-
挥发性有机污染物(volatile organic compounds,VOCs)是城市大气主要污染物之一,已取代SO2成为我国生态环境部制定的“十四五”城市空气质量考核新指标。VOCs在生产与生活中来源广泛,室内建材的释放,道路机动车的排放,化石燃料的燃烧以及工业生产排放的废气都是大气中VOCs的重要来源[1-2]。这些废气中的VOCs组分复杂,易造成次生污染,对大气环境影响突出,严重威胁着生态环境和人体健康[3]。因此,VOCs的高效治理是当前亟待解决的环境问题。
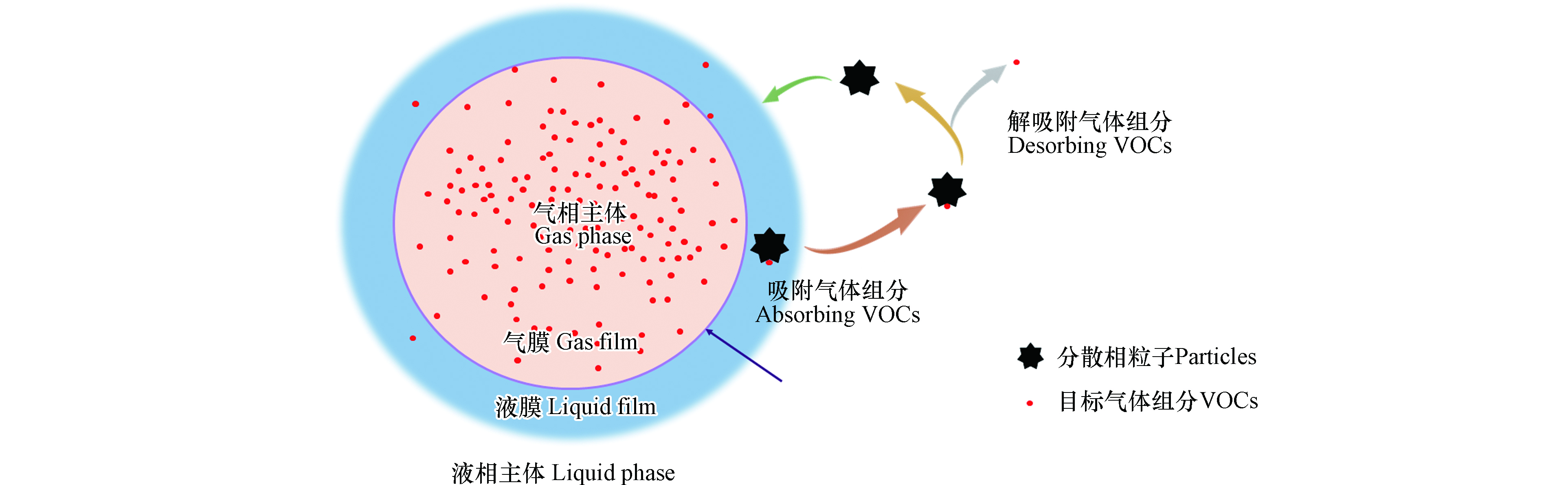
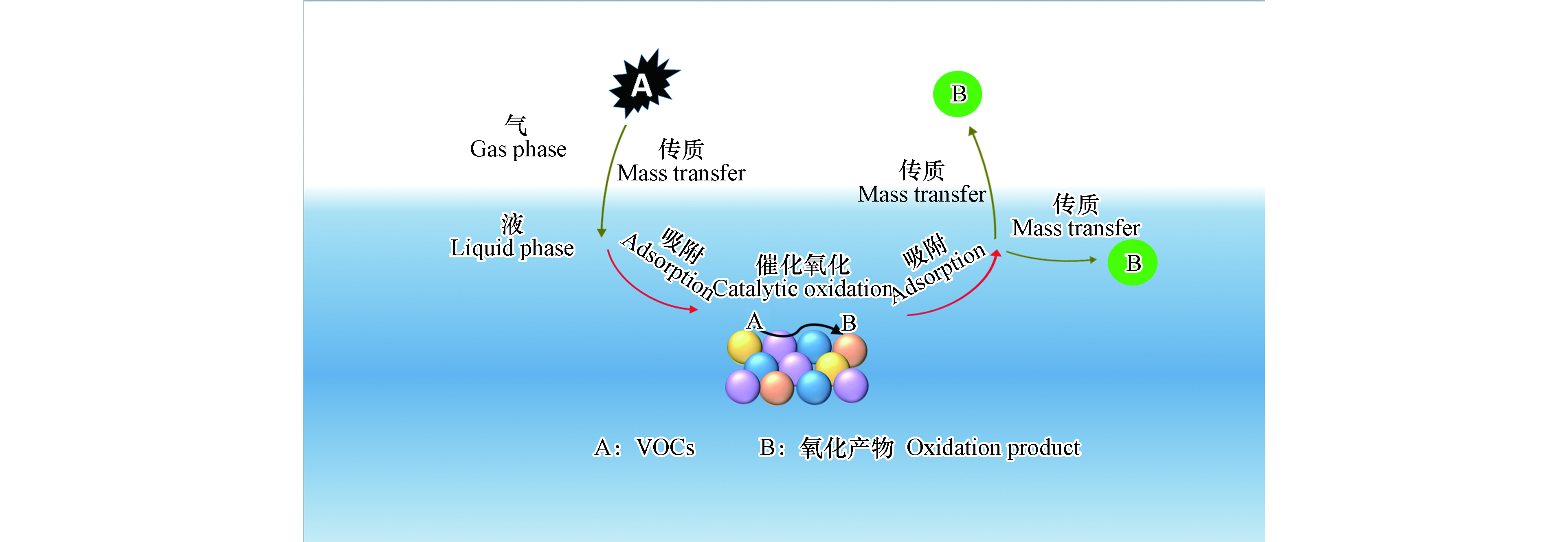
芬顿(Fenton)法以溶液中的二价铁离子或固体粒子为催化剂,通过催化过氧化氢分解生成具有强氧化性的羟基自由基,进而降解溶液中的有机污染物[4-5],是废水中有机污染物治理最具代表性和最有效的方法之一[6]。近年来,将芬顿氧化法应用于废气中VOCs的治理也取得了良好的效果[7]。芬顿法分为均相芬顿法和非均相芬顿法[8],由于催化剂状态的不同,VOCs在两种体系中的降解过程有一定差异。均相芬顿体系以溶液中的Fe2+为催化剂,VOCs首先通过气-液传质进入芬顿体系,然后被溶液中的羟基自由基氧化分解[9]。非均相芬顿体系以固相粒子为催化剂,VOCs的降解过程可分为“传质-吸附-催化氧化-解吸附-传质”五个步骤[10](图1),即VOCs经过气-液传质由气相进入溶液并吸附于催化剂表面,在催化剂表面发生催化氧化反应生成氧化产物(中间产物或二氧化碳),这些氧化产物经过解吸附和传质过程返回气相或进入溶液中。
VOCs的分子结构相对简单,且羟基自由基具有较强的氧化能力,当VOCs分子通过气-液传质进入芬顿反应体系后,催化降解效率往往较高。而疏水性VOCs极低的气-液传质速率限制了其进入芬顿体系中并被氧化降解,进而导致芬顿过程降解复杂混合VOCs的效率降低,成为当前VOCs治理过程中亟待解决的关键问题之一[11]。根据气-液传质的双膜理论,气-液相界面两侧各存在一个相对静止的膜,气相一侧称为气膜,液相一侧称为液膜,气液两相的传质速率取决于气膜和液膜内的传质速率。疏水性VOCs由于溶解度系数小,液膜传质阻力大,导致总传质效率低。因此,提高疏水性VOCs气-液传质效率的关键是提高疏水性VOCs的液膜传质效率。
近年来,通过改进反应器结构、加入分散相粒子和优化催化界面微结构等措施来增大气-液传质界面和传质驱动力、降低传质阻力,在提高芬顿体系中VOCs气-液传质效率方面取得了良好效果。此外,通过将实验与理论模拟相结合,构建传质模型,在传质增强机制等方面也开展了一系列研究。随着传质增强研究的深入,芬顿体系全过程多步骤的协同作用引起了人们的关注与重视,传质、吸附、催化等多步骤通过耦合作用协同提高芬顿体系对VOCs的降解能力,不仅实现了复杂混合VOCs的高效降解,而且在促进污染物矿化、实现目标VOCs的深度降解等方面也取得了良好的效果。
本文综述了芬顿体系降解VOCs过程中气-液传质增强途径,深入分析了相关传质增强机理,通过总结传质-吸附-催化协同作用促进芬顿体系高效降解VOCs的发展现状并分析其微观机制,提出了芬顿体系降解复杂混合VOCs过程的进一步研究方向,旨在为芬顿体系降解VOCs过程优化及高效传质-吸附-催化耦合粒子的制备提供参考。
-
反应介质是影响芬顿体系传质效率的主要因素之一。VOCs在芬顿体系中的传质效率不仅受其组成和浓度的影响,还与溶液pH[12]、温度、气体和液体流速[13-14]等条件密切相关。增强机械搅拌、提高气体流速[15]或提高反应温度等方法都可以在一定程度上提高传质效率[16]。通过改进反应介质条件可以增大化学反应速率,进而增大液膜两侧浓度差,提高液膜传质的驱动力。超声可以通过引发湍流[17]和剪力流[18]增大传质界面,并加速产物在反应位点的脱附过程,降低体系传质阻力[19]。光、电、磁等能量场也是提高传质速率的重要措施[17-18]。例如,在外加磁场的芬顿体系中,4-氯苯酚的传质效率得到了显著提高[20]。
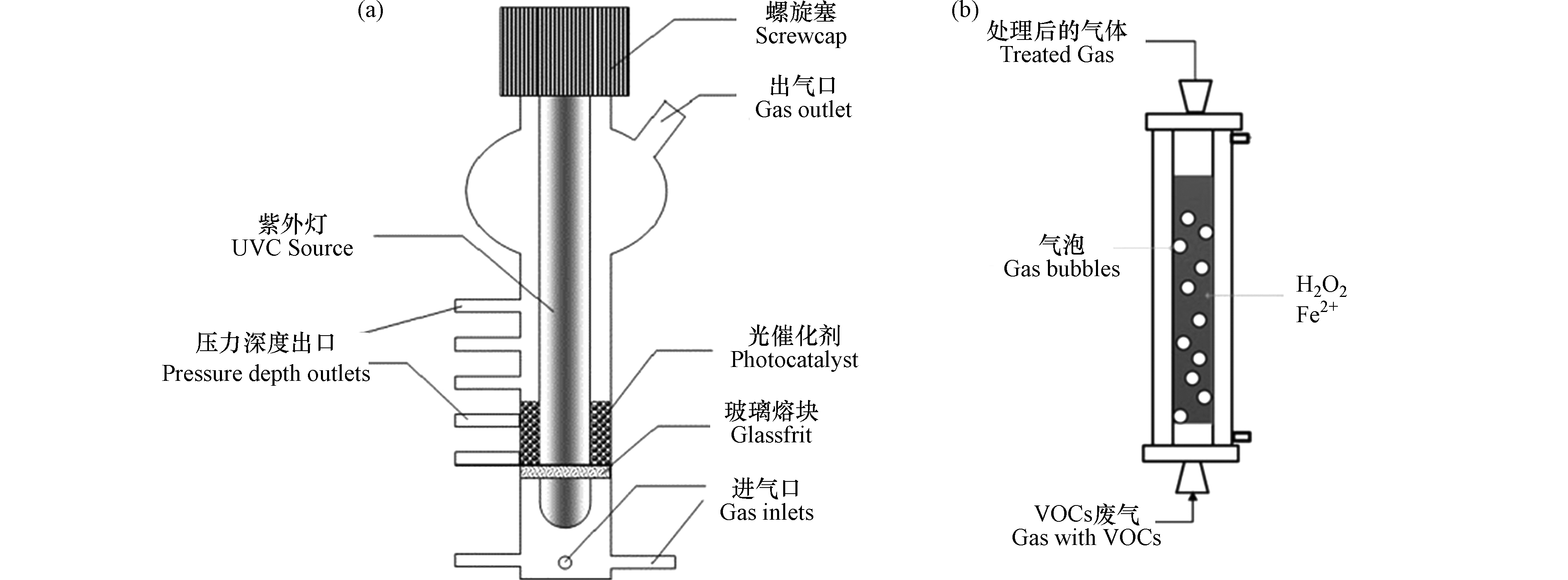
通过优化反应器结构,如增加曝气装置和微流控系统[21]等,可以增大传质界面 [15, 21] ,在一定程度上克服气-液传质阻力,加快反应物和中间产物的传质。用流化床反应器(图2a)[22]代替固定床或移动床反应器是增大体系传质效率的有效方法[15]。芬顿体系降解VOCs过程中常用的鼓泡反应装置(图2b)是一种可有效提高气液传质效率的多相反应器,气泡的运动促进了液相中物质的混合与传递[23],在促进芬顿体系传质和传热过程方面具有良好的效果[24]。
-
添加分散相粒子是加快芬顿体系中VOCs气-液传质速率的有效途径[25-28]。分散相粒子的引入是通过加快液膜传质速率强化气-液传质过程。活性炭、分子筛等微细颗粒可以通过改变溶液物理性质、气液界面形态、气液化学反应动力学参数等,降低VOCs在气-液传质过程中的液膜传质阻力,并有效提高气液接触面积和气-液传质速率[29-31]。如图3所示的分散相粒子加速气-液传质作用机制较好地通过双膜理论解释了分散相粒子的传质强化作用:微细粒子不断循环穿梭于液相主体和液膜之间,不仅产生流体扰动和微对流,而且通过在液膜吸附目标气体组分和在液相主体解吸附形成“捕获”效应(Grazing effect)[32],实现气-液传质强化作用[33]。在均相芬顿体系降解难溶性VOCs正辛烷的过程中加入活性炭颗粒,体系的体积传质系数增大了24倍[33]。将大量的碳基颗粒加入到电芬顿体系中,可形成“准均相”催化反应环境,也可以极大地促进体系传质效率的提高[28]。
体系中粒子加入量、颗粒直径和比表面积是影响气-液传质系数的重要因素[34-36]。在颗粒直径对传质增强效率影响的研究中发现,向体系中加入相同质量的分散相粒子,传质增强效率随颗粒尺寸减小而提高[32]。纳米与微米量级的微细粒子在体系传质增强方面都表现出良好的效果[35-36]。纳米粒子比表面积大,气液接触面积和颗粒表面吸附容量大,在芬顿体系中表现出较高的传质增强效率[37-38]。当体系中加入的粒子直径增大,运动的粒子具有较大的动能,可以通过与气泡碰撞将气泡分解,也表现出良好的传质增强能力,因此,微米级及更大的粒子也是良好的气-液传质增强颗粒[39]。粒子加入量也是影响传质增强效率的重要因素。在芬顿体系降解正辛烷的过程中加入不同质量的活性炭(0— 0.1%),体积传质系数随活性炭含量的提高而增大 [33] 。
-
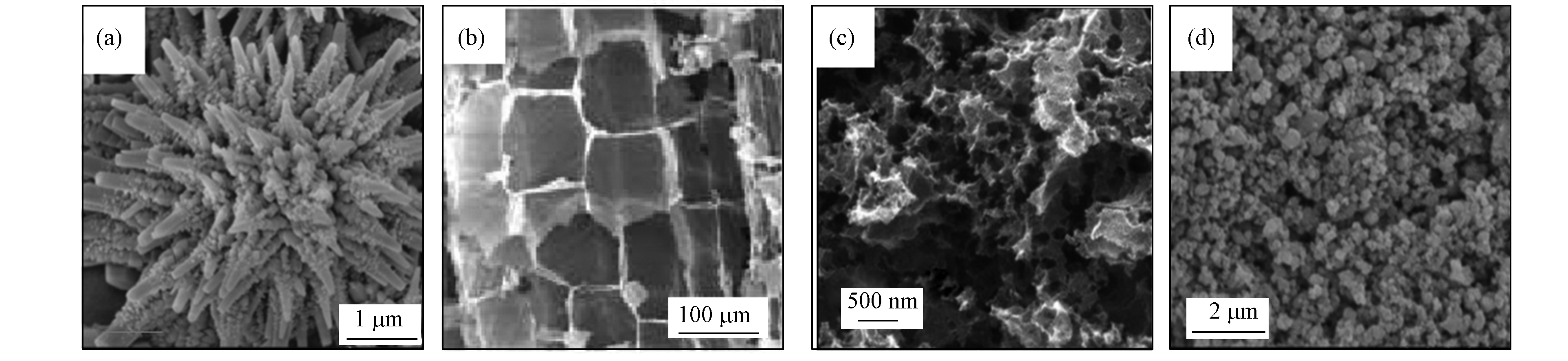
芬顿催化界面微结构可调控污染物分子的界面传质行为,是芬顿体系传质效率的重要影响因素。通过芬顿催化剂表面结构设计与化学修饰等方法优化催化界面结构是提高芬顿体系中污染物分子传质速率的有效途径。在不锈钢基体上制备层状阵列Fe2O3(图4a),利用层状结构可以缩短反应物与产物的扩散路径来提高体系传质效率[40]。在芬顿法降解有机污染物的过程中,层状多孔Fe2O3[41] (图4b)的超大比表面积和丰富孔结构可提高污染物分子的传质效率。
将催化剂负载于具有特定微结构的载体上,载体不仅可以作为分散相粒子提高气液传质效率,其特定的表面结构还可以增强纳米尺度上的传质,是提高传质效率的重要方法。有序介孔结构通过增加介质传质通道提高传质效率。将Fe负载于具有有序介孔结构的多孔碳材料上(图4c)并将其应用于芬顿体系降解苯酚,苯酚及其氧化产物的传质效率显著加快[42]。将纳米Fe2O3负载于KIT-6介孔分子筛(图4d)可以提高体系传质效率[43]。在3D石墨烯框架制备的液流电极上复合聚苯胺,可在界面区域诱导纳米尺度对流来增强体系纳米尺度的传质[44]。此外,将分散相粒子与催化剂复合,还可以实现传质、吸附、催化的多过程协同,在提高难溶性VOCs的降解效率方面有着广阔的前景[45-46]。
-
传质与吸附两个步骤在芬顿体系降解VOCs的过程中紧密相关。研究表明提高气-液传质效率可以提高体系对VOCs的吸附能力,体系吸附能力的提高也会促进传质效率的提高。在芬顿体系去除甲苯的实验中发现,体系气-液传质系数提高20倍后,甲苯的吸附去除效率提高了18倍[47]。芬顿体系对VOCs的吸附包括催化剂的吸附和溶液的溶解两部分,因此,增强催化材料的吸附性能是增大体系吸附容量的重要途径。例如,在芬顿体系中加入具有丰富孔隙结构和较强吸附能力的多孔复合催化材料可以促进污染物气-液传质效率的提高[11]。当体系吸附能力下降,VOCs的气-液传质效率也会相应降低。例如,当污染物在溶液中溶解并积累到一定浓度[48],或液相中总有机物含量增加到一定程度后[49],会阻碍体系对VOCs的吸附,VOCs的气-液传质阻力也会相应增大,导致气-液传质效率降低。
-
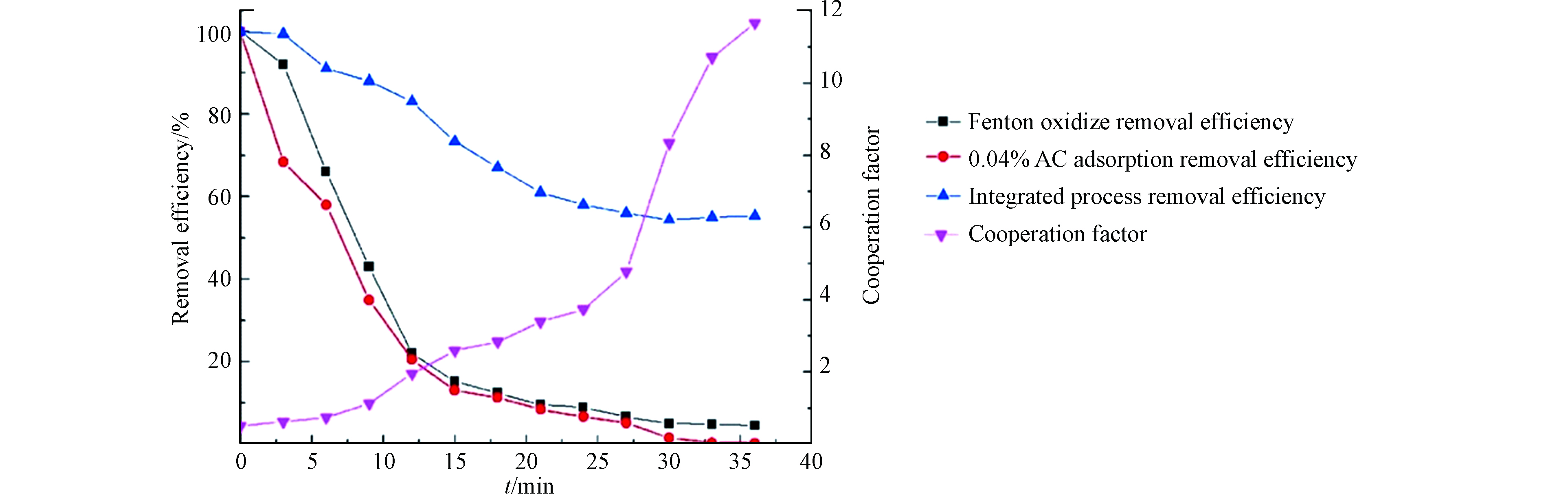
传质与催化是芬顿体系降解VOCs过程的关键步骤,这两个步骤具有耦合效应,相互促进协同提高芬顿体系对VOCs的去除效率。研究表明,通过在均相芬顿体系中加入分散相粒子可实现传质-催化的协同作用。如图5所示,在均相芬顿体系降解难溶性VOCs正辛烷的过程中,未引入活性炭颗粒的均相芬顿体系受传质效率低的限制,在反应开始后,正辛烷的去除率即急剧下降;当体系中引入活性炭颗粒但不加芬顿试剂,吸附总容量虽有所提高,但体系对正辛烷的吸收在10 min达到饱和;而在体系中同时加入芬顿试剂和活性炭颗粒,在传质-催化协同作用下,体系对正辛烷的降解效率保持在了较高的水平;通过计算传质与催化的协同增强因子对协同作用进行量化分析发现,随着反应的进行,协同增强因子逐渐增大,使得体系在H2O2被逐渐消耗的情况下仍然能够保持降解效率的基本稳定[33]。在芬顿体系中,提高传质效率可以促进目标污染物在溶液中的吸收,从而提高目标污染物的催化氧化效率;提高催化氧化效率可以通过降低体系中的污染物浓度来增大传质驱动力[49]。例如,通过对比研究Fe2+芬顿体系与 Fe3+芬顿体系在降解甲苯过程中传质速率的差异表明,在催化效率较高的芬顿体系(Fe2+)中甲苯的气液传质效率也更高[49];在芬顿体系降解甲苯和乙酸乙酯的过程中,通过提高有机物的氧化分解速率可以增大体系的气液传质系数[47]。
复合催化材料的制备是利用传质-催化协同作用构建高效芬顿体系的有效途径。例如,通过制备铃铛结构Fe3O4@C,将碳壳的传质增强作用和Fe3O4核的催化作用有机结合,在正辛烷降解过程中表现出了传质-催化协同促进作用[50];将Fe负载于ZSM-5型沸石用于芬顿体系去除废气中的甲苯,通过催化与传质协同作用也实现了甲苯高效深度降解[12]。
-
吸附与催化步骤在芬顿体系降解VOCs的过程中相互促进,具有协同效应。通过实验对比芬顿体系与纯水对废气中甲苯的吸收表明,芬顿体系对甲苯的吸附去除速率和吸附饱和容量均提高[49]。在均相芬顿体系降解苯胺过程中,向体系中加入具有丰富活性吸附位点的介孔活性炭,体系吸附性能提高后,苯胺的降解效率也大大提高[51]。
将芬顿催化剂负载于具有大比表面积的载体上,利用吸附与催化的耦合作用提高VOCs降解效率是构建高效芬顿体系的有效方法。在非均相芬顿体系降解萘的反应中,将铁锰二元氧化物与生物炭复合后萘的降解效率大大提高;且对比复合材料芬顿体系与生物炭芬顿体系对萘的吸附去除率发现,复合材料体系对萘的吸附去除率由23.4%提高到48.9%,证明了吸附-催化的协同作用既提高了铁锰二元氧化物的催化性能,又提高了生物炭的吸附能力[52]。
吸附与催化的协同效应不仅可以提高VOCs的降解效率,还可以促进VOCs的深度降解。在苯胺降解过程中,将具有高效催化性能的氧化亚铜与丰富活性吸附位点的氧化钇复合,构建吸附-催化协同体系后,体系降解苯胺的效率大大提高,氧化钇的高比表面积也有利于污染物和中间产物的吸附与进一步降解,实现了有机污染物的矿化[53-54]。
污染物分子与吸附位点结合后,还会影响其邻近活性催化位点的电子结构,从而对催化位点的催化性能产生影响。研究表明,被活性位点吸附的苯胺更易被溶液中的羟基自由基氧化并发生开环反应生成脂肪醛或脂肪醇类化合物,从而被进一步氧化降解为CO2[55]。不同目标污染物分子与表面不同的吸附位点结合后,对活性催化位点电子结构的影响不同,因此吸附过程与催化过程的耦合作用还与污染物的分子结构息息相关。分别对亲水性与疏水性目标污染物在FexOy-d-g-C3N4上的降解行为进行探究发现,亲水性目标污染物通过O-Fe键吸附在FexOy表面,疏水性目标污染物通过π-π或氢键吸附在g-C3N4上,亲水性污染物的羟基可作为氧空穴的电子供体,促进H2O2分解为羟基自由基,从而实现污染物的高效降解,而疏水性污染物与g-C3N4的相互作用导致H2O2的快速分解,H2O2浓度的快速降低限制了污染物的高效降解,这一结果表明,污染物分子的亲疏水特性决定了其在复合材料上的吸附方式,进而影响了污染物在体系中的降解速率[56]。
-
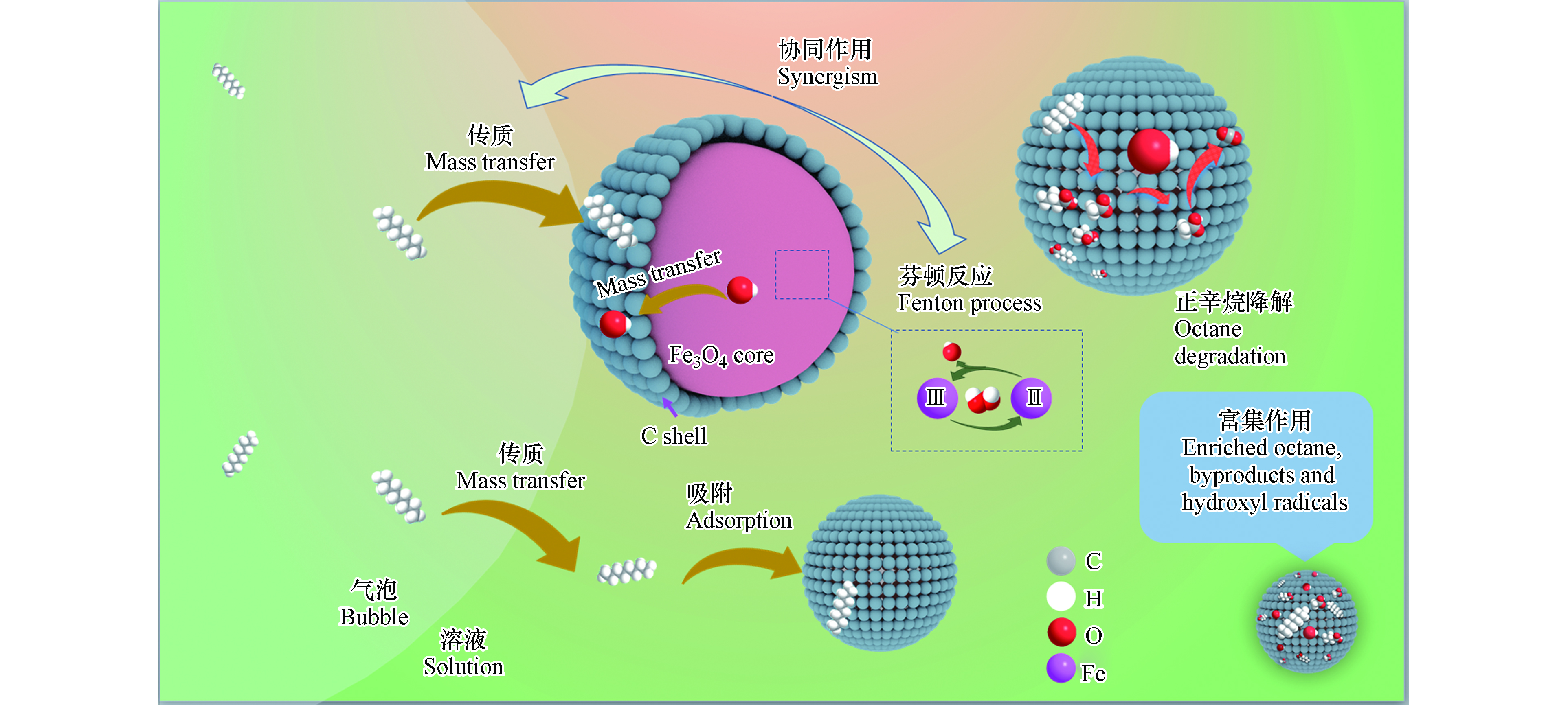
芬顿体系降解VOCs过程中传质-吸附-催化多步骤紧密相关、相互作用,利用其多步耦合效应,可以构建高效降解VOCs的芬顿体系。将核壳结构Fe3O4@C用于芬顿体系降解正辛烷,可以利用复合材料的特征结构将传质-吸附-催化多过程有机结合,实现正辛烷的高效降解[57]。
如图6所示,正辛烷通过气液传质进入芬顿体系并吸附于碳壳表面,与碳壳表面活性位点生成的羟基自由基发生一系列氧化反应,最终生成CO2和H2O,在这一过程中,核壳结构Fe3O4@C不仅提高了正辛烷的传质效率,还扮演“微反应器”的角色,富集正辛烷、中间产物和羟基自由基,增大反应物的局域浓度,将传质-吸附-催化多过程耦合,实现了污染物的高效深度降解。芬顿法降解废气中的甲苯的相关研究也表明,液相中催化氧化反应的进行增大了气液传质和吸附驱动力,传质-吸附-催化过程相互促进实现了废气中甲苯的高效去除[58]。将碳基颗粒加入到电化学体系后,在提高传质效率的同时吸附污染物分子,为污染物分子与其表面生成的氧化活性物种的反应提供了便利,也在复杂污染物去除方面表现出传质-吸附-催化的多步骤协同效应[28]。利用超声振动实现芬顿体系中的多步耦合,不仅可以加快物质在液相、气液界面和液固界面的传质,还可以促进物质在催化材料界面的传质,调控物质在其表面的吸附与解吸附性能,实现催化剂表面活性位点的有效暴露[12]。此外,目标污染物催化氧化生成的中间产物在材料表面吸附并富集,在活性位点附近发生进一步氧化[12],通过多步协同作用促进污染物的矿化,还可以实现目标VOCs的深度降解[47]。
-
研究表明,在芬顿体系降解污染物的速率预测模型构建中,引入传质、吸附、催化等过程的协同作用项可提高模型预测的准确度,这也进一步证明了协同作用在有机污染物降解中的重要作用。在降解正辛烷的芬顿体系中加入活性炭颗粒,将协同因子这一关键参数引入偏微分方程组,并通过不同固含率的芬顿实验对数学模型进行验证,结果显示,引入协同因子的数学模型与无协同因子项的数学模型相比,对出气口正辛烷浓度预测的准确度大大提高,其预测结果与实测数据基本吻合[59]。此外,在构建芬顿体系降解废气中甲苯的多釜串联模型中,引入体系气液传质和催化氧化的耦合作用后,所构建的动态模型也能够更准确预测体系中气液两相各物质浓度变化[60]。
-
本文针对复杂混合VOCs中难溶性组分溶解度系数小,气液传质效率低,导致在芬顿体系中降解效率低的问题,综述了芬顿体系降解VOCs过程中气-液传质增强途径及相关机理,总结了通过传质-吸附-催化多步协同作用促进芬顿体系高效降解VOCs的发展现状。虽然传质增强与协同作用对芬顿体系降解复杂混合VOCs的效率提升明显,但现阶段关于协同作用的微观机制研究仍缺乏,且协同作用下复杂混合VOCs在气液、液固界面的吸附、氧化、迁移机制仍不明确,使得在高效功能复合材料和高效芬顿体系综合设计与构建等方面难以发挥全面的指导作用,也导致协同作用在实际应用中受限。因此,芬顿体系治理VOCs需要在以下几个方面进一步开展研究:
(1)进一步明确芬顿体系中的传质-吸附-催化耦合作用的微观机制,特别是不同目标污染物及复杂混合VOCs的降解过程,为高效芬顿体系的构建提供理论依据;
(2)合理设计并制备应用于VOCs降解过程的传质-吸附-催化多步耦合功能复合材料,实现难溶性和难降解VOCs的高效降解和复杂混合VOCs的深度降解,并探索其在工业生产与日常生活中的推广应用;
(3)结合理论计算深入研究有机污染物在气液、液固界面的吸附、氧化、迁移机制;
(4)探索芬顿体系与新技术、新工艺的结合,拓宽芬顿体系在环境领域的应用范围。
芬顿催化氧化VOCs过程中的传质增强及协同作用研究进展
Mass transfer enhancement and synergistic effect during VOCs removal by Fenton oxidation
-
摘要: 芬顿(Fenton)法是降解挥发性有机污染物(volatile organic compounds,VOCs)最有效的方法之一。在芬顿法降解复杂混合VOCs的过程中,难溶性VOCs极低的气-液传质效率是限制其降解速率的关键因素。近年来,如何增强芬顿法降解VOCs过程的传质效率得到了广泛关注和研究。本文综述了芬顿体系降解VOCs过程中的传质增强方法和研究现状,分析了VOCs降解过程中传质-吸附-催化多步协同作用及机制,提出了芬顿体系高效、深度降解复杂混合VOCs的未来研究方向。Abstract: Fenton process is one of the most effective methods to degrade volatile organic compounds (VOCs). The degradation of hydrophobic VOCs during Fenton process has been limited by the extremely low gas-liquid mass transfer rate, and methods to enhance the mass transfer process have been widely concerned and studied over the decades. Here, we present a brief review on the mass transfer enhancement, and the synergy of the multistep including mass transfer, adsorption and chemical reaction during VOCs removal in Fenton system. Environmental implications and the prospects are also given.
-

-
-
[1] MONTERO-MONTOYA R, LÓPEZ-VARGAS R, ARELLANO-AGUILAR O. Volatile organic compounds in air: Sources, distribution, exposure and associated illnesses in children [J]. Annals of Global Health, 2018, 84(2): 225-238. doi: 10.29024/aogh.910 [2] NORRIS C, FANG L, BARKJOHN K K, et al. Sources of volatile organic compounds in suburban homes in Shanghai, China, and the impact of air filtration on compound concentrations [J]. Chemosphere, 2019, 231: 256-268. doi: 10.1016/j.chemosphere.2019.05.059 [3] ZHANG D C, LIU J J, JIA L Z, et al. Speciation of VOCs in the cooking fumes from five edible oils and their corresponding health risk assessments [J]. Atmospheric Environment, 2019, 211: 6-17. doi: 10.1016/j.atmosenv.2019.04.043 [4] GAO P, SONG Y, HAO M J, et al. An effective and magnetic Fe2O3-ZrO2 catalyst for phenol degradation under neutral pH in the heterogeneous Fenton-like reaction [J]. Separation and Purification Technology, 2018, 201: 238-243. doi: 10.1016/j.seppur.2018.03.017 [5] JUNG K W, LEE S Y, LEE Y J, et al. Ultrasound-assisted heterogeneous Fenton-like process for bisphenol A removal at neutral pH using hierarchically structured manganese dioxide/biochar nanocomposites as catalysts [J]. Ultrasonics Sonochemistry, 2019, 57: 22-28. doi: 10.1016/j.ultsonch.2019.04.039 [6] BABUPONNUSAMI A, MUTHUKUMAR K. A review on Fenton and improvements to the Fenton process for wastewater treatment [J]. Journal of Environmental Chemical Engineering, 2014, 2(1): 557-572. doi: 10.1016/j.jece.2013.10.011 [7] TOKUMURA M, WADA Y, USAMI Y, et al. Method of removal of volatile organic compounds by using wet scrubber coupled with photo-Fenton reaction - Preventing emission of by-products [J]. Chemosphere, 2012, 89(10): 1238-1242. doi: 10.1016/j.chemosphere.2012.07.018 [8] ZHAN J J, LI M F, ZHANG X J, et al. Aerosol-assisted submicron γ-Fe2O3/C spheres as a promising heterogeneous Fenton-like catalyst for soil and groundwater remediation: Transport, adsorption and catalytic ability [J]. Chinese Chemical Letters, 2020, 31(3): 715-720. doi: 10.1016/j.cclet.2019.09.001 [9] YAN L C, LIU J M, FENG Z H, et al. Continuous degradation of BTEX in landfill gas by the UV-Fenton reaction [J]. RSC Advances, 2016, 6(2): 1452-1459. doi: 10.1039/C5RA22585J [10] CHONG M N, JIN B, CHOW C W K, et al. Recent developments in photocatalytic water treatment technology: A review [J]. Water Research, 2010, 44(10): 2997-3027. doi: 10.1016/j.watres.2010.02.039 [11] XIE R J, LIU G Y, LIU D P, et al. Wet scrubber coupled with heterogeneous UV/Fenton for enhanced VOCs oxidation over Fe/ZSM-5 catalyst [J]. Chemosphere, 2019, 227: 401-408. doi: 10.1016/j.chemosphere.2019.03.160 [12] ZIYLAN A, INCE N H. Catalytic ozonation of ibuprofen with ultrasound and Fe-based catalysts [J]. Catalysis Today, 2015, 240: 2-8. doi: 10.1016/j.cattod.2014.03.002 [13] AKITA K, YOSHIDA F. Gas holdup and volumetric mass transfer coefficient in bubble columns. effects of liquid properties [J]. Industrial & Engineering Chemistry Process Design and Development, 1973, 12(1): 76-80. [14] YOSWATHANA N. Toluene degradation using aqueous absorption and Fenton's oxidation in pilot plant [J]. Journal of Food Agriculture and Environment, 2013, 11(2): 808-813. [15] TISA F, ABDUL RAMAN A A, WAN DAUD W M A. Applicability of fluidized bed reactor in recalcitrant compound degradation through advanced oxidation processes: A review [J]. Journal of Environmental Management, 2014, 146: 260-275. doi: 10.1016/j.jenvman.2014.07.032 [16] MOREIRA F C, BOAVENTURA R A R, BRILLAS E, et al. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters [J]. Applied Catalysis B:Environmental, 2017, 202: 217-261. doi: 10.1016/j.apcatb.2016.08.037 [17] MOLINA R, MARTÍNEZ F, MELERO J A, et al. Mineralization of phenol by a heterogeneous ultrasound/Fe-SBA-15/H2O2 process: Multivariate study by factorial design of experiments [J]. Applied Catalysis B:Environmental, 2006, 66(3/4): 198-207. [18] ROY K, MOHOLKAR V S. Sulfadiazine degradation using hybrid AOP of heterogeneous Fenton/persulfate system coupled with hydrodynamic cavitation [J]. Chemical Engineering Journal, 2020, 386: 121294. doi: 10.1016/j.cej.2019.03.170 [19] ZHONG X, ROYER S, ZHANG H, et al. Mesoporous silica iron-doped as stable and efficient heterogeneous catalyst for the degradation of C. I. Acid Orange 7 using sono-photo-Fenton process [J]. Separation and Purification Technology, 2011, 80(1): 163-171. doi: 10.1016/j.seppur.2011.04.024 [20] XIANG W, ZHANG B P, ZHOU T, et al. An insight in magnetic field enhanced zero-valent iron/H 2 O2 Fenton-like systems: Critical role and evolution of the pristine iron oxides layer [J]. Scientific Reports, 2016, 6: 24094. doi: 10.1038/srep24094 [21] PÉREZ J F, LLANOS J, SÁEZ C, et al. On the design of a jet-aerated microfluidic flow-through reactor for wastewater treatment by electro-Fenton [J]. Separation and Purification Technology, 2019, 208: 123-129. doi: 10.1016/j.seppur.2018.04.021 [22] LIM M, RUDOLPH V, ANPO M, et al. Fluidized-bed photocatalytic degradation of airborne styrene [J]. Catalysis Today, 2008, 131(1/2/3/4): 548-552. [23] de JESUS S S, MOREIRA NETO J, MACIEL FILHO R. Hydrodynamics and mass transfer in bubble column, conventional airlift, stirred airlift and stirred tank bioreactors, using viscous fluid: A comparative study [J]. Biochemical Engineering Journal, 2017, 118: 70-81. doi: 10.1016/j.bej.2016.11.019 [24] HERNÁNDEZ M, QUIJANO G, MUÑOZ R, et al. Modeling of VOC mass transfer in two-liquid phase stirred tank, biotrickling filter and airlift reactors [J]. Chemical Engineering Journal, 2011, 172(2/3): 961-969. [25] SU C C, PUKDEE-ASA M, RATANATAMSKUL C, et al. Effect of operating parameters on decolorization and COD removal of three reactive dyes by Fenton's reagent using fluidized-bed reactor [J]. Desalination, 2011, 278(1/2/3): 211-218. [26] RADJENOVIC J, SEDLAK D L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water [J]. Environmental Science & Technology, 2015, 49(19): 11292-11302. [27] WANG C, HUANG Y K, ZHAO Q, et al. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects [J]. Chemical Engineering Journal, 2014, 243: 1-6. doi: 10.1016/j.cej.2013.12.044 [28] YIN H S, GUO Q, LEI C, et al. Electrochemical-driven carbocatalysis as highly efficient advanced oxidation processes for simultaneous removal of humic acid and Cr(VI) [J]. Chemical Engineering Journal, 2020, 396: 125156. doi: 10.1016/j.cej.2020.125156 [29] CHAUVEAU R, GRÉVILLOT G, MARSTEAU S, et al. Values of the mass transfer coefficient of the linear driving force model for VOC adsorption on activated carbons [J]. Chemical Engineering Research and Design, 2013, 91(5): 955-962. doi: 10.1016/j.cherd.2012.09.019 [30] LEE J, KIM K, CHANG I S, et al. Enhanced mass transfer rate of methane in aqueous phase via methyl-functionalized SBA-15 [J]. Journal of Molecular Liquids, 2016, 215: 154-160. doi: 10.1016/j.molliq.2015.12.041 [31] ZHANG S, WANG D, FAN P P, et al. Enhancement of gas-to-liquid oxygen transfer in the presence of fine solid particles for air-exposed multiphase system [J]. Chemical Engineering Research and Design, 2015, 100: 434-443. doi: 10.1016/j.cherd.2015.04.024 [32] KARS R L, BEST R J, DRINKENBURG A A H. The sorption of propane in slurries of active carbon in water [J]. The Chemical Engineering Journal, 1979, 17(2): 201-210. doi: 10.1016/0300-9467(79)85014-5 [33] CHEN H Y, LIU J M, PEI Y P, et al. Study on the synergistic effect of UV/Fenton oxidation and mass transfer enhancement with addition of activated carbon in the bubble column reactor [J]. Chemical Engineering Journal, 2018, 336: 82-91. doi: 10.1016/j.cej.2017.10.185 [34] RAZZAK S A. Hydrodynamic studies in liquid-solid and gas-liquid-solid circulating fluidized beds[D]. Canada: University of Western Ontario, 2009. [35] KIM S D, KANG Y. Heat and mass transfer in three-phase fluidized-bed reactors—an overview [J]. Chemical Engineering Science, 1997, 52(21/22): 3639-3660. [36] KIM J K, JUNG J Y, KANG Y T. The effect of nano-particles on the bubble absorption performance in a binary nanofluid [J]. International Journal of Refrigeration, 2006, 29(1): 22-29. doi: 10.1016/j.ijrefrig.2005.08.006 [37] BALASUBRAMANIAN G, SEN S, PURI I K. Shear viscosity enhancement in water-nanoparticle suspensions [J]. Physics Letters A, 2012, 376(6/7): 860-863. [38] WANG A Q, CHEN Y W, ZHENG Z K, et al. In situ N-doped carbon-coated mulberry-like cobalt manganese oxide boosting for visible light driving photocatalytic degradation of pharmaceutical pollutants [J]. Chemical Engineering Journal, 2021, 411: 128497. doi: 10.1016/j.cej.2021.128497 [39] KIM S D, LEE D H, KIM D Y, et al. Liquid-liquid interfacial area and mass transfer characteristics in liquid-liquid three-phase fluidized beds [J]. Journal of the Kerean Institute of Chemical Engineers, 1993, 31(3): 311-317. [40] YANG Z H, WANG K, SHAO Z M, et al. In-situ preparation of Fe2O3 hierarchical arrays on stainless steel substrate for high efficient catalysis [J]. Journal of Solid State Chemistry, 2017, 246: 278-283. doi: 10.1016/j.jssc.2016.11.023 [41] LIN X C, XIE F, YU X D, et al. Ultraviolet light assisted hierarchical porous Fe2O3 catalyzing heterogeneous Fenton degradation of tetracycline under neutral condition with a low requirement of H2O2 [J]. Chemical Research in Chinese Universities, 2019, 35(2): 304-310. doi: 10.1007/s40242-019-8238-y [42] SHAO Y, CHEN H H. Heterogeneous Fenton oxidation of phenol in fixed-bed reactor using Fe nanoparticles embedded within ordered mesoporous carbons [J]. Chemical Engineering Research and Design, 2018, 132: 57-68. doi: 10.1016/j.cherd.2017.12.039 [43] 孙晓红, 陈佩佩, 胡旭东, 等. 纳米Fe2O3/KIT-6介孔结构材料的制备及非均相Fenton催化降解亚甲基蓝性能研究 [J]. 稀有金属材料与工程, 2015, 44(Sup1): 498-502. SUN X H, CHEN P P, HU X D, et al. Preparation of nanoscale Fe2O3/KIT-6 mesoporous structure catalysts for heterogeneous Fenton degradation of methylene blue [J]. Rare Metal Materials and Engineering, 2015, 44(Sup1): 498-502(in Chinese).
[44] JI Q H, YU D W, ZHANG G, et al. Microfluidic flow through polyaniline supported by lamellar-structured graphene for mass-transfer-enhanced electrocatalytic reduction of hexavalent chromium [J]. Environmental Science & Technology, 2015, 49(22): 13534-13541. [45] LEE J W, KIM J K, KANG T H, et al. Direct synthesis of hydrogen peroxide from hydrogen and oxygen over palladium catalyst supported on heteropolyacid-containing ordered mesoporous carbon [J]. Catalysis Today, 2017, 293/294: 49-55. doi: 10.1016/j.cattod.2016.10.008 [46] JEONG H E, KIM S, SEO M G, et al. Catalytic activity of Pd octahedrons/SiO2 for the direct synthesis of hydrogen peroxide from hydrogen and oxygen [J]. Journal of Molecular Catalysis A:Chemical, 2016, 420: 88-95. doi: 10.1016/j.molcata.2016.03.043 [47] XIE R J, JI J, GUO K H, et al. Wet scrubber coupled with UV/PMS process for efficient removal of gaseous VOCs: Roles of sulfate and hydroxyl radicals [J]. Chemical Engineering Journal, 2019, 356: 632-640. doi: 10.1016/j.cej.2018.09.025 [48] AZIZ A, KIM K S. Synergistic effect of UV pretreated Fe-ZSM-5 catalysts for heterogeneous catalytic complete oxidation of VOC: A technology development for sustainable use [J]. Journal of Hazardous Materials, 2017, 340: 351-359. doi: 10.1016/j.jhazmat.2017.07.019 [49] LIMA V N, RODRIGUES C S D, MADEIRA L M. Simultaneous treatment of toluene-containing gas waste and industrial wastewater by the Fenton process [J]. Science of the Total Environment, 2020, 749: 141497. doi: 10.1016/j.scitotenv.2020.141497 [50] ZHUANG Y, YUAN S Y, LIU J M, et al. Synergistic effect and mechanism of mass transfer and catalytic oxidation of octane degradation in yolk-shell Fe3O4@C/Fenton system [J]. Chemical Engineering Journal, 2020, 379: 122262. doi: 10.1016/j.cej.2019.122262 [51] KARTHIKEYAN S, GUPTA V K, BOOPATHY R, et al. A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: Kinetic and spectroscopic studies [J]. Journal of Molecular Liquids, 2012, 173: 153-163. doi: 10.1016/j.molliq.2012.06.022 [52] LI L, LAI C, HUANG F L, et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe-Mn binary oxides [J]. Water Research, 2019, 160: 238-248. doi: 10.1016/j.watres.2019.05.081 [53] CHEN C, JIA N, SONG K J, et al. Sulfur-doped copper-yttrium bimetallic oxides: A novel and efficient ozonation catalyst for the degradation of aniline [J]. Separation and Purification Technology, 2020, 236: 116248. doi: 10.1016/j.seppur.2019.116248 [54] XIE Y, WANG X Q, TONG W H, et al. FexP/biochar composites induced oxygen-driven Fenton-like reaction for sulfamethoxazole removal: Performance and reaction mechanism [J]. Chemical Engineering Journal, 2020, 396: 125321. doi: 10.1016/j.cej.2020.125321 [55] LOVATO M, BUFFELLI J R, ABRILE M, et al. Kinetics and efficiency of ozone for treatment of landfill leachate including the effect of previous microbiological treatment [J]. Environmental Science and Pollution Research, 2019, 26(5): 4474-4487. doi: 10.1007/s11356-018-1710-2 [56] WANG Y M, ZHANG P, LI T, et al. Enhanced Fenton-like efficiency by the synergistic effect of oxygen vacancies and organics adsorption on FexOy-d-g-C3N4 with Fe-N complexation [J]. Journal of Hazardous Materials, 2021, 408: 124818. doi: 10.1016/j.jhazmat.2020.124818 [57] ZHUANG Y, LIU J M, YUAN S Y, et al. Degradation of octane using an efficient and stable core-shell Fe3O4@C during Fenton processes: Enhanced mass transfer, adsorption and catalysis [J]. Applied Surface Science, 2020, 515: 146083. doi: 10.1016/j.apsusc.2020.146083 [58] HANDA M, LEE Y, SHIBUSAWA M, et al. Removal of VOCs in waste gas by the photo-Fenton reaction: Effects of dosage of Fenton reagents on degradation of toluene gas in a bubble column [J]. Journal of Chemical Technology & Biotechnology, 2013, 88(1): 88-97. [59] TOKUMURA M, SHIBUSAWA M, KAWASE Y. Dynamic simulation of degradation of toluene in waste gas by the photo-Fenton reaction in a bubble column [J]. Chemical Engineering Science, 2013, 100: 212-224. doi: 10.1016/j.ces.2012.12.010 [60] CHEN H Y, LIU J M, WU C D, et al. A comprehensive mathematical model for analyzing synergistic effect of oxidation and mass transfer enhancement during UV-Fenton removal of VOCs [J]. Chemosphere, 2021, 283: 131021. doi: 10.1016/j.chemosphere.2021.131021 -



 下载:
下载: