-
氟(F)是人体必需的微量元素,但氟离子是温泉水中典型的高浓度有害元素. 适宜浓度的含氟饮用水(0.5—1.5 mg·L−1)对人体有益,能维持人体的钙、磷正常代谢,防止患龋齿病. 但长期饮用过量含氟水会对人体免疫系统和组织产生威胁从而引发病变,如氟斑牙和氟骨症,甚至引发脑损伤、癌症等病症[1- 2]. 一般在地热温泉水流经的河流水样中,大都能检测到高含量的F离子[3-4],若长期以含氟河水为饮用水源,势必会对流域居民产生一定的威胁,因此地热温泉水中氟离子的净化处理,不仅对流域生态保护,而且对居民健康具有重要的意义.
当前含氟水的处理方法主要包括:电化学法、混凝沉淀法、膜过滤法、离子交换法和吸附法等[5- 6]. 其中吸附法具有吸附剂种类多、成本低、污染小、易操作等特点,是当前用于处理大体积、高浓度含氟废水的经典常用方法[7-8]. 吸附法的核心在于吸附剂的选择,它是决定吸附性能的关键. 当前已被报道的除氟吸附剂大致可分为几类:活性氧化铝[9]、金属氧化物[10-11]、生物质衍生的碳基吸附剂[12-13]、羟基磷灰石[14]等. 以生物质为原料的吸附剂应用广泛,吸附性能较好、成本较低、适用条件温和,常用于吸附剂. 茶叶属于天然植物吸附剂,具有网状结构、多孔、比表面积大、含有多种活性基团,是一类理想的除氟吸附剂[15].
本文选择了大红袍、武夷茶、日照绿茶、四川绿茶、泾阳砖茶、安华砖茶(拉萨本地使用较多)、桂花乌龙茶、茉莉花茶、铁观音、金丝皇菊等10种茶叶进行除氟对比研究,选择氟离子去除效果较好的茶叶吸附剂进行实验条件优化,采用动态吸附方式对氟离子吸附效果及吸附动力学和吸附等温线进行了探究,最后对实际温泉水中氟离子进行了净化处理,期望为高氟地热水净化提供技术支撑.
-
pHSJ-6L型pH计(上海仪电科学仪器股份有限公司)、BSA224S型电子天平(赛多利斯科学仪器)、ZNCL-G型智能磁力搅拌机(上海予申仪器有限公司)、Thermo Eutech 优特F090 ION 700型氟离子浓度测量仪(优特仪器有限公司)配置有CHN090 加液式复合氟离子电极和自动温度补偿ATC电极. 氟离子电极填充液、100 mg·L−1氟离子标准溶液和氟离子强度调节剂(TISAB Ⅱ总离子强度缓冲液)均购置于优特仪器有限公司并用于氟离子浓度测定. 日立S-4800扫描电子显微镜、赛默飞Nicolet iG50 傅立叶变换红外光谱仪和美国麦克仪器公司ASAP 2020比表面积分析仪用于茶叶吸附剂形貌和结构表征. 浓硝酸(优级纯)和氢氧化钠固体(分析纯)购置于国药试剂厂.
-
大红袍、武夷茶、日照绿茶、四川绿茶、泾阳砖茶、安华砖茶、桂花乌龙茶、茉莉花茶、铁观音、金丝皇菊均购置于拉萨本地超市. 茶叶经沸水反复浸泡8—10次,去除茶叶中的色素、茶多酚等可溶成分,期间多次换水,直至上清液为无色后,测定上清液中氟电位与空白信号一致后,将茶叶过滤、烘干、粉碎备用.
-
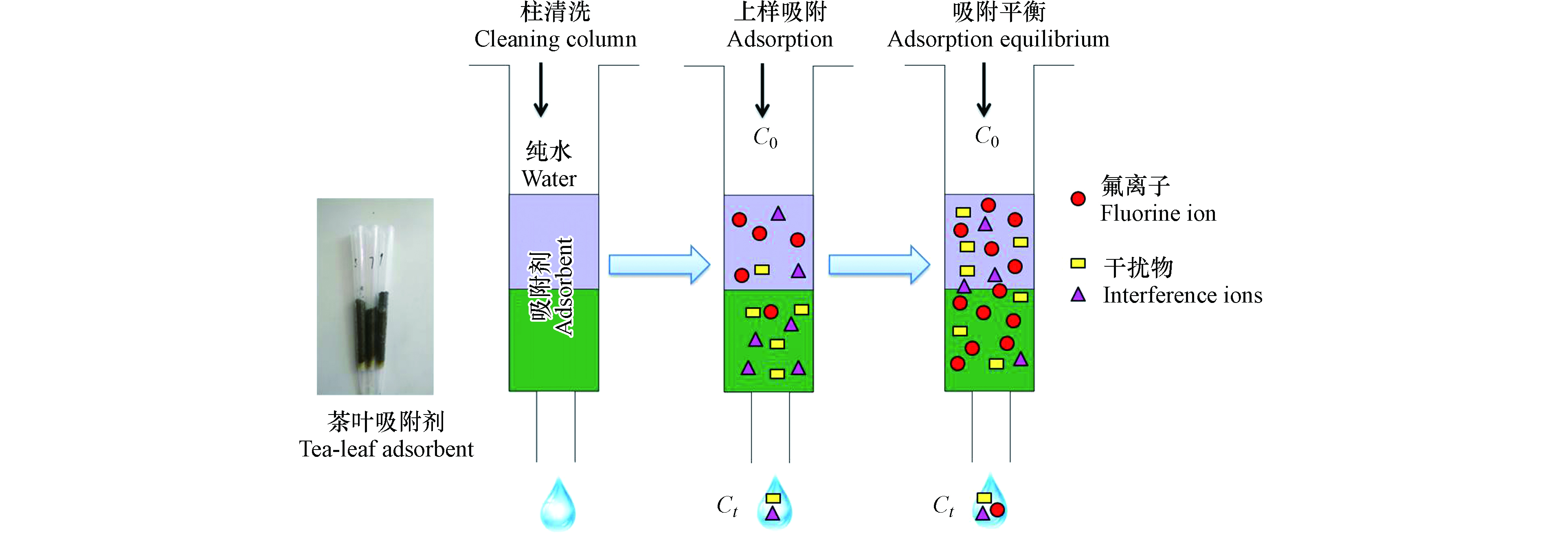
动态微柱模式是考察吸附剂性能的一种经典方法,本文利用10 mL枪头作为小型微柱,自制了动态微柱,如图1插图所示. 溶液通过自身重力流过微柱与吸附剂实现动态吸附,无需泵源,操作模式简单. 具体流程如下:准确称量废弃茶叶粉末0.5 g,装入10 mL枪头,枪头底部填入少量脱脂棉起过滤作用. 首先,用纯水持续冲洗去除色素后,直至流速稳定,此时上样流速约为0.67 mL·min−1. 用初始浓度为C0 (mg·L−1)的含氟离子溶液持续上样,一定时间间隔后收集3 mL流出液测定其氟离子平均浓度记作Ct (mg·L−1). 直至流出液浓度Ct达到初始浓度C0的90%,认为达到穿透终点,吸附达平衡,停止上样,其吸附过程如图1所示.
采用离子选择性电极法测定氟离子浓度. 氟电极电位E(mV)与溶液中氟离子浓度
(CF−) 满足能斯特(Nernst)方程. 即氟电位E(mV)与氟离子浓度的负对数值(−lgCF−) 呈正比.待测废水中常见的阴离子如NO−3 、SO2−4 和Cl−等干扰较小,主要干扰物为OH−,需要加入TIASB Ⅱ缓冲溶液掩蔽Fe3+和Al3+并保持pH值在5—6之间. 将100 mg·L−1氟标准储备液逐级稀释配置成0.1、1、5、10、20 mg·L−1的氟离子溶液,分别取3 mL氟离子标准溶液,加入3 mL TIASB Ⅱ,混合后采用氟电极测定其电位值.以标准溶液氟离子浓度的负对数为横坐标,电位为纵坐标作图,并得出线性方程E=k(−lgCF−) +b (y=58.37x+77.27)和相关系数(R2=0.9991),未知样品氟离子浓度通过标准曲线计算. -
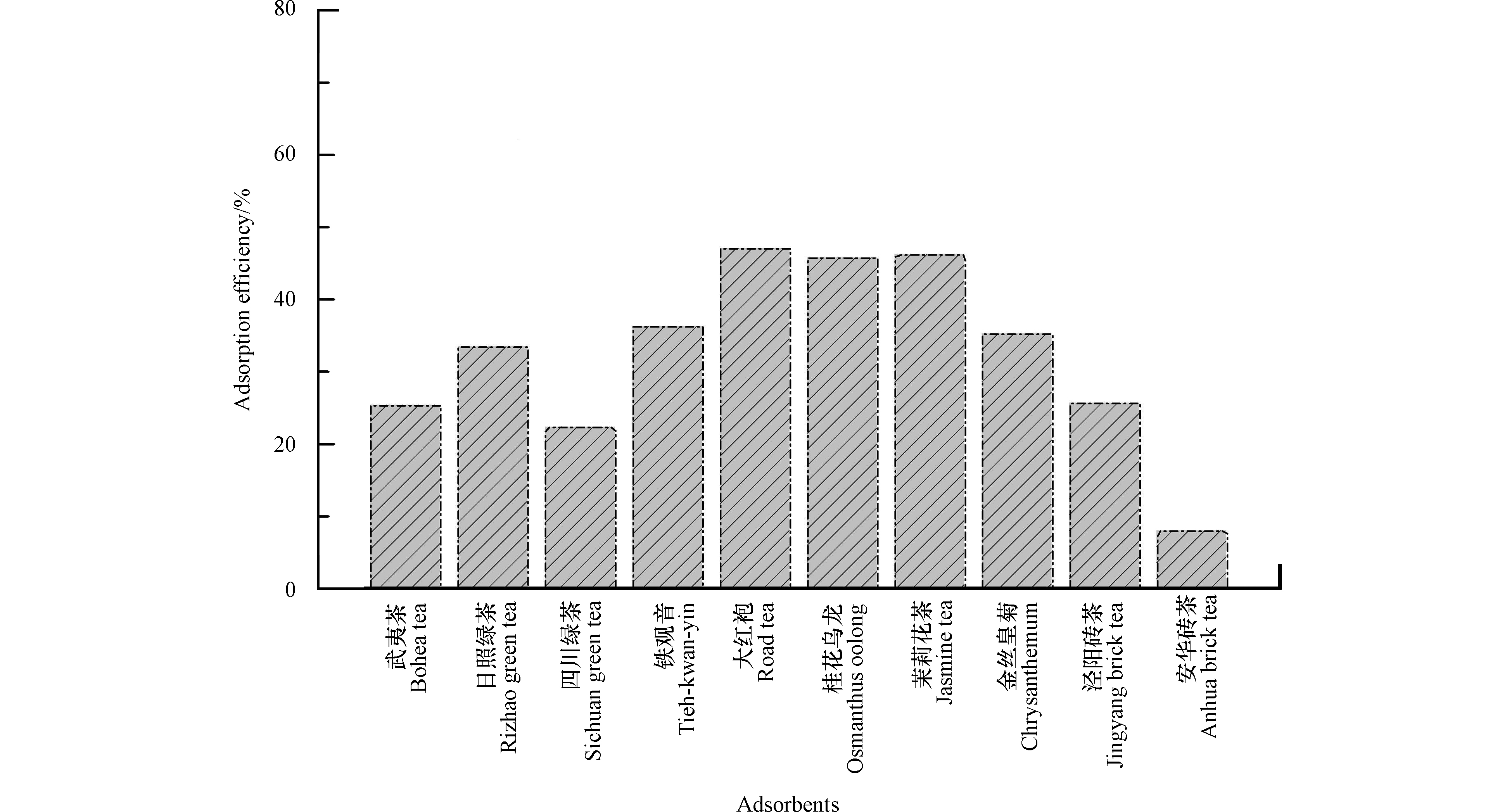
在初始氟离子浓度为5 mg·L−1、固液比为20 g·L−1、pH 3、室温条件下考察了10种茶叶对氟离子的吸附效率,结果如图2所示.
不同茶叶品种对氟离子吸附性能差异较大,吸附效果最好的3种茶叶分别是大红袍、桂花乌龙茶和茉莉花茶,分别为47.0%、45.7%和46.2%,吸附效率不存在显著差异(P>0.05,其它几种茶叶的吸附率在10%—35%之间. 已有研究表明,茶叶聚氟性能和规律存在差异,其因素主要包括茶树器官、生长周期、茶树品种、土壤生长环境、茶叶中铝离子含量及表面微孔结构等[16].
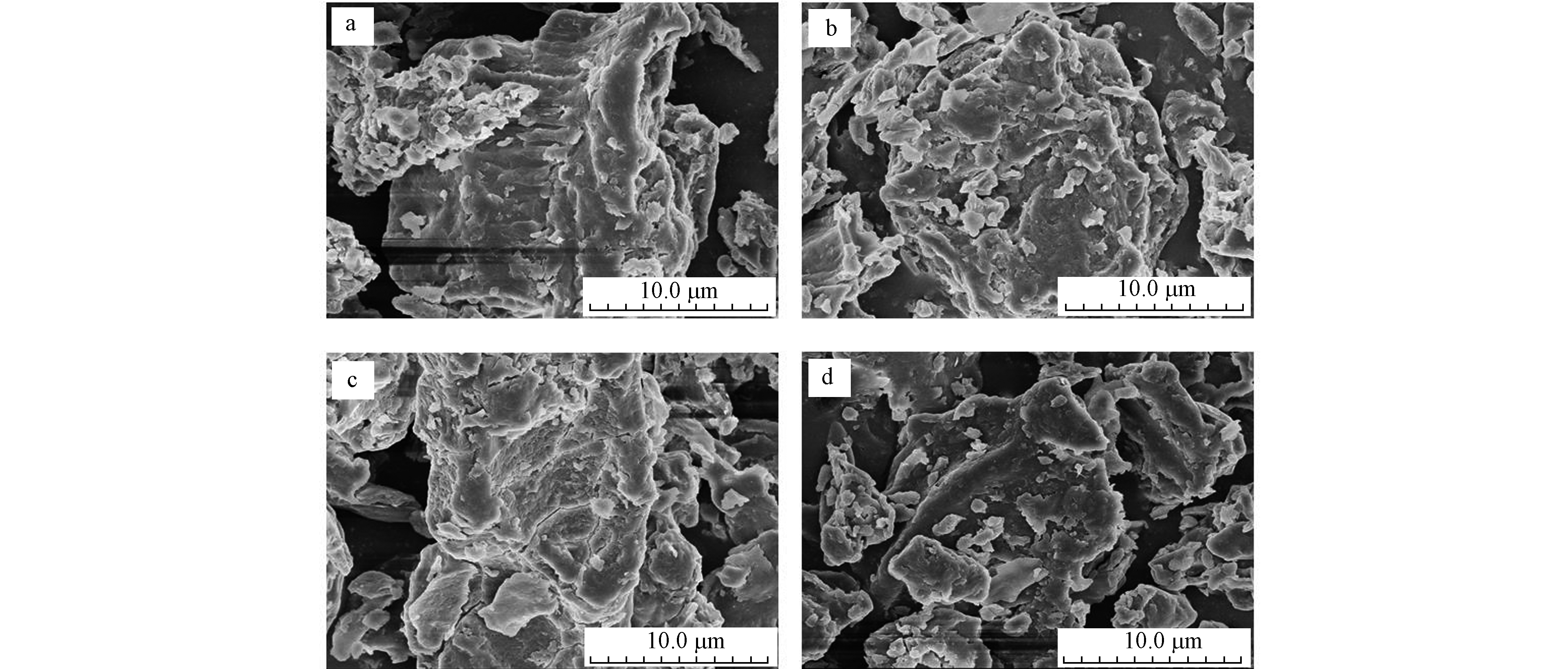
为进一步探究不同茶叶吸附氟离子的性能差异原因,考察了大红袍、桂花乌龙茶、茉莉花茶和四川绿茶吸附剂的表面微观形貌和比表面积(BET)特征. 扫描电镜图(SEM)如图3所示,茶叶表面粗糙,结构较为致密,不具有明显的孔结构但伴有粗糙褶皱,粗糙表面特征为丰富比表面积提供有力条件. 比表面积越大,对吸附越有利.
4种茶叶的BET表征结果列于表1. 从表1可见,4种茶叶的孔体积都较低,基本不具有孔结构特征. 由于茶叶结构较为致密,无孔结构,整体比表面积都较小,其中大红袍的比表面积最大,达1.788 m2·g−1,桂花乌龙茶和茉莉花茶的比表面积相当,四川绿茶的最低. 因而大红袍茶叶的比表面积在吸附过程中具有一定的优势. 因而在选择茶叶吸附剂时宜优选择大红袍茶叶作为氟离子吸附剂,在后续实验中,选择大红袍作为吸附剂进一步优化吸附条件.
-
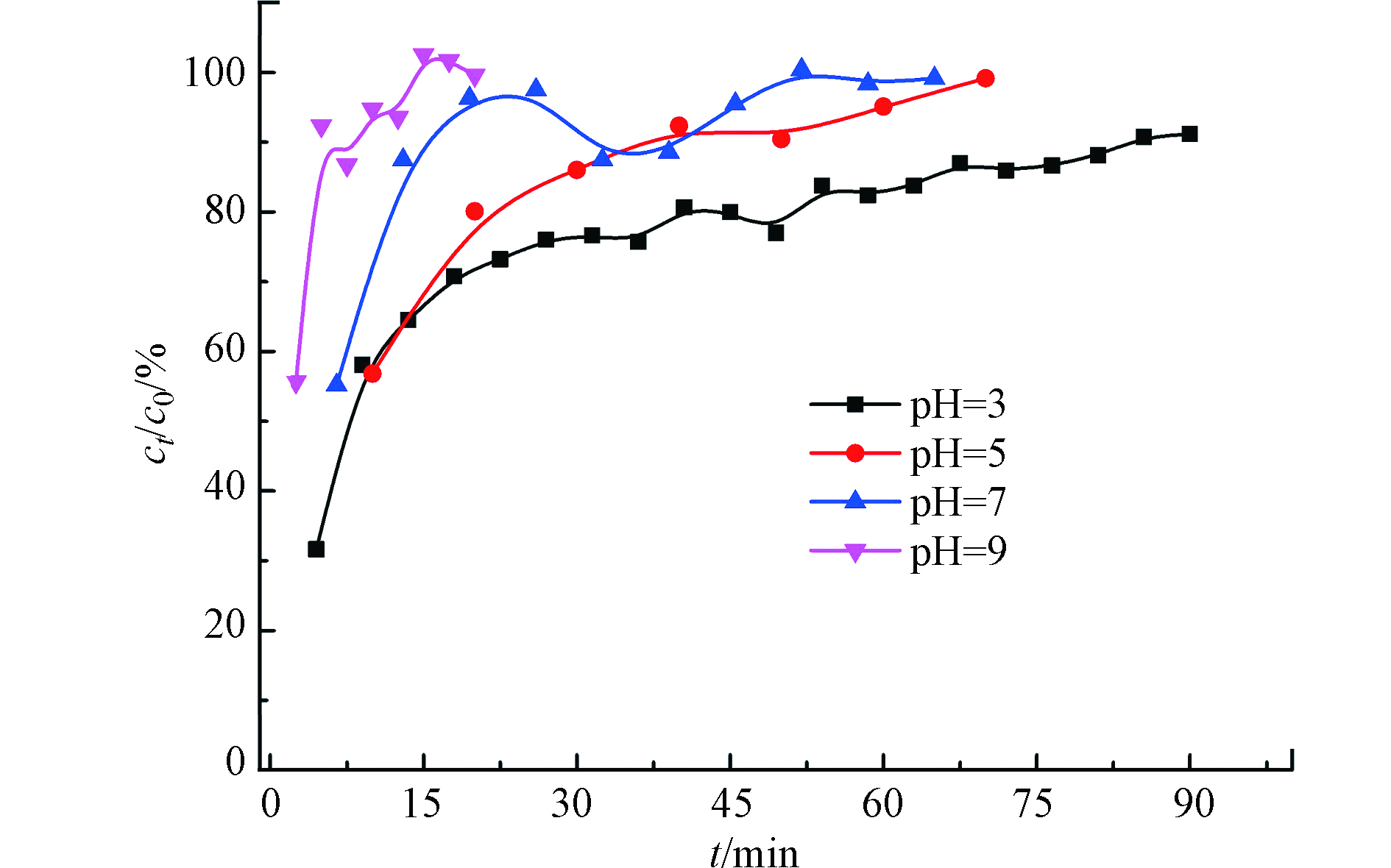
实际废水处理操作中,pH是影响吸附剂能否发挥优良性能的重要因素,当溶液的pH过低时,溶液中游离的氟离子很容易与氢离子结合生成氟化氢,氟化氢挥发;而pH过高时,溶液中游离的氟离子和溶液中游离的氢氧根形成竞争. 为了解pH对废弃茶叶吸附剂的影响程度,因此探究了废弃茶叶的最佳吸附pH,固定起始氟离子浓度为5.0 mg·L−1,吸附温度为25 ℃,按实验方法的步骤,结果如图4所示. pH越高,越早出现拐点,达到终点的时间越短,吸附氟离子的总量也就越少,所以pH越高,越不利于茶叶吸附剂吸附,因此,选择pH 3为茶叶吸附氟离子的最优pH.
在pH 3时,穿透曲线前期没有平缓的过渡阶段而是突然地开始上升, 30 min之后流出液浓度缓慢升高,最终在85 min处达到穿透终点. 因为茶叶吸附剂的吸附量略低,吸附位点不足,流出液的浓度明显增加,在实际氟离子吸附操作中可以加大吸附剂的投入量、减小上样流速和初始浓度获得更高吸附效率.
-
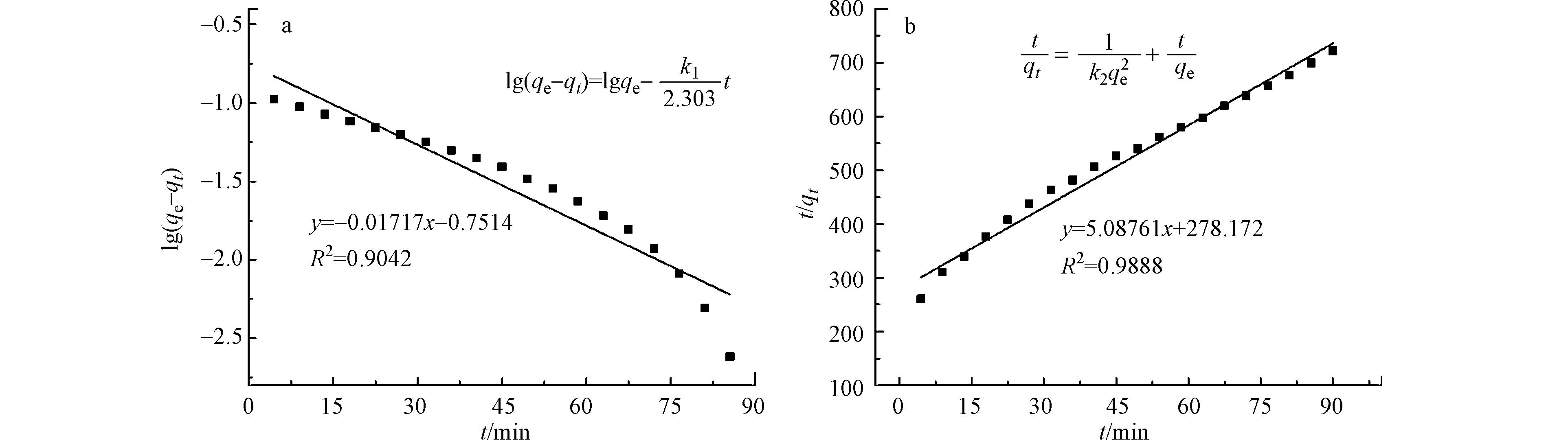
吸附动力学分析不仅可以评价反应速率还能通过模型拟合结果研究反应机制,是研究吸附机理的重要手段之一. Pseudo-first-order和Pseudo-second-order动力学模型是经典和常用的吸附模型,前者认为吸附过程主要受物理吸附控制,而后者则是化学吸附为主,其吸附模型方程[17]如图5所示. 在氟离子初始浓度为5 mg·L−1、pH 3、室温条件下考察了茶叶吸附剂吸附效率随时间的变化,对所得的实验数据进行了动力学吸附模型拟合,结果如图5所示,线性拟合后的动力学参数也列于表2中. 从图5拟合度(R2)来看, Pseudo-second-order(R2=0.9888)吸附模型较Pseudo-first-order(R2=0.9042)更高. 而从拟合后的吸附量来看,两种动力学模型拟合值与实验值都较为一致,但Pseudo-first-order动力学拟合后的吸附量(qe计算)与实验所得的吸附量(qe实验值)更接近.综上所述,废弃茶叶吸附氟离子过程更符合Pseudo-second-order动力学模型,表明吸附过程主要受化学吸附控制,其吸附机理涉及液膜扩散、表面吸附和颗粒内部扩散等综合作用[18].
-
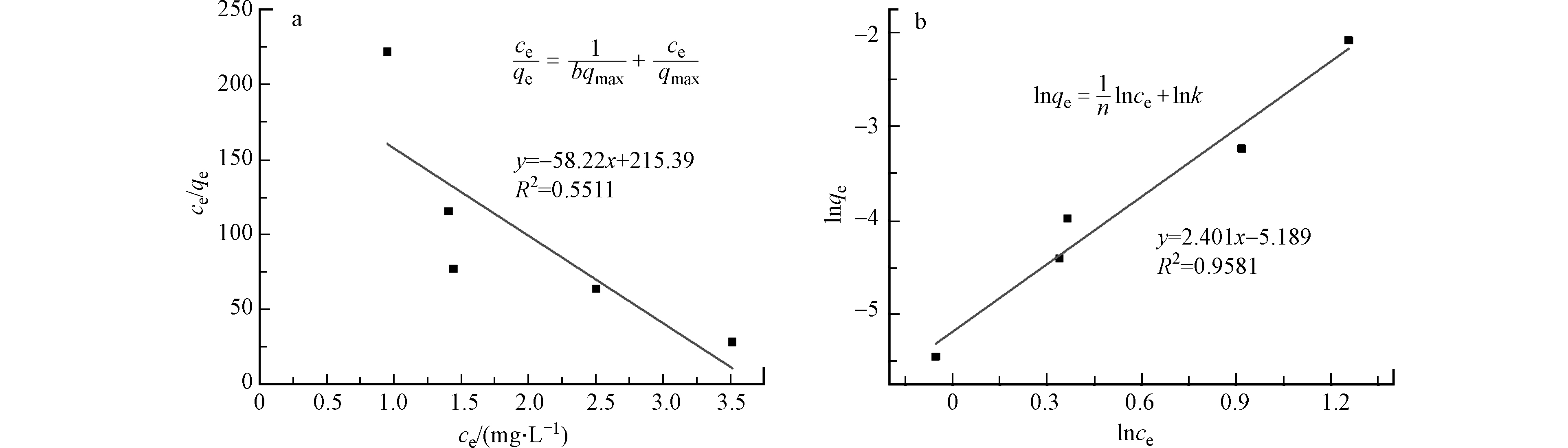
等温吸附模型拟合可以评估吸附过程是否为单分子层吸附. 为了研究废弃茶叶对氟离子的等温吸附模型,在pH 3、室温反应条件下考察了吸附量随不同起始浓度的变化. Langmuir模型与Freundlich是常用的等温吸附模型,前者主要是单层吸附而后者为多层吸附,其拟合吸附方程[19]如图6所示. 从图6中的相关性可知,Freundlich等温吸附模型的相关性(R2=0.9581)很高,而Langmuir模型的数据离散不呈线性 (R2=0.5511). 此外等温吸附模型拟合后的相关参数列于表3. 从表3可见Langmuir等温吸附模型计算后的最大吸附量(qmax)与实验所得吸附量(qe)差别较大,综合相关参数表明,吸附过程更符合Freundlich等温吸附模型,表明吸附过程是不均匀表面吸附,其吸附热与吸附量呈对数形式降低.
-
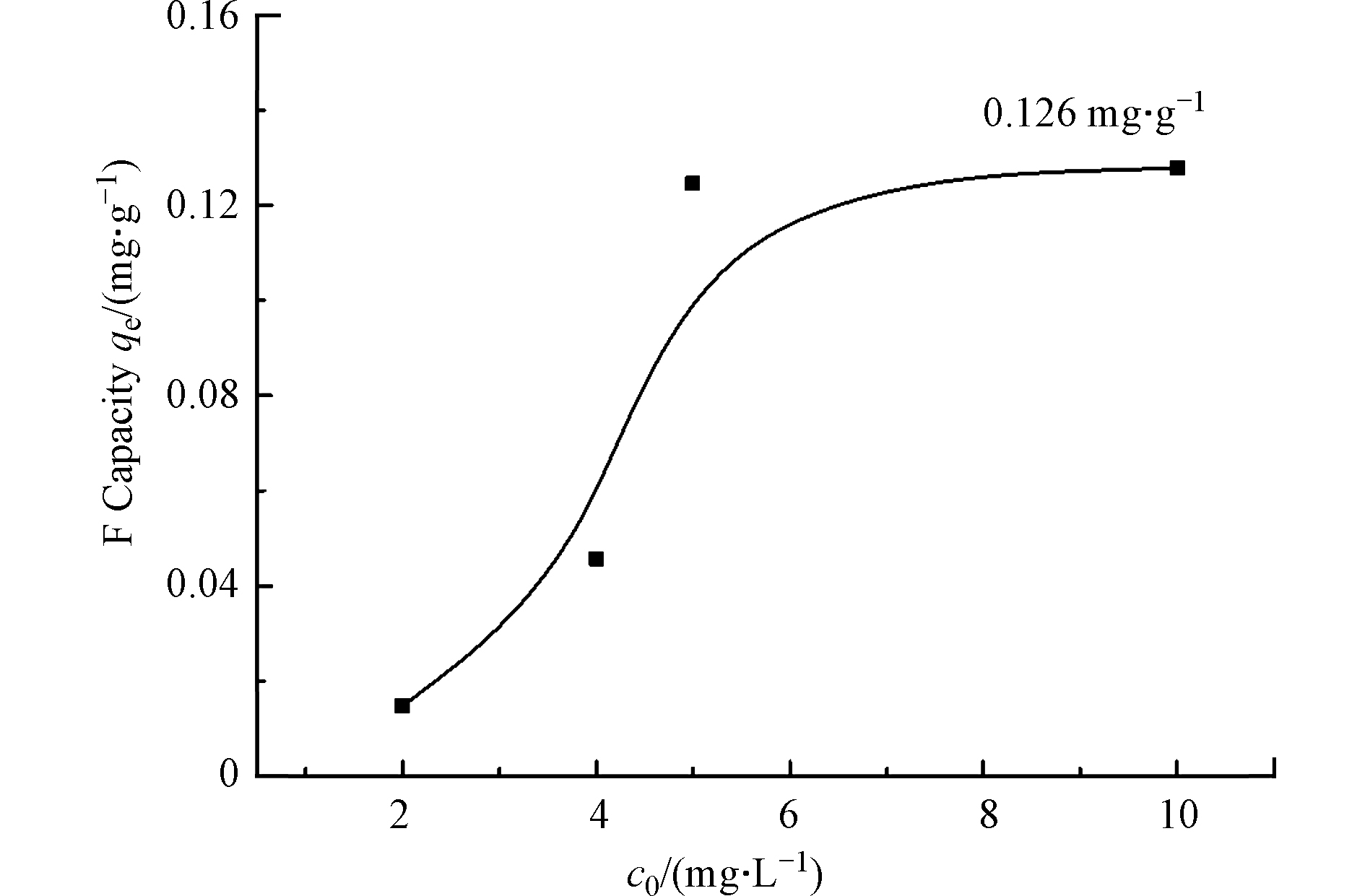
吸附容量指单位吸附剂吸附目标物的饱和吸附量(mg·g−1),是衡量吸附剂吸附能力的重要参数之一. 实验中采用动态吸附柱吸附探究废弃茶叶对氟离子的吸附容量,结果如图7. 从图7可见,随着浓度的升高废弃茶叶对氟离子的吸附量不断升高,当氟离子浓度达到一定程度后,茶叶吸附剂的位点不足,持续吸附能力下降因而趋近平缓,此时茶叶的吸附量达到最大,为0.126 mg·g−1. 对比文献中报道的生物吸附剂除氟吸附量,如芦苇残体(0.18 mg·g−1)[20]、富里酸-膨润土复合体(0.311 mg·g−1)[21]、荷叶基生物炭(0.85 mg·g−1)[22]、改性茶叶吸附剂(9.96 mg·g−1)[15]、改性桑叶(14.99 mg·g−1)[23],废弃茶叶的吸附容量较小,但是废弃茶叶具有来源丰富、易于获得、成本低廉、无需复杂前处理和改性过程的优点,针对吸附量和去除率低的问题,可以在实际操作中加大投放量来达到较好的吸附效果.
-
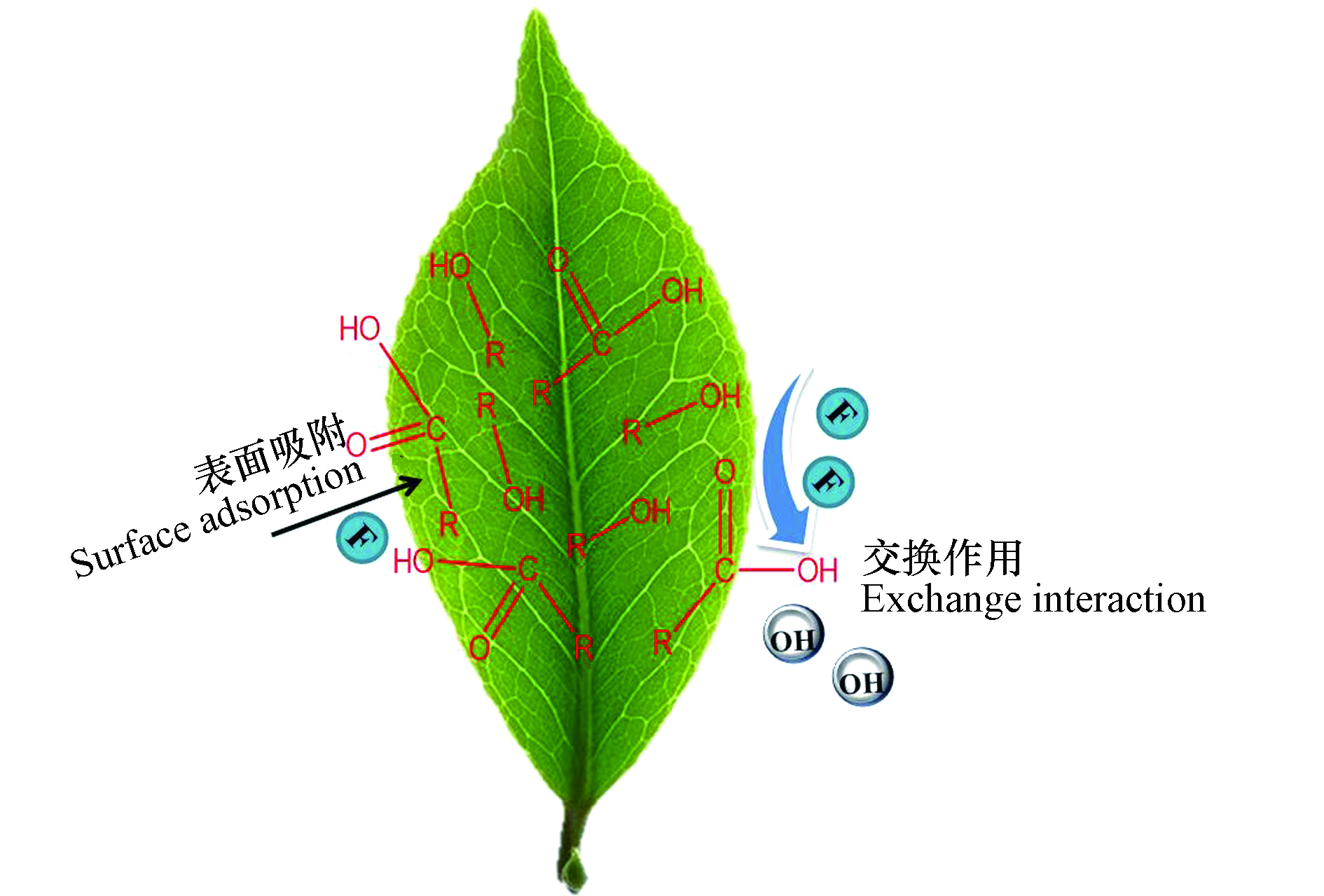
为了探究茶叶吸附剂吸附氟离子的吸附机制,对茶叶粉末吸附剂进行了红外光谱表征,其结果如图8所示. 大红袍茶叶吸附剂在400—4000 cm−1范围内存在多个吸收峰,与文献报道的其它茶叶的红外图谱形状一致[24]. 其中3378 cm−1处是—OH的伸缩振动峰;2924 cm−1处是C—H不对称伸缩振动峰;1647 cm−1处是—COO−不对称伸缩振动;1371 cm−1处是—COOH不对称伸缩振动;1238 cm−1是羧酸中的C—O伸缩振动;1075 cm−1处是C—O—C伸缩振动[25]. 由于茶叶吸附剂中含有大量茶多酚、纤维素和木质素成分,因而分子链上分布有大量的羟基和羧基活性基团.吸附氟离子后,峰形未有明显变化,但在3366、1638、1039 cm−1处有明显位移,表明—OH、—COOH和C—O基团参与了氟离子的吸附过程. 可以推测茶叶吸附剂中的羟基和羧基活性基团与氟离子发生电子共用和交换,结合动力学的研究和红外表征结果,可以推测茶叶吸附剂吸附氟离子的机理主要通过表面吸附和交换作用共同实现,其机理图如图9所示.
-
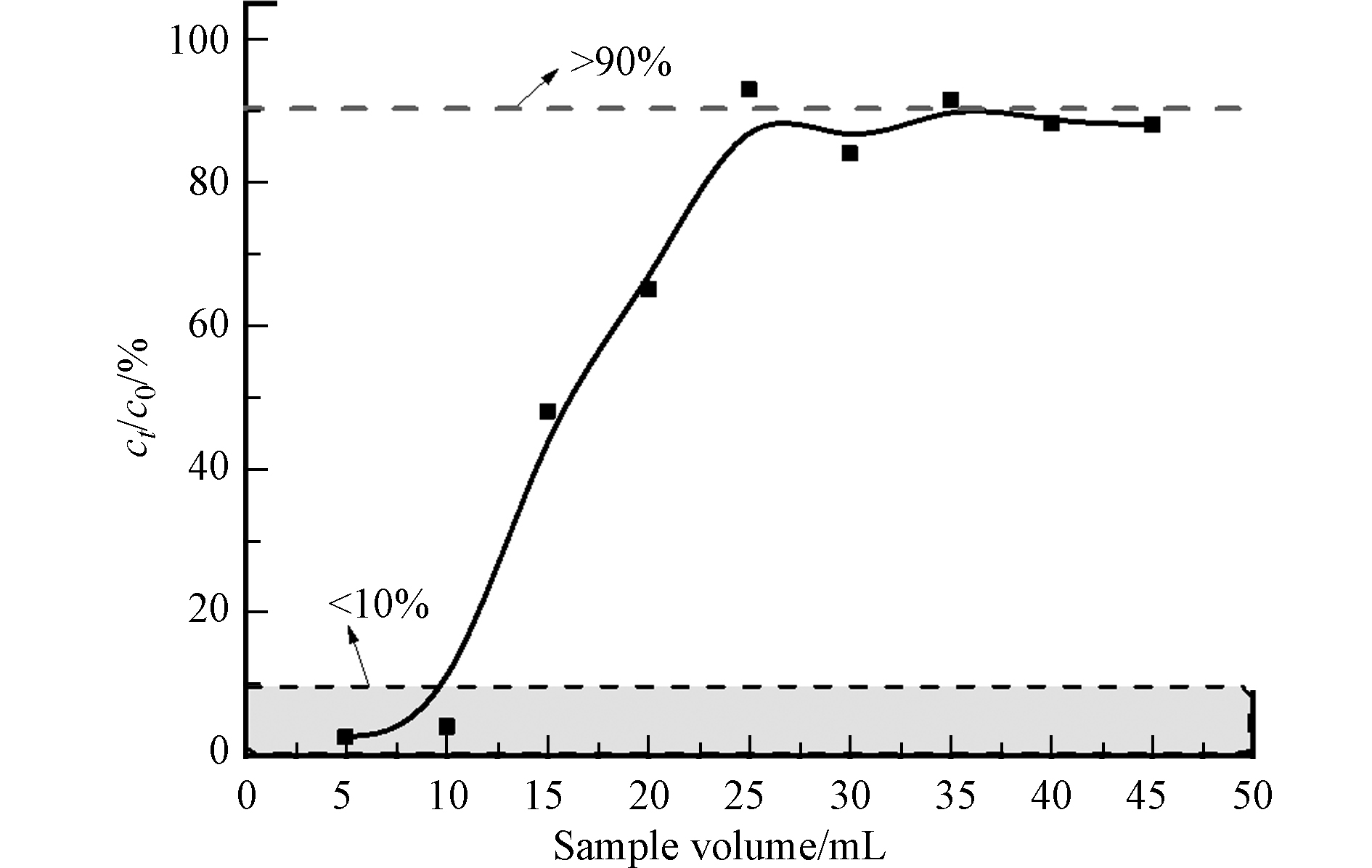
实际地热水选用西藏日多温泉水,温泉水中氟离子浓度高达7.6 mg·L−1. 为了获得更好的吸附效果,吸附剂填料1 g,在最优的条件下上样,并绘制了穿透曲线,结果如图10所示.
从图10可见,1 g的茶叶吸附剂能处理净化10 mL的高浓度含氟温泉水,使其流出液浓度小于1 mg·L−1,达到我国饮用水标准,表现出优良的性能. 当上样达到25 mL后, Ct/C0才达到90%. 由于实际地热水基质较为复杂,使得饱和吸附量较模拟地热水的吸附量降低至0.09 mg·g−1. 废弃茶叶吸附剂能净化处理大体积、高浓度的含氟温泉水,表现出优良的性能.
-
(1)本文对比了大红袍等10种常规茶叶吸附剂对氟离子的吸附效果,大红袍茶叶吸附性能最优,比表面积为1.788 m2·g−1,吸附机理主要是氟离子通过与茶叶中的羧基和羟基活性基团进行交换反应.
(2)pH是影响废弃茶叶吸附效果最重要的因素,pH 3时吸附效果最好,氟离子的吸附容量达到0.126 mg·g−1.
(3)1 g茶叶吸附剂能净化10 mL浓度高达7.6 mg·L−1的含氟废水,且茶叶吸附剂具有来源丰富、易于获得、成本低廉、无需复杂前处理等优点,在处理含氟废水方面具有实际应用价值.
废弃茶叶吸附剂去除水中的氟离子
Waste tea-leaves as adsorbent for the removal of fluoride from water solution
-
摘要: 地热温泉常含有高浓度的氟,给温泉利用带来不可忽视的环境问题,因此温泉水中氟离子吸附净化处理是重要的研究热点. 本文采用废弃茶叶作为吸附剂,氟离子为目标离子,优化了茶叶吸附剂种类、溶液pH对吸附效果的影响. 在pH 3、初始浓度为5 mg·L−1时,大红袍茶叶对氟离子的吸附容量达到0.126 mg·g−1. 进一步考察了吸附剂对氟离子的吸附机制,结果表明,氟离子吸附符合Pseudo-second-order动力学模型,等温线符合Freundlich模型,氟离子吸附过程主要通过与茶叶中的羟基和羧基活性基团发生交换作用. 茶叶氟吸附量虽较其它天然吸附剂略低但无需复杂改性和前处理,具有环境友好,成本低廉,来源丰富,易与获得等优点,在含氟水净化处理具有潜在应用和发展前景.Abstract: High temperature hot spring water always contains high concentration of fluoride, which brings environmental problems that cannot be ignored for hot spring utilization. Therefore, study on defluorinate by the adsorption in hot spring water is an important research topic. In this work, waste tea-leaves were used as adsorbents for the removal of fluoride from water solution. The effects of tea-leaves adsorbent types and pH on the adsorption efficiency were optimized. As the result, at pH of 3 and initial concentration of 5 mg·L−1, the sorption capacity of Road tea-leaves for fluoride reached 0.126 mg·g−1. The adsorption mechanism of the adsorbent for fluoride was further investigated. As the result, the kinetic conforms to the Pseudo-second-order sorption model, and the sorption isotherm fitted to Freundlich model, respectively. The adsorption process for fluoride was mainly through the exchange with hydroxyl and carboxyl groups in tea-leaves. Although the sorption capacity of tea-leaves adsorbent is slightly lower than that of other natural waste adsorbents, it merits without complex pretreatment, environment friendly, easy to obtain, indicates that tea-leaves adsorbent is easily applicable for the remediation of fluoride-containing water solution.
-
Key words:
- waste tea-leaves adsorbent /
- water solution /
- fluoride /
- adsorption
-
氟(F)是人体必需的微量元素,但氟离子是温泉水中典型的高浓度有害元素. 适宜浓度的含氟饮用水(0.5—1.5 mg·L−1)对人体有益,能维持人体的钙、磷正常代谢,防止患龋齿病. 但长期饮用过量含氟水会对人体免疫系统和组织产生威胁从而引发病变,如氟斑牙和氟骨症,甚至引发脑损伤、癌症等病症[1- 2]. 一般在地热温泉水流经的河流水样中,大都能检测到高含量的F离子[3-4],若长期以含氟河水为饮用水源,势必会对流域居民产生一定的威胁,因此地热温泉水中氟离子的净化处理,不仅对流域生态保护,而且对居民健康具有重要的意义.
当前含氟水的处理方法主要包括:电化学法、混凝沉淀法、膜过滤法、离子交换法和吸附法等[5- 6]. 其中吸附法具有吸附剂种类多、成本低、污染小、易操作等特点,是当前用于处理大体积、高浓度含氟废水的经典常用方法[7-8]. 吸附法的核心在于吸附剂的选择,它是决定吸附性能的关键. 当前已被报道的除氟吸附剂大致可分为几类:活性氧化铝[9]、金属氧化物[10-11]、生物质衍生的碳基吸附剂[12-13]、羟基磷灰石[14]等. 以生物质为原料的吸附剂应用广泛,吸附性能较好、成本较低、适用条件温和,常用于吸附剂. 茶叶属于天然植物吸附剂,具有网状结构、多孔、比表面积大、含有多种活性基团,是一类理想的除氟吸附剂[15].
本文选择了大红袍、武夷茶、日照绿茶、四川绿茶、泾阳砖茶、安华砖茶(拉萨本地使用较多)、桂花乌龙茶、茉莉花茶、铁观音、金丝皇菊等10种茶叶进行除氟对比研究,选择氟离子去除效果较好的茶叶吸附剂进行实验条件优化,采用动态吸附方式对氟离子吸附效果及吸附动力学和吸附等温线进行了探究,最后对实际温泉水中氟离子进行了净化处理,期望为高氟地热水净化提供技术支撑.
1. 实验方法 (Experimental section)
1.1 仪器与试剂
pHSJ-6L型pH计(上海仪电科学仪器股份有限公司)、BSA224S型电子天平(赛多利斯科学仪器)、ZNCL-G型智能磁力搅拌机(上海予申仪器有限公司)、Thermo Eutech 优特F090 ION 700型氟离子浓度测量仪(优特仪器有限公司)配置有CHN090 加液式复合氟离子电极和自动温度补偿ATC电极. 氟离子电极填充液、100 mg·L−1氟离子标准溶液和氟离子强度调节剂(TISAB Ⅱ总离子强度缓冲液)均购置于优特仪器有限公司并用于氟离子浓度测定. 日立S-4800扫描电子显微镜、赛默飞Nicolet iG50 傅立叶变换红外光谱仪和美国麦克仪器公司ASAP 2020比表面积分析仪用于茶叶吸附剂形貌和结构表征. 浓硝酸(优级纯)和氢氧化钠固体(分析纯)购置于国药试剂厂.
1.2 茶叶吸附剂的预处理
大红袍、武夷茶、日照绿茶、四川绿茶、泾阳砖茶、安华砖茶、桂花乌龙茶、茉莉花茶、铁观音、金丝皇菊均购置于拉萨本地超市. 茶叶经沸水反复浸泡8—10次,去除茶叶中的色素、茶多酚等可溶成分,期间多次换水,直至上清液为无色后,测定上清液中氟电位与空白信号一致后,将茶叶过滤、烘干、粉碎备用.
1.3 实验方法
动态微柱模式是考察吸附剂性能的一种经典方法,本文利用10 mL枪头作为小型微柱,自制了动态微柱,如图1插图所示. 溶液通过自身重力流过微柱与吸附剂实现动态吸附,无需泵源,操作模式简单. 具体流程如下:准确称量废弃茶叶粉末0.5 g,装入10 mL枪头,枪头底部填入少量脱脂棉起过滤作用. 首先,用纯水持续冲洗去除色素后,直至流速稳定,此时上样流速约为0.67 mL·min−1. 用初始浓度为C0 (mg·L−1)的含氟离子溶液持续上样,一定时间间隔后收集3 mL流出液测定其氟离子平均浓度记作Ct (mg·L−1). 直至流出液浓度Ct达到初始浓度C0的90%,认为达到穿透终点,吸附达平衡,停止上样,其吸附过程如图1所示.
采用离子选择性电极法测定氟离子浓度. 氟电极电位E(mV)与溶液中氟离子浓度
(CF−) (−lgCF−) NO−3 SO2−4 (−lgCF−) 2. 结果与讨论(Results and discussion)
2.1 不同废弃茶叶的吸附率对比
在初始氟离子浓度为5 mg·L−1、固液比为20 g·L−1、pH 3、室温条件下考察了10种茶叶对氟离子的吸附效率,结果如图2所示.
不同茶叶品种对氟离子吸附性能差异较大,吸附效果最好的3种茶叶分别是大红袍、桂花乌龙茶和茉莉花茶,分别为47.0%、45.7%和46.2%,吸附效率不存在显著差异(P>0.05,其它几种茶叶的吸附率在10%—35%之间. 已有研究表明,茶叶聚氟性能和规律存在差异,其因素主要包括茶树器官、生长周期、茶树品种、土壤生长环境、茶叶中铝离子含量及表面微孔结构等[16].
为进一步探究不同茶叶吸附氟离子的性能差异原因,考察了大红袍、桂花乌龙茶、茉莉花茶和四川绿茶吸附剂的表面微观形貌和比表面积(BET)特征. 扫描电镜图(SEM)如图3所示,茶叶表面粗糙,结构较为致密,不具有明显的孔结构但伴有粗糙褶皱,粗糙表面特征为丰富比表面积提供有力条件. 比表面积越大,对吸附越有利.
4种茶叶的BET表征结果列于表1. 从表1可见,4种茶叶的孔体积都较低,基本不具有孔结构特征. 由于茶叶结构较为致密,无孔结构,整体比表面积都较小,其中大红袍的比表面积最大,达1.788 m2·g−1,桂花乌龙茶和茉莉花茶的比表面积相当,四川绿茶的最低. 因而大红袍茶叶的比表面积在吸附过程中具有一定的优势. 因而在选择茶叶吸附剂时宜优选择大红袍茶叶作为氟离子吸附剂,在后续实验中,选择大红袍作为吸附剂进一步优化吸附条件.
表 1 废弃茶叶吸附剂的比表面积和孔径分布Table 1. BET specific surface area and pore size of the waste tea-leaves adsorbent吸附剂种类 Adsorbents 比表面积/(m2·g−1) BET surface area 孔体积/(cm3·g−1) Pore volume 平均孔径/nm Pore size 大红袍 1.788 0.011 24.43 桂花乌龙茶 0.811 0.0048 23.51 茉莉花茶 0.624 0.0049 31.46 四川绿茶 0.423 0.0045 42.08 2.2 pH对吸附效率的影响
实际废水处理操作中,pH是影响吸附剂能否发挥优良性能的重要因素,当溶液的pH过低时,溶液中游离的氟离子很容易与氢离子结合生成氟化氢,氟化氢挥发;而pH过高时,溶液中游离的氟离子和溶液中游离的氢氧根形成竞争. 为了解pH对废弃茶叶吸附剂的影响程度,因此探究了废弃茶叶的最佳吸附pH,固定起始氟离子浓度为5.0 mg·L−1,吸附温度为25 ℃,按实验方法的步骤,结果如图4所示. pH越高,越早出现拐点,达到终点的时间越短,吸附氟离子的总量也就越少,所以pH越高,越不利于茶叶吸附剂吸附,因此,选择pH 3为茶叶吸附氟离子的最优pH.
在pH 3时,穿透曲线前期没有平缓的过渡阶段而是突然地开始上升, 30 min之后流出液浓度缓慢升高,最终在85 min处达到穿透终点. 因为茶叶吸附剂的吸附量略低,吸附位点不足,流出液的浓度明显增加,在实际氟离子吸附操作中可以加大吸附剂的投入量、减小上样流速和初始浓度获得更高吸附效率.
2.3 吸附动力学
吸附动力学分析不仅可以评价反应速率还能通过模型拟合结果研究反应机制,是研究吸附机理的重要手段之一. Pseudo-first-order和Pseudo-second-order动力学模型是经典和常用的吸附模型,前者认为吸附过程主要受物理吸附控制,而后者则是化学吸附为主,其吸附模型方程[17]如图5所示. 在氟离子初始浓度为5 mg·L−1、pH 3、室温条件下考察了茶叶吸附剂吸附效率随时间的变化,对所得的实验数据进行了动力学吸附模型拟合,结果如图5所示,线性拟合后的动力学参数也列于表2中. 从图5拟合度(R2)来看, Pseudo-second-order(R2=0.9888)吸附模型较Pseudo-first-order(R2=0.9042)更高. 而从拟合后的吸附量来看,两种动力学模型拟合值与实验值都较为一致,但Pseudo-first-order动力学拟合后的吸附量(qe计算)与实验所得的吸附量(qe实验值)更接近.综上所述,废弃茶叶吸附氟离子过程更符合Pseudo-second-order动力学模型,表明吸附过程主要受化学吸附控制,其吸附机理涉及液膜扩散、表面吸附和颗粒内部扩散等综合作用[18].
表 2 Pseudo-first-order和Pseudo-second-order动力学拟合参数Table 2. Simulation of the Pseudo-first-order and Pseudo-second-order sorption kinetic and corresponding parametersPseudo-first-order Pseudo-second-order qe实验值/(mg·g−1) qe计算/ (mg·g−1) k1/min−1 R2 qe计算/(mg·g−1) k2/(g·mg−1·min−1) R2 0.125 0.177 0.0395 0.9042 0.197 0.093 0.9888 2.4 吸附等温线
等温吸附模型拟合可以评估吸附过程是否为单分子层吸附. 为了研究废弃茶叶对氟离子的等温吸附模型,在pH 3、室温反应条件下考察了吸附量随不同起始浓度的变化. Langmuir模型与Freundlich是常用的等温吸附模型,前者主要是单层吸附而后者为多层吸附,其拟合吸附方程[19]如图6所示. 从图6中的相关性可知,Freundlich等温吸附模型的相关性(R2=0.9581)很高,而Langmuir模型的数据离散不呈线性 (R2=0.5511). 此外等温吸附模型拟合后的相关参数列于表3. 从表3可见Langmuir等温吸附模型计算后的最大吸附量(qmax)与实验所得吸附量(qe)差别较大,综合相关参数表明,吸附过程更符合Freundlich等温吸附模型,表明吸附过程是不均匀表面吸附,其吸附热与吸附量呈对数形式降低.
表 3 Langmuir和Freundlich吸附等温线模型拟合相关参数Table 3. Simulation of the Langmuir and Freundlich sorption isotherm and corresponding parametersLangmuir Freundlich qe/(mg·g−1) qmax/(mg·g−1) b/(L·mg−1) R2 k n R2 0.126 0.017 0.270 0.5511 0.0056 0.416 0.9581 2.5 吸附容量
吸附容量指单位吸附剂吸附目标物的饱和吸附量(mg·g−1),是衡量吸附剂吸附能力的重要参数之一. 实验中采用动态吸附柱吸附探究废弃茶叶对氟离子的吸附容量,结果如图7. 从图7可见,随着浓度的升高废弃茶叶对氟离子的吸附量不断升高,当氟离子浓度达到一定程度后,茶叶吸附剂的位点不足,持续吸附能力下降因而趋近平缓,此时茶叶的吸附量达到最大,为0.126 mg·g−1. 对比文献中报道的生物吸附剂除氟吸附量,如芦苇残体(0.18 mg·g−1)[20]、富里酸-膨润土复合体(0.311 mg·g−1)[21]、荷叶基生物炭(0.85 mg·g−1)[22]、改性茶叶吸附剂(9.96 mg·g−1)[15]、改性桑叶(14.99 mg·g−1)[23],废弃茶叶的吸附容量较小,但是废弃茶叶具有来源丰富、易于获得、成本低廉、无需复杂前处理和改性过程的优点,针对吸附量和去除率低的问题,可以在实际操作中加大投放量来达到较好的吸附效果.
2.6 吸附机理
为了探究茶叶吸附剂吸附氟离子的吸附机制,对茶叶粉末吸附剂进行了红外光谱表征,其结果如图8所示. 大红袍茶叶吸附剂在400—4000 cm−1范围内存在多个吸收峰,与文献报道的其它茶叶的红外图谱形状一致[24]. 其中3378 cm−1处是—OH的伸缩振动峰;2924 cm−1处是C—H不对称伸缩振动峰;1647 cm−1处是—COO−不对称伸缩振动;1371 cm−1处是—COOH不对称伸缩振动;1238 cm−1是羧酸中的C—O伸缩振动;1075 cm−1处是C—O—C伸缩振动[25]. 由于茶叶吸附剂中含有大量茶多酚、纤维素和木质素成分,因而分子链上分布有大量的羟基和羧基活性基团.吸附氟离子后,峰形未有明显变化,但在3366、1638、1039 cm−1处有明显位移,表明—OH、—COOH和C—O基团参与了氟离子的吸附过程. 可以推测茶叶吸附剂中的羟基和羧基活性基团与氟离子发生电子共用和交换,结合动力学的研究和红外表征结果,可以推测茶叶吸附剂吸附氟离子的机理主要通过表面吸附和交换作用共同实现,其机理图如图9所示.
2.7 实际地热水
实际地热水选用西藏日多温泉水,温泉水中氟离子浓度高达7.6 mg·L−1. 为了获得更好的吸附效果,吸附剂填料1 g,在最优的条件下上样,并绘制了穿透曲线,结果如图10所示.
从图10可见,1 g的茶叶吸附剂能处理净化10 mL的高浓度含氟温泉水,使其流出液浓度小于1 mg·L−1,达到我国饮用水标准,表现出优良的性能. 当上样达到25 mL后, Ct/C0才达到90%. 由于实际地热水基质较为复杂,使得饱和吸附量较模拟地热水的吸附量降低至0.09 mg·g−1. 废弃茶叶吸附剂能净化处理大体积、高浓度的含氟温泉水,表现出优良的性能.
3. 结论(Conclusion)
(1)本文对比了大红袍等10种常规茶叶吸附剂对氟离子的吸附效果,大红袍茶叶吸附性能最优,比表面积为1.788 m2·g−1,吸附机理主要是氟离子通过与茶叶中的羧基和羟基活性基团进行交换反应.
(2)pH是影响废弃茶叶吸附效果最重要的因素,pH 3时吸附效果最好,氟离子的吸附容量达到0.126 mg·g−1.
(3)1 g茶叶吸附剂能净化10 mL浓度高达7.6 mg·L−1的含氟废水,且茶叶吸附剂具有来源丰富、易于获得、成本低廉、无需复杂前处理等优点,在处理含氟废水方面具有实际应用价值.
-
表 1 废弃茶叶吸附剂的比表面积和孔径分布
Table 1. BET specific surface area and pore size of the waste tea-leaves adsorbent
吸附剂种类 Adsorbents 比表面积/(m2·g−1) BET surface area 孔体积/(cm3·g−1) Pore volume 平均孔径/nm Pore size 大红袍 1.788 0.011 24.43 桂花乌龙茶 0.811 0.0048 23.51 茉莉花茶 0.624 0.0049 31.46 四川绿茶 0.423 0.0045 42.08 表 2 Pseudo-first-order和Pseudo-second-order动力学拟合参数
Table 2. Simulation of the Pseudo-first-order and Pseudo-second-order sorption kinetic and corresponding parameters
Pseudo-first-order Pseudo-second-order qe实验值/(mg·g−1) qe计算/ (mg·g−1) k1/min−1 R2 qe计算/(mg·g−1) k2/(g·mg−1·min−1) R2 0.125 0.177 0.0395 0.9042 0.197 0.093 0.9888 表 3 Langmuir和Freundlich吸附等温线模型拟合相关参数
Table 3. Simulation of the Langmuir and Freundlich sorption isotherm and corresponding parameters
Langmuir Freundlich qe/(mg·g−1) qmax/(mg·g−1) b/(L·mg−1) R2 k n R2 0.126 0.017 0.270 0.5511 0.0056 0.416 0.9581 -
[1] ZHEN J, JIA Y, LUO T, et al. Efficient removal of fluoride by hierarchical MgO microspheres: performance and mechanism study [J]. Applied Surface Science, 2015, 357: 1080-1088. doi: 10.1016/j.apsusc.2015.09.127 [2] OLADOJA N A, CHEN S, DREWES J E, et al. Characterization of granular matrix supported nano magnesium oxide as an adsorbent for defluoridation of groundwater [J]. Chemical Engineering Journal, 2015, 281: 632-643. [3] GUO Q, WANG Y, LIU W. Major hydrogeochemical processes in the two reservoirs of the Yangbajing geothermal field, Tibet, China [J]. Journal of Volcanology and Geothermal Research, 2007, 166(3-4): 255-268. doi: 10.1016/j.jvolgeores.2007.08.004 [4] 孙红丽, 马峰, 刘昭, 等. 西藏高温地热显示区氟分布及富集特征 [J]. 中国环境科学, 2015, 35(1): 251-259. SUN H L, MA F, LIU Z, et al. The distribution and enrichment characteristics of fluoride in geothermal active area in Tibet [J]. China Environmental Science, 2015, 35(1): 251-259(in Chinese).
[5] JAGTAP S, YENKIE M K, LABHSETWAR N, et al. Fluoride in drinking water and defluoridation of water [J]. Chemical Reviews, 2012, 112(4): 2454-2466. doi: 10.1021/cr2002855 [6] TANG D, ZHANG G. Efficient removal of fluoride by hierarchical Ce–Fe bimetal oxides adsorbent: thermodynamics, kinetics and mechanism [J]. Chemical Engineering Journal, 2016, 283: 721-729. doi: 10.1016/j.cej.2015.08.019 [7] 郑国河, 李剑超, 卢堂俊, 等. 镧掺杂纳米材料合成及其高氟选择性吸附特性 [J]. 环境化学, 2009, 28(6): 823-828. doi: 10.3321/j.issn:0254-6108.2009.06.009 ZHENG G H, LI J C, LU T J, et al. Synthesis of La doped nano materials used in high-selective defluorination [J]. Environmental Chemistry, 2009, 28(6): 823-828(in Chinese). doi: 10.3321/j.issn:0254-6108.2009.06.009
[8] VELAZQUEZ J L, VENCES A E, FLORES A J, et al. Water defluoridation with special emphasis on adsorbents-containing metal oxides and/or hydroxides: A review [J]. Separation and Purification Technology, 2015, 150: 292-307. doi: 10.1016/j.seppur.2015.07.006 [9] 段颖, 杨琰琰, 张小凤, 等. 两种盐复合改性活性氧化铝对水中氟的吸附特性 [J]. 环境化学, 2014, 33(11): 1950-1956. doi: 10.7524/j.issn.0254-6108.2014.11.005 DUAN Y, YANG Y Y, ZHANG X F, et al. Adsorption characteristics of floride on activated alumina modified with ferric and aluminum salt [J]. Environmental Chemistry, 2014, 33(11): 1950-1956(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.11.005
[10] 方文侃, 李小娣, 方菁, 等. 新型材料磁性氧化锆的除氟效能 [J]. 环境科学, 2019, 40(5): 2295-2301. FANG W K, LI X D, FANG J, et al. Fluoride removal efficiency of novel material: Magnetite core/zirconia shell nanocomposite [J]. Environmental Science, 2019, 40(5): 2295-2301(in Chinese).
[11] DOU X M, ZHANG Y S, WANG H J, et al. Performance of granular zirconium-iron oxide in the removal of fluoride from drinking water [J]. Water Research, 2011, 45(12): 3571-3578. doi: 10.1016/j.watres.2011.04.002 [12] SHARMA M, MONDAL D, SINGH N, et al. Seaweed-derived nontoxic functionalized graphene sheets as sustainable materials for the efficient removal of fluoride from high fluoride containing drinking water [J]. ACS Sustainable Chemistry and Engineering, 2017, 5(4): 3488-3498. doi: 10.1021/acssuschemeng.7b00198 [13] PRABHU SM, KOILRAJ P, SASAKI K. Synthesis of sucrose-derived porous carbon-doped ZrxLa1-x OOH materials and their superior performance for the simultaneous immobilization of arsenite and fluoride from binary systems [J]. Chemical Engineering Journal, 2017, 325: 1-13. doi: 10.1016/j.cej.2017.05.052 [14] 刘成, 胡伟, 李俊林, 等. 用于地下水除氟的羟基磷灰石制备及其除氟效能 [J]. 中国环境科学, 2014, 34(1): 58-64. LIU C, HU W, LI J L, et al. Preparation of the hydroxyapatite to remove fluorine from groundwater and its removal performance [J]. China Environmental Science, 2014, 34(1): 58-64(in Chinese).
[15] 田奇峰, 孙杰. 茶叶对氟的吸附研究 [J]. 化学与生物工程, 2011, 28(4): 29-31. doi: 10.3969/j.issn.1672-5425.2011.04.008 TIAN Q F, SUN J. Study on the adsorption of fluorine by tea [J]. Chemistry & Bioengineering, 2011, 28(4): 29-31(in Chinese). doi: 10.3969/j.issn.1672-5425.2011.04.008
[16] 龚受基, 刘仲华, 谭君. 茶树聚氟的规律及其调控研究进展 [J]. 茶叶通讯, 2013, 40(1): 18-21. doi: 10.3969/j.issn.1009-525X.2013.01.005 GONG S J, LIU Z H, TAN J. The law of fluoride condensity in tea tree and its regulation [J]. Tea Communication, 2013, 40(1): 18-21(in Chinese). doi: 10.3969/j.issn.1009-525X.2013.01.005
[17] CHAI L, WANG Y, ZHAO N, et al. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water [J]. Water Research, 2013, 47(12): 4040-4049. doi: 10.1016/j.watres.2013.02.057 [18] 汪爱河, 周康根, 刘行, 等. Mg-Al-Me(Me=La, Ce, Zr)复合氧化物制备及其除氟性能 [J]. 环境科学, 2016, 37(12): 4874-4881. WANG A H, ZHOU K G, LIU X, et al. Preparation of Mg-Al-me (me =La, ce, Zr) composite oxides for efficient fluoride uptake [J]. Environmental Science, 2016, 37(12): 4874-4881(in Chinese).
[19] LIN K Y A, LIU Y T, CHEN S Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies [J]. Journal of Colloid and Interface Science, 2016, 461: 79-87. doi: 10.1016/j.jcis.2015.08.061 [20] 董刚, 赵琦玥, 冯佳, 等. 芦苇秸秆对水体中氟离子的吸附研究 [J]. 东北师大学报(自然科学版), 2018, 50(2): 134-143. DONG G, ZHAO Q Y, FENG J, et al. Preparation of activated carbon from Phragmites australis Trin. to remove the fluoride in aqueous solutions [J]. Journal of Northeast Normal University (Natural Science Edition), 2018, 50(2): 134-143(in Chinese).
[21] 方敦, 田华婧, 叶欣, 等. 富里酸-膨润土复合体对氟的吸附特性 [J]. 环境科学, 2016, 37(3): 1023-1031. FANG D, TIAN H J, YE X, et al. Adsorption properties of fluorine onto fulvic acid-bentonite complex [J]. Environmental Science, 2016, 37(3): 1023-1031(in Chinese).
[22] 邱会华, 林婉琪. 荷叶基生物炭的制备及对水中氟离子的吸附研究 [J]. 广东石油化工学院学报, 2018, 28(3): 34-38. doi: 10.3969/j.issn.2095-2562.2018.03.008 QIU H H, LIN W Q. Preparation of Lotus leaf-based biochar and its adsorption property for fluorine ion [J]. Journal of Guangdong University of Petrochemical Technology, 2018, 28(3): 34-38(in Chinese). doi: 10.3969/j.issn.2095-2562.2018.03.008
[23] 秦文欣, 张庆乐, 李庆山, 等. 改性桑树叶对含氟废水吸附性能研究 [J]. 化工新型材料, 2017, 45(8): 202-204. QIN W X, ZHANG Q L, LI Q S, et al. Adsorption characteristics of fluoride in waste water on modified mulberry leave [J]. New Chemical Materials, 2017, 45(8): 202-204(in Chinese).
[24] 赵晓辉, 聂志矗, 张连水, 等. 茶叶及其组份的红外光谱研究 [J]. 光学学报, 2009, 29(2): 533-536. doi: 10.3788/AOS20092902.0533 ZHAO X H, NIE Z C, ZHANG L S, et al. Study on tea and its principal components by infrared spectroscopy [J]. Acta Optica Sinica, 2009, 29(2): 533-536(in Chinese). doi: 10.3788/AOS20092902.0533
[25] LI Y, YANG J L, JIANG Y. Trace rare earth element detection in food and agricultural products based on flow injection walnut shell packed microcolumn preconcentration coupled with inductively coupled plasma mass spectrometry [J]. Journal of Agricultural and Food Chemistry, 2012, 60(12): 3033-3041. doi: 10.1021/jf2049646 -






 下载:
下载: