-
抗生素也称抗菌素,是微生物产生的一类次级代谢产物,会抑制其他微生物的生长和存活[1]。抗生素按化学结构可分为β-内酰胺类、四环素类、大环内酯类、磺胺类和喹诺酮类五大类[2]。近几十年来,抗生素常作为人类感染性疾病的治疗剂。如今,抗生素也广泛应用于畜牧业和水产养殖[3]。然而,抗生素的广泛使用,特别是抗生素的过度使用或滥用引起了公众的关注。在抗生素的生产和应用过程中,大量的含抗生素废水产生并排放到环境中,造成严重污染[4]。残留的抗生素因具有毒性大、难生物降解等特点,传统的水处理方法(生物法、物理法、化学法)难以去除[5]。因此,在各种自然环境中经常检测到抗生素,如河水[6]、地下水[7]、地表水[8]、土壤[9]和饮用水[10]等。在自然环境中长期存在抗生素可能会导致产生和传播抗生素抗性基因(ARG)和抗生素抗性细菌(ARB),从而加速抗生素耐药性,进而对人类健康和生态系统造成威胁[11]。因此,如何将这些抗生素有效地去除仍然是人们急需解决的问题。
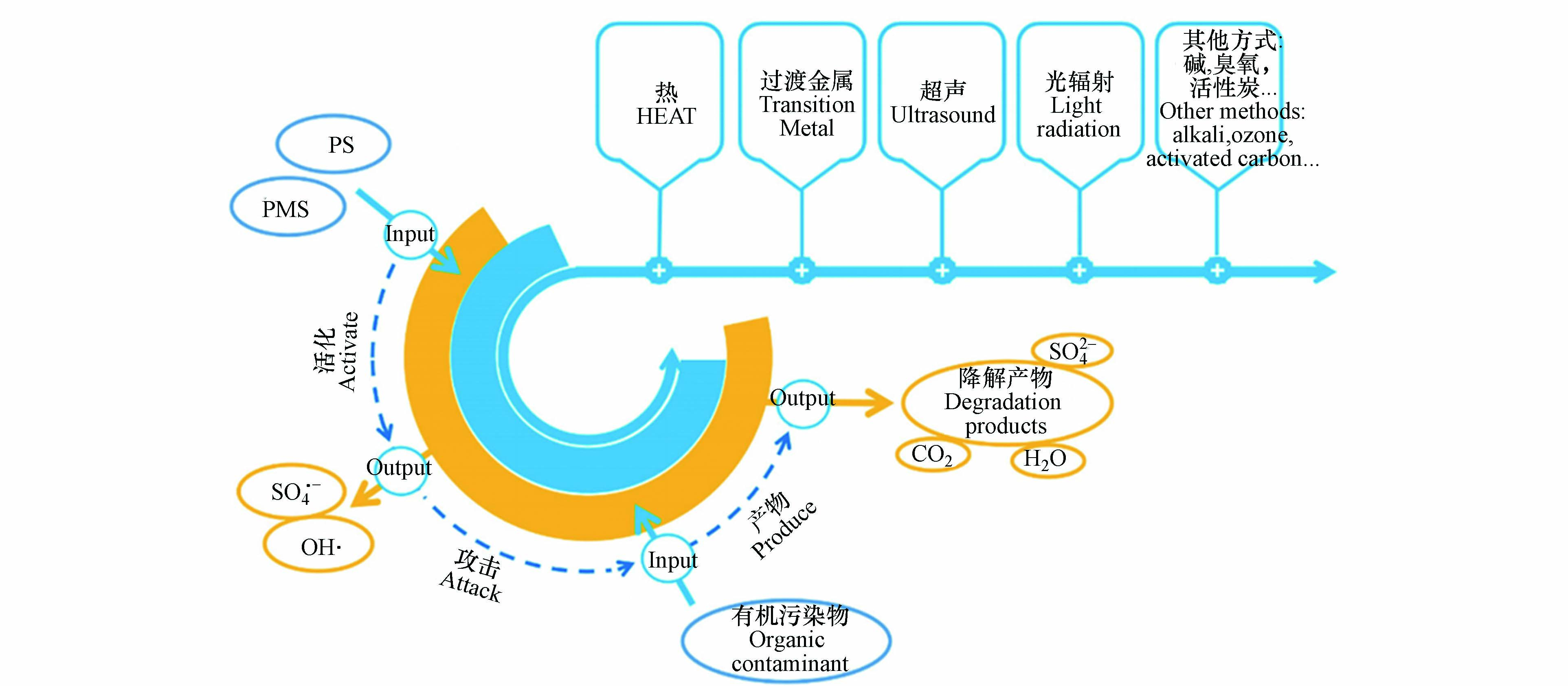
基于硫酸根自由基(
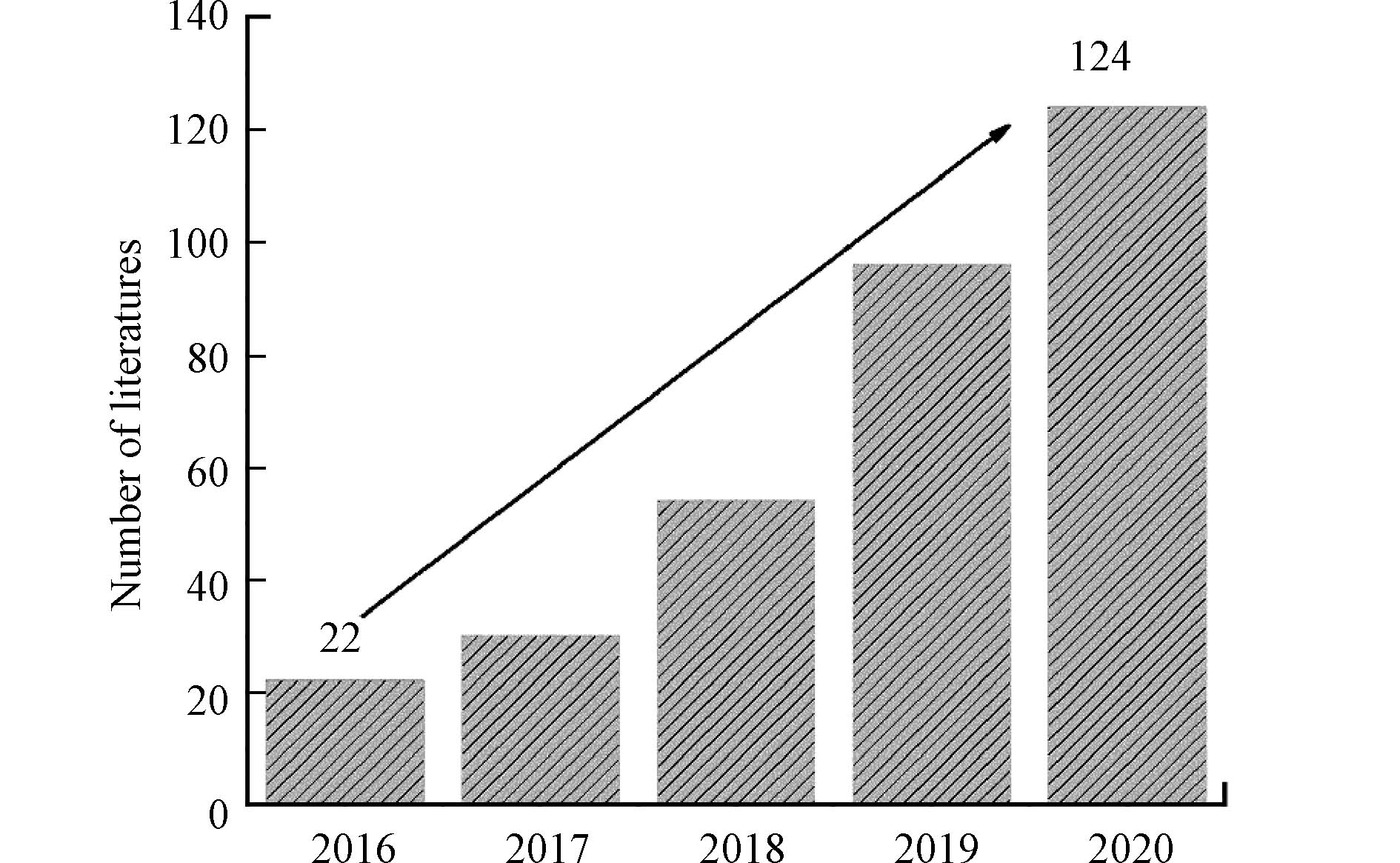
$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ )的高级氧化技术(sulfate radical-based advanced oxidation processes,SR-AOPs)是近年国内外新兴起的一类处理难降解有机污染物的新型高级氧化技术。$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 由于它的强氧化性在降解有机污染物方面表现出巨大的优势。与传统的高级氧化技术(基于·OH)相比,$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 选择性强,半衰期长(40 μs),氧化电位高(2.5—3.1 V),具有更宽的pH应用范围,能够有效地降解抗生素污染物分解为小分子物质,减轻抗生素对微生物的抑制作用,提高其生物降解能力和去除率。近年来,国内外众多学者对SR-AOPs进行了广泛的研究,可以通过Web of Science数据库已发表论文(近5年)的数量来大致反映,如图1所示。很明显,SR-AOPs应用于去除抗生素的文章数量2020年比2016年增加了超5倍。
本文重点分析、总结和比较过硫酸盐的各种活化方式,以及SR-AOPs在去除抗生素污染物的应用,讨论和分析了抗生素的降解效果和机理,以提供SR-AOPs去除抗生素的广泛概述。最后提出了SR-AOPs用于抗生素氧化降解的研究方向和一些挑战。
-
过硫酸盐包括过一硫酸氢盐(PMS,
${\rm{HS{O}}_{{5^ - }}} $ )和过二硫酸盐(PS或PDS,${{\rm{S}}_2}{{\rm{O}}_8}^{2 - } $ )。这两种过硫酸盐结构上与H2O2 相似,都含有O—O键。其中,PMS是由1个${\rm{HS{O}}_{{3^ - }}} $ 取代H2O2中的1个H原子所构成的非对称结构的过氧化物。而PDS则是 H2O2中的两个H原子都被${\rm{HS{O}}_{{3^ - }}} $ 所取代而得到的对称结构的过氧化物。H2O2中的H原子被$HS{O_{{3^ - }}} $ 取代后,O—O键的键长增加,键能减小。PMS和PDS的O—O键长分别为1.460 nm和1.497 nm。PDS的键能分别为140 kJ·mol−1,而PMS的键能一般在140—213.3 kJ·mol−1的范围内[12-13]。因此,理论上讲PDS比PMS需要更少的能量来断裂过氧键产生$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 。过氧化氢、过二硫酸根离子和过一硫酸根离子的结构式:

PMS是白色固体粉末,易溶于水(溶解度大于250 g·L−1)。pH < 6或pH = 12时稳定,当pH = 9时,它表现出最差的稳定性,其中一半的
${\rm{HS{O}}_{{5^ - }}} $ 分解为SO52−[14]。实验中常用的PMS是过一硫酸氢钾复合盐(KHSO5·0.5KHSO4·0.5K2SO4)。PDS一般为白色晶体,稳定性高,易溶于水(溶解度为730 g·L−1)。实验中常用的PDS是过硫酸钠(Na2S2O8)或过硫酸钾(K2S2O8)。PMS和PDS本身都具有较强氧化性,其标准氧化还原电势分别为1.82 V和2.01 V。它们单独用于降解有机物时,氧化效果并不明显且速率较慢。但是使用一定的活化方式将它们活化产生具备强氧化能力的
$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 和·OH,对有机物氧化降解能力显著增强。$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 与有机物反应的机理与·OH类似,能够通过电子转移、氢提取以及加成3种方式氧化降解有机物。其中$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 与芳香类化合物主要发生电子转移的反应(公式(1));与醇类、烷烃、醚和脂类化合物主要通过氢提取反应(公式(2));而与不饱和烯烃类化合物则常是通过加成反应(公式(3))。但是与·OH主要通过加成和氢提取的方式氧化降解有机物不同,$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 更倾向于通过电子转移的途径氧化降解有机物[15]。
-
UV被认为是活化过硫酸盐产生
$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 去除水中有机污染物的有效方式。目前,关于UV活化过硫酸盐的机理主要是通过紫外线的能量输入使O—O键断裂,其反应过程如下:关于UV活化过硫酸盐产生
$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 的波长有两种说法。Malato等[17]研究发现在波长小于270 nm的UV可使O—O键断裂。Maurino等[18]研究表明 UV 活化过硫酸盐的波长要小于295 nm。目前,254 nm常用作活化过硫酸盐的波长。例如,Zhou等[19]采用254 nm的UV活化过硫酸盐降解青霉素抗生素。研究结果发现,青霉素G,阿莫西林和羧苄青霉素与$ \text{S}{\text{O}}_{\text{4}}^{\text{•}{-}} $ 和$ \text{·OH} $ 反应的二阶速率常数估计分别为(3.90—9.32)×109 L·mol−1·s−1和(6.67—9.86)×109 L·mol−1·s−1。然而,也有一些研究者[20]采用280 nm波长的UV活化PDS降解环丙沙星,结果表明,在pH=6.8—7.2、环丙沙星浓度为3 μmol·L−1、PDS浓度为210 μmol·L−1 时,20 min内环丙沙星降解率达到97%。UV活化过硫酸盐被广泛应用于降解水中抗生素(如表1)。从表中可以看出,UV活化过硫酸盐能有效降解水中抗生素。现在也有一些研究采用UV活化过硫酸盐方法处理实际水样中的抗生素,并取得良好的效果。例如:饮用水[21]、模拟医院废水[22]、海洋养殖用水和海水[23]、合成海水[24]等。UV活化虽然被广泛应用于降解水中有机污染物,但其也存在一些不足:(1)目前大部分的UV活化过硫酸盐集中在紫外光,而紫外光只占太阳光谱的5%,需要外加设备提供光能量,处理成本高。(2)有机废水中的色度会降低紫外光的透射率,从而影响其活化效果。因此,UV活化过硫酸盐技术很难在工业废水处理中得到应用。 -
热活化过硫酸盐产生
$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 是研究较早的一种活化方法。过硫酸盐在热能(140—213.3 kJ·mol−1)作用下,O—O键可以断裂产生$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ 。该反应方程式如下:根据有关文献[31],在碱性pH值下,
$ \text{S}{\text{O}}_{\text{4}}^{\text{•-}} $ 在加热过程中也可以转变为$ \text{·}\text{OH} $ 。其反应过程如下:pH值是
$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 转变为$ \text{·}\text{OH} $ 的关键因素。一般在酸性条件下,反应体系主要是$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ ,7 < pH < 9时,$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 和$ \text{·}\text{OH} $ 共同存在,pH > 9时,$ \text{·}\text{OH} $ 主导反应进行。与PS热活化相比,对PMS热活化的研究很少。因此,对于PMS热活化需要进一步研究。Ji等[32]研究了在环境温度((20 ± 1) ℃)下活化PDS降解四环素,结果表明,在pH = 7.0, 只有20%的四环素被降解。可以看出,虽然环境温度也可以活化过硫酸盐,但对污染物的降解率非常低。因此,热活化过硫酸盐一般在较高温度下进行。Qian等[33]研究了在60 ℃下活化PDS降解头孢菌素类抗生素(CEFs),并研究了各种基质中的成分(Cl−、${\rm{HS{O}}_{{3^ - }}} $ 等)对活化过程的影响。结果表明,在pH = 7.0、CEFs浓度为0.1 mmol·L−1、PDS投加量为1.0 mmol·L−1时,各种基质中的成分的浓度高低对CEFs的降解效率有很大促进作用。另外,许多研究者对热活化过硫酸盐进行了研究(见表2)。温度的升高可以加速PDS分解为$ \text{S}{\text{O}}_{\text{4}}^{\text{•}-} $ ,进一步增加抗生素的降解效率和速率,其主要原因是升高的温度有利于基于热力学定律(阿伦尼乌斯方程)的氧化反应,并降低抗生素分解所需的活化能。从表2中可以看出,pH值也是去除抗生素的影响因素。当pH < 7.0或pH > 10时,多数抗生素无法完全去除。而且大多数抗生素可以在7.0 < pH < 8.0时完全降解。相比单独$ \text{S}{\text{O}}_{\text{4}}^{\text{•}-} $ 或$ \text{·}\text{OH} $ ,$ \text{S}{\text{O}}_{\text{4}}^{\text{•}-} $ 和$ \text{·}\text{OH} $ 共同存在对抗生素具有更好的去除率。虽然热活化是活化过硫酸盐有效的方式之一,具有操作简单,不会造成水体二次污染等优势。然而,需要加热设备,能耗较高,运行成本高等限制了该方式的大规模使用。 -
过渡金属离子(Fe2+、Ag+、Mn2+、Co2+、Cu2+等)可以通过电子转移的方式活化过硫酸盐产生
$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 。其中机理如下:相比UV和热活化过硫酸盐, 过渡金属离子活化过硫酸盐的反应通常在常温常压下即可进行, 无需外加能量。以Fe2+为例,它的无毒性、良好的活化性能等被广泛应用于活化过硫酸盐体系。表3列出一些Fe2+活化过硫酸盐去除抗生素的研究。从表中可以看出,不同浓度的抗生素使用的过硫酸盐和Fe2+的浓度不一样,并且具有较高的催化效率。然而,该体系存在着一些问题:(1)Fe2+活化往往会产生Fe3+,该离子催化活性低,并容易水解产生铁污泥。(2)Fe2+必须要极强的酸性环境,实际环境应用受到很大的限制。(3)溶液中游离的金属离子不易控制,难以回收,而且残留的金属离子可能会造成二次污染。因此,为了减少这些弊端,过渡金属离子活化过硫酸盐用于降解抗生素有两种常见方法:(1)在体系中添加各种络合剂或螯合剂,如柠檬酸、EDTA、EDDS等。(2)设计和制备高效、环保、稳定且易回收的非均相催化剂。
-
鉴于UV活化、热活化都需要外加能量、需要外加设备、成本高、难以实际应用,而基于过渡金属离子的均相催化方式受pH影响大、不易控制、难以回收而可能造成二次污染等问题。相比均相催化剂,非均相催化剂具有稳定、可重复利用、较宽的pH适用范围等优点,可以克服上述限制。目前,非均相过硫酸盐活化方式主要包括金属氧化物活化、零价金属活化、非金属碳基材料活化等。
-
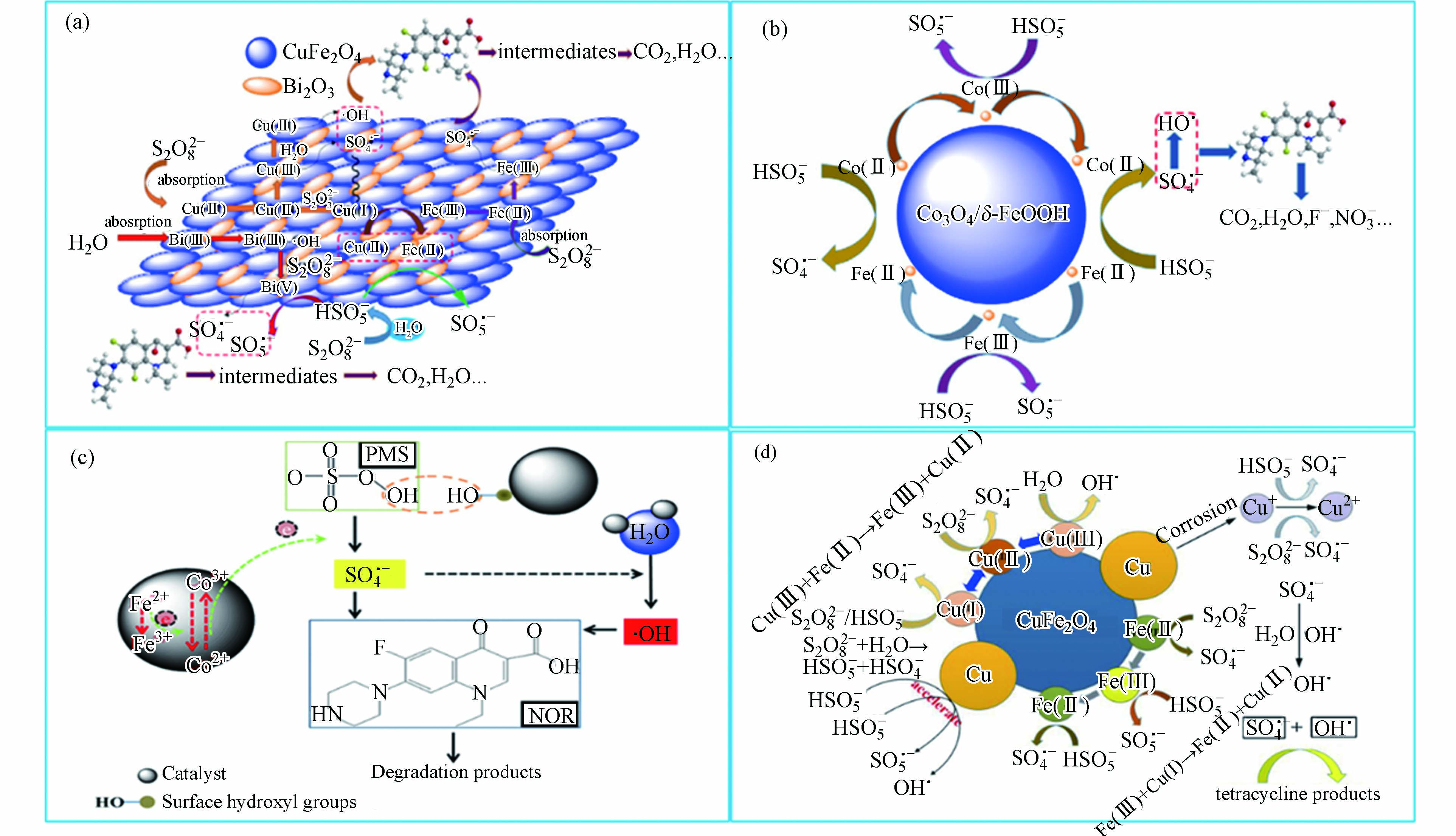
非均相活化的金属氧化物主要是过渡金属氧化物,它们活化过硫酸盐降解抗生素的机理和过渡金属离子相似[14]。一些金属氧化物及其复合材料活化过硫酸盐用于降解抗生素的机理研究如图3所示。从图3可以看出,非均相活化过硫酸盐反应主要发生在催化剂的表面,并且通过催化剂表面过渡金属离子的价态变化引发自由基的产生氧化降解抗生素。此外,基于还原机理,过硫酸盐的活化应与金属的氧化还原电位呈正相关。实际上,过硫酸盐的活化不仅和金属氧化还原电位有关,还跟金属是作为电子供体(介体)还是电子受体有关。例如,已证明CuO能够激活PMS[44]。然而,Cu2+对Cu+的氧化还原电势仅为0.17 V,远低于(
$\text{S}{\text{O}}_{\text{5}}^{\text{•}-}$ /$\text{HS}{\text{O}}_{\text{5}}^{{-}}$ (1.1 V)或$\text{S}{\text{O}}_{\text{5}}^{\text{•}-}$ /$\text{S}{\text{O}}_{\text{5}}^{{2-}}$ (0.81 V))。此外,非均相活化的性能随着材料的性质,特别是材料的表面性质而显著变化[14]。因此,许多研究者合成非均相催化剂活化过硫酸盐氧化降解抗生素,例如Co3O4纳米粒子[45]。表4列出一些金属氧化物活化过硫酸盐去除抗生素的研究。从表4可以看出,虽然不同浓度的抗生素使用的过硫酸盐和催化剂浓度不一样,但反应最佳pH基本在中性,这表明金属氧化物催化剂比较稳定、受pH影响小。此外,大多数研究集中在研究Fe基或Co基氧化物。这些氧化物表现出良好的活化性能,并且广泛关注的是Fe基或Co基氧化物的稳定性和重复利用性。因此,对于金属氧化物活化过硫酸盐,开发一种经济,稳定和高效的新型非均相催化剂成为其重要的课题之一。 -
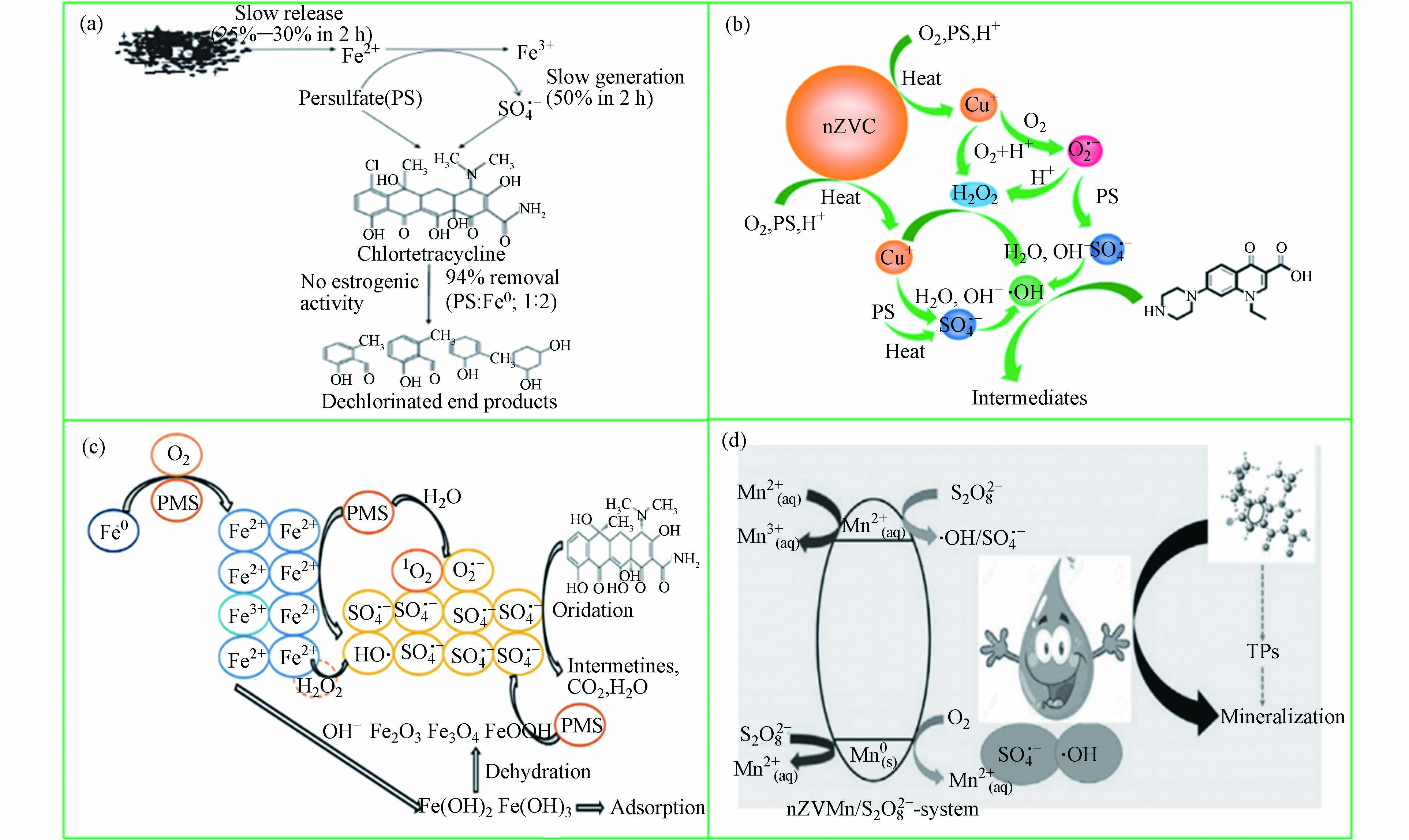
零价金属(Fe0、Al0、Mn0、Cu0等)既具有过渡金属离子的优势,又弥补了过渡金属离子反应速率过快、副反应较多等缺陷。以零价铁为例,Fe0活化过硫酸盐是利用Fe0作为Fe2+的来源,其中Fe0既是过硫酸盐的活化剂,又是还原反应的还原剂[57]。此外,Fe0作为一种非均相催化剂,可以缓慢释放Fe2+,从而控制反应速度,保证体系持续高效地降解抗生素。一些零价金属活化过硫酸盐用于降解抗生素的机理研究如图4所示。从图4可以看出,零价金属活化过硫酸盐反应主要通过释放不同价态过渡金属离子引发自由基(
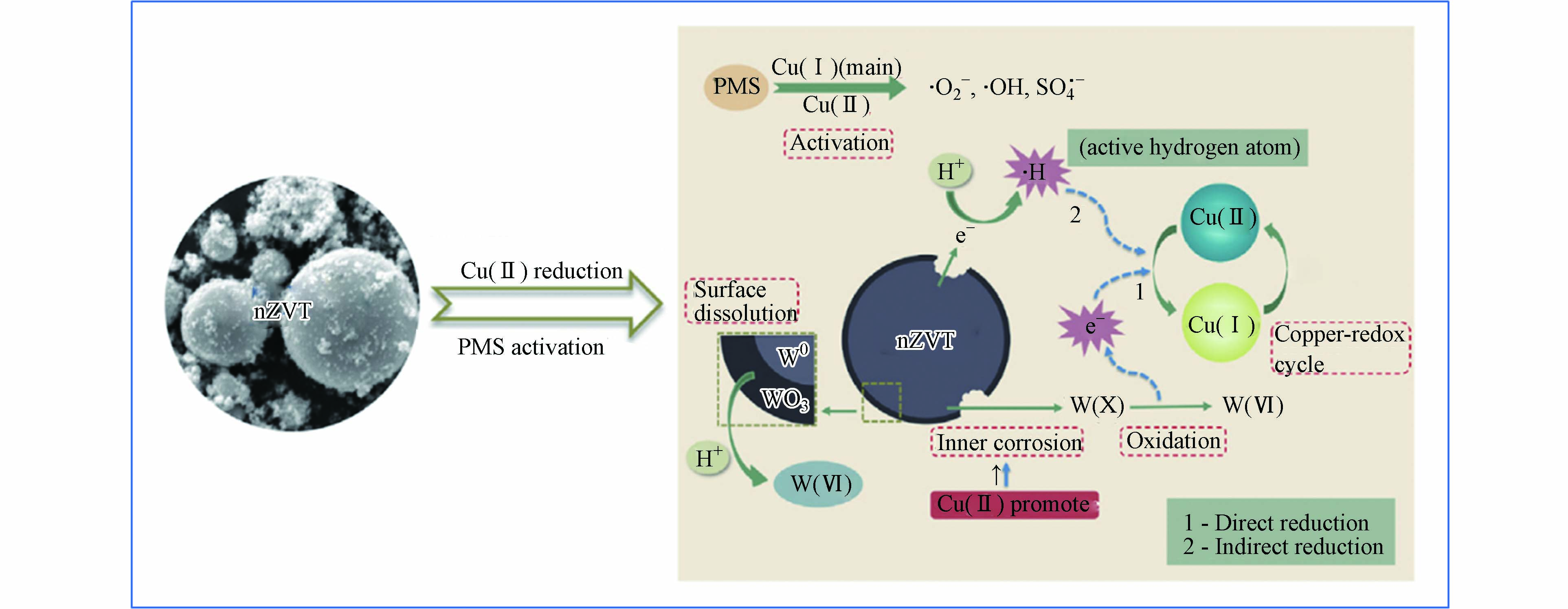
$ \text{S}{\text{O}}_{\text{4}}^{\text{•}-} $ 、$ \text{·}\text{OH} $ 和${\text{O}}_{\text{2}}^{\text{·}-}$ )的产生破坏抗生素的结构降解为小分子物质。表5列出一些零价金属活化过硫酸盐去除抗生素的研究。Cao等[58]采用零价铁活化PMS降解四环素。研究表明,在最佳条件下,处理5 min后TC去除效率达到88.5%。此外,与Fe2+/PMS体系相比,Fe0是一种高效且持久的活化剂,消耗较少的PMS,并产生较少的溶解铁离子。此外,Ye等[59]在零价金属活化过硫酸盐提出了一种新型策略,其反应机制如图5所示。纳米零价钨(nZVT)通过直接还原途径加速Cu(Ⅱ)/Cu(Ⅰ)的氧化还原循环,进而活化过硫酸盐产生自由基氧化降解水中四环素。因此,后续实验研究可以开展零价金属与其他活化技术联用,为体系提供持续稳定的金属离子,提高反应效率。 -
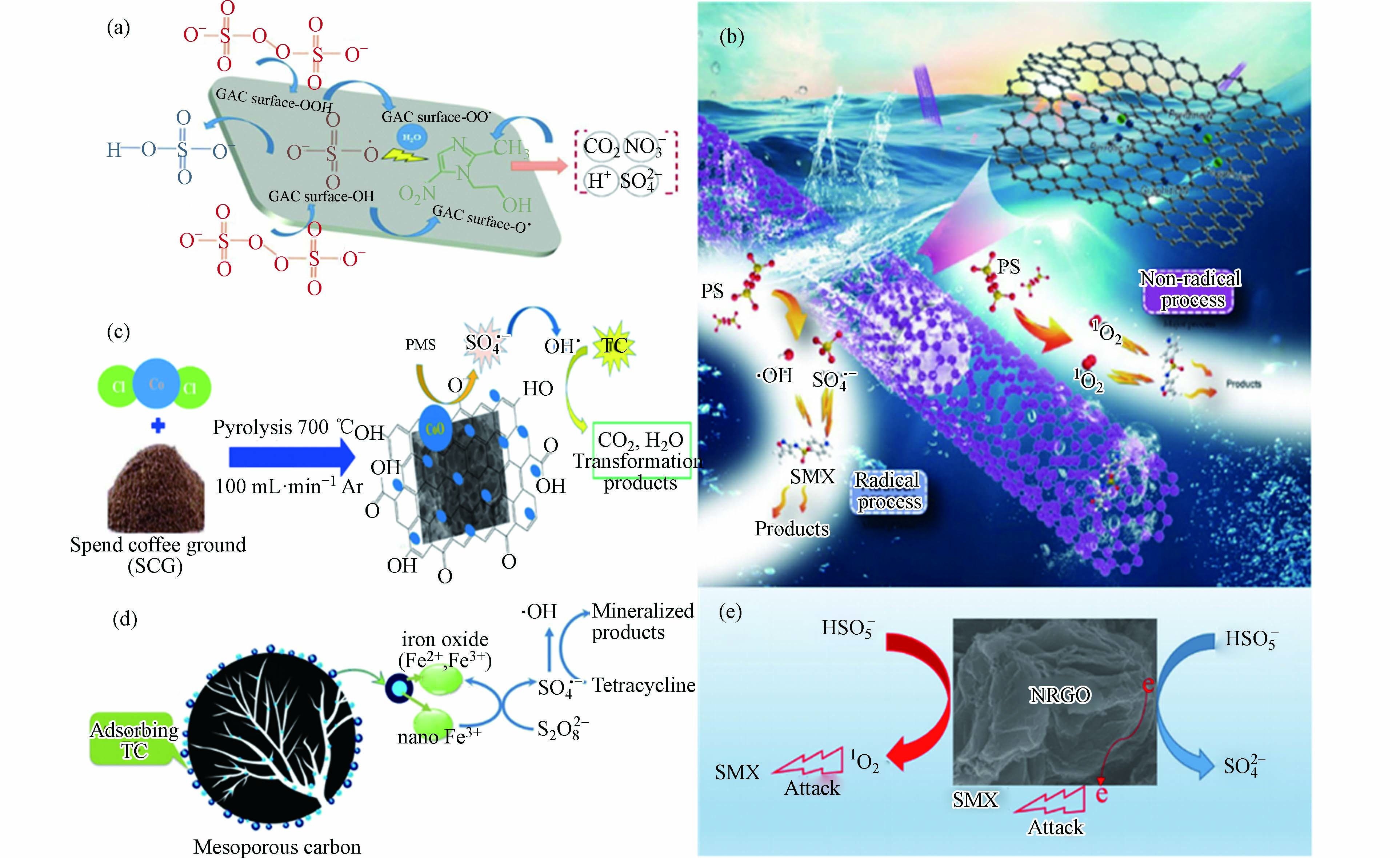
碳基材料具有较高的比表面积、发达的孔隙结构,较强的吸附性和导电性,同时表面还有着丰富的官能团如−OH、−C=O 和−COOH,易于功能化[66]。研究发现,活性炭(AC)、碳纳米管(CNT)、氧化石墨烯(GO)、还原氧化石墨烯(rGO)、生物炭(Biochar)等碳基材料都具有活化过硫酸盐分解产生
$ \text{S}{\text{O}}_{\text{4}}^{\text{•}-} $ 的能力。它们活化过硫酸盐降解抗生素的机理主要是电子转移(氧化还原反应)。推测多壁碳纳米管缺陷边缘的sp2共价碳网络和氧官能团进行了氧化还原循环,使电子转移到PDS上产生自由基。另一个机理可能是电子从碳质材料(例如芳族石墨烯结构)中撤出。碳质材料因此可以将电子输送给过硫酸盐,从而形成反应性自由基。一些碳基材料活化过硫酸盐用于降解抗生素的机理研究如图6。从图7(a)可知活性炭活化过硫酸盐机制如下公式:图6(c)和(d)分别展示了CoO负载于咖啡粉生物炭和Fe0负载于介孔碳上活化过硫酸盐氧化降解四环素。这两个反应虽然主要是通过CoO和Fe0活化过硫酸盐产生自由基(
$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 和$ \text{·}\text{OH} $ )来降解四环素。但是他们有几个优点:(1)将金属氧化物或零价金属负载于碳基材料上可以抑制金属离子的溢出;(2)可以通过碳基材料的高吸附性能提高反应速率和自由基的利用率。(3)可以优化反应条件(最佳pH在中性左右)。图6(b)和(e)主要展示了碳基材料通过两种途径氧化降解磺胺甲噁唑抗生素。一种途径是以自由基($\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ /$ \text{·}\text{OH} $ 为主)攻击污染物,另一种是以非自由基形式(单线态氧(1O2)为主)攻击污染物。然而,目前为止,关于非自由基途径的存在还是具有疑问的。有些学者认为非金属碳基材料活化过硫酸盐主要是通过电子转移进行的。Ren等[67]提出了一种电化学技术,用于研究CNT活化的PDS对有机物进行非自由基氧化的机理。结果发现,PDS最初被CNT催化以形成具有高氧化还原电位的CNT表面受限和活化的PDS(CNT-PDS*)复合物。然后,CNT-PDS*络合物选择性地从共吸附的酚类化合物中提取电子以引发氧化。表6总结了碳基材料活化PDS或PMS对水中抗生素的降解研究。碳质材料的改性可以显着提高去除抗生素的催化性能。常用的改性方法包括氮掺杂和金属(氧化物)掺杂。对于改性的含碳材料,sp2碳,氮掺杂剂和含氧官能团被认为是催化活性的起源[68]。Kang等[69]采用低温水热法制备出了掺杂N素的还原石墨烯(N-rGO)并用于活化PDS氧化降解抗生素磺胺氯哒嗪(SCP)。实验结果表明,室温(25 °C)时,当催化剂用量为0.2 g·L−1,PDS浓度为2 g·L−1,SCP初始浓度为20 mg·L−1时,经改性后的N-rGO活化PDS体系反应180 min后SCP即可得到完全的降解,比未改性的rGO/PDS体系降解率提高了35%。EPR实验结果也表明,N-rGO/PDS体系中SCP的降解是得益于$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 与·OH的共同作用。因此,后续对于碳基材料活化过硫酸盐氧化降解抗生素研究可以从以下三方面进行改善:(1)通过不同技术(化学探针技术、EPR技术、电化学技术等)对活化机理进行进一步的研究;(2)通过改进碳基材料的结构、成分组成、官能团等手段提高其对过硫酸盐的活化性能以达到高效去除抗生素的目的;(3)可以开展碳基材料与其他活化技术(光/电)联用,提高反应效率和对抗生素降解效果。 -
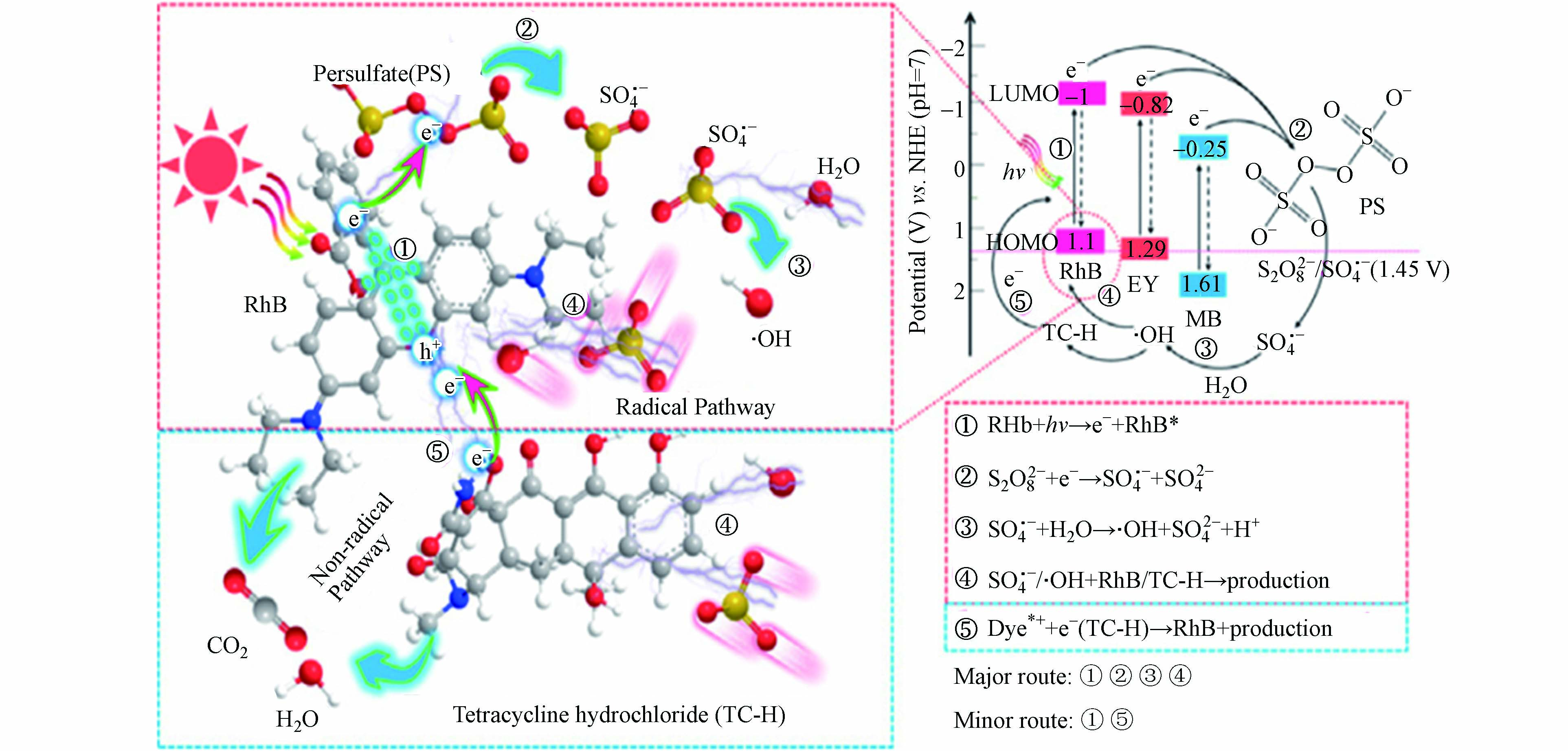
有机物活化是近几年才被发现的一种新型SR-AOPs活化方式。最初研究者在土壤修复过程中发现过硫酸盐能够被污染土壤中的某种或者多种物质活化,但矿物成分、溶解性的金属离子和一些有机物都有可能在这个过程中发挥作用。因此,对于具体产生作用的主体及其作用方式和机理并不清楚。有研究者确认了污染土壤中含有的一些有机物,甚至是污染物本身能够活化过硫酸盐产生强氧化性自由基[82]。Cai等[83]采用光激发染料(Dye)(罗丹明B(RhB)/曙红Y(EY)/亚甲基蓝(MB))活化过硫酸盐氧化降解四环素,其反应机制如图7所示。实验结果表明自由基反应是主要途径,这是由于染料的光生电子还原过硫酸盐引起的。染料介导的电子从污染物到氧化Dye(Dye*+)的转移是非自由基反应的原因。与EY和MB相比,RhB的负LUMO(最低未占用分子轨道)水平最低,促进了电子从RhB到PS的快速转移,从而导致了最高的活化效率。这项工作阐明了过硫酸盐活化的新途径,并为处理染料废水和含染料的复杂废水提供了新的“利用废物处理废物”策略。此外,有些学者还采用酮类、醇类、羧酸类、酚类、苯醌类等有机物活化过硫酸盐氧化降解有机污染物[84-86]。研究结果表明有机物活化过硫酸盐的效率与其分子中含有的官能团种类、位置、有机物本身的离子化程度及是否产生有机自由基(例如半苯醌有机自由基)有关。
-
除了上述各种过硫酸盐活化方式之外,近些年,还有学者采用了电化学活化、等离子体活化、微波活化、超声辐射活化等方式活化过硫酸盐氧化降解抗生素(表7)。从表7可以看出,这些活化方式都能有效活化过硫酸盐氧化降解抗生素。其中,电化学活化过硫酸盐研究最为广泛。然而,这些活化方式都需要反应装置,外加能量,这导致处理成本增加,不利于实际工程应用。此外,单一活化技术均存在不同程度的限制。近年来国内外研究者尝试用组合活化方式来协同强化过硫酸盐活化,提高抗生素的去除效率。一般组合方式包括活化方式的组合和双氧化剂体系的组合。针对抗生素,活化方式的组合有光-过渡金属离子活化[87]、超声-零价金属活化[88]、超声-金属氧化物活化[89]、光-金属氧化物活化[90]、电化学-金属氧化物活化[91]等。双氧化剂体系的组合主要是H2O2-PDS体系[92, 93]。活化方式的组合可以强化过硫酸盐活化效果,从而提高污染物去除效率,缩短氧化降解反应时间。而双氧化剂体系能较好地弥补单一活化方式的缺陷,强氧化剂相互之间形成了协同强化效应,提高了氧化体系的效能。
-
pH是研究过硫酸盐活化技术降解抗生素必不可少的影响因素。根据2.2节讨论,不同pH值时,主导反应自由基也不一样。因此,不同pH对抗生素的去除效果不一样。此外,pH对过硫酸盐非均相活化技术也有很大的影响。这主要原因是:(1)非均相催化剂的表面电荷与pH有关,这会影响它们的活化性能;(2)pH会影响非均相催化剂的存在形态,进而对其催化活性和氧化电位产生影响。最后,对于具有两性离子特性的抗生素,溶液的pH值会影响其种类以及表面电荷性质,会在不同抗生素形式之间产生吸引力或排斥力,最终影响降解效率。例如,Qi等[97]研究发现当溶液的pH值超过磺胺甲噁唑的酸度系数(pKa)5.7时,磺胺甲恶唑主要为负电荷形式,会产生排斥力,从而降低反应速率。因此,应根据过硫酸盐的活化方式调节适当的pH。
-
实际水体是复杂的基质,通常包含一些无机阴离子(如Cl−、
${\rm{HC{O}}_{{3^ - }}} $ 、${\rm{C{O}}_{{3^ - }}} $ 、${{\rm{H}}_2}{\rm{P{O}}_{{4^ - }}} $ 、${\rm{N{O}}_{{3^ - }}} $ 等)和天然有机物(如腐殖酸)[100]。这些无机离子可能会与$\text{S}{\text{O}}_{\text{4}}^{\text{•}-}$ 和$ \text{·}\text{OH} $ 反应产生低氧化还原电位的离子自由基,进而不同程度的影响抗生素的降解速率和效果[101]。此外,在这些无机阴离子中,${{\rm{H}}_2}{\rm{P{O}}_{{4^ - }}} $ 不仅可以淬灭自由基,还可以代替催化剂表面的羟基并吸收催化剂表面的过渡金属离子,形成内部球形络合物[102]。因此,与其他无机阴离子相比,磷酸盐对SR-AOPs去除抗生素的抑制作用更强。一般情况下,腐殖酸的存在会降低抗生素的降解效率。这可能与溶液的pH有关。在低pH下,腐殖酸清除了催化剂表面上的空穴;在高pH下,腐殖酸会猝灭自由基[14]。然而,有些天然有机物的成分(例如醌和苯酚)也可以有效活化过硫酸盐,提高抗生素去除效率。正如2.5节中所述。 -
反应过程产生的副产物也是影响SR-AOPs氧化降解抗生素的重要因素。副产物产生途径主要有两种:(1)氧化降解抗生素产生的中间产物;(2)一些水体中的成分自由基(氯自由基和硝酸根自由基)导致产生的氧化副产物(氯化副产物和硝化副产物)[103, 104]。副产物的产生不仅影响抗生素氧化降解速率和效果,还可能对环境和人类健康构成潜在威胁。因此,在后续的研究中需要完善抗生素降解副产物的毒性评价和分析方法,降低有毒物质的生成或消除副产物。
-
抗生素作为一类新兴的污染物,由于它们在自然环境中无处不在。因此,近年来受到越来越多的关注。而SR-AOPs作为一种新兴发展的水环境处理技术,其在抗生素废水修复中的应用受到了广泛关注和研究。在各种活化方法中,根据上述的概述,UV和金属氧化物活化是研究最广的方法。此外,碳基材料和有机物活化过硫酸盐氧化降解抗生素已展现出巨大的潜力。能量需求是影响SR-AOPs工程应用的重要因素。UV活化过硫酸盐可以在pH = 7的条件下使用,这可能推动其工程应用。然而,由于高能量需求,热活化和超声活化目前可能无法推广到实际应用。非均相催化剂活化需要进一步研究在实际废水条件下的性能和稳定性。而有机物的活化仅适用于含有相应化合物的特定废水。各种活化方式的经济成本也是一个重要因素。Zhu等[105]开发了一种新型材料海绵@ MoS2 @ GO(SMG)3D复合材料并构建SMG/Fe2+-PMS氧化体系用于中试试验去除磺胺嘧啶废水,研究发现连续反应16天后,磺胺嘧啶浓度从120 mg·L−1降至2.56 mg·L−1,去除率达到97.87%。此外,SMG/Fe2+-PMS氧化体系中PMS的经济成本仅为0.33美元。然而,对于Fe2+活化PDS,要考虑污泥的处理费用。因此,需要进一步研究以对各种活化方式的成本进行全面比较。最后,虽然SR-AOPs去除抗生素的研究取得了一些进展。然而,现有研究大多局限于处理模拟抗生素废水的小试试验,而实际工程应用研究(如中试试验)还很少,部分研究工作还有待加强:
(1)为了增强对水中抗生素的去除,深入研究一些组合活化技术或双氧化体系的反应机制;此外,可以根据抗生素结构和特点,选择有针对性的活化方式。
(2)可以将SR-AOPs与其他水处理技术(光催化、吸附、生物处理技术等)耦合增强抗生素的矿化作用,降低运行成本,提高处理效率。
(3) 采用过硫酸盐高级氧化技术去除抗生素抗性基因(ARG)和抗生素抗性细菌(ARB)的研究还比较少,需要进一步加强该方面实验研究。
(4)需要明确一些非均相催化剂(如非金属等)活化过硫酸盐的机理,可以利用一些跨学科方法和技术(如DFT计算或电化学等)对其进行深入研究。
(5)为了弥补一些非均相催化剂的不足(如活化效率低、易产生二次污染等),制备高效、环保、稳定且易回收的新型非均相催化剂。
(6)SR-AOPs要将TOC转化率或矿化率作为抗生素的去除标准,而非抗生素的去除率。此外,要更加完善抗生素降解中间产物的毒性评价和分析方法,降低有毒物质的生成,向绿色环保方向发展;
(7)进一步阐明SR-AOPs处理实际废水以及共存有机污染物等混合水体的作用机制,采取相应措施减少无机化合物等因素影响,分析如何降低SR-AOPs的处理成本,推动该技术的实际工程应用。
(8) 有机物本身活化过硫酸盐技术研究还处于起步阶段,需要进一步加强该方面的研究。这将会提高过硫酸盐活化氧化污染物的效率,减少活化剂的使用,极大地降低抗生素废水处理成本。
过硫酸盐的活化及其在氧化降解水中抗生素的机理和应用
Activation of persulfate and its mechanism and application in oxidative degradation of antibiotics in water
-
摘要: 抗生素是一类用于阻止和治疗微生物传染性疾病的人用和兽用药物,在人类和动物疾病治疗领域以及水产养殖业有着广泛的用途。近年来,抗生素作为一种新型污染物不断排入水体并且在水体中持续存在,对水生态环境以及人类健康造成了威胁。基于硫酸根自由基(
$\text{S}{\text{O}}_{\text{4}}^{\text{•}{-}}$ )的高级氧化技术因其快速高效、适用范围广等特点,其在处理抗生素废水已经成为国内外研究热点。本文综述了近年来国内外利用UV、热、过渡金属、金属氧化物、零价金属、碳基材料、有机物、组合方式等常规及新型活化方法活化过硫酸盐以及处理抗生素废水的研究进展,并讨论了不同活化方式对抗生素的降解效率及机理的影响,最后展望了过硫酸盐高级氧化技术应用于抗生素降解的研究方向和挑战。Abstract: Antibiotics are a class of human and veterinary drugs used to prevent and treat microbial infectious diseases. They are widely used in the treatment of human and animal diseases and in the aquaculture industry. In recent years, antibiotics, as a new type of pollutant, have been continuously discharged into water bodies and continue to exist in water bodies, posing a threat to the aquatic environment and human health. The sulfate radical-based advanced oxidation processes have become a research hotspot in the treatment of antibiotic wastewater due to its rapid, high-efficiency and wide application range. This article reviews the research progress in the treatment of antibiotic wastewater using conventional and new persulfate activation methods such as UV, heat, transition metals, metal oxides, zero-valent metals, carbon-based materials, organic compounds, and combination methods at domestic and foreign, and the effects of different activation methods on the degradation efficiency and mechanism of antibiotics are discussed. Finally, the research directions and challenges of the application of persulfate advanced oxidation technology to antibiotic degradation are prospected.-
Key words:
- antibiotics /
- persulfate /
- catalytic oxidation /
- activation method /
- mechanism analysis
-

-
图 5 在nZVT/Cu(Ⅱ)/PMS体系中产生氧化自由基的机制[59]
Figure 5. The mechanism of generating oxidative free radicals in the nZVT/Cu(Ⅱ)/PMS system [59]
表 1 UV活化过硫酸盐用于降解抗生素的研究进展
Table 1. Research progress of ultraviolet light activated persulfate for degradation of antibiotics
抗生素
Antibiotics源水
Source water抗生素浓度
Antibiotic concentration过硫酸盐浓度
Persulfate concentration波长/nm
WavelengthpH 反应时间/min
Reaction time降解率/%
Degradation rate研究者
Researcher磺胺二甲嘧啶 Milli-Q水 0.02 mmol·L−1 0.2 mmol·L−1 PDS 254 6.5 45 90 Gao等[25] 磺胺甲噁唑 去离子水 20 μmol·L−1 1 mmol·L−1 PDS 254 8 120 100 Yang等[26] 氯霉素 去离子水 31 μmol·L−1 0.25 mmol·L−1 PDS 254 6.07 60 100 Ghauch等[27] 环丙沙星 Milli-Q水 50 μmol·L−1 1 mmol·L−1 PDS 254 7 60 100 Moussa等[28] 甲砜霉素 去离子水 10 μmol·L−1 1 mmol·L−1 PDS 254 7 90 100 Wang等[29] 诺氟沙星 Milli-Q水 5 μmol·L−1 100 μmol·L−1 PDS 254 7 5 100 Xue等[30] 表 2 过硫酸盐热活化用于降解抗生素的研究进展
Table 2. Research progress of persulfate thermal activation for degradation of antibiotics
抗生素
Antibiotics源水
Source water抗生素浓度
Antibiotic concentration过硫酸盐浓度
Persulfate concentration温度/℃
TemperaturepH 反应时间/h
Reaction time降解率/%
Degradation rate研究者
Researcher磺胺二甲嘧啶 Milli-Q水 0.03 mmol·L−1 2.0 mmol·L−1 PDS 60 7.0 6 100 Fan等[34] 氯霉素 去离子水 0.2 mmol·L−1 16 mmol·L−1 PDS 70 5.4 2.7 96.3 Nie等[35] 氟康唑 超纯水 10 mg·L−1 20 mmol·L−1 PDS 60 3 4 87 Yang等[36] 四环素 人工地表水 0.03 mmol·L−1 2.0 mmol·L−1 PDS 70 7.0 1.5 100 Ji等[32] 卡巴多司 Milli-Q水 30 μmol·L−1 2.0 mmol·L−1 PDS 60 7.0 4 98.6 李轶涵等[37] 青霉素G 双重蒸馏水 0.02 mmol·L−1 0.5 mmol·L−1 PDS 60 5.0 1.25 97.25 Norzaee等[38] 头孢氨苄 去离子水 0.1 mmol·L−1 1.1 mmol·L−1 PDS 60 7.0 4 100 Qian等[39] 环丙沙星 Milli-Q水 30 μmol·L−1 2.0 mmol·L−1 PDS 60 7.0 3 92 Jiang等[40] 表 3 过渡金属离子活化过硫酸盐用于降解抗生素的研究进展
Table 3. Research progress of persulfate activated by transition metal ions for degradation of antibiotics
抗生素
Antibiotics源水
Source water抗生素浓度
Antibiotic concentration过硫酸盐浓度
Persulfate concentration过渡金属离子
Transition
metal ionpH 反应时间/h
Reaction time降解率/%
Degradation rate研究者
Researcher磺胺甲噁唑 去离子水 20 mg·L−1 25 mmol·L−1 PDS Fe2+ 2.5 mmol·L−1 3.3 4 100 Luo等[41] 环丙沙星/
磺胺甲噁唑Milli-Q水 30 μmol·L−1 600 μmol·L−1 PDS Fe2+ 600 μmol·L−1 6.0 4 95.6/95.8 Ji等[42] 阿莫西林 Milli-Q水 50 μmol·L−1 600 μmol·L−1 PDS Fe2+ 600 μmol·L−1 3 1 89.69 Matta等[43] 表 4 金属氧化物活化过硫酸盐用于降解抗生素的研究进展
Table 4. Research progress of metal oxide activated persulfate for degradation of antibiotics
抗生素
Antibiotics源水
Source water抗生素浓度
Antibiotic concentration过硫酸盐浓度
Persulfate concentration金属氧化物
Metal oxidepH 反应时间/min
Reaction
time降解率/%
Degradation
rate研究者
Researcher四环素 超纯水 20 mg·L−1 42.0 µmol·L−1
PDSNi0.6Fe2.4O4
350 mg·L−17 35 86 Guan等[50] 磺胺甲噁唑 超纯水 1.6 mg·L−1 40 mg·L−1
PMSα-Fe2O3
0.4 g·L−16.8 180 100 Feng等[51] 环丙沙星 超纯水 50 mg·L−1 1.0 g·L−1
PDSFe3O4
2.0 g·L−17 40 93.73 Jiang等[52] 磺胺二甲嘧啶 去离子水 50 mg·L−1 1 mmol·L−1
PMSCuCo2O4
0.1 g·L−15 30 98 Chen等[53] 头孢氨苄/
奥沙星— 10 mg·L−1 1 mmol·L−1
PDSCuO
0.5 g·L−18 30 80/92 Li等[54] 诺氟沙星 去离子水 25 μmol·L−1 0.5 mmol·L−1
PMSCuFe2O4
0.2 g·L−17 120 90 Wang等[55] 磺胺氯哒嗪 超纯水 5 mg·L−1 0.5 mmol·L−1
PMSCoFe2O4/Al2O3
1.0 mmol·L−17 60 97.8 Wang等[56] 洛美沙星 去离子水 10 mg·L−1 0.49 mmol·L−1
PMSCo3O4/δ-FeOOH
0.25 g·L−16.08 25 82 Zhang等[47] 表 5 零价金属活化过硫酸盐用于降解抗生素的研究进展
Table 5. Research progress of zero-valent metal activated persulfate for degradation of antibiotics
抗生素
Antibiotics源水
Source water抗生素浓度
Antibiotic concentration过硫酸盐浓度
Persulfate concentration零价金属
Zero-valent metalpH 反应时间/min
Reaction time降解率/%
Degradation rate研究者
Researcher四环素 去离子水 20 mg·L−1 0.08 mmol·L−1 PMS Fe0
0.01 g·L−13.0 5 88.5 Cao等[58] 环丙沙星 原地表水 2 mg·L−1 0.25 mmol·L−1 PDS Al0
1 g·L−13.0 120 73 Olmez-Hanci等[63] 环丙沙星 超纯水 10 mg·L−1 50 mg·L−1 PDS Mn0
1.0 g·L−12.0 80 95 S. Shah等[62] 氯霉素 去离子水 10 mg·L−1 0.2 mmol·L−1 PMS Fe0
0.5 g·L−17.0 120 95.2 Tan等[64] 磺胺嘧啶 去离子水 20 μmol·L−1 1 mmol·L−1 PDS Fe0
1 mmol·L−17.0 10 83.5 Yang等[65] 诺氟沙星 超纯水 31.32 μmol·L−1 1.0 mmol·L−1 PDS Cu0
0.05 g·L−17.0 5 95.46 Deng等[61] 表 6 碳基材料活化过硫酸盐用于降解抗生素的研究进展
Table 6. Research progress of activated persulfate of carbon-based materials for degradation of antibiotics
抗生素
Antibiotics源水
Source water抗生素浓度
Antibiotic concentration过硫酸盐浓度
Persulfate concentration碳基材料
Carbon-based materialspH 反应时间/min
Reaction time降解率/%
Degradation rate研究者
Researcher磺胺氯哒嗪 去离子水 20 mg·L−1 2 g·L−1 PDS N-rGO0.2 g·L−1 8.5 180 100 Kang等[69] 磺胺甲噁唑 去离子水 5 mg·L−1 1 mmol·L−1 PDS N-GP0.05 g·L−1 6 180 99.9 Hao等[75] 氯霉素 纯水 30 mg·L−1 10 mmol·L−1 PDS Co3O4/BC0.2 g·L−1 7 10 97.6 Xu等[76] 甲硝唑 去离子水 0.58 mmol·L−1 58 mmol·L−1PDS GAC5 g·L−1 3.9 240 80 Forouzesh等[70] 诺氟沙星 去离子水 15 μmol·L−1 0.5 mmol·L−1 PMS CoFe2O4/GO0.3 g·L−1 7 20 100 Chen等[77] 环丙沙星 去离子水 30 mg·L−1 2 mmol·L−1 PMS CuFe2O4/GO 0.2 g·L−1 7 60 98 Noroozi等[78] 四环素 去离子水 1 mmol·L−1 60 mmol·L−1 PDS Fe-SCG2.5 g·L−1 2.0 120 96 Nguyen等[79] 左氧氟沙星 去离子水 10 mg·L−1 1.8 g·L−1 PDS NiFe2O4/CS0.6 g·L−1 5 105 67 Wang等[80] 甲氧苄啶 — 0.02 mmol·L−1 0.6 mmol·L−1 PMS CuFe2O4/MWCNTs
0.2 g·L−17 24 90 Kong等[81] 表 7 其他活化过硫酸盐方式用于降解抗生素的研究进展
Table 7. Research progress of other methods of activating persulfate for degradation of antibiotics
活化方式
Activation method污染物
Pollutants研究结果
Research results参考文献
References电化学(EC) 环丙沙星
(CIP)当CIP浓度为20 mg·L−1,电流密度为2.75 mA·cm−2,PDS浓度0.84 mmol·L−1,
pH = 7,反应40 min后CIP去除率为90%Malakootian等[94] 四环素
(TC)当TC浓度为50 mg·L−1,电流密度为13.33 mA·cm−2,PDS浓度12.6 mmol·L−1,
pH = 4.42,反应240 min后TC去除率为81.1%Liu等[95] 气相表面放
电等离子体四环素
(TC)当TC浓度为40 mg·L−1,PDS与TC的摩尔比为20:1, 峰值电压为7 kV,风量为1.0 L·min−1,
pH = 5.3时,反应15 min后TC去除率为87.5%Tang等[96] 微波(MW) 磺胺甲噁唑
(SMX)当SMX浓度为0.5 mmol·L−1,PDS浓度0.25 mmol·L−1,
pH = 4.7,温度为90℃时,反应60min后SMX去除率为81.4%Qi等[97] 超声辐射 四环素
(TC)当TC浓度为0.052 mmol·L−1,PDS浓度4 mmol·L−1,pH = 10,
超声波频率为35 KHz和超声波功率为 500 W时,反应120min后TC去除率为96.5%Nasseri等[98] 磺胺二甲嘧啶
(SMT)当SMT浓度为50 mg·L−1,PMS浓度0.6 g·L−1,pH = 7.5,
超声波频率为20 KHz和超声波功率为 600 W时,反应30min后SMT去除率为99.6%Yin等[99] -
[1] DEMAIN A. L, SANCHEZ S. Microbial drug discovery: 80 years of progress [J]. The Journal of Antibiotics, 2009, 62: 5-16. doi: 10.1038/ja.2008.16 [2] MARTINEZ J. L. Environmental pollution by antibiotics and by antibiotic resistance determinants [J]. Environmental Pollution, 2009, 157: 2893-2902. doi: 10.1016/j.envpol.2009.05.051 [3] NISHA A R. Antibiotic Residues - A Global Health Hazard [J]. Veterinary World, 2008, 1(12): 375-377. [4] FOCAZIO M. J, KOLPIN D. W, BARNES K. K, et al. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States — II) Untreated drinking water sources [J]. Science of The Total Environment, 2008, 402: 201-216. doi: 10.1016/j.scitotenv.2008.02.021 [5] CHEN W R, DING Y, JOHNSTON C. Johnston, et al. Reaction of Lincosamide Antibiotics with Manganese Oxide in Aqueous Solution [J]. Environmental Science & Technology, 2010, 44: 4486-4492. [6] XU Y, GUO C, LUO Y, et al. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing [J]. China Environmental Pollution, 2016, 213: 833-840. doi: 10.1016/j.envpol.2016.03.054 [7] SZEKERES E, CHIRIAC C M, BARICZ A, et al. Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas [J]. Environment Pollution, 2018, 236: 734-744. doi: 10.1016/j.envpol.2018.01.107 [8] DANNER M C, ROBERTSON A, BEBRENDS V, et al. Antibiotic pollution in surface fresh waters: Occurrence and effects [J]. Science of The Total Environment, 2019, 664C: 793-804. [9] GERQUEIRA F, MATAMOROS V, BAYONA J, et al. Distribution of antibiotic resistance genes in soils and crops. A field study in legume plants (Vicia faba L. ) grown under different watering regimes [J]. Environmental Research, 2019, 170: 16-25. doi: 10.1016/j.envres.2018.12.007 [10] SANGANYADO E, GWENZI W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks [J]. Science of Total Environment, 2019, 669: 785-797. doi: 10.1016/j.scitotenv.2019.03.162 [11] KUNNERER K. Antibiotics in the aquatic environment – A review – Part II [J]. Chemosphere, 2009, 75: 435-441. doi: 10.1016/j.chemosphere.2008.12.006 [12] FLANAGAN J, GRIFFITH W, SKAPSKI A. The active principle of Caro's acid, HSO5–: X-ray crystal structure of KHSO5·H2O [J]. J. Chem. Soc. , Chem. Commun, 1984, 23: 1574-1575. [13] KOLTHOFF I, MILLER K. The chemistry of persulfate. I. The kinetics and mechanism of the decomposition of the persulfate ion in aqueous medium1 [J]. Journal of the American Chemical Society, 1951, 73(7): 1-30. [14] WANG J, WANG S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants [J]. Chemical Engineering Journal, 2018, 334: 1502-1517. doi: 10.1016/j.cej.2017.11.059 [15] TSITONAKI A, PETRI B, CRIMI M, et al. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review [J]. Critical Reviews in Environmental Science and Technology, 2010, 40: 55-91. doi: 10.1080/10643380802039303 [16] YANG Q, MA Y, CHEN F, et al. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water [J]. Chemical Engineering Journal, 2019, 378: 122-149. [17] MALATO S, FERNANDEZ-IBANEZ P, MALDONADO M I, et al. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends [J]. Catalysis Today, 2009, 147: 1-59. doi: 10.1016/j.cattod.2009.06.018 [18] MAURINO V, CALZA P, MINERO C, et al. Light-assisted 1, 4-dioxane degradation [J]. Chemosphere, 1997, 35: 2675-2688. doi: 10.1016/S0045-6535(97)00322-6 [19] ZHOU X, LIU D, ZHANG Y, et al. Degradation mechanism and kinetic modeling for UV/peroxydisulfate treatment of penicillin antibiotics [J]. Chemical Engineering Journal, 2018, 341: 93-101. doi: 10.1016/j.cej.2018.01.137 [20] YE J S, LIU J, OU H S, WANG L. Degradation of ciprofloxacin by 280 nm ultraviolet-activated persulfate: Degradation pathway and intermediate impact on proteome of Escherichia coli [J]. Chemosphere, 2016, 165: 311-319. doi: 10.1016/j.chemosphere.2016.09.031 [21] CUI C, JIN L, JIANG L, et al. Removal of trace level amounts of twelve sulfonamides from drinking water by UV-activated peroxymonosulfate [J]. Science of The Total Environment, 2016, 572: 244-251. doi: 10.1016/j.scitotenv.2016.07.183 [22] SERNA-GALVIS E, FERRARO F, SILVA-AGREDO J, et al. Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV 254 and UV 254/persulfate processes[J]. Water Research, 122(1): 128-138. [23] ZHANG Y, LI L, PAN Z, et al. Degradation of sulfamethoxazole by UV/persulfate in different water samples: Influential factors, transformation products and toxicity [J]. Chemical Engineering Journal, 2019, 379: 122354. [24] ZHU Y, WEI M, PAN Z, et al. Ultraviolet/peroxydisulfate degradation of ofloxacin in seawater: Kinetics, mechanism and toxicity of products [J]. Science of The Total Environment, 2020, 705: 135960. doi: 10.1016/j.scitotenv.2019.135960 [25] GAO Y Q, GAO N Y, DENG Y, et al. Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water [J]. Chemical Engineering Journal, 2012, 195/196: 248-253. doi: 10.1016/j.cej.2012.04.084 [26] YANG Y, LU X, JIANG J, et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate [J]. Water Research, 2017, 118: 196-207. doi: 10.1016/j.watres.2017.03.054 [27] GHAUCH A, BAALBAKI A, AMASHA M, et al. Contribution of persulfate in UV-254nm activated systems for complete degradation of chloramphenicol antibiotic in water [J]. Chemical Engineering Journal, 2017, 317: 1012-1025. doi: 10.1016/j.cej.2017.02.133 [28] MAHDI-AHMED M, CHIRON S. Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater [J]. Journal of Hazardous Materials, 2014, 265: 41-46. doi: 10.1016/j.jhazmat.2013.11.034 [29] WANG F, WANG W, YUAN S, et al. Comparison of UV/H2O2 and UV/PS processes for the degradation of thiamphenicol in aqueous solution [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2017, 348: 79-88. doi: 10.1016/j.jphotochem.2017.08.023 [30] XUE H, GAO S, ZHENG N, et al. Degradation of norfloxacin in aqueous solution with UV/peroxydisulfate [J]. Water Science and Technology, 2019, 79: 2387-2394. doi: 10.2166/wst.2019.240 [31] ZHAO D, LIAO X, YAN X, et al. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil [J]. Journal of Hazardous Materials, 2013, 254/255: 228-235. doi: 10.1016/j.jhazmat.2013.03.056 [32] JI Y, SHI Y, DONG W, et al. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution [J]. Chemical Engineering Journal, 2016, 298: 225-233. doi: 10.1016/j.cej.2016.04.028 [33] QIAN Y, LIU X, LI K, et al. Enhanced degradation of cephalosporin antibiotics by matrix components during thermally activated persulfate oxidation process [J]. Chemical Engineering Journal, 2019, 384: 123332. [34] FAN Y, JI Y, KONG D, et al. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process [J]. Journal of Hazardous Materials, 2015, 300: 39-47. doi: 10.1016/j.jhazmat.2015.06.058 [35] NIE M, YANG Y, ZHANG Z, et al. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution [J]. Chemical Engineering Journal, 2014, 246: 373-382. doi: 10.1016/j.cej.2014.02.047 [36] YANG J F, YANG L M, ZHANG S B, et al. Degradation of azole fungicide fluconazole in aqueous solution by thermally activated persulfate [J]. Chemical Engineering Journal, 2017, 321: 113-122. doi: 10.1016/j.cej.2017.03.103 [37] 李轶涵, 姜恬, 周旭, 等. 热活化过硫酸盐氧化降解水溶液中的抗生素卡巴多司和奥喹多司 [J]. 环境科学学报, 2019, 39: 3821-3831. LI Y H, JIANG T, ZHOU X, et al. Thermally activated persulfate oxidation of antibiotics carbadox and olaquindox in aqueous solution [J]. Acta Scientiae Circumstantiae, 2019, 39: 3821-3831(in Chinese).
[38] NORZAEE S, TAGHAVI M, DJAHED B, et al. Degradation of penicillin G by heat activated persulfate in aqueous solution [J]. Journal of Environmental Management, 2018, 215: 316-323. [39] QIAN Y, XUE G, CHEN J, et al. Oxidation of cefalexin by thermally activated persulfate: Kinetics, products, and antibacterial activity change [J]. Journal of Hazardous Materials, 2018, 354: 153-160. doi: 10.1016/j.jhazmat.2018.05.004 [40] JIANG C, JI Y, SHI Y, et al. Sulfate radical-based oxidation of fluoroquinolone antibiotics: Kinetics, mechanisms and effects of natural water matrices [J]. Water Research, 2016, 106: 507-517. doi: 10.1016/j.watres.2016.10.025 [41] LUO T, WAN J, MA Y, et al. Sulfamethoxazole degradation by Fe(II)-activated persulfate process: Insight into the reactive sites, products identification and degradation pathways [J]. Environmental Science:Processes & Impacts, 2019: 21. [42] JI Y, FERRONATO C, SALVADOR A, et al. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics [J]. Science of The Total Environment, 2014, 472: 800-808. doi: 10.1016/j.scitotenv.2013.11.008 [43] MATTA R, YOUNES H, HANNA R, et al. Sulfate radicals mediated oxidation of amoxicillin: Optimization of key parameters [J]. Journal of Environmental Management, 2019, 245: 375-383. doi: 10.1016/j.jenvman.2019.05.030 [44] DING Y, TANG H, ZHANG S, et al. Efficient degradation of carbamazepine by easily recyclable microscaled CuFeO2 mediated heterogeneous activation of peroxymonosulfate [J]. Journal of Hazardous Materials, 2016, 317: 686-694. doi: 10.1016/j.jhazmat.2016.06.004 [45] HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications [J]. Applied Catalysis B:Environmental, 2016, 181: 103-117. doi: 10.1016/j.apcatb.2015.07.024 [46] ZHANG H, SONG Y, NENGZI L C, et al. Activation of persulfate by a novel magnetic CuFe2O4/Bi2O3 composite for lomefloxacin degradation [J]. Chemical Engineering Journal, 2019, 379: 122362. [47] ZHANG H, WANG J, ZHANG X, et al. Enhanced removal of lomefloxacin based on peroxymonosulfate activation by Co3O4/δ-FeOOH composite [J]. Chemical Engineering Journal, 2019, 369: 834-844. doi: 10.1016/j.cej.2019.03.132 [48] CHEN L, ZUO X, ZHOU L, et al. Efficient heterogeneous activation of peroxymonosulfate by facilely prepared Co/Fe bimetallic oxides: Kinetics and mechanism [J]. Chemical Engineering Journal, 2018, 345: 364-374. doi: 10.1016/j.cej.2018.03.169 [49] LI Z, GUO C, LYU J, et al. Tetracycline degradation by persulfate activated with magnetic Cu/CuFe2O4 composite: Efficiency, stability, mechanism and degradation pathway [J]. Journal of Hazardous Materials, 2019, 373: 85-96. doi: 10.1016/j.jhazmat.2019.03.075 [50] GUAN R, YUAN X, WU Z, et al. Accelerated tetracycline degradation by persulfate activated with heterogeneous magnetic NixFe3−xO4 catalysts [J]. Chemical Engineering Journal, 2018, 350: 573-584. doi: 10.1016/j.cej.2018.05.195 [51] FENG Y, LIAO C, LI H, et al. Cu2O-promoted degradation of sulfamethoxazole by α-Fe2O3-catalyzed peroxymonosulfate under circumneutral conditions: synergistic effect, Cu/Fe ratios, and mechanisms [J]. Environmental Technology, 2018, 39: 1-11. doi: 10.1080/09593330.2017.1293164 [52] JIANG S, ZHU J, WANG Z, et al. Efficiency and mechanism of ciprofloxacin hydrochloride degradation in wastewater by Fe3O4 /Na2S2O8 [J]. Ozone:Science & Engineering, 2018, 40: 1-8. [53] CHEN C, LIU L, LI Y, et al. Insight into heterogeneous catalytic degradation of sulfamethazine by peroxymonosulfate activated with CuCo2O4 derived from bimetallic oxalate [J]. Chemical Engineering Journal, 2020, 384: 123257. doi: 10.1016/j.cej.2019.123257 [54] LI W, WU Y, GAO Y, et al. Mechanism of persulfate activation with CuO for removing cephalexin and ofloxacin in water [J]. Research on Chemical Intermediates, 2019, 45: 5549-5558. doi: 10.1007/s11164-019-03919-9 [55] WANG Y, TIAN D, CHU W, et al. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin [J]. Separation and Purification Technology, 2019, 212: 536-544. doi: 10.1016/j.seppur.2018.11.051 [56] WANG Q, SHAO Y, GAO N, et al. Activation of peroxymonosulfate by Al2O3-based CoFe2O4 for the degradation of sulfachloropyridazine sodium: Kinetics and mechanism [J]. Separation and Purification Technology, 2017, 189: 176-185. doi: 10.1016/j.seppur.2017.07.046 [57] LI R, JIN X, MEGHARAI M, et al. Heterogeneous Fenton oxidation of 2, 4-dichlorophenol using iron-based nanoparticles and persulfate system [J]. Chemical Engineering Journal, 2015, 264: 587-594. doi: 10.1016/j.cej.2014.11.128 [58] GAO J, LAI L, LAI B, et al. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism [J]. Chemical Engineering Journal, 2019, 364: 45-56. doi: 10.1016/j.cej.2019.01.113 [59] YE Q, XU H, ZHANG J, et al. Enhancement of peroxymonosulfate activation for antibiotics removal by nano zero valent tungsten induced Cu(II)/Cu(I) redox cycles [J]. Chemical Engineering Journal, 2020, 382: 123054. doi: 10.1016/j.cej.2019.123054 [60] PULICHARLA R, DROUINAUD R, BRAR S K, et al. Activation of persulfate by homogeneous and heterogeneous iron catalyst to degrade chlortetracycline in aqueous solution [J]. Chemosphere, 2018, 207: 543-551. doi: 10.1016/j.chemosphere.2018.05.134 [61] DENG J, XU M, CHEN Y, et al. Highly-efficient removal of norfloxacin with nanoscale zero-valent copper activated persulfate at mild temperature [J]. Chemical Engineering Journal, 2019, 366: 491-503. doi: 10.1016/j.cej.2019.02.073 [62] SHAH N S, KHAN J A, SAYED M, et al. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82− [J]. Chemical Engineering Journal, 2019, 356: 199-209. doi: 10.1016/j.cej.2018.09.009 [63] OLMEZ-HANCI T, ARSLAN-ALATON I, DOAN M, et al. Enhanced degradation of micropollutants by zero-valent aluminum activated persulfate: Assessment of toxicity and genotoxic activity [J]. Water Science and Technology, 2017, 76: 2017489. [64] CHAOQUN T, DONG Y, FU D, et al. Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: Kinetics and mechanism of radical generation [J]. Chemical Engineering Journal, 2017, 334: 1006-1015. [65] YANG S, CHE D. Degradation of aquatic sulfadiazine by Fe0/persulfate: Kinetics, mechanisms, and degradation pathway [J]. RSC Advances, 2017, 7: 42233-42241. doi: 10.1039/C7RA07920F [66] REN X, CHEN C, NAGATSU M, et al. Carbon nanotubes as adsorbents in environmental pollution management: A review [J]. Chemical Engineering Journal, 2011, 170: 395-410. doi: 10.1016/j.cej.2010.08.045 [67] REN W, XIONG L, YUAN X, et al. Activation of peroxydisulfate on carbon nanotubes: electron-transfer mechanism [J]. Environmental Science & Technology, 2019, 53: 14595-14603. [68] SUN H, KWAN C, SUVOROVA A, et al. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals [J]. Applied Catalysis B:Environmental, 2014, 154/155: 134-141. doi: 10.1016/j.apcatb.2014.02.012 [69] KANG J, DUAN X, ZHOU L, et al. Carbocatalytic activation of persulfate for removal of antibiotics in water solutions [J]. Chemical Engineering Journal, 2016, 288: 399-405. doi: 10.1016/j.cej.2015.12.040 [70] FOROUZESH M, EBADI A, AGHAEINEJAD-MEYBODI A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator [J]. Separation and Purification Technology, 2019, 210: 145-151. doi: 10.1016/j.seppur.2018.07.066 [71] SHANG Y, CHEN C, ZHANG P, et al. Removal of sulfamethoxazole from water via activation of persulfate by Fe3C@NCNTs including mechanism of radical and nonradical process [J]. Chemical Engineering Journal, 2019, 375: 122004. doi: 10.1016/j.cej.2019.122004 [72] NGUYEN V T, NGUYEN T B, CHEN C W, et al. Cobalt-impregnated biochar (Co-SCG) for heterogeneous activation of peroxymonosulfate for removal of tetracycline in water [J]. Bioresource Technology, 2019, 292: 121954. doi: 10.1016/j.biortech.2019.121954 [73] JIANG X, GUO Y, ZHANG L, et al. Catalytic degradation of tetracycline hydrochloride by persulfate activated with nano Fe0 immobilized mesoporous carbon [J]. Chemical Engineering Journal, 2018, 341: 392-401. doi: 10.1016/j.cej.2018.02.034 [74] WANG S, XU L, WANG J. Nitrogen-doped graphene as peroxymonosulfate activator and electron transfer mediator for the enhanced degradation of sulfamethoxazole [J]. Chemical Engineering Journal, 2019, 375: 122041. doi: 10.1016/j.cej.2019.122041 [75] CHEN H, CARROLL K C. Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene [J]. Environmental Pollution, 2016, 215: 96-102. doi: 10.1016/j.envpol.2016.04.088 [76] XU H, ZHANG Y, LI J, et al. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols [J]. Environmental Pollution, 2020, 257: 113610. doi: 10.1016/j.envpol.2019.113610 [77] CHEN L, DING D, LIU C, et al. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration [J]. Chemical Engineering Journal, 2017, 334: 273-284. [78] NOROOZI R, GHOLAMI M, FARZADKIA M, et al. Degradation of ciprofloxacin by CuFe2O4/GO activated PMS process in aqueous solution: performance, mechanism and degradation pathway [J]. International Journal of Environmental Analytical Chemistry, 2020: 1-22. [79] TRUC N, HUNG C M, NGUYEN B, et al. Efficient heterogeneous activation of persulfate by iron-modified biochar for removal of antibiotic from aqueous solution: a case study of tetracycline removal [J]. Catalysts, 2019, 49: 9-23. [80] WANG Z, ZHANG X, ZHANG H, et al. Synthesis of magnetic nickel ferrite/carbon sphere composite for levofloxacin elimination by activation of persulfate [J]. Separation and Purification Technology, 2019, 215: 528-539. doi: 10.1016/j.seppur.2019.01.063 [81] KONG J, LI R, WANG F, et al. Sulfate radical-induced transformation of trimethoprim with CuFe2O4/MWCNTs as a heterogeneous catalyst of peroxymonosulfate: mechanisms and reaction pathways [J]. RSC Advances, 2018, 8: 24787-24795. doi: 10.1039/C8RA04103B [82] AHMAD M, TEEL A L, WATTS R J. Persulfate activation by subsurface minerals [J]. Journal of Contaminant Hydrology, 2010, 115: 34-45. doi: 10.1016/j.jconhyd.2010.04.002 [83] CAI T, LIU Y, WANG L, et al. Activation of persulfate by photoexcited dye for antibiotic degradation: Radical and nonradical reactions [J]. Chemical Engineering Journal, 2019, 375: 122070. doi: 10.1016/j.cej.2019.122070 [84] OCAMPO A M. Persulfate activation by organic compounds[D]. Pullman: Washington State University, 2009. [85] AHMAD M, TEEL A, WATTS, R J. Mechanism of Persulfate Activation by Phenols[J]. Environmental Science & Technology 2013, 47: 5864-5871. [86] FANG G, GAO J, DIONYSIOU D D, et al. Activation of Persulfate by Quinones: Free Radical Reactions and Implication for the Degradation of PCBs [J]. Environmental Science & Technology, 2013, 47: 4605-4611. [87] NIE M, YAN C, XIONG X, et al. Degradation of chloramphenicol using a combination system of simulated solar light, Fe2+ and persulfate [J]. Chemical Engineering Journal, 2018, 348: 455-463. doi: 10.1016/j.cej.2018.04.124 [88] PAN Y, ZHANG Y, ZHOU M, et al. Synergistic degradation of antibiotic sulfamethazine by novel pre-magnetized Fe0/PS process enhanced by ultrasound [J]. Chemical Engineering Journal, 2018, 354: 777-789. doi: 10.1016/j.cej.2018.08.084 [89] HOU L, ZHANG H, XUE X. Ultrasound enhanced heterogeneous activation of peroxydisulfate by magnetite catalyst for the degradation of tetracycline in water [J]. Separation and Purification Technology, 2012, 84: 147-152. doi: 10.1016/j.seppur.2011.06.023 [90] KAUR B, KUNTUS L, TIKKER P, et al. Photo-induced oxidation of ceftriaxone by persulfate in the presence of iron oxides [J]. Science of The Total Environment, 2019, 676: 165-175. doi: 10.1016/j.scitotenv.2019.04.277 [91] TANG S, ZHAO M, YUAN D, et al. MnFe2O4 nanoparticles promoted electrochemical oxidation coupling with persulfate activation for tetracycline degradation [J]. Separation and Purification Technology, 2021, 255: 117690. doi: 10.1016/j.seppur.2020.117690 [92] WANG S, WANG J. Trimethoprim degradation by Fenton and Fe(II)-activated persulfate processes [J]. Chemosphere, 2018, 191: 97-105. doi: 10.1016/j.chemosphere.2017.10.040 [93] WU J, WANG B, CAGNETTA G, et al. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation [J]. Separation and Purification Technology, 2020, 239: 116534. doi: 10.1016/j.seppur.2020.116534 [94] MALAKOOTIAN M, AHMADIAN M. Removal of ciprofloxacin from aqueous solution by electro-activated persulfate oxidation using aluminum electrodes [J]. Water Science and Technology, 2019, 80(3): 587-596. doi: 10.2166/wst.2019.306 [95] LIU J, ZHONG S, SONG Y, et al. Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation [J]. Journal of Electroanalytical Chemistry, 2018, 809: 74-79. doi: 10.1016/j.jelechem.2017.12.033 [96] TANG S, YUAN D, RAO Y, et al. Persulfate activation in gas phase surface discharge plasma for synergetic removal of antibiotic in water [J]. Chemical Engineering Journal, 2018, 337: 446-454. doi: 10.1016/j.cej.2017.12.117 [97] QI C, LIU X, LIN C, et al. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity [J]. Chemical Engineering Journal, 2014, 249: 6-14. doi: 10.1016/j.cej.2014.03.086 [98] NASSERI S, MAHVI A H, SEYEDSALEHI M, et al. Degradation kinetics of tetracycline in aqueous solutions using peroxydisulfate activated by ultrasound irradiation: Effect of radical scavenger and water matrix [J]. Journal of Molecular Liquids, 2017, 241: 704-714. doi: 10.1016/j.molliq.2017.05.137 [99] YIN R, GUO W, WANG H, et al. Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: Performances and mechanisms [J]. Chemical Engineering Journal, 2018, 335: 145-153. doi: 10.1016/j.cej.2017.10.063 [100] WANG J, ZHUAN R. Degradation of antibiotics by advanced oxidation processes: An overview [J]. Science of The Total Environment, 2020, 701: 135023. doi: 10.1016/j.scitotenv.2019.135023 [101] WANG L, LAN X, PENG W, et al. Uncertainty and misinterpretation over identification, quantification and transformation of reactive species generated in catalytic oxidation processes: A review [J]. Journal of Hazardous Materials, 2020: 124436. [102] DUAN P, MA T, YUE Y, et al. Fe/Mn nanoparticles encapsulated in nitrogen-doped carbon nanotubes as a peroxymonosulfate activator for acetamiprid degradation [J]. Environmental Science:Nano, 2019, 6: 1799-1811. doi: 10.1039/C9EN00220K [103] LEI Y, LEI X, WESTERHOFF P, et al. Reactivity of Chlorine Radicals (Cl• and Cl2•–) with Dissolved Organic Matter and the Formation of Chlorinated Byproducts [J]. Environmental Science & Technology, 2021, 55: 689-699. [104] JI Y, WANG L, JIANG M, et al. The role of nitrite in sulfate radical-based degradation of phenolic compounds: An unexpected nitration process relevant to groundwater remediation by in-situ chemical oxidation [J]. Water Research, 2017, 123: 249-257. doi: 10.1016/j.watres.2017.06.081 [105] ZHU L, JI J, LIU J, et al. Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control [J]. Angewandte Chemie International Edition, 2020, 59: 13968-13976. doi: 10.1002/anie.202006059 -




 下载:
下载: