-
汞是一种全球性的环境污染物[1]。甲基汞作为最重要的有机汞形态,具有极强的神经毒性,可在生物体内累积并在食物链中放大,从而造成生态与健康风险。零价汞的大气半衰期为0.5—1 a,可随大气环流在全球范围内进行长距离传输。因此,在无明显汞排放源的偏远地区(如南北极、青藏高原),也观测到了一定程度的汞污染。经由人为源(如化石燃料燃烧、金属冶炼、垃圾焚烧等)排放到大气中的汞约为2000—3000 t·a−1[2]。而自然源(如火山喷发、森林大火、土壤与森林释放、海洋释放等)导致的一次与二次汞排放(沉降后的再排放)可达5500 t·a−1,约为人为源汞通量的2倍[2]。自《水俣公约》的实施,汞的使用和人为排放得到了有效的控制,大气汞浓度逐步降低。但汞自然源的一次与二次排放使得全球汞污染问题仍将长期存在。此外,在后水俣时代,除了受汞排放减少的控制外,生物体内汞累积将受到环境介质中汞分子转化等过程的显著影响[2]。因此,汞在环境多介质中的形态转化过程对汞的全球分布与生态健康效应具有深远影响。
汞还原是汞环境分子转化的重要过程,控制着汞的局域/长距离传输与甲基化。零价汞水溶性低,大气传输半衰期较长,汞的还原可促进汞从其它环境介质向大气气相的迁移,从而增强汞的区域与全球传输。另一方面,汞的还原与挥发可降低汞在土壤、水体、底泥和冰雪等介质中汞的含量,进而降低汞微生物甲基化的底物浓度与甲基汞的生成。此外,由于零价汞的甲基化率低于二价汞,汞的还原也可能降低微生物汞甲基化的效率[3-4]。
汞的还原反应在大气、地表水、底泥、土壤、冰雪等多种环境介质中均可发生,主要分为化学还原与微生物还原。本文介绍了汞的化学/微生物还原在汞生物地球化学循环中的重要作用;聚焦于微生物还原,详细总结了有氧与缺氧条件下汞微生物还原的途径与机制,提出了微生物还原汞其它可能的新机制,对相关领域的研究进行了展望。
-
氧化态汞在多种环境介质(如大气、土壤、地表水、底泥、冰雪)中普遍存在。相应地,在这些介质中均广泛存在汞的还原过程。
-
大气气相中活性气态汞(RGM)也被称为气态氧化汞(GOM),其可被大气还原性物质(如一氧化碳[5]、二氧化硫[6]等)还原。光照可显著促进气态氯化汞、氢氧化汞等RGM形态的还原[7]。近期一项研究计算了典型RGM化合物的光吸收截面,表明RGM可快速光解并发生汞的还原[8]。这一光还原是大气汞的主要还原过程之一,极大改变了大气中的汞动力学,模型计算结果表明该过程可导致大气汞寿命增加两倍[8]。大气水相中的氢氧化汞也可直接发生光解还原[7]。此外,水相中亚硫酸根[9]、低分子量有机酸[10]、铁矿物[11]等均可导致或促进均相与非均相体系中二价汞的暗还原或光还原。由于大气环境中微生物的丰度与活性较低,因此相比于大气化学还原过程,大气环境中汞的微生物还原并不重要。
-
地表水中汞的还原主要受日光照射、溶解性有机质(DOM)浓度与组成、微生物活性等因素的影响。对于表层水,溶解性气态汞(DGM)的日间变化表明,光照是控制汞还原的重要因素;而对于深层水体,由于光照的衰减,DOM参与的暗化学还原[12]或微生物还原[13]可能是汞还原的主要途径[14]。对北太平洋涡流和白令海的深层无光水体,DGM的生成与有机质的矿化有关,而有机质矿化主要来自于微生物的作用,暗示DGM的生成与微生物有关[15]。美国佛罗里达州淡水与近岸海水的微宇宙实验显示,水体中大于1 μm微生物的去除对汞还原影响较小,而0.3 μm滤膜过滤可显著抑制水样中汞的还原,提示这一还原过程主要与细菌有关[16]。对美国威斯康星州帕莱特湖(Pallette Lake)湖水进行加热灭菌后,零价汞的生成受到显著抑制,证明生物在汞还原中发挥重要作用[17]。类似地,另一项淡水与海水过滤实验显示,< 3 μm的微生物是天然水体中汞还原的主要贡献者;真核浮游植物可介导汞的生物还原,但其还原速率不足以解释所观察到的还原率,表明细菌在汞还原中起主导作用[18]。据估计,微生物介导的汞还原可占表层与深层北极海水中DGM来源的68%—90%,其重要性高于光还原[19]。
