-
全氟/多氟化合物(PFASs)是一类人工合成的有机化合物,高键能的碳氟键和多样的极性基团赋予了该类化合物极强的物理和化学稳定性、疏水疏油性和低浓度时就能表现出的极佳表面活性[1-3]。在过去70年里,该类物质被广泛应用于各类产品中,并随着产品的生产、使用和处置过程进入到环境中[4-5]。多项研究表明PFASs在人体和其他生物体内具有极强的蓄积能力[6],并可能造成如生殖毒性[7]、神经毒性[8]、甲状腺激素干扰[9]等多种不良健康效应, 其带来的生态环境问题和人体健康风险引起了广泛的社会关注。目前,PFASs相关研究成为了世界环境科学研究的热点。
近年来,国内外已有大量研究报道了PFASs在水体[10-11]、大气[12]、灰尘[13]、土壤[14]、沉积物[15-16]等环境介质以及生物体内[17-18]的广泛存在。特别是PFASs极性和水溶性较高,易通过水环境进行扩散和传播。因此,现有研究多集中于不同类型水体中PFASs的检测、赋存和行为。然而,一些离子型PFASs也存在以细小的大气颗粒物为载体的长距离迁移现象,已有研究在干湿沉降样品中检出了PFASs的存在[19],表明大气环境也是PFASs传输和转化的重要介质,尤其与人类接触密切的低层大气更是发生PFASs生物暴露的主要场所[20]。目前,大气细颗粒物(PM2.5)作为最受关注的一类大气污染物,具有成分复杂、高比表面积、高环境活性以及长距离传输性,并且其往往成为众多污染物的高浓度载体[21-22],PFASs也不例外。因此,开展大气环境PM2.5中PFASs赋存水平及其相关环境问题研究,尤其是对二者的复合污染引起的人类健康风险具有重要的现实意义。高效的分析方法是开展PM2.5中PFASs环境科学研究的前提,尽管目前在大量使用的以水环境为主的PFASs的固相萃取(SPE)-高效液相色谱(HPLC)-串联质谱(MS/MS)分析方法也在PM2.5中PFASs的分析检测中获得一定的应用,但仍然存在一些不尽如人意之处。
一方面,目前少量PM2.5中PFASs 污染赋存的研究仅涉及传统PFASs[23-24]。然而近年来随着部分传统PFASs被禁用或限用,巨大的市场需求促使大量新型PFASs被研发作为替代品使用,如氯代多氟醚磺酸(Cl-PFESAs)被作为PFOS的替代产品应用于电镀行业。研究表明Cl-PFESAs已在各种水环境介质中广泛存在,且具有比传统PFASs更高的生物毒性和更长的人体半衰期[25-26]。但目前已有的PFASs方法未对许多新型PFASs全面覆盖。另一方面,大气颗粒物存在复杂的样品基体,这些基体是否会对PFASs的分析产生影响仍然没有明确的研究,这样在一定程度上制约了大气颗粒物中PFASs的准确分析以及相关环境科学研究。因此,研究PM2.5中包括一些新型PFASs在内的PFASs的分析方法及其关键影响因素,对获得准确可靠结果,理解PFASs大气环境赋存、行为和远距离传输具有重要意义。
本研究关注包括Cl-PFESAs在内的16种PFASs,比较了不同采样膜对PM2.5及其中PFASs的富集效率,考察了对方法可能产生影响的基质效应,通过优化色谱-质谱参数,建立了基于弱阴离子交换(WAX)SPE-HPLC-MS/MS的分析方法,为后续大气环境中PFASs的污染研究提供有效的方法支撑。
-
高效液相色谱-三重四极杆质谱仪(HPLC:UltiMate 3000,美国Thermo Fisher公司;MS/MS:API 3200, 美国Applied Biosystems/MDS SCIEX公司),配有Analyst工作站;WAX固相萃取小柱(6 mL,150 mg,Waters公司);Milli-Q 超纯水系统 (美国Millipore公司)。
16种目标PFASs详细信息见表1,包括11种全氟羧酸类化合物(PFCAs),3种全氟磺酸类化合物(PFSAs)和2种Cl-PFESAs;同位素标记的全氟化合物内标物(MPFAC-MXA)包括13C4PFBA、13C4PFOA、13C2PFDA、13C2PFDoDA、18O2PFHxS和13C4PFOS,用来修正前处理过程和仪器分析中引起的误差,以上PFASs标准品和内标物均购自加拿大Wellington Laboratories。
甲醇(色谱纯,美国Merck公司),乙酸铵、氨水、冰醋酸(色谱纯,美国Alfar公司),实验用水由Milli-Q超纯水系统制备。
-
本研究采样点位于中国科学院生态环境研究中心综合楼楼顶,周围空旷且无特征排放源。2020年10月—11月期间,使用中流量大气颗粒物采样器(青岛崂应2050型)以100 L·min−1的流速采集PM2.5样品。采样前,滤膜预先在马弗炉500 ℃煅烧6 h去除有机物杂质,恒温恒湿条件下平衡24 h后称重保存在聚乙烯采样袋中备用。所有可能与样品发生接触的采样器部件和器皿均用甲醇进行清洗,以免造成污染。样品采集完成,滤膜称重后用铝箔包好置于采样袋中,在-20 ℃保存至处理。
取1/2采样膜剪成小块(0.5 cm×0.5 cm)放入15 mL离心管中,加入2 ng内标,混匀后老化30 min,随后加入8 mL甲醇,涡旋1 min,45 ℃下超声30 min,250 r ·min−1振荡提取16 h,以4000 r ·min−1离心10 min后取上清液至另一15 mL离心管。再加入6 mL甲醇按照上述步骤进行二次提取,合并上清液。将合并后的提取液氮吹至1 mL,去离子水稀释至50 mL后用WAX固相萃取小柱净化处理。
上样前,WAX小柱先用1%的氨水甲醇溶液和甲醇活化,然后加入8 mL去离子水转化溶剂。样品以2—3 滴·s−1的速度装载至WAX小柱,样品装载完成后,用4 mL醋酸缓冲溶液和8 mL去离子水清洗杂质,小柱在负压条件下抽干后,目标物依次用甲醇和1%的氨水甲醇溶液进行洗脱,洗脱液氮吹至约0.5 mL,用甲醇定容至1 mL,以4000 r ·min−1离心10 min后取上清液至进样瓶中待测。
-
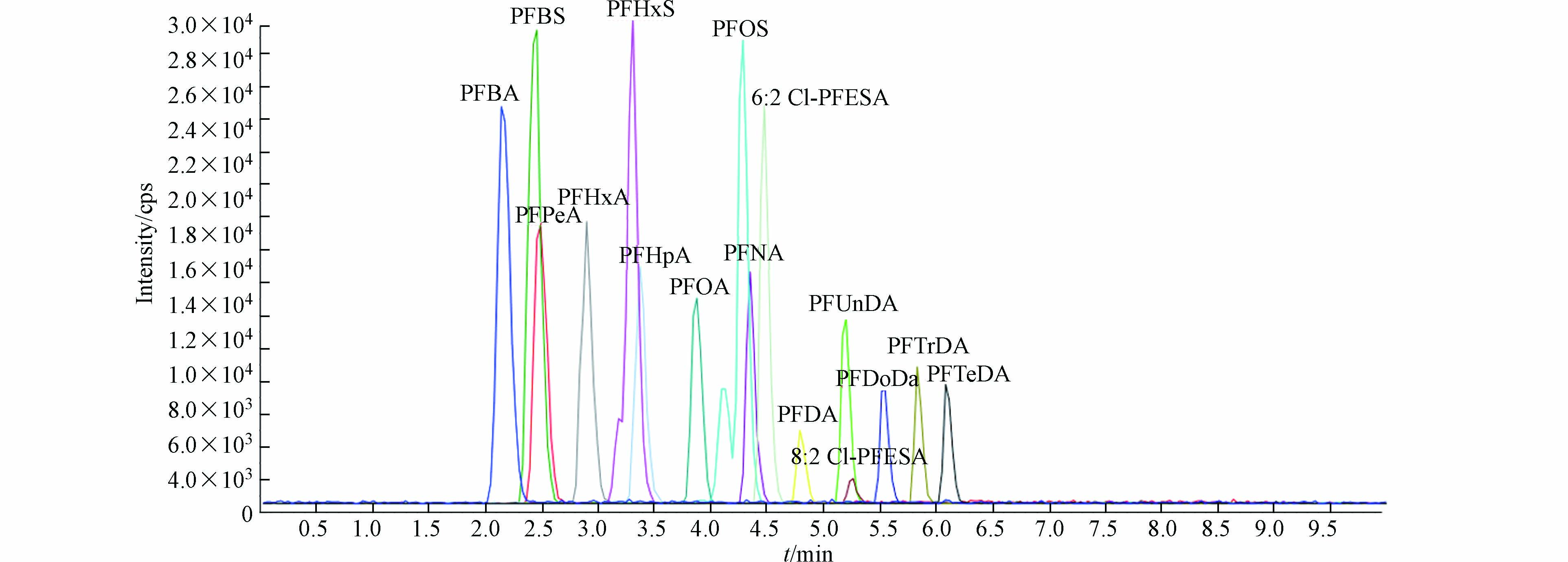
以Acclaim 120 C18色谱柱(4.6 mm×150 mm,5 μm)为分离柱,甲醇(流动相A)和50 mmol·L−1乙酸铵水溶液(流动相B)混合液进行梯度淋洗,10 min内可以对16种目标PFASs实现良好的分离(图1)。梯度洗脱程序为0—4 min,28%—5% B;4—7 min,5% B;7—7.1 min,5%—28% B;7.1—10 min,28% B;流速为1.0 mL ·min−1;进样量为10 μL。
质谱仪在ESI负离子模式使用多反应监测(MRM)运行,确定母离子和子离子的质量数后,对解簇电压(DP)、入口电压(EP)等参数进行优化,优化后的各质谱参数见表2。质谱具体参数如下:离子源温度350 ℃;气帘气0.20 MPa;碰撞气0.06 MPa;喷雾电压-1000 V;离子源温度350 ℃;雾化气0.60 MPa;辅助雾化气0.40 MPa。
-
本研究采用基质提取液添加法对基质效应(ME)进行评估。由于真实的PM2.5基质难以获得,本研究以降尘样品模拟PM2.5,取0.5 g降尘加入10 mL甲醇混匀配成悬浊液,将悬浊液加至处理好的采样膜后老化24 h作为模拟采样膜。具体步骤如下:分别取模拟PM2.5甲醇悬浊液、空白采样膜和模拟PM2.5采样膜3种基质,不加入任何目标物和内标,按照样品前处理方法进行富集净化,将提取液氮吹至1 mL后获得空白基质。在空白基质中加入2 ng的PFASs和内标,混匀后上机测定,得到加标后基质响应值(A);另取空白基质直接上机检测,获得空白基质响应值(B);另外取2 ng·mL−1的PFASs甲醇溶液进样分析获得标准溶液的响应值(S)。每种基质设置3个平行样品,不同介质的基质效应计算公式[27]如下:
当ME≈1时,表示不存在基质效应;当ME<1时,表示检测时存在基质抑制;当ME>1时,表示检测时存在基质增强。
-
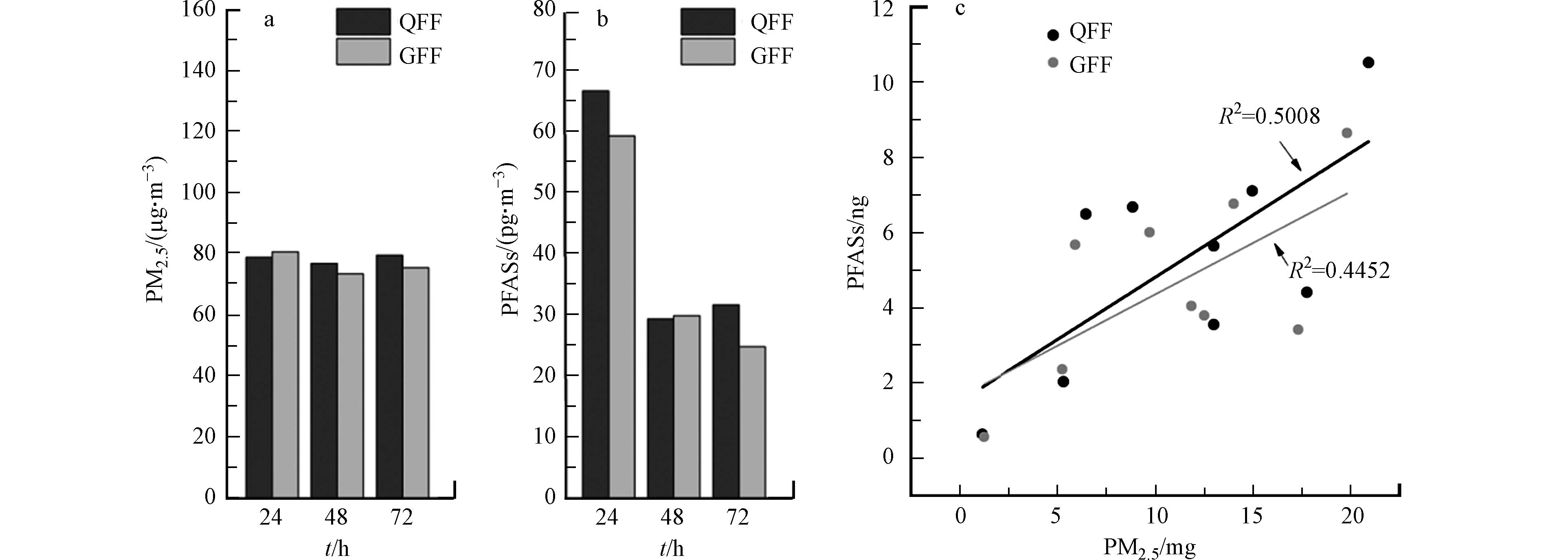
石英纤维滤膜(QFF)和玻璃纤维滤膜(GFF)是采集大气颗粒物最常用的滤膜,而目前尚未报道不同滤膜对PM2.5中PFASs采集效率的影响。本研究使用两台大气颗粒物采样器考察两种滤膜在相同气象条件和环境下对PM2.5及其中PFASs的采集效率,同时探究不同采样时长下两种采样膜对其富集效率的影响。
如图2a所示,不同采样时长下两种采样膜对PM2.5的采集效率相近,在48 h和72 h的采集时长下QFF的富集能力略高于GFF。但QFF在24 h和72 h的采集时长下对PFASs的富集能力明显高于GFF,48 h下二者富集能力相近(图2b)。本次研究对不同时长采集到的PM2.5和PFASs的质量数进行线性拟合,结果发现两种采样膜上二者均表现出正相关关系(图2c),说明当PM2.5浓度增加的同时,大气环境中PFASs的污染也会加剧,证实了PM2.5是PFASs在大气环境中的重要载体和介质。值得注意的是,QFF采集的PM2.5和PFASs二者之间的质量数拟合线性关系更好,表明QFF对于PM2.5和PFASs的采集效果更佳,并在浓度趋势监测分析中具备一致性和稳定性的优势。综合比较,QFF更适用于实际环境下PM2.5中PFASs的采集。
-
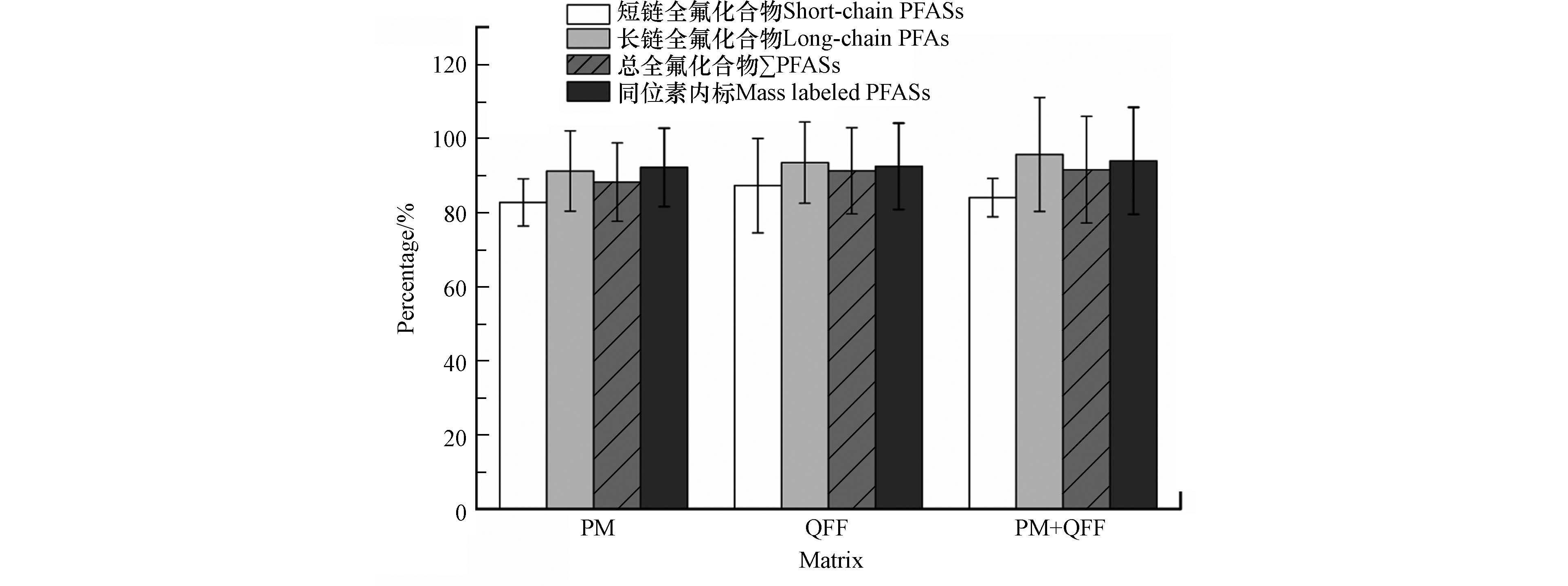
采样膜和PM2.5中的复杂基质可能在色谱分离后与待测目标组分在离子化过程中发生竞争,进而影响目标组分的离子化效率产生基质效应[28-29]。本研究采取基质提取液添加法对二者的基质效应进行评估,3种介质的基质效应如图3所示。
模拟PM2.5、QFF和模拟PM2.5采样膜中PFASs的基质效应分别为74.6%—106.8%、75.2%—111.4%和77.2%—124.0%,对同位素内标的基质效应分别为82.5%—120.3%、80.1%—124.0%和79.2%—128.4%。3种介质存在不同程度的基质效应,本方法利用内标法进行定量,可通过对应的同位素内标进行修正消除。另外,短链PFASs(C<8)与长链PFASs(C≥8)相比更容易受到信号抑制,特别是PFBA存在明显的基体抑制效应(75.2%—77.2%),但仍可通过其对应同位素内标13C4PFBA(86.4%—91.8%)在一定程度上进行消除,校正后PFBA的回收率达到92.5%—96.3%,满足实际样品分析测定的要求。
-
配制含2 ng·mL−1的PFASs同位素标记内标和系列不同浓度的PFASs混合标准溶液(0.05、0.1、0.2、0.5、1、2、5、10、20、50 ng·mL−1)进样分析,以PFASs的质量浓度为横坐标,以其与对应内标峰面积的比值为纵坐标绘制标准曲线。结果表明,16种PFASs在0.05—50 ng·mL−1范围内表现出较好的线性关系,在1/x2权重下的线性相关系数均达到0.99以上。分别以信噪比为3和10时对应的浓度作为仪器检出限(LODs)和定量限(LOQs),方法检出限(MDLs)和定量限(MQLs)分别由LODs、LOQs和相应的富集体积计算得出。如表3所示,16种PFASs的MDLs和MQLs分别为0.09—1.53 pg·m−3和0.23—5.13 pg·m−3。
本研究将在气象条件良好(非空气重污染预警)的情况下采集的PM2.5样品作为基质进行加标回收实验,评价方法的可靠性和精密度。分别向样品中加入20、40、200 μL混合标准溶液(浓度为50 ng·mL−1),按照优化后的前处理步骤和分析条件进行检测,最终样品提取液中加标浓度分别为1、2、10 ng·mL−1,每个加标浓度分别进行3次重复实验。16种PFASs的加标回收率在76.6%—122.3%之间,相对标准偏差为0.5%—13.1%。上述结果表明,本研究所用前处理方法和分析条件能满足PM2.5中包括Cl-PFESAs在内的痕量PFASs同时分析。
-
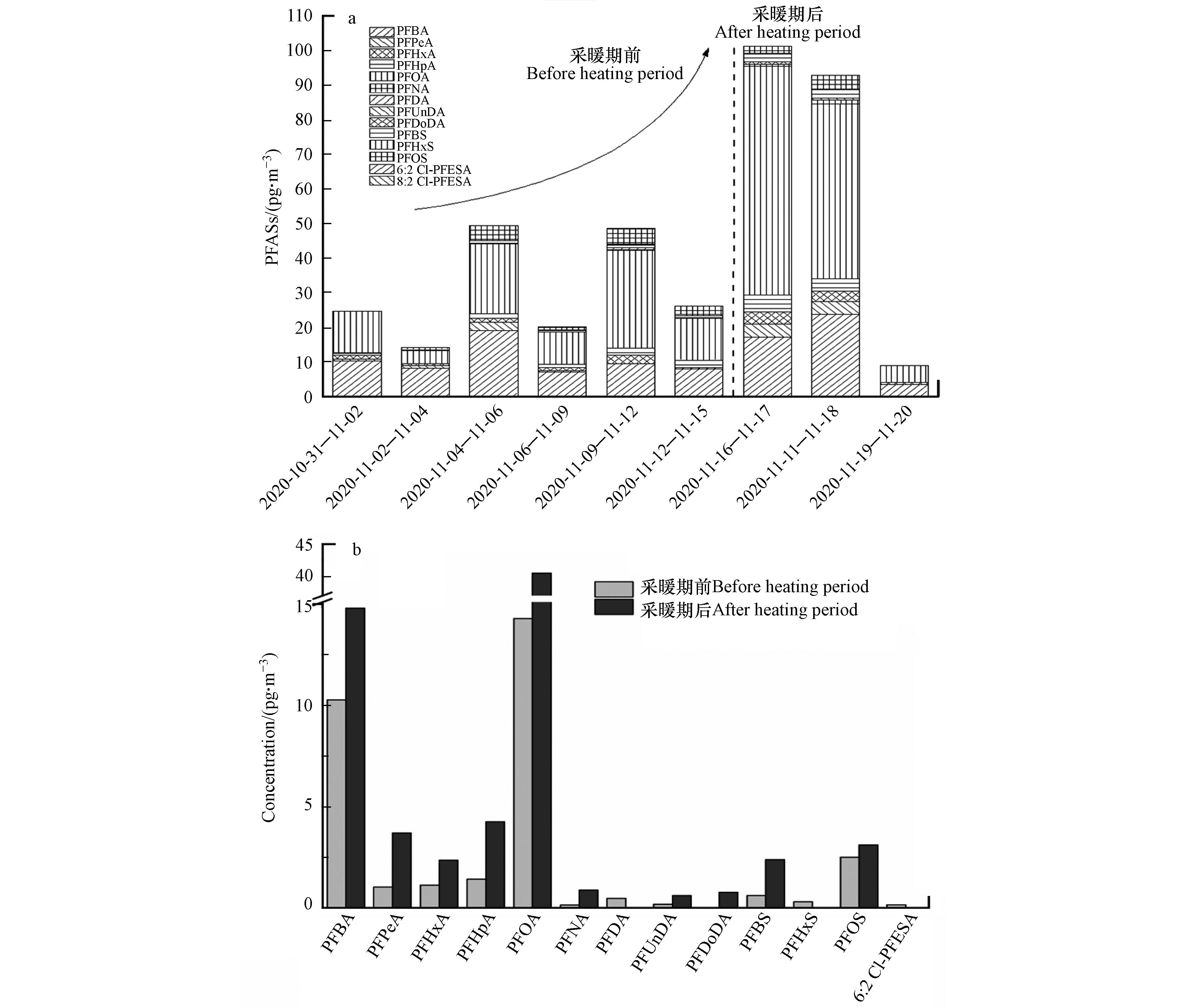
本研究在北京市冬季采暖期前后对PM2.5进行采集,并分析PFASs浓度和分布特征,结果表明PM2.5样品中∑PFASs浓度在8.9—101.3 pg·m−3之间,平均浓度为42.9 pg·m−3。该浓度高于之前深圳市报道的PFASs浓度(3.4—34 pg·m−3)[30],但低于上海市颗粒物中浓度(260—1980 pg·m−3)[31]。除PFTrDA、PFTeDA和8:2 Cl-PFESA外,其它13种PFASs均有检出,以PFOA和PFBA为主要污染物,平均浓度为23.1 pg·m−3和11.8 pg·m−3,其次为PFHpA、PFOS、PFPeA和PFHxA。PFOA作为北京市PM2.5中的主要污染物,也在其他研究中被报道为颗粒物中PFASs的主要成分。与其它地区的测定结果相比,本文采集PM2.5样品中PFOA浓度高于加拿大北极地区(1.4 pg·m−3)、日本筑波(2.6 pg·m−3)和中国南京市(11.6 pg·m−3)颗粒物中的浓度,远低于常熟工业区附近浓度(556 pg·m−3)[32-34]。PM2.5中以短链PFASs为主要污染物可能与该类物质较高的饱和蒸汽压有关,使其更容易从水和土壤等介质中迁移至大气环境[35]。此外,长链PFCAs(C>8)和PFSAs较低的浓度和检出率也可能与这些物质在当地有限的使用和排放有关[36]。
北京市采暖期前后PM2.5中PFASs浓度变化如图4所示。进入采暖期后,PM2.5中PFASs浓度显著升高,采暖期后∑PFASs平均浓度(67.7 pg·m−3)是采暖期前的两倍(30.5 pg·m−3)。另外,进入采暖期后除PFDA、PFHxS和6:2 Cl-PFESA外,其余PFASs浓度均有明显升高。之前的研究表明采暖期集中供热会造成大气污染物的排放增加,但是本研究中涉及到的PFASs浓度在采暖季节增加的具体原因目前还没有明确认识。值得注意的是,采暖期后第3次样品采集过程中(2020.11.19—2020.11.20)有降雨出现,且为本研究采样期间唯一的一次降雨过程,此次采集的样品中PFASs浓度远低于其余样品浓度,该结果可能与湿沉降对大气环境中PM2.5和PFASs的清除有关[37]。综上,冬季较少的降水次数和不利于污染物扩散的气象条件造成的PM2.5累积和其对一些有机物的富集能力增强有一定关系[38-39]。
-
本研究优化和建立了PM2.5中包含新型替代品Cl-PFESAs在内的16种PFASs的SPE-HPLC-MS/MS同时分析方法。该方法能够有效消除复杂基体的基质效应并对目标化合物进行富集。建立的分析方法灵敏度高,待测物的回收率满足实际样品的分析需求。应用该方法对北京市采暖期前后PM2.5中PFASs的浓度水平进行探究,结果表明北京市PM2.5中广泛存在PFSAs污染,以PFOA和PFBA为主要组分,并且采暖期后PFASs浓度明显升高。本研究为后续PM2.5中PFASs及其新型替代品的分析和污染防控提供重要方法支撑。
固相萃取-高效液相色谱-串联质谱法测定大气细颗粒物中全氟/多氟化合物
Determination of per- and polyfluoroalkyl substances in PM2.5 by high performance liquid chromatography-tandem mass spectrometry combined with solid phase extraction
-
摘要: 大气细颗粒物(PM2.5)中全氟/多氟化合物(PFASs)浓度的测定对于了解大气环境中PFASs的赋存水平和远距离传输具有重要意义。本研究通过比较采样膜对PM2.5以及PFASs的富集效率差异,选取了效果最佳的石英纤维滤膜(QFF)为采样膜。利用基质提取液添加法对基质效应进行评估,发现样品基质对短链PFASs存在一定的信号抑制,通过采用相对应的内标可消除部分影响。最终优化建立了固相萃取-高效液相色谱-串联质谱(SPE-HPLC-MS/MS)同时测定PM2.5中包括氯代多氟醚磺酸(Cl-PFESAs)在内的16种PFASs的方法。PM2.5样品经甲醇超声-振荡萃取后,选用弱阴离子交换(WAX)固相萃取柱富集净化,使用Acclaim 120 C18色谱柱,以甲醇和50 mmol·L−1乙酸铵水溶液为流动相对目标PFASs进行梯度洗脱和分离,经电喷雾离子源离子化后在负离子多重反应监测模式(MRM)下进行检测。在优化的分析条件下,16种PFASs的方法检出限(MDLs)为0.09—1.53 pg·m−3,加标回收率为76.6%—122.3%,相对标准偏差为0.5%—13.1%。该方法灵敏度好,准确度高,适用于实际环境PM2.5中PFASs的分析检测。对北京市采暖期前后PM2.5中PFASs进行测定,结果表明∑PFASs浓度在8.9—101.3 pg·m−3之间,以PFOA和PFBA为代表的全氟烷基羧酸(PFCAs)为主,冬季采暖期PM2.5中PFASs污染水平有一定升高,其原因有待进一步研究。
-
关键词:
- 高效液相色谱-串联质谱 /
- PFASs /
- PM2.5 /
- 基质效应 /
- 富集效率
Abstract: Determination of per- and polyfluoroalkyl substances (PFASs) in PM2.5 (particulate matter with an aerodynamic diameter less than 2.5 micrometres) is important for understanding their occurrence and long-range transportation in the atmospheric environment. In this study, quartz fiber filter (QFF) was selected as the appropriate filter based on comparable findings of enrichment efficiency of PM2.5 and PFASs. The matrix effects of PFASs in PM2.5 were evaluated by post-extraction spiking method. The results indicated that matrix suppression was found for the measurement of short-chain PFASs, but it could be eliminated by using their corresponding mass labeled standards. The method was developed successfully for simultaneous determination of 16 PFASs, including chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs), in PM2.5 by using solid phase extraction (SPE) and high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Target PFASs in PM2.5 samples were extracted with methanol using ultrasonic and shaking, and then purified using weak anion exchange (WAX) cartridges. An Acclaim 120 C18 column was applied to separate all compounds. The mobile phase was the mixture of methanol and 50 mmol·L−1 ammonium acetate, which were run in a gradient program. The PFASs were detected using electrospray ionization tandem mass spectrometry (ESI-MS/MS) operated in negative and multiple reactive monitoring (MRM) mode. Under the optimized analysis condition, the method detection limits (MDLs) for 16 PFASs ranged from 0.09 pg·m−3 to 1.53 pg·m−3. Average recoveries of 16 PFASs in PM2.5 ranged from 76.6% to 122.3%, with relative standard deviations (RSDs) of 0.5%—13.1%. Hence, this method is accurate, sensitive and suitable for analysis of PFASs in PM2.5 from real environment. Moreover, the method was used to determine PFASs in PM2.5 around heating period in Beijing. It was found that the concentrations of ∑PFASs in PM2.5 ranged from 8.9 pg·m−3 to 101.3 pg·m−3. Perfluorooctanoic acid (PFOA) and perfluorobutanoic acid (PFBA) were the predominant compounds. The concentrations of PFASs in heating period were higher than that before the heating period, the reason of which needs to be further studied.-
Key words:
- HPLC-MS/MS /
- PFASs /
- PM2.5 /
- matrix effect /
- enrichment efficiency
-

-
表 1 16种PFASs的英文名称、缩写、化学式和对应内标
Table 1. Full names, abbreviations, formulas and mass labeled standards of 16 PFASs
化合物名称
Full name缩写
Abbreviation化学式
Formula对应内标
Mass labeled standardPerfluorobutanoic acid PFBA C4F7O2H 13C4PFBA Perfluoropentanoic acid PFPeA C5F9O2H 13C4PFBA Perfluorohexanoic acid PFHxA C6F11O2H 13C4PFOA Perfluoroheptanoic acid PFHpA C7F13O2H 13C4PFOA Perfluorooctanoic acid PFOA C8F15O2H 13C4PFOA Perfluorononanoic acid PFNA C9F17O2H 13C4PFOA Perfluorodecanoic acid PFDA C10F19O2H 13C2PFDA Perfluoroundecanoic acid PFUnDA C11F21O2H 13C2PFDA Perfluorododecanoic acid PFDoDA C12F23O2H 13C2PFDoDA Perfluorotridecanoic acid PFTrDA C13F25O2H 13C2PFDoDA Perfluorotetradecanoic acid PFTeDA C14F27O2H 13C2PFDoDA Perfluorobutanesulfonic acid PFBS C4F9SO3H 18O2PFHxS Perfluorohexanesulfonic acid PFHxS C6F13SO3H 18O2PFHxS Perfluorooctanesulfonic acid PFOS C8F17SO3H 13C4PFOS 6:2 Chlorinated Polyfluoroalkyl ether sulfonic acid 6:2 Cl-PFESA C8ClF16SO4 13C4PFOS 8:2 Chlorinated Polyfluoroalkyl ether sulfonic acid 8:2 Cl-PFESA C10ClF20SO4 13C4PFOS 表 2 PFASs及其同位素内标的质谱参数
Table 2. MS parameters of the PFASs and mas labeled standards
化合物
Compound母离子
Precursor ion
(m/z)子离子
Product ion
(m/z)解簇电压/V
Declustering
potential入口电压/V
Entrance
potential碰撞能量/V
Collision
potential碰撞出口电压/V
Collision cell
exit potentialPFBA 212.8 168.8 −15.5 −10 −16.2 −4.7 PFPeA 262.8 218.9 −21.5 −10 −15.5 −5.8 PFHxA 312.8 269.0 −18.7 −10 −15.6 −7.8 PFHpA 362.8 319.0 −23 −10 −13 −9 PFOA 412.8 369.0* −16.5 −10 −15.2 −10.4 412.8 168.8 −20.6 −10 −24.5 −4.4 PFNA 462.8 419.1 −31.4 −10 −19.1 −12.1 PFDA 512.8 469.1 −24.4 −10 −24 −7.4 PFUnDA 562.8 519.1 −23.4 −10 −30 −8.5 PFDoDA 612.8 569.0 −24 −10 −25.6 −9.2 PFTrDA 662.8 619.0 −33.4 −10 −24 −10.3 PFTeDA 712.8 669.0 −52.9 −10 −21.1 −11.2 PFBS 298.8 79.9* −71.2 −10 −51.2 −3.5 298.8 99.0 −70.2 −10 −40 −5.1 PFHxS 398.8 79.9* −75.3 −10 −66 −3.1 398.8 99.0 −72.6 −10 −51.4 −2 PFOS 498.8 79.9* −89.1 −10 −86.1 −3.4 498.8 99.0 −82 −10 −62.7 −2 6: 2 Cl-PFESA 530.6 351.0* −66 −10 −41.6 −9.8 530.6 83.0 −56 −10 −50.6 −1.6 8: 2 Cl-PFESA 631.0 451.2* −31 −10 −35 −7 631.0 83.2 −75 −10 −53 −4.3 13C4PFBA 216.9 171.9 −24 −10 −13.5 −4.8 13C4PFOA 416.8 372.1 −25.4 −10 −13.5 −5.45 13C2PFDA 515.0 470.0 −22.3 −10 −18.2 −13.2 13C2PFDoDA 614.8 570.0 −25.6 −10 −22 −9.4 18O2PFHxS 403.0 102.9 −81.2 −10 −48 −2.5 13C4PFOS 502.8 79.9 −91.8 −10 −78.3 −3.6 * 定量离子 Quantitative ion. 表 3 PM2.5中16种PFASs的加标回收率、相对标准偏差、方法检出限和定量限
Table 3. Recoveries, RSDs, MDLs and MQLs of the 16 PFASs in PM2.5
PFAS 加标浓度水平Spiked concentration 方法检出限/(pg·m−3)
MDL方法定量限/(pg·m−3)
MQL1 ng·mL−1 2 ng·mL−1 10 ng·mL−1 回收率/% RSD/% 回收率/% RSD/% 回收率/% RSD/% PFBA 112.3 5.9 93.5 4.2 100.9 2.5 0.28 0.88 PFPeA 101.4 9.6 85.3 7.5 118.9 3.9 0.23 0.69 PFHxA 106.7 5.2 93.3 2.2 98.7 12.1 0.18 0.55 PFHpA 99.6 7.1 89 4.8 99.3 12.1 0.14 0.46 PFOA 110 5.1 94.7 8.5 104.1 12.7 0.18 0.60 PFNA 76.8 13.1 88.8 4.1 94.9 9.2 0.09 0.28 PFDA 105.7 7.4 79.2 12.4 97.5 12.6 0.18 0.60 PFUnDA 122.3 5.3 98.7 11.9 113.2 5.6 0.09 0.28 PFDoDA 115.3 4.4 96 4.3 110.5 5.0 0.18 0.69 PFTrDA 92.7 7.1 92.8 1.6 100.2 7.7 0.32 1.02 PFTeDA 87.8 8.2 91 3.8 93.7 7.3 1.53 5.13 PFBS 116.3 3.5 99.1 5.3 90.9 5.4 0.09 0.23 PFHxS 104.3 5.8 101.2 7.2 98.7 9.6 0.09 0.32 PFOS 104.7 8.7 95.1 8.7 99.2 4.9 0.09 0.28 C8 Cl-PFESA 104.6 8.2 120.3 3.5 119.2 1.5 0.09 0.32 C10 Cl-PFESA 76.6 11.2 96.3 4.5 80.8 0.5 0.28 0.88 -
[1] SUN H W, LI F S, ZHANG T, et al. Perfluorinated compounds in surface waters and WWTPs in Shenyang, China: Mass flows and source analysis [J]. Water Research, 2011, 45(15): 4483-4490. doi: 10.1016/j.watres.2011.05.036 [2] WANG Z Y, DEWITT J C, HIGGINS C P, et al. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? [J]. Environmental Science & Technology, 2017, 51(5): 2508-2518. [3] LINDSTROM A B, STRYNAR M J, LIBELO E L. Polyfluorinated compounds: Past, present, and future [J]. Environmental Science & Technology, 2011, 45(19): 7954-7961. [4] CHEN S, JIAO X C, GAI N, et al. Perfluorinated compounds in soil, surface water, and groundwater from rural areas in Eastern China [J]. Environmental Pollution, 2016, 211: 124-131. doi: 10.1016/j.envpol.2015.12.024 [5] FURL C V, MEREDITH C A, STRYNAR M J, et al. Relative importance of wastewater treatment plants and non-point sources of perfluorinated compounds to Washington State rivers [J]. Science of the Total Environment, 2011, 409(15): 2902-2907. doi: 10.1016/j.scitotenv.2011.04.035 [6] PAN C G, ZHAO J L, LIU Y S, et al. Bioaccumulation and risk assessment of per- and polyfluoroalkyl substances in wild freshwater fish from rivers in the Pearl River Delta region, South China [J]. Ecotoxicology and Environmental Safety, 2014, 107: 192-199. doi: 10.1016/j.ecoenv.2014.05.031 [7] 周圆圆, 沈娟, 毛愉婵, 等. 全氟及多氟类化合物与人类生殖关系的研究进展 [J]. 中华生殖与避孕杂志, 2018, 38(11): 940-945. ZHOU Y Y, SHEN J, MAO Y C, et al. Research progress of the relationship between perfluoroalkyl and polyfluoroalkyl substances and human reproduction [J]. Chinese Journal of Reproduction and Contraception, 2018, 38(11): 940-945(in Chinese).
[8] SHRESTHA S, BLOOM M S, YUCEL R, et al. Perfluoroalkyl substances, thyroid hormones, and neuropsychological status in older adults [J]. International Journal of Hygiene and Environmental Health, 2017, 220(4): 679-685. doi: 10.1016/j.ijheh.2016.12.013 [9] TSAI M S, LIN C C, CHEN M H, et al. Perfluoroalkyl substances and thyroid hormones in cord blood [J]. Environmental Pollution, 2017, 222: 543-548. doi: 10.1016/j.envpol.2016.11.027 [10] CHEN H T, REINHARD M, YIN T R, et al. Multi-compartment distribution of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in an urban catchment system [J]. Water Research, 2019, 154: 227-237. doi: 10.1016/j.watres.2019.02.009 [11] WANG Y, SHI Y L, CAI Y Q. Spatial distribution, seasonal variation and risks of legacy and emerging per- and polyfluoroalkyl substances in urban surface water in Beijing, China [J]. Science of the Total Environment, 2019, 673: 177-183. doi: 10.1016/j.scitotenv.2019.04.067 [12] WU J, JIN H B, LI L, et al. Atmospheric perfluoroalkyl acid occurrence and isomer profiles in Beijing, China [J]. Environmental Pollution, 2019, 255: 113129. doi: 10.1016/j.envpol.2019.113129 [13] TIAN Z X, KIM S K, SHOEIB M, et al. Human exposure to per- and polyfluoroalkyl substances (PFASs) via house dust in Korea: Implication to exposure pathway [J]. Science of the Total Environment, 2016, 553: 266-275. doi: 10.1016/j.scitotenv.2016.02.087 [14] HOUTZ E F, HIGGINS C P, FIELD J A, et al. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil [J]. Environmental Science & Technology, 2013, 47(15): 8187-8195. [15] DONG W H, LIU B L, SONG Y, et al. Occurrence and partition of perfluorinated compounds (PFCs) in water and sediment from the Songhua River, China [J]. Archives of Environmental Contamination and Toxicology, 2018, 74(3): 492-501. doi: 10.1007/s00244-017-0474-x [16] WU J Y, GU L, HUA Z L, et al. Per-, poly-fluoroalkyl substances (PFASs) pollution in benthic riverine ecosystem: Integrating microbial community coalescence and biogeochemistry with sediment distribution [J]. Chemosphere, 2021, 281: 130977. doi: 10.1016/j.chemosphere.2021.130977 [17] WANG Y, SHI Y L, VESTERGREN R, et al. Using hair, nail and urine samples for human exposure assessment of legacy and emerging per- and polyfluoroalkyl substances [J]. Science of the Total Environment, 2018, 636: 383-391. doi: 10.1016/j.scitotenv.2018.04.279 [18] MOODY C A, MARTIN J W, KWAN W C, et al. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek [J]. Environmental Science & Technology, 2002, 36(4): 545-551. [19] YAO Y M, CHANG S, SUN H W, et al. Neutral and ionic per- and polyfluoroalkyl substances (PFASs) in atmospheric and dry deposition samples over a source region (Tianjin, China) [J]. Environmental Pollution, 2016, 212: 449-456. doi: 10.1016/j.envpol.2016.02.023 [20] WANG Q, RUAN Y F, LIN H J, et al. Review on perfluoroalkyl and polyfluoroalkyl substances (PFASs) in the Chinese atmospheric environment [J]. Science of the Total Environment, 2020, 737: 139804. doi: 10.1016/j.scitotenv.2020.139804 [21] ZOU Y J, JIN C Y, SU Y, et al. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro [J]. Environmental Pollution, 2016, 212: 627-635. doi: 10.1016/j.envpol.2016.03.022 [22] 陈刚, 周潇雨, 吴建会, 等. 成都市冬季PM2.5中多环芳烃的源解析与毒性源解析 [J]. 中国环境科学, 2015, 35(10): 3150-3156. doi: 10.3969/j.issn.1000-6923.2015.10.036 CHEN G, ZHOU X Y, WU J H, et al. Source apportionment and toxicity quantitation of PM2.5-associated polycyclic aromatic hydrocarbons obtained from Chengdu, China [J]. China Environmental Science, 2015, 35(10): 3150-3156(in Chinese). doi: 10.3969/j.issn.1000-6923.2015.10.036
[23] 杨朔, 陈辉伦, 盖楠, 等. 北京市大气颗粒物中全氟烷基化合物的粒径分布特征 [J]. 岩矿测试, 2018, 37(5): 549-557. doi: 10.15898/j.cnki.11-2131/td.20180620074 YANG S, CHEN H L, GAI N, et al. Particle size distribution of perfluoroalkyl substances in atmospheric particulate matter in Beijing [J]. Rock and Mineral Analysis, 2018, 37(5): 549-557(in Chinese). doi: 10.15898/j.cnki.11-2131/td.20180620074
[24] LIU Y, LIU W J, XU Y S, et al. Characteristics and human inhalation exposure of ionic per- and polyfluoroalkyl substances (PFASs) in PM10 of cities around the Bohai Sea: Diurnal variation and effects of heating activity [J]. Science of the Total Environment, 2019, 687: 177-187. doi: 10.1016/j.scitotenv.2019.06.103 [25] WANG Y, VESTERGREN R, SHI Y L, et al. Identification, tissue distribution, and bioaccumulation potential of cyclic perfluorinated sulfonic acids isomers in an airport impacted ecosystem [J]. Environmental Science & Technology, 2016, 50(20): 10923-10932. [26] XU L, SHI Y L, LI C X, et al. Discovery of a novel polyfluoroalkyl benzenesulfonic acid around oilfields in Northern China [J]. Environmental Science & Technology, 2017, 51(24): 14173-14181. [27] 侯敏敏, 史亚利, 蔡亚岐. 液液提取-固相萃取-高效液相色谱-串联质谱测定人体血液中16种有机磷酸酯 [J]. 色谱, 2021, 39(1): 69-76. doi: 10.3724/SP.J.1123.2020.07033 HOU M M, SHI Y L, CAI Y Q. Determination of 16 organophosphate esters in human blood by high performance liquid chromatography-tandem mass spectrometry combined with liquid-liquid extraction and solid phase extraction [J]. Chinese Journal of Chromatography, 2021, 39(1): 69-76(in Chinese). doi: 10.3724/SP.J.1123.2020.07033
[28] 周悦榕, 李丹妮, 吴剑平, 等. 超高效液相色谱-电喷雾电离-串联质谱法测定猪粪便中6种抗生素残留的基质效应研究 [J]. 分析测试学报, 2017, 36(8): 1010-1017. doi: 10.3969/j.issn.1004-4957.2017.08.011 ZHOU Y R, LI D N, WU J P, et al. Study on matrix effects in analysis of six antibiotics in swine manure by ultra performance liquid chromatography-electrospray ionization-tandem mass spectrometry [J]. Journal of Instrumental Analysis, 2017, 36(8): 1010-1017(in Chinese). doi: 10.3969/j.issn.1004-4957.2017.08.011
[29] 孙腾飞, 向垒, 陈雷, 等. 环境水样及固相样品中全氟化合物分析方法研究进展 [J]. 分析化学, 2017, 45(4): 601-610. doi: 10.11895/j.issn.0253-3820.160817 SUN T F, XIANG L, CHEN L, et al. Research progresses of determination of perfluorinated compounds in environmental water and solid samples [J]. Chinese Journal of Analytical Chemistry, 2017, 45(4): 601-610(in Chinese). doi: 10.11895/j.issn.0253-3820.160817
[30] LIU B L, ZHANG H, YAO D, et al. Perfluorinated compounds (PFCs) in the atmosphere of Shenzhen, China: Spatial distribution, sources and health risk assessment [J]. Chemosphere, 2015, 138: 511-518. doi: 10.1016/j.chemosphere.2015.07.012 [31] GUO M J, LYU Y, XU T T, et al. Particle size distribution and respiratory deposition estimates of airborne perfluoroalkyl acids during the haze period in the megacity of Shanghai [J]. Environmental Pollution, 2018, 234: 9-19. doi: 10.1016/j.envpol.2017.10.128 [32] GE H, YAMAZAKI E, YAMASHITA N, et al. Size specific distribution analysis of perfluoroalkyl substances in atmospheric particulate matter–development of a sampling method and their concentration in meeting room/ambient atmosphere [J]. Aerosol and Air Quality Research, 2017, 17(2): 553-562. doi: 10.4209/aaqr.2016.07.0292 [33] STOCK N L, FURDUI V I, MUIR D C G, et al. Perfluoroalkyl contaminants in the Canadian Arctic: Evidence of atmospheric transport and local contamination [J]. Environmental Science & Technology, 2007, 41(10): 3529-3536. [34] YU N Y, GUO H W, YANG J P, et al. Non-target and suspect screening of per- and polyfluoroalkyl substances in airborne particulate matter in China [J]. Environmental Science & Technology, 2018, 52(15): 8205-8214. [35] 何鹏飞, 张鸿, 李静, 等. 深圳市大气中全氟化合物的残留特征 [J]. 环境科学, 2016, 37(4): 1240-1247. doi: 10.13227/j.hjkx.2016.04.007 HE P F, ZHANG H, LI J, et al. Residue characteristics of perfluorinated compounds in the atmosphere of Shenzhen [J]. Environmental Science, 2016, 37(4): 1240-1247(in Chinese). doi: 10.13227/j.hjkx.2016.04.007
[36] MENG J, ZHOU Y Q, LIU S F, et al. Increasing perfluoroalkyl substances and ecological process from the Yongding Watershed to the Guanting Reservoir in the Olympic host cities, China [J]. Environment International, 2019, 133: 105224. doi: 10.1016/j.envint.2019.105224 [37] CHEN H, ZHANG L, LI M Q, et al. Per- and polyfluoroalkyl substances (PFASs) in precipitation from mainland China: Contributions of unknown precursors and short-chain (C2C3) perfluoroalkyl carboxylic acids [J]. Water Research, 2019, 153: 169-177. doi: 10.1016/j.watres.2019.01.019 [38] 赵晴, 李岩岩, 贺克斌, 等. 2019年元宵节重污染期间济宁市PM2.5化学组分特征及污染成因分析 [J]. 环境化学, 2020, 39(4): 900-910. doi: 10.7524/j.issn.0254-6108.2019090902 ZHAO Q, LI Y Y, HE K B, et al. Analysis of PM2.5 chemical characteristics and causes during heavy pollution in Jining City around the Lantern Festival of 2019 [J]. Environmental Chemistry, 2020, 39(4): 900-910(in Chinese). doi: 10.7524/j.issn.0254-6108.2019090902
[39] 李杏茹, 白羽, 陈曦, 等. 北京冬季重污染过程大气细颗粒物化学组成特征及来源分析 [J]. 环境化学, 2018, 37(11): 2397-2409. doi: 10.7524/j.issn.0254-6108.2018011401 LI X R, BAI Y, CHEN X, et al. Chemical composition and source apportionment of PM2.5 during winter in Beijing [J]. Environmental Chemistry, 2018, 37(11): 2397-2409(in Chinese). doi: 10.7524/j.issn.0254-6108.2018011401
-



 下载:
下载: