-
地球上储存的可利用资源有限,若一直依靠石油和天然气等不可再生能源为原料合成高分子材料以及相关有机化工产品等,材料发展终将会面临原料逐渐枯竭的困境。硫酸钙晶须(calcium sulfate whisker,CSW)是以废石膏为原料制备的一种良好的合成材料添加剂,除了可以改善合成产品的各项性能指标之外,还能缓解目前废石膏大量堆存的问题。目前,我国各类工业副产废石膏存储量为704.3亿吨,石膏年产量1.53亿吨,但每年利用率仅为36%,约为0.56亿吨[1]。其中,磷石膏作为一种大宗固体废物,其年均排放量达8000余万吨,全球累计堆存量达60亿吨以上,但是利用率仅为40%[2-4]。此外,全球约有20个国家和地区的火电厂使用烟气脱硫系统控制SO2排放;据统计显示,在2010年,全球已有超过90多个国家产生脱硫石膏,脱硫石膏的生产量已超过2000万吨[5-6],对环境造成了不小的负担,因此亟须找寻一种合适的资源化方法以解决废石膏大量堆积问题。
废石膏成分较复杂,主要含有二水硫酸钙、半水硫酸钙、硫酸钙、二氧化硅及少量结晶金属等;其中结晶二水硫酸钙(CaSO4·2H2O)含量达到70%—80%[7],因此用废石膏制备硫酸钙晶须是石膏固体废弃物高附加值利用以及减少资源浪费的一种可行方式[8],也是废石膏资源化利用最具有研究价值的方向之一。若能将我国每年产出的废石膏制备成硫酸钙晶须资源化利用,不仅能解决大量堆积废石膏的去向问题,同时还能产生良好的经济效应。然而,当前废石膏的资源化利用范围较窄、利用率低、成本较高、市场前景堪忧、对环境影响突出等问题限制了其资源化进程。目前,大部分的废石膏被用作水泥缓凝剂[9]、石膏板[10]、土壤改良[11]、重金属稳定固化[12],用于矿井与路基回填,建筑石膏粉与石膏砌块[13]、砂浆[14]、建筑用材[15]等方面,总利用率不到30%,余下的废石膏大量堆积。由于废石膏组分的不确定性及多样性,大部分废石膏中的有害成分会对地表水和地下水体造成污染威胁、影响环境卫生。
本文总结了几种目前废石膏的资源化利用途径,并对比了其优缺点,提出利用废石膏制备硫酸钙晶须的高附加值利用途径,为废石膏的处理处置提供一种新的解决思路。
-
硫酸钙晶须是一种无水硫酸钙的纤维状单晶体,其尺寸稳定,平均长径比为80,硫酸钙晶须模型如下。

硫酸钙晶须(CSW)具有耐高温、抗化学腐蚀、韧性好、强度高、易进行表面处理、和橡胶塑料等聚合物的亲和力强等优点,重要的是其价格却仅为碳化硅晶须的1/200—1/300,因此具有很强的市场竞争力。硫酸钙晶须作为聚乙烯、聚丙烯、聚氯乙烯的添加剂,添加后所得制品性能良好,每吨塑料成本可降低190元到5300元左右。因此废石膏在制备硫酸钙晶须方面具有广阔的研究价值和市场前景[16]。
-
到目前为止,关于废石膏的处理技术的研究报道越来越多,许多研究学者根据其浸出成分,提出了不同的处理技术。废石膏的主要成分为CaSO4·2H2O,但也有例如Mg、K、Cl、F、B、Al、Fe、Si和Se等的其他元素的存在。此外,一些痕量可能存在有毒元素,例如砷,汞,镉和铅等。这些微量元素的数量取决于不同的生产加工工艺[17],如:磷石膏主要取决于磷矿的主要成分以及磷酸生产工艺,脱硫石膏主要取决于含硫烟气的原材料以及产品的生产工艺等。其常见的天然石膏和废石膏成分如表1所示[14]。
-
将废石膏用于改良盐碱土壤是一种方便而有效的资源化途径,其原理是利用废石膏溶解产生的Ca2+代换土壤胶体上的Na+,从而在降低土壤的碱化度和pH值的同时增加土壤的透水性。土壤胶体粒(由黏土与腐殖质形成),长期与盐碱土中Na2CO3、NaHCO3、NaCl等物质接触,形成含Na胶体粒子。含Na胶体粒子在土壤中水化度较大,有较好的分散性,能散布在土壤颗粒之间的细缝中,形成致密、不透水的含Na板结土层。不易透水的含Na板结土层中掺入 CaSO4后,因Ca2+比 Na+ 对土壤中胶体粒的吸附能力大得多,原已吸附的Na+会被Ca2+置换,所以土壤溶液中的Ca2+会和土壤胶体上附着的 Na+ 离子交换。含 Ca2+ 胶体微粒的外层不吸附水分子,胶体微粒能互相靠近、聚团,形成微粒团[18]。水分子渗入微粒之间时会使微粒团膨胀,然后在干燥过程中使土层龟裂。这过程反复进行后,土壤就形成团粒结构,从而使得土壤疏松增加,增加透水性,降低土壤碱度[19],有利于农作物生长和吸收水分、养分。但大部分废石膏中存在有害元素如砷,汞,镉,铅和硒的存在[17],增加了废石膏作为土壤改良剂的潜在风险。
-
由于近年来建筑行业的快速发展,市场对于建筑材料的需求量也越来越大。目前大部分的建筑材料来源于天然建材,造成了自然资源的过渡开发,且破坏了生态环境。若能利用废石膏作为建筑材料的添加剂,不但能大大缓解了废石膏对环境污染的压力,而且也能降低生态环境破坏的风险。Wu等[20]运用废石膏制成的硫铝酸钙水泥在大大提高废石膏的利用率的同时,还可以减少常规生产中约50%的二氧化碳排放量,在某种程度上减缓了温室效应的产生。Xu等[21]利用废石膏作为添加剂生产了硫铝酸钙水泥,发现废石膏的添加有助于在所有固化条件下产生更多的钙矾石和氢氧化铝,并在40 ℃时抑制了单硫酸盐和玄武岩的形成。Lei等[22]使用未经煅烧的废石膏制备了未经处理的废石膏基高强度建筑材料,分析了未经处理的废石膏的化学成分,物理性质和颗粒微观形态。研究了消解时间对未经处理的废石膏基高强度建筑材料力学性能的影响,结果表明,未经处理的废石膏基高强度建筑材料具有良好的力学性能,耐水性,抗冻性以及低收缩率,高水合度,组织致密等优点。Wu等[23]运用硅酸盐熟料改性废石膏制备防水砌块,研究了硅酸盐熟料的数量和粒径对改性废石膏材料力学性能,软化系数和吸水率的影响。结果表明,加入适量的硅酸盐熟料可以改善废石膏的耐水性和力学性能。除此之外,还有许多研究学者研究废石膏制备并运用于建筑材料方面,但由于废石膏的成分复杂且多变,不同的工艺可能存在不同的成分,故废石膏在建材方面的运用尚存较大局限性。
-
在矿石的开采过程中留下的许多采空区和矿坑,容易造成地质灾害和生态破坏,因此为废石膏作为回填材料进行回填,减少矿区地质灾害提供了可行思路。Ma等[24]运用废石膏和粉煤灰混合制备回填材料,发现制备出的回填材料机械强度可满足回填材料要求。Li等[25]运用添加了废石膏的赤泥渣的灌浆材料进行试验,结果表明,石膏的掺入可以降低赤泥灌浆材料的流动性,缩短凝结时间。由于废石膏的现有堆存量大,且磷石膏的产量会随着磷化工产品需求的增大而增大;此外,大部分烟气脱硫工艺均采用石膏脱硫,导致每日废石膏产量巨大,其中大部分废石膏中含有重金属及其他腐蚀性有害物质,因此将其作为回填材料使用具有较大的潜在风险,如在长时间的地表径流以及雨水冲刷等条件下可能会造成有毒有害的成分浸出,造成地下水和土壤污染。
-
废石膏经过改性或者加工后,可作为环境功能材料如吸附材料等使用。Kang等[26]运用烟气石膏作为环境功能性材料除氟,结果表明,废石膏通过钙和氟化物的结合去除氟化物,生成氟化钙,具有良好的除氟效果。Li等[27]运用Fe/脱硫石膏去除污水中和EDTA螯合的Pb(Ⅱ)。还原剂, Fang等[28]将半干式脱硫灰用作通过化学还原沉淀去除铬和钒的还原剂,获得了副产物石膏和含铬污泥。特别是在水处理方面运用研究较为广泛,表2为废石膏在不同类型水处理中的应用细节。将废石膏作为环境功能性材料相对来说是一种比较好的处理手段,但是最终会产生新的污染,可能还会造成二次固体废物污染,带来新的环境问题。
-
近10年来,许多关于运用废石膏合成墙板的研究报道成为了研究热点。有学者提出运用废石膏合成墙板是废石膏资源化的一个较好的利用途径之一。除此之外,运用相对比较广泛的资源化途径是合成高强度建筑材料,水泥、混泥土、沥青等。Wang等[32]研究人员研究应用废石膏生产CaCO3,在不同的反应条件下使用废石膏制备不同的CaCO3多晶型物。
-
废石膏中有价值成分的提取也是一种可行的资源化方式,Patricia Cordoba等[33]发现烟气脱硫石膏中含有稀有元素硒,提取稀贵重金属成分也成为废石膏处理的方法之一。
-
迄今为止,已经有许多晶体制备的方法,其中主要分为溶液法生长、熔体法生长和气相法生长,如表3所示。除了上述合成方法之外,还有生化学活化法[34]能够增强结晶过程中的成核能力等。目前常见的水热合成法如表4所示。
目前尚未有关于采用多物理场耦合协助对废石膏晶体生长及晶型转化制备硫酸钙晶须机理及产物定向调控机制研究的报道。但根据国内外同行的研究报道与我们的研究基础推断[46-47],人工晶体制备的主要过程为:把组成晶体的基元(原子、分子、离子)解离后,通过严格控制生长条件,在化学势驱动下,又重新将它们合并生长,由于多物理场均能提供不同形式的能量和条件,因此存在多物理场耦合作用可以对石膏晶体生长及晶型转化进行协助,增强硫酸钙晶须的制备。在对石膏施加电场时,电场释放的电子能够使脱硫石膏产生大量的高能量分子激发态和次级电子,能量较高的激发态和次级电子会发生一系列物理化学变化,如化学键的断裂、阈上电离、阈上解离、键软化、库仑爆炸、分子在强场中的重新取向以及新激发态的生成等;再施加磁场时,由于脱硫石膏中的均匀顺磁逆磁性物质,使其在磁场作用下定向分布;在施加超声场时,超声提供的能量能使脱硫石膏原有的晶格形状发生改变以及破碎;增加温度时,能提供石膏晶体生长以及晶型所需的能量。因此在废石膏制备硫酸钙晶须的过程中施加多物理场,并根据多物理场的强度,可以精确把握晶体生长及晶型转化过程。
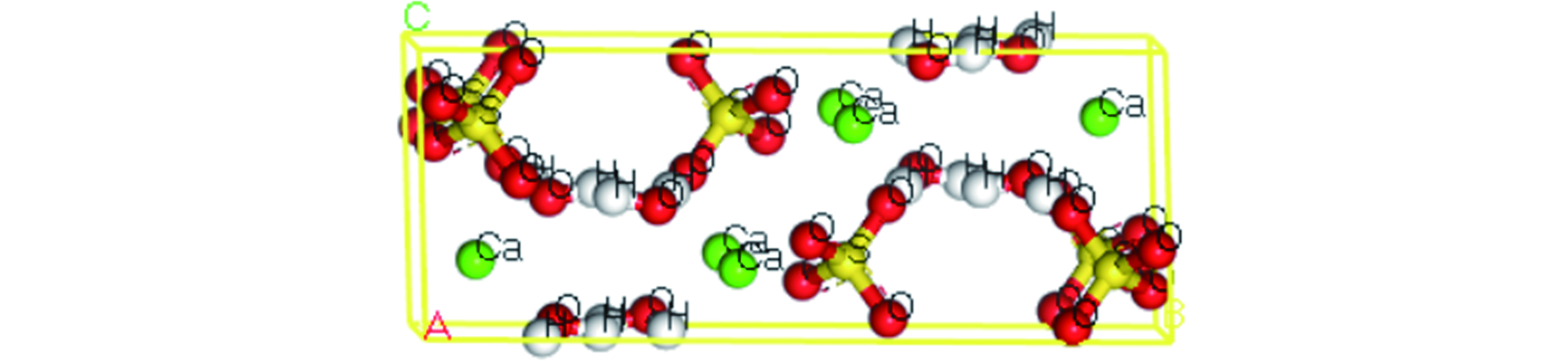
目前,关于针对石膏固体废弃物的高附加值资源化利用的相关研究较少,废石膏晶体球棍模型如图1所示。
其中石膏晶体转化主要是在合成过程控制晶核的形成条件,形成晶体结构不同的同质异构体;而石膏一共存在3种不同形式,分别为α石膏、β石膏和γ石膏,其中α石膏被认为是硫酸钙晶须的制备最佳原材料,制备硫酸钙晶须的主要方法有微波辅助合成[48]、水热合成[49]、常压酸化法[41]、反应性结晶[50]浓硝酸钙溶液法[43]等,通过改变外加条件强度实现β石膏、γ石膏向α石膏的转化,最终形成硫酸钙晶须。
近年来,国内外已开展大量的关于废石膏制备硫酸钙晶须晶体结构[38]、形态[39]、制备及合成机理[51]以及硫酸钙晶须改性功能的研究[52]。另外,还有大部分研究人员研究了关于酒石酸钾[53]、CuCl2[44]、CaCl2[54]、六偏磷酸钠/二氧化硅[55]、乙二醇[56]、
${{\rm{H}}_2}{\rm{SO}}_4^ - {\rm{NaC}}{{\rm{l}}^ - }{{\rm{H}}_2}{\rm{O}} $ [45]、十二水合磷酸氢二钠[57]、金属离子[58]、磷酸一氢钙[59]、磷酸钠[60]、油酸钠[61]以及一些稳定剂[62]、成瘾剂[63]、偶联剂[64]助熔剂[65]等物质的添加对硫酸钙晶须生成和生长的影响,这些添加剂可以定向控制晶体生长方向。控制晶体转化和晶须生长的外加条件手段主要有:原位刺激、纳微条件调控、外源控制等,例如,通过对活性废石膏前体进行水热处理(通过在150 ℃的温度下烧结废石膏进行6.0 h然后在室温下水合形成),制备长径比高达325的α-石膏晶须。在135 ℃下加热4.0 h;通过改变pH值或溶液组成来调节α-石膏的形貌;存在23.5%—35.5%质量分数的CaCl2也可以改变α-石膏的形貌。α-石膏晶须从长而细的六角棒到长短的六角柱;乙醇,酒石酸钾钠和柠檬酸钠的存在导致α-石膏晶须的长径比从1.7增加到4.8。十六烷基三甲基溴化铵(CTAB)在α-石膏晶须侧面上具有优先吸附和抑制作用[66],十二烷基磺酸钠(SDS)在α-石膏晶须的上侧面上,导致长径比从2—7增加到180—250[67]。但由于硫酸钙晶须生成速度慢、成本高、产量少、生成和生长过程中难以控制以及不稳定性等因素,导致石膏资源化利用制备硫酸钙晶须技术成为瓶颈问题。由于石膏的大量产生和堆积,硫酸钙晶须具有优异的性能和适用于各种实际应用(例如具有耐高温、抗化学腐蚀、韧性好、强度高、易进行表面处理、和橡胶塑料等聚合物的亲和力强等优点)的适应性[37],研究人员对合成具有良好结晶度和高纵横比的硫酸钙晶须(CSW)的兴趣日益浓厚,使得运用石膏制备硫酸晶须成为废石膏资源化利用研究热点之一。通过近期的研究报道分析可知,通常利用废石膏制备硫酸钙晶须的方法有微波合成、水热合成、常压酸化法、反应性结晶和浓硝酸钙溶液法等,但在这些制备方法中均存在合成晶相过程难以控制、制备条件苛刻、生成量少、晶须稳定性难以控制等问题导致晶须控制合成困难,如水热合成硫酸钙晶须所需温度较高,均在130°以上,而且晶体取向无法控制,晶须稳定性不好控制,产量较低[32];运用单物理场控制硫酸钙晶须的生长已有一定的研究[37, 68],Hazra等[69]运用单超声辅助废石膏制备过硫酸钙晶须效果较好,但目前仍没有关于在多物理场耦合辅助石膏制备硫酸钙晶须方面的报道,且石膏制备硫酸钙晶须的微观演变过程仍属于一个黑箱结构;因此,如果能够掌握废石膏制备硫酸钙晶须的演化规律并形成晶须的精确调控机制,则有望根据硫酸钙晶须的性质和价值,定向控制晶体生长和转化,达到硫酸钙晶须的最大量化生成和生长的目的,使废石膏固体废物资源化在制备硫酸钙晶须方面规模化应用。
多物理场的物理特性为石膏晶体的生长及晶型的转变,从而达到促使硫酸钙晶须的生成及生长做到精准微观调控,在石膏固体废物资源化高价值利用研究方面打开研究前景。多物理场包括电场、磁场、温度场、力场等,而多物理场耦合为两个及两个以上物理条件同时作用在同一样品上,由于多物理场存在能量传输和信息传递,所以会引起样品的许多变化,近年来,关于多物理场的研究越来越广泛; Guillon等[70]使用外部电场和磁场来合成和加工无机材料,发现电磁能可以是以不同方式用于加速或改善晶相形成和它的稳定的晶体颗粒状材料的排序,使其致密化和粗化(孔消除和晶粒长大),机械变形(可塑性和蠕变),说明多物理场在晶体生成和生长方面的研究越来越受欢迎。近年来,已经有少量单物理场协助石膏制备硫酸钙晶须方面的报道,如单超声辅助合成硫酸钙晶须[69],其中水热合成法只涉及到温度场[36],这些合成方法虽然能改善硫酸钙晶体及晶须的生成、生长及结构,但是产量方面和机理研究仍然不足,不能大规模化制备,运用石膏制备硫酸钙晶须的生成和生长微观精确调控的没有研究报道,而刺激硫酸钙晶须生长的外加条件的报道较少。实际上,多物理场所具有的优良特性不仅可以从微观量子结构改善晶体内部结构,而且能从宏观上刺激晶体的生成和生长,从而达到多物理场对石膏对制备硫酸钙晶须生成和生长的调控。2017年Dalvi-Isfahan等[71]应用超声场、电场和磁场协同作用控制冰核的生长,2014年Zhang等[72]报道了水热合成过程中,用氯化镁作为晶体促进剂,十二烷基硫酸钠作为分散剂,合成并控制硫酸钙晶须生长,但关于多物理场耦合作用对晶体的影响尚未有人研究。
-
由于硫酸钙晶须优越的性能和环保,无毒的特性而被进一步用于橡胶、涂料、塑料、胶黏剂、纺织、造纸等许多行业[73]。
-
硫酸钙晶须是合成材料中的一种良好的添加剂,由于其具有加大的机械强度,因此能做为如橡胶、塑料等合成材料的增强组元,在不同方面优化合成材料的性能。Lu等[74]制备硫酸钙晶须/聚四氟乙烯材料,发现具有该强度力学性能和良好的热学性能。Sheng等[75]使用硫酸钙晶须(CSW)作为增强剂来提高PG瓷砖的机械强度,所制得的具有1%(重量)CSW的PG瓷砖的弯曲强度可达27.2 MPa,与没有添加CSW的样品相比增加了80%;其中,机械强度的增强主要归因于这样的事实:瓷砖主体中分散的CSW充当“桥”并与石膏晶体牢固结合,从而形成完整的紧密链接的瓷砖网络。此外, Liu等[52]运用乙醇改性硫酸钙晶须,然后作为添加剂制备的聚丙烯片材产品,发现其具有出色的机械性能。Yang等[76]研究了硫酸钙晶须对聚乳酸复合材料力学性能,形态结构和热降解的影响,结果发现,玻璃化转变和冷结晶过程在低浓度的CSW时滞后,但随着晶须的进一步添加又加速,这也影响了实际结晶度的变化。然而,热稳定性显着提高,热分解动力学分析表明,随着CSW的增加,活化能降低,反应顺序几乎不变。多项研究表明,以硫酸钙晶须或者改性硫酸钙晶须为添加剂改性符合材料的性能,能在材料的机械性能方面提高0.6—3.5倍[77],还能作为防水增强添加剂[55],特别是再经改性后的硫酸钙晶须作为添加剂,合成材料的性能变化更为显著[78]。硫酸钙晶须不仅在合成有机复合材料中具有广泛的运用价值,在无机复合材料和无机金属材料[79]中同样具有显著的改性性能,如无机建筑材料[80]等,经过一系列物理测试结果表明,添加晶须大大改善了机械性能,并略微提高了热稳定性。除此之外,硫酸钙晶须还可作为沥青填料,在沥青中加入硫酸钙晶须,可显著提高沥青的软化温度。
-
除此之外,硫酸钙晶须还能直接或改性后作为吸附剂使用,并具有良好的吸附性能。Chen等[81]通过冷却重结晶法合成的硫酸钙晶须(CSW,二水合物或无水石膏)从水溶液中吸收As(Ⅲ) / As(Ⅴ)的行为表明As(Ⅴ)和CSW之间的相互作用机制是一个复杂的吸附过程,结合了表面溶解和化学沉淀。Fan等[82]运用不同改性的硫酸钙晶须制备沥青粘结剂,发现沥青粘结剂在高温性能、抗变形性能、温度敏感性性能、黏温特性、流变性能和低温性能等方面的影响。
-
由于硫酸钙晶须(CSW)具有较小的密度和巨大的比表面积,因此可以在环境工程领域中被用作过滤材料,可以过滤包括废气、水体中有害杂质等高温或具有腐蚀性的污染物,且在过滤之前不用进行冷却和中和操作,因此具有使用方便、过滤高效等优点;除此之外,硫酸钙晶须无毒,因此还可以广泛用于果汁、啤酒及药物的过滤[83]。
-
硫酸钙晶须还可代替石棉,添加到摩擦材料中,在刹车片和轮胎等部件中使用,使耐磨材料在达到耐磨要求的同时,安全性也能显著提高,并有效避免了在生产和使用过程中石棉对人体健康造成危害。王泽红等[84]用合成的硫酸钙晶须生产了不含石棉的摩擦片,并对其耐磨性能进行了检测,结果显示,含硫酸钙晶须的摩擦材料的耐磨性能显著提高。
-
硫酸钙晶须可作为无机纤维制造难燃纸张。美国曾有学者[85]采用碳酸钙晶须与多种无机物混合,制造出了性能优异的无机纸张,该纸张难燃且稳定性强,因此可作为防火材料用于室内外的装饰。
-
硫酸钙晶须除上述应用外,在隔热材料、轻质建材、铸造型砂及涂料等方面也有应用。
-
针对目前废石膏潜在风险及产量较大的问题,通过将现存的废石膏的处理方法进行对比,得出废石膏最佳的高附加值资源化利用的方法是制备硫酸钙晶须,并对硫酸钙晶须潜在的市场价值,经济价值,以及环境价值进行了系统性分析。除此之外,也分析了现有的硫酸钙晶须制备方法的局限性,包括产量低、成本高以及无法形成产业化等缺点,有待进一步研究开发新技术手段制备硫酸钙晶须,以降低硫酸钙晶须的生产成本,形成产业化生产。这样不仅能解决废石膏固体废物资源化利用问题,还能形成高价值生态企业链。然而硫酸钙晶须在制备方法和应用方面尚有不足,特别是在硫酸钙晶须的改性和运用硫酸钙晶须制备环境功能材料方面缺乏大量研究,有待相关专家学者的进一步探讨和研究。大量文献表明,电磁、温度、压力等物理场手段能够有效刺激晶体和晶须的变化,但相关理论还有待进一步的多物理场耦合手段精确定向调控晶体和晶须生长的相关研究,找出适合晶体和晶须生长的最佳耦合物理条件。
废石膏制备硫酸钙晶须的高附加值利用前景
The high value-added utilization prospect of calcium sulfate whiskers prepared from waste gypsum
-
摘要: 目前全球范围内石膏资源储量丰富,种类繁多,但开发利用率低。特别是脱硫石膏、磷石膏固有堆存量大,其每年年产量逐渐剧增,大部分废石膏中含有大量有害组分,不仅会对地表水和地下水体造成污染威胁、影响环境卫生,而且会造成资源的极大浪费;由于其处理成本高、具有较大的潜在风险,截至目前,仍没有较好的石膏处理途径。石膏的主要成分是硫酸钙,硫酸钙晶须是一种良好的材料合成添加剂,对材料的各项性能均有改善作用,因而具有广阔的研究价值和市场前景。本文通过对比石膏的多种处理方法和硫酸钙晶须的制备方法,突出硫酸钙晶须的运用前景、市场前景,表明其市场价值和环境价值,提出运用石膏制备硫酸钙晶须的新控制理论,为石膏制备硫酸钙晶须的高附加值资源化利用提供理论支撑。Abstract: At present, the global reserves of gypsum resources are abundant, but the development utilization rate is still in a low level. Especially for the large production and inherent stockpiles of desulfurized gypsum and phosphogypsum, and most of the waste gypsum poses a threat to surface water and groundwater, affects environmental sanitation, but it also a huge waste of energy resource. However, due to the high processing cost and high potential risk of gypsum, there hardly find an efficient way to treat them. In general, the main component of desulfurized gypsum is calcium sulfate, and the calcium sulfate whiskers is a promising additive, which could reach a promising improvement to the performance of materials with a broad research value and market prospects. In this paper, we compared several treatment methods of desulfurization gypsum and the preparation methods of calcium sulfate whiskers, highlights the application prospects and market prospects of calcium sulfate whiskers, and shows its social and environmental values, then proposes the use of desulfurization gypsum to prepare calcium sulfate whiskers. The new control theory shows that the use of flue gas desulfurization gypsum to prepare calcium sulfate whiskers is an efficient way to treat high value-added resources of desulfurization gypsum solid waste.
-

-
表 1 废石膏的主要成分(%)
Table 1. Main components of desulfurized gypsum (%)
CaSO4 Al2O3 Fe2O3 MgO SiO2 天然石膏 72.60 1.73 1.15 1.30 4.30 废石膏 74.00—80 0.70—1.00 0.50—1.00 1.00—2.00 2.70—3.00 表 2 脱硫石膏在不同类型水处理中的应用
Table 2. Application details of desulfurized gypsum in different types of water treatment
表 3 常见硫酸钙晶须的制备方法
Table 3. Preparation methods of common calcium sulfate whiskers
制备方法
Preparation methods制备机理
Preparation mechanism优缺点
Advantages and disadvantages溶液法生长 把硫酸钙基元溶于溶剂中,通过外加条件引发晶体和晶须生长的方法[35]。 是最为常见的方法,但产率低。平均长径比可达91左右[36]。 熔体法生长 把硫酸钙基元在高温下熔融,在冷却刺激晶体和晶须生长的方法。 对反应釜要求较高。 气相法生长 把硫酸钙基元汽化,通过外加条件,时汽化基元固化在晶核上的过程。 需要的温度较高。 微波合成 微波时控制晶核形成和晶核生长的唯一外加物理条件,为晶须生长提供能量[37]。 是一种比较前沿的研究方法,明显提高晶须产率。 水热合成 温控的溶液生长法[38],在外添加剂的作用下,改变结晶过程中的动力学以及结晶机理,常见的加酸、盐、有机物等。 温度是晶须生长所需能量的唯一外加条件,所需反应釜要求温度较高,且产率较低[33],目前最为常见的合成方法。一般制备的晶须直径为2 μm至6 μm,长径比大于80 μm [39]。 常压外添加剂酸化法 用酸酸化,如使用不同浓度的CuCl2在硫酸溶液中水热合成半水合硫酸钙晶须(HH-CSWs)[40]。 可以调节生成的晶须形体,但无法调节晶须的生长速率和方向,产率较低。 反应性结晶[41] 制造化学反应(添加结晶剂)。 在添加结晶剂的基础上,引发结晶。 浓硝酸钙溶液法 通过在大气压下将硫酸溶液滴加到浓硝酸钙溶液中,开发了一种合成硫酸钙晶须的创新方法[42]。 在合成晶须的同时,生成硝酸。 表 4 常见的水热合成法以及制备效果
Table 4. Common hydrothermal synthesis methods and preparation effects
具体方法
Specific Method制备效果
Preparation Effect以纯净废石膏为原料,以AlCl3为晶体改性剂,通过水热法制备硫酸钙晶须(CSWs) CSW的直径为2 μm至6 μm,长径比大于80 μm [37] 甘油-水反应体系中实现了石膏的晶相和形貌控制的结晶 产生了具有高纵横比(类似于1∶200)的α-CSH晶须[37] 通过在大气压下将硫酸溶液滴加到浓硝酸钙溶液中 生成了高纵横比(约93.5)的硫酸钙晶须,再生了硝酸(浓度为170 g·L−1)[42] 在固溶比为1∶60的20%H2SO4水热1 h溶液中 获得了具有光滑表面和高纵横比的晶须[43] 采用大气酸化法,由烟气脱硫(FGD)石膏合成了硫酸钙晶须(CSW) CSW的宽度范围是3—22 μm,长宽比范围是25—80[41] 以废石膏为原料,研究了CuCl2对 $ {\rm{H}}_2 {\rm{SO}}_4^ - {\rm{H}}_2 {\rm{O}} $ 生产的硫酸钙晶须的直径范围为1—3 m,平均长径比大于200[44] 在 ${\rm{H}}_2 {\rm{SO}}_4^ - {\rm{H}}_2 {\rm{O}} {\rm{NaCl}}^ - {\rm{H}}_2 {\rm{O}} $
130 ℃下使用10—70 g NaCl·kg-1石膏−0.01 mol·L−1${\rm{H}}_2 {\rm{SO}}_4^ - {\rm{H}}_2 {\rm{O}} $ 硫酸钙晶须的直径为3—5 pm,长度为200 μm至600 μm 反应性结晶利用废氯化钙制备硫酸钙晶须 使用废CaCl2制备具有稳定且结构化的微晶(长宽比高达190)的硫酸钙晶须[41] -
[1] 高强. 大型石膏制硫酸装置处理废硫酸和废石膏运行实践 [J]. 磷肥与复肥, 2020, 35(8): 41-43. doi: 10.3969/j.issn.1007-6220.2020.08.013 GAO Q. Operation practice of treating waste acid and waste gypsum in large sulfuric acid plant based gypsum [J]. Phosphate & Compound Fertilizer, 2020, 35(8): 41-43(in Chinese). doi: 10.3969/j.issn.1007-6220.2020.08.013
[2] 李纯, 薛鹏丽, 张文静, 等. 我国磷石膏处置现状及绿色发展对策 [J]. 化工环保, 2021, 1: 1-6. LI C, XUE P L, ZHANG W J. Disposal status of phosphogypsum in China and countermeasures of green development [J]. Environmental Protection of Chemical Industry, 2021, 1: 1-6(in Chinese).
[3] 罗大鹏, 雍毅, 侯江, 等. 磷石膏基α-半水石膏的制备及其在绿色建材中的应用 [J]. 磷肥与复肥, 2020, 35(11): 32-36. doi: 10.3969/j.issn.1007-6220.2020.11.011 LUO D P, YONG Y, HOU J, et al. Preparation on α-hemihydrate gypsum from phosphogypsum and its application in the green building materials [J]. Phosphate & Compound Fertilizer, 2020, 35(11): 32-36(in Chinese). doi: 10.3969/j.issn.1007-6220.2020.11.011
[4] 叶学东. 磷石膏综合利用现状及分析 [J]. 磷肥与复肥, 2013, 28(6): 5-8. doi: 10.3969/j.issn.1007-6220.2013.06.002 YE X D. Status and analysis of comprehensive utilization of phosphogypsum [J]. Phosphate & Compound Fertilizer, 2013, 28(6): 5-8(in Chinese). doi: 10.3969/j.issn.1007-6220.2013.06.002
[5] KORALEGEDARA N H, PINTO P X, DIONYSIOU D D, et al. Recent advances in flue gas desulfurization gypsum processes and applications-A review [J]. Journal of Environmental Management, 2019, 251: 12380. [6] PAN D, WU H, YANG L J. Investigation on the relationship between the fine particle emission and crystallization characteristics of gypsum during wet flue gas desulfurization process [J]. Journal of Environmental Sciences, 2017, 55: 303-310. doi: 10.1016/j.jes.2016.08.020 [7] SEO S K, KIM Y, CHU Y S, et al. Experimental study on the carbonation properties of dry desulfurized gypsum [J]. Journal of the Korean Ceramic Society, 2018, 55(1): 44-49. doi: 10.4191/kcers.2018.55.1.11 [8] TENG W L, WANG J S, WU J S, et al. Rapid synthesis of alpha calcium sulfate hemihydrate whiskers in glycerol-water solution by using flue-gas-desulfurization gypsum solid waste [J]. Journal of Crystal Growth, 2018, 496/497: 24-30. doi: 10.1016/j.jcrysgro.2018.05.008 [9] SHEN J S, XU Y D, YOU W G. Stabilized effect of desulfurized gypsum and steel slag blended clinker free cement on soft clay [J]. Bulletin of the Chinese Ceramic Society, 2018, 37(12): 3888-3891. [10] XU Z Y, WANG X, JIN B, et al. Study on mechanical properties of desulphurized gypsum-fly ash brick [J]. Non-metallic Mines, 2018, 41(6): 89-91. [11] TANG Y, ZHANG Q, WANG B, et al. Environmental effect of the amelioration of heavy soda saline soil using desulfurized gypsum [J]. Journal of Soil and Water Conservation, 2017, 31(2): 317-321. [12] WU C, CHEN C, TIAN M, et al. Study on mercury stability in desulfurized gypsum at coal-fired power plants [J]. Environmental Science and Technology, 2016, 39(8): 159-163. [13] YANG J F, CHENG J P, ZHAI W, et al. Study on aseismic performance of a new type of favricated steel frame with internal desulfurizition gypsum block wall [J]. Engineering Mechanics, 2019, 36(6): 147-156. [14] 张倩. 燃煤电厂烟气脱硫石膏的特征及综合利用途径分析 [J]. 资源信息与工程, 2017, 32(6): 103-104. doi: 10.3969/j.issn.2095-5391.2017.06.047 ZHANG Q. Analysis of characteristics and comprehensive utilization of flue gas desulfurization gypsum in coal-fired power plants [J]. Resource Information and Engineering, 2017, 32(6): 103-104(in Chinese). doi: 10.3969/j.issn.2095-5391.2017.06.047
[15] LIU Y X, ZHANG C M, ZHAO J, et al. Hydration characteristics and mechanism analysis of fgd gypsum [J]. Bulletin of the Chinese Ceramic Society, 2018, 37(8): 2583-2587. [16] YANG Y, ZHANG Q, CAI W, et al. Formation and application of hierarchical calcium silicate-calcium sulfate whiskers [J]. Materials & Design, 2018, 146: 172-179. [17] KORALEGEDARA N H, PINTO P X, DIONYSIOU D D, et al. Recent advances in flue gas desulfurization gypsum processes and applications-A review [J]. Journal of Environmental Management, 2019, 251: 1-13. [18] 李彦, 衣怀峰, 赵博, 等. 燃煤烟气脱硫石膏在新疆盐碱土壤改良中的应用研究 [J]. 生态环境学报, 2010, 19(7): 1682-1685. doi: 10.3969/j.issn.1674-5906.2010.07.029 LI Y, YI H F, ZHAO B, et al. Study on improving Xinjiang sodic soils amelioration with desulfurized gypsum [J]. Ecology and Environmental Sciences, 2010, 19(7): 1682-1685(in Chinese). doi: 10.3969/j.issn.1674-5906.2010.07.029
[19] ZHAO Y, WANG S, LI Y, et al. Effects of straw layer and flue gas desulfurization gypsum treatments on soil salinity and sodicity in relation to sunflower yield [J]. Geoderma, 2019, 352: 13-21. doi: 10.1016/j.geoderma.2019.06.004 [20] WU S, WANG W, REN C, et al. Calcination of calcium sulphoaluminate cement using flue gas desulfurization gypsum as whole calcium oxide source [J]. Construction and Building Materials, 2019, 228: 7-15. [21] XU L, WU K, LI N, et al. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement [J]. Journal of Cleaner Production, 2017, 161: 803-811. doi: 10.1016/j.jclepro.2017.05.055 [22] LEI D, GUO L, SUN W, et al. Study on properties of untreated FGD gypsum-based high-strength building materials [J]. Construction and Building Materials, 2017, 153: 765-773. doi: 10.1016/j.conbuildmat.2017.07.166 [23] WU Q, MA H, CHEN Q, et al. Preparation of waterproof block by silicate clinker modified FGD gypsum [J]. Construction and Building Materials, 2019, 214: 318-325. doi: 10.1016/j.conbuildmat.2019.04.053 [24] MA Y, NIE Q, XIAO R, et al. Experimental investigation of utilizing waste flue gas desulfurized gypsum as backfill materials [J]. Construction and Building Materials, 2020, 245: 234-245. [25] LI Z F, ZHANG J, LI S C, et al. Effect of different gypsums on the workability and mechanical properties of red mud-slag based grouting materials [J]. Journal of Cleaner Production, 2020, 245: 132-156. [26] KANG J H, GOU X Q, HU Y H, et al. Efficient utilisation of flue gas desulfurization gypsum as a potential material for fluoride removal [J]. Science of The Total Environment, 2019, 649: 344-352. doi: 10.1016/j.scitotenv.2018.08.416 [27] LI R, LI Q, SUN X, et al. Efficient and rapid removal of EDTA-chelated Pb(Ⅱ) by the Fe(Ⅲ)/flue gas desulfurization gypsum (FGDG) system [J]. Journal of Colloid and Interface Science, 2019, 542: 379-386. doi: 10.1016/j.jcis.2019.01.129 [28] FANG D, LIAO X, ZHANG X, et al. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater [J]. Journal of Hazardous Materials, 2018, 342: 436-445. doi: 10.1016/j.jhazmat.2017.08.060 [29] YAN Y, LI Q, SUN X, et al. Recycling flue gas desulphurization (FGD) gypsum for removal of Pb(Ⅲ) and Cd(Ⅱ) from wastewater [J]. Journal of Colloid and Interface Science, 2015, 457: 86-95. doi: 10.1016/j.jcis.2015.06.035 [30] VARCOE J, VAN LEEUWEN J A, CHITTLEBOROUGH D J, et al. Changes in water quality following gypsum application to catchment soils of the Mount Lofty Ranges, South Australia [J]. Organic Geochemistry, 2010, 41(2): 116-123. doi: 10.1016/j.orggeochem.2009.09.010 [31] JENKINS M B, SCHOMBERG H H, ENDALE D M, et al. Hydrologic transport of fecal bacteria attenuated by flue gas desulfurization gypsum [J]. Journal of Environmental Quality, 2014, 43(1): 297-302. doi: 10.2134/jeq2012.0132 [32] WANG B, PAN Z, DU Z, et al. Effect of impure components in flue gas desulfurization (FGD) gypsum on the generation of polymorph CaCO3 during carbonation reaction [J]. Journal of Hazardous Materials, 2019, 369: 236-243. doi: 10.1016/j.jhazmat.2019.02.002 [33] CORDOBA P, STAICU L C. Flue gas desulfurization effluents: An unexploited selenium resource [J]. Fuel, 2018, 223: 268-276. doi: 10.1016/j.fuel.2018.03.052 [34] QIN J, SHI W, YANG H, et al. Sonochemical activation calcium sulfate whisker with enhanced beta-nucleating ability for isotactic polypropylene [J]. Colloid and Polymer Science, 2013, 291(11): 2579-2587. doi: 10.1007/s00396-013-3004-z [35] LI Y, CHEN Y, TANG C, et al. Co-treatment of waste smelting slags and gypsum wastes via reductive-sulfurizing smelting for valuable metals recovery [J]. Journal of Hazardous Materials, 2017, 322: 402-412. doi: 10.1016/j.jhazmat.2016.10.028 [36] TENG W L, WANG J S, WU J S, et al. Rapid synthesis of alpha calcium sulfate hemihydrate whiskers in glycerol-water solution by using flue-gas-desulfurization gypsum solid waste [J]. Journal of Crystal Growth, 2018, 496: 24-30. [37] JIANG N, ZHANG C, XUE C, et al. In situ synthesis of hydrophobic calcium sulfate hemihydrate whiskers [J]. Materials Research Express, 2018, 5(7): 23-34. [38] FAN H, SONG X, LIU T, et al. Effect of Al3+ on crystal morphology and size of calcium sulfate hemihydrate: Experimental and molecular dynamics simulation study [J]. Journal of Crystal Growth, 2018, 495: 29-36. doi: 10.1016/j.jcrysgro.2018.05.013 [39] WANG X, JIN B, XU Z Y, et al. Effects of AlCl3 on the crystal morphology of calcium sulfate whisker prepared from FGD gypsum//WANG K (ed). 6th Annual International Conference on Material Science and Environmental Engineering[C]. 2019: 27-41. [40] ZHANG X T, WANG X, JIN B, et al. Crystal structure formation of hemihydrate calcium sulfate whiskers (HH-CSWs) prepared using FGD gypsum [J]. Polyhedron, 2019: 173. [41] SUN H, TAN D, PENG T, et al. Preparation of calcium sulfate whisker by atmospheric acidification method from flue gas desulfurization gypsum//LI J, DONG F (eds). Selected Proceedings of the Tenth International Conference on Waste Management and Technology[C]. 2016: 621-626 [42] MA B, XING P, WANG C, et al. A novel way to synthesize calcium sulfate whiskers with high aspect ratios from concentrated calcium nitrate solution [J]. Materials Letters, 2018, 219: 1-3. doi: 10.1016/j.matlet.2018.02.025 [43] TAN H, DONG F, BIAN L, et al. Preparation of anhydrous calcium sulfate whiskers from phosphogypsum in H2O-H2SO4 autoclave-free hydrothermal system [J]. Materials Transactions, 2017, 58(8): 1111-1117. doi: 10.2320/matertrans.M2017042 [44] WANG X, JIN B, YANG L, et al. Effect of CuCl2 on hydrothermal crystallization of calcium sulfate whiskers prepared from FGD gypsum [J]. Crystal Research and Technology, 2015, 50(8): 633-640. doi: 10.1002/crat.201500035 [45] WANG X, YANG L, ZHU X, et al. Preparation of calcium sulfate whiskers from FGD gypsum via hydrothermal crystallization in the H2SO4-NaCl-H2O system [J]. Particuology, 2014, 17: 42-48. doi: 10.1016/j.partic.2013.12.001 [46] 秦善. 晶体学基础[M]. 北京: 北京大学出版社, 2006: 134-144 QING S. Fundamentals of crystallography[M]. Beijing: Peking University Press, 2006: 134-144 (in Chinese).
[47] LI W Y, YANG S, LI Y A, et al. Synthesis of an MOF-based Hg2+-fluorescent probe via stepwise post-synthetic modification in a single-crystal-to-single-crystal fashion and its application in bioimaging [J]. Dalton Transactions, 2019, 48(44): 16502-16508. doi: 10.1039/C9DT02866H [48] YANG H C, TSAI T P. Microwave-assisted synthesis and thermal resistance of calcium sulfate whiskers [J]. Chemical Engineering Communications, 2017, 204(2): 232-237. doi: 10.1080/00986445.2016.1260010 [49] LIU C, ZHAO Q, WANG Y, et al. Hydrothermal synthesis of calcium sulfate whisker from flue gas desulfurization gypsum [J]. Chinese Journal of Chemical Engineering, 2016, 24(11): 1552-1560. doi: 10.1016/j.cjche.2016.04.024 [50] SONG X, ZHANG L, ZHAO J, et al. Preparation of calcium sulfate whiskers using waste calcium chloride by reactive crystallization [J]. Crystal Research and Technology, 2011, 46(2): 166-172. doi: 10.1002/crat.201000420 [51] WANG S, CHEN D, ZHANG K. Preparation, characterization, and formation mechanism of calcium sulfate hemihydrate whiskers [J]. Journal of Wuhan University of Technology-Materials Science Edition, 2018, 33(6): 1407-1415. doi: 10.1007/s11595-018-1983-9 [52] LIU C, ZHAO Q, WANG Y, et al. Surface modification of calcium sulfate whisker prepared from flue gas desulfurization gypsum [J]. Applied Surface Science, 2016, 360: 263-269. doi: 10.1016/j.apsusc.2015.11.032 [53] SUN X, ZHANG G, CUI P. Aspect ratio-controlled preparation of alpha-CaSO4 center dot 0.5H(2)O from phosphogypsum in potassium tartrate aqueous solution [J]. Rsc Advances, 2019, 9(38): 21601-21607. doi: 10.1039/C9RA03569A [54] MIAO M, FENG X, WANG G, et al. Direct transformation of FGD gypsum to calcium sulfate hemihydrate whiskers: Preparation, simulations, and process analysis [J]. Particuology, 2015, 19: 53-59. doi: 10.1016/j.partic.2014.04.010 [55] FENG X, ZHANG Y, WANG G, et al. Dual-surface modification of calcium sulfate whisker with sodium hexametaphosphate/silica and use as new water-resistant reinforcing fillers in papermaking [J]. Powder Technology, 2015, 271: 1-6. doi: 10.1016/j.powtec.2014.11.015 [56] ZHAO W, WU Y, XU J, et al. RETRACTED: Effect of ethylene glycol on hydrothermal formation of calcium sulfate hemihydrate whiskers with high aspect ratios [J]. Rsc Advances, 2015, 5(62): 50544-50548. doi: 10.1039/C5RA07712E [57] HAN Q, LUO K, LI H, et al. Influence of disodium hydrogen phosphate dodecahydrate on hydrothermal formation of hemihydrate calcium sulfate whiskers [J]. Particuology, 2014, 17: 131-135. doi: 10.1016/j.partic.2013.10.002 [58] MAO X, SONG X, LU G, et al. Effects of metal ions on crystal morphology and size of calcium sulfate whiskers in aqueous HCl solutions [J]. Industrial & Engineering Chemistry Research, 2014, 53(45): 17625-17635. [59] LU P, FEI D, DANG Y. Effects of calcium monohydrogenphosphate on the morphology of calcium sulfate whisker by hydrothermal synthesis [J]. Canadian Journal of Chemical Engineering, 2014, 92(10): 1709-1713. doi: 10.1002/cjce.22037 [60] CHEN C, WANG Y, YU L, et al. Hydration ability of hemihydrate calcium sulphate whiskers with different content of sodium phosphate// LIANG J, WU X, YANG W, et al. (eds). Progress in industrial and civil engineering Ⅲ, Pt 1[J]. Applied Mechanics and Materials 2014, 638-640: 1346-1349 [61] WANG X, LI X, LI X, et al. Influence of sodium oleate on morphology of semi-hydrated calcium sulfate whiskers and its infrared adsorption performance//LIU H, KURODA SI, ZHENG L (eds). Advances in textile engineering and materials Ⅳ[J]. Advanced Materials Research 2014, 1048: 479-482 [62] LIU H, WANG Y. Effect of stabilizer on morphology and stability of hemihydrate calcium sulfate whiskers//LI H, LIU Y F, GUO M, et al. (eds). Sustainable development of urban environment and building material, Pts 1-4[J]. Advanced Materials Research, 2012, 374-377: 1495. [63] LUO K B, LI C M, XIANG L, et al. Hydrothermal formation of hemi-hydrate calcium sulfate whiskers in the presence of additives//JIN F M, ZHOU Q, WU B, et al. (eds). 2nd International Symposium on Aqua Science, Water Resource and Low Carbon Energy[C]. 2010: 296. [64] WANG X, ZHU Y, HAN Y, et al. Toughening of polypropylene with calcium sulfate whiskers treated by coupling agents//GAI G (ed). Powder Technology and Application[C]. 2009: 225. [65] TAS A C. X-ray diffraction data for flux-grown calcium hydroxyapatite whiskers [J]. Powder Diffraction, 2001, 16(2): 102-106. doi: 10.1154/1.1330273 [66] CHEN R S, HOU S C, WANG J, et al. Influence of alkyl trimethyl ammonium bromides on hydrothermal formation of alpha-CaSO4 center dot 0.5H2O whiskers with high aspect ratios [J]. Crystals, 2017, 7(1): 56-64. [67] HOU S, WANG J, WANG X, et al. Effect of Mg2+ on hydrothermal formation of alpha-CaSO4 center dot 0.5H2O whiskers with high aspect ratios [J]. Langmuir, 2014, 30(32): 9804-9810. doi: 10.1021/la502451f [68] WANG Y, TAN D, LI X, et al. Research on affecting factor of preparation of calcium sulfate whisker with FGD gypsum//QIANG L (ed). Advanced development in automation, materials and manufacturing[J]. Applied Mechanics and Materials, 2014, 624: 82-85 [69] HAZRA C, BARI S, KUNDU D, et al. Ultrasound-assisted/biosurfactant-templated size-tunable synthesis of nano-calcium sulfate with controllable crystal morphology [J]. Ultrasonics Sonochemistry, 2014, 21(3): 1117-1131. doi: 10.1016/j.ultsonch.2013.12.020 [70] GUILLON O, ELSAESSER C, GUTFLEISCH O, et al. Manipulation of matter by electric and magnetic fields: Toward novel synthesis and processing routes of inorganic materials [J]. Materials Today, 2018, 21(5): 527-536. doi: 10.1016/j.mattod.2018.03.026 [71] DALVI-ISFAHAN M, HAMDAMI N, XANTHAKIS E, et al. Review on the control of ice nucleation by ultrasound waves, electric and magnetic fields [J]. Journal of Food Engineering, 2017, 195: 222-234. doi: 10.1016/j.jfoodeng.2016.10.001 [72] ZHANG X, WANG L. Study of calcium sulfate whiskers [J]. Asian Journal of Chemistry, 2014, 26(1): 17-20. doi: 10.14233/ajchem.2014.15192 [73] LI G, DU M, SU Y, et al. Research progress on preparation technology of calcium sulfate//ZENG J, LI J, ZHU H (eds). Chemical, Material and Metallurgical Engineering Ⅲ, Pts 1-3[J]. Advanced Materials Research, 2014: 998-1002. [74] LU Y, LI X, WU C, et al. Comparison between polyether titanate and commercial coupling agents on the properties of calcium sulfate whisker/poly (vinyl chloride) composites [J]. Journal of Alloys and Compounds, 2018, 750: 197-205. doi: 10.1016/j.jallcom.2018.03.301 [75] SHENG Z, ZHOU J, SHU Z, et al. Calcium sulfate whisker reinforced non-fired ceramic tiles prepared from phosphogypsum [J]. Boletin De La Sociedad Espanola De Ceramica Y Vidrio, 2018, 57(2): 73-78. doi: 10.1016/j.bsecv.2017.09.005 [76] YANG J, NIE S. Effects of calcium sulfate whisker on the mechanical property, morphological structure and thermal degradation of poly (lactic acid) composites [J]. Polymer Degradation and Stability, 2017, 144: 270-280. doi: 10.1016/j.polymdegradstab.2017.08.031 [77] ZHANG Q, MA P, YANG Y, et al. Reinforcement of recycled paint slag hybrid-filled lightweight calcium sulphate whisker/PVC foam composites [J]. Journal of Environmental Chemical Engineering, 2018, 6(1): 520-526. doi: 10.1016/j.jece.2017.12.025 [78] YUAN W, CUI J, XU S. Mechanical properties and interfacial interaction of modified calcium sulfate whisker/poly (vinyl chloride) Composites [J]. Journal of Materials Science & Technology, 2016, 32(12): 1352-1360. [79] ZHU Z, XU L, CHEN G. Effect of different whiskers on the physical and tribological properties of non-metallic friction materials [J]. Materials & Design, 2011, 32(1): 54-61. [80] JEGANMOHAN S R, CHRISTY T V, SOLOMON D G, et al. Influence of calcium sulfate whiskers on the tribological characteristics of automotive brake friction materials [J]. Engineering Science and Technology-an International Journal-Jestech, 2020, 23(2): 445-451. doi: 10.1016/j.jestch.2019.06.007 [81] CHEN X, YANG L, ZHANG J, et al. Exploration of As(Ⅲ)/As(Ⅴ) uptake from aqueous solution by synthesized calcium sulfate whisker [J]. Chinese Journal of Chemical Engineering, 2014, 22(11-12): 1340-1346. doi: 10.1016/j.cjche.2014.09.018 [82] FAN T, WANG X, GAO Y, et al. Investigating the interaction mechanism and effect of different calcium sulfate whiskers on performance of asphalt binder [J]. Construction and Building Materials, 2019, 224: 515-533. doi: 10.1016/j.conbuildmat.2019.07.093 [83] 师存杰, 张兴儒, 郭祖鹏, 等. 硫酸钙晶须的制备及其应用进展 [J]. 当代化工, 2010, 39(4): 436-438,441. doi: 10.3969/j.issn.1671-0460.2010.04.029 SHI C J, ZHANG X R, GUO Z P, et al. Preparation and application development of calcium sulfate whisker [J]. Contemporary Chemical Industry, 2010, 39(4): 436-438,441(in Chinese). doi: 10.3969/j.issn.1671-0460.2010.04.029
[84] 王泽红, 韩跃新, 袁致涛, 等. CaSO4晶须制备技术及应用研究 [J]. 矿冶, 2005(2): 38-41. doi: 10.3969/j.issn.1005-7854.2005.02.010 WANG Z H, HAN Y X, YUAN Z T, et al. Calcium sulfate whiskers preparation and its application [J]. Mining and Metallurgy, 2005(2): 38-41(in Chinese). doi: 10.3969/j.issn.1005-7854.2005.02.010
[85] HAAS K, LOTHAT P, HANS T, et al. Calcium sulfate whiskers: DE[P]. [1978-07-20] -



 下载:
下载:

