-
底泥中氧含量低,有机质含量高,厌氧细菌种类丰富。由于黑暗条件下不发生光还原,细菌介导的汞还原可能占据主要作用。零价汞是底泥中汞的重要赋存形态之一。程序升温热解析研究显示,加拿大两个湖泊中零价汞可达总汞的7.4%—28.4%[20]。放射性汞同位素与底泥的共培养研究显示,底泥中的二价汞可被还原为零价汞[21],但其还原途径并不明确。加拿大哈得孙湾(Hundson Bay)附近淡水湖泊底泥培养实验显示,甲醛处理显著抑制零价汞的生成,表明这一还原涉及微生物过程,而微生物丰度指示物谷氨酰胺合成酶基因的拷贝数与零价汞生成量的正相关关系进一步显示微生物在还原中发挥关键作用[22]。
-
土壤中汞的化学与微生物还原过程可能同时存在,其相对重要性依赖于研究位点的土壤组成与微生物活性。对于美国阿迪朗达克山脉(Adirondacks)落叶与针叶林土壤,日光中UV-B组分显著促进了汞的还原与释放,而辐照灭菌对汞的还原影响较小,表明在此地点汞的还原以化学过程为主,微生物过程的贡献较小[23]。对于加拿大新斯科舍(Nova Scotia)森林土壤,辐照灭菌显著降低了汞还原的比例,研究认为土壤中零价汞的还原包含两个关键过程:(1)快速的化学过程,其生成少部分零价汞;(2)较为缓慢的速率限制的微生物过程,其生成大部分零价汞[24]。对于采自美国田纳西州东叉白杨溪(East Fork Poplar Creek)的滨河土壤,黑暗淹水条件下汞的还原与土壤中微生物的Fe/Mn异化还原相关,提示微生物在土壤汞还原中可能起着重要作用[25]。瑞士瓦莱州工业污染土壤中检测到汞抗性基因(merA),且随着土壤汞浓度增加,微生物群落中merA丰度增加,表明汞的微生物还原可能是土壤中挥发性零价汞形成的重要途径[26]。我国哀牢山常绿阔叶林土壤汞同位素分析表明,微生物还原与有机质介导的暗化学还原可导致新鲜凋落物层和表层土壤中汞同位素组成的独特变化;此外,土壤微生物汞还原可能与土壤中氮的释放和固定有关[27]。
-
光照是引发冰雪表层二价汞还原的主要因素,其可能涉及雪中的有机质、氯离子以及铁等[28]。进一步研究发现,雪中汞的光还原主要是由日光组分中的UV-B引起,而非可见光和UV-A部分[29]。除了光照,微生物也可能在冰雪介质的汞还原中起一定的作用。研究表明,北极积雪中可培养细菌的31%具有抗汞能力,约四分之一的抗汞菌可将二价汞还原为零价汞[30]。据估计,在光还原受抑制的较深雪层中,微生物对汞的生物还原可能很重要[30]。
-
人们对有氧环境中好氧微生物的汞还原过程认识较早。在有氧的环境下,好氧微生物对汞的还原与挥发可显著减少微生物的汞暴露,维持微生物的正常生长,达到脱毒的目的[31]。目前已报道的好氧汞还原细菌主要为耐汞原核生物,包括好氧异养细菌(变形菌和某些革兰氏阳性菌)和古菌。这些耐汞原核生物的物种多样性高,广泛分布于汞含量较高的生态系统。
-
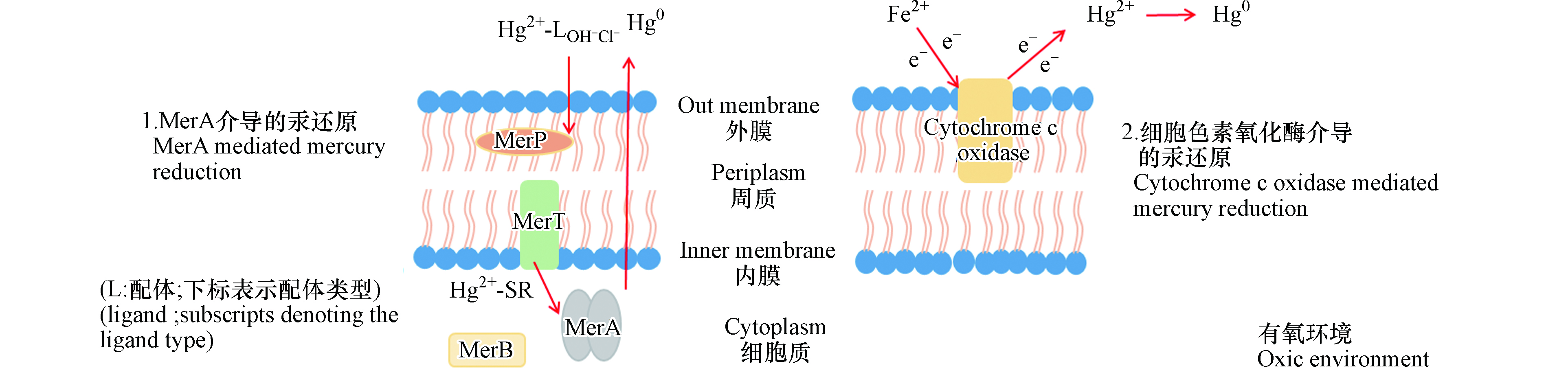
在汞的微生物还原中,mer操纵子介导的还原是最普遍的一种形式。来自不同环境的异养好氧微生物中约有1—10%具有mer操纵子[32]。mer操纵子介导的汞还原机制已经较为明确:在高浓度汞离子的诱导下,汞与Mer系列蛋白发生一系列的结合、转运和催化反应,使得汞离子还原为易挥发的零价汞排出胞外,起到脱毒的作用(如图1中MerA介导的汞还原)。
在整个mer操纵子还原过程中,MerR控制大多数Mer系列蛋白的启动。在某些微生物中,Mer系列蛋白也同时受MerD的调节。merP、merT、merC、merF、merE、merG基因控制转运蛋白的合成,合成的转运蛋白协同完成汞的细胞内外转运。其中MerP位于细胞周质中,MerT位于细胞内部,二者共同作用使汞离子从细胞周质转运至细胞内。对大多数细菌,MerT在汞离子胞内转运中起着重要作用。在mer操纵子介导的汞还原中,MerA是关键的汞还原酶。MerA以蛋白二聚体的形式发挥功能,二聚体的结合末端存在半胱氨酸残基,与进入细胞的汞离子结合,转运至MerA的活性中心;在这里NADPH作为还原剂提供电子,将FDA还原为FDAH-;进而FDAH-提供电子,将与半胱氨酸残基结合的汞离子还原为零价汞[33-34]。
Hg2++2RS-→Hg(SR)2 (其中RS-代表MerA中的半胱氨酸残基)
NADPH+FAD→NADP++FDAH-
Hg(SR)2+NADPH+H+→Hg0+NADP++2RSH
有些细菌的mer操纵子还含有merB。含有merB的微生物对于汞的抗性被称为广谱抗性。MerB可使有机汞的C—Hg键断裂生成汞离子,再通过MerA使汞离子还原为零价汞[35]。而仅有merA的微生物对汞的抗性被称为窄谱抗性,其仅能还原无机汞[36]。
-
在有氧条件下,除了在高浓度汞下发生的胞内mer系统介导的汞还原以外,细胞外膜上还存在着另一种形式的汞还原过程。与MerA介导的胞内汞还原不同,这种汞还原发生在细胞外膜上。嗜酸氧化亚铁硫杆菌存在依赖Fe2+的细胞色素C氧化酶的汞离子还原途径,而在汞敏感的菌株中则未观测到这一现象[37]。将纯化后的细胞色素C氧化酶与Fe2+和汞离子反应,约有39%的汞被还原;以灭活的细胞色素C氧化酶为对照组,其不发生汞还原。此外,在一定浓度内随着Fe2+浓度的升高,汞还原效果增强[38]。在此过程中,Fe2+引发细胞色素C氧化酶的还原,还原态的细胞色素C酶再将电子转移给汞离子,最终实现汞离子的还原(如图1中细胞色素氧化酶介导的汞还原)。
进一步研究汞与细胞色素C氧化酶的结合,发现可使汞发生还原的耐汞菌株中细胞色素C氧化酶与汞的结合量是汞敏感型菌株的4倍以上[39]。细胞色素C氧化酶由α、β、γ 的3个亚基组成,汞与α、β亚基的结合是细胞色素C氧化酶还原的第一步,推测这两个亚基起到了活性中心的作用,耐汞菌株与汞敏感型菌株中只有α亚基不同,且α亚基与汞结合的比例最高[39]。该研究结果表明,耐汞菌株的这种Fe2+依赖的细胞色素C氧化酶与汞的大量结合在汞还原中起到了关键作用。但目前对于细胞色素C氧化酶各亚基在汞还原中的具体作用仍待进一步研究。
-
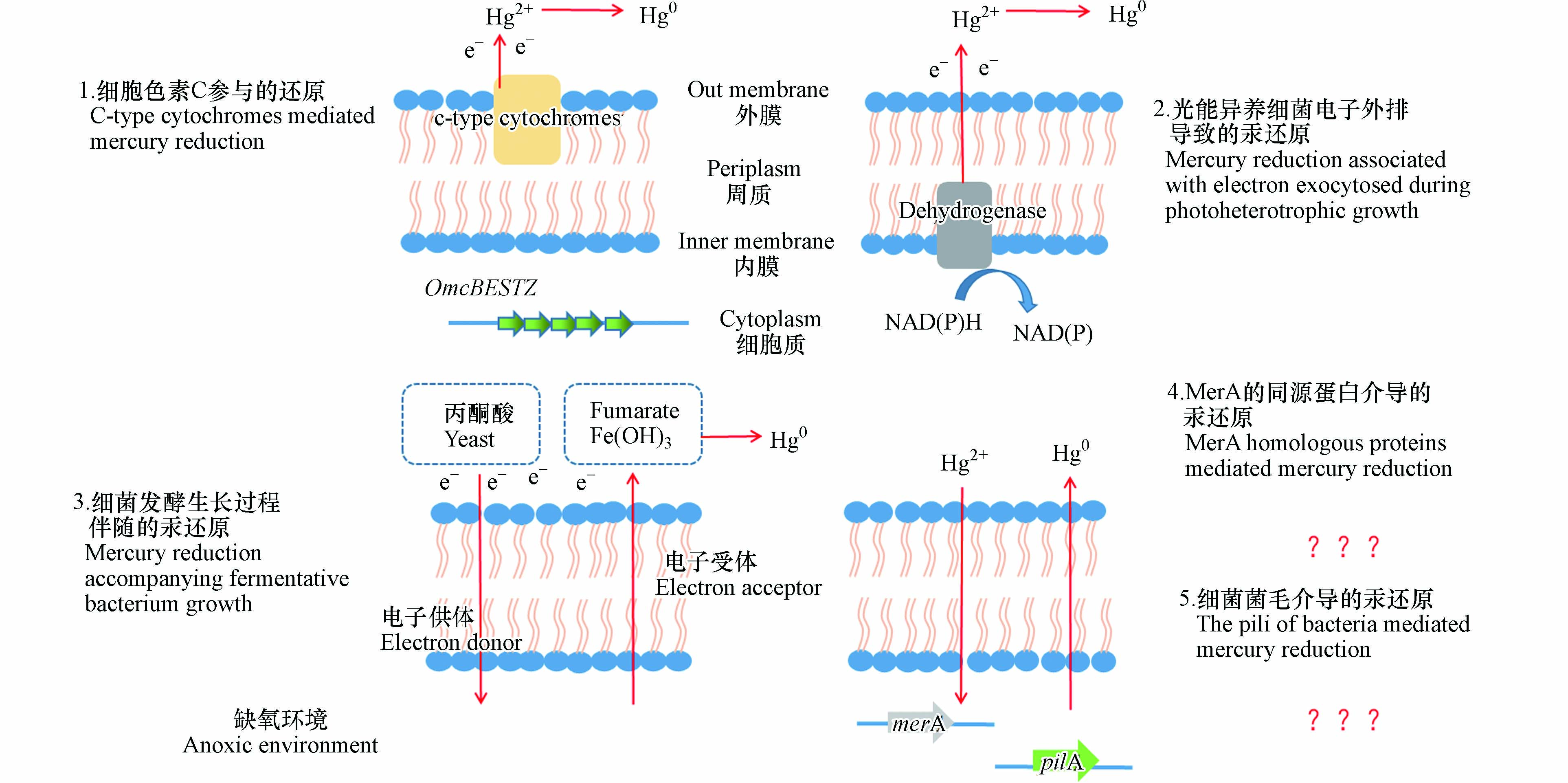
在黑暗缺氧环境中(如底泥、较深水体),零价汞也是汞的重要赋存形态[22]。缺氧环境中微生物可介导汞的还原[22]。目前,对于缺氧环境中微生物对汞的还原研究仍较少,其还原机制的认识也较为欠缺。例如,目前除了细胞色素C参与的汞还原机制阐述较为详细,光能异养细菌电子外排导致的汞还原、细菌发酵生长过程伴随的汞还原等途径的分子机制尚未明确。厌氧微生物对汞的还原涉及胞内电子的外排,一般为胞外还原过程,该还原区别于Mer操纵子诱导的还原,无需高浓度汞离子的激活,但在厌氧细菌中也不排除存在MerA同源蛋白导致汞的胞内还原。
-
在厌氧微生物的汞还原研究中发现,铁还原菌对汞离子的还原与细胞色素C有关[34]。与有氧条件下细胞色素C氧化酶介导的汞还原不同,厌氧细胞色素C对汞的还原过程无需Fe2+提供电子[34] 。据推测,细菌生长过程中电子外排到位于细胞外膜上的细胞色素C上,汞离子与细胞外膜直接接触时,发生还原[40](如图2中细胞色素C参与的汞还原)。铁还原菌Geobacer sulfurreducens PCA可以使汞离子还原为零价汞,且作为对照组的加热灭活菌液无法发生汞还原,表明活细菌在汞还原中起着关键作用[41]。进一步的研究表明,该细菌对汞离子的还原受到细菌数的影响。在其它条件适宜的情况下,零价汞的生成量随细胞数增加而增加:在细胞数达到1011 L-1时,细菌对汞的还原率最高,达到60%,细胞数更高则会对汞还原产生抑制[41]。敲除细胞色素基因OmcBESTZ的菌株对汞的还原率较野生型下降至20%左右,但仍有一定的汞还原能力[41]。该结果表明,铁还原菌对二价汞的还原涉及细胞色素C,但还存在其它的还原途径。
-
光能异养微生物是水生生态系统的关键参与者,也是汞生物转化与积累的重要一环[42]。在厌氧稻田、水体等环境中发现的Heliobacterium modesticaldum Ice1、紫色非硫细菌等多种光能异养微生物,其除了具有汞甲基化的功能外,也可将二价汞还原为零价汞。一般来说,在光能异养微生物的生长过程中,为了维持细菌胞内的氧化还原平衡,需要将多余电子排出胞外,同时以汞离子作为电子受体发生汞的还原[43](见图2中光能异养细菌电子外排导致的汞还原)。
以普遍存在于环境中光营养代谢为主要生长方式的紫色非硫细菌为模型,研究了光能异养细菌对汞的还原过程与机制[44]。在20 μmol photon·m−2·s−1光照(既可以满足细菌生长所需,又不会引发汞的非生物还原)下[44],以丁酸盐为碳源时,紫细菌生长过程中将多余电子外排,以汞离子为电子受体,将其还原为零价汞[44]。由于汞离子作为电子受体促进了胞内多余电子的外排,因此汞离子的存在反而促进了紫细菌的生长。当在系统中加入其它竞争电子受体如碳酸氢盐(20 mmol·L−1)、二甲基亚砜(20 mmol·L−1)时,汞离子的还原则受到抑制[44]。但是当加入100 μmol·L−1的碳酸氢盐时,汞离子的还原速度和还原量略微增加[44],这可能是由于细菌参与汞还原的通路受到碳酸氢盐的刺激而提高了还原效率。
-
在兼性厌氧细菌中还存在一种与细菌发酵生长相关的汞还原途径。与MerA途径不同,这种汞还原过程不需要高浓度汞离子激活。兼性厌氧细菌Shewanella oneidensis MR-1和Heliobacterium modesticaldum Ice1是两种代表细菌。已有研究探究了影响该汞还原途径的因素,但其具体分子机制尚不明确(如图2中细菌发酵生长过程伴随的汞还原)。
在Shewanella oneidensis MR-1中,这种汞还原途径需要电子供体和受体的参与[45]。分别以富马酸盐、氢氧化铁等为电子受体,可以将汞离子还原成零价汞,且以氢氧化铁为电子受体时还原速率更高[45]。以Heliobacterium modesticaldum Ice1为实验菌株,在黑暗厌氧条件下其对汞的还原,需要有机碳的氧化来提供电子,以酵母提取物和丙酮酸为碳源时还原效果最佳。
这种兼性厌氧细菌发酵生长过程中伴随的汞还原过程即使在低浓度汞条件下也很活跃,无需高浓度汞的诱导启动,可能是缺氧环境中汞还原的重要过程。当环境中汞浓度较低,mer操纵子不表达时,该还原过程就起到了主要的作用[46]。同时,需要注意的是,并非所有的异化金属还原菌都具有还原汞的能力[45]。
-
在有氧、高汞浓度环境中,抗汞微生物中的汞还原酶MerA可将汞离子还原为零价汞。在产生高毒性甲基汞的厌氧沉积物中是否存在着MerA同源蛋白介导的汞还原呢?目前这一问题尚无明确答案。已有研究利用合成的MerA蛋白高度保守序列引物,检测汞污染厌氧沉积物中MerA的多样性,结果显示在厌氧环境微生物中有着丰富的MerA多样性[47]。但是,该研究并未进一步证明在厌氧环境下MerA同源蛋白是否会介导汞离子的还原[47]。同时,厌氧汞甲基化细菌也被发现具有MerA同源蛋白[48]。这些研究为进一步理解厌氧细菌MerA同源蛋白介导的汞还原提供了新的思路(见图2中MerA的同源蛋白介导的汞还原)。
-
导电探针原子力显微镜研究发现,G. sulfurreducens PCA菌体四周存在菌毛[49]。这种菌毛具有很高的导电性,相当于一种微生物纳米导线。通过菌毛,微生物可以将表面的电子传递给铁氧化物,使铁氧化物发生还原。对G. sulfurreducens PCA表达菌毛的基因pilA进行敲除[50],发现敲除型无法还原铁氧化物,表明G. sulfurreducens PCA菌毛在铁氧化物还原中发挥重要作用[51]。
可以推测,G. sulfurreducens PCA等厌氧细菌中可能存在另外一种汞还原途径,即细菌通过菌毛将其生长过程中产生的电子传递给胞外汞离子或其它电子穿梭体(如铁矿物);这种直接电子传递或经由电子穿梭体的传递可引发汞的还原(见图2中细菌菌毛介导的汞还原)。目前,这一推测尚未得到进一步的验证。
-
微生物还原在汞的区域与全球循环中起着关键作用。这一还原过程还控制着汞甲基化底物浓度,对甲基汞的生成与生物累积有重要影响。已有研究揭示了微生物还原汞的不同机制。在同一种微生物中,这些还原机制可能协同共存,而非一种非此即彼的关系。目前,针对已知的还原机制,尚有一些方面有待厘清:(1)光能异养细菌电子外排和化能异养细菌的组成型表达中的汞还原分子机制尚不明确;(2)厌氧条件下铁还原菌G. sulfurreducens PCA介导的汞还原中,除了细胞色素C,尚存在的其它途径有待进一步验证。此外,一些可能的未知还原途径(如菌毛介导的电子传递、MerA的同源蛋白介导的还原)仍有待证实。
目前,汞的微生物还原研究多聚焦于汞离子,对其它汞形态研究较少。在实际环境中,汞离子易吸附于有机物、黏土、铁锰矿物等,在还原性硫存在下易形成硫化汞(HgS)[52]。这些无机汞形态的微生物还原是理解真实环境中汞的活化与转化行为的关键。最近的研究表明,“惰性”的硫化汞矿物可被微生物转化为零价汞,这一途径可导致汞的活化[53],但其机制尚待进一步明确。最近,微生物与金属矿物之间的电子转移过程日益受到重视[54]。微生物可通过外膜蛋白直接传递、电子穿梭体介导的间接传递以及纳米导线传递等机制[55-56]将电子传递给金属矿物半导体。金属矿物自身可发生表面溶解与转化,或将电子进一步传递给表面吸附的污染物,介导污染物的还原[55]。这一途径也可能引发硫化汞或铁锰矿物吸附汞离子的还原。
环境中汞的微生物还原过程及机制
Microbial reduction process and mechanism of mercury in the environment
-
摘要: 汞是一种全球性的环境污染物。汞的还原过程可降低地表环境汞浓度,促进汞的大气传输,对汞的区域/全球循环、甲基化及生物积累具有重要的作用。地表环境微生物还原是汞还原的重要过程,在多种环境介质中均可发生。本文介绍了多种环境介质中微生物汞还原在汞生物地球化学循环中的重要作用,详细总结了有氧与缺氧条件下微生物汞还原的途径与机制,提出了微生物还原汞的其它可能机制,并对相关领域的研究进行了展望。Abstract: Mercury (Hg) is a global environmental pollutant. The reduction of Hg2+ in multiple environmental matrices, which can reduce the Hg concentrations in terrestrial and marine systems and promote the transport of Hg in atmosphere, is of great importance in the regional/global cycle, methylation, and bioaccumulation of Hg. Microbial reduction is one of the important processes of Hg reduction, which can occur in a variety of environmental compartments. This review introduced the important role of microbial reduction in Hg biogeochemical cycle in a variety of environmental compartments, summarized the detailed pathways and mechanisms regarding microbial Hg2+ reduction under aerobic and anoxic conditions, and proposed other possible mechanisms of microbial Hg2+ reduction and future research perspective in related fields.
-
Key words:
- mercury /
- reduction /
- microorganisms /
- aerobic /
- anaerobic
-

-
-
[1] DRISCOLL C T, MASON R P, CHAN H M, et al. Mercury as a global pollutant: sources, pathways, and effects [J]. Environmental Science & Technology, 2013, 47(10): 4967-4983. [2] UNEP. Global Mercury Assessment [R]. Geneva, Switzerland, UNEP Chemicals and Health Branch. [3] COLOMBO M J, HA J, REINFELDER J R, et al. Anaerobic oxidation of Hg(0) and methylmercury formation by Desulfovibrio desulfuricans ND132 [J]. Geochimica Et Cosmochimica Acta, 2013, 112(7): 166-177. [4] HU H, LIN H, ZHENG W, et al. Oxidation and methylation of dissolved elemental mercury by anaerobic bacteria [J]. Nature Geoscience, 2013, 6(9): 751-754. doi: 10.1038/ngeo1894 [5] PONGPRUEKSA P, LIN C J, LINDBERG S E, et al. Scientific uncertainties in atmospheric mercury models Ⅲ: Boundary and initial conditions, model grid resolution, and Hg(Ⅱ) reduction mechanism [J]. Atmospheric Environment, 2008, 42(8): 1828-1845. doi: 10.1016/j.atmosenv.2007.11.020 [6] SEIGNEUR C, VIJAYARAGHAVAN K, LOHMAN K, et al. Modeling the atmospheric fate and transport of mercury over North America: Power plant emission scenarios [J]. Fuel Processing Technology, 2004, 85(6-7): 441-450. doi: 10.1016/j.fuproc.2003.11.001 [7] LIN C J, PEHKONEN S O. The chemistry of atmospheric mercury: A review [J]. Atmospheric Environment, 1999, 33(13): 2067-2079. doi: 10.1016/S1352-2310(98)00387-2 [8] SAIZ-LOPEZ A, SITKIEWICZ S, ROCA-SANJUÁN D, et al. Photoreduction of gaseous oxidized mercury changes global atmospheric mercury speciation, transport and deposition [J]. Nature Communications, 2018, 9(1): 4796. doi: 10.1038/s41467-018-07075-3 [9] MUNTHE J, XIAO Z F, LINDQVIST O. The aqueous reduction of divalent mercury by sulfite [J]. Water Air & Soil Pollution, 1991, 56(1): 621-630. [10] LIN S I, ARIYA P A. Reduction of oxidized mercury species by dicarboxylic acids (C2-C4): Kinetic and product studies [J]. Environmental Science & Technology, 2008, 42(14): 5150-5155. [11] LIN C J, PEHKONEN S O. Aqueous free radical chemistry of mercury in the presence of iron oxides and ambient aerosol [J]. Atmospheric Environment, 1997, 31(24): 4125-4137. doi: 10.1016/S1352-2310(97)00269-0 [12] ALBERTSJJ S J E, MILLER R W, NUTTER D E. Elemental mercury evolution mediated by humic acid [J]. Science, 1974, 184(4139): 895-896. doi: 10.1126/science.184.4139.895 [13] TPERETYAZH K O, CHARLET L, MURESAN B. Formation of dissolved gaseous mercury in a tropical lake (Petit-Saut reservoir, French Guiana) [J]. Science of the Total Environment, 2006, 364(1-3): 260-271. doi: 10.1016/j.scitotenv.2005.06.016 [14] MONPERRUS M, TESSIER E, AMOUROUX D, et al. Mercury methylation, demethylation and reduction rates in coastal and marine surface waters of the Mediterranean Sea [J]. Marine Chemistry, 2007, 107(1): 49-63. doi: 10.1016/j.marchem.2007.01.018 [15] KIM H, RHEE T S, HAHM D, et al. Contrasting distributions of dissolved gaseous mercury concentration and evasion in the North Pacific Subarctic Gyre and the Subarctic Front [J]. Oceanographic Research Papers, 2016, 110: 90-98. doi: 10.1016/j.dsr.2016.02.001 [16] BARKAY T, LIEBERT C, GILLMAN M. Environmental significance of the potential for mer(Tn21)-mediated reduction of Hg2+ to Hg0 in natural waters [J]. Applied & Environmental Microbiology, 1989, 55(5): 1196-1202. [17] VANDAL G M, FITZGERALD W F, ROLFHUS K R, et al. Modeling the elemental mercury cycle in Pallette Lake, Wisconsin, USA [J]. Water Air & Soil Pollution, 1995, 80(1-4): 529-538. [18] MASON R, MOREL F, HEMOND H. The role of microorganisms in elemental mercury formation in natural waters [J]. Heavy Metals in the Environment, 1995, 80(1-4): 775-787. [19] POULAIN A J, NI CHADHAIN S M, ARIYA P A, et al. Potential for mercury reduction by microbes in the High Arctic [J]. Applied & Environmental Microbiology, 2007, 73(7): 2230-2238. [20] BOUFFARD A, AMYOT M. Importance of elemental mercury in lake sediments [J]. Chemosphere, 2009, 74(8): 1098-1103. doi: 10.1016/j.chemosphere.2008.10.045 [21] GUEVARA S R, IEK S, REPINC U K, et al. Novel methodology for the study of mercury methylation and reduction in sediments and water using 197Hg radiotracer [J]. Analytical & Bioanalytical Chemistry, 2007, 387(6): 2185-2197. [22] BRAZEAU M L, BLAIS J M, PATERSON A M, et al. Evidence for microbially mediated production of elemental mercury (Hg0) in subarctic lake sediments [J]. Applied Geochemistry, 2013, 37: 142-148. doi: 10.1016/j.apgeochem.2013.07.020 [23] CHOI H D, HOLSEN T M. Gaseous mercury emissions from unsterilized and sterilized soils: The effect of temperature and UV radiation [J]. Environmental Pollution, 2009, 157(5): 1673-1678. doi: 10.1016/j.envpol.2008.12.014 [24] PANNU R, SICILIANO S D, O'DRISCOLL N J. Quantifying the effects of soil temperature, moisture and sterilization on elemental mercury formation in boreal soils [J]. Environmental Pollution, 2014, 193: 138-146. doi: 10.1016/j.envpol.2014.06.023 [25] POULIN B A A, GEORGE R, KATHRYN L, et al. Mercury transformation and release differs with depth and time in a contaminated riparian soil during simulated flooding [J]. Geochimica Et Cosmochimica Acta, 2016, 176: 118-138. doi: 10.1016/j.gca.2015.12.024 [26] OSTERWALDER S, HUANG J H, SHETAYA W H, et al. Mercury emission from industrially contaminated soils in relation to chemical, microbial, and meteorological factors [J]. Environmental Pollution, 2019, 250: 944-952. doi: 10.1016/j.envpol.2019.03.093 [27] LU Z, YUAN W, LUO K, et al. Litterfall mercury reduction on a subtropical evergreen broadleaf forest floor revealed by multi-element isotopes [J]. Environmental Pollution, 2020, 268: 115867. [28] DOMMERGUE A, GAUCHARD P A, BOUTRON C F, et al. The fate of mercury species in a sub-arctic snowpack during snowmelt [J]. Geophysical Research Letters, 2003, 30(12): 1621-1625. [29] LALONDE J D, DOYON M R, AUCLAIR J C. Photo-induced Hg(Ⅱ) reduction in snow from the remote and temperate Experimental Lakes Area (Ontario, Canada) [J]. Geophysical Research-Atmospheres, 2003, 108(6): 4200. [30] MOLLER A K, AL-SOUD W, SORENSEN S J, et al. Diversity and characterization of mercury-resistant bacteria in snow, freshwater and sea-ice brine from the High Arctic [J]. Fems Microbiology Ecology, 2011, 75(3): 390-401. doi: 10.1111/j.1574-6941.2010.01016.x [31] BARKAY T, GILLMAN M. Environmental significance of the potential for mer-mediated reduction of Hg2+to Hg0 in natural-waters [J]. Applied & Environmental Microbiology, 1985, 55(5): 1196-1202. [32] BARKAY T. Adaptation of aquatic microbial communities to Hg2+ stress [J]. Applied & Environmental Microbiology, 1987, 53(12): 2725-2732. [33] BARKAY T, SAOUTER E, HORN J. Mercury biotransformations and their potential for remediation of mercury contamination [J]. Biodegradation, 1992, 3(2-3): 147-159. doi: 10.1007/BF00129080 [34] BARKAY T, MILLER S M, SUMMERS A O. Bacterial mercury resistance from atoms to ecosystems [J]. FEMS Microbiology Reviews, 2003, 27(2-3): 355-384. doi: 10.1016/S0168-6445(03)00046-9 [35] BENISON G C, PAOLA D, JACOB E, et al. A stable mercury-containing complex of the organomercurial lyase MerB: Catalysis product releaseand direct transfer to MerA [J]. Biochemistry, 2004, 43(26): 8333-8345. doi: 10.1021/bi049662h [36] BOYD E S, TAMAR B. The mercury resistance operon: From an origin in a geothermal environment to an efficient detoxification machine [J]. Frontiers in Microbiology, 2012, 3: 349. [37] IWAHORI K, TAKEUCH F, KAMIMURA K, et al. Ferrous ion-dependent volatilization of mercury by the plasma membrane of Thiobacillus ferrooxidans [J]. Applied & Environmental Microbiology, 2000, 66(9): 3823-3827. [38] TSUYOSHI S, FUMIAKI T, ATSUNORI N, et al. Cytochrome c oxidase purified from a mercury-resistant strain of Acidithiobacillus ferrooxidans Volatilizes Mercury [J]. Bioscience & Bioengineering, 2001, 92(1): 44-49. [39] APPIA-AYME C, GUILIANI N, RATOUCHNIAK J, et al. Characterization of an operon encoding two c-type cytochromes, an aa(3)-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020 [J]. Environmental Microbiology Reports, 1999, 65(11): 4781-4787. [40] SHI L, RICHARDSON D J, WANG Z, et al. The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer [J]. ChemInform, 2009, 1(4): 220-227. [41] HAIYAN H, HUI L, WANG Z, et al. Mercury reduction and cell-surface adsorption by Geobacter sulfurreducens PCA [J]. Environmental Science & Technology, 2013, 47(19): 10922-10930. [42] MASON R P, REINFELDER J R, MOREL F. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom [J]. Environmental Science & Technology, 1996(30): 1835-1845. [43] GRÉGOIRE D S, POULAIN A J. A physiological role for Hg(Ⅱ) during phototrophic growth [J]. Nature Geoscience, 2016, 9(2): 121-125. doi: 10.1038/ngeo2629 [44] POULAIN A J, FINDLAY D, TELOR S, et al. Biological and photochemical production of dissolved gaseous mercury in a boreal lake [J]. Limnology Oceanography, 2004(49): 2265-2275. [45] WIATROWSKI H A, WARD P M, BARKAY T. Novel reduction of mercury (Ⅱ) by mercury-sensitive dissimilatory metal reducing bacteria [J]. Environmental Science & Technology, 2006, 40(21): 6690-6696. [46] LLOYDJRS V A, VAN-PRAAGH C V, LOVLEY D R. Direct and Fe(Ⅱ)-mediated reduction of technetium by Fe(Ⅲ)-reducing bacteria [J]. Applied & Environmental Microbiology, 2000, 66(9): 3743-3749. [47] CHADHAIN S M N, SCHAEFER J K, CRANE S, et al. Analysis of mercuric reductase (merA) gene diversity in an anaerobic mercury-contaminated sediment enrichment [J]. Environmental Microbiology Reports, 2010, 8(10): 1746-1752. [48] LU X, LIU Y, JOHS A, et al. Anaerobic mercury methylation and demethylation by Geobacter bemidjiensis Bem [J]. Environmental Science & Technology, 2016, 50(8): 4366-4373. [49] REGUERA G, MCCARTHY K D, MEHTA T, et al. Extracellular electron transfer via microbial nanowires [J]. Nature, 2005, 435(7045): 1098-1101. doi: 10.1038/nature03661 [50] DOIG P, TODD T, SASTRY P A, et al. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells [J]. Infection & Immunity, 1988, 56(6): 1641-1646. [51] NEVIN K P, LOVLEY D R. Lack of production of electron-shuttling compounds or solubilization of Fe(Ⅲ) during reduction of insoluble Fe(Ⅲ) oxide by Geobacter metallireducens [J]. Environmental Microbiology Reports, 2000, 66(5): 2248-2251. [52] WOLFENDEN S, CHARNOCK J M, HILTON J, et al. Sulfide species as a sink for mercury in lake sediments [J]. Environmental Science & Technology, 2005, 39(17): 6644-6648. [53] VÁZQUEZ-RODRíGUEZ A, HANSEL C, ZHANG T, et al. Microbial- and thiosulfate-mediated dissolution of mercury sulfide minerals and transformation to gaseous mercury [J]. Frontiers in Microbiology, 2015, 6: 596. [54] 王莹, 李芳柏, 刘同旭. 微生物—矿物间半导体介导电子传递机制研究进展 [J]. 地球科学进展, 2016, 31(4): 347-356. doi: 10.11867/j.issn.1001-8166.2016.04.0347. WANG Y, LI F B, LIU T X. Advances in the semiconductor-mediated electron transfer mechanism at microbe-mineral interface [J]. Advances in Earth Science, 2016, 31(4): 347-356(in Chinese). doi: 10.11867/j.issn.1001-8166.2016.04.0347.
[55] LIU T, LI X, ZHANG W, et al. Fe(Ⅲ) oxides accelerate microbial nitrate reduction and electricity generation by Klebsiella pneumoniae L17 [J]. Journal of Colloid Interface Science, 2014, 423: 25-32. doi: 10.1016/j.jcis.2014.02.026 [56] VARGAS M, KASHEFI K, BLUNT-HARRIS E L, et al. Microbiological evidence for Fe(Ⅲ) reduction on early Earth [J]. Nature, 1998, 1(30): 565-571. -



 下载:
下载: