-
微塑料具有粒径小、比表面积大的特点,吸附能力较强,是有机污染物的载体[1]. 目前,关于微塑料吸附有机污染物的研究多集中在单一水质条件对吸附的影响(如pH、盐度、温度和溶解性有机质等)[2]. 然而,环境中水质条件复杂多变,尚缺乏多种条件变化对微塑料吸附有机污染物影响的研究. 溶解性有机质(如腐殖酸)是重要的水质指标,可与有机污染物相互作用影响其水溶解度,还能被微塑料吸附,改变微塑料表面的化学性质,进而影响微塑料对污染物的吸附性能[3-4]. 不仅如此,其他水质条件(如pH和盐度)也会影响腐殖酸在微塑料上的吸附. 当溶液pH值高于微塑料的等电点时,微塑料表面带负电,与腐殖酸之间的静电斥力增加,抑制对腐殖酸的吸附;相反则促进吸附[5-6]. 无机盐还可以通过盐析和架桥等作用影响腐殖酸在微塑料上的吸附[7-8]. 这些都将进一步影响微塑料对污染物的吸附性能. 因此,有必要研究腐殖酸与其他水质条件相互作用对微塑料吸附有机污染物行为的影响与机制. 研究成果对于洞察环境中微塑料与有机污染物相互作用规律以及了解微塑料对环境中有机污染物迁移转化行为的影响具有重要的理论和实际意义.
多环芳烃是环境中普遍存在的典型持久性有机污染物. 菲是最常见的多环芳烃之一,属于非极性化合物. 阿特拉津是由三嗪环组成的有机氯农药,普遍存在于环境中. 无论是陆地动物还是水中的鱼类甚至是人类,长期接触阿特拉津都会有致癌的风险[9]. 因此,笔者选取了新制和老化聚乙烯(PE和APE)和聚苯乙烯(PS和APS)微塑料,两种不同极性的有机污染物(菲和阿特拉津),研究了水质条件(包括pH和水中重要的阳离子Ca2+和Na+)对微塑料吸附腐殖酸的影响,以及腐殖酸与水质(pH和阳离子)相互作用对微塑料吸附菲和阿特拉津的影响. 在此基础上,探究了影响机制.
-
PE和PS微塑料购于东莞市中诚塑胶原料经营部(粒径范围0.106 —0.180 mm). 甲醇浸泡48 h(每24 h更换1次甲醇)、干燥后备用. 将清洗后的PE和PS微塑料置于培养皿中,加入50 mLH2O2(18%, V/W),盖上石英片,暴露于紫外线下300 h[10]. 期间每24 h摇匀1次并及时补充溶液. 取出微塑料,清洗、干燥后过筛,得到粒径范围0.106—0.180 mm的老化微塑料,分别命名为APE和APS.
-
分别称取5 mg不同种类的微塑料到40 mL的Agilent瓶中. 随后加入40 mL不同初始浓度菲(0.1—1.1 mg·L−1)或阿特拉津(1—10 mg·L−1)溶液(为了避免溶剂效应,引入甲醇的体积浓度控制在0.2%以内). 盖上盖子,置于25 ℃恒温摇床上以150 r·min−1的速度,避光条件下振荡3d(吸附动力学预实验结果显示微塑料吸附菲和阿特拉津的达平衡时间在24—60 h之间). 然后取出样品瓶,静置30 min. 将样品瓶中溶液转移至玻璃离心管中在4000 r·min−1下离心20 min,取出上清液,采用高效液相色谱仪测定菲或阿特拉津的浓度. 所有实验做3个平行.
-
依据单因素吸附实验结果设置Ca2+/Na+离子浓度(0.1、10、25 mmol·L−1)和pH值(3、5和8;用0.1 mol·L−1的HCl或NaOH调节)并进行组合(见表1),配制含腐殖酸浓度为10 mg·L−1的溶液. 分别称取5 mg不同种类的微塑料到40 mL的Agilent瓶中. 随后加入40 mL含腐殖酸的溶液,盖上盖子. 后续操作同“1.2.1”. 采用TOC分析仪测定上清液中腐殖酸的浓度.
-
向“1.2.2”的溶液中加入菲或阿特拉津,使其浓度分别为1 mg·L−1和5 mg·L−1. 其他操作同“1.2.1”. 采用高效液相色谱仪测定上清液中菲或阿特拉津的浓度.
-
采用扫描电子显微镜(SEM)对微塑料表面形貌进行观察. 扫描电镜工作电压为20 kV.
采用X射线衍射(XRD)仪在5°—90°范围内对样品进行扫描,测定微塑料结晶度. 计算公式为:
菲和阿特拉津的浓度使用高效液相色谱测定,配C18(4.6 mm×150 mm)色谱柱,柱温25℃,进样量10 μL. 菲的测定采用荧光(2475FLR)检测器(激发和发射波长分别为255 nm和370 nm);流动相为10%超纯水与90%色谱纯甲醇,流速1 mL·min−1. 阿特拉津的测定采用紫外(2687-UV)检测器(检测波长220 nm);流动相为25%超纯水与75%色谱纯甲醇,流速1 mL·min−1.
用Freundlich吸附模型描述菲和阿特拉津在微塑料上的吸附
式中,Ce为水相菲和阿特拉津的平衡浓度,mg·L−1;Q为微塑料的平衡吸附量,mg·kg−1;Kf是吸附能力系数,(mg·kg−1)·(mg·L−1)−N;N表示吸附的非线性程度.
-
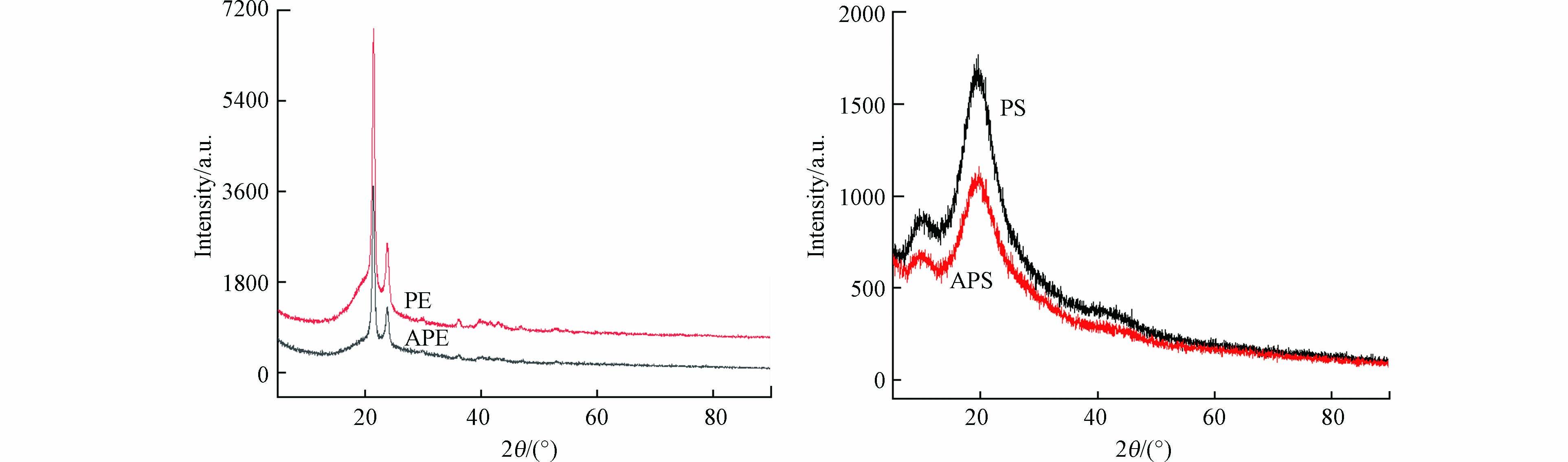
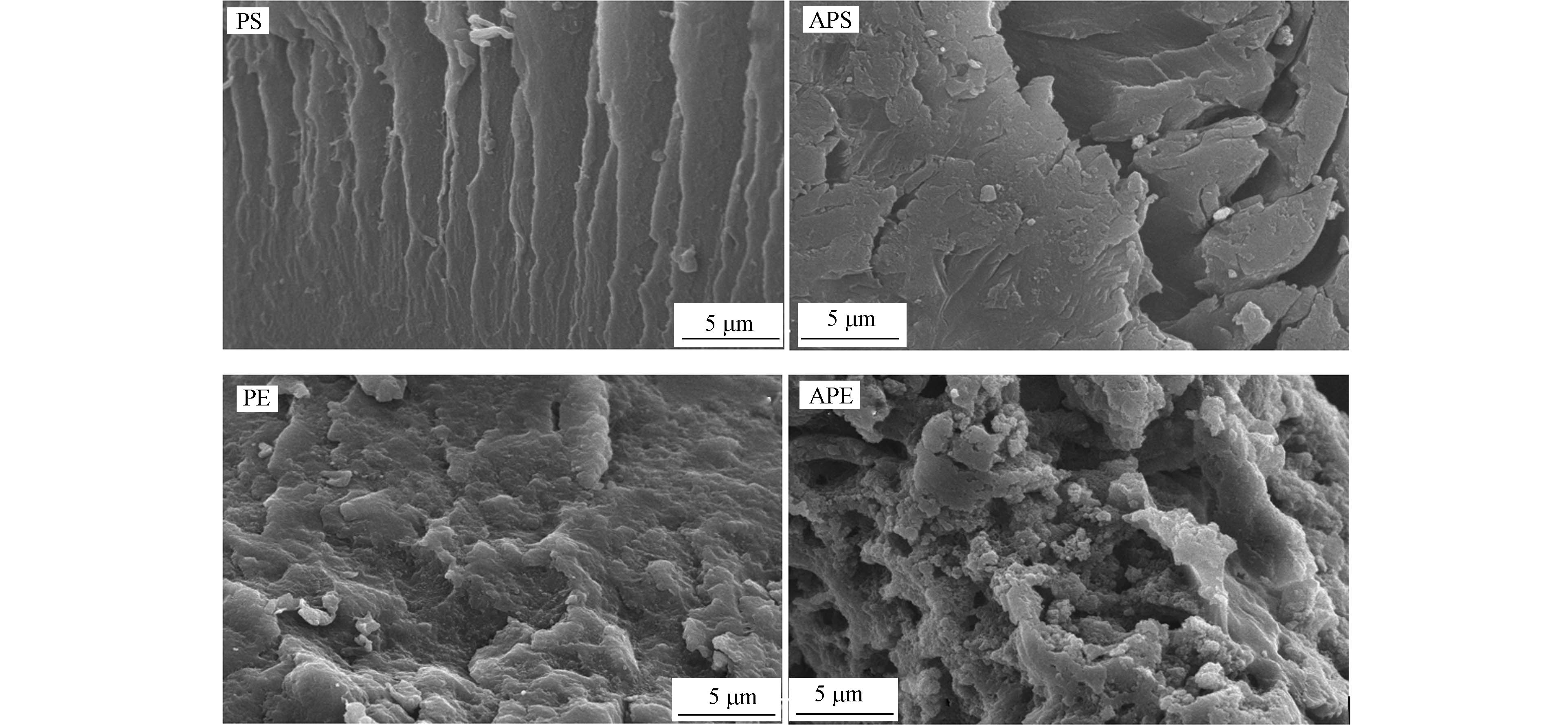
图1和图2分别为4种微塑料的SEM和XRD图谱. 可见,老化后PE和PS表面变得粗糙,孔隙增多. PE和APE的XRD图谱中有明显的结晶相衍射峰(细而尖),属于部分结晶聚合物[10]. 根据公式1计算得到PE的结晶度为43.58%,大于APE的结晶度(38.19%),说明老化降低了PE的结晶度. 这是因为老化过程中在PE主链或者侧链上引入了极性官能团,导致分子链的有序排列被打乱,在微塑料内部形成无定形区,结晶度下降[10]. PE的玻璃转化温度(Tg)在-100℃左右[11],低于室温,因此属于橡胶态聚合物. PS和APS的图谱为明显的非晶峰(矮而弥散),为非结晶聚合物[10]. 但是,PS的Tg在100℃左右[11],高于室温,因此属于玻璃态聚合物.
-
微塑料吸附菲和阿特拉津的等温线与Freundlich模型吻合良好(R2>0.96). 模型拟合结果见表2. 可见,这4种微塑料吸附菲和阿特拉津等温线的N值在0.851—0.995之间,接近1,说明老化前后PE和PS微塑料对这两种化合物的吸附由分配作用主导. 这与文献报道结果一致[12].
从lgKf数据可以看出,4种微塑料吸附菲的能力(0.805—1.422)均显著高于吸附阿特拉津的能力(0.611—0.688). 这是因为阿特拉津属于极性化合物,水溶解度较高(33 mg·L−1[12]);而菲属于非极性化合物,水溶解度较低(1.18 mg·L−1[12]),更易于吸附. 老化后,PE吸附菲的能力提高(增幅为4.9%),而PS吸附菲的能力下降(降幅为20.5%). 本研究的XRD结果显示,老化使PE的结晶度下降,无定形区域占比增大. 无定形PE的结构较为膨胀和柔韧,利于吸附. 而PS属于玻璃态. 老化后其表面极性增大,不利于吸附. 与菲不同,老化微塑料吸附阿特拉津的能力均高于新制微塑料(增幅为7.5%—8.8%). 阿特拉津属于极性化合物,老化后微塑料表面极性的增大是影响阿特拉津在微塑料上吸附量的主要原因[13].
-
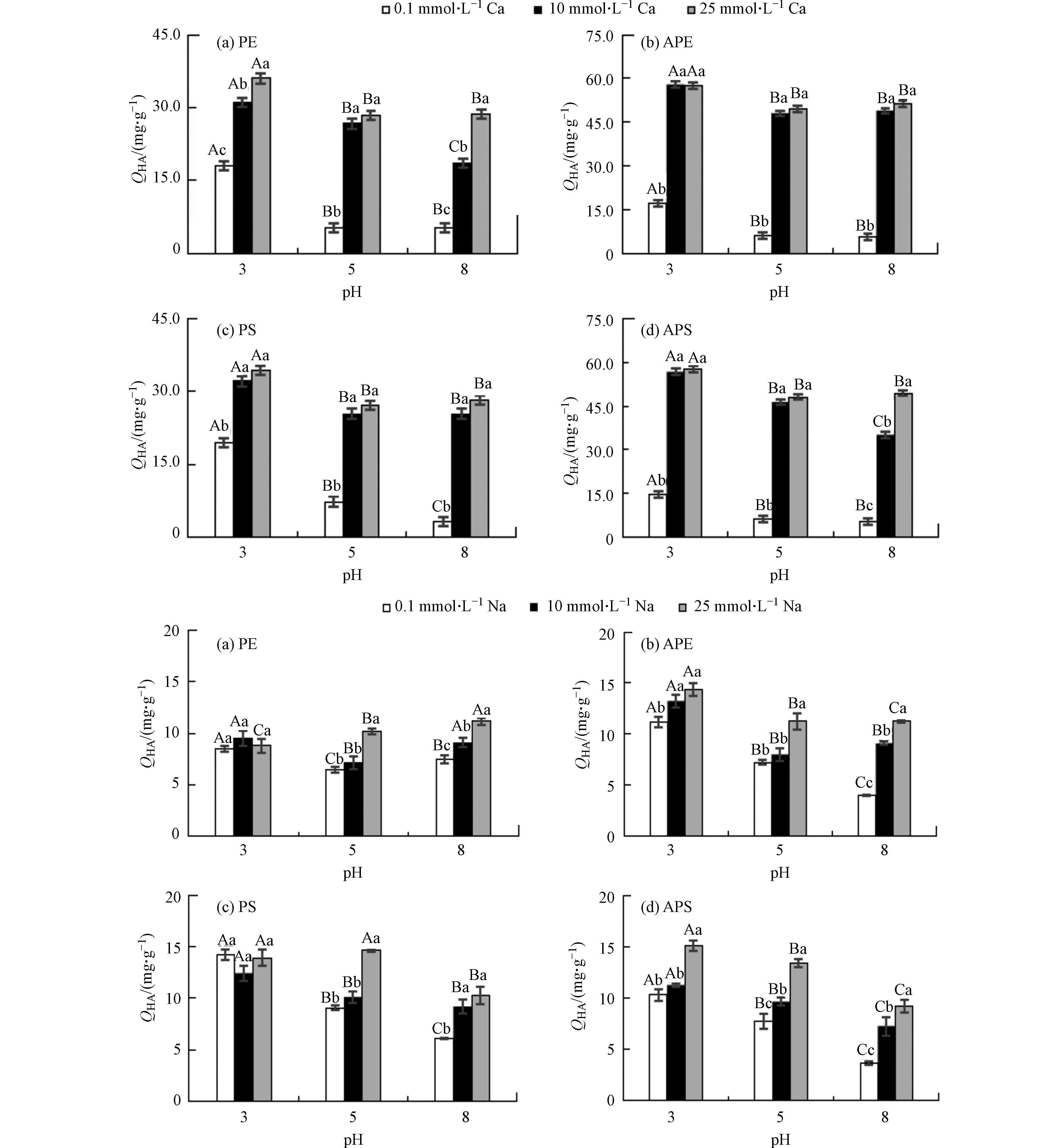
阳离子(Ca2+和Na+)浓度和pH值对微塑料吸附腐殖酸的影响如图3所示. 可见,相同pH值条件下,随Ca2+浓度从0.1 mmol·L−1增至10 mmol·L−1,微塑料对腐殖酸的吸附量显著增大(增幅可达1个数量级);继续增至25 mmol·L−1,微塑料对腐殖酸吸附量的增幅下降(不超过11%). 较高的Ca2+浓度可对微塑料吸附腐殖酸产生以下几个方面的影响:(1)盐析作用[8];(2)降低腐殖酸分子之间的排斥力,使微塑料表面能容纳更多的腐殖酸分子[14];(3)可在带负电的腐殖酸和微塑料之间起架桥作用,提高腐殖酸在微塑料上的吸附量[7].
与Ca2+相比,Na+浓度对这4种微塑料吸附腐殖酸的影响较小(变化幅度不超过1.8倍). 而且当离子浓度较高时(10 mmol·L−1和25 mmol·L−1),Na+对腐殖酸吸附量的影响程度与Ca2+的影响差异较大,较Ca2+存在时低约1—5倍. 这是因为二价Ca2+可在微塑料和腐殖酸之间起架桥作用[7],其离子浓度的增加可促进腐殖酸在微塑料上的吸附[15-16].
相比阳离子浓度,pH对微塑料吸附腐殖酸的影响相对较小. 阳离子浓度越低,微塑料吸附腐殖酸的量受pH的影响越大. 例如,当Ca2+和Na+浓度为0.1 mmol·L−1时,微塑料对腐殖酸的吸附量随pH增大而下降(降幅分别在70%—90%和16%—65%之间);当Ca2+和Na+浓度为10 mmol·L−1或25 mmol·L−1时,pH对腐殖酸在微塑料上吸附的影响下降(降幅均不超过38%). 因为当pH升高时,微塑料表面的负电荷密度增加,静电排斥作用抑制腐殖酸在微塑料上的吸附[17].
-
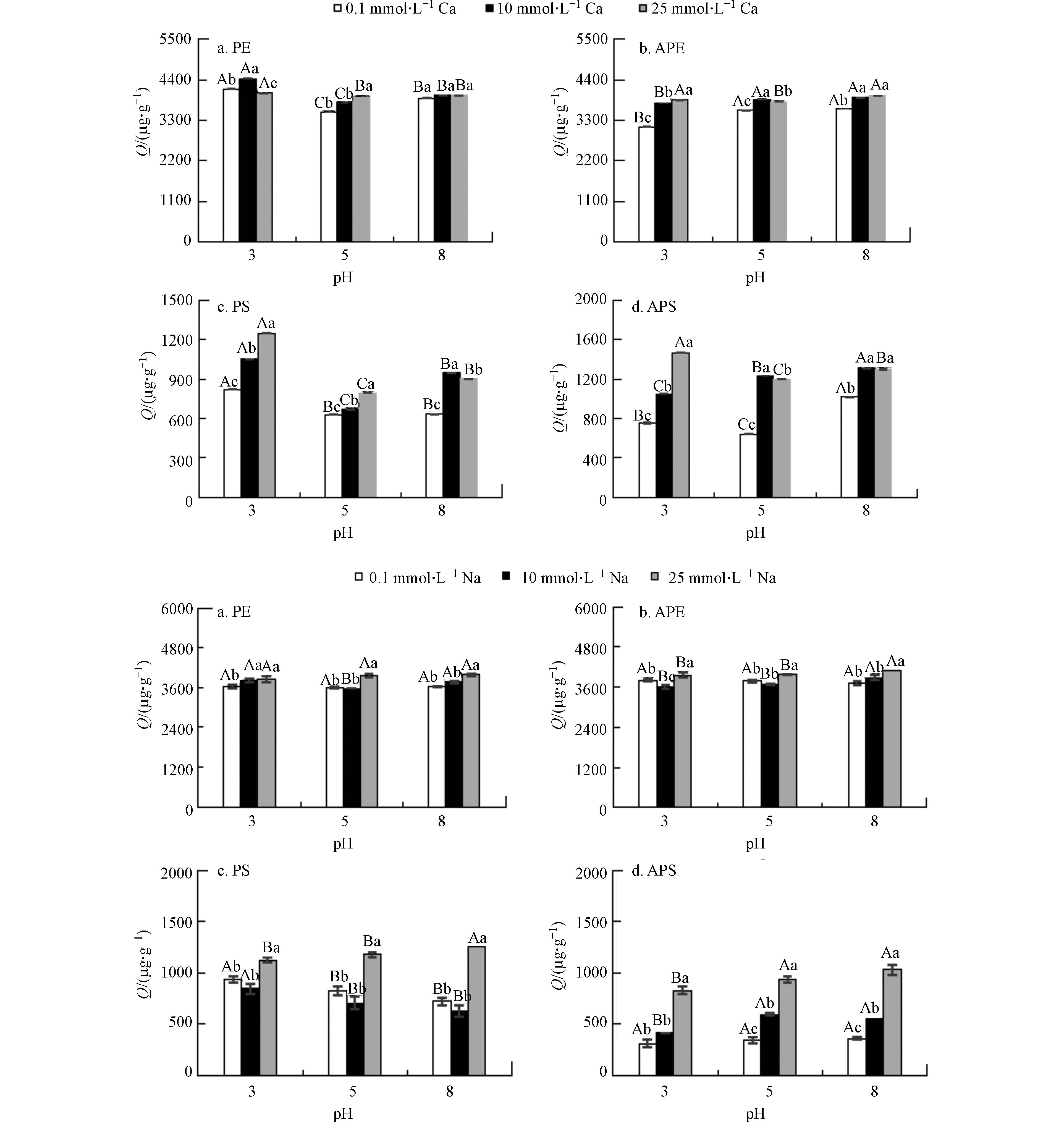
不同阳离子浓度和pH值条件下腐殖酸对微塑料吸附菲和阿特拉津的影响结果如图4和图5所示. 总体上微塑料对菲的吸附量随阳离子浓度的升高而升高(尤其是Ca2+),随pH的升高而下降.
这与微塑料对腐殖酸的吸附量随水质(阳离子和pH)的变化趋势相似. 将微塑料对菲的吸附量与对腐殖酸的吸附量数据做相关性分析,结果显示微塑料对菲的吸附量与对腐殖酸的吸附量之间均显著正相关(表3). 菲属于疏水性有机化合物,可以被腐殖酸吸附,增加其水溶解度[18]. 因此,微塑料对腐殖酸的吸附量越大,水相中腐殖酸的残留浓度越小,增溶作用越小,更有利于菲在微塑料上的吸附.
与菲相反,总体上微塑料对阿特拉津的吸附量随阳离子浓度的升高而下降,随pH的升高而升高. 相关性分析结果显示,微塑料对阿特拉津的吸附量与对腐殖酸的吸附量之间均显著负相关(表3). 阿特拉津属于极性有机化合物,水溶性高,因此腐殖酸对阿特拉津的增溶作用不明显. 不过,阿特拉津分子体积较大,可能是空间阻力制约了其吸附过程[12, 19].
-
(1)新制和老化PE和PS微塑料吸附菲和阿特拉津的等温线符合Freundlich模型(R2>0.96). 同种微塑料吸附菲的能力(lgKf)强于吸附阿特拉津的能力. 老化微塑料吸附菲和阿特拉津的能力>新制微塑料的吸附能力(APS吸附菲的能力<PS除外).
(2)Ca2+浓度对这4种微塑料吸附腐殖酸的影响显著高于Na+. 相同pH条件下,随阳离子浓度增加,微塑料对腐殖酸的吸附量显著增大. 相同阳离子浓度条件下,离子浓度越低,pH对腐殖酸在微塑料上吸附的影响越显著.
(3)腐殖酸与水质(阳离子和pH)相互作用显著影响微塑料对菲和阿特拉津的吸附. 相关性结果显示,微塑料对菲的吸附量与对腐殖酸的吸附量显著正相关;而阿特拉津刚好相反,呈显著负相关. 增溶作用和空间阻力分别是影响微塑料吸附菲和阿特拉津的主要机制.
腐殖酸与pH/阳离子作用对微塑料吸附菲和阿特拉津的影响
Effects of interactions between humic acid and pH/cation on the sorption of phenanthrene and atrazine on microplastics
-
摘要: 为了研究腐殖酸与pH/阳离子相互作用对微塑料吸附水中菲和阿特拉津产生的影响,选取了新制和老化聚乙烯(PE和APE)和聚苯乙烯(PS和APS)微塑料. 吸附等温线结果显示,微塑料对菲和阿特拉津的吸附均符合Freundlich模型(R2>0.96). 同种微塑料吸附菲的能力(lgKf=0.805—1.422)>吸附阿特拉津的能力(0.611—0.688),与污染物水溶性有关;老化微塑料吸附菲和阿特拉津的能力>新制微塑料的吸附能力(APS吸附菲的能力<PS除外),与微塑料表面极性和PE结晶度的变化有关. 相同pH条件下,Ca2+浓度对这4种微塑料吸附腐殖酸的影响显著高于Na+;随Ca2+/Na+浓度的增加,微塑料对腐殖酸的吸附量显著增大(增幅分别可达1个数量级和1.8倍). 相比Ca2+/Na+,pH对微塑料吸附腐殖酸的影响相对较小(随pH增大,降幅分别不超过90%和65%). 而且,相同Ca2+/Na+浓度条件下,离子浓度越低,pH对腐殖酸在微塑料上吸附的影响越显著. 腐殖酸与pH/阳离子相互作用显著影响微塑料对菲和阿特拉津的吸附. 总体上,微塑料对菲的吸附量随Ca2+/Na+浓度的升高而升高,随pH的升高而下降;对阿特拉津的影响则相反. 相关性分析结果显示,微塑料对腐殖酸的吸附量与对菲的吸附量显著正相关,但是与对阿特拉津的吸附量显著负相关. 增溶作用和空间阻力分别是影响微塑料吸附菲和阿特拉津的主要机制.Abstract: In order to study the effects of interactions between humic acid and pH/cation on the sorption of phenanthrene and atrazine on microplastics, virgin and aged polyethylene (PE and APE) and polystyrene (PS and APS) microplastics were selected. The sorption isotherms demonstrated that sorption of phenanthrene and atrazine on the microplastics fitted well with the Freundlich model (R2>0.96). The sorptive ability (lgKf) of the same kind of microplastics for phenanthrene (0.805—1.422) was higher than that for atrazine (0.611—0.688), which is related to their water solubility. The sorptive abilities of aged microplastics for phenanthrene and atrazine were higher than those of virgin microplastics except that sorptive ability of APS for phenanthrene was lower than that of PS. This is related to the changes of surface polarity of microplastics and crystallinity of PE. Under the same pH condition, effect of Ca2+ concentration on the amount of humic acid sorbed by microplastics was more significant than that of Na+. With the increase of Ca2+/ Na+ concentration, the amount of humic acid sorbed by microplastics increased obviously (up to one order of magnitude and 1.8 times increase, respectively). The effect of pH on the amount of humic acid sorbed by microplastics was less significant (no more than 90% and 65% decrease with pH, respectively) as compared to Ca2+/Na+. Under the condition of identical Ca2+/Na+ concentration, the lower the concentration of cation was, the more significant the effect of pH on the amount of humic acid sorbed by microplastics was. Interactions between humic acid and pH/cation significantly influenced the sorption of phenanthrene and atrazine on microplastics. In general, the amount of phenanthrene sorbed by microplastics increased with increasing Ca2+/Na+ concentration, but decreased with increasing pH. The effect on atrazine was the opposite. Correlation analysis results showed that the amount of humic acid sorbed by microplastics was significantly positively correlated with the amount of phenanthrene sorbed by microplastics, but significantly negatively correlated with the amount of atrazine sorbed by microplastics. Solubilization and steric resistance are the main mechanisms affecting the sorption of phenanthrene and atrazine by microplastics, respectively.
-
Key words:
- microplastics /
- sorption /
- organic pollutant /
- humic acid /
- pH/cation.
-

-
表 1 Ca2+/Na+浓度和pH值的设置
Table 1. Setting of Ca2+/Na+ concentration and pH value
序号
Number1 2 3 4 5 6 7 8 9 Ca2+/Na+/(mmol·L−1) 0.1 0.1 0.1 10 10 10 25 25 25 pH 3 5 8 3 5 8 3 5 8 表 2 微塑料吸附菲和阿特拉津的Freundlich模型参数
Table 2. Freundlich model parameters of sorption isotherms of phenanthrene and atrazine on microplastics
微塑料
Microplastics菲
Phenanthrene阿特拉津
AtrazineN lgKf R2 N lgKf R2 PE 0.995 1.356 0.992 0.928 0.623 0.968 APE 0.982 1.422 0.991 0.912 0.668 0.970 PS 0.851 1.013 0.970 0.927 0.611 0.969 APS 0.875 0.805 0.977 0.913 0.665 0.971 表 3 微塑料对菲/阿特拉津吸附量与对腐殖酸吸附量的相关性
Table 3. Relationship between sorption amounts of phenanthrene/atrazine and humic acid on microplastics
微塑料
Microplastics菲的r值 r
Value of phenanthrene阿特拉津的r值 r
Value of atrazineCa2+ Na+ Ca2+ Na+ PE 0.5713* 0.6153* −0.6021* −0.6399* APE 0.6571* 0.5948* −0.5166* −0.5364* PS 0.6934* 0.5979* −0.6167* −0.4742* APS 0.7703* 0.4035* −0.5020* −0.7578* 注:样品数n=18;*表示P<0.05. Note: Sample number n=18; * indicates P< 0.05 -
[1] ZHANG F, ZHAO D X, CHI J. Impact of different environmental particles on degradation of dibutyl phthalate in coastal sediments with and without Cylindrotheca closterium [J]. Environmental Pollution, 2020, 261: 114228. doi: 10.1016/j.envpol.2020.114228 [2] YU Y M, MO W Y, LUUKKONEN T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics - A review [J]. The Science of the Total Environment, 2021, 797: 149140. doi: 10.1016/j.scitotenv.2021.149140 [3] FU H Y, WEI C H, QU X L, et al. Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: A mechanism of pseudomicelle partition and environmental implications [J]. Environmental Pollution, 2018, 232: 402-410. doi: 10.1016/j.envpol.2017.09.053 [4] ZHANG H B, WANG J Q, ZHOU B Y, et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors [J]. Environmental Pollution, 2018, 243: 1550-1557. doi: 10.1016/j.envpol.2018.09.122 [5] ZHANG F, WANG Z, WANG S, et al. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations [J]. Chemosphere, 2019, 228: 195-203. doi: 10.1016/j.chemosphere.2019.04.115 [6] ABDURAHMAN A, CUI K Y, WU J, et al. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis [J]. Ecotoxicology and Environmental Safety, 2020, 198: 110658. doi: 10.1016/j.ecoenv.2020.110658 [7] CHEN W, OUYANG Z Y, QIAN C, et al. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight [J]. Environmental Pollution, 2018, 233: 1-7. doi: 10.1016/j.envpol.2017.10.027 [8] HYUNG H, KIM J H. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters [J]. Environmental Science & Technology, 2008, 42(12): 4416-4421. [9] SINGH S, KUMAR V, CHAUHAN A, et al. Toxicity, degradation and analysis of the herbicide atrazine [J]. Environmental Chemistry Letters, 2018, 16(1): 211-237. doi: 10.1007/s10311-017-0665-8 [10] LIU G Z, ZHU Z L, YANG Y X, et al. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater [J]. Environmental Pollution, 2019, 246: 26-33. doi: 10.1016/j.envpol.2018.11.100 [11] ANDRADY A L. The plastic in microplastics: A review [J]. Marine Pollution Bulletin, 2017, 119(1): 12-22. doi: 10.1016/j.marpolbul.2017.01.082 [12] ZHAO L F, RONG L L, XU J P, et al. Sorption of five organic compounds by polar and nonpolar microplastics [J]. Chemosphere, 2020, 257: 127206. doi: 10.1016/j.chemosphere.2020.127206 [13] LIU P, ZHAN X, WU X W, et al. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks [J]. Chemosphere, 2020, 242: 125193. doi: 10.1016/j.chemosphere.2019.125193 [14] LIANG L, LUO L, ZHANG S Z. Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano- and micro-scales [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2011, 384(1/2/3): 126-130. [15] ENGEL M, CHEFETZ B. Adsorption and desorption of dissolved organic matter by carbon nanotubes: Effects of solution chemistry [J]. Environmental Pollution, 2016, 213: 90-98. doi: 10.1016/j.envpol.2016.02.009 [16] WANG F, YAO J, CHEN H L, et al. Sorption of humic acid to functionalized multi-walled carbon nanotubes [J]. Environmental Pollution, 2013, 180: 1-6. doi: 10.1016/j.envpol.2013.04.035 [17] LIN D H, LI T T, YANG K, et al. The relationship between humic acid (HA) adsorption on and stabilizing multiwalled carbon nanotubes (MWNTs) in water: Effects of HA, MWNT and solution properties [J]. Journal of Hazardous Materials, 2012, 241/242: 404-410. doi: 10.1016/j.jhazmat.2012.09.060 [18] CAO H M, ZHANG P, JIA W L, et al. Adsorption of phenanthrene onto magnetic multi-walled carbon nanotubes (MMWCNTs) influenced by various fractions of humic acid from a single soil [J]. Chemosphere, 2021, 277: 130259. doi: 10.1016/j.chemosphere.2021.130259 [19] XIAO F, PIGNATELLO J J. Interactions of triazine herbicides with biochar: Steric and electronic effects [J]. Water Research, 2015, 80: 179-188. doi: 10.1016/j.watres.2015.04.040 -



 下载:
下载: