-
四溴双酚A(tetrabromobisphenol A,TBBPA)由于其高效的阻燃效率和优越的热稳定性,目前已成为世界上用量最大的阻燃剂。随着TBBPA产用量大幅增加,大量TBBPA的生产转向亚洲地区,中国已成为 TBBPA的主要生产基地[1]。根据国际溴工业理事会(BSEF)的统计,2019年TBBPA年产量为18万吨,其中亚洲消费量最高,达每年10.94万吨。TBBPA主要以简单的物理添加方式加入产品中,在产品的生产和使用中经挥发[2]、淋溶[3]、磨损[4]等方式进入环境,已经造成水环境的严重污染。国内受TBBPA污染较严重的区域为巢湖流域、珠江流域以及环渤海区域,其中巢湖流域检测到的TBBPA浓度最高可达4870 ng·L−1[5-9]。此外,TBBPA可能对人体产生肝肾[10]、神经等毒性[11]和内分泌干扰效应[12]。2017年,世界卫生组织国际癌症研究机构将其列为2A类致癌物[13]。如何有效实现水环境中TBBPA的高效去除已受到国内外学者的广泛关注。
目前TBBPA的去除方法主要包括物理吸附法[14]、生物降解法[15-16]、催化氧化法[17- 18]等。其中,多孔碳材料因其具有较大的比表面积和较高的疏水性,被认为是憎水性有机污染物的优良吸附剂。多孔碳材料由于价格低廉、吸附量高、易于分离等特点而被广泛应用于有机废水的处理。如,Li等[19]利用污水污泥制成多孔生物碳吸附TBBPA,发现吸附主要是均相和化学过程,主要受π-π相互作用和氢键的作用。Shao等[20]采用废弃烟头制备的多孔碳微球对双酚A(BPA)具有较好的吸附性能,最大吸附量为865 mg·g−1。但是通过改性进一步增强多孔碳微球吸附有机污染物的研究还鲜见报道。
本研究以葡萄糖为碳源,葡萄糖为单糖,与其他生物碳相比,葡萄糖完全碳化所需要的压强、温度和时间远小于其他生物炭,经水热法合成后可形成可形成粒径可控,绿色环保的多孔碳球材料;后采用球磨、H2O2氧化和氮掺杂改性多孔碳微球材料,通过表征分析和吸附实验研究改性多孔碳微球对TBBPA的吸附特征与吸附机理,探究环境因子pH与腐殖酸(HA)对吸附性能的影响,以期为提升多孔碳材料性能去除有机污染物提供数据支撑。
-
实验所用化学试剂:TBBPA(C15H12Br4O2,98%),氢氧化钠(NaOH,97%),葡萄糖(C6H12O6·H2O,99%),腐殖酸(HA,99%),碳酸氢钠(NaHCO3,99%),乙酰胺(C2H4NO,99%)均购自上海麦克林生物化学有限公司。
-
以葡萄糖为唯一碳源,采用一步水热合成法制备多孔碳微球[21]。把40 g葡萄糖粉末溶解在50 mL去离子水中配制饱和葡萄糖溶液,将配制好的溶液经搅拌、超声后置于具有聚四氟乙烯内衬的不锈钢水热反应釜中,密闭条件下,220 ℃水热反应14 h。混合液冷却至室温后,使用抽滤将其固液分离。将固相产物分别用去离子水和无水乙醇进行洗涤,直至洗涤液为无色。洗涤后的固相产物在80 ℃条件下真空干燥8 h,然后置于管式炉中在氮气氛围内800 ℃煅烧2 h。冷却后,收集到的黑色固体粉末为多孔碳微球。
-
C-N:在“1.2.1”节中饱和葡萄糖溶液中加入1 g乙酰胺,其余步骤同多孔碳微球的制备;C-H2O2:将5 g上述制备好的多孔碳微球投加到30%的H2O2溶液中,在磁力搅拌器中,以150 r·min−1搅拌1 h,反应温度为65 ℃。搅拌结束后收集粉末并用去离子水洗涤至中性,洗净后的材料在105 ℃下干燥8 h;C-球磨:将制备好的多孔碳微球与研磨质(石英球)以4.5∶1的比例混合,575 r·min−1速度下研磨1.6 h。
-
配制10 mg·L−1 TBBPA储备液,将50 mg吸附材料添加到盛有100 mL TBBPA溶液的锥形瓶中,并使用稀硝酸调节溶液的pH值为7(后续实验中,如无特殊说明,溶液pH均为7)。将装有混合液的锥形瓶置于恒温水浴震荡培养箱中,25 ℃条件下,150 r·min−1振荡24 h,分别在10 min、20 min、30 min、1 h、2 h、3 h、4 h、6 h时间点取出1.5 mL溶液,过0.45 µm微孔滤膜。过滤后的溶液,用高效液相色谱测定TBBPA浓度。流动相为甲醇(80 %)和水(20 %),流速为1 mL·min−1,柱温为30 ℃,紫外光检测波长为219 nm,进样量为10 µL。实验还评估了常见环境影响因素pH变化(3—9)和不同腐殖酸背景下对吸附性能和吸附量的影响。
材料对TBBPA的吸附性能计算公式如下:
其中,q为吸附平衡时材料对TBBPA的固相吸附浓度(mg·g−1),V为溶液体积(L),C0为污染物初始浓度(mg·L−1),Ce为吸附平衡时的液相浓度(mg·L−1),m为吸附剂质量(g)。
-
分别采用准一级动力学模型、准二级动力学模型对改性多孔碳微球吸附TBBPA的动力学过程进行拟合。准一级动力学模型、准二级动力学模型和颗粒内扩散模型分别如公式(2)、(3)、(4)所示。
式中,
$ {q}_{e} $ (mg·g−1)和$ {q}_{t} $ (mg·g−1)分别是平衡时和时间为t时的固相浓度,$ {k}_{1} $ (min−1)、$ {k}_{2} $ (g·mg−1·h−1)和 kd(mg·g−1·h−1/2)分别是准一级速率模型、准二级速率模型和颗粒内扩散模型速率常数;C(mg·g−1)为颗粒内扩散常数。 -
在污染物不同浓度梯度下投加一定量的吸附剂。将锥形瓶放入恒温震荡培养箱中,温度为25 ℃,转速控制在150 r·min−1。待吸附平衡后,用0.45 µm微孔滤膜过滤水样,测定并计算吸附平衡后TBBPA的液相平衡浓度及改性前后多孔碳微球材料对TBBPA平衡吸附容量。两种等温吸附模型公式如下:
Langmuir模型:
Freundlich模型:
式中,Ce(mg·L−1)为液相平衡浓度;qe(mg·g−1)代表固相平衡浓度;qmax(mg·g−1)代表Langmuir最大吸附容量;KL(L·mg−1)为Langmuir常数;KF为Freundlich吸附系数;n为非线性吸附系数。
采用Langmuir方程的平衡参数RL判断多孔碳微球材料对不同浓度TBBPA的吸附性能,若0<RL<1,为有利吸附;若RL>1,为不利吸附;若 RL=1,为线性吸附;RL=0,吸附不可逆[22]。
-
采用扫描电子显微镜SEM(Hitachi S4800,SEM)和比表面积分析仪(TriStar II 3020)表征改性前后碳材料的表观形貌、比表面积、孔径分布的变化;傅里叶变换红外光谱仪(FTIR;BRUKER TENSOR 27,Germany)和Boehm[23]滴定法分析多孔炭微球表面化学基团及含氧官能团的变化特征;X射线光电子能谱仪(Thermo ESCALAB 250XI)(XPS)测定碳材料表面元素和官能团变化;热重分析仪(TGA-50)测量样品的热稳定性。
-
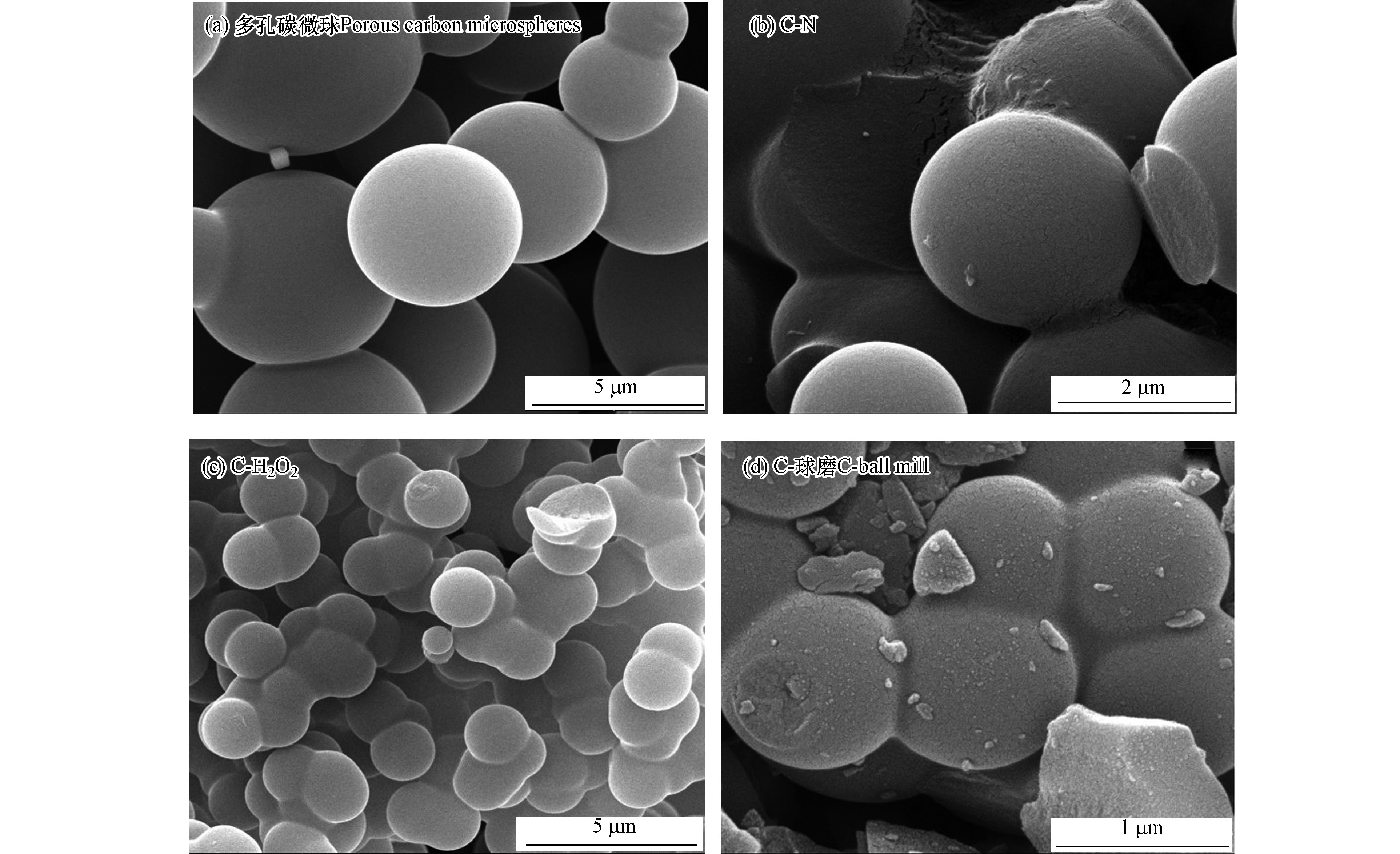
不同表面改性碳微球材料的表面形貌如图1所示,多孔碳微球为表面光滑,粒径3—5 μm,颗粒大小均匀的球形材料。C-N、C-H2O2和C-球磨粒径都有所减小,但未改变其球形结构;C-N球粒径在2—3 μm;C-H2O2粒径约2 μm,改性后表面粗糙度增加;C-球磨粒径约为1 μm,且在材料周围可以看见一些球形颗粒破碎后的残片。
-
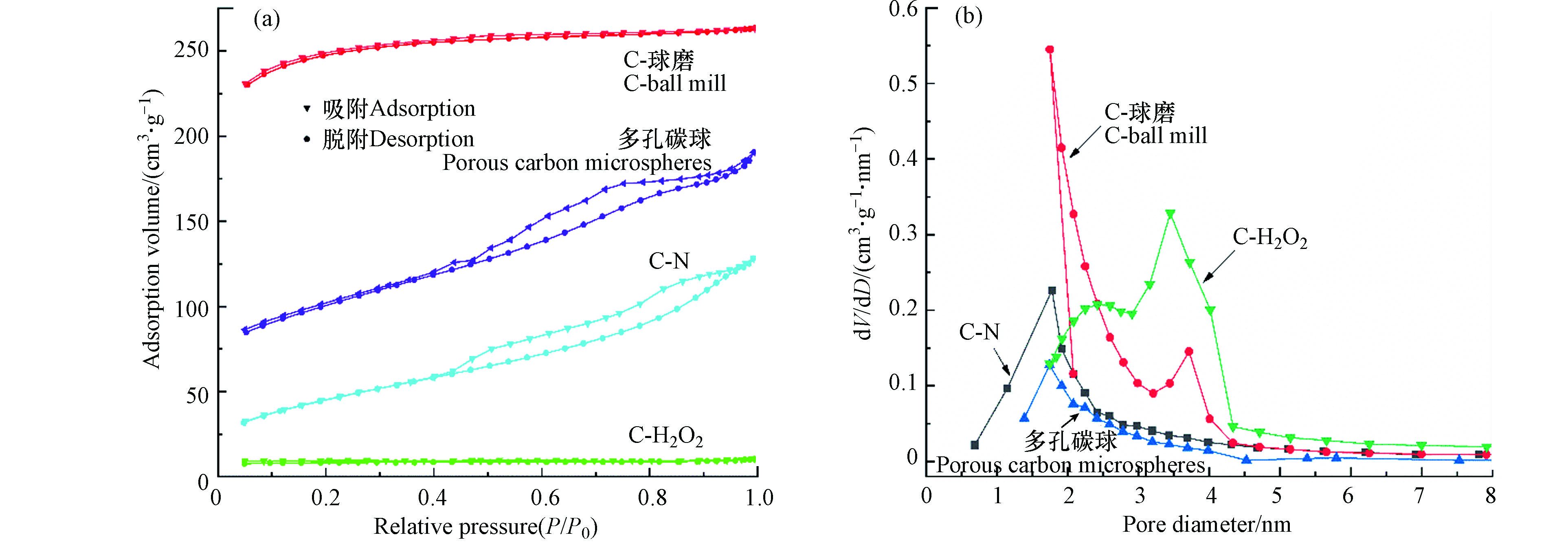
多孔碳微球、C-N、C-H2O2和C-球磨的N2吸-脱附等温线如图2a所示,多孔碳微球在相对压力(P/P0)大于0.4后等温线出现脱附滞后现象,倾向于Ⅳ型等温线的吸附-脱附特征,表明多孔碳微球材料存在介孔结构;滞后环的类型为H4型,表明多孔碳微球小孔嵌入了窄的狭缝状孔。C-N、C-H2O2和C-球磨的N2吸-脱附曲线基本重合,趋向于Ⅰ型,表明多孔碳微球材料具有丰富的微孔结构,C-球磨的吸附容量显著增加。C-球磨和C-H2O2材料在微孔和介孔孔径范围内均有峰出现,表明孔隙结构更发达(图2b )。
C-N、C-球磨总孔容和平均孔径增大,球磨改性后还出现了微孔孔径,其比表面积是多孔碳微球的1.14倍和2.6倍;C-H2O2的平均孔径增加,孔容减小,说明H2O2破坏了多孔碳微球表面的孔隙结构,造成一定量的微孔腐蚀和坍塌[24, 25],比表面积显著减小(为未改性前的24%)(表1)。
-
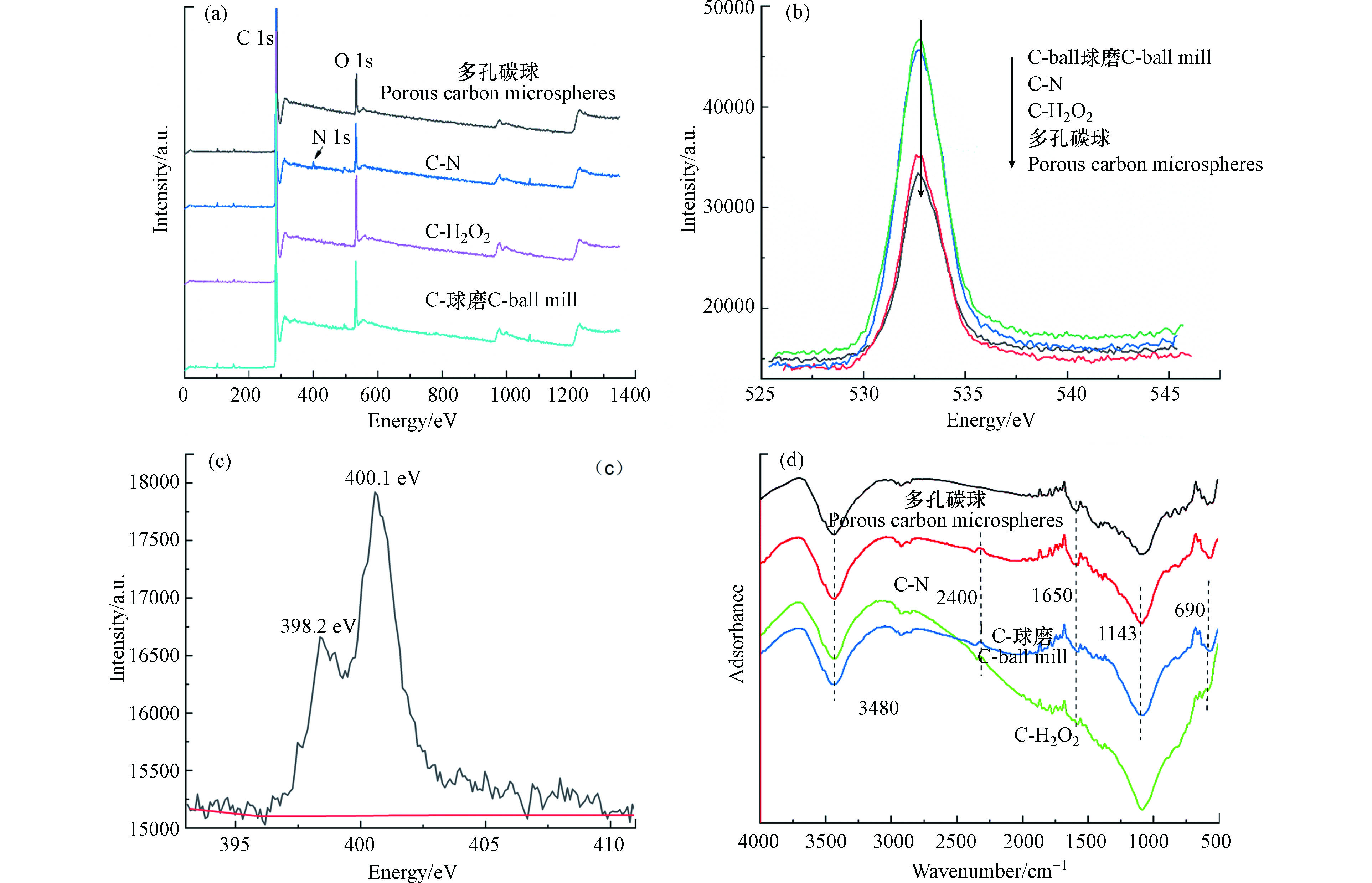
由4种材料的FT-IR谱图(图3d)可知,多孔碳微球在690、1143、1650、3480 cm−1附近有4处吸收峰;其中690 cm−1、3480 cm−1处出现的峰主要是由羟基的伸缩振动引起,1143 cm−1和1650 cm−1处主要是由—C—O键和羰基的伸缩振动引起。在1729 cm−1处出现了C==O吸收峰,可能存在羧基或酚羟基官能团[26]。C-H2O2在2400 cm−1附近有1个小的伸缩峰,存在不饱和的烃基与—N—O基团;C-H2O2和C-球磨在1143 cm−1处C—O的特征吸收峰增强,表明羧基或酚羟基官能团大幅度增加。XPS分析表明,C-N材料在398.2 eV和400.1 eV处,形成了大量的O—N和C—N基团(图3c),可能会形成石墨N导致活性位点增加[27];C-H2O2和C-球磨的氧含量是未改性前的1.9倍和1.8倍,可能是改性过程中引入了含氧官能团。
Boehm滴定发现,C-H2O2表面酸性含氧官能团总量最大,羧基、内酯基、酚羟基的含量都有所增加,羧基增加了3.0倍;C-球磨表面羧基含量提高了1.8倍,但酚羟基和内酯基含量减小,可能是改性过程中内酯基和酚羟基氧化转化成羧基[28-29](见表2)。研究表明碳材料表面增加的含氧官能团可以与有机物的酚羟基等官能团形成更多氢键,从而促进对有机污染物的吸附[30]。
-
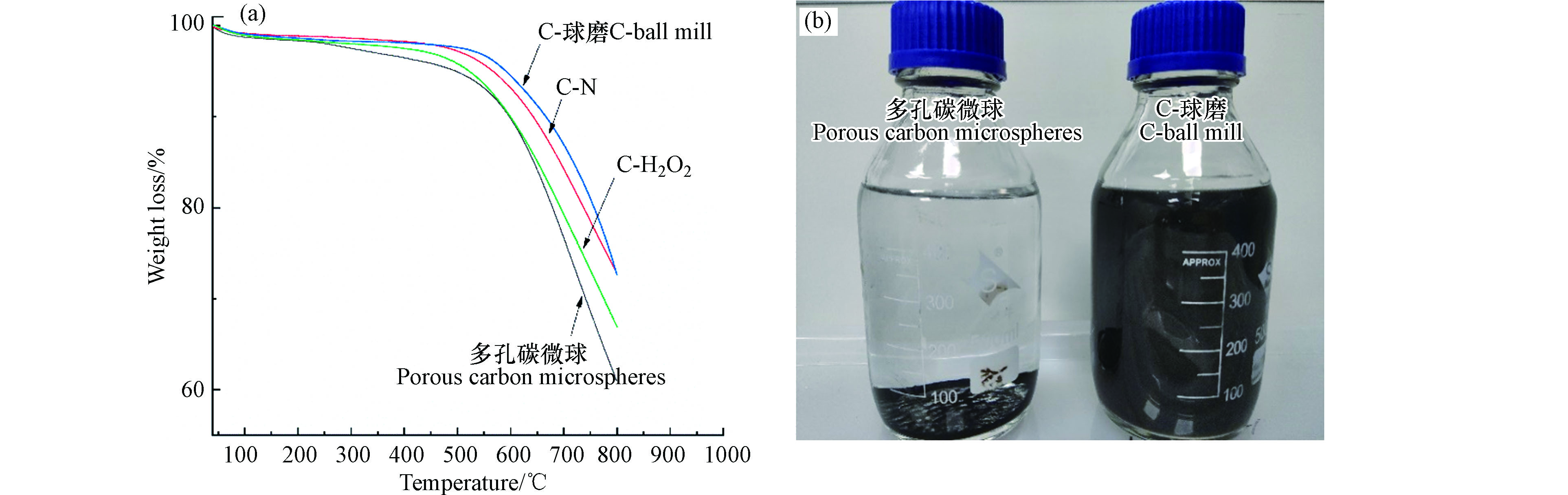
在相同加热温度下,C-N、C-H2O2、C-球磨的质量损失均低于多孔碳微球,其中800 ℃时,多孔碳微球的质量损失高达到40 %左右,C-H2O2的质量损失为30 %,C-N与C-球磨质量损失为20 %,表明改性增强了碳材料的热稳定性(图4a)。
多孔碳微球、C-N、C-H2O2和C-球磨在水中经24 h静置,多孔碳微球、C-N和C-H2O2自由沉降到瓶子底部,C-球磨分散均匀(图4b ),极大提高了材料在溶液内部的停留时间,为增强多孔碳微球材料吸附TBBPA的性能提供了可能[31]。
-
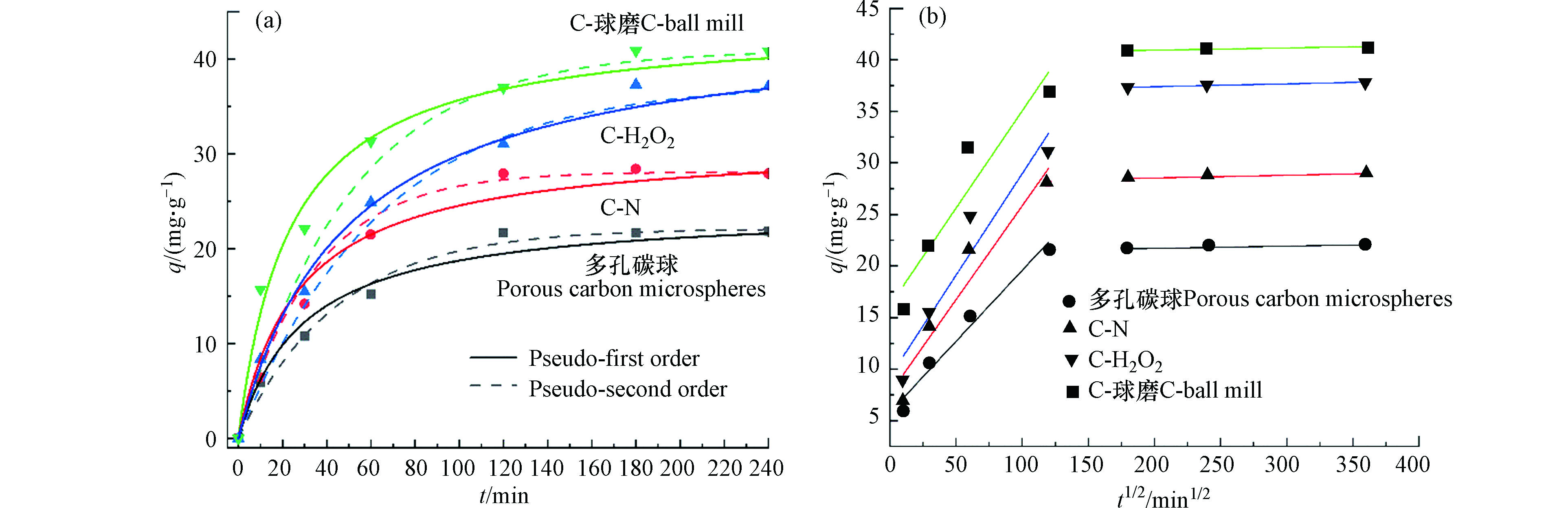
多孔碳微球、C-N、C-H2O2和C-球磨吸附TBBPA的准一级动力学和准二级动力学模型如图5(a)所示,准二级动力学拟合参数R2分别为0.997、0.995、0.998和0.990高于准一级动力学(表3),准二级方程包含如液膜扩散、表面吸附和颗粒内扩散以及电子共用或转移等吸附过程,而准一级方程只适用于描述吸附初始阶段,因此,准二级动力学模型能更合理地说明吸附过程,且以化学吸附为主[32-33]。C-N、C-H2O2、C-球磨的K2值分别增加了1.5倍、2.6倍和2.9倍,表明改性加快了多孔碳微球对TBBPA的吸附过程,比表面积和含氧官能团的增加可提高多孔碳微球对TBBPA的吸附速率[34]。颗粒内扩散拟合曲线表明,扩散过程主要分为颗粒内扩散和吸附平衡;多孔碳微球、C-N、C-H2O2和C-球磨内扩散拟合曲线均不过原点,表明颗粒内扩散不是唯一的控制步骤 [33, 35]。
-
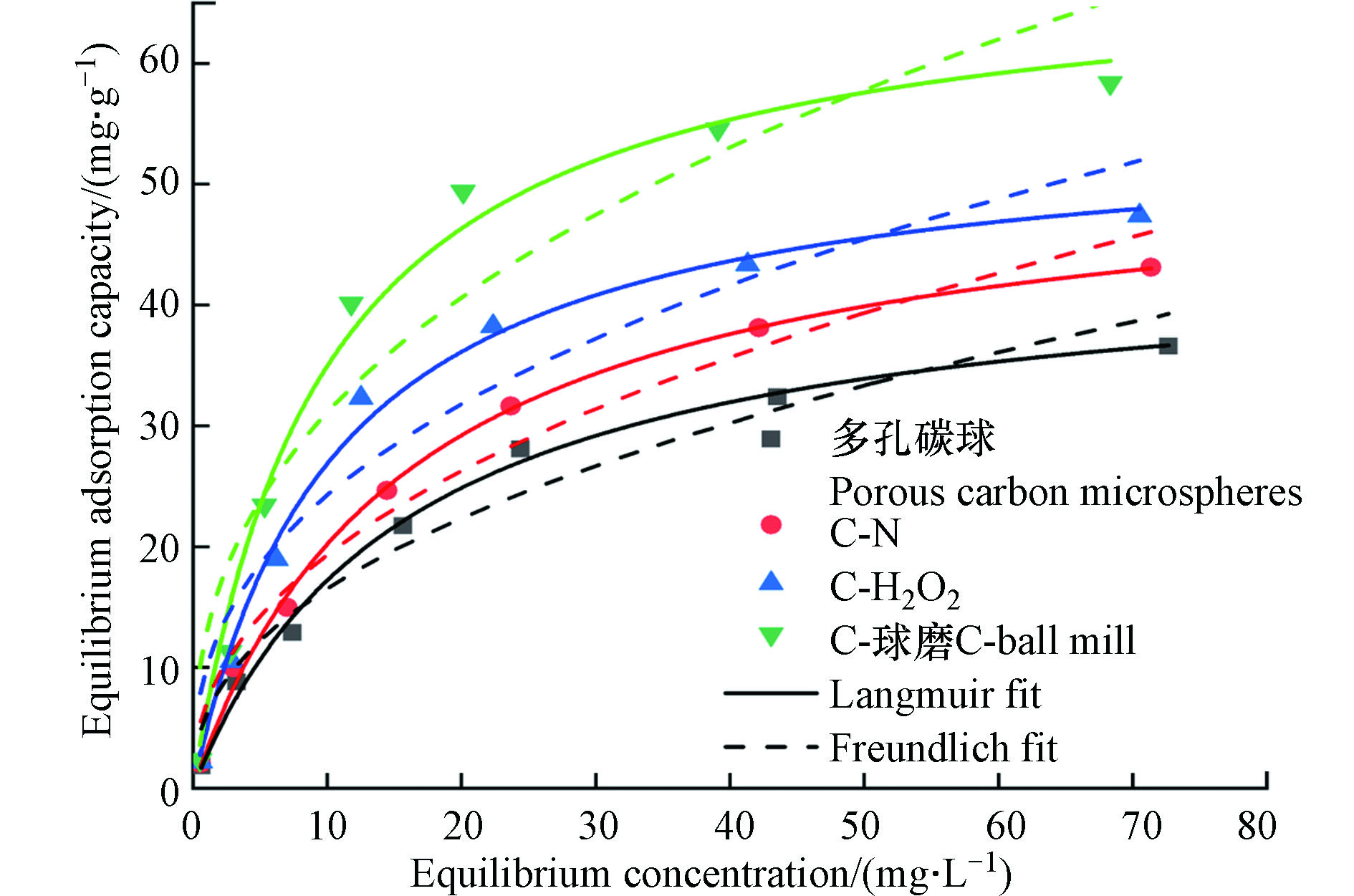
多孔碳微球、C-N、C-H2O2和C-球磨对TBBPA的最大吸附量分别为36.6、43.1、47.4 、58.35 mg·g−1,相对于未改性前分别提高了1.2倍、1.3倍和1.6倍(图6)。C-H2O2表面含氧官能团的增多,增强了与酚羟基等官能团形成氢键的能力,提升了吸附性能[30, 36]。球磨一方面显著提高了碳材料的比表面积,增加了吸附污染物的活性位点,另一方面,增加的含氧官能团增强了氢键和π-π电子供受体作用[37-39]。C-N吸附性能提高的可能原因是表面出现大量高活性和高稳定性的石墨N,形成了更多缺陷位点[40]。
多孔碳微球、C-N、C-H2O2和C-球磨对TBBPA吸附的Langmuir方程R2分别为0.994、0.996、0.992、0.985高于Freundlich模型(表4),吸附过程更加符合Langmuir等温吸附,为单分子层均匀吸附,Freundlich模型的拟合参数N小于1,说明吸附过程为协同非线性吸附[41]。由公式(7)计算得到多孔碳微球、C-N、C-H2O2和C-球磨的平衡参数RL分别为0.27、0.23、0.16和0.12,在0—1之间,说明改性过后的材料更加有利对TBBPA的吸附[22]。
-
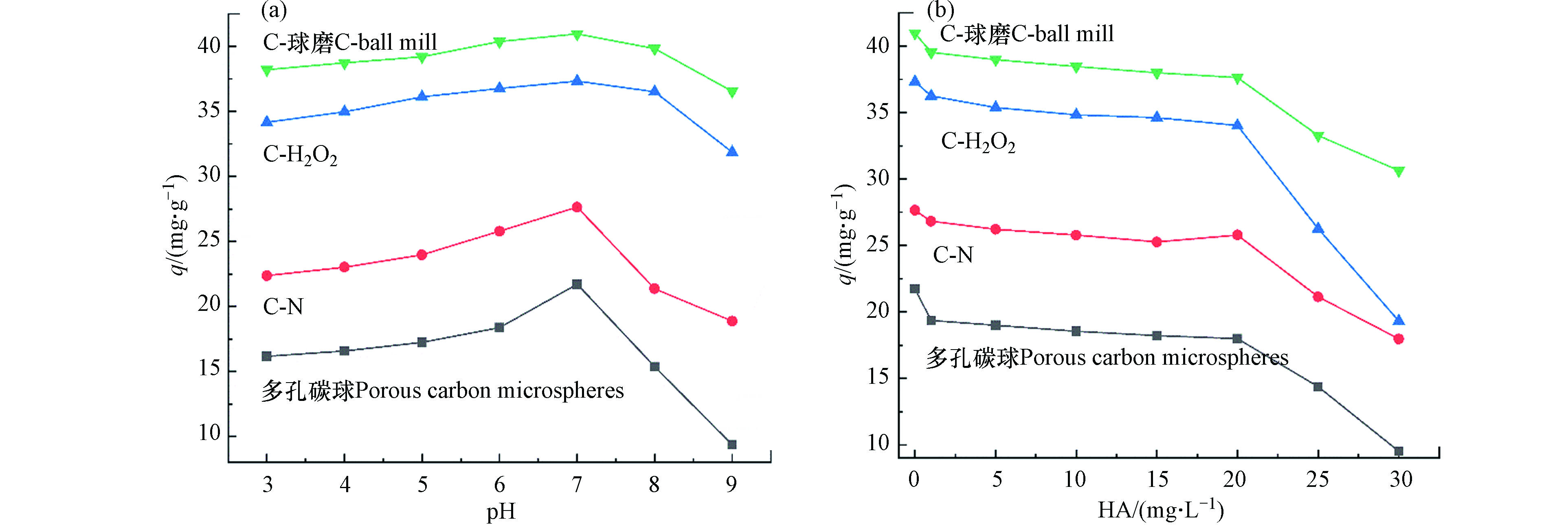
pH决定了吸附剂与吸附质分子的电荷状态,是影响碳材料在溶液中吸附能力的重要因素之一[42]。本研究探讨了不同pH值溶液碳微球对TBBPA吸附量的变化,结果表明,随着pH的增大,吸附量先增后减,当pH=7时4种多孔碳微球吸附量均达到最大(图7);pH<7时,随着pH的增加,溶液中H+不断减少,对TBBPA吸附量会逐渐增加,这可能与溶液中H+与TBBPA分子抢夺吸附位点有关[43];当pH>7时,4种多孔碳微球对TBBPA的吸附量减少,这是由于随着pH的增加,TBBPA被去质子化,以阴离子存在形式占据主导地位,且含碳吸附剂的IEPs(等电点)低于3.4,在碱性条件下其表面带有负电荷,形成静电斥力。随着pH的改变,改性材料吸附量的变化程度小于未改性的多孔碳微球,主要原因可能是表面改性后增多的含氧官能团(羧基)能起到缓冲pH变化的作用,表明改性拓宽了多孔碳材料去除水中TBBPA的pH适用范围。
-
添加HA后,C-N、C-H2O2、C-球磨的吸附量下降幅度相对缓慢,主要是由于氮掺杂和球磨改性碳材料的比表面积相对较大,能够提供更多的吸附位点[39, 44],H2O2改性大量增加了碳材料表面的含氧官能团,增强了碳材料与TBBPA的相互作用(氢键、π-π电子受体),强化了TBBPA的竞争吸附[11]。
当腐殖酸浓度小于20 mg·L−1时,多孔碳微球、C-N、C-H2O2、C-球磨对TBBPA的吸附量的变化小于4 mg·g−1,增加到30 mg·L−1时,吸附量分别减少了42%、31%、41%和19 %,这与以往的研究结果一致[45-46],可能是由于碳材料上的极性官能团与HA的氨基和羧基形成强氢键作用,以及碳材料的π电子共轭结构进一步促进其与HA形成强相互作用力[47-48],且吸附在碳材料表面的HA通过氢键作用吸附水分子形成水分子簇,阻碍碳材料对TBBPA的吸[49- 50]。
-
球磨、H2O2氧化和氮掺杂改性增强了多孔碳微球对TBBPA的去除性能,为进一步改性多孔碳微球材料去除有机污染物提供了数据支撑。球磨改性能明显增加多孔碳微球的比表面积、孔容积、表面官能团含量、热稳定性和分散性,对TBBPA的去除能力最强,吸附速率符合准二级动力学模型,Langmuir模型能更好的描述多孔碳微球对TBBPA的吸附过程,主要为单分子层均匀吸附,最大吸附量为58.35 mg·g−1,提高了1.6 倍,吸附速率为0.41 mg·(mg·min)−1,提升了2.9倍;吸附机理主要为氢键和π-π电子受体相互作用,且受pH和腐殖酸(DOM)的影响较小,拓宽了环境适用范围。
改性多孔碳微球的制备及去除水中四溴双酚A的性能
Preparation of modified porous carbon microspheres and performance of removing TBBPA from water
-
摘要: 为提高多孔碳微球对TBBPA的去除性能,采用氮掺杂、H2O2氧化和球磨对多孔碳微球进行表面改性,运用比表面积及孔隙度分析仪、傅里叶红外光谱(FT-IR)和X射线衍射仪(XPS)等方法表征改性前后多孔碳微球形貌、孔隙特征、官能团种类及含量和热稳定性等变化情况,通过吸附实验确定多孔碳微球的最佳改性方法,并探究吸附机理。结果表明,多孔碳微球、C-N、C-H2O2和C-球磨对TBBPA的最大吸附量分别为36.6 、43.1、47.4 、58.35 mg·g−1。吸附过程符合准二级动力学模型,Langmuir模型能够更好的描述多孔碳微球对TBBPA的吸附过程,主要为单分子层均匀化学吸附。其中C-球磨对TBBPA的吸附性能最佳,最大吸附量和吸附速率分别提高了1.6倍和2.9倍;球磨改性极大提高了碳材料的比表面积和含氧官能团,增加了吸附污染物的活性位点,强化了氢键和π-π电子供受体作用,且受pH和腐殖酸(HA)的影响较小,拓宽了环境适用范围。本研究以期为廉价碳材料去除有机污染物性能提供理论依据。Abstract: In order to improve the removal performance of porous carbon microspheres for TBBPA, nitrogen doping, H2O2 oxidation and ball milling were used to modify the surface of porous carbon microspheres. Specific surface area and porosity analyzer, Fourier infrared spectroscopy (FT-IR) were used. And X-ray diffractometer (XPS) methods to characterize the changes of porous carbon microsphere morphology, pore characteristics, functional group type and content, and thermal stability before and after modification, and determine the best modification method for porous carbon microspheres through adsorption experiments , And explore the adsorption mechanism. The results showed that the maximum adsorption capacity of porous carbon microspheres, C-N, C-H2O2 and C-ball mills for TBBPA were 36.6 mg·g−1, 43.1 mg·g−1, 47.4 mg·g−1, and 58.35 mg·g−1, respectively. The adsorption process conforms to the second-order kinetic model. The Langmuir model can better describe the adsorption process of TBBPA by porous carbon microspheres, which is mainly a uniform adsorption of monolayers. Among them, C-Ball Mill has the best adsorption performance for TBBPA, and the maximum adsorption capacity and adsorption rate are increased by 1.6 times and 2.9 times respectively. Ball mill modification greatly increases the specific surface area and oxygen-containing functional groups of carbon materials, and increases the adsorption of pollutants. The active site strengthens the role of hydrogen bond and π-π electron donor and acceptor, and is less affected by pH and humic acid (HA), which broadens the scope of environmental application. This research aims to provide a theoretical basis for the removal of organic pollutants by cheap carbon materials.
-
Key words:
- porous carbon microspheres /
- surface modification /
- TBBPA /
- adsorption
-

-
图 5 改性前后材料吸附 TBBPA 的动力学曲线(实线为准一级动力学拟合曲线;虚线为准二级动力学拟合曲线)(a)和颗粒内扩散方程拟合曲线(b)
Figure 5. The kinetic curve of TBBPA adsorption before and after modification (the dotted line is the first-order kinetic fitting curve; the solid line is the second-order kinetic fitting curve)(a)and the fitting curve of the intraparticle diffusion equation(b)
表 1 改性前后4种材料比表面积及孔隙结构
Table 1. Specific surface area and pore structure characteristics of the four materials before and after modification
材料种类
Material type比表面积/(m2·g−1)
Specific surface
Area外表面积/(m2·g−1)
Outer surface area
总孔容/(cm3·g−1)
Total pore volume微孔孔容/(cm3·g−1)
Micropore volume平均孔径/nm
Average pore size多孔碳微球 433.0 336.7 0.471 0.322 1.71 C-N 494.5 389.1 0.496 0.412 1.96 C-H2O2 106.7 64.2 0.085 0.041 2.85 C-球磨 1119.5 750.4 0.821 0.322 2.63 表 2 改性前后多孔碳微球表面含氧官能团的Boehm滴定结果(mmol·g−1)
Table 2. Oxygen functional groups on the surface of modified and unmodified porous carbon microspheres by Boehm titration method(mmol·g−1)
材料种类
Material type羧基
Carboxyl内酯基
Lactone酚羟基
Phenolic hydroxyl多孔碳微球 0.414 0.259 0.461 C-N 0.414 0.259 0.462 C-H2O2 1.239 0.387 0.526 C-球磨 0.741 0.016 0.368 表 3 改性前后材料吸附TBBPA动力学拟合参数
Table 3. Fitting parameters of TBBPA kinetics before and after modification
吸附材料
Adsorption material准一级动力学
Quasi-first order dynamics准二级动力学
Quasi-second order dynamic颗粒内扩散模型
Intraparticle diffusion modelK1 R2 K2 R2 Kd1 C1 R12 Kd2 C2 R22 多孔碳微球 0.022 0.960 0.142 0.997 0.830 0.503 0.966 0.064 4.580 0.890 C-N 0.002 0.987 0.206 0.995 1.172 0.475 0.979 0.066 7.134 0.997 C-H2O2 0.015 0.968 0.367 0.998 1.214 1.442 0.913 0.164 8.394 0.951 C-球磨 0.019 0.971 0.410 0.990 1.164 4.668 0.916 0.086 11.283 0.994 注: k1(min−1 )、k2(g·mg−1·h−1)分别为对应的准一级、准二级方程的参数;kd(mg·g−1·h−1/2)为颗粒内扩散方程速率参数;Ci为与边界层厚度有关的常数。 表 4 改性前后材料的吸附 TBBPA 的等温吸附线拟合参数
Table 4. Adsorption of materials before and after fitting parameters of TBBPA adsorption isotherms
吸附材料
Adsorption materialLangmuir Freundlich KL/(L·mg−1) qm/(mg·g−1) R2 KF/(L·mg−1) 1/N R2 多孔碳微球 0.061 44.74 0.994 6.04 2.30 0.95 C-N 0.063 52.74 0.996 7.00 2.26 0.96 C-H2O2 0.095 55.11 0.992 9.89 2.56 0.91 C-球磨 0.103 68.71 0.985 12.8 2.60 0.88 注:qm为理论最大吸附量;KL(L·mg−1)、KF(L·mg−1)和1/N为对应方程的吸附常数. -
[1] EMMA A, ZUIDE J, BOER J. Novel brominated flame retardants - A review of their occurrence in indoor air, dust, consumer goods and food [J]. Chemosphere, 2020, 255: 126816. doi: 10.1016/j.chemosphere.2020.126816 [2] NNOROM I C, OSIBANJO O. Sound management of brominated flame retarded (BFR) plastics from electronic wastes: State of the art and options in Nigeria [J]. Resources, Conservation & Recycling, 2008, 52(12): 1362-1367. [3] YU Y, WANG Z, WANG Q, et al. Excretion characteristics of tetrabromobisphenol-A in Wistar rats following mouth and nose inhalation exposure [J]. Chemosphere, 2017, 175 147-152. [4] YU Y, LI L, YU L, et al. Effect of exposure to decabromodiphenyl ether and tetrabromobisphenol A in combination with lead and cadmium on soil enzyme activity [J]. International Biodeterioration & Biodegradation, 2017, 117 45-51. [5] YANG S W, WANG S R, LIU H L, et al. Tetrabromobisphenol A: tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China [J]. Environmental Science and Pollution Research, 2012, 19(9): 4090-4096. [6] 程浩淼, 陈玉茹, 赵永岭, 等. 巢湖水域四溴双酚A的多介质迁移与归趋模拟 [J]. 中国环境科学, 2019, 39(1): 314-320. doi: 10.3969/j.issn.1000-6923.2019.01.036 CHEN H M, CHEN Y R, ZHAO Y L, et al. Simulation of multimedia transfer and fate of tetrabromobisphenol A in Lake Chaohu [J]. China Environmental Science, 2019, 39(1): 314-320(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.01.036
[7] FENG A H, CHEN S J, CHEN M Y, et al. Hexabromocyclododecane (HBCD) and tetrabromobisphenol A (TBBPA) in riverine and estuarine sediments of the Pearl River Delta in southern China, with emphasis on spatial variability in diastereoisomer- and enantiomer-specific distribution of HBCD [J]. Marine Pollution Bulletin, 2012, 64(5): 919-925. doi: 10.1016/j.marpolbul.2012.03.008 [8] 路风辉, 冯岸红, 陈满英, 等. 珠三角表层沉积物的有机碳及其与卤系阻燃剂的关系 [J]. 地球与环境, 2015, 43(1): 49-54. LU F H, FENG A H, CHEN M Y, et al. Total organic carbons and their correlations with halogenated flame retardants in surface sediments from the Pearl River Delta, China [J]. Earth and Environment, 2015, 43(1): 49-54(in Chinese).
[9] 张琳, 云霞, 那广水, 等. 环境水体中四溴双酚A的HPLC-MS/MS分析方法的建立与应用 [J]. 环境工程学报, 2011, 5(5): 1077-1080. ZHANG L, YUN X, NA G S, et al. Determination and application of TBBPA in water by high performance liquid chromatography-tandem mas spectrometry [J]. Chinese Journal of Environmental Engineering, 2011, 5(5): 1077-1080(in Chinese).
[10] YU Z, LIN Q, GU Y, et al. Bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in wild marine fish from the coastal waters of the northern South China Sea: Risk assessment for human health [J]. Ecotoxicology and Environmental Safety, 2019, 180: 742-748. doi: 10.1016/j.ecoenv.2019.05.065 [11] REN L, LI L, CHEN S, et al. Yolk-shell Fe/FeS@SiO2 particles with enhanced dispersibility, transportability and degradation of TBBPA [J]. Catalysis Today, 2019, 327: 2-9. doi: 10.1016/j.cattod.2018.10.023 [12] YU Y, YU Z, CHEN H, et al. Tetrabromobisphenol A: Disposition, kinetics and toxicity in animals and humans [J]. Environmental Pollution, 2019, 253: 909-917. doi: 10.1016/j.envpol.2019.07.067 [13] YU Y, LIU L, CHEN X, et al. Brominated flame retardants and heavy metals in common aquatic products from the pearl river delta, south china: Bioaccessibility assessment and human health implications [J]. Journal of Hazardous Materials, 2021, 403 124036. [14] 张萌, 吕耀斌, 朱一滔, 等. 去灰分对生物炭理化性质及芳香族污染物吸附的影响 [J]. 环境化学, 2020, 39(11): 3161-3170. ZHANG M, LYU Y B, ZHU Y T, et al. Impact of deaching treatment on biochar physicochemical properties and sorption mechanisms of aromatic pollutants [J]. Environmental Chemistry, 2020, 39(11): 3161-3170(in Chinese).
[15] UHNÁKOVÁ B, LUDWIG R, PĚKNICOVÁ J, et al. Biodegradation of tetrabromobisphenol A by oxidases in basidiomycetous fungi and estrogenic activity of the biotransformation products [J]. Bioresource Technology, 2011, 102(20): 9409-9415. doi: 10.1016/j.biortech.2011.07.036 [16] ZHU X, HUANG M, ZHANG Q, et al. Proposal of possible pathway of fluorene biodegrada-tion by Citrobacter sp. FL5 [J]. Applied Envrionmental Biotechnology, 2016, 1(1): 44-51. doi: 10.26789/AEB.2016.01.009 [17] YU Y, HUANG Z, DENG D, et al. Synthesis of millimeter-scale sponge Fe/Cu bimetallic particles removing TBBPA and insights of degradation mechanism [J]. Chemical Engineering Journal, 2017, 325 279-288. [18] 章琴琴, 丁世敏, 封享华, 等. Fenton法降解邻苯二甲酸二甲酯的动力学特征及其影响因素研究 [J]. 环境化学, 2020, 39(11): 3009-3016. doi: 10.7524/j.issn.0254-6108.2019082201 ZHANG Q Q, DING S M, FENG X H, et al. Study on the kinetic characteristics and influencing factors of degradation of dimethyl phthalate by Fenton treatmet [J]. Environmental Chemistry, 2020, 39(11): 3009-3016(in Chinese). doi: 10.7524/j.issn.0254-6108.2019082201
[19] LI T, HE Y, PENG X. Efficient removal of tetrabromobisphenol A (TBBPA) using sewage sludge-derived biochar: Adsorptive effect and mechanism [J]. Chemosphere, 2020, 251: 126370. doi: 10.1016/j.chemosphere.2020.126370 [20] SHAO P, PEI J, TANG H, et al. Defect-rich porous carbon with anti-interference capability for adsorption of bisphenol A via long-range hydrophobic interaction synergized with short-range dispersion force [J]. Journal of Hazardous Materials, 2021, 403: 123705. doi: 10.1016/j.jhazmat.2020.123705 [21] 张伟. 基于葡萄糖水热法制备功能性纳米材料及其应用的研究 [D]. 天津: 天津大学, 2012. ZHANG W. Preparation and application of functional nanomaterials base on the hydrothermal process of glucose[D]. Tianjin: Tianjin University, 2012(in Chinese).
[22] HAMEED B H, TAN I A W, AHMAD A L. Adsorption isotherm, kinetic modeling and mechanism of 2, 4, 6-trichlorophenol on coconut husk-based activated carbon [J]. Chemical Engineering Journal, 2008, 144(2): 235-224. doi: 10.1016/j.cej.2008.01.028 [23] HERNANDEZ-ORTIZ M, DURÁN-MUÑOZ H A, LOZANO-LÓPEZ J D, et al. Determination of the surface functionality of nanocarbon allotropes by boehm titration [J]. Surface Review and Letters, 2020, 27(8): 1950190. doi: 10.1142/S0218625X19501907 [24] 陈薇, 肖高, 郭杰, 等. 煤基活性炭表面改性对稀土负载型CeO_2/AC低温脱硝性能的影响 [J]. 环境工程学报, 2018, 12(7): 1959-1967. doi: 10.12030/j.cjee.201712188 CHEN W, XIAO G, GUO J, et al. Effect of surface modification of coal based activated carbon on low temperature denitration perform ance of rare-earth supported CeO2/AC [J]. Chinese Journal of Environmental Engineering, 2018, 12(7): 1959-1967(in Chinese). doi: 10.12030/j.cjee.201712188
[25] 刘寒冰, 杨兵, 薛南冬. 酸碱改性活性炭及其对甲苯吸附的影响 [J]. 环境科学, 2016, 37(9): 3670-3678. LIU H B, YANG B, XUE N D. Effects of acidic and basic modification on activated carbon for adsorption of toluene [J]. Environmental Science, 2016, 37(9): 3670-3678(in Chinese).
[26] 丁春生, 沈嘉辰, 缪佳, 等. 改性活性炭吸附饮用水中三氯硝基甲烷的研究 [J]. 中国环境科学, 2013, 33(5): 821-826. doi: 10.3969/j.issn.1000-6923.2013.05.008 DING C S, SHEN J C, LIAO J, et al. Adsorption of trichloronitromethane in drinking water by modified activated carbon [J]. China Environmental Science, 2013, 33(5): 821-826(in Chinese). doi: 10.3969/j.issn.1000-6923.2013.05.008
[27] JUNG H J, CHOI M Y. One-pot synthesis of graphitic and nitrogen-doped graphitic layers on nickel nanoparticles produced by pulsed laser ablation in liquid: Solvent as the carbon and nitrogen source [J]. Applied Surface Science, 2018, 457: 1050-1056. doi: 10.1016/j.apsusc.2018.07.036 [28] 刘丽, 石宝友, 盖克, 等. 化学改性活性炭对水中阿特拉津的吸附去除 [J]. 环境工程学报, 2012, 6(8): 2483-2488. LIU L, SHI B Y, GAI K, et al. Adsorption removal of atrazine from water by chemically modified activated carbons [J]. Chinese Journal of Environmental Engineering, 2012, 6(8): 2483-2488(in Chinese).
[29] 许琦, 侯亚芹, 郭倩倩, 等. 活性炭表面含氧官能团对燃煤烟气氮氧化物脱除的影响 [J]. 环境化学, 2020, 39(8): 2105-2111. doi: 10.7524/j.issn.0254-6108.2019060505 XU Q, HOU Y Q, GUO Q Q, et al. Effect of oxygen-containing functional groups on the removal of nitrogen oxides from coal-fired flue gas on activated carbon [J]. Environmental Chemistry, 2020, 39(8): 2105-2111(in Chinese). doi: 10.7524/j.issn.0254-6108.2019060505
[30] XIAO G, WEN R, YOU P, et al. Adsorption of phenol onto four hyper-cross-linked polymeric adsorbents: Effect of hydrogen bonding receptor in micropores on adsorption capacity [J]. Microporous and Mesoporous Materials, 2017, 239: 40-44. doi: 10.1016/j.micromeso.2016.09.044 [31] 付婷, 凌小佳, 丁莹, 等. 改性介孔碳对饮用水中3种指标吸附性能分析 [J]. 环境科学与技术, 2017, 40(10): 49-55. FU T, LING X J, DING Y, et al. Adsorption performance analysis of modified mesoporous carbon on three indexs in drinking water [J]. Environmental Science & Technology, 2017, 40(10): 49-55(in Chinese).
[32] 张华. 头孢类抗生素在改性活性炭上的吸附 [D]. 北京: 北京化工大学, 2015. ZHANG H. Adsorption of cephalosporin antibiotics on modified activated carbon[D]. Beijing: Beijing University of Chemical Technology, 2015(in Chinese).
[33] YANG Z, WU W, YU L, et al. Fabrication and characterization of magnetically responsive Fe3O4@TiO2 core-shell adsorbent for enhanced thallium removal [J]. Environmental Science and Pollution Research, 2020, 27(24): 30518-30529. doi: 10.1007/s11356-020-09144-x [34] 高珊珊, 赵竟博, 田家宇, 等. 化学改性对活性炭吸附磺胺甲恶唑和布洛芬的影响 [J]. 环境工程学报, 2015, 9(10): 4650-4654. doi: 10.12030/j.cjee.20151007 GAO S S, ZHAO J B, TIAN J Y, et al. Influence of chemical modification on activated carbon for adsorption of sulfamethoxazole and ibuprofen [J]. Chinese Journal of Environmental Engineering, 2015, 9(10): 4650-4654(in Chinese). doi: 10.12030/j.cjee.20151007
[35] YU Y, WANG C, GUO X, et al. Modification of carbon derived from Sargassum sp. by lanthanum for enhanced adsorption of fluoride [J]. Journal of Colloid And Interface Science, 2015, 441: 113-120. doi: 10.1016/j.jcis.2014.10.039 [36] LINBO Q, BAOLIANG C. Dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles [J]. Environmental Science & Technology, 2013, 47(15): 8759-8768. [37] TANG J, LV H, GONG Y, et al. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal [J]. Bioresource Technology, 2015, 196: 355-363. doi: 10.1016/j.biortech.2015.07.047 [38] WU T, CAI X, TAN S, et al. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions [J]. Chemical Engineering Journal, 2011, 173(1): 144-149. doi: 10.1016/j.cej.2011.07.050 [39] HONGHONG L, BIN G, FENG H, et al. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms [J]. Environmental Pollution, 2018, 233: 54-63. doi: 10.1016/j.envpol.2017.10.037 [40] 潘建, 潘顺龙, 刘志英, 等. 氮掺杂碳材料的制备及催化降解苯酚废水实验研究 [J]. 现代化工, 2020, 40(11): 131-135. PAN J, PAN S L, LIU Z Y, et al. Preparation of nitrogen-doped carbon materials and application in catalytic degradation of phenolcontaining wastewater [J]. Modern Chemical Industry, 2020, 40(11): 131-135(in Chinese).
[41] LI J M, MENG X G, HU C W, et al. Adsorption of phenol, p-chlorophenol and p-nitrophenol onto functional chitosan [J]. Bioresource Technology, 2009, 100(3): 1168-1173. doi: 10.1016/j.biortech.2008.09.015 [42] ZHANG G, FAN F, LI X, et al. Superior adsorption of thallium(I) on titanium peroxide: Performance and mechanism [J]. Chemical Engineering Journal, 2018, 331: 355-363. doi: 10.1016/j.cej.2017.08.100 [43] FASFOUS I I, RADWAN E S, DAWOUD J N. Kinetics, equilibrium and thermodynamics of the sorption of tetrabromobisphenol A on multiwalled carbon nanotubes [J]. Applied Surface Science, 2010, 256(23): 7246-7252. doi: 10.1016/j.apsusc.2010.05.059 [44] 方梦园, 赵天慧, 赵晓丽, 等. 碳纳米管对腐殖酸的吸附及其环境意义 [J]. 环境化学, 2020, 39(10): 2897-2906. doi: 10.7524/j.issn.0254-6108.2019111002 FANG M Y, ZHAO T H, ZHAO X L, et al. Effect of humic acid on adsorption and sedimentation of carboxylic multi-walled carbon nanotubes with different diameters [J]. Environmental Chemistry, 2020, 39(10): 2897-2906(in Chinese). doi: 10.7524/j.issn.0254-6108.2019111002
[45] JI L L, CHANG W, ZHANG S R, et al. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes [J]. Langmuir:the ACS journal of surfaces and colloids, 2009, 25(19): 11608-11613. doi: 10.1021/la9015838 [46] SEOKJOON K, YU L. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Attenuation of surface activity by humic and fulvic acids [J]. Environmental Science & Technology, 2006, 40(24): 7757-7763. [47] ZHANG M, SHU L, GUO X, et al. Impact of humic acid coating on sorption of naphthalene by biochars [J]. Carbon, 2015, 94: 946-954. doi: 10.1016/j.carbon.2015.07.079 [48] XIE M, CHEN W, XU Z, et al. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions [J]. Environmental Pollution, 2014, 186: 187-194. doi: 10.1016/j.envpol.2013.11.022 [49] FENGLING L, ZHAOYI X, HAIQIN W, et al. Enhanced adsorption of humic acids on ordered mesoporous carbon compared with microporous activated carbon [J]. Environmental Toxicology and Chemistry, 2011, 30(4): 793-800. doi: 10.1002/etc.450 [50] KASOZI, ZIMMER A, PETER N K, et al. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars) [J]. Environmental Science & Technology, 2010, 44(16): 6189-6195. -



 下载:
下载: