-
我国经济迅速发展的同时,大气中细微颗粒物(PM2.5)污染问题也逐渐备受人们关注。PM2.5中的主要成分为二次无机离子(sulfate-nitrate-ammonium, SNA)、地壳尘(GM)、有机碳(OC)、元素碳(EC),以及其他微量元素[1]。形成二次颗粒物的主要前体为二氧化硫(SO2)、氮氧化物(NOx)、氨气(NH3)和有机化合物(尤其是挥发性有机污染物,VOCs)[2]。自然界排入大气中的微量气体是还原态的,如氨气(NH3)、甲烷(CH4)、二氧化硫(SO2)等[3],但还原态的气体大都无法稳定存在[4],容易与氧化物发生反应生成铵根、硫酸根和硝酸根等离子,因此当这些气体回到地表时往往是该气体的氧化状态[5],如硫酸(H2SO4)、硝酸(HNO3)、硫酸盐(
${\rm{SO}}_4^{2-} $ )、硝酸盐(${\rm{NO}}_3^{-} $ )、二氧化碳(CO2)等[6-7],这些自然界的物理化学反应是生成PM2.5的重要过程[8]。NH3是大气中唯一能够大量存在的碱性气体,中国平均氨排放强度约为0.9 kg·(km2·a)−1(NH3),排放强度最大的地区分布在中东部地区和广东地区,华北地区的排放量约为1.90 kg·(km2·a)−1(NH3)[9]。由于NH3在大气中的浓度较小,也并不属于有毒有害气体,所以难以引起人们的重视[10-11]。但是,NH3容易被氢氧自由基(·OH)氧化成NO,进一步转化为NOx、HNO3等污染物[12],且容易与酸性物质反应生成铵根离子(
${\rm{NH}}_4^{+} $ )[13],${\rm{NH}}_4^{+} $ 与${\rm{SO}}_4^{2-} $ 发生不可逆的化学反应生成的(NH4)2SO4或NH4HSO4等颗粒态物质[14],${\rm{SO}}_4^{2-} $ 的成盐过程是生成颗粒物的有效途径,这种气-粒转化过程形成的二次气溶胶是大气中PM2.5的重要来源[15]。研究表明碱性环境容易促进SO2和二氧化氮(NO2)的转化,以及可以直接通过聚集作用与H2SO4、HNO3和OC的聚合成大分子,间接提高二次新粒子(secondary new particulate formation, NPF)的形成,提高大气中PM2.5的浓度[16]。邯郸市位于河北省南部,东临山东,西邻山西,南邻河南,地处四省交界,是全国空气污染最重的10个城市之一。2018年邯郸市PM2.5年均浓度为86.6 µg·m−3,为国家二级标准的2.4倍。大气中的NH3除了部分来源于自然界以外,很多来源于人类活动,如人类代谢过程、工业生产、肥料的使用等等[17]。近年来针对邯郸市PM2.5污染特征、化学成分及来源的研究较多。孟琛琛等对PM2.5的化学组分及其特征做了详细的研究,OC、EC和SNA是邯郸市PM2.5的主要成分[18];马思萌等指出,PM2.5对环境质量和人类健康有着很大的影响,这些影响均与PM2.5的化学组成直接相关[19];Zhao等研究表明,二次无机离子对PM2.5的形成有很大贡献[20]。但是对NH3的研究相对较少,人们通常用被动采样法采集大气中的NH3[21],邵生成等研究发现氨-铵气粒转化是推动气溶胶形成的重要因素,体现在低温高湿时NH3和NH4+转化速率较快[22]。
本研究对邯郸市包括NH3在内的主要大气污染物进行了长期在线监测,通过数据分析深入了解NH3的污染特征及其在PM2.5形成中扮演的角色和NH3参与反应的机理,以期为邯郸市未来的霾污染控制提供技术支持。
全文HTML
-
本研究的监测地点在河北省邯郸市南部偏东地区原河北工程大学能源与环境工程实验楼顶(36°34’N,114°29’E),属于城市文教住宅混合区,海拔约56 m,监测站距离地面约12 m。本研究在2015—2018年对PM2.5、NH3以及气象参数(气温、气压、湿度、能见度、风向、风速)进行了近4年的在线监测,同时对PM2.5进行了连续4年的采集和分析。剔除因仪器安装调试(如2015年1月NH3浓度数据)、日常维护及故障等导致的无效数据,在其他有效数据的小时浓度和日均浓度基础上进行数据分析。
-
本研究对NH3的监测采用具有较高灵敏度的化学发光分析仪(Thermo 17i,美国Thermo Fisher公司),监测时段为2015年2月1日—2018年12月31日。其原理如下:当样气进入反应室和内部臭氧发生器生成的臭氧混合,发生化学反应(3)。此反应产生特殊发光,发光强度和NO的浓度呈线性关系,从而被微处理器处理成NO的浓度数据。为检测Nt(NO+NO2+NH3)的浓度,需将NO2和NH3气体先转化为NO,转换后的分子一起与臭氧反应,生成的数据代表Nt(NTotal)浓度。它的工作原理如下式所示:
NO2的浓度由NOx模式下获取的信号减去NO模式下获取的信号决定,NH3浓度由Nt模式下获得的信号减去NOx模式下获得的信号决定,如公式(4)和公式(5)。
本研究对邯郸市PM2.5浓度采用在线监测方法,用双通道颗粒物在线检测仪(TEOM-1405D,美国Thermo Fisher公司)对PM2.5进行在线监测,监测时段为2015年1月1日—2018年12月31日。同时使用大流量采样器(VFC-PM2.5,美国Thermo Fisher公司)采集2015年和2017年代表月份(1、4、7、10月)的PM2.5样品,采集样品用于后期PM2.5的成分分析,剔除因仪器日常维护和故障后,离线样品可用率可达95%(1460个样品中1382个样品可用),具体仪器介绍及使用步骤见文献[23]。
PM2.5中的水溶性阴阳离子含量分别采用DX-600型号和ICS-2100型号的离子色谱仪进行分析,马笑等中有详细的仪器说明及实验介绍[24]。PM2.5中有机碳(OC)和元素碳(EC)含量由热/光碳分析检测仪(Model 2001A, 美国Desert Research Institute)以IMPROVE(Interagency Monitoring of Protected Visual Environment)分析协议规定的热光反射法进行分析。
-
为保证数据的可靠性,采样前需将石英膜置于铝箔纸内在550 ℃的马弗炉内灼烧4 h,此操作用于去除石英膜上的有机物,然后放入恒温恒湿箱(HWHS-150)存放备用;Teflon膜在恒温恒湿箱内平衡24 h后,用十万分之一天平(SartoriusBSA224S-CW)进行一次称量,并密封备用。采样后将样品先置于零下20 ℃的冰柜中保存,等测试样品时在实验室取出直径为2 cm的圆形试样,用超声波水浴、0.45 μm孔径过滤后离心,储存至化学分析。每月1号、15号和30号均保留一张空白膜与其他样品同时分析,通过计算空白膜和样品膜中的浓度差值,减小实验的系统误差。
1.1. 监测站点
1.2. 设备及数据
1.3. 质量控制
-
研究结果表明,2015—2018年邯郸市NH3平均浓度为15.8 µg·m−3。表1给出了国内外城市大气中NH3浓度对比,与国内其他城市相比,邯郸市的NH3浓度远高于上海(6.2 µg·m−3)、青岛(4.7 µg·m−3)、广东(7.3 µg·m−3)和香港(3 µg·m−3),与北京(22.8 µg·m−3)、南京(15.3 µg·m−3)和西安(18.6 µg·m−3)处于同一水平。与国外城市相比,邯郸市的NH3浓度远高于首尔(11.2 µg·m−3)、纽约(5.1 µg·m−3)和罗马(5.5 µg·m−3),但低于拉合尔(50.1 µg·m−3)和德里(40.7 µg·m−3)等南亚城市。从表1也可以看出,工业区大气中NH3浓度明显高于一般城市和农村地区,同一城市中城市和农村的NH3浓度没有明显规律。
-
NH3的年平均浓度为15.7 µg·m−3(表2),其中2016年最高(18.8 µg·m−3),比2015年(15.2 µg·m−3)高9.2%;2017年NH3浓度最低(13.8 µg·m−3),与2016年浓度相差36.2%。大气中NH3排放来源既有自然源,也有人为源,其中畜牧养殖和化肥施用是贡献最大的人为源排放[35],可占我国NH3总排放量的80%—90%[9],其中化肥中氮肥的主要成分是碳酸氢铵、尿素、氨水和硫酸铵,在大气中容易挥发生成NH3逸散到空气中[36]。2017年邯郸施肥量约为162.3万吨,比2016年(174.0万吨)降低7.2%,同时,2017年的大型牲畜(24.6万头)、猪(463.1万头)、羊(375.2万头)和家禽养殖量(7635.5万只)分别比2016年低41.9%、17.0%、66.6%和39.5%[37]。2017年氨浓度明显低于2016年,很大程度是2017年农业氨排放减少的影响。
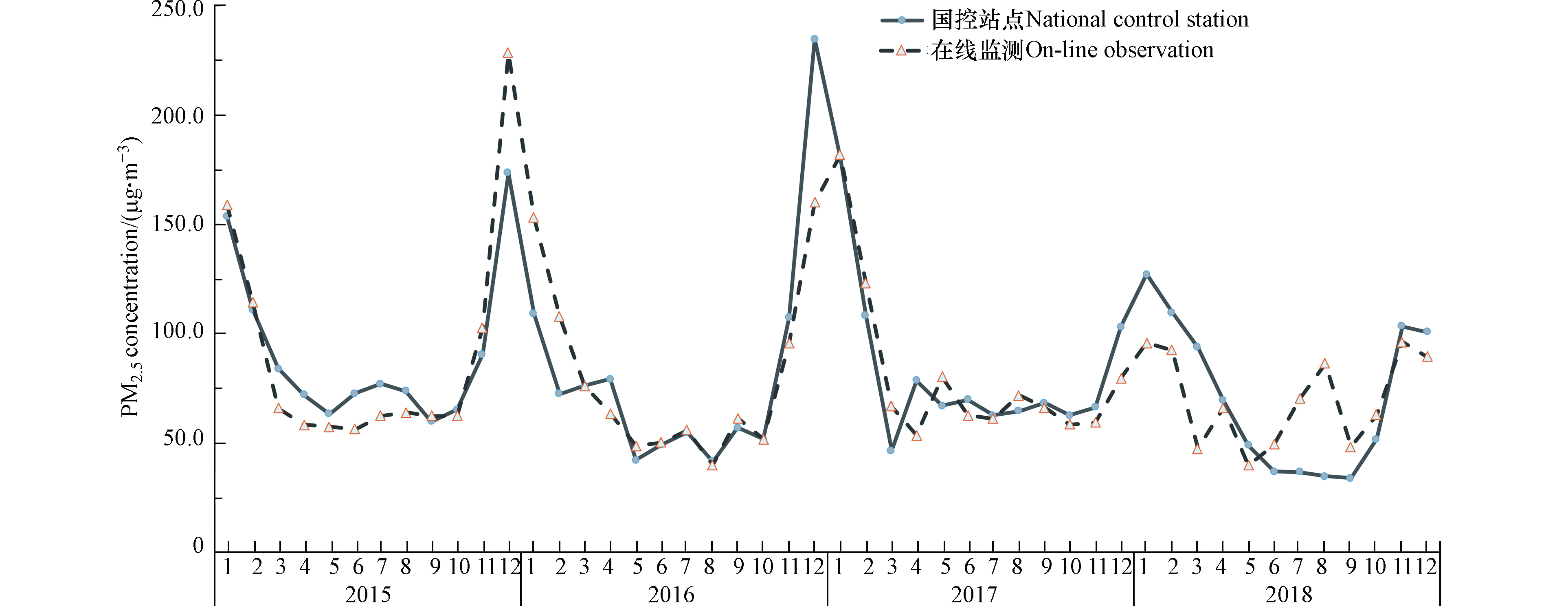
邯郸市2015—2018年PM2.5浓度总体呈下降趋势。2015—2018年PM2.5的年平均值分别为90.8、80.9、86.2、82.0 µg·m−3,分别为国家二级标准的2.6倍、2.3倍、2.5倍和2.3倍。其中,每年的11月到次年2月为重污染时段,3月到10月则属于污染较轻时段。图1给出了邯郸市监测站点的在线监测情况及其与距离监测站200 m的国控站点的数据进行对比,结果显示,站内仪器与国控站点的监测结果基本一致,冬季重污染时段结果略有偏差,2018年8月数据偏差较大,原因是仪器维修造成数据较不稳定导致偏差,此月数据在后面的研究中将不予采用。2015年—2018年冬季PM2.5浓度有明显下降趋势,2018年冬季PM2.5浓度(106.9 µg·m−3)比2015年平均浓度(163.5 µg·m−3)下降34.6%。邯郸市PM2.5浓度呈现出冬季(123.6 µg·m−3)>秋季(67.4 µg·m−3)>春季(56.8 µg·m−3)>夏季(56.2 µg·m−3)的特征。
-
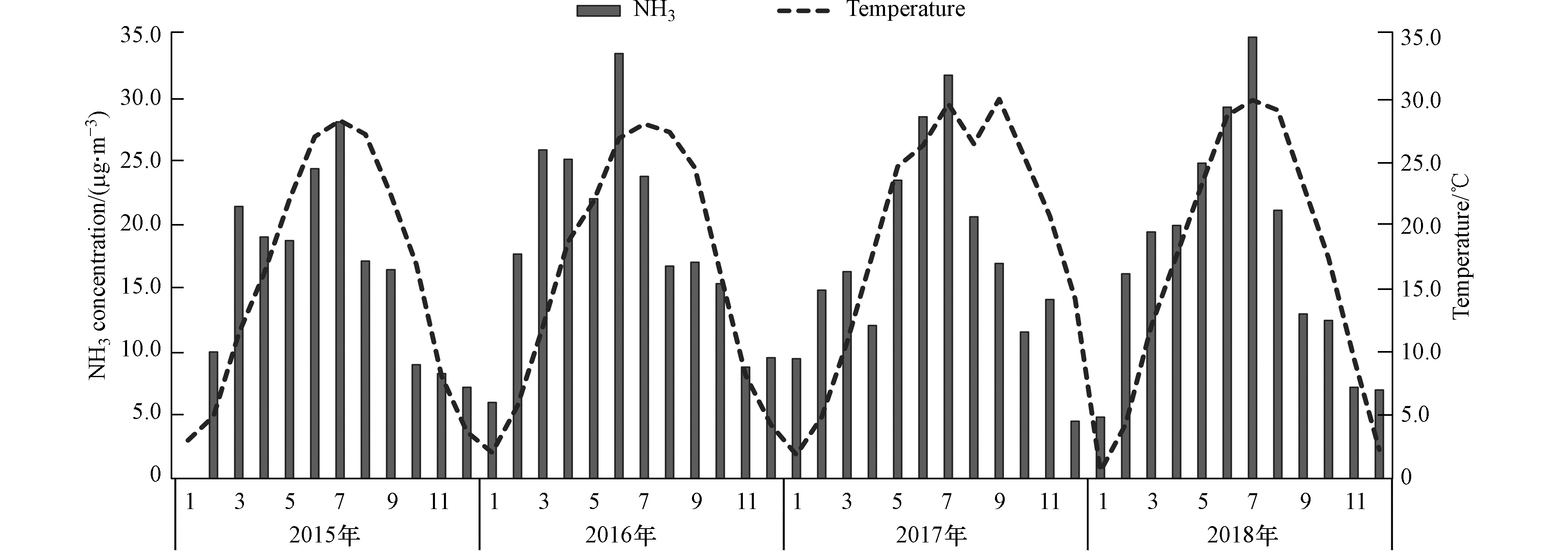
邯郸市NH3浓度总体呈现出夏季(22.8 µg·m−3)、春季(22.0 µg·m−3)浓度高,秋季(11.7 µg·m−3)、冬季(9.6 µg·m−3)浓度较低的特征。图2给出了邯郸市2015—2018年NH3浓度及温度季节变化,研究观测期间,定义3—5月为春季,6—8月为秋季,9—11月为秋季,12—2月为冬季。从图2可以看出,NH3的月均浓度有两个高值期,第一个浓度高值期出现在春季的3月,在近4年的监测中浓度分别为21.5、26.0、16.3、19.5 µg·m−3,由于春季农耕施肥,农业活动中肥料的主要成分磷酸氢二铵和尿素的挥发是NH3排放的主要来源[38];第二个浓度值高峰出现在夏季,在近4年监测中分别出现在7月(28.2 µg·m−3)、6月(33.6 µg·m−3)、7月(31.9 µg·m−3)和7月(34.9 µg·m−3),在夏季达到一年中NH3浓度的最大值。
NH3浓度变化与温度变化较为相似(r= 0.69),由此可见温度变化对NH3有一定影响。NH3的熔沸点较低,易挥发,较高温度可使NH3从排放源挥发释放,引起NH3排放量增大,同时夏季高温降低了大气颗粒物中挥发性铵盐(如硝酸铵)的稳定性[39],使反应逆向进行,从而增大NH3的浓度。
${\rm{NH}}_4^{+} $ 是二次无机气溶胶的成分之一,也是PM2.5的重要成分。表3反应了2015年和2017年${\rm{NH}}_4^{+} $ 的季节浓度特征,分别表现为2015年冬季(32.0 µg·m−3)>秋季(8.9 µg·m−3)>夏季(8.3 µg·m−3)>春季(6.8 µg·m−3)和2017年冬季(18.9 µg·m−3)>秋季(9.5 µg·m−3)>夏季(8.4 µg·m−3)>春季(6.8 µg·m−3),全年呈现单峰状态。2015年的${\rm{NH}}_4^{+} $ 对PM2.5的贡献率(${\rm{NH}}_4^{+} $ /PM2.5)为15.4%,2017年下降到13.6%。 -
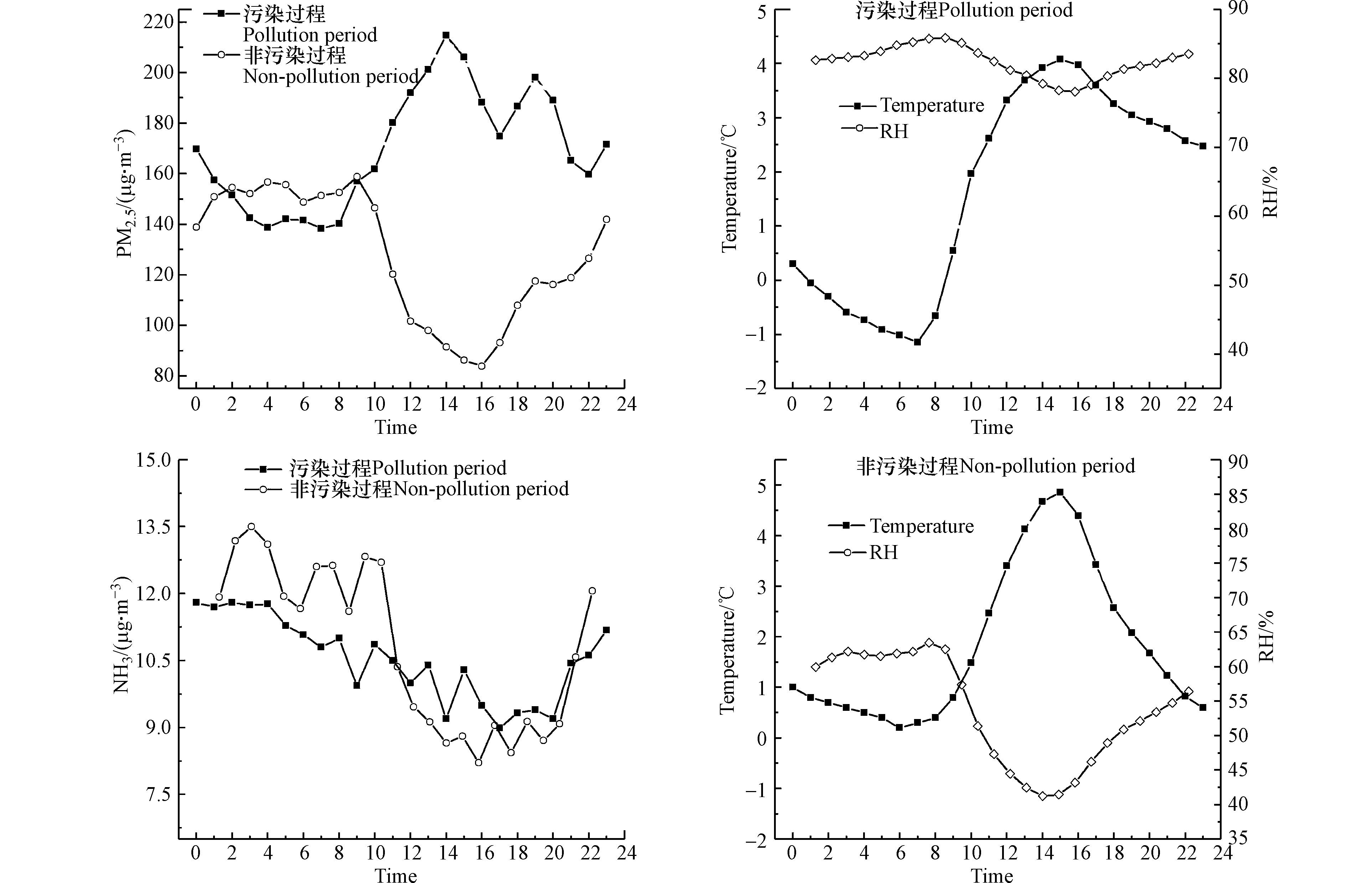
图3给出了2017年污染代表月1月的NH3、PM2.5及气象要素的时间序列演化,阴影部分描述的是该月的重污染时期,即PM2.5浓度大于200 µg·m−3(1月1日—1月8日、1月26日—1月28日,共11 d),在此时期PM2.5的平均浓度为231.8 µg·m−3,是非污染过程(129.0 µg·m−3)的1.8倍,同时污染过程的
${\rm{NH}}_4^{+} $ 也呈现出较高浓度(23.5 µg·m−3),是非污染过程(16.2 µg·m−3)的1.4倍。观测期间NH3平均浓度变化差异较小,污染过程浓度(7.5 µg·m−3)<非污染过程浓度(10.3 µg·m−3)。图4给出了2017年1月不同污染条件下NH3、PM2.5和温湿度的日变化曲线。总体来看,污染过程的湿度(82%)明显大于非污染过程(54%),两者相差53.2%。温度和湿度昼夜变化较为明显,呈现出夜间低温高湿,白昼高温低湿的变化趋势,夜间NH3浓度略高于白天(二者相差16.5%)。NH3是大气中
${\rm{NH}}_4^{+} $ 的唯一前提气体,NH3-${\rm{NH}}_4^{+} $ 转化机制是生${\rm{NH}}_4^{+} $ 的主要驱动因子,${\rm{NH}}_4^{+} $ 形成的NH4NO3和NH4Cl均属于半挥发性盐类,性质不稳定,两种铵盐均与大气中的前体气体以气粒平衡的形式存在,其气粒分配与气体含量、颗粒物化学组分和温度湿度有关。王晓琪等[40]研究表明,湿度是影响二次转化的重要因素,主要表现为湿度增大时气粒转化也随之增大,${\rm{NH}}_4^{+} $ 生成量增多,而2017年冬季湿度在污染时期湿度明显大于非污染时期,导致冬季污染时期${\rm{NH}}_4^{+} $ 日均浓度处于全年较高水平(25.5 µg·m−3)。 -
本研究根据PM2.5的日均浓度划分为严重污染时期HP(PM2.5日浓度超过150 µg·m−3)、中度污染时期PP(PM2.5日浓度介于75—150 µg·m−3)和清洁时期CP(PM2.5日浓度不足75 µg·m−3)。结果表明,2017年累计空气清洁天数达278 d(76%),比2015年(207 d)增长了33%,累积中污染天数为27 d(7%),比2015年(43 d)下降了59%。SNA在PM2.5中占有较大比例,与PM2.5存在线性关系,即PM2.5处于高浓度水平时,SNA的浓度也较高。在二次气溶胶污染过程中,高湿度条件下NH3更易与
${\rm{NO}}_3^{-} $ 、${\rm{SO}}_4^{2-} $ 等离子发生中和反应生成硝酸盐、硫酸盐等二次无机盐。Wang等[27]研究表明氨排放强度与NH3浓度及SNA浓度呈正相关,NH3-${\rm{NH}}_4^{+} $ 气粒转化率与SNA浓度,尤其是${\rm{NO}}_3^{-} $ 呈强正相关性,因此NH3与SNA的浓度有较为密切的关系[41],大气中的NH3在高PM2.5的情况下容易发生化学反应形成${\rm{NH}}_4^{+} $ 。表4给出了不同空气质量状况下NH3、
${\rm{NH}}_4^{+} $ 和PM2.5的浓度水平及气象参数条件,观测期间NH3/${\rm{NH}}_4^{+} $ 通常大于1,在轻度污染状态下该比值最高(平均为3.83),大气处于NH3富余状态[42],在轻污染状态下2015年和2017年的${\rm{NH}}_4^{+} $ 浓度仅为6.8 µg·m−3和7.3 µg·m−3。邯郸市四季的相对湿度呈现出夏(72.1%)>秋(68.9%)>春(56.9%)>冬(55.3%),NH3浓度与温度有强正相关性(r=0.69)。从热力学角度来看,NH4NO3和NH4Cl热稳定性差,与前提气体(HNO3、HCl和NH3)的可逆过程收到温度湿度的影响。其中NH4NO3具有强挥发性,当温度高于30 ℃时,有利于该盐分解,当温度低于15 ℃时,NH3又与HNO3发生反应生成NH4NO3颗粒。在CP、PP和HP污染事件中的平均温度分别为19.8、15.1、5.8 ℃,平均湿度分别为58.1%、62.8%、82.8%。定义总氨(NHx)为NH3和${\rm{NH}}_4^{+} $ 之和(NHx=NH3+${\rm{NH}}_4^{+} $ ),并将${\rm{NH}}_4^{+} $ /NHx比值定义为氨-铵的气固转化率[43-44]。表5给出了不同温湿度条件下的气固转化率情况,温度在−5—10 ℃、湿度在60%—100%时,NH3-${\rm{NH}}_4^{+} $ 气固转化率处于较高水平,HP时期的温湿度正处于此范围之内,这时更多的${\rm{NH}}_4^{+} $ 释放并为PM2.5的形成做出贡献,HP时期的PM2.5平均浓度是PP时期的2.3倍,CP时期的4.7倍。 -
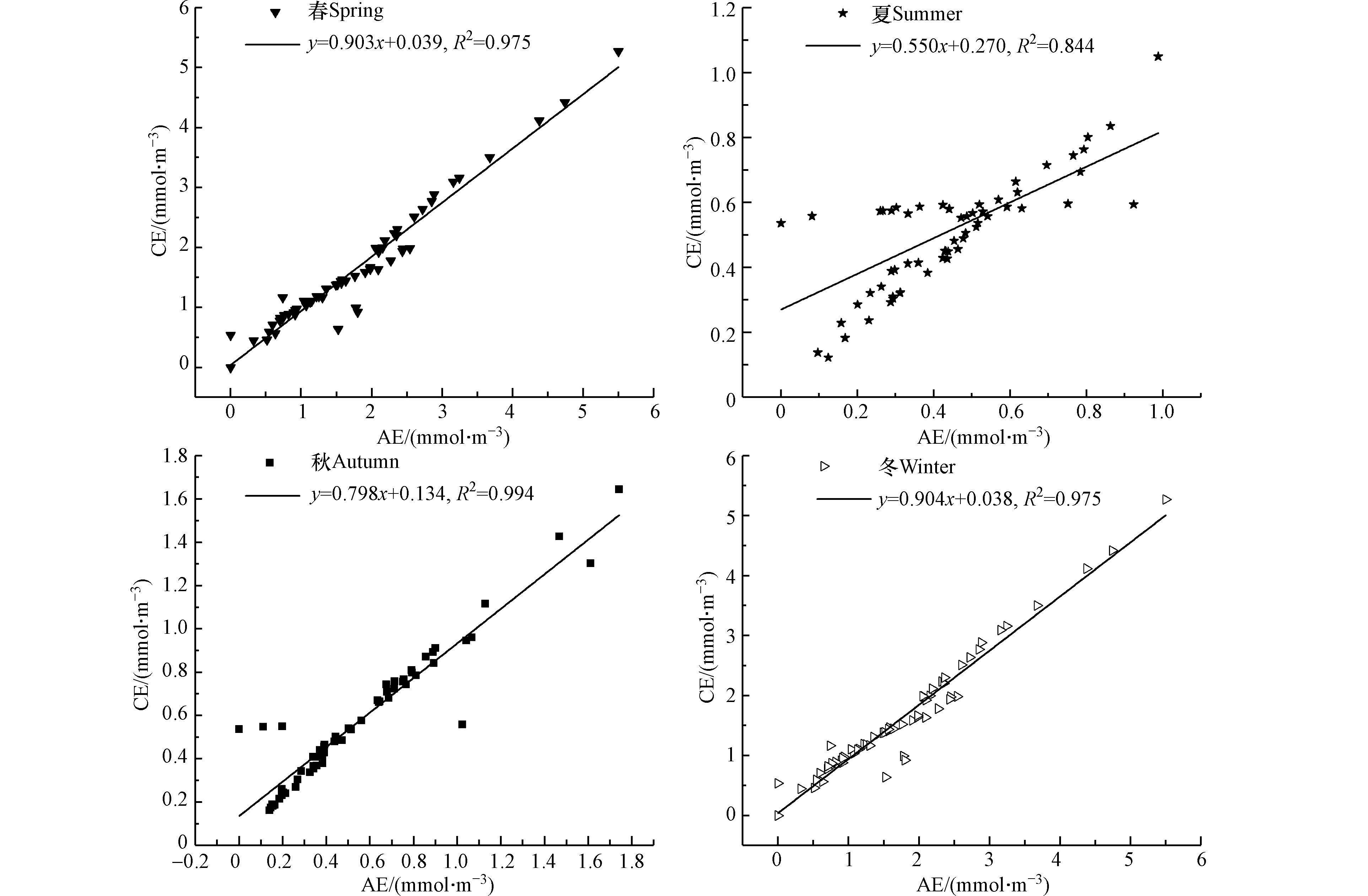
图5给出了邯郸市2015和2017年代表月份(1月、4月、7月和10月)PM2.5中阴阳离子的电荷平衡关系,其图中所有的阴阳离子均用电荷浓度来表示(µmol·m−3),本研究用AE来代表阴离子的电荷浓度,CE表示阳离子的电荷浓度,计算公式如下:
若颗粒物显示电中性,则根据电中性原理,正负电荷离子达到平衡,AE和CE的回归方程斜率为应等于1[7]。邯郸市PM2.5中阴阳离子的比值回归曲线显示,冬季和春季回归曲线的斜率最高分别为0.904和0.903,最接近电中性,夏季斜率最小,约为0.550,夏季
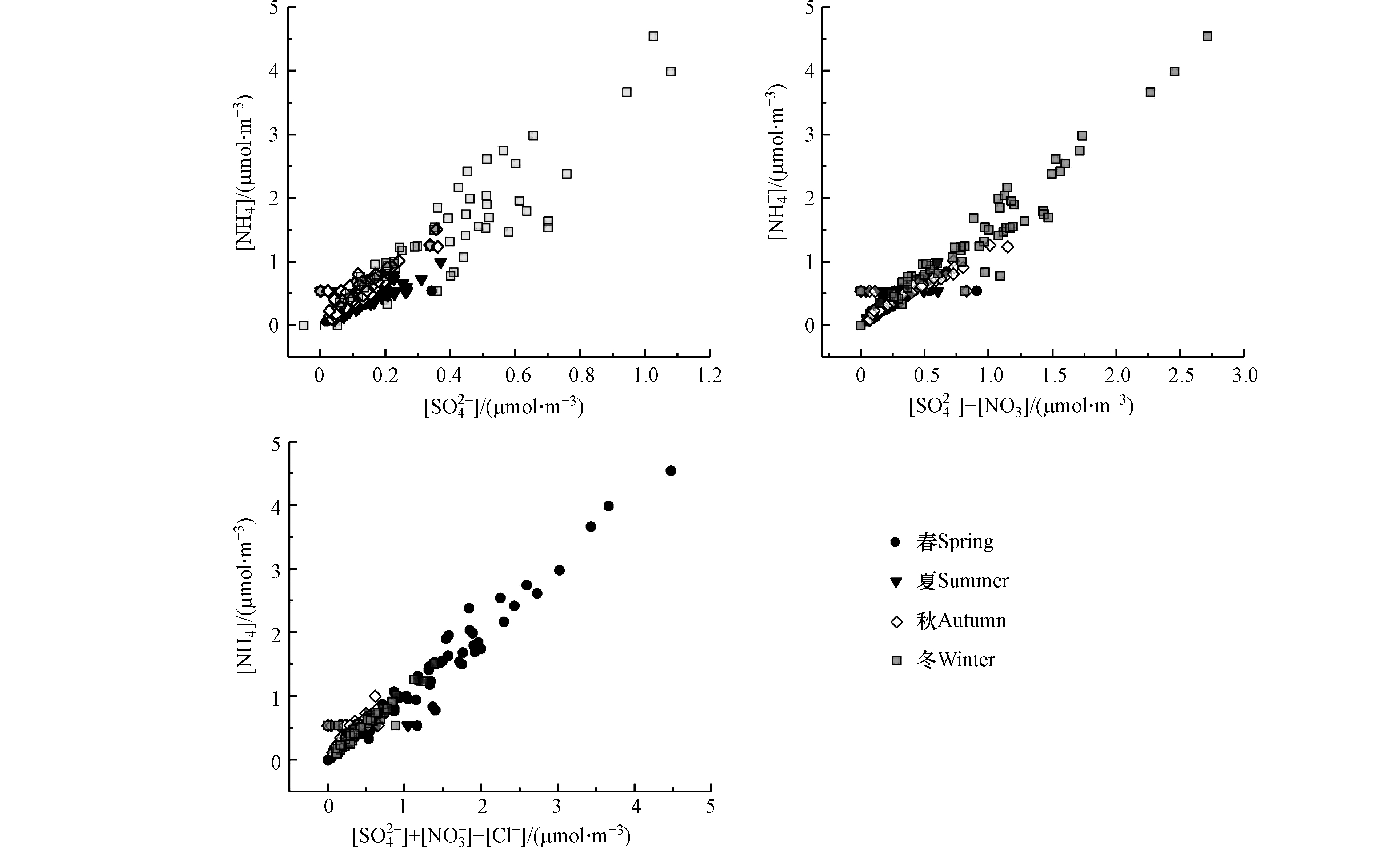
${\rm{NH}}_4^{+} $ 参与气粒转化,挥发到大气中可能是夏季CE/AE减小的主要原因,四季颗粒物均显酸性,夏秋季最为明显。图6给出了2015年和2017年代表月份PM2.5中
${\rm{NH}}_4^{+} $ 与主要酸性离子拟合状态,其中[ ]代表离子的电荷浓度。模拟结果显示${\rm{NH}}_4^{+} $ 与${\rm{SO}}_4^{2-} $ 、${\rm{NO}}_3^{-} $ 和Cl−阴离子拟合效果较好且季节性明显,四个季节中${\rm{SO}}_4^{2-} $ 离子均与${\rm{NH}}_4^{+} $ 离子拟合良好,且春秋季最佳,${\rm{SO}}_4^{2-} $ 主要以(NH4)2SO4形式存在。秋冬两季${\rm{NH}}_4^{+} $ 可同时满足${\rm{NO}}_3^{-} $ 和${\rm{SO}}_4^{2-} $ ,两种阴离子分别以NH4NO3、(NH4)2SO4形式存在,但春夏季拟合效果较差。春冬季节绝大多数酸性离子以中性盐的形式存在,${\rm{NH}}_4^{+} $ 可满足${\rm{SO}}_4^{2-} $ 、${\rm{NO}}_3^{-} $ 和Cl−分别生成(NH4)2SO4、NH4NO3和NH4Cl(此处暂不考虑${\rm{SO}}_4^{2-} $ 的一次水解),夏秋季节Cl−主要以NH4Cl和KCl的形式存在(Cl−和K+在夏秋两季中相关性为0.85)。以${\rm{NH}}_4^{+} $ 为代表的二次无机盐是PM2.5的关键成分,在污染中起着重要作用。
2.1. 邯郸市NH3污染水平
2.2. NH3和PM2.5污染特征
2.2.1. 年际浓度特征
2.2.2. 季节变化特征
2.2.3. 日变化特征
2.3.
NH3-${\rm{NH}}_4^{+} $ 气粒转化对PM2.5的影响
2.4.
${\rm{NH}}_4^{+} $ 成盐及其对高浓度PM2.5的贡献
-
(1)2015—2018年NH3平均浓度为15.8 µg·m−3,邯郸市NH3浓度总体呈现出夏季(22.8 µg·m−3)、春季(22.0 µg·m−3)浓度高,秋季(11.7 µg·m−3)、冬季(9.6 µg·m−3)浓度较低的特征。重污染过程(PM2.5>200 µg·m−3)的NH3浓度(7.5 µg·m−3)<非污染过程(10.3 µg·m−3),NH3浓度与温度有强正相关性(r=0.69)。污染过程的湿度(82.4%)明显大于非污染过程(53.8%),这与PM2.5和NH4+的浓度变化规律相类似。
(2)PM2.5中阴阳离子的比值在冬春季节处于较高水平(0.904和0.903),正负电荷离子达到平衡。冬春季绝大多数酸性离子以中性盐的形式存在,
${\rm{NH}}_4^{+} $ 可满足${\rm{SO}}_4^{2-} $ 、${\rm{NO}}_3^{-} $ 和Cl−分别生成(NH4)2SO4、NH4NO3和NH4Cl,夏秋季节Cl−主要以NH4Cl和KCl的形式存在,以${\rm{NH}}_4^{+} $ 为代表的二次无机盐是PM2.5的关键成分,在污染中起着重要作用。(3)温度在−5—10 ℃、湿度在60%—100%时,NH3-
${\rm{NH}}_4^{+} $ 气固转化率处于较高水平,重污染时期(HP)的平均温度为5.8 °C,平均湿度为82.8%,更多的${\rm{NH}}_4^{+} $ 在此温湿条件下被释放出来,HP时期的PM2.5平均浓度是污染时期(PP)的2.3倍,清洁时期(CP)的4.7倍。

















 下载:
下载: