-
胞外电子传递(extracellular electron transfer,EET)是指微生物胞内代谢过程所产生的电子传递至细胞外的电子受体,或是细胞外的电子供体将电子传递至胞内进行代谢的过程。这种胞外呼吸的方式最初是通过研究异化金属还原菌还原铁、锰矿物而发现的[1-2]。电子流动是微生物新陈代谢的固有特征,具有胞外电子转移能力的微生物称为电活性微生物(electroactive microorganisms)[3]。EET是元素生物地球化学循环与能量交换的重要驱动力,其研究受到广泛关注[4]。EET过程结合了物质流与能量流,可用于生物能源的制备以及废弃物的资源化利用[5-7],这使得电活性微生物不仅深刻影响地球化学循环,也具有了生态修复、工业生产和资源利用上的巨大潜力。
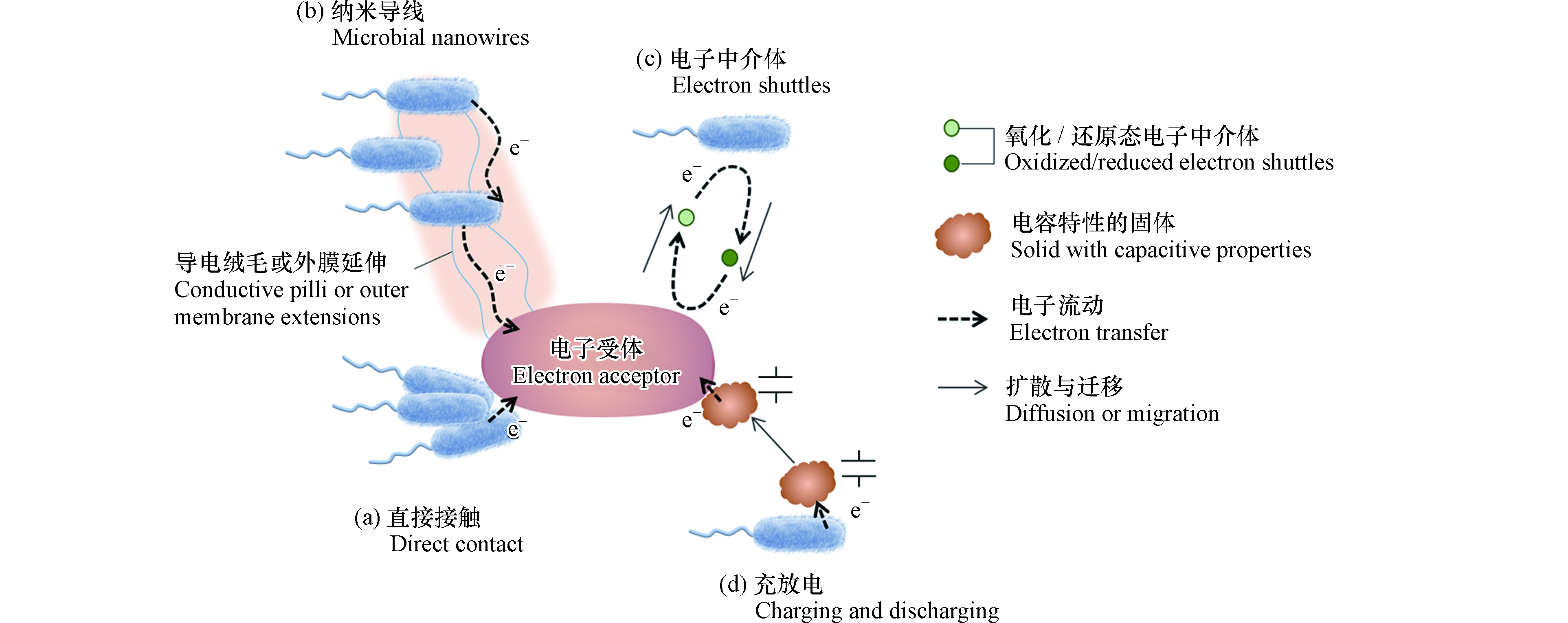
随着人们对于胞外电子传递过程的研究日渐深入,电活性微生物的胞外电子传递反应机制被逐渐认识。图1显示了微生物向胞外传递电子的几种方式。直接电子传递(direct electron transfer)在微生物细胞与胞外电子受体直接接触的前提下,微生物通过(a)细胞膜上的活性蛋白(如细胞色素c)或(b)生成纳米导线(导电菌毛)的方式将电子传递给胞外受体[8]。直接电子传递发生的前提是微生物电子传递部位与电子受体的距离在几个纳米范围。间接电子传递(mediated electron transfer)指微生物自身分泌(c)电子中介体(electron shuttles),或利用外源添加电子中介体实现胞外呼吸[8]。电子中介体也被称为氧化还原介体、电子穿梭体,在反应中充当电子载体,其特点是可在微生物与电子受体之间往返且可被循环利用。电子中介体在环境中具有扩散迁移能力,因此可以突破微生物与胞外电子受体间距离的限制。一些难溶或不溶物质具有赝电容性(d),可以储存来自微生物的电子,当环境条件变化或与氧化性物质接触,电子可重新释放,传递至最终电子受体[9]。
胞外电子传递过程涉及电子供体、受体和电子中介体,这些物质涵盖有机物和无机物,其中不乏难溶于水的物质。同时,一些通常被归类为“可溶性”的底物也可能在细胞表面被呼吸转化。原因是:(1)底物在其存在环境中可能与固体表面作用,实际上不溶;例如二甲基亚砜虽然可溶于水,但在海洋环境中常与悬浮颗粒物结合,而难以进入微生物胞内代谢,希瓦氏菌可通过外膜蛋白进行胞外电子传递以利用二甲基亚砜[10];(2)底物虽然可溶但分子量较大,无法通过细胞膜[11];(3)底物本身或其代谢副产物有毒[12]。例如在地下水、泥炭地、沉积物等多相环境中,固相碳材料、矿物、腐殖质、高分子聚合物和难溶有机污染物等物质无法进入细胞质膜和周质空间。因此,本文通过介绍胞外电子传递过程中典型的固体无机物-微生物界面和难溶有机物-微生物界面,总结物质表面性质对微生物EET途径和效率的影响,有助于更好的利用微生物胞外电子传递过程来进行污染修复及资源回收。
-
固相的无机物常常通过胞外电子传递的方式被微生物利用。其中,环境修复领域常用的一些碳材料具备促进微生物胞外电子传递的功能;矿物-微生物反应是生物地球化学循环的重要一环;金属-微生物反应常见于人工形成的电化学系统电极界面或者腐蚀现象。这里通过讨论碳材料、矿物和金属揭示无机物性质与微生物胞外电子传递间的互作关系。
-
碳材料被广泛应用于土壤改良、污染修复等领域,有些碳材料既可以作为呼吸代谢的终端电子受体,也可作为电子供体。不同碳材料的导电能力差异很大,从导体(如石墨)到绝缘体(如金刚石)。与微生物发生相互作用的碳材料根据其导电的方式可分为两类,一类是赝电容性导电途径,如生物炭等材料通过其丰富的表面活性官能团储存和释放电子,这种方式实质上是材料表面官能团与微生物之间的氧化还原作用[13-14]。另一类是共轭导电途径,是指石墨烯、碳纳米管等具有类似石墨的片层结构的碳材料,这类材料构型具有离域大π键,通过p电子的自由运动导电。尺寸在纳米级的共轭碳材料可附着或嵌入细胞外膜上,充当直接电子传递通道[15]。下面以生物炭和碳纳米管/石墨烯为例分别讨论两类碳材料在胞外电子传递中的作用。
-
生物炭(biochar,又称生物质炭,生物黑炭)是生物质材料在缺氧或少氧条件下热裂解产生的芳香化氟碳固体物质,其表面官能团、表面电性、孔隙率、比表面积和芳香度等理化性质因原材料及制备条件的不同而存在差异[16]。以生物炭为代表的具有赝电容性的碳材料广泛应用于土壤改良、污染物吸附、碳捕获等领域[17]。由于生物炭通常呈碱性,常作为改良剂和菌剂载体被施加于水体或土壤中,改善微生物群落的组成和结构。
生物炭的导电率低,属于半导体材料[15],但可以通过表面含O官能团(如醌基、酚基等芳环结构)发生氧化还原反应或者呈现赝电容性[13-14,18]。O/C比可以很好地反映生物炭官能团的电子存储能力[15]。研究表明热解温度越低的生物炭氧化还原活性部分含量越高,其电子接受能力相应更高[17]。还原态的生物炭可作为微生物或其他物质的电子供体,这得益于其表面官能团具有释放电子的能力[18-19]。Kappler等发现生物炭可将从微生物处获得的电子转移至水铁矿[20]。Xu等报道了生物炭作为电子供体,通过氧化表面的含氧官能团(—C—O—,—C=O)进行Cr(Ⅵ)还原[21]。这种基于含氧官能团氧化还原的充放电有别于一般电子中介体的作用,因为生物炭作为固体材料在环境中迁移能力较弱。并且很大一部分官能团的氧化还原不可循环,此前研究表明生物炭材料通常具有一定的使用寿命,其氧化还原能力随反应时间增长而降低[19,22]。
生物炭表面通常带负电,不利于长程吸附同样带负电的微生物,但其的高孔隙率、高比表面积为微生物吸附、定植和生物膜生长提供支撑[23-24];Yu等发现在Geobacter sulfurreducens厌氧培养过程中加入生物炭可使生物量显著增加[25]。
-
纳米尺度的共轭碳材料兼具大的表面积和特殊的电化学性能,为细胞粘附和存活提供了新的“生物界面”[26],引导细胞极化和排列,改变生物膜结构和促进细胞分化[27]。
碳纳米管(carbon nanotube),具有优越的导电性[28]。用碳纳米管修饰传统碳材料(如玻碳、碳纸、碳布、碳毡)可形成多孔电极,一方面提高微生物在其表面的粘附,另一方面增强细胞色素c与电极的相互作用[29]。 Xie等设计了一种由碳纳米管-纺织品复合材料制成三维大孔电极,这种电极为微生物提供更多活性位点,促进电子从外电极向阳极的传递[30-32]。Li等对单壁碳纳米管和产乙酸微生物之间的界面反应进行研究,发现单壁碳纳米管可以实现材料与微生物界面的直接电子传递[33]。
石墨烯(graphene)是一种以sp²杂化连接的碳原子紧密堆积成单层二维蜂窝状晶格结构的材料。氧化石墨烯可用作Shewanella的电子受体,在有氧的条件下也可被还原成石墨烯[34]。Igarashi等发现将氧化石墨烯加入到乙醇氧化菌Geobacter metallireducens(电子供体菌)和产甲烷菌Methanosarcina barkeri(电子受体菌)的共培养体系中,可促进种间电子传递以及甲烷的产生[35]。石墨烯常用作电极修饰材料,可通过电化学剥离在石墨电极表面原位形成石墨烯[36],或通过电沉积的方式将石墨烯负载在碳毡材料上[37]。石墨烯修饰电极有利于微生物在电极表面生长,促进直接电子转移,同时可能刺激电子中介体的分泌,提高反应动力学[37]。
-
矿物是土壤的主要成分,微生物通过胞外电子传递过程与具有氧化还原活性的矿物发生能量交换,用于微生物代谢和生长[5]。关于铁、锰矿物与微生物界面胞外电子传递的研究最为广泛。放射性核素在生物转化过程中的矿化同样涉及电活性菌的胞外电子传递。
-
铁氧化物包括铁的氧化物、氢氧化物和羟基氧化物,主要价态为+2和+3价。目前环境中存在的已知铁氧化物共12种,其中针铁矿、赤铁矿和磁铁矿在土壤中大量沉积[38]。锰氧化矿物广泛分布于土壤、沉积物等自然环境中,主要以Mn(Ⅲ/Ⅳ)氧化物形式存在,包括水钠锰矿、水羟锰矿、锰钡矿等。铁(锰)氧化物作为厌氧微生物呼吸作用最终电子受体的研究较多,其中最为典型的是以Geobacter [39-40]和Shewanella [2]为代表的胞外电子传递机制的研究。在没有分子氧(O2)和其它终端电子受体的情况下,微生物氧化有机物或氢(H2),将释放的电子转移到含有Fe(Ⅲ)、Mn(Ⅲ/Ⅳ)的矿物中进行呼吸。利用Shewanella oneidensis [41]、Geobacter sulfurreducens [42]、Bacillus subtilis [43]等模式菌株的研究揭示了影响微生物与铁(锰)矿物之间电子转移的因素。矿物自身的结晶度、粒径、表面电荷、氧化还原电位(Eh)及其赋存环境的pH值使其表现出不同的氧化还原能力。
自然界中矿物的粒径分布广,包括纳米颗粒、胶体、颗粒状或块状。其中纳米级的铁矿物显示出高反应活性[9]。Liu等研究了纳米颗粒聚集体和稳定分散的纳米颗粒,研究表明外膜细胞色素蛋白OmcA与聚集体的界面接触面积控制着矿物还原速率,当纳米矿物胶束聚集成的孔喉尺寸与OmcA一样大时,还原速率提高[44]。
在自然环境中,结晶度差的矿物生物氧化还原速率通常高于结晶度好的矿物[45-49]。这是由于结晶程度低的矿物通常结构松散[50],而且具有较小的粒径和较大的比表面积,因此暴露更多的反应位点[9]。水铁矿是一种结晶度较差(短程有序)的氢氧化铁矿物,其初级颗粒直径在低纳米(小于6 nm)范围内,具有较大的比表面积和较高的反应性[9]。Cutting等发现两种结晶度不同但表面积几乎相同的合成铁矿其Fe(Ⅲ)初始还原速率存在很大差异,结晶度较差的水铁矿初始还原速率高于结晶度较好的赤铁矿[51]。在微生物还原过程中,三价铁矿物向结晶度更高的二价铁矿物转变,其氧化还原活性也随之降低[52]。
许多矿物具有一定的半导体性质,外膜蛋白和矿物导带电位间的电势差可以实现微生物与矿物间的电子传递过程[53]。表1总结了6种常见铁矿物的表面物理性质。
-
电活性微生物可通过生物还原、生物矿化、生物吸附和生物积累的方法来修复放射性核素污染。其中核素作为电活性微生物在厌氧条件下的最终电子受体是生物还原法的常见形式。Geobacter [60]、Shewanella [61]和Desulfovibrio [62]可利用U(Ⅵ)作为电子受体,胞内产生的电子可以通过细胞色素c蛋白传递至胞外的U(Ⅵ),从而将其还原为不溶或难溶的U(Ⅳ)进行固定[63]。基于菌毛缺陷株和减少外膜细胞色素c含量的基因缺陷菌的对照实验,Cologgi等发现导电菌毛在Geobacter的U(Ⅵ)还原过程中具有重要作用[64]。高导电性的菌毛可与吸附在其它颗粒物表面的U(Ⅵ)接触,发生直接电子传递。同时铀还原的固相产物沿着菌毛沉淀,有效防止细胞周质矿化,有利于维持细胞呼吸活力[65]。此外,Shewanella也可在黄素类电子中介体的介导下还原U(Ⅵ)[66-67]。
-
金属具有优越的导电性,在生物电化学系统中常常用作电极材料,有利于降低体系的欧姆内阻 [68]。金属材料用作电极时比传统的碳基材料强度更高,但缺点是通常金属电化学窗口较窄,且表面更光滑,附着的生物量小,不利于生物膜生长。常将金属电极制成网状、纤维毡状,以提高微生物的附着[69]。用于研究微生物电子转移的金属电极有铜、不锈钢、金、铂、镍和钛等材料。金、铂等贵金属具有优越的导电性和高稳定性,但价格较高,适合于实验室进行的机制研究,实际应用需要考虑成本问题。铜电极导电性能良好且价格适中,但长期使用容易被生物腐蚀,而且该过程中产生的铜离子会造成微生物中毒[70]。不锈钢电极耐腐蚀且价格低廉,但生物附着量比较低[71]。
微生物造成金属腐蚀的现象广泛存在,造成了巨大的经济损失。胞外电子传递可以解释金属的微生物腐蚀发生的过程。硫还原菌和硝酸盐还原菌都能够从碳钢和不锈钢材料获取电子[72]。Sherar等发现硫还原菌在缺乏有机碳源的条件下产生导电菌毛,附着在金属铁表面以获取电子[73]。在硝酸盐还原菌的培养基中加入电子中介体将导致更严重的碳钢和不锈钢腐蚀[74]。
-
有机物在胞外电子传递中扮演着重要角色。有机物的碳原子拥有多变的成键方式,可呈现出不同导电性、电子接收、储存和传递性质。参与微生物胞外电子传递的有机反应界面可分为天然有机质和人工合成材料,这里主要介绍腐殖质、硝基芳烃、有机卤素化合物以及高分子聚合物。可以进入微生物细胞内的有机物不在讨论范围内。
-
腐殖质是生物残体经分解、氧化及合成而形成的有机高分子化合物。它广泛分布在土壤、沉积物和水生环境中,是天然有机质的重要组成部分[75]。腐殖质是土壤和沉积物中的主要电活性物质,其丰富的氧化还原活性基团对于驱动电子转移过程具有重要作用[76]。腐殖质涵盖的氧化还原电位范围广(图2),能够介导多种氧化还原反应。根据腐殖质成分、分子量以及在不同pH条件下的溶解度,腐殖质通常分为三类:黄腐酸,腐殖酸和胡敏素[77];其中黄腐酸在酸性和碱性条件下均可溶,腐殖酸在碱性条件下可溶,在pH<2的酸溶液中沉淀,而胡敏素在任何pH条件下均不可溶。不同溶解性的腐殖质迁移能力有差异,溶解态腐殖质由于尺寸大,不能进入微生物细胞内,在胞外电子传递过程中主要充当电子中介体,而固相腐殖质可作为电子受体接收和储存电子[76]。
-
电子中介体的显著特征包括迁移能力和氧化还原可逆。腐殖质混合物结构复杂,不同来源的腐殖质结构存在差异。因此学者有时采用蒽醌-2,6-二磺酸盐(AQDS)等单一醌类物质模拟可溶性腐殖质[78];此前发现AQDS介导的胞外电子传递距离可以拓展至厘米级[79]。
腐殖质的电化学活性来自以醌基为主的氧化还原活性基团[78]。Scott等发现地杆菌与腐殖质进行电子交换时,电子自旋共振/顺磁共振结果显示腐殖质反应主要体现为醌基的氧化还原[80]。Nurmi等通过循环伏安法发现AQDS、甲萘醌、泛醌-5等6种模型醌类化合物与天然腐殖质样品的特征峰相似[81]。Cory和McKnight等对醌类荧光分布与实验室氧化还原梯度函数进行评估,表明醌类部分氧化还原状态函数指征很好地代表了溶解态腐殖质的荧光分布[82]。
腐殖质中存在某些非醌基团同样具有电子传递能力。特定pH条件下,醌基团可被质子化形成酚基;但醌基质子化的腐殖质并未失去介导电子传递的能力。腐殖酸中的非醌氧化还原官能团占总定量电子传递能力的25%—44% [83]。这些非醌基团可能包括一些含氧、含硫基团、如二甲基砜、3-甲硫基-丙酸、n-甲基苯胺、1-甲基-2,5-吡咯烷二酮等[84],其具体机制需要进一步研究。
-
相比于溶解态的腐殖质,环境中的固相腐殖质(胡敏素、吸附态腐殖酸和黄腐酸)更为常见,但体系复杂、研究较少[76]。由于这些腐殖质很难在环境中扩散和迁移,不能起到电子中介体的传输功能。但是由于表面基团丰富且具有氧化还原活性,在厌氧环境下,固相电子受体常常代替氧气成为电活性微生物的电子受体,在一定条件下会起到存储电子的功能。
先前的研究指出湖泊中氧化态和还原态固相腐殖质的相对丰度随沉积物深度呈现梯度变化,深层缺氧环境的腐殖质呈现出更高的电子接受能力[85]。Roden等通过孵育实验和电子自旋共振测量证明,铁还原菌可以将电子转移到湿地沉积物中的固相腐殖质中[86]。固相腐殖质参与胞外电子传递的过程与生物炭、活性炭等半导体碳材料的作用类似,即体现出赝电容特性[87-88]。Klüpfel等提出还原态固相腐殖质在与氧化剂接触的条件下释放电子,固相腐殖质的还原(充电)和氧化(放电)仅在一定程度上可逆[89]。电子中介体是指氧化还原完全可逆、且能够在菌体和电子受体之间往返移动,与赝电容功能不同,这一点在当前的文献中有一些混淆。Zhao等发现胞外电子传递过程可以提高固相腐殖质中C和H的含量,并降低O含量[90];腐殖酸在这个过程中芳香度增加而极性降低,虽然腐殖化程度没有变化,但固相腐殖质介导电子传递的效率降低。这是由于固相腐殖质的一些表面基团发生了不可逆的氧化还原。
固相腐殖质在一些条件下能够加速污染物的还原转化。Zhang等将胡敏素固定在石墨电极上进行五氯酚酸生物脱氯,相比溶解态的电子中介体,固相腐殖质的生物脱氯效率更高[91],且循环实验证明胡敏素的促进作用较稳定。固相腐殖质的作用机制与功能仍有巨大潜力等待发掘。
-
聚合物是一类人工合成的高分子物质,常被用于生物电化学系统中电极的修饰。由于聚合物结构设计灵活,可系统地调节电子传输性能。包括使修饰后的电极具备好的生物相容性[92]、合适的氧化还原电势和亲/疏水性,改善导电性,降低微生物与电极间的过电势。与生物相互作用的聚合物,依据其性质可分为氧化还原聚合物和导电聚合物两类。
-
氧化还原聚合物的电子接收和传递能力来自于其单体中具有氧化还原活性的物质。常见的氧化还原活性物质包括锇、二茂铁[93]、紫精[94]、以及醌类。这类聚合物将具有氧化还原活性的物质固定在电极表面,相比游离的电子中介体,不仅缩短了反应传输路径来提高电子传递效率[92];还可以通过设计获得有利于生物膜功能的表面性质,如正电性、适宜的表面润湿性和生物相容性。
含锇聚合物是研究比较早的氧化还原聚合物,主要用于研究与酶的相互作用,近年来也初步用于研究微生物细胞。一般含锇聚合物分子量很大,无法进入胞内,但通常带有正电荷,可粘附带负电的菌体。同时作为胞外氧化还原介体介导胞外电子转移 [95]。研究表明电子很可能通过聚合物与锇活性中心之间的电子跳跃方式转移[96]。
含醌聚合物是近年来生物电化学系统的常用材料。Giroud等提出利用1-[双(2-萘醌)氨基甲基]与碳纳米管耦合,可以增强酶生物燃料电池的电催化的能力[97]。Hasan 等利用萘醌聚合物修饰碳毡电极,显著促进了Shewanella oneidensis MR-1细胞的产电[98]。Wang等通过电聚合法在石墨毡表面合成固相氢醌,一方面合成的产物具有赝电容特性,可提升电子存储效率,另一方面含氧基团的增加可能提升了电极表面润湿性,促进了生物膜的生长和附着[99]。
-
导电聚合物通常是主链具有共轭主电子体系,可通过掺杂达到导电态。导电聚合物可为细胞提供稳定的生存环境,以提高微生物的适应性。典型的共轭聚合物,包括聚苯胺[100]、聚吡咯[101]、聚噻吩[102],均被报道可以作为促进胞外电子传递的材料。Wu等采用枝接聚苯胺的方法制备了低电荷转移电阻的电极,获得了26倍于裸电极的电流密度[100];Song等开发了一种原位聚合方法,将聚吡咯涂覆在单个Shewanella oneidensis、Escherichia coli、Ochrobacterium anthropic和Streptococcus thermophilus的表面,不仅显著增强了胞外电子转移能力,还提高了菌的生存能力[103]。然而,需要注意的是,导电聚合物的电导率受掺杂的影响显著,没有掺杂的聚合物导电能力不佳,欧姆阻抗显著增加。此外,导电聚合物通常在酸性条件下表现出较高的电导率,因此实际应用中需要考虑体系的pH值是否对微生物活性有影响[104].
-
硝基芳烃常被用作炸药和工业生产的原料,是一类容易被吸附于土壤中的难降解有机污染物。这类污染物疏水性较强,常被归类为不溶性底物。硝基芳烃很难通过氧化反应去除,某些条件下可能生成比母化合物毒性更大的副产物。研究发现,缺氧或厌氧条件下电活性微生物能够有效地还原去除硝基芳烃。Luan等通过分析硝基苯和针铁矿体系的反应动力学,提出Shewanella oneidensis MR-1通过胞外电子传递还原硝基苯的可能途径[10]。随后Liu等在基因水平上研究了S. oneidensis MR-1对2,6-二硝基甲苯的厌氧还原机制,发现跨膜电子传递链Mtr呼吸途径是厌氧还原发生的关键[105]。
最新研究表明,Shewanella oneidensis MR-1可以采取胞外还原和胞内还原两种方式利用芳香族化合物。Wang等发现,一些小分子芳香族化合物,如硝基苯、2, 4, 6-三硝基甲苯和1-叔丁醇-4硝基苯,通过胞外和胞内两种方式还原。胞外还原依赖于Mtr呼吸途径,胞内还原则需要硝基芳烃进入细胞内,被细胞质中的硝基还原酶NfnB还原[106]。大分子芳香族化合物,如2, 5-二叔丁硝基苯、2-硝基联苯、2, 2’-二硝基联苯则只能依赖Mtr呼吸途径发生胞外还原。带有二苯环或者长碳链烷基结构的芳香族化合物分子尺寸和疏水性进一步提高,这很可能是诱导Shewanella oneidensis MR-1在胞外还原硝基芳烃的原因[105-106]。
-
有机卤化物常见于受污染的土壤和地下水。例如四氯乙烯、三氯乙烯是常用溶剂和脱脂剂,具有难溶、易挥发、毒性大的特点。一些Geobacter属于有机卤化物呼吸微生物,Sung等分离并鉴定了一种地杆菌菌株Geobacter lovleyi strain SZ,能够还原四氯乙烯和三氯乙烷[107]。Amos在四氯乙烯污染土壤中检测到Geobacter lovleyi strain SZ,并验证了它在四氯乙烯原位还原修复中发挥作用[108]。Wagner等从基因水平探究了该菌株能够利用四氯乙烯和三氯乙烷为电子受体的原因,与其他Geobacter的基因组相比,Geobacter lovleyi strain SZ通过基因获取(gene acquisitions)获得带有有机卤化物呼吸所必须基因的质粒,因此能够呼吸降解四氯乙烯和三氯乙烷[109]。
-
胞外电子供体或受体的表面性质对胞外电子传递的影响体现在微生物活性、胞外电子传递途径和微生物粘附的三个方面。良好的表面生物相容性可提高反应界面的生物活性。反应物的氧化还原电位决定了胞外电子传递的途径。
-
生物相容性较好的材料对微生物刺激小,有利于微生物维持较高的活性[69]。Zhang等制备了竹炭管电极,发现其单位比表面活菌数量比石墨阳极高出63%,代替传统石墨阳极可使微生物燃料电池最大功率密度提升50% [110]。氮掺杂能够进一步提升碳材料的生物相容性。一些铁矿具有良好的表面生物相容性,因此常作为提高EET效率的材料[111]。Wang等通过水热法在碳纸表面合成了α-FeOOH纳米晶须,制成具有生物相容性好和电阻低的阳极材料[112]。另外,部分高分子聚合物[113-114]、纳米颗粒[115]都被报道具有提升传统阳极生物相容性的效果。金属材料虽然具有高强度、高导电性的优秀性能,但其附着的生物量相对较少[71]。
-
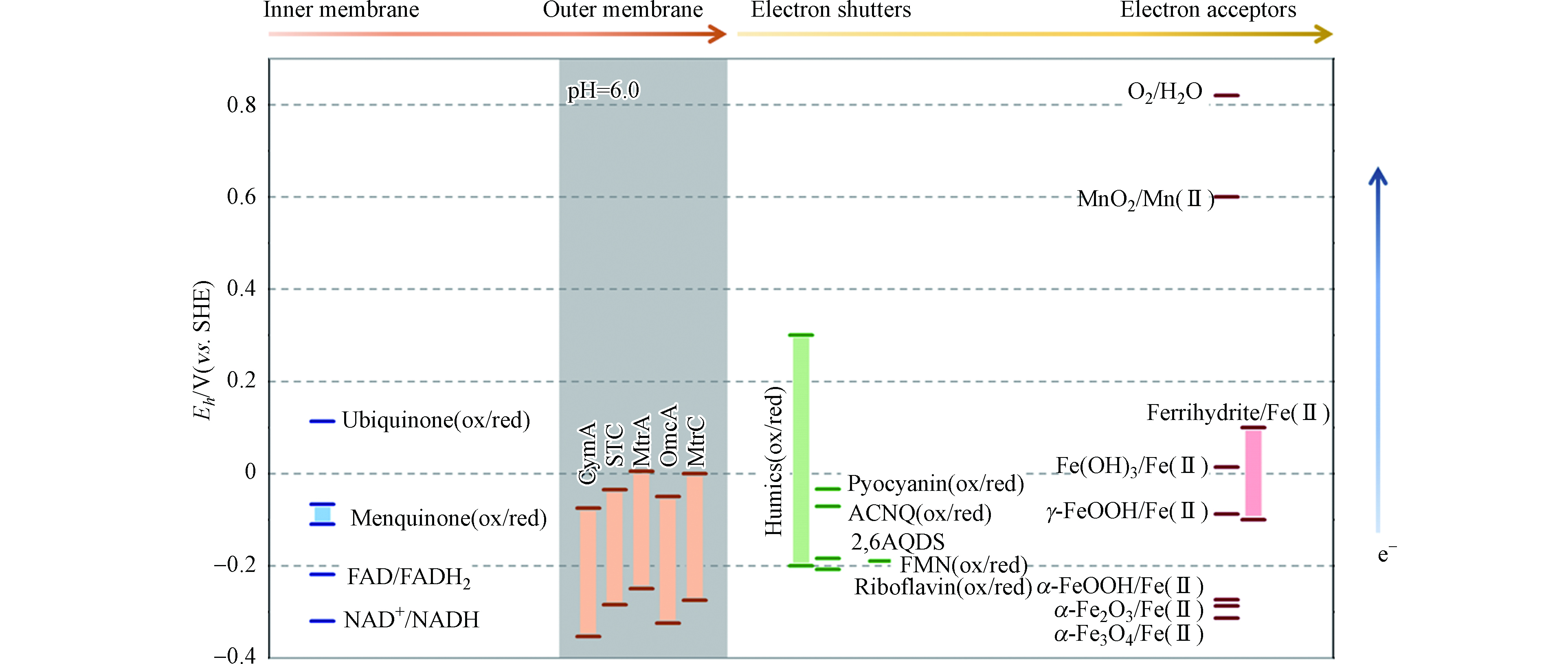
氧化还原电位差是电子在电子供体和受体间传递的驱动力。参与胞外电子传递的膜蛋白、电子中介体和胞外电子供体和受体具有一定的氧化还原电势范围,它们共同构成了电子传递链,决定电子流动的途径和方向[116-117]。图2总结了典型的微生物内膜、外膜蛋白、胞外电子中介体和电子受体氧化还原电位的分布。
-
通过设定电极电位,可以研究不同氧化还原电位对于微生物电子传递的影响[121]。Grobbler等发现,在- 0.19 V、+ 0.21 V、+ 0.71 V阳极电势条件下,Shewanella oneidensis MR-1呼吸方式体现出由游离黄素主导的间接电子传递,到直接电子传递为主的变化。同时不同的阳极电势使生物膜蛋白丰度差异化[122]。Korth等提出外生电子的可用能量的热力学评估需要考虑底物与细胞内电子载体之间的能量差,阳极电势 ≥ 0.2 V时,EET反应动力学的速率、NAD+ / NADH比值以及对可用能量的利用均达到最高水平[123]。通过比较Shewanella oneidensis MR-1在低(0 V)、中(+ 0.2 V)、高(+ 0.5 V)的3种电位条件下的生理生化指标,Hirose等发现模式菌MR-1仅在高电位下利用NADH依赖途径和甲酸盐依赖途径来提高生物量[124],表明电活性微生物可以主动响应电极电位。
-
反应位点增多可以提高电子传递效率。电子受体的粒径减小、孔隙率增大可使比表面积增大,反应位点增多、改善微生物粘附进而形成生物膜[118];微生物表面附着分为三个阶段[125-126]:(1)在长程力(范德华力和静电引力)作用下微生物靠近表面并初始吸附;(2)微生物与表面之间通过极性和疏水相互作用稳定吸附(定植);(3)生物膜生长。通过提高孔隙率和粗糙度,调控适宜的表面润湿性、增大正电性,可以促进粘附,利于生物膜的形成。
-
高孔隙度或较粗糙的表面通过捕获效应改善微生物粘附。粗糙表面具有较高的表面能,促进微生物粘附从而启动游离细胞在材料表面的初始附着,粘附的微生物通过代谢表面结合底物开始生长和繁殖,有助于反应启动和生物膜形成[127]。微生物细胞被胞外聚合物所包围,Xiao等研究表明减少微生物的胞外聚合物包裹将提高胞外电子传递电流密度[128]。而亚微米尺度的孔隙有助于减少生物膜底部的传质限制,减少微生物胞外聚合物阻塞,从而促进高效的胞外电子传递[129]。
-
超疏水表面常用于工业上阻碍生物污垢的形成[130-132],这是由于高疏水表面会造成高表面张力,不利于微生物的初步定植和生物膜的形成[133]。Guo等发现疏水官能团-CH3修饰的电极表面生物膜增厚,覆盖程度降低,生物量较低且微生物群落组成与亲水电极表面有显著差异[134]。如果在一定程度上提高电极表面的亲水性,降低表面自由能,有利于微生物粘附。但是,超亲水结构由于表面的高度水合,会形成紧密吸附的水层,因而无法进一步提供较高的生物粘合力,有时也用作防止生物吸附的屏障[135-136]。
腐殖质中的有机官能团显示出不同的对外亲/疏水性,暴露在水体中的固体(如矿物)容易吸附腐殖质并形成腐殖质附着层,从而改变相应反应界面的亲/疏水性。Yuan等以不同腐殖质固定在玻碳电极上构成的体系为模型,研究推出EET与腐殖质的电子接受能力和润湿性呈正相关,与腐殖质极性呈负相关,与给电子量和Zeta电位呈弱相关[137]。
电极亲疏水性的变化对EET活性和途径也产生影响。此前有研究表明疏水和亲水的电极表面,Shewanella loihica PV-4外膜上的细胞色素c的氧化还原状态明显不同,亲水电极使细胞色素c倾向于处于还原状态[138-139]。Lin等通过修饰水铁矿表面获得惰性疏水层,然后进行微生物的还原试验,发现水溶性的黄素等电子中介体在疏水层扩散缓慢,疏水的电子受体促使微生物调节自身呼吸方式,提出脂溶性电子介体在疏水界面呼吸过程中起到重要作用[140]。
-
在中性条件下,多数微生物的表面带有负电荷,这是由于细胞外膜主要由蛋白质和氨基酸构成,膜上的氨基酸倾向于发生羧基解离而带负电荷[141]。当材料带有正电荷,静电吸引使得微生物比较容易地附着在材料表面,阳极表面电荷越正,越有利于电活性生物膜的形成[134]。基于这种机制,部分抗菌材料采用控制表面电荷的方式增强病原菌与材料间的静电斥力,从而提升抗菌效果[142-143]。Shebl等制备了表面电荷范围在−30 — +30 mV的六种磁性纳米铁,测试纳米粒子对于生物膜的破坏率,结果显示表面正电性的纳米粒子表现出较好的抗菌性能[144]。
-
综合考虑不同反应界面的特性与共性,有助于深入理解微生物的EET行为与环境中反应物的关系,并用于资源回收、污染控制/修复领域的技术创新。未来以下三点可能需要进一步研究:
(1)从定义上来看,固相腐殖质和生物炭比较类似,属于赝电容的功能。由于固相腐殖质的组成、表面性质和电子接受-给出能力仍不明晰,其在有机污染物降解和电化学系统中的应用潜力需要进一步发掘。
(2)导电聚合物的高电导率仅体现在掺杂的前提下,通常在酸性条件下效果比较好,需要明确给出电极表面导电聚合物的欧姆内阻。
(3)微生物通过EET还原疏水有机污染物的机制仍未明确,微生物利用硝基芳烃的方式包括EET和胞内硝基还原酶的作用。已有研究表明微生物主动响应电极表面疏水性调控胞外电子传递的方式,微生物是否能够通过主动响应硝基芳烃的疏水性来调控胞外和胞内途径的依赖程度需要进一步探讨。
微生物的胞外电子传递界面
The interface of microbial extracellular electron transfer
-
摘要: 微生物胞外电子传递(Extracellular electron transfer,EET)在地球生物化学循环、生态修复、废水处理以及资源再生等领域发挥着重要作用。自然界中胞外电子传递的界面性质各异,导致反应速率和效率明显不同。本文介绍了胞外电子传递过程涉及的无机物-微生物界面和有机物-微生物界面,总结了反应物表面性质与微生物的互作规律:反应物表面的氧化还原活性决定其电子接受/释放能力,从根本上影响胞外电子传递发生的可能性;微生物与反应物之间的氧化还原电势差决定了电子传递方向;表面电荷、润湿性、表面粗糙度、孔隙度和生物相容性综合影响微生物在固体表面的吸附、粘附、生物膜生长及活性,从而影响胞外电子传递的效率;导电性影响电子传输速率。本综述旨在通过对比各种反应界面,认识不同反应物界面间的共性与特性。这些认识有助于系统理解微生物胞外电子传递与环境的关系,为其在工程中的应用提供理论指导。Abstract: Microbial extracellular electron transfer (EET) plays an important role in the fields of earth biochemical cycle, ecological restoration, wastewater treatment and resource regeneration. In nature, the interface properties of extracellular electron transfer are different, resulting in significantly different reaction rates and efficiencies. In this article, we introduce the microbe-inorganic substance interface and the microbe-organic substance interface involved in the process of extracellular electron transfer, and summarize the interaction rules between surface properties and microorganisms. The redox activities of reactants determine its electron accepting and releasing abilities, and fundamentally affects the possibility of extracellular electron transfer. The redox potential difference between the microorganism and reactants determines the direction of electron transfer. The surface charge, wettability, surface roughness, porosity and biocompatibility of the solid surface comprehensively affect the adsorption, adhesion, biofilm growth and activity of microorganisms on the solid-phase surface, thereby affecting the efficiency of extracellular electron transfer. The conductivity of the solid interface affects the electron transfer rate. This review aims to understand the commonalities and characteristics of different reaction interfaces by comparing various reaction interfaces. These understandings help to systematically understand the relationship between the EET behavior of microorganisms and environment, provide theoretical guidance for its application in engineering.
-

-
表 1 常见铁矿物的表面物理性质
Table 1. Surface properties of common iron minerals
矿物
Mineral化学式
Chemical formulaBET比表面积/(m2·g−1)
BET specific surface area导带电位[53] /(V vs. SHE)
Conduction band potential等电点
Isoelectric point针铁矿Goethite α-FeOOH 11—50[54− 55] −0.02 7.5—8.2[54] 赤铁矿Hematite α-Fe2O3 9—40[55− 56] −0.01 8.4—8.5[56] 磁铁矿Magnetite Fe3O4 6[55] +0.48 6.4—6.8 [55] 纤铁矿Lepidocrocite γ- FeOOH 25[57] — 7—10 [57] 水铁矿Ferrihydrite — 30—300[58] −0.1—+0.1[59] 8.0[58] 磁赤铁矿Maghemite γ-Fe2O3 — + 0.15 — -
[1] LOVLEY D R, PHILLIPS E J P. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese [J]. Applied & Environmental Microbiology, 1988, 54(6): 1472-1480. [2] MYERS C R , NEALSON K H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor [J]. Science, 1988, 240(4857): 1319-1321. doi: 10.1126/science.240.4857.1319 [3] XIAO X , YU H Q. Molecular mechanisms of microbial transmembrane electron transfer of electrochemically active bacteria [J]. Current Opinion in Chemical Biology, 2020, 59: 104-110. doi: 10.1016/j.cbpa.2020.06.006 [4] LOGAN B E, ROSSI R, RAGAB A, et al. Electroactive microorganisms in bioelectrochemical systems [J]. Nature Reviews. Microbiology, 2019, 17(5): 307-319. doi: 10.1038/s41579-019-0173-x [5] SHI L, DONG H, REGUERA G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals [J]. Nature Reviews Microbiology, 2016, 14(10): 651-662. doi: 10.1038/nrmicro.2016.93 [6] LOGAN B E, RABAEY K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies [J]. Science, 2012, 337(6095): 686-690. doi: 10.1126/science.1217412 [7] ZHENG Y, WANG H, LIU Y, et al. Methane-dependent mineral reduction by aerobic methanotrophs under hypoxia [J]. Environmental Science & Technology Letters, 2020, 7(8): 606-612. [8] LOVLEY D R. Electromicrobiology [J]. Annual Review of Microbiology, 2012, 66: 391-409. doi: 10.1146/annurev-micro-092611-150104 [9] KAPPLER A, BRYCE C, MANSOR M, et al. An evolving view on biogeochemical cycling of iron[J]. Nature Reviews Microbiology, 2021, 19: 360-374. [10] GRALNICK J A, VALI H, LIES D P, et al. Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(12): 4669-4674. doi: 10.1073/pnas.0505959103 [11] WOOD J M, WANG H K. Microbial resistance to heavy metals [J]. Environmental Science & Technology, 1983, 17(12): 582-590. [12] LUAN F B, BURGOS W D, XIE L, et al. Bioreduction of nitrobenzene, natural organic matter, and hematite by Shewanella putrefaciens CN32 [J]. Environmental Science & Technology, 2010, 44(1): 184-190. [13] LOGAN B E, HAMELERS B, ROZENDAL R, et al. Microbial fuel cells: Methodology and technology [J]. Environmental Science & Technology, 2006, 40(17): 5181-5192. [14] JIANG D , LI B. Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): A design suitable for large-scale wastewater treatment processes [J]. Biochemical Engineering Journal, 2009, 47(1-3): 31-37. doi: 10.1016/j.bej.2009.06.013 [15] ZHANG P, ZHENG S L, LIU J, et al. Surface properties of activated sludge-derived biochar determine the facilitating effects on Geobacter co-cultures [J]. Water Research, 2018, 142: 441-451. doi: 10.1016/j.watres.2018.05.058 [16] ANTAL M J , GRONLI M. The art, science, and technology of charcoal production [J]. Industrial & Engineering Chemistry Research, 2003, 42(8): 1619-1640. [17] QIU L, DENG Y F, WANG F, et al. A review on biochar-mediated anaerobic digestion with enhanced methane recovery [J]. Renewable & Sustainable Energy Reviews, 2019, 115: 14. [18] SUN T R, LEVIN B D A, GUZMAN J J L, et al. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon [J]. Nature Communications, 2017, 8: 14873. doi: 10.1038/ncomms14873 [19] CHACÓN F J, CAYUELA M L, ROIG A, et al. Understanding, measuring and tuning the electrochemical properties of biochar for environmental applications [J]. Reviews in Environmental Science and Bio/Technology, 2017, 16(4): 695-715. doi: 10.1007/s11157-017-9450-1 [20] KAPPLER A, WUESTNER M L, RUECKER A, et al. Biochar as an electron shuttle between bacteria and Fe(Ⅲ) minerals [J]. Environmental Science & Technology Letters, 2014, 1(8): 339-344. [21] XU X, HUANG H, ZHANG Y, et al. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption [J]. Environmental Pollution, 2019, 244: 423-430. doi: 10.1016/j.envpol.2018.10.068 [22] SCHIEVANO A, BERENGUER R, GOGLIO A, et al. Electroactive biochar for large-scale environmental applications of microbial electrochemistry [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(22): 18198-18212. [23] YAO Y, GAO B, INYANG M, et al. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential [J]. Bioresource Technology, 2011, 102(10): 6273-6278. doi: 10.1016/j.biortech.2011.03.006 [24] CHAUDHURI, S K and LOVLEY D R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells [J]. Nature Biotechnology, 2003, 21(10): 1229-1232. doi: 10.1038/nbt867 [25] YU L P, WANG Y Q, YUAN Y, et al. Biochar as electron acceptor for microbial extracellular respiration [J]. Geomicrobiology Journal, 2016, 33(6): 530-536. doi: 10.1080/01490451.2015.1062060 [26] GASIOROWSKI J Z, MURPHY C J, NEALEY P F. Biophysical cues and cell behavior: the big impact of little things [J]. Annual Review of Biomedical Engineering, 2013, 15: 155-176. doi: 10.1146/annurev-bioeng-071811-150021 [27] CHEN W Q, WENG S N, ZHANG F, et al. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies [J]. ACS Nano, 2013, 7(1): 566-575. doi: 10.1021/nn304719q [28] YAN F F, HE Y R, WU C, et al. Carbon nanotubes alter the electron flow route and enhance nitrobenzene reduction by Shewanella oneidensis MR-1 [J]. Environmental Science & Technology Letters, 2014, 1(1): 128-132. [29] WANG J X, LI M X, SHI Z J, et al. Direct electrochemistry of cytochrome c at a glassy carbon electrode modified with single-wall carbon nanotubes [J]. Analytical Chemistry, 2002, 74(9): 1993-1997. doi: 10.1021/ac010978u [30] XIE X, HU L B, PASTA M, et al. Three-dimensional carbon Nanotube−Textile anode for high-performance microbial fuel cells [J]. Nano Letters, 2011, 11(1): 291-296. doi: 10.1021/nl103905t [31] XIE X, MENG Y, HU L B, et al. Carbon nanotube-coated macroporous sponge for microbial fuel cell electrodes [J]. Energy Environ. Sci., 2012, 5(1): 5265-5270. doi: 10.1039/C1EE02122B [32] XIE X, ZHAO W T, LEE H R, et al. Enhancing the nanomaterial bio-interface by addition of mesoscale secondary features: Crinkling of carbon nanotube films to create subcellular ridges [J]. ACS Nano, 2014, 8(12): 11958-11965. doi: 10.1021/nn504898p [33] LI Z, XIONG W, DE VILLERS B J T, et al. Extracellular electron transfer across bio-nano interfaces for CO2 electroreduction [J]. Nanoscale, 2021, 13(2): 1093-1102. doi: 10.1039/D0NR07611B [34] WANG G M, QIAN F, SALTIKOV C W, et al. Microbial reduction of graphene oxide by Shewanella [J]. Nano Research, 2011, 4(6): 563-570. doi: 10.1007/s12274-011-0112-2 [35] IGARASHI K, MIYAKO E, KATO S. Direct interspecies electron transfer mediated by graphene oxide-based materials [J]. Frontiers in Microbiology, 2019, 10: 3068. [36] TANG J, CHEN S, YUAN Y, et al. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells [J]. Biosensors and Bioelectronics, 2015, 71: 387-395. doi: 10.1016/j.bios.2015.04.074 [37] LIU J, QIAO Y, GUO C X, et al. Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells [J]. Bioresource Technology, 2012, 114: 275-280. doi: 10.1016/j.biortech.2012.02.116 [38] 鲁安怀, 王长秋, 李艳, 环境矿物学研究进展(2011—2020年)[J]. 矿物岩石地球化学通报, 2020, 39(5): 881-898, 1068. LU A, WANG C, LI Y. Ressearch progress of environmental mineralogy (2011—2020)[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2020, 39(5): 881-898, 1068. (in Chinese).
[39] LOVLEY D R, STOLZ J F, NORD G L, et al. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism [J]. Nature, 1987, 330(6145): 252-254. doi: 10.1038/330252a0 [40] LOVLEY D R, CHAPELLE F H, PHILLIPS E J P. Fe(Ⅲ)-reducing bacteria in deeply buried sediments of the Atlantic Coastal Plain [J]. Geology, 1990, 18(10): 954-957. doi: 10.1130/0091-7613(1990)018<0954:FIRBID>2.3.CO;2 [41] PIRBADIAN S, BARCHINGER S E, LEUNG K M, et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(35): 12883-12888. doi: 10.1073/pnas.1410551111 [42] TAN Y, ADHIKARI R Y, MALVANKAR N S, et al. The low conductivity of Geobacter uraniireducens pili suggests a diversity of extracellular electron transfer mechanisms in the genus Geobacter [J]. Frontiers in Microbiology, 2016, 7: 980-990. [43] CHEN L X, CAO C L, WANG S H, et al. Electron communication of Bacillus subtilis in harsh environments [J]. Science, 2019, 12: 260-269. doi: 10.1016/j.isci.2019.01.020 [44] LIU J C, PEARCE I, SHI L, et al. Particle size effect and the mechanism of hematite reduction by the outer membrane cytochrome OmcA of Shewanella oneidensis MR-1 [J]. Geochimica et Cosmochimica Acta, 2016, 193: 160-175. doi: 10.1016/j.gca.2016.08.022 [45] LOVLEY D R, PHILLIPS E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments [J]. Applied and Environmental Microbiology, 1986, 51(4): 683-689. doi: 10.1128/aem.51.4.683-689.1986 [46] WU T, KUKKADAPU R K, GRIFFIN A M, et al. Interactions between Fe(Ⅲ)-oxides and Fe(Ⅲ)-phyllosilicates during microbial reduction 1: synthetic sediments [J]. Geomicrobiology Journal, 2016, 33(9): 793-806. doi: 10.1080/01490451.2015.1117546 [47] WU T, GRIFFIN A M, GORSKI C A, et al. Interactions between Fe(Ⅲ)-oxides and Fe(Ⅲ)-phyllosilicates during microbial reduction 2: Natural subsurface sediments [J]. Geomicrobiology Journal, 2017, 34(3): 231-241. doi: 10.1080/01490451.2016.1174758 [48] LOVLEY D R, PHILLIPS E J P. Availability of ferric iron for microbial reductionin bottom sediments of the freshwater tidal potomac river [J]. Applied and Environmental Microbiology, 1986, 52(4): 751-757. doi: 10.1128/aem.52.4.751-757.1986 [49] JAISI D P, DONG H L, LIU C X. Influence of biogenic Fe(Ⅱ) on the extent of microbial reduction of Fe(Ⅲ) in clay minerals nontronite, illite, and chlorite [J]. Geochimica Et Cosmochimica Acta, 2007, 71(5): 1145-1158. doi: 10.1016/j.gca.2006.11.027 [50] MIOT J, BENZERARA K, MORIN G, et a. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria [J]. Geochimica et Cosmochimica Acta, 2009, 73(3): 696-711. doi: 10.1016/j.gca.2008.10.033 [51] CUTTING R S, COKER V S, FELLOWES J W, et al. Mineralogical and morphological constraints on the reduction of Fe(Ⅲ) minerals by Geobacter sulfurreducens [J]. Geochimica et Cosmochimica Acta, 2009, 73(14): 4004-4022. doi: 10.1016/j.gca.2009.04.009 [52] AEPPLI M, VRANIC S, KAEGI R, et al. Decreases in iron oxide reducibility during microbial reductive dissolution and transformation of ferrihydrite [J]. Environmental Science & Technology, 2019, 53(15): 8736-8746. [53] NAKAMURA R, KAI F, OKAMOTO A, et al. Mechanisms of long-distance extracellular electron transfer of metal-reducing bacteria mediated by nanocolloidal semiconductive iron oxides [J]. Journal of Materials Chemistry A, 2013, 1(16): 5148-5157. doi: 10.1039/c3ta01672b [54] 朱朝菊, 向文军, 罗和青, 等. 铝掺杂针铁矿的制备、表征及吸附氟的特性 [J]. 无机化学学报, 2017, 33(12): 2215-2224. doi: 10.11862/CJIC.2017.266 ZHU Z J, XIANG W J, LUO H Q, et al. Preparation, characterization and fluoride adsorption characteristics of goethite and Al-doped goethite [J]. Chinese Journal of Inorganic Chemistry, 2017, 33(12): 2215-2224(in Chinese). doi: 10.11862/CJIC.2017.266
[55] 贾小红, 王甫, 任燕, 等. SO2、NO2与针铁矿、赤铁矿、磁铁矿的非均相反应 [J]. 地球化学, 2021, 50(1): 88-97. JIA X, WANG F, REN Y, et al. Heterogeneous reactions of SO2 and NO2 with goethite, hematite, and magnetite [J]. Geochimica, 2021, 50(1): 88-97(in Chinese).
[56] 向文军, 朱朝菊, 魏世勇. 几种赤铁矿的制备、表征及其对氟的吸附性能研究 [J]. 化学研究与应用, 2018, 30(2): 290-296. XIANG W, ZHU Z, WEI S Y. Preparation, characterization and fluoride adsorption characteristics of three hematite samples [J]. Chemical Research and Application, 2018, 30(2): 290-296(in Chinese).
[57] 方敦, 王锐, 许海娟, 等. 硅/铝掺杂纤铁矿的表面性质及对F-的吸附性能 [J]. 环境污染与防治, 2020, 42(3): 287-292. FANG D, WANG R, XU H J, et al. The surface properties and adsorption performance for F- of Si-or Al-doped lepidocrocite [J]. Environmental Pollution and Control, 2020, 42(3): 287-292(in Chinese).
[58] YU G, FU F, YE C, et al. Behaviors and fate of adsorbed Cr(VI) during Fe(II)-induced transformation of ferrihydrite-humic acid co-precipitates [J]. Journal of Hazardous Materials, 2020, 392: 122272. doi: 10.1016/j.jhazmat.2020.122272 [59] BIRD L J, BONNEFOY V, NEWMAN D K. Bioenergetic challenges of microbial iron metabolisms [J]. Trends in Microbiology, 2011, 19(7): 330-340. doi: 10.1016/j.tim.2011.05.001 [60] LOVLEY D R, GIOVANNONI S J, WHITE D C, et al. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals [J]. Archives of Microbiology, 1993, 159(4): 336-344. doi: 10.1007/BF00290916 [61] MARSHALL M J, BELIAEV A S, DOHNALKOVA A C, et al. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis [J]. PLoS Biology, 2006, 4(8): 1324-1333. [62] LOVLEY D R, PHILLIPS E J P, WIDMAN P K. Reduction of uranium by Desulfovibrio desulfuricans [J]. Applied and Environmental Microbiology, 1992, 58(3): 850-856. doi: 10.1128/aem.58.3.850-856.1992 [63] VELZEN L V. Environmental remediation and restoration of contaminated nuclear and norm sites[M]. America: Woodhead Publishing 2015: 185-236. [64] COLOGGI D L, LAMPA-PASTIRK S, SPEERS A M, et al. Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism [J]. Proc Natl Acad Sci U S A, 2011, 108(37): 15248-15252. doi: 10.1073/pnas.1108616108 [65] DUMMI MAHADEVAN G, ZHAO F. A concise review on microbial remediation cells (MRCs) in soil and groundwater radionuclides remediation [J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 314(3): 1477-1485. doi: 10.1007/s10967-017-5612-4 [66] SUZUKI Y, KITATSUJI Y, OHNUKI T, et al. Flavin mononucleotide mediated electron pathway for microbial U(VI) reduction [J]. Physical Chemistry Chemical Physics, 2010, 12(34): 10081-10087. doi: 10.1039/c0cp00339e [67] YAMASAKI S, TANAKA K, KOZAI N, et al. Effect of flavin compounds on uranium(VI) reduction- kinetic study using electrochemical methods with UV-vis spectroscopy [J]. Applied Geochemistry, 2017, 78: 279-286. doi: 10.1016/j.apgeochem.2017.01.014 [68] SUN, H, SUN G Q, WANG S L, et al. Pd electroless plated Nafion(R) membrane for high concentration DMFCs [J]. Journal of Membrane Science, 2005, 259(1a2): 27-33. [69] HINDATU Y, ANNUAR M S M, GUMEL A M. Mini-review: Anode modification for improved performance of microbial fuel cell [J]. Renewable and Sustainable Energy Reviews, 2017, 73: 236-248. doi: 10.1016/j.rser.2017.01.138 [70] ZHU X, LOGAN B E. Copper anode corrosion affects power generation in microbial fuel cells [J]. Journal of Chemical Technology & Biotechnology, 2014, 89(3): 471-474. [71] HOU J Z, LIU S, YANG, et al. Three-dimensional macroporous anodes based on stainless steel fiber felt for high-performance microbial fuel cells [J]. Journal of Power Sources, 2014, 258: 204-209. doi: 10.1016/j.jpowsour.2014.02.035 [72] LI Y, XU D, CHEN C, et al. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review [J]. Journal of Materials Science & Technology, 2018, 34(10): 1713-1718. [73] SHERAR B W A, POWER I M, KEECH P G, et al. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion [J]. Corrosion Science, 2011, 53(3): 955-960. doi: 10.1016/j.corsci.2010.11.027 [74] JIA R, YANG D, XU D, et al. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm [J]. Bioelectrochemistry, 2017, 118: 38-46. doi: 10.1016/j.bioelechem.2017.06.013 [75] SCHMIDT M W I, TORN M S, ABIVEN S, et al. Persistence of soil organic matter as an ecosystem property [J]. Nature, 2011, 478(7367): 49-56. doi: 10.1038/nature10386 [76] 蔡茜茜, 袁勇, 胡佩, 等. 腐殖质电化学特性及其介导的胞外电子传递研究进展 [J]. 应用与环境生物学报, 2015, 21(6): 996-1002. CAI X X, YUAN Y, HU P, et al. Progress in study of humic substances: Electrochemical redox characterization and extracellular respiration [J]. Chinese Journal of Applied & Environmental Biology, 2015, 21(6): 996-1002(in Chinese).
[77] ZHENG Y, KAPPLER A, XIAO Y, et al. Redox-active humics support interspecies syntrophy and shift microbial community [J]. Science China Technological Sciences, 2019, 62(10): 1695-1702. doi: 10.1007/s11431-018-9360-5 [78] NEWMAN D K and KOLTER R. A role for excreted quinones in extracellular electron transfer [J]. Nature, 2000, 405(6782): 94-97. doi: 10.1038/35011098 [79] BAI Y G, MELLAGE A, CIRPKA O A, et al. AQDS and redox-active NOM enables microbial Fe(Ⅲ)-mineral reduction at cm-scales [J]. Environmental Science & Technology, 2020, 54(7): 4131-4139. [80] SCOTT D T, MCKNIGHT D M, BLUNT-HARRIS E L, et al. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms [J]. Environmental Science & Technology, 1998, 32(19): 2984-2989. [81] NURMI J T and TRATNYEK P G. Electrochemical properties of natural organic matter (NOM), fractions of NOM, and model biogeochemical electron shuttles [J]. Environmental Science & Technology, 2002, 36(4): 617-624. [82] CORY R M, MCKNIGHT D M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter [J]. Environmental Science & Technology, 2005, 39(21): 8142-8149. [83] HERNáNDEZ-MONTOYA V, ALVAREZ L H, MONTES-MORáN M A, et al. Reduction of quinone and non-quinone redox functional groups in different humic acid samples by Geobacter sulfurreducens [J]. Geoderma, 2012, 183-184: 25-31. doi: 10.1016/j.geoderma.2012.03.007 [84] TAN W, WANG G, ZHAO X, et al. Molecular‐weight‐dependent redox cycling of humic substances of paddy soils over successive anoxic and oxic alternations [J]. Land Degradation & Development, 2019, 30(9): 1130-1144. [85] KAPPLER A, BENZ M, SCHINK B, et al. Electron shuttling via humic acids in microbial iron(Ⅲ) reduction in a freshwater sediment [J]. FEMS Microbiology Ecology, 2004, 47(1): 85-92. doi: 10.1016/S0168-6496(03)00245-9 [86] RODEN E E, KAPPLER A, BAUER I, et al. Extracellular electron transfer through microbial reduction of solid-phase humic substances [J]. Nature Geoscience, 2010, 3(6): 417-421. doi: 10.1038/ngeo870 [87] LAU M P, SANDER M, GELBRECHT J, et al. Solid phases as important electron acceptors in freshwater organic sediments [J]. Biogeochemistry, 2015, 123(1-2): 49-61. doi: 10.1007/s10533-014-0052-5 [88] TAN W, XI B, WANG G, et al. Microbial-accessibility-dependent electron shuttling of in situ solid-phase organic matter in soils [J]. Geoderma, 2019, 338: 1-4. doi: 10.1016/j.geoderma.2018.11.037 [89] KLüPFEL L, PIEPENBROCK A, KAPPLER A, et al. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments [J]. Nature Geoscience, 2014, 7(3): 195-200. doi: 10.1038/ngeo2084 [90] ZHAO J L, WANG L, TANG L L, et al. Changes in bacterial community structure and humic acid composition in response to enhanced extracellular electron transfer process in coastal sediment [J]. Archives of Microbiology, 2019, 201(7): 897-906. doi: 10.1007/s00203-019-01659-3 [91] ZHANG D D, ZHANG C F, ZHILING L, et al. Electrochemical stimulation of microbial reductive dechlorination of pentachlorophenol using solid-state redox mediator (humin) immobilization [J]. Bioresource Technology, 2014, 164: 232-240. doi: 10.1016/j.biortech.2014.04.071 [92] PANKRATOVA G, PANKRATOV D, et al. Following nature: Bioinspired mediation strategy for gram-positive bacterial cells [J]. Advanced Energy Materials, 2019, 9(16): 1900215.1-1900215.6. [93] NISHIO K, NAKAMURA R, LIN X J, et al. Extracellular electron transfer across bacterial cell membranes via a cytocompatible redox-active polymer [J]. Chemphyschem, 2013, 14(10): 2159-2163. doi: 10.1002/cphc.201300117 [94] KANEKO M, ISHIKAWA M, SONG J, et al. Cathodic supply of electrons to living microbial cells via cytocompatible redox-active polymers [J]. Electrochemistry Communications, 2017, 75: 17-20. doi: 10.1016/j.elecom.2016.12.002 [95] HASAN K, PATIL S A, LEECH D, et al. Electrochemical communication between microbial cells and electrodes via osmium redox systems [J]. Biochemical Society Transactions, 2012, 40(6): 1330-1335. doi: 10.1042/BST20120120 [96] PATIL S A, HASAN K, LEECH D, et al. Improved microbial electrocatalysis with osmium polymer modified electrodes [J]. Chemical Communications, 2012, 48(82): 10183-10185. doi: 10.1039/c2cc34903e [97] GIROUD F, MILTON R D, TAN B X, et al. Simplifying enzymatic biofuel cells: Immobilized naphthoquinone as a biocathodic orientational moiety and bioanodic electron mediator [J]. ACS Catalysis, 2015, 5(2): 1240-1244. doi: 10.1021/cs501940g [98] HASAN K, GRATTIERI M, WANG T, et al. Enhanced bioelectrocatalysis of Shewanella oneidensis MR-1 by a naphthoquinone redox polymer [J]. ACS Energy Letters, 2017, 2(9): 1947-1951. doi: 10.1021/acsenergylett.7b00585 [99] WANG G , FENG C. Electrochemical polymerization of hydroquinone on graphite felt as a pseudocapacitive material for application in a microbial fuel cell [J]. Polymers, 2017, 9(12): 220. doi: 10.3390/polym9060220 [100] WU W, NIU H, YANG D, et al. Polyaniline/carbon nanotubes composite modified anode via graft polymerization and self-assembling for microbial fuel cells [J]. Polymers, 2018, 10(7): 759. doi: 10.3390/polym10070759 [101] SUMISHA A , HARIBABU K. Modification of graphite felt using nano polypyrrole and polythiophene for microbial fuel cell applications-a comparative study [J]. International Journal of Hydrogen Energy, 2018, 43(6): 3308-3316. doi: 10.1016/j.ijhydene.2017.12.175 [102] RAJENDRAN R, DHAKSHINA MOORTHY G P, KRISHNAN H, et al. A study on polythiophene modified carbon cloth as anode in microbial fuel cell for lead removal [J]. Arabian Journal for Science and Engineering, 2021: 1-7. [103] SONG R B, WU Y C, LIN Z Q, et al. Living and conducting: Coating individual bacterial cells with In Situ formed polypyrrole [J]. Angewandte Chemie (International Ed. in English), 2017, 56(35): 10516-10520. doi: 10.1002/anie.201704729 [104] KANEKO M, ISHIHARA K, NAKANISHI S. Redox-active polymers connecting living microbial cells to an extracellular electrical circuit [J]. Small (Weinheim an Der Bergstrasse, Germany), 2020, 16(34): e2001849. doi: 10.1002/smll.202001849 [105] LIU D F, MIN D, CHENG L, et al. Anaerobic reduction of 2, 6-dinitrotoluene by Shewanella oneidensis MR-1: Roles of Mtr respiratory pathway and NfnB [J]. Biotechnology and Bioengineering, 2017, 114(4): 761-768. doi: 10.1002/bit.26212 [106] WANG H F, ZHAO H P, ZHU L Z. Structures of nitroaromatic compounds induce Shewanella oneidensis MR-1 to adopt different electron transport pathways to reduce the contaminants [J]. Journal of Hazardous Materials, 2020, 384: 121495. doi: 10.1016/j.jhazmat.2019.121495 [107] SUNG Y, FLETCHER K E, RITALAHTI K M, et al. Geobacter lovleyi sp. nov. strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium [J]. Applied and Environmental Microbiology, 2006, 72(4): 2775-2782. doi: 10.1128/AEM.72.4.2775-2782.2006 [108] AMOS B K, SUNG Y, FLETCHER K E, et al. Detection and quantification of Geobacter lovleyi strain SZ: implications for bioremediation at tetrachloroethene- and uranium-impacted sites [J]. Applied and Environmental Microbiology, 2007, 73(21): 6898-6904. doi: 10.1128/AEM.01218-07 [109] WAGNER D D, HUG L A, HATT J K, et al. Genomic determinants of organohalide-respiration in Geobacter lovleyi, an unusual member of the Geobacteraceae [J]. BMC Genomics, 2012, 13(1): 200. doi: 10.1186/1471-2164-13-200 [110] ZHANG J, LI J, YE D, et al. Tubular bamboo charcoal for anode in microbial fuel cells [J]. Journal of Power Sources, 2014, 272: 277-282. doi: 10.1016/j.jpowsour.2014.08.115 [111] HSU L H H, DENG P, ZHANG Y X, et al. Nanostructured interfaces for probing and facilitating extracellular electron transfer [J]. Journal of Materials Chemistry. B, 2018, 6(44): 7144-7158. doi: 10.1039/C8TB01598H [112] WANG L, SU L, CHEN H, et al. Carbon paper electrode modified by goethite nanowhiskers promotes bacterial extracellular electron transfer [J]. Materials Letters, 2015, 141(feba15): 311-314. [113] DING Q, CAO Y, LI F, et al. Construction of conjugated polymer-exoelectrogen hybrid bioelectrodes and applications in microbial fuel cells [J]. Chinese Journal of Biotechnology, 2021, 37(1): 1-14. [114] ZHOU X, LV F, HUANG Y, et al. Biohybrid conjugated polymer materials for augmenting energy conversion of bioelectrochemical systems [J]. Chemistry-a European Journal, 2020, 26(66): 15065-15073. doi: 10.1002/chem.202002041 [115] DONG G W, WANG H H, YAN Z Y, et al. Cadmium sulfide nanoparticles-assisted intimate coupling of microbial and photoelectrochemical processes: Mechanisms and environmental applications [J]. The Science of the Total Environment, 2020, 740: 140080. doi: 10.1016/j.scitotenv.2020.140080 [116] KORTH B, MASKOW T, PICIOREANU C, et al. The microbial electrochemical Peltier heat: an energetic burden and engineering chance for primary microbial electrochemical technologies [J]. Energy & Environmental Science, 2016, 9(8): 2539-2544. [117] WAGNER R C, CALL D F, LOGAN B E. Optimal set anode potentials vary in bioelectrochemical systems [J]. Environmental Science & Technology, 2010, 44(16): 6036-6041. [118] FIRER-SHERWOOD M, PULCU G S, ELLIOTT S J. Electrochemical interrogations of the Mtr cytochromes from Shewanella: Opening a potential window [J]. JBIC Journal of Biological Inorganic Chemistry, 2008, 13(6): 849-854. doi: 10.1007/s00775-008-0398-z [119] STRAUB, BENZ, SCHINK. Iron metabolism in anoxic environments at near neutral pH [J]. FEMS Microbiology Ecology, 2001, 34(3): 181-186. doi: 10.1111/j.1574-6941.2001.tb00768.x [120] WEBER K A, ACHENBACH L A, COATES J D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction [J]. Nature Reviews. Microbiology, 2006, 4(10): 752-764. doi: 10.1038/nrmicro1490 [121] BEBLAWY S, BURSAC T, PAQUETE C, et al. Extracellular reduction of solid electron acceptors by Shewanella oneidensis [J]. Molecular Microbiology, 2018, 109(5): 571-583. doi: 10.1111/mmi.14067 [122] GROBBLER C, VIRDIS B, NOUWENS A, et al. Effect of the anode potential on the physiology and proteome of Shewanella oneidensis MR-1 [J]. Bioelectrochemistry (Amsterdam, Netherlands), 2018, 119: 172-179. doi: 10.1016/j.bioelechem.2017.10.001 [123] KORTH B, HARNISCH F. Spotlight on the energy harvest of electroactive microorganisms: The impact of the applied anode potential [J]. Frontiers in Microbiology, 2019, 10: 1352. doi: 10.3389/fmicb.2019.01352 [124] HIROSE A, KASAI T, AOKI M, et al. Electrochemically active bacteria sense electrode potentials for regulating catabolic pathways [J]. Nature Communications, 2018, 9(1): 1083. doi: 10.1038/s41467-018-03416-4 [125] GARRETT T R, BHAKOO M, ZHANG Z. Bacterial adhesion and biofilms on surfaces [J]. Progress in Natural Science, 2008, 18(9): 1049-1056. doi: 10.1016/j.pnsc.2008.04.001 [126] LI C C , CHENG S A. Functional group surface modifications for enhancing the formation and performance of exoelectrogenic biofilms on the anode of a bioelectrochemical system [J]. Critical Reviews in Biotechnology, 2019, 39(8): 1015-1030. doi: 10.1080/07388551.2019.1662367 [127] MARSHALL T A, MORRIS K, LAW G T W, et al. Incorporation of uranium into hematite during crystallization from ferrihydrite [J]. Environmental Science & Technology, 2014, 48(7): 3724-3731. [128] XIAO Y, ZHANG E H, ZHANG J D, et al. Extracellular polymeric substances are transient media for microbial extracellular electron transfer [J]. Science Advances, 2017, 3(7): e1700623. doi: 10.1126/sciadv.1700623 [129] PONS L, DELIA M L, BERGEL A. Effect of surface roughness, biofilm coverage and biofilm structure on the electrochemical efficiency of microbial cathodes [J]. Bioresource Technology, 2011, 102(3): 2678-2683. doi: 10.1016/j.biortech.2010.10.138 [130] HIZAL F, RUNGRAENG N, LEE J, et al. Nanoengineered superhydrophobic surfaces of aluminum with extremely low bacterial adhesivity [J]. ACS Applied Materials & Interfaces, 2017, 9(13): 12118-12129. [131] WANG H, SONG L J, JIANG R J, et al. Super-repellent photodynamic bactericidal hybrid membrane [J]. Journal of Membrane Science, 2020, 614: 118482. doi: 10.1016/j.memsci.2020.118482 [132] ANJUM, A S, K C SUN, M ALI, et al. Fabrication of coral-reef structured nano silica for self-cleaning and super- hydrophobic textile applications [J]. Chemical Engineering Journal, 2020, 401: 125859. doi: 10.1016/j.cej.2020.125859 [133] BOKS N P, NORDE W, van der MEI H C, et al. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces [J]. Microbiology, 2008, 154(10): 3122-3133. doi: 10.1099/mic.0.2008/018622-0 [134] GUO K, FREGUIA S, DENNIS P G, et al. Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems [J]. Environmental Science & Technology, 2013, 47(13): 7563-7570. [135] KOBAYASHI M, TERAYAMA Y, YAMAGUCHI H, et al. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes [J]. Langmuir, 2012, 28(18): 7212-7222. doi: 10.1021/la301033h [136] WANG Z X, ELIMELECH M, LIN S H. Environmental applications of interfacial materials with special wettability [J]. Environmental Science & Technology, 2016, 50(5): 2132-2150. [137] YUAN Y, CAI X X, WANG Y Q, et al. Electron transfer at microbe-humic substances interfaces: Electrochemical, microscopic and bacterial community characterizations [J]. Chemical Geology, 2017, 456: 1-9. doi: 10.1016/j.chemgeo.2017.02.020 [138] DING C M, LV M L, ZHU Y, et al. Wettability-regulated extracellular electron transfer from the living organism of Shewanella loihica PV-4 [J]. Angewandte Chemie (International Ed. in English), 2015, 54(5): 1446-1451. doi: 10.1002/anie.201409163 [139] ZHAO C, DING C, LV M, et al. Hydrophilicity boosted extracellular electron transfer in Shewanella loihica PV-4 [J]. RSC Advances, 2016, 6(27): 22488-22493. doi: 10.1039/C5RA24369F [140] LIN X, YANG F, YOU L X, et al. Liposoluble quinone promotes the reduction of hydrophobic mineral and extracellular electron transfer of Shewanella oneidensis MR-1 [J]. The Innovation, 2021,2 (2): 100104. [141] YEUNG T, GILBERT G E, SHI J L, et al. Membrane phosphatidylserine regulates surface charge and protein localization [J]. Science, 2008, 319(5860): 210-213. doi: 10.1126/science.1152066 [142] KE Y, LIU C, ZHANG X, et al. Surface modification of polyhydroxyalkanoates toward enhancing cell compatibility and antibacterial activity [J]. Macromolecular Materials and Engineering, 2017, 302(11): 1700258. doi: 10.1002/mame.201700258 [143] WU J, ZHAO S, XU S, et al. Acidity-triggered charge-reversible multilayers for construction of adaptive surfaces with switchable bactericidal and bacteria-repelling functions [J]. Journal of Materials Chemistry B, 2018, 6(45): 7462-7470. doi: 10.1039/C8TB02093K [144] CHAROENSRI K, RODWIHOK C, WONGRATANAPHISAN D, et al. Investigation of functionalized surface charges of thermoplastic Starch/Zinc oxide nanocomposite films using polyaniline: the potential of improved antibacterial properties [J]. Polymers, 2021, 13(3): 425. doi: 10.3390/polym13030425 -



 下载:
下载: