-
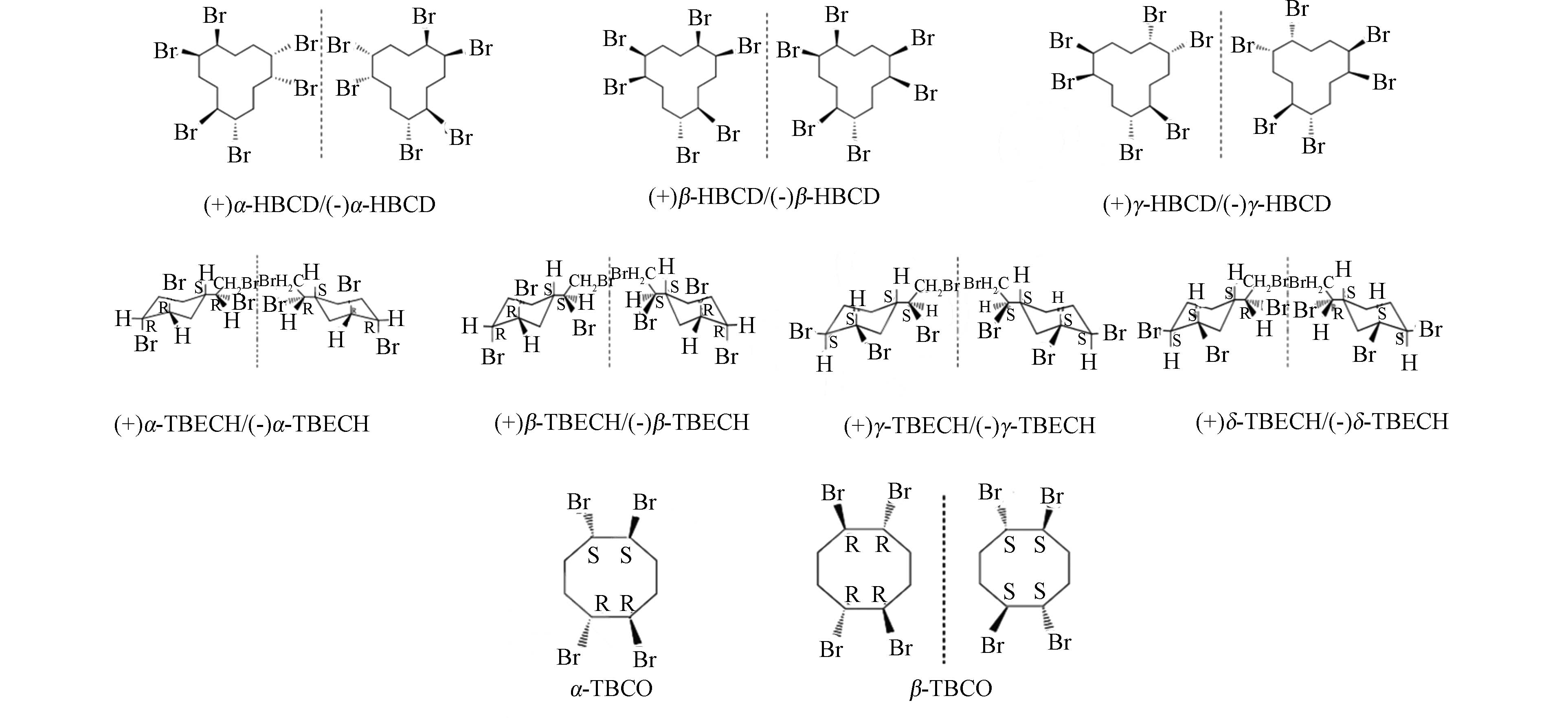
溴代阻燃剂是一类包括脂肪族、脂环族、芳香族及芳香-脂肪族的含溴化合物.其中脂环族溴代阻燃剂(cycloaliphatic brominated flame retardants, CBFRs)的阻燃效果好于芳香族溴代阻燃剂,热稳定性能优于脂肪族溴代阻燃剂,而一度作为多溴联苯醚(PBDEs)的替代品被广泛应用于纺织、建材、电子、电气、交通、化工、石油等领域中[1].脂环族溴代阻燃剂主要包括六溴环十二烷(hexabromocyclododecane,HBCD),1,2-二溴-4-(1,2-二溴乙基)-环己烷(tetrabromoethylcyclohexane,TBECH),和1,2,5,6-四溴环辛烷(1,2,5,6-Tetrabromocyclooctane,TBCO). 目前关于CBFRs研究最多的是六溴环十二烷,其作为全球第三大溴系阻燃剂,在生产、使用、运输及废弃物报废处理等过程进入环境.迄今为止,人们已经在许多环境介质、生物体如鸟类[2]、水生动物[3-4]、植物[5-6]、人体[7],甚至在遥远的北极圈的生物体[8]内都检测到HBCD. 随着HBCD增列《关于持久性有机污染物的斯德哥尔摩公约》持久性有机污染物,其生产和使用受到限制,人们会选择TBECH和TBCO这样结构和性质相似、但知名度较低、认识尚不深入的溴代阻燃剂.虽然目前TBECH、TBCO的产量远低于HBCD,但可以预见在不远的未来,这类阻燃剂的产量、使用量将会上升,从而导致其环境水平的增加[9-10],因此有必要充分认识这类阻燃剂的环境和生态效应[11-16]. TBECH、TBCO和HBCD一样,具有溴代的脂环结构,包含多种异构体和对映体(图1),但碳环数、溴取代数、取代位置、空间结构存在差异.表1展示了HBCD、TBECH、TBCO的理化性质,其中部分参数来自EPI Suite和SPARC软件的计算. 相比HBCD,TBECH和TBCO的分子量较小,熔点较低,稳定性较差;蒸气压和溶解度都较高,说明两者的挥发性和水溶性更大;lgKow都大于4,说明三者同样具有较强的亲脂特性和生物蓄积潜力.三者结构和性质的异同对它们的生物富集、代谢及毒性效应的影响尚不清楚. 研究表明,异构体的生物富集、代谢及毒性效应均存在显著差异[13, 17-22].只有在异构体水平进行研究,才能全面准确地对CBRFs的生态和健康风险做出评价.
本文对HBCD、TBECH、TBCO及其异构体的生物富集、代谢及毒性效应进行综述,并预测了这3种污染物的研究趋势.
-
工业级HBCD主要含有α-HBCD、β-HBCD、γ-HBCD等3种异构体,其比例分别为10%—13%,1%—12%和75%—89%,其中γ-HBCD是工业品中最主要的异构体,另外工业品HBCD中还含有少量的δ-HBCD和ε-HBCD[29].HBCD可以在从生产到废物处理的整个生命周期中进入环境,表2展示了HBCD在各个污染源区环境介质中的水平.天津[30]聚苯乙烯生产厂附近粉尘、土壤和沉积物中均检测出较高的水平的HBCD,在中国香港[31]污水处理厂的污泥中,HBCD浓度比在悬浮颗粒物中的浓度高出近2个数量级,说明其容易在污泥中发生富集,α-HBCD为主要异构体.HBCD在华北塑料垃圾处理区[5]的土壤中的浓度大于处理厂路边和农田土壤中的浓度,γ-HBCD是土壤中主要的异构体.其比例可能受到HBCD加工过程中热异构化和环境介质中非生物/生物转化的影响.同样发现在中国渤海[32]附近一家HBCD生产企业和捷克源区[33]周围的土壤和沉积物中,HBCD异构体比例与工业品相似,都以γ-HBCD为主,推测HBCD在土壤等不透光封闭性较强的介质中不易发生异构体转化,此外在捷克源区的产品仓库和工厂外大气、消费产品和建造材料中都检测出了以γ-HBCD为主的HBCD,其比例与工业品相似,可见上述介质中的污染直接来源于HBCD工业品的生产和使用;在捷克源区的房屋室内和室外背景空气中观察到了工厂空气中95%为γ-HBCD,而背景空气中40%为γ-HBCD,表明存在不同异构体的大气迁移和γ-HBCD到α-HBCD的光转化,工业活动中HBCD的大量生产和使用正在造成持续的环境污染.上述研究表明,HBCD的生产和使用,导致HBCD进入大气、水体和土壤等环境介质。进入环境的HBCD,其异构体比例可能发生变化[30-33].
HBCD作为溴代阻燃剂,已被确定为室内和室外环境中的全球污染物. 研究表明,不同国家、地区的大气气相和颗粒相中都可以检测到HBCD[33-38].瑞典斯德哥尔摩[36]室内灰尘中HBCD的平均浓度为190 ng·g−1. 中国大连大气[37]中73%—97%的HBCD存在于颗粒相. 在深圳[38]室内大气颗粒相中,96%以上的HBCD都存在于PM10. 无论是气相还是颗粒相,α-HBCD都是主要组分,推测α-HBCD更容易被吸附在灰尘等颗粒物表面. 捷克源区[33]房屋室内和室外背景空气中的HBCD都以α-HBCD为主,γ-HBCD的比例明显小于工业品,推测HBCD在空气中发生异构体转化,室外空气中的污染可能来自于暖通空调系统、装饰性聚苯乙烯和绝缘材料. 可见,大气颗粒物是HBCD的重要归趋,HBCD的室内浓度高于室外浓度. 此外,据报道来自中国[39]的空气样本中,δ-HBCD浓度高达15%,超过了在商品HBCD中的比例.但是并不确定δ-HBCD的来源.
工业废水的排放、大气沉降等过程会造成HBCD持续的水体污染.在韩国[40]工业园区附近的湖泊中,HBCD在沉积物中的浓度与中国天津市大沽河[41]和中国贵屿连河[42]中的浓度相似,但比日本九头龙河[43]沉积物中的浓度低1个数量级,在该湖泊附近的小溪中也检测到了HBCD. 有研究报道我国沉积物中HBCD的浓度从东到西呈现下降趋势. 天津大沽河[41]中HBCD的总浓度最高,其次是天津港[41]和长江[44]. 大沽河、天津港、天津地区HBCD总浓度较高的原因可能是受城市和重工业活动的影响. 在东江和珠江口的沉积物中,HBCD的最高平均浓度达到64.7 ng·g−1 dw[45]. 从全国HBCD的分布来看,天津、长江和广东的HBCD总浓度高于其他地区,这主要是由于这些地区是人口密集区和工业活动密集区并对HBCD的市场需求较高. 在渤海南部海域未过滤水体中,α-HBCD、β-HBCD和γ-HBCD浓度范围分别为1.23—1800 ng·L−1、0.85—1120 ng·L−1、1.10—2150 ng·L−1,α-HBCD和γ-HBCD是主要成分[32]. 结合相关研究[32, 43, 46]发现我国渤海南岸的浓度最高,同时也是世界上浓度最高的,因为我国渤海南岸靠近HBCD生产地. 研究发现沉积物中HBCD的异构体组成与工业品中的相似,均以γ-HBCD为主,而在活性污泥中则以α-HBCD为主,其原因是β-和γ-HBCD均可以转化为α-HBCD以及微生物对α-HBCD的降解速率最慢[47].
污水处理厂的污物处置[47]、垃圾填埋地的渗漏、塑料及电子垃圾的不当处置[5]都会导致土壤中HBCD的污染. 除此之外,土壤中的HBCD还可能来自大气中的干湿沉降[33].HBCD由于其疏水性,可以与固体颗粒如土壤、沉积物和污泥有很强的结合. 研究发现不同类型土壤之间也存在显著差异. Tang等[48]分析了浙江省宁波市的垃圾填埋场、工业区、居住区、交通区、菜地和农田土壤,发现在废物倾卸场地和工业区的HBCD浓度较高,HBCD在菜地和农田土壤中的污染极有可能是由扩散源引起的. 与潍坊和宁波相比,除了广东清远,广东的工业区和电子垃圾回收站HBCD浓度最高[49],与印度、越南、马来西亚、印度尼西亚和柬埔寨的露天倾倒场土壤的平均值(<2.4 ng·g−1 dw)相当[50]. 华南地区HBCD浓度较低的原因可能与采样点有关. Li等[51]报道从制造设施开始HBCD浓度呈下降趋势. 山东、浙江和广东土壤中HBCD异构体分布相似,其中γ-HBCD为主要成分,α-HBCD和β-HBCD次之[48-49, 52].土壤中HBCD的异构体组成与工业品中的相似,均以γ-HBCD为主[47].表3展示了HBCD在不同地区大气、水、土壤等介质中的环境水平.
-
TBECH有4种异构体,分别是α-TBECH、β-TBECH、γ-TBECH、δ-TBECH,其中商业品(t-TBECH)中以α-TBECH和β-TBECH为主要组分,二者比例约为 1∶1[26],而β-TBECH是环境中较为常见的异构体[53, 54].商业品TBCO有2种异构体:α-TBCO、β-TBCO,其比例分别为34%和66%[55].
与大量关于HBCD的报道相比,环境中TBECH和TBCO的报道非常有限,而与TBECH相比,TBCO的报道更是少之又少. 作为新型溴代阻燃剂,TBECH和TBCO在建筑材料、电子设备、家具用品中的使用,使其很容易挥发到室内空气中,进而污染室外空气. 如今已在挪威和英国[56]的室内大气和灰尘中检测到了TBECH,办公室布置相比家庭,具有较快的器具及电子产品更换速率,使得办公室大气中TBECH浓度普遍高于家庭房屋中的浓度,α-TBECH为主要异构体;室内灰尘中TBECH的浓度相比大气要高得多,在家庭和办公室室内灰尘中,β-TBECH为主要异构体,可能是由于β-TBECH相比α-TBECH具有较低的挥发性,更容易在灰尘中积累.意大利罗马[57]家庭和工作场所的室内灰尘样本(33.7—228 ng·g−1),美国加利福尼亚州[58](<0.64—360 ng·g−1;54%的检出率)在室内环境中测得的TBECH平均浓度是迄今为止最高的. TBECH在瑞典斯德哥尔摩[36]室内和室外大气、室内灰尘中也有所检出,公寓中的浓度是办公室浓度的4倍,这与上述英国家庭和办公室内浓度的结论不同,说明TBECH的浓度可能很大程度上受到室内装修布置的影响. 同样在捷克[59]发现不同房屋大气中TBECH的浓度差距很大,室内最高浓度比第二高浓度高出10倍;其夏季浓度高于冬季,夏季和冬季的室外平均浓度分别为96、2.2 pg·m−3,体现出了在夏季TBECH更高的挥发性.在英国西米德兰兹[34]也发现夏季TBECH的浓度高于冬季,小于英国[56]室内空气浓度的报道,其检出量是瑞典空气的10—20倍.在瑞典新建幼儿园[60]空气和灰尘、中国北方电子垃圾回收区大气[61]中检测到TBECH和TBCO异构体,后者以β-TBECH和β-TBCO为主要组分,TBCO在后者中的浓度为30 pg·m−3. 有报道发现TBECH具有持久性[62]和长距离迁移性[63],在偏远的北极和南极地区已经测量到TBECH的存在. 在北极和亚北极[64]检测到了TBECH的大气沉积.在来自挪威北极地区[65]的被动空气样本中,α-TBECH和β-TBECH是检出率最高的卤化阻燃剂,每种异构体的浓度分别为29—65、17—46 pg·m−3. 虽然无法获得北极其他地区(例如北美)空气中TBECH的浓度,但在加拿大北极地区的一些海洋生物群中检测到了β-TBECH,表明其具有远距离运输的潜力[66]. 最近一个报告指出,在南极洲西部的南设得兰群岛大气样本中检测出α-TBECH、β-TBECH和β-TBCO,主要分布在颗粒相中,浓度范围分别为nd—0.39、nd—1.3、nd—0.21 pg·m−3.这意味着TBCO可能也具有环境持久性和长距离迁移能力[9].
通过污染源的排放和大气沉积,TBECH和TBCO会进入地表水、沉积物、底泥和土壤.如今已在中国北方电子垃圾回收区[61]的水、土壤中检测出TBECH,且以β-TBECH为主;TBCO在土壤和灰尘中的浓度分别为<0.0493—0.0890 ng·g−1dw、7.38 ng·g−1,以β-TBCO为主. 中国香港水域[67]地表沉积物中的TBECH浓度比该水域中HBCD的浓度低约1个数量级,且发现两者浓度存在很强的相关性,推测两者的排放源或排放途径相似.中国香港污水处理厂[31]中废水(包括颗粒相和溶解相)和污泥中TBECH的浓度比该研究中HBCD相应浓度低一个数量级,底泥中浓度高出悬浮颗粒物两个数量级,且都以α-TBECH为主,意味着TBECH的污染和扩散程度小于HBCD。TBECH与HBCD具有相似的理化性质,与固体颗粒如污泥、沉积物有很强的结合.新加坡[68]地表水和悬浮沉积物中TBECH的浓度与中国香港污水处理厂的报道不同,底泥沉积物中的浓度低于悬浮沉积物,可见TBECH在地表水体中并未发生大量沉积.在德国北海地区[69]的沉积物中检测到高达12 ng·g−1dw的TBCO,这意味着TBCO从河流到北海沉积区的迁移率很高.可见,作为HBCD的替代品,TBECH和TBCO已经在大气、水体、土壤等多种环境介质中被检出.表4展示了TBECH在不同地区大气、水、土壤等介质中的环境水平.
-
生物体能通过环境暴露和食物链传递蓄积HBCD. 一系列调查结果表明,HBCD在水生和陆地生态系统中都存在生物富集现象.
有关海洋生态系统的调查发现,在2005年至2015年之间,中国南海江豚(Neophocaena phocaenoides)和海豚(Sousa chinensis)[70]中HBCD的浓度范围分别为97.2—6260 ng·g−1lw和447—45800 ng·g−1lw(lipid weight,以脂质重计),明显高于其生长环境,且HBCD主要积累在脂肪中,含量逐年增长. 在渤海[71]生态系统所有生物样品中HBCD的浓度范围在3.45—461 ng·g−1dw之间,在污染最重的鲻鱼(Liza haematocheila)中高达332 ng·g−1dw. 中国香港水域[67]海洋食物网中的生物体中HBCD的浓度范围在3.01—93.2 ng·g−1lw之间,生物放大因子(BMF,捕食者和猎物之间平均脂质标准化浓度的比率)都大于1,意味着HBCD发生了生物富集和食物链放大,该现象同样在渤海[71]沿岸和南极洲乔治王岛[72]的生态系统中被发现. 在乔治王岛生态系统的所有样品中均检测到HBCD,α-HBCD和γ-HBCD在所有样品中占主导地位,α-HBCD的TMF(营养级放大因子,描述平均脂质归一化浓度与营养级之间的相关性)显著大于1. 上述调查结果证明了HBCD在海洋生物中普遍存在生物富集和食物链放大现象. 目前认为α-HBCD的富集能力最强[67, 70-71],有报道称γ-和β-HBCD不具有生物富集能力[71].
有关淡水生生态系统的调查发现,在韩国河流的鰕虎鱼(Gobiidae)[47]中,HBCD的浓度范围在24.96—66.71 ng·g−1lw之间. 韩国工业园区鲫鱼(Carassius carassius)[73]不同组织HBCD的积累量为:肝脏>卵>血液>肌肉,不同组织中HBCD的浓度普遍高于水体和沉积物中的浓度,所有组织中的生物富集因子(BCF)都大于1,意味着在鲫鱼中发生了生物积累. 韩国工业园区附近的湖泊[40]中,HBCD在鱼类和贝类中的平均浓度分别为1.55 ng·g−1ww(wet weight,以湿重计)、60.9 ng·g−1lw,且α-HBCD相比γ-HBCD在鱼类和贝类中更占优势,其生物群沉积物累积因子(BSAF,生物体内的浓度经过脂质归一化,沉积物中的浓度经过TOC归一化)在0.79—3.21,此外还观察到近岸的生物样本(平均值:2.42)中BSAF高于远离海岸的生物样本(平均值:1.73). 在华南某电子垃圾场的捕食性鱼类鲮鱼(Cirrhinus molitorella)和北方蛇头鱼(Ophicephalus argus)[74]中,HBCD的浓度分别为0.07—96.9、0.18—240 ng·g−1ww,BCF分别高达6225和6431. 不同组织HBCD的积累量为:脂肪>肝脏>鳃>肌肉,积累量与组织脂质含量正相关,说明HBCD更容易蓄积在生物脂肪中. 在电子拆解区水域[75]中,苹果螺(Ampullaria gigas spix)和草鱼(Ctenopharyngodon idellus)体内HBCD的平均浓度分别为5.02 ng·g−1lw和45.9 ng·g−1lw,HBCD在水生食物网中的TMF为6.36,表明存在生物放大. 上述调查结果说明HBCD在淡水生物中也普遍存在生物富集现象,浓度水平与在海洋生物中的相同. 研究发现,在鲤鱼(Cyprimus carpio morpha moblis)[76]中存在α-和γ-HBCD的特异性富集,α-HBCD在不同组织中的BCF均显著高于β-和γ-HBCD. 在天津渤海的海洋生物中,鲱鸥(Larus argentatus)体内HBCD的浓度最高(1111 ng·g−1 lw),在渤海的海洋和淡水食物网中α-HBCD的浓度和HBCD的总浓度随着营养水平的增加而增加,说明在所有异构体中α-HBCD的生物富集潜力较强[77].
与大量的HBCD在水生生物中的富集报道相比,HBCD在陆生生态系统中生物积累研究相对较少,主要集中于植物[5, 78].研究者在户外松针(Pinus armandi Franch, 208 ng·g−1dw)[79]、南极的苔藓(Andreaea depressinervis、Sanionia uncinate, 0.63—930 pg·g−1dw)和地衣(Himantormia lugubris、Usnea antarctica, 0.1—21.1 pg·g−1dw)[80]中均检测出HBCD,说明植物能够吸收空气中的HBCD,同时也意味着HBCD能够进行远距离大气传输. Zhu等[81-82]的研究也证明植物既可以通过叶片吸收空气中的HBCD,也可以通过植物根吸收土壤中的HBCD,根吸收的HBCD能够传输到地上部分. 在珠江三角洲各类蔬菜[83]、自然生态系统红树林[78]中观察到HBCD在植物组织中分布为茎>根>叶.
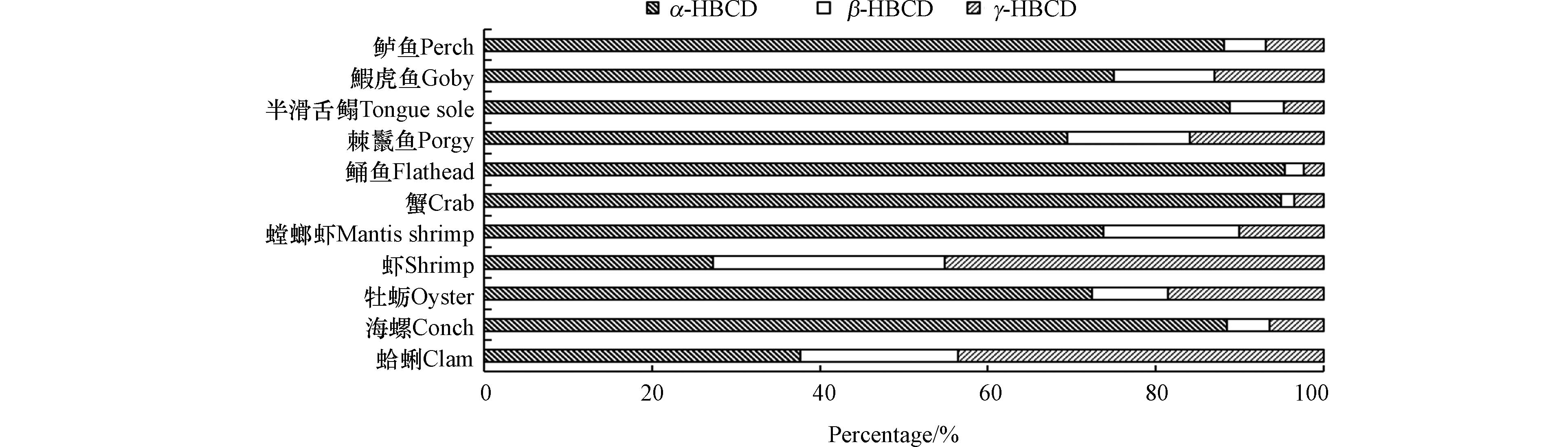
无论是水生还是陆生生物,对HBCD的吸收均显示出显著的异构体差异性.相比β- HBCD、γ-HBCD,α-HBCD具有较高的水溶性[78]、TMF[71]、BCF[73]、生物积累因子(BAF)和摄取速率常数[84],较长的半衰期[84-86],较低的降解率[87],且β- HBCD、γ-HBCD会在生物体内异构化[76, 84, 88-89]为α-HBCD,因此,α-HBCD在生物体内中更占优势. 大量研究发现,HBCD异构体在生物体内的浓度大小为α-HBCD>γ-HBCD>β-HBCD,该规律被发现存在于草鱼(Ctenopharyngodon idellus)[75]、斑马鱼(Brachydanio rerio var)[85]、蚯蚓(Eisenia fetida)[90]、鸭(Anas platyrhynchos)[86]、牛(Bos taurus)[91]、加拿大顶级捕食性鱼类(Salvelinus namaycush、Stizostedion vitreum、Salvelinus fontinalis)[92]、日本虎斑猛水蚤(Tigriopus japonicas)[93]、捕食性鱼类鲮鱼和北方蛇头鱼[74]以及渤海生态系统和韩国河流的鱼类[47, 71]中. 也有不符合上述积累顺序的,如在渤海的虾(Fenneropenaeus chinensis)和蛤蜊(Ruditapes philippinarum)中,HBCD异构体浓度大小γ-HBCD>α-HBCD>β-HBCD,这可能是由于虾和蛤蜊位于食物链底层,直接受到其环境中异构体比例的影响[71]. 陆生植物的研究显示,大部分植物[5, 78-82]中α-HBCD为主要异构体,但在珠江三角洲蔬菜[83]中,γ-HBCD为主要异构体,α-HBCD的比重与土壤接近,蔬菜根部对HBCD不具有异构体选择性吸收. 有学者发现,在沿渤海生态系统中,3种HBCD异构体在不同生物中的分布比例存在差异(图2)[71]. 沿着虾、蛤蜊、鰕虎鱼(Acanthogobius flavimanus)、鲈鱼(Lateolabrax japonicus)这一食物链,随营养级的增加,α-HBCD的比例增大,γ-HBCD的比例降低,由此推测出α-HBCD具有生物放大作用.
-
TBECH和TBCO可通过饮食摄入、皮肤接触或呼吸吸入等途径进入生物体内.目前,国内外关于二者在环境生物中积累和分布的研究主要集中于水生生态系统. 一项关于太湖食物网[94]的研究发现,β-TBECH在10种水生生物中的浓度范围为(276±628) pg·g−1ww,而α-TBECH仅在银鱼(Protosalanx hyalocranius)中被检测到((134±208)pg·g−1ww). 迄今为止,已在鲱鸥(Larus argentatus)[53, 95-96]中持续检测到TBECH的存在. Ruan[70]的研究发现,中国南海海豚脂中的TBECH从2005年到2015年的十年间显著增长,江豚(Neophocaena phocaenoides)和海豚(Sousa chinensis)中TBECH的浓度范围为<0.8—125、<0.8—362 ng·g−1lw,其中α-TBECH为主要异构体,其浓度比该研究中HBCD的浓度低约2个数量级,但高于环境中浓度. 中国香港附近水域[67]的海洋食物网中,TBECH的浓度范围在<0.8—13.9 ng·g−1lw,α-TBECH为主要异构体,在软体动物中的浓度明显高于甲壳类和鱼类中的浓度,表明不同生物对TBECH的积累存在差异. 但是TBECH的BMF都大于1,与HBCD相比,营养级放大因子较低,说明TBECH具有生物放大潜力但低于HBCD.TBECH在甲壳和软体动物中的BSAF均小于1,意味着TBECH在底泥生物中并未发生生物富集. 对于不同异构体,γ-TBECH和δ-TBECH的BMF显著高于α-TBECH和β-TBECH,即γ-TBECH和δ-TBECH的生物放大潜力相对较高,但一般环境中以α-TBECH和β-TBECH为主要组分,因此生物体中也以α-TBECH和β-TBECH为主要组分. 研究表明,在鲸类动物体中,α-TBECH占主导地位.最近的调查结果显示,食物样品中β-TBECH浓度远大于英国室内空气和尘埃,在野生鲭鱼(Scomberomorus niphonius)和金枪鱼(Thunnini)中发现TBECH的浓度分别为6.0、39.48 ng·g−1lw,β-TBECH是主要异构体;在食物和母乳[97]中出现频率最多、含量最高的EFR(新型阻燃剂)是β-TBECH,浓度为2.5 ng·g−1 lw,与α-HBCD的浓度(2.1 ng·g−1 lw)相当.
TBCO在水生生物体内亦有发现.在北美五大湖的水鸟银鸥(Larus argentatus)蛋[53]中检测出TBCO. 在德国湾的黄盖鲽(Limanda limanda)[69]中检测到TBCO,其最高浓度为12 ng·g−1 ww. 在安大略湖的鳟鱼(Salvelinus namaycush)和圆虾虎鱼(Neogobius melanostomus)[98]中检测到TBCO浓度分别为0.033、0.009 ng·g−1ww. 2003年欧洲环境署科学小组的研究报告指出,TBCO符合欧盟的vPvB筛选标准,即具有显著的持久性和生物累积性[99].研究发现TBCO在海水中及淡水中的半衰期大于60 d,在海洋及淡水沉积物中半衰期大于180 d. 然而目前对TBCO生物积累的认识还非常缺乏. 在日本青鳉(Oryzias latipes)[16]胚胎试验中发现TBCO在胚胎中的累积速率常数为1.7—1.8 d−1,生物富集因子最高达到1.3×104,显著大于HBCD意味着其具有较强的生物富集能力,从而影响胚胎发育. 在德国北海地区,TBCO在底栖鱼类[69]中浓度最高为12 ng·g−1ww,其BSAF为2.9,接近于已知的持久性生物有机污染物BDE-47(2,2',4,4'-四溴联苯醚)和BDE-100(2,2',4,4',6-五溴联苯醚)的BSAF,表明该研究中底栖鱼类对TBCO具有生物富集作用. 但TBCO异构体水平的生物积累知之甚少,植物作为环境中重要的生物介质,对有机污染物的环境行为有着不可忽视的作用.目前关于植物中TBECH、TBCO的吸收、传输和转化过程知之甚少.
-
异构化是改变化合物的结构而分子量不变的过程.HBCD的立体结构复杂,主要包含3种立体异构体,分别为α- HBCD、β- HBCD和γ-HBCD. 不同HBCD异构体对生物产生的毒性效应存在差异,因此了解不同异构体的生物异构化现象十分必要. 早期的研究显示,HBCD在生物中不存在异构体转化现象.如Essslinger等[100]用含纯γ-HBCD的食物喂养镜鲤(Cyprinus carpio morpha noblis),并未观察到γ-HBCD异构化为α-HBCD的证据. 随着研究的深入,有关HBCD异构体在不同生物体内的异构化的报道不断涌现. Gannon等[101]用含有98% γ-HBCD的商品HBCD喂养大鼠(Fischer F344、Sprague Dawley、Wistar),在中、高剂量投喂下的大鼠组织中均检测到10%左右的α-HBCD,证明了γ-HBCD向α-HBCD转化的生物异构化. 与γ-HBCD相比,α-HBCD更易分布在肝脏.在母鸡(Gallus domesticus)[2]和斑马鱼(Danio rerio)[87]体内也发现了γ-HBCD转化为α-HBCD的现象. γ-HBCD不仅可以转化为α-HBCD,也可以转化为β-HBCD. Szabo等[102]观察到雌鼠(C57BL/6)的肝和脑组织中的γ-HBCD转变为β-HBCD,以及脂肪和粪便中的γ-HBCD转变为α-HBCD和β-HBCD,该现象也在母鸡(Lohmann Brown)[103]肝脏中被发现,生物异构化更容易发生在脑、肝脏等具有更长保留时间的组织中. 也有一些研究[104]发现雌鼠(C57BL/6)的脂肪和肝脏中β-HBCD可转化为γ-HBCD.
不仅γ-HBCD可以转化为α-HBCD,β-HBCD也可以转化为α-HBCD.Law等[89]发现在虹鳟鱼(Oncorhynchus mykiss)幼体内β-HBCD和γ-HBCD能异构化为α-HBCD.同样,在镜鲤(Cyprimus carpio morpha moblis)[76]和蚯蚓(Metaphire guillelmi、Eisenia fetida)[84, 90]中,发现了β-HBCD和γ-HBCD能异构化为α-HBCD,与β-HBCD相比,γ-HBCD异构化程度更高,且在鲤鱼内脏和肌肉中更容易发生生物异构化. 该异构化现象也在雄性大鼠(Sprague–Dawley)[88]、小麦(Triticum aestivum L.)[81]和黑麦草(Lolium perenne L.)[82]中被发现. Abdallah等[105]研究了鼠和鳟鱼(Oncorhynchus mykiss)肝亚细胞组分对α-HBCD、β-HBCD、γ-HBCD的体外生物转化,在鳟鱼S9馏分(肝匀浆上清液)中检测到δ-HBCD说明发生了异构体转化,但具体异构化机制尚不清楚,而鼠S9馏分测定中不存在δ-HBCD,说明鼠体外生物异构化现象与鳟鱼存在明显差异. 一项生物体外转化试验表明鸡肝和猫肝微粒体中CYP酶不介导HBCD生物异构化和对映选择性代谢[106-107].
表5总结了HBCD在不同生物中的异构体转化,γ-HBCD可以转变为α-HBCD和β-HBCD,β-HBCD可以转变为α-HBCD和γ-HBCD. 以上所有的生物异构化现象都在玉米(Zea mays L.)根[6]中被观察到,而针对α-HBCD的生物异构化现象尚未被发现.
-
HBCD在生物体内可以通过各种生化反应进行代谢. 相关研究包括一些污染场地生物样品测试和实验室暴露研究.结果表明,HBCD的生物代谢速度和代谢产物均存在明显的物种特异性[105, 108-109],不同异构体的代谢产物亦存在显著差异. 目前关于HBCD代谢途径的模型生物主要集中在以小鼠、大鼠为代表的哺乳动物[88, 105, 109-111]和微生物[19, 112-118],少量关于禽类、鱼类和植物等[2, 5,76]的降解研究,以及一些体外实验,如动物肝微粒体[106-107]对HBCD的降解.
在小鼠和大鼠中,HBCD主要通过还原性脱溴、羟基化[88, 105, 109-110]、氧化脱氢、开环等途径进行代谢[88]. Brandsma等[109]在喂食HBCD的Wistar鼠组织中检测出五溴环十二碳烯(PBCDe)、四溴环十二碳烯(TBCDe)、单羟基化的六溴环十二烷(OH-HBCD)、二羟基化的六溴环十二烷(di-OH-HBCD)、二羟基化的五溴环十二碳烯(di-OH-PBCDe). 在野外采集的海燕(Asterinidae)蛋中检测出到OH-HBCD,斑海豹(Phoca vitulina)油脂中检测出OH-HBCD和单羟基的五溴环十二碳烯(OH-PBCDe),但在比目鱼(Pleuronectiformes)组织中却未检测到羟基代谢产物. Hakk比较了HBCD不同异构体的大鼠(Sprague–Dawley)代谢,发现总代谢量为β- > γ- > α-HBCD.α-HBCD没有脱溴代谢或生物异构化,而是形成了两种羟基化的代谢产物[88];β-和γ-HBCD发生了明显的生物异构化、氧化、脱氢、还原脱溴以及开环反应. β-HBCD的代谢产物除α-和γ-HBCD外,还包括OH-HBCD,三羟基取代的六溴环十二烷(tri-OH-HBCD),PBCDe,二硫代羟基四溴环十二碳二烯(dithio-TeBCDe),二羟基取代的三溴环十二碳三烯(di-OH-triBCDeee),巯基二溴甲基酯(dibromomethyl mercapturate);γ-HBCD的代谢产物除α-和β-HBCD外,还包括OH-HBCD,PBCDe,二羟基化的五溴环十二碳烯(DiOH-PBCDe),二硫代羟基五溴环十二烷(Dithio-PBCD),单羟基化的四溴环十二碳二烯(OH-TeBCDe),和四溴十二烷二甲酸(tetrabromodecane dicarboxylic acid). 可见,β-HBCD和γ-HBCD的代谢产物存在较大差异.对成年C57BL/6雌鼠[110]的α-和γ-HBCD代谢研究发现,α-HBCD暴露下检测到OH-HBCD以及两种谷胱甘肽结合产物. 可见,HBCD不仅能发生Ⅰ相代谢,还能发生Ⅱ相代谢. 与此不同的是,在γ-HBCD暴露下检测到的是单羟基化的五溴环十二烷(OH-PBCD),OH-PBCDe,di-OH-PBCDe,二羟基化的五溴环十二碳二烯(di-OH-PBCDee),六溴十二烷二酸,三溴壬烯酸,三溴单烯酸甲酯和四巯基脲酸甲酯(methyl-mercapturateoftetraBCDee).图3给出了大鼠对α-HBCD(A)和γ-HBCD(B)不同的代谢途径. 一些代谢产物可以作为不同异构体暴露的生物标志物. 例如,暴露于不同异构体的C57BL/6雌鼠尿液中,己烯基谷胱甘肽可以作为α-HBCD暴露的生物标志物,而溴化壬烯酸或十二烷二酸可以作为γ-HBCD暴露的生物标志物[110].Abdallah等[105]研究了鼠和鳟鱼(Oncorhynchus mykiss)肝亚细胞组分对HBCD的体外生物转化,发现鼠肝亚细胞的生物转化明显快于鳟鱼,代谢产物包括有3种五溴环十二碳烯异构体、2种四溴环十二碳烯异构体、OH-HBCD、二羟基化的六溴环十二烷(diOH-HBCD)、OH-PBCD和单羟基化的四溴环十二烷(OH-TBCD),而β-和γ-HBCD的降解没有明显的对映体选择性. 表6总结了文献报道的鼠类对HBCD异构体的代谢产物.
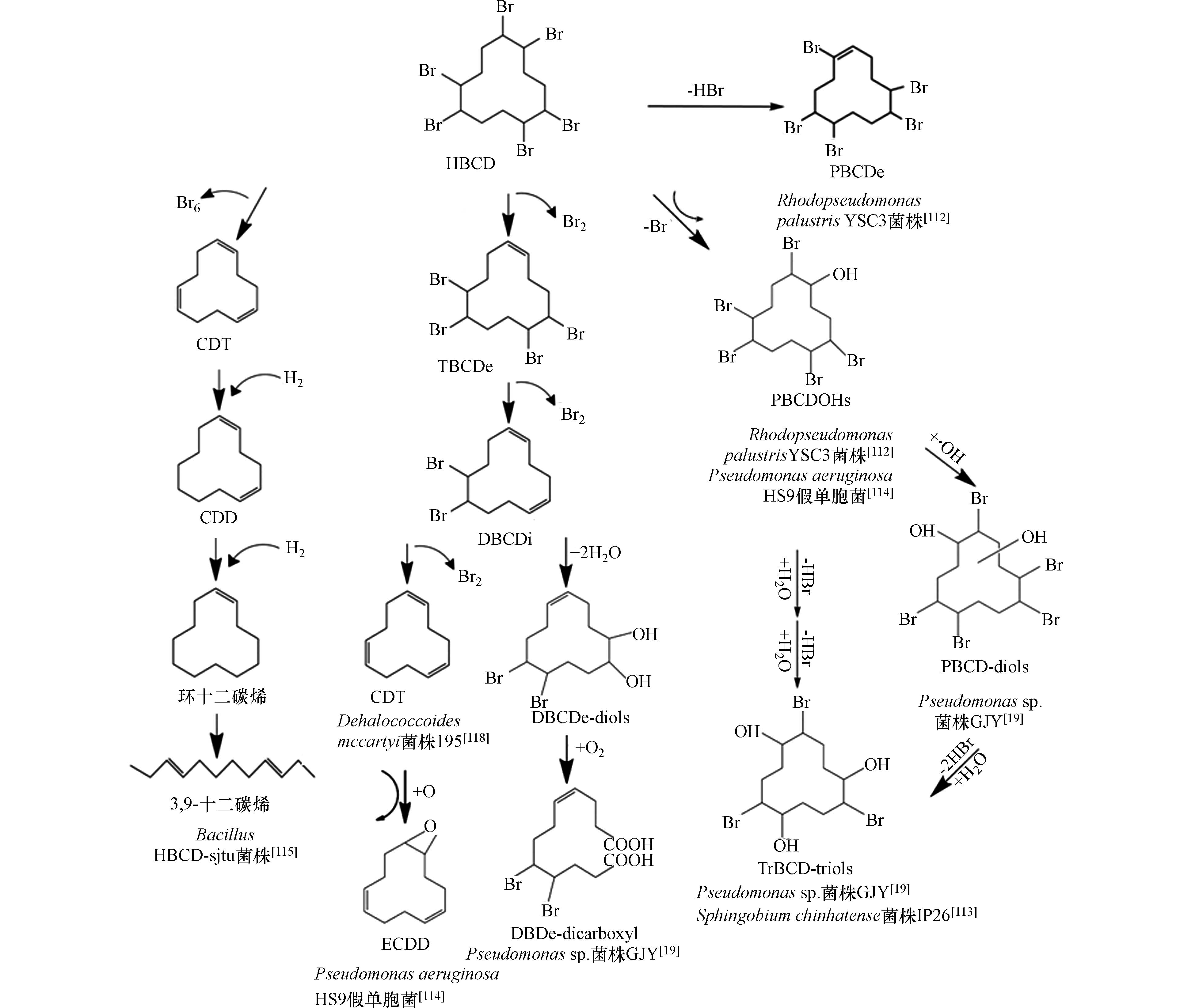
微生物对HBCD的代谢包括脱溴、羟基化、氧化[114-115]、开环[19]等途径,但是没有关于Ⅱ相代谢反应的报道.Dehalococcoides mccartyi菌株195能够对HBCD逐级脱溴降解,形成TBCDe、二溴环十二碳二烯(DBCDi)、1,5,9-环十二碳三烯(CDT)[118]. Pseudomonas aeruginosa HS9假单胞菌对HBCD的降解途径[114]有两条,第一条途径先将HBCD转化为TBCDe、二溴环十二碳二烯(DBCDi),然后再脱溴为顺、反、反-1,5,9-环十二碳三烯(CDT),CDT再氧化成1,2-环氧-5,9-环十二烯(ECDD);第二条途径是通过脱溴和羟基化,生成2,5,6,9,10-五溴环十二醇(PBCDOHs). Bacillus HBCD-sjtu菌株[115]对HBCD的降解产物有CDT、1,5-环十二碳烯(CDD)、环十二碳烯和3,9-十二碳烯. Rhodopseudomonas palustrisYSC3菌株[112]对HBCD的降解,形成了两种主要产物:PBCDOHs和五溴环十二烯(PBCDEs). Heeb等人研究了Sphingobium chinhatense菌株IP26[113]对HBCD异构体对映体的转化,所有异构体、对映体均能够被细菌转化,其中(-)-β-HBCD转化的速度最快,而α-HBCD对映体转化速度最慢. 转化产物包括3种羟基取代物,7种五溴环十二醇(PeBCD-ols)异构体,11种四溴环十二二醇(TeBCD-diols)和3个三溴环十二三醇(TrBCD-triols). 细菌内(+)-α-HBCD、(-)-β-HBCD和(-)-γ-HBCD的转化速度快于其他对映体. 降解体系中首先产生的是PeBCD-ols, 然后是TeBCD-diols和TrBCD-triols,意味着羟基化和脱溴反应是逐级进行的. Geng等[19]发现,在有氧条件下,Pseudomonas sp.菌株GJY降解α-HBCD、β-HBCD、γ-HBCD的主要相同代谢产物是五溴环十二醇(PBCD-ols)、五溴环十二二醇(PBCD-diols)、三溴环十二三醇(TrBCD-triols)、二溴环十二二醇(DBCDe-diols)、二溴十二烷烯二羧酸(DBDe-dicarboxyl),β-HBCD、γ-HBCD共有的代谢产物还有PBCDe,仅在γ-HBCD中检测到四溴环十二碳二烯(TeBCDe)、DBCDi,并未发现HBCD的羟基化产物. 表7总结了文献报道的微生物对HBCD异构体的代谢产物. 图4总结了不同菌株降解HBCD的可能途径. 不同菌株对α-HBCD、β-HBCD、γ-HBCD的代谢速率不同,针对3种异构体的降解研究发现,Pseudomonas sp.菌株GJY[19]对α-HBCD的降解能力最强,Dehalococcoides mccartyi菌株195[118]对HBCD异构体的降解速率大小顺序为α-HBCD>β-HBCD>γ-HBCD,而Sphingobium chinhatense菌株IP26[113]对α-HBCD的降解能力最弱.
HBCD的羟基化、脱溴代谢不仅发生于哺乳动物和微生物体内,而且普遍存在于鸟类、鱼类以及植物中. 例如,Fournier等[2]用喂食的方法,对母鸡(Gallus domesticus)进行γ-HBCD暴露,发现其代谢产物包括TBCDe、di-OH-TBCDe、OH-DBCDe.Zheng等[106-107]研究了鸡肝和猫肝微粒体中CYP酶对α-HBCD、β-HBCD、γ-HBCD的代谢转化,发现两种微粒体中的主要代谢物均为OH-HBCD、di-OH-HBCD、OH-PBCD和di-OH-PBCD. Zhang等[76]着重研究了镜鲤(Cyprimus carpio morpha moblis)组织中的脱溴代谢产物,检测到四溴环十二碳烯(TBCDe)、三溴环十二碳二烯(TriBCDi)和三溴环十二碳三烯(TriBCDie),但未检测出五溴环十二碳烯(PBCDe).
HBCD在植物体内可发生异构化、脱溴和羟基化反应,与谷胱甘肽结合生成谷胱甘肽结合产物[119]. Huang等[5]在塑料垃圾填埋场采集到植物样品中检测到OH-HBCD,PBCDe、OH-PBCDe、Di-OH-HBCD. 温室水培实验结果表明[6],玉米(Zea mays L.)对HBCD的代谢产物包括OH-HBCD、diOH-HBCD、PBCDe、OH-PBCDs、TBCDe以及HBCD-谷胱甘肽加合物. α-HBCD暴露显著降低玉米CYP蛋白含量,抑制CYP酶活性,而γ-HBCD对CYP酶活性有明显的诱导作用[20].不同异构体的玉米CYP450酶体外降解速率为γ-HBCD>α-HBCD,羟基化代谢产物为OH-HBCD、OH-PBCDs和OH-TBCDs.
Heeb等[120]研究了脱卤酶LinB对δ-HBCD的体内转化并与计算机模拟结果进行比较,检测出的代谢产物有五溴环十二烷醇(PBCDols)、四溴环十二烷二醇(TBCDdiols),通过体内试验和计算机模拟综合描述了δ-HBCD的逐步酶促脱卤羟基化降解途径.
-
与大量的HBCD的生物转化研究相比,TBECH的相关研究非常有限,TBCO的更是罕见,目前尚没有关于3种污染物代谢转化速率的比较. Marteinson等人用每日喂食的方法,将美国红隼(Falcosparverius)[13]暴露在β-TBECH中,分别于第28天和第82天测定美国红隼中的β-TBECH及其潜在的降解产物. 在红隼肝脏、血浆、脂肪组织中未检测到任何β-TBECH及可能的异构化、羟基化、脱溴代谢产物. 推测其原因是β-TBECH的快速代谢和/或快速排出. Nyholm等[121]也发现,不同浓度的TBECH在斑马鱼中的半衰期只有0.9—1.3 d,远远小于多溴联苯醚(PBDE)、HBCD等溴代阻燃剂. 暴露于TBECH的斑马鱼(Danio rerio)、鳟鱼(Salmo trutta)中均未检测到TBECH的降解产物[121-122]. 以上研究意味着,TBECH的任何代谢物都可能与母体一样快速或更快速地被生物体清除. 迄今为止,TBECH的生物代谢产物的表征仅限于体外实验. 例如,Chu等[123]发现,TBECH在大鼠肝微粒体中快速耗竭,孵育1 h后,α-HBCD和β-TBECH均减少了40%,意味着二者能够被大鼠肝微粒体降解且具有相似的降解速率. 在大鼠肝微粒体中检测到2种羟基化代谢产物,OH-TBECH、di-OH-TBECH,但未检测到脱溴代谢产物. 一项人肝微粒体[21]的降解研究表明,TBECH的降解反应包括P450催化下的羟基化、脱溴以及α-氧化反应,降解产物包括 OH-TriTBECH、di-OH-TriTBECH和α-(1,2-二溴环己基)-乙酸. β-TBECH较其他异构体具有更快的降解速率.
Wong等[124]发现,α-TBECH,β-TBECH和β-TBCO在土壤中迅速降解,α-TBECH的降解速率大于β-TBECH. 针对对映体的研究发现,E2-α-TBECH(在色谱上第二个出峰的α-TBECH对映体)的降解速率大于E1-α-TBECH;E2-β-TBCO(在色谱上第二个出峰的β-TBCO对映体)的降解速率大于E1-β-TBCO(在色谱上第一个出峰的β-TBCO对映体). 遗憾的是,该工作没有对降解产物进行进一步分析.
-
HBCD具有肝脏、神经、免疫、甲状腺、内分泌干扰、生殖发育等毒性效应[8, 125-126]. HBCD是中至强的甲状腺干扰物.HBCD长期暴露对甲状腺激素三碘甲状腺原氨酸(T3)和四碘甲状腺原氨酸(T4)的分泌与合成有抑制作用[85],能干扰甲状腺激素受体基因(TRβ)的转录[85]及其代谢酶的活性[127],可能导致甲状腺的肥大[18, 128],此外HBCD对促肾上腺激素释放基因(Crh)具有抑制作用[85]. 神经发育毒性是HBCD的另一个较为敏感的毒性终点. HBCD具有抑制神经递质传递[129-130]、影响神经信号传导[131]、影响神经元发育[130, 132]、诱导细胞凋亡[130, 133-134]的能力. HBCD还具有内分泌干扰效应,能通过降低性激素结合球蛋白增加男性游离雄激素指数[135],具有类雌激素效应[136],能与性激素产生拮抗作用[137],影响生殖系统[138-139]. 此外HBCD在生物体中具有免疫、生长发育、遗传、代谢、肝脏等毒性效应,能够促进脂肪形成基因的表达[140],破坏与免疫和炎症相关的基因表达[18],造成免疫力下降和生长缓慢[141],其引起的代谢功能异常可能导致肥胖症的发展[142]. HBCD容易在肝脏中积累[143],引起肝脏中CAR核受体的活化[18],诱导肝脏重量增加并可能导致肝纤维化[18]. 相关的细胞代谢毒性还能引起DNA损伤[117],影响生物体酶的活性[144, 145]及对多种物质的代谢或运输[18, 105],改变氧化应激关键反应和调控昼夜节律基因的表达[18],影响细胞凋亡和自噬过程[146],引起氧化应激和代谢稳态受损[145],造成代谢网络的破坏[111, 117]. 此外有研究探讨了HBCD对秀丽隐杆线虫(Caenorhabditis elegans)的急性和慢性暴露的毒性效应. 在HBCD的急性毒性浓度暴露下,最敏感的表现是运动行为的减弱,其次是活性氧和细胞凋亡水平的提高[147],其慢性毒性也包含以上表现[148].
大量研究表明,HBCD的毒性效应亦具有异构体选择性. Palace等[149]用喂食的方法将虹鳟鱼分别暴露于3种HBCD异构体56 d,其中食物中α-HBCD、β-HBCD和γ-HBCD的浓度分别为(29.14±1.95)、(11.84±4.62)、(22.84±2.26) ng·g−1 lw,发现不同异构体暴露均可以减少碘的吸收并加速甲状腺激素的转化效率,从而提高T4到T3的转化率,最终影响虹鳟鱼的代谢,其中γ-HBCD的毒害程度最严重. Marteinson等[150]发现,以800 ng·g−1 ww HBCD的饮食饲养21 d后,红隼(Falco sparverius)雏鸟蛋中的HBCD以α-HBCD为主,但羽毛中的以γ-HBCD为主. 他们认为羽毛中的α-HBCD能够反映HBCD的身体负荷,并发现羽毛中的α-HBCD浓度与血浆游离三碘甲状腺素与游离甲状腺素的比值呈负相关,能够反应HBCD暴露引起的甲状腺功能障碍,而β-和γ-HBCD含量则没有显著相关性.
Du等[3]的研究发现,暴露在0.1、1.0 mg·L−1水体中,HBCD的3种异构体均能引起斑马鱼(Oncorhynchus mykiss)胚胎发育过程的氧化应激、细胞凋亡、胚胎发育异常,其毒性大小顺序为γ-HBCD> β-HBCD> α-HBCD. 但Hong等[4]研究了HBCD异构体对海洋青鳉鱼(Oryzias melastigma)胚胎的发育毒性,结果表明在高暴露浓度(200 μg·L−1)下胚胎发育、氧化应激、细胞凋亡,毒性大小顺序为α-HBCD/γ-HBCD>β-HBCD,3种异构体在海水青鳉鱼胚胎的发育毒性顺序与淡水中斑马鱼胚胎中顺序不同,突出了使用淡水和盐水中物种进行毒性评价的重要性. Hong等[93]研究了HBCD异构体对日本桡足动物(Tigriopus japonicas)不同发育阶段的毒性,发现不同异构体的急性致死毒性大小为β-HBCD>α-HBCD>γ-HBCD;在成足期的发育延迟毒性表现为α-HBCD/γ-HBCD>β-HBCD,且F1代对所有3种异构体都比F0代更敏感.在30 μg·L−1或100 μg·L−1的α-HBCD、β-HBCD、γ-HBCD暴露下,产生氧化应激、细胞凋亡、内分泌干扰等毒性效应,其大小顺序为α-HBCD/β-HBCD>γ-HBCD,可见在同一生物体中急性致死毒性与发育毒性存在异构体特异性.
Huang等[151]进行了HBCD异构体在肝细胞中的体外毒性试验,结果显示异构体对L02和HepG2细胞的毒性大小为β-HBCD≥γ-HBCD>α-HBCD. HBCD对两种细胞的毒性机制有所不同:细胞内氧化还原状态的稳定性在诱导HepG2细胞毒性中起重要作用;DNA损伤是抑制L02细胞增殖的核心.HepG2对HBCD的代谢能力优于L02,这可能是HBCD在HepG2细胞中具有更大毒性的原因. Shi等[152]比较了HBCD异构体对SH-SY5Y人成神经细胞瘤细胞的毒性效应,发现3种HBCD同分异构体均能降低细胞活力、增加LDH的释放、破坏细胞骨架及诱导SH-SY5Y细胞发生凋亡. 分析与细胞凋亡相关基因和蛋白(Bax、caspase-3、caspase-9、Cyt c、Bcl-2、XIAP)的表达、细胞周期停滞、DNA损伤、ATP消耗,发现HBCD同分异构体神经毒性大小顺序为β-HBCD > γ-HBCD > α-HBCD. 3种HBCD异构体诱导的细胞凋亡与caspase蛋白表达水平遵循相同的顺序,表明HBCD同分异构体诱导的神经毒性是一种结构选择性caspase依赖性凋亡. 3种HBCD异构体均能引起胞内ROS和Ca2+水平均显著增加. 胞内ROS增加的大小顺序为β-HBCD > γ-HBCD > α-HBCD,与它们的毒性大小顺序一致,而胞内Ca2+水平增加的大小顺序为γ-HBCD > β-HBCD > α-HBCD,与它们的毒性大小顺序不一致. 因此ROS可能是影响HBCD同分异构体神经毒性的关键因素. Hamers等[137]发现β-HBCD和γ-HBCD能与雌激素受体发生拮抗反应,而α-HBCD的反应则不明显;相反在测定与雄激素受体的拮抗作用试验中,α-HBCD的反应程度最强烈. 可见针对不同受体,不同异构体的内分泌干扰毒性效应明显不同. Pseudomonas sp.菌株GJY[19]对HBCD不同异构体的生物降解所体现出的细胞毒性顺序也为β-HBCD≥γ-HBCD >α-HBCD.
代谢组学方法被用于研究HBCD的毒性机制. 例如:Szabo等[111]研究了C57BL/6雌性小鼠急性饮食暴露HBCD异构体后血清代谢组学的特征,发现所有暴露组(3、10、30 mg·kg−1)都表现出了参与有氧能量代谢、脂肪分解、神经发育和氨基酸代谢的代谢物变化,意味着HBCD对生物体的生理功能影响广泛. 根据代谢组学分析不同异构体暴露对小鼠内源性代谢的影响存在显著差异:α-HBCD的暴露能够引起小鼠体内学习和记忆中的兴奋性神经递质谷氨酸水平明显降低,而γ-HBCD的暴露能够导致小鼠的神经递质前体苯丙氨酸水平明显降低. 针对α-HBCD的代谢产物变化包括内源性乙酰乙酸酯、亮氨酸和酪氨酸的增加,谷氨酸、乳酸、丙酮酸和丝氨酸的下降;针对γ-HBCD的代谢产物变化包括甘氨酸、胆碱的增加,苏氨酸、2-羟基丁酸酯的减少;在所有实验组中,血清3-羟丁酸、肌酸和牛磺酸含量都显著升高,说明这三者都是可以预测HBCD暴露的生物标志物;α-HBCD和γ-HBCD均能引起特异性和独特的代谢产物效应,这些效应在CM-HBCD(混合物)的暴露中明显表现出来. 代谢组学分析方法表明每个非对映异构体和异构体混合物的生物反应不同,异构体特殊的代谢产物不仅能够成为其潜在的生物标志物,且对发现HBCD暴露与疾病和功能障碍之间的潜在机制具有重要意义.
综上所述,不同HBCD异构体具有不同的生物毒性效应,且毒性效应存在种间差异性,同时受物种的不同发育阶段、不同的毒性终点影响.
-
与HBCD类似,TBECH亦具有甲状腺、内分泌干扰、生殖发育、免疫、运动行为以及神经等毒性效应. TBECH能够干扰甲状腺激素水平,伴随着T4水平的降低和T3水平的升高,发生甲状腺细胞结构变异[14],引起甲状腺轴病变[101, 153],导致甲状腺肥大和生物体重下降[101, 153]. 与大多数内分泌干扰物不同,TBECH的内分泌干扰毒性具有复杂的、多模式的性激素干扰作用.例如,在鱼体、鸟类等细胞试验中TBECH类似于雄性激素激活剂[11, 13, 154-155],但在某些条件下TBECH类似于雄性激素拮抗剂[156],可通过影响性激素的分泌导致生物体性别发育异常[154]. 内分泌干扰毒性还包括胰脏、脾脏和卵巢毒性[101],影响分泌酶的含量,改变卵泡形态、数量从而导致各种性激素分泌的改变. 此外TBECH还具有生殖发育、免疫、运动行为、神经等毒性效应. 可由母体转移到卵中,干扰生物体内性腺的发育和分化[157]. 能够诱导促发炎和促细胞凋亡基因表达,降低胚胎孵化率和幼虫心率,引起胚胎发育畸形和运动行为异常[22]. 免疫毒性表现为巨噬细胞减少[158]、免疫细胞数量的改变和脾细胞增殖减少[101]. 神经毒性表现为能够降低其动作电位活性[159]. TBECH还具有肝脏、肾脏毒性[153],能够引起氧化应激,降低鸟类肝细胞活性和细胞存活率[160],且对肝脏代谢功能的影响存在性别差异[101]. Larsson等[156]在人体HepG2肿瘤细胞试验中发现,TBECH是一种有效的雄激素激动剂,并表明低水平接触该化合物会导致雄激素效应和潜在的内分泌紊乱. 迄今为止,有关TBECH毒性效应的研究主要集中于饮食暴露的影响、少量对水生生物影响的致毒机制,以及基于细胞实验的体外测试.
TBECH的毒性效应亦具有异构体选择性. Khalaf等[161]在TBECH异构体对人体肝细胞的毒性研究中发现,γ-TBECH、δ-TBECH对肝细胞的激活作用强于α-TBECH、β-TBECH. 可见TBECH具有内分泌干扰效应,不同浓度及不同异构体的毒性效应也会存在差异.
相比于其他异构体,对β-TBECH毒性效应研究最多,主要集中在甲状腺、内分泌干扰、生殖发育、神经、氧化胁迫等毒性效应的研究. Park等[14]在研究β-TBECH对褐鳟(Salmo trutta)甲状腺毒性中发现,高浓度β-TBECH饲养的暴露组((118.4±3.1) pmol·g−1 lw)能够造成甲状腺轴发生病变,改变甲状腺上皮细胞结构,使总甲状腺水平降低,干扰甲状腺稳态. Marteinson等[162]以0.239 ng·g−1·d−1的β-TBECH暴露量饲养红隼,研究发现β-TBECH不仅引起了雄性红隼体内总甲状腺素的血浆浓度升高,而且能引起成年红隼(Falco sparverius)的脂肪和体重的增加,还观察到两性在求爱期间表现出飞行行为的减少和进食行为的增加,结果表明β-TBECH是一种可能的致肥剂. Stojak等[159]发现了β-TBECH能够以剂量依赖方式降低大鼠神经元动作电位的活性. Marteinson等[13]报道了β-TBECH暴露(0.239 ng·g−1·d−1)导致红隼(Falco sparverius)产卵量和卵重量减少,卵育性变差,繁殖能力变低,母体在卵中沉积的雌二醇增加,雄性后代减少,这意味着β-TBECH具有生殖毒性. Porter等[160]发现β-TBECH暴露能导致禽类肝细胞MT4mRNA表达的上调,但t-TBECH并没有,MTs的急性诱导被认为是抗氧化损伤和自由基的保护机制,β-TBECH和t-TBECH能够降低肝细胞的活力,引起硫氧还蛋白抗氧化酶(TXN)的表达的上调,肝细胞的正常氧化还原状态受到干扰,这表明β-TBECH和t-TBECH暴露能够引起禽类肝细胞的氧化胁迫. Huang等[163]研究了TBECH非对映异构体对玉米(Zea mays L.)的氧化胁迫和DNA损伤,结果表明TBECH可以诱导O2·和H2O2的产生,引起植物组蛋白的磷酸化,抗氧化酶基因过表达,且基于异构体水平存在选择性表达,β-TBECH的氧化损伤作用最为显著.
-
关于TBCO的毒性效应研究非常有限,缺乏针对TBCO不同异构体的毒性研究. TBCO的毒性效应主要集中在内分泌干扰和生殖发育毒性. Saunders等[15]研究了TBCO对日本青鳉(Oryzias latipes)的卵子的产量和受精率的影响,并以下丘脑-垂体-性腺-肝脏(HPGL)的34个基因的转录量丰度作为整体内分泌紊乱的标志,研究TBCO的内分泌干扰机制.研究暴露在低浓度58 μg·g−1 TBCO的鱼卵日产量普遍低于对照组,且随时间推移累计产卵量与对照组相比显著存在不同,青鳉累积繁殖力降低18%,而较高浓度607 μg·g−1 TBCO的暴露组与对照组无明显不同,表明低浓度TBCO暴露对青鳉具有生殖毒性. TBCO对部分性腺基因表达的影响分别具有浓度依赖性和性别特异性,暴露在较高TBCO浓度的环境中,性腺标志基因以及参与HPGL激素类固醇生成和调节网络的基因转录会下调,且TBCO的浓度越大对雌激素基因表达的影响越大,结果证实了TBCO对内分泌的潜在干扰. TBCO对青鳉肝脏中基因转录产物的丰度有显著影响,表明TBCO有潜在的肝脏毒性.酵母雌激素/雄激素筛查试验结果表明,TBCO是人类雌激素和雄激素受体(hERα/ hARα)的拮抗剂,可通过与雌激素受体和雄激素受体作用调控内分泌,并干扰17-β雌二醇与睾酮的合成[164]. Mankidy等[12]发现TBCO会导致猪睾丸原代细胞雄性激素睾酮(T)和雌二醇(E2)的分泌增加.Sun等[16]将日本青鳉(Oryzias latipes)的胚胎作为研究对象,结合转录组学和蛋白质组学方法识别TBCO对日本青鳉早期生命阶段的致毒性途径. 研究发现100 μg·L−1 TBCO暴露,对胚胎孵化天数和孵化成功率均产生影响. 在孵化过程中TBCO的富集造成了蛋白质丰度的降低. 根据转录组和蛋白质组的反应,预测TBCO存在损害视力和心肌收缩的风险,具有干扰生物体的生殖发育毒性. Essen等[165]把斑马鱼(Danio rerio)的胚胎作为研究对象,暴露在2、20、200 μg·L−1的TBCO溶液中,发现TBCO会导致浓度依赖性的死亡率增加、心率降低以及脊柱弯曲和未充气鳔的发生率增加,但其作用机理尚需进一步研究.
时晓丽等[17]比较了HBCD、TBCO、TBECH的对SH-SY5Y细胞的毒性并探讨了3种污染物的致毒机制,发现3种阻燃剂均能引起SH-SY5Y细胞的凋亡,并遵循线粒体凋亡途径. 诱导产生ROS是3种阻燃剂共同的致毒机制,同时HBCD和TBCO的毒性还遵循Ca2+介导的细胞凋亡途径,而对于TBCO,Ca2+介导的细胞凋亡在诱导毒性方面比ROS更重要. 此研究对不同结构的CBFRs的毒性效应进行比较,虽然结果显示TBECH和TBCO的神经细胞毒性均低于HBCD,但是对于其它种类生物的毒性效应是否遵循相同的规律有待更多的研究.
-
CBFRs可以在生产、使用、运输及废弃物报废处置的整个生命周期中进入大气、水体以及土壤等环境介质,并通过空气和水的流动以及生物的携带进行传输。近年来,随着HBCD生产和使用的限制,预计会有更多的TBECH和TBCO作为其替代品进入环境,导致他们环境水平的增加。本文综述了CBFRs的环境暴露水平、生物富集、转化及毒性效应,重点介绍了CBFRs异构体在不同环境介质中的分布,在生物中的富集、代谢及毒性效应的差异性。针对目前研究的现状以及存在的问题,提出以下总结和展望:
(1)与大量的HBCD在环境介质中的时空分布、在环境生物中的富集、代谢、食物链放大和毒性效应研究相比,有关TBECH和TBCO的研究主要集中于在大气颗粒物及水体沉积物中的分布及在水生生物中的富集和毒性效应,其在陆生生态系统中的研究相当匮乏.为了综合评价TBECH、TBCO作为替代品的风险,开展二者的在陆生生态系统的环境行为和生物效应的研究势在必行.
(2)作为HBCD潜在的替代品,应该具有更低的生物富集、食物链放大和和毒性效应.目前有关CBFRs的相关研究各自独立,缺乏与HBCD在同样条件下的直接比较,难以对TBECH和TBCO是否适合做HBCD的替代物做出正确评价.因此需要在同一污染场地对三者的生物富集、放大和毒性效应同时进行监测,或在在实验室相同暴露条件的情况下对三者进行研究,同时考察不同种类的生物富集、放大和毒性的差异。
(3)TBECH与TBCO具有多种异构体和对映体,异构体和对映体是否存在选择性的生物吸收、是否像HBCD一样存在生物异构化反应,异构体及对映体代谢和毒性效应研究均不够全面. 因此,在异构体和对映体水平对以上问题进行研究,是未来工作的重要方向.
(4)污染物的生物转化是影响污染物的归趋及毒性效应的重要因素.虽然目前研究表明,TBECH与TBCO有较快的生物转化和排出速率,但有限的转化产物的鉴定仅限于体外研究,缺乏体内数据的支持以及对转化途径、转化机制的探索,更缺少在异构体和对映体层面的探索. 因此,有必要开展TBECH与TBCO在多种生物物种中的体内转化研究,探索转化速率与它们结构的关系。由于代谢物质缺乏相应的标准品,如何对它们进行定性分析和定量检测,是未来研究亟待解决的关键问题。
(5)CBFRs在人体内均有检出,具有肝脏、神经、免疫、甲状腺、内分泌干扰、生殖发育等毒性效应。但目前研究只关注了CBFRs原型的毒性效应,它们的一些代谢产物可能呈现比原型更大的生物毒性。因此,未来在加强CBFRs人体暴露风险评估的同时,开展CBFRs转化产物的毒性效应研究十分必要。
脂环族溴代阻燃剂的生物富集、代谢及毒性效应研究进展
Research progress on bioconcentration, metabolism and toxicity of cycloaliphatic brominated flame retardant isomers
-
摘要: 以六溴环十二烷(HBCD)为代表的脂环族溴代阻燃剂(CBFRs)被广泛应用于纺织、建材、电子、电气、化工、交通、建材等领域.随着HBCD作为《关于持久性有机污染物的斯德哥尔摩公约》增列持久性有机污染物,与HBCD具有相似结构和性能的四溴环己烷(TBECH)和四溴环辛烷(TBCO)等CBFRs被当作HBCD的潜在替代产品.迄今为止,HBCD、TBECH和TBCO已在大气、水体、土壤等多种环境介质和生物体中被检出,它们在生物体内的代谢转化以及内分泌干扰、神经、生殖、发育等毒性效应亦受到广泛关注.值得指出的是,所有CBFRs均含有同分异构体,表现出异构体选择性的生物富集、代谢和毒性效应.遗憾的是,目前相关研究还十分匮乏.本文从CBFRs的环境暴露水平、生物富集、毒性效应、以及CBFRs的生物转化等方面展开综述,特别强调了从异构体水平研究HBCD及其替代物的必要性.本文有助于全面了解CBFRs生物富集、代谢及毒性效应,对于正确认识和准确评价CBFRs的生态和健康风险具有重要的科学意义.Abstract: Cycloaliphatic brominated flame retardants (CBFRs), represented by hexabromocyclododecane (HBCD) are widely used in textile, building materials, electronics, electrical, chemical, transportation, building materials and other fields. With the addition of HBCD in the “Stockholm Convention on Persistent Organic Pollutants” as persistent organic pollutants, other types of CBFRs, such as tetrabromocyclohexane (TBECH) and 1,2,5,6-Tetrabromocyclooctane (TBCO), with similar structure and performance to HBCD are regarded as potential substitutes for HBCD. So far, HBCD, TBECH and TBCO have been detected in the atmosphere, water, soil and other environmental media and organisms. Their metabolic transformation in organisms, endocrine interference, nerves, reproduction, development and other toxic effects have been drawn much attention. It should be mentioned that all CBFRs contain isomers, showing isomer-selective bioaccumulation, metabolism, and toxicity. Unfortunately, research at the level of isomers is scarce. In this article, the environmental exposure level, bioconcentration, biotransformation and toxic effects of CBFRs have been reviewed, and the necessity of studying HBCD and its substitutes at the level of isomers has been emphasized. This article helps to fully understand the bioaccumulation, metabolism and toxic effects of CBFRs, which has important scientific significance for the correct understanding and accurate evaluation of the ecological and health risks of CBFRs.
-

-
表 1 HBCD、TBECH、TBCO的理化性质
Table 1. The physical and chemical properties of HBCD, TBECH and TBCO
化合物
Compound分子式
Molecular formula分子量
Molecular weight熔点/℃
Melting point蒸汽压/Pa(21℃)
Vapor pressure溶解度/(μg·L−1)
Solubility稳定性
StabilitylgKow
(25 ℃)tHBCD C12H18Br6 641.7 175—195[23] 7.23×10−7[24] 65.6
(20 ℃) [24]160 ℃
重排
240 ℃
脱溴5.77[25] tTBECH C8H12Br4 427.8 70—77 1.40×10−2 a[26] 69.2a 123 ℃ 5.24a[26] 2.31×10−3 b 2420b 重排[27] 4.41b tTBCO C8H12Br4 427.8 102—124[28] 9.43×10−3 a 69.2a 123 ℃ 5.24a 2.13×10−3 b 2640b 重排[27] 4.37b 注:a. EPI Suite计算的理化性质;b. SPARC计算的理化性质.
Note: a. Physical and chemical properties calculated by EPI Suite; b. Physical and chemical properties calculated by SPARC.表 2 污染源区HBCD的浓度
Table 2. Concentration of HBCD in the pollution source area
地区
Area环境介质
Environmental mediumHBCD浓度
HBCD concentration参考文献
Reference天津聚苯乙烯生产厂 粉尘 328—31752 ng·g−1 dw [30] 土壤 2.19—1730 ng·g−1 dw 沉积物 23.5—716 ng·g−1 dw 中国华北塑料垃圾处理区 土壤 11.0—6240 ng·g−1 [5] 中国渤海附近HBCD生产企业 土壤 4.20—11700 ng·g−1 [32] 沉积物 1.52—6740 ng·g−1 水 3.28—5080 ng·L−1 中国香港污水处理厂 水 13.6—45.4 ng·L−1 [31] 污泥 108—402 ng·g−1 dw 捷克源区 土壤 (15.0 ± 35.5 )ng·g−1 [33] 沉积物 (2.59 ± 3.22) ng·g−1 仓库大气 (1960±1060) ng·m−3 仓外大气 (53.9±17.3) ng·m−3 消费产品、建造材料 (65±505) μg·g−1 室内空气 (4.30 ± 4.96) pg·m−3 室外背景空气 (3.83 ± 5.25) pg·m−3 注:dw,dry weight. 干重. Note: dw, dry weight. Dry weight. 表 3 大气、水、土壤等环境介质中HBCD的浓度
Table 3. Concentration of HBCD in environmental media such as air, water and soil
地区
Area环境介质
Environmental mediumHBCD浓度
HBCD concentration参考文献
Reference英国西米德兰兹 大气 100 pg·m−3 [34] 法国巴黎 大气 16—58 pg·m−3 [35] 瑞典斯德哥尔摩 室内灰尘 190 ng·g−1 [36] 室内空气 3.1 pg·m−3 室外空气 0.066 pg·m−3 中国大连 大气 15.47—43.57 pg·m−3 [37] 中国深圳 室内大气颗粒相 31.6—94.1 pg·m−3 [38] 室外大气颗粒相 5.36 pg·m−3 中国天津大沽河 水体沉积物 83.7 ng·g−1dw [41] 中国天津港 水体沉积物 60.8 ng·g−1 dw [41] 中国长江 水体沉积物 23.28 ng·g−1 dw [44] 韩国工业园区附近湖泊 水体沉积物 143 ng·g−1dw [40] 中国贵屿连河 水体沉积物 117 ng·g−1dw [42] 日本九头龙河 水体沉积物 1527 ng·g−1dw [43] 东江和珠江口 水体沉积物 64.7 ng·g−1 dw [45] 渤海南部海域 水 3.28—5080 ng·L−1 [32] 中国宁波的垃圾填埋场 废物倾卸场地土壤 60.74 ng·g−1 dw [48] 工业区土壤 37.9 ng·g−1 dw 交通区土壤 31.8 ng·g−1 dw 居民区土壤 14.1 ng·g−1 dw 菜地土壤 11.0 ng·g−1 dw 农田土壤 7.75 ng·g−1dw 中国广东 清远土壤 106 ng·g−1 dw [49] 工业区土壤 0.31—9.99·ng·g−1 dw 电子废物回收站土壤 0.22—2.34 ng·g−1 dw 表 4 大气、水、土壤等环境介质中TBECH的浓度
Table 4. Concentration of TBECH in environmental media such as air, water and soil
地区
Area环境介质
Environmental mediumTBECH浓度
TBECH concentration参考文献
Reference挪威 家庭空气 77.9 pg·m−3 [56] 教室空气 46.6 pg·m−3 英国 家庭空气 173 pg·m−3 办公室空气 320 pg·m−3 家庭灰尘 21.4 ng·g−1 办公室灰尘 41 ng·g−1 意大利罗马 室内灰尘 33.7—228 ng·g-1 [57] 美国加利福尼亚州 室内灰尘 <0.64—360 ng·g-1 [58] 瑞典斯德哥尔摩 室内大气 43 pg·m−3 [36] 室外大气 0.48 pg·m−3 室内灰尘 0.9 ng·g−1 捷克 室内大气 100 pg·m−3 [59] 英国西米德兰兹 室外大气 2.5—9.3 pg·m−3 [34] 中国北方电子垃圾回收区 室外大气 39.5 pg·m−3 [61] 水 0.423—0.710 ng·L−1 土壤 0.0486—0.309 ng·g−1·dw 中国香港水域 地表沉积物 <0.5—5.10 ng·g−1·dw [67] 中国香港污水处理厂 废水 1.47—3.33 ng·L−1 [31] 污泥 13.2—23.5 ng·g−1·dw 新加坡 地表水 0.099—0.47 ng·L−1 [68] 悬浮沉积物 3.86—4.65 ng·g−1·dw 表 5 HBCD在不同生物中的异构体转化
Table 5. Transformation of HBCD isomers in different biological samples
生物样本
Biological samples转化现象
Transformations参考文献
Reference大鼠 γ-HBCD异构化为α-HBCD [101] 母鸡 [2] 斑马鱼 [87] 镜鲤、蚯蚓 — [100] 大鼠 β-HBCD异构化为γ-HBCD [104] 大鼠肝脏和脑组织 γ-HBCD异构化为β-HBCD [102] 大鼠脂肪组织和粪便 γ-HBCD异构化为α-和β-HBCD [102] 母鸡肝脏 [103] 虹鳟鱼幼体 β-、γ-HBCD异构化为α-HBCD [89] 镜鲤 [76] 蚯蚓 [84, 90] 雄性大鼠 [88] 小麦 [81] 黑麦草 [82] 玉米根 β-和γ-HBCD异构化为α-HBCD;β-HBCD和γ-HBCD相互转化 [6] 注:—表示无转化现象. Note: —indicates no transformation. 表 6 HBCD异构体在鼠中的代谢产物
Table 6. Metabolites of diastereomers of HBCD in mice
污染物
Pollution种类
Species代谢产物
Metabolites参考文献
Referenceα-HBCD Sprague–Dawley OH-HBCD、OH-HBCDee [88] α-HBCD C57BL/6 OH-HBCD、谷胱甘肽复合物 [110] β-HBCD Sprague–Dawley OH-HBCD、tri-OH-HBCD、PBCDe、dithio-TeBCDe [88] di-OH-triBCDeee、巯基二溴甲基酯 γ-HBCD Sprague–Dawley OH-HBCD、PBCDe、OH-TeBCDe、OH-PBCD [88] OH-PBCDe、DiOH-PBCDe、Dithio-PBCD 四溴二烷二羧酸 γ-HBCD C57BL/6 OH-PBCD、OH-PBCDe、DiOH-PBCDe、diOH-PBCDee [110] 六溴十二烷二酸、三溴壬烯酸 三溴硝基苯甲酸甲硫醇酯、四甲硫醇酯 表 7 HBCD异构体在菌株中的代谢转化产物
Table 7. Metabolites of HBCD isomers in microorganisms
菌株
Strain代谢产物
Metabolites参考文献
ReferenceDehalococcoides mccartyi菌株195 TBCD、DBCD、CDT [118] Pseudomonas aeruginosa HS9假单胞菌 TBCDe、DBCDi、CDT、ECDD、PBCDOHs [114] Bacillus HBCD-sjtu菌株 CDT、CDD、环十二碳烯、3,9-十二碳烯 [115] Rhodopseudomonas palustrisYSC3菌株 PBCDOHs、PBCDEs [112] Sphingobium chinhatense菌株IP26 PeBCD-ols、TeBCD-diols、TrBCD-triols [113] Pseudomonas sp.菌株GJY PBCD-ols、PBCD-diols、TrBCD-triols、DBCDe-diols、DBDe-dicarboxyl [19] -
[1] 陈海, 孙云娜, 魏东洋. 溴代阻燃剂的环境危害 [J]. 广东化工, 2011, 38(8): 91-92. doi: 10.3969/j.issn.1007-1865.2011.08.045 CHEN H, SUN Y N, WEI D Y. Bromination flame retardants environmental damage [J]. Guangdong Chemical Industry, 2011, 38(8): 91-92(in Chinese). doi: 10.3969/j.issn.1007-1865.2011.08.045
[2] FOURNIER A, FEIDT C, MARCHAND P, et al. Kinetic study of γ-hexabromocyclododecane orally given to laying hens (Gallus domesticus) [J]. Environmental Science and Pollution Research, 2012, 19(2): 440-447. doi: 10.1007/s11356-011-0573-6 [3] DU M M, ZHANG D D, YAN C Z, et al. Developmental toxicity evaluation of three hexabromocyclododecane diastereoisomers on zebrafish embryos [J]. Aquatic Toxicology, 2012, 112/113: 1-10. doi: 10.1016/j.aquatox.2012.01.013 [4] HONG H Z, SHEN R, LIU W X, et al. Developmental toxicity of three hexabromocyclododecane diastereoisomers in embryos of the marine medaka Oryzias melastigma [J]. Marine Pollution Bulletin, 2015, 101(1): 110-118. doi: 10.1016/j.marpolbul.2015.11.009 [5] HUANG H L, WANG D, WAN W N, et al. Hexabromocyclododecanes in soils and plants from a plastic waste treatment area in North China: Occurrence, diastereomer- and enantiomer-specific profiles, and metabolization [J]. Environmental Science and Pollution Research, 2017, 24(27): 21625-21635. doi: 10.1007/s11356-017-9792-9 [6] HUANG H L, ZHANG S Z, LV J, et al. Experimental and theoretical evidence for diastereomer- and enantiomer-specific accumulation and biotransformation of HBCD in maize roots [J]. Environmental Science & Technology, 2016, 50(22): 12205-12213. [7] FROMME H, BECHER G, HILGER B, et al. Brominated flame retardants - Exposure and risk assessment for the general population [J]. International Journal of Hygiene and Environmental Health, 2016, 219(1): 1-23. doi: 10.1016/j.ijheh.2015.08.004 [8] KOCH C, SCHMIDT-KÖTTERS T, RUPP R, et al. Review of hexabromocyclododecane (HBCD) with a focus on legislation and recent publications concerning toxicokinetics and -dynamics [J]. Environmental Pollution, 2015, 199: 26-34. doi: 10.1016/j.envpol.2015.01.011 [9] ZHAO J P, WANG P, WANG C, et al. Novel brominated flame retardants in West Antarctic atmosphere (2011-2018): Temporal trends, sources and chiral signature [J]. Science of the Total Environment, 2020, 720: 137557. doi: 10.1016/j.scitotenv.2020.137557 [10] MARTEINSON S C, BODNARYK A, FRY M, et al. A r0065view of 1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane in the environment and assessment of its persistence, bioaccumulation and toxicity [J]. Environmental Research, 2021, 195: 110497. doi: 10.1016/j.envres.2020.110497 [11] ASNAKE S, PRADHAN A, BANJOP-KHARLYNGDOH J, et al. 1, 2-Dibromo-4-(1, 2 dibromoethyl) cyclohexane (TBECH)-mediated steroid hormone receptor activation and gene regulation in chicken LMH cells [J]. Environmental Toxicology and Chemistry, 2014, 33(4): 891-899. doi: 10.1002/etc.2509 [12] MANKIDY R, RANJAN B, HONARAMOOZ A, et al. Effects of novel brominated flame retardants on steroidogenesis in primary porcine testicular cells [J]. Toxicology Letters, 2014, 224(1): 141-146. doi: 10.1016/j.toxlet.2013.10.018 [13] MARTEINSON S C, LETCHER R J, GRAHAM L, et al. The flame retardant β-1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane: Fate, fertility, and reproductive success in American kestrels (Falco sparverius) [J]. Environmental Science & Technology, 2012, 46(15): 8440-8447. [14] PARK B J, PALACE V, WAUTIER K, et al. Thyroid axis disruption in juvenile brown trout (Salmo trutta) exposed to the flame retardant β-tetrabromoethylcyclohexane (β-TBECH) via the diet [J]. Environmental Science & Technology, 2011, 45(18): 7923-7927. [15] SAUNDERS D M V, PODAIMA M, WISEMAN S, et al. Effects of the brominated flame retardant TBCO on fecundity and profiles of transcripts of the HPGL-axis in Japanese medaka [J]. Aquatic Toxicology, 2015, 160: 180-187. doi: 10.1016/j.aquatox.2015.01.018 [16] SUN J X, TANG S, PENG H, et al. Combined transcriptomic and proteomic approach to identify toxicity pathways in early life stages of Japanese medaka (Oryzias latipes) exposed to 1, 2, 5, 6-tetrabromocyclooctane (TBCO) [J]. Environmental Science & Technology, 2016, 50(14): 7781-7790. [17] 时晓丽. 脂环族溴代阻燃剂对 SH-SY5Y 细胞的神经毒性研究 [D]. 北京: 中国科学院大学, 2019. SHI X L. In vitro study on the neurotoxicity of cycloaliphatic brominated flame retardants in SH-SY5Y cells[D]. Beijing: University of Chinese Academy of Sciences, 2019( in Chinese).
[18] FARMAHIN R, GANNON A M, GAGNÉ R, et al. Hepatic transcriptional dose-response analysis of male and female Fischer rats exposed to hexabromocyclododecane [J]. Food and Chemical Toxicology, 2019, 133: 110262. doi: 10.1016/j.fct.2018.12.032 [19] GENG J Y, HAN M, YANG X, et al. Different biotransformation of three hexabromocyclododecane diastereoisomers by Pseudomonas sp. under aerobic conditions [J]. Chemical Engineering Journal, 2019, 374: 870-879. doi: 10.1016/j.cej.2019.05.232 [20] HUANG H L, WANG D, WEN B, et al. Roles of maize cytochrome P450 (CYP) enzymes in stereo-selective metabolism of hexabromocyclododecanes (HBCDs) as evidenced by in vitro degradation, biological response and in silico studies [J]. Science of the Total Environment, 2019, 656: 364-372. doi: 10.1016/j.scitotenv.2018.11.351 [21] NGUYEN K H, ABOU-ELWAFA ABDALLAH M, MOEHRING T, et al. Biotransformation of the flame retardant 1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane (TBECH) in vitro by human liver microsomes [J]. Environmental Science & Technology, 2017, 51(18): 10511-10518. [22] WANG X, WEI L, WANG Y, et al. Evaluation of development, locomotor behavior, oxidative stress, immune responses and apoptosis in developing zebrafish (Danio rerio) exposed to TBECH (tetrabromoethylcyclohexane) [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2019, 217: 106-113. [23] HEEB N, SCHWEIZER W B, HAAG R, et al. Hexabromocyclododecanes: From Smart Molecules to Persistent Pollutants [J]. Chimia, 2008, 62(11): 936-936. doi: 10.2533/chimia.2008.936 [24] MARVIN C H, TOMY G T, ARMITAGE J M, et al. Hexabromocyclododecane: Current understanding of chemistry, environmental fate and toxicology and implications for global management [J]. Environmental Science & Technology, 2011, 45(20): 8613-8623. [25] SCHENKER U, MACLEOD M, SCHERINGER M, et al. Improving data quality for environmental fate models: A least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds [J]. Environmental Science & Technology, 2005, 39(21): 8434-8441. [26] HOWARD P H, MUIR D C G. Identifying new persistent and bioaccumulative organics among chemicals in commerce [J]. Environmental Science & Technology, 2010, 44(7): 2277-2285. [27] ARSENAULT G, LOUGH A, MARVIN C, et al. Structure characterization and thermal stabilities of the isomers of the brominated flame retardant 1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane [J]. Chemosphere, 2008, 72(8): 1163-1170. doi: 10.1016/j.chemosphere.2008.03.044 [28] RIDDELL N, ARSENAULT G, KLEIN J, et al. Structural characterization and thermal stabilities of the isomers of the brominated flame retardant 1, 2, 5, 6-tetrabromocyclooctane (TBCO) [J]. Chemosphere, 2009, 74(11): 1538-1543. doi: 10.1016/j.chemosphere.2008.11.026 [29] BERGMAN Å, HEINDEL J, JOBLING S, et al. State-of-the-science of endocrine disrupting chemicals, 2012 [J]. Toxicology Letters, 2012, 211: S3. [30] ZHU H K, ZHANG K, SUN H W, et al. Spatial and temporal distributions of hexabromocyclododecanes in the vicinity of an expanded polystyrene material manufacturing plant in Tianjin, China [J]. Environmental Pollution, 2017, 222: 338-347. doi: 10.1016/j.envpol.2016.12.029 [31] RUAN Y F, ZHANG K, LAM J C W, et al. Stereoisomer-specific occurrence, distribution, and fate of chiral brominated flame retardants in different wastewater treatment systems in Hong Kong [J]. Journal of Hazardous Materials, 2019, 374: 211-218. doi: 10.1016/j.jhazmat.2019.04.041 [32] ZHANG Y Q, LU Y L, WANG P, et al. Transport of Hexabromocyclododecane (HBCD) into the soil, water and sediment from a large producer in China [J]. Science of the Total Environment, 2018, 610/611: 94-100. doi: 10.1016/j.scitotenv.2017.08.039 [33] OKONSKI K, MELYMUK L, KOHOUTEK J, et al. Hexabromocyclododecane: Concentrations and isomer profiles from sources to environmental sinks [J]. Environmental Science and Pollution Research, 2018, 25(36): 36624-36635. doi: 10.1007/s11356-018-3381-4 [34] DRAGE D S, NEWTON S, de WIT C A, et al. Concentrations of legacy and emerging flame retardants in air and soil on a transect in the UK West Midlands [J]. Chemosphere, 2016, 148: 195-203. doi: 10.1016/j.chemosphere.2016.01.034 [35] RAUERT C, SCHUSTER J K, ENG A, et al. Global atmospheric concentrations of brominated and chlorinated flame retardants and organophosphate esters [J]. Environmental Science & Technology, 2018, 52(5): 2777-2789. [36] NEWTON S, SELLSTRÖM U, de WIT C A. Emerging flame retardants, PBDEs, and HBCDDs in indoor and outdoor media in Stockholm, Sweden [J]. Environmental Science & Technology, 2015, 49(5): 2912-2920. [37] LI Y, ZHU X H, WANG L X, et al. Levels and gas-particle partitioning of hexabromocyclododecanes in the urban air of Dalian, China [J]. Environmental Science and Pollution Research, 2018, 25(27): 27514-27523. doi: 10.1007/s11356-018-2793-5 [38] ZHU Y S, YANG W D, LI X W, et al. Airborne particle-bound brominated flame retardants: Levels, size distribution and indoor-outdoor exchange [J]. Environmental Pollution, 2018, 233: 1104-1112. doi: 10.1016/j.envpol.2017.10.013 [39] LI H R, MO L G, YU Z Q, et al. Levels, isomer profiles and chiral signatures of particle-bound hexabromocyclododecanes in ambient air around Shanghai, China [J]. Environmental Pollution, 2012, 165: 140-146. doi: 10.1016/j.envpol.2012.02.015 [40] LEE S, MOON H B. Multi-matrix distribution and contamination profiles of HBCDD isomers in a man-made saltwater lake near industrial complexes with high flame retardant consumption in Korea [J]. Marine Pollution Bulletin, 2021, 172: 112812. doi: 10.1016/j.marpolbul.2021.112812 [41] ZHANG Y W, RUAN Y F, SUN H W, et al. Hexabromocyclododecanes in surface sediments and a sediment core from Rivers and Harbor in the northern Chinese city of Tianjin [J]. Chemosphere, 2013, 90(5): 1610-1616. doi: 10.1016/j.chemosphere.2012.08.037 [42] LI H R, la GUARDIA M J, LIU H H, et al. Brominated and organophosphate flame retardants along a sediment transect encompassing the Guiyu, China e-waste recycling zone [J]. Science of the Total Environment, 2019, 646: 58-67. doi: 10.1016/j.scitotenv.2018.07.276 [43] OH J K, KOTANI K, MANAGAKI S, et al. Levels and distribution of hexabromocyclododecane and its lower brominated derivative in Japanese riverine environment [J]. Chemosphere, 2014, 109: 157-163. doi: 10.1016/j.chemosphere.2014.01.074 [44] LI H H, SHANG H T, WANG P, et al. Occurrence and distribution of hexabromocyclododecane in sediments from seven major river drainage basins in China [J]. Journal of Environmental Sciences, 2013, 25(1): 69-76. doi: 10.1016/S1001-0742(12)60010-2 [45] FENG A H, CHEN S J, CHEN M Y, et al. Hexabromocyclododecane (HBCD) and tetrabromobisphenol A (TBBPA) in riverine and estuarine sediments of the Pearl River Delta in Southern China, with emphasis on spatial variability in diastereoisomer- and enantiomer-specific distribution of HBCD [J]. Marine Pollution Bulletin, 2012, 64(5): 919-925. doi: 10.1016/j.marpolbul.2012.03.008 [46] HARRAD S, ABDALLAH M A E, COVACI A. Causes of variability in concentrations and diastereomer patterns of hexabromocyclododecanes in indoor dust [J]. Environment International, 2009, 35(3): 573-579. doi: 10.1016/j.envint.2008.10.005 [47] JO H, SON M H, SEO S H, et al. Matrix-specific distribution and diastereomeric profiles of hexabromocyclododecane (HBCD) in a multimedia environment: Air, soil, sludge, sediment, and fish [J]. Environmental Pollution, 2017, 226: 515-522. doi: 10.1016/j.envpol.2017.04.093 [48] TANG J F, FENG J Y, LI X H, et al. Levels of flame retardants HBCD, TBBPA and TBC in surface soils from an industrialized region of East China [J]. Environmental Science:Processes & Impacts, 2014, 16(5): 1015-1021. [49] GAO S T, WANG J Z, YU Z Q, et al. Hexabromocyclododecanes in surface soils from E-waste recycling areas and industrial areas in South China: Concentrations, diastereoisomer- and enantiomer-specific profiles, and inventory [J]. Environmental Science & Technology, 2011, 45(6): 2093-2099. [50] CAO X H, LU Y L, ZHANG Y Q, et al. An overview of hexabromocyclododecane (HBCDs) in environmental media with focus on their potential risk and management in China [J]. Environmental Pollution, 2018, 236: 283-295. doi: 10.1016/j.envpol.2018.01.040 [51] LI H H, ZHANG Q H, WANG P, et al. Levels and distribution of hexabromocyclododecane (HBCD) in environmental samples near manufacturing facilities in Laizhou Bay area, East China [J]. Journal of Environmental Monitoring, 2012, 14(10): 2591-2597. doi: 10.1039/c2em30231d [52] YI S, LIU J G, JIN J, et al. Assessment of the occupational and environmental risks of hexabromocyclododecane (HBCD) in China [J]. Chemosphere, 2016, 150: 431-437. doi: 10.1016/j.chemosphere.2016.01.047 [53] GAUTHIER L T, POTTER D, HEBERT C E, et al. Temporal trends and spatial distribution of non-polybrominated diphenyl ether flame retardants in the eggs of colonial populations of great lakes herring gulls [J]. Environmental Science & Technology, 2009, 43(2): 312-317. [54] SHOEIB M, AHRENS L, JANTUNEN L, et al. Concentrations in air of organobromine, organochlorine and organophosphate flame retardants in Toronto, Canada [J]. Atmospheric Environment, 2014, 99: 140-147. [55] RIDDELL N, ARSENAULT G, LOUGH A, et al. The three-dimensional structural characterization of hexachlorocyclopentenyl-dibromocyclooctane (HCDBCO) [J]. Chemosphere, 2008, 73(4): 479-483. doi: 10.1016/j.chemosphere.2008.06.047 [56] TAO F, ABDALLAH M A E, HARRAD S. Emerging and legacy flame retardants in UK indoor air and dust: Evidence for replacement of PBDEs by emerging flame retardants? [J]. Environmental Science & Technology, 2016, 50(23): 13052-13061. [57] SIMONETTI G, di FILIPPO P, RICCARDI C, et al. Occurrence of halogenated pollutants in domestic and occupational indoor dust [J]. International Journal of Environmental Research and Public Health, 2020, 17(11): 3813. doi: 10.3390/ijerph17113813 [58] BROWN F R, WHITEHEAD T P, PARK J S, et al. Levels of non-polybrominated diphenyl ether brominated flame retardants in residential house dust samples and fire station dust samples in California [J]. Environmental Research, 2014, 135: 9-14. doi: 10.1016/j.envres.2014.08.022 [59] MELYMUK L, BOHLIN-NIZZETTO P, KUKUČKA P, et al. Seasonality and indoor/outdoor relationships of flame retardants and PCBs in residential air [J]. Environmental Pollution, 2016, 218: 392-401. doi: 10.1016/j.envpol.2016.07.018 [60] PERSSON J, WANG T, HAGBERG J. Temporal trends of decabromodiphenyl ether and emerging brominated flame retardants in dust, air and window surfaces of newly built low-energy preschools [J]. Indoor Air, 2019, 29(2): 263-275. doi: 10.1111/ina.12528 [61] HONG W J, JIA H L, DING Y S, et al. Polychlorinated biphenyls (PCBs) and halogenated flame retardants (HFRs) in multi-matrices from an electronic waste (e-waste) recycling site in Northern China [J]. Journal of Material Cycles and Waste Management, 2018, 20(1): 80-90. doi: 10.1007/s10163-016-0550-8 [62] MUIR D, HOWARD P H, MEYLAN W. Screening chemicals in commerce to identify possible persistent and bioaccumulative organohalogen chemicals: new results [J]. Organohalogen Compounds, 2007, 69: 1053-1056. [63] TOMY G T, PLESKACH K, ARSENAULT G, et al. Identification of the novel cycloaliphatic brominated flame retardant 1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane in Canadian arctic beluga (Delphinapterus leucas) [J]. Environmental Science & Technology, 2008, 42(2): 543-549. [64] NEWTON S, BIDLEMAN T, BERGKNUT M, et al. Atmospheric deposition of persistent organic pollutants and chemicals of emerging concern at two sites in northern Sweden [J]. Environmental Science. Processes & Impacts, 2014, 16(2): 298-305. [65] CARLSSON P, VRANA B, SOBOTKA J, et al. New brominated flame retardants and dechlorane plus in the Arctic: Local sources and bioaccumulation potential in marine benthos [J]. Chemosphere, 2018, 211: 1193-1202. doi: 10.1016/j.chemosphere.2018.07.158 [66] FERNIE K J, CHABOT D, CHAMPOUX L, et al. Spatiotemporal patterns and relationships among the diet, biochemistry, and exposure to flame retardants in an apex avian predator, the peregrine falcon [J]. Environmental Research, 2017, 158: 43-53. doi: 10.1016/j.envres.2017.05.035 [67] RUAN Y F, ZHANG X H, QIU J W, et al. Stereoisomer-specific trophodynamics of the chiral brominated flame retardants HBCD and TBECH in a marine food web, with implications for human exposure [J]. Environmental Science & Technology, 2018, 52(15): 8183-8193. [68] WANG Q, KELLY B C. Occurrence and distribution of halogenated flame retardants in an urban watershed: Comparison to polychlorinated biphenyls and organochlorine pesticides [J]. Environmental Pollution, 2017, 231: 252-261. doi: 10.1016/j.envpol.2017.07.092 [69] SÜHRING R, BUSCH F, FRICKE N, et al. Distribution of brominated flame retardants and dechloranes between sediments and benthic fish—A comparison of a freshwater and marine habitat [J]. Science of the Total Environment, 2016, 542: 578-585. doi: 10.1016/j.scitotenv.2015.10.085 [70] RUAN Y F, LAM J C W, ZHANG X H, et al. Temporal changes and stereoisomeric compositions of 1, 2, 5, 6, 9, 10-hexabromocyclododecane and 1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane in marine mammals from the South China sea [J]. Environmental Science & Technology, 2018, 52(5): 2517-2526. [71] ZHANG Y Q, LU Y L, WANG P, et al. Biomagnification of Hexabromocyclododecane (HBCD) in a coastal ecosystem near a large producer in China: Human exposure implication through food web transfer [J]. Science of the Total Environment, 2018, 624: 1213-1220. doi: 10.1016/j.scitotenv.2017.12.153 [72] KIM J T, CHOI Y J, BARGHI M, et al. Occurrence, distribution, and bioaccumulation of new and legacy persistent organic pollutants in an ecosystem on King George Island, maritime Antarctica [J]. Journal of Hazardous Materials, 2021, 405: 124141. doi: 10.1016/j.jhazmat.2020.124141 [73] WANG W T, CHOO G, CHO H S, et al. The occurrence and distribution of hexabromocyclododecanes in freshwater systems, focusing on tissue-specific bioaccumulation in crucian carp [J]. Science of the Total Environment, 2018, 635: 470-478. doi: 10.1016/j.scitotenv.2018.03.262 [74] TANG B, ZENG Y H, LUO X J, et al. Bioaccumulative characteristics of tetrabromobisphenol A and hexabromocyclododecanes in multi-tissues of prey and predator fish from an e-waste site, South China [J]. Environmental Science and Pollution Research, 2015, 22(16): 12011-12017. doi: 10.1007/s11356-015-4463-1 [75] ZHU C F, WANG P, LI Y M, et al. Trophic transfer of hexabromocyclododecane in the terrestrial and aquatic food webs from an e-waste dismantling region in East China [J]. Environmental Science. Processes & Impacts, 2017, 19(2): 154-160. [76] ZHANG Y W, SUN H W, RUAN Y F. Enantiomer-specific accumulation, depuration, metabolization and isomerization of hexabromocyclododecane (HBCD) diastereomers in mirror carp from water [J]. Journal of Hazardous Materials, 2014, 264: 8-15. doi: 10.1016/j.jhazmat.2013.10.062 [77] ZHANG Y W, SUN H W, LIU F, et al. Hexabromocyclododecanes in limnic and marine organisms and terrestrial plants from Tianjin, China: Diastereomer- and enantiomer-specific profiles, biomagnification, and human exposure [J]. Chemosphere, 2013, 93(8): 1561-1568. doi: 10.1016/j.chemosphere.2013.08.004 [78] LI H W, HU Y X, SUN Y X, et al. Bioaccumulation and translocation of tetrabromobisphenol A and hexabromocyclododecanes in mangrove plants from a national nature reserve of Shenzhen City, South China [J]. Environment International, 2019, 129: 239-246. doi: 10.1016/j.envint.2019.05.034 [79] ZHU H K, SUN H W, YAO Y M, et al. Legacy and alternative brominated flame retardants in outdoor dust and pine needles in mainland China: Spatial trends, dust-plant partitioning and human exposure [J]. Environmental Pollution, 2018, 243: 758-765. doi: 10.1016/j.envpol.2018.08.097 [80] KIM J T, CHOI Y J, BARGHI M, et al. Occurrence and distribution of old and new halogenated flame retardants in mosses and lichens from the South Shetland Islands, Antarctica [J]. Environmental Pollution, 2018, 235: 302-311. doi: 10.1016/j.envpol.2017.12.080 [81] ZHU H K, SUN H W, ZHANG Y W, et al. Uptake pathway, translocation, and isomerization of hexabromocyclododecane diastereoisomers by wheat in closed Chambers [J]. Environmental Science & Technology, 2016, 50(5): 2652-2659. [82] ZHU H K, SUN H W, YAO Y M, et al. Fate and adverse effects of hexabromocyclododecane diastereoisomers (HBCDDs) in a soil-ryegrass pot system [J]. Chemosphere, 2017, 184: 452-459. doi: 10.1016/j.chemosphere.2017.05.166 [83] LÜ H, MA X J, HUANG X J, et al. Distribution, diastereomer-specific accumulation and associated health risks of hexabromocyclododecanes (HBCDs) in soil-vegetable system of the Pearl River Delta region, South China [J]. Journal of Environmental Management, 2019, 248: 109321. doi: 10.1016/j.jenvman.2019.109321 [84] LI B, YAO T Q, SUN H W, et al. Diastereomer- and enantiomer-specific accumulation, depuration, bioisomerization, and metabolism of hexabromocyclododecanes (HBCDs) in two ecologically different species of earthworms [J]. Science of the Total Environment, 2016, 542: 427-434. doi: 10.1016/j.scitotenv.2015.10.100 [85] GUO Z, ZHANG L J, LIU X Y, et al. The enrichment and purification of hexabromocyclododecanes and its effects on thyroid in zebrafish [J]. Ecotoxicology and Environmental Safety, 2019, 185: 109690. doi: 10.1016/j.ecoenv.2019.109690 [86] XIA W, WANG J M, YANG H, et al. Bioaccumulation and distribution of hexabromocyclododecane isomers in duck tissues [J]. Bulletin of Environmental Contamination and Toxicology, 2018, 100(6): 754-759. doi: 10.1007/s00128-018-2342-4 [87] DU M M, LIN L F, YAN C Z, et al. Diastereoisomer- and enantiomer-specific accumulation, depuration, and bioisomerization of hexabromocyclododecanes in zebrafish (Danio rerio) [J]. Environmental Science & Technology, 2012, 46(20): 11040-11046. [88] HAKK H. Comparative metabolism studies of hexabromocyclododecane (HBCD) diastereomers in male rats following a single oral dose [J]. Environmental Science & Technology, 2016, 50(1): 89-96. [89] LAW K, PALACE V P, HALLDORSON T, et al. Dietary accumulation of hexabromocyclododecane diastereoisomers in juvenile rainbow trout (Oncorhynchus mykiss) Ⅰ: Bioaccumulation parameters and evidence of bioisomerization [J]. Environmental Toxicology and Chemistry, 2006, 25(7): 1757-1761. doi: 10.1897/05-445R.1 [90] LI B, CHEN H, SUN H W, et al. Distribution, isomerization and enantiomer selectivity of hexabromocyclododecane (HBCD) diastereoisomers in different tissue and subcellular fractions of earthworms [J]. Ecotoxicology and Environmental Safety, 2017, 139: 326-334. doi: 10.1016/j.ecoenv.2017.01.004 [91] SHIN E S, JEONG Y, BARGHI M, et al. Internal distribution and fate of persistent organic contaminants (PCDD/Fs, DL-PCBs, HBCDs, TBBPA, and PFASs) in a Bos Taurus [J]. Environmental Pollution, 2020, 267: 115306. doi: 10.1016/j.envpol.2020.115306 [92] SU G Y, MCGOLDRICK D J, CLARK M G, et al. Isomer-specific hexabromocyclododecane (HBCDD) levels in top predator fish from across Canada and 36-year temporal trends in lake Ontario [J]. Environmental Science & Technology, 2018, 52(11): 6197-6207. [93] HONG H Z, LV D, LIU W X, et al. Toxicity and bioaccumulation of three hexabromocyclododecane diastereoisomers in the marine copepod Tigriopus japonicas [J]. Aquatic Toxicology, 2017, 188: 1-9. doi: 10.1016/j.aquatox.2017.04.010 [94] ZHENG G M, WAN Y, SHI S N, et al. Trophodynamics of emerging brominated flame retardants in the aquatic food web of lake Taihu: Relationship with organism metabolism across trophic levels [J]. Environmental Science & Technology, 2018, 52(8): 4632-4640. [95] CHEN D, LETCHER R J, BURGESS N M, et al. Flame retardants in eggs of four gull species (Laridae) from breeding sites spanning Atlantic to Pacific Canada [J]. Environmental Pollution, 2012, 168: 1-9. doi: 10.1016/j.envpol.2012.03.040 [96] SU G Y, LETCHER R J, MOORE J N, et al. Spatial and temporal comparisons of legacy and emerging flame retardants in herring gull eggs from colonies spanning the Laurentian Great Lakes of Canada and United States [J]. Environmental Research, 2015, 142: 720-730. doi: 10.1016/j.envres.2015.08.018 [97] TAO F, ABOU-ELWAFA ABDALLAH M, ASHWORTH D C, et al. Emerging and legacy flame retardants in UK human milk and food suggest slow response to restrictions on use of PBDEs and HBCDD [J]. Environment International, 2017, 105: 95-104. doi: 10.1016/j.envint.2017.05.010 [98] KURT-KARAKUS P B, MUIR D C G, de JOURDAN B, et al. Bioaccumulation of selected halogenated organic flame retardants in lake Ontario [J]. Environmental Toxicology and Chemistry, 2019, 38(6): 1198-1210. doi: 10.1002/etc.4413 [99] FISK P R, GIRLING A E, WILDEY R J. Prioritisation of flame retardants for environmental risk assessment [R]. Environment Agency, United Kingdom, 2003. [100] ESSLINGER S, BECKER R, MÜLLER-BELECKE A, et al. HBCD stereoisomer pattern in mirror carps following dietary exposure to pure γ-HBCD enantiomers [J]. Journal of Agricultural and Food Chemistry, 2010, 58(17): 9705-9710. doi: 10.1021/jf101469q [101] GANNON A M, NUNNIKHOVEN A, LISTON V, et al. Rat strain response differences upon exposure to technical or alpha hexabromocyclododecane [J]. Food and Chemical Toxicology, 2019, 130: 284-307. doi: 10.1016/j.fct.2019.05.003 [102] SZABO D T, DILIBERTO J J, HAKK H, et al. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: Effect of dose, timing, route, repeated exposure, and metabolism [J]. Toxicological Sciences, 2010, 117(2): 282-293. doi: 10.1093/toxsci/kfq183 [103] RATEL J, PLANCHE C, MERCIER F, et al. Liver volatolomics to reveal poultry exposure to γ-hexabromocyclododecane (HBCD) [J]. Chemosphere, 2017, 189: 634-642. doi: 10.1016/j.chemosphere.2017.09.074 [104] SANDERS J M, KNUDSEN G A, BIRNBAUM L S. The fate of β-hexabromocyclododecane in female C57BL/6 mice [J]. Toxicological Sciences, 2013, 134(2): 251-257. doi: 10.1093/toxsci/kft121 [105] ABDALLAH M A E, UCHEA C, CHIPMAN J K, et al. Enantioselective biotransformation of hexabromocyclododecane by in vitro rat and trout hepatic sub-cellular fractions [J]. Environmental Science & Technology, 2014, 48(5): 2732-2740. [106] ZHENG X B, ERRATICO C, ABDALLAH M A E, et al. In vitro metabolism of BDE-47, BDE-99, and α-, β-, γ-HBCD isomers by chicken liver microsomes [J]. Environmental Research, 2015, 143: 221-228. doi: 10.1016/j.envres.2015.10.023 [107] ZHENG X B, ERRATICO C, LUO X J, et al. Oxidative metabolism of BDE-47, BDE-99, and HBCDs by cat liver microsomes: Implications of cats as sentinel species to monitor human exposure to environmental pollutants [J]. Chemosphere, 2016, 151: 30-36. doi: 10.1016/j.chemosphere.2016.02.054 [108] ESSLINGER S, BECKER R, MAUL R, et al. Hexabromocyclododecane enantiomers: Microsomal degradation and patterns of hydroxylated metabolites [J]. Environmental Science & Technology, 2011, 45(9): 3938-3944. [109] BRANDSMA S H, van der VEN L T M, de BOER J, et al. Identification of hydroxylated metabolites of hexabromocyclododecane in wildlife and 28-days exposed Wistar rats [J]. Environmental Science & Technology, 2009, 43(15): 6058-6063. [110] HAKK H, SZABO D T, HUWE J, et al. Novel and distinct metabolites identified following a single oral dose of α- or γ-hexabromocyclododecane in mice [J]. Environmental Science & Technology, 2012, 46(24): 13494-13503. [111] SZABO D T, PATHMASIRI W, SUMNER S, et al. Serum metabolomic profiles in neonatal mice following oral brominated flame retardant exposures to hexabromocyclododecane (HBCD) alpha, gamma, and commercial mixture [J]. Environmental Health Perspectives, 2017, 125(4): 651-659. doi: 10.1289/EHP242 [112] CHANG T H, WANG R, PENG Y H, et al. Biodegradation of hexabromocyclododecane by Rhodopseudomonas palustris YSC3 strain: A free-living nitrogen-fixing bacterium isolated in Taiwan [J]. Chemosphere, 2020, 246: 125621. doi: 10.1016/j.chemosphere.2019.125621 [113] HEEB N V, GRUBELNIK A, GEUEKE B, et al. Biotransformation of hexabromocyclododecanes with hexachlorocyclohexane-transforming Sphingobium chinhatense strain IP26 [J]. Chemosphere, 2017, 182: 491-500. doi: 10.1016/j.chemosphere.2017.05.047 [114] HUANG L, WANG W W, SHAH S B, et al. The HBCDs biodegradation using a Pseudomonas strain and its application in soil phytoremediation [J]. Journal of Hazardous Materials, 2019, 380: 120833. doi: 10.1016/j.jhazmat.2019.120833 [115] SHAH S B, HUANG L, HU H Y, et al. Characterization of environmentally friendly degradation of hexabromocyclododecane by a Bacillus strain HBCD-sjtu [J]. International Biodeterioration & Biodegradation, 2019, 145: 104794. [116] WANG R, LIN C Y, CHEN S H, et al. Using high-throughput transcriptome sequencing to investigate the biotransformation mechanism of hexabromocyclododecane with Rhodopseudomonas palustris in water [J]. Science of the Total Environment, 2019, 692: 249-258. doi: 10.1016/j.scitotenv.2019.07.140 [117] YANG K L, ZHONG Q, QIN H M, et al. Molecular response mechanism in Escherichia coli under hexabromocyclododecane stress [J]. Science of the Total Environment, 2020, 708: 135199. doi: 10.1016/j.scitotenv.2019.135199 [118] ZHONG Y, WANG H L, YU Z Q, et al. Diastereoisomer-specific biotransformation of hexabromocyclododecanes by a mixed culture containing Dehalococcoides mccartyi strain 195 [J]. Frontiers in Microbiology, 2018, 9: 1713. doi: 10.3389/fmicb.2018.01713 [119] ZHANG Q, YAO Y M, WANG Y, et al. Plant accumulation and transformation of brominated and organophosphate flame retardants: A review [J]. Environmental Pollution, 2021, 288: 117742. doi: 10.1016/j.envpol.2021.117742 [120] HEEB N V, MAZENAUER M, WYSS S, et al. Kinetics and stereochemistry of LinB-catalyzed δ-HBCD transformation: Comparison of in vitro and in silico results [J]. Chemosphere, 2018, 207: 118-129. doi: 10.1016/j.chemosphere.2018.05.057 [121] NYHOLM J R, NORMAN A, NORRGREN L, et al. Uptake and biotransformation of structurally diverse brominated flame retardants in zebrafish (Danio rerio) after dietary exposure [J]. Environmental Toxicology and Chemistry, 2009, 28(5): 1035-1042. doi: 10.1897/08-302.1 [122] GEMMILL B, PLESKACH K, PETERS L, et al. Toxicokinetics of tetrabromoethylcyclohexane (TBECH) in juvenile brown trout (Salmo trutta) and effects on plasma sex hormones [J]. Aquatic Toxicology, 2011, 101(2): 309-317. doi: 10.1016/j.aquatox.2010.11.003 [123] CHU S G, GAUTHIER L T, LETCHER R J. Alpha and beta isomers of tetrabromoethylcyclohexane (TBECH) flame retardant: Depletion and metabolite formation in vitro using a model rat microsomal assay [J]. Environmental Science & Technology, 2012, 46(18): 10263-10270. [124] WONG F, KURT-KARAKUS P, BIDLEMAN T F. Fate of brominated flame retardants and organochlorine pesticides in urban soil: Volatility and degradation [J]. Environmental Science & Technology, 2012, 46(5): 2668-2674. [125] DARNERUD P O. Toxic effects of brominated flame retardants in man and in wildlife [J]. Environment International, 2003, 29(6): 841-853. doi: 10.1016/S0160-4120(03)00107-7 [126] LILIENTHAL H, van der VEN L T M, PIERSMA A H, et al. Effects of the brominated flame retardant hexabromocyclododecane (HBCD) on dopamine-dependent behavior and brainstem auditory evoked potentials in a one-generation reproduction study in Wistar rats [J]. Toxicology Letters, 2009, 185(1): 63-72. doi: 10.1016/j.toxlet.2008.12.002 [127] van der VEN L T M, VERHOEF A, van de KUIL T, et al. A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in wistar rats [J]. Toxicological Sciences, 2006, 94(2): 281-292. doi: 10.1093/toxsci/kfl113 [128] FERY Y, MUELLER S O, SCHRENK D. Development of stably transfected human and rat hepatoma cell lines for the species-specific assessment of xenobiotic response enhancer module (XREM)-dependent induction of drug metabolism [J]. Toxicology, 2010, 277(1/2/3): 11-19. [129] MARIUSSEN E, de FONNUM F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles [J]. Neurochemistry International, 2003, 43(4/5): 533-542. [130] SAEGUSA Y, FUJIMOTO H, WOO G H, et al. Transient aberration of neuronal development in the hippocampal dentate gyrus after developmental exposure to brominated flame retardants in rats [J]. Archives of Toxicology, 2012, 86(9): 1431-1442. doi: 10.1007/s00204-012-0824-4 [131] REFFATTO V, RASINGER J D, CARROLL T S, et al. Parallel in vivo and in vitro transcriptomics analysis reveals calcium and zinc signalling in the brain as sensitive targets of HBCD neurotoxicity [J]. Archives of Toxicology, 2018, 92(3): 1189-1203. doi: 10.1007/s00204-017-2119-2 [132] IBHAZEHIEBO K, IWASAKI T, SHIMOKAWA N, et al. 1, 2, 5, 6, 9, 10-αHexabromocyclododecane (HBCD) impairs thyroid hormone-induced dendrite arborization of Purkinje cells and suppresses thyroid hormone receptor-mediated transcription [J]. The Cerebellum, 2011, 10(1): 22-31. doi: 10.1007/s12311-010-0218-1 [133] AL-MOUSA F, MICHELANGELI F. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells [J]. PLoS One, 2012, 7(4): e33059. doi: 10.1371/journal.pone.0033059 [134] AL-MOUSA F, MICHELANGELI F. The sarcoplasmic-endoplasmic Reticulum Ca2+-ATPase (SERCA) is the likely molecular target for the acute toxicity of the brominated flame retardant hexabromocyclododecane (HBCD) [J]. Chemico-Biological Interactions, 2014, 207: 1-6. doi: 10.1016/j.cbi.2013.10.021 [135] JOHNSON P I, STAPLETON H M, MUKHERJEE B, et al. Associations between brominated flame retardants in house dust and hormone levels in men [J]. Science of the Total Environment, 2013, 445/446: 177-184. doi: 10.1016/j.scitotenv.2012.12.017 [136] DOROSH A, DĚD L, ELZEINOVÁ F, et al. Assessing oestrogenic effects of brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on MCF-7 cells [J]. Folia Biologica, 2011, 57(1): 35-39. [137] HAMERS T, KAMSTRA J H, SONNEVELD E, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants [J]. Toxicological Sciences, 2006, 92(1): 157-173. doi: 10.1093/toxsci/kfj187 [138] FERNIE K J, MARTEINSON S C, BIRD D M, et al. Reproductive changes in American kestrels (Falco sparverius) in relation to exposure to technical hexabromocyclododecane flame retardant [J]. Environmental Toxicology and Chemistry, 2011, 30(11): 2570-2575. doi: 10.1002/etc.652 [139] MARTEINSON S C, KIMMINS S, LETCHER R J, et al. Diet exposure to technical hexabromocyclododecane (HBCD) affects testes and circulating testosterone and thyroxine levels in American kestrels (Falco sparverius) [J]. Environmental Research, 2011, 111(8): 1116-1123. doi: 10.1016/j.envres.2011.08.006 [140] XIE X N, YU C X, REN Q D, et al. Exposure to HBCD promotes adipogenesis both in vitro and in vivo by interfering with Wnt6 expression [J]. Science of the Total Environment, 2020, 705: 135917. doi: 10.1016/j.scitotenv.2019.135917 [141] 王玲, 郑明刚, 仝艳丽, 等. 六溴环十二烷(hexabromocyclododecane, HBCD)暴露对生长阶段文昌鱼的毒性及其几种重要酶活性的影响 [J]. 环境科学学报, 2011, 31(5): 1086-1091. WANG L, ZHENG M G, TONG Y L, et al. Effect of immune-related enzymes in amphioxus exposed to waterborne hexabromocyclododecane(HBCD) [J]. Acta Scientiae Circumstantiae, 2011, 31(5): 1086-1091(in Chinese).
[142] YANAGISAWA R, KOIKE E, WIN-SHWE T T, et al. Impaired lipid and glucose homeostasis in hexabromocyclododecane-exposed mice fed a high-fat diet [J]. Environmental Health Perspectives, 2014, 122(3): 277-283. doi: 10.1289/ehp.1307421 [143] SZABO D T, DILIBERTO J J, HAKK H, et al. Toxicokinetics of the flame retardant hexabromocyclododecane alpha: Effect of dose, timing, route, repeated exposure, and metabolism [J]. Toxicological Sciences, 2011, 121(2): 234-244. doi: 10.1093/toxsci/kfr059 [144] DONG H K, LU G H, YAN Z H, et al. Responses of antioxidant and biotransformation enzymes in Carassius carassius exposed to hexabromocyclododecane [J]. Environmental Toxicology and Pharmacology, 2018, 62: 46-53. doi: 10.1016/j.etap.2018.06.009 [145] SHI Y J, XU X B, CHEN J, et al. Antioxidant gene expression and metabolic responses of earthworms (Eisenia fetida) after exposure to various concentrations of hexabromocyclododecane [J]. Environmental Pollution, 2018, 232: 245-251. doi: 10.1016/j.envpol.2017.09.039 [146] JIN Y Y, SHANG Y, ZHANG D P, et al. Hexabromocyclododecanes promoted autophagy through the PI3K/Akt/mTOR pathway in L02 cells [J]. Journal of Environmental Management, 2019, 244: 77-82. [147] 陈海波, 李辉, 刘勇弟. 六溴环十二烷急性暴露对秀丽隐杆线虫的毒性效应 [J]. 华东理工大学学报(自然科学版), 2019, 45(1): 87-94. CHEN H B, LI H, LIU Y D. Toxicological effects of hexabromocyclododecane (HBCD) by acute exposure on nematode Caenorhabditis elegans [J]. Journal of East China University of Science and Technology, 2019, 45(1): 87-94(in Chinese).
[148] WANG X L, YANG J, LI H, et al. Chronic toxicity of hexabromocyclododecane(HBCD) induced by oxidative stress and cell apoptosis on nematode Caenorhabditis elegans [J]. Chemosphere, 2018, 208: 31-39. doi: 10.1016/j.chemosphere.2018.05.147 [149] PALACE V P, PLESKACH K, HALLDORSON T, et al. Biotransformation enzymes and thyroid axis disruption in juvenile rainbow trout (Oncorhynchus mykiss) exposed to hexabromocyclododecane diastereoisomers [J]. Environmental Science & Technology, 2008, 42(6): 1967-1972. [150] MARTEINSON S C, EULAERS I, JASPERS V L B, et al. Transfer of hexabromocyclododecane flame retardant isomers from captive American kestrel eggs to feathers and their association with thyroid hormones and growth [J]. Environmental Pollution, 2017, 220: 441-451. doi: 10.1016/j.envpol.2016.09.086 [151] HUANG X M, CHEN C, SHANG Y, et al. In vitro study on the biotransformation and cytotoxicity of three hexabromocyclododecane diastereoisomers in liver cells [J]. Chemosphere, 2016, 161: 251-258. doi: 10.1016/j.chemosphere.2016.07.001 [152] SHI X L, ZHA J M, WEN B, et al. Diastereoisomer-specific neurotoxicity of hexabromocyclododecane in human SH-SY5Y neuroblastoma cells [J]. Science of the Total Environment, 2019, 686: 893-902. doi: 10.1016/j.scitotenv.2019.06.008 [153] CURRAN I H A, LISTON V, NUNNIKHOVEN A, et al. Toxicologic effects of 28-day dietary exposure to the flame retardant 1, 2-dibromo-4-(1, 2-dibromoethyl)-cyclohexane (TBECH) in F344 rats [J]. Toxicology, 2017, 377: 1-13. doi: 10.1016/j.tox.2016.12.001 [154] LIU P Y, MENG T, LI Y Y, et al. Tetrabromoethylcyclohexane affects gonadal differentiation and development in the frog Pelophylax nigromaculatus [J]. Aquatic Toxicology, 2017, 192: 40-47. doi: 10.1016/j.aquatox.2017.09.009 [155] PRADHAN A, KHARLYNGDOH J B, ASNAKE S, et al. The brominated flame retardant TBECH activates the zebrafish (Danio rerio) androgen receptor, alters gene transcription and causes developmental disturbances [J]. Aquatic Toxicology, 2013, 142/143: 63-72. doi: 10.1016/j.aquatox.2013.07.018 [156] LARSSON A, ERIKSSON L A, ANDERSSON P L, et al. Identification of the brominated flame retardant 1, 2-dibromo-4-(1, 2-dibromoethyl)cyclohexane as an androgen agonist [J]. Journal of Medicinal Chemistry, 2006, 49(25): 7366-7372. doi: 10.1021/jm060713d [157] NYHOLM J R, NORMAN A, NORRGREN L, et al. Maternal transfer of brominated flame retardants in zebrafish (Danio rerio) [J]. Chemosphere, 2008, 73(2): 203-208. doi: 10.1016/j.chemosphere.2008.04.033 [158] WANG X, WEI L, ZHU J B, et al. Tetrabromoethylcyclohexane (TBECH) exhibits immunotoxicity in murine macrophages [J]. Environmental Toxicology, 2020, 35(2): 159-166. doi: 10.1002/tox.22852 [159] STOJAK B L, van GINKEL R A, IVANCO T L, et al. Acute β-tetrabromoethylcyclohexane (β-TBECH) treatment inhibits the electrical activity of rat Purkinje neurons [J]. Chemosphere, 2019, 231: 301-307. doi: 10.1016/j.chemosphere.2019.05.102 [160] PORTER E, CRUMP D, EGLOFF C, et al. Use of an avian hepatocyte assay and the avian Toxchip Polymerse chain reaction array for testing prioritization of 16 organic flame retardants [J]. Environmental Toxicology and Chemistry, 2014, 33(3): 573-582. doi: 10.1002/etc.2469 [161] KHALAF H, LARSSON A, BERG H, et al. Diastereomers of the brominated flame retardant 1, 2-dibromo-4-(1, 2 dibromoethyl)cyclohexane induce androgen receptor activation in the HepG2 hepatocellular carcinoma cell line and the LNCaP prostate cancer cell line [J]. Environmental Health Perspectives, 2009, 117(12): 1853-1859. doi: 10.1289/ehp.0901065 [162] MARTEINSON S C, FERNIE K J. Is the current-use flame retardant, DBE-DBCH, a potential obesogen?Effects on body mass, fat content and associated behaviors in American kestrels [J]. Ecotoxicology and Environmental Safety, 2019, 169: 770-777. doi: 10.1016/j.ecoenv.2018.11.104 [163] HUANG H L, LV L, WANG D, et al. Biochemical and molecular responses of maize (Zea mays L. ) to 1, 2-dibromo-4-(1, 2 dibromoethyl) cyclohexane (TBECH) diastereomers: Oxidative stress, DNA damage, antioxidant enzyme gene expression and diversity of root exudates [J]. Science of the Total Environment, 2021, 753: 141872. doi: 10.1016/j.scitotenv.2020.141872 [164] SAUNDERS D M V, HIGLEY E B, HECKER M, et al. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO [J]. Toxicology Letters, 2013, 223(2): 252-259. doi: 10.1016/j.toxlet.2013.09.009 [165] van ESSEN D, DEVOY C, MILLER J, et al. Effects of the brominated flame retardant, TBCO, on development of zebrafish (Danio rerio) embryos [J]. Chemosphere, 2021, 266: 129195. doi: 10.1016/j.chemosphere.2020.129195 -



 下载:
下载: