-
自2019年底新型冠状病毒肺炎(COVID-19,简称“新冠肺炎”)疫情暴发以来,许多场所(医院、污水处理厂、市政)均存在含氯消毒剂的大规模、大剂量使用[1-2],例如武汉市仅1个月就投放了1963.58 t消毒药剂[3],其他地区污水处理厂也将氯消毒剂使用量提升到平时的2.5—3.5倍,出现过度消毒现象[1]。大量余氯会与水体的有机物反应形成有害的氯化消毒副产物(DBPs),而后进入自然水体中[4]。其中大多数DBPs具有生物毒性[5-6],可能会对水生生物产生危害。
目前,国内外对于DBPs的风险评估主要侧重于在饮用水中DBPs对人体健康的影响[7-10]。已有研究表明DBPs对细菌[11]、大鼠[12]以及人类[8-9]的生长发育均会有负面影响。卤乙酸(HAAs)作为最常见的DBPs之一[13],存在于饮用水和通过采用次氯酸盐消毒的废水中[8],与三卤甲烷(THMs)相比,HAAs的毒性更高,在环境中存在的时间更长[11]。目前关于HAAs的毒理学研究多集中于鱼类[14-15]和大型溞[16-17],而有关藻类的毒理学研究较少。
藻类作为初级生产者,是水生生态系统的重要组成部分,通过水生食物链之间的能量传递,从而影响整个水生态系统[18],然而,关于DBPs对浮游植物的毒理学研究成果较为缺乏,需要进一步的研究来评估DBPs对水生态环境的影响。本文以我国淡水水域中常见的浮游植物小球藻作为模式生物[18],通过分析污水厂全年加氯消毒后出水的的DBPs检测情况,选择加氯消毒后出水中占比最高的一氯乙酸(CAA)为目标污染物,探究其对小球藻的毒性效应,可以为水环境中CAA的生态风险评估和环境污染控制提供理论支持。
-
CAA标准品购于坛墨质检科技股份有限公司,超氧化物歧化酶(SOD)、还原型谷胱甘肽(GSH)、过氧化氢酶(CAT)活性测定试剂盒和丙二醛(MDA)含量测定试剂盒购于苏州科铭生物技术有限公司。
-
小球藻(Chlorella vulgaris , FACHB-8)购于中国科学院水生生物研究所淡水藻种库。培养条件: 以 BG11 培养基(pH=7.1)为基础培养基,置于光照培养箱(GZX-250BSH-Ⅲ,上海新苗医疗器械制造有限公司)内进行扩培。温度设置在(25±1)℃,光照强度为8000 lux,光暗比为12 h:12 h,每天定期摇荡3次。
-
(1)DBPs测定
本文中测定的DBPs主要包括THMs、HAAs、卤乙醛(CH)、卤代酮(HKs)、三氯硝基甲烷(TCNM)和卤乙腈(HANs)等饮用水中检出频率较高的DBPs。其中氯消毒后水样中的THMs、CH、HKs、TCNM和HANs根据US EPA 551.1标准方法测定,利用萃取剂甲基叔丁基醚(MTBE)进行液-液萃取后,采用1 mL注射器吸取上层有机相1 mL过0.22 µmPTFE膜后,装入气相小瓶测定。HAAs 根据US EPA 552标准方法先酸化甲醇衍生化后测定其衍生产物。
利用配有ECD检测器的GC(GC 2010,岛津,日本)测定分析。选用30 μm×0.25 mm 的HP-5柱(安捷伦,美国)分离,膜厚0.25 μm,载气N2(高纯氮,无锡鑫锡仪选用科技)。分流进样模式下进样体积1 µL[19]。
(2)生物量的测定
每次取10 mL胁迫组和对照组样品,用血球计数板( XB-K-25) 在显微镜下进行藻细胞计数。
(3) 最大光化学量子产量(Fv/Fm)的测定
Fv/Fm的测定采用 PHYTO-PAM 手持浮游植物分析仪(PHYTO-PAM Phytoplankton Analyzer, Walz, Effeltrich, Germany)。在室温下,取一定量藻液加入石英小杯中暗适应,15 min后读取其Fv/Fm值。
(4)半数效应浓度 EC50的确定
本研究中采用96 h生物量的 EC50来评价CAA的毒性,即以96 h的生物量来计算EC50值。通过Logisitic拟合计算公式为:
式中,x为暴露浓度,y为抑制率。
(5)叶绿素a含量的测定
参考GB17378-2007丙酮萃取-分光光度法提取叶绿素。再通过公式(2)计算叶绿素a的浓度:
式中,D630、D645、D663和 D750为对应波长吸光度,V1为丙酮体积(mL),V为藻液体积(L),δ 为比色皿量程(cm)。
小球藻比生长速率μ:
式中,μ为比生长速率(d−1),Et和E0为t时刻和初始时的叶绿素a的浓度,Δt为胁迫时间(4 d)。
(6)小球藻细胞内抗氧化酶及MDA含量的测定
藻细胞数与提取液体积(mL)比为1000∶1。具体测定方法按照苏州科铭酶测定试剂盒的方法进行。
-
挑选生长健康并且处于对数增长期的小球藻在无菌环境下进行暴露实验。对照组(CK)CAA浓度为0,实验组CAA梯度分为低(50 μg·L−1、500 μg·L−1)、中(5 mg·L−1、10 mg·L−1、25 mg·L−1)、高(50 mg·L−1、500 mg·L−1)共7个浓度。初始实验时选取藻浓度为106 个·mL−1,每个处理设置3个平行组,分别在0、6、12、24、36、48、72、96 h进行取样,每次取10 mL胁迫组藻液和对照组藻液,测定藻胞生物量,取96 h胁迫组藻液计算半数效应浓度(EC50),测定各处理组中叶绿素a含量,抗氧化物酶活及MDA含量。
-
采用OriginPro 2018处理数据作图,利用IBM SPSS Statistics 25统计分析,使用Dunnett's t-test法进行显著性差异分析,以*表示显著差异。
-
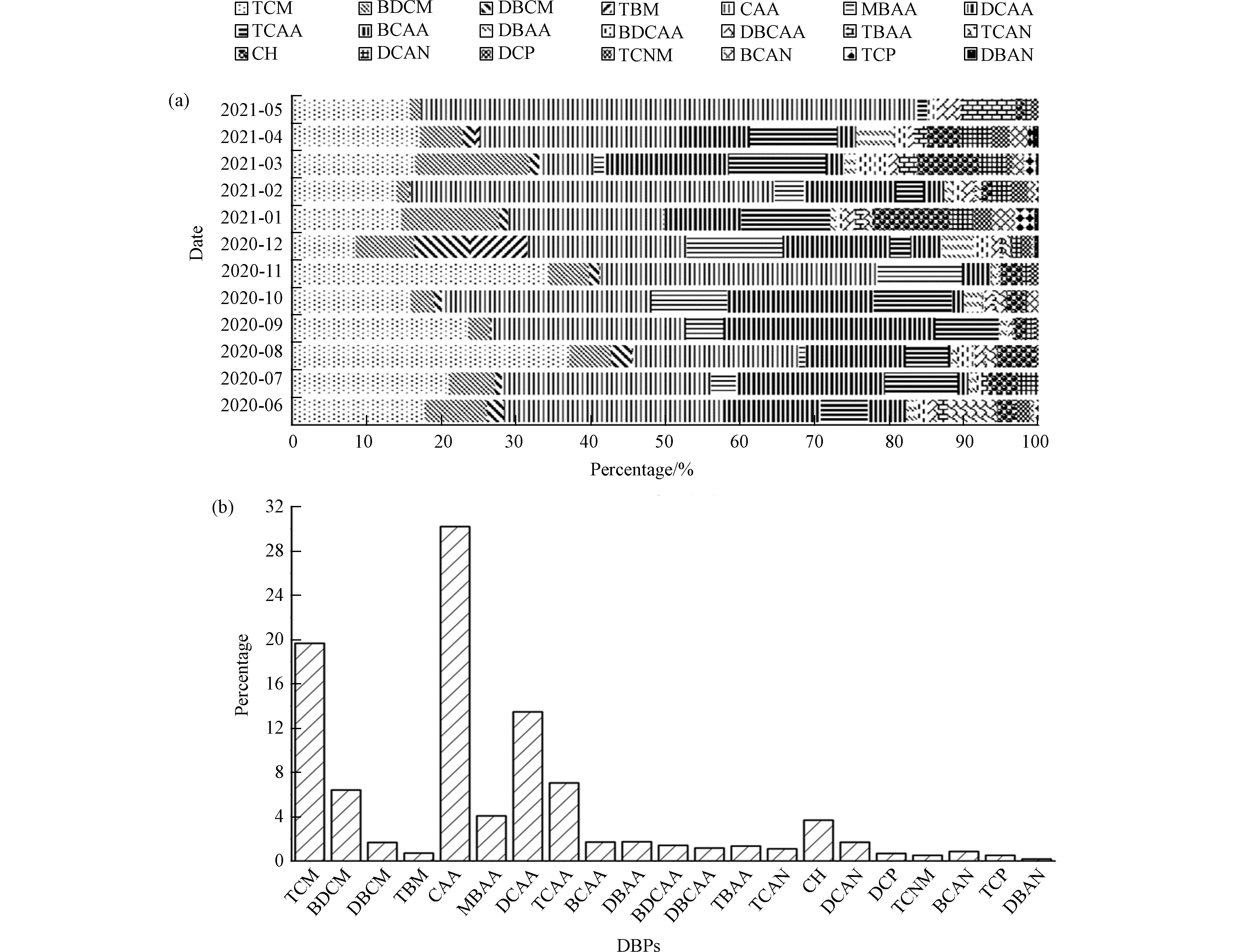
从2020年6月至2021年5月,对以次氯酸钠作为消毒剂的江苏省无锡市某污水厂尾水中DBPs进行监测,采样频率为每月1次。该污水处理厂尾水中监测出了三氯甲烷(TCM 47.10 μg·L−1)、一溴二氯甲烷(BDCM 21.62 μg·L−1)、二溴一氯甲烷(DBCM 6.49 μg·L−1)、三溴甲烷(TBM 4.27 μg·L−1)、一氯乙酸(CAA 67.84 μg·L−1)、一溴乙酸(MBAA 14.50 μg·L−1)、二氯乙酸(DCAA 39.06 μg·L−1)、三氯乙酸(TCAA 21.31 μg·L−1)、溴氯乙酸(BCAA 4.51 μg·L−1)、二溴乙酸(DBAA 5.48 μg·L−1)、一溴二氯乙酸(BDCAA 3.84 μg·L−1)、二溴一氯乙酸(DBCAA、3.00 μg·L−1)、三溴乙酸(TBAA 2.31 μg·L−1)、三氯乙腈(TCAN 2.69 μg·L−1)、水合氯醛(CH 12.30 μg·L−1)、二氯乙腈(DCAN 5.09 μg·L−1)、二氯丙酮(DCP 2.21 μg·L−1)、三氯硝基甲烷(TCNM 1.38 μg·L−1)、溴氯乙腈(BCAN 3.40 μg·L−1)、三氯丙酮(TCP 2.31 μg·L−1)和二溴乙腈(DBAN 0.78 μg·L−1)等消毒副产物,与疫情前污水厂消毒后DBPs总浓度(95.8 μg·L−1)相较[20],疫情期间测得的DBPs总检出量(271.47 μg·L−1)显著增加,为疫情前总浓度的2.83倍。
由图1可以看出,其中疫情期间DBPs检出量占比5%以上的TCM、BDCM、CAA、DCAA、TCAA五种DBPs占总检出的76.85%,而CAA占比最高,为30.22%,远高出其它DBPs的检出量。前期的研究中发现CAA是一种高度稳定的物质,会在环境中积累[21],并且CAA对水生生物有很强的毒性作用,其进入环境将会对水生态有较大的影响。
-
(1)CAA对小球藻生长的影响
在不同浓度CAA胁迫下,小球藻的藻密度和叶绿素浓度的变化趋势基本一致,对小球藻的生长具有抑制作用。从图2(a)和(b)可以看出,随着暴露时间的延长,抑制程度与CAA胁迫浓度呈正相关。在低浓度(疫情期间检出浓度范围)胁迫下,小球藻的藻细胞密度和叶绿素浓度随时间依然呈上升趋势,但与对照组相比,均有一定程度的降低。从36 h起,低浓度组(50 μg·L−1、500 μg·L−1)与对照组相比开始出现显著差异,随着暴露时间的延长,显著性差异更明显,到96 h时,低浓度组小球藻密度和叶绿素 浓度与对照组相比分别下降了6.68%、13.80%和40.41%、42.34%。在中、高浓度胁迫下,小球藻的藻细胞密度和叶绿素浓度随时间逐渐下降。从6 h起,中(5 mg·L−1、10 mg·L−1、25 mg·L−1)、高(50 mg·L−1、500 mg·L−1)浓度组与对照组相比,已经开始出现显著差异,并且随着暴露时间越长,显著性差异更明显,到96 h时,与对照组相比,中、高浓度组小球藻密度分别下降了25.82%、44.53%、62.34%、98.48%和 98.97%,叶绿素浓度分别下降了56.07%、66.55%、71.83%、99.54%和99.84%。叶绿素含量能较好地反映微藻生长发育状态,实验发现其与藻密度变化趋势基本一致。可以看出,在低浓度CAA的短时间暴露下,会对小球藻细胞内藻类色素合成的相关酶类进行激活,从而增加小球藻体内代谢速率使得在短时间内使得色素合成增多,但长期的胁迫反而会抑制小球藻的生长。另外,由于高浓度CAA的胁迫,使得小球藻细胞内活性氧大量积累,进一步破坏了小球藻细胞内叶绿体结构,影响了小球藻光捕获化合物的形成,从而阻碍小球藻叶绿素的合成。总体上来说,中、高浓度CAA会造成小球藻藻细胞的破坏和叶绿素a的合成。
(2)CAA对小球藻Fv/Fm的影响
Fv/Fm经常被用来描述植物的光合生理状况,反应植物的潜在最大光合能力,用来判定植物的健康程度。不同CAA胁迫浓度下,小球藻Fv/Fm的变化情况如图2(c)所示,可以看出随着CAA暴露浓度越高,小球藻的最大光合利用速率越低。低浓度(50 μg·L−1、500 μg·L−1)CAA胁迫组暴露96 h后,藻液的Fv/Fm值与对照组相比没有显著差异;而中浓度(5 mg·L−1、10 mg·L−1、25 mg·L−1)CAA胁迫组中,Fv/Fm与对照组相比均有显著性下降,与对照组相比分别下降了13.04%、40.58%、52.17%,而在高浓度(50 mg·L−1、500 mg·L−1)CAA暴露下,小球藻的Fv/Fm值过低,均低于仪器的检出值,说明此时的小球藻藻细胞被破坏,已经失去了活性。
CAA对小球藻的毒性可用半数效应浓度 EC50 来表示。本研究中采用96 h生物量的 EC50来评价CAA的毒性,即以96 h的生物量来计算EC50值。根据公式(1)计算得到CAA对小球藻的96 h的EC50值为14.73 mg·L−1,与疫情期间污水厂的实际检出量67.84 μg·L−1相较,高出实际检出浓度约217倍。根据表1[22]可知,CAA属于中毒物质。
CAA胁迫组比生长速率均低于对照组,且暴露浓度越高,小球藻的比生长速率(μ)越小(表2)。对照组小球藻的μ值为0.54 d−1,在中、低浓度CAA胁迫下,μ逐渐降低,但依然大于0,然而50 mg·L−1和500 mg·L−1浓度暴露4 d 后,μ 值为负,且胁迫浓度越高,μ 值越小,其比生长速率数值分别为−0.83 d−1和−1.08 d−1。在中、低浓度下,虽然小球藻的生长速率相比对照组来说有所下降,但是仍处于正生长状态,这说明小球藻对低浓度的CAA有一定的耐受力,但是在高浓度CAA暴露胁迫下,小球藻的生长受到了严重的抑制,甚至死亡,这说明高浓度CAA已超出了小球藻的耐受范围,小球藻细胞无法适应高浓度CAA的胁迫。
-
(1)CAA 对小球藻抗氧化系统的影响
CAT酶和SOD酶是生物体抗氧化酶体系的基本组成,通过协同作用清除植物体内的活性氧自由基,用来保护在逆境胁迫下,活性氧自由基造成的细胞膜膜脂过氧化而对植物细胞造成的伤害[23]。而GSH则是植物体内清除自由基的抗氧化物质,它通过谷胱甘肽过氧化物酶(GPx)将H2O2还原为H2O,其自身则会被氧化为氧化型谷胱甘肽(GSSG),GSSG又会在谷胱甘肽还原酶(GR)的催化下,被还原成GSH,不断循环这一过程,使得植物体内的自由基被清除。植物细胞内的抗氧化物质含量越高,其对外界胁迫的耐受性则越大[24]。
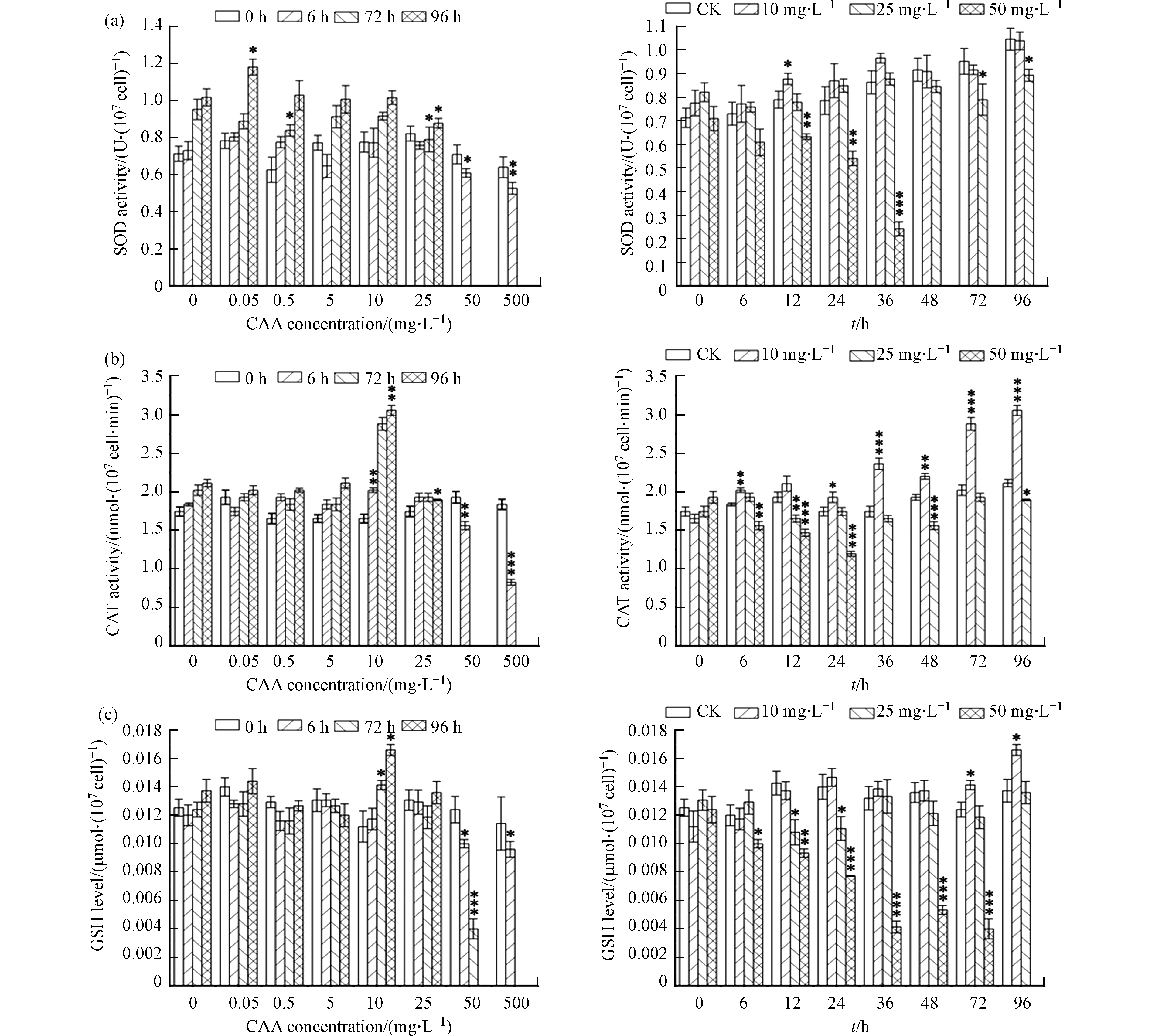
在不同CAA浓度胁迫下,小球藻SOD、CAT酶活性和GSH含量的变化趋势基本一致。从图3可以看出,小球藻细胞内的SOD、CAT酶活性和GSH含量随胁迫浓度增加呈先上升后下降的变化趋势,且随着暴露时间的延长,显著性差异更明显。
在低浓度(50 μg·L−1、500 μg·L−1)胁迫下,小球藻CAT酶活性和GSH含量无明显变化,但SOD酶活性受到明显的诱导作用,在96 h时达到峰值,为对照组的1.16倍。在10 mg·L−1胁迫浓度下,从时间变化和浓度变化两个角度来看,小球藻SOD、CAT酶活性和GSH含量均达到单峰曲线的顶点,这时,小球藻CAT酶活和GSH含量性分别为对照组的1.52倍和 1.21倍。在高浓度(50 mg·L−1、500 mg·L−1)胁迫下,随着时间的延长,小球藻的SOD、CAT酶活性和GSH含量逐渐降低,96 h后由于小球藻酶活过低而未能检出。从而说明如果植物细胞内的活性氧浓度过高,其所产生的过量的活性氧自由基会降低细胞内抗氧化酶活性。所得的实验结论与刘青青等[25]以不同浓度的氰化钾胁迫斜生栅藻的结果一致.
生物体的抗氧化酶活性随着外源物质暴露浓度的增加先升高随后逐渐降低的趋势称为“钟形”曲线[26],本实验中,小球藻细胞内的SOD、CAT酶活性和GSH含量随胁迫浓度的变化符合“钟形”曲线。Stebbing称这种在低毒状况下出现的应激反应为“毒物兴奋效应”,该效应对于保护生物机体避免抗氧化损伤具有重要意义[27]。
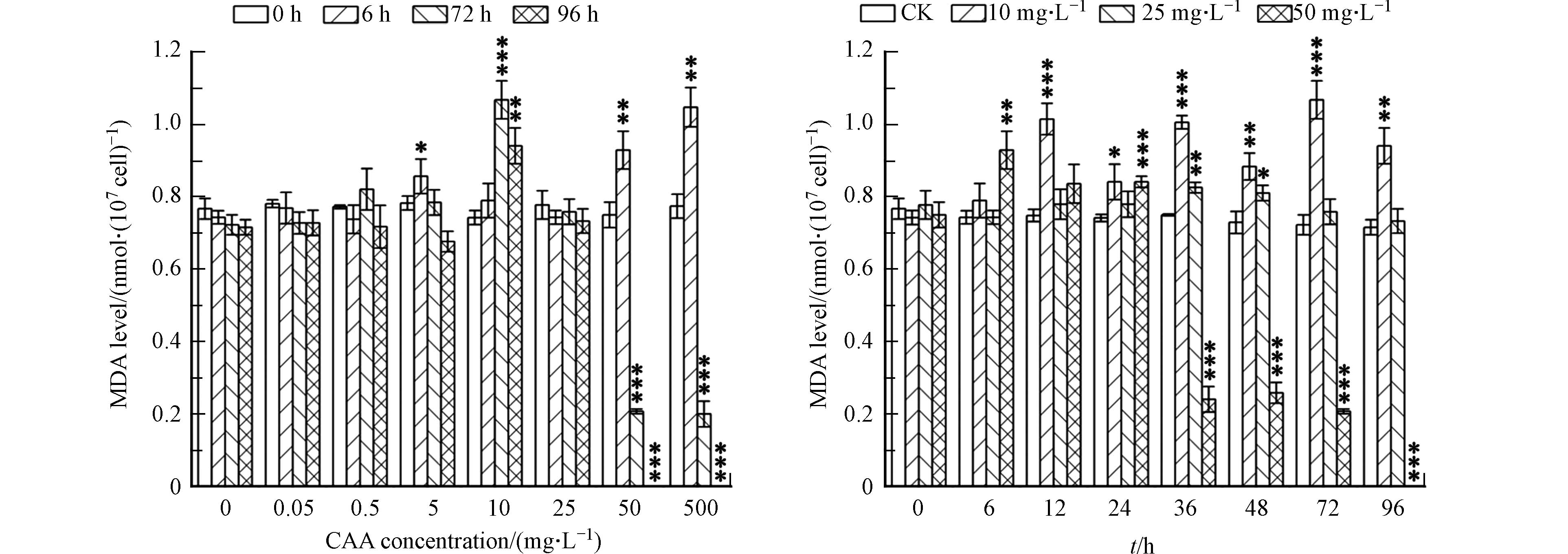
(2) CAA对小球藻MDA的影响
丙二醛MDA是膜脂过氧化的关键产物,代表细胞膜脂过氧化的程度,在细胞内的累积量通常被用作膜脂过氧化程度的指标[28]。由图4可知,在暴露时间为6 h时,低浓度(50 μg·L−1、500 μg·L−1)胁迫下MDA含量变化不明显,说明低浓度小球藻细胞膜脂过氧化程度不明显;当CAA浓度为5 mg·L−1时,MDA 含量明显增加,是对照组的1.15倍;高浓度胁迫下MDA含量继续增长,在500 mg·L−1胁迫浓度下,MDA含量为对照组的1.41倍。而在96 h后,低浓度胁迫下MDA含量变化不明显,中浓度胁迫下的MDA含量先增长后降低,10 mg·L−1小球藻细胞内的MDA含量达到峰值,为对照组的1.32倍;而高浓度胁迫下MDA含量呈快速下降趋势,96 h时MDA含量低于检出限。
一般来说,植物体内存在着氧化-还原的动态平衡,通过体内活性氧自由基的产生与保护酶系统及非保护酶系统有效的清除来保证植物不会引起氧化伤害。但是,当植物受到外界胁迫时,细胞内会累积大量的活性氧,从而影响抗氧化酶的活性,会造成细胞膜脂的过氧化。而SOD酶作为抗氧化体系的第一道防线,它通过催化O2-·发生歧化反应生成H2O2和O2,再通过CAT酶去除细胞内的 H2O2 [23,25] ,使得植物体内形成氧化-还原的动态平衡。根据实验结果得出,在低浓度CAA胁迫下,小球藻体内的两种抗氧化酶活性及GSH的含量与对照组相比均出现了上升,这表明小球藻已经出现应激反应,为了维持活性氧产生与清除的动态平衡,通过提升抗氧化酶活性来缓解活性氧对植株造成的伤害[21] 。
另外,MDA代表了细胞膜脂过氧化的程度,实验结果显示,在低浓度CAA胁迫下,MDA含量无明显变化,是由于在低浓度胁迫下,小球藻体内的活性氧清除系统被激活,抗氧化酶活性相对较高,会对体内的活性氧自由基进行清除,从而减轻了细胞膜脂过氧化的程度,使得MDA含量与对照组相较无较大变化。而在受到中、高浓度CAA胁迫时MDA含量明显上升,是因为在高浓度CAA胁迫下,细胞受到外界的损害程度加深,细胞内的活性氧自由基累积量越来越多,超出体系内抗氧化酶活清除的能力,破坏了植物体内的氧化-还原的动态平衡,导致小球藻体内的MDA含量升高,这也说明中、高浓度CAA加剧了小球藻藻细胞脂膜过氧化程度。
疫情期间污水厂尾水中DBPs含量为本研究中设定的低浓度范围,此浓度短时间的暴露,会刺激小球藻相关酶类的活性,但长时间暴露也会逐渐影响小球藻的生理活性,因此对水体中藻类生态会产生一定的影响。
-
(1)疫情期间(2020年6月至2021年5月)污水厂尾水中检测出21种消毒副产物,总浓度达271.47 μg·L−1,为疫情前总浓度的2.83倍。其中检出量占比在5%以上的 TCM 、BDCM、CAA、DCAA、TCAA 的5种DBPs占总检出的76.85%,而CAA占比最高,为30.22%。
(2)低浓度(疫情期间检出浓度范围)的CAA暴露,会导致小球藻的生长速率和叶绿素a合成速率会有所减缓;而中、高浓度暴露下,小球藻的生长停滞,最后完全死亡。中、低浓度CAA胁迫会诱导SOD、CAT活性和GSH含量的上升,减少 MDA 的积累;而高浓度胁迫下,SOD、CAT活性和GSH含量持续下降,MDA含量在6 h时达到最大值,随后下降。CAA对小球藻的96 h的EC50值为14.734 mg·L−1。
(3)疫情期间污水厂排放尾水中的DBPs含量,长期暴露会逐渐影响小球藻的生理活性,对水体中藻类生态产生一定的影响。
污水厂尾水中消毒副产物对小球藻的生长生理影响
Effects of disinfection by-products in sewage treatment plant effluent on the growth and physiology of Chlorella vulgaris
-
摘要: 通过检测无锡某污水厂疫情期间排放尾水中消毒副产物的种类和浓度,发现一氯乙酸(CAA)占全年总检出量的30.22%。采用毒性标准实验方法研究CAA对小球藻生长和抗氧化系统的影响。结果表明在CAA胁迫下,小球藻生长速率减慢,最大光化学量子产量(Fv/Fm)较空白组出现明显下降,96 h叶绿素 a 浓度明显降低。CAA对小球藻的96 h半数效应浓度(EC50)为14.734 mg·L-1。中、低浓度CAA胁迫会诱导超氧化物歧化酶(SOD)、过氧化氢酶(CAT)活性和还原型谷胱甘肽(GSH)含量的上升,减少丙二醛(MDA)含量的积累;而高浓度胁迫下,SOD、CAT活性和GSH含量持续下降,MDA含量先升高再下降。Abstract: This study investigated the species and concentration of disinfection by-products in a sewage treatment plant effluent in Wuxi during the COVID-19 pandemic. It was found that chloroacetic acid (CAA) accounted for 30.22% of the total amount of disinfection by-products detected in the whole year. The effects of CAA on the growth and antioxidant system of Chlorella vulgaris were studied via standard toxicity tests. The results showed that upon the exposure of CAA, the growth rate of Chlorella vulgaris was slowed down, the maximum photochemical quantum yield (Fv/Fm) decreased significantly compared to the control group, and the concentration of chlorophyll-a was significantly decreased at 96 h. The EC50 for 96 h-exposure of CAA was found to be 14.734 mg·L-1. The activities of superoxide dismutase (SOD) and catalase (CAT) and the content of reduced glutathione (GSH) were increased while the accumulation of MDA was reduced upon the exposure of medium and low concentrations of CAA. However, high concentration exposure could lead to a decreasing trend in SOD and CAT activities as well as GSH content, while the content of MDA was first increased and then decreased.
-

-
表 1 藻类生长抑制毒性分级标准
Table 1. Toxicity grading standard of algae growth inhibition
96h-EC50/(mg·L−1) <1 1—10 10—100 >100 毒性分级 极高毒 高毒 中毒 低毒 表 2 不同CAA胁迫浓度对小球藻的比生长速率( μ )的影响
Table 2. Growth rate ( μ ) of Chlorella vulgaris under CAA exposure
暴露浓度 Exposure concentration CK 50 μg·L−1 500 μg·L−1 5 mg·L−1 10 mg·L−1 25 mg·L−1 50 mg·L−1 500 mg·L−1 比生长速率(μ)/ d−1 0.54 0.45 0.41 0.35 0.32 0.26 −0.83 −1.08 -
[1] CHU W H, FANG C, DENG Y, et al. Intensified disinfection amid COVID-19 pandemic poses potential risks to water quality and safety [J]. Environmental Science & Technology, 2021, 55(7): 4084-4086. [2] LI Z G, SONG G F, BI Y H, et al. Occurrence and distribution of disinfection byproducts in domestic wastewater effluent, tap water, and surface water during the SARS-CoV-2 pandemic in China [J]. Environmental Science & Technology, 2021, 55(7): 4103-4114. [3] 叶利兰, 甘春娟, 陈垚, 等. 疫情防控期间含氯消毒剂大量使用对水生生物的影响综述 [J]. 环境污染与防治, 2021, 43(5): 644-648. YE L L, GAN C J, CHEN Y, et al. Effect of chlorinated disinfectants usage on aquatic organism during the epidemic control: A review [J]. Environmental Pollution & Control, 2021, 43(5): 644-648(in Chinese).
[4] 楚文海, 沈杰, 栾鑫淼, 等. 疫情防控期间污水处理厂强化消毒下的水环境次生风险实证研究 [J]. 给水排水, 2020, 56(6): 1-5, 14. doi: 10.13789/j.cnki.wwe1964.2020.06.001 CHU W H, SHEN J, LUAN X M, et al. Study on secondary risk of water environment under enhanced disinfection of wastewater treatment plant during epidemic prevention and control [J]. Water & Wastewater Engineering, 2020, 56(6): 1-5, 14(in Chinese). doi: 10.13789/j.cnki.wwe1964.2020.06.001
[5] ZHONG Y, GAN W H, DU Y, et al. Disinfection byproducts and their toxicity in wastewater effluents treated by the mixing oxidant of ClO2/Cl2 [J]. Water Research, 2019, 162: 471-481. doi: 10.1016/j.watres.2019.07.012 [6] 李悦宁, 贺凯, 王婷, 等. 日本消毒副产物及其前体物的现状及研究进展 [J]. 环境化学, 2018, 37(8): 1820-1830. doi: 10.7524/j.issn.0254-6108.2018022602 LI Y N, HE K, WANG T, et al. Trend and progress on disinfection byproducts and their precursors in Japan [J]. Environmental Chemistry, 2018, 37(8): 1820-1830(in Chinese). doi: 10.7524/j.issn.0254-6108.2018022602
[7] LIU C, MESSERLIAN C, CHEN Y J, et al. Trimester-specific associations of maternal exposure to disinfection by-products, oxidative stress, and neonatal neurobehavioral development [J]. Environment International, 2021, 157: 106838. doi: 10.1016/j.envint.2021.106838 [8] SIMMONS J E, RICHARDSON S D, TEUSCHLER L K, et al. Research issues underlying the four-lab study: Integrated disinfection by-products mixtures research [J]. Journal of Toxicology and Environmental Health. Part A, 2008, 71(17): 1125-1132. doi: 10.1080/15287390802181906 [9] LIU J Q, GIBB M, PRADHAN S H, et al. Synergistic cytotoxicity of bromoacetic acid and three emerging bromophenolic disinfection byproducts against human intestinal and neuronal cells [J]. Chemosphere, 2022, 287: 131794. doi: 10.1016/j.chemosphere.2021.131794 [10] SRIVASTAV A L, PATEL N, CHAUDHARY V K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement [J]. Environmental Pollution, 2020, 267: 115474. doi: 10.1016/j.envpol.2020.115474 [11] ROBERTS J F, van EGMOND R, PRICE O R. Toxicity of haloacetic acids to freshwater algae [J]. Ecotoxicology and Environmental Safety, 2010, 73(1): 56-61. doi: 10.1016/j.ecoenv.2009.09.013 [12] NAROTSKY M G, BEST D S, MCDONALD A, et al. Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids [J]. Reproductive Toxicology, 2011, 31(1): 59-65. doi: 10.1016/j.reprotox.2010.08.002 [13] QIN L T, LIU M, ZHANG X, et al. Concentration addition, independent action, and quantitative structure-activity relationships for chemical mixture toxicities of the disinfection by products of haloacetic acids on the green alga Raphidocelis subcapitata [J]. Environmental Toxicology and Chemistry, 2021, 40(5): 1431-1442. doi: 10.1002/etc.4995 [14] LIN T, ZHOU D J, DONG J, et al. Acute toxicity of dichloroacetonitrile (DCAN), a typical nitrogenous disinfection by-product (N-DBP), on zebrafish (Danio rerio) [J]. Ecotoxicology and Environmental Safety, 2016, 133: 97-104. doi: 10.1016/j.ecoenv.2016.06.047 [15] FISHER D, YONKOS L, ZIEGLER G, et al. Acute and chronic toxicity of selected disinfection byproducts to Daphnia magna, Cyprinodon variegatus, and Isochrysis galbana [J]. Water Research, 2014, 55: 233-244. doi: 10.1016/j.watres.2014.01.056 [16] MELO A, FERREIRA C, FERREIRA I M P L V O, et al. Acute and chronic toxicity assessment of haloacetic acids using Daphnia magna [J]. Journal of Toxicology and Environmental Health. Part A, 2019, 82(18): 977-989. doi: 10.1080/15287394.2019.1676959 [17] PARK K Y, CHOI S Y, LEE S H, et al. Comparison of formation of disinfection by-products by chlorination and ozonation of wastewater effluents and their toxicity to Daphnia magna [J]. Environmental Pollution, 2016, 215: 314-321. doi: 10.1016/j.envpol.2016.04.001 [18] MILOLOŽA M, BULE K, UKIĆ Š, et al. Ecotoxicological determination of microplastic toxicity on algae Chlorella sp. : Response surface modeling approach [J]. Water, Air, & Soil Pollution, 2021, 232(8): 1-16. [19] 刘宝明. 组合工艺对地表水中消毒副产物前体物控制效果研究[D]. 无锡: 江南大学, 2020. LIU B M. Study on the control effect of combined process on the precursors of disinfection byproduct[D]. Wuxi: Jiangnan University, 2020(in Chinese).
[20] LI Z G, LIU X Y, HUANG Z J, et al. Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China [J]. Water Research, 2019, 157: 247-257. doi: 10.1016/j.watres.2019.03.072 [21] KARPOV G V, VASILIEV E S, VOLKOV N D, et al. Structure of monochloroacetic acid anions in water from mass spectral data [J]. Chemical Physics Letters, 2020, 760: 138001. doi: 10.1016/j.cplett.2020.138001 [22] 魏复盛, 国家环境保护总局, 水和废水监测分析方法编委会. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. State Environmental Protection Administration. Determination methods for examination of water and wastewater [M]. Beijing: China Environment Science Press, 2002(in Chinese).
[23] HE N, LIU Z W, SUN X, et al. Phytotoxicity, bioaccumulation, and degradation of nonylphenol in different microalgal species without bacterial influences [J]. International Journal of Molecular Sciences, 2020, 21(4): 1338. doi: 10.3390/ijms21041338 [24] 王辅明, 朱祥伟, 马永鹏, 等. 低浓度五氯酚暴露对稀有鮈鲫体内SOD活性、GSH和HSP70含量的影响 [J]. 生态毒理学报, 2009, 4(3): 415-421. WANG F M, ZHU X W, MA Y P, et al. Effects of low concentration of pentachlorophenol exposure on SOD activity, GSH and HSP70 content in rare minnow(Gobiocypris rarus) [J]. Asian Journal of Ecotoxicology, 2009, 4(3): 415-421(in Chinese).
[25] 刘青青, 张闪闪, 邹华, 等. 斜生栅藻与氰化钾的相互作用 [J]. 环境化学, 2018, 37(3): 552-558. doi: 10.7524/j.issn.0254-6108.2017073001 LIU Q Q, ZHANG S S, ZOU H, et al. Interactions between Scenedesmus obliquus and potassium cyanide [J]. Environmental Chemistry, 2018, 37(3): 552-558(in Chinese). doi: 10.7524/j.issn.0254-6108.2017073001
[26] DING J N, LU G H, LIANG Y. Evaluation of the interactive effects of lead, zinc and benzo(k)fluoranthene on crucian carp, Carassius carassius, using a multiple biomarker approach [J]. Bulletin of Environmental Contamination and Toxicology, 2014, 92(5): 534-539. doi: 10.1007/s00128-014-1220-y [27] STEBBING A R D. Tolerance and hormesis—increased resistance to copper in hydroids linked to hormesis [J]. Marine Environmental Research, 2002, 54(3/4/5): 805-809. [28] 冯凡, 赵中华, 陈晨, 等. 铜绿微囊藻对有机毒物菲的生理生态响应研究 [J]. 长江流域资源与环境, 2018, 27(9): 2031-2041. FENG F, ZHAO Z H, CHEN C, et al. Physi-ecological responses of Microcystis aeruginosa to phenanthrene exposure [J]. Resources and Environment in the Yangtze Basin, 2018, 27(9): 2031-2041(in Chinese).
-



 下载:
下载: