-
微生物污染问题作为全球饮用水供给安全中的重大挑战,在缺乏强效饮用水消毒措施的不发达国家和地区尤为严重[1–3]。传统的饮用水消毒手段存在一些局限,主要是消毒不彻底,极易生成有毒害副产物等,例如臭氧消毒不仅成本高、难储存,易腐蚀管道,而且会产生多余的消毒副产物(disinfection byproducts,DBPs)[3–5];紫外消毒存在着光穿透率低、易受水质状况影响、紫外灯耗电量大,以及微生物易光复活和暗复活等问题;加氯消毒则无法灭活两虫等抗氯微生物,而且会生成较多的卤代消毒副产物 [6–8]。为了应对传统消毒方法的局限性,近年来一些先进的消毒技术作为替代方案应运而生[9–11]。
在各种先进的消毒技术中,基于硫酸根自由基(SO4−•,通过活化过一硫酸盐(peroxymonosulfate , PMS)和过二硫酸盐(peroxo-disulfate,PDS))的高级氧化工艺(advanced oxidation processes, AOPs)具有较好的应用前景,主要基于以下几方面原因:(1)SO4•−具有宽pH适应范围,在pH = 3—11均具有较高的氧化电位(E0(SO4−•/SO42−) = +2.60 — +3.10 VNHE),能够氧化灭活多种微生物,并且能降解胞内基因来抑制微生物假死复活[12];(2)SO4−•的活化方式多样,如可以通过光、热、碱、超声或投加催化剂活化过硫酸盐来获得SO4−•,且过硫酸盐能够溶于水来渗透进土壤来进行修复[12–14];(3)与加氯和臭氧工艺相比,SO4−•消毒后产生的副产物数量较少[15];(4)SO4−•具有较高的选择性,在含溶解有机物、阴离子、阳离子等不同水环境中受影响较小,并能保持较高的消毒效果[12–13]。虽然基于SO4−•的AOPs用于消毒已经有较好的文献报道[16–20],但到目前为止仍没有相关的文章综述总结和比较基于SO4−•的AOPs用于灭活不同微生物的效率及其适用性。
当采用基于SO4−•的AOPs处理工艺对废水进行消毒时,不同水质环境和操作条件对于评估和优化该工艺至关重要。以往的研究评估了pH值、共存的溶解性有机物 (dissolved organic matter, DOM)阴离子、催化剂用量、温度和光强对消毒效率的影响[21–23],因此需要进行全面的概述来总结基于SO4−•的最佳消毒工艺条件。此外,目前对基于SO4−•的AOPs消毒工艺灭活微生物的机理仍存在一些争论:从过程角度来看,灭菌主要活性物种究竟是SO4−•,还是OH•、O2•−、1O2、激发态电子等仍然存在争议;从微生物的角度来看,有报告指出活性物种最先氧化微生物的壁或膜, 导致其不可逆转的损伤,最终影响膜的通透性和生理功能[17, 24-25];或有文献也强调活性物种还会攻击细胞内成分,甚至影响到微生物的酶与基因组[26–28]。因此,总结分析基于SO4−•的消毒工艺将提高对该技术的基础理解,并为指导其工程应用提供帮助。本文总结了各种基于SO4−•工艺的灭菌反应体系及其灭菌机理,同时讨论了水质环境的影响以及副产物的生成,进一步对该方向的研究需求和未来发展方向作出展望。
-
PS可被铁、钴、铜、锰等多种金属活化,其中如溶解态铁、螯合铁和固态铁等铁基催化剂具有较为实际的应用前景[13, 29-33]。将铁类物种与PS结合以产生用于微污染物降解的自由基已在文献中得到充分证明[13]。这种组合还可用于对水中不同类型微生物的灭活[31-33]。表1总结了包括亚铁离子(Fe2+)、零价铁(ZVI = Zero valent iron)、铁矿物和氧化铁(Fe2O3)在内的不同铁基活化PS的消毒效率。Wordofa等研究了Fe2+/PDS系统对大肠杆菌O157:H7的灭活作用,在3 mmol·L−1 S2O82−+3 mmol·L−1 Fe2+投加量条件下,130 min后灭活率达到95%,灭活速率常数为0.025 min−1[16]。而Qi等对Fe2+/PDS系统进行优化,在投加量为40 mmol·L−1 S2O82− + 13.3 mmol·L−1 Fe2+,即 1:0.33的最佳条件下,120 s后(7.77 ± 0.57)lg CFU·mL−1的大肠杆菌O157:H7和(7.25 ± 0.36)lg CFU·mL−1的李斯特菌全部灭活[31]。Xia 等使用天然磁黄铁矿活化PDS使大肠杆菌K-12失活,达到了0.47 lg CFU·min−1的失活速率常数并在15 min内使7 lg CFU·mL−1细胞完全失活[33]。 RodriguezChueca等比较了使用Fe2+、Fe2O3、ZVI活化PDS和PMS以灭活大肠杆菌[34]。在很短的反应时间内PMS与Fe2+、Fe2O3和ZVI的偶联反应便使大肠杆菌完全失活,同时,PDS诱导的细菌灭活率低于PMS[34]。
上述结果表明,无论是溶解态还是固态,基元态还是复合态,铁基材料都能有效激活PDS或PMS生成活性物种用于微生物失活。将铁物种用于PS的活化和消毒的优点包括:(1)铁基材料种类丰富且易于获得;(2)与钴、铜等金属相比,使用铁基材料相对安全;(3)固态铁很容易从处理过的水中分离提取出来。与铁介导的活化PS灭菌机理相关的争论之一是除了活性物种直接攻击外,铁物种尤其是固态铁是否可以增强微生物的失活:在一些情况下观察到某些固体铁可通过界面正价铁来对带负电的微生物发生静电吸附作用[11, 34],吸附过程导致二价铁渗透到胞内会增强微生物的失活 [35]。
-
除了铁基介导的电子转移途径外,还可以通过紫外线、可见光等光介导的能量激活PS,如表2所示 [17, 24, 26]。Sun等报告了采用UV/PDS对大肠杆菌、MS2噬菌体和枯草芽孢杆菌孢子的灭活,当PDS剂量为0.3 mmol·L−1时,使4 lg CFU·mL−1的大肠杆菌、MS2噬菌体和枯草芽孢杆菌孢子失活所需紫外线剂量分别为8.8、30、30 mJ·cm−2 [36]。UV/PDS对大肠杆菌和MS2的灭活效率高于单独UV,但在灭活孢子作用不显著[36]。值得注意的是,消毒所需的紫外线剂量远低于降解有机污染物所需的剂量,这表明采用UV/PDS进行消毒是实际可行的。Wen等报道了使用UV/PMS工艺对真菌孢子(T. harzianum)进行灭活比单独使用UV的效果更好,当PMS剂量为0.1 mmol·L−1时,使2 lg CFU·mL−1的T. harzianum孢子失活所需的紫外线剂量为35 mL·cm−2[37]。除了能量密度相对较高的紫外光,可见光也被应用于活化PDS进行深度消毒。Wang等报道了在不投加催化剂条件下可见光活化PS灭活水中大肠杆菌 [17],当PDS剂量为2.0 mmol·L−1,将反应溶液暴露于带有420 nm截止滤光片的太阳模拟器中,在40 min内约6 lg CFU·mL−1大肠杆菌细胞被灭活[17];引入Fe3O4基的磁性水热炭在太阳光/PDS体系中可进一步增强该过程,在相同的实验条件下,灭活效率从6 lg CFU·mL−1提高到8 lg CFU·mL−1[24]。在大型塑料瓶中太阳能可以提高PMS和PMS/Fe2+体系对大肠杆菌的灭活率。当PMS和Fe2+剂量分别为36 μmol·L−1和18 μmol·L−1时,太阳光/PMS工艺灭活5 lg CFU·mL−1的大肠杆菌所需太阳能为650 Wh·m−2,而在添加了Fe2+后,太阳光能耗降低到450 Wh·m−2[38]。
以上结果表明,光介导活化PDS/PMS可有效杀灭多种微生物。使用光介导的过程有几个优点:例如,光辐射被认为是一种无化学物质的能量输入,它可以减少如在使用铁基催化剂时的铁污泥等副产物。实际上,可以较为容易地将紫外线或可见光灯安装到现有的水处理机组中,并且不会占用很多空间。此外,由于太阳能是一种自然资源而且容易获得,利用太阳能更具可持续性。太阳能介导的PDS/PMS活化在水的深度消毒方面很有前景,这在碳中和背景下发展绿色清洁技术有很深的意义,为发展中国家减少污染排放提供了帮助。
-
据报道,PS也能够被氢氧化钠(NaOH)活化,用于灭活大肠杆菌O157:H7[31]。研究进行了两组实验,当使用40 mmol·L−1 PS和30 mmol·L−1 NaOH时,120 s后使6.21 lg CFU·mL−1的大肠杆菌失活[31];当化学剂量增加至500 mmol·L−1 PS和350 mmol·L−1 NaOH时,120 s后8.64 lg CFU·mL−1的大肠杆菌失活[31]。与之前的铁介导或光辅助过程相比,碱基介导的研究较少,其主要原因可能是将溶液pH值调整为强碱性进行处理后需再将pH调整回来,这在实际运用中可能是不可行的。然而,该工艺可能会适用于某些特殊工业废水或某些特殊地区的地下水处理。
-
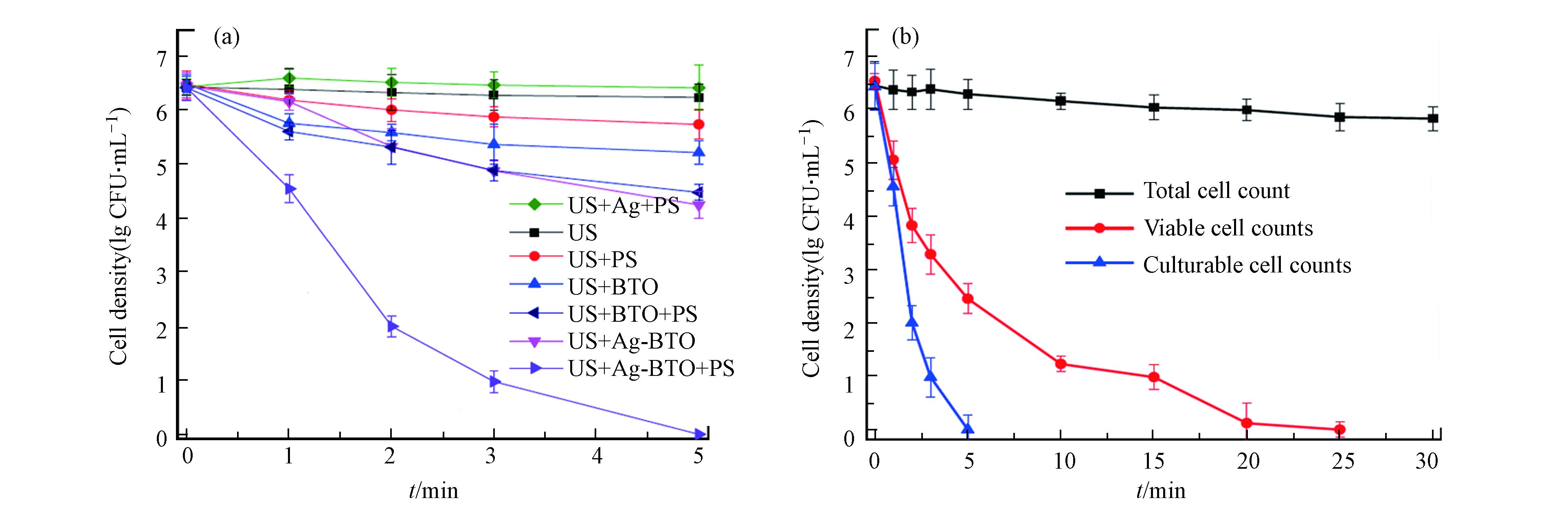
普遍报道的铁介导法和光辅助法都有其固有的局限性,除此以外,近年来一些新型的PDS活化法也被报道用于水的深度消毒。Xia等报道了压电催化过硫酸盐活化体系进行消毒,他们合成了银改性的钛酸钡(Ag-BTO)并将其用作压电催化剂,在超声振动(US)下激活PDS以灭活大肠杆菌[39]。新开发的US/Ag-BTO/PDS工艺可以在5 min内使6.2 lg CFU·mL−1的大肠杆菌完全进入存活但不可培养(VBNC = viable but non-culturable)状态,并在20 min处理后彻底灭活VBNC状态细菌[39]。如图1所示,US/Ag-BTO/PDS体系的消毒性能比其他对照组有更好的消毒效果[39]。在该体系中,压电由超声驱动,可通过超声破坏细菌细胞膜而强化活性物种穿透,而且压电电子也能高效活化过硫酸盐,实现过硫酸盐的高效利用和催化灭菌性能。
-
基于SO4−•的AOPs工艺消毒效率受催化剂和PDS/PMS的用量、接触时间、溶液pH、共存的阴离子或阳离子等操作和环境条件的影响。这些因素对消毒效率的影响在不同的工艺过程中大致呈相同的趋势,即随着剂量和接触时间的增加而增加,但在如碱基活化PDS体系较特殊的过程中会有一些例外。本节对上述提到的活化方法中相关因素的影响进行了总结。
-
在Wordofa等用Fe2+/PDS体系对大肠杆菌进行灭活的研究中,灭活速率常数k几乎随着过硫酸盐的用量增加而呈线性增加,而诱导时间与过硫酸盐的用量增加呈反指数衰减关系[16]。速度常数k在稳定前也随Fe2+用量的增加而迅速增加,而诱导时间则随Fe2+浓度的增加而呈指数衰减[16]。在Xia等使用天然磁黄铁矿/PDS体系灭活大肠杆菌的研究中,随着PDS从0.2 mmol·L−1增加到1.0 mmol·L−1,NP剂量从0.15 g·L−1增加到1.0 g·L−1,灭活效率也得到提高。而由于过量的铁对活性物质的消耗,高浓度的铁会抑制消毒效率[33]。同样,在Sun等使用动力学模型模拟证明中,随着PDS剂量从0 mmol·L−1增加到10 mmol·L−1,MS2噬菌体的失活效率增加,然而大肠杆菌和杆菌孢子的失活效率受PDS剂量的影响较小[36]。与“CT值”类似的是在使用光辅助活化工艺时,灭活效率也随着紫外或可见光通量的增加而增加(光通量=光强度×辐照时间)。
-
根据相关研究报道,消毒效率会受到pH值的显著影响。例如在Xia等报告中,天然磁黄铁矿/PDS系统对大肠杆菌的灭活效率随着pH值由3.0增加到9.0而逐渐降低[33];在Wang等的报告中,随着pH值从4.0增加到9.0,可见光/PDS体系的消毒效率逐渐降低[17];在Wordofa等的研究中,在Fe2+/PDS体系中的大肠杆菌灭活速率随着pH值从5.0增加到9.0而降低,这主要是因为碱性条件下Fe2+形成絮体发生沉降导致过硫酸盐活化效率降低。他们还发现溶液pH值对失活速率常数的影响比活化时长更加显著[16]。
pH值对消毒过程的影响有以下几个方面:(1)pH值影响溶液中铁离子的形态;(2)pH值影响PDS/PMS活化过程中活性物种的生成;(3)pH值也会影响微生物的表面电荷及其对活性物种的亲和力。大多数天然水和再生水的pH值一般在6.5—8.5之间,在这个范围内铁介导的过程可能无法很好地进行,而光辅助催化和新型压电催化在这个pH范围内能体现出其使用的优势,以及在某些碱性较强的工业废水或极端条件下,碱活化的PDS/PMS体系可能会成为深度消毒的良好选择。
-
溶解氧(DO = dissolved oxygen)、DOM(dissolved organic matter)、无机阴离子和阳离子以及悬浮固体(SS = suspended solid)等水基质会在不同程度上会影响SO4−•的消毒效率。如1—20 mg·L−1的碳酸氢盐能够降低对大肠杆菌的消毒效率,在降低溶解氧含量时会降低天然磁黄铁矿/PDS体系内的消毒效率[33]。在大多数研究报道中DOM会降低体系内的消毒效率,但在天然磁黄铁矿/PDS体系中DOM对大肠杆菌的消毒效果具有双重作用,其研究表明,在添加1 mg·L−1的DOM后,大肠杆菌的灭活率提高,但随着DOM进一步增加至5 mg·L−1和20 mg·L−1,大肠杆菌的灭活率显著降低[33]。低浓度DOM的增强效应归因于DOM中的对苯二酚和醌基团可以激活PDS生成SO4−•和HO•等活性物种[33]。与光催化相比,铁基催化对水基质成分相对更敏感,因为铁物种会活跃的参与到氧化还原反应和络合反应中,因此在实际应用中应当考虑到目标水质状况来选择合适的处理手段。
-
目前已经有文献提出了SO4−•灭活微生物的机理,但现有文献中的观点仍存在有争议的部分。本章将从两个角度总结灭活机理:(1)从反应过程角度,重点介绍了活性物种是如何产生的,负责微生物灭活的主要物种以及主要途径是什么;(2)从微生物角度,重点介绍了微生物的哪些部位(如细胞膜、酶、基因组等)受到活性物种的攻击而导致失活。
-
在紫外光、可见光活化的PDS或PMS的过程中,活性物种的生成是相对简单清晰的。如在UV/PDS体系中,Sun等计算了不同活性物质相对于大肠杆菌、MS2和杆菌孢子的CT值,结果表明消毒效果为HO• > SO4−• > CO3−• O2−•[36]。但与之相反的是,在Wang等的研究中,SO4−•对通过可见光/PDS体系使大肠杆菌失活的贡献程度远远大于HO•和O2−•[17]。这是因为与自由基相比,SO4−•具有相对较长的寿命和更强的氧化能力。不同研究中结果的不一致性是由于其对活性物质不同的量化方法,例如在Sun等的研究中使用动力学模型来预测每种活性物质的贡献[36],而在Wang等的研究中则使用自由基抑制剂达到同样的目的。
在铁介导的过程中,由于铁物种的参与,自由基的化学过程相对更加复杂。在Fe2+/PDS体系中,Wordofa等通过实验和构建模型的方法证明SO4−•导致大肠杆菌O157:H7细胞灭活的速度比HO•快5倍[16],且同时提出SO4−•的优势可能与其与大肠杆菌O157:H7细胞膜表面的富电子基团的高度选择性有关[16]。在天然磁黄铁矿/PDS体系中,根据抑制剂实验和原位ROS的测定,灭活大肠杆菌的活性物质贡献为SO4−• > HO• >H2O2[33],研究证明PDS能够有效地结合到天然磁黄铁矿表面,形成电荷转移复合物从而介导活性物种的产生。在Wang等使用可见光/PDS/hydro-char体系灭活大肠杆菌的工作中,SO4−•被认为是导致大肠杆菌灭活的主要活性物质[17]。在该体系中,SO4−•主要通过3种途径产生:(1)通过可见光激活PDS;(2)通过表面Fe(Ⅱ)激活PDS;(3)通过光催化产生的电子激活PDS。在关于铁介导的PDS/PMS活化体系的一个新的观点是,高价态铁物种可能参与到此类灭活过程,尤其是在接近中性的pH下。在对有机污染物的降解过程中这些高价态铁与自由基一样具有不可忽略的贡献[40-41]。例如,一些研究预测Fe(Ⅳ)可能会参与到微生物的去除过程[42],但迄今为止没有直接证据证明,因此这个问题值得进一步研究。
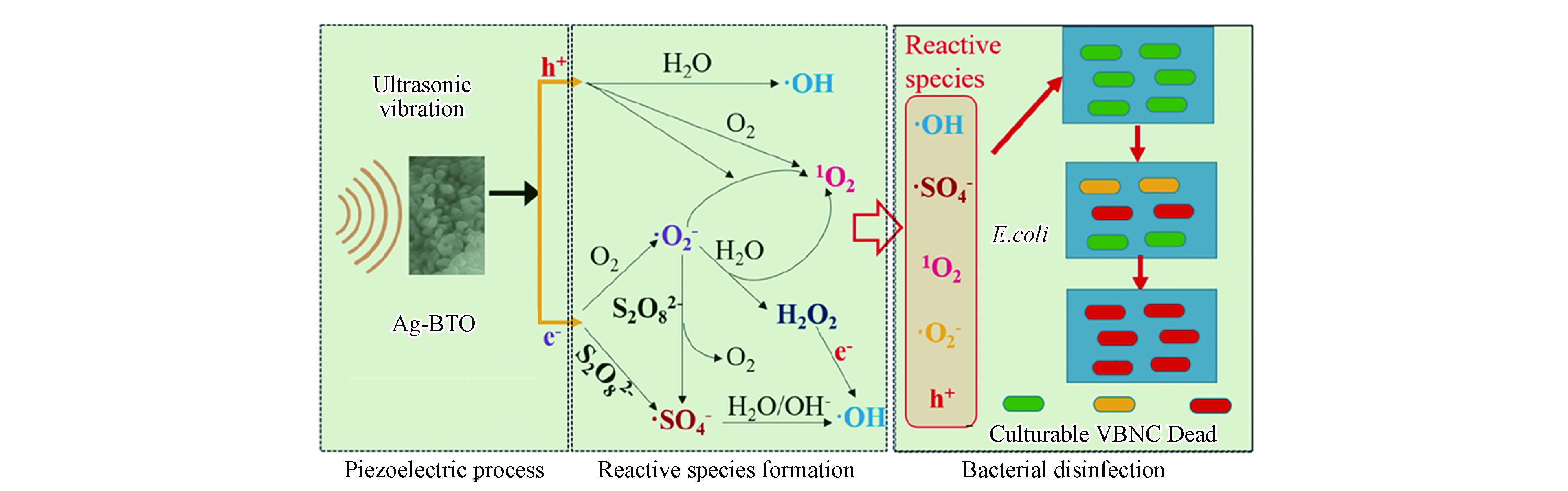
在新型压电催化系统中,Xia等报道了通过压电催化产生电子和超氧自由基活化PDS连续生成SO4−•和HO•对大肠杆菌进行高效灭活[39]。图2显示了活性物种的产生途径[39]。其中值得关注的是通过使用US/Ag-BTO/PS体系灭活大肠杆菌的过程中发现了超声波和自由基氧化之间的协同作用,如压电电子和超声本身可以帮助活化过硫酸盐来强化自由基生成。
-
过往的研究提供了一些关于微生物是如何被破坏和灭活的信息,它们是基于SO4−•的消毒工艺处理前后微生物从宏观到微观的特征而得出的。例如Wang等报道了大肠杆菌的细胞膜受到了被可见光激活PDS所产生的活性物质的破坏,随后CAT(catalase)和SOD(superoxide dismutase)等一些抗氧化酶被诱导进而使基因组DNA受损,导致细胞死亡[17]。在他们接下来的工作中使用水热炭增强了该过程,并得到了相似的机制,即细菌灭活过程被确定为从外膜开始最终到细胞内成分降解 [24]。Xiao等研究了用于大肠杆菌灭活的ZVI/PDS体系,通过扫描电子显微镜(SEM)拍摄的受试细菌的形态图像,表明SO4−•在细胞壁或细胞膜上引发氧化反应,影响膜的通透性和生理功能,最终导致其不可逆损伤[32]。 Xia等利用天然磁黄铁矿/PDS工艺系统地研究了大肠杆菌细胞破坏过程,由外膜、肽聚糖层和细胞质膜组成的细菌包膜首先受到系统中产生的活性物质的攻击[33]。利用BacLight kit显微荧光法直接观察天然磁黄铁矿/PS体系中细胞膜的通透性变化,同时由于ATP水平急剧下降,细胞几乎在瞬间因代谢停滞而失活。此外,他们的研究还提到反应性物种通过表现为质子载体或通过抑制呼吸链中的酶来抑制ATP的形成,活性物种在穿透细胞包膜后,通过与细胞质蛋白、基因组等多种生物分子发生反应损伤细胞,最后还观察到DNA条带的荧光强度逐渐减弱可知基因组DNA被破坏 [33]。但作者同时指出,处理后的细胞可能处于一种可存活但不可培养(VBNC)的状态,这种状态仍可能对水体造成健康风险[33]。Xia等提出了一种新的压电催化强化消毒工艺来处理VBNC状态下的微生物[39]。研究人员通过跟踪ATP(adenosine triphosphate)水平检测了可培养状态的大肠杆菌以及VBNC大肠杆菌细胞的代谢活性,并通过荧光光谱仪检测大肠杆菌的成分变化证明大肠杆菌在可培养状态和VBNC状态均被有效灭活 [39]。因此,基于SO4−•的AOPs能够通过氧化协同其他外加能量如光,超声等作用实现对微生物胞膜到胞内物质的裂解矿化。
-
基于SO4−•的工艺已被发展用于水的深度处理,但消毒过程中产生的消毒副产物却很少被提及。事实上在任何涉及消毒的化学过程中,都不可避免地会形成DBPs(disinfection by-products),虽然基于SO4−•的工艺手段中产生的DBPs可能与氯、氯胺或二氧化氯等消毒过程中形成的DBPs不同,但它们也同样会造成一定的健康风险。本节将对基于SO4−•的深度水消毒处理过程中形成的潜在DBPs进行总结。
-
使用基于SO4−•的深度水处理过程中会形成无机副产物,典型的无机副产物是由PDS或PMS分解生成的硫酸盐。当使用PDS时由于1 mol的PDS可产生2 mol硫酸盐,无机副产物的形成尤其严重。由于摄入含有高硫酸盐含量的饮用水会对胃肠道产生影响,世卫组织建议向卫生当局通报硫酸盐浓度超过500 mg·L−1的饮用水源[42- 43]。然而硫酸盐在美国环保局中按二级最大污染物水平(secondary maximum contaminant level = SMCL)标准分类,饮用水中硫酸盐的SMCL为250 mg·L−1 [44]。但假设所有剂量的PDS或PMS最终全部分解为硫酸盐,并以250 mg·L−1倒推,则PDS和PMS的最大剂量仅为1.3 mmol·L−1和2.6 mmol·L−1。但在许多已报道的文献中,PDS/PMS的剂量均超过了该计算值,这表明在实际应用此类技术时,过量硫酸盐的形成可能会成为值得注意的问题。
而除了硫酸盐,用于自由基生成的催化剂也可能是这些过程的副产物。除了上文提到的铁泥外,其他金属或非金属材料也可能成为有害副产物。例如在PDS/PMS活化中,钴表现出比铁更优越的催化性能,但钴金属有毒,不能留在任何处理过的饮用水中。在实际应用时,金属或非金属材料的浸出始终是一个主要问题。
此外,当使用SO4−•进行水的深度处理时,氯酸盐的形成可能成为另一个问题。氯离子在水中普遍存在,而氯化物能够被SO4−•氧化成氯酸盐,这对饮用处理水的人群造成了极大的健康问题[45]。世卫组织和美国环境保护局将饮用水中氯酸盐的水质健康参考水平标准分别定为0.7 mg·L−1和0.21 mg·L−1 [46]。因此在使用基于SO4−•工艺处理含高浓度氯化物的水时需要对氯酸盐的形成应予以关注。
-
在基于SO4−•的工艺用于水的深度消毒处理时还会产生有机副产物。SO4−•被认为是一种绿色环保的自由基,与如活性氯和活性溴等的卤素自由基相比,SO4−•产生的有机副产品数量有限。然而,最近的一项研究表明在用SO4−•处理水中有机污染物和灭菌的过程中会普遍产生有机硫酸盐[47]。其中一些有机硫酸盐,例如硫酸苯酯(phenyl sulfate)和硫酸对甲酚酯(p-cresyl sulfate)均是有害的尿毒症毒素。因此在使用基于SO4−•的水深度消毒处理的过程中,应进一步调查关注有机硫酸盐的形成、去向及其毒性。
同时,水中普遍存在的氯化物可通过SO4−•氧化为活性氯物种,如Cl•、Cl2•−和ClO•[46],这些活性氯物种通过取代反应和加成反应与水中溶解的有机物反应形成如三卤甲烷、卤乙酸、水合氯醛等的氯化副产物[48]。这些氯化副产品是会对人类健康构成严重威胁的致癌物[49]。而由于自由基转化,还可能会形成溴化和碘化副产物。因此在对水使用基于SO4−•的深度消毒处理时需要对卤化副产物的形成、迁移和毒性进行评估。
-
本文综述了基于SO4−•的AOPs应用于消毒的研究进展。本文首先回顾了过往研究中各种基于SO4−•工艺对不同微生物的灭活作用,这些工艺根据其驱动力主要分为三大类:铁介导、光辅助和新型过硫酸盐活化(例如压电催化),针对各类当前的研究进展和主要发现进行总结,并讨论了各类工艺的深度消毒处理的优缺点。接着本文概述了不同操作因素和环境条件对消毒性能的影响,包括过硫酸盐和催化剂的用量、接触时间、光照通量、pH值、溶解氧和其他水基质成分等,对上述不同因素造成影响的原因进行了讨论。进一步概述了基于SO4−•消毒的微生物灭活机理,并从两个角度对这些机理进行了综述,即从反应过程角度总结了活性物种是如何形成的,以及每种活性物种对微生物失活的贡献,并且从微生物的角度总结了微生物的损伤及灭活过程。最后,本文回顾了基于SO4−•工艺应用中无机和有机消毒副产物的形成,通过机理解释和讨论指出了与副产物形成有关的一些被忽视的问题。该综述还为基于SO4−•工艺的饮用水处理过程中工程设计和运行提供了建议。
(1)目前的研究仅局限于对少数微生物的失活,如大肠杆菌、MS2噬菌体和杆菌孢子,这对基于SO4−•的消毒方法在广谱致病微生物灭活方面的适用性和优点提供的信息较为有限。建议对更多与人类健康相关的微生物进行调查。此外对不同微生物进行的比较较少,考虑到SO4−•的选择性,建议在分子水平或借助一些微生物学工具对不同微生物的失活进行比较以供将来研究。
(2)在涉及铁物种活化的过程中,高价铁(如Fe(Ⅳ)和Fe(Ⅴ))对微生物失活的作用尚不清楚。因此,目前在铁介导活化方面对不同工艺过程下的SO4−•、HO−•以及H2O2、O2−•、和1O2等其他活性物质的相对贡献的判断可能不准确。建议对高价铁(如Fe(Ⅳ)和Fe(Ⅴ))等对微生物失活的贡献进行定性和定量的研究说明。
(3) 目前,基于SO4−•工艺中消毒副产物的形成往往被忽略,需要对微生物本身或共存的有机物产生的有机副产物和无机副产物进行更多的研究,以及分析探究更多关于基于SO4−•工艺处理前后水的毒理学变化的更基本的资料。
(4)基于SO4−•的AOP处理技术,虽然文献报道可以通过增加反应物浓度提升消毒效率,但如此也会引发一些技术应用问题,如吨水处理费用会远高于UV和氯,且大量硫酸盐会高于各类水质标准,甚至会导致饮用水后续管网腐蚀或污水排入河流中导致底泥生成H2S气体等。针对这些应用局限性,可以考虑引入微纳气泡或结合UV来协同提高过硫酸盐的活化效率及杀菌速率,一方面能有效降低反应物的投加浓度,另一方面还可以高效抑制微生物的复活。
(5)综合该工艺的优缺点,基于SO4−•的AOPs仍然在再生水、地下水或是应急场所中的消毒应用有发展前景。一是再生水,由于再生水中还含有较多持久性污染物,SO4−•可以同时降解某些污染物,并且可以灭活多种病原微生物;二是地下水,如紫外和臭氧无法穿透土壤,而过硫酸盐是可以溶解渗透的;且过硫酸盐是可以高效灭活本身具有抗氯性的真菌及阿米巴等微生物;三是某些特殊应急场所有可能处于复杂pH范围,而SO4−•具有广泛pH适应范围。
考虑到对过硫酸盐剂量和pH条件较严格的要求,在诸如使用点或入口水处理装置等一些小型处理装置中基于SO4−•的深度消毒水处理工艺的应用似乎比在大型水处理厂中的应用更有前景。在这种情况下,利用太阳能等自然资源加强基于SO4−•的消毒过程可能成为实现可持续发展目标——“清洁的水和卫生”要求的直接现实途径。
基于硫酸根自由基的微生物灭活技术研究进展
Sulfate radical-based advanced oxidation processes for water disinfection
-
摘要: 基于硫酸根自由基(SO4−•)的高级氧化工艺(advanced oxidation processes , AOPs),因其具有强氧化性及广泛pH适应范围,对不同微生物如抗氯菌、真菌及病毒等均具有广谱灭菌性,且在反应过程中仅产生极少量卤代副产物,因此在再生水、地下水或是应急场所中病原微生物灭活应用方面具有十分广阔的发展前景。本文概述了基于SO4−•的不同AOPs(如金属辅助、光辅助、碱辅助及压电催化)对各类致病微生物和指示微生物的灭活动力学响应,并揭示了其对病原微生物的灭活机制,主要为先破坏细胞膜通透性,进而引发酶和基因组损伤,从而有效抑制并彻底灭活病原微生物。此外,本文还总结和讨论了水质参数(如pH、温度和各种水基质)对基于SO4−•的AOPs消毒效果的影响,并对该体系中副产物的生成进行了调研和讨论。最后在结论中总结了综述中的关键点,并对将该工艺应用于实际工程中的知识缺口、研究需求和设计需要做出展望。Abstract: Sulfate radical (SO4−•)-based advanced oxidation processes (AOPs) have emerged as promising technologies for inactivation of pathogenic microorganisms in water and wastewater, due to the high oxidation potential of SO4−• towards different microorganisms, such as chlorine-resistant bacteria, fungi and viruses; strong oxidation at wide range of pH adaptation, and the negligible formation of undesired halogenated byproducts, which is suitable to be applied in reclaimed water, underground water or emergency places. This work provides an overview on the kinetic responses of various pathogenic/indicator microorganisms in different SO4−•-based AOPs (e.g., metal-assisted, light-assisted, and piezo-catalytic ones) and the mechanisms responsible for the inactivation, mainly including membrane permeability is first destroyed, which leads to enzyme and genome damage, thus effectively inhibiting and inactivating pathogenic microorganisms. The effects of water matrix (e.g., pH, temperature, and various water matrix) on the disinfection efficacies have also been reviewed and discussed. The formation of undesired byproducts in the SO4−•-based AOPs was also reviewed and discussed. Key points from the review are summarized in the conclusive remarks. Knowledge gaps, research needs, and design requirements of engineering applications of these processes in real-world practice are proposed as future perspectives.
-
Key words:
- sulfate radical /
- advanced oxidation processes /
- microorganisms /
- disinfection
-
微生物污染问题作为全球饮用水供给安全中的重大挑战,在缺乏强效饮用水消毒措施的不发达国家和地区尤为严重[1–3]。传统的饮用水消毒手段存在一些局限,主要是消毒不彻底,极易生成有毒害副产物等,例如臭氧消毒不仅成本高、难储存,易腐蚀管道,而且会产生多余的消毒副产物(disinfection byproducts,DBPs)[3–5];紫外消毒存在着光穿透率低、易受水质状况影响、紫外灯耗电量大,以及微生物易光复活和暗复活等问题;加氯消毒则无法灭活两虫等抗氯微生物,而且会生成较多的卤代消毒副产物 [6–8]。为了应对传统消毒方法的局限性,近年来一些先进的消毒技术作为替代方案应运而生[9–11]。
在各种先进的消毒技术中,基于硫酸根自由基(SO4−•,通过活化过一硫酸盐(peroxymonosulfate , PMS)和过二硫酸盐(peroxo-disulfate,PDS))的高级氧化工艺(advanced oxidation processes, AOPs)具有较好的应用前景,主要基于以下几方面原因:(1)SO4•−具有宽pH适应范围,在pH = 3—11均具有较高的氧化电位(E0(SO4−•/SO42−) = +2.60 — +3.10 VNHE),能够氧化灭活多种微生物,并且能降解胞内基因来抑制微生物假死复活[12];(2)SO4−•的活化方式多样,如可以通过光、热、碱、超声或投加催化剂活化过硫酸盐来获得SO4−•,且过硫酸盐能够溶于水来渗透进土壤来进行修复[12–14];(3)与加氯和臭氧工艺相比,SO4−•消毒后产生的副产物数量较少[15];(4)SO4−•具有较高的选择性,在含溶解有机物、阴离子、阳离子等不同水环境中受影响较小,并能保持较高的消毒效果[12–13]。虽然基于SO4−•的AOPs用于消毒已经有较好的文献报道[16–20],但到目前为止仍没有相关的文章综述总结和比较基于SO4−•的AOPs用于灭活不同微生物的效率及其适用性。
当采用基于SO4−•的AOPs处理工艺对废水进行消毒时,不同水质环境和操作条件对于评估和优化该工艺至关重要。以往的研究评估了pH值、共存的溶解性有机物 (dissolved organic matter, DOM)阴离子、催化剂用量、温度和光强对消毒效率的影响[21–23],因此需要进行全面的概述来总结基于SO4−•的最佳消毒工艺条件。此外,目前对基于SO4−•的AOPs消毒工艺灭活微生物的机理仍存在一些争论:从过程角度来看,灭菌主要活性物种究竟是SO4−•,还是OH•、O2•−、1O2、激发态电子等仍然存在争议;从微生物的角度来看,有报告指出活性物种最先氧化微生物的壁或膜, 导致其不可逆转的损伤,最终影响膜的通透性和生理功能[17, 24-25];或有文献也强调活性物种还会攻击细胞内成分,甚至影响到微生物的酶与基因组[26–28]。因此,总结分析基于SO4−•的消毒工艺将提高对该技术的基础理解,并为指导其工程应用提供帮助。本文总结了各种基于SO4−•工艺的灭菌反应体系及其灭菌机理,同时讨论了水质环境的影响以及副产物的生成,进一步对该方向的研究需求和未来发展方向作出展望。
1. 不同SO4−•工艺对微生物的灭活(Microorganism inactivation by various SO4−• based processes)
1.1 铁基催化活化PS灭活微生物
PS可被铁、钴、铜、锰等多种金属活化,其中如溶解态铁、螯合铁和固态铁等铁基催化剂具有较为实际的应用前景[13, 29-33]。将铁类物种与PS结合以产生用于微污染物降解的自由基已在文献中得到充分证明[13]。这种组合还可用于对水中不同类型微生物的灭活[31-33]。表1总结了包括亚铁离子(Fe2+)、零价铁(ZVI = Zero valent iron)、铁矿物和氧化铁(Fe2O3)在内的不同铁基活化PS的消毒效率。Wordofa等研究了Fe2+/PDS系统对大肠杆菌O157:H7的灭活作用,在3 mmol·L−1 S2O82−+3 mmol·L−1 Fe2+投加量条件下,130 min后灭活率达到95%,灭活速率常数为0.025 min−1[16]。而Qi等对Fe2+/PDS系统进行优化,在投加量为40 mmol·L−1 S2O82− + 13.3 mmol·L−1 Fe2+,即 1:0.33的最佳条件下,120 s后(7.77 ± 0.57)lg CFU·mL−1的大肠杆菌O157:H7和(7.25 ± 0.36)lg CFU·mL−1的李斯特菌全部灭活[31]。Xia 等使用天然磁黄铁矿活化PDS使大肠杆菌K-12失活,达到了0.47 lg CFU·min−1的失活速率常数并在15 min内使7 lg CFU·mL−1细胞完全失活[33]。 RodriguezChueca等比较了使用Fe2+、Fe2O3、ZVI活化PDS和PMS以灭活大肠杆菌[34]。在很短的反应时间内PMS与Fe2+、Fe2O3和ZVI的偶联反应便使大肠杆菌完全失活,同时,PDS诱导的细菌灭活率低于PMS[34]。
表 1 铁基活化SO4−•致微生物失活Table 1. Microorganism inactivation by SO4−•-based processes activated by iron species体系Process 目标微生物Target microorganisms 实验条件Experimental conditions 消毒效率Disinfection efficiency 参考文献Reference Fe2+/PDS O157:H7大肠杆菌 3 mmol·L−1 PDS、3 mmol·L−1 FeSO4、3 mmol·L−1 NH2OH和pH7的饮用水 约130 min达到95%的灭活率 [16] Fe2+/PDS O157:H7大肠杆菌和李斯特菌 40 mmol·L−1 PDS 和 13.3 mmol·L−1 Fe2+ 120 s后(7.77 ± 0.57 )lg CFU·mL−1的大肠杆菌和(7.25 ± 0.36) lg CFU·mL−1的李斯特菌失活 [31] ZVI/PDS 大肠杆菌BL21和粪肠球菌 大肠杆菌测试中:3 mmol·L−1 PDS、 0.6 g·L−1 ZVI粪肠球菌测试中:1 mmol·L−1 PDS、0.2 g·L−1 ZVI以及 pH 为5.5 80 min后90%的大肠杆菌失活;12 min后75%的粪肠球菌失活 [11] 天然黄铁矿/PDS 大肠杆菌K-12 0.5 mmol·L−1 PDS、1g·L−1 NP 以及 pH 为5.0 15 min内7 lg CFU·mL−1大肠杆菌失活 [33] Fe2+, Fe2O3, nZVI/PDS和PMS 大肠杆菌 0.5 mmol·L−1 PDS、 0.5 mmol·L−1 PMS、1 mmol·L−1 Fe(Ⅱ)、1 mmol·L−1 Fe2O3以及50 mg·L−1 ZVI 5 min内PMS与Fe2+、Fe2O3和ZVI的偶联反应使大肠杆菌完全失活 [34] 上述结果表明,无论是溶解态还是固态,基元态还是复合态,铁基材料都能有效激活PDS或PMS生成活性物种用于微生物失活。将铁物种用于PS的活化和消毒的优点包括:(1)铁基材料种类丰富且易于获得;(2)与钴、铜等金属相比,使用铁基材料相对安全;(3)固态铁很容易从处理过的水中分离提取出来。与铁介导的活化PS灭菌机理相关的争论之一是除了活性物种直接攻击外,铁物种尤其是固态铁是否可以增强微生物的失活:在一些情况下观察到某些固体铁可通过界面正价铁来对带负电的微生物发生静电吸附作用[11, 34],吸附过程导致二价铁渗透到胞内会增强微生物的失活 [35]。
1.2 光介导活化PS灭活微生物
除了铁基介导的电子转移途径外,还可以通过紫外线、可见光等光介导的能量激活PS,如表2所示 [17, 24, 26]。Sun等报告了采用UV/PDS对大肠杆菌、MS2噬菌体和枯草芽孢杆菌孢子的灭活,当PDS剂量为0.3 mmol·L−1时,使4 lg CFU·mL−1的大肠杆菌、MS2噬菌体和枯草芽孢杆菌孢子失活所需紫外线剂量分别为8.8、30、30 mJ·cm−2 [36]。UV/PDS对大肠杆菌和MS2的灭活效率高于单独UV,但在灭活孢子作用不显著[36]。值得注意的是,消毒所需的紫外线剂量远低于降解有机污染物所需的剂量,这表明采用UV/PDS进行消毒是实际可行的。Wen等报道了使用UV/PMS工艺对真菌孢子(T. harzianum)进行灭活比单独使用UV的效果更好,当PMS剂量为0.1 mmol·L−1时,使2 lg CFU·mL−1的T. harzianum孢子失活所需的紫外线剂量为35 mL·cm−2[37]。除了能量密度相对较高的紫外光,可见光也被应用于活化PDS进行深度消毒。Wang等报道了在不投加催化剂条件下可见光活化PS灭活水中大肠杆菌 [17],当PDS剂量为2.0 mmol·L−1,将反应溶液暴露于带有420 nm截止滤光片的太阳模拟器中,在40 min内约6 lg CFU·mL−1大肠杆菌细胞被灭活[17];引入Fe3O4基的磁性水热炭在太阳光/PDS体系中可进一步增强该过程,在相同的实验条件下,灭活效率从6 lg CFU·mL−1提高到8 lg CFU·mL−1[24]。在大型塑料瓶中太阳能可以提高PMS和PMS/Fe2+体系对大肠杆菌的灭活率。当PMS和Fe2+剂量分别为36 μmol·L−1和18 μmol·L−1时,太阳光/PMS工艺灭活5 lg CFU·mL−1的大肠杆菌所需太阳能为650 Wh·m−2,而在添加了Fe2+后,太阳光能耗降低到450 Wh·m−2[38]。
表 2 光介导活化SO4−•致微生物失活Table 2. Microorganism inactivation by SO4−•-based processes activated by light体系Process 目标微生物Target microorganisms 实验条件Experimental conditions 消毒效率Disinfection disinfection 参考文献Reference UV/PDS 大肠杆菌、MS2噬菌体、枯草芽孢杆菌孢子 0.3 mmol·L−1 PDS,大肠杆菌、噬菌体MS2、枯草芽孢杆菌孢子紫外光剂量分别为8.8、30、30 mL·cm−2 3种微生物在30 min内均达到4 lg CFU·mL−1失活 [36] UV/PDS T. harzianum孢子 0.1 mmol·L−1 PMS, 紫外光剂量为35 mL·cm−2 达到2 lg CFU·mL−1失活 [37] 可见光/PDS 大肠杆菌 2 mmol·L−1 PDS, 30 °C, pH 6.0, λ ≥ 420 nm 40 min内6 lg CFU·mL−1大肠杆菌失活 [17] 可见光/ hydrochar /PS 大肠杆菌 200 mg·L−1 MHC; 2 mmol·L−1 PS; 25 °C, pH 6.0, λ ≥ 420 nm 40 min后8 lg CFU·mL−1大肠杆菌失活 [24] 太阳光/PMS;太阳光/Fe2+/PMS 大肠杆菌 装有蒸馏水的塑料瓶中加入18 μmol·L−1 Fe2+, 290 μmol·L−1 H2O2, 和 36 μmol·L−1 PMS 650 Wh·m−2太阳光/PMS体系和450 Wh·m−2太阳光/Fe2+/PMS体系达到5 lg CFU·mL−1失活 [38] 以上结果表明,光介导活化PDS/PMS可有效杀灭多种微生物。使用光介导的过程有几个优点:例如,光辐射被认为是一种无化学物质的能量输入,它可以减少如在使用铁基催化剂时的铁污泥等副产物。实际上,可以较为容易地将紫外线或可见光灯安装到现有的水处理机组中,并且不会占用很多空间。此外,由于太阳能是一种自然资源而且容易获得,利用太阳能更具可持续性。太阳能介导的PDS/PMS活化在水的深度消毒方面很有前景,这在碳中和背景下发展绿色清洁技术有很深的意义,为发展中国家减少污染排放提供了帮助。
1.3 碱法活化PS灭活微生物
据报道,PS也能够被氢氧化钠(NaOH)活化,用于灭活大肠杆菌O157:H7[31]。研究进行了两组实验,当使用40 mmol·L−1 PS和30 mmol·L−1 NaOH时,120 s后使6.21 lg CFU·mL−1的大肠杆菌失活[31];当化学剂量增加至500 mmol·L−1 PS和350 mmol·L−1 NaOH时,120 s后8.64 lg CFU·mL−1的大肠杆菌失活[31]。与之前的铁介导或光辅助过程相比,碱基介导的研究较少,其主要原因可能是将溶液pH值调整为强碱性进行处理后需再将pH调整回来,这在实际运用中可能是不可行的。然而,该工艺可能会适用于某些特殊工业废水或某些特殊地区的地下水处理。
1.4 压电催化活化PS灭活微生物
普遍报道的铁介导法和光辅助法都有其固有的局限性,除此以外,近年来一些新型的PDS活化法也被报道用于水的深度消毒。Xia等报道了压电催化过硫酸盐活化体系进行消毒,他们合成了银改性的钛酸钡(Ag-BTO)并将其用作压电催化剂,在超声振动(US)下激活PDS以灭活大肠杆菌[39]。新开发的US/Ag-BTO/PDS工艺可以在5 min内使6.2 lg CFU·mL−1的大肠杆菌完全进入存活但不可培养(VBNC = viable but non-culturable)状态,并在20 min处理后彻底灭活VBNC状态细菌[39]。如图1所示,US/Ag-BTO/PDS体系的消毒性能比其他对照组有更好的消毒效果[39]。在该体系中,压电由超声驱动,可通过超声破坏细菌细胞膜而强化活性物种穿透,而且压电电子也能高效活化过硫酸盐,实现过硫酸盐的高效利用和催化灭菌性能。
2. 操作条件与环境条件影响(Effects of operational andenvironmental conditions)
基于SO4−•的AOPs工艺消毒效率受催化剂和PDS/PMS的用量、接触时间、溶液pH、共存的阴离子或阳离子等操作和环境条件的影响。这些因素对消毒效率的影响在不同的工艺过程中大致呈相同的趋势,即随着剂量和接触时间的增加而增加,但在如碱基活化PDS体系较特殊的过程中会有一些例外。本节对上述提到的活化方法中相关因素的影响进行了总结。
2.1 CT值的影响
在Wordofa等用Fe2+/PDS体系对大肠杆菌进行灭活的研究中,灭活速率常数k几乎随着过硫酸盐的用量增加而呈线性增加,而诱导时间与过硫酸盐的用量增加呈反指数衰减关系[16]。速度常数k在稳定前也随Fe2+用量的增加而迅速增加,而诱导时间则随Fe2+浓度的增加而呈指数衰减[16]。在Xia等使用天然磁黄铁矿/PDS体系灭活大肠杆菌的研究中,随着PDS从0.2 mmol·L−1增加到1.0 mmol·L−1,NP剂量从0.15 g·L−1增加到1.0 g·L−1,灭活效率也得到提高。而由于过量的铁对活性物质的消耗,高浓度的铁会抑制消毒效率[33]。同样,在Sun等使用动力学模型模拟证明中,随着PDS剂量从0 mmol·L−1增加到10 mmol·L−1,MS2噬菌体的失活效率增加,然而大肠杆菌和杆菌孢子的失活效率受PDS剂量的影响较小[36]。与“CT值”类似的是在使用光辅助活化工艺时,灭活效率也随着紫外或可见光通量的增加而增加(光通量=光强度×辐照时间)。
2.2 pH值的影响
根据相关研究报道,消毒效率会受到pH值的显著影响。例如在Xia等报告中,天然磁黄铁矿/PDS系统对大肠杆菌的灭活效率随着pH值由3.0增加到9.0而逐渐降低[33];在Wang等的报告中,随着pH值从4.0增加到9.0,可见光/PDS体系的消毒效率逐渐降低[17];在Wordofa等的研究中,在Fe2+/PDS体系中的大肠杆菌灭活速率随着pH值从5.0增加到9.0而降低,这主要是因为碱性条件下Fe2+形成絮体发生沉降导致过硫酸盐活化效率降低。他们还发现溶液pH值对失活速率常数的影响比活化时长更加显著[16]。
pH值对消毒过程的影响有以下几个方面:(1)pH值影响溶液中铁离子的形态;(2)pH值影响PDS/PMS活化过程中活性物种的生成;(3)pH值也会影响微生物的表面电荷及其对活性物种的亲和力。大多数天然水和再生水的pH值一般在6.5—8.5之间,在这个范围内铁介导的过程可能无法很好地进行,而光辅助催化和新型压电催化在这个pH范围内能体现出其使用的优势,以及在某些碱性较强的工业废水或极端条件下,碱活化的PDS/PMS体系可能会成为深度消毒的良好选择。
2.3 水质的影响
溶解氧(DO = dissolved oxygen)、DOM(dissolved organic matter)、无机阴离子和阳离子以及悬浮固体(SS = suspended solid)等水基质会在不同程度上会影响SO4−•的消毒效率。如1—20 mg·L−1的碳酸氢盐能够降低对大肠杆菌的消毒效率,在降低溶解氧含量时会降低天然磁黄铁矿/PDS体系内的消毒效率[33]。在大多数研究报道中DOM会降低体系内的消毒效率,但在天然磁黄铁矿/PDS体系中DOM对大肠杆菌的消毒效果具有双重作用,其研究表明,在添加1 mg·L−1的DOM后,大肠杆菌的灭活率提高,但随着DOM进一步增加至5 mg·L−1和20 mg·L−1,大肠杆菌的灭活率显著降低[33]。低浓度DOM的增强效应归因于DOM中的对苯二酚和醌基团可以激活PDS生成SO4−•和HO•等活性物种[33]。与光催化相比,铁基催化对水基质成分相对更敏感,因为铁物种会活跃的参与到氧化还原反应和络合反应中,因此在实际应用中应当考虑到目标水质状况来选择合适的处理手段。
3. SO4−•致微生物灭活机理(Mechanisms of microorganism inactivation by SO4−• based processes)
目前已经有文献提出了SO4−•灭活微生物的机理,但现有文献中的观点仍存在有争议的部分。本章将从两个角度总结灭活机理:(1)从反应过程角度,重点介绍了活性物种是如何产生的,负责微生物灭活的主要物种以及主要途径是什么;(2)从微生物角度,重点介绍了微生物的哪些部位(如细胞膜、酶、基因组等)受到活性物种的攻击而导致失活。
3.1 活性物种的生成与贡献度
在紫外光、可见光活化的PDS或PMS的过程中,活性物种的生成是相对简单清晰的。如在UV/PDS体系中,Sun等计算了不同活性物质相对于大肠杆菌、MS2和杆菌孢子的CT值,结果表明消毒效果为HO• > SO4−• > CO3−• O2−•[36]。但与之相反的是,在Wang等的研究中,SO4−•对通过可见光/PDS体系使大肠杆菌失活的贡献程度远远大于HO•和O2−•[17]。这是因为与自由基相比,SO4−•具有相对较长的寿命和更强的氧化能力。不同研究中结果的不一致性是由于其对活性物质不同的量化方法,例如在Sun等的研究中使用动力学模型来预测每种活性物质的贡献[36],而在Wang等的研究中则使用自由基抑制剂达到同样的目的。
在铁介导的过程中,由于铁物种的参与,自由基的化学过程相对更加复杂。在Fe2+/PDS体系中,Wordofa等通过实验和构建模型的方法证明SO4−•导致大肠杆菌O157:H7细胞灭活的速度比HO•快5倍[16],且同时提出SO4−•的优势可能与其与大肠杆菌O157:H7细胞膜表面的富电子基团的高度选择性有关[16]。在天然磁黄铁矿/PDS体系中,根据抑制剂实验和原位ROS的测定,灭活大肠杆菌的活性物质贡献为SO4−• > HO• >H2O2[33],研究证明PDS能够有效地结合到天然磁黄铁矿表面,形成电荷转移复合物从而介导活性物种的产生。在Wang等使用可见光/PDS/hydro-char体系灭活大肠杆菌的工作中,SO4−•被认为是导致大肠杆菌灭活的主要活性物质[17]。在该体系中,SO4−•主要通过3种途径产生:(1)通过可见光激活PDS;(2)通过表面Fe(Ⅱ)激活PDS;(3)通过光催化产生的电子激活PDS。在关于铁介导的PDS/PMS活化体系的一个新的观点是,高价态铁物种可能参与到此类灭活过程,尤其是在接近中性的pH下。在对有机污染物的降解过程中这些高价态铁与自由基一样具有不可忽略的贡献[40-41]。例如,一些研究预测Fe(Ⅳ)可能会参与到微生物的去除过程[42],但迄今为止没有直接证据证明,因此这个问题值得进一步研究。
在新型压电催化系统中,Xia等报道了通过压电催化产生电子和超氧自由基活化PDS连续生成SO4−•和HO•对大肠杆菌进行高效灭活[39]。图2显示了活性物种的产生途径[39]。其中值得关注的是通过使用US/Ag-BTO/PS体系灭活大肠杆菌的过程中发现了超声波和自由基氧化之间的协同作用,如压电电子和超声本身可以帮助活化过硫酸盐来强化自由基生成。
3.2 微生物的损伤和灭活
过往的研究提供了一些关于微生物是如何被破坏和灭活的信息,它们是基于SO4−•的消毒工艺处理前后微生物从宏观到微观的特征而得出的。例如Wang等报道了大肠杆菌的细胞膜受到了被可见光激活PDS所产生的活性物质的破坏,随后CAT(catalase)和SOD(superoxide dismutase)等一些抗氧化酶被诱导进而使基因组DNA受损,导致细胞死亡[17]。在他们接下来的工作中使用水热炭增强了该过程,并得到了相似的机制,即细菌灭活过程被确定为从外膜开始最终到细胞内成分降解 [24]。Xiao等研究了用于大肠杆菌灭活的ZVI/PDS体系,通过扫描电子显微镜(SEM)拍摄的受试细菌的形态图像,表明SO4−•在细胞壁或细胞膜上引发氧化反应,影响膜的通透性和生理功能,最终导致其不可逆损伤[32]。 Xia等利用天然磁黄铁矿/PDS工艺系统地研究了大肠杆菌细胞破坏过程,由外膜、肽聚糖层和细胞质膜组成的细菌包膜首先受到系统中产生的活性物质的攻击[33]。利用BacLight kit显微荧光法直接观察天然磁黄铁矿/PS体系中细胞膜的通透性变化,同时由于ATP水平急剧下降,细胞几乎在瞬间因代谢停滞而失活。此外,他们的研究还提到反应性物种通过表现为质子载体或通过抑制呼吸链中的酶来抑制ATP的形成,活性物种在穿透细胞包膜后,通过与细胞质蛋白、基因组等多种生物分子发生反应损伤细胞,最后还观察到DNA条带的荧光强度逐渐减弱可知基因组DNA被破坏 [33]。但作者同时指出,处理后的细胞可能处于一种可存活但不可培养(VBNC)的状态,这种状态仍可能对水体造成健康风险[33]。Xia等提出了一种新的压电催化强化消毒工艺来处理VBNC状态下的微生物[39]。研究人员通过跟踪ATP(adenosine triphosphate)水平检测了可培养状态的大肠杆菌以及VBNC大肠杆菌细胞的代谢活性,并通过荧光光谱仪检测大肠杆菌的成分变化证明大肠杆菌在可培养状态和VBNC状态均被有效灭活 [39]。因此,基于SO4−•的AOPs能够通过氧化协同其他外加能量如光,超声等作用实现对微生物胞膜到胞内物质的裂解矿化。
4. 消毒副产物的形成(Formation of disinfection by-products)
基于SO4−•的工艺已被发展用于水的深度处理,但消毒过程中产生的消毒副产物却很少被提及。事实上在任何涉及消毒的化学过程中,都不可避免地会形成DBPs(disinfection by-products),虽然基于SO4−•的工艺手段中产生的DBPs可能与氯、氯胺或二氧化氯等消毒过程中形成的DBPs不同,但它们也同样会造成一定的健康风险。本节将对基于SO4−•的深度水消毒处理过程中形成的潜在DBPs进行总结。
4.1 无机副产物的形成
使用基于SO4−•的深度水处理过程中会形成无机副产物,典型的无机副产物是由PDS或PMS分解生成的硫酸盐。当使用PDS时由于1 mol的PDS可产生2 mol硫酸盐,无机副产物的形成尤其严重。由于摄入含有高硫酸盐含量的饮用水会对胃肠道产生影响,世卫组织建议向卫生当局通报硫酸盐浓度超过500 mg·L−1的饮用水源[42- 43]。然而硫酸盐在美国环保局中按二级最大污染物水平(secondary maximum contaminant level = SMCL)标准分类,饮用水中硫酸盐的SMCL为250 mg·L−1 [44]。但假设所有剂量的PDS或PMS最终全部分解为硫酸盐,并以250 mg·L−1倒推,则PDS和PMS的最大剂量仅为1.3 mmol·L−1和2.6 mmol·L−1。但在许多已报道的文献中,PDS/PMS的剂量均超过了该计算值,这表明在实际应用此类技术时,过量硫酸盐的形成可能会成为值得注意的问题。
而除了硫酸盐,用于自由基生成的催化剂也可能是这些过程的副产物。除了上文提到的铁泥外,其他金属或非金属材料也可能成为有害副产物。例如在PDS/PMS活化中,钴表现出比铁更优越的催化性能,但钴金属有毒,不能留在任何处理过的饮用水中。在实际应用时,金属或非金属材料的浸出始终是一个主要问题。
此外,当使用SO4−•进行水的深度处理时,氯酸盐的形成可能成为另一个问题。氯离子在水中普遍存在,而氯化物能够被SO4−•氧化成氯酸盐,这对饮用处理水的人群造成了极大的健康问题[45]。世卫组织和美国环境保护局将饮用水中氯酸盐的水质健康参考水平标准分别定为0.7 mg·L−1和0.21 mg·L−1 [46]。因此在使用基于SO4−•工艺处理含高浓度氯化物的水时需要对氯酸盐的形成应予以关注。
4.2 有机副产物的形成
在基于SO4−•的工艺用于水的深度消毒处理时还会产生有机副产物。SO4−•被认为是一种绿色环保的自由基,与如活性氯和活性溴等的卤素自由基相比,SO4−•产生的有机副产品数量有限。然而,最近的一项研究表明在用SO4−•处理水中有机污染物和灭菌的过程中会普遍产生有机硫酸盐[47]。其中一些有机硫酸盐,例如硫酸苯酯(phenyl sulfate)和硫酸对甲酚酯(p-cresyl sulfate)均是有害的尿毒症毒素。因此在使用基于SO4−•的水深度消毒处理的过程中,应进一步调查关注有机硫酸盐的形成、去向及其毒性。
同时,水中普遍存在的氯化物可通过SO4−•氧化为活性氯物种,如Cl•、Cl2•−和ClO•[46],这些活性氯物种通过取代反应和加成反应与水中溶解的有机物反应形成如三卤甲烷、卤乙酸、水合氯醛等的氯化副产物[48]。这些氯化副产品是会对人类健康构成严重威胁的致癌物[49]。而由于自由基转化,还可能会形成溴化和碘化副产物。因此在对水使用基于SO4−•的深度消毒处理时需要对卤化副产物的形成、迁移和毒性进行评估。
5. 总结与展望(Conclusions and future perspectives)
本文综述了基于SO4−•的AOPs应用于消毒的研究进展。本文首先回顾了过往研究中各种基于SO4−•工艺对不同微生物的灭活作用,这些工艺根据其驱动力主要分为三大类:铁介导、光辅助和新型过硫酸盐活化(例如压电催化),针对各类当前的研究进展和主要发现进行总结,并讨论了各类工艺的深度消毒处理的优缺点。接着本文概述了不同操作因素和环境条件对消毒性能的影响,包括过硫酸盐和催化剂的用量、接触时间、光照通量、pH值、溶解氧和其他水基质成分等,对上述不同因素造成影响的原因进行了讨论。进一步概述了基于SO4−•消毒的微生物灭活机理,并从两个角度对这些机理进行了综述,即从反应过程角度总结了活性物种是如何形成的,以及每种活性物种对微生物失活的贡献,并且从微生物的角度总结了微生物的损伤及灭活过程。最后,本文回顾了基于SO4−•工艺应用中无机和有机消毒副产物的形成,通过机理解释和讨论指出了与副产物形成有关的一些被忽视的问题。该综述还为基于SO4−•工艺的饮用水处理过程中工程设计和运行提供了建议。
(1)目前的研究仅局限于对少数微生物的失活,如大肠杆菌、MS2噬菌体和杆菌孢子,这对基于SO4−•的消毒方法在广谱致病微生物灭活方面的适用性和优点提供的信息较为有限。建议对更多与人类健康相关的微生物进行调查。此外对不同微生物进行的比较较少,考虑到SO4−•的选择性,建议在分子水平或借助一些微生物学工具对不同微生物的失活进行比较以供将来研究。
(2)在涉及铁物种活化的过程中,高价铁(如Fe(Ⅳ)和Fe(Ⅴ))对微生物失活的作用尚不清楚。因此,目前在铁介导活化方面对不同工艺过程下的SO4−•、HO−•以及H2O2、O2−•、和1O2等其他活性物质的相对贡献的判断可能不准确。建议对高价铁(如Fe(Ⅳ)和Fe(Ⅴ))等对微生物失活的贡献进行定性和定量的研究说明。
(3) 目前,基于SO4−•工艺中消毒副产物的形成往往被忽略,需要对微生物本身或共存的有机物产生的有机副产物和无机副产物进行更多的研究,以及分析探究更多关于基于SO4−•工艺处理前后水的毒理学变化的更基本的资料。
(4)基于SO4−•的AOP处理技术,虽然文献报道可以通过增加反应物浓度提升消毒效率,但如此也会引发一些技术应用问题,如吨水处理费用会远高于UV和氯,且大量硫酸盐会高于各类水质标准,甚至会导致饮用水后续管网腐蚀或污水排入河流中导致底泥生成H2S气体等。针对这些应用局限性,可以考虑引入微纳气泡或结合UV来协同提高过硫酸盐的活化效率及杀菌速率,一方面能有效降低反应物的投加浓度,另一方面还可以高效抑制微生物的复活。
(5)综合该工艺的优缺点,基于SO4−•的AOPs仍然在再生水、地下水或是应急场所中的消毒应用有发展前景。一是再生水,由于再生水中还含有较多持久性污染物,SO4−•可以同时降解某些污染物,并且可以灭活多种病原微生物;二是地下水,如紫外和臭氧无法穿透土壤,而过硫酸盐是可以溶解渗透的;且过硫酸盐是可以高效灭活本身具有抗氯性的真菌及阿米巴等微生物;三是某些特殊应急场所有可能处于复杂pH范围,而SO4−•具有广泛pH适应范围。
考虑到对过硫酸盐剂量和pH条件较严格的要求,在诸如使用点或入口水处理装置等一些小型处理装置中基于SO4−•的深度消毒水处理工艺的应用似乎比在大型水处理厂中的应用更有前景。在这种情况下,利用太阳能等自然资源加强基于SO4−•的消毒过程可能成为实现可持续发展目标——“清洁的水和卫生”要求的直接现实途径。
-
表 1 铁基活化SO4−•致微生物失活
Table 1. Microorganism inactivation by SO4−•-based processes activated by iron species
体系Process 目标微生物Target microorganisms 实验条件Experimental conditions 消毒效率Disinfection efficiency 参考文献Reference Fe2+/PDS O157:H7大肠杆菌 3 mmol·L−1 PDS、3 mmol·L−1 FeSO4、3 mmol·L−1 NH2OH和pH7的饮用水 约130 min达到95%的灭活率 [16] Fe2+/PDS O157:H7大肠杆菌和李斯特菌 40 mmol·L−1 PDS 和 13.3 mmol·L−1 Fe2+ 120 s后(7.77 ± 0.57 )lg CFU·mL−1的大肠杆菌和(7.25 ± 0.36) lg CFU·mL−1的李斯特菌失活 [31] ZVI/PDS 大肠杆菌BL21和粪肠球菌 大肠杆菌测试中:3 mmol·L−1 PDS、 0.6 g·L−1 ZVI粪肠球菌测试中:1 mmol·L−1 PDS、0.2 g·L−1 ZVI以及 pH 为5.5 80 min后90%的大肠杆菌失活;12 min后75%的粪肠球菌失活 [11] 天然黄铁矿/PDS 大肠杆菌K-12 0.5 mmol·L−1 PDS、1g·L−1 NP 以及 pH 为5.0 15 min内7 lg CFU·mL−1大肠杆菌失活 [33] Fe2+, Fe2O3, nZVI/PDS和PMS 大肠杆菌 0.5 mmol·L−1 PDS、 0.5 mmol·L−1 PMS、1 mmol·L−1 Fe(Ⅱ)、1 mmol·L−1 Fe2O3以及50 mg·L−1 ZVI 5 min内PMS与Fe2+、Fe2O3和ZVI的偶联反应使大肠杆菌完全失活 [34] 表 2 光介导活化SO4−•致微生物失活
Table 2. Microorganism inactivation by SO4−•-based processes activated by light
体系Process 目标微生物Target microorganisms 实验条件Experimental conditions 消毒效率Disinfection disinfection 参考文献Reference UV/PDS 大肠杆菌、MS2噬菌体、枯草芽孢杆菌孢子 0.3 mmol·L−1 PDS,大肠杆菌、噬菌体MS2、枯草芽孢杆菌孢子紫外光剂量分别为8.8、30、30 mL·cm−2 3种微生物在30 min内均达到4 lg CFU·mL−1失活 [36] UV/PDS T. harzianum孢子 0.1 mmol·L−1 PMS, 紫外光剂量为35 mL·cm−2 达到2 lg CFU·mL−1失活 [37] 可见光/PDS 大肠杆菌 2 mmol·L−1 PDS, 30 °C, pH 6.0, λ ≥ 420 nm 40 min内6 lg CFU·mL−1大肠杆菌失活 [17] 可见光/ hydrochar /PS 大肠杆菌 200 mg·L−1 MHC; 2 mmol·L−1 PS; 25 °C, pH 6.0, λ ≥ 420 nm 40 min后8 lg CFU·mL−1大肠杆菌失活 [24] 太阳光/PMS;太阳光/Fe2+/PMS 大肠杆菌 装有蒸馏水的塑料瓶中加入18 μmol·L−1 Fe2+, 290 μmol·L−1 H2O2, 和 36 μmol·L−1 PMS 650 Wh·m−2太阳光/PMS体系和450 Wh·m−2太阳光/Fe2+/PMS体系达到5 lg CFU·mL−1失活 [38] -
[1] MUSTAFA A, SCHOLZ M, KHAN S, et al. Application of solar disinfection for treatment of contaminated public water supply in a developing country: Field observations [J]. Journal of Water and Health, 2013, 11(1): 135-145. doi: 10.2166/wh.2012.119 [2] MARA D. Sanitation: what's the real problem? [J]. IDS Bulletin, 2012, 43(2): 86-92. doi: 10.1111/j.1759-5436.2012.00311.x [3] HUTTON G, HALLER L, BARTRAM J. Global cost-benefit analysis of water supply and sanitation interventions [J]. Journal of Water and Health, 2007, 5(4): 481-502. doi: 10.2166/wh.2007.009 [4] NIEUWENHUIJSEN M J, TOLEDANO M B, EATON N E, et al. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: A review [J]. Occupational and Environmental Medicine, 2000, 57(2): 73-85. doi: 10.1136/oem.57.2.73 [5] ZHANG C, LI Y, SHUAI D M, et al. Progress and challenges in photocatalytic disinfection of waterborne Viruses: A review to fill current knowledge gaps [J]. Chemical Engineering Journal, 2019, 355: 399-415. doi: 10.1016/j.cej.2018.08.158 [6] PICHEL N, VIVAR M, FUENTES M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods [J]. Chemosphere, 2019, 218: 1014-1030. doi: 10.1016/j.chemosphere.2018.11.205 [7] ELLIS K V. Water disinfection: A review with some consideration of the requirements of the third world [J]. Critical Reviews in Environmental Control, 1991, 20(5/6): 341-407. [8] DENG L Z, MUJUMDAR A S, PAN Z L, et al. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review [J]. Critical Reviews in Food Science and Nutrition, 2020, 60(15): 2481-2508. doi: 10.1080/10408398.2019.1649633 [9] LAZAROVA V, SAVOYE P, JANEX M L, et al. Advanced wastewater disinfection technologies: State of the art and perspectives [J]. Water Science and Technology, 1999, 40(4/5): 203-213. [10] LOEB S, HOFMANN R, KIM J H. Beyond the pipeline: Assessing the efficiency limits of advanced technologies for solar water disinfection [J]. Environmental Science & Technology Letters, 2016, 3(3): 73-80. [11] XIAO R Y, LIU K, BAI L, et al. Inactivation of pathogenic microorganisms by sulfate radical: Present and future [J]. Chemical Engineering Journal, 2019, 371: 222-232. doi: 10.1016/j.cej.2019.03.296 [12] LEE J, von GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks [J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [13] MATZEK L W, CARTER K E. Activated persulfate for organic chemical degradation: A review [J]. Chemosphere, 2016, 151: 178-188. doi: 10.1016/j.chemosphere.2016.02.055 [14] ZHOU Z, LIU X T, SUN K, et al. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review [J]. Chemical Engineering Journal, 2019, 372: 836-851. doi: 10.1016/j.cej.2019.04.213 [15] TSITONAKI A, PETRI B, CRIMI M, et al. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review [J]. Critical Reviews in Environmental Science and Technology, 2010, 40(1): 55-91. doi: 10.1080/10643380802039303 [16] WORDOFA D N, WALKER S L, LIU H Z. Sulfate radical-induced disinfection of pathogenic Escherichia coli O157: H7 via iron-activated persulfate [J]. Environmental Science & Technology Letters, 2017, 4(4): 154-160. [17] WANG W J, WANG H N, LI G Y, et al. Catalyst-free activation of persulfate by visible light for water disinfection: Efficiency and mechanisms [J]. Water Research, 2019, 157: 106-118. doi: 10.1016/j.watres.2019.03.071 [18] MORENO-ANDRÉS J, FARINANGO G, ROMERO-MARTÍNEZ L, et al. Application of persulfate salts for enhancing UV disinfection in marine waters [J]. Water Research, 2019, 163: 114866. doi: 10.1016/j.watres.2019.114866 [19] AHN S, PETERSON T D, RIGHTER J, et al. Disinfection of ballast water with iron activated persulfate [J]. Environmental Science & Technology, 2013, 47(20): 11717-11725. [20] 徐梓淞, 宋雄伟, 黄闻宇, 等. 不同活化过硫酸盐体系的机理分析及不同无机阴离子的作用: 以两种有机染料为例 [J]. 环境化学, 2022, 41(4): 1412-1424. doi: 10.7524/j.issn.0254-6108.2020122103 XU Z S, SONG X W, HUANG W Y, et al. Mechanism analysis of different activated persulfate systems and effects of different inorganic anions: a case study of two organic dyes [J]. Environmental Chemistry, 2022, 41(4): 1412-1424(in Chinese). doi: 10.7524/j.issn.0254-6108.2020122103
[21] MORENO-ANDRÉS J, PEPERZAK L. Operational and environmental factors affecting disinfection byproducts formation in ballast water treatment systems [J]. Chemosphere, 2019, 232: 496-505. doi: 10.1016/j.chemosphere.2019.05.152 [22] COWIE B E, PORLEY V, ROBERTSON N. Solar disinfection (SODIS) provides a much underexploited opportunity for researchers in photocatalytic water treatment (PWT) [J]. ACS Catalysis, 2020, 10(20): 11779-11782. doi: 10.1021/acscatal.0c03325 [23] MEIERHOFER R, LANDOLT G. Factors supporting the sustained use of solar water disinfection—Experiences from a global promotion and dissemination programme [J]. Desalination, 2009, 248(1/2/3): 144-151. [24] WANG W J, WANG H N, LI G Y, et al. Visible light activation of persulfate by magnetic hydrochar for bacterial inactivation: Efficiency, recyclability and mechanisms [J]. Water Research, 2020, 176: 115746. doi: 10.1016/j.watres.2020.115746 [25] XIA D H, YIN R, SUN J L, et al. Natural magnetic pyrrhotite as a high-Efficient persulfate activator for micropollutants degradation: Radicals identification and toxicity evaluation [J]. Journal of Hazardous Materials, 2017, 340: 435-444. doi: 10.1016/j.jhazmat.2017.07.029 [26] MA H K, ZHANG L L, HUANG X M, et al. A novel three-dimensional galvanic cell enhanced Fe2+/persulfate system: High efficiency, mechanism and damaging effect of antibiotic resistant E. coli and genes [J]. Chemical Engineering Journal, 2019, 362: 667-678. doi: 10.1016/j.cej.2019.01.042 [27] 韩仪, 黄明杰, 周涛, 等. 氧化铜活化过硫酸盐的界面反应机理 [J]. 环境化学, 2020, 39(3): 735-744. doi: 10.7524/j.issn.0254-6108.2019110101 HAN Y, HUANG M J, ZHOU T, et al. Interfacial reaction mechanism of copper oxide activating persulfate [J]. Environmental Chemistry, 2020, 39(3): 735-744(in Chinese). doi: 10.7524/j.issn.0254-6108.2019110101
[28] SERNA-GALVIS E A, VÉLEZ-PEÑA E, OSORIO-VARGAS P, et al. Inactivation of carbapenem-resistant Klebsiella pneumoniae by photo-Fenton: Residual effect, gene evolution and modifications with citric acid and persulfate [J]. Water Research, 2019, 161: 354-363. doi: 10.1016/j.watres.2019.06.024 [29] XIAO S, CHENG M, ZHONG H, et al. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review [J]. Chemical Engineering Journal, 2020, 384: 123265. doi: 10.1016/j.cej.2019.123265 [30] HOU K J, PI Z J, YAO F B, et al. A critical review on the mechanisms of persulfate activation by iron-based materials: Clarifying some ambiguity and controversies [J]. Chemical Engineering Journal, 2021, 407: 127078. doi: 10.1016/j.cej.2020.127078 [31] QI H, HUANG Q G, HUNG Y C. Efficacy of activated persulfate in inactivating Escherichia coli O157: H7 and Listeria monocytogenes [J]. International Journal of Food Microbiology, 2018, 284: 40-47. doi: 10.1016/j.ijfoodmicro.2018.06.021 [32] XIAO R Y, BAI L, LIU K, et al. Elucidating sulfate radical-mediated disinfection profiles and mechanisms of Escherichia coli and Enterococcus faecalis in municipal wastewater [J]. Water Research, 2020, 173: 115552. doi: 10.1016/j.watres.2020.115552 [33] XIA D H, LI Y, HUANG G C, et al. Activation of persulfates by natural magnetic pyrrhotite for water disinfection: Efficiency, mechanisms, and stability [J]. Water Research, 2017, 112: 236-247. doi: 10.1016/j.watres.2017.01.052 [34] 陈妍希, 严登明, 朱明山. 外场效应强化过硫酸盐氧化技术去除有机污染物的研究进展 [J]. 环境科学研究, 2022, 35(1): 131-140. CHEN Y X, YAN D M, ZHU M S. Recent progress in removal of organic pollutants by external-field effect enhanced persulfate oxidation processes [J]. Research of Environmental Sciences, 2022, 35(1): 131-140(in Chinese).
[35] YIN R, SUN J L, XIANG Y Y, et al. Recycling and reuse of rusted iron particles containing core-shell Fe-FeOOH for ibuprofen removal: Adsorption and persulfate-based advanced oxidation [J]. Journal of Cleaner Production, 2018, 178: 441-448. doi: 10.1016/j.jclepro.2018.01.005 [36] SUN P Z, TYREE C, HUANG C H. Inactivation of Escherichia coli, bacteriophage MS2, and Bacillus spores under UV/H2O2 and UV/peroxydisulfate advanced disinfection conditions [J]. Environmental Science & Technology, 2016, 50(8): 4448-4458. [37] WEN G, DENG X L, WAN Q Q, et al. Photoreactivation of fungal spores in water following UV disinfection and their control using UV-based advanced oxidation processes [J]. Water Research, 2019, 148: 1-9. doi: 10.1016/j.watres.2018.10.028 [38] OZORES DIEZ P, GIANNAKIS S, RODRÍGUEZ-CHUECA J, et al. Enhancing solar disinfection (SODIS) with the photo-Fenton or the Fe2+/peroxymonosulfate-activation process in large-scale plastic bottles leads to toxicologically safe drinking water [J]. Water Research, 2020, 186: 116387. doi: 10.1016/j.watres.2020.116387 [39] XIA D H, TANG Z Y, WANG Y C, et al. Piezo-catalytic persulfate activation system for water advanced disinfection: Process efficiency and inactivation mechanisms [J]. Chemical Engineering Journal, 2020, 400: 125894. doi: 10.1016/j.cej.2020.125894 [40] WANG Z, JIANG J, PANG S Y, et al. Is sulfate radical really generated from peroxydisulfate activated by iron(II) for environmental decontamination? [J]. Environmental Science & Technology, 2018, 52(19): 11276-11284. [41] 谷得明, 郭昌胜, 冯启言, 等. 基于硫酸根自由基的高级氧化技术及其在环境治理中的应用 [J]. 环境化学, 2018, 37(11): 2489-2508. doi: 10.7524/j.issn.0254-6108.2018012102 GU D M, GUO C S, FENG Q Y, et al. Sulfate radical-based advanced oxidation processes and its application in environmental remediation [J]. Environmental Chemistry, 2018, 37(11): 2489-2508(in Chinese). doi: 10.7524/j.issn.0254-6108.2018012102
[42] WANG Z, QIU W, PANG S Y, et al. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment [J]. Chemical Engineering Journal, 2019, 371: 842-847. doi: 10.1016/j.cej.2019.04.101 [43] LEE C H. Use of high-valent metal species produced by the Fenton (-like) reactions in water treatment[M]//Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability. Cham: Springer International Publishing, 2019: 89. [44] MERIDE Y, AYENEW B. Drinking water quality assessment and its effects on residents health in Wondo genet campus, Ethiopia [J]. Environmental Systems Research, 2016, 5: 1. doi: 10.1186/s40068-016-0053-6 [45] SONG W, LI J, WANG Z Y, et al. A mini review of activated methods to persulfate-based advanced oxidation process [J]. Water Science and Technology, 2019, 79(3): 573-579. doi: 10.2166/wcc.2018.168 [46] HOU S D, LING L, DIONYSIOU D D, et al. Chlorate formation mechanism in the presence of sulfate radical, chloride, bromide and natural organic matter [J]. Environmental Science & Technology, 2018, 52(11): 6317-6325. [47] BOLYARD M, FAIR P S, HAUTMAN D P. Occurrence of chlorate in hypochlorite solutions used for drinking water disinfection [J]. Environmental Science & Technology, 1992, 26(8): 1663-1665. [48] MARRON E L, van BUREN J, CUTHBERTSON A A, et al. Reactions of α, β-unsaturated carbonyls with free chlorine, free bromine, and combined chlorine [J]. Environmental Science & Technology, 2021, 55(5): 3305-3312. [49] YIN R, LING L, SHANG C. Wavelength-dependent chlorine photolysis and subsequent radical production using UV-LEDs as light sources [J]. Water Research, 2018, 142: 452-458. doi: 10.1016/j.watres.2018.06.018 -






 DownLoad:
DownLoad: