-
随着现代工业的迅速发展,越来越多的有机污染物(例如工业化学品、药品等)被释放在水生环境中[1-3]。然而,大部分有机污染物在水体中具有持久性特点,不易从自然生态系统中去除,给生态系统和人类健康带来严重威胁[4-5]。因此,迫切需要开发更高效的有机污染物降解技术。基于硫酸盐自由基(
${\rm{SO}}_4^{-}\cdot $ )的高级氧化工艺因其能高效、快速降解或矿化水体中难降解有机化合物的特点,近年来被广泛应用于废水的治理[6]。过一硫酸盐(PMS)或过硫酸盐(PS)活化产生的${\rm{SO}}_4^{-}\cdot $ 具有高的氧化还原电位、宽的pH适用范围以及长的半衰期[7]等特点;且与过硫酸盐活化特性相比,PMS中的O—O键更容易被激活,更有利于水中有机污染物降解。PMS的活化方法主要包括热活化[8]、紫外光活化[9]、碳材料活化[10-11]和过渡金属活化[12-13]等。热活化、紫外光活化方式反应条件苛刻,能耗高。过渡金属活化存在金属离子容易浸出、容易造成二次污染等问题。在碳基材料中,生物炭由于其制备方便、价格低廉、比表面积大、富含官能团、热稳定性和化学稳定性高等优点,在有机污染物消除方面得到广泛的研究[14-15]。然而,材料难回收、易造成二次污染、污染物降解不彻底等问题限制了生物炭的应用。将生物炭与磁性介质结合制备磁性生物炭,赋予生物炭磁响应特性,是目前的研究热点[16-17]。Wang等[18]以松木絮为原料制备了磁性生物炭,并用于吸附废水中的Hg2+,生物炭上负载的γ-F2O3有助于吸附材料从水中快速分离。Sewu等[19]研究了两种不同的磁性前躯体(α-FeOOH和FeCl3)对杉木生物炭磁响应特性的影响。研究表明,前者具有较高的磁饱和度、铁磁化性和可回收率。
同时,为了进一步提高污染物的去除效率,目前不少研究者尝试采用磁性生物炭对过一硫酸盐进行活化,发现磁性生物炭对PMS具有较强的活化能力及应用前景[20-22]。鉴于此,本文综述了磁性生物炭的制备方法、理化性质及其作为PMS激活剂在有机污染物降解方面的应用。从自由基途径和非自由基途径分析了磁性生物炭活化PMS的机理,同时提出磁性生物炭在活化PMS方面有待深入研究的问题,并对未来的研究前景进行了展望。
-
磁性生物炭的制备方法多样,主要包括浸渍热解法、液相沉淀法、水热/溶剂热合成法、液相还原法、高能球磨法、溶胶凝胶法等。这些制备方法的优缺点及磁性物质存在形式归纳如表1。表2对磁性生物炭的特征及其应用进行了分类。
-
浸渍热解法是指将生物质或预处理的生物炭浸泡在铁、钴等过渡金属盐溶液中,超声或搅拌一段时间,再将干燥后的样品置于氮气或其他无氧、限氧条件下的管式炉或马弗炉中热解获得磁性生物炭。Xu等[24]将木屑和双氰胺浸渍在FeCl3·6H2O溶液中,再经热解法制备了磁性生物炭,并用于活化PMS降解双酚A。结果表明,60 min内双酚A的降解率和矿化率分别为97%和68.9%。此外,热解温度会影响磁性生物炭中铁氧化物种的形成。Wang等[31]发现磁性生物炭中的铁氧化物种类随热解温度的升高(300—600 ℃)而变化。结果表明,铁氧化物按温度的形成顺序依次为赤铁矿、磁铁矿、钨矿和零价铁。
-
液相沉淀法是制备磁性生物炭比较常用的方法,将生物炭分散到含有过渡金属的溶液中,在一定温度下加入氢氧化钠或氢氧化铵溶液进行搅拌,通过调节pH值使其产生沉淀,再经过过滤、洗涤、干燥等过程制得磁性生物炭。崔志文等[25]在450 ℃和N2条件下热解制备小麦秸秆生物炭,先将其浸渍在KOH溶液中进行碱改性,再浸渍在FeCl3溶液中,用NaOH溶液调节pH值至11,经过静置,超声,搅拌,烘干等过程制备碱和磁复合改性小麦秸秆生物炭。经过改性后,其比表面积从7.37 m2·g−1增加到152.24 m2·g−1,含氧官能团大量增加,生物炭上的磁性物质以Fe3O4存在。
-
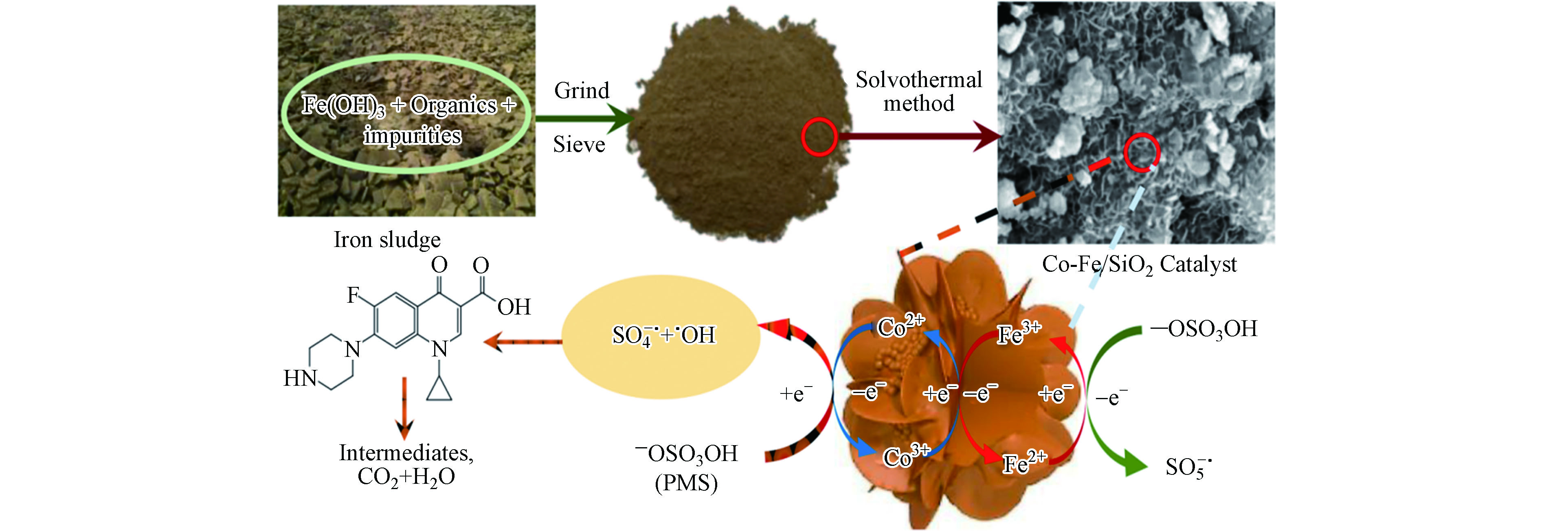
水热合成法是指以水作为溶剂,在密闭容器高压釜内,控制温度在100—240 ℃,物料经溶解和再结晶的制备方法。溶剂热法是在水热法的基础上发展起来的,它与水热反应的不同之处在于以有机物代替水作为溶剂。袁健等[26]用水热法从含铁污泥中成功地合成了磁性生物炭,将所得磁性炭作为水体Cd2+及Pb2+吸附剂,将污染物完全去除。近年来,一些研究者用有机物代替水作为溶剂成功地制备磁性生物炭。例如,Zhu等[27]以铁污泥、CoCl2、醋酸钠为原料,乙二醇为溶剂成功制备了磁性生物炭复合材料,将所得磁性炭用于活化PMS降解环丙沙星。循环使用4次后仍能保持100%的降解率,对其他类型的污染物如苯酚、双酚A、亚甲基蓝等均具有优异的催化性能。
-
液相还原法主要用于制备纳米零价铁负载生物炭复合材料,制备核心是在溶液中加入还原剂,促使高价态的铁还原成低价态铁。吴鸿伟等[32]采用液相还原法制备了纳米零价铁负载的生物炭,并将其用于降解头孢噻肟。结果表明,50 min内头孢噻肟的去除率为92%,归因于改性生物炭与纳米零价铁的之间协同作用。Li等[28]以FeSO4·7H2O和玉米棒为原料,NaBH4为还原剂合成了纳米零价铁/生物炭材料。由Fe0和含氧集团(即C=O)介导的超氧化物自由基和单线态氧是纳米零价铁/生物炭活化PMS降解三氯乙烯的主要的活性氧物种。
-
溶胶凝胶法制备磁性生物炭是指将金属盐溶于水中,形成均匀的溶液,然后加入其他组分,在一定温度下反应形成凝胶,最后经干燥处理制成产品。例如,李玉梅等[29]将硝酸铁和硝酸镧溶于去离子水中,形成均匀溶液,然后加入柠檬酸和玉米秸秆粉末搅拌均匀,超声辅助一段时间后在70 ℃下水浴加热搅拌形成溶胶,并于105 ℃干燥形成凝胶,制得生物炭/LaFeO3磁性复合材料。溶胶凝胶法一般可用于制备双金属氧化物负载的铁氧体/生物炭复合材料,该方法具有磁性颗粒结晶度高,分散性好等优点。
-
高能球磨法是通过球磨机对生物炭材料进行强烈的搅拌、研磨,最终形成粉体的方法。Shan等[30]采用球磨法制备了两种超细磁性生物炭/Fe3O4和活性炭AC/Fe3O4杂化材料,研究发现,球磨2 h制备的磁性生物炭材料对水中的四环素具有较高去除率。Fu等[21]将水杨树炭C400与K2FeO4混合球磨1 h后,分别在600 ℃、700 ℃和800 ℃下热解制备3种改性磁性生物炭。由扫描电子显微镜(SEM)和透射电子显微镜(TEM)图可知,所合成的磁性生物炭均具有石墨化多孔结构,这种独特的结构促进了磁性生物炭对PMS的活化能力,提高了污染物的降解性能。高能球磨法能明显降低反应活化能、细化晶粒、极大提高粉末活性和改善颗粒分布均匀性。
-
根据磁性颗粒与生物炭基质之间相对位置的差异,磁性生物炭的基本结构可大致分为负载结构、镶嵌结构和包覆结构[33]。负载结构比较常见,其制备工艺成熟,覆盖性广。镶嵌结构性质稳定,重复利用性强,但较为少见。包覆结构以碳质材料为外壳将磁性颗粒内核包裹起来,使磁性内核不易被氧化。磁性生物炭的形貌结构与制备所用的原料、煅烧温度等因素密切相关。不同的原材料所合成的磁性生物炭形貌不尽相同,但大多数磁性生物炭表面光滑,孔隙结构发达,与生物炭基体化学结合的磁性物质往往分布在生物炭孔隙或表面。Yi等[34]采用钢酸洗废液(铁盐)、甘蔗蔗渣、稻草、花生壳和药草残渣成功合成了4种磁性生物炭,由SEM表征可以清晰看到4种不同原料制得的磁性生物炭具有不一样的表观形态。甘蔗渣制备的磁性生物炭具有丰富的孔隙,氧化铁分布在骨骼结构的表面和孔隙内部;稻草和药草残渣为原料制备的样品骨骼与纹路清晰,铁氧化物分布在生物炭表面;由花生壳合成的磁性炭呈现片状结构,孔隙不清晰。而不同煅烧温度则会影响磁性介质的粒径大小、孔径以及磁性颗粒团聚情况[21],也会影响氧化物的种类[31]。
目前,成功研制的磁性生物炭主要包括铁基[35]、铁氧体复合材料[36]、钴基[23]以及非金属元素掺杂改性的磁性生物炭[37-38]等。采用X射线衍射(XRD)、X射线电子能谱(XPS)等表征方法可以确定磁性生物炭中铁的主要形式为Fe0、FeO、Fe3O4、α-Fe2O3、γ- Fe2O3等。磁性介质通过改变表面电荷[39]、直接参与反应[40]、引入其他金属(如锰[41]、铜锌[36])等提高磁性生物炭的性能,从而进一步提高其吸附降解污染物的能力。
-
比表面积是衡量吸附剂吸附容量的重要指标。磁性生物炭的比表面积与其孔隙率、磁性物种的负载量等密切相关。Liang等[42]发现所制备的磁性炭的比表面积随着Fe3O4/BC质量比的增加先递增后减小,过多Fe3O4的负载会导致磁性颗粒的聚集。另外,磁性生物炭的比表面积还受到热解温度的影响,随着热解温度的升高而增加[43-45]。为了增加磁性生物炭比表面积,一些研究者在磁性生物炭制备过程添加造孔剂。例如,Ifthikar等[46]通过加入ZnCl2造孔剂,使磁性生物炭的比表面积增加了2.5倍,孔容增加了1倍。
-
磁性生物炭表面的官能团在污染物的催化降解中起着关键作用。含氧官能团可作为催化位点(如C = O[47])或电子媒介体(如—COOH 和—OH[48])影响磁性生物炭的催化活性。氮物种则通过改变材料的缺陷程度来增加炭基材料的催化活性。利用红外光谱(FT-IR)可以对磁性生物炭表面官能团进行定性分析。Zhang等[49]通过FT-IR研究生物炭表面官能团,研究表明,O—H与C=O的伸缩振动分别位于3436 cm−1和1735 cm−1处,同时在580 cm−1处检测到Fe—O键的振动峰,表明了铁氧化物的存在,间接证实了磁性生物炭的成功合成。此外,利用XPS可以进一步验证磁性生物炭表面元素组成及化学价态的变化规律。C1s谱图的C—C/C=C、C—O和O—C=O分别位于结合能284.49 、285.9 、288.99 eV处[34]。Rong等[50]利用XPS对O1s光谱进行分析,结果表明,530.3、531.6、533、534 eV分别对应于Fe—O,C—OH,C—C=O和C—O—C;氮物种主要由吡啶氮(398.6 eV)、吡咯氮(400 eV)和石墨氮(401 eV)组成;Fe 2p3/2 谱中,结合能711 eV 和713 eV对应于Fe3+,709.8 eV对应于Fe2+。
-
随着化工产业与印染纺织业的发展及其废水的大量排放,有机染料成为水中高检出污染物。染料废水因其浓度高、可生化性差和水质变化大等特点,一直备受研究者的关注。部分含氯染料废水如罗丹明B的催化降解过程通常存在自由基途径和以单线态氧为主的非自由基途径,而具有羟基给电子基团的污染物如酸性橙则更容易被
${\rm{SO}}_4^{-}\cdot $ 氧化降解。Huang等[51]对猪废水混凝污泥热解所制备的生物炭进行磁改性,研究其活化PMS去除罗丹明B的可行性。在pH = 3—10范围内,罗丹明B的去除率较好,归因于合适的pH有利于1O2、·OH和 HO2·的生成,从而促进罗丹明B的降解。Fu等[52]制备了一种石墨化分层多孔磁性生物炭,用于活化PMS降解酸性橙废水。自由基淬灭实验表明,表面结合${\rm{SO}}_4^{-}\cdot $ 在MnFe2O4/MS+PMS系统中发挥关键作用。独特的石墨化分层多孔结构不仅为污染物与PMS之间的电子转移途径提供了纽带,还促进了1O2的产生,提升了酸性橙废水的降解效率。 -
含酚废水是目前世界上污染范围广、危害大的工业废水之一,具有毒性大、难降解的特点,是环境中水污染的重要来源。将基于
${\rm{SO}}_4^{-}\cdot $ 的高级氧化技术应用于含酚废水的处理,具有高效、快速的优点。含酚污染物受到自由基的攻击后快速降解为小分子有机物。Yang等[53]以木质素为原料,CO2取代N2作为热解的反应介质制备了钴浸渍生物炭催化剂(CoIB),并用于活化PMS降解对乙酰氨基酚废水。实验结果表明,与N2相比,以CO2作为反应介质合成的催化剂具有更大的比表面积和孔容。Co物种和碳基体的协同效应促进了CoIB对PMS的活化,从而提高了对乙酰氨基酚的催化降解性能。Zhang等[54]同样以木质素为前驱体,采用简便的热解方法,合成了一种新型铁纳米粒子包覆在氮和硫共掺杂的磁性多孔碳材料,用于活化PMS高效降解1-萘酚。分批加入氧化剂反应20 min,1-萘酚完全被降解。材料中Fe2+/Fe3+的转化循环促进了${\rm{SO}}_4^{-}\cdot $ 和·OH的形成。同时,N和S元素的掺杂增加了反应的活性中心,加速了1-萘酚的有效分解。 -
抗生素主要存在于医药废水中,抗生素污染诱发的细菌耐药性引起的人们的广泛关注。由于其难降解性和生物累积性,抗生素废水的处理成为水环境治理中一个亟待解决的问题。具有羟基(—OH)给电子基团的抗生素(如环丙沙星)和具有酰胺基(—NH2)给电子基团的抗生素通常具有较低的电离能量。当磁性生物炭耦合PMS降解这类废水时,这些污染物更倾向于被
${\rm{SO}}_4^{-}\cdot $ 催化氧化。Luo等[23]通过在不同温度下对浸渍钴的山羊粪便废弃物进行碳化,成功地合成了表面负载钴/生物炭,在活化PMS降解环丙沙星污染物过程中表现出优异的催化性能和稳定性。自由基淬灭实验和电子顺磁共振波谱(EPR)结果表明,${\rm{SO}}_4^{-}\cdot $ 是Co-GMC-900+PMS体系的主要活性自由基。Co-GMC-900良好的稳定性得益于优良的磁性、山羊粪的固有椭球结构以及钴纳米粒子与石墨化生物炭之间的相互作用增强。当磁性生物炭中的铁含量较少时,污染物可能被由生物基体与PMS产生的1O2催化降解。Li等[55]通过液相还原法制备了铁/生物炭多相催化剂复合材料,生物炭基体与PMS形成单线态氧1O2,通过羟基化攻击磺胺嘧啶使其完全被去除。除此之外,磁性生物炭+PMS体系对泰乐菌素具有良好的催化降解性能[51]。 -
环境内分泌干扰素广泛存在于大气和水环境中,对人体健康和水环境造成了不良影响。双酚A、邻苯二甲酸二甲酯等邻苯二甲酸酯类是目前常用的有机化工产品或原料,因其破坏人体的代谢过程而受到广泛关注。这类污染物容易受到
${\rm{SO}}_4^{-}\cdot $ 和·OH的攻击,发生羟基化、脱羧等反应而被快速降解。Jiang等[56]制备了一种多孔纳米纤维结构且含有零价铁的功能化生物炭复合材料(Fe-BC-700),并用于激活PMS高效去除双酚A。在最佳条件(0.2 g·L−1 PMS和0.15 g·L−1催化剂)下,Fe-BC-700可在5 min内完全去除20 mg·L−1的双酚A。高效的双酚A去除率归因于Fe物种活化PMS生成${\rm{SO}}_4^{-}\cdot $ 、纳米纤维-介孔碳结构的电子转移和生物炭中固有的持久性自由基的作用。Gan等[57]将CoFe2O4负载在生物炭载体上形成磁性复合材料,并应用于活化过一硫酸盐降解邻苯二甲酸二甲酯。邻苯二甲酸二甲酯受到${\rm{SO}}_4^{-}\cdot $ 和·OH的攻击后,先生成羧酸化合物中间体和羟基化中间产物,最后矿化成CO2、H2O。Dai等[47]采用一种简便的碳热氧化还原方法合成出类石墨烯二维磁性生物炭,并用于活化过一硫酸盐降解双酚A废水,生物炭中的C=O基团是活化PMS的活性位点之一。Wan等[58]通过水热碳化结合微波辐照和NH3活化,制备了一种由纤维素衍生的分级Fe/生物炭,并用酒石酸进行处理。酒石酸的引入不仅增加了催化剂中C=O基团含量,而且减轻了生物炭复合材料的铁离子浸出。 -
Liu等[59]以稻草为生物炭原料,将CoFe2O4负载在氮改性的生物炭上形成磁性生物炭复合材料,在活化PMS降解广谱氯乙酰胺类除草剂-异丙甲草胺过程中表现出优异的催化性能。Li等[28]研究了不同生物炭原料(玉米秸秆和玉米芯)、不同煅烧温度(300 ℃和600 ℃)对纳米零价铁负载生物炭催化剂活化过一硫酸盐降解三氯乙烯的影响。实验结果表明,以玉米芯为原料,在600 ℃下热解所得的磁性生物炭材料获得较高的三氯乙烯降解效率,20 min内去除率可达100%。Fu等[21]将Fe3O4负载在石墨化分级多孔生物炭上形成磁性复合材料,并用于活化PMS降解有机污染物,反应30 min,对羟基苯甲酸几乎完全被去除。
-
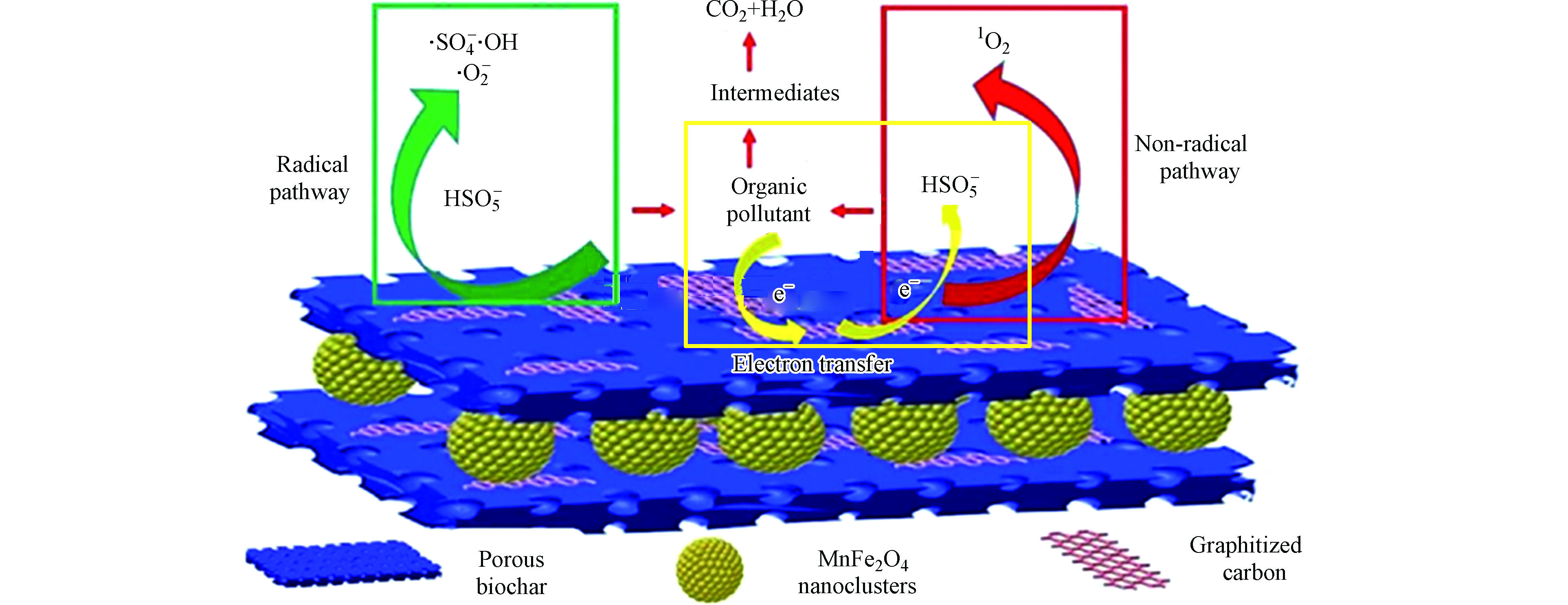
磁性生物炭活化PMS主要分为自由基途径和非自由基途径(图1)。由于磁性炭中存在半醌等含氧官能团和铁等金属离子,过一硫酸盐能被激活产生自由基和非自由基。价态较低的金属离子可以有效的激活PMS产生氧化能力较强的硫酸根自由基(
${\rm{SO}}_4^{-}\cdot $ )和羟基自由基(·OH)。生物炭除了自身固有的持久性自由基,还可以通过特殊的石墨化结构将电子传递,激活PMS生成活性自由基SO4.-和·OH。此外,过一硫酸盐还可以通过自身的分解产生同样具有催化活性的单线态氧1O2。磁性生物炭活化PMS的具体反应如下[60]: -
自由基途径是指在磁性生物炭的活化下,PMS产生
${\rm{SO}}_4^{-}\cdot $ 和·OH的过程(图2)。Zhu等[27]采用简便的水热法合成了一种磁性Co-Fe/SiO2层状催化剂,并作为一种有效的过一硫酸盐活化剂降解环丙沙星。与其他Co/Fe单金属氧化物和离子相比,Co-Fe/SiO2在10 min内具有较好的催化性能,环丙沙星降解效率为98%。由自由基捕获实验和电子顺磁共振波谱可知,在PMS活化过程中,体系中产生了${\rm{SO}}_4^{-}\cdot $ 和·OH,而${\rm{SO}}_4^{-}\cdot $ 是主要活性自由基。最近,有研究者发现表面结合自由基对磁性生物炭催化体系的重要作用。例如,Fu等[52]证实了表面结合${\rm{SO}}_4^{-}\cdot $ 在MnFe2O4/MS和PMS系统中的关键作用。研究发现,当加入${\rm{SO}}_4^{-}\cdot $ 和·OH捕获剂乙醇、·OH捕获剂叔丁醇后,污染物去除率变化很小,表明溶液中的${\rm{SO}}_4^{-}\cdot $ 和·OH都不是该体系的主要活性自由基。但加入苯酚后,苯酚作为表面结合${\rm{SO}}_4^{-}\cdot $ 和·OH的淬灭剂,体系去除效率降到19%,表明表面结合自由基发挥主要作用。除此之外,生物炭中固有的持久性自由基在有机污染物的降解中起着重要作用[56]。自由基的种类随着溶液中pH的变化而变化[24]。在酸性和中性条件下,${\rm{SO}}_4^{-}\cdot $ 和·OH对污染物的降解起主导作用,而在碱性条件下起主要作用的是·OH或1O2(式4、5)。 -
非自由基途径是指过一硫酸盐进行自分解生成单线态氧1O2的过程以及表面电子传递途径。研究表明[61-62],生物炭材料中的酮基(C=O)有利于1O2的生成,是磁性生物炭材料活化PMS体系中的重要活性位点。研究系统中是否存在单线态氧主要采用EPR或淬灭实验进行定性分析。Li等[22]采用L-组氨酸、Wang等[63]以糠醇为淬灭剂均发现,1O2在磁性生物炭材料活化PMS体系中发挥了关键作用。Xu等[24]通过EPR检测到1O2的信号峰,而Fu等[21]的研究发现1O2在磁性生物炭活化PMS系统中的作用很小。因此,单线态氧在PMS活化体系中的贡献率需要针对具体的催化体系进行探讨。
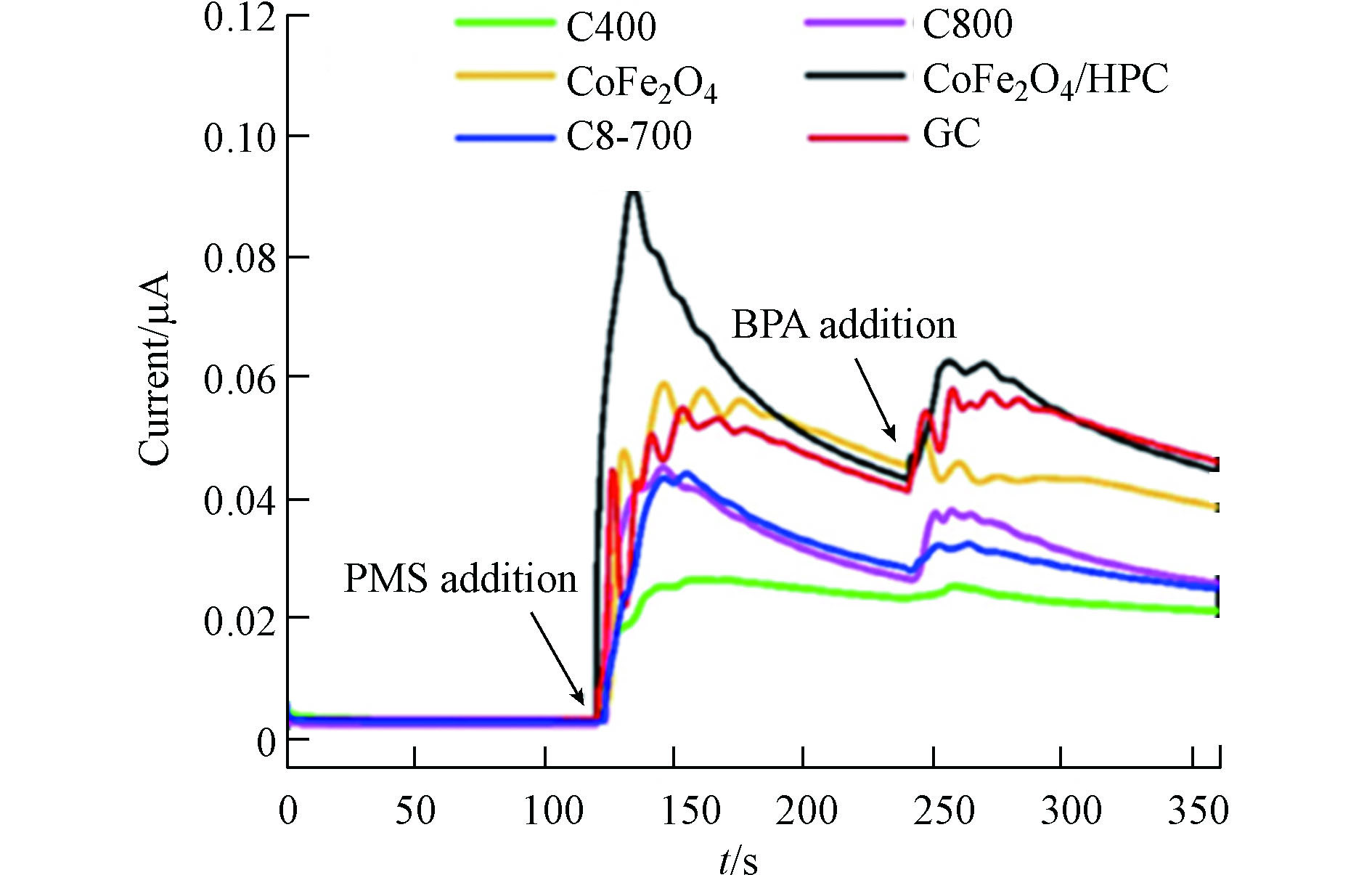
表面电子传递途径是以PMS为电子受体,催化剂为传递介质,有机污染物为电子供体,电子从污染物流经磁性生物炭再传输到PMS上,PMS得到电子被激活,从而降解污染物的过程。可采用电化学阻抗谱、线性伏安法和瞬态电流谱分析PMS活化体系中是否存在表面电子传递途径。Fu等[21]以NaClO4为电子转移淬灭剂,辅以电化学阻抗谱和线性伏安法证明了系统中存在电子转移介导的非自由基途径。Li等[22]通过瞬态电流图指出(图3),当PMS和BPA注入时,磁性复合材料CoFe2O4/HPC电极和存在石墨化结构的GC材料电极均引起明显的电流响应,而CoFe2O4电极只在PMS注入时引起电流响应,说明在CoFe2O4/HPC活化PMS体系中污染物通过生物炭中的石墨化结构将电子传递给PMS诱导非自由基途径。
-
虽然磁性生物炭在活化PMS降解水中有机污染物方面表现出良好的应用前景,但以下问题仍有待深入探讨:
(1)目前的研究只针对单一污染物的去除,而实际水环境中污染物复杂多样。因此,磁性生物炭活化过一硫酸盐对水中复合污染物的去除有待深入研究。
(2)在现阶段的研究中,主要采用静态实验研究磁性生物炭活化PMS对有机污染物的降解情况。模拟实际水环境中的动态吸附降解,可以为磁性生物炭活化PMS的实际应用提供指导性建议。
(3)磁性生物炭在活化PMS过程中的表面催化机理、电子转移机制仍需进一步研究,自由基、1O2和电子转移仍缺乏定量分析。
(4)如何提高磁性生物炭在实际废水处理过程中的稳定性和回收利用性能依然有待深入探索。
-
在综述中,系统地介绍了磁性生物炭的制备方法和基本理化性质,详细介绍了磁性生物炭活化过一硫酸盐在有机污染物如染料废水、抗生素废水、酚类物质、环境内分泌干扰素、除草剂等方面的应用。围绕自由基途径(
${\rm{SO}}_4^{-}\cdot $ 和·OH为主)和非自由基途径(1O2和电子转移)等两个方面解析了磁性生物炭活化PMS机理。尽管磁性生物炭在环境修复中的应用越来越多,但一些研究空白和不确定性仍然存在。为了弥补空缺,需要更多的相关调查和深入研究。
磁性生物炭的制备及其活化过一硫酸盐的研究进展
Research progress on preparation and peroxymonosulfate activation of magnetic biochar
-
摘要: 硫酸根自由基高级氧化技术因其具有高效、快速降解污染物的特点,近年来在水体中新兴有机污染物去除方面受到广泛关注。同时,磁性生物炭凭借其比表面积大、富含官能团和可磁性分离的特性有望成为应用于过一硫酸盐(PMS)活化的新生催化剂。本文综述了磁性生物炭复合材料常用的制备方法及其在形貌、比表面积和官能团方面的理化性质,着重介绍了磁性生物炭在活化PMS降解有机污染物方面的应用,探讨了磁性生物炭活化PMS的自由基和非自由基机理。最后,对磁性生物炭活化PMS遇到的问题及发展趋势进行了探讨。Abstract: In recent years, utilizing sulfate radical-based advanced oxidation processes to remove emerging organic pollutants in water has received widespread attention due to their high degradation efficiency toward pollutants. Meanwhile, magnetic biochar has been emerged as promising catalysts to activate peroxymonosulfate (PMS) because of its large surface area, abundant functional groups and good recyclable feature. The preparation methods and physicochemical properties (including morphology, specific surface area and functional group) of magnetic biochar were introduced in this study. Moreover, the applications of magnetic biochar for PMS activation were investigated in detail. The mechanisms including radicals and non-radical pathway were also discussed. Finally, the problems and development trend of magnetic biochar for PMS activation in the field of environmental catalysis were pointed out.
-
Key words:
- magnetic biochar /
- peroxymonosulfate /
- preparation /
- organic pollutant
-

-
表 1 磁性生物炭制备方法的优缺点及磁性物质存在形式
Table 1. Advantages and disadvantages of methods for preparing magnetic biochar
制备方法
Preparation method优点
Advantage缺点
Disadvantage磁性物质
Magnetic substances浸渍热解法 赋磁与热解一步完成,工艺简单 活性组分容易流失 钴、Fe2O3、铁氧体、Fe3O4 液相沉淀法 反应过程简单,成本低 产品分散性差,容易团聚 Fe3O4、γ-Fe2O3 水热/溶剂热合成法 无需烧结,粒子纯度高、分散性好 设备要求高、技术难度大 Fe3O4 液相还原法 原理简单,经济高效 操作复杂,推广难度较大 Fe0 溶胶-凝胶法 粒子结晶度高、分散性好 样品比表面积较小 铁氧体 高能球磨法 晶粒细化,颗粒分布均匀 设备要求较高,能耗大 Fe3O4、Fe0 表 2 不同磁性生物炭的特征及应用
Table 2. The characterization and application of different magnetic biochar
磁性生物炭
Magnetic biochar制备方法
Preparation method原材料
Material比表面积/
(m2·g−1)
SBET孔径/
nm
Dp孔容/
(cm3·g−1)
Vp降解率/%
Degradation参考文献
ReferencesCo-GMC-900 浸渍热解法 山羊粪肥、
Co(NO3)2·6H2O305.1 3.8 0.255 环丙沙星(20 mg·L−1):25.2% [23] Fe0.5−N-C 浸渍热解法 木屑、FeCl3·6H2O、双氰胺 215.25 — — 双酚A(10 mg·L−1):97% [24] Fe-BC-450 液相沉淀法 小麦秸秆、FeCl3、KOH、NaOH 152.24 7.12 0.13 Cd2+: 87.7% [25] Fe-SSBC 水热法 含 FeSO4活性污泥 114.57 32 0. 99 Cd2+ (8 mg·L−1):100%
Pb2+ (70 mg·L−1):100%[26] Co-Fe/SiO2 LC 溶剂热法 铁污泥、乙二醇、CoCl2、醋酸钠 145.9 3.2 0.116 环丙沙星(10 mg·L−1):98% [27] Fe-CB600 液相还原法 玉米棒、FeSO4·7H2O、NaBH4 22 3.73 0.205 三氯乙烯(0.1 mmol·L−1): 100% [28] BC/FL 溶胶凝胶法 玉米秸秆、硝酸铁、硝酸镧、柠檬酸 34.67 7.82 0.041 亚甲基蓝(30 mg·L−1): 92.3% [29] Biochar/Fe3O4 高能球磨法 椰子壳、Fe3O4 365 1.1 0.54 四环素: 99% [30] Fe3O4/MC800 高能球磨+热解法 水杨树、K2FeO4 350 1.2、2.5 0.28 对羟基苯甲酸(10 mg·L−1):
100%[21] -
[1] SHARMA J, MISHRA I M, DIONYSIOU D D, et al. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway [J]. Chemical Engineering Journal, 2015, 276: 193-204. doi: 10.1016/j.cej.2015.04.021 [2] 徐萍, 王娜, 文志潘, 等. 新型纳米CeO2催化类Fenton降解盐酸四环素 [J]. 环境化学, 2020, 39(3): 601-609. doi: 10.7524/j.issn.0254-6108.2019103003 XU P, WANG N, WEN Z P, et al. Degradation of tetracycline hydrochloride via a heterogeneous Fenton-like catalyzed by nano-CeO2 [J]. Environmental Chemistry, 2020, 39(3): 601-609(in Chinese). doi: 10.7524/j.issn.0254-6108.2019103003
[3] LI K S, LU X Y, ZHANG Y, et al. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants [J]. Environmental Research, 2020, 185: 109409. doi: 10.1016/j.envres.2020.109409 [4] PETRIE B, BARDEN R, KASPRZYK-HORDERN B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring [J]. Water Research, 2015, 72: 3-27. doi: 10.1016/j.watres.2014.08.053 [5] LI Y, XIA Y, LIU K L, et al. Constructing Fe-MOF-derived Z-scheme photocatalysts with enhanced charge transport: Nanointerface and carbon sheath synergistic effect [J]. ACS Applied Materials & Interfaces, 2020, 12(22): 25494-25502. [6] XIAO R Y, LUO Z H, WEI Z S, et al. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies [J]. Current Opinion in Chemical Engineering, 2018, 19: 51-58. doi: 10.1016/j.coche.2017.12.005 [7] LIN K Y A, ZHANG Z Y. Degradation of Bisphenol A using peroxymonosulfate activated by one-step prepared sulfur-doped carbon nitride as a metal-free heterogeneous catalyst [J]. Chemical Engineering Journal, 2017, 313: 1320-1327. doi: 10.1016/j.cej.2016.11.025 [8] LIU Y X, WANG Y, WANG Q, et al. Simultaneous removal of NO and SO2 using vacuum ultraviolet light (VUV)/heat/peroxymonosulfate (PMS) [J]. Chemosphere, 2018, 190: 431-441. doi: 10.1016/j.chemosphere.2017.10.020 [9] SHAD A, CHEN J, QU R J, et al. Degradation of sulfadimethoxine in phosphate buffer solution by UV alone, UV/PMS and UV/H2O2: Kinetics, degradation products, and reaction pathways [J]. Chemical Engineering Journal, 2020, 398: 125357. doi: 10.1016/j.cej.2020.125357 [10] ZHAO Q X, MAO Q M, ZHOU Y Y, et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications [J]. Chemosphere, 2017, 189: 224-238. doi: 10.1016/j.chemosphere.2017.09.042 [11] OUYANG D, CHEN Y, YAN J C, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1, 4-dioxane: Important role of biochar defect structures [J]. Chemical Engineering Journal, 2019, 370: 614-624. doi: 10.1016/j.cej.2019.03.235 [12] LI J, XU M J, YAO G, et al. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation [J]. Chemical Engineering Journal, 2018, 348: 1012-1024. doi: 10.1016/j.cej.2018.05.032 [13] XU H D, JIANG N, WANG D, et al. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways [J]. Applied Catalysis B: Environmental, 2020, 263: 118350. doi: 10.1016/j.apcatb.2019.118350 [14] ZHU S S, HUANG X C, MA F, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms [J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. [15] HOSLETT J, GHAZAL H, KATSOU E, et al. The removal of tetracycline from water using biochar produced from agricultural discarded material [J]. Science of the Total Environment, 2021, 751: 141755. doi: 10.1016/j.scitotenv.2020.141755 [16] YIN Z B, XU S, LIU S, et al. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium [J]. Bioresource Technology, 2020, 300: 122680. doi: 10.1016/j.biortech.2019.122680 [17] WANG H, ZHAO W, CHEN Y N, et al. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange [J]. Bioresource Technology, 2020, 315: 123834. doi: 10.1016/j.biortech.2020.123834 [18] WANG H B, LIU Y, IFTHIKAR J, et al. Towards a better understanding on mercury adsorption by magnetic bio-adsorbents with γ-Fe2O3 from pinewood sawdust derived hydrochar: Influence of atmosphere in heat treatment [J]. Bioresource Technology, 2018, 256: 269-276. doi: 10.1016/j.biortech.2018.02.019 [19] SEWU D D, TRAN H N, OHEMENG-BOAHEN G, et al. Facile magnetic biochar production route with new goethite nanoparticle precursor [J]. Science of the Total Environment, 2020, 717: 137091. doi: 10.1016/j.scitotenv.2020.137091 [20] LI K, MA S L, XU S J, et al. The mechanism changes during bisphenol A degradation in three iron functionalized biochar/peroxymonosulfate systems: The crucial roles of iron contents and graphitized carbon layers [J]. Journal of Hazardous Materials, 2021, 404: 124145. doi: 10.1016/j.jhazmat.2020.124145 [21] FU H C, ZHAO P, XU S J, et al. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism [J]. Chemical Engineering Journal, 2019, 375: 121980. doi: 10.1016/j.cej.2019.121980 [22] LI Y, MA S L, XU S J, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4 [J]. Chemical Engineering Journal, 2020, 387: 124094. doi: 10.1016/j.cej.2020.124094 [23] LUO J M, BO S F, QIN Y N, et al. Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation [J]. Chemical Engineering Journal, 2020, 395: 125063. doi: 10.1016/j.cej.2020.125063 [24] XU L, FU B R, SUN Y, et al. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application [J]. Chemical Engineering Journal, 2020, 400: 125870. doi: 10.1016/j.cej.2020.125870 [25] 崔志文, 任艳芳, 王伟, 等. 碱和磁复合改性小麦秸秆生物炭对水体中镉的吸附特性及机制 [J]. 环境科学, 2020, 41(7): 3315-3325. CUI Z W, REN Y F, WANG W, et al. Adsorption characteristics and mechanism of cadmium in water by alkali and magnetic composite modified wheat straw biochar [J]. Environmental Science, 2020, 41(7): 3315-3325(in Chinese).
[26] 袁健, 钱雅洁, 薛罡, 等. 活性污泥水热碳化法制备磁性炭及对水体Cd2+及Pb2+的去除 [J]. 环境工程, 2020, 38(2): 55-62. YUAN J, QIAN Y J, XUE G, et al. Removal of cadmium and lead in water by magnetic carbon prepared from activated sludge with hydrothermal carbonization [J]. Environmental Engineering, 2020, 38(2): 55-62(in Chinese).
[27] ZHU S J, XU Y P, ZHU Z G, et al. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation [J]. Chemical Engineering Journal, 2020, 384: 123298. doi: 10.1016/j.cej.2019.123298 [28] LI Z, SUN Y Q, YANG Y, et al. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater [J]. Journal of Hazardous Materials, 2020, 383: 121240. doi: 10.1016/j.jhazmat.2019.121240 [29] 李玉梅, 王畅, 张连科, 等. 生物炭/铁酸镧磁性复合材料的制备及对亚甲基蓝的吸附性能 [J]. 环境污染与防治, 2020, 42(7): 826-832. LI Y M, WANG C, ZHANG L K, et al. Preparation of biochar/LaFeO3 magnetic composite material and adsorption properties for methylene blue [J]. Environmental Pollution & Control, 2020, 42(7): 826-832(in Chinese).
[30] SHAN D N, DENG S B, ZHAO T N, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling [J]. Journal of Hazardous Materials, 2016, 305: 156-163. doi: 10.1016/j.jhazmat.2015.11.047 [31] WANG S S, ZHAO M Y, ZHOU M, et al. Biomass facilitated phase transformation of natural hematite at high temperatures and sorption of Cd2+ and Cu2+ [J]. Environment International, 2019, 124: 473-481. doi: 10.1016/j.envint.2019.01.004 [32] 吴鸿伟, 冯启言, 杨虹, 等. 改性生物炭负载纳米零价铁去除水体中头孢噻肟 [J]. 环境科学学报, 2017, 37(7): 2691-2698. WU H W, FENG Q Y, YANG H, et al. Nanoscale zero valent iron stabilized on modified biochar to remove cefotaxime from aqueous solutions [J]. Acta Scientiae Circumstantiae, 2017, 37(7): 2691-2698(in Chinese).
[33] 吴明山, 马建锋, 杨淑敏, 等. 磁性生物炭复合材料研究进展 [J]. 功能材料, 2016, 47(7): 7028-7033. doi: 10.3969/j.issn.1001-9731.2016.07.006 WU M S, MA J F, YANG S M, et al. Progress of the magnetic biochar composite materials [J]. Journal of Functional Materials, 2016, 47(7): 7028-7033(in Chinese). doi: 10.3969/j.issn.1001-9731.2016.07.006
[34] YI Y Q, TU G Q, ZHAO D Y, et al. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor [J]. Chemical Engineering Journal, 2019, 360: 212-220. doi: 10.1016/j.cej.2018.11.205 [35] WANG K, SUN Y B, TANG J C, et al. Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: Role of biochar electron transfer capacity under high pyrolysis temperature [J]. Chemosphere, 2020, 241: 125044. doi: 10.1016/j.chemosphere.2019.125044 [36] HEO J, YOON Y, LEE G, et al. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4-biochar composite [J]. Bioresource Technology, 2019, 281: 179-187. doi: 10.1016/j.biortech.2019.02.091 [37] ZHANG Y T, LIU N, YANG Y D, et al. Novel carbothermal synthesis of Fe, N co-doped oak wood biochar (Fe/N-OB) for fast and effective Cr(VI) removal [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: 124926. doi: 10.1016/j.colsurfa.2020.124926 [38] ZHANG C, LU J, WU J. One-step green preparation of magnetic seaweed biochar/sulfidated Fe0 composite with strengthen adsorptive removal of tetrabromobisphenol A through in situ reduction [J]. Bioresource Technology, 2020, 307: 123170. doi: 10.1016/j.biortech.2020.123170 [39] BOMBUWALA DEWAGE N, LIYANAGE A S, PITTMAN C U Jr, et al. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar [J]. Bioresource Technology, 2018, 263: 258-265. doi: 10.1016/j.biortech.2018.05.001 [40] ZHONG D L, ZHANG Y R, WANG L L, et al. Mechanistic insights into adsorption and reduction of hexavalent chromium from water using magnetic biochar composite: Key roles of Fe3O4 and persistent free radicals [J]. Environmental Pollution, 2018, 243: 1302-1309. doi: 10.1016/j.envpol.2018.08.093 [41] ZHANG L K, GUO J Y, HUANG X M, et al. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb(ii) and Cd(ii) [J]. RSC Advances, 2019, 9(1): 365-376. doi: 10.1039/C8RA09061K [42] LIANG S, SHI S Q, ZHANG H H, et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution [J]. Science of the Total Environment, 2019, 695: 133886. doi: 10.1016/j.scitotenv.2019.133886 [43] YIN Z H, LIU Y G, LIU S B, et al. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17β-estradiol and copper [J]. Science of the Total Environment, 2018, 639: 1530-1542. doi: 10.1016/j.scitotenv.2018.05.130 [44] ZHU Y, YI B J, HU H Y, et al. The relationship of structure and organic matter adsorption characteristics by magnetic cattle manure biochar prepared at different pyrolysis temperatures [J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104112. doi: 10.1016/j.jece.2020.104112 [45] YI Y Q, TU G Q, ERIC TSANG P, et al. Insight into the influence of pyrolysis temperature on Fenton-like catalytic performance of magnetic biochar [J]. Chemical Engineering Journal, 2020, 380: 122518. doi: 10.1016/j.cej.2019.122518 [46] IFTHIKAR J, WANG J, WANG Q L, et al. Highly efficient lead distribution by magnetic sewage sludge biochar: Sorption mechanisms and bench applications [J]. Bioresource Technology, 2017, 238: 399-406. doi: 10.1016/j.biortech.2017.03.133 [47] DAI X H, FAN H X, YI C Y, et al. Solvent-free synthesis of a 2D biochar stabilized nanoscale zerovalent iron composite for the oxidative degradation of organic pollutants [J]. Journal of Materials Chemistry A, 2019, 7(12): 6849-6858. doi: 10.1039/C8TA11661J [48] YAN J C, HAN L, GAO W G, et al. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene [J]. Bioresource Technology, 2015, 175: 269-274. doi: 10.1016/j.biortech.2014.10.103 [49] ZHANG H, XUE G, CHEN H, et al. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment [J]. Chemosphere, 2018, 191: 64-71. doi: 10.1016/j.chemosphere.2017.10.026 [50] RONG X, XIE M, KONG L S, et al. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation [J]. Chemical Engineering Journal, 2019, 372: 294-303. doi: 10.1016/j.cej.2019.04.135 [51] HUANG Z Y, WANG T L, SHEN M X, et al. Coagulation treatment of swine wastewater by the method of in situ forming layered double hydroxides and sludge recycling for preparation of biochar composite catalyst [J]. Chemical Engineering Journal, 2019, 369: 784-792. doi: 10.1016/j.cej.2019.03.136 [52] FU H C, MA S L, ZHAO P, et al. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism [J]. Chemical Engineering Journal, 2019, 360: 157-170. doi: 10.1016/j.cej.2018.11.207 [53] YANG M T, DU Y C, TONG W C, et al. Cobalt-impregnated biochar produced from CO2-mediated pyrolysis of Co/lignin as an enhanced catalyst for activating peroxymonosulfate to degrade acetaminophen [J]. Chemosphere, 2019, 226: 924-933. doi: 10.1016/j.chemosphere.2019.04.004 [54] ZHANG T, LI C Y, SUN X T, et al. Iron nanoparticles encapsulated within nitrogen and sulfur co-doped magnetic porous carbon as an efficient peroxymonosulfate activator to degrade 1-naphthol [J]. Science of the Total Environment, 2020, 739: 139896. doi: 10.1016/j.scitotenv.2020.139896 [55] LI Z, SUN Y Q, YANG Y, et al. Comparing biochar-and bentonite-supported Fe-based catalysts for selective degradation of antibiotics: Mechanisms and pathway [J]. Environmental Research, 2020, 183: 109156. doi: 10.1016/j.envres.2020.109156 [56] JIANG S F, LING L L, CHEN W J, et al. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors [J]. Chemical Engineering Journal, 2019, 359: 572-583. doi: 10.1016/j.cej.2018.11.124 [57] GAN L, ZHONG Q, GENG A B, et al. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation [J]. Science of the Total Environment, 2019, 694: 133705. doi: 10.1016/j.scitotenv.2019.133705 [58] WAN Z H, SUN Y Q, TSANG D C W, et al. Sustainable impact of tartaric acid as electron shuttle on hierarchical iron-incorporated biochar [J]. Chemical Engineering Journal, 2020, 395: 125138. doi: 10.1016/j.cej.2020.125138 [59] LIU C, CHEN L W, DING D H, et al. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor [J]. Applied Catalysis B: Environmental, 2019, 254: 312-320. doi: 10.1016/j.apcatb.2019.05.014 [60] YANG Q, MA Y H, CHEN F, et al. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water [J]. Chemical Engineering Journal, 2019, 378: 122149. doi: 10.1016/j.cej.2019.122149 [61] SUN H W, PENG X X, ZHANG S P, et al. Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water [J]. Bioresource Technology, 2017, 241: 244-251. doi: 10.1016/j.biortech.2017.05.102 [62] HUANG B C, JIANG J, HUANG G X, et al. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate [J]. Journal of Materials Chemistry A, 2018, 6(19): 8978-8985. doi: 10.1039/C8TA02282H [63] WANG J, KOU L D, ZHAO L, et al. One-pot fabrication of sludge-derived magnetic Fe, N-codoped carbon catalysts for peroxymonosulfate-induced elimination of phenolic contaminants [J]. Chemosphere, 2020, 248: 126076. doi: 10.1016/j.chemosphere.2020.126076 -



 下载:
下载: