-
随着水域富营养化现象频发,藻类爆发性生长繁殖,其产生的嗅味物质等次级代谢产物污染了水源水,造成了经济与环境损失,影响着饮用水的供水安全,引发了社会各界的关注[1]。在水体污染物中,嗅味物质是国内外饮用水处理中较为容易见到也是十分难解决的问题之一[2]。饮用水中出现嗅味将引起群众大范围恐慌,嗅味目前已被列为水厂出厂水和管网水的必检项目之一。
水中的嗅味问题在各个地方有不同的成因,所形成的臭味种类也不同,但土臭素(GSM)和2-甲基异莰醇(2-MIB)是引起水质问题的众多恶臭物质中最突出的,饮用水中二者的含量均不能超过10 ng·L−1。常规水处理工艺,如混凝、沉淀、过滤、消毒等,无法有效减少水中的GSM和2-MIB,不能达到《生活饮用水卫生标准》要求[3]。常用控制嗅味物质的方法包括活性炭吸附、生物降解、高级氧化、臭氧氧化等。其中高级氧化技术(AOPs)被普遍认为是一种高效降解GSM和2-MIB的方法,其中紫外联用高级氧化(UV-AOPs)技术是近年来研究的热点[4]。UV-AOPs技术实质是通过利用某些活性物质较强的氧化能力,如氯自由基、羟基自由基,氧化降解污染物,将其分解成水、二氧化碳等无机小分子[5]。该技术具有氧化电位高、选择性低、碱干扰小、对大多数有机污染物降解迅速、操作过程简单、反应条件温和等优点。
本文通过对比不同UV-AOPs工艺降解嗅味物质的效能,考察了光照强度、氧化剂投加量、目标物初始浓度、反应时间、pH、腐殖酸浓度及水体所含无机阴离子(SO42-、Cl−、HCO3−)等对降解效果的影响,并对比了在实际水体中UV-AOPs工艺对嗅味物质的去除效果,以期为UV-AOPs工艺去除嗅味物质在实际运用中提供理论参考与技术支持。
-
土臭素(GSM)、2-甲基异莰醇(2-MIB)购自日本Wako,纯度大于98%,30%双氧水(H2O2)、硫代硫酸钠(Na2S2O3)、次氯酸钠(NaClO)、氯化钠(NaCl)、碳酸氢钠(NaHCO3)、无水硫酸钠(Na2SO4)、磷酸二氢钠(NaH2PO4),腐殖酸(HA)购自美国Aldrich-sigma公司。
-
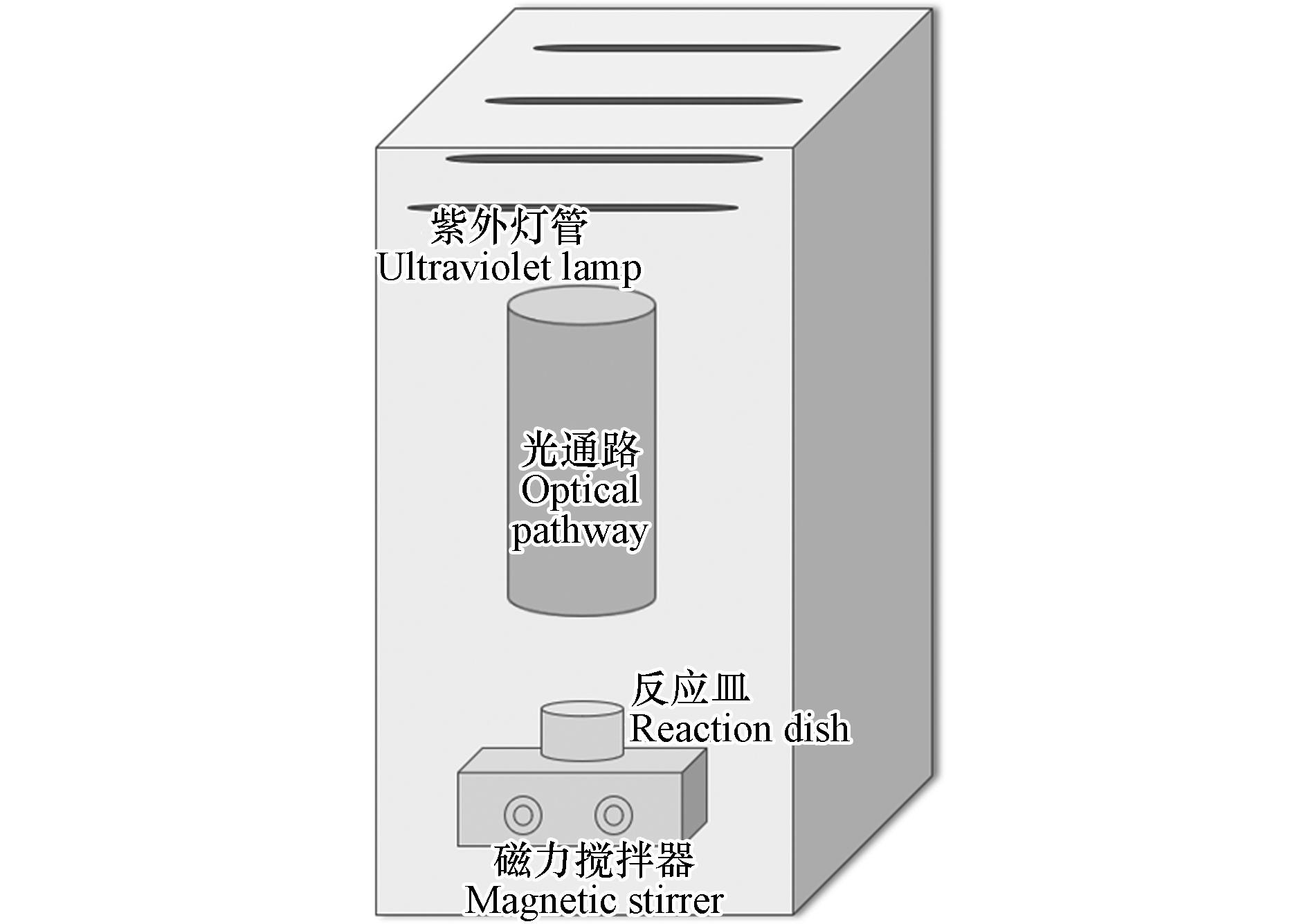
实验主要依托平行光束仪进行,黑色不透光箱体装置中设有4根紫外灯管,每根灯管的发射波长为254 nm,功率为10 W,其中1、2、3、4根灯管的光强分别为8.9×10−5、2.0×10−4、2.1×10−4、2.2×10−4 W·cm−2,实验装置见图1。
实验操作流程如下:
(1)打开UV灯管预热稳定30 min,用光强测定仪监测反应皿上液面的光照强度,核对并计算达到所需紫外剂量的光照时间;
(2)配制所需浓度的目标污染物缓冲液置于反应皿中,将反应皿置于光通路的正下方;
(3)把所需浓度的氧化剂(如H2O2、NaClO水溶液)加入反应皿中,开启磁力搅拌器混合1 min,打开遮光板开始计时,到达设计反应时间后关闭遮光板以停止反应,滴入硫代硫酸钠终止反应,取40 mL水样测定GSM和2-MIB浓度。其中,H2O2储备液浓度为1000 mg·L−1,HOCl储备液浓度为620 mg·L−1。
-
本实验中的光照强度采用光强测定仪进行测定,H2O2浓度检测使用便携式过氧化氢测定仪,pH值检测使用FE28型pH计,氯浓度采用滴定法[6]。
硫酸盐、氯化物的检测采用离子色谱法。准备电导率小于0.5 μS·cm−1的二次去离子水,并经0.45 μm微孔滤膜进行过滤。碳酸氢钠、碳酸钠淋洗贮备液浓度分别为0.17 mol·L−1、0.18 mol·L−1;碳酸氢钠、碳酸钠淋洗使用液浓度分别为0.0017 mol·L−1、0.0018 mol·L−1。硫酸根离子和氯离子标准贮备液浓度均为1000.0 mg·L−1。进样量约为1.0—2.0 mL·min−1。
GSM和2-MIB的测定参照国标《GB/T32470-2016》。使用顶空固相微萃取-气相色谱-质谱法,通过固相微萃取纤维吸附待测样中的GSM和2-MIB,顶空富集样品后通过气相色谱-质谱联用仪分离测定。气相色谱仪进样口压力56.5 kPa,进样口温度:250 ℃;程序升温:起始温度60 ℃保持2.5 min,以8 ℃·min−1速率升至250 ℃,保持5 min;质谱仪离子源:电子电离源(EI),离子源温度:230 ℃,接口温度:280 ℃,离子化能量:70 eV,扫描模式:选择离子检测(SIM)。
-
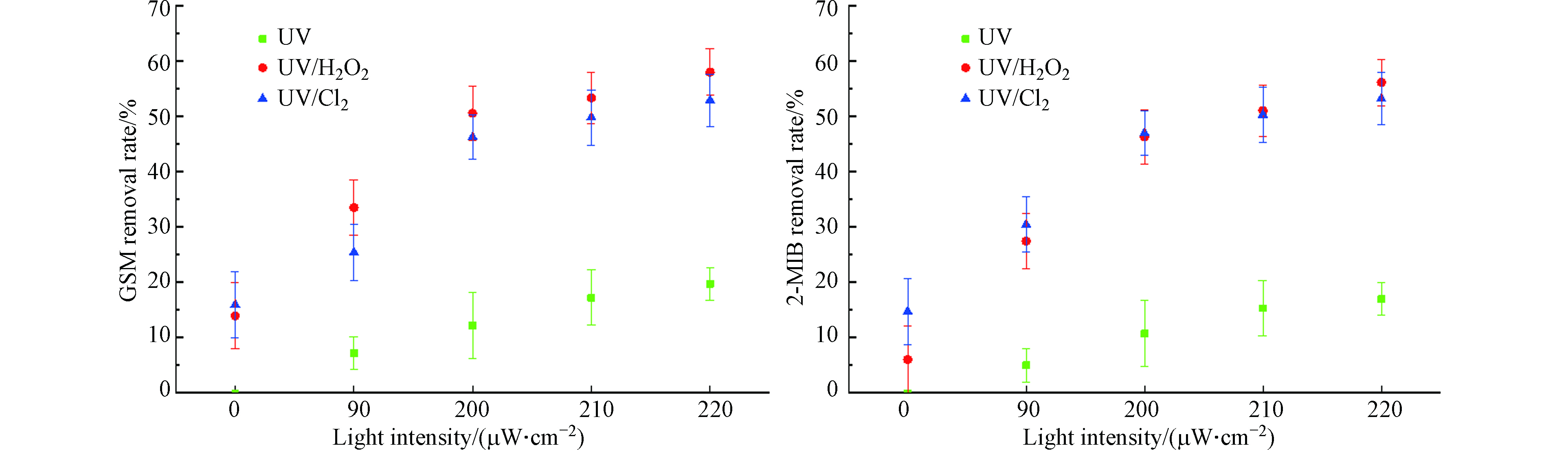
使用目标污染物浓度为100 ng·L−1的GSM和2-MIB混标溶液,降解时间40 min,加入8 mg·L−1的H2O2与NaClO,分别打开0、1、2、3、4根灯管,光照强度分别为0、90、200、210、220 μW·cm−2,分析2-MIB和GSM的降解情况。去除效果如图2所示。
当光照强度从90 μW·cm−2提高至220 μW·cm−2时,UV、UV/H2O2、UV/Cl2工艺对GSM的去除率分别从7.2%、33.5%、25.4%升至19.7%、58.0%、53.0%;对2-MIB的去除率分别从5.0%、27.5%、30.5%升至17.0%、56.2%、53.3%。随着光照强度的提高,各工艺对GSM和2-MIB的去除率不断升高。当光照强度为0 μW·cm−2,即单独投加H2O2时,对GSM的去除率为14%,2-MIB的去除率为6.1%;单独投加NaClO时,GSM的去除率为16%,2-MIB的去除率为14.8%。单独投加H2O2或ClO−对GSM和2-MIB的去除效果一般。在相同的光照强度下,UV/H2O2工艺对GSM和2-MIB的去除效果要优于UV/Cl2工艺[7]。随着光照强度的增加,反应体系内光子量在逐渐增多,自由基数量增多,对嗅味物质的反应速率增大,对污染物的去除量增大。在实际工程中,可通过增加紫外灯管数或增大功率来提高光照强度,加强GSM和2-MIB的去除效果。
-
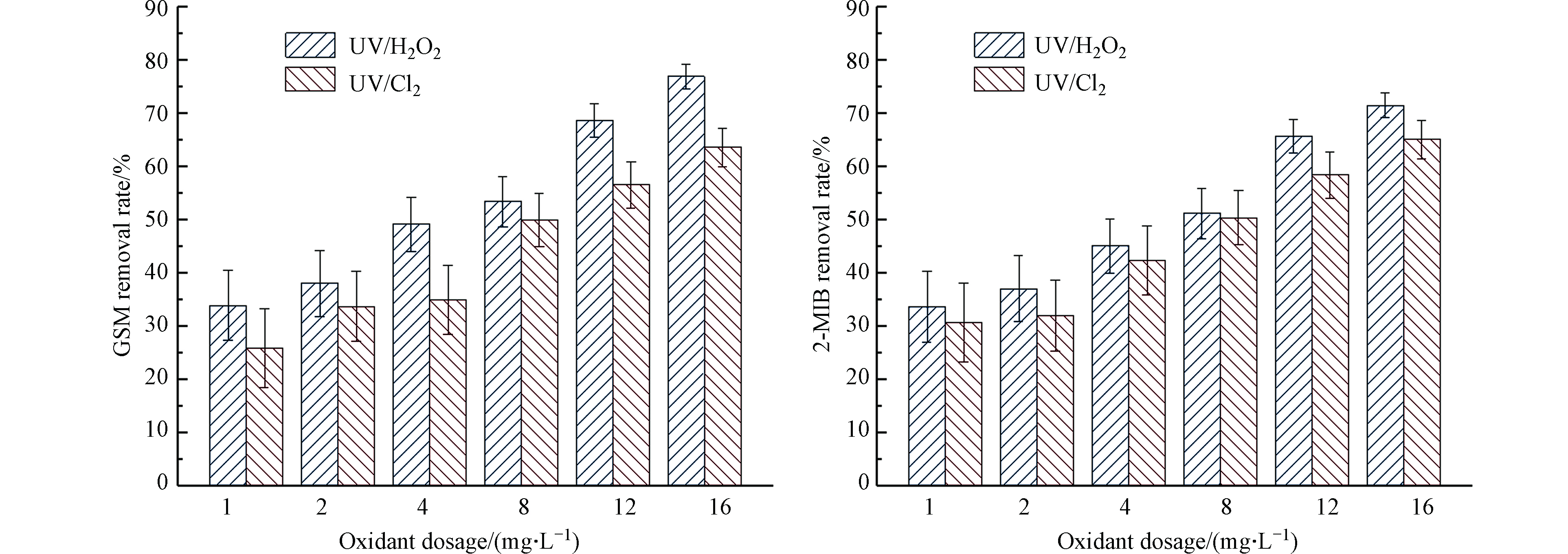
固定紫外灯光照强度为210 μW·cm−2,向反应皿中加入GSM和2-MIB浓度均为100 ng·L−1的混标溶液,反应时间为40 min,分别加入1、2、4、8、12、16 mg·L−1的H2O2与NaClO,分析GSM和2-MIB的降解情况。其降解效果如图3所示。
当H2O2浓度从1 mg·L−1增加至16 mg·L−1时,对GSM的去除率从33.8%升至76.9%,对2-MIB的去除率从33.6%升至71.4%;当氯的浓度从1 mg·L−1增加至16 mg·L−1时,对GSM的去除率从25.9%升至63.5%,对2-MIB的去除率从30.6%升至65%。增大氧化剂的量,GSM和2-MIB的去除率随之升高。在氧化剂投加量相同的条件下,UV/H2O2对GSM和2-MIB的去除率要高于UV/Cl2对二者的去除率。在水体为中性或弱碱性的实验条件下,UV/Cl2工艺中自由氯的主要形态是OCl−,其会影响自由基的生成,使体系中的自由基浓度降低[8],选用的紫外波长距离OCl−的最大吸收波长较远,影响其自由基的形成。同时OCl−与·OH的反应速率比HOCl高出5个数量级,进而导致体系氧化能力下降[9-10],故去除率略低。在降解相同浓度的GSM和2-MIB时,UV/H2O2工艺去除GSM的效果略优于2-MIB;UV/Cl2工艺去除2-MIB的效果略优于GSM。
当氧化剂的用量小于某个限值时,氧化剂的用量越多,生成的自由基越多。H2O2的投加量过多会对羟基自由基起捕获作用,而H2O2比羟基自由基的氧化性小,因此使GSM和2-MIB的降解速率常数降低[11]。在本实验中随氧化剂投加量的增加,GSM和2-MIB的去除率升高,未出现该情况。实际工程中,氧化剂的最佳投加量应根据实际工程去除效果进行试验得出。
-
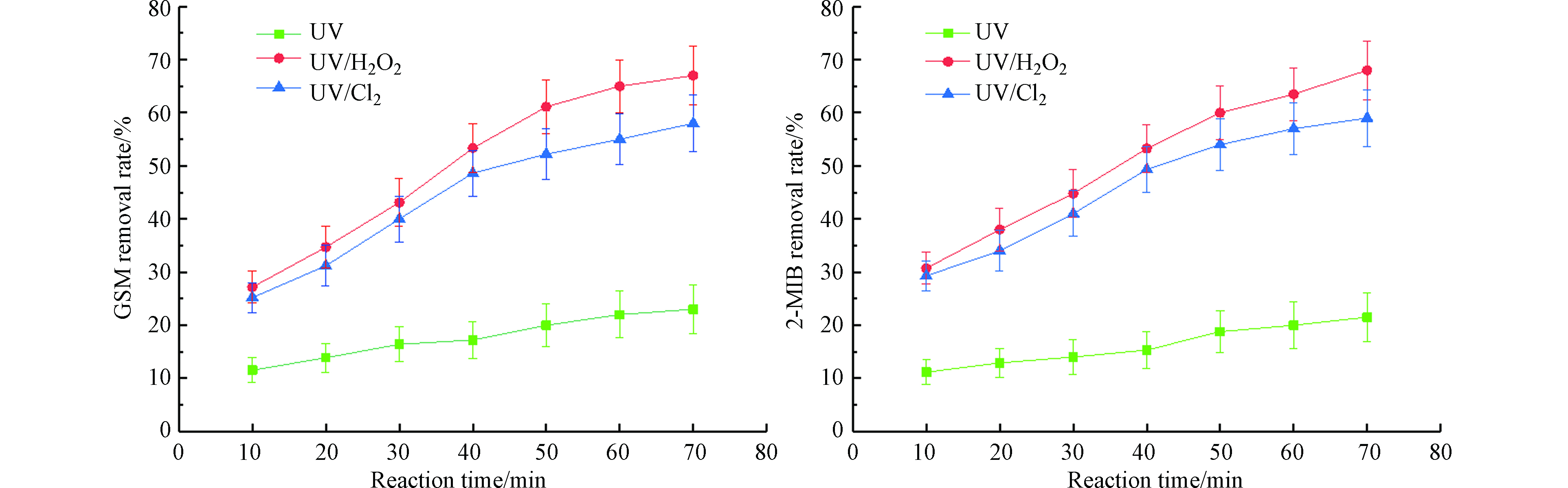
固定紫外灯光照强度为210 μW·cm−2,投加8 mg·L−1的H2O2与NaClO,目标污染物为100 ng·L−1的GSM和2-MIB混标溶液,降解时间为10、20、30、40、50、60、70 min,比较GSM和2-MIB的去除效果。其降解效果如图4所示。
当反应时间从10 min升至70 min时,3种工艺对GSM和2-MIB的去除效果都在提升,UV工艺对GSM的去除率从11.5%升至23%,对2-MIB的去除率从11.1%升至21.5%;UV/H2O2工艺对GSM的去除率从27.2%升至67.0%,对2-MIB的去除率从23.7%升至65.0%;UV/Cl2工艺对GSM的去除率从28.2%升至58.0%,对2-MIB的去除率从29.3%升至60.0%。随着反应时间的增加,各工艺对GSM和2-MIB的去除率不断提升;UV/H2O2工艺去除效果的增加幅度高于UV工艺及UV/Cl2工艺,随着反应时间的增加,3种不同的UV-AOPs工艺对GSM和2-MIB去除效果增加的幅度均减弱。反应时间为10 min时,UV/Cl2工艺对GSM和2-MIB的去除效果优于UV/H2O2工艺,但随着反应时间的增加,UV/H2O2工艺对二者的去除效果逐渐优于UV/Cl2工艺。
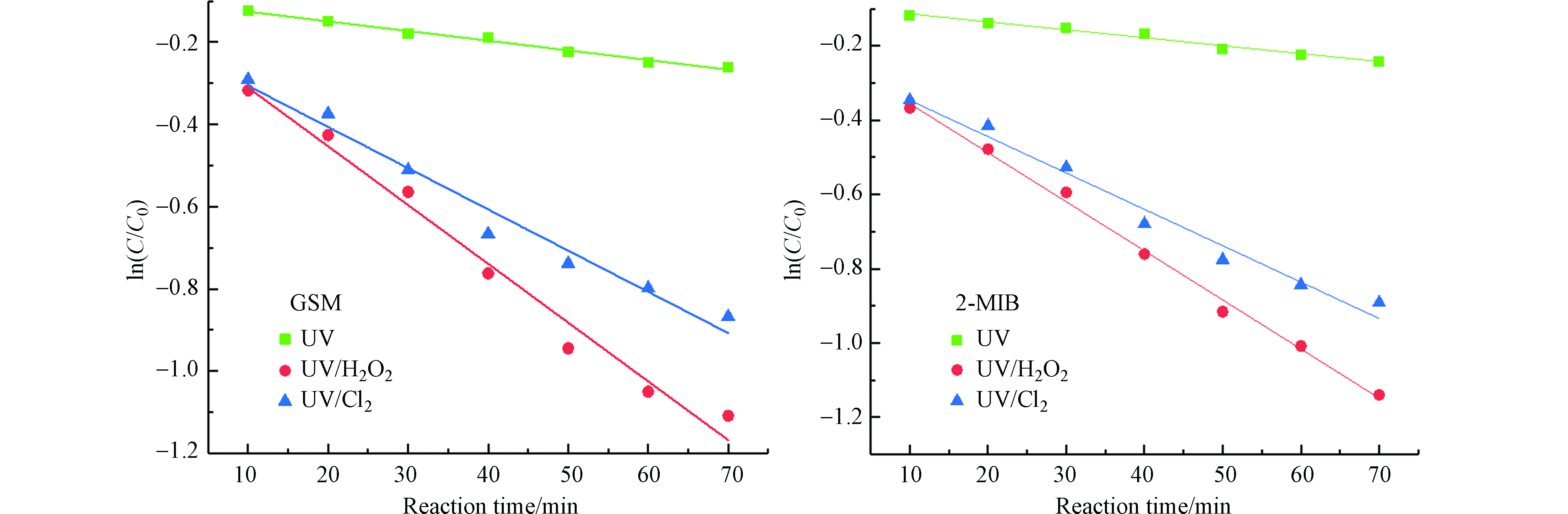
由图5和表1可知,在该实验条件下,各反应的降解曲线呈一级反应动力学的形式,UV/H2O2工艺降解GSM和2-MIB的反应速率常数略大于UV/Cl2工艺。这是由于在水体为中性或弱碱性的实验条件下,UV/Cl2工艺所受的影响较大,此时OCl−占据优势地位,其对·OH有一定的清除作用,使体系中的自由基大量淬灭,影响反应速率。
-
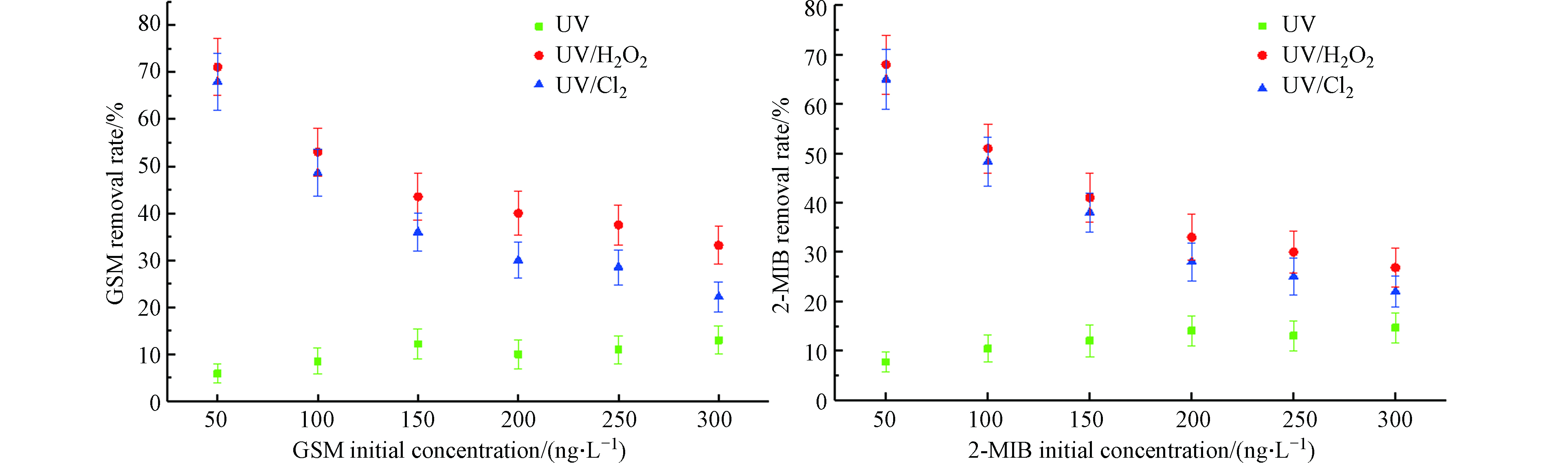
固定紫外灯光照强度为210 μW·cm−2,投加8 mg·L−1的H2O2与NaClO,降解时间为40 min,投加不同初始浓度50、100、150、200、250、300 ng·L−1的GSM和2-MIB混标溶液,比较GSM和2-MIB的去除效果。其降解效果如图6所示。
当目标污染物初始浓度从50 ng·L−1升至300 ng·L−1时,UV工艺对GSM的去除率从5.9%升至13.0%,2-MIB的去除率从7.7%升至14.6%;UV/H2O2工艺对GSM的去除率从71.1%降低至33.2%,2-MIB的去除率从68.0%降低至26.8%;UV/Cl2工艺对GSM的去除率从68.0%降低至22.2%,2-MIB的去除率从65.0%降低至22.0%。增加目标污染物初始浓度,UV/H2O2、UV/Cl2工艺对嗅味物质的去除率也随之降低,但UV工艺对嗅味物质的去除率反而略微提高,推测随着GSM和2-MIB浓度的增加,UV的利用率也得到提高[12]。不断增加目标污染物初始浓度时,UV/H2O2和UV/Cl2工艺对嗅味物质去除效果减少的幅度随之变小,污染物去除总量是不断提高的。
-
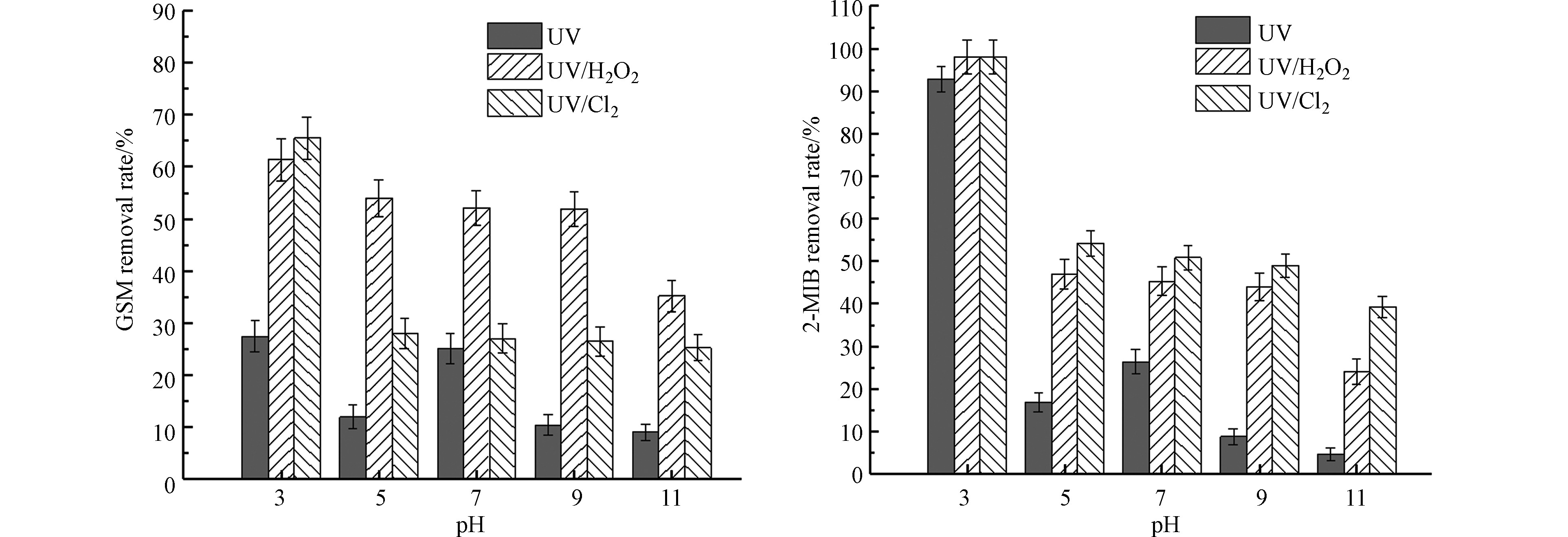
固定紫外灯光照强度为210 μW·cm−2,氧化剂H2O2与NaClO的浓度为8 mg·L−1,降解时间为40 min,初始浓度为100 ng·L−1的GSM和2-MIB混标溶液,控制溶液pH分别为3、5、7、9、11,对比GSM和2-MIB的去除效果。其降解效果如图7所示。
随着pH的升高,UV/Cl2工艺对GSM去除效果的衰减远大于UV/H2O2工艺。不同酸碱条件下,3种UV-AOPs工艺对GSM和2-MIB的降解效率不同。当pH为3时,UV、UV/H2O2、UV/Cl2工艺对2-MIB的去除率分别为92.8%、98.0%、98.0%。当水体环境为酸性时,即使不加氧化剂,UV工艺对2-MIB的去除效果也较好。当pH从酸性升至中性,UV工艺对GSM和2-MIB的去除率先降低再升高,pH从中性升高至碱性时,UV工艺对GSM和2-MIB的去除率降低。随着pH的升高,UV/H2O2、UV/Cl2工艺对GSM和2-MIB的去除率不断降低。当pH值从3升至11时,二者对GSM的去除率分别为35.2%、25.2%,分别降低了26.2%、40.4%;对2-MIB的去除率分别为24.1%、39.2%,分别降低了73.9%、58.8%。
不同pH条件下自由基的不同存在形式对结果产生了一定的影响[13]。当pH升高时,HOCl的比例减少,OCl−的比例增加;pH为5时,游离氯主要以HOCl的形式存在,而在pH大于7的条件下,游离氯主要以OCl−的形式存在。游离氯作为自由基的淬灭剂,在pH升高的条件下,对自由基的淬灭作用增强,体系中自由基含量降低,使2-MIB和GSM的去除率降低[14]。一般来说,UV/H2O2高级氧化工艺在酸性条件下具有更强的氧化性[15]。过氧化氢是弱酸,在酸性及中性环境下较稳定,碱性环境中H2O2会发生一定程度的解离,影响参与反应的H2O2的浓度,降低自由基的生成量,影响反应效率。
-
天然水体中含有成分复杂、含量各异的无机阴离子(如SO42-、Cl−、HCO3−),部分无机阴离子可能会影响高级氧化工艺对嗅味物质的去除效果。固定紫外灯光照强度为210 μW·cm−2,氧化剂H2O2与NaClO的浓度为8 mg·L−1,降解时间40 min,初始浓度为100 ng·L−1的GSM和2-MIB混标溶液,投加0.5 mmol·L−1的硫酸钠、氯化钠与碳酸氢钠,对比GSM与2-MIB的去除效果。其降解效果如图8所示。
添加SO42-后,UV工艺对GSM和2-MIB的去除率基本没有变化;而UV/H2O2和UV/Cl2工艺对GSM和2-MIB的去除率升高。在紫外光的辐照下,硫酸根离子与羟基自由基发生反应,生成硫酸根自由基,导致对嗅味物质的去除效果增加[16]。用含氯化钠、高氯酸钠、硝酸钠、硫酸钠的废水实施芬顿实验,发现氯离子、硝酸根离子和高氯酸盐离子不影响芬顿试剂的氧化效果,而在硫酸盐离子的存在下,芬顿试剂的氧化效率得到了提高[17]。
添加Cl−后,UV和UV/H2O2工艺对GSM和2-MIB的去除率减小,UV/Cl2工艺对GSM和2-MIB的去除率略微增加。氯基自由基的反应速率常数为10−3—10−1 L·mol−1·s−1,羟基自由基的反应速率常数为107—1010 L·mol−1·s−1[18],但Cl−与·OH或Cl·反应后生成一系列氯基自由基(Cl·、Cl2·、ClOH·等),故去除效果得到部分提高。
在3种离子中,投加HCO3−离子后对嗅味物质去除效果的影响最为显著。HCO3−是一种有效的·OH淬灭剂,它会使·OH的浓度降低,其存在是AOPs实际应用中的最不利条件之一[19]。同时,HCO3−和·OH反应产生的碳酸根将与H2O2反应,进一步降低UV-AOPs的氧化效率。
在实际应用中,溶液中的主要阴离子HCO3−和Cl−等对·OH有淬灭作用,它们的大量存在会致使UV/H2O2工艺的氧化剂投加量增大或反应时间延长。
-
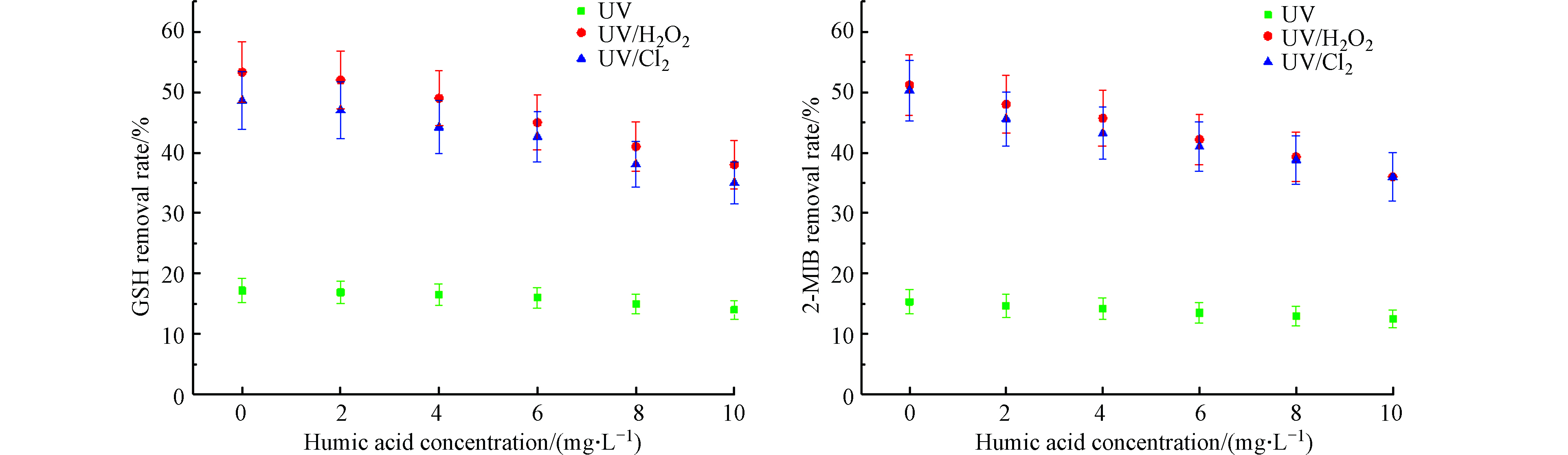
腐殖酸(HA)广泛存在于天然水体中,其对AOPs工艺去除水体中嗅味物质的效能存在一定的影响。固定紫外灯光照强度为210 μW·cm−2,H2O2与NaClO的浓度为8 mg·L−1,降解时间40 min,初始浓度100 ng·L−1的GSM和2-MIB混标溶液,分别投加0、2、4、6、8、10 mg·L−1的腐殖酸,比较对GSM和2-MIB的去除效果。其降解效果如图9所示。
结构复杂的腐殖酸,对UV-AOPs工艺去除致嗅物质的效果存在一定影响。当HA投加量从0 mg·L−1升到10 mg·L−1时,UV、UV/H2O2、UV/Cl2工艺对GSM的去除率分别为14%、38%、35%,降低了3.2%、15.3%、13.6%;对2-MIB的去除率分别为12.5%、36%、36%,降低了2.8%、15.2%、14.3%。在投加HA的条件下,不同UV-AOPs工艺对GSM和2-MIB的去除率均受到抑制,且抑制程度随HA的增多而增大。其中,HA对UV/H2O2和UV/Cl2工艺降解嗅味物质的抑制作用较为显著。HA可以吸收紫外线并形成能级跃迁至激发状态,产生较多活性物质,如·OH等自由基,增大了体系中氧化剂的含量,促进氧化降解[20];HA存在一定的不饱和化学键,可吸收部分紫外光,因而降低了被激发氧化剂的光子数,使产生自由基的效率降低,进而抑制了GSM和2-MIB等的去除[21];腐殖酸可作为活性自由基受体对自由基进行消耗,且会升高溶液色度,减小紫外光的穿透性,降低其利用率,抑制高级氧化作用[22]。
-
通过顶空固相微萃取(HS-SPME)对处理后出水形成的中间产物进行富集来探究UV/H2O2工艺对GSM和2-MIB的实际降解途径及中间产物。GSM和2-MIB的初始浓度为1 μg·L−1,H2O2的投加量约为13.0 mg·L−1。取样并加入0.1 mol·L−1的Na2S2O3溶液,快速停止反应。通过全扫描GC-MS鉴定GSM和2-MIB的氧化降解中间产物,质谱结果与NIST 11.0数据库进行匹配。
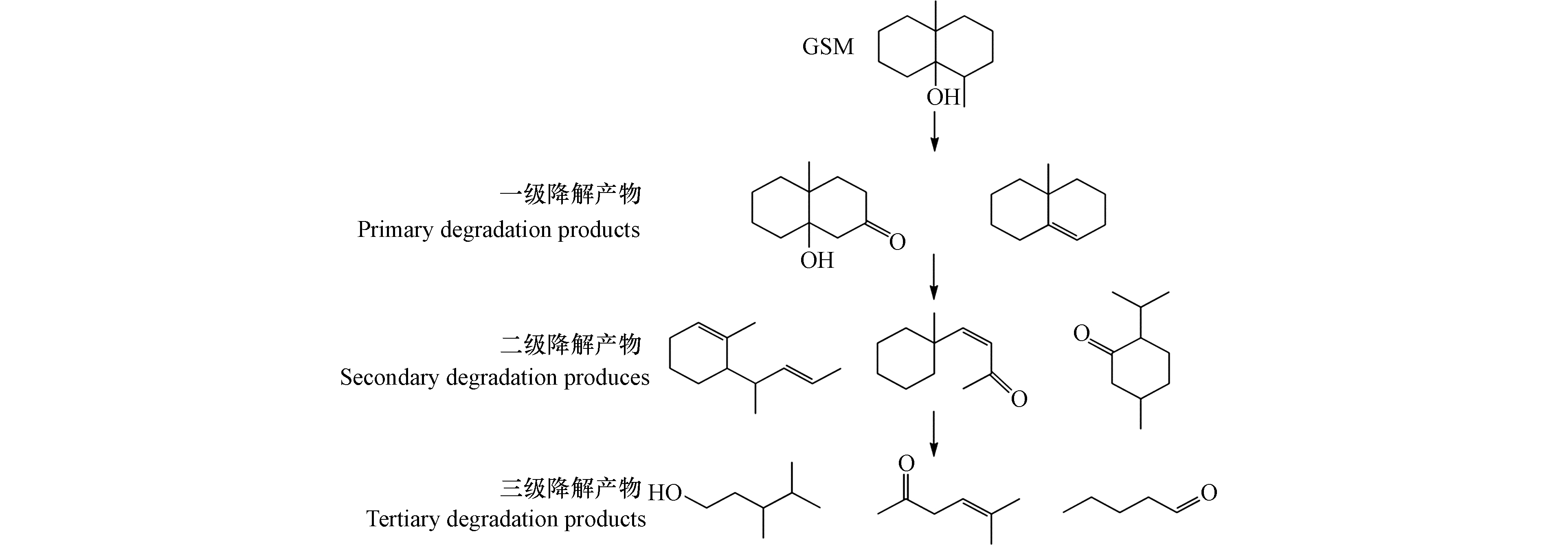
对比色谱图和质谱图分析结果,GSM可能的降解途径如图10所示,GSM中间产物仍存在原来的环状结构。光催化降解GSM中间产物的途径与此较为相似[23]。·OH在GSM侧链上发生氧化脱氢与去甲基反应,将自由电子移至GSM上对其化学结构进行破坏,产生的一级降解产物存在环内不稳定双键。·OH与一级降解产物的加成反应和电子转移使C—C键断裂,改变双环结构,形成单环结构的二级氧化降解产物。·OH继续加成反应,C—C或C=C键断裂,导致环结构断裂,生成三级氧化降解产物,如小分子醛、酮等。醛、酮等小分子产物最终被·OH矿化成水和二氧化碳。
对比色谱图和质谱图分析结果,2-MIB可能的降解途径如图11所示,·OH先氧化2-MIB侧链,通过脱水脱甲基反应改变2-MIB的结构,生成含酮基及环内双键的一级降解产物。·OH继续氧化该产物,进行加成反应并将自由电子转移至桥环内部,致使桥环结构断开,形成二级单环醛酮类分子。·OH通过环内加成反应转移电子,打破环结构的化学键,生成三级醛、酮和酸等小分子产物。·OH继续降解链状小分子产物,矿化成CO2和H2O。这与双氧水/臭氧氧化降解2-MIB生成中间产物的降解途径较为相似[24].
采用GC-MS法对GSM和2-MIB降解的中间产物进行全扫描鉴别,结果与GSM和2-MIB的前线轨道分析结果一致。
-
为了探究不同UV-AOPs工艺对实际水体水质指标的去除效果,并验证以上实验结果。选取3个实际水厂的原水及滤后水进行实验,紫外灯光照强度为210 μW·cm−2,氧化剂H2O2与NaClO的浓度为8 mg·L−1,降解时间为50 min,水体的水质指标以及处理后的水质指标如表2所示。
经高级氧化工艺处理后GSM、2-MIB、浊度、UV254、耗氧量、DOC、SUVA等水质指标均得到进一步去除,水质得到明显改善,UV/H2O2工艺对实际水体的处理效果优于UV/Cl2工艺对实际水体的处理效果。当GSM的浓度为20—30 ng·L−1时,UV/H2O2和UV/Cl2工艺的处理效果均能满足饮用水标准规定;当2-MIB浓度较高时,UV/H2O2和UV/Cl2工艺对2-MIB的降解效能差强人意,需要增加紫外光剂量或氧化剂投加量以改善去除效果。由于实际水体的复杂性,UV/H2O2和UV/Cl2工艺对嗅味物质的部分去除效果未达到实验室效果,因此高级氧化技术在实际运用中,应根据实际水体水质情况选择适宜的工况条件。UV/H2O2和UV/Cl2工艺对浊度及有机物浓度的降低均有一定的效果,且UV/H2O2技术的去除效果更稳定。污染物的降解会消耗电能,而电耗与净化水的成本相关,根据国际理论与应用化学联合会(IUPAC)提出的高级氧化技术处理水中低浓度污染物的电能效率评价指标公式计算[25],在该实验条件下,UV、UV/H2O2、UV/Cl2工艺降解GSM的平均电耗为1440、241.68、345.6 kW·h·m−3,降解2-MIB的平均电耗为1645.71、261.82、352.65 kW·h·m−3。相较而言,UV/H2O2技术降解嗅味物质及去除部分污染物的效果更优。
-
(1)UV、UV/H2O2、UV/Cl2工艺对GSM和2-MIB均有一定的去除效果,其中UV/H2O2工艺对GSM和2-MIB的去除效果较优。在氧化剂投加量增加、光照强度增大、反应时间增长及目标污染物初始浓度降低的条件下,三种工艺对GSM和2-MIB的去除率也会相应升高。
(2)3种工艺去除GSM和2-MIB的效果受水体中pH值、无机阴离子、腐殖酸等条件的影响。pH越高,去除GSM和2-MIB的效果越差。SO42-可以促进UV/H2O2、UV/Cl2工艺去除嗅味物质,HCO3−的存在会抑制二者对嗅味物质的去除,Cl−会促进UV/Cl2工艺去除嗅味物质,抑制UV/H2O2工艺去除嗅味物质。腐殖酸浓度过高对UV/H2O2、UV/Cl2工艺去除GSM和2-MIB的抑制作用更为显著。
(3)根据不同UV-AOPs工艺对实际水体水质指标的实验结果,其对GSM、2-MIB、浊度、UV254、耗氧量、DOC、SUVA等水质指标均有一定的去除效果,同时电能消耗较低,相比其他两种工艺,UV/H2O2工艺对实际水体的处理效果较优。
紫外高级氧化工艺降解土臭素(GSM)和2-甲基异莰醇(2-MIB)的对比
Comparative study on degradation of GSM and 2-MIB by ultraviolet advanced oxidation processes
-
摘要: 紫外(UV)、紫外/过氧化氢(UV/H2O2)、紫外/氯(UV/Cl2)等3种不同的紫外高级氧化工艺(UV-AOPs)可在一定程度上去除土臭素(GSM)和2-甲基异莰醇(2-MIB),其中UV/H2O2工艺对GSM和2-MIB的去除效果更好。光照强度、氧化剂投加量、反应时间、目标污染物初始浓度、pH值、无机阴离子及腐殖酸均会影响UV-AOPs工艺去除GSM和2-MIB的效果。提高光照强度、氧化剂投加量、反应时间会促进3种工艺对GSM和2-MIB的去除;目标污染物初始浓度、pH值及腐殖酸的浓度过高会抑制3种工艺对GSM和2-MIB的去除;SO42−对去除嗅味物质有促进作用,HCO3−会抑制对嗅味物质的去除。通过对产物降解途径的分析,UV/H2O2工艺降解GSM和2-MIB会导致其中的环结构断裂,将小分子链状产物最终矿化成CO2和H2O。通过比较不同UV-AOPs工艺对实际水体不同指标的处理效能得出,UV/H2O2工艺对实际水体的处理效果优于UV及UV/Cl2工艺。Abstract: Ultraviolet (UV), ultraviolet/hydrogen peroxide (UV/H2O2), ultraviolet/chlorine (UV/Cl2) three different ultraviolet advanced oxidation processes (UV-AOPs) for geosmin (GSM) and 2-methylisoborneol (2-MIB) has a certain removal effect, and the UV/H2O2 process has a better removal effect on GSM and 2-MIB. The light intensity, dosage of oxidant, reaction time, initial concentration of target pollutants, pH, inorganic anions and humic acid will all affect the effect of UV-AOPs process in removing GSM and 2-MIB. Among them, the light intensity, dosage of oxidant, and reaction time will promote the removal of GSM and 2-MIB by the three processes; the high concentration of the initial concentration of target pollutants, pH and humic acid will inhibit the effects of the three processes on GSM and 2-MIB. SO42− enhances the removal of odorous substances, and HCO3- can abate the removal of odorous substances. Through the analysis of the degradation pathway of the products, the degradation of GSM and 2-MIB by UV / H2O2 process will lead to the ring structure fracture, and the small molecular chain products will eventually be mineralized into CO2 and H2O. By comparing the treatment efficiency of different UV-AOPs processes on different indicators of the real waters, it is concluded that the UV/H2O2 process has better treatment effects on the real waters than the UV and UV/Cl2 processes.
-

-
表 1 不同UV-AOPs降解GSM和2-MIB的拟一级反应动力学参数
Table 1. Quasi-first-order reaction kinetic parameters for degradation of GSM and 2-MIB by different UV-AOPs
嗅味物质
Odorants工艺
Method一级反应动力学方程
First order kinetic equationK/min-1 R2 GSM UV $ \mathrm{l}\mathrm{n}\left(C/{C}_{0}\right)=-0.0024t-0.1021 $ 0.0024 0.989 UV/H2O2 $ \mathrm{l}\mathrm{n}\left(C/{C}_{0}\right)=-0.0143t-0.1671 $ 0.0143 0.9822 UV/Cl2 $ \mathrm{l}\mathrm{n}\left(C/{C}_{0}\right)=-0.0100t-0.2054 $ 0.0100 0.9741 2-MIB UV $ \mathrm{l}\mathrm{n}\left(C/{C}_{0}\right)=-0.0021t-0.0925 $ 0.0021 0.9788 UV/H2O2 $ \mathrm{l}\mathrm{n}\left(C/{C}_{0}\right)=-0.0132t-0.2235 $ 0.0132 0.9956 UV/Cl2 $ \mathrm{l}\mathrm{n}\left(C/{C}_{0}\right)=-0.0098t-0.2488 $ 0.0098 0.9783 表 2 不同UV-AOPs工艺对实际水体的处理效能
Table 2. Treatment efficiency of different UV-AOPs processes for real water
名称Name GSM/
(ng·L−1)2-MIB/
(ng·L−1)浊度/NTU
TurbidityUV254 耗氧量/(mg·L−1)
Oxygen
consumption可溶性有机碳/
(g·kg−1)
DOC比紫外吸光度
SUVA北控水厂 水厂原水 20.99 115.23 4.50 0.048 2.58 2.93 1.655 原水经UV/H2O2处理后 2.51 28.24 4.11 0.039 2.05 2.87 1.367 原水经UV/Cl2处理后 5.25 42.02 4.18 0.041 2.21 2.90 1.407 水厂滤后水 7.54 85.66 0.22 0.027 1.6 2.76 0.987 滤后水经UV/H2O2处理后 1.20 17.40 0.20 0.021 1.25 2.56 0.810 滤后水经UV/Cl2处理后 3.73 34.67 0.21 0.022 1.33 2.62 0.846 众兴水厂 水厂原水 15.89 81.67 2.58 0.069 3.84 4.25 1.634 原水经UV/H2O2处理后 2.41 20.14 2.30 0.056 3.27 4.09 1.359 原水经UV/Cl2处理后 6.74 31.82 2.34 0.058 3.52 4.13 1.408 水厂滤后水 10.40 50.62 0.46 0.049 2.14 3.10 1.576 滤后水经UV/H2O2处理后 1.92 8.54 0.40 0.038 1.7 2.91 1.311 滤后水经UV/Cl2处理后 4.23 15.90 0.41 0.040 1.79 2.98 1.341 白浪河水厂 水厂原水 28.42 106.5 4.30 0.048 2.4 3.62 1.336 原水经UV/H2O2处理后 2.46 68.19 3.86 0.039 1.89 3.49 1.110 白浪河水厂 原水经UV/Cl2处理后 4.31 84.10 3.92 0.041 1.99 3.50 1.176 水厂滤后水 14.81 44.06 2.32 0.020 1.42 2.23 0.901 滤后水经UV/H2O2处理后 1.22 12.52 2.01 0.015 1.14 2.16 0.700 滤后水经UV/Cl2处理后 6.84 19.62 2.08 0.016 1.26 2.18 0.742 -
[1] 林晓娟, 高姗, 仉天宇, 等. 海水富营养化评价方法的研究进展与应用现状 [J]. 地球科学进展, 2018, 33(4): 373-384. doi: 10.11867/j.issn.1001-8166.2018.04.0373 LIN X J, GAO S, ZHANG T Y, et al. Researching progress and application status of eutrophication evaluation method of seawater [J]. Advances in Earth Science, 2018, 33(4): 373-384(in Chinese). doi: 10.11867/j.issn.1001-8166.2018.04.0373
[2] 李勇, 张晓健, 陈超. 我国饮用水中嗅味问题及其研究进展 [J]. 环境科学, 2009, 30(2): 583-588. doi: 10.3321/j.issn:0250-3301.2009.02.045 LI Y, ZHANG X J, CHEN C. Review on the tastes and odors compounds in drinking water of China [J]. Environmental Science, 2009, 30(2): 583-588(in Chinese). doi: 10.3321/j.issn:0250-3301.2009.02.045
[3] 宋武昌, 李星, 贾瑞宝, 等. 微污染引黄水库水中有机物特性研究 [J]. 北京工业大学学报, 2015, 41(1): 131-136. SONG W C, LI X, JIA R B, et al. Investigation of characteristics of organics in the micro-polluted Yellow River reservoir water [J]. Journal of Beijing University of Technology, 2015, 41(1): 131-136(in Chinese).
[4] 朱欢欢, 孙韶华, 冯桂学, 等. 紫外联用高级氧化技术处理饮用水应用进展 [J]. 水处理技术, 2019, 45(3): 1-7,13. ZHU H H, SUN S H, FENG G X, et al. Research progress of ultraviolet combined advanced oxidation technology for drinking water treatment [J]. Technology of Water Treatment, 2019, 45(3): 1-7,13(in Chinese).
[5] 刘俊逸, 张宇, 张蕾, 等. 含酚工业废水处理技术的研究进展 [J]. 工业水处理, 2018, 38(10): 12-16. doi: 10.11894/1005-829x.2018.38(10).012 LIU J Y, ZHANG Y, ZHANG L, et al. Research progress in the treatment technologies of industrial wastewater containing phenol [J]. Industrial Water Treatment, 2018, 38(10): 12-16(in Chinese). doi: 10.11894/1005-829x.2018.38(10).012
[6] 张燚. 真空紫外/氯技术去除饮用水致嗅物质2-MIB和GSM的研究[D]. 西安: 西安建筑科技大学, 2018. ZHANG Y. Study on removing odorants of 2-MIB and GSM in drinking water by vacuum ultraviolet combined with chlorine[D]. Xi'an: Xi'an University of Architecture and Technology, 2018(in Chinese).
[7] CHANG M W, CHUNG C C, CHERN J M, et al. Dye decomposition kinetics by UV/H2O2: Initial rate analysis by effective kinetic modelling methodology [J]. Chemical Engineering Science, 2010, 65(1): 135-140. doi: 10.1016/j.ces.2009.01.056 [8] 喻杰, 叶志伟, 党文悦, 等. 紫外波长对UV/Cl2高级氧化去除水中有机物的影响 [J]. 环境工程学报, 2019, 13(3): 577-585. doi: 10.12030/j.cjee.201809143 YU J, YE Z W, DANG W Y, et al. Effect of ultraviolet wavelength on organic matter removal from water by UV/Cl2 advanced oxidation [J]. Chinese Journal of Environmental Engineering, 2019, 13(3): 577-585(in Chinese). doi: 10.12030/j.cjee.201809143
[9] 李慧琴. 基于紫外的高级氧化技术在水处理中的应用进展 [J]. 化工设计通讯, 2020, 46(11): 28-30. doi: 10.3969/j.issn.1003-6490.2020.11.015 LI H Q. Recent advances of ultraviolet light-based advanced oxidation processes in water treatment [J]. Chemical Engineering Design Communications, 2020, 46(11): 28-30(in Chinese). doi: 10.3969/j.issn.1003-6490.2020.11.015
[10] 王婷, 吴乾元, 王文龙, 等. 紫外线/氯高级氧化降解甲基异噻唑啉酮 [J]. 环境工程学报, 2017, 11(1): 21-26. doi: 10.12030/j.cjee.201509106 WANG T, WU Q Y, WANG W L, et al. Degradation of methylisothiazolinone by UV/chlorine advanced oxidation process [J]. Chinese Journal of Environmental Engineering, 2017, 11(1): 21-26(in Chinese). doi: 10.12030/j.cjee.201509106
[11] 高乃云, 祝淑敏, 马艳, 等. 2, 4, 6-三氯酚的UV/H2O2光化学降解 [J]. 中南大学学报(自然科学版), 2013, 44(3): 1262-1268. GAO N Y, ZHU S M, MA Y, et al. UV/H2O2 photochemical degradation of 2, 4, 6-trichlorophenol [J]. Journal of Central South University (Science and Technology), 2013, 44(3): 1262-1268(in Chinese).
[12] 章丽萍, 崔炎炎, 贾泽宇, 等. 等离子体高级氧化技术去除饮用水中土霉味物质 [J]. 中国给水排水, 2019, 35(5): 36-42. ZHANG L P, CUI Y Y, JIA Z Y, et al. Removal of earthy/musty odor compounds in drinking water using an advanced oxidation process based on plasma [J]. China Water & Wastewater, 2019, 35(5): 36-42(in Chinese).
[13] KIM T K, MOON B R, KIM T, et al. Degradation mechanisms of geosmin and 2-MIB during UV photolysis and UV/chlorine reactions [J]. Chemosphere, 2016, 162: 157-164. doi: 10.1016/j.chemosphere.2016.07.079 [14] 孙昕, 张燚, 史路肖, 等. 真空紫外/氯处理饮用水典型致嗅物质 [J]. 环境科学, 2018, 39(4): 1654-1660. SUN X, ZHANG Y, SHI L X, et al. Removing typical odorants in drinking water by vacuum ultraviolet combined with chlorine [J]. Environmental Science, 2018, 39(4): 1654-1660(in Chinese).
[15] GOGATE P R, PANDIT A B. A review of imperative technologies for wastewater treatment II: Hybrid methods [J]. Advances in Environmental Research, 2004, 8(3/4): 553-597. [16] 成建国. 羟基自由基快速氧化降解饮用水中致嗅物质研究[D]. 大连: 大连海事大学, 2018. CHENG J G. Study on fast oxidative degradation of odor compounds in drinking water by hydroxyl radicals[D]. Dalian, China: Dalian Maritime University, 2018(in Chinese).
[17] TRUONG G L, LAAT J D, LEGUBE B. Effects of chloride and sulfate on the rate of oxidation of ferrous ion by H2O2 [J]. Water Research, 2004, 38(9): 2384-2394. doi: 10.1016/j.watres.2004.01.033 [18] 邱丽佳. H2O2及O3氧化两种典型蓝藻致嗅物质和灭藻效应研究[D]. 北京: 北京建筑大学, 2017. QIU L J. Oxidation of odor compound and inactivation efficiency of two kinds of typical cyanobacteria by hydrogen peroxide and ozone[D]. Beijing: Beijing University of Civil Engineering and Architecture, 2017(in Chinese).
[19] 潘晶, 孙铁珩, 李海波. 水中无机阴离子对UV/H2O2降解LAS的影响及机理 [J]. 环境科学, 2007, 28(11): 2539-2543. doi: 10.3321/j.issn:0250-3301.2007.11.023 PAN J, SUN T H, LI H B. Effects and mechanisms of inorganic anions in water on degradation of LAS by UV/H2O2 combination process [J]. Environmental Science, 2007, 28(11): 2539-2543(in Chinese). doi: 10.3321/j.issn:0250-3301.2007.11.023
[20] RAZAVI B, BEN ABDELMELEK S, SONG W H, et al. Photochemical fate of atorvastatin (lipitor) in simulated natural waters [J]. Water Research, 2011, 45(2): 625-631. doi: 10.1016/j.watres.2010.08.012 [21] 骆靖宇, 李学艳, 李青松, 等. 紫外活化过硫酸钠去除水体中的三氯卡班 [J]. 中国环境科学, 2017, 37(9): 3324-3331. doi: 10.3969/j.issn.1000-6923.2017.09.015 LUO J Y, LI X Y, LI Q S, et al. Degradation of triclocarban aqueous solution through UV irradiation-activated sodium persulfate process [J]. China Environmental Science, 2017, 37(9): 3324-3331(in Chinese). doi: 10.3969/j.issn.1000-6923.2017.09.015
[22] HO L, NEWCOMBE G, CROUÉ J P. Influence of the character of NOM on the ozonation of MIB and geosmin [J]. Water Research, 2002, 36(3): 511-518. doi: 10.1016/S0043-1354(01)00253-6 [23] FOTIOU T, TRIANTIS T M, KALOUDIS T, et al. Photocatalytic degradation of water taste and odour compounds in the presence of polyoxometalates and TiO2: Intermediates and degradation pathways [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2014, 286: 1-9. doi: 10.1016/j.jphotochem.2014.04.013 [24] 李学艳, 高乃云, 沈吉敏, 等. O3/H2O2降解水中致嗅物质2-MIB的效能与机理 [J]. 环境科学学报, 2009, 29(2): 344-352. doi: 10.3321/j.issn:0253-2468.2009.02.018 LI X Y, GAO N Y, SHEN J M, et al. Efficiency and mechanism of degradation of 2-MIB by O3/H2O2 in water [J]. Acta Scientiae Circumstantiae, 2009, 29(2): 344-352(in Chinese). doi: 10.3321/j.issn:0253-2468.2009.02.018
[25] 高乃云, 张晏晏, 马艳. UV/TiO2去除水中磺胺甲唑的动力学及影响因素分析 [J]. 中国环境科学, 2013, 33(11): 1958-1964. GAO N Y, ZHANG Y Y, MA Y. Kinetics and impact factors of sulfamethoxazole(SMX) degradation in drinking water by UV/TiO2 [J]. China Environmental Science, 2013, 33(11): 1958-1964(in Chinese).
-



 下载:
下载: