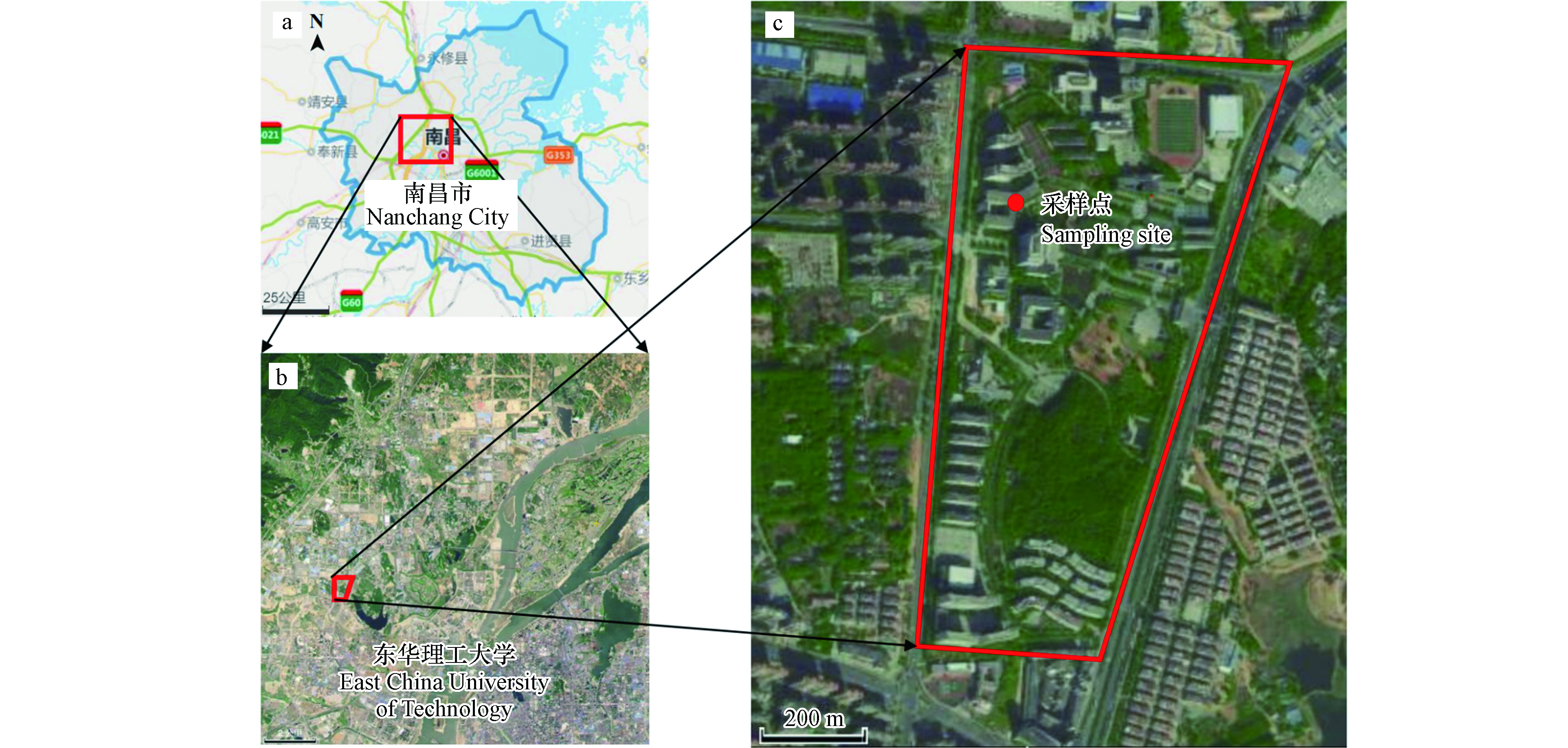
本研究于2019年8月7日至8月31日分昼(7:30—20:00)夜(20:00—翌日7:30)在东华理工大学地学楼顶楼(距离地面约20 m,图1)共采集42个NOy干沉降样本。样本采集过程简要如下:在矩形开口塑料箱(长宽为55.5 cm×44.4 cm,用10%盐酸浸泡过夜,超纯水清洗干净)内装入550 mL的超纯水,放置于楼顶。由于白天光照强,采样箱水分蒸发严重,在收集样品时补充550 mL超纯水,清洗箱底后再收集。晚上则直接收集干沉降样本。若下雨,则用盖子盖住采样箱,舍弃当次样品,清洗采样箱,雨停后继续收集。样品收集后,用0.22 μm的一次性针孔Millex-GP滤头过滤并称重,冰冻保存待分析。采样期间,气象数据(环境温度(°C)、相对湿度(RH))和污染物浓度(NO2、SO2、CO、O3和PM2.5)如图2所示。
-
硝酸盐浓度用紫外分光光度法测定。简要步骤如下:使用岛津UV-2600型紫外分光光度计,在220 nm和275 nm波长处用10 nm石英比色皿测得吸光值A(A = A220−2A275),根据测得不同浓度硝酸盐的吸光度和对应的硝酸盐浓度绘制标准曲线,计算采集样本中硝酸盐氮浓度。本方法最低检出浓度为0.08 mg·L−1,测量上限为4 mg·L−1,同一样本多次测定结果的相对标准偏差为4.8%。
硝酸盐氮氧同位素(δ18O-NO3−(‰, vs. VSMOW)=(R样品/ R标准−1) × 1000‰, δ15N-NO3−(‰, vs. Air-N2)=(R样品/ R标准−1) × 1000‰,R样品和R标准分别是样品和标准中18O/16O和15N/14N的质量比值)采用反硝化细菌法测定[21-22]。将含有40 nmol NO3−的样本注入培养好的反硝化细菌(金色假单胞菌,ATCC 13985)瓶中。由于金色假单胞菌缺乏N2O还原酶,且能定量地将NO3−全部转化为N2O。然后使用GasBench-Ⅱ和连续流同位素比值质谱仪(IRMS; Thermo Fisher DELTAV advantage, Thermo Fisher Scientific, Inc., USA)测量N2O的δ15N和δ18O值。国际硝酸盐参考标准USGS32、USGS34、USGS35、IAEA-N3[23]和实验室硝酸盐标准品用于数据校准。该方法测得标准品中δ15N-NO3−和δ18O-NO3−的标准偏差分别优于0.2‰和0.5‰。详细操作参考Luo等[1,4-5,19,24]。
-
NOy干沉降通量估算公式如下:
式中,C为样品浓度 (mg·L−1);t为采样时长 (h);S为集尘箱面积 (m2);V为样品体积 (mL);F为沉降通量 (μg·h−1·m−2).
-
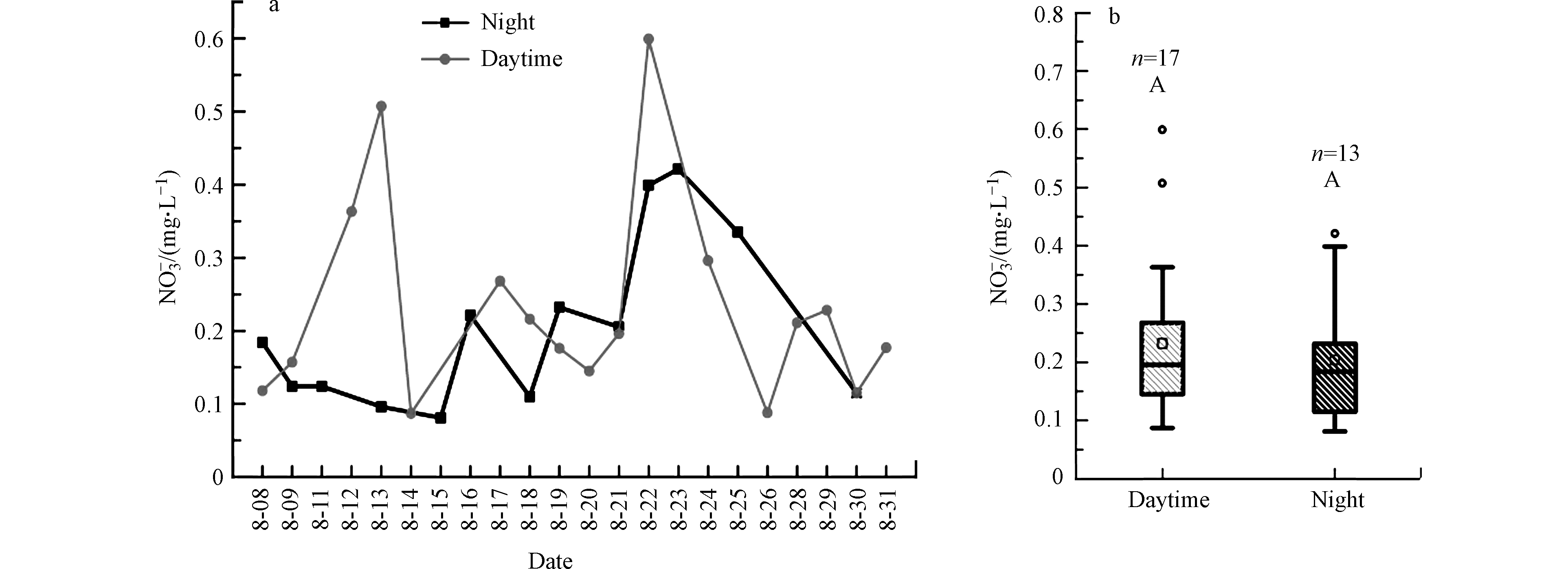
本研究NOy干沉降样本中NO3−浓度的范围为0.08—0.60 mg·L−1 (平均值(0.22±0.13) mg·L−1)(图3),与何瑞亮等[25]在重庆市夏季干沉降得到的数据(范围为0.29—0.41 mg·L−1)相类似。
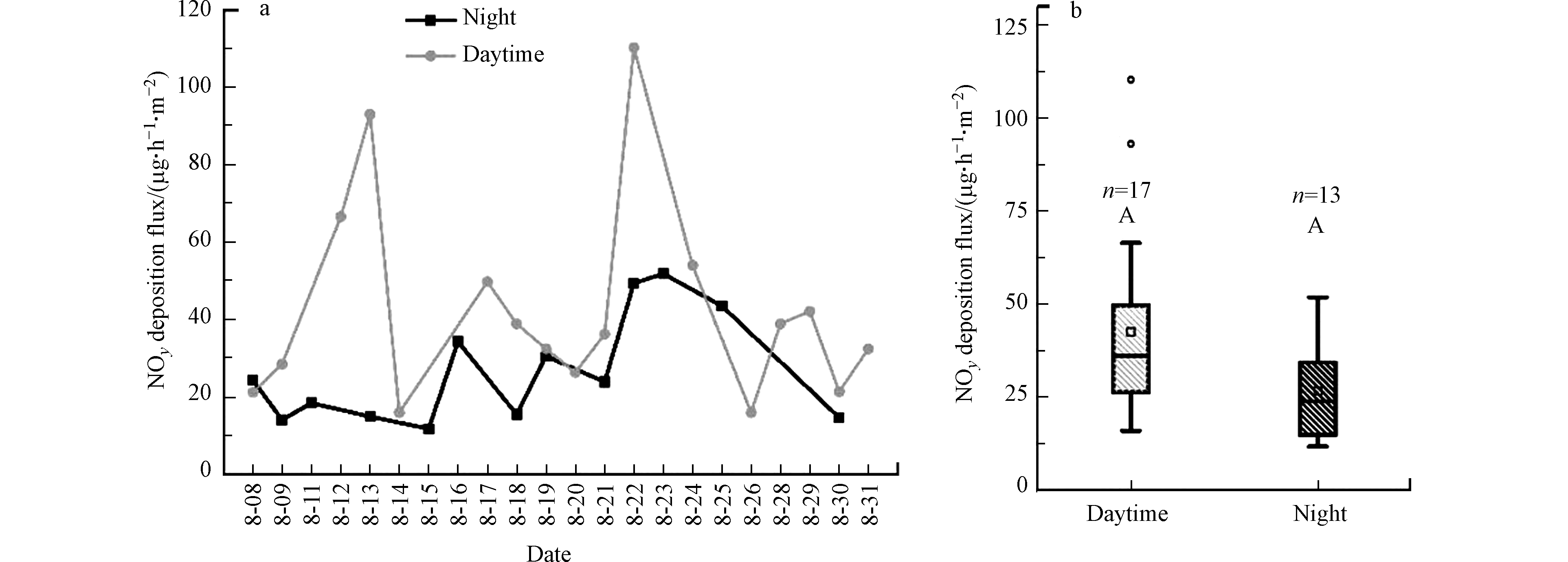
昼夜NO3−浓度变化范围分别是0.09—0.60 mg·L−1 (平均浓度为(0.23±0.14) mg·L−1)和0.08—0.42 mg·L−1 (平均值为(0.20 ±0.12) mg·L−1),但是昼夜无显著性差异(P=0.56)。NOy干沉降通量为11.9—110.2 μg·h−1·m−2(均值(35.7 ±22.9) μg·h−1·m−2)(图4),高于何瑞亮等[25]在重庆夏季估算的NOy干沉降沉降通量(范围为12.1—16.7 μg·h−1·m−2)。这可能与区域大气NOx污染程度不同有关。NOy干沉降沉降通量在白天16.0—110.2 μg·h−1·m−2(平均值(42.6±26.1) μg·h−1·m−2)高于晚上的沉降通量11.9—51.9 μg·h−1·m−2(平均值(26.7±14.1) μg·h−1·m−2)(图4b),这可能与昼夜温度有关。例如,张艳等[26]的研究中表明在白天太阳强烈照射,近地面温度高于高层大气,形成上下空气层温度分布不均,从而大气状态不稳定,湍流活动性强,导致较大的干沉积速率,从而形成较大的沉降通量。值得注意的是,在8月12日、13日和22日的白天,NOy干沉降沉降通量远高于其他观测日(图4a),这可能与气体湍流活动和PM2.5浓度等有关,也可能与白天采样过程中水分蒸发严重有关。具体原因须进一步研究。
-
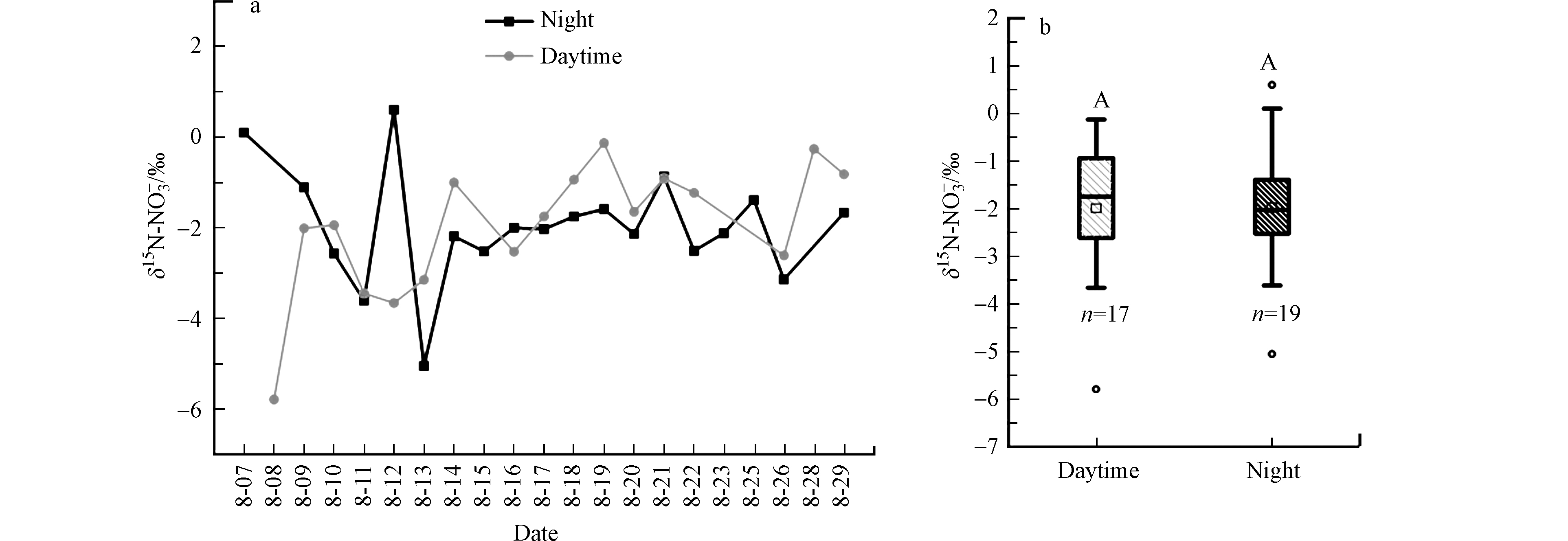
δ15N-NO3−值的范围是−5.8‰—+0.6‰(均值(−2.0‰±1.3‰))(图5a)。本研究δ15N-NO3−值的范围明显小于前人报道的大气降雨和气溶胶中δ15N-NO3−的范围(−15.8‰—+12.7‰)(表1)。本研究中δ15N-NO3−的均值低于成都夏季气溶胶中δ15N-NO3−值(+2.1‰±6.1‰)[27]和广州降雨中δ15N-NO3−值(+4.8‰±3.2‰)[28],但是与百慕大降雨中δ15N-NO3−值(−2.1‰±1.5‰)[16]相似。不同区域报道的夏季δ15N-NO3−值不同,说明了这些区域大气中NOx来源不同。尽管有些NO3−形成路径会引起δ15N分馏不同,例如在300K温度下,N2O5+H2O路径形成的δ15N-HNO3值比δ15N-NO2值高25.5‰,相反NO3+VOCs路径形成的δ15N-HNO3值比δ15N-NO2值低18.0‰。但是,NO2+OH路径形成的δ15N-HNO3值稍低于δ15N-NO2值(−3‰—0‰)[6]。
前人研究显示,南昌8月份NO2+OH路径形成的HNO3贡献了60%以上的气溶胶NO3−,N2O5+H2O和NO3+VOCs等路径的贡献量分别为26%和12%[4]。根据氮同位素质量守恒定律,HNO3形成路径引起的δ15N分馏为3.5‰,该分馏值远远低于不同来源δ15N-NOx的差异(如燃煤产生的δ15N-NOx值为(13.5‰±4.9‰)[8-9],机动车尾气(−4.3‰±4.5‰) [10-12],闪电固氮(−0.5‰—+1.4‰)[13],土壤微生物排放δ15N-NOx值为(−28.9‰±8.2‰)[10,14-15])。
本研究中南昌夏季干沉降样本中δ15N-NO3−值主要受来源影响。考虑分馏作用的影响,南昌市夏季干沉降样本中δ15N-NO3−值与汽车尾气排放的δ15N-NOx相似,说明南昌市夏季大气干沉降样本中NO3−主要来源于汽车尾气。另外,南昌夏季干沉降样本中δ15N-NO3−值位于闪电固氮(−0.5‰—+1.4‰)[13],土壤微生物排放δ15N-NOx值(−28.9‰±8.2‰)[10,14-15]之间,暗示闪电固氮和土壤微生物固氮也可能对南昌市夏季大气中NOx存在一定的贡献。例如,对广州夏季降雨中δ15N-NO3−值分析后,陈法锦等[28]认为雷电对广州夏季大气中NOx存在一定的贡献。对中国北方地方北隍城岛和北京市夏季气溶胶中δ15N-NO3−值分析认为,夏季土壤微生物活动释放的NOx对大气气溶胶NO3−贡献高达10%左右[17,32]。
如图5b所示,南昌市夏季白天δ15N-NO3−值(范围−5.8‰—−0.1‰,均值(−2.0‰±1.4‰))与晚上δ15N-NO3−值(−5.0‰—+0.6‰,均值(−2.0‰±1.2‰))相同。该趋势与Luo等[19]在晚春北京观测的气溶胶δ15N-NO3−值的昼夜变化趋势一致,但是与杨周等[27]在成都夏季的研究结果(夜间δ15N-NO3−值高于白天)不相符。杨周等[27]认为,成都白天气溶胶δ15N-NO3−值低于晚上是与白天农业活动强,土壤释放的NOx通量较大有关。Luo等[19]认为,晚春的北京气溶胶δ15N-NO3−值没有昼夜差异与昼夜NOx来源和不同路径形成NO3−引起的15N分馏有关。但是如图5a所示,本研究干沉降样本中δ15N-NO3−值出现昼夜交替的现象,表明南昌市夏季干沉降样本中δ15N-NO3−值也同时与昼夜NOx来源差异和不同路径形成NO3−引起的15N分馏有关。
-
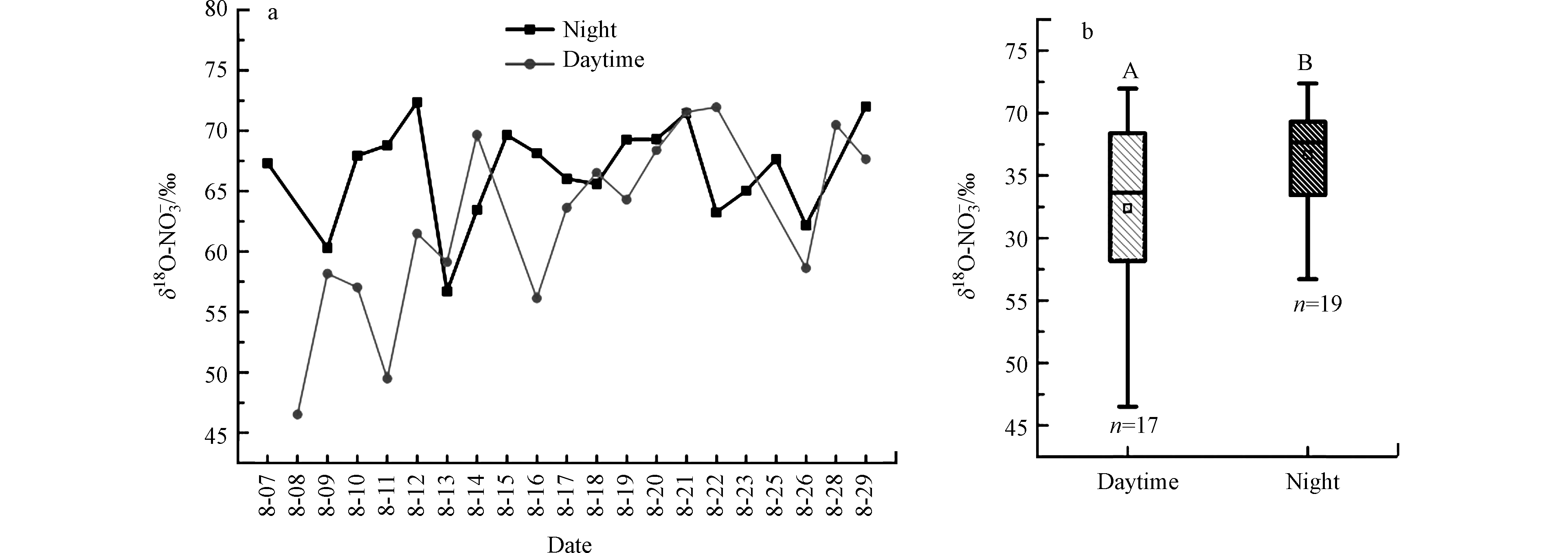
南昌市夏季NOy干沉降样本中δ18O-NO3−值的范围是46.5‰—72.4‰,平均值(64.6‰±6.3‰)(图6),该值与北半球夏季气溶胶和降雨中δ18O-NO3−值的范围相似(表1),对南昌结合模型和气溶胶δ18O-NO3−值,Luo等[4]研究认为南昌市夏季NO2+OH路径形成的NO3−占气溶胶中总NO3−的60%以上,说明南昌市夏季大气中NO3−以NO2+OH路径形成主导。本研究中,尽管δ18O-NO3−值在白天和晚上出现了交替(图6a),但是干沉降样本中δ18O-NO3−值在晚上(66.7‰±4.1‰)高于白天(62.4‰±7.5‰),这说明白天NO3−的主要形成路径与晚上存在一定的差异。
大气中的NOx主要是NO和NO2两种形式,NO和NO2在光化学作用下,氧原子与臭氧迅速发生交换,因此大气中的硝酸盐氧同位素由氧化反应途径决定。在NO3−形成路径中,不同的前体氧化物有不同的δ18O值(例如,Luo等[4]估算了南昌市夏季8月份δ18O-OH、δ18O-NO2、δ18O-NO3和δ18O-N2O5值分别为(−51.0‰±0.5‰)、(92.7‰±7.7‰)、(97.0‰±8.2‰)和(95.0‰±8.0‰))。根据氧同位素质量守恒,南昌市夏季8月份白天NO2+OH路径形成的δ18O-HNO3值(45.0‰±0.2‰)低于晚上N2O5+H2O路径和NO3+VOCs路径形成的δ18O-HNO3(分别为(77.7‰±6.6‰)和(97.0‰±8.2‰))。由于南昌夏季的白天日照时间长,太阳辐射作用强,羟基自由基浓度高[4],因此白天NO2+OH路径生成的HNO3高,从而降低了白天NOy干沉降样本中δ18O-NO3−值。相反在晚上,由于羟基自由基不再生成,理论上N2O5+H2O路径和NO3+VOCs路径主导HNO3形成。根据图6a,可以得出白天与夜晚δ18O-NO3−值出现重叠,由此说明,存在另外一种可能,因为采样时间较长(12 h),而大气气相HNO3和颗粒NO3−有相对长的寿命(—2 d)[33],即晚上收集的NOy干沉降样本可能继承了白天的δ18O-NO3−信号,相反白天收集的NOy干沉降样本也可能受到晚上形成HNO3的影响。
-
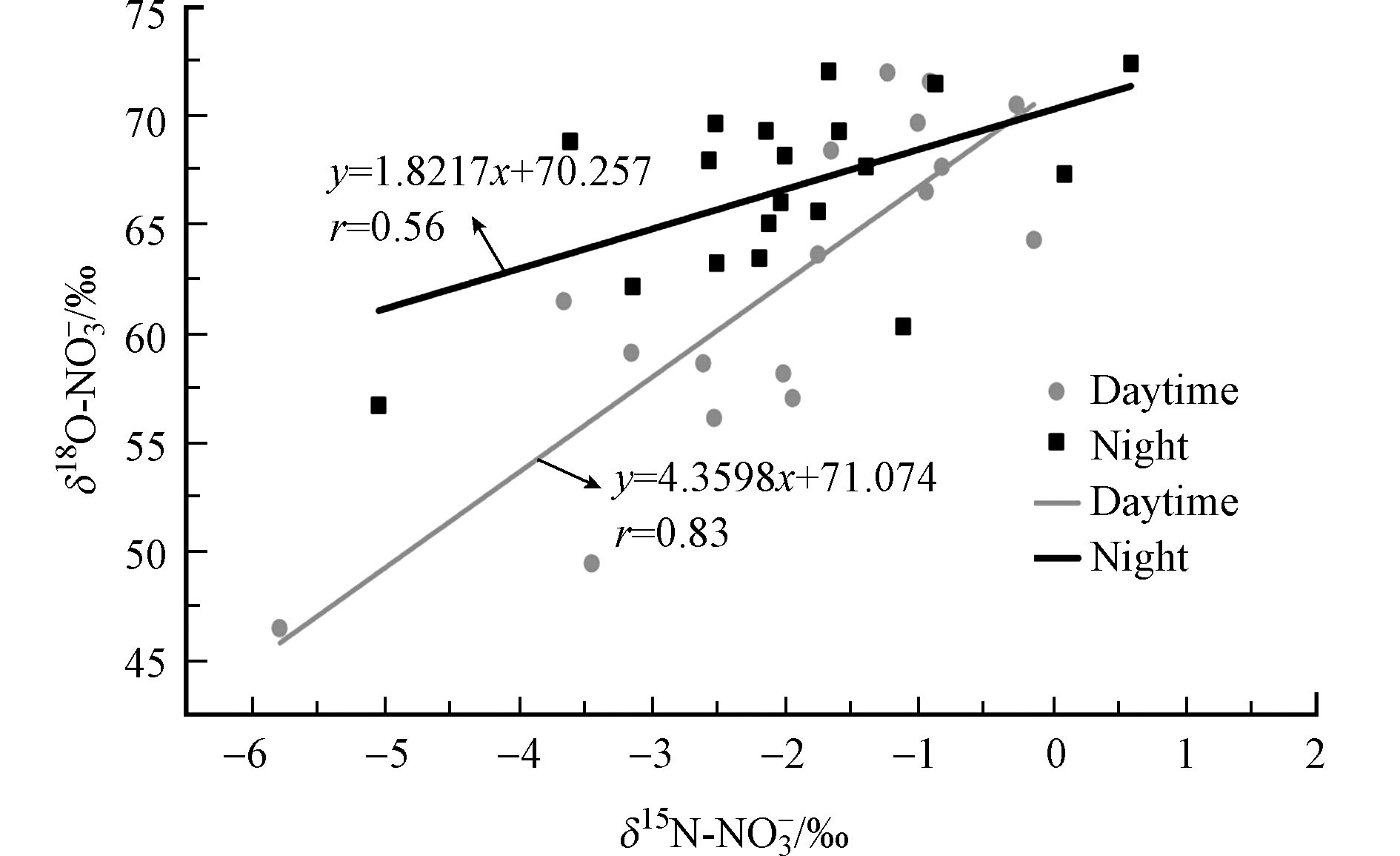
NOy干沉降样品中,δ15N-NO3−值和δ18O-NO3−值存在显著的正相关关系(P<0.05) (图7),该趋势与Elliott等[34]报道的美国东部地区降雨、气溶胶和气态HNO3中的δ15N-NO3−值和δ18O-NO3−值的线性相关性一致。
相反,在大西洋百慕大和南海东沙岛采集雨水样本中δ15N-NO3−值和δ18O-NO3−值却存现负相关性[16,31,35]。而Fang等[7]在广州市连续2年的降雨观测显示,δ15N-NO3−值和δ18O-NO3−值之间没有正或负相关性。不同研究区域δ15N-NO3−值和δ18O-NO3−值之间的相关性的差异可能与区域性的NOx来源,NO3−形成路径引起的15N分馏和NO3−形成路径产生的δ18O-NO3−值不同有关[1,5-6,9-11,18,34]。本研究所有NOy干沉降样本采集于夏季的8月份,NOx来源变化应该不大。因此,δ15N-NO3−值和δ18O-NO3−值正相关关系(图7)主要由不同NO3−形成路径引起的15N分馏和NO3−形成路径产生的δ18O-NO3−值不同引起。低的δ15N-NO3−值和δ18O-NO3−值说明了NO2+OH路径是主要的HNO3形成路径,高的δ15N-NO3−值和δ18O-NO3−值说明了N2O5+H2O则是主要的HNO3形成路径,相对低的δ15N-NO3−值和高的δ18O-NO3−值则表明了NO3+VOCs路径是主要的HNO3形成路径。
-
南昌市夏季NOy干沉降通量为11.9—110.2 μg·h−1·m−2(均值(35.7±22.9) μg·h−1·m−2),昼夜无显著性差异。NOy干沉降样本中δ15N-NO3−值的范围是−5.8‰—+0.6‰(均值(−2.0‰±1.3‰)),白天δ15N-NO3−值(范围−5.8‰—−0.1‰,均值(−2.0‰±1.4‰))与晚上δ15N-NO3−值(−5.0‰—+0.6‰,均值(−2.0‰±1.2‰))相同。结合氮同位素分馏和不同来源的NOx的氮同位素,表明汽车尾气是南昌市夏季大气中NOx的主要来源。NOy干沉降样本中δ18O-NO3−值的范围是46.5‰—72.4‰(平均值(64.6‰±6.3‰)),在白天(62.4‰±7.5‰)低于晚上(66.7‰±4.1‰),说明了昼夜NO3−形成路径差异性的存在。δ15N-NO3−值和δ18O-NO3−值之间的相关性进一步说明昼夜间NO3−形成路径的差异。
南昌市夏季NOy干沉降及其氮氧同位素昼夜变化特征
Nyctohemeral variations of NOy
-
摘要: 大气活性氮沉降对陆地和海洋生态系统有重要的影响,对于气溶胶和降雨中硝酸盐沉降通量和硝酸盐氮氧同位素的研究较多,但对NOy(NO、NO2、NO3、N2O5、HNO3和NO3−)干沉降通量及其氮氧同位素的研究很少。本研究于2019年8月7日—2019年8月31日在江西省南昌市东华理工大学分昼夜采集了NOy干沉降样品,分析了干沉降样本中硝酸盐浓度,δ15N-NO3−和δ18O-NO3−值,基于干沉降样本中硝酸盐浓度估算了大气NOy干沉降通量。结果表明,南昌市夏季白天NOy干沉降通量(16.0—110.2 μg·h−1·m−2,均值(42.6±26.1)μg·h−1·m−2)高于夜晚(11.9—51.9 μg·h−1·m−2,均值(26.7±14.1) μg·h−1·m−2)。NOy干沉降样品中δ18O-NO3−值在白天(均值为(62.4‰±7.5‰))稍低于夜晚(均值为(66.7‰±4.1‰)),表明白天NOy参与的化学过程与晚上不同。NOy干沉降样品中δ15N-NO3−在白天(−5.8‰—−0.1‰,均值为(−2.0‰±1.4‰))与晚上(−5.0‰—+0.6‰,均值为(−2.0‰±1.2‰))类似,表明机动车尾气是南昌市夏季大气中NOx的主要来源。Abstract: Atmospheric reactive nitrogen deposition has important effects on terrestrial and marine ecosystems. Although many studies have focused on the nitrate deposition fluxes and nitrate dual isotopic compositions of aerosol and precipitation, rarely studies reported the NOy (NO2, N2O5, HNO3 and NO3−) dry deposition fluxes and nitrogen and oxygen isotopic compositions. In this study, the NOy dry deposition samples were collected during the daytime and at night on the campus of East China University of Technology in Nanchang City from August 7 to 31, 2019. Concentration of nitrate, values of δ15N-NO3− and δ18O-NO3− in dry deposition samples were analyzed, and the dry deposition fluxes of NOy were estimated. The estimated NOy dry deposition fluxes during the daytime (from 16.0—110.2 μg·h−1·m−2 with average of(42.6±26.1) μg·h−1·m−2 ) were higher than these during night (from 11.9—51.9 μg·h−1·m−2 with mean of (26.7±14.1) μg·h−1·m−2). Values of δ18O-NO3− in daytime (with average of 62.4‰ ±7.5‰) were lower than these during night (with average of 66.7‰±4.1‰), indicated that the NOy involved atmospheric photochemical oxidation processes in daytime were different from these at night. δ15N-NO3− values in daytime (from −5.8‰ to −0.1‰, with mean of (−2.0‰±1.4‰)) were similar to these at night (from −5.0‰ to +0.6‰, with average of (−2.0‰±1.2‰)), suggested that vehicle exhaust was the main NOx source of in the atmosphere of Nanchang City in summer.
-
Key words:
- Nanchang City /
- NOy dry deposition /
- nitrate /
- nitrogen and oxygen isotope
-

-
表 1 与其他地区硝酸盐氮、氧同位素比较
Table 1. Comparison of δ15N-NO3− and δ18O-NO3− in This Study with Other Regions
研究点
Research site采样时间
(夏季)
Sample time
(Summer)采样类型
Type of samplingδ15N-NO3−
范围/‰
Range of
δ15N-NO3−δ15N-NO3−
平均值/‰
Average of
δ15N-NO3−δ18O-NO3−
范围/‰
Range of
δ18O-NO3−δ18O-NO3−
平均值/‰
Average of
δ15N-NO3−参考文献
References南昌 2019-07—2019-08 NOy干沉降 −5.8—+0.6 −2.0±1.3 46.5—72.4 64.6±6.3 本研究 贵阳(小雨) 2001-06—2001-07 降雨 −3.8—+8.5 2 — — [29] 贵阳(暴雨) 2001-06—2001-07 降雨 −2.9—+10.1 4.1 — — [29] 九龙江流域 2005-05—2005-08 降雨 −7.5— −0.3 −3.6 — — [20] 成都 2014-08 气溶胶 −15.8—+8.1 +2.1±6.1 21.6—76.5 59.2±13.6 [27] 广州 2007-07—2007-09 降雨 0—+12.7 +4.8±3.2 — — [28] 百慕大群岛 2000—2001暖季 降雨 −4.8—+1.6 −2.1±1.5 60.3—78.5 68.6±3.6 [16] 广东 2008-04—2008-09 降雨 −3.9—+7.9 3.8 33.4—81.5 63.5 [7] 广东 2009-04—2009-09 降雨 +0.5—+10.1 4.1 47.2—86.2 66.4 [7] 日本秋田县 2010夏季 气溶胶 — −2.5±1.5 — — [30] 南昌 2018夏季 气溶胶 −1.9—+10.2 +3.1±2.4 50.7—70.5 60.7±3.9 [4] 百慕大(沿海) 2009—2011暖季 降雨 −6.2—+8.9 −0.8±3.0 50.5—77.7 68.2±5.1 [31] 百慕大(大陆) 2009—2011暖季 降雨 −5.2—0 −3.0±2.1 64.6—77.3 71.3±4.4 [31] -
[1] LUO L, ZHU R G, SONG C B, et al. Changes in nitrate accumulation mechanisms as PM2.5 levels increase on the North China Plain: A perspective from the dual isotopic compositions of nitrate [J]. Chemosphere, 2021, 263: 127915. doi: 10.1016/j.chemosphere.2020.127915 [2] LI H Y, ZHANG Q, ZHENG B, et al. Nitrate-driven urban haze pollution during summertime over the North China Plain [J]. Atmospheric Chemistry and Physics, 2018, 18(8): 5293-5306. doi: 10.5194/acp-18-5293-2018 [3] THIEMENS M H, JACKSON T. Pressure dependency for heavy isotope enhancement in ozone formation [J]. Geophysical Research Letters, 1990, 17(6): 717-719. doi: 10.1029/GL017i006p00717 [4] LUO L, PAN Y Y, ZHU R G, et al. Assessment of the seasonal cycle of nitrate in PM2.5 using chemical compositions and stable nitrogen and oxygen isotopes at Nanchang, China [J]. Atmospheric Environment, 2020, 225: 117371. [5] LUO L, KAO S J, WU Y F, et al. Stable oxygen isotope constraints on nitrate formation in Beijing in springtime [J]. Environmental Pollution, 2020, 263: 114515. [6] WALTERS W W, MICHALSKI G. Theoretical calculation of oxygen equilibrium isotope fractionation factors involving various NOy molecules, [rad]OH, and H2O and its implications for isotope variations in atmospheric nitrate [J]. Geochimica et Cosmochimica Acta, 2016, 191: 89-101. doi: 10.1016/j.gca.2016.06.039 [7] FANG Y T, KOBA K, WANG X M, et al. Anthropogenic imprints on nitrogen and oxygen isotopic composition of precipitation nitrate in a nitrogen-polluted city in southern China [J]. Atmospheric Chemistry and Physics, 2011, 11(220): 1313-1325. [8] HEATON T H E. 15N/14N ratios of NOx from vehicle engines and coal‐fired power stations [J]. Tellus:Series B, Chemical and Physical Meteorology, 1990, 42(3): 304-307. [9] FELIX J D, ELLIOTT E M, SHAW S L. Nitrogen isotopic composition of coal-fired power plant NOx:Influence of emission controls and implications for global emission inventories [J]. Environmental Science and Technology, 2012, 46(6): 3528-3535. doi: 10.1021/es203355v [10] FELIX J D, ELLIOTT E M. Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures [J]. Atmospheric Environment, 2014, 92: 359-366. [11] WALTERS W W, GOODWIN S R, MICHALSKI G. Nitrogen stable isotope composition (δ15N) of vehicle-emitted NOx [J]. Environmental Science and Technology, 2015, 49(4): 2278-2285. doi: 10.1021/es505580v [12] MILLER D J, WOJTAL P K, CLARK S C, et al. Vehicle NOx emission plume isotopic signatures: Spatial variability across the eastern United States [J]. Journal of Geophysical Research:Atmospheres, 2017, 122(8): 4698-4717. doi: 10.1002/2016JD025877 [13] THOMAS H. The isotopic composition of the ammonia and the nitrate ion in rain [J]. Geochimica et Cosmochimica Acta, 1957, 12(1、2): 97-102. [14] LI D J, WANG X M. Nitrogen isotopic signature of soil-released nitric oxide (NO) after fertilizer application [J]. Atmospheric Environment, 2008, 42(19): 4747-4754. doi: 10.1016/j.atmosenv.2008.01.042 [15] FELIX J D, ELLIOTT E M. The agricultural history of human‐nitrogen interactions as recorded in ice core δ15N‐NO3- [J]. Geophysical Research Letters, 2013, 40(8): 1642-1646. doi: 10.1002/grl.50209 [16] HASTINGS M G, SIGMAN D M, LIPSCHULTZ F. Isotopic evidence for source changes of nitrate in rain at Bermuda [J]. Journal of Geophysical Research:Earth Surface, 2003, 108(D24): 4790. [17] ZONG Z, WANG X P, TIAN C G, et al. First assessment of NOx sources at a regional background site in North China using isotopic analysis linked with modeling [J]. Environmental Science and Technology, 2017, 51(11): 5923-5931. doi: 10.1021/acs.est.6b06316 [18] CHANG Y H, ZHANG Y L, TIAN C G, et al. Nitrogen isotope fractionation during gas-to-particle conversion of NOx to NO3− in the atmosphere – implications for isotope-based NOx source apportionment [J]. Atmospheric Chemistry and Physics, 2018, 18: 11647-11661. doi: 10.5194/acp-18-11647-2018 [19] LUO L, WU Y F, XIAO H Y, et al. Origins of aerosol nitrate in Beijing during late winter through spring [J]. Science of The Total Environment, 2019, 653: 776-782. doi: 10.1016/j.scitotenv.2018.10.306 [20] 陈能汪, 洪华生, 张珞平. 九龙江流域大气氮湿沉降研究 [J]. 环境科学, 2008, 29(1): 38-46. doi: 10.3321/j.issn:0250-3301.2008.01.007 CHEN N W, HONG H S, ZHANG L P. Wet deposition of atmospheric nitrogen in Jiulong River watershed [J]. Environmental Science, 2008, 29(1): 38-46(in Chinese). doi: 10.3321/j.issn:0250-3301.2008.01.007
[21] SIGMAN D M, CASCIOTTI K L, ANDREANI M, et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater [J]. Analytical Chemistry, 2001, 73(17): 4145-4153. doi: 10.1021/ac010088e [22] CASCIOTTI K L, SIGMAN D M, HASTINGS M G, et al. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method [J]. Analytical Chemistry, 2002, 74(19): 4905-4912. doi: 10.1021/ac020113w [23] BÖHLKE J K, MROCZKOWSKI S J, COPLEN T B. Oxygen isotopes in nitrate: New reference materials for 18O: 17O: 16O measurements and observations on nitrate-water equilibration [J]. Rapid Communications in Mass Spectrometry, 2003, 17(16): 1835-1846. doi: 10.1002/rcm.1123 [24] LUO L, KAO S J, BAO H Y, et al. Sources of reactive nitrogen in marine aerosol over the Northwest Pacific Ocean in spring [J]. Atmospheric Chemistry and Physics, 2018, 18(9): 6207-6222. doi: 10.5194/acp-18-6207-2018 [25] 何瑞亮, 蒋勇军, 张远瞩, 等. 重庆市近郊大气无机氮、硫沉降特征及其来源分析 [J]. 生态学报, 2019, 39(16): 6173-6185. HE R L, JIANG Y J, ZHANG Y Z, et al. Characteristics and sources of atmospheric inorganic nitrogen and sulfur deposition in the suburbs of Chongqing [J]. Acta Ecologica Sinica, 2019, 39(16): 6173-6185(in Chinese).
[26] 张艳, 王体健, 胡正义, 等. 典型大气污染物在不同下垫面上干沉积速率的动态变化及空间分布 [J]. 气候与环境研究, 2004, 9(4): 591-604. doi: 10.3878/j.issn.1006-9585.2004.04.05 ZHANG Y, WANG T J, HU Z Y, et al. Temporal variety and spatial distribution of dry deposition velocities of typical air pollutants over different landuse types [J]. Climatic and Environmental Research, 2004, 9(4): 591-604(in Chinese). doi: 10.3878/j.issn.1006-9585.2004.04.05
[27] 杨周, 李晓东, 雷国良, 等. 成都市PM2.5中无机组分与硝酸盐氮氧同位素变化特征 [J]. 地球与环境, 2020, 48(1): 10-16. YANG Z, LI X D, LEI G L, et al. Characteristics of inorganic components and nitrate nitrogen-oxygen isotopes of PM2.5 in the Chengdu City [J]. Earth And Environment, 2020, 48(1): 10-16(in Chinese).
[28] 陈法锦, 贾国东, 陈建芳, 等. 广州夏季雨水硝酸盐δ15N变化特征 [J]. 地球化学, 2010, 39(2): 154-158. CHEN F J, JIA G D, CHEN J F, et al. The variation of nitrate δ15N in summertime rainwater in Guangzhou [J]. Geochimica, 2010, 39(2): 154-158(in Chinese).
[29] XIAO H Y, LIU C Q. Sources of nitrogen and sulfur in wet deposition at Guiyang, southwest China [J]. Atmospheric Environment, 2002, 36(33): 5121-5130. doi: 10.1016/S1352-2310(02)00649-0 [30] KAWASHIMA H, KURAHASHI T. Inorganic ion and nitrogen isotopic compositions of atmospheric aerosols at Yurihonjo, Japan: Implications for nitrogen sources [J]. Atmospheric Environment, 2011, 45(35): 6309-6316. doi: 10.1016/j.atmosenv.2011.08.057 [31] ALTIERI K E, HASTINGS M G, GOBEL A R, et al. Isotopic composition of rainwater nitrate at Bermuda: The influence of air mass source and chemistry in the marine boundary layer [J]. Journal of Geophysical Research:Atmospheres, 2013, 118(19): 11,304-11,316. doi: 10.1002/jgrd.50829 [32] SONG W, WANG Y L, YANG W, et al. Isotopic evaluation on relative contributions of major NOx sources to nitrate of PM2.5 in Beijing [J]. Environmental Pollution, 2019, 248: 183-190. doi: 10.1016/j.envpol.2019.01.081 [33] LIANG J Y, HOROWITZ L W, JACOB D J, et al. Seasonal budgets of reactive nitrogen species and ozone over the United States, and export fluxes to the global atmosphere [J]. Journal of Geophysical Research:Earth Surface, 1998, 103(D11): 13435-13450. doi: 10.1029/97JD03126 [34] ELLIOTT E M, KENDALL C, BOYER E W, et al. Dual nitrate isotopes in dry deposition: Utility for partitioning NOx source contributions to landscape nitrogen deposition [J]. Journal of Geophysical Research:Earth Surface, 2009, 114(G4): 425-453. [35] YANG, J Y T, HSU S C, DAI M H, et al. Isotopic composition of water-soluble nitrate in bulk atmospheric deposition at Dongsha Island: Sources and implications of external N supply to the northern South China Sea [J]. Biogeosciences, 2014, 11(7): 1833-1846. doi: 10.5194/bg-11-1833-2014 -



 下载:
下载: