-
铁作为地球丰度元素,是地球岩石和土壤的重要组成部分,不仅参与了植物的光合作用、新陈代谢、呼吸过程中电子的转移和氮的还原等重要过程[1-2],也参与了生物体内多种生命过程. 如细胞色素P450酶是广泛存在的含亚铁血红素单加氧酶,其主要活性位点就是亚铁卟啉蛋白[3]. 铁是多种功能材料的催化活性中心,还有助于构建材料的吸附位点. 例如Li等[4]制备的铁基金属有机骨架材料(MOF)上丰富的铁活性位点能高选择性地吸附CO2. Qin等[5]采用共沉淀法构建的具有氧空位缺陷的铁基改性生物炭材料,能实现对水中汞的优异吸附. 香蒲(Cattail)作为一种能超富集铁的沼生草本植物,主要通过根部对金属进行富集[6-8],已被广泛地应用于土壤修复[9-10].
生物炭是由农作物秸秆、木屑等生物质经过热裂解制备成富含炭的固体物质[11],因其在土壤修复、减缓气候变化、废弃物资源化和新能源开发领域具有良好应用价值而受到广泛关注[12-15]. 生物炭具有较大的比表面积、多孔结构和丰富的表面官能团,是一种能替代活性炭的优良吸附剂[6,16-18]. 目前,随着土壤重金属污染形势加剧,富集了金属的植物数量也日益增多,由此转化的金属负载型生物炭材料也越来越易得[19-21]. 如Paul等[19]监测发现由蔬菜废弃物制备的生物炭材料中Zn、Cu、Ni和Pb的含量分别高达120.2、16.8、34.3、12.3 g·kg−1,在经堆肥处理后这些金属含量分别提高至250.0、32.2、60.7、25.2 g·kg−1. 这些废弃植物为原料所制备的生物炭材料性质可能会随着富集金属元素的类型或含量的变化而发生改变. 如Wang等[21]发现,超富集Fe,Mn和Zn的生物炭能有效活化过硫酸盐,且其持久性自由基、含氧官能团、缺陷位点和类石墨结构方面随着热化学转化行为的不同存在着显著差异.
据报道,生物炭表面—C—OH、—COOH和—COOR等官能团的含量和类别,会随着负载的金属元素的不同类型而发生变化,以实现生物炭对污染物的去除作用[22-23]. 例如Wu等[24]以水稻秸秆为原料,将其浸泡在氯化铁和氯化亚铁的混合溶液中,并在氮气氛围下搅拌后热裂解制得铁负载型生物炭材料,能有效促进盐碱地土壤对磷的吸附. 曾凤美等[25]采用热活化法辅以加压超声浸渍技术将硅酸盐水泥颗粒(PC)和Fe2O3与稻壳生物炭(RHC)混合,得到具有高选择性吸附磷的复合多孔炭材料(Fe-PC/RHC).负载了铁氧化物颗粒的生物炭复合材料对底物吸附性能的提升,主要是通过增加其比表面积和孔结构等物理途径来实现. Wu等[20]通过热解Mn超富集植物(美洲商陆)制备负载MnOx生物炭,却发现生物炭的含氧官能团的含量随着MnOx的引入而增加. 然而,由超富集Fe的植物制备的铁负载型生物炭材料的表面官能团是否会发生变化以及铁的富集对吸附性能的影响,还有待进一步研究.
本文将通过原位富集铁的香蒲根制备铁生物炭,采用SEM/EDX、XRD和FTIR等手段对样品的形貌、化学组成和官能团结构进行分析表征,并重点探究自富集途径对生物炭表面性质(比表面积、孔径和基团类型)的影响,并以阳离子染料结晶紫为目标污染物,重点探讨了铁香蒲根生物炭的吸附机制. 该研究将应用在污水处理领域中的生物炭吸附材料的选择提供理论基础.
-
冰醋酸、无水乙醇、福尔马林、二甲苯、双氧水、七水硫酸亚铁、高锰酸钾、氯化钠、硝酸、氢氟酸、氢氧化钠、盐酸、亚铁氰化钾铁、中性红等均为分析纯,实验用水为蒸馏水. 磺酰罗丹明B (sulfonyl rhodamine B,SRB)、结晶紫(crystal violet,CV)、金橙II(golden orange II,OII)、罗丹明B(rhodamine B,RhB). 甲醛(5 mL)-乙醇(70% 无水乙 醇 90 mL)-乙酸(5 mL)溶液(FAA)。
Nano ZS90型纳米粒度及Zeta电位仪(Malvern,英国);JSM-7500F型X射线衍射仪(XRD)(Rigaku,日本);JSM-7500F型冷场发射扫描电子显微镜(SEM)(JEOL,日本);JW-BK112型比表面积及孔径分析仪(BET)(JWGB,中国);Nicolet iS50傅立叶变换红外光谱仪(ATR-FTIR)(Thermo Fisher Scientific,美国);U-3010紫外分光光度计(Hitachi,日本);Spectr AA-600型原子吸收光谱仪(AAS)(Varian,美国). X-射线荧光分析仪(XRF)(EDX-7000,日本岛津).
-
从三峡大学求索溪中选取宽叶香蒲,待其自净两周后,取长势相同的植株进行铁胁迫水培试验. 每隔一周浇灌1次100 mol·L−1的FeSO4·7H2O溶液. 两个月后分别收集植物的根、茎和叶部,切碎并烘干. 然后,在氮气气氛下以17 ℃·min−1升到700 ℃烧2 h,自然冷却,研磨成粉末,最后过80目筛网.
生物炭中铁的含量通过原子吸收光谱仪进行测定,结果见表1. 取生物炭样品0.1 g放入消解罐,向其中加入5 mL硝酸和1 mL氢氟酸,放入烘箱中160 ℃消解10 h至溶液透明,将消解罐放在石墨电热板上180 ℃加热,赶酸至溶液剩余约1 mL,将剩下样品加入25 mL比色管中用3%硝酸溶液定容后,用火焰法测定消解后样品中总Fe元素的含量. 根据表1可知,Fe主要富集在香蒲根部,因此本文采用的生物炭材料是富集了铁(Iron)的香蒲根(Cattail root),铁富集浓度为100 mol·L−1,将其标记为CRI-100. 未进行富集的香蒲根为空白组,标记为CRI-0.
其中,Cv是原子吸收光谱仪测得的浓度(mg·L−1);m为所称取的生物炭质量(g);Cm是不同部位所制备的生物炭中铁的浓度(mg·g−1).
比表面积(specific surface area,SSA)是决定生物炭吸附性能的关键因素[26],通过对比发现,实验组的比表面积普遍大于空白组,说明经过铁富集能有效地增大其比表面积. 预示着铁富集对其吸附性能有利. 此外,实验所采用的香蒲根(120.84 m2·g−1)远远大于空白根(8.05 m2·g−1),为保证与香蒲根生物炭具有相近的比表面积,对照试验中外负载型铁生物炭是由空白茎制备的(119.99 m2·g−1)而非空白根.
外负载型生物炭样品:以空白香蒲茎生物炭(CL)作为载体,将其浸入到不同初始浓度为的柠檬酸铁溶液中,搅拌12 h,离心,用大量水洗涤,以除去表面上残留的铁离子. 根据铁浓度(0.2、2、10 mol·L−1)分别标记为CL-0.2M,CL-2M和CL-10M. 此外,采用超声分散法将Fe2O3直接和空白茎生物炭粉末混合,制得负载Fe2O3生物炭,命名为C-Fe2O3.
-
材料选取为添加铁元素2个月后的植物,将新鲜的植株取根部洗净,裁剪后放入FAA溶液中固定24 h以上. 再按照70%乙醇→85%乙醇→95%乙醇→100%乙醇→100%乙醇→50%乙醇+50%二甲苯→100%二甲苯→100%二甲苯→蜡杯中过夜(37 ℃)中逐级脱水. 之后采用纯蜡浸泡材料,至于60 ℃烘箱,4 h换1次蜡,重复3次后包埋,放置3—4 d风干. 用切片机切4 μm厚片,置于载玻片,置于37 ℃展片台上伸展,放置3—4 d干燥. 用纯二甲苯至脱蜡完全,以下步骤每级5 min,50%二甲苯+50%乙醇→100%乙醇→95%乙醇→85%乙醇→70%乙醇→50%乙醇,用普鲁士蓝染液染色20 min; 蒸馏水洗涤,再用1%伊红对比染色1 min,蒸馏水洗涤,最后用95%乙醇至无水乙醇脱水、二甲苯透明、中性树胶封片. 将晾干后带有样品的载玻片置于Olympus CX41电子显微镜下观察并拍照.
-
在pH=6.2条件下,在50 mL结晶紫(CV)(5×10−5 mol·L−1)水溶液中加入10 mg香蒲根生物炭样品,其吸附动力学曲线满足一级动力学反应:
其中,C0和Ct分别是0时和t时的CV剩余浓度. 通过UV-Vis光谱法,测定CV在590 nm最大吸收波长处的吸光度值,计算得到C0和Ct,拟合直线的斜率为吸附动力学常数(kobs).
-
在CRI-100生物炭和CV的悬浮液中,分别添加不同含量的NaF和EDTA-Na,测定该体系吸附对CV的动力学曲线. 同时,采用氟离子选择性电极法测定了生物炭样品表面羟基位点数:将0.05 g样品分散到10−5 mol·L−1的NaF溶液中,搅拌3 h后测定氟离子浓度,与初始浓度10−5 mol·L−1的差值即为样品表面活性位点的浓度[27].
-
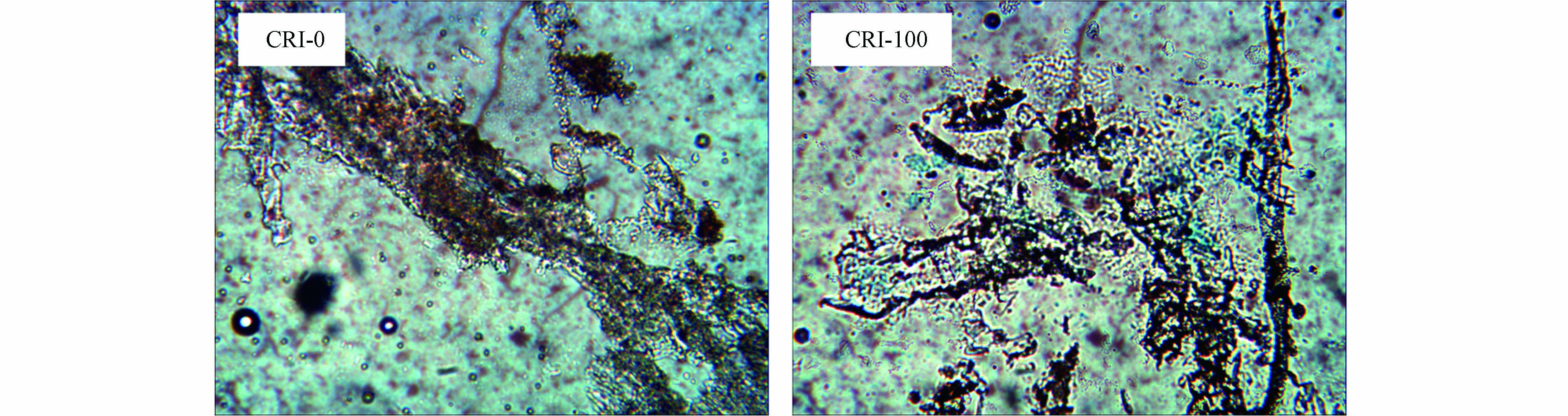
根据根部组织切片图确定铁在香蒲根上分布状况(图1). 通过普鲁士蓝和中性红对香蒲根进行染色,结果发现,CRI-0组只有中性红染成肉红色的细胞质和细胞外基质而未见与普鲁士蓝络合铁后的蓝色,说明未经胁迫的香蒲根中不含铁,只含有植物细胞. 而在CRI-100组中可以看见染色特别明显的蓝色成分,说明经铁胁迫后,铁沉积在了香蒲根细胞上.
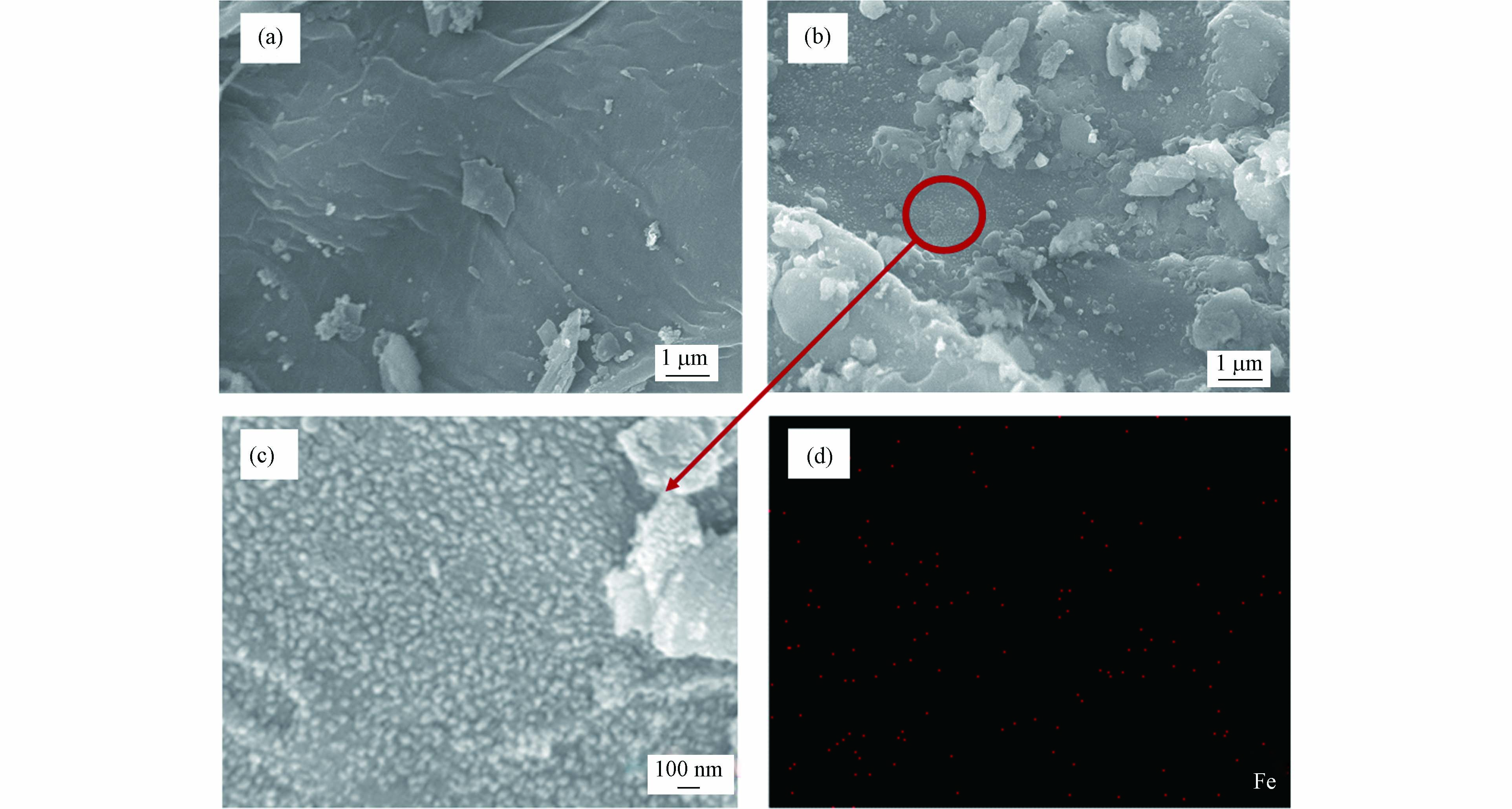
图2a和b可以观察到CRI-0的表面比较光滑平整,CRI-100的表面粗糙. 图2b的局部放大的照片显示有大量颗粒物质均匀地镶嵌在生物炭表面(图2c),由图2d可以看到CRI-100表面铁元素分布均匀,因此推测这些凸起的颗粒有可能为铁颗粒物质.
EDS数据发现富集铁后CRI-100表面铁含量为5.14%(表2),X射线荧光光谱(XRF)数据也发现铁的含量从CRI-0的7.262%升至CRI-100的16.124%(表3),这说明两生物炭样品都含有铁,只是前者表面含铁量过低EDS无法检测到. 这些结果说明通过香蒲根自富集途径得到的铁生物炭表面形成了分布均匀的铁颗粒,这可能是引起CRI-100比表面积增加的主要原因.
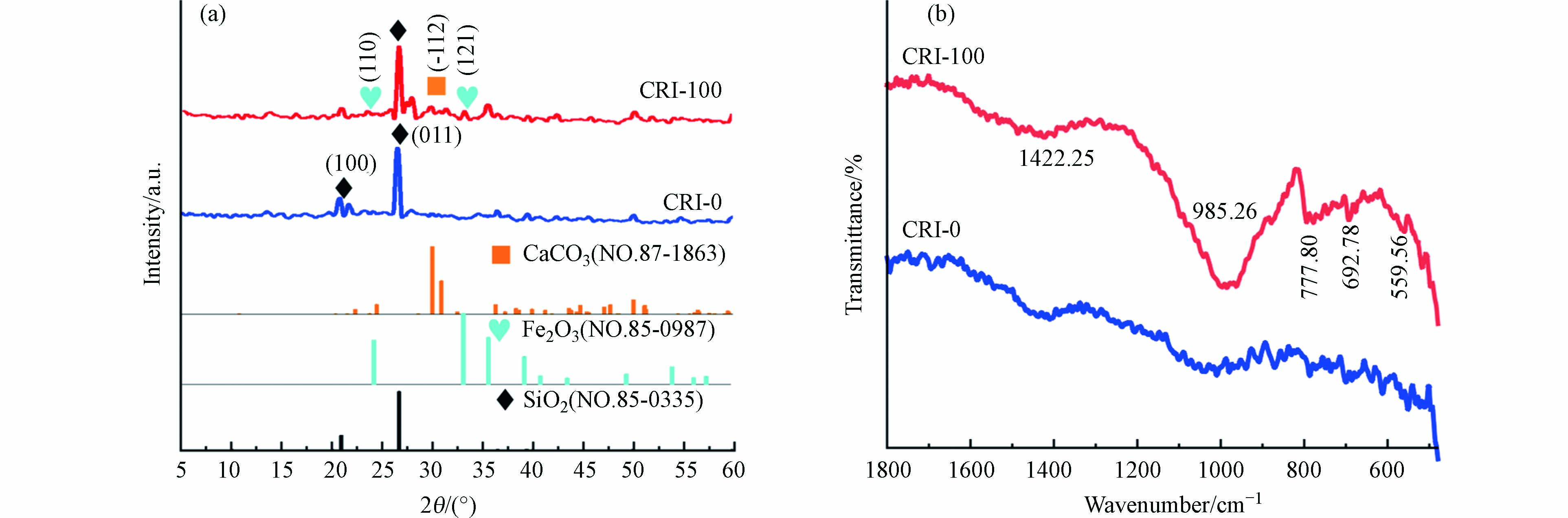
从图3a可以看出,CRI-0主要衍射角2θ为20.87º和26.65º处对应为SiO2(100)和(011)衍射峰(JCPDS No.70-3755). 2θ在29.78º和31.43º峰归属为CaCO3的(-122)和(-202)晶面(JCPDS No.29-0305)[28]. 结果说明,CRI-100除了含SiO2和CaCO3成分外,在35.68º处还有Fe2O3的(121)晶面的特征峰(JCPDS No.25-1402)[29],进一步证明镶嵌在生物炭表面的铁颗粒为Fe2O3. 通过FT-IR识别CRI-0和CRI-100存在样品表面的官能团(图3b). 1422 cm−1是由生物炭表面C—H键变形振动引起的[18],985 cm−1处的吸收峰是Si—OH的伸缩振动引起的,881 cm−1处的吸收峰是Si—O—Si的特征峰[19]. 778 cm−1处的吸收峰为CO32-的特征吸收峰. 693 cm−1处为Fe—OH的振动峰[11],559 cm−1吸收峰是由Fe—O的晶格振动引起,再次证明CRI-100存在着Fe2O3,与XRD的结论一致.
-
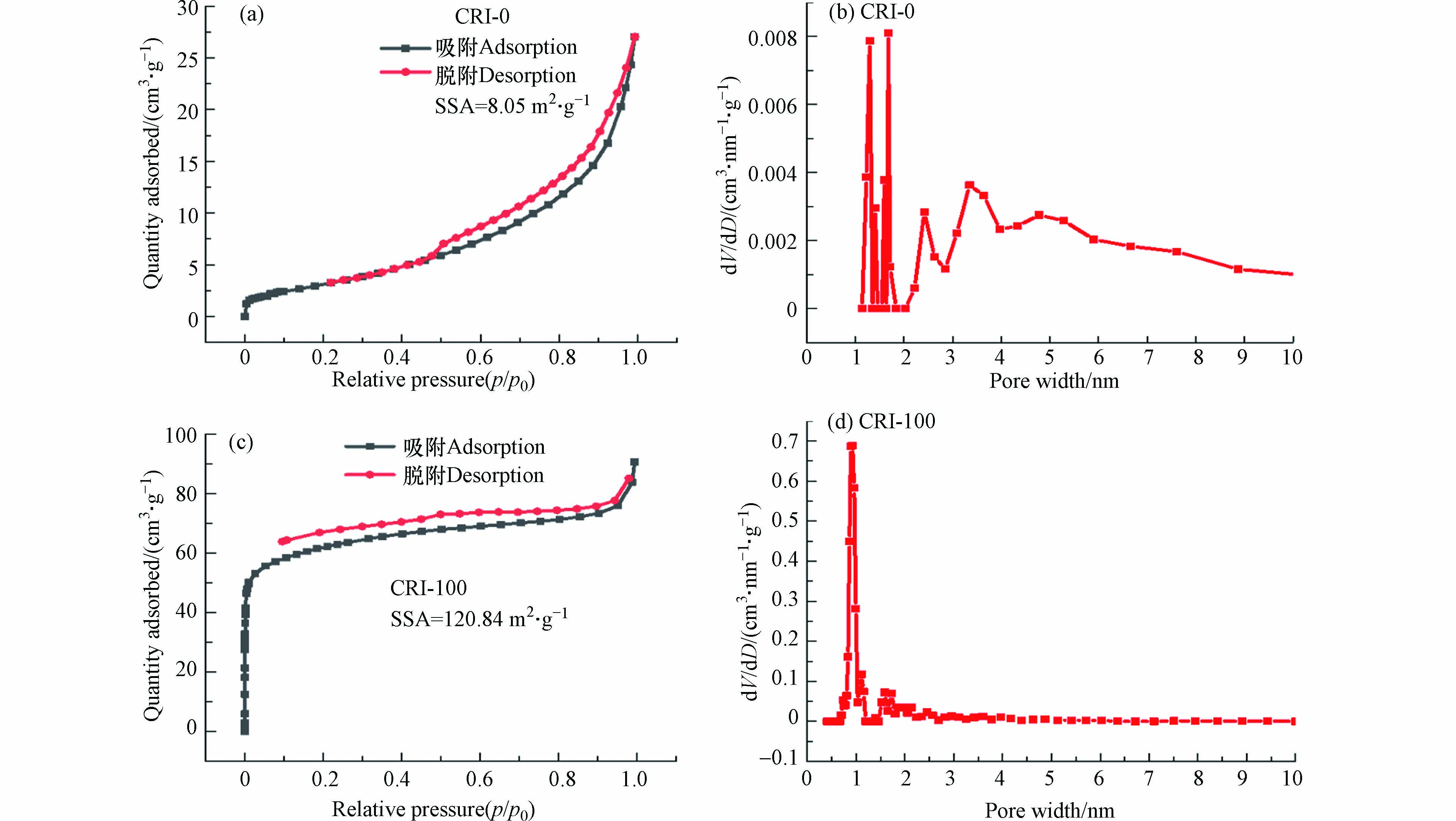
通过N2吸附/解吸等温线研究了样品的比表面积和表面孔径结构. 吸附和解吸等温线的形状通过IUPAC(International Union of Pure and Applied Chemistry)分为Ⅰ、Ⅱ、 Ⅲ、Ⅳ和Ⅴ型. 低压下的吸附和解吸等温线之间的较大差异可能是由不规则介孔(例如瓶状孔和狭缝孔)的存在,这阻碍了N2从孔中的解吸[30]. 在图4a、c可以看出,CRI-0和CRI-100生物炭吸附/解吸等温线均可以描述为Ⅳ型等温线,并且磁滞回线属于H3型,这种磁滞回线与中孔中的毛细管凝结有关,在较高的相对压力范围内没有吸附极限. 正是由于表面Fe2O3的形成,CRI-100的比表面积(120.84 m2·g−1)比CRI-0(8.05 m2·g−1)大15倍[23]. 相比CRI-0孔径分布,CRI-100微孔(1—2 nm),窄中孔(2—7 nm)数量均显着增加(如图4b、d所示),这说明CRI-100表面凹凸不平的颗粒能有效控制生物炭上孔的扩张,从而形成含量更多的微孔结构[31]. 因此,与未负载的CRI-0相比,CRI-100拥有更大的比表面积和孔径,预示着CRI-100具有更好吸附能力.
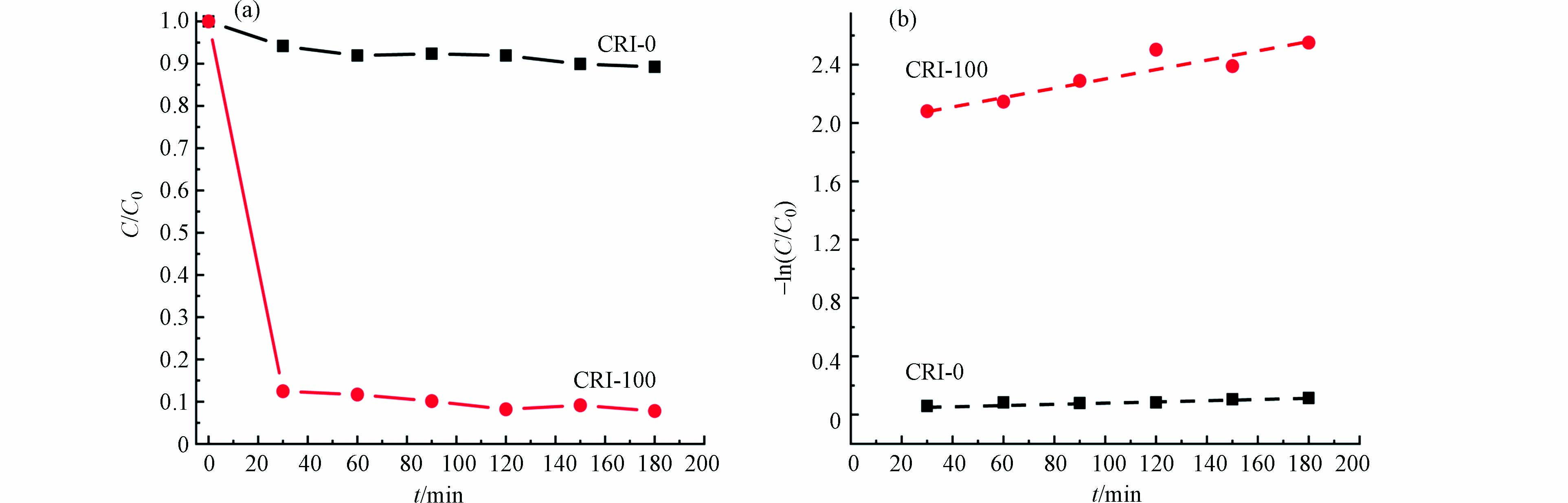
两种生物炭材料的吸附性能是通过对阳离子染料CV的吸附动力学来表征. 由图5a可以看出,CRI-0对CV的吸附能力明显弱于CRI-100(图5a),其吸附动力学常数为0.00032 min−1;但CRI-100对CV的吸附动力学常数为0.00314 min−1,后者是前者反应速率的9.8倍,说明CRI-100对CV的去除效果强于CRI-0. 由于CRI-100和CRI-0在比表面积(15.01)和铁含量(19.99)的比值都远大于9.8,说明比表面积和含铁量与生物炭的吸附性能之间不是简单的线性差异. 比表面积对吸附性能的影响无疑是重大的[32],然而铁的存在又是怎样影响生物炭的吸附性能需进一步探究.
-
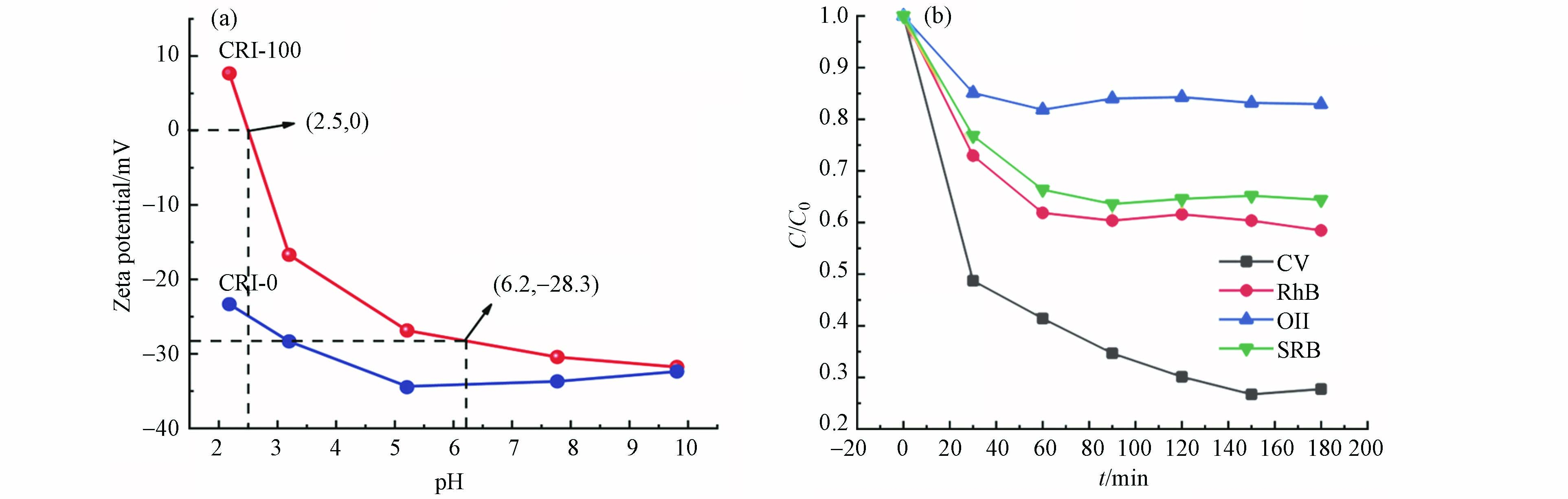
为分析其吸附机理, 首先测试了样品的表面电荷(图6a). CRI-0无等电点,CRI-100吸附剂的等电点是2.5,即在pH>2.5时,CRI-100表面带负电荷. 而在实验条件pH=6.2时,CRI-100吸附剂表面电荷为-28.3 mV,一方面说明CRI-100在水体系中处于较稳定的分散状态[33],另一方面验证了带负电荷的生物炭对阳离子染料CV的静电吸附作用. 选择其他染料,如磺酰罗丹明B(SRB)、罗丹明B(RhB)和阴离子染料金橙II(OII)进行了吸附验证. 结果发现,在pH6.2时,对SRB和RhB吸附率约为38%,这是由于此时SRB(pKa=1.5)、RhB(pKa=3.7)[26]表面带有部分正电荷,与带负电荷的CRI-100通过静电吸引力而吸附. 而CRI-100对带负电荷的OII(pKa=11.4)[26]相排斥不易吸附. 以上结果说明CRI-100主要是通过静电作用实现对染料的去除. 但有趣的是,尽管CRI-0在较大pH范围也同样带着稳定的负电荷,但它对CV的吸附效果(−10%)却远不及CRI-100(-90%,图5a). 因此,推测这种显著差异主要来源于CRI-100表面所镶嵌的铁纳米颗粒.
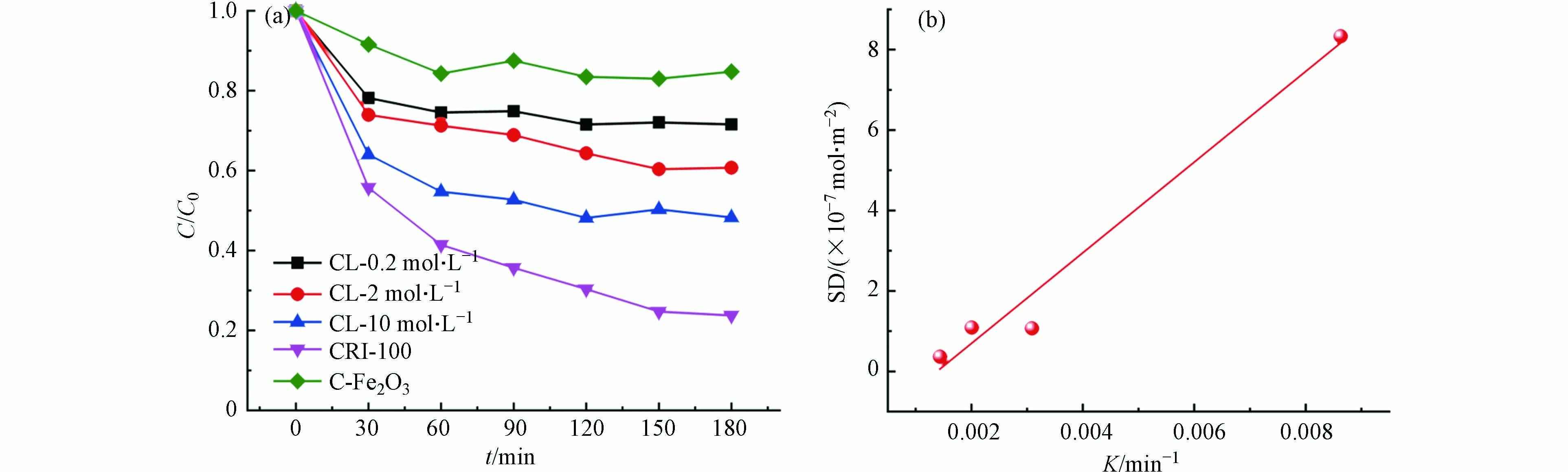
为弄清楚CRI-100表面铁位点的作用,进行了一系列对照试验. 为探究不同负载方式(外负载与富集)和不同含铁量对生物炭吸附性能的影响,分别测定了不同铁外负载制备的铁生物炭样品的比表面积,铁元素含量,生物炭表面的羟基位点数目(SD)及其对CV的反应动力学常数,结果见表4. 发现于其他所有样品相比,CRI-100的铁含量、比表面积和羟基位点密度均为最大,且对CV具有最大吸附效率(-90%,图5a).
从图7a可见,随着外负载铁含量的增加,CL-0.2M,CL-2M和CL-10M对CV的吸附动力学常数随之增加,分别为0.00144、0.00201、0.00309 min−1,说明外负载铁含量能促进对CV吸附. 但是,当铁含量相同时,CL-10M(Fe:76.45 mg·g−1)对CV吸附效果却依然没有CRI-100(Fe:65.57 mg·g−1)效果好(两样品比表面积相近),说明原位富集方式是使CRI-100具备良好吸附性能的关键步骤. 与CRI-100(SSA:120.84 m2·g−1;Fe:65.57 mg·g−1)相比,铁含量相近条件下,比表面积更大C-Fe2O3(SSA:367.72 m2·g−1;Fe:64.19 mg·g−1)几乎对CV不吸附,这证明自富集方式铁赋予了生物炭强的吸附能力. 另外,CRI-100的羟基位点密度最大,说明其单位面积上羟基位点数最多,且其与吸附性能呈正相关(R2=0.977,图7b),说明生物炭的羟基位点密度越大,吸附性能越好. 所以,生物炭表面上羟基位点数目才是影响其吸附性能的关键因素.
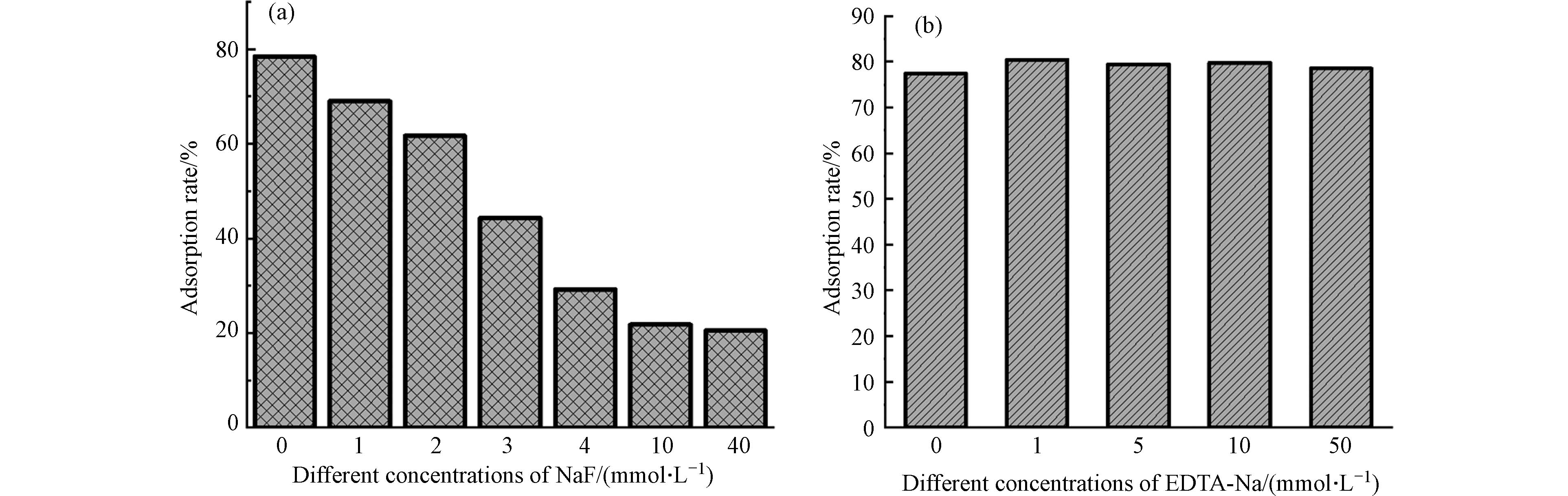
试验外加NaF和EDTA-Na对CRI-100吸附性能的影响. 由图8a可以看出,随着NaF浓度的增加,CRI-100对CV的吸附效果在逐渐降低,这是由于NaF中的氟离子占用了生物炭表面的吸附羟基位点[34]. EDTA-Na是铁位点良好的络合剂,然而添加EDTA-Na后对CRI-100的吸附性能几乎没有影响,说明生物炭对CV吸附作用位点主要是CRI-100表面带负电的羟基位点而非铁位点. 同时还在表4中发现不同负载方式可使生物炭产生不同量的羟基位点数,其中CRI-100表面的羟基位点数最多是由原位富集铁赋予的.
-
本文通过热裂解富集铁的香蒲根制备出铁负载型生物炭材料CRI-100. 研究结果发现CRI-100材料对水中有毒有机染料CV显示出优异的吸附性能. 表征结果说明铁是以Fe2O3纳米颗粒的形态镶嵌在生物炭表面,这不仅呈15倍地增加了生物炭的比表面积,同时有助于产生更多的带负电荷的羟基位点. CRI-100表面羟基位点是吸附去除CV的重要作用位点,而该位点的大量形成是由自富集方式赋予的. 该研究为将污染型植物转化为生物炭吸附材料的可行性提供实验基础.
原位富集铁元素增强生物炭的吸附性能
The enhanced adsorption performance of biochar via in-situ enrichment approach
-
摘要: 负载金属型生物炭材料具有良好的吸附性能, 但其吸附机制尚有待研究. 本文通过香蒲根(Cattail root)原位富集铁(Iron)元素, 再经热裂解得到负载铁生物炭(CRI-100)材料. 该材料对水中有毒有机染料结晶紫(crystal violet, CV)的吸附效率比空白生物炭(CRI-0)高9.8倍. 通过SEM/EDX、XRD和FTIR等表征, 结果显示自富集上去的铁以直径约40 nm的Fe2O3纳米颗粒均匀镶嵌在CRI-100表面. 实验证明,这种铁纳米颗粒的形成,使CRI-100比表面积(120.84 m2·g−1)比未富集铁的生物炭CRI-0(8.05 m2·g−1)高15倍, 且促使其表面产生了更多羟基位点, 用于CV的静电吸附. 本研究将为金属富集型植物转化为生物炭吸附剂的后续应用提供理论依据.Abstract: Metal-loaded biochar has good adsorption properties, but the understanding of such adsorption remains limited. In this study, the iron-loading biochar (CRI-100) were prepared through in-situ enrichment on cattail root and then pyrolysis. CRI-100 exhibited the adsorption efficiency of Crystal Violet (CV), a toxic organic dye in water, was 9.8 times higher than that of the blank biochar (CRI-0). On the basis of SEM/EDX, XRD and FTIR characterizations, the self-enriched iron exists in the form of Fe2O3 nanoparticles with a diameter of about 40 nm, which were evenly embedded on the surface of CRI-100. The presence of these nanoparticles rendered the specific surface area (120.84 m2·g−1) of CRI-100 was 15 times higher than that of CRI-0 without iron (8.05 m2·g−1), and increased the amount of surface hydroxyl sites that were applied to the electrostatic adsorption of CV. This study will provide a theoretical basis for the subsequent applications of metal-enriched plants into biochar adsorbents.
-
Key words:
- biochar /
- self-enriched iron /
- number of hydroxyl sites /
- positive pollutants
-

-
表 1 由植物各部位富集制备的铁生物炭比表面积和铁含量
Table 1. Specific surface area of biochar enriched iron at different part of plants
样品
SampleCRI-0 CRI-100 根
Root茎
Stem叶
Leaf根
Root茎
Stem叶
Leaf比表面积/ (m2·g−1) 8.05 119.99 128.31 120.84 246.89 274.24 铁含量/ (mg·g−1) 3.28 0.136 0.151 65.57 0.235 0.354 表 2 CRI生物炭EDS结果
Table 2. Results of CRI biochar EDS
CRI-0 CRI-100 元素
Element重量百分比/%
Weight percentage原子百分比/%
Atomic percentage元素
Element重量百分比/%
Weight percentage原子百分比/%
Atomic percentageC 7.72 11.26 C 9.38 15.82 O 68.86 75.35 O 48.66 61.61 Si 9.76 7.43 Si 15.67 11.30 Ca 13.65 5.96 Ca 12.12 6.13 Fe — — Fe 14.18 5.14 总量 100.00 — 总量 100.00 — 表 3 CRI生物炭XRF结果
Table 3. Results of CRI biochar XRF
CRI-0 CRI-100 元素
Element含量百分比/%
Content percentageElement 含量百分比/%
Content percentageSi 48.493 Si 37.924 Ca 31.396 Ca 34.160 K 8.388 Fe 16.124 Fe 7.262 K 7.122 S 2.594 S 2.174 表 4 不同处理生物炭的表征
Table 4. The characterization of different treated biochar
编号
Number比表面积/ (m2·g−1)
Specific surface area铁含量/(mg·g−1)
Iron content aCV的kobsb /min−1
CV of kobs羟基位点密度c /(mol·m−2)
Superficial densityCRI-0 8.05 3.28 0.00032 — CRI-100 120.84 65.57 0.00314 8.333×10−7 CL-0.2M 244.47 21.04 0.00144 3.64×10−8 CL-2M 221.28 59.34 0.00201 1.09×10−7 CL-10 M 329.44 76.45 0.00309 1.07×10−7 C-Fe2O3 367.72 64.19 — — a: 采用AAS测定; b: 吸附动力学常数; c: 氟离子选择性电极法测定.
a: Measured by AAS; b: Adsorption kinetic constant; c: Fluoride ion selective electrode method. -
[1] RYAN-KEOGH T J, DELIZO L M, SMITH W O Jr, et al. Temporal progression of photosynthetic-strategy in phytoplankton in the ross sea, Antarctica [J]. Journal of Marine Systems, 2017, 166: 87-96. doi: 10.1016/j.jmarsys.2016.08.014 [2] BROWNING T J, AL-HASHEM A A, HOPWOOD M J, et al. Iron regulation of north Atlantic eddy phytoplankton productivity [J]. Geophysical Research Letters, 2021, 48(6): e2020GL091403. [3] 李永康, 马雪祺, 冯婧娴, 等. 细胞色素P450酶在植物次生代谢产物生物合成中的研究进展[J/OL]. 分子植物育种: 1-8. [2022-06-12].
[4] LI Y W, YAN H, HU T L, et al. Two microporous Fe-based MOFs with multiple active sites for selective gas adsorption [J]. Chemical Communications, 2017, 53(15): 2394-2397. doi: 10.1039/C6CC09923H [5] QIN S N, FAN H D, JIA L, et al. Molecular structure analysis and mercury adsorption mechanism of iron-based modified biochar [J]. Energy & Fuels, 2022, 36(6): 3184-3200. [6] DU J, ZHANG L, ALI A, et al. Research on thermal disposal of phytoremediation plant waste: Stability of potentially toxic metals (PTMs) and oxidation resistance of biochars [J]. Process Safety and Environmental Protection, 2019, 125: 260-268. doi: 10.1016/j.psep.2019.03.035 [7] LI H G, WATSON J, ZHANG Y H, et al. Environment-enhancing process for algal wastewater treatment heavy metal control and hydrothermal biofuel production: A critical review [J]. Bioresource Technology, 2020, 298: 122421. doi: 10.1016/j.biortech.2019.122421 [8] LIU K H, ZHANG H C, LIU Y F, et al. Investigation of plant species and their heavy metal accumulation in manganese mine tailings in Pingle Mn mine, China [J]. Environmental Science and Pollution Research International, 2020, 27(16): 19933-19945. doi: 10.1007/s11356-020-08514-9 [9] HAMAD M T M H. Comparative study on the performance of Typha latifolia and Cyperus papyrus on the removal of heavy metals and enteric bacteria from wastewater by surface constructed wetlands [J]. Chemosphere, 2020, 260: 127551. doi: 10.1016/j.chemosphere.2020.127551 [10] XU X Y, MILLS G L. Do constructed wetlands remove metals or increase metal bioavailability? [J]. Journal of Environmental Management, 2018, 218: 245-255. [11] ZHU H, TAN X, TAN L, et al. Biochar derived from sawdust embedded with molybdenum disulfide for highly selective removal of Pb2+ [J]. ACS Applied Nano Materials, 2018, 1(6): 2689-2698. doi: 10.1021/acsanm.8b00388 [12] DU J, ZHANG L, LIU T, et al. Thermal conversion of a promising phytoremediation plant (Symphytum officinale L. ) into biochar: Dynamic of potentially toxic elements and environmental acceptability assessment of the biochar [J]. Bioresource Technology, 2019, 274: 73-82. doi: 10.1016/j.biortech.2018.11.077 [13] MESQUITA A M, GUIMARÃES I R, de CASTRO G M M, et al. Boron as a promoter in the goethite (α-FeOOH) phase: Organic compound degradation by Fenton reaction [J]. Applied Catalysis B:Environmental, 2016, 192: 286-295. doi: 10.1016/j.apcatb.2016.03.051 [14] OLIVEIRA F R, PATEL A K, JAISI D P, et al. Environmental application of biochar: Current status and perspectives [J]. Bioresource Technology, 2017, 246: 110-122. doi: 10.1016/j.biortech.2017.08.122 [15] WANG S S, GAO B, LI Y C, et al. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests [J]. Journal of Hazardous Materials, 2017, 322: 172-181. doi: 10.1016/j.jhazmat.2016.01.052 [16] KAZEMI SHARIAT PANAHI H, DEHHAGHI M, OK Y S, et al. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications [J]. Journal of Cleaner Production, 2020, 270: 122462. doi: 10.1016/j.jclepro.2020.122462 [17] LIANG J, LI X M, YU Z G, et al. Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II) [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5049-5058. [18] 张睿媛, 王欣, 喻惠玲, 等. 利用植物提取液制备铁基纳米粒子及其在水污染处理中的研究进展 [J]. 环境化学, 2022, 41(2): 683-693. doi: 10.7524/j.issn.0254-6108.2021070203 ZHANG R Y, WANG X, YU H L, et al. Production of iron-based nanoparticles using plant extracts and its application in contaminated water treatment [J]. Environmental Chemistry, 2022, 41(2): 683-693(in Chinese). doi: 10.7524/j.issn.0254-6108.2021070203
[19] PAUL S, KAUSER H, JAIN M S, et al. Biogenic stabilization and heavy metal immobilization during vermicomposting of vegetable waste with biochar amendment [J]. Journal of Hazardous Materials, 2020, 390: 121366. doi: 10.1016/j.jhazmat.2019.121366 [20] WU P, CUI P X, ZHANG Y, et al. Unraveling the molecular mechanisms of Cd sorption onto MnOx-loaded biochar produced from the Mn-hyperaccumulator Phytolacca americana [J]. Journal of Hazardous Materials, 2022, 423: 127157. doi: 10.1016/j.jhazmat.2021.127157 [21] WANG X H, ZHANG P, WANG C P, et al. Metal-rich hyperaccumulator-derived biochar as an efficient persulfate activator: Role of intrinsic metals (Fe, Mn and Zn) in regulating characteristics, performance and reaction mechanisms [J]. Journal of Hazardous Materials, 2022, 424: 127225. doi: 10.1016/j.jhazmat.2021.127225 [22] HUANG Y S, HU H. The interaction of perrhenate and acidic/basic oxygen-containing groups on biochar surface: A DFT study [J]. Chemical Engineering Journal, 2020, 381: 122647. doi: 10.1016/j.cej.2019.122647 [23] 程瑜炜, 凌晨, 王正晓, 等. 聚胺壳聚糖微球对焦磷酸络合废水中镍的高效选择性去除特性与机制 [J]. 环境化学, 2022, 40(8): 2530-2539. CHENG Y W, LING C, WANG Z X, et al. Efficient removal of Ni(Ⅱ) from pyrophosphate-plating wastewater using the polyamine-grafted chitosan beads [J]. Environmental Chemistry, 2022, 40(8): 2530-2539(in Chinese).
[24] WU L P, ZHANG S R, WANG J, et al. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests [J]. Environmental Pollution, 2020, 261: 114223. doi: 10.1016/j.envpol.2020.114223 [25] 曾凤美, 孙丰文, 孙恩惠, 等. Fe2O3/PC功能化复合多孔炭材料的制备及除磷机理 [J]. 环境科学学报, 2021, 41(9): 3487-3496. ZENG F M, SUN F W, SUN E H, et al. Fabrication of Fe2O3/PC functionalized biochar composites for phosphate removal from wastewater: Adsorption properties and mechanism [J]. Acta Scientiae Circumstantiae, 2021, 41(9): 3487-3496(in Chinese).
[26] FANG Y F, ZHOU A, YANG W, et al. Complex formation via hydrogen bonding between rhodamine B and montmorillonite in aqueous solution [J]. Scientific Reports, 2018, 8: 229. doi: 10.1038/s41598-017-18057-8 [27] 徐艳, 杨宏国, 牛慧斌, 等. 磁性纳米颗粒Fe3O4的醇改性制备机理及应用 [J]. 高等学校化学学报, 2021, 42(8): 2564-2573. XU Y, YANG H G, NIU H B, et al. Preparation mechanism and application of alcohol-modified Fe3O4 magnetic nanoparticles [J]. Chemical Journal of Chinese Universities, 2021, 42(8): 2564-2573(in Chinese).
[28] MOHSEN E, JABER J, MEHDI M A, et al. Synthesis and characterization of Fe3O4@SiO2–polymer-imid–Pd magnetic porous nanospheres and their application as a novel recyclable catalyst for Sonogashira–Hagihara coupling reactions [J]. Journal of the Iranian Chemical Society, 2014, 11(2): 499-510. doi: 10.1007/s13738-013-0323-4 [29] ZHANG H, XUE G, CHEN H, et al. Magnetic biochar catalyst derived from biological sludge and ferric sludge using hydrothermal carbonization: Preparation, characterization and its circulation in Fenton process for dyeing wastewater treatment [J]. Chemosphere, 2018, 191: 64-71. doi: 10.1016/j.chemosphere.2017.10.026 [30] ZHANG J J, SHAO J G, JIN Q Z, et al. Effect of deashing on activation process and lead adsorption capacities of sludge-based biochar [J]. Science of the Total Environment, 2020, 716: 137016. doi: 10.1016/j.scitotenv.2020.137016 [31] LI C Y, FU M, WANG Y, et al. In situ synthesis of Co2P-decorated red phosphorus nanosheets for efficient photocatalytic H2 evolution [J]. Catalysis Science & Technology, 2020, 10(7): 2221-2230. [32] DOU S, KE X X, SHAO Z D, et al. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin [J]. Chemosphere, 2022, 287: 131962. doi: 10.1016/j.chemosphere.2021.131962 [33] POCHAPSKI D J, CARVALHO dos SANTOS C, LEITE G W, et al. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2021, 37(45): 13379-13389. doi: 10.1021/acs.langmuir.1c02056 [34] BISWAS A, PRATHIBHA C. Nanocomposite of ceria and trititanate nanotubes as an efficient defluoridating material for real-time groundwater: Synthesis, regeneration, and leached metal risk assessment [J]. ACS Omega, 2021, 6(47): 31751-31764. doi: 10.1021/acsomega.1c04424 -



 下载:
下载: