-
三维氧化石墨烯(3D-GO)是在氧化石墨烯的基础上,通过水热自组装方法合成的具有高孔隙密度、比表面积大、导电性能强等优异性能的新型材料[1]。基于这些优异性能,3D-GO在许多领域得到了广泛地应用,如扩充电池电容[2]、药物缓控释放[3]和环境污染修复等[4]。尤其值得关注的是,3D-GO的高比表面积和孔隙密度使其对多种类型的污染物,如重金属、酚类[5]等均表现出优良的吸附能力。有研究发现游离金属离子在高pH下可以通过中和负电荷促进碳纳米管对邻氯苯甲酸的吸附[6];还有研究发现在石墨烯-碳纳米管三维材料合成过程中添加Fe2+可以促进对铅的吸附[7]。然而,金属离子嫁接在3D-GO上对有机污染物吸附的影响还未见报道。
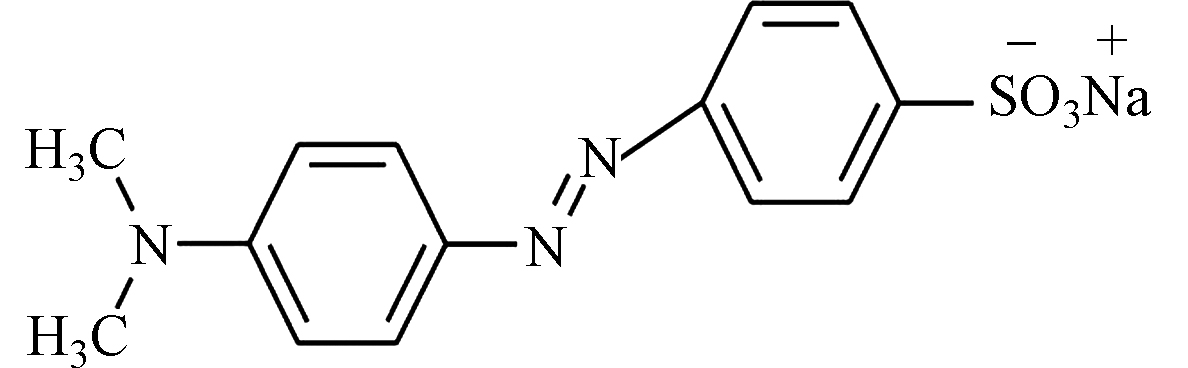
染料废水因具有色度深、有机污染物含量高、组分复杂、生物毒性大、难生物降解等特点,研究处理染料废水成为当今环保领域的热点和难点[8-9],甲基橙(MO)是一种难降解的偶氮类-阴离子型染料,具有致突变和致癌性,危害水生生物和人体健康[10]。
本研究选取不同电荷的金属阳离子(Ag+、Ca2+),通过水热合成嫁接于3D-GO上。选取甲基橙(MO)作为模型有机污染物,系统探究金属离子嫁接对3D-GO吸附MO的影响机理。
全文HTML
-
氧化石墨烯(纯度>99%)、硝酸银、硝酸钙(分析纯),购于上海阿拉丁生化科技股份有限公司;甲基橙(纯度>99%),购于上海阿拉丁生化科技股份有限公司,其基本性质见表1。
-
Autosorb-IQ2比表面积分析仪:美国康塔公司;640-IR型傅立叶红外光谱仪:美国瓦里安公司;元素分析仪:德国元素分析系统公司;QuantaFEG250扫描电镜:美国赛默森公司;UV-2600型紫外-可见分光光度计:日本岛津株式会社;Naobrook 90plus-PALS-Zeat电位分析仪:美国Bruker海文仪器有限公司。
-
采用水热自组装还原法制备3D-GO并对其进行金属阳离子的嫁接。具体实验操作如下:通过超声将二维氧化石墨烯(2D-GO)分散在超纯水中,获得浓度为5 mg·L−1的氧化石墨烯悬浮液。分别配制浓度为400 mg·L−1和100 mg·L−1的Ag+、Ca2+的溶液,将5 mg·L−1的2D-GO溶液与金属离子溶液分别以15∶1(金属离子占比7%)和20∶1(金属离子占比5%)的比例进行混合并再次超声。混合悬浮液置于反应釜中于150 ℃下恒温加热6 h,冷却水洗后得到不同阳离子嫁接浓度的3D-GO,分别以5%-Ag、7%-Ag、5%-Ca、7%-Ca表示。此外,将未加入金属离子的同浓度的2D-GO溶液以同样流程制备3D-GO作为对照。
-
利用扫描电子显微镜(SEM)观察材料的微观外貌;利用比表面积分析仪测定样品的比表面积和孔体积;元素分析仪分析金属离子嫁接后材料元素含量变化;傅立叶红光谱(FT-IR)分析金属离子嫁接对材料表面官能团的影响;Zeta电位分析pH对材料表面电荷的影响。
-
通过批量吸附实验分别获取在pH=2和5时材料对MO的吸附等温线,以探究不同pH下甲基橙的吸附机理。其中,在pH=2时甲基橙主要以中性分子形式存在,pH=5时甲基橙则主要以负电离子形式存在。具体步骤如下:将一定质量的材料加入40 mL带聚四氟乙烯盖的玻璃瓶中,分别加入初始浓度为2—60 mg·L−1的MO溶液。吸附平衡48 h后,3000 r·min−1离心15 min,静置取上清液,利用紫外可见分光光度计测定其中MO浓度。pH=2时,选取最大吸收波长526 nm测定,pH=5时,选取波长为464 nm测定;试验结果采用Langmuir和Freundlich吸附等温线模型进行拟合。
Langmuir吸附等温线模型假设吸附过程处于均相单层界面[11]。公式为:
其中,Ce(mg·L−1)是吸附平衡时溶液的浓度;Qe(mg·g−1)是平衡时的吸附量;Qm(mg·g−1)为最大吸附容量,KL(L·mg−1)为与吸附能量相关的平衡常数。
Freundlich吸附等温线模型是可逆非均相的吸附,不受单层吸附能力的影响[12]。公式为:
其中,Ce(mg·L−1)是吸附平衡时溶液的浓度,Qe(mg·g−1)是平衡时的吸附量,n为非线性指数,KF为吸附平衡常数(mg1-n·Ln ·g−1)。
通过Freundlich模型进行点位能量分布计算,公式为:
其中,E*(kJ·mol−1)为平衡浓度为Ce时发生吸附的最低键能,公式为:
式中,Ce(mg·L−1)是吸附平衡时溶液的浓度,Cs(mg·L−1)是MO的溶解度,为5000 mg·L−1,R是通用气体常数,为8.314 × 10−3 kJ· (mol·K)−1,T为绝对温度,293.15 K。
1.1. 实验材料
1.2. 实验仪器
1.3. 金属离子嫁接三维氧化石墨烯的制备
1.4. 材料表征
1.5. 批量吸附实验
-
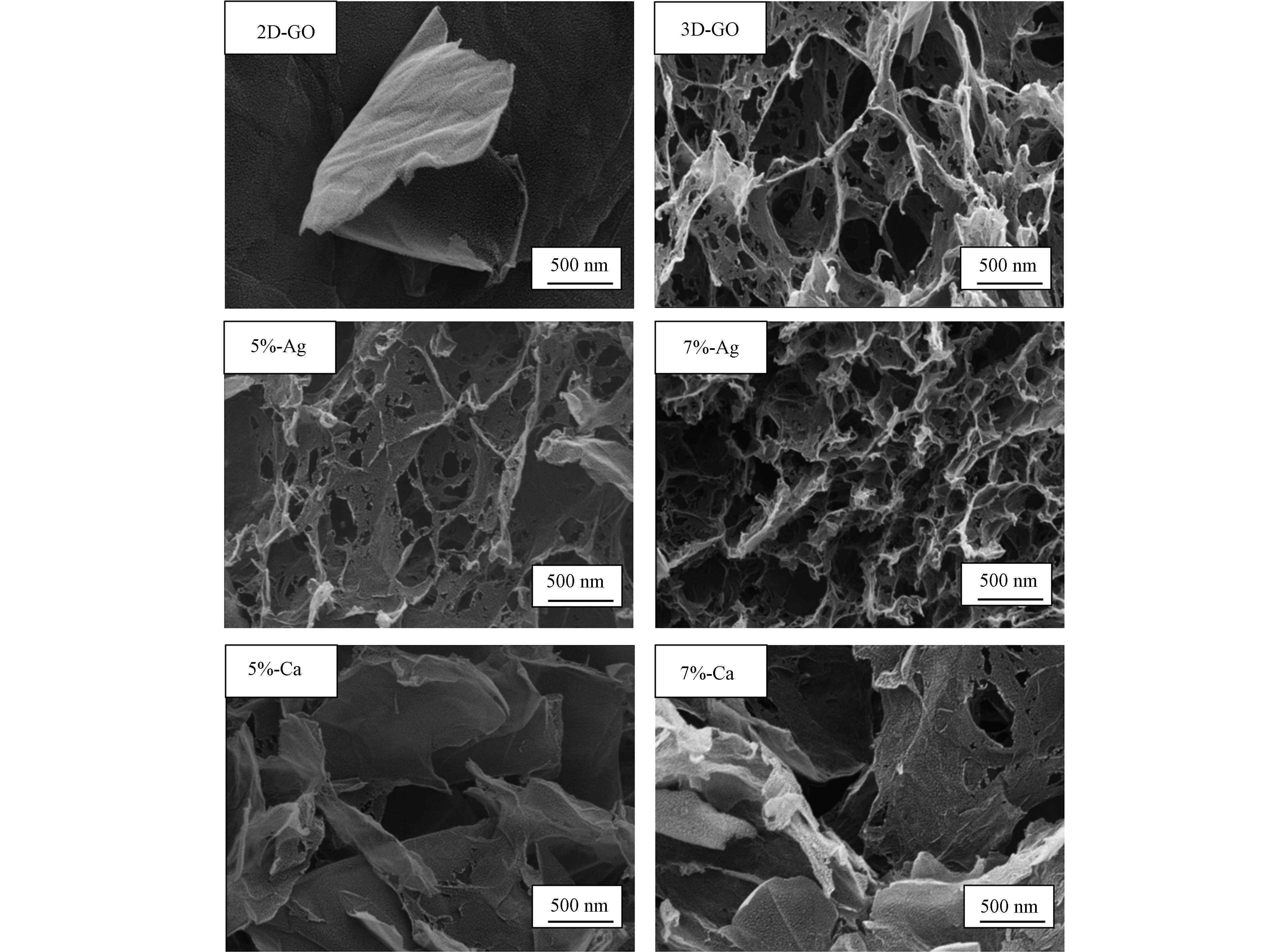
如图1所示,2D-GO表面有明显的褶皱状,片层之间因为π-π键的作用产生明显的堆叠痕迹,呈现出经典的氧化石墨烯结构[13-14]。经过水热法制备的3D-GO则具有光滑且蓬松的特点,且片层之间相互连接,可以明显观察到三维的多孔立体结构。Ag+嫁接对材料表面孔隙结构影响较大,且随着金属离子嫁接浓度的提高,材料表面孔结构变得愈加破碎;而Ca2+的嫁接造成原本的一些三维结构塌缩,使其的孔隙结构退化。
为进一步探究金属阳离子嫁接对三维氧化石墨烯材料表面性质的影响,通过氮气吸脱附实验比较了嫁接前后材料比表面积和孔体积。如表2所示,2D-GO的比表面积和孔体积较其他材料低,3D-GO的比表面积和孔体积均为最高。金属离子嫁接后的三维氧化石墨烯材料比表面积和孔体积下降,尤其是Ca2+的嫁接使比表面积和孔体积下降更为明显:Ca-5%和Ca-7%材料的比表面积分别下降了49.2%和74.8%,孔体积分别下降32.2%和58.8%。以上现象进一步表明金属离子在嫁接过程中会对三维材料表面结构产生破坏,并与SEM图所显示的现象作为互补验证。
-
为了研究金属离子嫁接前后材料官能团的变化,本研究通过元素分析测定了各材料的元素含量。其中金属元素含量通过扣除其他元素百分比计算所得,结果如表3所示。从表3中可以看出,金属离子嫁接处理后的材料均检测出金属元素,说明金属离子嫁接成功,但结合到材料上的金属量低于其初始加入量(5%和7%)。Ca2+的嫁接使得材料的氧含量相比于3D-GO均呈现出下降的趋势,表明Ca2+在嫁接的过程中对表面含氧官能团如—COOH和—OH[15]有一定的消耗。
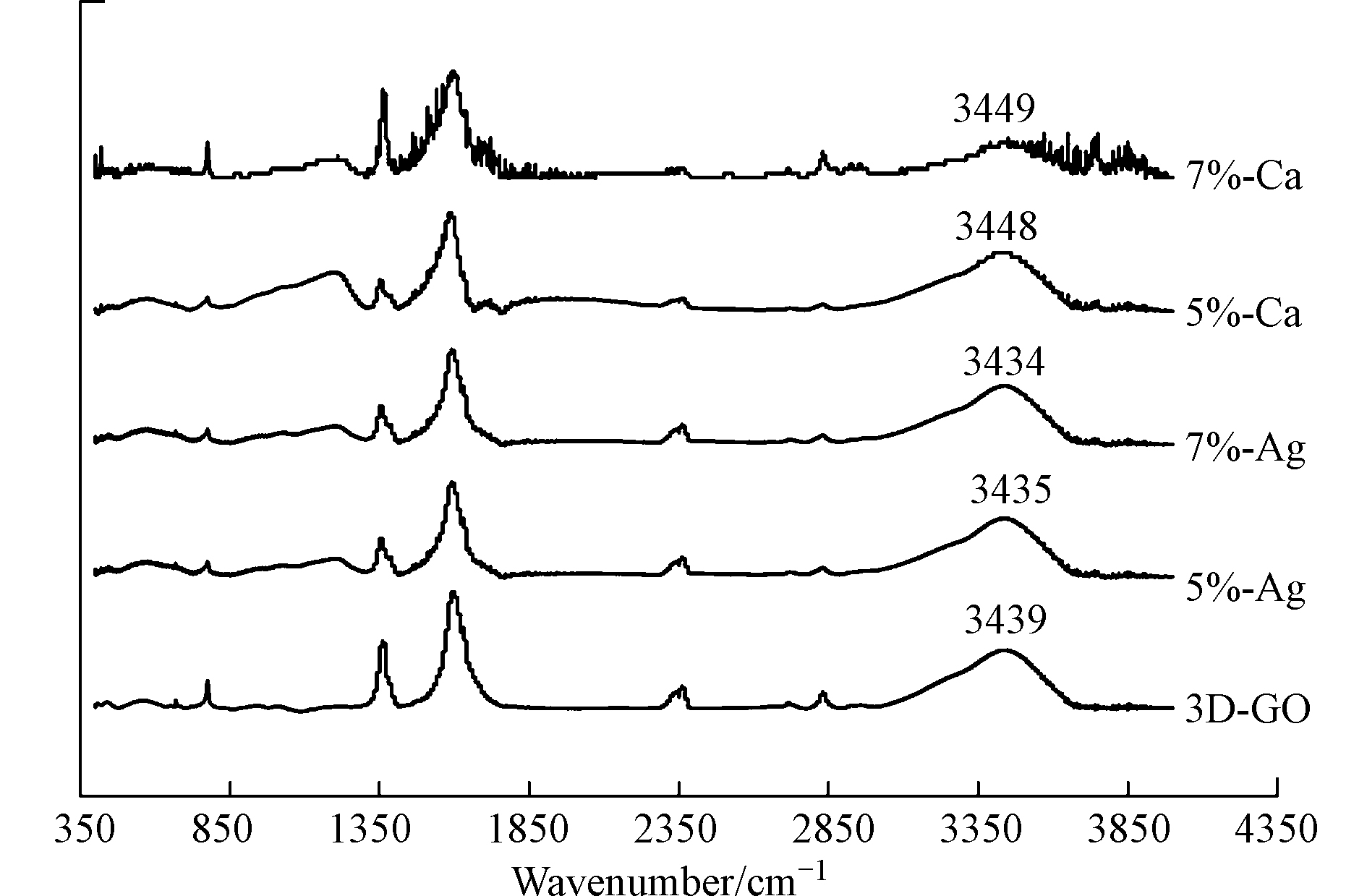
为进一步验证金属离子对材料表面含氧官能团的影响,通过比较红外光谱反映材料表面主要官能团的变化。图2傅立叶红外光谱中,3439 cm−1处—OH特征宽吸收峰、1350 cm−1处C—O与O—H相互作用特征吸收峰说明材料表面具有丰富的羟基[16],且嫁接不同金属离子的三维氧化石墨烯基材料的—OH特征吸收峰发生了变化。其中嫁接了Ag+的材料的—OH位置稍微发生位移;而嫁接Ca2+的材料尤其是Ca-7%则可以看出—OH位置峰型变化明显且强度减弱。
这是因为在嫁接进不同金属离子后,在金属离子的作用下材料表面的一部分的—OH会和金属离子形成化学键,导致羟基的特征吸收峰发生变化[17]。其中Ca2+由于价态更高,与—OH氧原子的作用更强,因此对—OH特征吸收峰影响更大。三维石墨烯表面丰富的含氧官能团如—OH可通过形成氢键而吸附离子型有机污染物;甲基橙具有6个氢键受体,因此氢键作用可能是两者间的吸附作用之一。此前也有研究者指出黏胶基活性碳纤维表面的羟基和甲基橙形成的氢键是两者发生吸附的主要机理[18]。
-
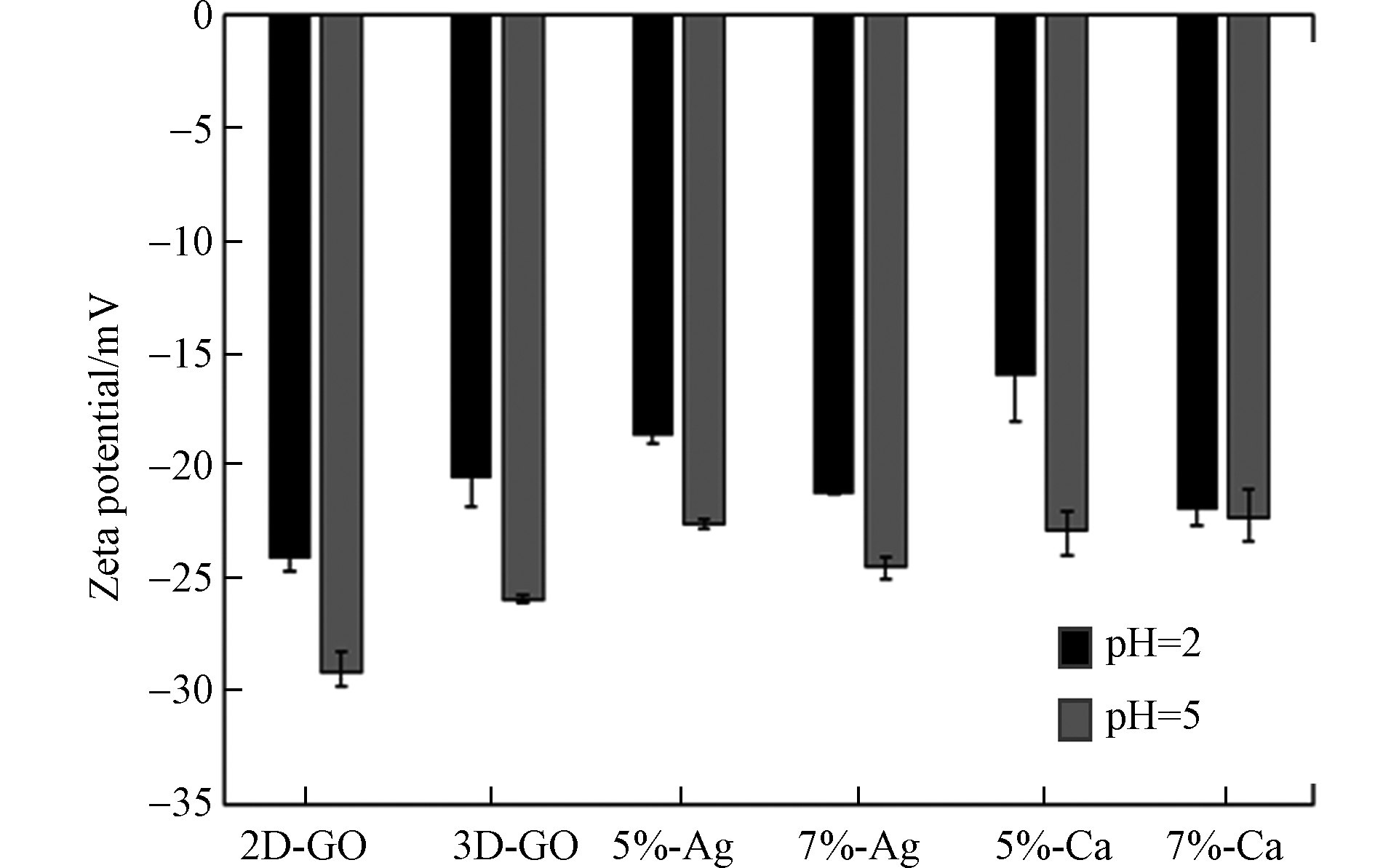
Zeta电位用来分析材料在液体环境中带电性质,以探究材料带电性质对MO吸附的影响。如图3所示,pH=2和pH=5条件下,各碳材料表面均带负电荷,且pH=5时各碳材料表面的负电荷均比pH=2条件下更加丰富。这是因为材料在较高pH条件下,溶液中—OH离子较多,碳材料表面羟基上的H+更易解离,材料因此比较低pH时表面携带更多负电荷。三维氧化石墨烯材料的负电荷较2D-GO更少,说明水热合成过程中部分含氧官能团被还原;金属离子嫁接后,各碳材料的负电荷整体上少于3D-GO。以上现象说明含氧官能团在与金属离子反应的过程中进一步被破坏,与上文中元素分析和红外光谱表征结果相互印证。
-
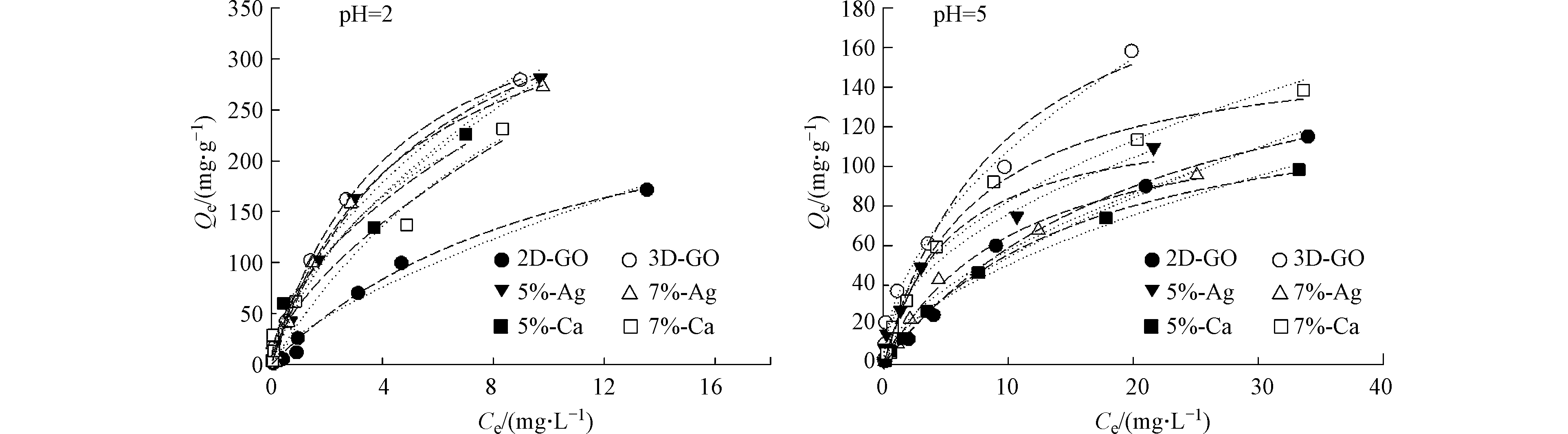
为了探究金属离子嫁接对材料吸附MO的影响,比较嫁接后材料的吸附能力,在pH=2和pH=5的条件下进行了吸附实验。采用Langmuir和Freundlich模型分别对吸附等温线进行拟合(图4),表4为相应拟合结果。由吸附等温线和拟合结果可知,pH=2条件下的吸附量显著高于pH=5条件下的吸附。如前文所述,pH=2时材料表面负电荷更少,且此时MO以中性分子形态存在,静电排斥小;pH=5时材料表面电荷更多,且MO以负电性离子形态存在,较大的静电排斥使其吸附量较低[19]。
在pH=2时,2D-GO吸附量最低,是因为其比表面积小,孔结构欠发达。离子的嫁接对吸附的作用不明显,Ag+嫁接的材料和3D-GO基本相同,而Ca2+嫁接的材料从吸附趋势上要低于3D-GO。原因在于Ca2+嫁接比Ag+造成了更明显的比表面积下降(表2),因此Ag+嫁接的氧化石墨烯对MO的吸附要大于Ca2+嫁接的材料。
在pH=5时,2D-GO的吸附量仍为最低,3D-GO的吸附量整体大于金属离子嫁接的氧化石墨烯,Ag+嫁接后的材料与原始的材料相比,比表面积下降,吸附降低;随着Ag+嫁接的浓度提高,材料的孔隙结构更加破碎,不利于甲基橙的吸附;虽然钙离子嫁接材料的比表面积随着嫁接量的增加而下降(表2),而吸附量却增加。这是由于嫁接到材料表面的Ca2+有着更多的正电荷,可以在材料表面与MO之间起到架桥作用[19],减弱静电排斥的影响,表明高价态的金属离子更能对吸附产生贡献。值得注意的是,此时材料对MO的最终吸附量不仅仅取决材料的比表面积。例如,7%-Ca的比表面积约为3D-GO的1/4,但是最终的吸附量高于3D-GO吸附量的1/2;且随着Ca2+嫁接量的提高,吸附量上升。这表明金属离子嫁接的材料对MO的吸附同时取决于比表面积和嫁接金属离子的种类和含量,一方面金属离子嫁接降低了材料表面的比表面积和材料的孔体积,减少吸附量;另一方面多价金属可能引入部分吸附位点,增加吸附量。因此,吸附的作用是材料自身比表面积和金属离子共同作用的结果,金属离子对碳基材料引起的吸附促进作用不可忽视。
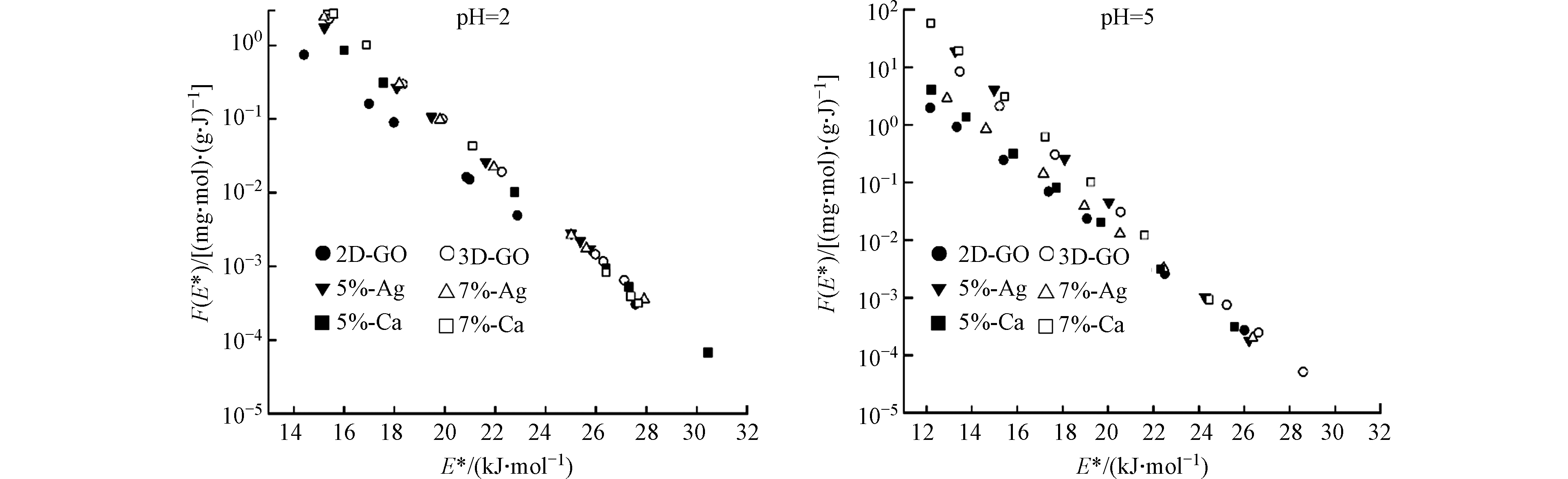
两种pH条件下材料吸附的位点能量分布如图5所示。所有材料的吸附位点能量主要分布于10—30 kJ·mol−1的范围内。其中,2D-GO的吸附位点能量普遍要低于其他几种材料,也进一步解释了2D-GO的吸附量最低的结果。在pH=2时,经过金属离子嫁接的材料与原始3D-GO位点能量分布差异不大,因为此时MO呈未解离的分子态,静电在吸附中未起作用;而在pH=5时,Ag-5%的能量分布函数F(E*)与3D-GO相差不大,Ag-7%的F(E*)要低于3D-GO,这一点与吸附等温线趋势一致。原因在于单价的Ag对碳材料表面位点具有遮蔽作用。Ca-5%在低能位点区域(约12—22 kJ·mol−1)的F(E*)数值低于3D-GO,而Ca2+的浓度提高到7%时,F(E*)的数值高于3D-GO。这说明Ca2+的添加,即使遮蔽了部分吸附位点,也同时因为Ca为多价金属,赋予材料更多的吸附位点[20]。
2.1. 金属离子嫁接对3D-GO表面形貌和比表面积的影响
2.2. 金属离子嫁接对3D-GO表面官能团的影响
2.3. 金属离子嫁接对3D-GO表面电位的影响
2.4. MO在各碳材料上的吸附等温线
-
通过水热合成法制备了Ag+和Ca2+嫁接的三维氧化石墨烯材料,研究分析嫁接后材料的表面特性改变以及对甲基橙吸附的影响,得出如下结论:
(1)扫描电镜照片、比表面积和孔体积以及红外光谱证明金属离子的嫁接对三维氧化石墨烯表面具有破坏作用;这表明在进行三维氧化石墨烯的合成时,应尽可能的保证材料纯度,因为残存的金属催化剂可能会对材料表面性质以及材料吸附性能造成很大的影响。
(2)由于比表面积的下降,金属嫁接后的材料对甲基橙的吸附量降低。但是金属的嫁接提高了甲基橙在三维氧化石墨烯单位面积上的吸附量,尤其是Ca2+对单位面积吸附量提高的能力更强。金属尤其是多价金属产生的架桥作用对甲基橙在碳材料表面吸附的促进不可忽视。



 下载:
下载: