-
近年来,工业快速发展、人为活动加剧,大量含有重金属的废弃物通过各种途径进入环境造成污染,导致“镉米”、儿童血铅超标等事件频发,引起了广泛关注[1]。铅是常见的重金属元素,能够持久存在和具有高蓄积性,不像有机污染物可以通过降解消除,容易对生态环境和人体健康造成严重危害[2]。例如,铅能够降低土壤品质,影响植物正常生长[3];铅能够在鲇鱼脏器组织中积累,对其氧化机能、生长性能和代谢水平等具有负面影响[4];严重的是,铅能够在人体器官和组织中蓄积并产生伤害,如伤害内脏器官及神经系统,特别是容易造成儿童学习困难、智力低下等长期不良影响[5]。
吸附-固定是一种常用的铅污染修复方法[6],吸附材料的选择是关键。常用的吸附材料有生物质炭[7]、黏土矿物[8]和铁锰氧化物[9-10]等,而这些吸附材料对铅的吸附能力小,使用量大。腐殖质是一类以碳为骨架的高分子有机复合物,富含羧基、酚羟基等酸性官能团,对重金属具有较强吸附能力[11-12]。腐殖质在环境中广泛存在、原材料易得,具有环境友好、高重金属容量等特点。因此,腐殖质在重金属污染修复领域受到了广泛的研究和关注。
腐殖质是生物质在挤压、增温、缺氧等条件下经热解、排气等过程形成的有机弱酸混合物,主要以胡敏酸和富里酸为主。腐殖质具有丰富的羧基和酚羟基能够与重金属结合,其结合机制已得到深入研究,主要通过羧基和酚羟基吸附、螯合和络合重金属[11,13]。另外,腐殖质能与土壤形成矿物-有机复合体,改善土壤理化性质,增加吸附重金属结合点位。这些作用可以有效降低重金属的移动性和毒性。由于腐殖质原材料来源广泛(如风化煤、褐煤、泥炭和、污泥和畜禽粪便等)、储量丰富(国内储量超过1000亿吨)[11,14-16],制备方法简单、阳离子交换量大等,因此,腐殖质在去除水体和钝化土壤重金属应用方面具有较大潜力。
腐殖质吸附的研究有很多但多数集中于“碱溶酸析”法提取的胡敏酸对其吸附能力和吸附点位的研究[17-19],而对于吸附方式和吸附过程研究较少。“碱溶酸析”法制备的腐殖质具有低pH(甚至<2),不适于酸性水体和土壤污染修复。为了制备高pH腐殖质并更好地描述其对铅的吸附过程与方式,利用不同絮凝方法制备的腐殖质对铅溶液进行了动力学和热力学吸附试验,研究反应时间、浓度和絮凝方法对吸附的影响,确定其对重金属铅的吸附性能和机制。另外,通过钝化试验探究腐殖质对土壤Pb的固定作用,确定其钝化效果,评估风化煤提取腐殖质应用于水体和土壤修复的潜力。
-
材料及试剂:风化煤原料购自山东创新腐植酸创新科技有限公司,硝酸(HNO3,优级纯)、盐酸(HCl,优级纯)、硝酸铅(Pb(NO3)2,分析纯)、硝酸钠(NaNO3,分析纯)、氯化钙(CaCl2,分析纯)、氢氧化钠(NaOH,分析纯)等试剂购自国药集团化学试剂有限公司,铅标准溶液(1000 mg·L−1)购自国家有色金属及电子材料分析测试中心。试验中所用水均是去离子水。
仪器:水浴恒温振荡器(常州国宇,SHA-2),pH计(雷磁,PHS-3C),离心机(可成,L3-5K),超声机(昆山禾创,KH5200B),火焰原子吸收分光光度计(AAS,德国耶拿,NOVAA400P),元素分析仪(德国Elementar,Vario Micro cube),扫描电镜(SEM,S800,TESCAN,USA)。
-
制备方法:称取一定量的风化煤与0.1 mol·L−1 NaOH按固液比1∶10 (g∶mL)的比例充分混合,常温超声30 min,静置6 h后将上层悬浊液倒出,按此步骤再重复提取5次,将所有提取溶液均匀混合。将混合均匀的溶液平均分成3份,采用不同的絮凝方法处理[12]:(1)用6 mol·L−1 HCl调节溶液pH≤2.0,静置6 h,3000 r·min−1离心15 min后将上清液倒出,用去离子水对沉淀物质洗涤3次,去除盐分及水溶腐殖质,冻干备用。制备的固体物质记为HA;(2)80 g·L−1 CaCl2添加入提取液中,CaCl2的添加量根据溶液阳离子交换量而定。静置-离心-洗涤-冻干流程与(1)相同。制备的物质记为Ca-HA;(3)80 g·L−1 CaCl2-1%阳离子聚丙烯酰胺(CPAM)联合加入溶液中,CaCl2的添加量同(2)。其中,CPAM添加量为CaCl2的5%,作用是加快腐殖质絮凝沉淀。具体操作过程与(2)相同,制备的物质记为Ca-CPAM-HA。其中,离心结束后的上清液可加入悬浊液重复利用。
理化性质表征分析(1)元素分析:元素分析仪测定80 ℃烘至恒重样品元素含量;(2)灰分:马弗炉中800 ℃煅烧4 h,根据前后质量差计算灰分;(3)官能团分析:傅里叶转换红外光谱仪(FT-IR)在波长400—4000 cm−1测定分析腐殖质官能团;(4)羧基含量:采用国际腐殖质协会(IHSS)推荐方法滴定测定羧基[20];(5)pH:pH计测定,固液比为1∶10(g∶mL);(6)表观测定:扫描电镜(SEM)观测样品表面形态及微观结构。
-
称取20 mg腐殖质于50 mL离心管中,加入30 mL用0.1 mol·L−1 HNO3或者NaOH调节pH值、以1 mmol·L−1 NaNO3为背景电解质的Pb(NO3)2溶液。吸附实验于25 ℃水浴恒温振荡器中进行,振荡速度120 r·min−1。振荡结束后离心过0.45 μm水系滤膜,AAS测定Pb浓度。Ca-HA和Ca-CPAM-HA为碱性,为防止因腐殖质的强缓冲性导致溶液pH过高Pb2+沉淀,故吸附试验溶液初始pH均调为2.0。
-
将3%(质量分数)HA添加于处理好的采集于湖南两处的表层土壤中(0—30 cm),充分混匀,室温下保持土壤湿润老化培育1个月。两土壤分别标为S1和S2,土壤基本性质已在之前文章中说明[21]。老化培育结束后,自然风干,利用0.11 mol·L−1 CH3COOH溶液提取酸溶态Pb[22],过0.45 μm膜,AAS测定Pb含量。设定空白对照试验,每个样品3个平行样。
-
不溶腐殖质对Pb2+的吸附量根据溶液吸附前后Pb2+浓度、溶液体积和其质量计算(方程1);HA对酸溶态Pb的钝化能力可根据钝化前后酸溶态Pb含量及土壤质量计算(方程2)。
其中,q是腐殖质对Pb2+的吸附量,mg·g−1;C0、C1分别是吸附前后Pb2+浓度,mg·L−1;w是酸溶态Pb降低量,mg·kg−1;C2、C3分别是钝化前后提取液中Pb2+浓度,mg·L−1;V是溶液体积,mL;m1是腐殖质质量,mg;m2是土壤质量,g。
Excel用于数据计算、处理,Origin 8.0用于数据处理与绘制曲线。
-
吸附模型常被用来描述发生在固-液体系的吸附过程并用来探究吸附方式与机制,其主要分为吸附动力学和吸附热力学模型。
-
吸附动力学模型常被用来描述固-液体系的动态过程,有助于分析吸附过程及机制。准一级、准二级、内扩散和Elovich 4个吸附动力学模型常被用于描述腐殖质吸附Pb2+的动力学过程。准一级动力学模型是基于吸附容量而提出的,适用于描述固-液体系初始扩散阶段控制的动态吸附过程[23];准二级动力学模型适于描述吸附剂对吸附质间的吸附由化学反应控制,如离子交换作用[24];内扩散模型常被用来分析动态吸附的初期过程,根据预期的内扩散行为确定吸附过程的控制步骤[25];Elovich动力学模型可用于描述整个动态吸附过程,适于分析活化能变化较大的非均相化学吸附[26]。4个模型的方程如下所示:
其中,qt是腐殖质在反应时间t(h)对Pb2+的吸附量,mg·g−1;q1、q2是准一级、准二级方程计算的动态吸附达到平衡时腐殖质对Pb2+的理论吸附量,mg·g−1;k1(h−1)、k2(g·(mg·h)−1)和ki(mg·g−1·h−1/2)分别是准一级、准二级和内扩散方程吸附参数;C是内扩散方程参数,mg·g−1;α是初始吸附速率,mg·(mg·h)−1;β是Elovich方程常数,g·mg−1。
-
吸附热力学模型常被用于分析吸附质在固-液体系间的分配,有助于分析吸附方式。Langmiur、Freundlich、Temkin和Elovich模型是常用的描述溶质在固-液体系分配的模型。Langmuir模型是适用于描述单分子层的吸附-解吸过程,分析固体界面覆盖率,计算吸附剂对吸附质的理论最大吸附量[27];Freundlich模型是基于固体表面能量的非均质性而提出并发展的,常被用于描述固-液体系非理想吸附过程的非均质吸附[27];Temkin模型是根据吸附热能随着覆盖率增大而线性降低的假设而提出的,该模型强调了中等浓度溶液环境下吸附剂-吸附质间的相互作用[27];Elovich模型则是基于多层吸附这一假设而提出来的,即假定吸附能不均匀的分布在固体表面[28]。4个模型方程如下:
其中,qe是吸附达到平衡时吸附量,mg·g−1;Ce是Pb2+的平衡浓度,mg·L−1;qL、qE分别是Langmiur、Elovich方程计算的理论最大吸附量,mg·g−1;kL(L·mg−1)、kF(mg(1−n)·Ln·g−1)、kT(L·mg−1)与kE(L·mg−1)分别是Langmiur、Freundlich、Temkin和Elovich方程的平衡吸附常数;1/n是Freundlich方程常数;b是Tenkin方程常数,J·mol−1。
-
风化煤、HA、Ca-HA和Ca-CPAM-HA的pH、元素组成、灰分和羧基含量等已在前期成果中展现[12]。相较于风化煤,HA的pH和灰分含量降低,而Ca-HA和Ca-CPAM-HA的升高;HA的碳和氧元素含量增大,而Ca-HA和Ca-CPAM-HA的碳元素含量降低、氧元素含量没有明显变化;羧基含量均增大,其中HA的增加幅度大于Ca-HA和Ca-CPAM-HA。HA是HCl提供强酸性条件下制备,H+能够替换与羧基、羟基等官能团结合的阳离子(如Ca2+),具有富集作用;Ca-HA和Ca-CPAM-HA是在碱性条件下以CaCl2为絮凝剂制备,Ca2+与官能团结合絮凝沉淀,保留了碱性,但对腐殖质具有稀释作用[12]。因此,造成了HA、Ca-HA和Ca-CPAM-HA的理化性质存在差异。
修复材料的应用需满足相应标准。经三酸(HNO3-HCl-HF)消解、测定分析,风化煤及其提取的腐殖质重金属含量均低于《食用农产品产地环境质量评价标准》(HJ/T332—2006),如风化煤含Cd、Pb和Cu分别是0.15、2.81、6.51 mg·kg−1,HA含Cd、Pb和Cu分别是0.15、3.23、6.09 mg·kg−1,满足应用修复。
-
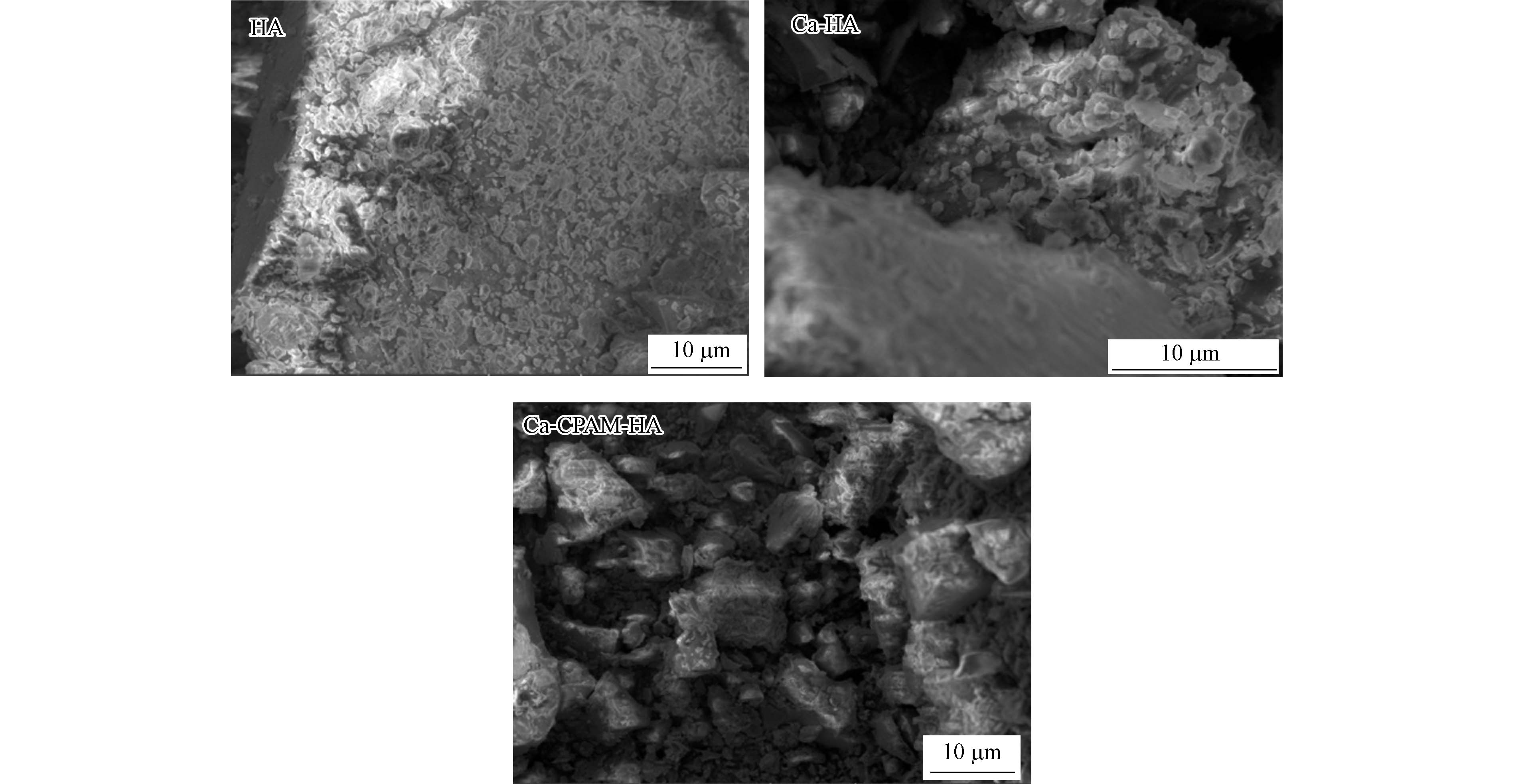
HA、Ca-HA和Ca-CPAM-HA的扫描电镜照片(图1)显示,三者之间存在相同点和不同点。相同点:表面粗糙含有丰富小颗粒;不同点:表面粗糙程度不同且团块大小差异明显。与Ca-HA与Ca-CPAM-HA相比,HA表面颗粒均匀细密、多孔,表明HA具有更强的吸附能力[29]。
-
酸性官能团是腐殖质吸附固定铅的主要作用基团[30],特别是羧基。研究表明,腐殖质对阳离子重金属的吸附能力与羧基含量呈正比[31]。Tan汇总了前人研究成果得出腐殖质中羧基的含量范围是2.4—5.4 mol·kg−1[13],本研究中HA、Ca-HA和Ca-CPAM-HA的羧基含量也在此范围内[12]。从红外光谱谱图分析得出,HA、Ca-HA和Ca-CPAM-HA均含有羧基[32],然而三者的红外光谱图存在区别,如HA、Ca-HA和Ca-CPAM-HA的对称和反对称羧基伸缩频率间距分别是233 cm−1(1609—1376 cm−1)、172 cm−1(1560—1388 cm−1)和192 cm−1(1575—1383 cm−1),这可能是由于絮凝方法造成的。
-
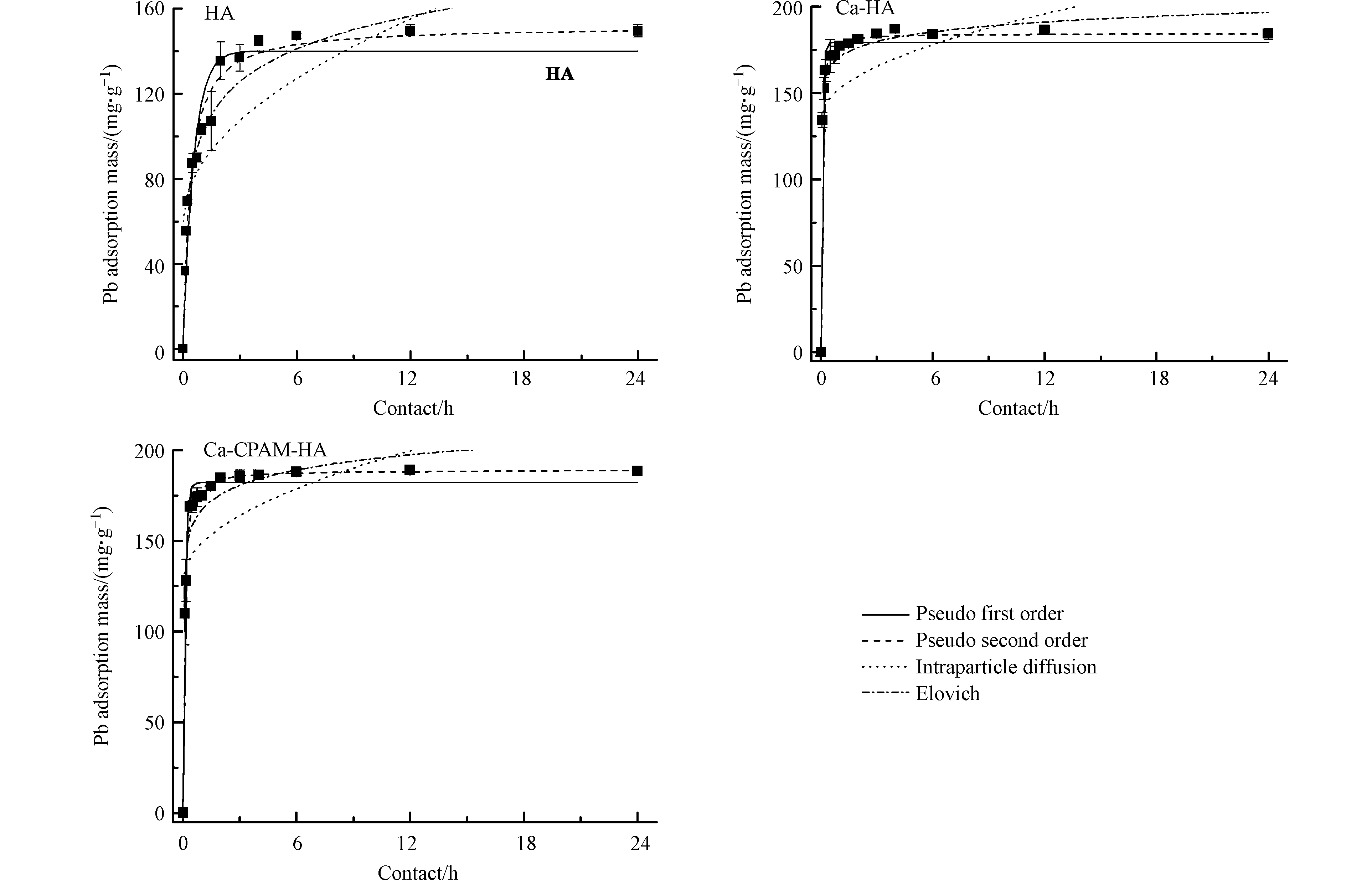
反应时间对HA、Ca-HA和Ca-CPAM-HA吸附Pb2+具有明显影响。吸附初始阶段,HA、Ca-HA和Ca-CPAM-HA对Pb2+的吸附量迅速增大,随着时间增长增加缓慢,分别在6、3、6 h达到吸附-解吸平衡(图2)。HA、Ca-HA和Ca-CPAM达到吸附-解吸平衡时溶液pH值分别为3.80±0.20、5.00±0.15与4.90±0.20。羧基和酚羟基的电离常数(pKa)分别是3.0和9.0,表明酚羟基的电离度非常小,因此可忽略其对Pb2+的吸附量[13]。
为了分析HA、Ca-HA和Ca-CPAM对Pb2+的吸附过程及探究其吸附机制,将吸附数据拟合准一级、准二级、内扩散和Elovich方程,关键参数如表1所示。根据测定的实际吸附量qe、理论平衡吸附量q与相关性R2,可知准二级动力学方程较好地描述HA、Ca-HA和Ca-CPAM-HA对Pb2+的吸附过程,表明整个吸附过程以化学吸附为主[24]。同时,内扩散模型(R2<0.6)和Elovich方程(R2>0.9)的拟合结果也验证支持了HA、Ca-HA和Ca-CPAM-HA吸附Pb2+的整个过程以化学吸附为主这一结论[25-26]。
-
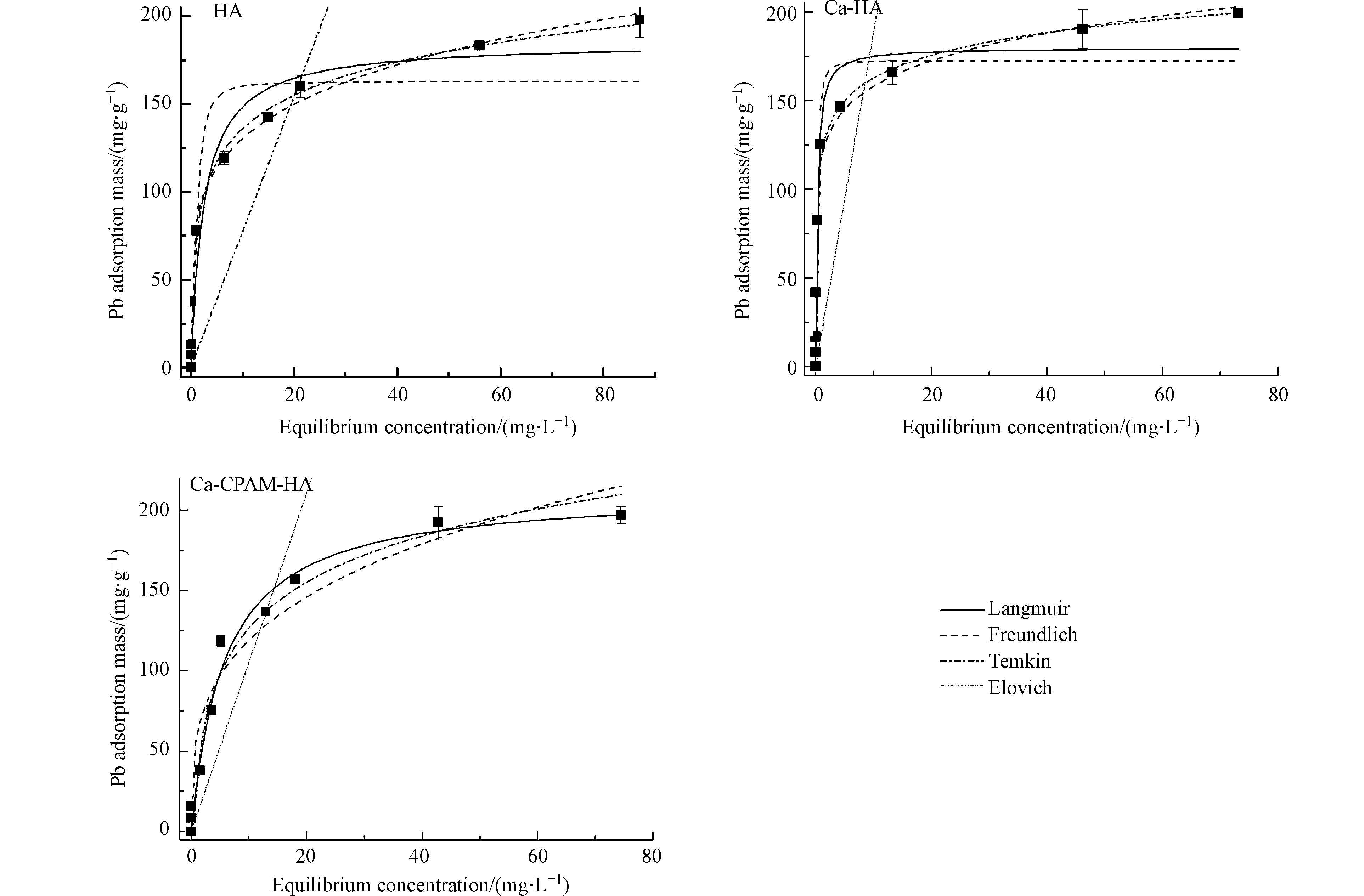
浓度是影响吸附容量的重要因素:HA、Ca-HA和Ca-CPAM-HA对Pb2+的吸附容量在低浓度Pb2+时随着浓度升高而迅速增加,然后增加缓慢直至最大吸附容量(图3)。
为了解析HA、Ca-HA和Ca-CPAM-HA对Pb2+的吸附性能及方式,利用Langmuir、Freundlich、Temkin和Elovich等4个吸附热力学方程对吸附数据进行拟合,相关关键参数列于表2。从表2可看出,4个热力学方程对HA、Ca-HA与Ca-CPAM-HA吸附Pb2+的数据拟合相关系数存在差异,表明三者的吸附方式不同。除了Elovich方程(R2<0.70),其它方程对吸附数据均呈现出较好的拟合效果(R2>0.90),说明HA、Ca-HA和Ca-CPAM-HA对Pb2+不被多层吸附控制[28]。对于HA吸附Pb2+,Langmuir方程的拟合效果最好,说明HA对Pb2+的吸附以单分子层吸附为主[27];Freundlich和Temkin方程均能较好地描述Ca-HA对Pb2+的吸附,说明吸附是在非均质表面进行且吸附质浓度以中等浓度作用为主[27];Temkin方程能较好地描述Ca-CPAM-HA吸附Pb2+,表明在吸附过程中吸附能力随着吸附反应的进行而降低且中等浓度溶液起控制作用[27]。因此,HA、Ca-HA和Ca-CPAM-HA对Pb2+的吸附方式是不同的,可能是由于絮凝方法导致的。
本实验是在相同条件下进行,即每6 h调节1次pH,吸附过程中保持溶液pH=4.5±0.2。Langmuir方程常被用于计算理论最大吸附容量,计算得出HA、Ca-HA和Ca-CPAM-HA对Pb2+的理论最大吸附容量分别是212.3、179.8、185.2 mg·g−1,高于常见的黏土矿物、活性炭和农林废弃物等[7,33-35](表3)。三者对Pb2+吸附后的平衡pH=4.5±0.2,则起吸附作用的主要是羧基用[13]。HA、Ca-HA和Ca-CPAM-HA的羧基含量分别是3.15、2.57、2.74 mol·kg−1,与计算的理论最大吸附容量成正相关,这与Shi等的研究结果相吻合[31]。此外,HA、Ca-HA和Ca-CPAM-HA对低浓度Pb2+具有高去除效果,特别是初始浓度不高于50 mg·L−1时去除率超过99%。吸附完成后的腐殖质需要有效处理,不然会造成二次污染。因腐殖质主要是有机质,因此可采用煅烧处理,减少污染且能够将重金属回收利用。因此,不溶性腐殖质对去除污染水体中Pb2+具有较大应用潜力。
-
腐殖质可通过丰富的酸性官能团与重金属阳离子结合,可通过分析吸附Pb2+前后的红外光谱伸缩频率变化确定作用基团[29]。分析HA、Ca-HA和Ca-CPAM-HA吸附Pb2+前后的FTIR光谱谱图可知(图4),FTIR谱图中反对称羧基伸缩振动频率分别由1609 cm−1变为1589 cm−1(右移20 cm−1)、1560 cm−1变为1578 cm−1(左移18 cm−1)和1575 cm−1变为1589 cm−1(左移14 cm−1),酚羟基、羟基等官能团伸缩频率变化不明显,表明羧基是吸附固定Pb2+的主要官能团。本试验条件下(pH=4.5±0.2)酚羟基几乎不发生解离,这也侧面证明了羧基是主要作用基团[13]。通过动力学过程分析可知,准二级方程较好地描述整个动态吸附过程,说明吸附过程以化学吸附为主[23],内扩散方程拟合结果(R2<0.6)侧面验证了此结论。另外,分析过程中发现,HA吸附Pb2+后FTIR光谱发生红移(右移20 cm−1),而Ca-HA(左移18 cm−1)与Ca-CPAM-HA(左移14 cm−1)发生蓝移,这可能是由于絮凝方法不同造成的[36]。因此,HA、Ca-HA和Ca-CPAM-HA对Pb2+的化学吸附主要通过羧基实现的。
-
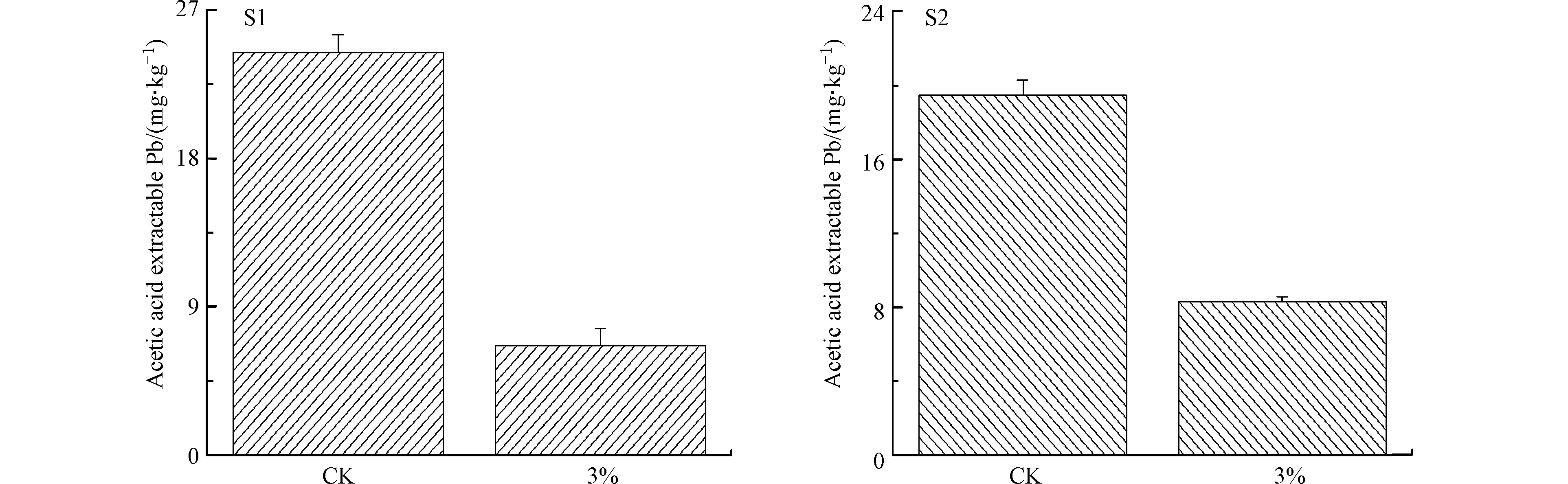
酸溶态重金属常被用于反映其生物有效性[37-39]。添加3% HA处理后,S1和S2中酸溶态Pb的含量分别由24.45 mg·kg−1和19.50 mg·kg−1降至6.67 mg·kg−1与8.61 mg·kg−1,降低幅度分别为72.7%和55.8%(图5)。S1和S2中酸溶态Pb降低程度不同可能由土壤性质差异造成的。通过结果可知,HA能够有效降低土壤酸溶态Pb含量,这与之前的研究结果相一致[12,40]。因此,腐殖质可用于重金属污染土壤修复。
不溶性腐殖质作为铅污染土壤修复剂具有明显的优势及潜力,具体体现在以下几点:1)原材料广泛,制备方法简单,吸附能力强。制备腐殖质的原材料来源广泛,如本研究采用的风化煤在我国的储量超过1000亿t。制备方法操作简便,制备的不溶性腐殖质酸性官能团丰富,如HA的羧基为3.15 mol·kg−1,对Pb2+具有强吸附能力,高于许多常见的吸附剂[7,33-35];2)结构稳定,环境中停留时间长。通过13C-NMR研究发现,腐殖质在土壤中的平均停留时间(MRT)超过1000 a[41]。另外,研究发现MRT与含碳量呈正比,即碳含量越高MRT越长[42],反映腐殖质对Pb具有持久的固定能力;3)环境友好,安全性高。腐殖质本身就是土壤有机质的重要组成部分(占80%以上),其含量能够反映土壤肥力。本研究采用的风化煤重金属含量低,如Cd、Pb和Cu含量分别是0.14、3.34、6.38 mg·kg−1,远低于国家农用田标准[43],安全性好。同时,腐殖质能够与土壤矿物成分结合形成矿物-有机复合体[44],环境安全性好,如Ca-HA(pH=10.09)和Ca-CPAM-HA(pH=9.62)施于酸性土壤中提升土壤pH但不像石灰破坏土壤结构,不产生负面环境影响。可见,腐殖质作为土壤修复剂能够满足不同土壤环境的应用,即在技术和经济上可行且不产生负面环境效应[45]。因此,风化煤提取的不溶性腐殖质在土壤重金属修复方面具有较大应用潜力。
-
(1)与传统“碱溶酸析”方法制备的HA相比,CaCl2和CaCl2-阳离子聚丙烯酰胺(CPAM)制备的Ca-HA和Ca-CPAM-HA具有低C和羧基含量,但是具有高pH和灰分
(2)准二级方程较好地描述整个吸附动力学过程,结合FTIR光谱谱图分析,可知HA、Ca-HA和Ca-CPAM-HA对Pb2+的化学吸附主要通过羧基实现的
(3)HA、Ca-HA和Ca-CPAM-HA对Pb2+均具有强吸附-固定能力。通过Langmuir方程拟合计算,HA、Ca-HA和Ca-CPAM-HA对Pb2+的理论最大吸附量分别是212.3、179.8、185.2 mg·g−1;能够明显钝化土壤Pb,如HA对S1和S2中酸溶态Pb的降低幅度分别为72.7%和55.8%。
风化煤提取的不溶腐殖质对铅的吸附性能及应用潜力
Insoluble humic substances derived from leonardite for lead adsorption and application potential
-
摘要: 以不同絮凝方法从风化煤中提取的不溶性腐殖质为研究对象,表征其理化性质和表观形态结构,通过吸附和钝化试验探究不溶性腐殖质对铅(Pb)的吸附、钝化性能。结果表明,传统“碱溶酸析”法制备的腐殖质(记为HA)具有较低pH和灰分含量,较高的碳和羧基含量;碱性条件下采用CaCl2和CaCl2-阳离子聚丙烯酰胺(CPAM)絮凝制备的腐殖质(分别记为Ca-HA和Ca-CPAM-HA)具有较高pH和灰分含量,较低的碳和羧基含量。HA、Ca-HA和Ca-CPAM-HA对铅离子(Pb2+)的吸附分别在6、3、6 h达到吸附-解吸平衡,吸附量随着Pb2+浓度增加而增大。准二级动力学方程较好地描述整个动力学吸附过程,结合傅立叶变换红外吸收光谱谱图(FTIR),表明吸附过程以化学吸附为主且羧基是主要作用官能团。采用热力学方程对吸附数据进行拟合发现,HA、Ca-HA和Ca-CPAM-HA对Pb2+的吸附方式存在差异。利用Langmuir方程对数据拟合量化,表明HA、Ca-HA和Ca-CPAM-HA对Pb2+均具有较高吸附能力,分别是212.3、179.8、185.2 mg·g-1。添加3% HA降低土壤1(S1)和土壤2(S2)中酸溶态Pb含量幅度分别为72.7%和55.8%。风化煤来源广、储量大、价廉易得,以其为原料制备环境适应性好、吸附容量大的不溶腐殖质,可用于吸附固定重金属,具有良好应用前景。Abstract: In this study, the HCl, CaCl2 or CaCl2-cationic polyacrylamide (CPAM) was used as a flocculant to produce humic substances (named as HA, Ca-HA and Ca-CPAM-HA, respectively) to explore the adsorption capacity in wastewater and immobilization performance in soil for lead (Pb). The results revealed that, compared with Ca-HA and Ca-CPAM-HA, the HA had a lower pH and ash content, and higher carbon and carboxyl content. Kinetic adsorption studies showed that the Pb2+ onto HA, Ca-HA and Ca-CPAM-HA reached equilibrium was 6, 3 and 6 h, respectively, and the adsorption capacity increased with initial Pb2+ concentration. Kinetic adsorption data were better fitted to pseudo-second model than other models, together with FTIR spectroscopic data, indicating the adsorption processes of Pb2+ onto HA, Ca-HA and Ca-CPAM-HA were mainly controlled by chemisorption through carboxyl groups. Four adsorption isotherm models were used to fit the adsorption data to describe the distribution of Pb2+ in solid-liquid system, showing preparation procedures influenced the adsorption process. The maximum adsorption capacity of Pb2+ onto HA, Ca-HA and Ca-CPAM-HA was 212.3, 179.8 and 185.2 mg·g-1 calculated from Langmuir, respectively. HA reduced acetic acid extractable Pb by 72.7% and 55.8% in soil 1 (S1) and soil 2 (S2). The leonardite can be easily obtained as abundant across in China, which can be used as resources to prepare the insoluble humic substances to remediate water and soil pollution.
-
Key words:
- humic substances /
- lead /
- adsorption /
- immobilization /
- leonardite
-

-
表 1 不溶腐殖质吸附Pb2+动力学参数
Table 1. Parameters of kinetic models for Pb2+ adsorption onto leonardite-derived humic substances
吸附剂
Adsorbentqe/
(mg·g−1)准一级方程
Pseudo-first model准二级方程
Pseudo-second modelElovich方程 内扩散方程
Intraparticle diffusionq1/(mg·g−1) R2 q2/(mg·g−1) R2 R2 R2 HA 147.2 140.1 0.917 151.7 0.973 0.948 0.592 Ca-HA 184.4 179.4 0.978 184.6 0.998 0.977 0.177 Ca-CPAM-HA 188.0 182.1 0.975 189.3 0.987 0.931 0.238 表 2 不溶腐殖质吸附Pb2+热力学参数
Table 2. Parameters of adsorption isotherms Pb2+ adsorption onto leonardite-derived humic substances
模型
Model吸附剂
Adsorbent参数 Parameters qL /(mg·g−1) kL/(L·mg−1) R2 Langmuir HA 212.3 0.173 0.982 Ca-HA 179.8 3.591 0.929 Ca-CPAM-HA 185.2 0.402 0.935 Freundlich n kF /(mg(1−n)·Ln·g−1) R2 HA 4.97 80.02 0.963 Ca-HA 8.00 118.624 0.951 Ca-CPAM-HA 3.40 60.485 0.946 Temkin kT /(L·mg−1) b /(J·mol−1) R2 HA 2.650 90.252 0.963 Ca-HA 6.530 134.408 0.954 Ca-CPAM-HA 0.075 5.977 0.977 Elovich qE /(mg·g−1) kE /(L·mg−1) R2 HA 192.44 0.040 0.510 Ca-HA 115.36 0.163 0.243 Ca-CPAM-HA 178.8 0.059 0.680 表 3 腐殖质与其它吸附材料对Pb2+的吸附对比
Table 3. Comparison of adsorption capacities of humic substances with other adsorbents
-
[1] 肖瑶, 吴中杰, 崔美, 等. 生物炭-膨润土共改性及其铅离子吸附与稳定化研究 [J]. 无机材料学报, 2021, 36(10): 1083-1090. doi: 10.15541/jim20200745 XIAO Y, WU Z J, CUI M, et al. Co-modification of biochar and bentonite for adsorption and stabilization of Pb2+ ions [J]. Journal of Inorganic Materials, 2021, 36(10): 1083-1090(in Chinese). doi: 10.15541/jim20200745
[2] 陈晓晨, 韩泽亮, 张剑宇, 等. 中国典型土壤中铅的生物可给性的影响因素分析与健康风险评估 [J]. 生态环境学报, 2021, 30(1): 165-172. CHEN X C, HAN Z L, ZHANG J Y, et al. Study on the influencing factors of Pb bioaccessibility in typical soils in China and the human health risk assessment [J]. Ecology and Environmental Sciences, 2021, 30(1): 165-172(in Chinese).
[3] 厉有为, 梁婵娟. 三种油料作物对土壤Pb污染的耐受性与积累 [J]. 环境化学, 2021, 40(5): 1602-1610. doi: 10.7524/j.issn.0254-6108.2020010601 LI Y W, LIANG C J. Tolerance and accumulation of lead in three oil crops to lead pollution in soil [J]. Environmental Chemistry, 2021, 40(5): 1602-1610(in Chinese). doi: 10.7524/j.issn.0254-6108.2020010601
[4] 彭涛. 水体中铅的浓度对南方鲇的生理生态学影响 [D]. 重庆: 西南大学, 2013. PENG T. Ecophysiological effects of the water-borne lead (Pb) concentrations on southern catfish (Silurus meridionalis) [D]. Chongqing: Southwest University, 2013 (in Chinese).
[5] MAYANS L. Lead poisoning in children [J]. American Family Physician, 2019, 100(1): 24-30. [6] 汪振文, 王会才, 杨继斌, 等. 吸附法去除水中重金属复合污染物的研究状况 [J]. 稀有金属, 2020, 44(1): 87-99. WANG Z W, WANG H C, YANG J B, et al. Removal of heavy metal complex pollutants in water by adsorption [J]. Chinese Journal of Rare Metals, 2020, 44(1): 87-99(in Chinese).
[7] 王鑫宇, 张曦, 孟海波, 等. 温度对生物炭吸附重金属特性的影响研究 [J]. 中国农业科技导报, 2021, 23(2): 150-158. WANG X Y, ZHANG X, MENG H B, et al. Impact of temperature on adsorption characteristics of biochar on heavy metals [J]. Journal of Agricultural Science and Technology, 2021, 23(2): 150-158(in Chinese).
[8] 钱琪所, 赵娟, 谢立灏, 等. 改良黏土对重金属离子的吸附特性及防渗性能 [J]. 环境科学与技术, 2020, 43(2): 96-101. QIAN Q S, ZHAO J, XIE L H, et al. Adsorption characteristics of heavy metal and permeability by sludge activated carbon modified clay [J]. Environmental Science & Technology, 2020, 43(2): 96-101(in Chinese).
[9] 艾翠玲, 雷英杰, 张国春, 等. 纳米铁氧化物吸附处理重金属废水的研究进展 [J]. 化工环保, 2015, 35(6): 593-598. doi: 10.3969/j.issn.1006-1878.2015.06.008 AI C L, LEI Y J, ZHANG G C, et al. Research progresses on adsorption of heavy metals from wastewater using nano iron oxides [J]. Environmental Protection of Chemical Industry, 2015, 35(6): 593-598(in Chinese). doi: 10.3969/j.issn.1006-1878.2015.06.008
[10] 尹仁文, 陈正行, 李娟, 等. 米渣蛋白对镉的吸附效果及其对土壤中镉的钝化作用研究 [J]. 农业工程学报, 2019, 35(2): 221-228. doi: 10.11975/j.issn.1002-6819.2019.02.028 YIN R W, CHEN Z X, LI J, et al. Adsorption of cadmium in aqueous solution and passivation of cadmium in soil by rice dreg protein [J]. Transactions of the Chinese Society of Agricultural Engineering, 2019, 35(2): 221-228(in Chinese). doi: 10.11975/j.issn.1002-6819.2019.02.028
[11] 孟凡德, 袁国栋, 韦婧, 等. 风化煤提取的胡敏酸对镉的吸附性能及其应用潜力 [J]. 浙江大学学报(农业与生命科学版), 2016, 42(4): 460-468. MENG F D, YUAN G D, WEI J, et al. Humic acid from leonardite for cadmium adsorption and potential applications [J]. Journal of Zhejiang University (Agriculture and Life Sciences), 2016, 42(4): 460-468(in Chinese).
[12] MENG F D, YUAN G D, WEI J, et al. Leonardite-derived humic substances are great adsorbents for cadmium [J]. Environmental Science and Pollution Research, 2017, 24(29): 23006-23014. doi: 10.1007/s11356-017-9947-8 [13] TAN K H. Humic matter in soil and the environment: principles and controversies, 2nd edn [M]. Boca Raton: CRC Press, 2014. [14] COLES C A, YONG R N. Humic acid preparation, properties and interactions with metals lead and cadmium [J]. Engineering Geology, 2006, 85(1-2): 26-32. doi: 10.1016/j.enggeo.2005.09.024 [15] de SOUZA F, BRAGANÇA S R. Extraction and characterization of humic acid from coal for the application as dispersant of ceramic powders [J]. Journal of Materials Research and Technology, 2018, 7(3): 254-260. doi: 10.1016/j.jmrt.2017.08.008 [16] TAHIR M M, KHURSHID M, KHAN M Z, et al. Lignite-derived humic acid effect on growth of wheat plants in different soils [J]. Pedosphere, 2011, 21(1): 124-131. doi: 10.1016/S1002-0160(10)60087-2 [17] LIU A G, GONZALEZ R D. Modeling adsorption of copper(II), cadmium(II) and lead(II) on purified humic acid [J]. Langmuir, 2000, 16(8): 3902-3909. doi: 10.1021/la990607x [18] BAKER H, KHALILI F. Analysis of the removal of lead(II) from aqueous solutions by adsorption onto insolubilized humic acid: Temperature and pH dependence [J]. Analytica Chimica Acta, 2004, 516(1-2): 179-186. doi: 10.1016/j.aca.2004.03.068 [19] HABIBUL N, CHEN W. Structural response of humic acid upon binding with lead: A spectroscopic insight [J]. Science of the Total Environment, 2018, 643: 479-485. doi: 10.1016/j.scitotenv.2018.06.229 [20] International Humic Substances Society. Acidic functional groups of IHSS samples, 2021. https://humic-substances.org/acidic-functional-groups-of-ihss-samples/#products. [21] MENG F D, YUAN G D, WEI J, et al. Humic substances as a washing agent for Cd-contaminated soils [J]. Chemosphere, 2017, 181: 461-467. doi: 10.1016/j.chemosphere.2017.04.127 [22] RAURET G, LÓPEZ-SÁNCHEZ J F, SAHUQUILLO A, et al. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials [J]. Journal of Environmental Monitoring, 1999, 1(1): 57-61. doi: 10.1039/a807854h [23] SIMONIN J P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics [J]. Chemical Engineering Journal, 2016, 300: 254-263. doi: 10.1016/j.cej.2016.04.079 [24] HO Y S. Review of second-order models for adsorption systems [J]. Journal of Hazardous Materials, 2006, 136(3): 681-689. doi: 10.1016/j.jhazmat.2005.12.043 [25] OFOMAJA A E. Intraparticle diffusion process for lead(II) biosorption onto Mansonia wood sawdust [J]. Bioresource Technology, 2010, 101(15): 5868-5876. doi: 10.1016/j.biortech.2010.03.033 [26] WU F C, TSENG R L, JUANG R S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems [J]. Chemical Engineering Journal, 2009, 150(2/3): 366-373. [27] ARAÚJO C S T, ALMEIDA I L S, REZENDE H C, et al. Elucidation of mechanism involved in adsorption of Pb (II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms [J]. Microchemical Journal, 2018, 137: 348-354. doi: 10.1016/j.microc.2017.11.009 [28] GUBERNAK M, ZAPAȽA W, KACZMARSKI K. Analysis of amylbenzene adsorption equilibria on an RP-18e chromatographic column [J]. Acta Chromatographica, 2003(13): 38-59. [29] MENG F D, YUAN G D, LARSON S L, et al. Removing uranium (Ⅵ) from aqueous solution with insoluble humic acid derived from leonardite [J]. Journal of Environmental Radioactivity, 2017, 180: 1-8. doi: 10.1016/j.jenvrad.2017.09.019 [30] ORSETTI S, MARCO-BROWN J L, ANDRADE E M, et al. Pb(Ⅱ) binding to humic substances: An equilibrium and spectroscopic study [J]. Environmental Science & Technology, 2013, 47(15): 8325-8333. [31] SHI W J, LÜ C, HE J, et al. Nature differences of humic acids fractions induced by extracted sequence as explanatory factors for binding characteristics of heavy metals [J]. Ecotoxicology and Environmental Safety, 2018, 154: 59-68. doi: 10.1016/j.ecoenv.2018.02.013 [32] PICCOLO A, ZACCHEO P, GENEVINI P G. Chemical characterization of humic substances extracted from organic-waste-amended soils [J]. Bioresource Technology, 1992, 40(3): 275-282. doi: 10.1016/0960-8524(92)90154-P [33] ETCI Ö, BEKTAŞ N, ÖNCEL M S. Single and binary adsorption of lead and cadmium ions from aqueous solution using the clay mineral beidellite [J]. Environmental Earth Sciences, 2010, 61(2): 231-240. doi: 10.1007/s12665-009-0338-4 [34] IMAMOGLU M, TEKIR O. Removal of copper (Ⅱ) and lead (Ⅱ) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks [J]. Desalination, 2008, 228(1/2/3): 108-113. [35] 邵云, 陈静雯, 王温澎, 等. 四种有机物料对Pb2+的吸附特性 [J]. 农业环境科学学报, 2017, 36(9): 1858-1867. doi: 10.11654/jaes.2017-0253 SHAO Y, CHEN J W, WANG W P, et al. Adsorption of Pb2+ by different organic materials in aqueous solution [J]. Journal of Agro-Environment Science, 2017, 36(9): 1858-1867(in Chinese). doi: 10.11654/jaes.2017-0253
[36] MENG F D, ZHANG Y W, CAI Y B, et al. Kinetic and thermodynamic features of Pb(Ⅱ) removal from aqueous solution by leonardite-derived humic acid [J]. Water, Air, & Soil Pollution, 2021, 232(7): 1-12. [37] SHAHID M, PINELLI E, DUMAT C. Review of Pb availability and toxicity to plants in relation with metal speciation: role of synthetic and natural organic ligands [J]. Journal of Hazardous Materials, 2012, 219-220: 1-12. doi: 10.1016/j.jhazmat.2012.01.060 [38] JANOŠ P, VÁVROVÁ J, HERZOGOVÁ L, et al. Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study [J]. Geoderma, 2010, 159(3-4): 335-341. doi: 10.1016/j.geoderma.2010.08.009 [39] KOUKAL B, GUÉGUEN C, PARDOS M, et al. Influence of humic substances on the toxic effects of cadmium and zinc to the green alga Pseudokirchneriella subcapitata [J]. Chemosphere, 2003, 53(8): 953-961. doi: 10.1016/S0045-6535(03)00720-3 [40] RONG Q, ZHONG K, HUANG H, et al. Humic acid reduces the available cadmium, copper, lead, and zinc in soil and their uptake by tobacco [J]. Applied Sciences, 2020, 10(3): 1077. doi: 10.3390/app10031077 [41] WANG M C, CHANG S H. Mean residence times and characteristics of humic substances extracted from a Taiwan soil [J]. Canadian Journal of Soil Science, 2001, 81(3): 299-307. doi: 10.4141/S00-068 [42] RODRÍGUEZ-MURILLO J C, ALMENDROS G, KNICKER H. Humic acid composition and humification processes in wetland soils of a Mediterranean semiarid wetland [J]. Journal of Soils and Sediments, 2017, 17(8): 2104-2115. doi: 10.1007/s11368-017-1663-y [43] 国家环境保护总局. 中华人民共和国环保行业标准: 食用农产品产地环境质量评价标准HJ/T 332—2006 [S]. 北京: 中国环境科学出版社, 2007. State Environmental Protection Administration of the People's Republic of China. Environmental Protection Standard of the People's Republic of China: Farland environmental quality evaluation standards for edible agricultural products. HJ/T 332—2006 [S]. Beijing: China Environment Science Press, 2007 (in Chinese).
[44] YUAN G D, THENG B K G. Clay-organic interactions in soil environments// HUANG PM, SUMNER M, LI YC. Handbook of soil science: Resource management and environmental impacts, 2nd edn [M]. Boca Raton: CRC Press, Taylor & Francis Group, 2011: 2-1–2-20. [45] YUAN G D. Nanomaterials to the rescue [J]. Nano Today, 2008, 3(1-2): 61. -



 下载:
下载: