-
纳米银(silver nanoparticles, AgNPs)具有优异抗菌性能,被广泛应用在纺织品、食品包装、医疗及净水设备等产品中[1–5]. 目前,AgNPs是商品化程度最高的纳米材料[6-7],占据全球纳米材料消费品市场50%以上份额,预计到2025年其市场规模将达到980亿美元[8].
随着AgNPs需求增加,在其生产、存储、使用及处置过程中,会不可避免地进入到环境中[9]. 例如可通过织物释放、污水灌溉、活性污泥施用及大气沉降等多种途径进入水体、沉积物和土壤[10–12],其潜在的生态环境风险受到广泛关注[8,13-14]. 已有研究表明,进入环境中的AgNPs可对鱼类、藻类、植物、土壤微生物及无脊椎动物等产生危害[13,15–17]. 水体中大部分AgNPs最终会进入沉积物和土壤介质[10], 在水流作用下发生迁移, 甚至进入地下水系统对饮用水安全构成威胁[18]. 因此,了解AgNPs的相关性质以及进入环境介质后的迁移规律,是科学评估AgNPs环境行为及生态安全的基础和前提. 大量研究表明AgNPs表面包覆层以及环境中的有机质、无机矿物及微生物是影响其迁移的重要因素. 本文针对近年来已发表的不同表面稳定剂、有机质、土壤矿物及微生物等对AgNPs性质以及在多孔介质中迁移产生的影响进行了全面的总结,并就目前研究中存在的问题和后续研究方向进行了展望.
-
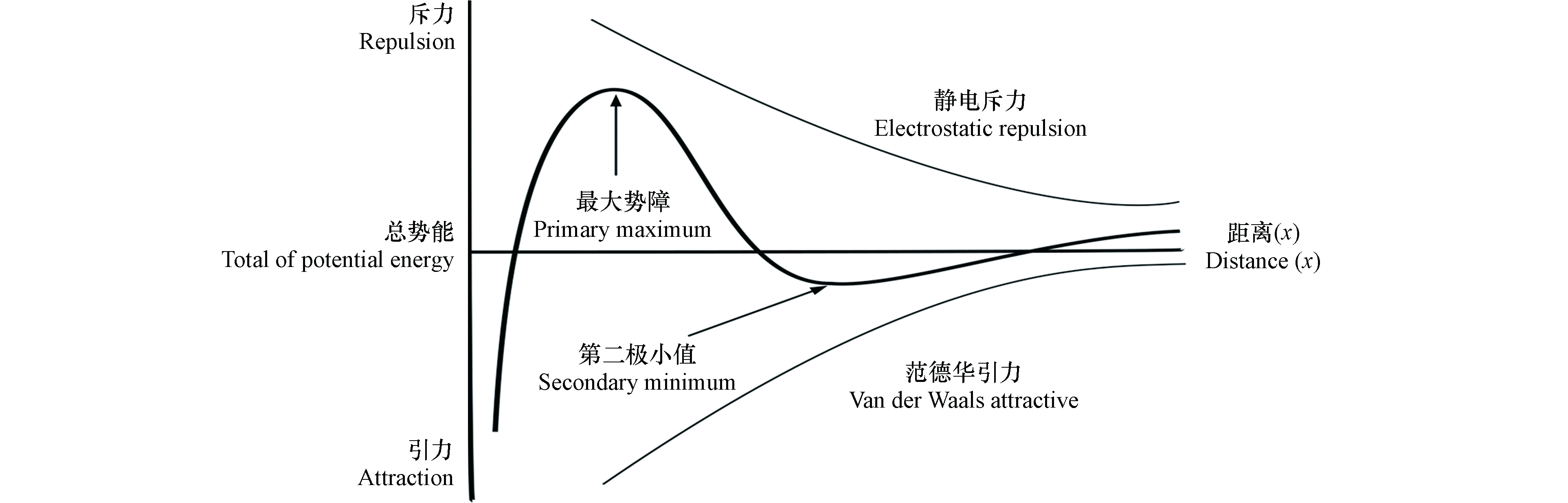
AgNPs在多孔介质中的行为通常包括两个过程:迁移(transport)和滞留(attachment). 迁移指AgNPs由溶液向固相颗粒表面传输过程,主要受AgNPs粒径、介质孔隙率和溶液流速等因素的影响. 滞留是指AgNPs附着在固相颗粒表面,主要受背景液、胶体表面以及介质表面化学性质的影响,这些因素可引起AgNPs和介质表面间作用力的变化,从而影响其在多孔介质表面的滞留. 经典的Derjaguin–Landau–Verwey–Overbeek(DLVO)理论包括范德华力和静电作用力(图1),可准确描述简单体系中AgNPs和多孔介质间的作用力大小. 当AgNPs从较远处向多孔介质表面靠近过程中,范德华力占据一定优势,总势能为负值,此时存在一个较浅的第二极小值(secondary minimum),AgNPs在此位置产生可逆沉降[19];当AgNPs 进一步向多孔介质表面靠近,静电斥力开始产生作用,此时总势能逐渐变为正值,并在一定位置时达到最大值(primary maximum);当AgNPs继续接近多孔介质表面,“跃过”最大势障后,总势能快速降为负值,这时引起AgNPs在多孔介质表面不可逆沉降[20]. 当AgNPs表面和石英砂介质表面存在大分子包覆物或特殊官能团时,必须考虑非DLVO作用力,如疏水力、空间位阻力等对AgNPs迁移的影响. 范德华力只与物质本身的性质有关,静电作用力受水化学条件和表面电荷影响最大,而疏水力、空间位阻力等分别与材料表面亲疏水基团和包覆层厚度等因素相关. 此外,AgNPs迁移过程中还可能存在阻隔作用(blocking)和截留作用(straining)[21-22]. 阻隔作用是指AgNPs沉降在多孔介质表面后,由于AgNPs间存在的斥力作用,抑制了后面AgNPs在多孔介质上的滞留。截留作用指AgNPs迁移过程中被多孔介质较小的空隙拦截,导致其滞留在介质中.
-
AgNPs具有较高的表面能和热力学不稳定性,易于相互聚集和交联团聚[24]. 为了控制AgNPs的尺寸、形貌,增强其稳定性,在制备过程中需加入一定量的稳定剂. 稳定剂通过巯基(—SH)、氰基(—CN)、羧基(—COOH)和氨基(—NH2)等基团结合在AgNPs表面[25],增强AgNPs间的静电斥力、空间位阻作用力以维持材料的稳定性和活性[26–28]. 静电作用稳定剂主要包括带电化合物,如硼氢化钠(sodium borohydride, NaBH4)、柠檬酸(citrate)以及表面活性剂类,如十六烷基三甲基氯化铵(hexadecyltrimethylammonium chloride, CTAC),十二烷基硫酸钠(sodium dodecyl sulfate, SDS)、十二烷基苯磺酸钠(sodium dodecyl benzene sulfonate, SDBS)等. 空间位阻作用稳定剂主要包括非离子型聚合物,如聚乙烯吡咯烷酮(polyvinylpyrrolidone, PVP),吐温20(Tween20);另外还包含可电离的大分子物质,如牛血清白蛋白(bovine serum albumin, BSA),支化聚乙烯亚胺(branched polyethyleneimine, BPEI)等,这些大分子物质除提供空间位阻作用力外还存在一定的静电作用力. 稳定剂具有不同的空间体积和表面官能团,可显著改变AgNPs表面的Zeta电位、亲疏水性、包覆层厚度等,进而影响其在环境中的稳定性以及迁移行为[29–31].
-
表面无稳定剂的AgNPs(Bare-AgNPs)释放到环境后稳定性降低,在多孔介质中具有较弱的迁移能力[10,32-33]. 本课题组研究[34]发现在低离子浓度下(0.5 mmol·L−1 KNO3),只有约8.6%的Bare-AgNPs可穿过石英砂多孔介质,大部分都滞留在石英砂柱中. 在迁移过程中Bare-AgNPs易发生团聚,通过截留作用,增加其在多孔介质中的滞留. 由于缺少稳定剂,Bare-AgNPs表面溶解产生的少量Ag+可与石英砂表面的硅烷醇(Si—OH)结合[35],进一步减弱其在石英砂多孔介质中的迁移. Bare-AgNPs表面虽然不含包覆层,但合成过程中通常会吸附溶液中一定量的负电荷离子,使其表面呈现电负性[36], 降低表面Zeta电位. 当多孔介质表面含有正电荷位点时,通过静电引力作用可限制Bare-AgNPs迁移,而在表面为负电荷的石英砂介质中迁移时,由于静电斥力作用,Bare-AgNPs仍具有一定的迁移能力[29,34].
-
静电稳定机制主要通过加入小分子带电化合物来改变AgNPs表面Zeta电位,增强AgNPs之间静电斥力,以达到稳定AgNPs的效果. 柠檬酸是较常用的AgNPs静电稳定剂[28,37-38]. 由于柠檬酸的去质子化,使柠檬酸包覆AgNPs(Citrate-AgNPs)表面具有较低的Zeta电位,因而具有比Bare-AgNPs更好的稳定性和迁移能力[10,39]. 同样,NaBH4稳定的AgNPs(NaBH4-AgNPs)在玻璃珠多孔介质中具有较强的迁移能力,经过2个孔体积(Pore volume, PV)即可达到穿透平衡[40]. 静电稳定剂包覆AgNPs间具有很强的静电斥力,可通过阻隔效应减少后进入的AgNPs在石英砂介质表面滞留[29],Park等[41]发现增加阴离子表面活性剂SDS浓度,可增强AgNPs稳定性以及阻隔效应,促进AgNPs的迁移.
-
空间位阻稳定剂主要通过大分子物质结合在AgNPs表面,为其提供物理位阻以减少颗粒间团聚. 一般认为,空间位阻作用比静电作用稳定的AgNPs具有更强的稳定性及迁移能力[29,33,42-43]. PVP是使用最为广泛的一种非离子型高分子稳定剂[28],通过其表面N和O元素结合在AgNPs表面[44]. 相同条件下,PVP-AgNPs在流出液中的最高浓度与流入液浓度之比(C/C0)(可达0.61, 而Citrate-AgNPs只有0.45[10]. 高素娟等[45]对比了PVP-AgNPs和Bare-AgNPs在天然湖水沉积物中长期(60 d)迁移,发现PVP-AgNPs在沉积物中的迁移能力显著高于Bare-AgNPs. 在土壤多孔介质中,Hoppe等[46]发现聚氧乙烯甘油三油酸酯和Tween20稳定的AgNPs比Citrate-AgNPs具有更强的稳定性和迁移能力. 另外,BSA稳定AgNPs(BSA-AgNPs)同样比Citrate-AgNPs具有更强的迁移能力[4]. 相对于静电稳定AgNPs,大分子包覆物在AgNPs和多孔介质间提供了较强的空间位阻效应,更有利于促进AgNPs迁移. 但部分研究发现,AgNPs表面包覆物可与多孔介质相互作用从而产生一定的滞留. 如PVP和石英砂介质上的Si—OH结合[44],使PVP-AgNPs在石英砂介质上产生一定的滞留[47],但由于PVP-AgNPs间较强空间位阻斥力,通过阻隔效应可以有效减少其在介质上滞留. 另外,离子型高聚物稳定剂BPEI包覆在AgNPs上(BPEI -AgNPs),不仅为其提供较强的空间位阻斥力,还由于胺基质子化使其表面带有较高正电荷[48]. BPEI-AgNPs在表面为负电荷的石英砂介质中依然具有极强迁移能力,主要由于BPEI-AgNPs间存在较强静电斥力和空间位阻,通过阻隔效用,可减少其在多孔介质中的滞留[29]. 虽然高聚物稳定剂为AgNPs提供了较强空间位阻斥力,但随着AgNPs的老化,AgNPs表面包覆层出现脱落. Zhu等[47]观察到PVP-AgNPs表面硫化后(S-AgNPs),PVP部分脱落,使AgNPs稳定性减弱,但形成的S-AgNPs表面Zeta电位降低,迁移能力增强.
稳定剂分子量及浓度的差异会影响AgNPs表面包覆层厚度及AgNPs和多孔介质间的结合. Wang等[49]发现增加溶液中PVP含量,可增强PVP-AgNPs在土壤多孔介质中的迁移能力,更高浓度PVP不仅在AgNPs和石英砂间提供更强空间位阻斥力,部分PVP还可和多孔介质表面滞留位点结合,减少PVP-AgNPs和多孔介质的结合. Lin等[30]也发现溶液中自由的稳定剂可以吸附在多孔介质表面,减弱AgNPs和多孔介质的结合,促进AgNPs迁移. Liang等[50]发现增加聚氧乙烯甘油三油酸酯和Tween20在AgNPs溶液中的含量,虽然对AgNPs在石英砂柱中的迁出率影响较小,但增加了柱中滞留AgNPs向下迁移能力,主要由于稳定剂占据了更多的滞留位点减小了AgNPs在石英砂介质上的最大滞留量. 另外,随着分子量的增加,稳定剂在AgNPs表面形成更厚的包覆层[51],提供更强空间位阻斥力. 例如,Lin[30]发现更高分子量(200 kDa)阿拉伯胶(Gum arabic, GA)比小分子量(10 kDa)PVP更能促进AgNPs的迁移.
-
pH的变化会影响AgNPs表面Zeta电位,从而影响AgNPs的迁移. Taghavy等[52]发现在pH=7条件下,约17%的Citrate-AgNPs滞留在石英砂柱中,而在酸性条件下(pH=4), AgNPs的滞留显著增加(88%),同时Citrate-AgNPs的溶解有所增加,约有6%的Citrate-AgNPs转化为Ag+. 在其它迁移实验中同样发现降低pH可增加Citrate-AgNPs在石英砂及土壤多孔介质中的滞留[53-54]. 在高pH条件下,Citrate-AgNPs表面Zeta电位下降,和多孔介质间静电斥力增加,促进了Citrate-AgNPs迁移;低pH条件下,由于柠檬酸羧基的质子化[53],使Citrate-AgNPs表面Zeta电位升高,减弱其与多孔介质间静电斥力,迁移能力降低. 另外,由于柠檬酸分子量较小(192 g·mol−1),无法在AgNPs间形成有效空间位阻效应[42],低pH条件下,AgNPs间静电斥力降低,易形成团聚并通过截留作用滞留在介质中[53]. 但也有研究发现NaBH4-AgNPs在纯玻璃珠多孔介质中迁移受pH影响并不明显,而在赤铁矿覆盖的玻璃珠多孔介质中,当溶液pH小于赤铁矿等电点(6.6—6.9)时,多孔介质表面带正电荷,通过静电作用降低了带负电荷NaBH4-AgNPs的迁移[40].
pH变化虽然可以影响静电稳定AgNPs的迁移,但在大部分情况下对空间位阻作用稳定AgNPs迁移的影响较小,例如,通过聚氧乙烯甘油三油酸酯和Tween20包覆的AgNPs在较低pH条件下(pH=5.2)依然保持较好的稳定性和较强的迁移能力[33]. pH的变化对PVP-AgNPs的稳定性几乎没有影响[55],且在多个pH条件下(pH=4、9 和11)都具有较强的迁移能力[56]. 而部分实验发现,聚氧乙烯甘油三油酸酯和Tween20包覆AgNPs在高pH下(pH=10)的迁出率可达86.3%,而低pH条件下(pH=4)只有0.2%。究其原因,较高的pH降低了AgNPs和石英砂介质表面的Zeta电位,两者静电斥力增加,促进了AgNPs迁移;另一方面,低pH时,多孔介质表面粗糙度对AgNPs滞留影响增加[57].
溶液中阳离子的屏蔽和压缩双电层效应可升高AgNPs表面Zeta电位,降低AgNPs之间以及AgNPs和多孔介质间静电斥力,使AgNPs更容易团聚和沉降,降低其迁移. Ellis等[58]发现Citrate-AgNPs在超纯水中具有良好的稳定性和迁移能力,但在硬水中(包含Na+、Ca2+、Mg2+、K+)易于发生团聚,迁移能力下降. Lin等[40]也发现NaBH4-AgNPs迁移能力随溶液中Na+增加而降低. 部分实验发现离子强度对空间位阻作用稳定AgNPs迁移影响并不明显. 例如,PVP-AgNPs在静态水柱和石英砂多孔介质迁移过程中,增加阳离子浓度,对其迁移影响很小[58-59],主要由于PVP-AgNPs在高离子强度下具有较强的稳定性. 但是,Thio等[42]发现较高的Na+离子浓度使PVP-AgNPs表面Zeta电位升高,产生团聚,使其在石英砂介质上的沉降增多. Ren和Smith[60]发现,溶液中K+浓度增至50 mg·L−1时,BSA-AgNPs在石英砂介质中的滞留量由24.3%增加到65.0%. 对于聚氧乙烯甘油三油酸酯和Tween20包覆AgNPs,增加阳离子浓度后,可减弱其在石英砂及土壤等多孔介质中的迁移[50,61-62].
高价阳离子对AgNPs迁移影响更明显[38,42]. 低离子强度下,可使Citrate-AgNPs和PVP-AgNPs发生团聚[46,49],增加AgNPs在土壤和石英砂多孔介质中的滞留[34,63]. 例如,1 mmol·L−1 的Ca2+条件下, Citrate-AgNPs在石英砂介质上的沉降显著增加[42],而聚氧乙烯甘油三油酸酯和Tween20包覆AgNPs滞留在砂壤土中的滞留可达96%以上[64]. 主要是由于高价阳离子(Ca2+)相对于一价阳离子具有更强的压缩双电层效应[65–67]. 此外,Ca2+可在PVP-AgNPs和石英砂介质表面形成架桥作用,进一步限制PVP-AgNPs的迁移[66]. 然而,聚氧乙烯甘油三油酸酯和Tween20包覆AgNPs在10 mmol·L−1 Ca2+条件下依然可以快速迁出[68],主要由于岩石多孔介质的空隙较大,溶液的流速较高.
-
地表环境介质(水、沉积物和土壤)中存在大量溶解性有机质(dissolved organic matter, DOM)[69–71],DOM表面官能团的去质子化过程使其表面带有一定量的负电荷,与AgNPs结合后可改变AgNPs表面性质及Zeta电位[31],影响AgNPs的迁移。海藻酸钠是一种天然阴离子多糖,在自然水环境下表面电位通常为负. Hou等[72]发现少量海藻酸钠吸附在Bare-AgNPs上,降低其表面Zeta电位,与石英砂间静电斥力增加,促进Bare-AgNPs迁移,而海藻酸钠吸附在PVP-AgNPs表面后增强了其疏水性,增加了PVP-AgNPs在石英砂表面的滞留. 阴离子表面活性剂SDS可和PVP也可发生作用,增加PVP-AgNPs表面Zeta电位绝对值,通过静电斥力作用促进PVP-AgNPs迁移. 当溶液中SDS浓度大于其临界胶束浓度(critical micelle concentration, CMC)时,对PVP-AgNPs迁移促进作用更明显,主要因为SDS形成胶束后在介质上滞留对PVP-AgNPs阻隔效应增强[44].
腐殖酸(humic acid, HA)等大分子有机质广泛分布于水体、沉积物以及土壤等各种地表环境介质中,表面含有大量长链烷烃、一定量的芳香基和羧基等官能团[73],不仅可以增强AgNPs表面静电斥力作用,同时还可吸附在AgNPs表面,表现出一定的空间位阻效应,影响AgNPs在多孔介质中迁移. Yang等[74]发现溶液中HA不仅可以和PVP结合, 减少PVP和石英砂结合,还可吸附到AgNPs内核表面,通过空间位阻和静电斥力共同作用增强PVP-AgNPs迁移能力. HA包覆在Citrate-AgNPs表面,增强了Citrate-AgNPs与土壤介质间的斥力作用及其稳定性[75],减少其在多孔介质中的截留. HA通过其表面羧基(COO—)结合在NaBH4-AgNPs表面[76],提供一定的空间位阻斥力作用,促进NaBH4-AgNPs迁移. Park等[41]发现HA可使SDS-AgNPs更加稳定,同时增加其和石英砂间的空间位阻斥力,促进SDS-AgNPs迁移,并且该促进作用随着HA浓度增加而增强. 溶解性黑碳(dissolved black carbon, DBC)在水体中普遍存在[77],其表面芳香微域结构和含氧官能团是有机物和重金属吸附/络合的高能位点[78],生物炭基DBC可吸附在AgNPs表面,通过空间位阻效应促进AgNPs迁移[34]. 同样,Degenkolb等[79]发现,在土壤中老化后的AgNPs表面含有大量土壤有机质(soil organic matter, SOM),为AgNPs提供强大的空间位阻效应,促进AgNPs迁移.
在有些实验中发现,DOM和AgNPs表面不同稳定剂间结合能力具有一定的差异性. Ellis等[58]发现溶液中加入萨旺尼河富里酸(suwannee river fulvic acid, SRFA)后,增强了PVP-AgNPs稳定性和迁移能力,而对Citrate-AgNPs的影响并不明显. DOM和PVP-AgNPs结合能力强,为其提供较强空间位阻和静电斥力作用,而和Citrate-AgNPs结合能力较弱. 另外,高价态阳离子存在时,使DOM和AgNPs间的作用更加复杂. Thio等[42]发现在含HA的AgNPs溶液中加入Ca2+后,由于Ca2+在AgNPs及介质表面起到架桥作用使其沉降增加. Wang等[80]发现溶液中的HA可通过Ca2+架桥作用和PVP-AgNPs结合,形成较大络合物(HA—Ca—PVP-AgNPs),减弱了PVP-AgNPs在黄泥土壤中的迁移. Akaighe等[32]也发现虽然HA和DOM吸附在AgNPs表面,增强其稳定性;但在Ca2+存在条件下,可和有机质表面的羧基或酚羟基结合,形成架桥作用增加PVP-AgNPs团聚,减弱其在石英砂多孔介质中的迁移.
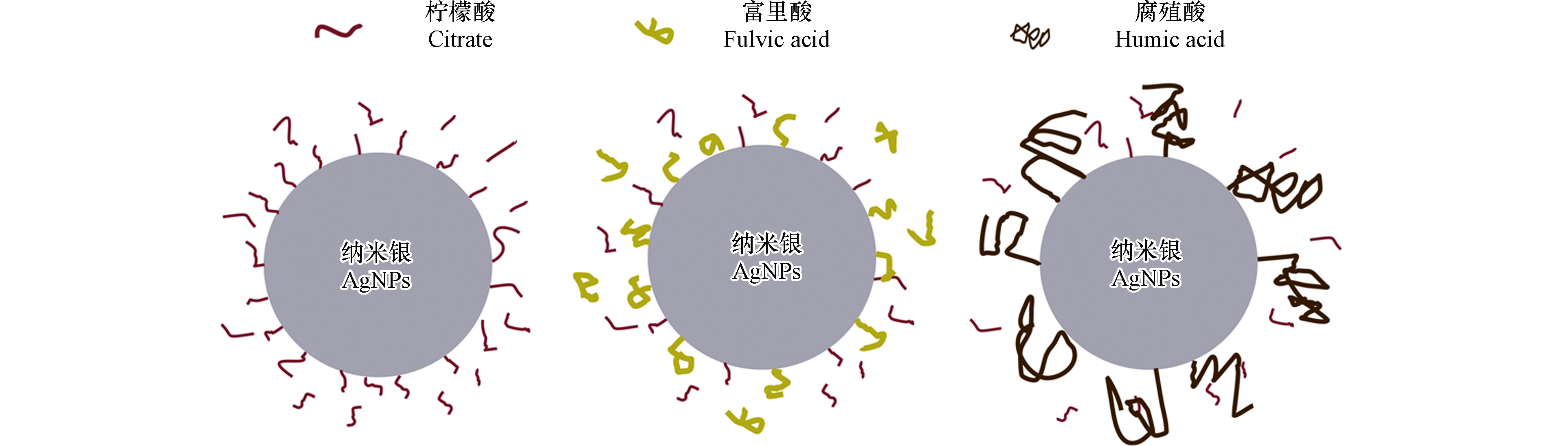
由于环境中有机质的竞争吸附,AgNPs表面稳定剂可被环境中有机质取代,进而影响其在多孔介质中的迁移. Yang等[44]发现SDBS、半胱氨酸以及乙二胺四乙酸(Ethylenediaminotetraacetic acid,EDTA)3种有机质可部分取代PVP-AgNPs表面PVP,影响其在多孔介质中的迁移. Chen等[66]发现在自然石英砂多孔介质中存在的硫化物也可取代PVP-AgNPs表面的PVP,取代后减弱了PVP-AgNPs的迁移能力. 溶液中HA和富里酸还可部分取代Citrate-AgNPs表面的柠檬酸(图2),减弱其稳定性[37],增加其在多孔介质中的滞留. 有些实验发现有机质虽然可以取代AgNPs表面稳定剂,但对AgNPs迁移的影响并不明显. 例如,Yang等[74]发现半胱氨酸通过表面巯基(—SH)和AgNPs结合,取代了PVP-AgNPs表面部分PVP,引起PVP-AgNPs团聚,但PVP-AgNPs在石英砂介质上的沉降并没有明显增加,主要是PVP-AgNPs水合粒径虽然增加,但在石英砂表面的沉降速率降低. 也有研究指出HA可以少量取代PVP-AgNPs上的PVP[47],由于置换量较少,对PVP-AgNPs迁移影响不明显。
-
在环境条件下,多孔介质表面附着大量有机质,这些表面有机质对AgNPs迁移产生重要影响[54]. Wang等[34]发现生物炭基DBC占据Bare-AgNPs在石英砂上的沉降位点后,可使Bare-AgNPs的C/C0达到90%左右. 溶液中HA同样可以覆盖石英砂介质表面AgNPs的沉降位点,促进PVP-AgNPs和Bare-AgNPs迁移,并且随着HA浓度增加,促进迁移效应更加明显[72]. 硫酸葡聚糖(Dextran sulfate)覆盖在石英砂表面(Dextran sulfate-Sand)后,介质表面的Zeta电位降至-90 mV左右,Citrate-AgNPs和多孔介质间的静电斥力增强,在介质上的滞留量减少. N-乙酰半胱氨酸和SDBS在多孔介质上的有利位点(铁氧化物)附近聚集,可减少PVP-AgNPs在介质上的沉降,同时还可增加PVP-AgNPs从多孔介质中再次释放的能力[44]. SOM也可以占据多孔介质表面的有利沉降位点并提供一定的空间位阻作用促进PVP-AgNPs迁移[80-81].
多孔介质表面的有机质可以和AgNPs或其表面的有机质结合,限制AgNPs在多孔介质中迁移[82]. Lau等[37]发现石英砂表面有机质可以和PVP的吡咯结构及氧原子发生络合作用,使PVP-AgNPs在表面含有HA及FA石英砂上的滞留量达328—440 ng·cm−2. Rahmatpour等[83]发现钙质土壤中有机质的含量与PVP-AgNPs在土壤多孔介质中的滞留正相关,主要是PVP羰基和土壤有机物极性官能团之间氢键及范德华力作用导致PVP-AgNPs滞留增加. 聚氧乙烯甘油三油酸酯和Tween20包覆AgNPs可通过与多孔介质表面有机质的羟基作用减弱其迁移能力[84]. 大量研究发现Ca2+可通过架桥作用使AgNPs和多孔介质表面有机质结合,影响AgNPs迁移. 例如,河水老化AgNPs表面含有的Ca2+可和石英砂介质表面DOM结合,限制AgNPs迁移[79]. 在水流侵蚀地表土壤过程中发现,Citrate-AgNPs在含SOM较高土壤中滞留较多,主要是因为土壤中存在大量Ca2+,使土壤表面有机质和Citrate-AgNPs结合,起到固定Citrate-AgNPs的作用.
有机质和多孔介质结合后可改变多孔介质表面的亲疏水性,并影响AgNPs在多孔介质中的迁移. Lau等[37]发现PVP-AgNPs在Dextran sulfate-Sand中的滞留量是Citrate-AgNPs的43倍,PVP-AgNPs的疏水性较强[30],而疏水作用可能是引起PVP-AgNPs滞留的重要原因. Song等[31]也发现相对于其它稳定剂包覆的AgNPs,PVP-AgNPs在十八烷基三氯硅烷覆盖的玻璃珠多孔介质滞留增加最明显,PVP和十八烷基三氯硅烷疏水作用同样增加了PVP-AgNPs的滞留. 另外,石英砂表面的SOM和AgNPs表面的Tween20也可通过疏水作用增加AgNPs在石英砂多孔介质中的滞留[84].
-
土壤等多孔介质表面存在铁氧化物、铝氧化物、黏土等矿物,这些矿物表面含有大量正电荷位点,通过静电作用会显著影响带负电荷的AgNPs迁移. 例如,钙质砂表面Zeta电位为正值,砂柱中加入钙质砂后使多孔介质表面Zeta电位升高,AgNPs和介质间静电斥力减小,增加了PVP-AgNPs滞留[84]. 玻璃多孔介质表面铁氧化物也通过静电吸引作用增加PVP-AgNPs滞留[66]. Tian等[18]发现石英砂介质表面金属矿物通过静电作用增加了SDBS-AgNPs滞留,且滞留受第二极小值控制,降低离子强度后滞留的SDBA-AgNPs仍可迁出. 红壤土中含有的针铁矿(goethite)不仅通过静电作用吸附PVP-AgNPs,并且形成AgNPs—goethite络合物增加其滞留[49]. Wang等[85]研究了AgNPs在我国10种类型表层土壤(0—20 cm)中的滞留情况,发现AgNPs在土壤中的滞留量和土壤铁氧化物含量正相关. 虽然大部分实验证明铁氧化物增加了AgNPs在多孔介质中的滞留,但Yang等[44]发现多孔介质被铁氧化物覆盖后减少了PVP-AgNPs表面PVP和石英砂的结合,从而促进了PVP-AgNPs的迁移. 除铁氧化物,土壤等多孔介质表面其它矿物同样会影响AgNPs的迁移. Wang等[80]对比了黏性红壤土(clay loam red soil)、砂壤土(sandy loam fluvo-aquic soil)和黄泥土(loam huangni soil)的3种土壤对PVP-AgNPs迁移的影响,发现PVP-AgNPs在粘性红壤土中的滞留量最高,红壤土中不仅含有较高的铁氧化物(5.1%),还有最多的黏土矿物(27%). Cornelis[81]研究了PVP-AgNPs在11中天然土壤中的迁移,发现PVP-AgNPs的滞留量和土壤中的铁氧化物及铝氧化物正相关, 主要由于铁氧化物或黏土矿物边缘位置吸附了更多的PVP-AgNPs. 除PVP-AgNPs外,黏土矿物还可增加Citrate-AgNPs、Bare-AgNPs以及聚氧乙烯甘油三油酸酯和Tween20包裹AgNPs在多孔介质中的滞留[46,84,86]. Hedberg等[33]发现黏土对Bare-AgNPs和聚氧乙烯甘油三油酸酯和Tween20包裹AgNPs有极强的吸附能力,经过48h,可吸附99%以上的AgNPs.
AgNPs或其表面稳定剂与矿物相互作用是影响其滞留的另一个重要因素. 以PVP-AgNPs为例,PVP分子上吡咯环羰基和黏土矿表面羟基通过氢键作用结合可增加PVP-AgNPs在多孔介质上的滞留[29,49,81,83]. 另外,黏土成分容易结合二价阳离子(Ca2+),并形成Clay—Ca—PVP-AgNPs,增加PVP-AgNPs在多孔介质中滞留[49,62]. 多孔介质表面的矿物还可和AgNPs形成异质团聚从而影响AgNPs在多孔介质中的滞留. 磁赤铁矿和PVP-AgNPs可形成异质团聚,比单独PVP-AgNPs的团聚程度要高[81],可影响PVP-AgNPs在介质中的迁移. Rahmatpour等[83]还发现土壤表面黏土矿物和PVP-AgNPs可形成异质团聚,增加了PVP-AgNPs的滞留. 聚氧乙烯甘油三油酸酯和Tween20包裹AgNPs在黏土中的迁移能力减弱,也是由于AgNPs和黏土形成了异质团聚[87].
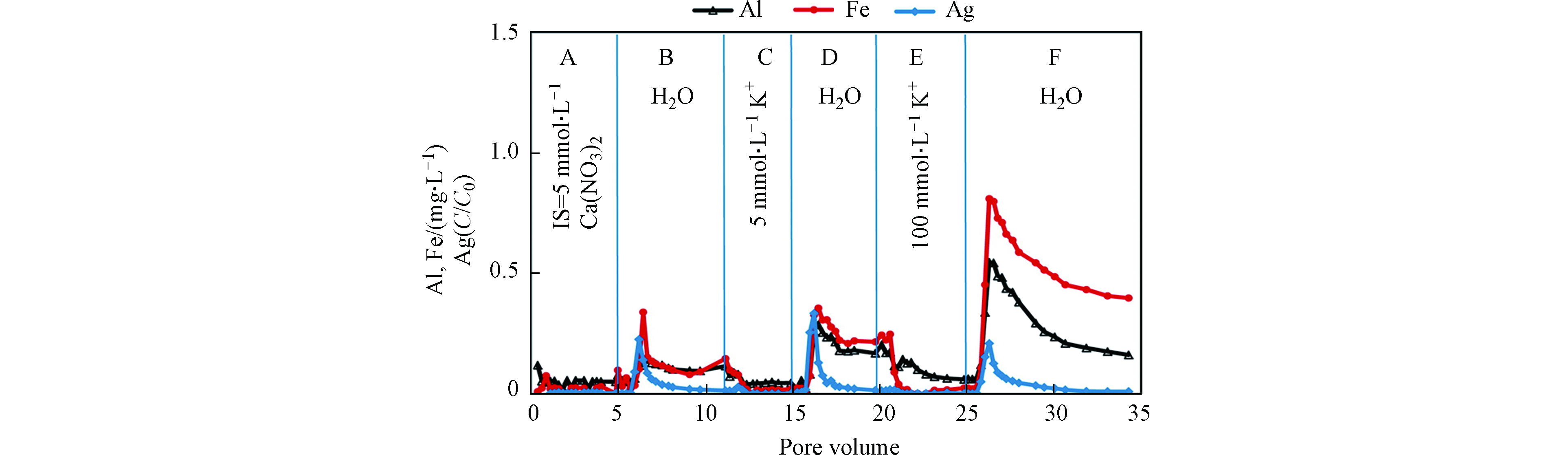
在迁移过程中,多孔介质表面无机矿物和AgNPs形成的异质团聚体共迁移. Degenkolb等[79]在Citrate-AgNPs迁移实验中发现,迁出溶液中AgNPs含量和Al含量呈正比,Hoppe等[87]也在聚氧乙烯甘油三油酸酯和Tween20包覆AgNPs的土柱释放实验中发现,迁出溶液中AgNPs和Al的释放量正相关. 产生以上现象的主要原因是AgNPs和黏土形成了异质团聚从土壤表面脱落,增加了AgNPs的迁移. 改变迁移柱中水化学条件也可影响无机矿物和AgNPs异质团聚的释放,例如,Liang等[61]在柱实验中发现通过离子交换或降低离子强度,可使黏土矿物及铁氧化物从土壤多孔介质上释放,并和AgNPs共迁出(图3). Makselon等[62]发现当溶液中的离子强度由1 mmol·L−1 降低到 0.2 mmol·L−1时,迁出溶液中可以检测到更多的Al和Fe,表明壤质砂土中的矿物胶体从土柱中释放并作为AgNPs的载体共同迁移. 另外,通过改变土柱的物理因素,如孔隙水流速,同样可以促进土壤矿物胶体和AgNPs结合体迁出[88].
在部分实验中发现,多孔介质表面矿物对AgNPs滞留的影响较小. 例如,Adrian等[65]发现多孔介质中加入黏土矿物对表面活性剂包裹AgNPs的迁移几乎没有影响,主要由于表面活性剂包裹AgNPs表面Zeta电位较低,和多孔介质间静电斥力较强. 多孔介质中含有水铁矿或高岭土对BPEI-AgNPs迁移几乎没有影响[29],主要是BPEI-AgNPs表面Zeta电位为正,无机矿物表面正电荷对其迁移影响较小,另外,由于静电和空间位阻作用力的存在,使BPEI-AgNPs间具有极强的斥力作用,通过介质表面的阻隔效应,减弱了多孔介质表面矿物对其迁移的影响.
-
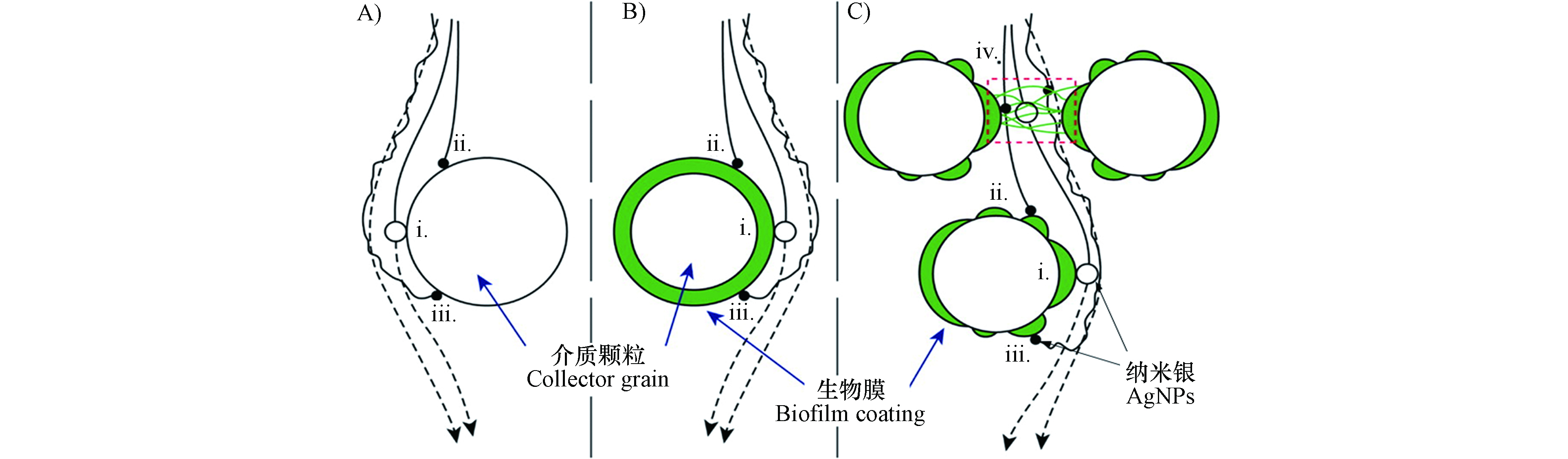
土壤微生物可通过胞外聚合物(Extracellular polymeric substances, EPS)或生物膜影响AgNPs在多孔介质中的迁移. 据报道铜绿假单胞菌(Pseudomonas aeruginosa PAO1)生物膜覆盖的石英砂相对于洁净石英砂更能促进PVP-AgNPs迁移[59], 主要由于细菌产生的EPS和PVP-AgNPs之间产生了更多的静电和空间位阻斥作用(图4). 相对于无生物膜的石英砂,PVP-AgNPs在覆盖生物膜的石英砂柱中的穿透曲线斜率较高,并且随着生物膜的生长穿透曲线斜率明显上升,可能是生物膜对PVP-AgNPs迁移形成了尺寸排阻效应(Size exclusion). 但在有些研究中发现生物膜或EPS限制了AgNPs的迁移. Degenkolb等[79]发现天然池塘中的过滤柱表面生物膜限制了Citrate-AgNPs迁移,主要是石英砂表面生物膜对Citrate-AgNPs吸附的结果. 在研究革兰氏阴性大肠杆菌(Escherichia coli,E.coli)和PVP-AgNPs共迁移实验中发现,大肠杆菌表面存在大量羧基及膦酸酯官能团,表面Zeta电位为负,自身在多孔介质中具有较强的迁移能力[69,89],但大肠杆菌却增加了PVP-AgNPs在玻璃多孔介质中的滞留,主要由于PVP-AgNPs在大肠杆菌上的吸附很少,大肠杆菌释放的EPS升高了多孔介质表面Zeta电位使PVP-AgNPs和多孔介质间的静电斥力减弱[66],另外多孔介质上EPS在PVP-AgNPs和多孔介质间起到架桥作用,限制了PVP-AgNPs迁移. 而在一些研究中发现,生物膜对AgNPs迁移的影响具有一定的选择性,Xiao等[38]研究了Citrate-AgNPs和PVP-AgNPs两种不同稳定剂包覆AgNPs在革兰氏阴性铜绿假单胞菌(Gram-negative Pseudomonas aeruginosa)和革兰氏阳性蜡样芽孢杆菌(Gram-positive Bacillus cereus)生物膜覆盖玻璃多孔介质中的迁移,在无阳离子存在时,两种AgNPs的滞留几乎不受生物膜的影响,而在Ca2+存在条件下,只有Citrate-AgNPs在石英砂介质滞留量增加,PVP-AgNPs由于具有较强稳定性,生物膜对其迁移几乎没有影响.
-
稳定剂包覆AgNPs后,使其表面具有不同的表面官能团和包覆厚度,可显著改变AgNPs的表面Zeta电位、亲疏水性等,这些性质是影响AgNPs迁移行为的重要因素,且不同类型稳定剂和水环境化学条件使AgNPs表面性质和稳定性产生差异,影响AgNPs进入环境后的迁移行为. 地表环境介质中含有大量的溶解性有机质、土壤胶体矿物以及土壤微生物,而这些物质又和AgNPs或其表面包覆物相互作用,进一步影响了AgNPs 在土壤多孔介质中的迁移.
尽管近年来关于AgNPs在土壤等多孔介质中的迁移行为受到越来越多的关注,但这些研究依然存在一些不足,还有诸多问题需要进一步研究和解决. 在研究中使用的AgNPs大部分是实验室自行合成,不同的合成方法会造成AgNPs形貌、粒径、残留物以及包覆物在AgNPs表面分布等方面的差异,而这些差异会影响AgNPs的迁移,进而影响不同AgNPs迁移实验间数据对比,甚至产生矛盾的结论. 现有实验选用的多孔介质主要是以洁净的石英砂为主,而天然石英砂或土壤表面通常含有有机质、矿物以及微生物膜等物质,多孔介质表面这些物质对AgNPs迁移起着非常重要的作用. 在研究AgNPs在多孔介质迁移实验的设计中,大部分实验周期较短,通常忽略AgNPs迁移过程中的转化问题,而AgNPs在土壤等环境多孔介质中的迁移实际上是一个长期的过程,在这个过程中AgNPs极有可能会发生转化,AgNPs的转化必然影响其在多孔介质中的迁移. 现有的表征技术如X射线显微断层扫描虽然可对AgNPs在柱中的分布和转化进行原位表征,但很难做到动态跟踪AgNPs在柱中的迁移情况. 另外,石英晶体微天平虽然可以实时观察AgNPs在介质表面的结合、分布等情况,但很难真实地考察多孔介质异质性、粒径分布以及截留等作用对AgNPs迁移的影响. 因此,对AgNPs迁移实验最好制定标准的材料或者合成方法,最好选择真实环境中的多孔介质(例如,原位土壤)来进行AgNPs迁移实验的研究,另外应该开发新的研究方法或者表征技术,以便更好地观察AgNPs在迁移过程中的转化等情况.
纳米银在多孔介质中的迁移过程与机制研究进展
Research progress on the transport process and mechanism of silver nanoparticles in porous media
-
摘要: 纳米银(AgNPs)作为消费品中最常用的人工纳米材料,由于其优异的抗菌性能,在织物、医疗设备和食品及饮料包装中广泛使用. AgNPs可通过大气沉降、地表水径流、污水灌溉和生物污泥的土地施用等多种途径进入土壤等多孔介质,甚至进入地下水. AgNPs进入环境后可在动物和植物体内累积并产生毒性效应,对生态环境构成危害. 因此,全面了解AgNPs在土壤等多孔介质中的迁移过程对正确评估其环境归趋和生态效应具有重要的理论和现实意义. 本文针对近年来已发表的不同表面稳定剂、环境有机质、土壤矿物及微生物等对AgNPs性质及在多孔介质中迁移过程中产生的影响进行了全面的总结,并就目前研究中存在的问题和后续研究的发展方向进行了展望.Abstract: Silver nanoparticles (AgNPs) are the most commonly used engineered nanomaterials in consumer products, serving primarily as antimicrobial agents in fabrics, medical devices and food and beverage packaging. AgNPs enter the soil via multiple pathways, including atmospheric deposition, stormwater runoff, wastewater irrigation and land application of wastewater treatment biosolids. They are potential to migrate in porous media into ground water. Additionally, they may accumulate in plants and animals, and then pose potential threats to ecological environment. Thus, it is of critical significance to thoroughly understand the transport of AgNPs in soils and to assess their environmental behaviors, fate, and ecological effects. This article curates the related references which have been published in recent years, and summarizes the effects of different stabilizing agents, organic matters, soil minerals and microorganisms on the properties of AgNPs and their transport in porous media. Finally, the advancement direction was prospected.
-
Key words:
- AgNPs /
- stabilizing agents /
- porous media /
- transport
-

-
-
[1] BENN T M, WESTERHOFF P. Nanoparticle silver released into water from commercially available sock fabrics [J]. Environmental Science & Technology, 2008, 42(11): 4133-4139. [2] DAVIES R L, ETRIS S F. The development and functions of silver in water purification and disease control [J]. Catalysis Today, 1997, 36(1): 107-114. doi: 10.1016/S0920-5861(96)00203-9 [3] LIU J F, JIANG G B. Silver Nanoparticles in the Environment[M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 2015. [4] REN D J, SMITH J A. Retention and transport of silver nanoparticles in a ceramic porous medium used for point-of-use water treatment [J]. Environmental Science & Technology, 2013, 47(8): 3825-3832. [5] TALEBIAN S, WALLACE G G, SCHROEDER A, et al. Nanotechnology-based disinfectants and sensors for SARS-CoV-2 [J]. Nature Nanotechnology, 2020, 15(8): 618-621. doi: 10.1038/s41565-020-0751-0 [6] HANSEN S F, HANSEN O F H, NIELSEN M B. Advances and challenges towards consumerization of nanomaterials [J]. Nature Nanotechnology, 2020, 15(12): 964-965. doi: 10.1038/s41565-020-00819-7 [7] PATIÑO J E, KUHL T L, MORALES V L. Direct measurements of the forces between silver and Mica in humic substance-rich solutions [J]. Environmental Science & Technology, 2020, 54(23): 15076-15085. [8] TEMIZEL-SEKERYAN S, HICKS A L. Emerging investigator series: Calculating size- and coating-dependent effect factors for silver nanoparticles to inform characterization factor development for usage in life cycle assessment [J]. Environmental Science:Nano, 2020, 7(9): 2436-2453. doi: 10.1039/D0EN00675K [9] LIU C, LENG W N, VIKESLAND P J. Controlled evaluation of the impacts of surface coatings on silver nanoparticle dissolution rates [J]. Environmental Science & Technology, 2018, 52(5): 2726-2734. [10] HE J Z, WANG D J, ZHOU D M. Transport and retention of silver nanoparticles in soil: Effects of input concentration, particle size and surface coating [J]. Science of the Total Environment, 2019, 648: 102-108. doi: 10.1016/j.scitotenv.2018.08.136 [11] PACHAPUR V L, LARIOS A D, CLEDÓN M, et al. Behavior and characterization of titanium dioxide and silver nanoparticles in soils [J]. Science of the Total Environment, 2016, 563/564: 933-943. doi: 10.1016/j.scitotenv.2015.11.090 [12] TOURINHO P S, van GESTEL C A M, LOFTS S, et al. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates [J]. Environmental Toxicology and Chemistry, 2012, 31(8): 1679-1692. doi: 10.1002/etc.1880 [13] MARAMBIO-JONES C, HOEK E M V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment [J]. Journal of Nanoparticle Research, 2010, 12(5): 1531-1551. doi: 10.1007/s11051-010-9900-y [14] ROELOFS D, MAKAMA S, de BOER T E, et al. Surface coating and particle size are main factors explaining the transcriptome-wide responses of the earthworm Lumbricus rubellus to silver nanoparticles [J]. Environmental Science:Nano, 2020, 7(4): 1179-1193. doi: 10.1039/C9EN01144G [15] BLASER S A, SCHERINGER M, MACLEOD M, et al. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles [J]. Science of the Total Environment, 2008, 390(2/3): 396-409. [16] FORSTNER C, ORTON T G, WANG P, et al. Wastewater treatment processing of silver nanoparticles strongly influences their effects on soil microbial diversity [J]. Environmental Science & Technology, 2020, 54(21): 13538-13547. [17] 王震宇, 赵建, 李娜, 等. 人工纳米颗粒对水生生物的毒性效应及其机制研究进展 [J]. 环境科学, 2010, 31(6): 1409-1418. WANG Z Y, ZHAO J, LI N, et al. Review of ecotoxicity and mechanism of engineered nanoparticles to aquatic organisms [J]. Environmental Science, 2010, 31(6): 1409-1418(in Chinese).
[18] TIAN Y, GAO B, SILVERA-BATISTA C, et al. Transport of engineered nanoparticles in saturated porous media [J]. Journal of Nanoparticle Research, 2010, 12(7): 2371-2380. doi: 10.1007/s11051-010-9912-7 [19] REDMAN J A, WALKER S L, ELIMELECH M. Bacterial adhesion and transport in porous media: Role of the secondary energy minimum [J]. Environmental Science & Technology, 2004, 38(6): 1777-1785. [20] SHEN C Y, LI B G, HUANG Y F, et al. Kinetics of coupled primary- and secondary-minimum deposition of colloids under unfavorable chemical conditions [J]. Environmental Science & Technology, 2007, 41(20): 6976-6982. [21] BRADFORD S A, SIMUNEK J, BETTAHAR M, et al. Modeling colloid attachment, straining, and exclusion in saturated porous media [J]. Environmental Science & Technology, 2003, 37(10): 2242-2250. [22] BECKER M D, WANG Y G, PENNELL K D, et al. A multi-constituent site blocking model for nanoparticle and stabilizing agent transport in porous media [J]. Environmental Science:Nano, 2015, 2(2): 155-166. doi: 10.1039/C4EN00176A [23] HAHN M W, O'MELIAE C R. Deposition and reentrainment of Brownian particles in porous media under unfavorable chemical conditions: Some concepts and applications [J]. Environmental Science & Technology, 2004, 38(1): 210-220. [24] OLENIN A Y, KRUTYAKOV Y A, KUDRINSKII A A, et al. Formation of surface layers on silver nanoparticles in aqueous and water-organic media [J]. Colloid Journal, 2008, 70(1): 71-76. doi: 10.1134/S1061933X08010110 [25] SI S, MANDAL T K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study [J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2007, 13(11): 3160-3168. [26] AMRI N E, ROGER K. Polyvinylpyrrolidone (PVP) impurities drastically impact the outcome of nanoparticle syntheses [J]. Journal of Colloid and Interface Science, 2020, 576: 435-443. doi: 10.1016/j.jcis.2020.04.113 [27] PRYSHCHEPA O, POMASTOWSKI P, BUSZEWSKI B. Silver nanoparticles: Synthesis, investigation techniques, and properties [J]. Advances in Colloid and Interface Science, 2020, 284: 102246. doi: 10.1016/j.cis.2020.102246 [28] TOLAYMAT T M, EL BADAWY A M, GENAIDY A, et al. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers [J]. Science of the Total Environment, 2010, 408(5): 999-1006. doi: 10.1016/j.scitotenv.2009.11.003 [29] EL BADAWY A M, HASSAN A A, SCHECKEL K G, et al. Key factors controlling the transport of silver nanoparticles in porous media [J]. Environmental Science & Technology, 2013, 47(9): 4039-4045. [30] LIN S H, CHENG Y W, LIU J, et al. Polymeric coatings on silver nanoparticles hinder autoaggregation but enhance attachment to uncoated surfaces [J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2012, 28(9): 4178-4186. doi: 10.1021/la202884f [31] SONG J E, PHENRAT T, MARINAKOS S, et al. Hydrophobic interactions increase attachment of gum Arabic- and PVP-coated Ag nanoparticles to hydrophobic surfaces [J]. Environmental Science & Technology, 2011, 45(14): 5988-5995. [32] AKAIGHE N, DEPNER S W, BANERJEE S, et al. Transport and deposition of Suwannee River Humic Acid/Natural Organic Matter formed silver nanoparticles on silica matrices: The influence of solution pH and ionic strength [J]. Chemosphere, 2013, 92(4): 406-412. doi: 10.1016/j.chemosphere.2012.12.077 [33] HEDBERG J, OROMIEH A G, KLEJA D B, et al. Sorption and dissolution of bare and coated silver nanoparticles in soil suspensions: Influence of soil and particle characteristics [J]. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 2015, 50(9): 891-900. [34] WANG K K, ZHANG Y Q, SUN B B, et al. New insights into the enhanced transport of uncoated and polyvinylpyrrolidone-coated silver nanoparticles in saturated porous media by dissolved black carbons [J]. Chemosphere, 2021, 283: 131159. doi: 10.1016/j.chemosphere.2021.131159 [35] QUANG D V, SARAWADE P B, HILONGA A, et al. Preparation of silver nanoparticle containing silica micro beads and investigation of their antibacterial activity [J]. Applied Surface Science, 2011, 257(15): 6963-6970. doi: 10.1016/j.apsusc.2011.03.041 [36] CHENG K L. The negative charge of nanoparticles [J]. Microchemical Journal, 2006, 82(1): 119-120. doi: 10.1016/j.microc.2005.11.002 [37] LAU B L T, HOCKADAY W C, IKUMA K, et al. A preliminary assessment of the interactions between the capping agents of silver nanoparticles and environmental organics [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2013, 435: 22-27. [38] XIAO Y, WIESNER M R. Transport and retention of selected engineered nanoparticles by porous media in the presence of a biofilm [J]. Environmental Science & Technology, 2013, 47(5): 2246-2253. [39] SAGEE O, DROR I, BERKOWITZ B. Transport of silver nanoparticles (AgNPs) in soil [J]. Chemosphere, 2012, 88(5): 670-675. doi: 10.1016/j.chemosphere.2012.03.055 [40] LIN S H, CHENG Y W, BOBCOMBE Y, et al. Deposition of silver nanoparticles in geochemically heterogeneous porous media: Predicting affinity from surface composition analysis [J]. Environmental Science & Technology, 2011, 45(12): 5209-5215. [41] PARK C M, HEO J, HER N, et al. Modeling the effects of surfactant, hardness, and natural organic matter on deposition and mobility of silver nanoparticles in saturated porous media [J]. Water Research, 2016, 103: 38-47. doi: 10.1016/j.watres.2016.07.022 [42] THIO B J R, MONTES M O, MAHMOUD M A, et al. Mobility of capped silver nanoparticles under environmentally relevant conditions [J]. Environmental Science & Technology, 2012, 46(13): 6985-6991. [43] ZHANG H, ZHANG C. Transport of silver nanoparticles capped with different stabilizers in water saturated porous media [J]. Journal of Materials and Environmental Science, 2014, 5(1): 231-236. [44] YANG X Y, YIN Z Y, CHEN F M, et al. Organic matter induced mobilization of polymer-coated silver nanoparticles from water-saturated sand [J]. Science of the Total Environment, 2015, 529: 182-190. doi: 10.1016/j.scitotenv.2015.05.066 [45] 高素娟, 方涛, 王广召, 等. 纳米银在水-沉积物中的迁移机制研究 [J]. 水生生物学报, 2015, 39(2): 375-381. doi: 10.7541/2015.49 GAO S J, FANG T, WANG G Z, et al. The transportation of silver nanoparticles between water and sediments [J]. Acta Hydrobiologica Sinica, 2015, 39(2): 375-381(in Chinese). doi: 10.7541/2015.49
[46] HOPPE M, MIKUTTA R, UTERMANN J, et al. Retention of sterically and electrosterically stabilized silver nanoparticles in soils [J]. Environmental Science & Technology, 2014, 48(21): 12628-12635. [47] ZHU T R, LAWLER D F, CHEN Y Q, et al. Effects of natural organic matter and sulfidation on the flocculation and filtration of silver nanoparticles [J]. Environmental Science:Nano, 2016, 3(6): 1436-1446. doi: 10.1039/C6EN00266H [48] EL BADAWY A M, SCHECKEL K G, SUIDAN M, et al. The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles [J]. Science of the Total Environment, 2012, 429: 325-331. doi: 10.1016/j.scitotenv.2012.03.041 [49] WANG D J, GE L Q, HE J Z, et al. Hyperexponential and nonmonotonic retention of polyvinylpyrrolidone-coated silver nanoparticles in an Ultisol [J]. Journal of Contaminant Hydrology, 2014, 164: 35-48. doi: 10.1016/j.jconhyd.2014.05.007 [50] LIANG Y, BRADFORD S A, SIMUNEK J, et al. Sensitivity of the transport and retention of stabilized silver nanoparticles to physicochemical factors [J]. Water Research, 2013, 47(7): 2572-2582. doi: 10.1016/j.watres.2013.02.025 [51] KYRYCHENKO A, KORSUN O M, GUBIN I I, et al. Atomistic simulations of coating of silver nanoparticles with poly(vinylpyrrolidone) oligomers: Effect of oligomer chain length [J]. The Journal of Physical Chemistry C, 2015, 119(14): 7888-7899. doi: 10.1021/jp510369a [52] TAGHAVY A, MITTELMAN A, WANG Y G, et al. Mathematical modeling of the transport and dissolution of citrate-stabilized silver nanoparticles in porous media [J]. Environmental Science & Technology, 2013, 47(15): 8499-8507. [53] KUMAHOR S K, HRON P, METREVELI G, et al. Transport of citrate-coated silver nanoparticles in unsaturated sand [J]. Science of the Total Environment, 2015, 535: 113-121. doi: 10.1016/j.scitotenv.2015.03.023 [54] KUMAHOR S K, HRON P, METREVELI G, et al. Transport of soil-aged silver nanoparticles in unsaturated sand [J]. Journal of Contaminant Hydrology, 2016, 195: 31-39. doi: 10.1016/j.jconhyd.2016.10.001 [55] EL BADAWY A M, LUXTON T P, SILVA R G, et al. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions [J]. Environmental Science & Technology, 2010, 44(4): 1260-1266. [56] FLORY J, KANEL S R, RACZ L, et al. Influence of pH on the transport of silver nanoparticles in saturated porous media: Laboratory experiments and modeling [J]. Journal of Nanoparticle Research, 2013, 15(3): 1484. doi: 10.1007/s11051-013-1484-x [57] LIANG Y, ZHOU J N, DONG Y W, et al. Evidence for the critical role of nanoscale surface roughness on the retention and release of silver nanoparticles in porous media [J]. Environmental Pollution, 2020, 258: 113803. doi: 10.1016/j.envpol.2019.113803 [58] ELLIS L J A, VALSAMI-JONES E, LEAD J R, et al. Impact of surface coating and environmental conditions on the fate and transport of silver nanoparticles in the aquatic environment [J]. Science of the Total Environment, 2016, 568: 95-106. doi: 10.1016/j.scitotenv.2016.05.199 [59] MITZEL M R, TUFENKJI N. Transport of industrial PVP-stabilized silver nanoparticles in saturated quartz sand coated with Pseudomonas aeruginosa PAO1 biofilm of variable age [J]. Environmental Science & Technology, 2014, 48(5): 2715-2723. [60] REN D J, SMITH J A. Proteinate-capped silver nanoparticle transport in water-saturated sand [J]. Journal of Environmental Engineering, 2013, 139(6): 781-787. doi: 10.1061/(ASCE)EE.1943-7870.0000684 [61] LIANG Y, BRADFORD S A, SIMUNEK J, et al. Retention and remobilization of stabilized silver nanoparticles in an undisturbed loamy sand soil [J]. Environmental Science & Technology, 2013, 47(21): 12229-12237. [62] MAKSELON J, ZHOU D, ENGELHARDT I, et al. Experimental and numerical investigations of silver nanoparticle transport under variable flow and ionic strength in soil [J]. Environmental Science & Technology, 2017, 51(4): 2096-2104. [63] YECHESKEL Y, DROR I, BERKOWITZ B. Silver nanoparticle (Ag-NP) retention and release in partially saturated soil: Column experiments and modelling [J]. Environmental Science:Nano, 2018, 5(2): 422-435. doi: 10.1039/C7EN00990A [64] BRAUN A, KLUMPP E, AZZAM R, et al. Transport and deposition of stabilized engineered silver nanoparticles in water saturated loamy sand and silty loam [J]. Science of the Total Environment, 2015, 535: 102-112. doi: 10.1016/j.scitotenv.2014.12.023 [65] ADRIAN Y F, SCHNEIDEWIND U, BRADFORD S A, et al. Transport and retention of surfactant- and polymer-stabilized engineered silver nanoparticles in silicate-dominated aquifer material [J]. Environmental Pollution, 2018, 236: 195-207. doi: 10.1016/j.envpol.2018.01.011 [66] CHEN F M, YUAN X M, SONG Z F, et al. Gram-negative Escherichia coli promotes deposition of polymer-capped silver nanoparticles in saturated porous media [J]. Environmental Science:Nano, 2018, 5(6): 1495-1505. doi: 10.1039/C8EN00067K [67] XIA T J, QI Y, LIU J, et al. Cation-inhibited transport of graphene oxide nanomaterials in saturated porous media: The hofmeister effects [J]. Environmental Science & Technology, 2017, 51(2): 828-837. [68] NEUKUM C, BRAUN A, AZZAM R. Transport of engineered silver (Ag) nanoparticles through partially fractured sandstones [J]. Journal of Contaminant Hydrology, 2014, 164: 181-192. doi: 10.1016/j.jconhyd.2014.05.012 [69] BRADFORD S A, SIMUNEK J, WALKER S L. Transport and straining of E. coli O157: H7 in saturated porous media[J]. Water Resources Research, 2006, 42(12),doi:10.1029/2005WR004805, 2006. [70] JEREZ J, FLURY M, SHANG J Y, et al. Coating of silica sand with aluminosilicate clay [J]. Journal of Colloid and Interface Science, 2006, 294(1): 155-164. doi: 10.1016/j.jcis.2005.07.017 [71] SALEH N B, PFEFFERLE L D, ELIMELECH M. Influence of biomacromolecules and humic acid on the aggregation kinetics of single-walled carbon nanotubes [J]. Environmental Science & Technology, 2010, 44(7): 2412-2418. [72] HOU J, ZHANG M Z, WANG P F, et al. Transport, retention, and long-term release behavior of polymer-coated silver nanoparticles in saturated quartz sand: The impact of natural organic matters and electrolyte [J]. Environmental Pollution, 2017, 229: 49-59. doi: 10.1016/j.envpol.2017.05.059 [73] STEVENSON F J. Humus chemistry genesis, composition, reactions[M]. John Wiley & Sons, 1994. [74] YANG X Y, LIN S H, WIESNER M R. Influence of natural organic matter on transport and retention of polymer coated silver nanoparticles in porous media [J]. Journal of Hazardous Materials, 2014, 264: 161-168. doi: 10.1016/j.jhazmat.2013.11.025 [75] CHINNAPONGSE S L, MACCUSPIE R I, HACKLEY V A. Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters [J]. Science of the Total Environment, 2011, 409(12): 2443-2450. doi: 10.1016/j.scitotenv.2011.03.020 [76] KANEL S R, FLORY J, MEYERHOEFER A, et al. Influence of natural organic matter on fate and transport of silver nanoparticles in saturated porous media: Laboratory experiments and modeling [J]. Journal of Nanoparticle Research, 2015, 17(3): 1-13. [77] NAKANE M, AJIOKA T, YAMASHITA Y. Distribution and sources of dissolved black carbon in surface waters of the Chukchi Sea, Bering Sea, and the north Pacific Ocean [J]. Frontiers in Earth Science, 2017, 5: 34. doi: 10.3389/feart.2017.00034 [78] QU X L, FU H Y, MAO J D, et al. Chemical and structural properties of dissolved black carbon released from biochars [J]. Carbon, 2016, 96: 759-767. doi: 10.1016/j.carbon.2015.09.106 [79] DEGENKOLB L, METREVELI G, PHILIPPE A, et al. Retention and remobilization mechanisms of environmentally aged silver nanoparticles in an artificial riverbank filtration system [J]. Science of the Total Environment, 2018, 645: 192-204. doi: 10.1016/j.scitotenv.2018.07.079 [80] WANG D J, JAISI D P, YAN J, et al. Transport and retention of polyvinylpyrrolidone-coated silver nanoparticles in natural soils [J]. Vadose Zone Journal, 2015, 14(7): 1-13. [81] CORNELIS G, PANG L P, DOOLETTE C, et al. Transport of silver nanoparticles in saturated columns of natural soils [J]. Science of the Total Environment, 2013, 463/464: 120-130. doi: 10.1016/j.scitotenv.2013.05.089 [82] JACOBSON A R, MCBRIDE M B, BAVEYE P, et al. Environmental factors determining the trace-level sorption of silver and thallium to soils [J]. Science of the Total Environment, 2005, 345(1/2/3): 191-205. [83] RAHMATPOUR S, SHIRVANI M, MOSADDEGHI M R, et al. Retention of silver nano-particles and silver ions in calcareous soils: Influence of soil properties [J]. Journal of Environmental Management, 2017, 193: 136-145. [84] ADRIAN Y F, SCHNEIDEWIND U, BRADFORD S A, et al. Transport and retention of engineered silver nanoparticles in carbonate-rich sediments in the presence and absence of soil organic matter [J]. Environmental Pollution, 2019, 255: 113124. doi: 10.1016/j.envpol.2019.113124 [85] WANG R, DU H, WANG Y J, et al. Retention of silver nanoparticles and silver ion to natural soils: Effects of soil physicochemical properties [J]. Journal of Soils and Sediments, 2018, 18(7): 2491-2499. doi: 10.1007/s11368-018-1918-2 [86] MAHDI K N M, COMMELIN M, PETERS R J B, et al. Transport of silver nanoparticles by runoff and erosion - A flume experiment [J]. Science of the Total Environment, 2017, 601/602: 1418-1426. doi: 10.1016/j.scitotenv.2017.06.020 [87] HOPPE M, MIKUTTA R, UTERMANN J, et al. Remobilization of sterically stabilized silver nanoparticles from farmland soils determined by column leaching [J]. European Journal of Soil Science, 2015, 66(5): 898-909. doi: 10.1111/ejss.12270 [88] MAKSELON J, SIEBERS N, MEIER F, et al. Role of rain intensity and soil colloids in the retention of surfactant-stabilized silver nanoparticles in soil [J]. Environmental Pollution, 2018, 238: 1027-1034. doi: 10.1016/j.envpol.2018.02.025 [89] NGWENYA B T, CURRY P, KAPETAS L. Transport and viability of Escherichia coli cells in clean and iron oxide coated sand following coating with silver nanoparticles [J]. Journal of Contaminant Hydrology, 2015, 179: 35-46. doi: 10.1016/j.jconhyd.2015.05.005 -



 下载:
下载: