-
磺胺类抗生素是人工合成的广谱型抗菌药物,因杀菌性强、使用方便等优点被广泛应用于人类医疗和畜禽养殖中。当人类或动物服用后,仅有20%左右的磺胺抗生素能被吸收,剩余部分则以母体或代谢产物的形式随排泄物进入环境[1]。磺胺嘧啶(SDZ)作为一种常用的磺胺类药物,在世界各地的水环境中经常被检测到[2],可能对水生生物构成生态风险。由于磺胺抗生素固有的N、S杂环结构使其具有较强的生化稳定性,采用传统的处理工艺与技术难以有效的将其去除[3]。因此,探寻高效的磺胺类抗生素处理方法成为研究难点。
基于活化过一硫酸盐(PMS)产生硫酸根自由基SO4·-的高级氧化技术因氧化能力强、稳定性好、pH值耐受范围广、便于运输储存等优点在难降解有机物处理领域得到广泛关注[4-5]。目前活化PMS的方法主要有过渡金属离子活化、紫外活化、热活化以及超声活化[6]等,其中以零价铁(Fe0)为主要活性点位的过渡金属活化法因较高的氧化还原电位、环保及低成本等特性备受广大研究者青睐,但金属团聚及反应后催化剂难回收等问题限制了其广泛应用。因此,开发新型、绿色高效的Fe0基支撑材料具有广泛而深远的现实意义。
生物炭作为一种由废弃生物质高温分解而来的碳材料, 不仅来源广泛、价格低廉、制备过程简单,其巨大比表面积、丰富的含氧官能团、杂化的碳结构还能为PMS提供新的活性点位[7],是一种颇具潜力的Fe0基氧化还原介体。目前,已有研究者将污泥[8]、秸秆[9]和草药残渣[10]等工业及林业废弃物与高价铁源混合制备Fe0功能的生物炭复合材料,而以绵羊粪便为原料制备生物炭并作为Fe0支撑材料的研究未有报道。不同种类的铁物质对活化PMS降解污染物的机制有较大差异,现有研究多集中在Fe0、Fe2+和Fe3+参与的自由基途径耦合生物炭产生的非自由基途径来降解有机物[11-13]。关于Fe3C和Fe0两种铁物质在生物炭上的形成以及协同作用的污染物降解机制并不明确。
因此,本研究以绵羊粪便为生物炭来源,通过共沉淀法制备磁性羊粪生物炭(Fe3O4-SMB),再将其在3种不同温度(500 ℃、600 ℃、700 ℃)下热解得到Fe@SMB复合材料。通过SEM、EDS、XRD、Raman、BET等方法对材料的形貌、石墨化程度、比表面积和表面化学性质进行表征,揭示Fe0/Fe3C在生物炭上的产生机制,并通过序批实验考察不同PMS和催化剂用量、温度、阴离子、pH等因素对SDZ降解的影响。结合猝灭实验实验和XPS测试阐明Fe0/Fe3C双铁功能生物炭复合材料活化PMS的主要活性点位及反应机理。最后,通过稳定性评价该催化剂的应用潜力,为Fe0/Fe3C-生物炭在硫酸根自由基高级氧化技术实际工程的应用提供理论和技术支撑。
-
原料羊粪产自四川当地农场。过硫酸氢钾 (KHSO5·0.5KHSO4·0.5K2SO4)、磺胺嘧啶(SDZ)购自中国阿拉丁化学有限公司。甲醇(MeOH)、乙醇(EtOH)、叔丁醇(TBA)、L-组氨酸(L-H)、对苯醌(p-BQ)、苯酚(phenol)、硝基苯(NB)、氯化钠(NaCl)、碳酸钠(NaCO3)、硝酸钠(NaNO3)、磷酸二氢钠(NaH2PO4)由成都市科隆化学品有限公司提供。实验所用化学试剂均为分析级,稀释溶解用水为超纯水(电阻率≥18.25 MΩ·cm)。
-
(1)羊粪生物炭的制备:剔除羊粪中杂质后粉碎过100目筛。称取适量过筛后的羊粪粉末于石英舟中后压实放于管式炉中。在N2氛围保护下,以恒定升温速率(10 ℃·min−1)加热至400 ℃后恒温1 h。冷却后的生物炭加入适量的1 mol·L−1硫酸溶液后于室温下振荡。振荡结束后用去离子水反复清洗生物炭至溶液近中性后于100 ℃ 烘箱中烘干。所得生物炭命名为SMB-400。
(2)运用共沉淀法制备磁性生物炭复合材料Fe3O4-SMB,具体步骤如下:①分别加入2.50 g SMB-400、0.5 g FeSO4·7H2O和1.61 g Fe(NO3)·9H2O于100 mL去离子水中,水浴加热至60 ℃搅拌0.5 h。②加入浓度为1 mol·L−1的NaOH溶液使pH升高至10—11,沉淀铁氧化物并继续搅拌溶液0.5 h。③将静置后的悬浮液过滤,并将沉淀物用去离子水和乙醇反复清洗,于烘箱中烘干后命名为Fe3O4-SMB。
(3)铁功能化生物炭(Fe@SMBs)制备:将Fe3O4-SMB置于管式炉中在N2氛围保护下,以10 °C ·min−1的升温速率分别升温至500、600、700 °C后恒温1 h,得到铁功能化生物炭,命名为Fe@SMB500、Fe@SMB600、Fe@SMB700。
-
通过X射线衍射仪(Panalytical Empyrean powder X-ray Cu Ka (45 kV/40 mA,λ =0.15406 nm))、拉曼光谱(Raman)对催化剂晶体结构、石墨及缺陷程度进行表征。通过配备有X射线能谱仪(EDS)的场发射扫描电子显微镜(FE-SEM)表征催化剂的形貌以及表面元素。利用Micromeritics Gemini装置(ASAP 2460 3.01,Micromeritics,Co.,Norcross,GA,USA)在77 K下用氮气吸附-脱附等温线和Brunauer,Emmett和Teller法测定了催化剂的比表面积和孔径分布。用X射线光电子能谱仪(XPS)研究了样品的表面元素组成和价态变化,并在C1s峰的284.8 eV处对所有光谱的结合能进行了校正。
-
本实验采用间歇性动力学实验考察不同催化剂活化PMS降解SDZ的动力学影响和机理探究。未有另外说明时,溶液的初始 pH 值不经调节,反应溶液温度控制在25 ℃。将称好的生物炭以及一定浓度的过硫酸氢钾溶液同时加入到SDZ(10 mg·L−1,100 mL)中并用秒表开始计时。在设定取样时间点取出1.0 mL的反应溶液,经0.22 μm的滤膜快速过滤后加入离心管中并迅速以1.0 mL甲醇溶液进行猝灭。在考察溶液初始pH值对Fe@SMB700去除SDZ性能的影响时,用1 mol·L−1NaOH或HNO3调节溶液pH;在考察催化剂的稳定性时,通过磁铁回收溶液中反应后的催化剂,并用去离子水洗涤后于60 ℃真空干燥箱中干燥后用于下一次反应;在猝灭实验中,甲醇、叔丁醇、硝基苯、苯酚、对苯醌和L-组氨酸作为猝灭剂,以清除反应中的活性物质。使用配有C18色谱柱(150 mm×4.6 mm, 5 um)高效液相色谱(UPLC)检测反应溶液中的SDZ浓度,进样量为10 μL,流动相为乙腈和0.1%的冰乙酸(40:60),流速为0.7 mL·min−1,检测波长为270 nm。本研究所有实验均进行3次,结果呈现为具有标准偏差的平均值。
-
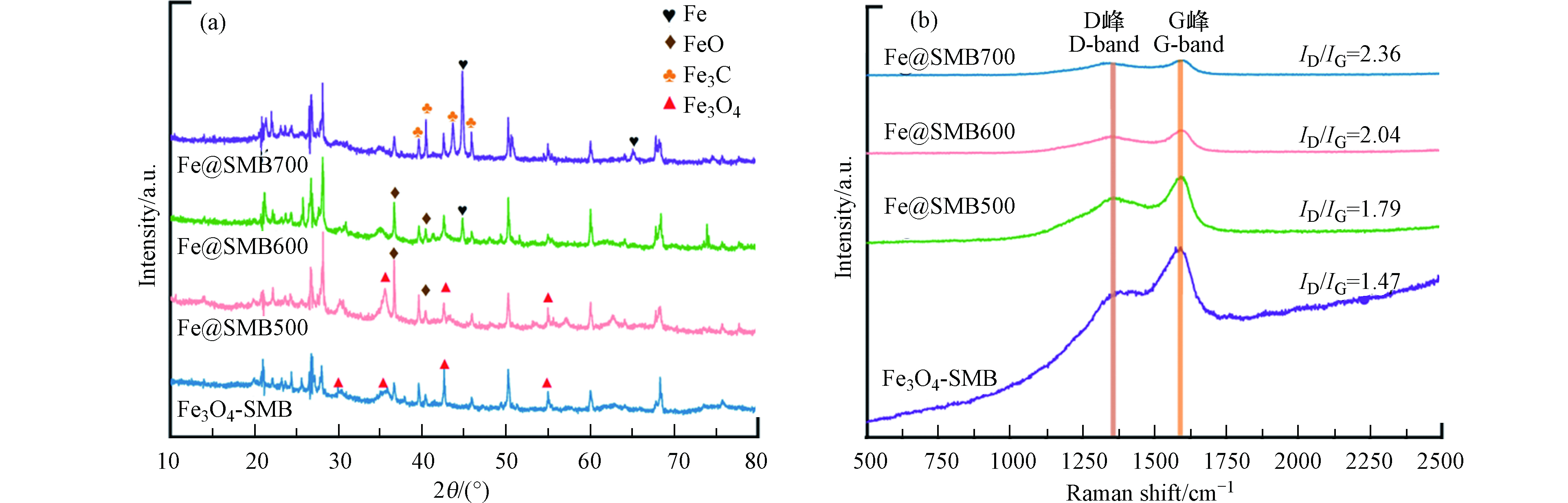
从4种催化剂的XRD图谱(图1a)可以清楚地观察到Fe3O4-SMB、Fe@SMB500、Fe@SMB600和Fe@SMB700具有明显不同的特征衍射峰。2θ=23.1°处的峰归因于SiO2,2θ=26.3 ° 处的衍射峰是典型的(002)面石墨碳峰(JCPDS NO.41-1487)。位于30.1°、35.4°、43.0°和56.9°的衍射峰,分别归属于Fe3O4的(220)、(311)、(400)和(511)晶面(JCPDS NO.72-2303)。2θ=44.7°归属于金属Fe(JCPDS No.06-0696)。2θ=36.3°和41.7°的峰对应于FeO(JCPDS No.06-0615)。位于37.7°、39.9°、43.8°和46.0°的衍射峰对应于Fe3C (JCPDS 76-1877)的(112)、(200)、(210)和(211)平面。从图1a中可以看出,Fe3O4-SMB、Fe@SMB500、Fe@SMB600和Fe@SMB700在2θ=26.3°处均有明显的衍射峰,表明4种生物炭都产生了石墨化结构[14],而Fe@SMB700的石墨峰最为明显,表明高温有利于石墨碳的形成;通过共沉淀法在Fe3O4-SMB上成功负载了大量的Fe3O4,当其被热解至500 ℃时,Fe3O4被部分还原FeO;当热解温度升高到600 ℃时,FeO的信号峰减弱,随之产生金属Fe的信号峰。当温度进一步升高到700 ℃时,FeO晶相消失,金属Fe信号峰显著增强,同时在Fe@SMB700中发现了Fe3C,形成了一种双铁功能化的催化材料(Fe0/Fe3C-SMB700)。Mangarella等[15]的研究表示,Fe3C的主要形成原因是高温下炽热炭的还原。上述结果表明,生物炭在较高热解温度下产生的还原性物质能将高价铁物种还原成Fe0,并促进Fe3C的形成,从而影响催化剂的催化性能和催化机理。
碳材料的缺陷边缘不仅可以提供更多的吸附和催化活性点位[16],同时其石墨化结构有利于电荷的转移,因此对不同催化剂的碳结构进行拉曼光谱分析,结果如图1b所示。4种催化剂在1350 cm−1(D峰)和1580 cm−1(G峰)左右均出现明显特征峰,其中D峰是由于材料中存在缺陷等造成的,G峰则是sp2键的面内伸缩震动所致。D峰与G峰面积比值(ID/IG)反映了材料的石墨化和缺陷程度。随着热解温度的升高,ID/IG从1.47增加到2.36,表明较高的碳化温度有利于形成更多的缺陷活性点位,这可能与高温条件下生物炭表面形成了大量的孔隙,增加了无序度有关[17]。
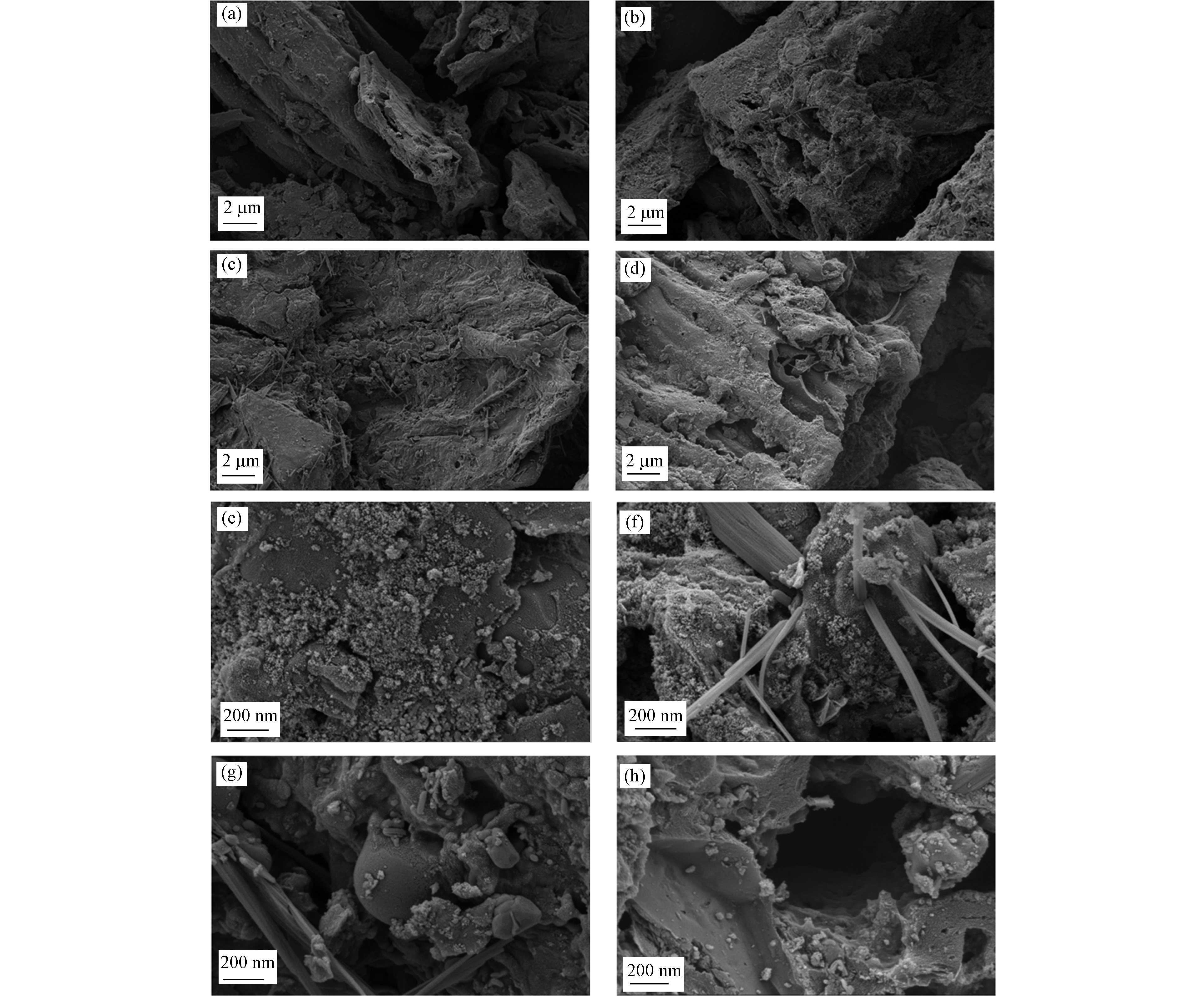
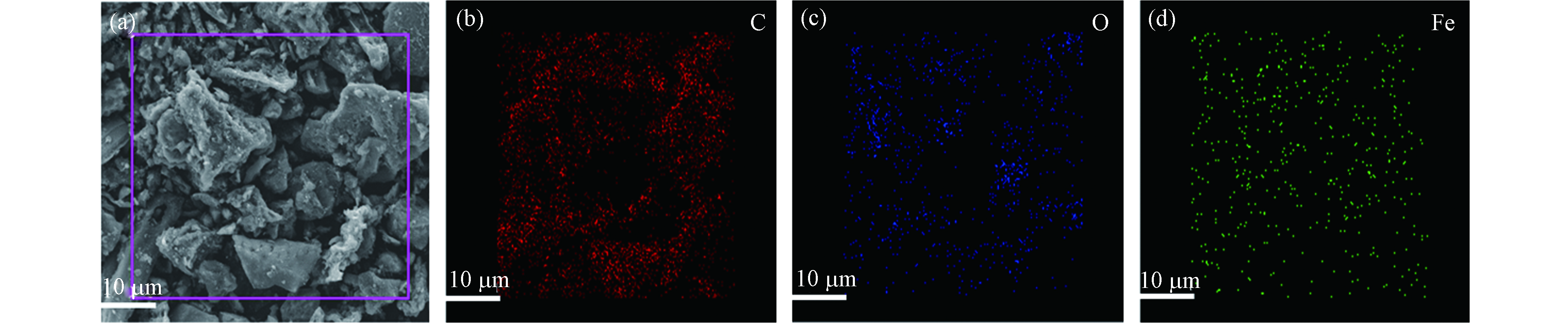
4种催化剂表面形貌特征如SEM图所示。由图2a可知,Fe3O4-SMB表面呈致密的片状结构,无中微孔产生。经过不同温度的热解处理之后,催化剂的表面形貌发生了巨大的变化,不同的热解温度形成了不同的碳层结构。如图2b,当Fe3O4-SMB在500 ℃的条件下热解后,生成了较多纳米纤维,表面粗糙不平,有明显的中孔结构。由图2c可知,Fe@SMB600表面纳米纤维进一步增加,出现介孔结构,且表面产生了许多球状的凸起,由XRD分析结果推测其中可能包裹着FeO纳米粒子。由图2d可知,Fe0/Fe3C-SMB700表面褶皱且凹凸不平,单位面积中微孔数量明显增多。分析认为,在较高热解温度下,生物炭中的有机物解产生的热能进一步打开内部堵塞的孔道,形成褶皱及多孔结构,这种分层褶皱及丰富的孔隙结构有利于反应物传质至其表面发生催化反应[18]。图2e—h则展现了碳材料表面金属纳米粒子的变化,由图2可知,随着热解温度的升高,碳材料表面的金属纳米粒子体积逐渐变小,且较为规整的平铺或嵌入碳层中。当温度上升到700 ℃,Fe0/Fe3C-SMB700的金属纳米粒子大多镶嵌、包裹在碳层中。此外,从Fe0/Fe3C-SMB700的SEM-EDS图谱图像(图3)可以观察到碳层结构中的C(图3b)和O(图3c)元素的连续分布,而Fe(图3d)呈离散分布。与Fe元素相比,C和O在碳骨架上的含量高且分布均匀,验证了Fe纳米粒子存在于生物炭表面或内部。
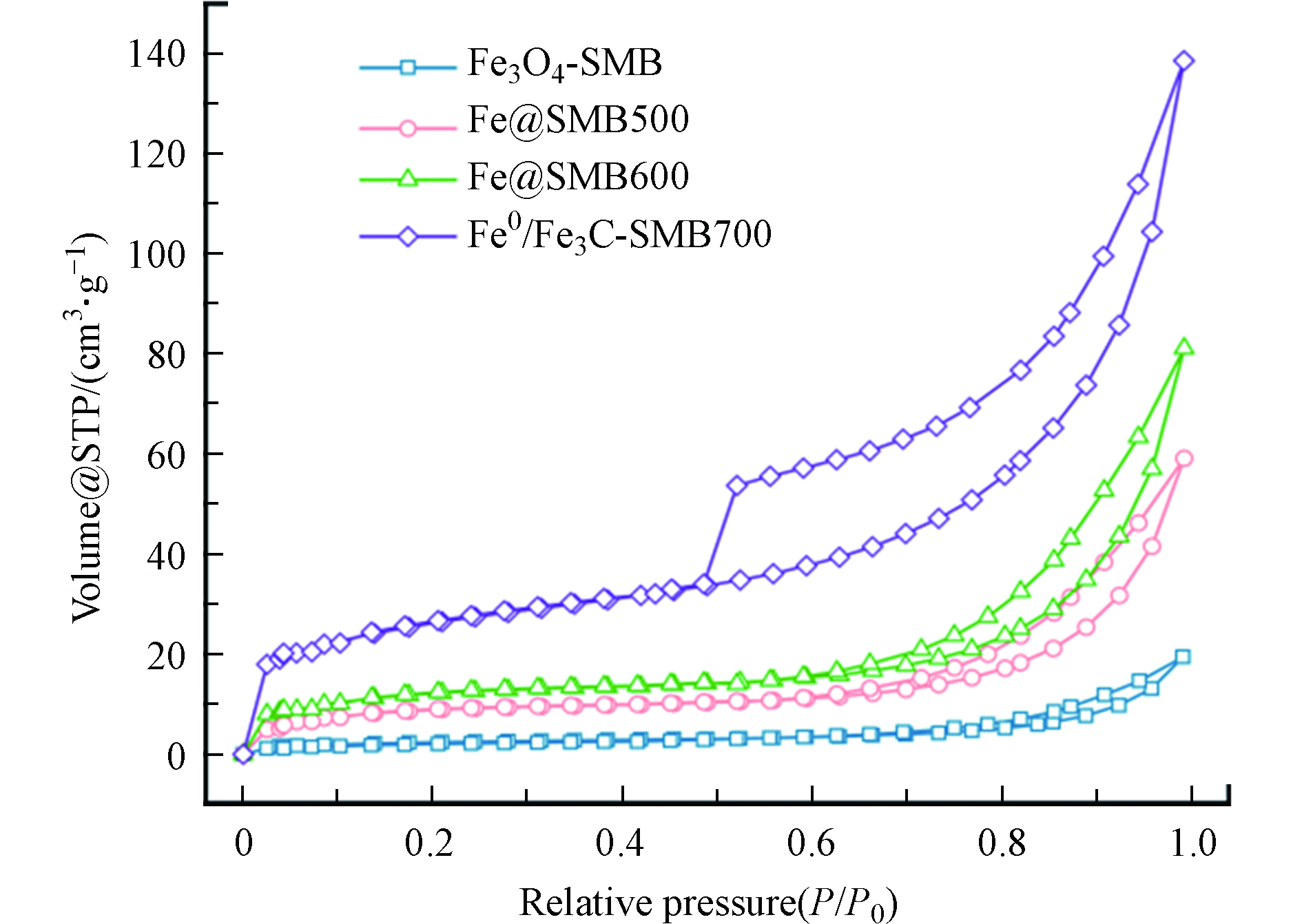
用N2吸附脱附曲线测定了Fe@SMBs的比表面积和孔结构。如图4所示,Fe3O4-SMB呈Ⅱ型等温线,反映非孔性或者大孔吸附剂上典型的物理吸附过程。Fe@SMB500、Fe@SMB600、Fe0/Fe3C-SMB700均表现为Ⅳ型等温线,在P/P0=0.5—1.0处出现明显的滞后曲线,表明Fe@SMB500、Fe@SMB600、Fe0/Fe3C-SMB700均存在中孔和微孔结构,这与SEM结果一致。Fe@SMB500、Fe@SMB600的吸脱附等温线产生了H3型的介孔回滞环,表明其孔结构不规整,而Fe0/Fe3C-SMB700的吸脱附等温线产生了H1型的介孔回滞环,在0—0.1的相对压力范围内有一个陡峭的吸附拐点,表明其含有丰富的微孔结构[17]。通过计算得到Fe@SMBs的比表面积和孔径分布,结果如表1所示。随着热解温度的升高,Fe0/Fe3C-SMB700较Fe@SMB600、Fe@SMB500、Fe3O4-SMB拥有更大的比表面积和总孔容,这为反应暴露更多的活性点位,有利于加快传质速率。
-
不同催化剂降解SDZ性能如图5所示。如图5a所示,在无PMS存在的情况下,SDZ的去除取决于催化剂的吸附作用,50 min内Fe3O4-SMB、Fe@SMB500、Fe@SMB600、Fe0/Fe3C-SMB700对SDZ的吸附效率分别为15%、19%、24%和27%,表明吸附对SDZ的去除有一定的贡献,但不是主要作用。而Fe0/Fe3C-SMB700具有最佳的吸附性能,这可能与其在高温下产生的较大的比表面积和丰富的孔隙结构有关。单独的PMS存在时SDZ浓度几乎不变,50 min内去除率仅为4%,说明在没有催化剂的情况下,PMS很难被激活。当催化剂和PMS共存时,50 min内Fe0/Fe3C-SMB700、Fe@SMB600、Fe@SMB500和Fe3O4-SMB对SDZ的去除率分别为88%、85%、82%和75%。为了全面比较4种复合材料的性能,引入了拟一级动力学模型,拟合结果如图5b所示。从图5c可以看出,Fe3O4-SMB、Fe@SMB500、Fe@SMB600和Fe0/Fe3C-SMB700的表观速率常数(kobs)呈上升趋势,分别为0.0327、0.0395、0.0423、0.0509 min−1。分析认为在较高温度下产生的Fe0/Fe3C-SMB700具有更高的无序度、石墨化程度及介孔比,为吸附和催化提供更多的活性点位,同时双铁活性金属进一步强化催化效率。因此,选择Fe0/Fe3C-SMB700作为代表催化剂来探究影响因素以及降解机理。
-
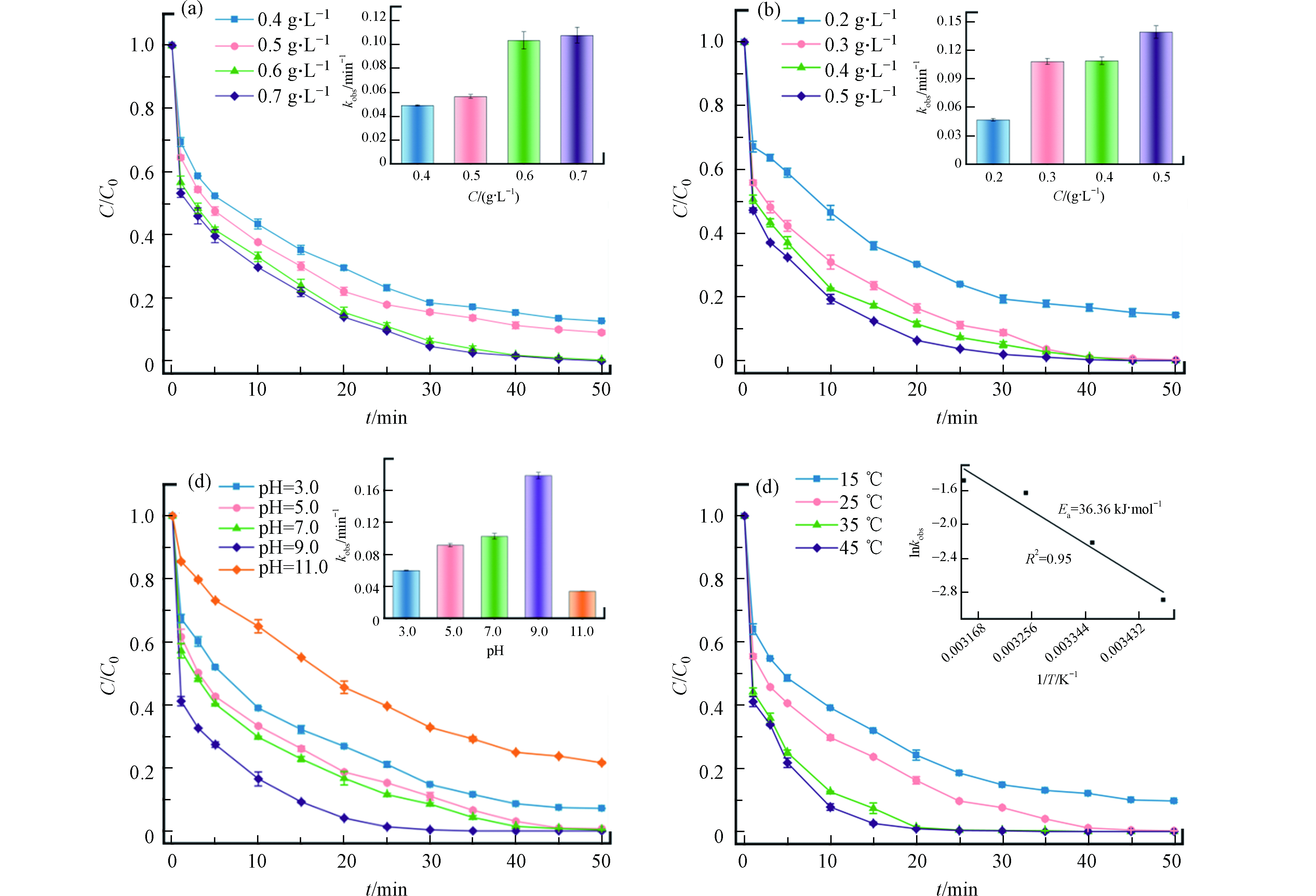
Fe0/Fe3C-SMB700的投加量对活化PMS降解SDZ效率影响如图6a所示,当催化剂用量从0.4 g·L−1增加到0.6 g·L−1时,50 min内SDZ的去除率从88%上升到100%,相应的表观速率常数(kobs)从0.0490 min−1上升到0.1033 min−1,这与较多催化投加量提供更多的反应活性点位有关;当Fe0/Fe3C-SMB700投加量进一步上升到0.7 g·L−1时,SDZ可在45 min内被完全去除,但其kobs(kobs=0.1076 min−1)较0.6 g·L−1时仅上升0.0043,这可能是由于PMS浓度的限制[19]。因此,在随后的实验中均使用0.6 g·L−1的催化剂。
不同PMS投加量对降解SDZ影响如图6b所示,当PMS浓度从0.2 g·L−1上升到0.3 g·L−1时,50 min内SDZ的去除率从86%提升到了100%,对应的kobs值从0.048 min−1上升至0.1039 min−1。当PMS浓度继续上升至0.4 g·L−1时,SDZ能在40 min内被完全降解,但其kobs值(kobs=0.1122 min−1)仅上升0.008,分析认为,当PMS浓度为0.2—0.3 g·L−1时,较大的PMS浓度有利于生成更多活性物质,提高SDZ降解速率,但当PMS浓度超过此范围,过量的PMS会因为自由基的竞争(式1—3)和清除反应(式4—5)降低污染物的去除效率[20]。选择合适的PMS剂量,既可以节约成本,且能达到更好的处理效果,因此在后续实验中使用0.3 g·L−1的PMS。
-
溶液的pH对SDZ去除效率有着重要的影响,由图6c可知,当pH=3.0—9.0时,SDZ的降解率随pH的增加而增大,相应的kobs值从0.0602 min−1增加到0.1735 min−1。当pH=9.0时,SDZ的降解效率最佳,在30 min内被完全去除。而当pH=11.0时,SDZ的去除率最差,50 min内SDZ仅去除了79%。分析认为:在偏酸性条件下,高浓度的H+会清除SO4·-和·OH(式 6—7)[21],降低反应速率。随着pH值的上升,H+的抑制作用减弱,SDZ降解率逐上升;当pH=9.0时,SDZ以去质子状态(SDZ−)存在,有利于SO4·-的亲电攻击,进一步提高去除效率[22];而当pH=11.0时,PMS发生自分解反应且SO4·-可能与高浓度OH−反应生成氧化电位较低的·OH,从而影响SDZ降解效率(式 8)[6]。综上所述,在中性以及弱碱性环境下,PMS能得到最好的催化效果,这与以往的一些研究结果是一致的[12,23]。
-
不同溶液温度对SDZ降解率由图6d所示,随着温度升高,SDZ降解效率显著提高。当溶液温度上升至45 ℃时,SDZ在30 min内能被完全降解,反应的kobs则从15 ℃时的0.0557 min−1增加到了0.2271 min−1,这可能是在高温下增强了PMS活化,加快了SO4·-的产生[18]。根据阿伦尼乌斯方程(式9)计算得到Fe0/Fe3C-SMB700的活化能为36.36 kJ·mol·L−1,略低于以往的一些研究[24],说明Fe0/Fe3C-SMB700具有较好的催化活性,本氧化降解反应较易发生。
-
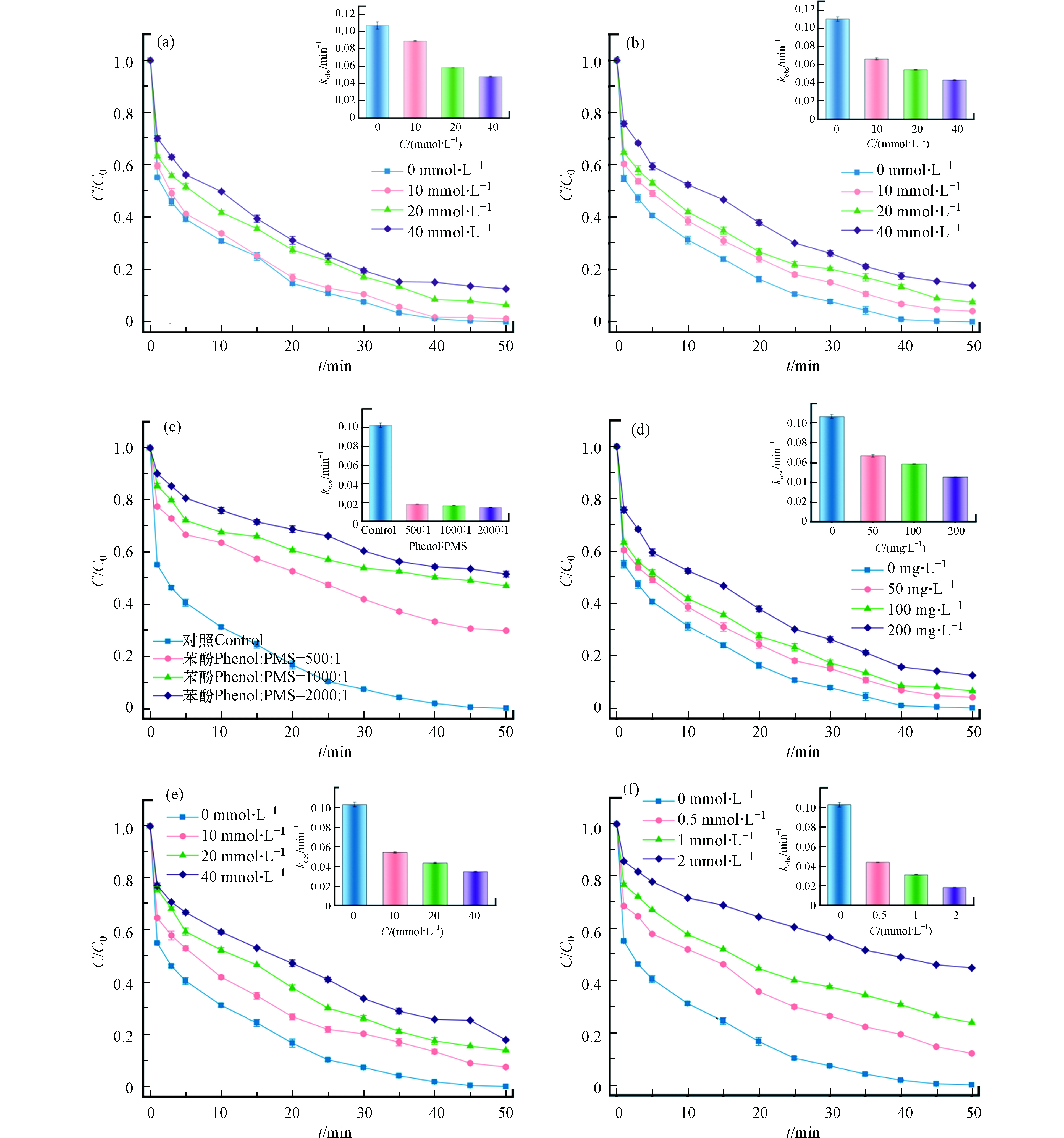
自然水体中广泛存在着大量的阴离子,这些阴离子会不同程度参与到SDZ的降解过程中。因此本实验选择水体中常见的4种阴离子Cl−、CO32-、H2PO4−和NO3−,考察其不同浓度(0、10、20、40 mmol·L−1)对反应过程的影响。Cl−对SDZ降解率的影响如图7a所示,当Cl−浓度分别为0、10、20、40 mmol·L−1时,对应的kobs值分别为0.1045、0.1149、0.1278、0.1777 min−1。由此可见,Cl−对SDZ的降解具有促进作用。分析认为,带负电荷的Cl−可以作为电子供体向 PMS提供电子,促进Fe0/Fe3C-SMB700催化PMS产生SO4·-和·OH。与此同时,大量的Cl−易被PMS氧化成具有较强氧化能力的HOCl/Cl2(式10—11)[25] ,且反应生成Cl·可继续氧化生成Cl2·-(式12)[26],进一步提高催化效率。
图7b的结果显示,CO32-对SDZ的降解反应有强烈的抑制作用。当CO32-的浓度从0 mmol·L−1上升至40 mmol·L−1时,50 min内SDZ降解效率从100%降至60%,kobs从0.1077 min−1减小至0.0216 min−1。原因可能是CO32-能有效捕获SO4·-和·OH,从而降低反应效率(式13—14)。CO32-与SO4·-、·OH反应的动力学常数分别为6.1×106 mol−1
$ \cdot $ s−1和3.9×108 mol−1$ \cdot $ s−1[27],因此与SO4·-相比,CO32-对·OH的抑制作用更强。通常,水中存在的H2PO4−会对过硫酸盐体系生成的自由基产生淬灭作用(式15—16)[28]。本次实验得到了相同的结果,如图7c所示,SDZ去除率随着H2PO4−浓度的升高而降低,50 min内其kobs值从0.1095 min−1降至0.0493 min−1。相较CO32-而言,H2PO4−对反应的抑制效果并不明显。可能是由于H2PO4−与SO4·-和·OH之间的反应速率是相对缓慢的,其反应的kobs分别为7.0×104 mol−1·s−1和(1—2)×104 mol−1·s−1。
图7d展示了NO3−对Fe0/Fe3C-SMB700/PMS反应体系的影响。从图中可以看到当NO3−的浓度从0 mmol·L−1增加到20 mmol·L−1,50 min内SDZ的降解率下降了10%,SDZ的降解并未受到明显的抑制,这与Yi等的实验结果一致[29]。造成这种现象的原因可能是由于与其他阴离子相比,NO3−具有一定程度的氧化能力,所以不能消耗高氧化电位的自由基。但当NO3−的浓度到40 mmol·L−1,SDZ的降解受到明显的抑制,50 min内,SDZ仅降解了80%,这一实验现象归因于当NO3−浓度较高时,其会同SO4·-反应生成氧化电位更低的NO3·(式17)[30]。
-
铁碳复合材料在催化PMS的过程中通常表现出不同的激活机制,包括自由基途径和非自由基途径。其中自由基途径包括SO4·-、·OH和O2·-等,非自由基途径又包括电子转移途径和1O2 等。因此,通过猝灭实验对催化反应中可能产生的降解途径进行分析,有助于了解Fe0/Fe3C-SMB700/PMS体系降解SDZ过程中不同部分的贡献以及机理。
甲醇(MeOH)和叔丁醇(TBA)通常用来判断体系中SO4·-和·OH的生成情况。其中MeOH能有效地淬灭反应溶液中的SO4·-和·OH,而TBA只能淬灭·OH[31]。结果如图8a、8b所示,当MeOH和TBA的初始浓度从0 mmol·L−1增加到 40 mmol·L−1时,SDZ的去除率分别从100%下降至86%和88%,可以看出MeOH和TBA对自由基的抑制作用并不明显,可能是由于MeOH为亲水化合物,不能很好地猝灭存在于固-液界面的自由基,而Fe0/Fe3C-SMB700表面丰富的微孔和介孔的存在为活性物种的转移提供了有利条件。而对于MeOH较TBA无明显的抑制优势可能与黏性的TBA堵塞催化剂孔道或是阻止碳表面的电子转移[32]有关。因此,需要进一步实验来探明Fe0/Fe3C-SMB700/PMS反应体系中SO4·-和·OH的生成情况。
苯酚 (phenol)与SO4·-和·OH均有较高的反应速率常数,且极性较弱,因此苯酚能有效地消除催化剂表面自由基。由图8c可见,当苯酚/PMS物质的量比为2000:1的时,50 min内SDZ的去除率仅为49%,且反应的kobs从0.1030 min−1降至0.0150 min−1,说明SO4·-和·OH对SDZ的降解有着重要作用。另外,由于硝基苯(NB)具有疏水性,且其与·OH的反应速率是SO4·-的3000倍[33]。因此,可以通过添加硝基苯来探明催化剂表面键合的·OH的作用。如图8d所示,当硝基苯的浓度为200 mg·L−1时,SDZ的去除率为88%。由此可见,Fe0/Fe3C-SMB700/PMS反应体系中,SO4·-的作用远大于·OH。
将对苯醌(p-BQ)作为抑制剂鉴定体系中可能存在的O2·-,结果如图8e所示。在对苯醌(p-BQ)存在时,SDZ的降解反应受到了一定的抑制。当p-BQ的浓度为40 mmol·L−1时,50 min内SDZ被降解了83%,说明O2·-参与了SDZ降解反应。而组氨酸(L-H)作为1O2有效捕获剂,L-H对SDZ降解的影响反映了非自由基途径中1O2对PMS活化的贡献。如图8f所示,当L-H的浓度为2 mmol·L−1时,50 min内SDZ仅被降解了55%,说明SDZ的降解过程中,1O2具有突出的贡献。
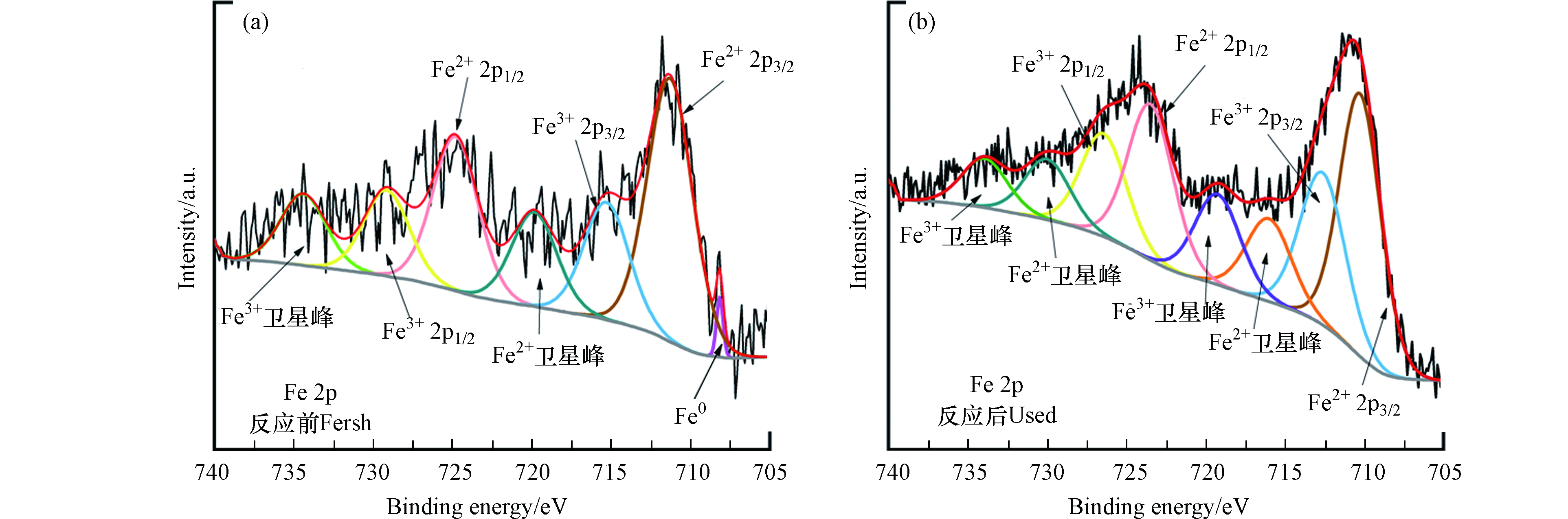
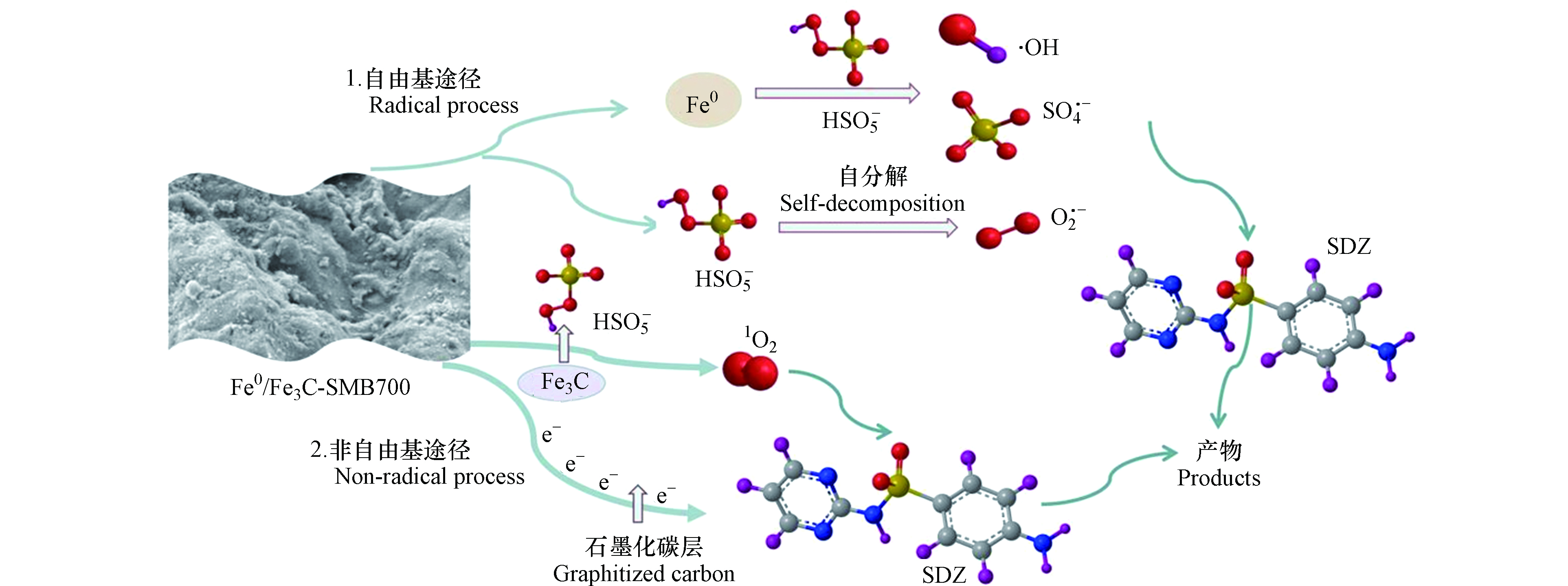
为了进一步探索反应机理,比较了新鲜的和使用过的两种Fe0/Fe3C-SMB700的Fe 2p的光谱 (图9a、9b)。反应前的Fe0/Fe3C-SMB700在708.1 eV的特征峰归属于Fe0;711.29 eV和724.8 eV的特征峰分别由Fe2+的2p2/3和2p1/2轨道自旋产生;Fe3+的2p2/3和2p1/2轨道的特征峰分别位于715.29 eV和729.1 eV。Fe2+和Fe3+可能是Fe0/Fe3C-SMB700保存过程中Fe0被氧化产生。反应后的Fe0/Fe3C-SMB700表面Fe0的峰消失了,且Fe2+含量略有减少,而Fe3+含量增加。这意味着Fe0参与了催化降解反应,且部分Fe2+转化为Fe3+。基于上述分析,提出了两种详细的自由基途径,分别是Fe0激活PMS产生活性物种以及PMS自分解产生O2·-。(1)Fe0可以直接活化PMS生成SO4·-和·OH(式18—19),随之产生的Fe2+也可以进一步催化PMS分解生成SO4·-(式20)。此过程中产生的SO4·-还可以与水反应生成·OH(式21)。(2)研究表明PMS能自分解生成O2·-(式22—23)[34],p-BQ猝灭实验也证实了反应体系中O2·-的存在。此外,HSO5−/SO4·-的还原电位(2.5—3.1 V)大于O2·-/O2的还原电位(-0.33 V),O2·-还可以还原HSO5−生成SO4·-(式24)。
在非自由基机理中,1O2对SDZ降解的贡献十分显著。Fe0/Fe3C-SMB700的Fe3C纳米颗粒可以显著改变碳在碳壳中的电子分布[35],产生更多的活性碳位,从而还原PMS形成SO5·-这种暂态物种(式25)。SO5·-通过自分解、与水反应可形成大量的1O2 (式26—28)[36]。另外,Fe0/Fe3C-SMB700表面的sp2杂化碳结构具有大量可自由移动的π电子,这些π电子可以通过电子转移激活和破坏PMS中的O—O键,对SDZ的降解也有一定的作用。如图10所示,上述分析结果产生的SO4·-、·OH、O2·-和1O2 共同参与SDZ的高效降解过程。
-
催化剂的有效回收能增加其利用率,从图11a可以看到,Fe0/Fe3C-SMB700可通过外置磁铁快速与反应溶液分离。催化剂的稳定性也是衡量其催化性能的重要指标之一,通过循环实验和反应溶液浸出铁离子浓度测定对催化剂稳定性进行分析,结果分别如图11b—c所示。5次循环后,SDZ的降解率从100%下降至82%,反应液中浸出铁则由0.94 mg·L−1降至0.09 mg·L−1。假设浸出铁均为Fe2+离子,研究了Fe2+/PMS体系对SDZ的降解效果。如图11d所示,均相过程在前15 min具有较快的反应速率,30% SDZ被降解,但15 min后降解速率变慢,50 min内SDZ的降解率仅为42%。对照实验表明,非均相过程(Fe0/Fe3C-SMB700/PMS)优于均相过程(Fe2+/PMS)。因此,在Fe0/Fe3C-SMB700PMS体系中SDZ的降解效果主要来源于非均相催化降解。
随着循环次数的增加SDZ的降解效率逐渐降低可能是由于Fe0/Fe3C-SMB700表面吸附了SDZ中间产物所致。据报道,降解过程产生的中间产物可能会钝化活性位点并且堵塞催化剂的多孔结构[37]。为了进一步验证此推测,对反应前后Fe0/Fe3C-SMB700进行XPS分析。如图12a—b所示,新鲜和已使用的O 1s图谱在531.1 eV、532.2 eV 和 534.2 eV出现的3个特征峰分别是晶格氧(Olatt),表面氧(Osurf)和吸附氧(Oads),其中Oads通常由C=O,—OH和—COOH组成[38]。氧化反应后Oads从8.6%增加到53.4%,证实了Fe0/Fe3C-SMB700的表面产生了中间产物。反应后Osurf从42.8%降至29.8%,Osurf在反应过程中可以增强催化酸性[23],这也解释了随着循环次数增多,降解效率下降的现象。此外,还研究了新鲜和使用过的Fe@SMB700的C 1s的XPS谱。从图12c—d可以看出,新鲜和使用过的Fe0/Fe3C-SMB700均可以在284.8 eV(sp2-C)、286.2 eV(C-OH)和289.0 eV(COOH)的3个结合能位置处进行拟合。反应后,sp2-C含量从56.6%上升至77.7%,这一现象表明,吸附在Fe0/Fe3C-SMB700表面的中间体主要含有C=C。
-
(1)以羊粪生物炭为原料通过共沉淀法制备的Fe3O4-SMB在500、600、700 ℃下快速热解成功得到了Fe@SMBs复合材料。
(2)表征结果显示,Fe0/Fe3C-SMB700较Fe@SM600、Fe@SMB500和Fe3O4-SMB有更强的催化活性,与其较大的比表面积、高度石墨化结构,以及产生大量的Fe0和Fe3C有关。
(3)在最适投加量条件下(0.3 g·L−1PMS和0.6 g·L−1Fe0/Fe3C-SMB700),10 mg·L−1的SDZ能在50 min内被完全去除。在中性及弱碱性环境中,Fe0/Fe3C-SMB700活化PMS的能力最佳。
(4)Fe0/Fe3C-SMB700具有较好的可回收性和稳定性,在循环使用5次时仍能达到82%的SDZ去除效率,造成SDZ降解效率降低的原因可能是降解过程产生的中间产物堵塞了反应活性位点。
(5)猝灭实验和XPS分析结果表明,自由基途径(SO4·-、·OH和O2·-)和非自由基途径(1O2和电荷转移)共同作用于SDZ的降解。其中自由基途径以Fe0为主要活性点位,非自由基途径以Fe3C产生的1O2为主。
Fe0/Fe3C羊粪生物炭复合材料的制备及其活化过一硫酸盐降解磺胺嘧啶
Preparation of Fe0/Fe3C sheep manure biochar composites for activating peroxymonosulfate to degrade sulfadiazine
-
摘要: 以Fe3O4-羊粪生物炭为原料,热解制备铁功能化的生物炭复合材料(Fe0/Fe3C-SMB700)用于活化过一硫酸盐(PMS)降解磺胺嘧啶(SDZ)。结果表明,在700 ℃下制备的Fe0/Fe3C-SMB700,因双铁Fe0/Fe3C功能化结构、高度石墨化及层状多孔结构表现出最强的催化活性。在最优反应条件下(0.3 g·L−1 PMS和0.6 g·L−1催化剂),10 mg·L-1的SDZ在50 min内被完全去除。Fe0/Fe3C-SMB700可通过外部磁铁进行有效回收,并且经过5次循环使用仍能保持良好的催化活性。猝灭实验及X射线光电子能谱分析结果表明,自由基与非自由基途径均对SDZ的降解有着重要影响,其中自由基途径以Fe0激活PMS产生SO4·−为主,非自由基途径主要通过Fe3C改变碳电子分布产生1O2,而Fe0/Fe3C-SMB700的石墨碳层结构为非自由基途径提供了良好的电子转移场所。综上,本研究通过以废制废的方式制备双铁功能化的催化材料,不仅实现了粪源废物的资源化利用,同时为有机废水高效的处理方法提供了技术支撑。Abstract: Fe3O4-sheep manure biochar was used for the preparation of iron-functionalized biochar composite (Fe0/Fe3C-SMB700) by pyrolysis for the degradation of sulfadiazine (SDZ) by activating peroxymonosulfate (PMS) . The results showed that Fe0/Fe3C-SMB700, prepared at 700 °C, exhibited the strongest catalytic activity due to the functionalized structure of double iron (Fe0/Fe3C), high graphitization and layered porous structure. Under the optimal reaction conditions (0.3 g·L−1 PMS and 0.6 g·L−1 catalyst) , 10 mg·L−1 SDZ was completely removed within 50 min. Fe0/Fe3C-SMB700 could be effectively recovered by external magnets and retained good catalytic activity after 5 cycles of use. The results of quenching experiments and X-ray photoelectron spectroscopy showed that both radical pathway and non-radical pathway had important effects on the degradation of SDZ, in which the free radical pathway was dominated by SO4·−produced by Fe0 activating PMS, and the non-radical pathway mainly caused by Fe3C changing the carbon electron distribution to produce 1O2, while the graphitic carbon layer structure of Fe0/Fe3C-SMB700 provided a good electron transfer site for the non-radical pathway. Consequently, this study prepares a catalytic material with double iron functionalization by waste-to-waste method, which not only realizes the resource utilization of manure-sourced waste but also provides the technical support for the efficient treatment method of organic wastewater.
-
Key words:
- peroxymonosulfate /
- sulfadiazine /
- sheep manure biochar /
- Fe0 /
- Fe3C
-

-
表 1 样品的比表面积和孔隙参数
Table 1. Specific surface areas and pore parameters of different composites
样品
Sample比表面积/(m2·g−1)
SBET总孔容/(cm3·g−1)
Total pore volume微孔孔容/(cm3·g−1)
Micropore volume平均孔径/nm
DpFe3O4-SMB 8.3 0.006 0 9.7 Fe@SMB500 45.6 0.011 0.003 9.2 Fe@SMB600 76.2 0.036 0.042 9.1 Fe0/Fe3C-SMB700 136.2 0.122 0.068 8.9 -
[1] CHENG D L, NGO H H, GUO W S, et al. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches [J]. Journal of Hazardous Materials, 2020, 387: 121682. doi: 10.1016/j.jhazmat.2019.121682 [2] STANGE C, TIEHM A. Occurrence of antibiotic resistance genes and microbial source tracking markers in the water of a karst spring in Germany [J]. Science of the Total Environment, 2020, 742: 140529. doi: 10.1016/j.scitotenv.2020.140529 [3] WANG L, YOU L X, ZHANG J M, et al. Biodegradation of sulfadiazine in microbial fuel cells: Reaction mechanism, biotoxicity removal and the correlation with reactor microbes [J]. Journal of Hazardous Materials, 2018, 360: 402-411. doi: 10.1016/j.jhazmat.2018.08.021 [4] WANG S Z, WANG J L. Peroxymonosulfate activation by Co9S8@ S and N co-doped biochar for sulfamethoxazole degradation [J]. Chemical Engineering Journal, 2020, 385: 123933. doi: 10.1016/j.cej.2019.123933 [5] CHEN X W, VIONE D, BORCH T, et al. Nano-MoO2 activates peroxymonosulfate for the degradation of PAH derivatives [J]. Water Research, 2021, 192: 116834. doi: 10.1016/j.watres.2021.116834 [6] GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review [J]. Chemical Engineering Journal, 2017, 31: 41-62. [7] DIAO Z H, ZHANG W X, LIANG J Y, et al. Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism [J]. Chemical Engineering Journal, 2021, 409: 127684. doi: 10.1016/j.cej.2020.127684 [8] ZANG T C, WANG H, LIU Y H, et al. Fe-doped biochar derived from waste sludge for degradation of rhodamine B via enhancing activation of peroxymonosulfate [J]. Chemosphere, 2020, 261: 127616. doi: 10.1016/j.chemosphere.2020.127616 [9] ZHANG Y, JIANG Q, JIANG S M, et al. One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine [J]. Chemical Engineering Journal, 2021, 420: 129868. doi: 10.1016/j.cej.2021.129868 [10] GAO J, HAN D Q, XU Y, et al. Persulfate activation by sulfide -modified nanoscale iron supported by biochar (S-nZVI/BC) for degradation of ciprofloxacin [J]. Separation and Purification Technology, 2020, 235: 116202. doi: 10.1016/j.seppur.2019.116202 [11] LI W, ZHANG Y L, ZHAO P J, et al. Enhanced kinetic performance of peroxymonosulfate/ZVI system with the addition of copper ions: Reactivity, mechanism, and degradation pathways [J]. Journal of Hazardous Materials, 2020, 393: 122399. doi: 10.1016/j.jhazmat.2020.122399 [12] JIANG S F, LING L L, CHEN W J, et al. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors [J]. Chemical Engineering Journal, 2019, 359: 572-583. doi: 10.1016/j.cej.2018.11.124 [13] FU H C, ZHAO P, XU S J, et al. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism [J]. Chemical Engineering Journal, 2019, 375: 121980. doi: 10.1016/j.cej.2019.121980 [14] MALDONADO-HODAR F J, MORENO-CASTILLA C, RIVERA-UTRILLA J, et al. Catalytic graphitization of carbon aerogels by transition metals [J]. Langmuir, 2000, 16(9): 4367-4373. doi: 10.1021/la991080r [15] MANGARELLA M C, EWBANK J L, DUTZER M R, et al. Synthesis of embedded iron nanoparticles in Fe3C-derived carbons [J]. Carbon, 2014, 79: 74-84. doi: 10.1016/j.carbon.2014.07.044 [16] DUAN X G, SUN H Q, KANG J, et al. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons [J]. Acs Catalysis, 2015, 5(8): 4629-4636. doi: 10.1021/acscatal.5b00774 [17] GONG Y N, LI D L, LUO C Z, et al. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors [J]. Green Chemistry, 2017, 19(17): 4132-4140. doi: 10.1039/C7GC01681F [18] FU H C, MA S L, ZHAO P, et al. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism [J]. Chemical Engineering Journal, 2019, 360: 157-170. doi: 10.1016/j.cej.2018.11.207 [19] LUO J M, BO S F, QIN Y N, et al. Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation [J]. Chemical Engineering Journal, 2020, 395: 125063. doi: 10.1016/j.cej.2020.125063 [20] YOU Y, ZHAO Z J, SONG Y R, et al. Synthesis of magnetized nitrogen-doped biochar and its high efficiency for elimination of ciprofloxacin hydrochloride by activation of peroxymonosulfate [J]. Separation and Purification Technology, 2021, 258: 117977. doi: 10.1016/j.seppur.2020.117977 [21] AHMADI M, GHANBARI F. Combination of UVC-LEDs and ultrasound for peroxymonosulfate activation to degrade synthetic dye: influence of promotional and inhibitory agents and application for real wastewater [J]. Environmental Science and Pollution Research, 2018, 25(6): 6003-6014. doi: 10.1007/s11356-017-0936-8 [22] YANG J F, HE M, WU T F, et al. Sulfadiazine oxidation by permanganate: Kinetics, mechanistic investigation and toxicity evaluation [J]. Chemical Engineering Journal, 2018, 349: 56-65. doi: 10.1016/j.cej.2018.05.018 [23] GUO W Q, ZHAO Q, DU J S, et al. Enhanced removal of sulfadiazine by sulfidated ZVI activated persulfate process: Performance, mechanisms and degradation pathways [J]. Chemical Engineering Journal, 2020, 388: 124303. doi: 10.1016/j.cej.2020.124303 [24] ZHU S J, WANG W, XU Y P, et al. Iron sludge-derived magnetic Fe0/Fe3C catalyst for oxidation of ciprofloxacin via peroxymonosulfate activation [J]. Chemical Engineering Journal, 2019, 365: 99-110. doi: 10.1016/j.cej.2019.02.011 [25] JAVIER RIVAS F, SOLIS R R. Chloride promoted oxidation of tritosulfuron by peroxymonosulfate [J]. Chemical Engineering Journal, 2018, 349: 728-736. doi: 10.1016/j.cej.2018.05.117 [26] MA J, YANG Y, JIANG X, et al. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water [J]. Chemosphere, 2018, 190: 296-306. doi: 10.1016/j.chemosphere.2017.09.148 [27] ZHOU T, ZOU X, MAO J, et al. Decomposition of sulfadiazine in a sonochemical Fe-catalyzed persulfate system: Parameters optimizing and interferences of wastewater matrix [J]. Applied Catalysis B-Environmental, 2016, 185: 31-41. doi: 10.1016/j.apcatb.2015.12.004 [28] MA D M, YANG Y, LIU B F, et al. Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation [J]. Chemical Engineering Journal, 2021, 408: 127992. doi: 10.1016/j.cej.2020.127992 [29] YI Q Y, TAN J L, LIU W Y, et al. Peroxymonosulfate activation by three-dimensional cobalt hydroxide/graphene oxide hydrogel for wastewater treatment through an automated process [J]. Chemical Engineering Journal, 2020, 400: 125965. doi: 10.1016/j.cej.2020.125965 [30] GUO Y P, ZENG Z Q, LIU Y J, et al. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics [J]. Journal of Materials Chemistry A, 2018, 6(9): 4055-4067. doi: 10.1039/C7TA09814F [31] BENNER J, TERNES T A. Ozonation of metoprolol: Elucidation of oxidation pathways and major oxidation products [J]. Environmental Science & Technology, 2009, 43(14): 5472-5480. [32] WANG H Z, GUO W Q, LIU B H, et al. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism [J]. Water Research, 2019, 160: 405-414. doi: 10.1016/j.watres.2019.05.059 [33] NETA P, HUIE R E, ROSS A B. Rate constants for reactions of inorganic radicals in aqueous solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. doi: 10.1063/1.555808 [34] LI Y, MA S L, XU S J, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4 [J]. Chemical Engineering Journal, 2020, 387: 124094. doi: 10.1016/j.cej.2020.124094 [35] HUANG Z Y, WU P X, LIU C H, et al. Multiple catalytic reaction sites induced non-radical/radical pathway with graphene layers encapsulated Fe-N-C toward highly efficient peroxymonosulfate (PMS) activation [J]. Chemical Engineering Journal, 2021, 413: 127507. doi: 10.1016/j.cej.2020.127507 [36] TAKDASTAN A, KAKAVANDI B, AZIZI M, et al. Efficient activation of peroxymonosulfate by using ferroferric oxide supported on carbon/UV/US system: A new approach into catalytic degradation of bisphenol A [J]. Chemical Engineering Journal, 2018, 331: 729-743. doi: 10.1016/j.cej.2017.09.021 [37] HUANG G X, WANG C Y, YANG C W, et al. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 nanospheres: Synergism between Mn and Fe [J]. Environmental Science & Technology, 2017, 51(21): 12611-12618. [38] DING D H, YANG S J, CHEN L W, et al. Degradation of norfloxacin by CoFe alloy nanoparticles encapsulated in nitrogen doped graphitic carbon (CoFe@N-GC) activated peroxymonosulfate [J]. Chemical Engineering Journal, 2020, 392: 123725. doi: 10.1016/j.cej.2019.123725 -



 下载:
下载: