-
氟喹诺酮类抗生素(fluoroquinolone antibiotics,FQs)作为全球最广泛的抗生素处方药之一,被广泛应用于防治人类及动物疾病。与许多抗生素类似,FQs不能被完全吸收,30%—90%以母体形式或代谢物形式进入各类环境中,对生态系统、食品卫生和人类安全造成威胁[1-3]。有调查发现,再生水中检测到的FQs最高浓度达1190 ng·L−1,地下水中FQs的浓度为未检出—503 ng·L−1 [4]。根据美国食品和药物管理局和欧洲药品管理局,长时间接触低水平的FQs会造成长期(长达数月或数年)的严重的致残性和不可逆的药物反应,从而影响到多个器官和感官,这种情况被称为“氟喹诺酮相关的残疾”(FADS)[5]。因此,寻找有效的处理手段以高效去除FQs成为亟待解决的问题。据报道,目前许多学者已经尝试将传统水处理技术、生物降解、化学氧化法、吸附技术等用于去除抗生素[6]。然而,每种处理方法都有其局限性,如污泥寿命有限,产生有毒中间体[7],且共同的缺点是处理成本高。相比之下,吸附法因其成本低、操作简便、去除率高而成为一种高效的抗生素废水处理方法[8]。
石墨烯(graphene,G)独特的蜂窝状二维晶体结构赋予其特殊的物理特性、化学可调性,如为其碳主链的修饰或功能化提供了无限的可能性[9]。同时,石墨烯因极高的电荷载流子迁移率,已经吸引了众多学者的研究兴趣。目前有研究学者为了解决吸附剂分离回收难的问题而将磁性铁氧体和石墨烯材料复合,其中CoFe2O4因具有较高的饱和磁化强度、矫顽力、良好的机械硬度及化学稳定性等显著特性[10-11]而被广泛使用。有研究表明,在微波场中,石墨烯是良好的介电损耗型材料,而CoFe2O4属于介电损耗型和磁损耗型吸波材料[12],石墨烯和CoFe2O4结合可增加相对介电常数,改善电磁波的阻抗匹配,同时CoFe2O4和石墨烯之间的界面极化和相关弛豫对微波利用率的提高有一定贡献。在此基础上,本文通过接枝阿仑膦酸对材料进行磷酸化改性,磷酸基团特殊的磷氧四面体结构可增加其与石墨烯的接触面积,使磷酸基团负载于石墨烯表面而非边缘,同时也提高了材料表面酸性基团的数量,一定程度上增加了材料对亲水性药物的吸附量。
本文采用微波法合成磷酸化石墨烯@CoFe2O4(PG@CoFe2O4)。相较于传统加热法,微波具有绿色、安全、低耗、耗时短等优点[13],且微波辅助加热过程中大量的微波能直接与反应体系内的溶剂或试剂耦合,避免出现加热不均匀的现象[14]。基于此,本文研究PG@CoFe2O4、pH、共存离子等对FQs吸附的影响,同时结合吸附等温线和吸附动力学参数探讨了PG@CoFe2O4吸附FQs的可能的吸附机理。
-
试剂:6种氟喹诺酮类标准品:环丙沙星(CIP,83.1%)、恩诺沙星(ENR,99.7%)、左氧沙星(LEV,≥98.0%)、氟罗沙星(FLE,98.4%)、司帕沙星(SPA,99.0%)、丹诺沙星(DAN),及乙酰丙酮钴[Co(acac)2]均购于美国Sigma-Aldrich公司。将上述标准品用色谱纯甲醇制成200 mg·L−1的单标储备液,储存于4 ℃的冰箱。继续用色谱纯甲醇将单标储备液混合稀释成1.0 mg·L−1 的工作标准溶液;阿仑膦酸购于东京化成工业株式会社;石墨烯购于江苏先丰纳米材料有限公司;乙二醇[(CH2OH)2]购于上海麦克林公司;氢氧化钠(NaOH)、冰醋酸(CH3COOH)、无水乙醇(C2H5OH)、氯化钠(NaCl)、盐酸(HCl)均为分析纯,购于国药集团化学试剂有限公司;实验室用水为Milli-Q所制的超纯水;
仪器:TSQ Quantum Ultra三重四极杆超高效液相色谱质谱联用仪(LC-MS)(Quantum ultra,赛默飞世尔科技有限公司);微波合成仪(UWave-2000,上海新仪微波化学有限公司);多点磁力搅拌器(CJB-S-10D,河南爱博特科技发展有限公司);pH计(PHS-3C,上海仪电科学仪器股份有限公司);冷冻干燥箱(FD-1C-50,北京博医康实验仪器有限公司)。
-
称取40.0 mg石墨烯均匀分散于含有去离子水和乙二醇(40 mL,体积比为1∶3)的混合溶液中。准确称取0.5143 g Co(acac)2和1.0812 g FeCl3·6H2O于上述溶液中经搅拌至溶解。将上述溶液转移至四颈烧瓶中再置于微波合成仪中,在N2保护下,pH值调至10,120 ℃下反应1 h。缓慢冷却至室温,而后加入10 mL 8.0 mg·mL−1的阿仑膦酸溶液,再于80 ℃下反应30 min。缓慢冷却后于外加磁场作用下分离磁性初产品,然后用无水乙醇和去离子水交替洗涤3次。冷冻干燥后得最终磁性纳米颗粒PG@CoFe2O4,最终得到0.4801 g PG@CoFe2O4材料。
-
在室温下,定量移取含有1.0 mg·L−1 FQs的水样100 mL,调节相应的pH。加入20.0 mg PG@CoFe2O4的同时开始计时。磁力搅拌一段时间后,移取1 mL上述溶液经0.45 μm滤膜过滤于液质瓶中,经LC-MS进行定量分析。上述实验重复3次。去除率Y(%)和吸附容量q(mg·g−1)计算公式如下:
式中,C0为溶液初始FQs浓度(mg·L−1),Ce为溶液平衡时FQs浓度(mg·L−1),V为溶液体(L),m为吸附剂质量(g),q为PG @CoFe2O4对FQs的吸附量(mg·g−1),Y为去除率(%)。
-
利用LC-MS对FQs进行定量分析。色谱柱:Zorbax Eclipse XDB-C18反相色谱柱(3.0 mm×50 mm,1.8 μm);流动相:0.1%的甲酸(A)和甲醇(B),梯度洗脱程序:0 min—1.0 min 10% B—75% B;1.0 min—6.0 min 75% B;6.0 min—6.5 min 75% B—10% B;6.5 min—9.0 min 10% B;柱温:30 ℃;流速为:0.2 mL·min−1;进样量为3.0 μL。
-
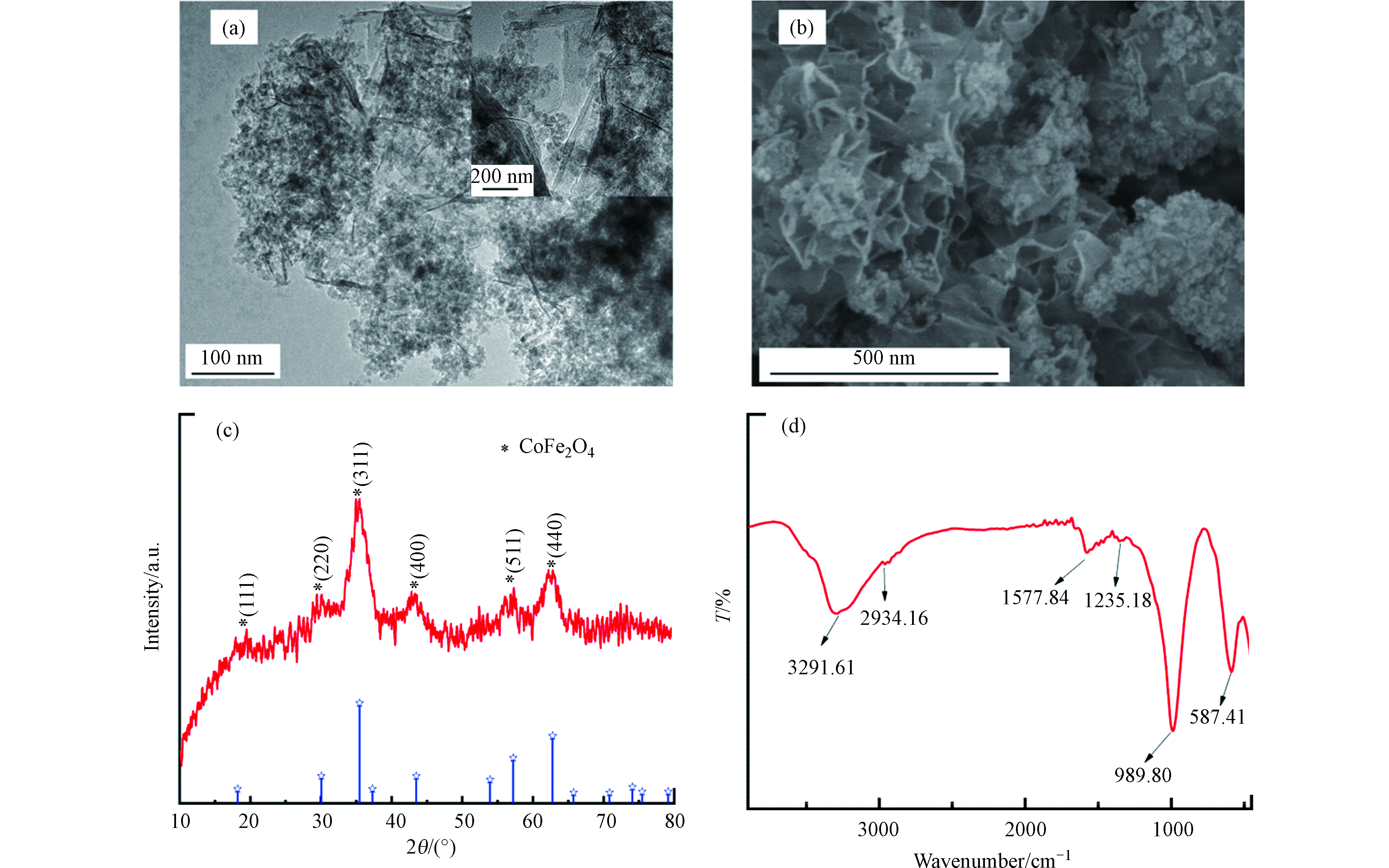
利用扫描电镜(SEM)对PG@CoFe2O4的微观形貌进行表征,结果见图1a。从图1a可看出,石墨烯的褶皱状片层结构,说明负载磁性粒子不会破坏石墨烯的结构。CoFe2O4纳米粒子较均匀地分散在磷酸化石墨烯的表面和片层之间,CoFe2O4纳米粒子未发生明显团聚现象。磷酸功能化磁性石墨烯的表面粗糙程度有利于其对FQs的吸附。图1b透射电镜(TEM)的表征结果。从图1b可以看出,石墨烯边缘存有褶皱,CoFe2O4较均匀地分散在石墨烯上。此外,片层结构较薄,可能是磷酸基团的引入避免了片与片之间的堆积。
PG@CoFe2O4的XRD谱图如图1c所示。从图1c中可以观察到,在2θ=18.5°、30.1°、35.4°、43.1°、57.1°、62.6°等处出现了CoFe2O4的特征衍射峰,分别对应于CoFe2O4的(111)、(220)、(311)、(400)、(511)、(440)立方尖晶石晶面,与CoFe2O4的标准图谱(JCPDS22—1086)基本吻合。故X-射线衍射仪的分析结果可初步说明本实验CoFe2O4成功被负载在石墨烯上。
图1d是PG@CoFe2O4的FTIR图谱。PG@CoFe2O4在3291.61cm−1处存在一个宽且强的吸收峰,这是水分子中O—H的伸缩振动峰所致。PG@CoFe2O4在1577.84 cm−1和2934.16 cm−1处的特征峰归属于石墨分子碳骨架结构中的C=C的伸缩振动和C—H拉伸振动峰。磷酸基团的引入,使PG@CoFe2O4在989.80 cm−1和1235.18 cm−1出现了P—O和P=O的伸缩振动峰[15]。同时,在587.41 cm−1处出现了尖锐的吸收峰,该峰由Co—O或 Fe—O的振动产生,说明CoFe2O4已负载于GO表面[16]。
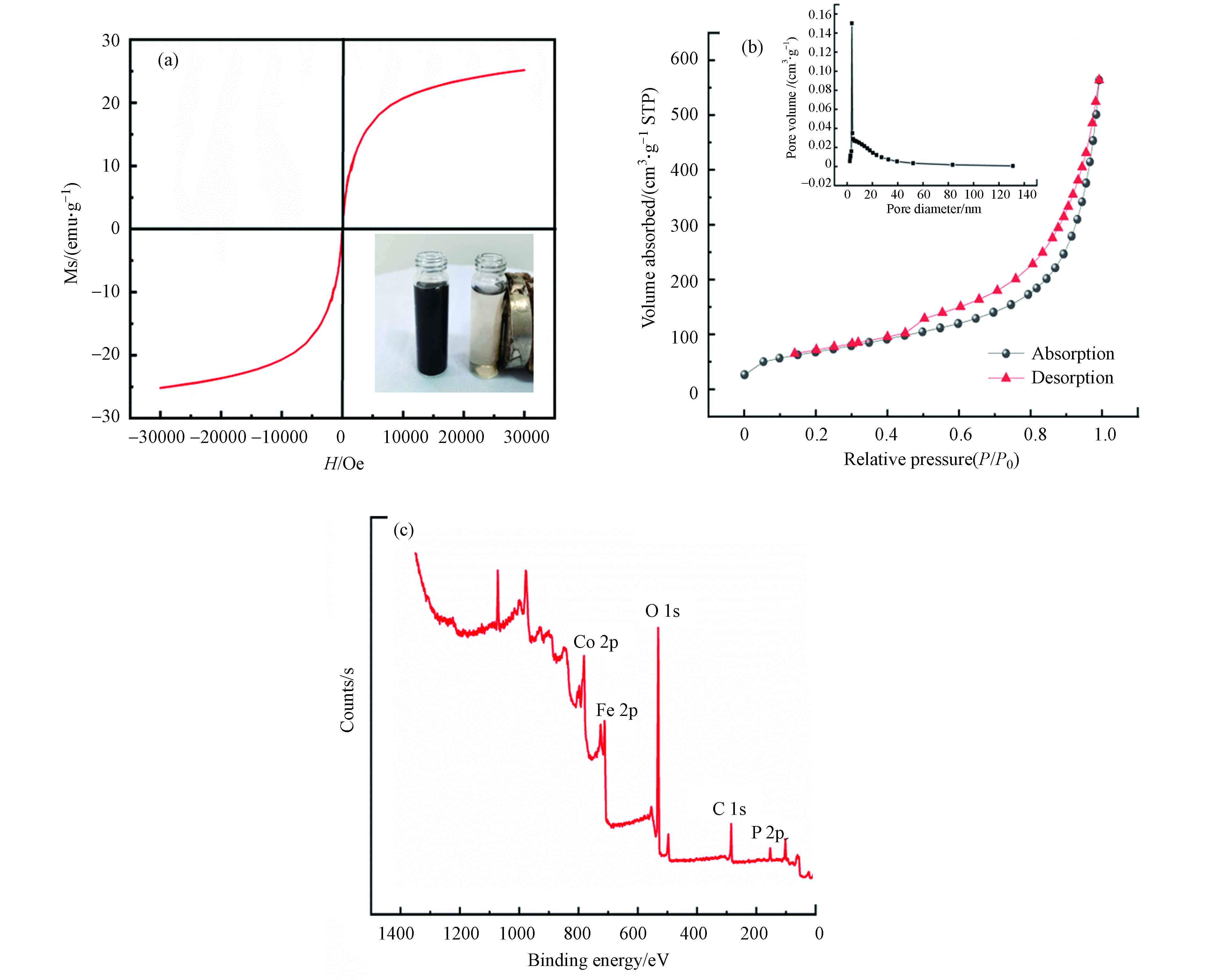
图2a是PG@CoFe2O4的磁化强度曲线。右下角插图是PG@CoFe2O4分散于水中被磁铁吸引的前后情况。在室温条件下,PG@CoFe2O4的磁化强度随着外加磁场强度的增加而增加。但到了一定程度后,PG@CoFe2O4的磁化强度趋于平稳,最终达到饱和磁化强度为25.18 emu·g−1
通过BET比表面积测试法得到了PG@CoFe2O4的N2吸附-脱附等温线,如图2b所示。PG@CoFe2O4的N2吸脱附曲线存在明显的迟滞环,表明其属于标准的Ⅳ型介孔材料的等温线。PG@CoFe2O4的比表面积和孔体积分别为250.20 m2·g−1和0.87 cm3·g−1,这不仅提供更多的活性位点,也增加了吸附剂与目标污染物的接触机会,故PG@CoFe2O4表现出优良的吸附效果。同时,PG@CoFe2O4的较高比表面积提供更多丰富的活性位点用于电磁波的有效反射和散射,促进了电磁波的多重吸收过程。
图2c是PG@CoFe2O4的XPS谱图。从图2c中可看出,C、O、Fe、Co及P这5个元素的特征峰值分别出现在284.4、530.78、711.3、780.96、132.68 eV处,证明了PG@CoFe2O4材料合成成功。根据XPS结果计算出C、O、Fe、Co及P的含量分别为30.69%、51.25%、8.33%、5.66%和2.33%。C 1s特征峰的出现说明了石墨烯与CoFe2O4复合成功,P 2p特征峰的出现说明成功对材料进行了磷酸化。
-
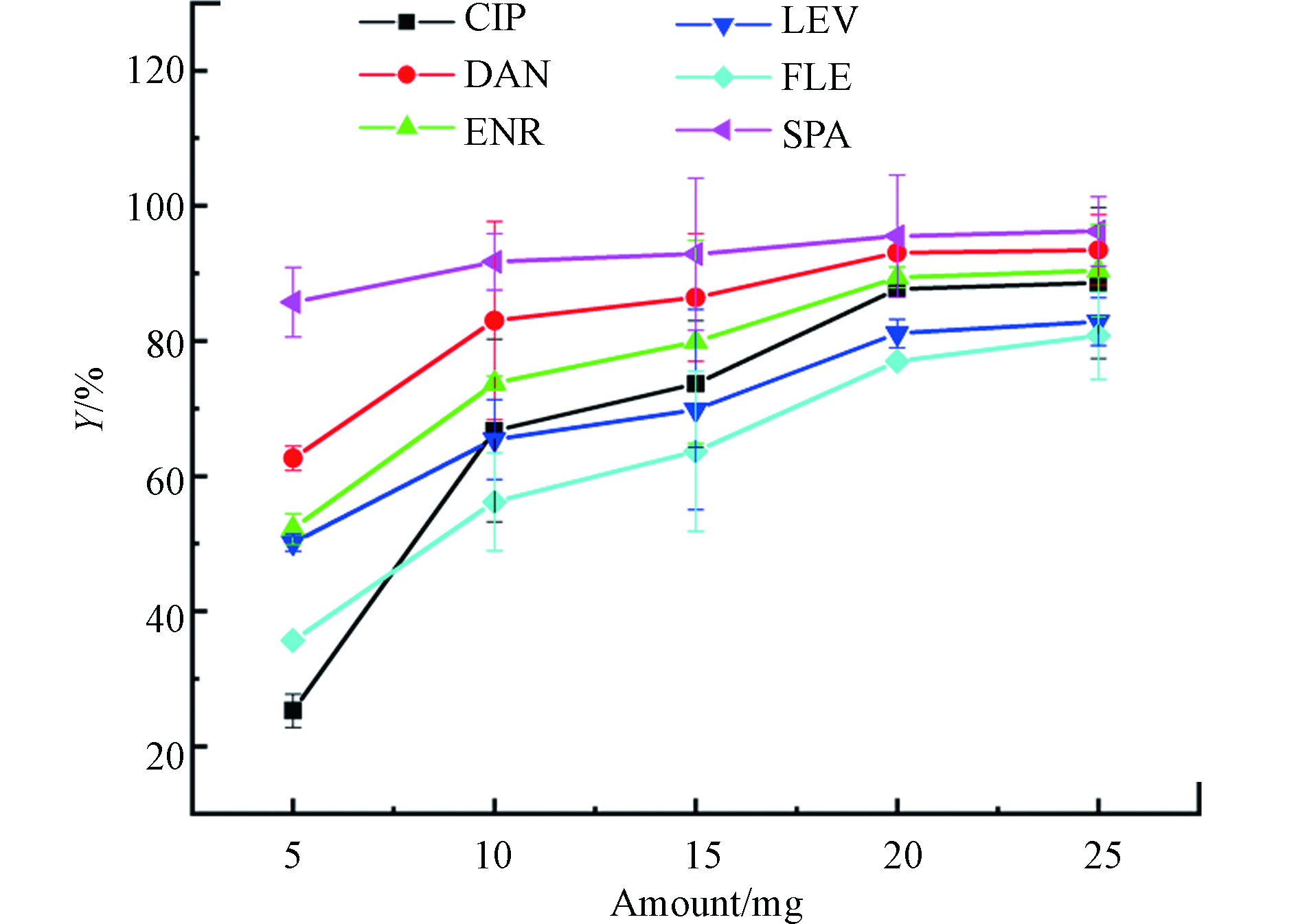
图3为不同PG@CoFe2O4投加量对吸附性能的影响。结果表明,当投加量从5.0 mg增加到20.0 mg时,FQs的去除率不断增加,这是因为随着吸附剂的增加,吸附位点也相应增多,从而FQs与PG@CoFe2O4的接触机率增加。当吸附剂投加量从20.0 mg增至25.0 mg时,去除率没有明显的增加。因此,考虑到PG@CoFe2O4对FQs的去除率和经济效益,选择20.0 mg作为后续实验吸附剂用量。
-
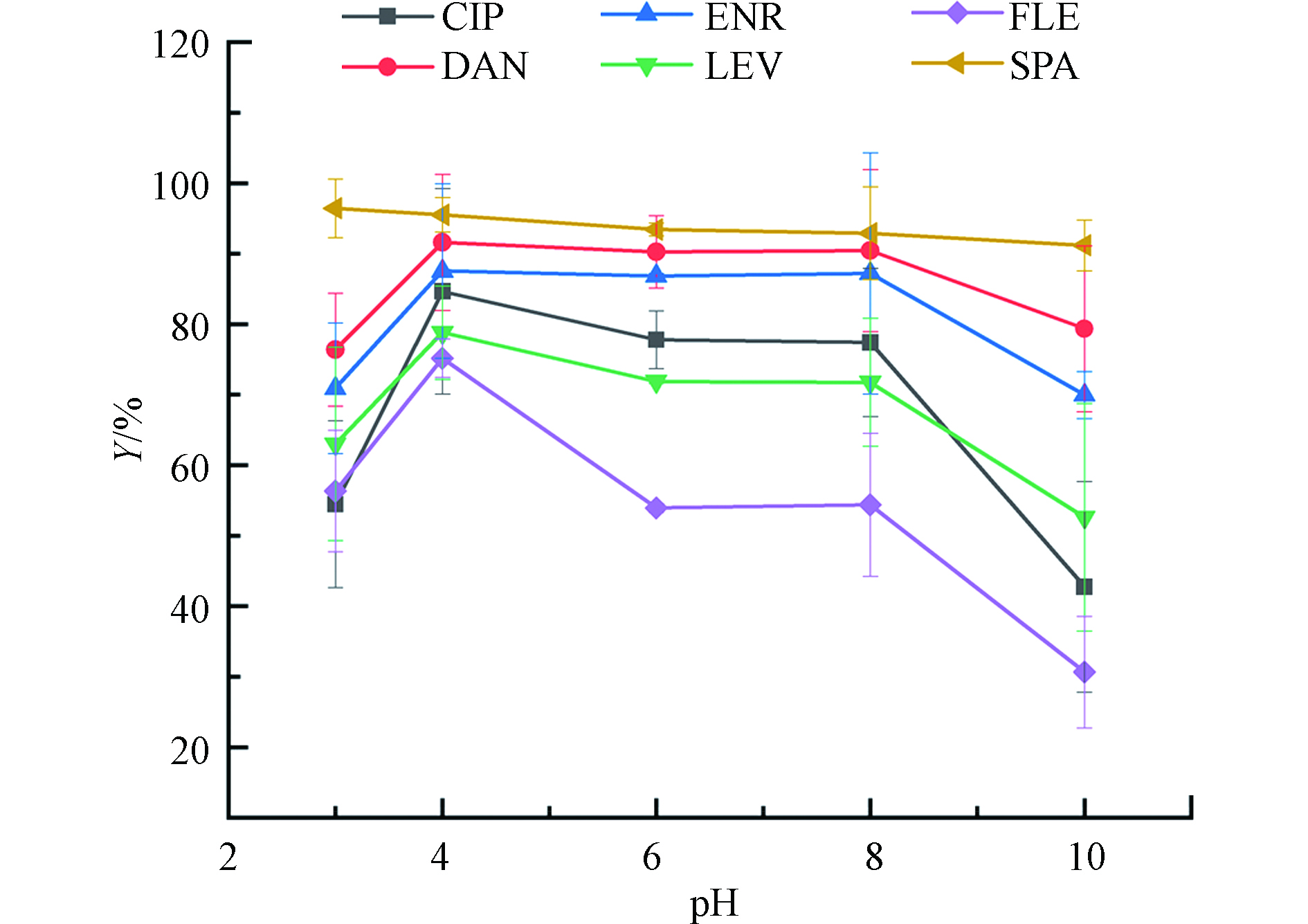
溶液的pH可能会影响污染物的存在形态,以及改变吸附剂的表面电荷,这可能会对吸附性能有很大的影响,因此有必要研究不同pH下FQs的去除情况。图4为溶液初始pH(2.0—10.0)对PG@CoFe2O4吸附FQs造成的影响结果。从图4中可看出,pH为4.0时,PG@CoFe2O4对6种FQs的去除率达到最大化,分别为84.7%、91.6%、87.5%、78.8%、75.2%和95.2%。一般来说,FQs通常有两个pKa值,CIP、LEV、FLE、SPA、ENR和DAN的pKa1为6.14、5.33、5.46、6.09、6.20和6.18,pKa2分别为8.85、8.07、8.00、8.79、8.13和8.78。
当pH<4.0时,溶液中
${\rm{H}}^+ $ 较多,PG@CoFe2O4表面的活性位置更多的被${\rm{H}}^+$ 占据,导致吸附效果不佳。由于阿仑膦酸中双磷酸基团的引入,使得PG@CoFe2O4表面负电荷偏多。在pH=4.0的溶液中,磷酸基团发生水解,增加了离子交换的几率,同时FQs以阳离子形式存在,其中氟原子、胺基等强吸电子基团与磷酸基团发生静电吸引作用而使去除率达到最高水平。当pH在6.0—8.0范围内时,以阳离子形式存在的FQs含量减少,而兼性离子含量增加。FQs抗生素所特有的基团(—F、—OH)都是很好的吸电子基团,而随着pH的升高,特有基团去质子化的程度增加,电子接收能力降低,FQs与PG@CoFe2O4中石墨化结构(给电子体)之间π-π电子供体受体作用受到抑制[17],故FQs的吸附效果下降。而从图中看出FLE的吸附效果下降明显,是因为FLE结构中含有两个氟原子,诱导力较强。8.0<pH<10.0时,FQs主要以阴离子形式存在,静电斥力导致吸附效果降低。 -
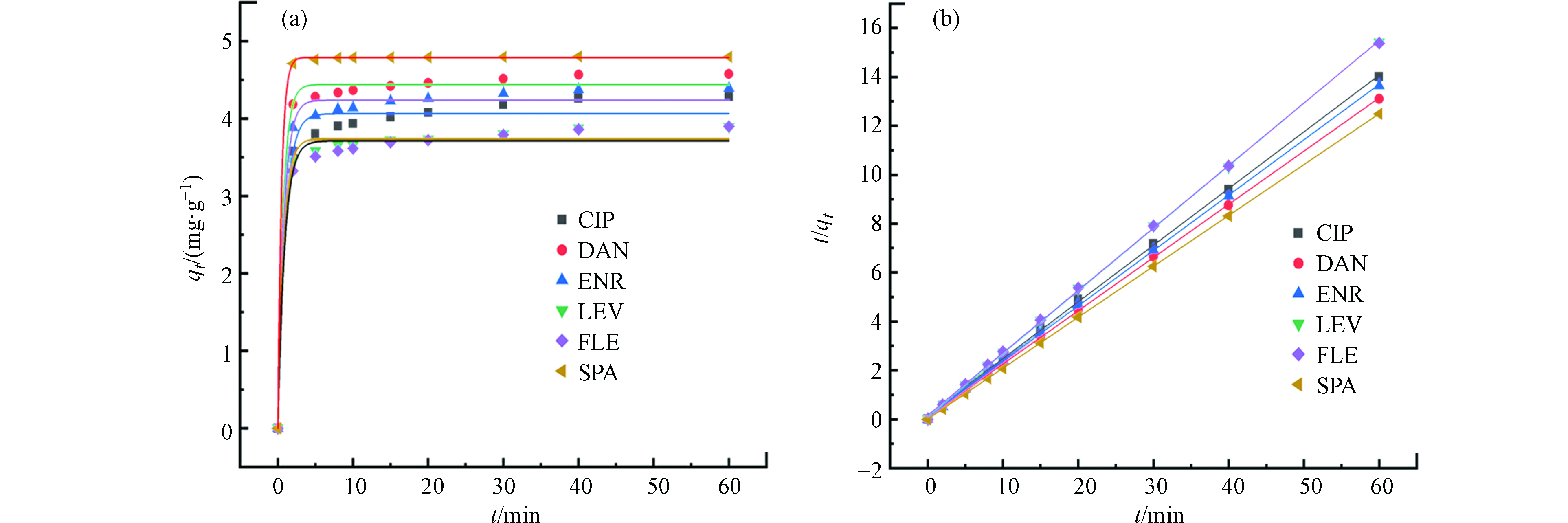
为了研究PG@CoFe2O4对FQs的吸附动力学特性,本文选用准一级动力学方程(3)和准二级动力学方程(4)对吸附数据进行动力学拟合,得到准一级拟合曲线和准二级拟合曲线(图5)。
式中,Qt和Qe分别为吸附时间为t和吸附达到平衡时的吸附量(mg·g−1);k1为准一级吸附速率常数(min−1);k2为准二级吸附速率常数(g·(mg·min)−1)。
一般认为R2越接近1该模型与实验结果越符合。表1显示,准一级模型和准二级模型均能较好地描述吸附过程,但准二级模型(0.9996—0.9998)比准一级模型(0.9795—0.9999)具有更高的相关系数,且准二级模型的qe值和实验值相似,说明PG@CoFe2O4吸附FQs的机理更符合准二级模型,即吸附过程主要以化学吸附为主,物理吸附为辅[18],该结果与文献报道的ENR[19]、CIP[20]等拟合结论一致。
-
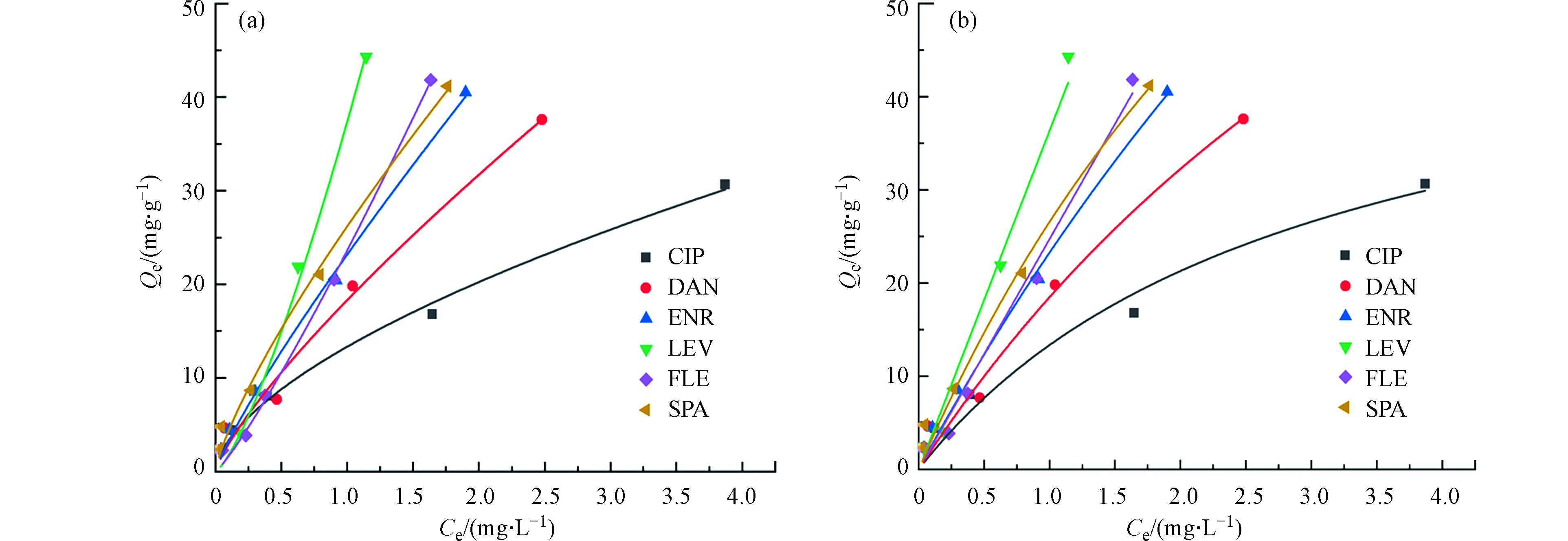
Freundlich模型解释的是不均匀表面上的多层物理吸附,而Langmuir模型解释的是单层均匀表面上的吸附[21]。本文分别利用Freundlich和Langmuir模型考察了PG@CoFe2O4对FQs的吸附行为。Freundlich模型和Langmuir模型方程如下:
式中,Qe为PG@CoFe2O4对FQs的平衡吸附量(mg·g−1),Ce为吸附平衡时溶液中FQs浓度(mg·L−1),KF为Freundlich模型常数,n为吸附强度常数,KL为Langmuir模型常数,Qm为饱和吸附容量(mg·g−1)。
图6和表2分别给出了Freundlich模型和Langmuir模型及各参数拟合结果。在本研究中,Freundlich模型较好地拟合了实验数据,说明本实验的吸附过程存在物理吸附,与已报道的文献结论一致[22-23]。研究表明,Freundlich常数0.1<1/n <0.5时,表明吸附容易发生;n=1时为线性吸附;1/n>2时,表明吸附较难[24]。Freundlich模型中环丙、丹诺、恩诺、司帕的1/n值均小于1,左氧和氟罗1/n值分别为1.33和1.16均小于2,说明6种FQs在PG@CoFe2O4表面的多相多层吸附过程是简单有利的。通过Freundlich模型得出PG@CoFe2O4对6种FQs吸附能力顺序为LEV>FLE>ENR>SPA>DAN>CIP,由于6种FQs不同的辛醇水分配系数(log Kow)疏水分配作用会导致PG@CoFe2O4对6种FQs产生不同的吸附效果。有研究也发现,由于ENR和CIP的log Kow分别为0.54和-0.86,故导致ENR和CIP的最大吸附容量存在较大差异[25],也验证了本实验的结果。
-
在已处理的废水和自然水体等现实水体中,存在
${{\rm{HCO}}}_3^- $ 、${\rm{SO}}_4^{2-} $ 、${\rm{Cl}}^{-}$ 等各种无机阴离子、腐殖酸、蛋白质、碳水化合物等溶解性有机物以及金属离子。它们的存在可能会竞争性的占据吸附位点,从而一定程度上影响吸附效果,因此考察了其对FQs去除效率的影响。 -
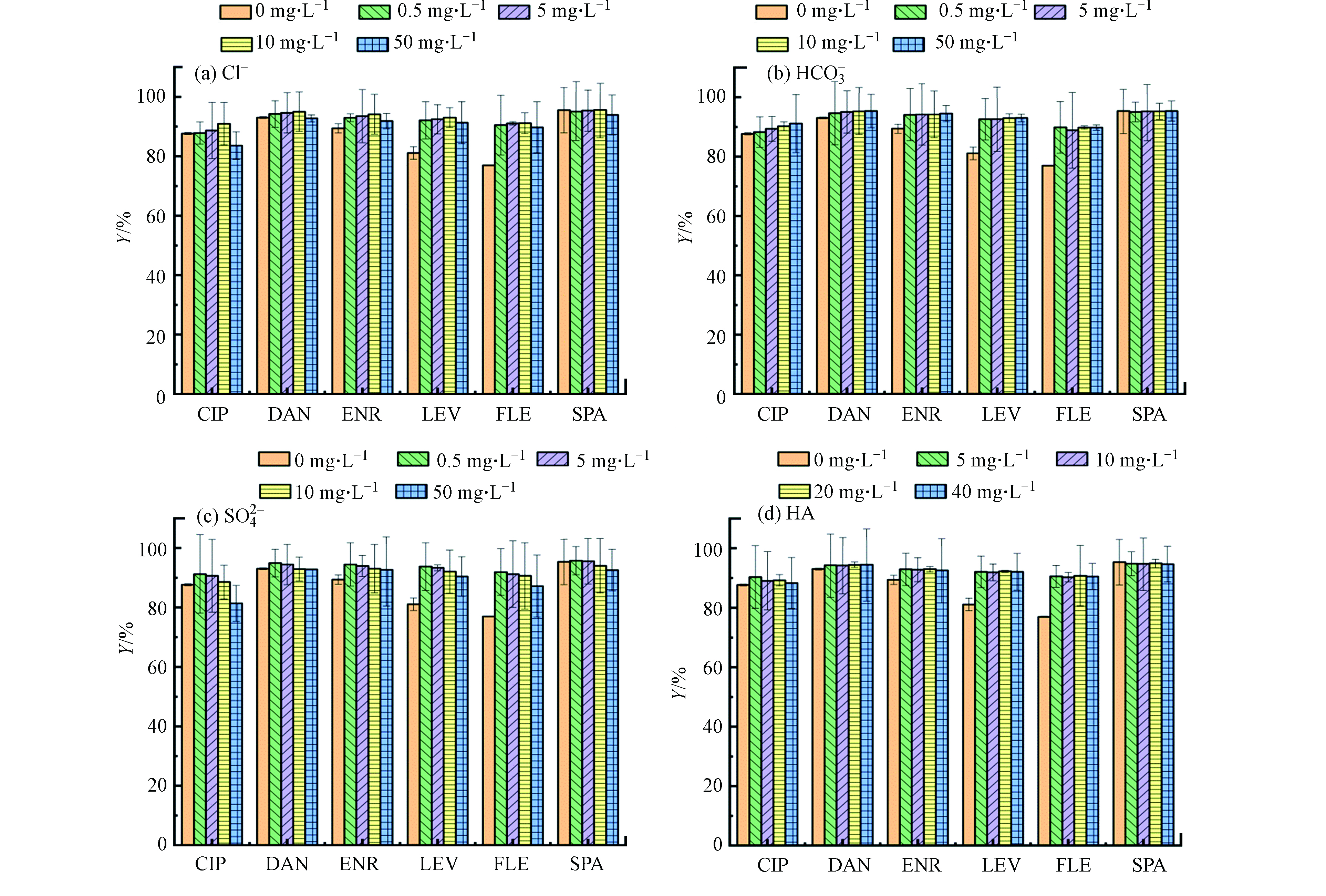
在溶液中加入NaCl(0—50 mg·L−1)并进行一系列批次试验。如图7a所示,随着离子强度从0 mg·L−1增加到10 mg·L−1,CIP、DAN、ENR、LEV、FLE和SPA的去除率呈上升趋势,分别提高了3.6%、2.1%、4.8%、11.9%、14.1%和0.1%,这可以归因于静电屏蔽效应和盐析效应[25-26]。首先,随着离子强度的增加,静电排斥力被屏蔽,因此吸附能力增强。其次,盐析效应可能是吸附的另一个原因,这可促进FQs向PG@CoFe2O4材料表面扩散,最终去除率得到提升。然而当NaCl浓度升至50 mg·L−1时,吸附去除率略有下降,可能是高浓度的Na+会中和材料表面的负电荷抑制静电吸引作用。这一结果也证明了静电相互作用是吸附机理之一。
图7b和7c显示了共存阴离子
${\rm{HCO}}_3^{-} $ 和${\rm{SO}}_4^{2-} $ 对FQs吸附去除率的影响。结果表明,在浓度(0—50 mg·L−1)情况下,${\rm{HCO}}_3^{-} $ 几乎不影响FQs吸附去除率,但高浓度的${\rm{HCO}}_3^{-} $ 对FLE的去除产生一定的抑制作用。高浓度${\rm{SO}}_4^{2-}$ 的存在对PG@CoFe2O4吸附FQs的影响总体呈一定的抑制趋势。也就是说,电荷是阴离子影响关键参数,${\rm{SO}}_4^{2-} $ 带两个负电荷多于${\rm{HCO}}_3^{-} $ 所带电荷,对周围吸附位点电荷的分布影响更大,从而减弱FQs与PG@CoFe2O4之间的静电引力。 -
水体中70%的有机质由腐殖酸(HA)组成,因此选择腐殖酸为代表来研究对FQs吸附去除率的影响。图7d展示了吸附去除率随HA浓度变化的情况,结果显示即使在高浓度的HA情况下,PG@CoFe2O4仍对FQs有着较高的吸附性能,因此可以说HA几乎不干扰PG@CoFe2O4对FQs的吸附,仅显示出一定的抑制作用。有文献报道,HA可以通过疏水作用及π-π作用被吸附到化学还原石墨烯上[27],进而和FQs在PG@CoFe2O4上发生竞争吸附,这就对吸附去除率有所下降进行了很好的说明。
-
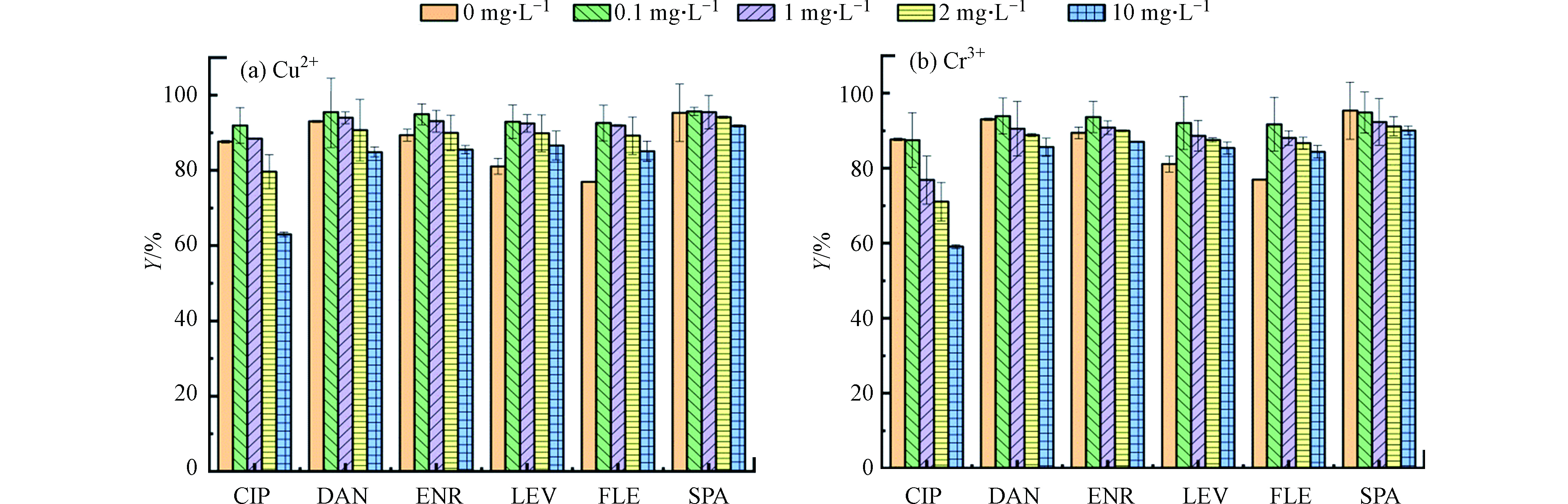
本研究选择了典型的重金属离子(Cu2+和Cr3+)作为影响离子。图8a和8b分别出示了Cu2+和Cr3+对吸附去除FQs的影响。如结果所示,Cu2+和Cr3+的存在对FQs去除的影响相似,均表现为低浓度促进FQs的吸附,随着浓度的增加FQs去除率逐渐降低,这与其他报道的体系中金属阳离子对抗生素的吸附能力有明显的促进不同[28-29]。
有文献报道[30],在吸附过程中,金属离子会从以下几个方面对FQs的吸附产生影响:(1)离子强度变化;(2)金属离子吸附在吸附剂表面[31];(3)在吸附剂与FQs之间形成阳离子桥接[32];(4)FQs中—F、—COOH及C=O容易和金属离子形成重金属离子—FQs络合物[33]。从本实验得到的结果来看,(2)和(4)用来描述金属离子影响FQs吸附的过程更为贴切。首先,当Cu2+和Cr3+在溶液中浓度较低时,竞争作用较弱,该环境下其主要和FQs络合生成络合物,从而促进FQs在PG@CoFe2O4表面的吸附。其次,Cu2+会形成内球配合物优先与吸附剂结合,从而与FQs竞争吸附位点,因此随着Cu2+和Cr3+浓度的增加,FQs的吸附去除受到了抑制。一些研究通过Cu2+和CIP的较高络合常数发现,Cu2+存在时可显著改变CIP的环境行为,即Cu2+ 会和CIP形成络合物而促进亦或抑制CIP的吸附[34],而本实验的显著改变体现在CIP的吸附受到显著抑制。
-
自然水体中存在阴离子(Cl−、
${\rm{SO}}_4^{2-} $ 等)、阳离子(${\rm{NH}}_4^{+} $ 、K+、Ca2+、Mg2+、Na+等)、腐殖酸等,本文着重研究实际水样中Cl−、${\rm{SO}}_4^{2-} $ 、Cu2+、Cr3+对FQs吸附效果的影响。为了研究PG@CoFe2O4在实际水样中的应用性,本研究选择苏州地区的两处地表水样作为样品。通过加标的方法(加标浓度为1.0 mg·L−1),在上述实验得到的最佳条件下进行吸附试验。从表3的数据可以看出,对太湖和石湖两种实际水样中6种FQs的去除率均在90%以上,说明天然水中的共存物质(Cl−、${\rm{SO}}_4^{2-} $ 等)通过协同作用有助于PG@CoFe2O4吸附FQs。从实验结果看出,本文制备的PG@CoFe2O4可作为高效吸附剂用于实际生产生活中FQs的去除。 -
(1)本文采用微波辐射法合成了PG@CoFe2O4用于吸附环境水体中的6种FQs。实验数据表明,在室温条件下,PG@CoFe2O4投加量为20.0 mg,溶液pH 4.0时,PG@CoFe2O4能在60 min内对6种FQs去除率达到最高水平,DAN、CIP、ENR、SPA、FLE和LEV的最大去除率分别为91.6%、84.7%、87.5%、95.5%、75.2%和78.8%。
(2)准二级动力学模型和Freundlich模型能较好地拟合吸附过程,说明吸附过程是以化学吸附为主,物理吸附为辅的多相吸附过程。Cl−对吸附有双重作用,
${\rm{HCO}}_3^{-} $ 、${\rm{SO}}_4^{2-} $ 和HA对吸附的干扰作用较小,而Cu2+和Cr3+抑制了吸附过程。(3)鉴于pH影响因素实验,静电作用对FQs在PG@CoFe2O4上的吸附起着关键作用。其次,PG@CoFe2O4的片状褶皱结构及大的脱位π-电子系统,同时FQs分子中存在吸电子能力极强的氟基团、芳香环和不饱和结构,这使得π-π电子供体-受体作用和孔道填充效应成为吸附机理之一。再者,FQs吸附实验中也伴随有氢键作用。
微波法制备磷酸化石墨烯@CoFe2O4及其对氟喹诺酮类抗生素的吸附
Synthesis of phosphorylated graphene@CoFe2O4 by microwave method for the adsorption of fluoroquinolone antibiotics
-
摘要: 本研究通过微波法制备了磷酸功能化磁性石墨烯(PG@CoFe2O4)材料,并用其吸附水中6种氟喹诺酮类抗生素(FQs)。采用扫描电镜、透射电镜、X射线衍射、磁滞回线等对复合材料进行系统的表征。系统考察了
${{\rm{Cl}}^ - } $ 、${{{\rm{HCO}}}_3^-} $ 、${{\rm{SO}}_4^{2-}}$ 和腐殖酸(HA)等对FQs吸附的影响。实验结果显示,在pH=4.0的情况下,PG@CoFe2O4投加量20.0 mg,30 min内6种目标物的去除率最高可达到95.5%。低浓度${{\rm{Cl}}^ - } $ 促进吸附而高浓度则抑制吸附,${{{\rm{HCO}}}_3^-} $ 、${{\rm{SO}}_4^{2-}} $ 和HA几乎不影响吸附过程,而${{\rm{Cr}}^{3+} } $ 和${{\rm{Cu}}^{2+} } $ 抑制FQs的吸附。准一级动力学模型和Freundlich模型拟合效果较好,说明吸附过程以化学吸附为主。两种实际水样中FQs的去除率均高达89.6%以上。实验表明,PG@CoFe2O4对含有氨基和卤素基团的污染物存在特异性吸附。Abstract: Phosphorylated magnetic graphene (PG@CoFe2O4) were fabricated by microwave radiation method and used to adsorb six fluoroquinolone antibiotics (FQs) in water. The composites were systematically characterized by X-ray diffraction (XRD), Fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The effects of${{\rm{Cl}}^ - } $ ,${{{\rm{HCO}}}_3^-} $ ,${{\rm{SO}}_4^{2-}} $ and humic acid (HA) on the adsorption of FQs were systematically investigated. The experimental results showed that the maximum removal efficiency of six targets could recah up to 95.5% within 30 min based on the condition of pH=4.0, PG@CoFe2O4 20.0 mg. Also, low concentrations of${{\rm{Cl}}^ - } $ promoted adsorption while high concentrations inhibited adsorption,${{{\rm{HCO}}}_3^-} $ ,${{\rm{SO}}_4^{2-}} $ and HA hardly affected the adsorption process, while${{\rm{Cr}}^{3+} } $ and${{\rm{Cu}}^{2+} } $ inhibited the adsorption of FQs. Besides, the quasi-first-order kinetic model and Freundlich model fit well, indicating that the adsorption process is dominated by chemisorption. The removal efficiency of FQs in both actual water samples were above 89.6%. The experiments showed that the PG@CoFe2O4 was specific for the adsorption of pollutants containing amino and halogen groups. -

-
表 1 FQs在PG@CoFe2O4上的吸附动力学参数
Table 1. Kinetic parameters for adsorption of FQs on PG@CoFe2O4
FQs 拟一级动力学模型 Quasi-first-order kinetic model 拟二级动力学模型 Quasi-second-order kinetic model qe/(mg·g−1) k1/min−1 R2 qe/(mg·g−1) k2/(g·(mg·min)−1) R2 CIP 4.0657 1.0325 0.9846 4.3154 0.3307 0.9995 DAN 4.4430 1.4097 0.9948 4.5956 0.5816 0.9998 ENR 4.2407 1.2265 0.9923 4.4163 0.4939 0.9998 LEV 3.7409 1.2737 0.9924 3.9079 0.5058 0.9997 FLE 3.7134 1.1053 0.9885 3.9162 0.4086 0.9996 SPA 4.7926 2.0622 0.9999 4.8054 7.0416 1.0000 表 2 FQs在PG@CoFe2O4上的吸附等温线拟合参数
Table 2. Adsorption isotherm fitting parameters for adsorption of FQs on PG@CoFe2O4
FQs Freundlich Langmuir KF 1/n R2 qm/(mg·g−1) KL R2 CIP 0.2069 0.6029 0.9938 52.7382 0.3381 0.9602 DAN 0.0766 0.7927 0.9795 126.5833 0.1708 0.9756 ENR 0.0637 0.8536 0.9956 213.9142 0.1219 0.9909 LEV 0.0039 1.3270 0.9880 413.9253 0.0002 0.9474 FLE 0.0079 1.1575 0.9965 315.8589 0.0003 0.9865 SPA 0.1192 0.7805 0.9895 141.8438 0.2302 0.9814 表 3 两种实际水样对FQs在PG@CoFe2O4上吸附的影响
Table 3. Influence of two actual water samples on FQs adsorption on PG@CoFe2O4
实际水样
Actual water samples基础数据/(mg·L−1)
Basic data去除率/%
Removal rateCIP DAN ENR LEV FLE SPA 水样1
Water sample 1TOC 12.95 89.62 94.49 93.48 93.85 94.99 95.91 ${\rm{SO}}_4^{2-} $ 46.39 Cl- 46.39 Cr3+ — Cu2+ — 水样2
Water sample 2TOC 10.51 89.83 94.55 93.62 93.85 94.97 96.12 ${\rm{SO}}_4^{2-} $ 50.89 Cl- 35.51 Cr3+ — Cu2+ — -
[1] CHEN J, YING G G, DENG W J. Antibiotic residues in food: Extraction, analysis, and human health concerns [J]. Journal of Agricultural and Food Chemistry, 2019, 67(27): 7569-7586. doi: 10.1021/acs.jafc.9b01334 [2] KOVALAKOVA P, CIZMAS L, MCDONALD T J, et al. Occurrence and toxicity of antibiotics in the aquatic environment: A review [J]. Chemosphere, 2020, 251: 126351. doi: 10.1016/j.chemosphere.2020.126351 [3] FELSEN C B, DODDS ASHLEY E S, BARNEY G R, et al. Reducing fluoroquinolone use and Clostridioides difficile infections in community nursing homes through hospital-nursing home collaboration [J]. Journal of the American Medical Directors Association, 2020, 21(1): 55-61. doi: 10.1016/j.jamda.2019.11.010 [4] DING G Y, CHEN G L, LIU Y D, et al. Occurrence and risk assessment of fluoroquinolone antibiotics in reclaimed water and receiving groundwater with different replenishment pathways [J]. Science of the Total Environment, 2020, 738: 139802. doi: 10.1016/j.scitotenv.2020.139802 [5] RICHARDS G A, BRINK A J, FELDMAN C. Rational use of the fluoroquinolones [J]. South African Medical Journal, 2019, 109(6): 378-381. doi: 10.7196/SAMJ.2019.v109i6.14002 [6] YANG W J, FULIDONG F, MA J W, et al. Research progress of antibiotic pollution and treatment technologies in China [J]. E3S Web of Conferences, 2020, 194: 04004. doi: 10.1051/e3sconf/202019404004 [7] TZENG T W, LIU Y T, DENG Y, et al. Removal of sulfamethazine antibiotics using cow manure-based carbon adsorbents [J]. International Journal of Environmental Science and Technology, 2016, 13(3): 973-984. doi: 10.1007/s13762-015-0929-4 [8] LUO J W, LI X, GE C J, et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems [J]. Bioresource Technology, 2018, 263: 385-392. doi: 10.1016/j.biortech.2018.05.022 [9] ZHU Y W, MURALI S, CAI W W, et al. Graphene and graphene oxide: Synthesis, properties, and applications [J]. Advanced Materials, 2010, 22(35): 3906-3924. doi: 10.1002/adma.201001068 [10] EL-SHOBAKY G A, TURKY A M, MOSTAFA N Y, et al. Effect of preparation conditions on physicochemical, surface and catalytic properties of cobalt ferrite prepared by coprecipitation [J]. Journal of Alloys and Compounds, 2010, 493(1/2): 415-422. [11] ATI A, OTHAMAN Z, SAMAVATI A. Influence of cobalt on structural and magnetic properties of nickel ferrite nanoparticles [J]. Journal of Molecular Structure, 2013, 1052: 177-182. doi: 10.1016/j.molstruc.2013.08.040 [12] 匡嘉敏. CoFe2O4/rGO复合纳米材料的制备及其性能研究[D]. 吉林: 东北电力大学, 2017. KUANG J M. Study on the preparation and properties of cobalt ferrite/reduced graphene oxide nanocomposites[D]. Jilin, China: Northeast Dianli University, 2017(in Chinese).
[13] MENG L Y, WANG B, MA M G, et al. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials [J]. Materials Today Chemistry, 2016, 1/2: 63-83. doi: 10.1016/j.mtchem.2016.11.003 [14] RATHI A K, GAWANDE M B, ZBORIL R, et al. Microwave-assisted synthesis – Catalytic applications in aqueous media [J]. Coordination Chemistry Reviews, 2015, 291: 68-94. doi: 10.1016/j.ccr.2015.01.011 [15] 张志宾, 张昊岩, 邱燕芳, 等. 磷酸化石墨烯吸附铀的性能研究 [J]. 中国科学:化学, 2019, 49(1): 195-206. doi: 10.1360/N032018-00153 ZHANG Z B, ZHANG H Y, QIU Y F, et al. Adsorption of uranium by phosphorylated graphene oxide [J]. Scientia Sinica (Chimica), 2019, 49(1): 195-206(in Chinese). doi: 10.1360/N032018-00153
[16] LU X F, YANG L, BIAN X J, et al. Rapid, microwave-assisted, and one-pot synthesis of magnetic palladium-CoFe2O4 -graphene composite nanosheets and their applications as recyclable catalysts [J]. Particle & Particle Systems Characterization, 2014, 31(2): 245-251. [17] 戴江栋. 多孔碳基材料的可控制备及其高效分离抗生素行为和机理研究[D]. 镇江: 江苏大学, 2016. DAI J D. Controlled preparation of porous carbon-based materials and study on behavior and mechanism of high-efficiency antibiotic separation[D]. Zhenjiang, China: Jiangsu University, 2016(in Chinese).
[18] BULUT E, OZACAR M, SENGIL I A. Equilibrium and kinetic data and process design for adsorption of Congo Red onto bentonite [J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 613-622. [19] CHOWDHURY S, SIKDER J, MANDAL T, et al. Comprehensive analysis on sorptive uptake of enrofloxacin by activated carbon derived from industrial paper sludge [J]. The Science of the Total Environment, 2019, 665: 438-452. doi: 10.1016/j.scitotenv.2019.02.081 [20] ZHAO J, LIANG G W, ZHANG X L, et al. Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water [J]. The Science of the Total Environment, 2019, 688: 1205-1215. doi: 10.1016/j.scitotenv.2019.06.287 [21] TANG L, YU J F, PANG Y, et al. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal [J]. Chemical Engineering Journal, 2018, 336: 160-169. doi: 10.1016/j.cej.2017.11.048 [22] 周俊, 李燕, 管益东, 等. 杨木生物炭对水溶液中3种磺胺类抗生素的混合吸附 [J]. 环境工程, 2021, 39(3): 1-6,13. doi: 10.13205/j.hjgc.202103001 ZHOU J, LI Y, GUAN Y D, et al. Mixed sorption of three aqueous sulfonamides onto the biochar derived from poplar wood chips [J]. Environmental Engineering, 2021, 39(3): 1-6,13(in Chinese). doi: 10.13205/j.hjgc.202103001
[23] HUANG P, GE C J, FENG D, et al. Effects of metal ions and pH on ofloxacin sorption to cassava residue-derived biochar [J]. Science of the Total Environment, 2018, 616/617: 1384-1391. doi: 10.1016/j.scitotenv.2017.10.177 [24] AKSU Z, DÖNMEZ G. Binary biosorption of cadmium(II) and nickel(II) onto dried Chlorella vulgaris: Co-ion effect on mono-component isotherm parameters [J]. Process Biochemistry, 2006, 41(4): 860-868. doi: 10.1016/j.procbio.2005.10.025 [25] 王琦, 胡碧波, 阳春, 等. 烷基功能化磁性介孔硅的制备及其对氟喹诺酮类抗生素的吸附 [J]. 环境工程学报, 2020, 14(9): 2450-2462. doi: 10.12030/j.cjee.202001019 WANG Q, HU B B, YANG C, et al. Fabrication of alkyl-functionalized magnetic mesoporous silica and its adsorption of fluoroquinolone antibiotics [J]. Chinese Journal of Environmental Engineering, 2020, 14(9): 2450-2462(in Chinese). doi: 10.12030/j.cjee.202001019
[26] PENG X M, HU F P, ZHANG T, et al. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: A batch and fixed-bed column study [J]. Bioresource Technology, 2018, 249: 924-934. doi: 10.1016/j.biortech.2017.10.095 [27] ZHAO J, WANG Z Y, WHITE J C, et al. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation [J]. Environmental Science & Technology, 2014, 48(17): 9995-10009. [28] GU X Y, TAN Y Y, TONG F, et al. Surface complexation modeling of coadsorption of antibiotic ciprofloxacin and Cu(Ⅱ) and onto goethite surfaces [J]. Chemical Engineering Journal, 2015, 269: 113-120. doi: 10.1016/j.cej.2014.12.114 [29] PEI Z G, SHAN X Q, KONG J J, et al. Coadsorption of ciprofloxacin and Cu(II) on montmorillonite and kaolinite as affected by solution pH [J]. Environmental Science & Technology, 2010, 44(3): 915-920. [30] LI X Z, BI E P. The impacts of Cu(II) complexation on gatifloxacin adsorption onto goethite and hematite [J]. Journal of Environmental Quality, 2020, 49(1): 50-60. doi: 10.1002/jeq2.20016 [31] HUANG D F, XU Y B, YU X Q, et al. Effect of cadmium on the sorption of tylosin by polystyrene microplastics [J]. Ecotoxicology and Environmental Safety, 2021, 207: 111255. doi: 10.1016/j.ecoenv.2020.111255 [32] MA J, XIONG Y C, DAI X H, et al. Coadsorption behavior and mechanism of ciprofloxacin and Cu(Ⅱ) on graphene hydrogel wetted surface [J]. Chemical Engineering Journal, 2020, 380: 122387. doi: 10.1016/j.cej.2019.122387 [33] ZHANG H C, HUANG C H. Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with goethite [J]. Chemosphere, 2007, 66(8): 1502-1512. doi: 10.1016/j.chemosphere.2006.08.024 [34] 童非, 顾雪元. 重金属离子与典型离子型有机污染物的络合效应研究 [J]. 中国环境科学, 2014, 34(7): 1776-1784. TONG F, GU X Y. Study on complexation effect between heavy metal cations and typical ionic organic pollutants [J]. China Environmental Science, 2014, 34(7): 1776-1784(in Chinese).
-



















 下载:
下载: