-
自从20世纪70年代末引入电化学高级氧化工艺(EAOPs)以来,EAOPs在处理水中的难生物降解和有毒有机污染物方面引起了世界各国科学团体的广泛关注[1-2]。EAOPs是一种基于原位生成羟基自由基(·OH)强氧化剂的技术,·OH可无选择性破坏废水中有机物成分。在各种水处理方法中,电化学氧化法在处理工业废水和目标污染物去除方面的应用越来越广泛,相对于其它高级氧化技术来说,具有1)与环境兼容;2)无需添加化学试剂;3)用途广泛;4)易于自动化;5)条件温和及设备简单;6)安全性高等优点,但也存在着能耗大,运行费用过高和电流效率低等缺点[3-4]。
抗生素作为一种广泛用于细菌感染的药物,替代品非常有限,但是抗生素在使用过程中只有20%—30%的抗生素在人和牲畜的身体系统中被代谢,其余以其原有的形态或中间产物的形式进入或排泄到水环境中。尽管此类新兴污染物带来的环境及健康威胁已逐步体现,但是由于人们对此类产品的依赖性和消费水平,此类化学物的消耗并不会随之下降[5]。根据相关研究报告称,地表水、地下水和污水处理厂的废水中抗生素的含量范围通常在几ng·L−1到100 mg·L−1,其中以制药厂及医院废水最为突出,尽管水体中浓度相对较低,但随着生物体的积累,抗生素浓度会随着时间的推移而越来越大,最终对生态系统产生深远和不可逆转的影响[6]。
由于其生物难降解特性和技术自身限制,污水处理厂中使用的传统物理化学和生物处理方法难以从废水中有效去除抗生素类污染物[7],电化学氧化法技术在应用于降解废水中新兴抗生素的应用研究成为一种适合的选择,而阳极材料是直接影响电催化效率的关键因素[8]。掺硼金刚石(boron-doped diamond, BDD)电极以其自身优异的物理及化学性质被认作为电催化氧化有机持久性污染物最为理想的电极材料[9-10],相对于传统的金属氧化物涂层阳极,BDD阳极在有机污染物的处理方面能够达到较高的去除率甚至可将有机物完全矿化为CO2和H2O,并同时保持较高的电流效率[11-13]。但是,如何实现高效去除水中存在的新兴抗生素类污染物,探讨电催化过程关键因素对降解动力学的影响机制是电催化氧化应用的重要前提。
本部分以当前环境中常见的一种抗生素药物,甲氧苄啶(trimethoprim,TMP)为研究对象,BDD电极为阳极,重点考察抗生素TMP在BDD电极上的电催化降解及动力学行为,分析降解过程中施加电流密度、溶液pH、TMP浓度、支持电解质浓度对抗生素污染物、化学需氧量去除率及降解过程动力学的影响规律,根据降解过程中的中间产物,提出TMP的可能降解路径,为电催化氧化技术和BDD阳极电催化降解水中新兴抗生素类污染物提供实验依据和技术支持。
-
BDD薄膜阳极通过热丝化学气相沉积法在钛基体上沉积得到。H2和CH4的混合气体作为气源,同时由H2带出的B(OCH3)3液体作为硼源,硼掺杂度通过控制调节通入B(OCH3)3的流速进行控制。甲烷含量为1.0 %(体积分数),反应器压强为4.0 kPa,总气体流量保持在标准状态下300 mL·min−1,基体温度保持在(750±20)℃,具体制备过程参见文献[14],所有制备条件均已进行优化。BDD薄膜的结晶度及相成分用XRD(Rigaku D/Max 2550,Japan)进行分析,SEM(SU8020,HITACHI,Japan)用以观察薄膜表面形貌及结构用金刚石薄膜质量。
电化学测试在上海辰华CHI760电化学工作站的三电极系统中进行,Ti/BDD电极作为研究电极,金属箔片作为对电极,饱和甘汞电极(SCE)为参比电极。循环伏安曲线用来确定制备的电极材料的电势窗口及背景电流,溶液为0.1 mol·L−1 H2SO4溶液,扫速为10 mV·s−1。
实验所有电化学测试均在室温状态下进行,溶液的配制均使用去离子水(电导率为18.25 Ω·cm,摩尔超纯水机自制)。每一组电催化氧化TMP实验均在室温下进行,将预定量的TMP抗生素和支持电解质溶解在1 L去离子水中,然后将混合物磁力搅拌1 h,用H2SO4或NaOH调节模拟废水的初始pH。
-
电化学降解抗生素TMP实验在无隔膜的单室电解槽中进行,1.5 cm2的Ti/BDD电极作为工作电极,3 cm×3 cm的Ti网作为对电极,电极间距为2.0 cm,采用恒压恒流电源固定电解过程的电流密度,整个实验过程中借助磁力搅拌器进行连续混合,同时在每次降解实验前对BDD电极在0.1 mol·L−1 H2SO4极化20 min去除表面污染物。当电源接通时,电催化氧化反应开始计时,每隔一定的时间取样,用来进行化学需氧量(chemical oxygen demand,COD)测试和高效液相色谱(HPLC)测量,并且在降解过程中记录电解槽的槽压变化。
溶液的COD值变化通过571型化学需氧量分析仪(上海仪电科学仪器股份有限公司)进行检测,TMP浓度及降解过程中羧酸分子中间产物的变化使用配置Inertsil ODS-SP, C18 柱 (4.6 mm×150 mm×5 μm)的LC-20A高效液相色谱仪(日本岛津公司)进行定性或定量分析,流动相为乙腈:水(体积比30:70),流速为0.8 mL·min−1,检测波长274 nm。采用HPLC-MS 2020液相色谱-质谱联用仪(日本岛津公司)检测降解过程中的可能形成的中间产物,质谱扫描采用正离子模式,m/z扫描范围为100—500。TMP溶液的紫外可见吸收光谱测试在UV-2400紫外可见吸收光谱(日本岛津公司)上进行,波长变化范围为200—800 nm。
电流效率定义为用于有机物氧化的电流与总电流值的比值,通过以下关系求得:
式中,COD0和CODΔt分别为时间为0和Δt时的COD值(mg·L−1),I(A)为电解电流;Δt(s)为电解时间,F为法拉第常数,96500(C·mol−1);V(L)为溶液体积。
-
采用热丝化学气相沉积法在金属钛基体上制备钛基BDD电极,如图1a为钛基BDD膜电极的SEM图。由图a1可以看出,BDD薄膜完全覆盖在基体金属钛上,金刚石颗粒结晶状况较好,晶体大小分布均匀且连续,晶粒尺寸范围在2—3 μm左右。图1b为钛基BDD电极的XRD谱图,除了明显的金属Ti衍射峰外,在2θ=43.9°和75.3°处存在明显的金刚石的(111)和(220)衍射峰,晶格取向以(111)取向为主,同时I(111)/I(220)值要大于金刚石的标准值,尖锐的(111)衍射峰也表明了BDD电极具有良好的结晶度及质量,这一特点进一步证明了BDD电极沉积到金属钛基体上[15]。此外,可以进一步观察到TiC的衍射峰,这是由金刚石沉积过程中的化学反应决定的,气相沉积过程中,Ti的高活性使得金刚石沉积以前首先由碳原子作用生成TiC相,这也与文献报道相一致[16]。
同时,对钛基BDD电极的化学性质进行了进一步的研究。图2为BDD电极在扫速为10 mV·s−1下,0.1 mol·L−1 H2SO4溶液下的线性扫描曲线图。由图2可以看出,BDD电极拥有较高的析氧电位(~2.3 V)和析氢电位(~−1.1 V),即,制备的BDD电极具有较宽的电势窗口(~3.4 V),与文献报道的沉积在单晶Si、Nb及钛基体上的BDD的对应值相近[17],较高的析氧电位有利于提升BDD电极电催化降解有机污染物的效率。
-
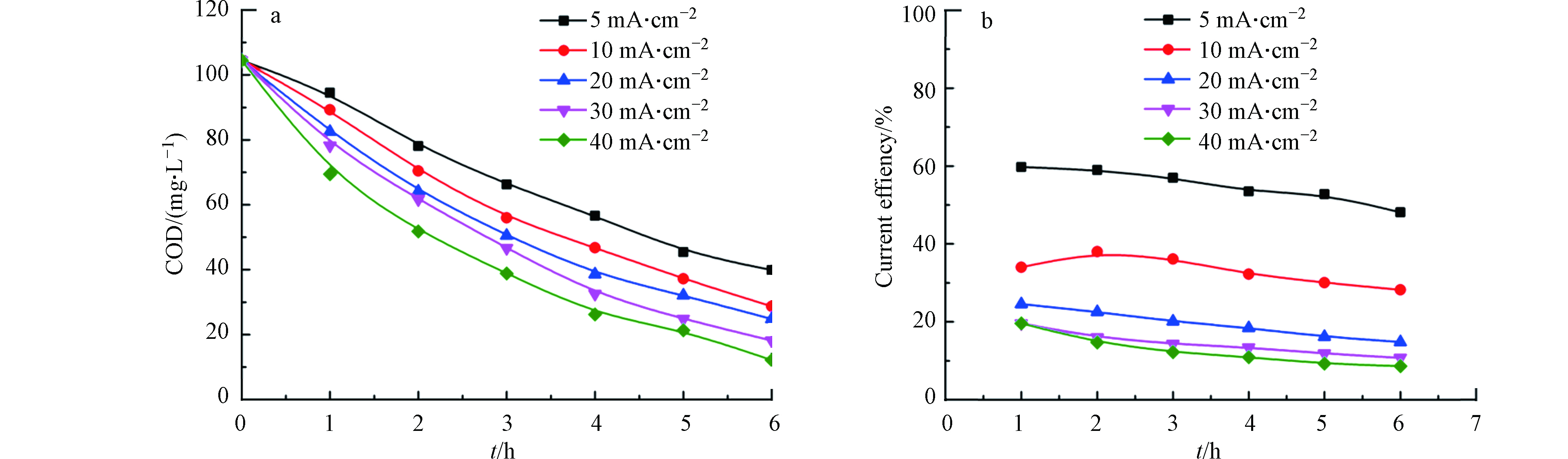
施加电流密度是电化学氧化过程中的一个关键实验参数[18],本部分通过改变施加到BDD电极上的电流密度,研究其对TMP降解速率及动力学的影响。图3显示了不同电流密度下50 mg·L−1 TMP抗生素在降解过程中的浓度随电解时间的变化关系。由图3可知,TMP浓度随着电解过程的进行逐渐降低,表明TMP在BDD电极上发生了电化学降解。最终,5、10、20、30、40 mA·cm−2电流密度下反应6 h后,TMP的去除率分别为69.1%、77.2%、86.3%、91.7%、95.1%。矿化过程TMP溶液的浓度(C0和Ct分别为初始浓度及对应降解时间的浓度)的相对对数与电解时间呈近似的线性关系,表明TMP在BDD电极上的降解符合准一级反应动力学,计算得到不同电流密度下对应的表观速率常数(kapp)分别为0.192、0.248、0.332、0.405、0.491 h−1,也即是,高电流密度下有利于提升TMP的降解动力学反应速率.
图4为降解过程中TMP溶液的COD值及电流效率的变化趋势。随着电流密度的增加,COD值降低速率逐步提升,5、10、20、30、40 mA·cm−2电流密度下,反应6 h后,TMP溶液的COD去除率分别为61.8%、72.6%、76.3%、82.6%、88.4%,其中,30 mA·cm−2和40 mA·cm−2电流密度下有机物的COD去除率已差别不大。电流密度的提升可以促进有机物的矿化,这可归结于高电流密度提升电催化过强阳极表面产生的强氧化性活性物质的数量[19]。但是,高电流密度在促进氧化活性物质的产生的同时,也加剧了析氧副反应的发生,电流效率却随电流密度的增加而逐渐降低,能耗也随之增加,消耗了额外的电能,因此在电催化过程要根据需要选择合适的电流密度[20],最终确定降解过程的最佳电流密度为30 mA·cm−2。此外,电流效率值(8.6%–59.8%)始终低于100%,表明TMP的氧化是在高于极限电流密度的电流密度下进行的,TMP的氧化过程受传质控制。
不同阶段TMP溶液的紫外可见吸收光谱如图5所示。初始阶段的TMP溶液的紫外可见吸收光谱在紫外区的275 nm附近呈现出对应于苯环的吸收峰,该特征峰强度随电解时间逐渐下降,证明有机物的苯环在BDD电极上进行了开环反应,造成苯环特征峰强度的下降。值得注意的是,TMP浓度的下降速率均要明显快于COD值及特征吸收峰的降低速率,这与TMP降解过程中形成的中间产物和降解路径有关,有机物在电极表面矿化过程并不是一步转化为CO2和H2O,当初始有机物消耗的同时,电解过程形成的其他中间产物,如多羟基酚、羧酸小分子等仍然存在于溶液体系中[21]。
-
电催化氧化有机污染物过程的效率高度依赖于溶液pH,阳极表面和整体溶液产生的各种氧化物种的性质也强烈依赖于废水的pH值。图6显示了不同pH下50 mg·L−1 TMP抗生素在BDD电极上降解过程中的浓度随电解时间的变化关系。由图可知,随着pH的降低,有机物被降解的速率逐步增加,最终,pH=1、3、5、7、9下,TMP的去除率分别为97.6%、91.7%、84.8%、79.7%、68.7%。根据矿化过程TMP溶液的浓度的相对对数与电解时间呈近似的线性关系及准一级反应动力学,计算得到不同pH下对应的表观速率常数kapp分别0.605、0.405、0.302、0.259、0.193 h−1,低溶液pH有利于提升TMP溶液的降解动力学反应速率。
图7分别为降解过程中TMP溶液的COD值变化趋势及对应的电流效率。由图7可以看出,随着pH的降低,溶液COD去除效率逐步提升。pH=1、3、5、7、9下,TMP溶液的COD去除率分别为88.0%、82.7%、74.7%、65.4%、56.4%。由于有中间产物的生成,COD的去除率要稍慢于有机物的浓度的下降速率。由图7可知,降解过程的电流效率随着pH的降低,呈逐渐增加的趋势,随着时间的进行,TMP降解的电流效率有不同强弱的递减,这与降解过程中不断消耗的有机物有关。由电催化氧化有机污染物的过程实验可以得出,有机物的降解效率依赖于溶液酸碱度,酸性条件下的电解速率明显高于碱性条件下,并且酸度值越高(即pH值越小),有机物浓度和COD值下降的越快。具体原理为,有机物的电催化降解过程是以电极表面产生的强氧化羟基自由基(·OH)为媒介,酸性条件有利于·OH的产生[22];同时,酸性条件还可抑制·OH的分解和析氧反应的发生,进而提高·OH的利用率;此外,TMP在酸性条件下氨基容易形成质子化化合物,较未质子化化合物,具有更强的·OH亲核反应活性,最终低pH显著提升了TMP的电催化降解效率,因此,确定最优为pH 1,但强酸性的溶液体系可能对反应器带来一定的腐蚀。
-
图8a为pH=3下,不同浓度的TMP溶液在30 mA·cm−2电流密度下,溶液的浓度随降解时间的变化趋势图。由图8可以看出,高浓度有机物需要更长的时间来达到完全的矿化,在浓度25、50、75、100 mg·L−1下,TMP的去除率分别为100%、91.7%、88.6%和81.8%。也即是,TMP浓度越低,有机物去除越彻底,这与一定电流密度下,电极表面产生的强氧化物种一定有关[23]。此时,不同浓度下对应的动力学反应速率分别为0.786、0.405、0.376、0.299 h−1。
图8b,c分别为降解过程中TMP溶液的COD值变化趋势及对应的电流效率。在浓度25、50、75、100 mg·L−1下,TMP溶液的COD去除率分别为97.1%、82.7%、78.8%、72.0%,表明污染物的降解速率随着初始浓度的增加而增加。由于有中间产物的生成,COD的去除率要稍慢于有机物的浓度的下降速率。由图8c可知,电流效率随着有机物浓度从小到大依次递增,随着电解时间的进行,电流效率有不同强弱的递减,这与有机物浓度随着时间进行逐渐降低,电催化氧化受传质控制有关[24]。以上结果说明,以上不同初始TMP浓度的电催化降解速率受传质控制,此时电化学氧化速率快于扩散速率,从而使TMP的降解速率与溶液初始浓度成正比。因此,BDD电极上的电催化氧化更适合于高浓度有机污染物。
-
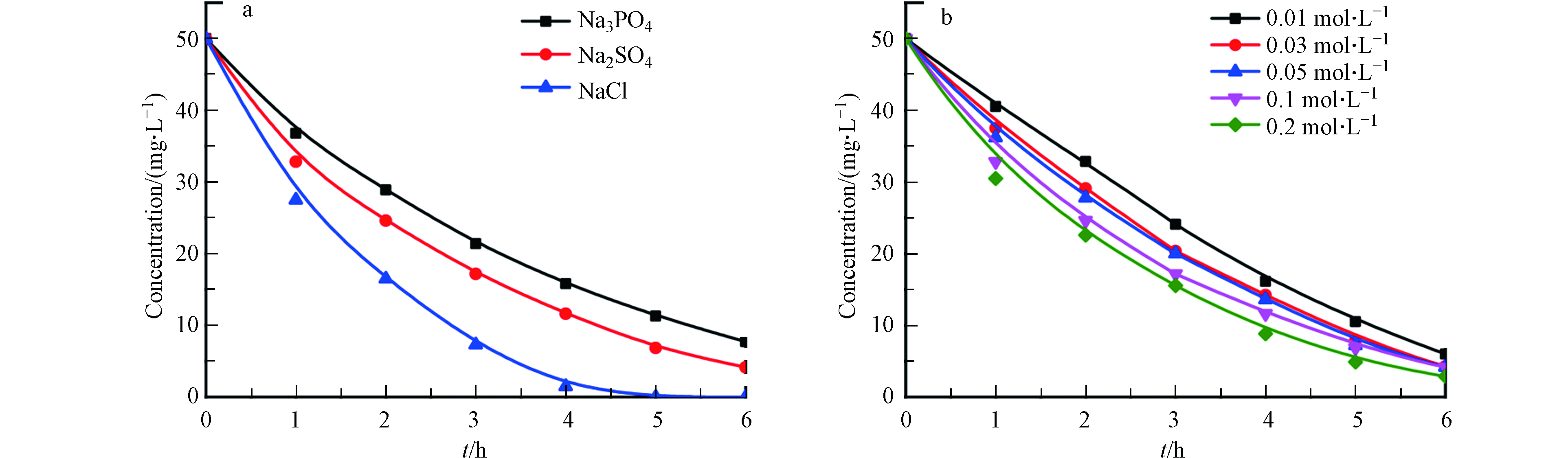
在电化学氧化反应中,电解质主要发挥导电作用,促进电子在电极表面与溶液间的传递,有的电解质还会发挥生成强氧化性物质的作用。图9a为不同支持电解质类型下,TMP浓度的变化趋势,结果显示,Na2SO4相对Na3PO4电解质具有较快的动力学降解速率,这与SO42-在电场一定电流密度下会生成具有一定氧化性的过硫酸根,与·OH形成协同作用,有利于有机物的降解过程[25]。
此外,在NaCl溶液体系下,TMP具有最快的动力学降解速率,这与Cl−在阳极作用下形成强氧化性Cl2和次氯酸根有关,额外层面上加剧了TMP的电催化降解[26]。但是,含氯电解质在电催化降解过程中通常会生成大量的活性氯和含氯有机中间产物,而这些副产物通常比母体溶液的毒性还要强,产生更严重的二次污染[27]。因此,本部分我们选择Na2SO4作为支持电解质。
图9b为不同浓度Na2SO4支持电解质浓度下,TMP浓度随电解时间的变化趋势,由图9b可以看出,有机物TMP浓度呈下降趋势,在硫酸钠浓度0.01、0.03、0.05、0.1、0.2 mol·L−1下,TMP的去除率分别为88.0%、91.4%、91.6%、91.7%、94.2%,随着Na2SO4浓度的提升TMP的降解动力学反应速率逐步提升。但是,图中曲线接近重合,整体差别不大,因此电解速率几乎不受电解质浓度的影响,Na2SO4在电解液中只发挥导电性作用[28],在高浓度的支持电解质下,电子的转移效率得到了提升,从而使得电解得速率得到了微弱的提高,但是其强化效果较弱,综合考虑降解效果及试剂成本,最终选择Na2SO4浓度为0.03 mol·L−1。
-
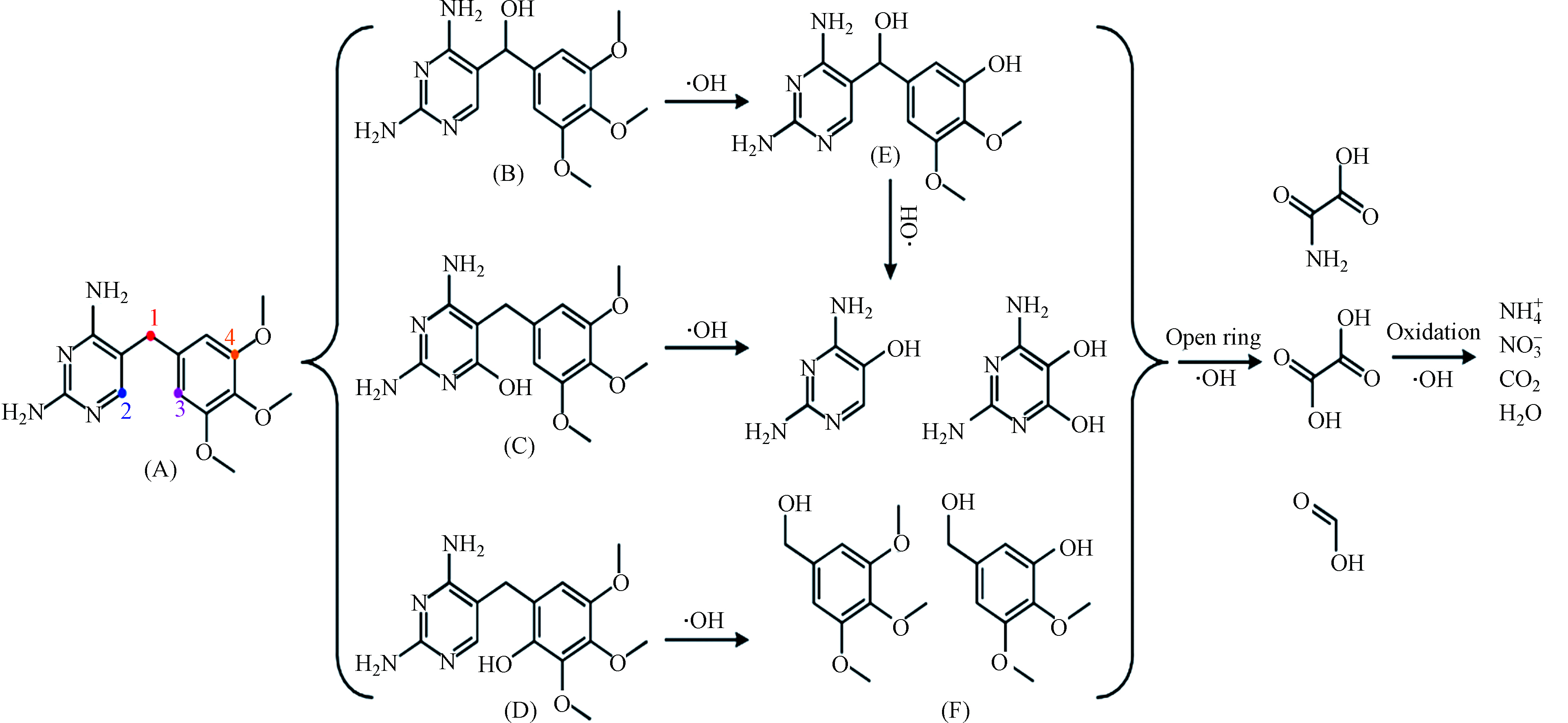
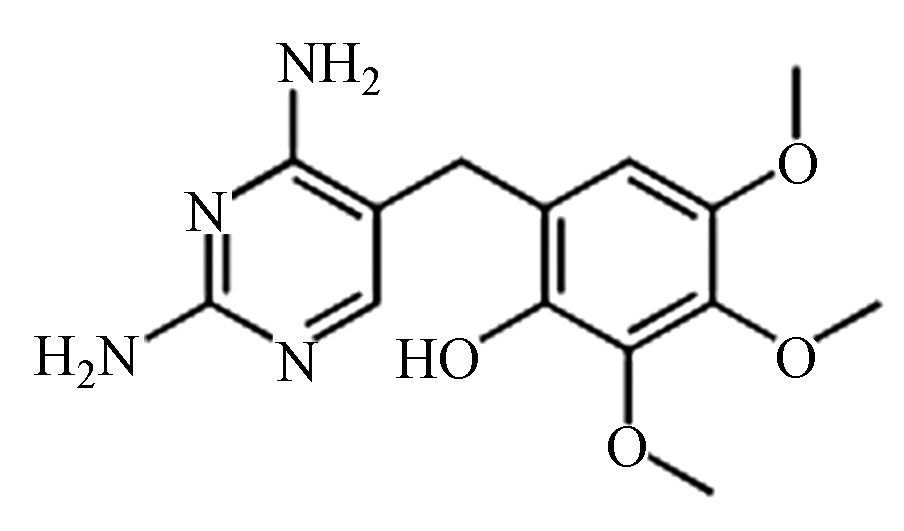
为了进一步探索TMP在BDD电极上电催化降解过程及其降解路径,采用高效液相色谱及液相质谱联用手段对于降解过程中的中间产物进行的研究,结果如表1所示,由于降解过程中可能存在许多同分异构体,对中间产物的判断造成一定的干扰,推断有机物可能的降解路径的具体结果如图10所示。羟基自由的诱发机制是BDD阳极电催化过程的主要降解途径,总体来说,可能的降解路径主要包括羟基化反应、脱甲氧基化反应、裂解和开环反应[29-30]。首先,羟基自由基可以进攻位置1,发生羟基化反应,形成化合物B。此外,羟基自由基也可以进攻取代苯环的甲氧基位置,生成化合物E。还有,由于空间位阻效应的影响[31],羟基自由基可进攻TMP的2和3位置,发生羟基自由基的加成反应,分别生成化合物C和D。此后,生成的化合物发生在羟基自由基作用下发生裂解反应,生成小分子苯环化合物。最终,生成的小分子苯环中间产物及有机胺继续在羟基自由基的强氧化性作用下发生去开环、羧基化及氧化作用,最终被进一步氧化分解,生成NO3−、CO2和H2O,实现有机物的矿化。
首先,羟基自由基可以进攻位置1,发生羟基化反应,形成化合物B。此外,羟基自由基也可以进攻取代苯环的甲氧基位置,生成化合物E。还有,由于空间位阻效应的影响[31],羟基自由基可进攻TMP的2和3位置,发生羟基自由基的加成反应,分别生成化合物C和D。此后,生成的化合物发生在羟基自由基作用下发生裂解反应,生成小分子苯环化合物。最终,生成的小分子苯环中间产物及有机胺继续在羟基自由基的强氧化性作用下发生去开环、羧基化及氧化作用,最终被进一步氧化分解,生成NO3−、CO2和H2O,实现有机物的矿化。
-
本部分选取了抗生素TMP为模拟有机物,使用BDD作为阳极,研究该有机物在电极表面的降解过程及反应动力学。电流密度、pH、有机物浓度和支持电解质的浓度对于降解过程有着不同的影响。高电流密度可加快强氧化性活性物种的产生,促进有机物的降解,但对应析氧副反应的加剧降低了过程的电流效率;低溶液pH有利于强氧化性活性物种的产生,提升有机物的降解动力学;由于电催化过程受传质控制,有机物浓度的提高有利于促进降解动力学;支持电解质发挥导电和产生强氧化性活性物质作用,Na2SO4浓度对有机物的矿化影响不大;最终得出TMP在BDD电极上的优化降解条件:电流密度 30 mA·cm−2,pH=1,TMP浓度100 mg·L−1,0.03 mol·L−1 Na2SO4电解质。同时,根据抗生素TMP电催化降解过程的中间产物,提出了其在BDD电极上的机理及可能的降解途径,主要包括羟基化反应、脱甲氧基化反应、裂解和开环反应,最终实现TMP的完全矿化。
掺硼金刚石阳极电催化降解甲氧苄啶抗生素及其动力学研究
Electrochemical degradation of antibiotic trimethoprim on boron doped diamond anode and kinetics
-
摘要: 水中新兴抗生素类污染物是环境工作者关注的重要问题,如何高效去除抗生素及其去除动力学是需要重点探讨的研究内容。本文以掺硼金刚石为阳极,着重研究抗生素甲氧苄啶在BDD电极上的电催化降解及动力学行为,分析降解过程中施加电流密度、溶液pH、TMP浓度、支持电解质类型和浓度对降解动力学的影响规律,根据降解过程中的中间产物,提出TMP的可能降解路径。结果表明,高电流密度加快强氧化性活性物种的产生,可促进有机物的降解,但析氧副反应的加剧降低了过程的电流效率;低溶液pH有利于强氧化性活性物种的产生,加快有机物的降解动力学;由于电催化过程受传质控制,有机物的浓度的提高有利于促进降解动力学;由于活性氯的产生,电解质中氯离子的存在可加快有机物的去除,同时支持电解质Na2SO4浓度对有机物的矿化影响不大。TMP在BDD电极上的可能的降解路径主要包括羟基化反应、脱甲氧基化反应、裂解和开环反应,最终实现TMP完全矿化为CO2、NO3−和H2O.Abstract: Emerging antibiotic contaminants in water are considered as an important environmental issue. How to efficiently remove the antibiotics and kinetics are of great concern and need to be discussed. Electrochemical degradation of antibiotic trimethoprim (TMP) on boron doped diamond (BDD) anode was conducted in this paper, and operating parameters including applied current density, pH, pollutant concentration and supporting electrolyte concentration on the mineralization kinetics were discussed. Moreover, the possible degradation pathway of TMP during electrochemical oxidation was also proposed based on the degradation mechanism and intermediate products. The results reveal that high current density accelerates the generation of highly oxidizing active species and promotes the degradation of TMP. However, the occurrence of oxygen evolution side reaction under high current reduces the current efficiency of the process. Low pH is favorable for the generation of highly oxidizing active species and accelerates the degradation kinetics. Due to the fact that the electrocatalytic process is controlled by mass transfer process, the increase of TMP concentration is beneficial to promote the degradation kinetics. The presence of chloride ion accelerates the removal of TMP benefitting from the generation of active chlorine while the concentration of sodium sulfate exhibits little effect on the mineralization efficiency. Finally, the possible degradation pathway of TMP on BDD electrode was proposed, which mainly includes the hydroxylation, demethoxylation, cleavage and open ring reactions, realizing the mineralization into CO2, NO3- and H2O.
-

-
表 1 TMP降解过程的中间产物信息
Table 1. Information of intermediate products during TMP degradation
化合物
Compound分子式
Formulam/z 保留时间/min Retention time 中间产物
Intermediate productTMP (A) C14H18N4O3 291.1 4.5 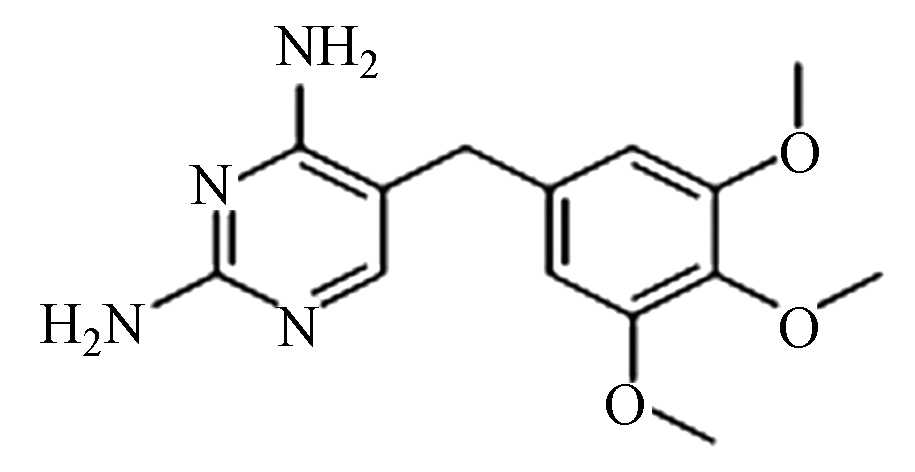
(B)/(C)/(D) C14H18N4O4 307.1 4.0/4.8/5.3 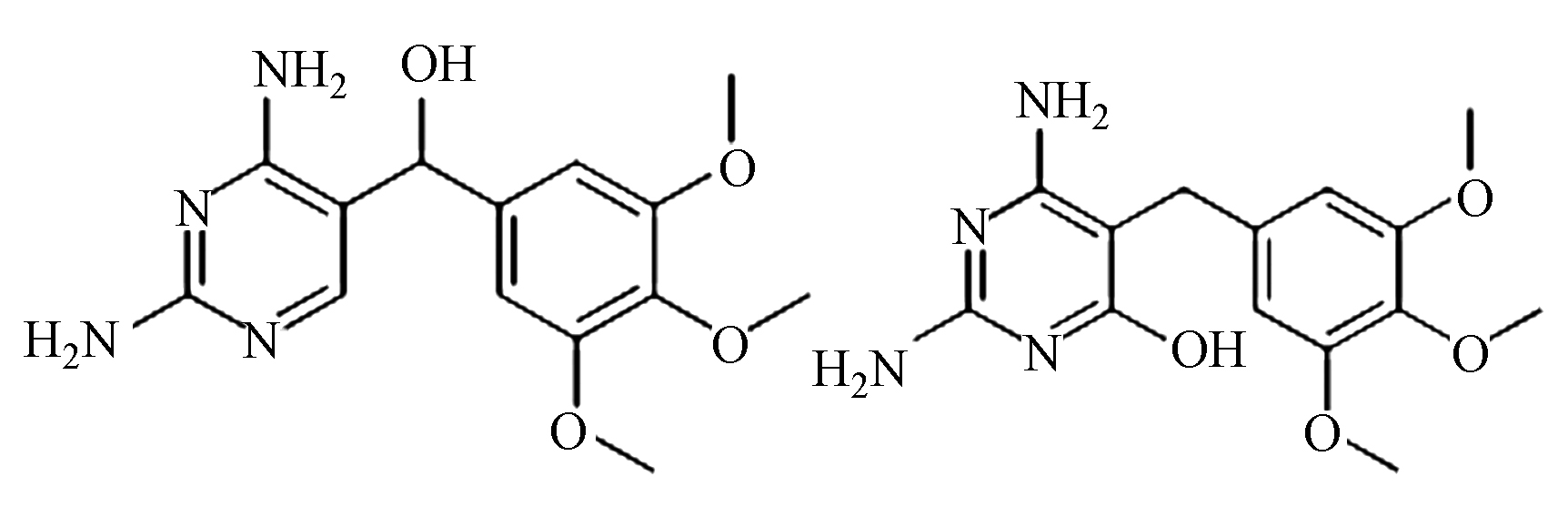
(E) C13H16N4O4 293.1 2.2/3.2 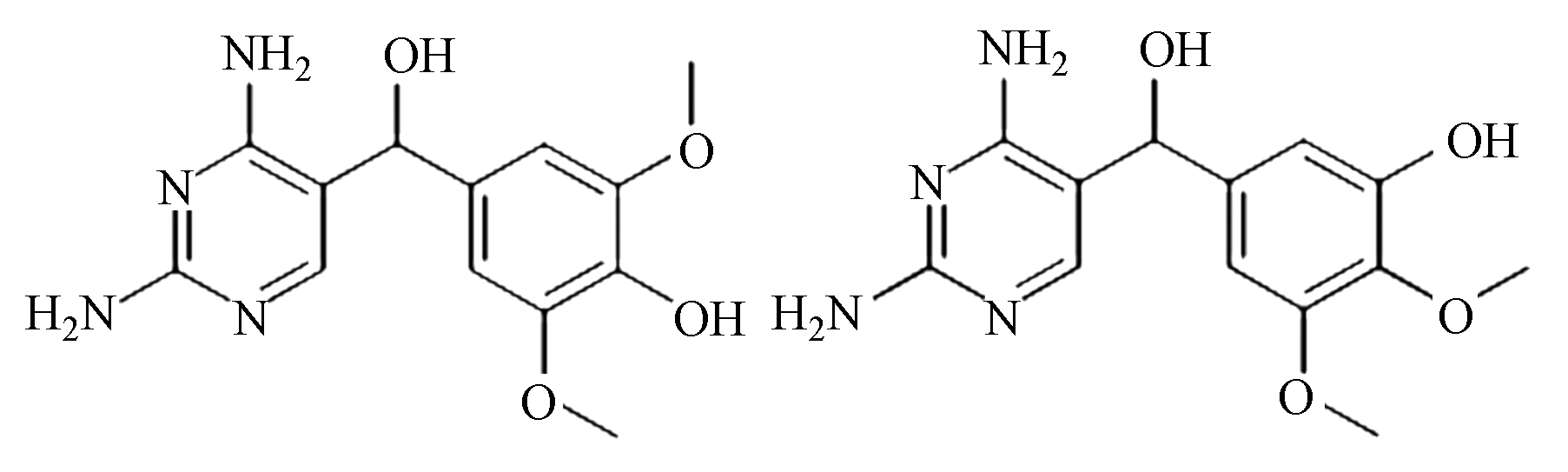
(F) C10H14O4/C4H6N4 199.1/110.1 6.5/1.2 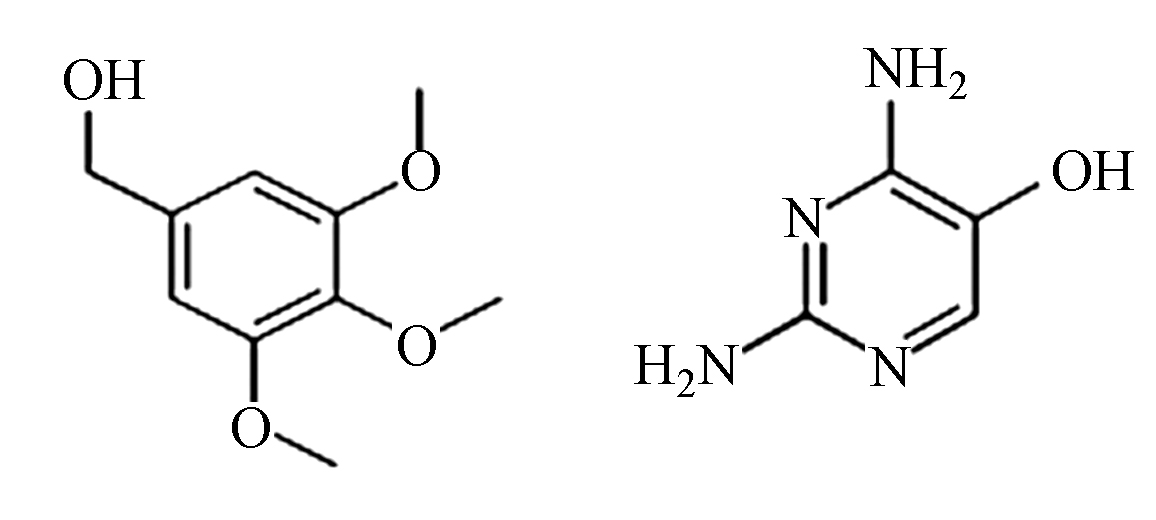
-
[1] 孙怡, 于利亮, 黄浩斌, 等. 高级氧化技术处理难降解有机废水的研发趋势及实用化进展[J]. 化工学报, 2017, 68(5)∶1743—1756. SUN Y, YU L L, HUANG H B, et al. Research trend and practical development of advanced oxidation process on degradation of recalcitrant organic wastewater[J]. CIESC Journal 2017, 68(5): 1743-1756(in Chinese).
[2] 冯雪梅, 卫新来, 陈俊, 等. 高级氧化技术在废水处理中的应用进展 [J]. 应用化工, 2020, 49(4): 993-996,1001. doi: 10.3969/j.issn.1671-3206.2020.04.042 FENG X L, WEI X L, CHEN J, et al. Progress in the application of advanced oxidation technology in wastewater treatment [J]. Applied Chemical Industry, 2020, 49(4): 993-996,1001(in Chinese). doi: 10.3969/j.issn.1671-3206.2020.04.042
[3] KAUR R, KUSHWAHA J P, SINGH N. Amoxicillin electro-catalytic oxidation using Ti/RuO2 anode: Mechanism, oxidation products and degradation pathway [J]. Electrochimica Acta, 2019, 296: 856-866. doi: 10.1016/j.electacta.2018.11.114 [4] CHAPLIN B P. The prospect of electrochemical technologies advancing worldwide water treatment [J]. Accounts of Chemical Research, 2019, 52(3): 596-604. doi: 10.1021/acs.accounts.8b00611 [5] SY A, YLA B, CSA B, et al. Rapid decontamination of tetracycline hydrolysis product using electrochemical CNT filter: Mechanism, impacting factors and pathways [J]. Chemosphere, 2020, 244: 125525. doi: 10.1016/j.chemosphere.2019.125525 [6] MUIR D C, HOWARD P H. Are there other persistent organic pollutants? A challenge for environmental chemists [J]. Environmental Science & Technology, 2006, 40(23): 7157-7166. [7] LI N, ZHOU L, JIN X, et al. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework [J]. Journal of Hazardous Materials, 2019, 366: 563-572. doi: 10.1016/j.jhazmat.2018.12.047 [8] 卓琼芳, 杨波, 邓述波, 等. 用于有机物降解的电化学阳极材料 [J]. 化学进展, 2012(4): 628-636. ZHUO Q F, YANG B, DENG S B, et al. Electrochemical anodic materials used for degradation of organic pollutants [J]. Progress in Chemistry, 2012(4): 628-636(in Chinese).
[9] YANG N, YU S, MACPHERSON J V, et al. Conductive diamond: synthesis, properties, and electrochemical applications [J]. Chemical Society Reviews, 2019, 48(1): 157-204. doi: 10.1039/C7CS00757D [10] 李彩霞, 张素素, 何平, 等. 掺杂金刚石电极及电化学应用研究进展 [J]. 功能材料, 2017, 48(8): 8007-8013. LI C X, ZHANG S S, HE P, et al. Research progress of doped diamond electrode and its electrochemical application [J]. Journal of Functional Materials, 2017, 48(8): 8007-8013(in Chinese).
[11] HE Y, LIN H, GUO Z, et al. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants [J]. Separation and Purification Technology, 2019, 212: 802-821. doi: 10.1016/j.seppur.2018.11.056 [12] GONZáLEZ T, DOMíNGUEZ J, PALO P, et al. Development and optimization of the BDD-electrochemical oxidation of the antibiotic trimethoprim in aqueous solution [J]. Desalination, 2011, 280(1-3): 197-202. doi: 10.1016/j.desal.2011.07.012 [13] NIDHEESH P V, DIVYAPRIYA G, OTURAN N, et al. Environmental applications of boron-doped diamond electrodes: 1. Applications in water and wastewater treatment [J]. ChemElectroChem, 2019, 6(8): 2124-2142. doi: 10.1002/celc.201801876 [14] HE Y P, HUANG W M, CHEN R L, et al. Anodic oxidation of aspirin on PbO2, BDD and porous Ti/BDD electrodes: Mechanism, kinetics and utilization rate[J]. Separation and Purification Technology 2015, 156: 124-131. [15] 蒋欢, 王婷, 郑彤, 等. 水体中磺胺甲恶唑在BDD电极体系中的电化学氧化机理 [J]. 北京大学学报(自然科学版), 2019, 55(3): 482-488. doi: 10.13209/j.0479-8023.2019.019 JIANG H, WANG T, ZHENG T, et al. Electrochemical oxidation mechanism of sulfamethoxazole in BDD electrode system [J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2019, 55(3): 482-488(in Chinese). doi: 10.13209/j.0479-8023.2019.019
[16] ZHANG J, YU X, ZHAO Z Y, et al. Influence of pore size of Ti substrate on structural and capacitive properties of Ti/boron doped diamond electrode [J]. Journal of Alloys and Compounds, 2019, 777: 84-93. doi: 10.1016/j.jallcom.2018.10.120 [17] MA L, ZHANG M Q, ZHU C W, et al. Electrochemical oxidation of reactive brilliant orange X-GN dye on boron-doped diamond anode [J]. Journal of Central South University, 2018, 25(8): 1825-1835. doi: 10.1007/s11771-018-3872-y [18] MEI R, WEI Q, ZHU C, et al. 3D macroporous boron-doped diamond electrode with interconnected liquid flow channels: A high-efficiency electrochemical degradation of RB-19 dye wastewater under low current [J]. Applied Catalysis B:Environmental, 2019, 245: 420-427. doi: 10.1016/j.apcatb.2018.12.074 [19] 蒋绍阶, 盛贵尚, 刘静, 等. 掺硼金刚石三电极系统处理垃圾渗滤液实验研究 [J]. 环境工程学报, 2014, 8(11): 4800-4805. JIANG S J, SHENG G S, LIU J, et al. Experimental research on treatment of landfill leachate by boron-doped diamond anode in three electrodes system [J]. Chinese Journal of Environmental Engineering, 2014, 8(11): 4800-4805(in Chinese).
[20] LUU T L, TIEN T T, DUONG N B, et al. Study the treatment of tannery wastewater after biological pretreatment by using electrochemical oxidation on BDD/Ti anode [J]. Desalination and Water Treatment, 2019, 137: 194-201. doi: 10.5004/dwt.2019.23352 [21] HE Y, ZHANG P, HUANG H, et al. Electrochemical degradation of herbicide diuron on flow-through electrochemical reactor and CFD hydrodynamics simulation [J]. Separation and Purification Technology, 2020, 251: 117284. doi: 10.1016/j.seppur.2020.117284 [22] 王慧晴, 李燕, 司友斌, 等. 电催化氧化降解水体中抗生素磺胺 [J]. 环境工程学报, 2018, 12(3): 779-787. doi: 10.12030/j.cjee.201709210 WANG H Q, LI Y, SI Y B, et al. Electro-catalytic oxidative degradation of sulfonamide in water [J]. Chinese Journal of Environmental Engineering, 2018, 12(3): 779-787(in Chinese). doi: 10.12030/j.cjee.201709210
[23] 石秋俊, 刘安迪, 唐柏彬, 等. Ni掺杂Sb-SnO2瓷环粒子电极电催化氧化磺胺嘧啶 [J]. 环境科学, 2020, 41(4): 1725-1733. SHI Q J, LIU A D, TANG B B, et al. Electrocatalytic oxidation of sulfadiazine with Ni-doped Sb-SnO2 ceramic ring particle electrode [J]. Environmental Science, 2020, 41(4): 1725-1733(in Chinese).
[24] OLIVEIRA K, VEROLI A B, RUOTOLO L. Using modulated current for energy minimization in the electrochemical treatment of effluents containing organic pollutants [J]. Journal of Hazardous Materials, 2020, 399: 123053. doi: 10.1016/j.jhazmat.2020.123053 [25] GANIYU S O, MARTíNEZ-HUITLE C A. Nature, mechanisms and reactivity of electrogenerated reactive species at thin-film boron-doped diamond (BDD) electrodes during electrochemical wastewater treatment [J]. ChemElectroChem, 2019, 6(9): 2379-2392. doi: 10.1002/celc.201900159 [26] CHONG M C, LEE W, HONG S W, et al. Effects of anode materials and chloride ions on current efficiency of electrochemical oxidation of carbohydrate compounds [J]. Journal of The Electrochemical Society, 2019, 166(13): H628-H634. doi: 10.1149/2.0801913jes [27] YANG Y. Recent advances in the electrochemical oxidation water treatment: Spotlight on byproduct control [J]. Frontiers of Environmental Science & Engineering, 2020, 14(5): 85. [28] DA SILVA S W, NAVARRO E M O, RODRIGUES M A S, et al. Using p-Si/BDD anode for the electrochemical oxidation of norfloxacin [J]. Journal of Electroanalytical Chemistry, 2019, 832: 112-120. doi: 10.1016/j.jelechem.2018.10.049 [29] 王慧娴, 罗建中, 梁子豪, 等. 甲氧苄啶在有机酸络合Fe2+活化过硫酸钠体系中的降解机制 [J]. 环境化学, 2018, 37(10): 2257-2266. doi: 10.7524/j.issn.0254-6108.2017121401 WANG H X, LUO J Z, LIANG Z H, et al. Degradation mechanism of trimethoprim by organic acid chelating Fe2+-activated sodium peroxydisulfate [J]. Environmental Science, 2018, 37(10): 2257-2266(in Chinese). doi: 10.7524/j.issn.0254-6108.2017121401
[30] AMORIM K D, ROMUALDO L L, ANDRADE L S. Electrochemical degradation of sulfamethoxazole and trimethoprim at boron-doped diamond electrode: Performance, kinetics and reaction pathway [J]. Separation & Purification Technology, 2013, 120: 319-327. [31] ZHU X P, SHI S Y, WEI J J, et al. Electrochemical oxidation characteristics of p-substituted phenols using a boron-doped diamond electrode [J]. Environmental Science & Technology, 2007, 41(18): 6541-6546. -




 下载:
下载: