-
近年来全球气候变暖问题愈发严重,有研究指出,在所有与高温有关的死亡中,有三分之一以上(37%)可归因于人为导致的全球变暖 [1]。目前普遍认为温室气体的大量排放是导致全球变暖的主要原因[2]。氢氟碳化合物(HFCs)作为第三代制冷剂,由于其不含氯元素,不会对臭氧层产生破坏,所以被广泛用于冰箱、空调中,用来替代第一代制冷剂氯氟烃(CFCs)、第二代制冷剂氢氯氟烃(HCFCs)。但是,研究发现大多数HFCs具有极高的全球变暖潜力值(GWP),且在大气中具有较长的寿命,其中,典型HFCs如1,1-二氟乙烷(HFC-152a)的GWP值为142,大气寿命是1.5 a。因此早在1997年《京都议定书》已将HFCs列为非CO2类温室气体,并且《蒙特利尔议定书(基加利修正案)》对HFCs的使用提出了更严格的要求。中国于2021年4月16日正式加入此议定书,决定加强对HFCs生产和消费方面的管控[3]。目前,中国致力于2030年达到“碳达峰”,2060年实现“碳中和”[4],所以对于HFCs的减排处理刻不容缓。
目前HFCs常用的处理方法主要有两种,消除处理法和资源化转化法[5-6]。消除处理法不能有效利用宝贵的氟资源,能耗高,同时会释放CO2加剧全球温室效应,不符合碳减排的大环境。而资源化转化法将HFCs转化为具有更高附加值的产品,是目前处理HFCs最有效的手段。其中,通过HFCs脱HF制备氢氟烯烃(HFOs)是最有价值的转化路线。
研究发现HFCs脱HF反应催化剂的活性位点为Lewis酸位点[7-11]。常用的Lewis酸催化剂主要包括氟化的Cr基、Mg基和Al基催化剂。Lim等[12]采用溶胶-凝胶法制备了Cr2O3催化剂,在1,1,1,2,3-五氟丙烷(HFC-245eb)脱HF生成2,3,3,3-四氟丙烯(HFO-1234yf)反应中,初始HFC-245eb转化率达到了80.1%,但由于其表面酸性太强导致催化剂快速积碳失活;加之Cr属于重金属元素,过度使用对环境会造成破环,逐渐被限制使用;Tang等[13]采用硬模板法制备了MgF2催化剂并对其HFC-152a脱HF反应进行研究,在反应600 h后转化率仍保持在45%以上;Mg基催化剂的酸性较弱导致活性低,且在温度高于300 ℃时容易出现烧结等问题,通常需要加入一些助剂来增加其酸性和比表面积;Al基催化剂兼具高活性和高性价比等优点,是目前HFCs气相脱HF制备HFOs反应最常用的催化剂[14-15]。Al基催化剂主要为AlF3催化剂。Yang等[16]制备了立方型AlF3催化剂,在350 ℃下1,1,1,3,3-五氟丙烷(HFC-245fa)脱HF反应中转化率为17.5%,对1,3,3,3-四氟丙烯(反式)(HFC-1234ze(E))的选择性保持在80%。但与Cr基催化剂相似,过强的Lewis酸容易引发积碳,从而导致催化剂失活[17]。因此,亟需寻找一种新型催化剂来缓解目前脱HF反应催化剂存在的活性与稳定性间的制约关系。
本文选用几种固体硫酸盐催化剂研究其对HFC-152a脱HF的反应性能,结果表明Al2(SO4)3催化剂表现出良好的催化性能。进一步研究发现结晶水含量与Al2(SO4)3催化剂的催化性能呈负相关。最后结合系列表征探讨了催化剂表面性能以及酸性对反应活性的影响,对Al2(SO4)3催化剂在HFC-152a脱HF反应中的催化机理做出假设。
-
取10 g工业硫酸盐Al2(SO4)3·18H2O (99% AR)、Fe2(SO4)3·xH2O (99% AR)、BaSO4 (99% AR)、CaSO4 (99% AR)、MgSO4 (99% AR)经过马弗炉400 ℃空气气氛焙烧4 h。将焙烧后的样品于20 MPa压力下压片,筛分至20—40目,即制得催化剂。经XRD测试后发现购买的Al2(SO4)3·18H2O晶相主要由Al2(SO4)3·17H2O组成,故后续采用Al2(SO4)3·17H2O来表示。
-
取定量工业Al2(SO4)3·17H2O (99% AR),分别在马弗炉中200 ℃、300 ℃、400 ℃、500 ℃和600 ℃空气气氛焙烧4 h。将焙烧后的样品于20 MPa压力下压片,筛分至20—40目,制得的催化剂记ASO-air-200、ASO-air-300、ASO-air-400、ASO-air-500、ASO-air-600。为了排除制备和装填催化剂过程中空气水分的干扰,ASO-air-300和ASO-air-400催化剂继续原位在N2气氛中分别在300 ℃和400 ℃焙烧2 h,制得催化剂记为ASO-N2-300和ASO-N2-400。
-
HFC-152a脱HF反应活性评价在实验室自行搭建的固定床反应装置中进行。反应管(内径12 mm,外径16 mm)使用纯镍(N6,Ni≥99.6%)材质制成。惰性气体N2和原料气HFC-152a经过质量流量计(MFC)和流量显示仪(Sevenstar-D08-3)来调节流速,并在流量计之前安装止逆阀。原料气和氮气混合进入反应管,采用厦门宇电控温仪来控制炉体和管内的反应温度。反应尾气从反应器底部流出,通过碱洗瓶以及装有无水氯化钙的U型干燥管后,在JieDao-GC1690气相色谱仪上进行在线检测,检测器采用热导检测器(TCD),载气为H2,柱温为60 ℃,检测器温度为110 ℃,桥电流100 mA,采用内标法定量,反应气体中添加N2作为内标物质,测定HFC-152a的转化率和氟乙烯(VF)的选择性。
-
X射线衍射(XRD):XRD表征测试在X'Pert PRO PANalytical型X射线衍射仪上的双测角仪中进行。使用的辐射是带有Ni滤光片的Cu Kα射线源(λ = 0.1542 nm),石墨单色器,管压30 kV,管流30 mA。扫描步长,扫描速率及扫描范围分别为0.008°(2θ),0.5(°)·min−1和2θ = 10°—80°。
热失重分析(TG-DTG):采用Perkin Elmer仪器进行热重分析(TGA)。将催化剂样品放在铂坩埚中,在N2气氛下(40 mL·min−1)从室温加热至600 ℃(10 ℃·min−1)。
N2物理吸附-脱附(BET):使用康塔Quantachrome Quadrasorb SI型气体吸附仪,先经过180 ℃脱气处理12 h,然后在-196 ℃下测试N2吸脱附。使用Brunauer-Emmett-Teller(BET)和BJH(Barrett-Joyner-Halenda)模型分析材料的织构性质。
吡啶红外(Py-IR):将样品压入自支撑圆盘中(2 cm2,10 mg·cm−2),之后将样品放入红外池中,该红外池配有KBr窗口并连接到真空管。首先将催化剂在350 ℃真空原位干燥60 min,然后样品冷却至50 ℃,并将吡啶引入池中。平衡30 min后,将催化剂在真空下处理30 min以除去物理吸附的吡啶。红外光谱在200 ℃的解吸温度下测试。在4000—400 cm−1范围内测量红外光谱。
原位红外(In-situ DRIFTS):DRIFTS表征在Thermo Fisher Nicolet iS50红外光谱仪上进行。样品先经N2(50 mL·min−1)气氛400 ℃脱水处理4 h,随后降至室温。样品在N2(50 mL·min−1)气氛下分别在25 、50 、150、250 、300 ℃采集背景。在室温通入原料混合气(HFC-152a = 5 mL·min−1,N2 = 45 mL·min−1)吸附30 min,之后N2吹扫30 min,在相应的温度下采集谱图。
-
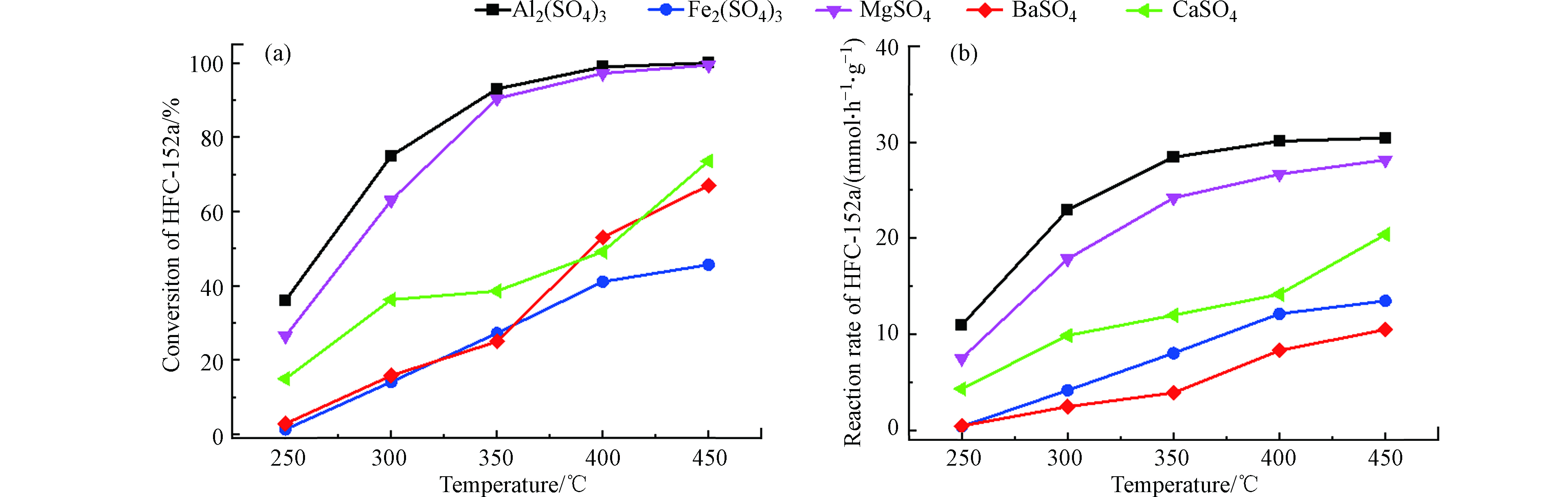
工业硫酸盐经过焙烧脱水处理后,进行HFC-152a脱HF反应,反应温度为250—450 ℃,各种硫酸盐催化剂的转化率和反应速率如图1所示。从图1中可以看出,随着温度的升高,所有的硫酸盐催化剂的转化率和反应速率随之上升,并且Al2(SO4)3和MgSO4的增加速率要快于其他硫酸盐催化剂。当温度增加到400 ℃时,Al2(SO4)3和MgSO4的反应转化率相近,接近100%,但从图1(b)可以看出,Al2(SO4)3对应的反应速率更高(30.44 mmol·h−1·g−1),具有更优的催化性能,故以Al2(SO4)3为研究对象进一步探索各种因素对其催化活性的影响。
-
有文献指出Al2(SO4)3中的结晶水可在390 ℃完全脱出[18-20],为了探究Al2(SO4)3·17H2O脱水过程,对其进行热重分析和对比不同温度焙烧处理后Al2(SO4)3·xH2O的XRD表征。图2(a)为Al2(SO4)3·17H2O的TG-DTG分析。从图2(a)中可以看到,Al2(SO4)3·17H2O的脱水主要分为3个过程,分别处于30—70 ℃,70—160 ℃和300—390 ℃。经过失重计算,在200 ℃时Al2(SO4)3·17H2O的质量损失为37.17 %,在所有Al位点失去结晶水数量相同的前提下,相当于13个水分子的损失量(理论质量损失:36.09%)。当温度位于300—390 ℃时,Al2(SO4)3继续失重,在该温度范围内,Al2(SO4)3继续脱掉3.34%的结晶水。然而继续升高温度未发现失重现象,即经过390—400 ℃焙烧处理可确保大部分Al2(SO4)3·17H2O转变成无水Al2(SO4)3。通过对不同温度下焙烧的Al2(SO4)3·17H2O进行XRD表征,进一步确定结晶水含量,如图2(b)所示。由图2可以看出,未焙烧处理的Al2(SO4)3主体由Al2(SO4)3·17H2O组成,经过200 ℃焙烧处理后Al2(SO4)3的物相为Al2(SO4)3·5H2O和少量Al2(SO4)3·17H2O的混合物,300 ℃和400 ℃焙烧处理后的Al2(SO4)3的晶相中仅含有少量的Al2(SO4)3·17H2O,主体结构为无水Al2(SO4)3。400 ℃焙烧处理得到的无水Al2(SO4)3信号强度最高,表明400 ℃是得到无水Al2(SO4)3的最佳焙烧温度,与TG研究结果一致。
-
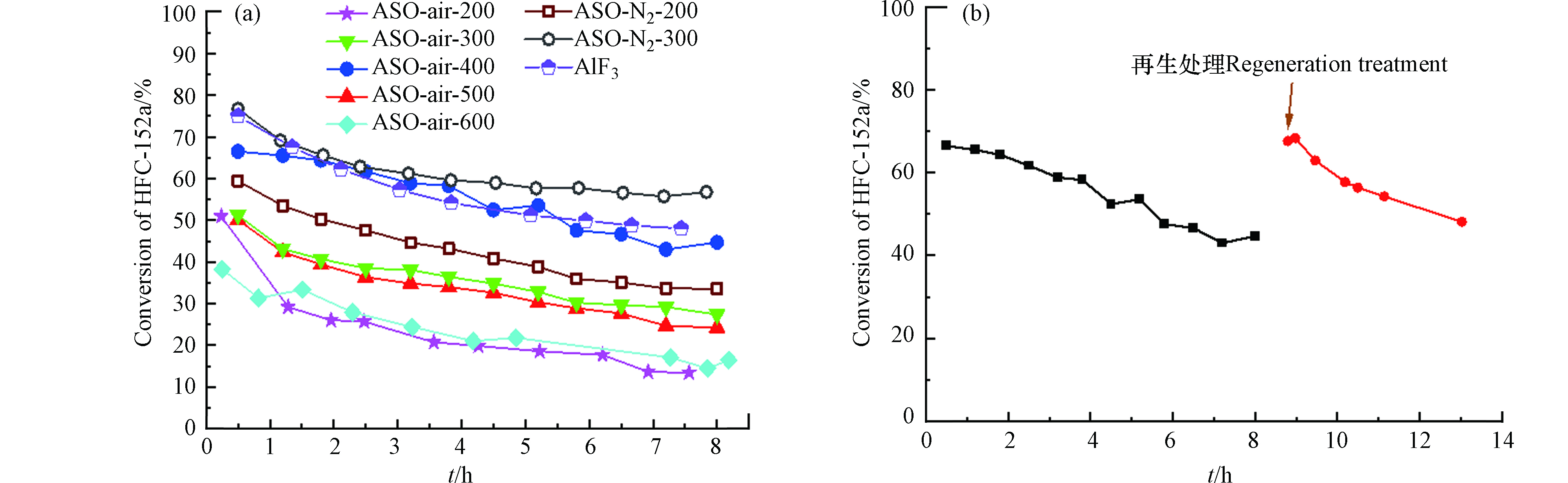
不同温度焙烧后的Al2(SO4)3·17H2O(简写ASO)对应的活性转化曲线如图3(a)所示。从图3(a)可以看出,随着焙烧温度的增加,活性出现先增加后降低的趋势,呈火山型曲线,其中400 ℃焙烧处理的Al2(SO4)3(ASO-air-400)对应的活性最高(反应8 h时转化率为44.71%),随着焙烧温度进一步增加,催化剂活性降低,这可能是催化剂烧结所致[21-23]。为了避免空气中水分的干扰,图中ASO-N2-400是指在经过400 ℃空气焙烧之后的催化剂在反应管内继续原位400 ℃通入N2焙烧2 h,发现催化剂的转化率进一步提升(8 h时为56.78%),证实结晶水对Al2(SO4)3催化剂的活性确实起抑制作用。同时相比传统的AlF3催化剂,Al2(SO4)3系催化剂具有更好的稳定性。例如ASO-N2-400与AlF3催化剂具有相近的初活性,但在相同的测试时间范围内。AlF3对应的转化率降低了约20%,而ASO-N2-400的约为10%,这是因为AlF3具有更强的Lewis酸强度易生成积碳所致。图3(a)中催化剂的活性随着反应时间的延长,呈现下降趋势,所以对失活原因进行了考察。如图3(b)所示,ASO-air-400催化剂经过8 h的反应后活性明显下降。通过在反应管中通入氧气进行烧炭处理1 h后评价发现,反应活性恢复,得到再生,说明催化剂的失活主要由积碳导致。对于脱HF反应,Lewis酸位点即是反应活性中心也是积碳中心,并且酸性越强,积碳越严重[24-25]。所有催化剂对氟乙烯(VF)的选择性皆接近100%,在此未列出。
-
为了考察Al2(SO4)3催化剂焙烧前后以及反应前后形貌的变化,对其进行SEM表征。图4(a,b)是Al2(SO4)3·17H2O的SEM图。从图4可以看出,Al2(SO4)3·17H2O呈致密的板状结构,尺寸约为2—10 μm。经400 ℃的焙烧处理后,从图4(c,d)中可以看出,催化剂仍呈板状结构,但是在催化剂的表面出现尺寸不均一的孔结构,这是因为在焙烧过程中结晶水的脱出会对催化剂结构造成一定的破坏,从而在催化剂体相和表面制造较多的孔结构,增加反应分子与活性位点的接触机率。但是从图4(e,f)可以发现,反应后的催化剂形貌与新鲜催化剂(图4c,d)存在明显的区别。催化剂呈现尺寸较大的块状结构,并且可以清晰地发现其表面由尺寸较小的圆润颗粒组成,这表明催化剂表面在反应过程中会与HFC-152a或HF反应,导致表面被刻蚀生成小尺寸颗粒。
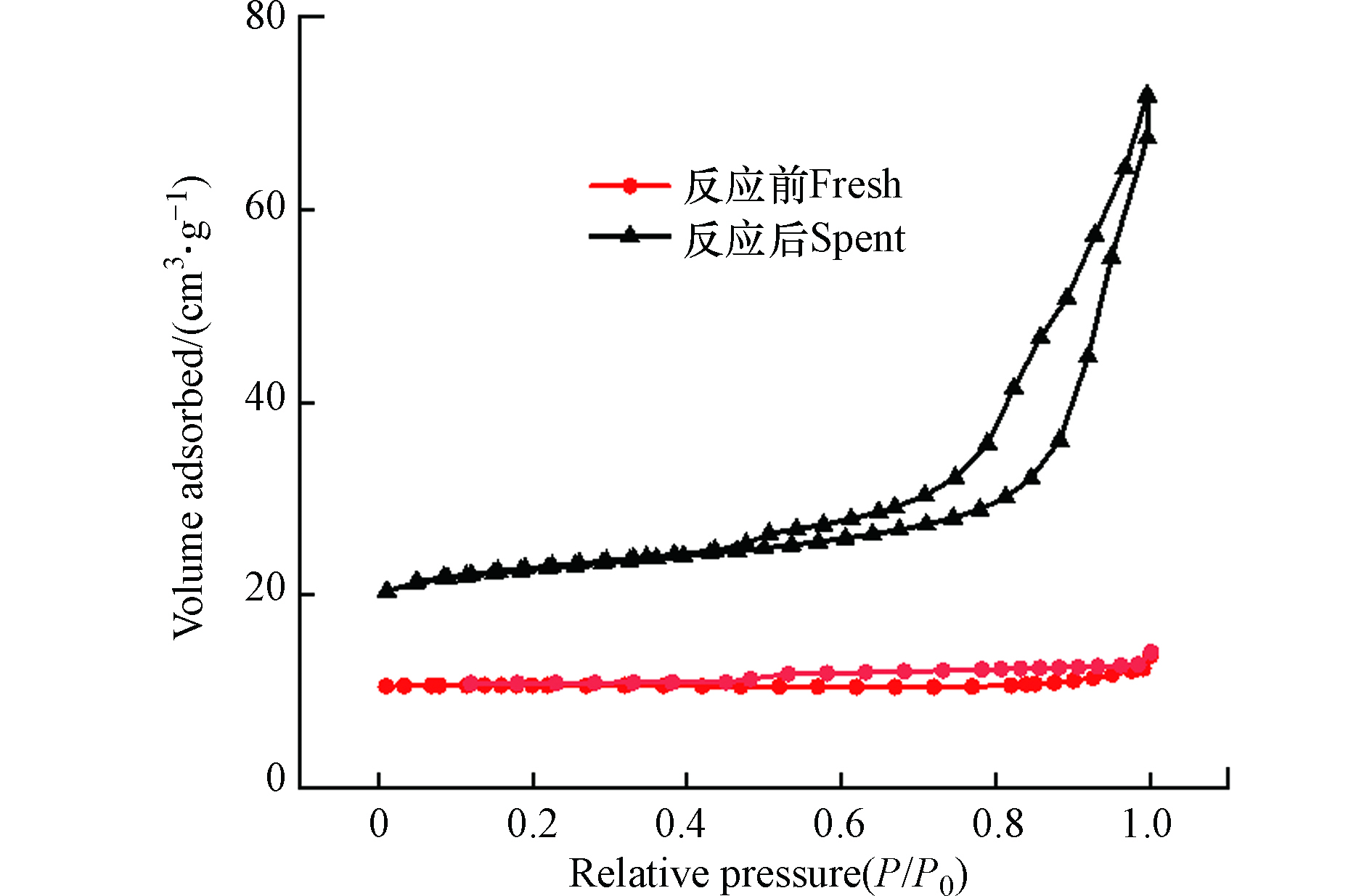
将反应前后的催化剂进行N2物理吸脱附表征测试,如图5所示,反应前后的催化剂吸附等温线都呈Ⅲ型等温线,H3回滞环,将计算的孔结构数据总结于表1中。虽然反应前后的催化剂对应的比表面积都较低,但是由于含氟气体的刻蚀作用(图4e,f),反应后催化剂的比表面积增加了10倍,进而有助于暴露更多的活性位点。反应前后催化剂的孔容都较低,说明催化剂起活性作用的活性中心主要来自于催化剂的外表面。通过图5中吸附等温线的H3型回滞环可知,催化剂存在介孔,并且孔径分布较宽,其主要由颗粒间的裂隙孔组成。反应后的催化剂平均孔径减少了近一半,这也是因为组成催化剂的颗粒尺寸变小所致。
-
经已有的研究可知,催化剂的Lewis酸位点是催化剂的活性位点[17],为了考察催化剂在反应前后酸性的变化,所以对反应前后的ASO-air-400催化剂进行了吡啶红外测试。在进行测试之前,样品先在350 ℃条件下真空处理1 h,除去结晶水对催化剂酸性的影响。从图6可以看出,反应前后都在相近的位置出现了特征峰,其中,1453、1493、1580 、1621 cm−1处归属于Lewis酸位点,1540 cm−1处归属为Brønsted酸位点[26]。将反应前后催化剂的Lewis酸量和Brønsted酸量总结于表2中。相比新鲜的ASO-air-400催化剂,反应后的ASO-air-400催化剂含有更高含量的Lewis酸位点,大约是新鲜催化剂的2倍,但是相对应的,Brønsted酸位点的量变成新鲜催化剂的一半,这一方面表明在反应过程中Brønsted酸位点的—OH物种被F物种取代,从而导致反应后的催化剂Lewis酸位点增加,与Al2O3氟化过程过程相似[8,11]。另一方面则是失去结晶水的ASO-air-400催化剂暴露的不饱和配位点部分被F物种占据,由于F元素具有较强的电负性,有助于增强Al物种的Lewis酸性。结合之前的SEM(图4e,f)表征,表明催化剂表面刻蚀的过程即为表面—OH基被F取代的过程,同时增加了催化剂的比表面积和Lewis酸位点,有助于增加催化剂的催化活性。新鲜的ASO-air-400催化剂比表面积极低,催化剂表面—OH数量有限,所以导致整个氟化过程较快,无法准确获得初始活性的数据及催化剂的诱导期。
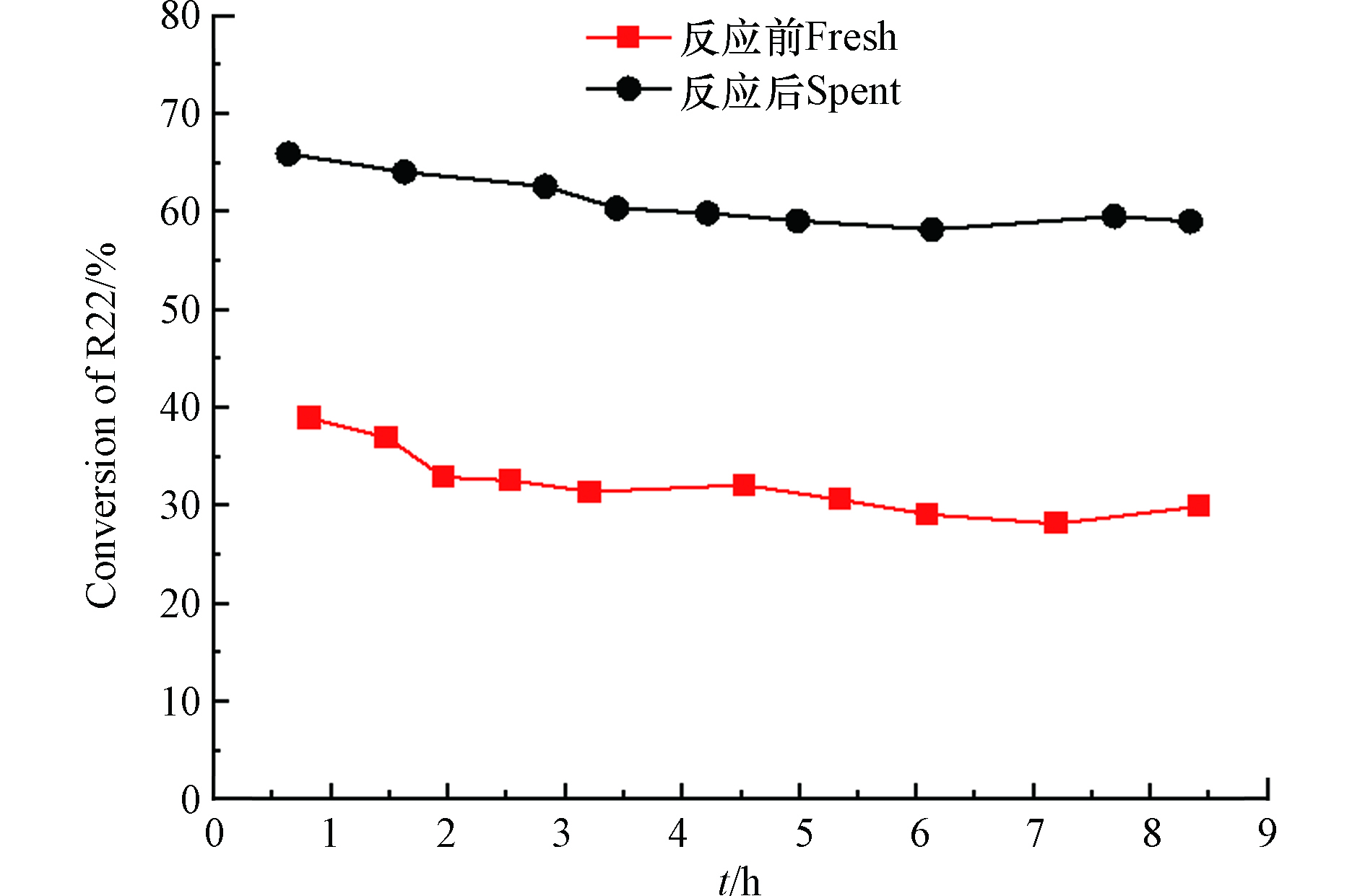
除了吡啶红外测试之外,选用常用的二氟一氯甲烷(R22)歧化反应考察反应前后ASO-air-400催化剂的酸性变化,反应活性越高,证明催化剂酸性越强,如图7所示。经HFC-152a反应后的ASO-air-400催化剂对应的R22转化率约是新鲜催化剂的2倍。再次证明ASO-air-400催化剂在HFC-152a脱HF的反应中由于含氟气体的氟化处理,增加了催化剂的酸性,进而提高Al2(SO4)3催化剂在R22歧化反应中的反应活性。
-
原位红外表征可以较好地模拟催化反应过程,为了研究ASO-air-400催化剂在HFC-152a脱HF反应中的催化机理,对其进行原位红外表征。样品首先在N2气氛下,400 ℃处理60 min以排除空气中水分的干扰。如图8所示,位于1128 cm−1处的特征峰属于C—F的拉伸振动[27-30],随着温度的升高,催化剂促进C—F键断裂导致对应的峰强度减弱。1670 cm−1处红外特征峰归属于Al—F物种的振动峰[31],可以发现该物种对应的特征峰强度随着温度的增加而增加,这是因为温度的增加可促进—OH被F取代。通过观察高波数范围内(3600—3710 cm−1)—OH负峰的变化趋势也可以印证这一观点。
从原位红外表征推测ASO-air-400催化剂在HFC-152a气相催化裂解中的催化机理,如下图9所示。首先在升温条件下,铝不饱和配位会吸附原料中的氟,缺电子的氧原子会吸附原料中的氢原子生成O—H。之后C—F和C—H拉伸振动至断裂,缺少F和H的HFC-152a不稳定,自发生成C=C以趋于稳定,而催化剂上不稳定的O—H和Al—F会自动将F和相邻的H生成共价键,最后另一产物氟化氢脱附下来,完成整个催化循环过程。
-
(1)对焙烧处理后的工业硫酸盐样品进行评价筛选,发现Al2(SO4)3催化剂活性最好。结合TG-DTG、XRD表征分析以及评价得出400 ℃焙烧后的无水Al2(SO4)3活性最优,分析出结晶水对催化活性有抑制作用。通过焙烧脱出结晶水后可以增加Al物种的不饱和配位点,进而有助于增加催化剂的Lewis酸性。
(2)在HFC-152a脱HF反应中,Al2(SO4)3催化剂会逐渐被氟化,导致Brønsted酸位点减少,同时增加Lewis酸位点和比表面积,进而有助于提高催化剂的催化活性。
硫酸铝催化剂在强温室气体1, 1-二氟乙烷气相催化裂解脱HF反应中的性能研究
Application of aluminum sulphate catalysts in the gas-phase catalytic cracking of potent greenhouse gas 1, 1-difluoroethane
-
摘要: 通过评价系列硫酸盐催化剂对1,1-二氟乙烷(HFC-152a)气相催化裂解脱HF反应的催化活性,筛选出活性最优的硫酸铝催化剂,并采用热失重分析(TG-DTG)、X射线衍射(XRD)、扫描电镜(SEM)、N2物理吸附-脱附(BET)、吡啶红外(Py-IR)等手段对不同焙烧温度处理硫酸铝催化剂进行表征。结果表明,通过对比不同焙烧温度的硫酸铝催化剂发现结晶水含量对催化活性有抑制作用。反应后的催化剂具有更高的Lewis酸量,一方面是由于脱出结晶水有助于增加Al物种不饱和配位点,而其中部分不饱和位点被F物种占据,进而有助于增强Lewis酸性;另一方面,催化剂表面Brønsted酸位点同样易被F物种取代,具有一定的刻蚀作用,所以在增加Lewis酸量的同时,也增加了催化剂的比表面积,提高反应分子与活性位点的接触机率。最后通过采用原位红外表征(In-situ DRIFTS)研究硫酸铝催化剂在HFC-152a气相催化裂解中的催化机理。Abstract: The catalytic activity of series sulfate catalysts for the gas-phase catalytic cracking of 1,1-difluoroethane (HFC-152a) was evaluated, and the aluminum sulfate catalysts exhibited the optimal activity. The catalytic properties of aluminum sulfate catalysts were characterized by thermal weight loss analysis (TG-DTG), X-ray diffraction (XRD), scanning electron microscopy (SEM), N2 adsorption-desorption, pyridine infrared (Py-IR). Comparation the catalytic activity of series aluminum sulfate treated with different calcination temperatures, the results showed that crystalline water had an opposite impact on the activity. Moreover, the spent catalysts had higher Lewis acid sites compared with fresh catalysts. On the one hand, the removal of crystalline water helped to increase the unsaturated coordination sites of aluminum species, then some of these unsaturated sites were occupied by fluorine species, which in turn helped to enhance the Lewis acidity of Al species; on the other hand, the Brønsted acid sites on the surface of catalyst were also susceptible to replace by fluorine species, which had a certain etching effect. Accordingly, the amount of Lewis acid sites as well as the specific surface area of the catalyst were improved, increasing the chance of contact between the reacting molecules and the active sites. Lastly, the catalytic mechanism of HFC-152a gas-phase catalytic cracking over aluminum sulfate catalyst was revealed by in-situ infrared characterization (In-situ DRIFTS).
-
Key words:
- 1-difluoroethane /
- dehydrofluorination /
- Lewis acid catalysis /
- aluminum sulfate /
- greenhouse gases
-

-
表 1 反应前后ASO-air-400催化剂的织构性质
Table 1. Texture properties of ASO-air-400 catalyst before and after the reaction
ASO-air-400 比表面积/(m2·g−1)
Surface area孔容/(cm3·g−1)
Pore volume平均孔径/nm
Average pore diameterFresh 1.60 0.01 38.97 Used 17.90 0.08 17.50 表 2 Py-IR测定的反应前后ASO-air-400催化剂的酸量
Table 2. Acid content of fresh and Used ASO-air-400 catalyst determined by pyridine Fourier infrared absorption spectroscopy
ASO-air-400 布朗斯特酸量/(μmol·g−1)
Brønsted acid content路易斯酸量/(μmol·g−1)
Lewis acid content总酸量/(μmol·g−1)
Total acid contentFresh 15.83 15.83 31.66 Used 8.60 35.05 43.65 -
[1] VICEDO-CABRERA A M, SCOVRONICK N, SERA F, et al. The burden of heat-related mortality attributable to recent human-induced climate change [J]. Nature Climate Change, 2021, 11(6): 492-500. doi: 10.1038/s41558-021-01058-x [2] 李晟. 高质量发展视角下产业结构升级对我国碳减排的影响 [J]. 可持续发展, 2021, 11(1): 149-159. doi: 10.12677/SD.2021.111018 LI S. The impact of industrial structure upgrading on my country’s carbon emission reduction from the perspective of high-quality development [J]. Sustainable Development, 2021, 11(1): 149-159(in Chinese). doi: 10.12677/SD.2021.111018
[3] 张朝晖, 陈敬良, 高钰, 等. 《蒙特利尔议定书》基加利修正案对制冷空调行业的影响分析 [J]. 制冷与空调, 2017, 17(1): 1-7,15. ZHANG Z H, CHEN J L, GAO Y, et al. Analysis on the influence of Kigali Amendment to Montreal Protocol to refrigeration and air-conditioning industry [J]. Refrigeration and Air-Conditioning, 2017, 17(1): 1-7,15(in Chinese).
[4] 倪吉, 杨奇. 实现碳中和, 对化工意味着什么 [J]. 中国石油和化工, 2020(11): 26-31. doi: 10.3969/j.issn.1008-1852.2020.11.006 NI J, YANG Q. What carbon neutrality means for the chemical industry [J]. China Petroleum and Chemical Industry, 2020(11): 26-31(in Chinese). doi: 10.3969/j.issn.1008-1852.2020.11.006
[5] 贾文志, 刘聪, 刘行, 等. 温室气体氢氟烃的处理与利用 [J]. 化工生产与技术, 2016, 23(4): 1-6, 8. doi: 10.3969/j.issn.1006-6829.2016.04.001 JIA W Z, LIU C, LIU X, et al. Treatment and utilization of greenhouse gases-HFCs [J]. Chemical Production and Technology, 2016, 23(4): 1-6, 8(in Chinese). doi: 10.3969/j.issn.1006-6829.2016.04.001
[6] 韩文锋, 靳碧波, 周强, 等. 三氟甲烷(HFC-23)的资源化转化利用 [J]. 化工进展, 2014, 33(2): 483-492. HAN W F, JIN B B, ZHOU Q, et al. Conversion and resource utilization of waste CHF3 gas [J]. Chemical Industry and Engineering Progress, 2014, 33(2): 483-492(in Chinese).
[7] PATIL P T, DIMITROV A, KIRMSE H, et al. Non-aqueous Sol-gel synthesis, characterization and catalytic properties of metal fluoride supported palladium nanoparticles [J]. Applied Catalysis B:Environmental, 2008, 78(1/2): 80-91. [8] LI G L, NISHIGUCHI H, ISHIHARA T, et al. Catalytic dehydrofluorination of CF3CH3(HFC143a) into CF2CH2(HFC1132a) [J]. Applied Catalysis B:Environmental, 1998, 16(4): 309-317. doi: 10.1016/S0926-3373(97)00087-8 [9] GANDLER J R, YOKOYAMA T. The E2 transition state: Elimination reactions of 2-(2, 4-dinitrophenyl)ethyl halides [J]. Journal of the American Chemical Society, 1984, 106(1): 130-135. doi: 10.1021/ja00313a027 [10] SONG T Y, DONG Z X, SONG J D, et al. Dehydrochlorination of 1, 1, 2-trichloroethane over SiO2-supported alkali and transition metal catalysts: Tunable selectivity controlled by the acid - base properties of the catalysts [J]. Applied Catalysis B:Environmental, 2018, 236: 368-376. doi: 10.1016/j.apcatb.2018.04.018 [11] MALLIKARJUNA R V N, SUBRAMANIAN M A. Fluoroolefin manufacturing process: US6031141[P]. [2000-02-29]. [12] LIM S, KIM M S, CHOI J W, et al. Catalytic dehydrofluorination of 1, 1, 1, 2, 3-pentafluoropropane (HFC-245eb) to 2, 3, 3, 3-tetrafluoropropene (HFO-1234yf) using in situ fluorinated chromium oxyfluoride catalyst [J]. Catalysis Today, 2017, 293/294: 42-48. doi: 10.1016/j.cattod.2016.11.017 [13] TANG H D, DANG M M, LI Y Z, et al. Rational design of MgF2 catalysts with long-term stability for the dehydrofluorination of 1, 1-difluoroethane (HFC-152a) [J]. RSC Advances, 2019, 9(41): 23744-23751. doi: 10.1039/C9RA04250D [14] RÜDIGER S, ELTANANY G, GROß U, et al. Real Sol-gel synthesis of catalytically active aluminium fluoride [J]. Journal of Sol-Gel Science and Technology, 2007, 41(3): 299-311. doi: 10.1007/s10971-006-9008-0 [15] CHRISTE K O, DIXON D A, MCLEMORE D, et al. On a quantitative scale for Lewis acidity and recent progress in polynitrogen chemistry [J]. Journal of Fluorine Chemistry, 2000, 101(2): 151-153. doi: 10.1016/S0022-1139(99)00151-7 [16] YANG H, WU S, CHEN Z F, et al. Catalytic performance for the conversion of potent fluorinated greenhouse gases by aluminium fluorides with different morphology [J]. Catalysis Letters, 2021, 151(7): 2065-2074. doi: 10.1007/s10562-020-03446-y [17] JIA W Z, WU Q, LANG X W, et al. Influence of lewis acidity on catalytic activity of the porous alumina for dehydrofluorination of 1, 1, 1, 2-tetrafluoroethane to trifluoroethylene [J]. Catalysis Letters, 2015, 145(2): 654-661. doi: 10.1007/s10562-014-1409-z [18] ÇıLGı G K, CETIŞLI H. Thermal decomposition kinetics of aluminum sulfate hydrate [J]. Journal of Thermal Analysis and Calorimetry, 2009, 98(3): 855-861. doi: 10.1007/s10973-009-0389-5 [19] CHOU K S, SOONG C S. Kinetics of the multistage dehydration of aluminum sulfate hydrate [J]. Thermochimica Acta, 1984, 81: 305-310. doi: 10.1016/0040-6031(84)85135-7 [20] CHOU K S, SOONG C S. Kinetics of the thermal decomposition of aluminum sulfate [J]. Thermochimica Acta, 1984, 78(1/2/3): 285-295. [21] BARTHOLOMEW C H, RAHMATI M, REYNOLDS M A. Optimizing preparations of Co Fischer-Tropsch catalysts for stability against sintering [J]. Applied Catalysis A:General, 2020, 602: 117609. doi: 10.1016/j.apcata.2020.117609 [22] ZARUBINA V, MELIÁN-CABRERA I. On the geometric trajectories of pores during the thermal sintering of relevant catalyst supports [J]. Scripta Materialia, 2021, 194: 113679. doi: 10.1016/j.scriptamat.2020.113679 [23] PAN C, GUO Z L, DAI H, et al. Anti-sintering mesoporous Ni-Pd bimetallic catalysts for hydrogen production via dry reforming of methane [J]. International Journal of Hydrogen Energy, 2020, 45(32): 16133-16143. doi: 10.1016/j.ijhydene.2020.04.066 [24] WANG Z K, HAN W F, TANG H D, et al. CaBaFx composite as robust catalyst for the pyrolysis of 1-chloro-1, 1-difluoroethane to vinylidene fluoride [J]. Catalysis Communications, 2019, 120: 42-45. doi: 10.1016/j.catcom.2018.11.011 [25] WANG J C, HAN W F, WANG S C, et al. Synergistic catalysis of carbon-partitioned LaF3–BaF2 composites for the coupling of CH4 with CHF3 to VDF [J]. Catalysis Science & Technology, 2019, 9(6): 1338-1348. [26] LI H R, LIU C L, WANG Y, et al. Synthesis, characterization and n-hexane hydroisomerization performances of Pt supported on alkali treated ZSM-22 and ZSM-48 [J]. RSC Advances, 2018, 8(51): 28909-28917. doi: 10.1039/C8RA04858D [27] MAITY J, JACOB C, DAS C K, et al. Direct fluorination of Twaron fiber and investigation of mechanical thermal and morphological properties of high density polyethylene and Twaron fiber composites [J]. Journal of Applied Polymer Science, 2008, 107(6): 3739-3749. doi: 10.1002/app.27510 [28] PENG T, CAI R Q, CHEN C F, et al. Surface modification of Para-aramid fiber by direct fluorination and its effect on the interface of aramid/epoxy composites [J]. Journal of Macromolecular Science, Part B, 2012, 51(3): 538-550. doi: 10.1080/00222348.2011.609777 [29] OU Y P, JIAO Q J, LI N, et al. Pyrolysis of ammonium perfluorooctanoate (APFO) and its interaction with nano-aluminum [J]. Chemical Engineering Journal, 2021, 403: 126367. doi: 10.1016/j.cej.2020.126367 [30] LIANG J, ROSELIUS M. FTIR study of a perfluoroacyl fluoride chemisorption onto alumina [J]. Journal of Fluorine Chemistry, 1994, 67(2): 113-117. doi: 10.1016/0022-1139(93)02958-H [31] LIMCHAROEN A, LIMSUWAN P, PAKPUM C, et al. Characterisation of C—F polymer film formation on the air-bearing surface etched sidewall of fluorine-based plasma interacting with Al2O3–TiC substrate [J]. Journal of Nanomaterials, 2013, 2013: 1-6. -



 下载:
下载: