-
三唑酮(Triadimefon,TDF),又名粉锈宁、百里通,是一种广泛用于植物病虫害防治的三唑类杀菌剂[1],具有高效、低毒、残留期长、内附性强等特点[2–4]。2015年我国TDF商品使用量为1.43万吨[5],受农业面源污染影响,TDF在不同水环境中被频繁检出。付岩[6]发现浙江诸暨稻田周围地表水中TDF的浓度高达到12 μg·L−1,附近鱼塘和河流也会受到影响,检出浓度为0.1—0.4 μg·L−1。刘娜等[7]调查了太湖流域饮用水源地、入湖河口及湖体中丰水期的TDF暴露水平,发现TDF的检出率为100%,检出浓度为0.002—0.007 μg·L−1。游明华[8]对九龙江的16个点位进行检测,发现丰水期TDF的检出率为43.8%。刘园等[9]调查了贵阳市4个主要集中式饮用水源地冬季枯水期的有机氯农药残留情况,发现TDF的检出率为36.4%,检出浓度为0.185—5.22 μg·L−1。Stamatis等[10]根据欧盟风险评估技术指南推荐的方法,计算出三唑酮的预测无观察效应浓度(predicted no effect concentration,PNEC)为0.34 μg·L−1。根据计算出的PNEC值,说明部分水环境中检出的三唑酮的浓度会对水生生物存在较高风险,可能会影响生态健康安全。
TDF不仅对水生生物具有急性致死、致畸以及慢性毒性效应[5,11–14],还能引起大鼠的运动活动增加和单胺代谢改变而发生病变[15],其在小鼠和大鼠的肝脏中具有致瘤性[16–17]。2006年欧盟(EU)将TDF列入具有内分泌-生殖干扰毒性的农药及其代谢产物名单。TDF不仅会破坏水生态环境,还对人类饮水安全和健康存在潜在的威胁[18]。常规水处理工艺是以降低原水浊度、除细菌等为主要目的,对TDF等农药的去除效果并不理想[19]。紫外/氯(UV/Cl)工艺被认为是一种有效的新兴高级氧化工艺,可用于微污染物降解[20–24],该体系具有高效、转化完全、降解反应时间短等优点。在UV/Cl体系中,氯发生光解反应,产生具有高氧化电位的羟基自由基(HO·)和包括Cl·和Cl2·–等的活性氯(reactive chlorine species,RCS)[25]。HO·和Cl·可以通过电子转移,脱氢和加成反应与有机污染反应[26–27]。在该体系中可以生成一级羟基自由基HO·和Cl·以及ClO·和Cl2·–,其过程如下式(1—5)所示[26,28–29]。HO·和RCS(即Cl·、ClO·和Cl2·–)共同作用,通过其高氧化性降解微污染物。
本研究在UV/Cl体系中降解TDF,考察了不同反应条件下TDF的降解效果,分别探究不同TDF浓度,不同次氯酸钠(NaClO)浓度,不同pH,溴离子(Br-)浓度和叔丁醇(t-BuOH)对降解效果的影响。利用超高效液相色谱质谱联用仪(UPLC-MS/MS)全扫描模式检测了降解反应中间产物,并推断了可能的降解路径,为去除水环境中的三唑类杀菌剂提供了技术和理论支持。
-
超高效液相色谱质谱联用仪(Waters Open Architecture ACQUITY QSM-BSM-2777C-FLR,Waters公司,美国)配备C18反相色谱柱(ACQUITYUPLC®BEH C18,1.7 μm,50 mm×2.1 mm,Waters,USA)用于TDF定量及中间产物鉴定,TDF标准品购自AccuStandard公司,详细信息见表1。乙腈(色谱纯)购自Fisher Scientific公司(Poole UK),甲酸(Formic acid,FA)(色谱纯)购自Sigma-Aldrich公司(美国)。溴化钠(NaBr)、t-BuOH、过硫酸盐(Na2S2O8)、磷酸二氢钾(KH2PO4)、磷酸氢二钠(Na2HPO4)、氢氧化钠(NaOH)和浓硫酸(H2SO4)购自国药控股化学试剂有限公司(中国)。实验用超纯水由Milli-Q系统(Millipore,MA,美国)制备。尼龙针式滤头(0.22 μm)购自Waters公司(美国)。磷酸盐缓冲溶液由0.2 mol·L−1的磷酸二氢钾与0.2 mol·L−1的磷酸氢二钠制备。
-
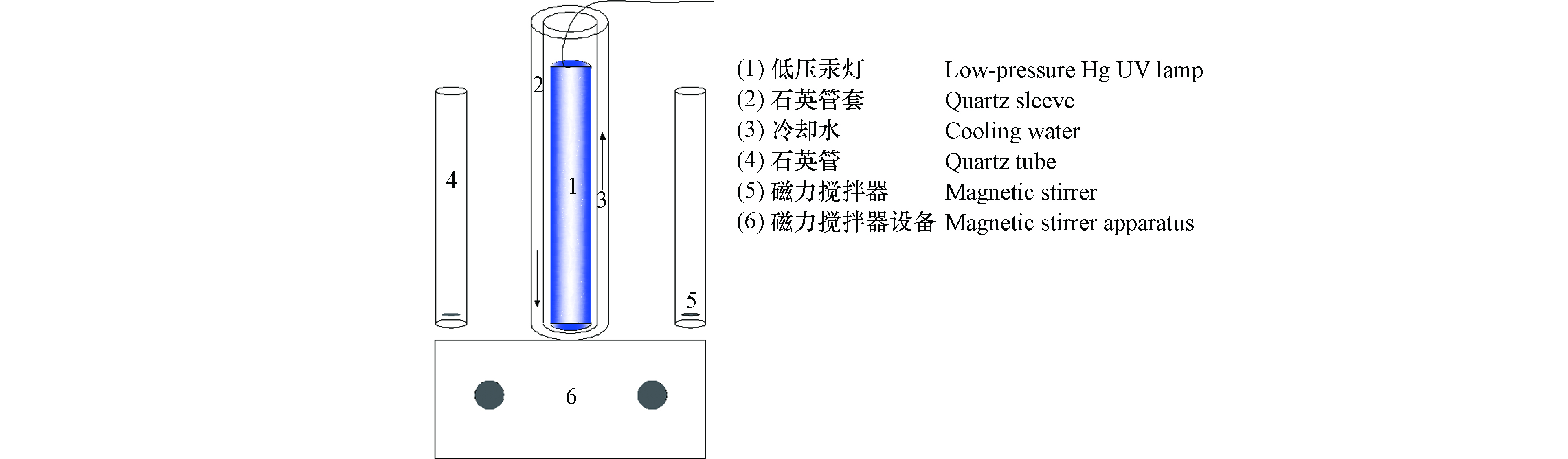
UV/Cl降解TDF反应在光反应仪(见图1)(XPA-7,南京胥江机电厂)中进行,石英管的有效体积为50 mL。将低压汞灯(功率为11 W,发射波长254 nm,中国飞利浦公司)放置在石英管套中,实验开始前预热30 min。根据文献中的测量方法[30],测定紫外辐照剂量为0.12 mW·cm−2。TDF初始浓度2 μmol·L−1,加入一定量新制备的NaClO溶液,磁力搅拌(300 r·min−1)将溶液充分混合,放置于紫外线照射下,反应开始并计时。在一定的时间间隔内取样,并利用1 mmol·L−1的Na2S2O3猝灭残余自由基,样品经0.22 μm尼龙针式滤头过滤后上机检测。
-
UPLC-MS/MS测定TDF浓度,使用反相色谱柱分离目标物,进样量为5 μL,流动相A为0.1%FA水溶液(V/V),流动相B为乙腈,液相洗脱梯度见表2。柱温保持在40 oC。Xevo T-QS三重四极杆串联质谱仪采用多反应监测(multiple reaction monitoring,MRM)与电喷雾正离子源(ESI+)模式对目标化合物进行定量分析。氮气作为脱溶剂和雾化气体,毛细管电压为1 kV,离子源和脱溶温度分别为150 ℃和500 ℃,TDF质谱参数详见表3。
-
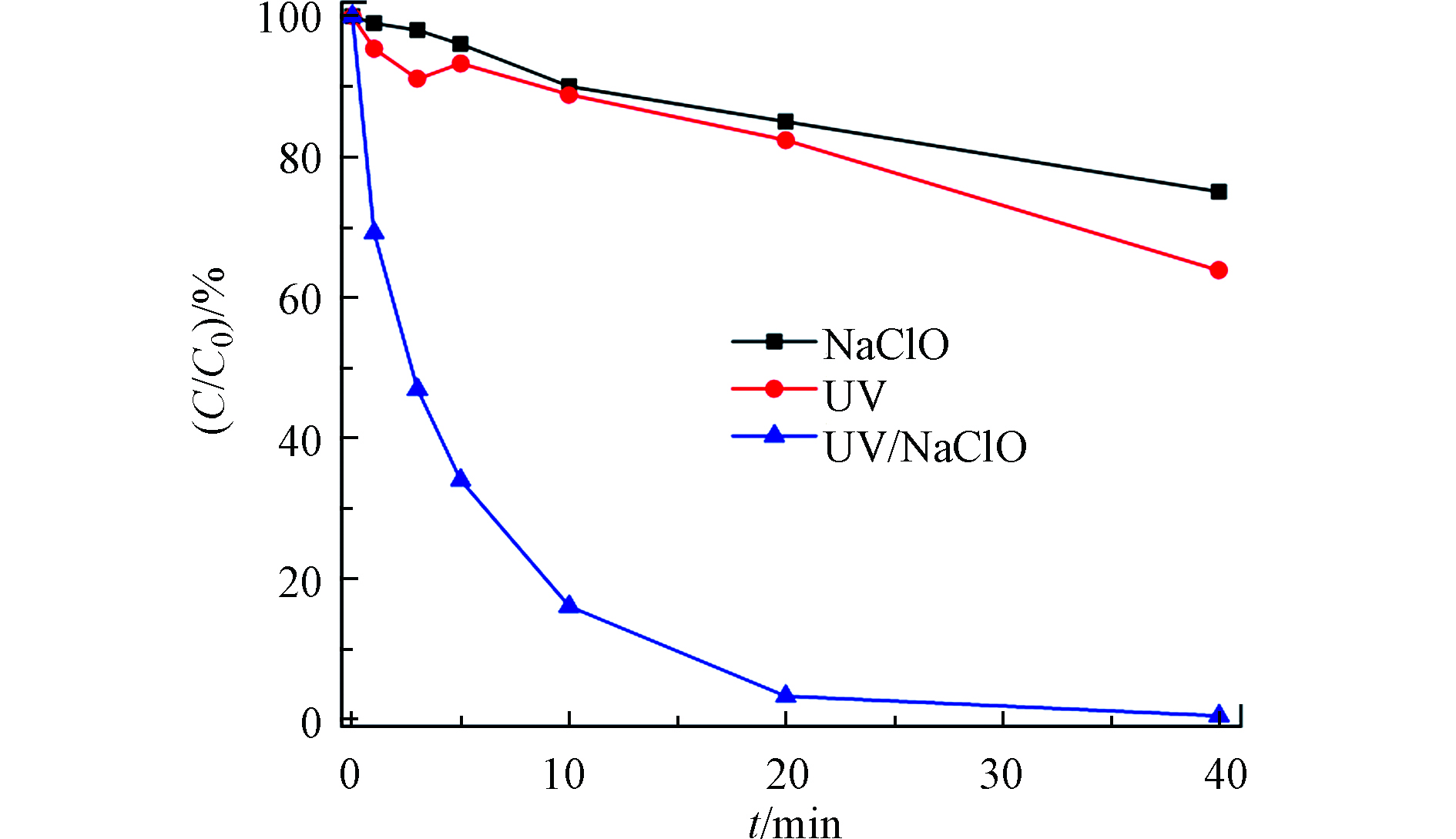
当[TDF]0=2 μmol·L−1,[NaClO]0=5000 μmol·L−1,初始pH为7,反应温度为(25±1)℃时,对比单独UV,单独NaClO和UV/Cl联合体系这3种不同反应条件下目标化合物的降解效果,探究不同反应条件对目标物降解效果的影响。降解效果如图2所示,单独使用UV照射或单独添加NaClO时,TDF的降解效果相对较差,单独使用UV照射20 min,TDF降解了17.7%(kobs=0.0084 min−1),这与之前报道的TDF可在日光下降解的结果一致[31–34]。单独使用NaClO反应20 min后,TDF降解了15.1%(kobs=0.0083 min−1)。在UV/Cl反应体系中,TDF在20 min内降解了98.8%,TDF在UV/Cl体系中的表观反应速率(kobs)为0.164 min−1,是单独使用UV或NaClO的19倍。在UV/Cl体系中,NaClO经过UV光解反应产生HO·和RCS。HO·氧化还原电位为2.8 V,是一种非选择性强氧化剂,可通过脱氢,亲电加成和电子转移与有机物反应[35];RCS是选择性氧化剂,尽管Cl·(2.4 V)、
${\text{Cl}}_{\text{2}}^{\cdot{-}}$ (2.0 V)[26]和ClO·(1.5—1.8 V)[36]的氧化还原电位低于HO·,但RCS更容易通过电子转移与一些富电子物质反应(如氯苯、苯酚和苯甲酸等)[27,37]。在UV/Cl体系中,UV,OCl−和自由基共同作用下降解目标污染物。 -
在pH为7,温度为(25±1)℃的反应条件下,在TDF和NaClO的初始浓度分别为2 μmol·L−1和5000 μmol·L−1时,探究不同反应物初始浓度对TDF降解效果的影响,并计算反应速率和反应级数。
TDF和NaClO的基本动力学方程为:
式中,r为反应速率;
$ \left[\text{TDF}\right] $ 和$ \left[\text{NaClO}\right] $ 分别为TDF和NaClO的浓度;α和β分别为反应级数;${{k}}_{\text{TDF}\cdot\text{NaClO}}$ 为TDF和NaClO的反应速率常数。当NaClO的初始浓度比TDF的初始浓度高出两个数量级时,认为在整个反应过程中NaClO的浓度不发生变化,即式(6)中的[NaClO]为一个常数[38–39],则式(6)简化为式(7):
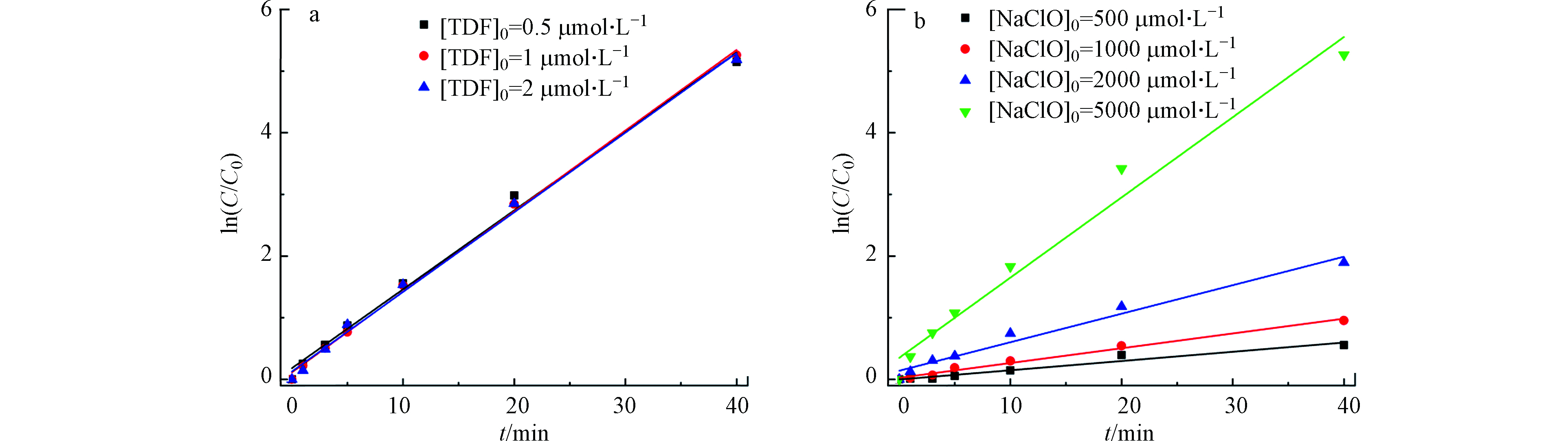
当NaClO的初始浓度为5000 μmol·L−1,TDF的浓度分别为为0.5、1、2 μmol·L−1时,根据TDF浓度随时间的变化计算出ln(C0/C)-t的关系(见图3a)。从图3a可以看出,TDF的反应速率不随TDF浓度的增加而变化,说明TDF的浓度对降解反应没有影响,认定TDF的反应级数为0,即α=0。因此,式(6)可简化为:
式(9)可变形为:
当TDF的初始浓度为2 μmol·L−1,分别考察NaClO初始浓度为500、1000、2000、5000 μmol·L−1时TDF的降解情况。图3b展示了不同NaClO初始浓度条件下TDF的ln(C0/C)-t关系图。
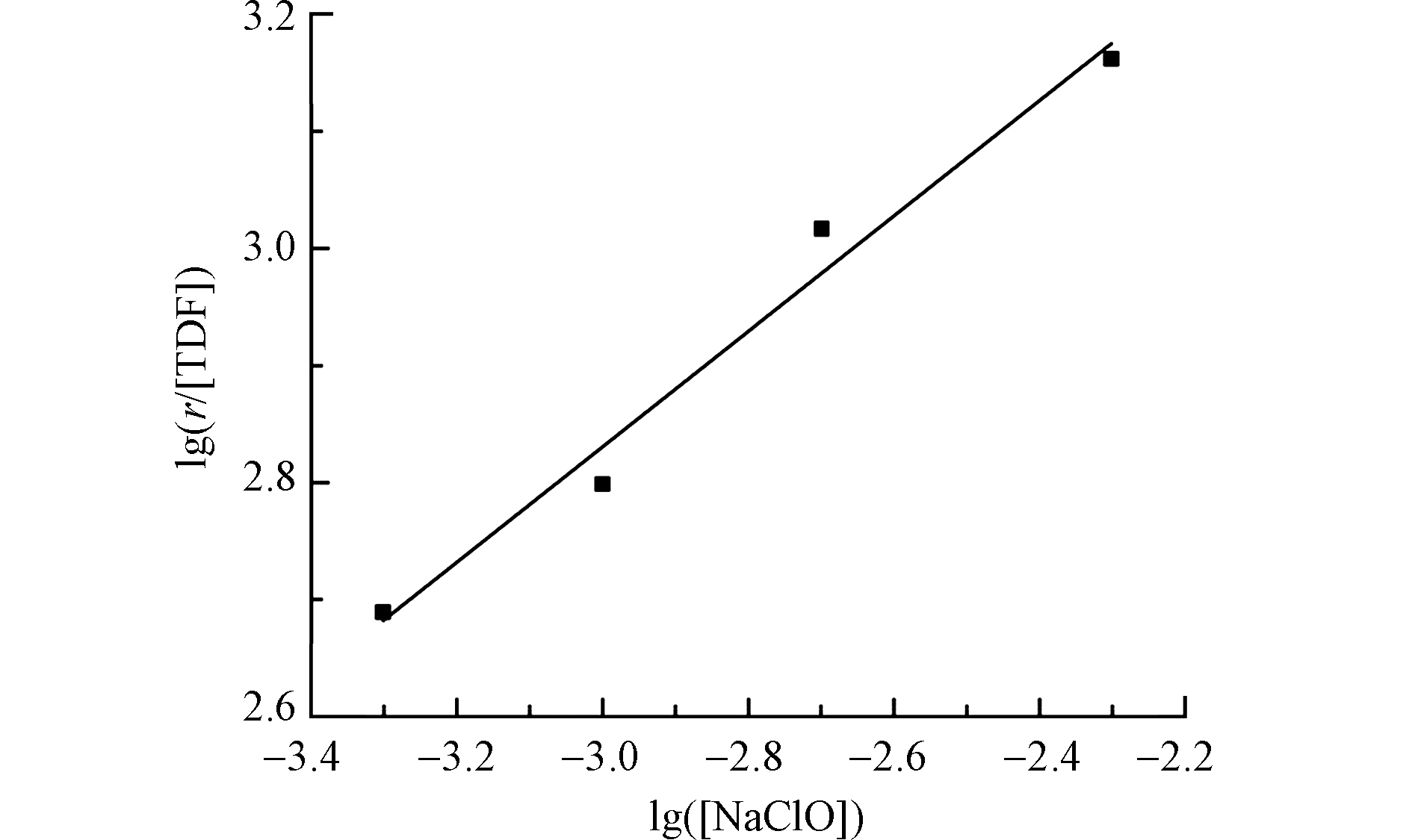
根据不同NaClO初始浓度下TDF的降解反应速率r,进一步可得得
$\text{lg}\left(\dfrac{{r}}{\left[\text{TDF}\right]}\right)$ 与$\text{lg}(\left[\text{NaClO}\right])$ 的关系见图4。图中拟合线性方程R2为0.98,这表明在UV/Cl体系中,当TDF的初始浓度一定时,NaClO的反应级数为1级,即β=1。综上,式(6)可简化为:
从式(12)中可知,UV/Cl体系降解TDF的反应级数为1。
-
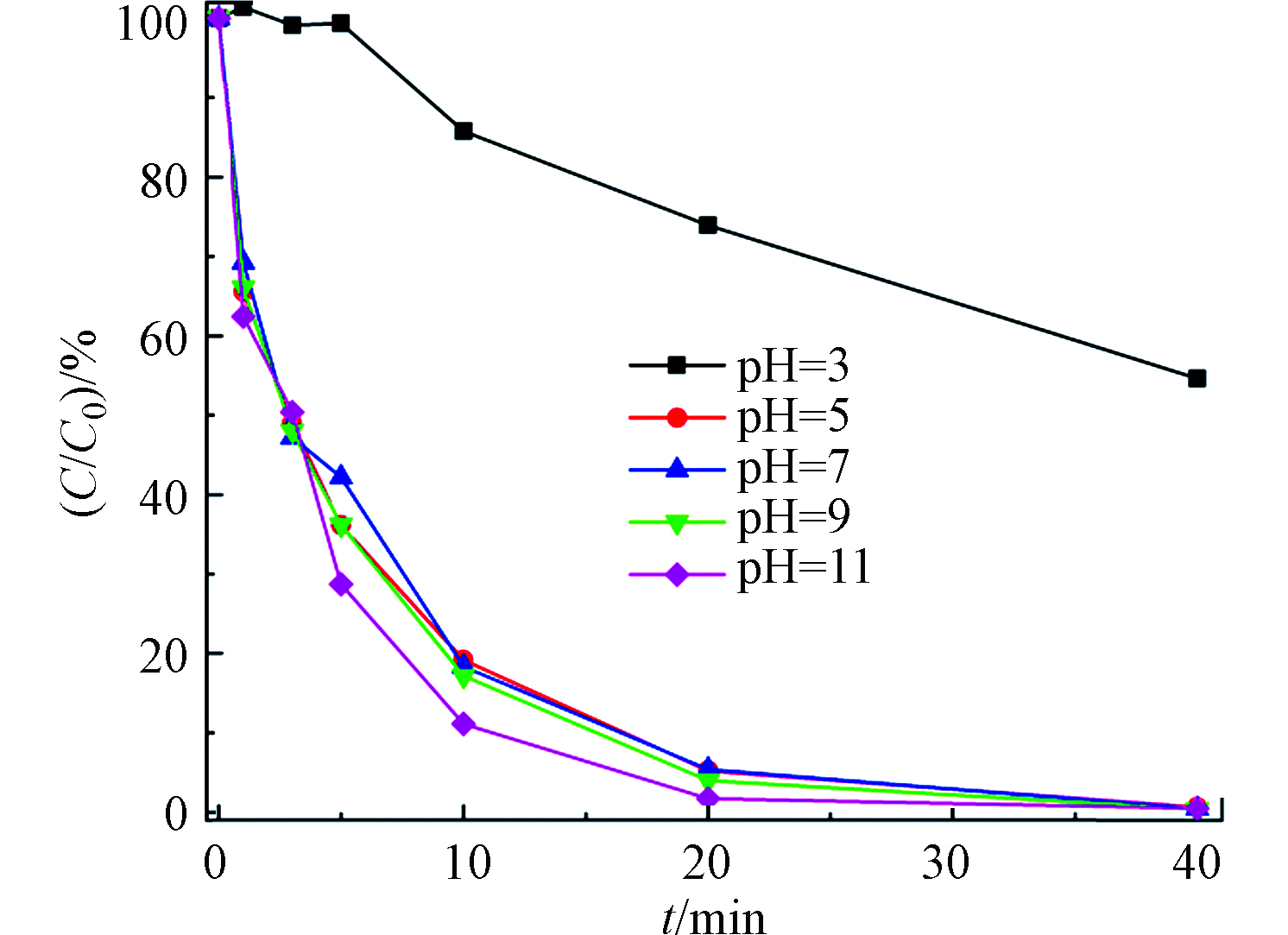
在[TDF]0=2 μmol·L−1,[NaClO]0=5000 μmol·L−1,温度为(25±1)℃的实验条件下,探讨反应体系的不同pH值对降解反应的影响。结果表明,pH对TDF在UV/Cl体系中的降解有较大的影响(图5)。在pH为3时,TDF的降解速率受到了一定的抑制作用,kobs为0.0150 min−1;当pH为5、7、9时,TDF的反应速率相近,kobs分别为0.154、0.158、0.165 min−1;当pH为11时,反应速率继续增大,此时kobs为0.211 min−1。TDF在碱性条件下降解速度更快可能有两方面原因:(1)TDF在酸性溶液中的稳定性高,而在碱性溶液中易水解[40];(2)在碱性条件下,ClO-的分布大于HClO[41],而ClO-比HClO有更高的亲电性[42],容易与TDF的苯环反应。
-
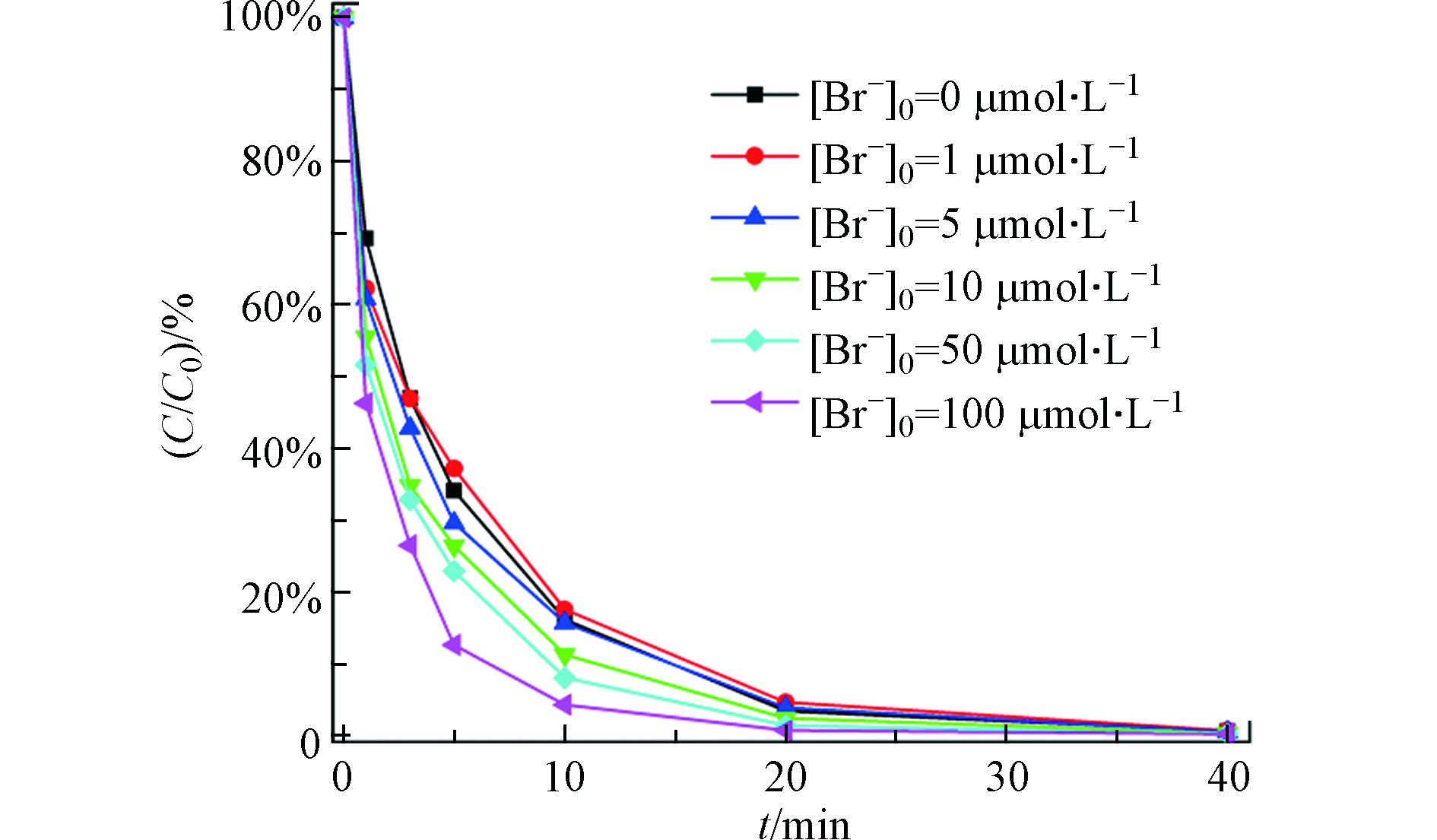
溴离子不仅在自然水体中广泛存在[43-44],而且在很多污染物的光化学反应中有多重作用[45-46]。在[TDF]0=2 μmol·L−1,[NaClO]0=5000 μmol·L−1,温度为(25±1)℃的实验条件下,考察不同初始浓度Br−对TDF降解反应的影响,结果如图6所示。随着溶液中Br−初始浓度的增加,TDF的降解反应呈现出先抑制后促进的现象。Br−浓度为0 μmol·L−1时,kobs为0.164 min−1,当Br-的初始浓度从1 μmol·L−1增加到100 μmol·L−1时,TDF的kobs从0.147 min−1增加到0.241 min−1。Br-呈现先抑制后促进的现象可能和其在光催化反应中的多重作用有关:(1)Br−可以与HClO快速反应生成HBrO(式13)[42],HBrO的亲电性比HClO高[42],更容易与TDF反应,促进降解反应的发生;(2)HBrO可以直接光解产生HO·和Br·,Br·可进一步与HO·,Cl·等离子反应生成反应活性更低的含溴自由基(式14—19)[42,47],从而抑制TDF的降解反应速率。
-
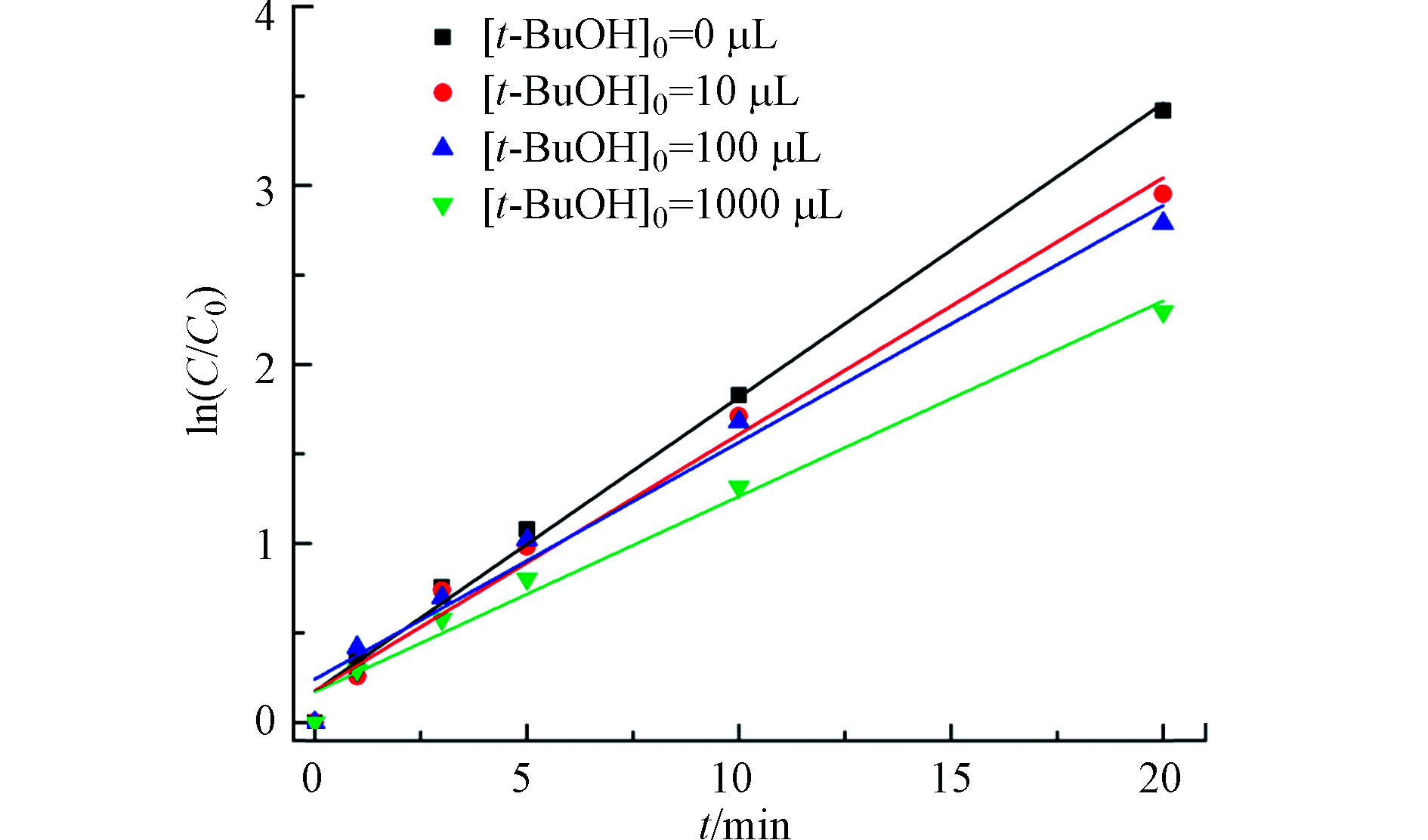
t-BuOH能选择性的与HO·和Cl·反应,因此常用作HO·和Cl·的猝灭剂[37],t-BuOH与HO·和Cl·的反应速率常数分别为6.0×108 (mol·L−1)−1s−1和1.9×108 (mol·L−1)−1s−1[48]。在[TDF]0=2 μmol·L−1,[NaClO]0=5000 μmol·L−1,温度为(25±1)℃的实验条件下,考察不同初始体积t-BuOH对TDF降解反应的影响。结果如图7所示,在UV/Cl体系中加入t-BuOH的初始体积从0增加到1000 μL,kobs从0.164 min−1降低到0.109 min−1,下降了33.5%,证明在UV/Cl体系中有HO·和Cl·参与反应,该结果与之前的研究报道一致[49]。猝灭剂加入后,反应速率有所下降,但降解速度远高于单独UV或单独NaClO,这表明UV/Cl体系中存在其他活性组分有助于TDF降解,该活性组分主要是是Cl2·−和ClO·。
-
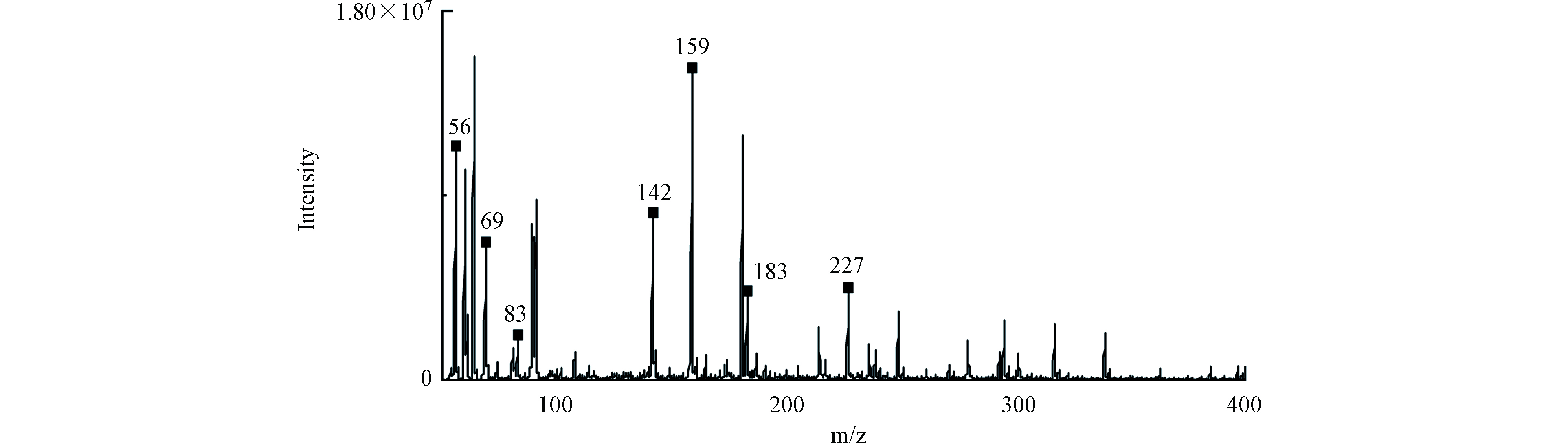
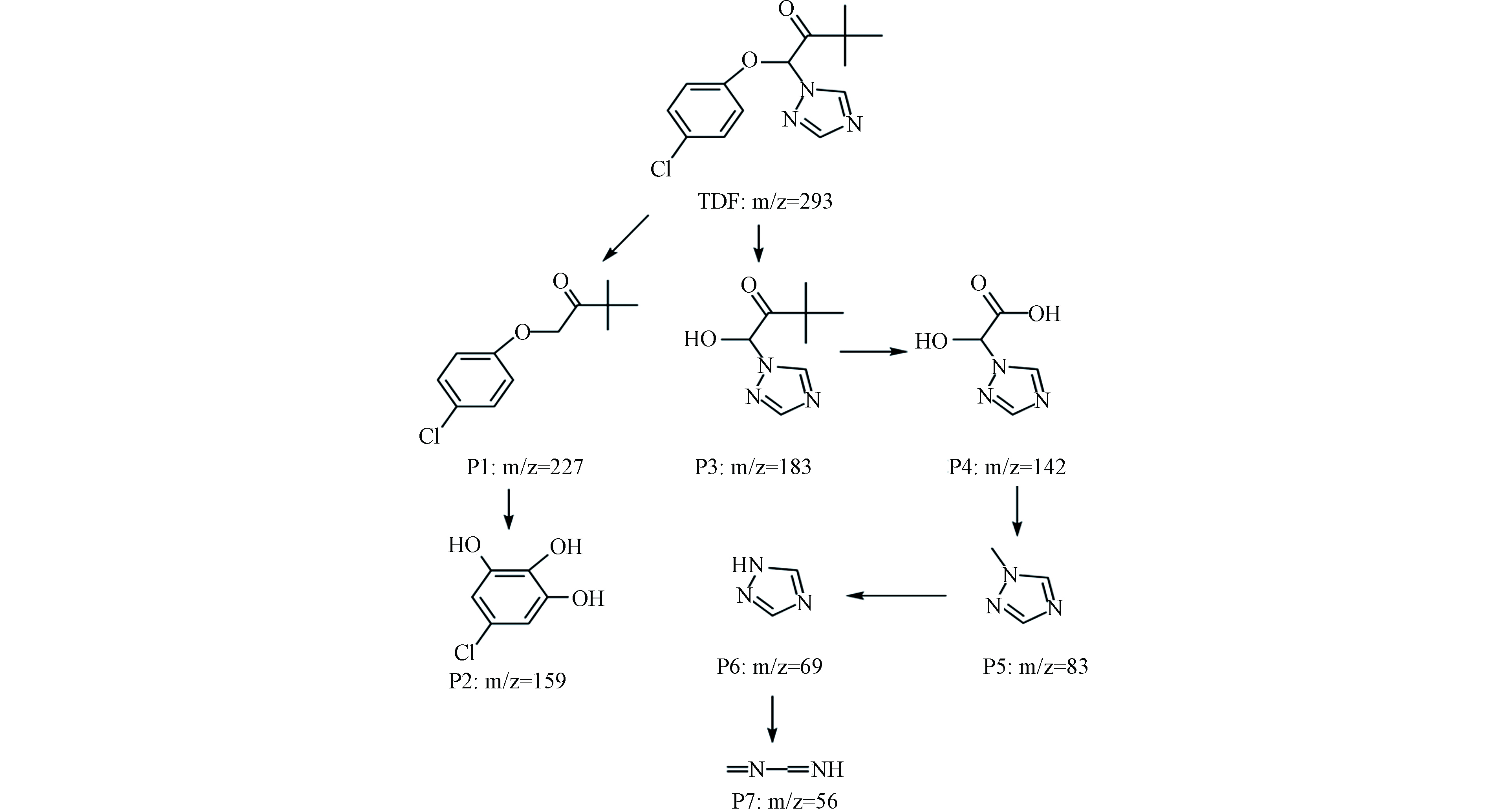
在[TDF]0=10 μmol·L−1,[NaClO]0=500 μmol·L−1,温度为(25±1)℃的实验条件下,利用UPLC-MS/MS对样品进行全扫描,分别在0、10、20、40 min取样检测TDF在降解过程的中间产物。根据TDF降解中间产物的二级质谱信息,发现7种可能的降解中间产物(P1—P7),并进一步推断了TDF可能的降解路径,如图8所示。TDF降解过程中的反应机理包括亲电取代和消除等。
在UV/Cl体系中存在的HO·可以与有机物支链发生加成或消除反应[50]。TDF(m/z=293)与自由基反应导致C—N键断裂,生成P1(m/z=227);P1水解生成对氯苯酚后与溶液中的羟基自由基发生取代反应,生成P2(m/z=159)。TDF也可以发生类似水解生成P3(m/z=183),P3在HO·发生取代反应,生成P4(m/z=142),并进一步在HO·的作用下生成P5(m/z=83),P6(m/z=69)和P7(m/z=56),详细的降解路径如图9所示。
-
(1)通过不同体系对下对TDF的降解比较,发现与单独使用UV和单独使用NaClO相比,UV/Cl体系下对TDF的降解效果大幅提高,降解速率为0.164 min−1,是单独使用UV或NaClO的19倍;
(2)UV/Cl体系下对TDF降解反应动力学方程总反应级数为1。pH对降解反应有较大影响,pH为3时,降解反应速率最低,kobs为0.0150 min−1;pH在11时,降解反应速率最高,kobs为0.211 min−1;
(3)Br-对降解反应存在先抑制后促进的现象;t-BuOH对降解反应呈现抑制作用,这是因为t-BuOH能猝灭溶液中生成的HO·和Cl·;
(4)通过UPLC-MS/MS质谱全扫描分析,共鉴定出7种可能的降解中间产物,TDF的降解机理主要包括亲电取代和消除。
UV/Cl降解水中三唑酮的影响因素和机理
Influencing factors and mechanism of UV/Cl degradation of triazolone in water
-
摘要: 采用UV/Cl高级氧化技术去除水中三唑酮(TDF),并研究了TDF在UV/Cl体系中的降解动力学、影响因素和降解机理。对比单独UV、单独次氯酸钠(NaClO)和UV/Cl的3种条件下TDF的降解效果,发现UV/Cl体系能有效降解TDF。考察了TDF的初始浓度、NaClO初始浓度、不同初始pH、溴离子(Br-)和叔丁醇(t-BuOH)对降解效果的影响。结果表明,UV/Cl降解TDF符合一级动力学方程。pH对降解反应有较大影响,在pH为3时,反应速率最慢,表观反应动力学常数(kobs)为0.0150 min−1;pH为11时,反应速率最快,kobs为0.211 min−1。Br−初始浓度为1 μmol·L−1时对降解反应有一定的抑制作用,当Br−初始浓度高于5 μmol·L−1时,Br−初始浓度越高对降解反应的促进作用越强。在UV/Cl体系中加入猝灭剂t-BuOH后,kobs降低了33.5%,表明UV/Cl体系中有HO·和Cl·参与反应。通过超高效液相色谱质谱联用仪(UPLC-MS/MS)全扫描模式鉴定了TDF降解的中间体,推断出7种可能的降解中间产物,UV/Cl降解TDF的反应机理包括亲电取代和消除等。Abstract: The UV/Cl was used to remove triazolone (TDF) in water, and the degradation kinetics and degradation mechanism of TDF in the UV/Cl system were studied. By comparing the degradation effects of UV alone, sodium hypochlorite (NaClO) alone, and UV/Cl system, it was found that the UV/Cl system can degrade TDF effectively. The influence factors of NaClO, TDF initial concentration, pH values, bromide ion (Br−) initial concentration and isopropanol (t-BuOH) were investigated in the degradation process. The results showed that the degradation of TDF by UV/Cl conformed to the first-order kinetic equation. pH has a great influence on the degradation reaction, the degradation rate was the slowest and fastest when the pH at 3 and 11, and the apparent reaction kinetic constant (kobs) was 0.0150 min−1 and 0.211 min−1, respectively; the inhibitory effect of the degradation reaction was found when the initial concentration of Br− was 1 μmol·L−1. When the initial concentration of Br− was higher than 5 μmol·L−1, the higher the initial concentration of Br−, the stronger the promotion effect on the degradation reaction. After added the quencher isopropanol (t-BuOH) to the UV/Cl system, the kobs was decreased by 33.5%. This indicated that HO· and Cl· were involved in the UV/Cl system. The intermediates of TDF degradation were identified by UPLC-MS/MS in full-scan mode, and seven possible degradation intermediates were proposed. The reaction mechanisms of UV/Cl system including electrophilic substitution and elimination.
-
Key words:
- UV/Cl /
- triadimefon /
- reaction kinetics /
- degradation mechanism
-

-
表 1 三唑酮的理化性质
Table 1. Physicochemical properties of triadimefon
目标物
Compound结构
Structure分子式
Molecular formula分子量
Molecular weightCAS 水溶解性/(g·100 mL−1)
Water solubility三唑酮 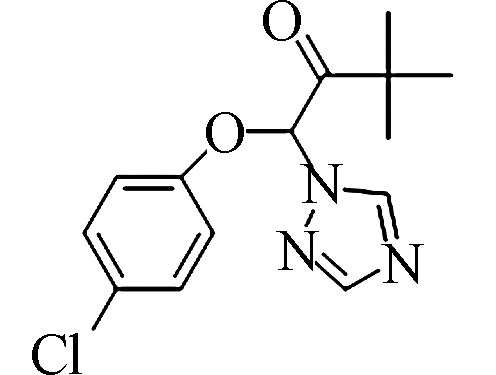
C14H16ClN3O2 293.7 43121-43-3 0.026 表 2 三唑酮液相洗脱梯度条件
Table 2. Triazolone liquid elution gradient conditions
时间/min
Time流速/(mL·min−1)
Flow rate流动相A/%
Mobile phase A流动相B/%
Mobile phase B0.00 0.400 80.0 20.0 0.50 0.400 80.0 20.0 3.50 0.400 5.0 95.0 4.70 0.400 5.0 95.0 4.80 0.400 80.0 20.0 5.50 0.400 80.0 20.0 表 3 TDF的UPLC-MS/MS参数
Table 3. UPLC-MS/MS parameters for the TDF
目标化合物Compound 母离子
Parent ion
(m/z)保留时间/min
Retention time子离子
Product ion
(m/z)锥孔电压/V
Cone voltage碰撞电压/V
Collision voltageTDF 294.06 2.61 197.08 16 14 225.02 16 12 -
[1] FENG J L, ZHAI M X, LIU Q, et al. Residues of organochlorine pesticides (OCPs) in upper reach of the Huaihe River, East China [J]. Ecotoxicology and Environmental Safety, 2011, 74(8): 2252-2259. doi: 10.1016/j.ecoenv.2011.08.001 [2] WAN Y, HU J Y, LIU J L, et al. Fate of DDT-related compounds in Bohai Bay and its adjacent Haihe Basin, North China [J]. Marine Pollution Bulletin, 2005, 50(4): 439-445. doi: 10.1016/j.marpolbul.2004.11.037 [3] LIU S Y, CHANG J H, ZHAO Y, et al. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon [J]. Environmental Toxicology and Pharmacology, 2011, 32(3): 472-477. doi: 10.1016/j.etap.2011.09.002 [4] LI M, LI S Y, YAO T T, et al. Waterborne exposure to triadimefon causes thyroid endocrine disruption and developmental delay in Xenopus laevis tadpoles [J]. Aquatic Toxicology, 2016, 177: 190-197. doi: 10.1016/j.aquatox.2016.05.018 [5] 刘娜, 金小伟, 穆云松, 等. 三唑酮在水环境中的环境行为、毒性效应及生态风险 [J]. 生态毒理学报, 2017, 12(4): 65-75. LIU N, JIN X W, MU Y S, et al. Review of environmental behavior, toxicity and ecological risk of triadimefon in the aquatic environment [J]. Asian Journal of Ecotoxicology, 2017, 12(4): 65-75(in Chinese).
[6] 付岩. 典型农药在稻田及周围水环境中对微生物群落的影响研究[D]. 杭州: 浙江大学, 2015. FU Y. Effects of typical pesticides on microbial community diversity in paddy ecosystems[D]. Hangzhou: Zhejiang University, 2015(in Chinese).
[7] 刘娜, 金小伟, 薛荔栋, 等. 太湖流域药物和个人护理品污染调查与生态风险评估 [J]. 中国环境科学, 2017, 37(9): 3515-3522. doi: 10.3969/j.issn.1000-6923.2017.09.039 LIU N, JIN X W, XUE L D, et al. Concentrations distribution and ecological risk assessment of pharmaceuticals and personal care products in Taihu Lake [J]. China Environmental Science, 2017, 37(9): 3515-3522(in Chinese). doi: 10.3969/j.issn.1000-6923.2017.09.039
[8] 游明华. 天然水中9种三唑类农药的检测方法及其非生物降解研究[D]. 厦门: 厦门大学, 2008. YOU M H. Determination and abiotic degradation of nine trizole pesticides in natural aquatic environments[D]. Xiamen: Xiamen University, 2008(in Chinese).
[9] 刘园, 杨卫萍, 魏琛, 等. 枯水期贵阳市饮用水源农药污染特征及健康风险 [J]. 地球与环境, 2015, 43(6): 653-659. LIU Y, YANG W P, WEI C, et al. Pollution characteristics and health risk assessment of pesticide in drinking water of Guiyang City, China during withered water period [J]. Earth and Environment, 2015, 43(6): 653-659(in Chinese).
[10] STAMATIS N, HELA D, TRIANTAFYLLIDIS V, et al. Spatiotemporal variation and risk assessment of pesticides in water of the lower catchment basin of acheloos river, western Greece [J]. The Scientific World Journal, 2013, 2013: 231610. [11] HERMSEN S A B, van den BRANDHOF E J, van der VEN L T M, et al. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies [J]. Toxicology in Vitro, 2011, 25(3): 745-753. doi: 10.1016/j.tiv.2011.01.005 [12] PAPIS E, BERNARDINI G, GORNATI R, et al. Triadimefon causes branchial arch malformations in Xenopus laevis embryos [J]. Environmental Science and Pollution Research International, 2006, 13(4): 251-255. doi: 10.1065/espr2006.01.014 [13] 刘少颖. 三唑酮对斑马鱼的胚胎发育和内分泌—生殖毒性[D]. 杭州: 浙江大学, 2011. LIU S Y. Embryonic developmental and endocrine-reproductive toxicity of triadimefon on zebrafish[D]. Hangzhou: Zhejiang University, 2011(in Chinese).
[14] 范博, 樊明, 刘征涛, 等. 稀有鮈鲫物种敏感性及其在生态毒理学与水质基准中的应用 [J]. 环境科学研究, 2019, 32(7): 1153-1161. FAN B, FAN M, LIU Z T, et al. Species sensitivity and application in ecotoxicology and water quality criterion for Gobiocypris rarus [J]. Research of Environmental Sciences, 2019, 32(7): 1153-1161(in Chinese).
[15] WALKER Q D, MAILMAN R B. Triadimefon and triadimenol: Effects on monoamine uptake and release [J]. Toxicology and Applied Pharmacology, 1996, 139(2): 227-233. doi: 10.1006/taap.1996.0161 [16] ROSS J A, LEAVITT S A. Analysis of the mutations induced by conazole fungicides in vivo [J]. Mutagenesis, 2010, 25(3): 231-234. doi: 10.1093/mutage/gep068 [17] ROSS J A, BLACKMAN C F, THAI S F, et al. A potential microRNA signature for tumorigenic conazoles in mouse liver [J]. Molecular Carcinogenesis, 2010, 49(4): 320-323. [18] 周明毅. 紫外辐照过硫酸钠降解水中有机氯农药的研究[D]. 贵阳: 贵州大学, 2019. ZHOU M Y. Degradation of organochlorine pesticides in water by UV irradiation-activated sodium persulfate process[D]. Guiyang: Guizhou University, 2019(in Chinese).
[19] 周明毅, 魏琛, 盛贵尚, 等. 紫外活化过硫酸钠降解水中三唑酮的效能 [J]. 环境工程学报, 2019, 13(4): 810-817. doi: 10.12030/j.cjee.201810017 ZHOU M Y, WEI C, SHENG G S, et al. Degradation of triadimefon in water by UV irradiation-activated sodium persulfate process [J]. Chinese Journal of Environmental Engineering, 2019, 13(4): 810-817(in Chinese). doi: 10.12030/j.cjee.201810017
[20] YE B, LI Y, CHEN Z, et al. Degradation of polyvinyl alcohol (PVA) by UV/chlorine oxidation: Radical roles, influencing factors, and degradation pathway [J]. Water Research, 2017, 124: 381-387. doi: 10.1016/j.watres.2017.05.059 [21] KONG X J, JIANG J, MA J, et al. Degradation of atrazine by UV/chlorine: Efficiency, influencing factors, and products [J]. Water Research, 2016, 90: 15-23. doi: 10.1016/j.watres.2015.11.068 [22] GUO K H, WU Z H, SHANG C, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water [J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. [23] HUANG N, WANG T, WANG W L, et al. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation [J]. Water Research, 2017, 114: 246-253. doi: 10.1016/j.watres.2017.02.015 [24] QIN L, LIN Y L, XU B, et al. Kinetic models and pathways of ronidazole degradation by chlorination, UV irradiation and UV/chlorine processes [J]. Water Research, 2014, 65: 271-281. doi: 10.1016/j.watres.2014.07.041 [25] JIN J, EL-DIN M G, BOLTON J R. Assessment of the UV/Chlorine process as an advanced oxidation process [J]. Water Research, 2011, 45(4): 1890-1896. doi: 10.1016/j.watres.2010.12.008 [26] FANG J Y, FU Y, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system [J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. [27] MÁRTIRE D O, ROSSO J A, BERTOLOTTI S, et al. Kinetic study of the reactions of chlorine atoms and Cl2•- radical anions in aqueous solutions. II. toluene, benzoic acid, and chlorobenzene [J]. The Journal of Physical Chemistry A, 2001, 105(22): 5385-5392. doi: 10.1021/jp004630z [28] WATTS M J, LINDEN K G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water [J]. Water Research, 2007, 41(13): 2871-2878. doi: 10.1016/j.watres.2007.03.032 [29] CONNICK R E. The interaction of hydrogen peroxide and hypochlorous acid in acidic solutions containing chloride ion [J]. Journal of the American Chemical Society, 1947, 69(6): 1509-1514. doi: 10.1021/ja01198a074 [30] BOLTON J R, STEFAN M I, SHAW P S, et al. Determination of the quantum yields of the potassium ferrioxalate and potassium iodide-iodate actinometers and a method for the calibration of radiometer detectors [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2011, 222(1): 166-169. doi: 10.1016/j.jphotochem.2011.05.017 [31] NAG S K, DUREJA P. Photodegradation of azole fungicide triadimefon [J]. Journal of Agricultural and Food Chemistry, 1997, 45(1): 294-298. doi: 10.1021/jf960074n [32] Da SILVA J P, da SILVA A M, KHMELINSKII I V, et al. Photophysics and photochemistry of azole fungicides: Triadimefon and triadimenol [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2001, 142(1): 31-37. doi: 10.1016/S1010-6030(01)00489-0 [33] Da SILVA J P, Da SILVA A M. Comparative study of the dissipation of triadimefon in greenhouse and field conditions [J]. Toxicological & Environmental Chemistry, 1998, 66(1/2/3/4): 229-236. [34] Da SILVA J P, VIEIRA FERREIRA L F, da SILVA A M. Aqueous photochemistry of pesticides triadimefon and triadimenol [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2003, 154(2/3): 293-298. [35] WU Z H, FANG J Y, XIANG Y Y, et al. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways [J]. Water Research, 2016, 104: 272-282. doi: 10.1016/j.watres.2016.08.011 [36] ALFASSI Z B, HUIE R E, MOSSERI S, et al. Kinetics of one-electron oxidation by the ClO radical [J]. International Journal of Radiation Applications and Instrumentation. Part C. Radiation Physics and Chemistry, 1988, 32(1): 85-88. doi: 10.1016/1359-0197(88)90018-5 [37] WANG W L, WU Q Y, HUANG N, et al. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species [J]. Water Research, 2016, 98: 190-198. doi: 10.1016/j.watres.2016.04.015 [38] BEN W W, SHI Y W, LI W W, et al. Oxidation of sulfonamide antibiotics by chlorine dioxide in water: Kinetics and reaction pathways [J]. Chemical Engineering Journal, 2017, 327: 743-750. doi: 10.1016/j.cej.2017.06.157 [39] EL NAJJAR N H, DEBORDE M, JOURNEL R, et al. Aqueous chlorination of levofloxacin: Kinetic and mechanistic study, transformation product identification and toxicity [J]. Water Research, 2013, 47(1): 121-129. doi: 10.1016/j.watres.2012.09.035 [40] 刘毅华, 郭正元, 杨仁斌, 等. 三唑酮的酸性、中性和碱性水解动力学研究 [J]. 农村生态环境, 2005, 21(1): 67-68,71. LIU Y H, GUO Z Y, YANG R B, et al. Hydrolysis dynamics of triadimefon in aquatic environment different in pH value [J]. Rural Eco-Environment, 2005, 21(1): 67-68,71(in Chinese).
[41] 冯家豪. 次氯酸钠氧化降解水杨酸的反应研究[D]. 新乡: 河南师范大学, 2015. FENG J H. Study on the reaction of salicylic acid oxidation by sodium hypochlorite[D]. Xinxiang: Henan Normal University, 2015(in Chinese).
[42] HEEB M B, CRIQUET J, ZIMMERMANN-STEFFENS S G, et al. Oxidative treatment of bromide-containing waters: Formation of bromine and its reactions with inorganic and organic compounds—A critical review [J]. Water Research, 2014, 48: 15-42. doi: 10.1016/j.watres.2013.08.030 [43] 卢宁, 黄鑫, 高乃云, 等. 青草沙水库原水中的溴离子和溴酸盐生成势 [J]. 净水技术, 2011, 30(3): 10-12,19. doi: 10.3969/j.issn.1009-0177.2011.03.003 LU N, HUANG X, GAO N Y, et al. Bromonium ion and its bromate formation potential in raw water of Qingcaosha Reservoir [J]. Water Purification Technology, 2011, 30(3): 10-12,19(in Chinese). doi: 10.3969/j.issn.1009-0177.2011.03.003
[44] MAGAZINOVIC R S, NICHOLSON B C, MULCAHY D E, et al. Bromide levels in natural waters: Its relationship to levels of both chloride and total dissolved solids and the implications for water treatment [J]. Chemosphere, 2004, 57(4): 329-335. doi: 10.1016/j.chemosphere.2004.04.056 [45] CHENG S S, ZHANG X R, YANG X, et al. The multiple role of bromide ion in PPCPs degradation under UV/chlorine treatment [J]. Environmental Science & Technology, 2018, 52(4): 1806-1816. [46] CRIQUET J, RODRIGUEZ E M, ALLARD S, et al. Reaction of bromine and chlorine with phenolic compounds and natural organic matter extracts - Electrophilic aromatic substitution and oxidation [J]. Water Research, 2015, 85: 476-486. doi: 10.1016/j.watres.2015.08.051 [47] Von GUNTEN U, OLIVERAS Y. Advanced oxidation of bromide-containing waters: Bromate formation mechanisms [J]. Environmental Science & Technology, 1998, 32(1): 63-70. [48] GILBERT B C, STELL J K, PEET W J, et al. Generation and reactions of the chlorine atom in aqueous solution [J]. Journal of the Chemical Society, Faraday Transactions 1:Physical Chemistry in Condensed Phases, 1988, 84(10): 3319. doi: 10.1039/f19888403319 [49] 何群英. UV/氯高级氧化技术处理内分泌干扰素泼尼松龙[D]. 长沙: 湖南大学, 2018. HE Q Y. Treatment of endocrine disruptor prednisolone by UV/chlorine advanced oxidation process[D]. Changsha: Hunan University, 2018(in Chinese).
[50] MINISCI F, CITTERIO A, Giordano C. Electron-transfer processes: Peroxydisulfate, a useful and versatile reagent in organic chemistry [J]. Accounts of Chemical Research, 1983, 16: 27-32. doi: 10.1021/ar00085a005 -



 下载:
下载: