-
天然水中过量的磷主要来自冶金、化工、造纸厂、水产养殖和市政等污水的过量排放[1],并导致了严重的水体富营养化及生态恶化现象[2]. 研究表明,当水体磷浓度大于0.03 mg·L–1,富营养化速率将显著加快[3]. 最近, 加拿大Schindler等通过连续37年的生态系统实验发现,控制湖泊富营养化的重点应该集中在降低水中磷的浓度,而不是氮的输入[4]. 因此,有效去除过量的磷酸盐在控制水体富营养化方面具有重要意义.
常用的除磷方法有混凝、化学沉淀、生物法、离子交换和吸附法[5-8]. 在这些方法中,吸附法由于其成本低,操作简单,易回收以及在微污染水中的吸附效率高而被广泛使用[9-10]. 目前,已有多种吸附剂被证实对磷酸盐具备有效的吸附能力,例如活性氧化铝、粉末状沸石、铝土矿残留物、改性生物质、离子交换剂等[11]. 然而,常规吸附剂存在吸附容量低,吸附选择性差的问题,难以满足日益严格的磷酸盐排放标准,甚至在竞争激烈的背景下吸附量可能降低至零[12-13]. 因此,开发对磷酸盐具有吸附容量高且吸附选择性强的新型吸附剂对于实际应用至关重要.
近年来,一类新型的微孔材料——金属有机骨架(MOFs)因具有极高的比表面积、均匀且可调的孔径等特性在催化、气体存储、分离和吸附等方向的应用中引起了广泛关注[14]. MOFs是由过渡金属离子和有机连接物组成的网状结构,层次有序,可以显著提高比表面积、增加材料活性位点[15]. 此外,与其他具有分级结构的吸附剂不同,MOFs的合成程序大大简化,节省了生产成本[16]. 然而,大多数MOFs水热稳定性较差,这限制了其在水处理中的进一步应用[17]. 最近,研究人员开发了一类具有优异的化学和水热稳定性的锆基MOFs,例如UiO-66[18]. 它是由Zr6O4(OH)4簇和对苯二甲酸酯(1,4-苯二甲酸,BDC)有机配体相连合成的,分子式为[Zr6O4(OH)4(BDC)12][19]. 由于高度的拓扑连接性和锆-氧之间的强配位键,无论在酸性还是碱性条件下,UiO-66都具备重要的水热稳定性能[20]. 更重要的是,即使在低浓度污染物条件下,UiO-66骨架的大量羟基也可以提供活性配位点并显示出对目标离子的特异性亲和力. 例如,Wang等报道,UiO-66可作为一种高效吸附剂在更宽的pH范围内去除废水中的砷[21]. 当前研究为UiO-66在水处理中的应用提供了一定的参考价值和理论依据.
本研究系统考察了UiO-66对水中磷酸盐的吸附性能与吸附机理. 采用SEM、XRD、FT-IR、TGA和N2吸附-脱附等方法对UiO-66的理化性质进行表征,并考察了UiO-66对磷酸盐的吸附等温线、pH效应、动力学和竞争离子效应. 此外,还进行了循环吸附和再生实验,以评估UiO-66在实际水体中应用的可行性.
-
材料和试剂:四氯化锆(ZrCl4,98%,阿拉丁(上海)试剂有限公司),盐酸和浓硫酸(分析纯,上海凌峰化学试剂有限公司),溴化钾(光谱纯),对苯二甲酸(99%),其他药品均为分析纯,购买国药集团上海化学试剂有限公司.
仪器:真空干燥箱(XMTD-8222,上海精宏实验设备有限公司)、鼓风烘箱(XMTD-8222,上海精宏实验设备有限公司)、控温磁力搅拌器(85-2,江苏怡仪器科技有限公司)、恒温水浴摇床(LSHZ-300,太仓市强乐实验设备有限公司)、数控超声波清洗器(KQ-300DB,昆山市超声仪器有限公司)、紫外可见分光光度计(UV-1800,上海菁华科技仪器有限公司)、pH计(PB-10,赛多利斯科学仪器公司).
-
UiO-66采用溶剂热法合成,参考文献[22]的合成方法,按照ZrCl4:对苯二甲酸摩尔比为1:1,溶剂体积比为3:1,具体步骤如下:
用分析天平分别称取0.400 g ZrCl4和0.280 g对苯二甲酸,分别加入45 mL和15 mL的DMF,超声溶解20 min;将上述溶解完全的溶液混合,再次超声2—3 min,然后将混合均匀的溶液转移到反应内衬里,放入不锈钢反应釜中,密封拧紧,最后放入到预先加热到120 ℃鼓风烘箱里,静态反应24 h;反应完成后,关闭烘箱自然冷却到室温,倒出反应物,将得到的反应物利用离心机进行分离,然后加入40 mL DMF和甲醇各洗涤3次,每次间隔浸泡12 h,并分离;最后一次分离后,然后放入真空烘箱,150 ℃干燥24 h.
-
采用扫描电子显微镜(SEM, SU1510,日本日立)观察UiO-66微观形貌.采用X射线衍射仪(XRD,XRD-6100,日本岛津)进行晶体结构的测定,判断UiO-66的晶体形态. 测定条件为:扫描范围为5°—80°,扫描速度为7(°)·min–1. 采用氮气脱附-吸附仪(Autosorb-iQ-AG-MP,美国康塔)测定材料比表面积和孔径分布. 脱气条件:以10 ℃·min–1速率,先升高到80 ℃,保持1 h,然后再升高到120 ℃,保持12 h. 采用同步热重-差热分析仪(LABSYS EVO TG-DTA/DSC,法国塞塔拉姆)测定UiO-66热稳定性. 采用红外光谱仪(Nicolet iS5,美国Thermo Fisher)分析UiO-66的特征官能团. 采用酸碱滴定仪(ET18,德国Mettler Toledo)测定UiO-66表面电性变化及等电位点.
称取0.1 g的UiO-66倒入烧杯,加入50 mL,0.1 mol·L−1的硝酸钠溶液,利用缓冲溶液校准仪器,通过酸碱中和原理计算得到HCl(0.1 mol·L–1)和NaOH(0.1 mol·L–1)的浓度;然后利用酸碱滴定仪边搅拌边向溶液中滴加盐酸,直至pH=1.5停止,记录消耗HCl体积,将该溶液搅拌一整夜,次日边搅拌边滴加NaOH溶液直到pH=11停止,得到加入NaOH溶液体积和pH值之间关系图,根据下面公式1计算氢离子浓度Ht
式中,Ca、Cb分别为加入的酸的浓度,碱的浓度;V0、Va、Vb分别为溶液初始体积,加入酸的体积,碱的体积. 根据计算的Ht和pH值制图,可得到Ht-pH之间的关系图.
-
采用超纯水配置100 mL一定初始浓度C0的KH2PO4溶液,用0.1 mol·L–1 NaOH溶液和0.1 mol·L–1 HNO3溶液调节pH,投入一定量的UiO-66,再放入温度为25 ℃,转速为160 r·min–1的恒温振荡器中反应24 h,然后取出并经0.22 μm的滤膜过滤,采用紫外可见分光光度计测定溶液中磷酸盐的浓度,计算去除率(%)和吸附量qe. 无特殊说明时,C0为20 mg·L–1,吸附剂投加量为0.25 g·L–1,pH=6,反应时间t=24 h.
(1) 静态吸附实验
在相同条件下分别测定初始pH(2、3、4、5、6、7、8、9、10、11、12)、初始磷浓度(10、20、30、40、50、60、70、80 mg·L–1)、共存阴离子(Cl–、NO3–、SO42–,n(Cl–/NO3–/SO42–)/n(P)物质的量比分别为0、5、10、20、40、60)、反应时间(0、1、3、5、8、12、16、20、30、40、60、80、100、120、150、180、210、240、270、300、360、420、480、540、600、660 min),500 mL溶液条件下溶液中剩余磷浓度,吸附量计算如下式(2)所示:
式中,C0、Ce分别为磷酸盐初始浓度,平衡浓度,mg·L–1;qe为磷酸盐平衡吸附量,mg·g–1;V为磷酸盐溶液体积,L;W为吸附剂的质量,g.
使用Langmuir和Freundlich等温模型拟合非线性回归形式的吸附等温线数据,将其线性化后得到下述公式。
Langmuir 模型公式:
Freundlich 模型公式:
式中,qm为最大吸附量,mg·g–1;KL为Langmuir平衡常数,L·mg–1,KF为Freundlich吸附特征常数,L·g–1;n为Freundlich吸附特征常数.
动力学机理分析使用伪一级和伪二级两种吸附模型线性公式,如下:
伪一级动力学模型公式:
伪二级动力学模型公式:
式中,t为吸附时间,min;qt为吸附剂在t时刻的吸附量,mg·g–1;k1为伪一级吸附动力学方程的系数,min–1;k2为伪二级吸附动力学方程的系数(g·mg–1·min–1).
(2)循环吸附和循环吸附-再生实验
循环吸附实验:相同条件下(C0为2 mg·L–1,吸附剂投加量为0.25 g·L–1,pH=6)将溶液振荡3 h当吸附达到平衡后,测定溶液中磷酸盐的浓度,然后将吸附后的UiO-66用离心机进行分离至于烧杯中,最后放入真空烘箱干燥24 h,循环上述吸附过程.
循环吸附-再生实验:相同条件下(C0为20 mg·L–1,吸附剂投加量为0.25 g·L–1,pH=6)将溶液振荡3 h吸附平衡后,测定溶液中磷酸盐的浓度,然后将吸附后的UiO-66用离心机进行分离,加入150 mL DMF超声1 h,再次用离心机分离,将分离后的UiO-66用蒸馏水洗涤2遍之后倒入玻璃烧杯中,最后放入真空烘箱干燥24 h,以备再次使用.
-
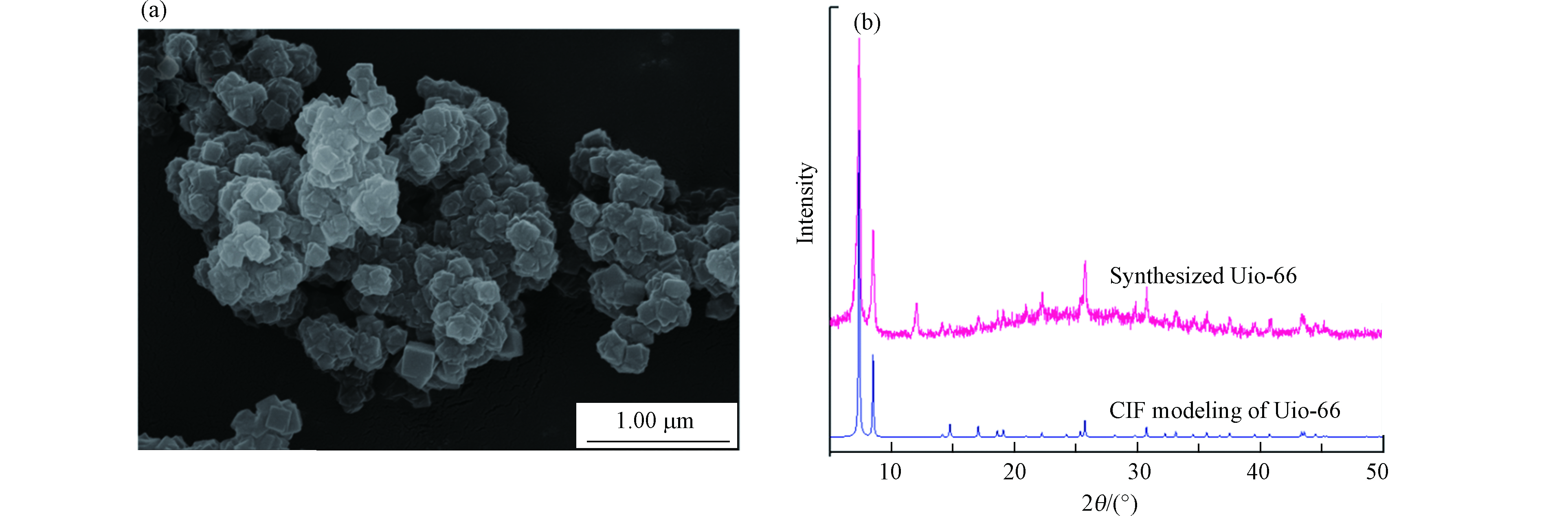
通过扫描电镜(SEM)观察UiO-66的形貌结构,如图1a所示. 由图1可以看出,合成的UiO-66微晶体呈堆积共生状,具有尖锐的边缘结构,尺寸大小与文献[23]报道类似. 根据图1b,UiO-66的XRD图谱分别在2θ 7.4°、8.5°、14.2°、17.1°、22.3°、25.7°、33.2°处出现明显的特征衍射峰,分别与(111)、(002)、(113)、(004)、(115)、(224)、(137)晶面相对应,并与UiO-66的CIF模拟XRD谱图基本吻合,说明合成的UiO-66特征峰与文献[24]报道相吻合,呈现良好的晶型特征.
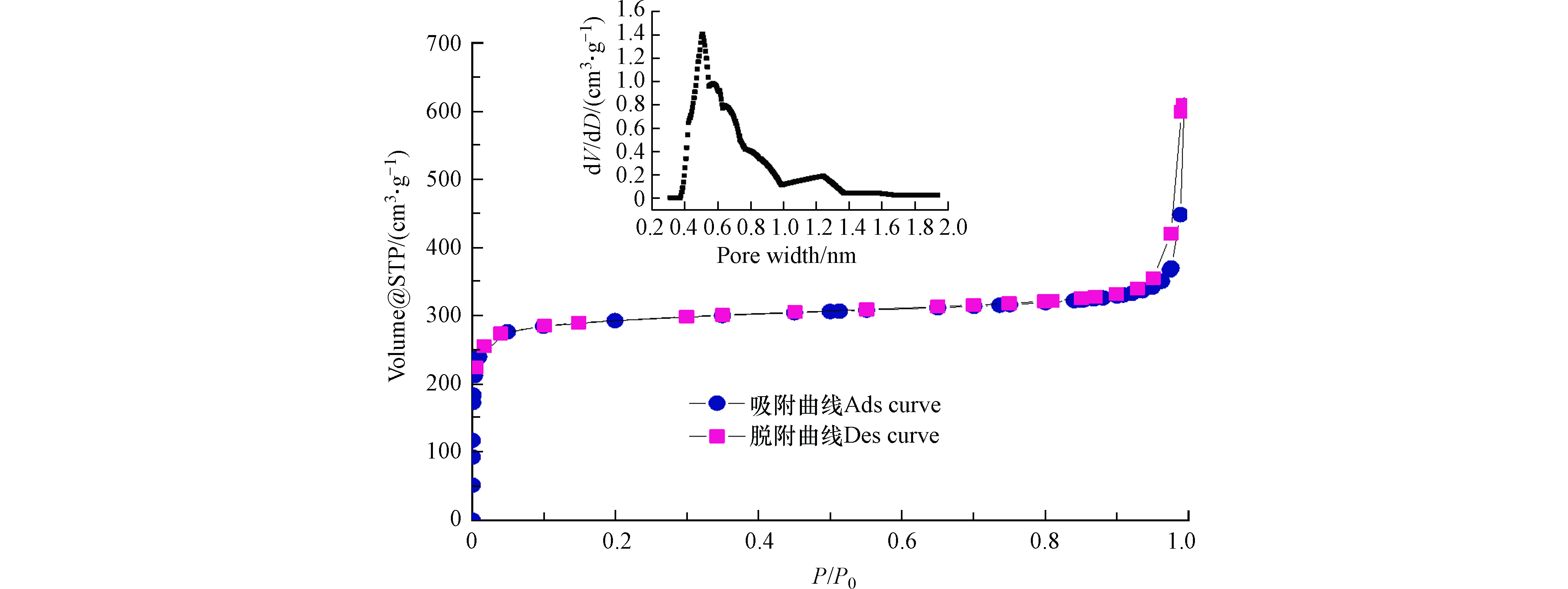
UiO-66的氮气吸附-脱附曲线和孔径分布如图2所示.在相对低压(P/P0<0.1)阶段观察到N2吸附量迅速增高,表明该阶段吸附以微孔填充作用为主,证实了吸附剂UiO-66内部大量狭窄微孔(孔径<2 nm)的存在. 在中压阶段(0.2<P/P0<0.8),由于微孔的吸附饱和,N2吸附量达到相对稳定的水平(接近水平). 当接近饱和蒸汽压时(0.9<P/P0<1),N2吸附量迅速增加,并且在吸附和解吸线之间出现了小的磁滞回线,这可能是由于非微孔表面(如中孔、大孔及外表面)的多层吸附所导致的吸附质凝聚现象. 可见,UiO-66的N2吸附-脱附等温线为典型微孔吸附剂的等温线类型,即为Ⅰ型吸附等温线. 经计算,UiO-66的BET结果为1156.6 m2·g−1(表面积为80.7 m2·g−1,微孔面积为1075.9 m2·g−1),十分接近文献报告结果(1187 m2·g−1)[24]. 此外, UiO-66的微孔孔径主要分布在0.3—0.6 nm之间,孔容为0.422 m2·g−1.
UiO-66的傅里叶红外光谱如图3a所示,1576 cm−1和1398 cm−1为—COO−的特征吸附峰,1506 cm−1为苯环—C=C—键的特征吸附峰,746 cm−1和662 cm–1处的特征峰是由UiO-66中Zr—O键引起,官能团的类型和出现的位置均与文献[24]一致,说明成功制备了UiO-66材料. UiO-66热重分析如图3b所示. UiO-66的重量随温度变化过程可分为3个阶段:第一阶段为30—130 ℃,重量减少了22.1%,这是由于UiO-66孔道内部水分或溶剂的蒸发;第二阶段为130—320 ℃,重量缓慢下降了8.1%,主要因为UiO-66发生脱羟基作用所致[25];第三阶段为320—580 ℃,重量下降29.3%,配体与金属离子之间的配位键断裂从而导致结构坍塌所致. 可见,UiO-66在200 ℃之前结构稳定,具有良好的稳定性.
-
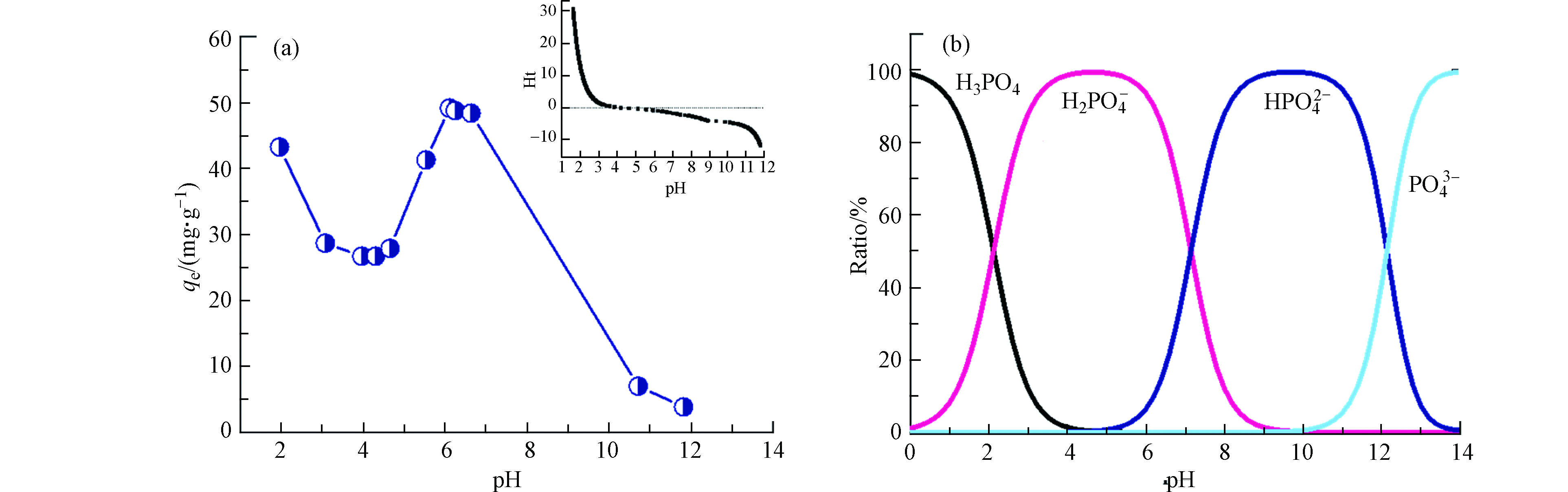
溶液pH是影响吸附剂吸附性能的重要参数之一. 由图4a可见,当pH<4.5时磷酸盐吸附量随着pH值的降低而逐渐增大;4.5<pH<6.2,UiO-66的吸附量随着pH值增大逐渐升高,pH=6.2达最大值;当pH>6.2,吸附量开始逐渐降低,尤其当pH>10.7吸附量迅速降低. UiO-66对磷酸盐的吸附主要是由于—OH的配体交换,酸性条件下大量H+会促使UiO-66表面质子化,使其表面所带正电荷增加(式7),从而促进其对带负电荷磷酸盐的吸附[26]. 此外,根据公式1可得等电位点Ht=0(Ca=0.0933 mol·L–1, Va=24.301 mL, Cb =0.0975 mol·L–1,Vb=23.203 mL,V0=50 mL)对应的pH为4.5,此时UiO-66表面电荷接近于零,材料对水中离子吸附能力较弱,磷酸盐吸附量在pH=4.5附近呈现出明显的吸附波谷. 当溶液pH值超越4.5时,虽然此时材料表面呈电负性,静电排斥作用会阻碍材料对磷酸盐的吸附,但是根据磷酸盐形态分布曲线(图4b),当pH大于等电位点时HPO42−浓度开始增加,同时与两个−OH作用可促进材料对磷酸盐的吸附(式8).
当pH≥6.2磷酸盐吸附量再次达到最大,此时随着pH值继续增大,溶液中—OH浓度越来越高,与磷酸盐形成明显的竞争吸附,因此吸附量呈明显下降趋势.
-
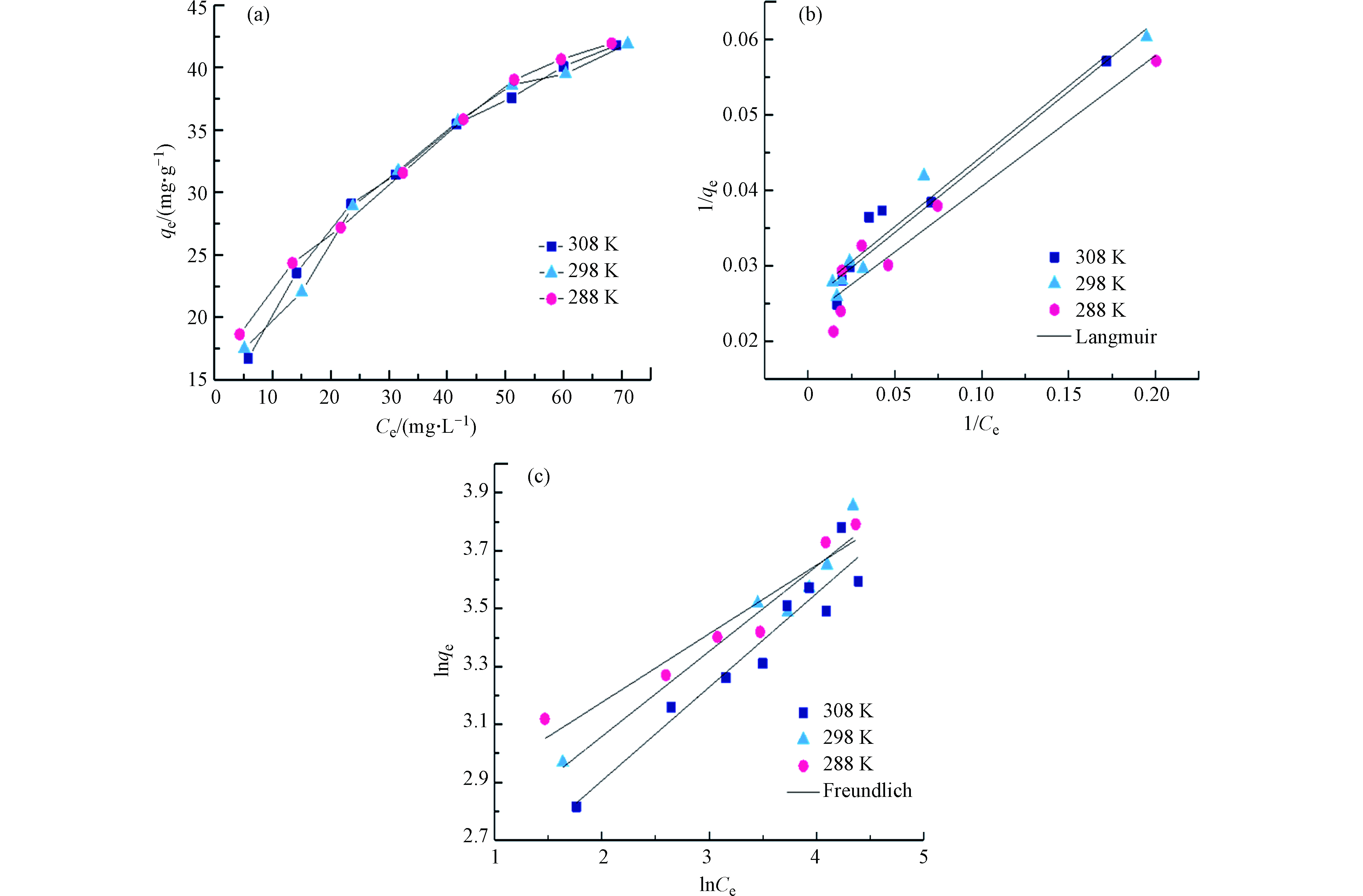
通过吸附等温实验来评价UiO-66对磷酸盐的吸附容量. 在不同温度条件下(288 、298 、308 K),UiO-66吸附磷酸盐变化趋势如图5a所示. 不同温度梯度的吸附结果表明温度对磷酸盐吸附效果的影响并不明显,这是因为材料对磷酸盐的吸附可能依靠羟基的配体交换作用,属于化学吸附,由于化学键力作用较强,所受温度影响不大. 采用Langmuir和Freundlich模型对UiO-66吸附等温线进行线性拟合,如图5b和5c,相关拟合参数见表1. 3个不同温度下,经Langmuir模型拟合的饱和吸附量分别为43.066、39.777、38.595 mg·g–1,与实验结果较为接近,再次证实温度对UiO-66吸附过程影响较小. 同时,与Freundlich模型相比,Langmuir模型拟合的相关系数R2更高,所以Langmuir模型更适合描述UiO-66对磷酸盐的吸附过程,再次表明该吸附为化学吸附过程,材料与磷酸盐之间具有较强的作用力,表明该材料可能具有良好的应用前景. 此外,将本研究所得吸附结果与相关文献对比表明(表2),与其他常规吸附剂相比,UiO-66对磷酸盐吸附量具有明显优势[27-34].
-
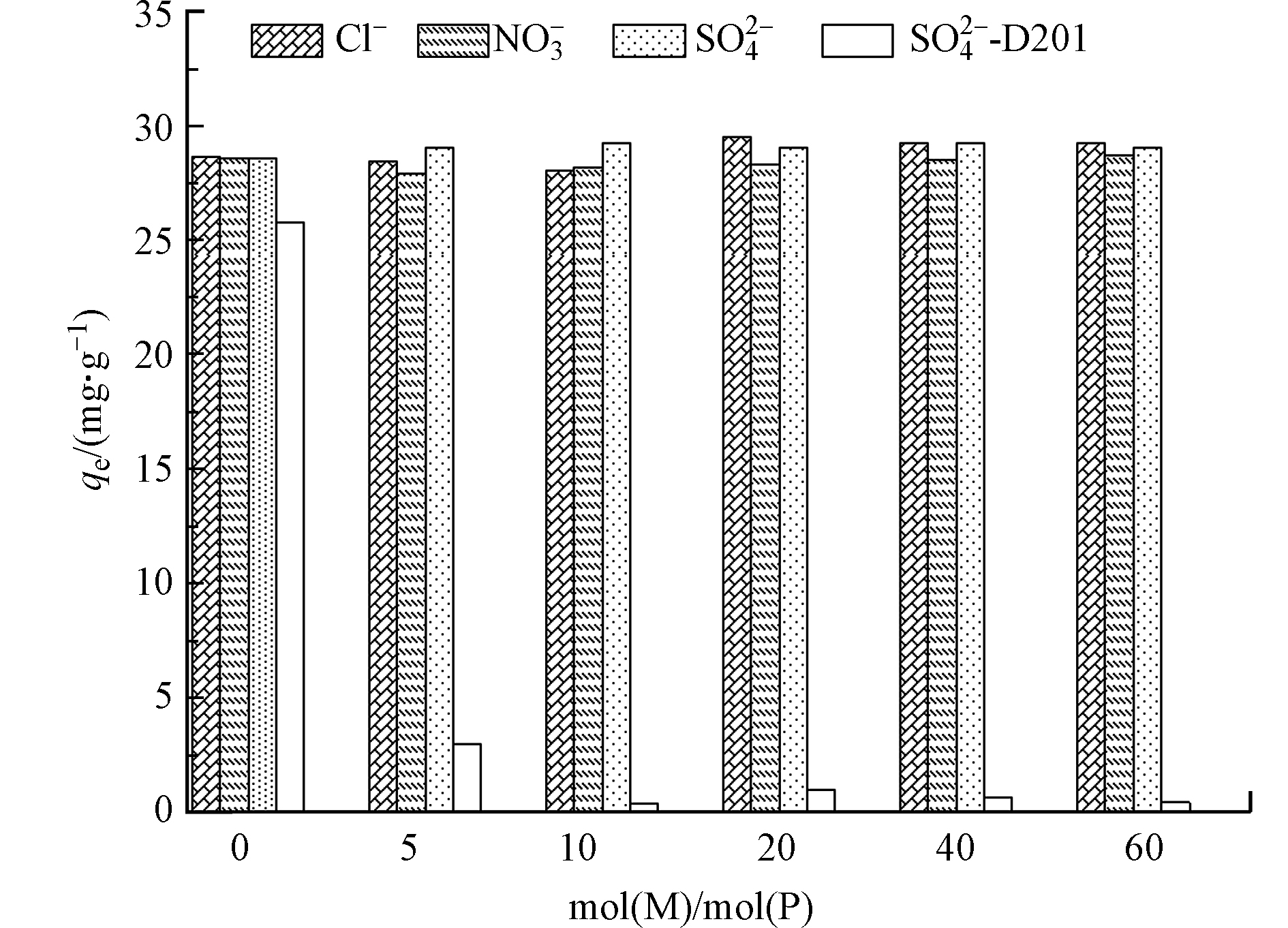
磷污染水体中普遍存在大量竞争离子,会显著降低材料的实际应用效果.为了进一步探究UiO-66对磷酸盐的选择吸附性能,本实验考察了水中常见的Cl–、SO42–和NO3–对UiO-66的吸附影响行为,并选用常见的商用阴离子吸附剂D201作为对比,如图6所示. 在相同条件下,D201对磷酸盐的吸附量较UiO-66略低,而加入竞争离子之后吸附量呈现大幅下降,最终接近于零. 对于UiO-66,体系中背景离子浓度高达磷酸盐浓度60倍时,其存在对UiO-66的吸附量仍然没有明显影响,说明共存离子对UiO-66和磷酸盐结合作用力的影响微乎其微,证实了UiO-66对磷酸盐具备良好的吸附亲和力和吸附选择性,明显优于现有商用阴离子吸附剂. 一般来说,化学配位吸附被分为外层配位(弱结合)和内层配位(强结合)[35],本吸附过程中,磷酸盐在UiO-66表面形成了内层配位络合物,具有较强的吸附作用力,因此在高浓度背景离子体系中仍能表现出优异的吸附选择性.
-
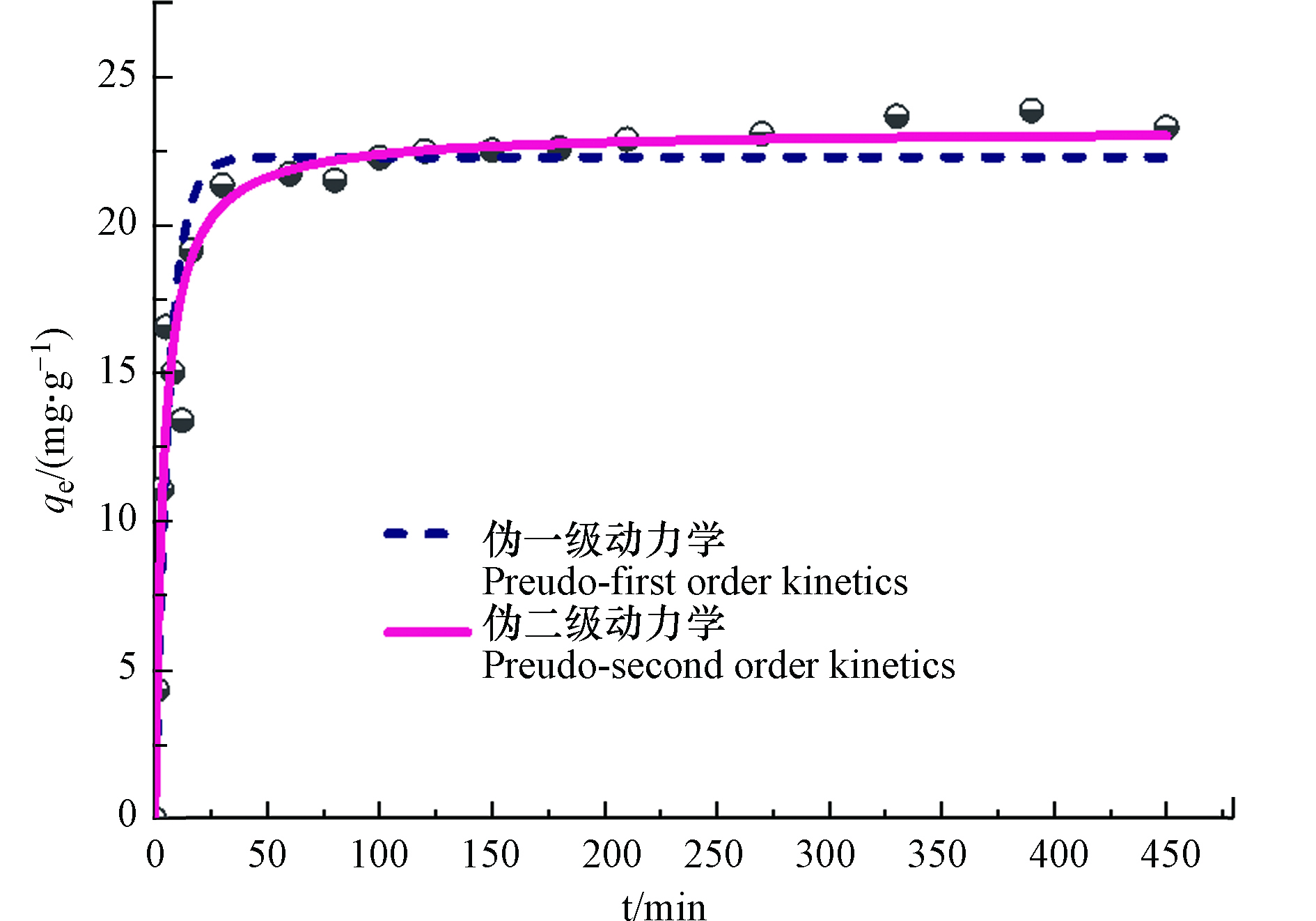
UiO-66吸附速率随时间变化的趋势如图7所示. 可以看出,在初始10 min内,磷酸盐吸附量迅速增加,10—50 min之内吸附速率变缓,约50 min左右即可达到吸附平衡. 这可能是由于反应初始阶段吸附剂表面含有丰富的吸附位点,随着吸附量的增加,可利用的吸附位点减少,吸附速率缓慢下降直至吸附平衡. 采用经典的伪一级动力学(式5)和伪二级动力学模型(式6)对实验数据进行拟合,拟合参数见表3. 结果表明,与伪一级动力学相比,伪二级动力学拟合相关系数R2更大,更能好的拟合该吸附过程. 由于伪二级动力学模型一般用于描述化学吸附过程[36],再次证明UiO-66与磷酸盐之间的化学吸附作用. UiO-66能够快速达到吸附平衡,这主要是由于其吸附过程主要在粉末颗粒表面,受到的位阻作用更小,吸附速率较常规吸附剂具有明显优势.
-
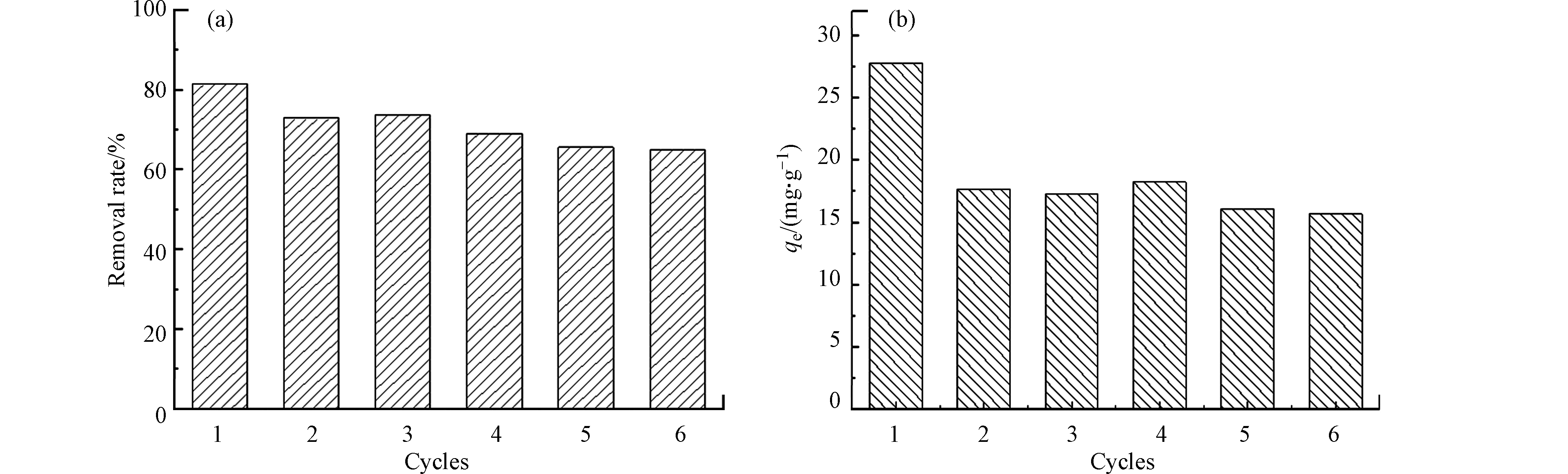
循环吸附和循环吸附-再生性能是评价吸附剂实际应用价值的重要参数. 对UiO-66进行了6次循环吸附和循环吸附-再生实验,如图8所示.为了评估材料对磷酸盐的深度处理能力,在循环吸附实验中将磷酸盐初始浓度设置为2.0 mg·L–1,以匹配富营养化水体或二次出水中磷酸盐的实际浓度. 循环吸附实验结果如图8a所示,前3次循环的磷酸盐吸附率别为82%、73%、73.6%,当循环吸附进行到第6次时,去除率仍保持在60%以上,说明UiO-66可用于反复多次去除低浓度磷酸盐溶液,对于深度处理实际含磷废水具有潜在的应用价值.
吸附-脱附再生实验中常用的脱附溶液为碱性溶液[12],但由于UiO-66在强碱性条件下结构可能会被破坏,且对磷酸盐吸附作用力较强,使其脱附过程较为困难,现有脱附研究较少,如Wang等[21]在探究UiO-66对水中砷酸根的去除性能时并未考察其脱附性能. 所以本研究尝试利用有机溶剂DMF进行超声脱附,对UiO-66的循环再生进行初步探究,通过比较再生前后的吸附量来评价材料再生性能. 如图8b所示,第一次吸附量为27.8 mg·L–1,经再生后第二次吸附量为第一次的63%,虽然吸附量发生了明显下降,但当循环再生进行到第6次时,UiO-66对磷酸盐的吸附量仍保持在第1次吸附的50%以上,吸附性能基本保持稳定. 对循环吸附6次的UiO-66进行XRD表征,峰型没有发生明显变化,说明UiO-66在多次吸附-脱附之后晶体结构保留,因此具有较好的再生性能.
-
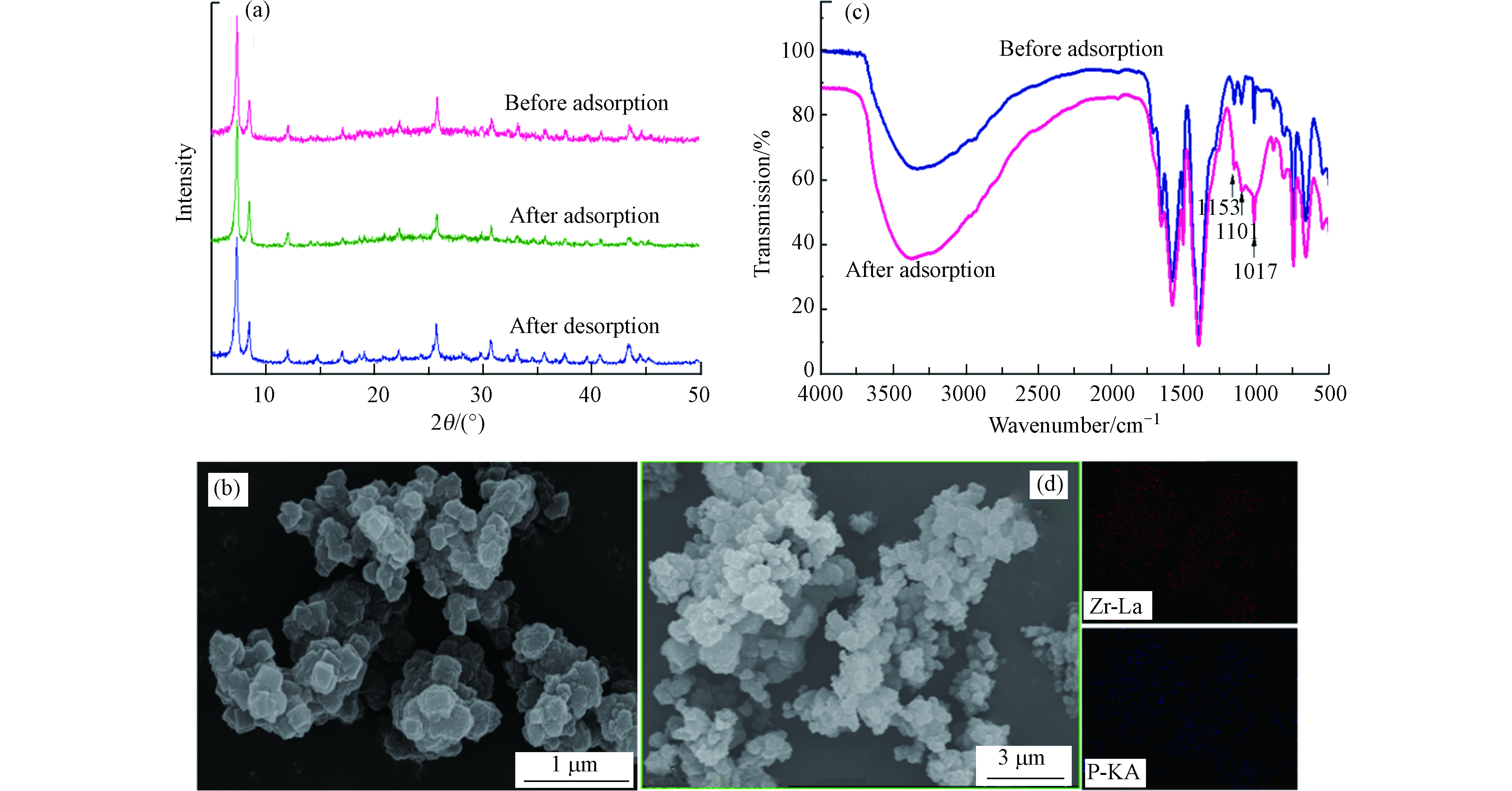
为进一步解析UiO-66对磷酸盐的吸附机理,对吸/脱附前后的UiO-66进行了XRD、SEM及FT-IR表征. 如图9a所示,吸/脱附前后XRD衍射峰的形状、位置和数目均未发生明显变化,说明吸附后UiO-66晶体结构保持稳定. 采用DMF脱附后UiO-66的SEM(图9b)结果显示其形貌结构与原UiO-66基本一致,说明DMF脱附过程不会破坏UiO-66原有晶型结构. 红外FT-IR结果如图9c,在1153 cm–1处出现的新特征峰为P=O键,在1101 cm–1和1017 cm–1出现的峰分别为P—O的对称和非对称振动特征峰,表明磷酸盐已被吸附在UiO-66表面[37-38]. 图9d为UiO-66吸附磷酸盐后的SEM-mapping表征,结果表明吸附的P分布与Zr(Ⅳ)元素分布可以很好的重叠,说明UiO-66内部Zr6O4(OH)4金属簇表面羟基为磷酸盐吸附的主要活性位点[39],与上述分析结果一致.
-
采用溶剂热法合成了一种水热稳定性良好的金属有机框架UiO-66,并系统考察了其对水中磷酸盐的吸附行为与机理. 通过SEM、XRD、FT-IR、TGA、氮气吸附-脱附等方法证实合成的UiO-66具有良好的晶型结构和极大的比表面积,稳定性良好,有利于其在水处理中应用. 静态吸附研究表明,UiO-66吸附磷酸盐受pH影响明显,最佳pH范围为5.5-7.0之间;温度对磷酸盐吸附过程几乎没有影响, Langmuir模型拟合的饱和吸附量可达43.1 mg·g–1;在强竞争体系下,UiO-66的吸附量保持不变,说明UiO-66对磷酸盐具有优异的吸附选择性;吸附过程可在50 min达到吸附平衡,动力学数据符合伪二级动力学模型. 经过6次循环吸附-脱附之后,UiO-66吸附量仍保持在50%以上,说明其具有较好的再生性能. 吸附机理分析表明,UiO-66对磷酸盐的强选择性吸附主要基于磷酸盐与Zr6O4(OH)4金属簇表面羟基的配体交换作用实现. 与商用材料D201相比,合成材料UiO-66对磷酸盐具有高度的吸附亲和力和优异的吸附选择性,并在含磷水体治理方面具备潜在的应用价值.
一种水热稳定的金属有机骨架UiO-66高效捕获水中磷酸盐的性能及机理
High-affinity phosphate sequestration by a hydrothermal-stable metal-organic framework UiO-66 from water
-
摘要: 本研究采用溶剂热法制备了一种水热性能稳定的金属有机框架UiO-66,并系统考察了其对水中磷酸盐的高效捕获性能. 通过表征手段证实,合成的UiO-66具有良好的晶型结构和极大的比表面积,且稳定性良好. UiO-66吸附磷酸盐的过程受pH影响明显,最佳吸附pH值介于5.5—7.0. Langmuir吸附等温模型对吸附过程拟合更好,饱和吸附量可达43.066 mg∙g−1,且由于吸附过程为化学吸附,主要依靠羟基的配体交换作用,因此温度影响不大. 与商用阴离子吸附剂D201相比,高浓度背景离子的加入对UiO-66吸附过程无明显影响,表明UiO-66对磷酸盐具有优异的吸附选择性和高效捕获性能. 伪二级动力学模型可以很好地描述UiO-66对磷酸盐的吸附过程. 经过6次循环吸附-脱附,UiO-66仍能有效去除水中磷酸盐,并可通过DMF溶液超声解吸实现有效再生. 结果表明,UiO-66作为一种新兴的吸附剂有望实现磷酸盐在水中的深度高效去除.Abstract: A hydrothermal stable metal-organic framework UiO-66 is prepared and applied as adsorbents to capture phosphate from water. As indicated by SEM, XRD and BET analysis, the synthesized UiO-66 has an extremely large specific surface area, good crystal structure as well as good stability. Phosphate adsorption on UiO-66 is significantly affected by pH values, the optimal pH is between 5.5 and 7.0. Langmuir adsorption isotherm model fits the adsorption process better, and saturated adsorption capacity is calculated to be 43.066 mg∙g−1. The temperature has little effect on phosphate adsorption due to the chemical adsorption between UiO-66 and phosphate which mainly relies on ligand exchange of the hydroxyl groups. Compared with commercial anionic adsorbent D201, the addition of high-concentration background ions has no significant effect on adsorption process, indicating that UiO-66 has excellent adsorption selectivity towards phosphate. The pseudo second-order kinetic model can give a good description of phosphate adsorption on UiO-66. After six cycles of adsorption-regeneration, UiO-66 can still effectively remove phosphate from water, and can be regenerated by ultrasonic desorption of DMF solution. The results demonstrated that UiO-66 is expected to be an emerging adsorbent for phosphate removal from water.
-
Key words:
- eutrophication /
- MOF /
- phosphate /
- adsorption
-

-
图 9 (a) UiO-66吸/脱附前后XRD表征;(b) UiO-66脱附后SEM表征;(c) UiO-66吸附前后FT-IR图;(d) UiO-66吸附磷酸盐后SEM-mapping图
Figure 9. (a) XRD characterization of UiO-66 before and after adsorption/desorption;(b) SEM characterization of UiO-66 after desorption;(c) FT-IR diagram of UiO-66 before and after adsorption;(d) SEM-mapping diagram of UiO-66 after phosphate adsorption
表 1 UiO-66吸附等温线拟合参数
Table 1. Adsorption isotherm fitting parameters of UiO-66
温度/K
TemperatureLangmuir Freundlich qm/(mg·g−1) KL/(L·g−1) R2 KF/(L·g−1) n R2 288 43.066 0.133 0.921 14.937 0.236 0.906 298 39.777 0.135 0.962 11.845 0.294 0.933 308 38.595 0.139 0.920 9.582 0.338 0.911 表 2 不同吸附剂对磷酸盐的吸附量对比
Table 2. Comparison of phosphate adsorption capacity for different adsorbents
Materials T/ K pH Qmax/(mg·g−1) 参考文献Ref. ZrO2·xH2O 295±2 6.5 <18 [27] ZrO(OH)2·(Na2O)0.05·1.5H2O — 5.0 <30 [28] magnetic Fe-Zr binary oxide — 4.0 13.65 [29] mesoporous ZrO2 293 6.7—6.9 29.7 [30] Fe-Zr binary oxide 298±1 5.5±0.1 33.4 [31] Zirconia-functionalized SBA-15 298 6.2 <18 [32] Zr(Ⅳ)-loaded activated carbon — 2—4 <8 [33] Zr(Ⅳ)-doped mesoporous silica 298 7—10 <4 [34] UiO-66 (Zr-MOFs) 288 6 43.066 本研究 表 3 吸附动力学拟合参数
Table 3. Adsorption kinetics fitting parameters of UiO-66
温度/K
Temperature伪一级动力学Pseudo first order kinetics 伪二级动力学Pseudo second order kinetics qe/(mg·g−1) k1/min−1 R2 qe/(mg·g−1) k2/( g·mg·min–1) R2 298 22.289 0.164 0.914 23.203 0.012 0.96 -
[1] PENN C, BOWEN J M. Design and construction of phosphorus removal structures for improving water quality[M]. The United States: Springer International Publishing, 2017. [2] 何思琪, 周亚义, 林建伟, 等. 氢氧化镧改良沉积物对水中磷的吸附特征 [J]. 环境化学, 2018, 37(11): 2565-2574. doi: 10.7524/j.issn.0254-6108.2018011104 HE S Q, ZHOU Y Y, LIN J W, et al. Adsorption characteristics of phosphate in water on lanthanum hydroxide-amended sediments [J]. Environmental Chemistry, 2018, 37(11): 2565-2574(in Chinese). doi: 10.7524/j.issn.0254-6108.2018011104
[3] ZAMPARAS M, GIANNI A, STATHI P, et al. Removal of phosphate from natural waters using innovative modified bentonites[J]. Applied Clay Science, 2012, 62–63: 101–106. [4] SCHINDLER D W, CARPENTER S R, CHAPRA S C, et al. Reducing phosphorus to curb lake eutrophication is a success [J]. Environmental Science & Technology, 2016, 50(17): 8923-8929. [5] 隋克俭, 李家驹, 李鹏峰, 等. 溶气气浮工艺用于城镇污水处理厂二级出水的深度除磷研究 [J]. 环境工程, 2020, 38(7): 66-70,65. SUI K J, LI J J, LI P F, et al. Study on deep dephosphorization of effluent from urban sewage treatment plant by dissolved air floatation process [J]. Environmental Engineering, 2020, 38(7): 66-70,65(in Chinese).
[6] PAHUNANG R R, BALLESTEROS F C, DE LUNA M D G, et al. Optimum recovery of phosphate from simulated wastewater by unseeded fluidized-bed crystallization process [J]. Separation and Purification Technology, 2019, 212: 783-790. doi: 10.1016/j.seppur.2018.11.087 [7] ZENG W, ZHANG L M, FAN P C, et al. Community structures and population dynamics of “Candidatus Accumulibacter” in activated sludges of wastewater treatment plants using ppk1 as phylogenetic marker [J]. Journal of Environmental Sciences (China), 2018, 67: 237-248. doi: 10.1016/j.jes.2017.09.001 [8] WAN J, WU B, LO I M C. Development of Fe0/Fe3O4 composites with tunable properties facilitated by Fe2+ for phosphate removal from river water [J]. Chemical Engineering Journal, 2020, 388(January): 124242. [9] CHEN L, LI Y, SUN Y, et al. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability [J]. Chemical Engineering Journal, 2019, 360: 342-348. doi: 10.1016/j.cej.2018.11.234 [10] ZHOU K, WU B, SU L, et al. Enhanced phosphate removal using nanostructured hydrated ferric-zirconium binary oxide confined in a polymeric anion exchanger[J]. Chemical Engineering Journal, 2018, 345(December 2017): 640–647. [11] 李聪, 钟溢健, 解庆林, 等. 不同吸附材料处理水中砷的效应分析 [J]. 现代化工, 2018, 38(7): 21-25. LI C, ZHONG Y J, XIE Q L, et al. Effect analysis on arsenic removal from water by different adsorption materials [J]. Modern Chemical Industry, 2018, 38(7): 21-25(in Chinese).
[12] QIU H, NI W, ZHANG H, et al. Fabrication and evaluation of a regenerable HFO-doped agricultural waste for enhanced adsorption affinity towards phosphate [J]. Science of the Total Environment, 2020, 703(PTa2): 135493.1-135493.11. [13] ZHANG Q R, BOLISETTY S, CAO Y P, et al. Selective and efficient removal of fluoride from water: In situ engineered amyloid fibril/ZrO2 hybrid membranes [J]. Angewandte Chemie (International Ed. in English), 2019, 58(18): 6012-6016. doi: 10.1002/anie.201901596 [14] 陈丹丹, 衣晓虹, 王崇臣. 机械化学法制备金属-有机骨架及其复合物研究进展 [J]. 无机化学学报, 2020, 36(10): 1805-1821. doi: 10.11862/CJIC.2020.212 CHEN D D, YI X H, WANG C C. Preparation of metal-organic frameworks and their composites using mechanochemical methods [J]. Chinese Journal of Inorganic Chemistry, 2020, 36(10): 1805-1821(in Chinese). doi: 10.11862/CJIC.2020.212
[15] CHENG K, SVEC F, LV Y, et al. Hierarchical micro- and mesoporous Zn-based metal–organic frameworks templated by hydrogels: Their use for enzyme immobilization and catalysis of knoevenagel reaction [J]. Small, 2019, 15(44): 1-10. [16] XU H Q, WANG K C, DING M L, et al. Seed-mediated synthesis of metal-organic frameworks [J]. Journal of the American Chemical Society, 2016, 138(16): 5316-5320. doi: 10.1021/jacs.6b01414 [17] GHALEI B, WAKIMOTO K, WU C Y, et al. Rational tuning of zirconium metal–organic framework membranes for hydrogen purification [J]. Angewandte Chemie International Edition, 2019, 58(52): 19034-19040. doi: 10.1002/anie.201911359 [18] HUANG K, WANG B, GUO S, et al. Micropatterned ultrathin MOF membranes with enhanced molecular sieving property [J]. Angewandte Chemie International Edition, 2018, 57(42): 13892-13896. doi: 10.1002/anie.201809872 [19] BAI Y, DOU Y B, XIE L H, et al. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications [J]. Chemical Society Reviews, 2016, 45(8): 2327-2367. doi: 10.1039/C5CS00837A [20] 李昭, 顾博翰, 蒋自展, 等. UiO-66系列材料在CO2吸附存储与分离中的应用研究进展 [J]. 化工新型材料, 2018, 46(11): 216-221. LI Z, GU B H, JIANG Z Z, et al. Research progress of UiO-66 series materials in CO2 adsorption storage and separation [J]. New Chemical Materials, 2018, 46(11): 216-221(in Chinese).
[21] WANG C, LIU X, CHEN J P, et al. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66 [J]. Scientific Reports, 2015, 5: 1-10. doi: 10.9734/JSRR/2015/14076 [22] 韩易潼, 刘民, 李克艳, 等. 高稳定性金属有机骨架UiO-66的合成与应用 [J]. 应用化学, 2016, 33(4): 367-378. doi: 10.11944/j.issn.1000-0518.2016.04.150439 HAN Y T, LIU M, LI K Y, et al. Preparation and application of high stability metal-organic framework UiO-66 [J]. Chinese Journal of Applied Chemistry, 2016, 33(4): 367-378(in Chinese). doi: 10.11944/j.issn.1000-0518.2016.04.150439
[23] FANG S Y, ZHANG P, GONG J L, et al. Construction of highly water-stable metal-organic framework UiO-66 thin-film composite membrane for dyes and antibiotics separation[J]. Chemical Engineering Journal, 2020, 385(August 2019): 123400. [24] CAVKA J H, JAKOBSEN S, OLSBYE U, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability [J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. doi: 10.1021/ja8057953 [25] CHAVAN S M, SHEARER G C, SVELLE S, et al. Synthesis and characterization of amine-functionalized mixed-ligand metal-organic frameworks of UiO-66 topology [J]. Inorganic Chemistry, 2014, 53(18): 9509-9515. doi: 10.1021/ic500607a [26] SAFARI G H, ZARRABI M, HOSEINI M, et al. Trends of natural and acid-engineered pumice onto phosphorus ions in aquatic environment: Adsorbent preparation, characterization, and kinetic and equilibrium modeling [J]. Desalination and Water Treatment, 2015, 54(11): 3031-3043. doi: 10.1080/19443994.2014.915385 [27] CHUBAR N I, KANIBOLOTSKYY V A, STRELKO V V, et al. Adsorption of phosphate ions on novel inorganic ion exchangers [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2005, 255(1/2/3): 55-63. [28] CHITRAKAR R, TEZUKA S, SONODA A, et al. Selective adsorption of phosphate from seawater and wastewater by amorphous zirconium hydroxide [J]. Journal of Colloid and Interface Science, 2006, 297(2): 426-433. doi: 10.1016/j.jcis.2005.11.011 [29] LONG F, GONG J L, ZENG G M, et al. Removal of phosphate from aqueous solution by magnetic Fe-Zr binary oxide [J]. Chemical Engineering Journal, 2011, 171(2): 448-455. doi: 10.1016/j.cej.2011.03.102 [30] LIU H L, SUN X F, YIN C Q, et al. Removal of phosphate by mesoporous ZrO2 [J]. Journal of Hazardous Materials, 2008, 151(2/3): 616-622. [31] REN Z M, SHAO L N, ZHANG G S. Adsorption of phosphate from aqueous solution using an iron-zirconium binary oxide sorbent [J]. Water, Air, & Soil Pollution, 2012, 223(7): 4221-4231. [32] TANG Y Q, ZONG E M, WAN H Q, et al. Zirconia functionalized SBA-15 as effective adsorbent for phosphate removal [J]. Microporous and Mesoporous Materials, 2012, 155: 192-200. doi: 10.1016/j.micromeso.2012.01.020 [33] HASHITANI H, OKUMURA M, FUJINAGA K. Preconcentration method for phosphate in water using activated carbon loaded with zirconium [J]. Fresenius' Zeitschrift Für Analytische Chemie, 1987, 326(6): 540-542. [34] DELANEY P, MCMANAMON C, HANRAHAN J P, et al. Development of chemically engineered porous metal oxides for phosphate removal [J]. Journal of Hazardous Materials, 2011, 185(1): 382-391. doi: 10.1016/j.jhazmat.2010.08.128 [35] LI J, ZHANG S W, CHEN C L, et al. Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles [J]. ACS Applied Materials & Interfaces, 2012, 4(9): 4991-5000. [36] 王凯, 邱广明, 魏利强, 等. 氨基功能化P(St-HEMA)磁性微球的制备及对Pb(Ⅱ)的吸附性能 [J]. 功能材料, 2020, 51(3): 3176-3181,3188. WANG K, QIU G M, WEI L Q, et al. Amino functional P (St-HEMA) the preparation of magnetic microspheres and adsorption properties of Pb(Ⅱ) [J]. Functional Materials, 2020, 51(3): 3176-3181,3188(in Chinese).
[37] MA Y, ZHENG Y M, CHEN J P. A zirconium based nanoparticle for significantly enhanced adsorption of arsenate: Synthesis, characterization and performance [J]. Journal of Colloid and Interface Science, 2011, 354(2): 785-792. doi: 10.1016/j.jcis.2010.10.041 [38] ZHU X Y, LI B, YANG J, et al. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67 [J]. ACS Applied Materials & Interfaces, 2015, 7(1): 223-231. [39] ZHAO X, LIU D, HUANG H, et al. The stability and defluoridation performance of MOFs in fluoride solutions [J]. Microporous and Mesoporous Materials, 2014, 185: 72-78. doi: 10.1016/j.micromeso.2013.11.002 -



 下载:
下载: