-
传统阻燃剂多溴二苯醚(PBDEs)由于持久性、生物毒性、生物积累性以及长距离迁移性,在全球范围内被禁止生产和使用[1]. 新型溴代阻燃剂(NBFRs)属于添加型阻燃剂,作为PBDEs的优良替代品被广泛用于电子产品生产、建筑业和纺织业中[2]. 常见的NBFRs有十溴二苯基乙烷(DBDPE),五溴苯(PBBz)和五溴甲苯(PBT)等. 值得注意的是,2005年我国开始生产DBDPE,但其产量以每年80%的速度增长,成为在中国最受欢迎的NBFRs[3]. 近年来,随着NBFRs的用量增多,大量NBFRs在灰尘[4]、土壤[5]、母乳[6]、血清[7]等介质中被检出. NBFRs可以通过呼吸、饮食和皮肤接触等途径进入人体,从而带来潜在健康危害. 动物实验的研究表明,DBDPE可通过增加斑马鱼幼鱼体内三碘甲状腺原氨酸和甲状腺素的含量,破坏甲状腺激素功能[8]. DBDPE也可在内分泌系统积累,从而对大鼠的生殖功能[9]、肝脏[10]和心血管系统[11]产生毒害作用. 流行病学数据表明,人体血清中五溴乙苯(PBEB)、DBDPE水平与甲状腺激素显著相关,PBT、1, 2-双(2,4,6-三溴苯氧基)乙烷(BTBPE)水平与肝损伤指标显著相关[12].
目前NBFRs主要使用气相色谱-三重四极杆串联质谱法(GC-MS/MS)检测,搭配负化学离子源(NCI)或电子轰击离子源(EI)分析. 但分析方法仍存在难点,由于GC-EI-MS/MS方法灵敏度较低,难以分析高溴代的NBFRs[13];GC-NCI-MS/MS虽然灵敏度较高,但选择性差,难以分离同位素标记的化合物及其单体,且基质复杂的样品会严重影响测试效果[2].
血清是评估人体内暴露的重要生物标志物. 目前对于血清中NBFRs的研究相对较少,并且人体血清的NBFRs检测存在着含量低、样品量少、前处理程序繁琐和基质复杂等问题,给NBFRs的分析带来了挑战. 因此,建立高效灵敏的NBFRs分析方法以评估其潜在的毒性和未知的健康风险尤为迫切. 基于EI源和MRM模式的GC-MS/MS方法具有较好的选择性,在分析复杂基质时具有明显优势,本研究使用GC-MS/MS同时测定9种NBFRs,并优化了人体血清样品中NBFRs的前处理方法. 该方法较好地解决了高溴代阻燃剂响应低、高温条件易降解等难点问题,为研究血清中NBFRs的浓度水平提供技术支持.
-
气相色谱/三重四极杆型质谱联用仪(GCMS-TQ8050NX,Shimadzu),配有电子轰击源(Electron Impact, EI)和GCMS solution工作站;固相萃取装置(Supelco);氮吹浓缩仪;固相萃取柱HLB (200 mg,Waters).
丙酮、乙酸乙酯(农残级,Fisher Scientific);二氯甲烷、正己烷(农残级,J.T.Baker);弗罗里土(Supleco)经140 ℃活化后保存于干燥器中;无水硫酸钠(天津市科密欧化学试剂有限公司)经660 ℃活化后保存于干燥器中;中性硅胶(60—100目,Merck)经550 ℃活化后保存于干燥器中;Milli-Q超纯水(Millipore).
9种NBFRs的基本信息如表1所示. PBBz、PBT、PBEB、2,3-二溴丙基-2,4,6-三溴苯氧基醚(DPTE)、六溴苯(HBBz)、乙基己基四溴苯甲酸酯(EHTBB)、BTBPE、双(2-乙基己基)四溴邻苯二甲酸酯(BEHTEBP)、DBDPE和同位素标记的十溴二苯基乙烷(13C-DBDPE)购自Wellington公司. 3,3′,4,4′-四溴二苯醚(BDE-77)、2,2′,3,3′,4,4′-六溴二苯醚(BDE-128)和2,2′,3,4,4′,5′-六溴二苯醚(BDE-138)购自AccuStandard公司[14].
血清样品来源于山东省千佛山医院[15].
-
取1 mL 血清样品于10 mL的玻璃试管中,加入1 ng提取内标(BDE-77,BDE-128和13C-DBDPE)和3 mL 乙酸乙酯,充分混匀后使用水平摇床老化20 min,超声提取20 min,然后在4000 r·min−1的转速下−6 ℃冷冻离心10 min,上层清液转移至另一干净玻璃试管中;向残渣中加入3 mL 乙酸乙酯,涡旋充分混匀后摇床振荡20 min,离心转移上层清液,并重复2次,转移并合并上层清液,在温和的氮气流下浓缩至干,用3 mL正己烷复溶,在−20 ℃冰箱中冷冻16 h以上. 将冷冻后的提取液在4000 r·min−1的转速下−6 ℃冷冻离心10 min,取上清液等待净化.
提取液使用HLB固相萃取小柱和弗罗里土-硅胶复合柱净化. HLB柱依次用5 mL甲醇、5 mL二氯甲烷和5 mL正己烷活化. 加载样品后,用12 mL二氯甲烷洗脱目标物,并在温和的氮气流下浓缩至1 mL,等待进一步净化处理;弗罗里土-硅胶柱(填料从下往上依次为0.2 g 无水硫酸钠、0.5 g 中性硅胶、1 g弗罗里土、0.5 g 中性硅胶、0.5 g 无水硫酸钠)依次使用6 mL 二氯甲烷: 丙酮 (1: 1,V/V)、6 mL 正己烷活化,加载样品后,使用10 mL 二氯甲烷 ∶ 正己烷(1:5, V/V)洗脱目标物. 最后,洗脱液在温和的氮气流下浓缩近干,转移至棕色进样小瓶,添加1 ng进样内标(BDE-138),待上机测试.
-
血清样品经过前处理后使用GCMS-TQ8050NX(Shimadzu)进行检测.
进样器选择:Autosampler AOC-20i, Shimadzu.
色谱条件:色谱柱选用DB-5MS(15 m×0.25 mm×0.1 μm,Agilent J&W Scientific);色谱柱升温程序为初始温度90 ℃(保持3 min),然后以20 ℃·min−1速率升温至325 ℃(保持6 min);进样方式为不分流进样,载气为氦气,流速为1.5 mL·min−1.
质谱条件:EI源,离子源温度为280 ℃,接口温度为300 ℃,采集模式为多反应监测(MRM)模式,分别优化了目标物的定性定量离子对及碰撞电压(表2). 检测器初始电压设为1.4 kV,采集DBDPE和 13C-DBDPE时,检测器电压为1.9 kV.
-
配制6个不同浓度的NBFRs混合标准溶液(CS1—CS6),其中PBBz、PBT、PBEB、DPTE、HBBz、EHTBB、BTBPE、BEHTEBP的浓度分别为0.2、1、5、10、20、50 ng·mL−1;DBDPE的浓度分别为1、5、25、50、100、250 ng·mL−1. 标准溶液使用1.3节的仪器条件进行分析,并使用内标法对标准曲线定量. 按照样品分析的全部步骤,重复7次空白实验,对于空白实验中检出的目标物,将各测定结果换算为样品中的浓度或含量,计算7次平行测定的标准偏差计算方法检出限(MDL);对于空白实验中未检出的目标物,对浓度值或含量为估计方法检出限值3—5倍的样品进行7次平行测定,计算平行测定的标准偏差计算MDL[16]. 结果见表3,标准曲线的相关系数(R2)均大于0.99. 除DBDPE外,其他8种化合物均未在空白实验中检出. 该方法整体MDL值较低,能在较宽的线性范围进行准确定量.
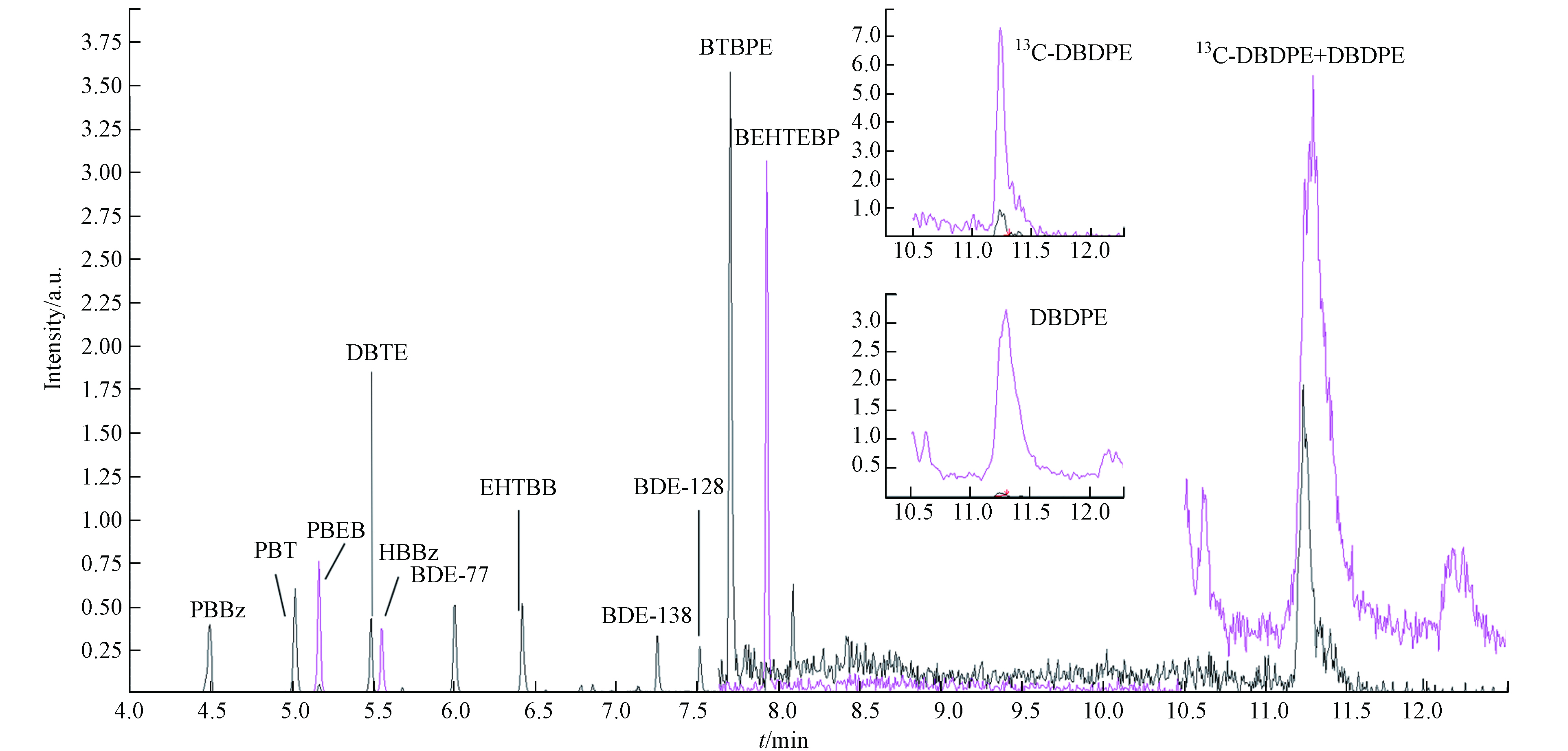
将标准溶液CS2每天进样6次,进样量1 μL,重复3 d,用于考察该方法的重现性. 标准溶液色谱图如图1,结果显示,除BTBPE和BEHTEBP之外,各化合物峰面积的日间相对标准偏差(RSD)均小于10%(表3),表明该方法具有良好的重现性.
-
首先设计空白实验,使用相同体积的超纯水,按照前处理流程,进行3次重复实验. 将参与者的血清样品混合,作为基质,并向基质中分别添加绝对量为0.05 ng、0.2 ng、1 ng的NBFRs混合标准溶液(DBDPE的绝对量分别为0.25 ng、1 ng、5 ng),进行基质加标回收实验,每个绝对量设计3次重复实验,以验证正确度. 同时设计3个不添加NBFRs混合标准溶液的基质作为对照实验.
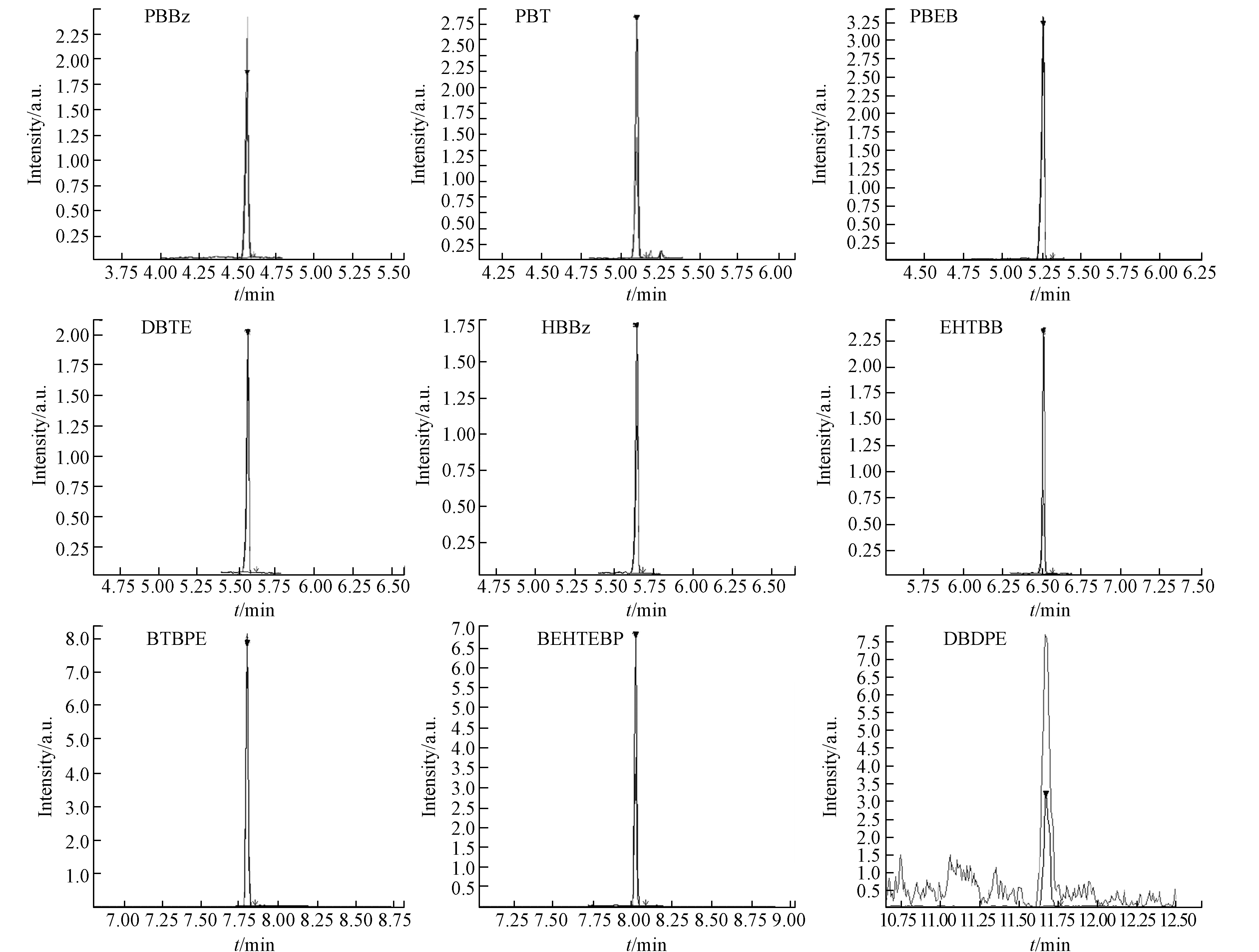
在空白实验中,DBDPE的浓度低于对照实验的10%,其他8种目标物均未检出. 基质加标实验的回收率在74%—136%,RSD<21%,结果见表4. 添加0.2 ng NBFRs混合标准溶液(DBDPE为5 ng)的基质加标实验色谱图见图2,各目标物均峰型良好,表明该前处理及仪器分析方法能够满足检测要求.
本研究与类似研究相比,具有样品用量少,稳定性好等优势. 目前,由于血清中NBFRs含量低,样品使用量偏高,例如一项我国成年人群血清研究使用5 mL血清分析[17],另一项关于血清中溴代阻燃剂检测方法研究的样品用量为2 mL [18]. 而本研究血清使用量为1 mL,并达到了较好的检测效果. 另一方面,一项巴基斯坦人群血清研究选取了2.5 ng和12.5 ng作为基质加标浓度[7],与之相比,本研究基质加标实验选取的浓度较低,能更准确地检测实际样品.
-
依据本研究建立的前处理及仪器分析方法,分析12个人体血清样本中NBFRs的含量,结果见表5. DBDPE在人体血清样品中被广泛检出,内标物BDE-77,BDE-138和13C-DBDPE的回收率分别在72%—96%,95%—116%和73%—126%之间.
-
配有EI源的GC-MS/MS能够较好地测定人体血清样品中9种NBFRs的含量,仪器方法具有良好的灵敏度和重现性,线性范围宽. 前处理方法所需样品量少,净化效果好,目标物均获得了较高的回收率. 实际血清样品测定结果显示,内标物回收率稳定在72%—126%之间. 与国内外的分析方法相比,该方法具有稳定性高,净化效果好等优势,可广泛用于人体血清中NBFRs的分析.
气相色谱-三重四极杆质谱法测定人体血清中新型溴代阻燃剂
Determination of novel brominated flame retardants in human serum by gas chromatography-triple quadrupole mass spectrometry
-
摘要: 新型溴代阻燃剂(NBFRs)作为多溴二苯醚(PBDEs)的替代品,具有优良的防火性能. 但有研究发现,NBFRs可能存在神经毒性和发育毒性等作用,从而对人体造成潜在的健康风险. 血清作为人体内暴露的重要生物标志物,对评估健康风险具有重要意义. 目前关于人体血清中NBFRs的健康风险研究较少,且血清中NBFRs含量低,前处理程序繁琐,基质复杂,给NBFRs的分析检测带来了很大困难. 本研究建立并优化了人体血清中五溴苯(PBBz)、五溴甲苯(PBT)及十溴二苯基乙烷(DBDPE)等9种NBFRs的前处理,及气相色谱-三重四极杆质谱检测方法. 血清样品经乙酸乙酯提取后,再经HLB固相萃取柱和弗罗里土-硅胶复合柱净化. 采用电子轰击离子源(EI)和多反应监测(MRM)模式进行仪器分析. 实验结果表明,血清样品中9种NBFRs的平均加标回收率在74%—136%之间,相对标准偏差小于21%. 实际血清样品测试发现,内标物的回收率稳定在72%—126%之间,具有良好的检测效果. 该方法前处理流程简单、稳定性好、灵敏度高,能广泛用于人体血清中NBFRs的分析.
-
关键词:
- 新型溴代阻燃剂 /
- 人体血清 /
- 气相色谱-三重四极杆质谱.
Abstract: Novel brominated flame retardants (NBFRs) are considered as the commercial replacements for polybrominated diphenyl ethers (PBDEs). The potential neurotoxicity and developmental toxicity of NBFRs could cause adversely effects to human health. Serum is regarded as an important biomarker for human exposure, which presented great significance in assessing human health risks. Few studies reported the health risks of NBFRs in human serum. The analysis and detection of NBFRs were limited by the low concentration, complicated pretreatment processes and matrix effect. In this study, gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) was established and optimized to detected the 9 NBFRs, mainly including pentabromobenzene (PBBz), pentabromotoluene (PBT) and decabromodiphenylethane (DBDPE). Serum samples were extracted by ethyl acetate, then cleaned up with HLB solid phase extraction column and Florisil-silica composite column. Electron impact (EI) ion source and multiple reaction monitoring (MRM) mode were applied for instrumental analysis. The average recoveries of the 9 NBFRs in serum samples were between 74% and 136%, and the relative standard deviation was less than 21%. Actual serum samples were collected to detect the 9 NBFRs, and the average recoveries of the internal standards ranged from 72% to 126%, which showed satisfactory performance. The developed methodology presented simple procedures, excellent stability and notable sensitivity, which was an efficient approach for the analysis of NBFRs in human serum.-
Key words:
- novel brominated flame retardants /
- human serum /
- GC-MS/MS.
-

-
表 1 9种NBFRs的名称、IUPAC名称、简称、分子式、相对分子质量和CAS号
Table 1. Chemical names, IUPAC names, abbreviations, molecular formula, relative molecular mass and CAS No. of 9 NBFRs
名称
Chemical namesIUPAC 名称
IUPAC names简称
Abbreviations分子式
Molecular formula相对分子质量/ (g·mol−1)
Relative molecular massCAS号
CAS No.五溴苯 1,2,3,4,5-五溴苯 PBBz C6HBr5 472.6 608-90-2 五溴甲苯 1,2,3,4,5-五溴-6-甲苯 PBT C7H3Br5 486.6 87-83-2 五溴乙苯 1,2,3,4,5-五溴-6-乙苯 PBEB C8H5Br5 500.6 85-22-3 2,3-二溴丙基2,4,6-三溴丙基醚 1,3,5-三溴-2-(2,3-二溴丙氧基)苯 DPTE C9H7Br5O 530.7 35109-60-5 六溴苯 1,2,3,4,5,6-六溴苯 HBBz C6Br6 551.5 87-82-1 乙基己基四溴苯甲酸酯 2-乙基己基-2,3,4,5-四溴苯甲酸酯 EHTBB C15H18Br4O2 549.9 183658-27-7 1,2-双(2,4,6-三溴苯氧基)乙烷 1,3,5-三溴-2-[2-(2,4,6-
三溴苯氧基)乙氧基]苯BTBPE C14H8Br6O2 687.6 37853-59-1 双(2-乙基己基)四溴邻苯二甲酸酯 双(2-乙基己基) 3,4,5,6-四溴苯-
1,2-二羧酸盐BEHTEBP C24H34Br4O4 706.1 26040-51-7 十溴二苯基乙烷 1,2,3,4,5-五溴-6-[2-(2,3,4,5,6-五溴苯基)乙基]苯 DBDPE C14H4Br10 971.2 84852-53-9 表 2 9种NBFRs的质谱条件
Table 2. Mass spectrometry parameters of the 9 NBFRs
化合物
Compound保留时间/min
Retention time定量离子对
Quantitative ion碰撞电压/eV
Collision energy定性离子对
Qualitative ion碰撞电压/eV
Collision energyPBBz 4.574 392.80>311.70 15 473.70>313.60 42 PBT 5.109 487.70>406.60 18 406.80>246.80 33 PBEB 5.262 499.70>484.40 18 501.70>486.50 21 DPTE 5.580 330.00>141.00 36 330.00>221.90 30 HBBz 5.648 391.80>231.80 39 551.50>473.00 30 EHTBB 6.511 420.80>392.50 21 437.80>420.50 24 BTBPE 7.800 687.60>358.90 9 356.70>198.20 30 BEHTEBP 8.029 464.60>380.60 42 466.60>382.30 33 DBDPE 11.658 971.30>484.80 36 486.50>324.80 45 表 3 9种NBFRs的相对标准偏差、相关系数、方法检出限
Table 3. Relative standard deviation, correlation coefficients, method limit of detection (MDL) of the 9 NBFRs
化合物
CompoundRSD/% 相关系数(R2)
Correlation coefficients方法检出限/ (ng·mL−1)
MDLPBBz 3.2 0.9997 0.13 PBT 2.2 0.9998 0.07 PBEB 5.4 0.9997 0.06 DPTE 8.3 0.9998 0.15 HBBz 6.8 0.9999 0.20 EHTBB 0.4 0.9983 0.09 BTBPE 11.9 0.9996 0.24 BEHTEBP 10.4 0.9996 0.35 DBDPE 9.9 0.9988 0.09 表 4 9种NBFRs的基质加标回收率(n=3)
Table 4. Matrix spiked recoveries of the 9 NBFRs (n=3)
化合物
Compound绝对量 0.05 ng
Spiked 0.05 ng绝对量 0.2 ng
Spiked 0.2 ng绝对量 1 ng
Spiked 1 ng回收率/%
RecoveryRSD/% 回收率/%
RecoveryRSD/% 回收率/%
RecoveryRSD/% PBBz 102 3.2 88 6.2 94 4.1 PBT 86 3.7 85 4.9 79 2.6 PBEB 88 9.6 83 2.6 74 0.9 DPTE 123 2.6 120 5.5 106 3.5 HBBz 97 4.0 94 3.4 91 5.2 EHTBB 129 3.2 125 8.1 109 11.3 BTBPE 86 20.3 95 4.0 89 16.8 BEHTEBP 136 7.0 128 12.9 126 4.8 DBDPE 109 10.9 115 3.5 101 17.2 表 5 人体血清样品测试结果 (ng·mL−1)
Table 5. Results of human serum samples
化合物
Compound1 2 3 4 5 6 7 8 9 10 11 12 PBBz ND ND ND ND ND ND ND ND ND ND ND ND PBT ND ND ND ND ND ND ND ND ND ND ND ND PBEB ND ND ND ND ND ND ND ND ND ND ND ND DPTE ND ND ND ND ND ND ND ND ND ND ND ND HBBz ND ND ND ND ND ND ND ND ND ND ND ND EHTBB ND ND ND ND ND ND ND ND ND ND ND ND BTBPE ND ND ND ND ND ND ND ND ND ND ND ND BEHTEBP ND ND ND ND ND ND ND ND ND ND ND ND DBDPE 1.298 1.012 0.135 0.034 0.419 0.346 0.085 0.056 0.464 0.187 0.286 0.281 BDE-77 Recovery/% 85 78 90 87 93 85 96 92 72 85 84 85 BDE-138 Recovery/% 109 99 116 110 102 101 106 105 95 98 99 97 13C-DBDPE Recovery/% 117 95 126 80 74 73 100 75 94 88 90 77 ND:未检出. ND: Not detected. -
[1] SALAMOVA A, HERMANSON M H, HITES R A. Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site [J]. Environmental Science & Technology, 2014, 48(11): 6133-6140. [2] WANG Y W, SUN Y M, CHEN T, et al. Determination of polybrominated diphenyl ethers and novel brominated flame retardants in human serum by gas chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry [J]. Journal of Chromatography B, 2018, 1099: 64-72. doi: 10.1016/j.jchromb.2018.09.015 [3] SHI Z X, ZHANG L, LI J G, et al. Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment [J]. Chemosphere, 2018, 198: 522-536. doi: 10.1016/j.chemosphere.2018.01.161 [4] WANG J D, WANG Y W, SHI Z X, et al. Legacy and novel brominated flame retardants in indoor dust from Beijing, China: Occurrence, human exposure assessment and evidence for PBDEs replacement [J]. Science of the Total Environment, 2018, 618: 48-59. doi: 10.1016/j.scitotenv.2017.11.049 [5] XIAN H, HAO Y F, LV J Y, et al. Novel brominated flame retardants (NBFRs) in soil and moss in Mt. Shergyla, southeast Tibetan Plateau: Occurrence, distribution and influencing factors [J]. Environmental Pollution, 2021, 291: 118252. doi: 10.1016/j.envpol.2021.118252 [6] CHEN T, HUANG M R, LI J, et al. Polybrominated diphenyl ethers and novel brominated flame retardants in human milk from the general population in Beijing, China: Occurrence, temporal trends, nursing infants' exposure and risk assessment [J]. Science of the Total Environment, 2019, 689: 278-286. doi: 10.1016/j.scitotenv.2019.06.442 [7] ALI N, MEHDI T, MALIK R N, et al. Levels and profile of several classes of organic contaminants in matched indoor dust and serum samples from occupational settings of Pakistan [J]. Environmental Pollution, 2014, 193: 269-276. doi: 10.1016/j.envpol.2014.07.009 [8] WANG X C, LING S Y, GUAN K L, et al. Bioconcentration, biotransformation, and thyroid endocrine disruption of decabromodiphenyl ethane (DBDPE), A novel brominated flame retardant, in zebrafish larvae [J]. Environmental Science & Technology, 2019, 53(14): 8437-8446. [9] LI X Y, LIU J H, ZHOU G Q, et al. BDE-209 and DBDPE induce male reproductive toxicity through telomere-related cell senescence and apoptosis in SD rat [J]. Environment International, 2021, 146: 106307. doi: 10.1016/j.envint.2020.106307 [10] SUN Y M, WANG Y W, LIANG B L, et al. Hepatotoxicity of decabromodiphenyl ethane (DBDPE) and decabromodiphenyl ether (BDE-209) in 28-day exposed sprague-dawley rats [J]. Science of the Total Environment, 2020, 705: 135783. doi: 10.1016/j.scitotenv.2019.135783 [11] JING L, SUN Y M, WANG Y W, et al. Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats [J]. Chemosphere, 2019, 223: 675-685. doi: 10.1016/j.chemosphere.2019.02.115 [12] ZHAO X Z, CHEN T, YANG B, et al. Serum levels of novel brominated flame retardants (NBFRs) in residents of a major BFR-producing region: Occurrence, impact factors and the relationship to thyroid and liver function [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111467. doi: 10.1016/j.ecoenv.2020.111467 [13] LI J, CHEN T, WANG Y W, et al. Simple and fast analysis of tetrabromobisphenol A, hexabromocyclododecane isomers, and polybrominated diphenyl ethers in serum using solid-phase extraction or QuEChERS extraction followed by tandem mass spectrometry coupled to HPLC and GC [J]. Journal of Separation Science, 2017, 40(3): 709-716. doi: 10.1002/jssc.201600969 [14] ZHAO J P, WANG P, WANG C, et al. Novel brominated flame retardants in West Antarctic atmosphere (2011-2018): Temporal trends, sources and chiral signature [J]. Science of the Total Environment, 2020, 720: 137557. doi: 10.1016/j.scitotenv.2020.137557 [15] HAN X, MENG L L, LI Y M, et al. Associations between exposure to persistent organic pollutants and thyroid function in a case-control study of East China [J]. Environmental Science & Technology, 2019, 53(16): 9866-9875. [16] 中华人民共和国生态环境部. 环境监测分析方法标准制订技术导则: HJ 168—2020[S]. 北京: 中国环境科学出版社, 2020. Ministry of Ecology and Environment of the People's Republic of China. Technical guideline for the development of environmental monitoring analytical method standards: HJ 168—2020[S]. Beijing: China Environment Science Press, 2020 (in Chinese).
[17] LIANG S, XU F, TANG W B, et al. Brominated flame retardants in the hair and serum samples from an e-waste recycling area in southeastern China: The possibility of using hair for biomonitoring [J]. Environmental Science and Pollution Research International, 2016, 23(15): 14889-14897. doi: 10.1007/s11356-016-6491-x [18] CEQUIER E, MARCÉ R M, BECHER G, et al. Determination of emerging halogenated flame retardants and polybrominated diphenyl ethers in serum by gas chromatography mass spectrometry [J]. Journal of Chromatography A, 2013, 1310: 126-132. doi: 10.1016/j.chroma.2013.08.067 -



 下载:
下载: