-
氟是常见的水体污染物,过量摄入会导致氟中毒,不但会损害人体骨骼和牙齿健康,还可能使人体肾脏受损和甲状腺激素紊乱[1-2]. 氟污染已对全球多个国家造成了严重威胁,全球有超过2亿人处于氟中毒的危险中,我国是氟中毒较为严重的国家之一[3-4]. 由于严重的毒副作用,世界卫生组织(WHO)规定饮用水中的氟离子(F–)浓度不得超过1.5 mg·L−1(ISBN 978-92-4-154995-0),我国《生活饮用水卫生标准》规定水中氟化物不应超过1.0 mg·L−1(GB 5749-2006). 因此,对水体中的F–进行监测和管理是保障水环境安全的重要内容,研究准确、快速、高灵敏度的水体F–检测方法十分必要.
目前F–的检测方法主要包括离子选择电极法、离子色谱法、分子吸收光谱法、比色法和荧光检测法等[5-8]. 其中,荧光检测法因其灵敏度高、检测实时、操作简便等优点,近年来引起了研究者的广泛关注[8-11]. 荧光检测技术的关键是其探针,探针的性能很大程度上决定了方法的检测灵敏度、速度和选择性. 目前可用于F–检测的荧光探针以硅基/硼基/脲基有机合成小分子或聚合物、无机半导体量子点为主[8, 12-15]. 这些探针不但种类较为有限,还存在着合成过程复杂、F–响应时间长、检测限高,以及在水溶液中适用性较差等问题[12-14].
碳量子点是一种新兴的零维荧光碳纳米材料,具有荧光性质可控、稳定性高、水溶性好、合成方法简单、毒性低等优点[16-20],在荧光检测领域显示了巨大的应用潜力. 在环境分析方面,碳量子点已被成功用于检测水体、血液等环境样品中的污染物[21-25]. 但目前关于碳量子点的荧光检测研究主要集中于重金属等阳离子型污染物[21-23],对F–等阴离子型污染物的研究相对较少.
本文以柠檬酸为碳源、尿素为氮源,采用简单的水热法制备了掺氮荧光碳量子点(NCDs),并利用多种表征技术对其结构组成和光学性质进行了表征. NCDs与铝离子(Al3+)作用后会发生荧光淬灭,而F–与Al3+的配位反应可置换出与NCDs结合的Al3+,使NCDs荧光恢复. 利用荧光“开启”效应,建立了F–的快速检测方法,研究了方法的灵敏度、选择性和稳定性,以及其在实际水体样品中的应用性能.
-
分析纯一水合柠檬酸(C6H8O7·H2O)、尿素(CH4N2O)、无水乙醇(C2H5OH)、氢氧化钠(NaOH)、盐酸(HCl)和硫酸(H2SO4)购自国药集团化学试剂有限公司. 分析纯六水合氯化铝(AlCl3·6H2O)、氟化钠(NaF)、氯化钠(NaCl)、溴化钠(NaBr)、硝酸钠(NaNO3)、溴酸钠(NaBrO3)、碳酸钠(Na2CO3)、氯化钾(KCl)、氯化镁(MgCl2)、氯化钙(CaCl2)购自上海Sigma-Aldrich公司. 生物试剂级硫酸奎宁购自上海阿拉丁生化科技股份有限公司.
-
利用水热法制备NCDs,具体制备过程如下:将0.21 g一水合柠檬酸和0.18 g尿素溶于5 mL去离子水中,超声至形成澄清溶液. 将上述溶液转移至含有聚四氟乙烯内衬的高压反应釜中,置于鼓风烘箱中加热,于160 ℃反应4 h. 离心收集反应产物,经乙醇洗涤、冷冻干燥后,得到固体NCDs样品. NCDs储备液配置于纯水中,并于4 ℃避光保存.
-
采用JEM-2100型透射电子显微镜(TEM,日本JEOL公司)观察NCDs形貌. 利用Vario MICRO cube型元素分析仪(德国Elementar公司)测定NCDs中碳、氮、氧、氢等元素含量. 使用NEXUS870型傅里叶变换红外光谱仪(FT-IR,美国Nicolet公司)解析NCDs的官能团性质. 利用PHI5000 VersaProbe型X射线光电子能谱仪(XPS,日本ULVAC-PHI公司)分析NCDs的表面元素组成和元素形态. 使用Zetasizer Nano ZS型纳米粒度电位仪(英国Malvern公司)测定NCDs的Zeta电位. 采用UV-2700型紫外-可见分光光谱仪(日本Shimadzu公司)和Aqualog型荧光光谱仪(日本Horiba公司)分别采集NCDs的紫外-可见吸收光谱(UV-vis)和荧光光谱. 以硫酸奎宁的H2SO4溶液为标准样品,采用参比法测定NCDs的荧光量子产率. 使用FLS-980型稳态瞬态荧光光谱仪分析(英国Edinburgh公司)测定NCDs的荧光寿命.
-
在10 mL离心管中加入适量NCDs溶液和AlCl3溶液,使溶液中NCDs浓度为1 mg·L−1、Al3+浓度为0—140 μmol·L−1,并用NaOH将溶液pH值调节为7.0. 溶液配置完成1 min后,采集上述溶液在激发波长(Ex)为340 nm处的二维荧光光谱. 利用相同的方法配置NCDs、AlCl3和NaF的混合溶液(pH 7.0),使NCDs浓度为1 mg L−1、Al3+浓度为100 μmol·L−1、F–浓度范围为0—500 μmol·L−1,并在Ex为340 nm处采集溶液的荧光光谱. 为研究NCDs荧光检测F–的稳定性和选择性,考察了光照、溶液pH值,以及常见水体阴阳离子对检测过程的影响. 在光稳定性实验中,持续采集NCDs、NCDs/Al3+、NCDs/Al3+/F–混合溶液在Ex为340 nm、发射波长(Em)为440 nm处的荧光强度,采集时长为1 h,采集频率为每分钟1次. 在pH影响实验中,采用NaOH和HCl调节溶液pH值,pH值设定范围为4.0—9.0. 在阴阳离子影响实验中,氯离子(Cl–)、溴离子(Br–)、硝酸根(NO3–)、硫酸根(SO42–)、溴酸根(BrO3–)、碳酸根(CO32–)、钾离子(K+)、镁离子(Mg2+)、钙离子(Ca2+)等离子的浓度为50 μmol·L−1和300 μmol·L−1. 所有实验均设置3组平行样.
-
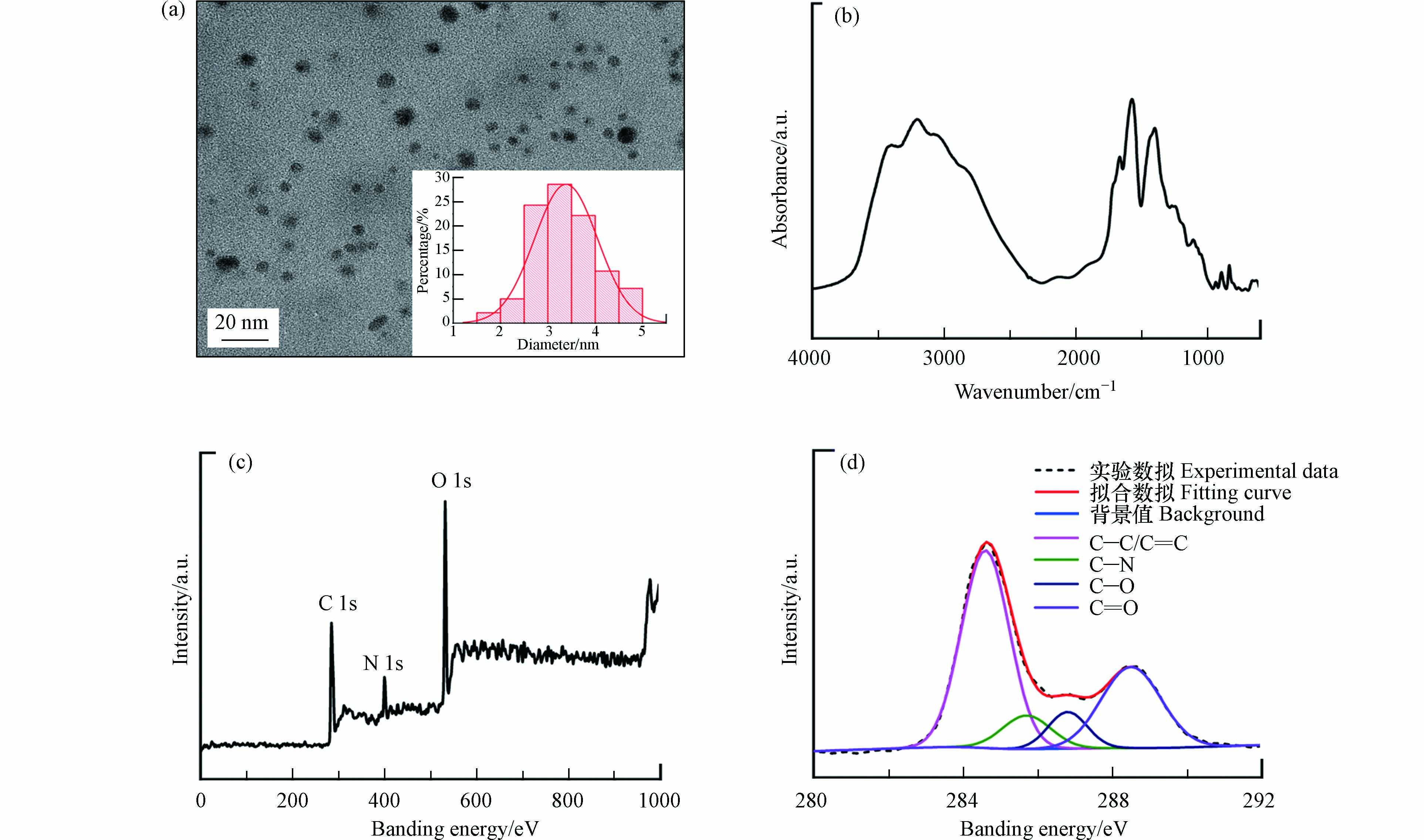
图1a为NCDs的TEM图像,可以看出本研究所合成的NCDs具有类球形结构,且颗粒分散性良好、尺寸较为均一(粒径范围为1.5—5.0 nm). 对NCDs的粒径进行统计分析,得到平均粒径为3.4 nm.
-
元素分析结果显示,NCDs中氮质量分数高达40.80% wt.,证实了氮元素的成功掺杂. NCDs中其它主要元素分别为碳(35.18%)、氧(17.65%)和氢(6.37%). 图1b为NCDs的FT-IR图谱. NCDs在1580 cm−1处的C=C伸缩振动峰和1440、1400、1340 cm−1处的C—H弯曲振动峰来源于其碳骨架结构[26];1260 cm−1处的吸收峰对应于C—N的伸缩振动,验证了NCDs中氮元素及含氮官能团的存在[27];1690 cm−1和1180 cm−1处的峰分别来源于C=O和C—O的伸缩振动[26, 28],表明NCDs还具有羧基、羟基等含氧官能团.
NCDs的XPS分析结果如图1c和d所示. 在NCDs的XPS全谱扫描图中出现了3个尖锐的特征结合能峰(图1c),分别对应于C1s(285 eV)、N1s(400 eV)和O1s(532 eV),表明NCDs表面富含碳、氮、氧元素. C1s峰可进一步分为4个子峰(图1d),对应于不同化学状态的碳原子,具体为:非氧化碳原子(C=C/C—C,结合能为284.5 eV)、与氮相连的碳原子(C—N,结合能为285.7 eV)、羟基/烷氧基中的碳原子(C—O,结合能为286.8 eV)和羰基/羧基中的碳原子(C=O,结合能为288.5 eV)[26, 28]. 结果与FT-IR表征结果一致,进一步证明了NCDs中存在含氮官能团以及羟基、羧基等含氧官能团.
-
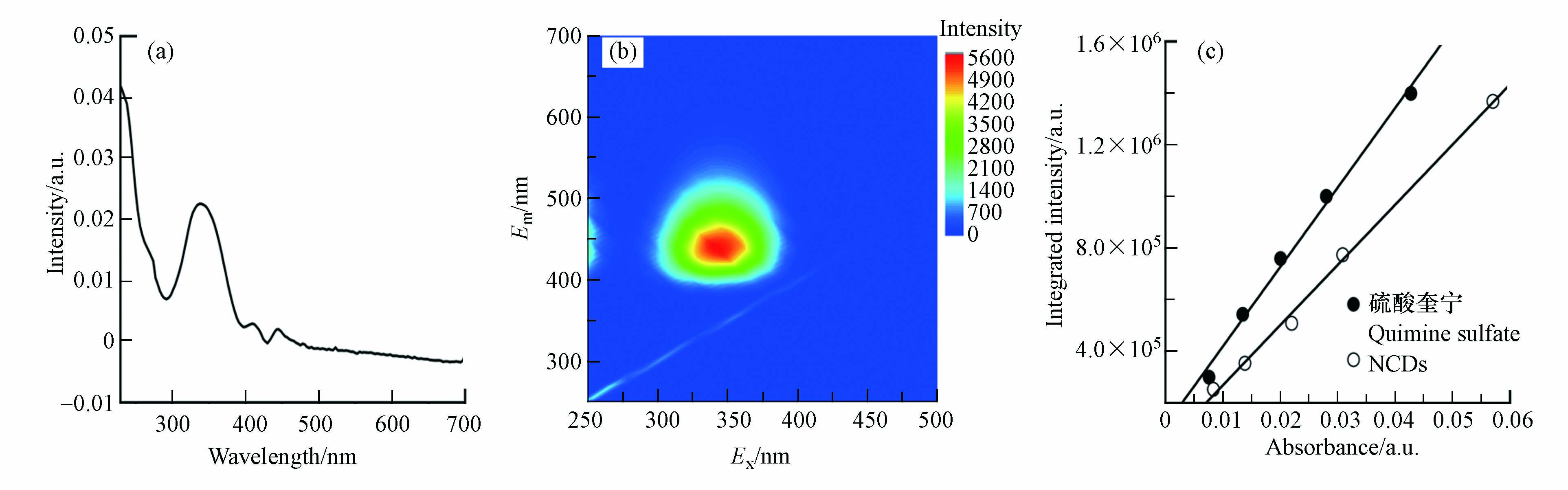
图2a为NCDs的UV-vis光谱图. 在低于230 nm和300—380 nm的波段范围内,NCDs均具有明显光吸收;其中小于230 nm的吸收峰归因于C=C基团的π-π*跃迁,通常不产生强荧光信号[29];而峰值位于340 nm处的吸收峰则可能伴随着荧光发射的产生. NCDs的三维荧光光谱分析结果印证了这一点. 如图2b所示,当Ex为300—380 nm时,NCDs产生了很强的荧光发射,Em范围为400—500 nm. NCDs的荧光峰值位于Ex = 340 nm/Em = 440 nm处,可能是产生于NCDs表面态电子-空穴对的辐射复合[17]. 值得注意的是,当Ex在300 nm至380 nm之间变化时,NCDs的Em峰始终位于440 nm左右,没有明显位移,说明NCDs具有不依赖于Ex的荧光发射特性,这可能源于其较为均一的粒径和表面状态分布[17]. 碳量子点的荧光量子产率是衡量其发光性能的重要指标. 以硫酸奎宁为参比,根据NCDs荧光强度与吸光度的关系,测定了NCDs的荧光量子产率(图2c),发现NCDs的量子产率高达41%,表明其具有优异的荧光发射本领.
-
碳量子点通常带负电,故绝大多数基于碳量子点的荧光传感器被用于检测阳离子型污染物 [21-23] . 研究显示,通过与碳量子点官能团的作用,金属等阳离子可引发量子点的荧光淬灭. 若能利用阴离子与金属的强配位作用,使金属离子从碳量子点脱附,则可恢复碳量子点的荧光,进而实现阴离子的荧光“开启”检测. Al3+作为一种环境友好的金属离子,与F–之间存在极强的配位能力. 基于此,以Al3+为介导离子,设计了NCDs检测F–的实验.
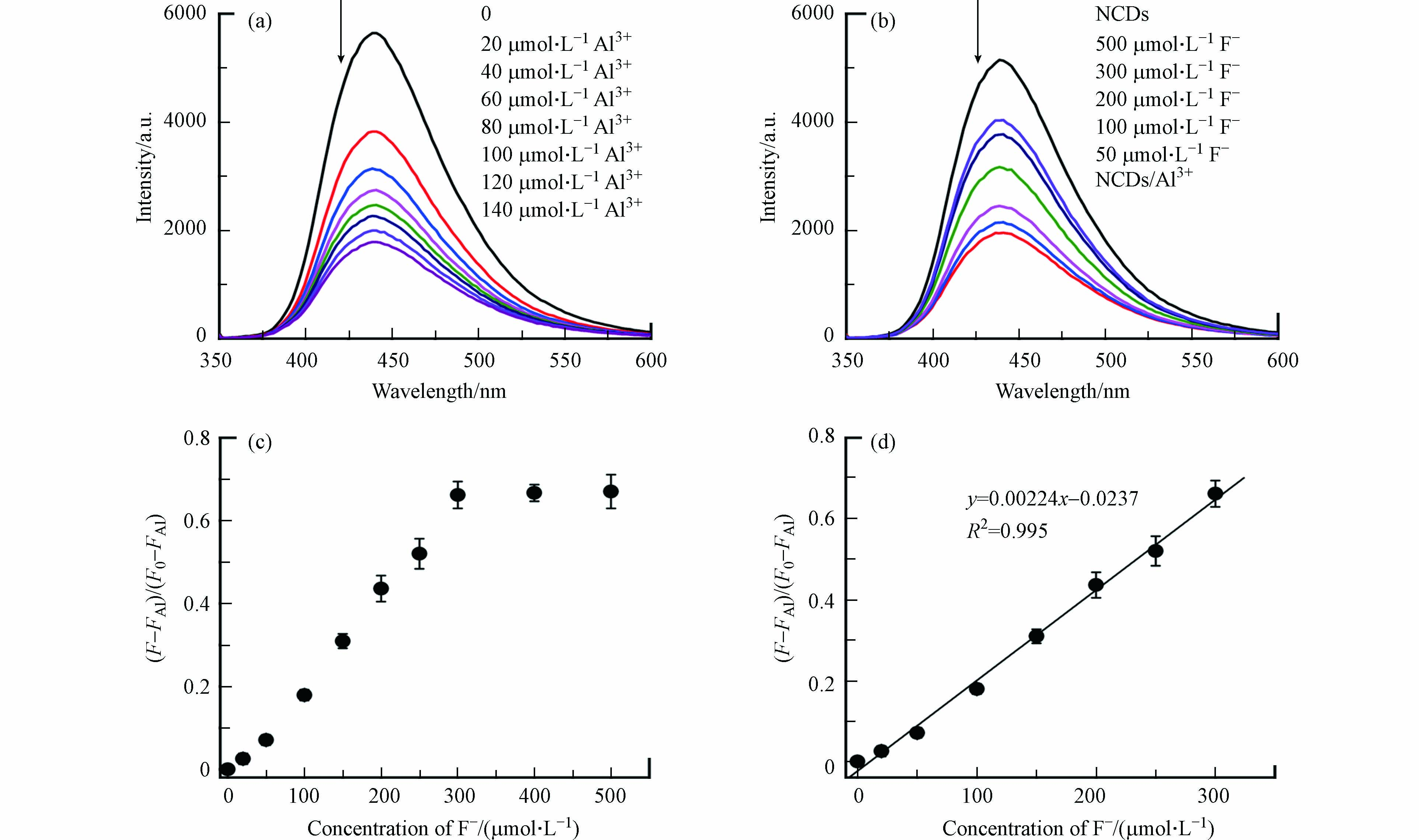
首先研究了Al3+对NCDs荧光的影响. 如图3a所示,当Al3+的浓度从0逐渐增加至140 μmol·L−1时,NCDs的荧光强度逐渐降低,证实了Al3+对NCDs的荧光淬灭效应.图3b显示了加入F–后,NCDs/Al3+溶液(Al3+浓度为100 μmol·L−1)的荧光变化情况. 可以看出,随着F–浓度的升高,NCDs/Al3+溶液的荧光强度逐渐变强,表明F–确实能够恢复NCDs被Al3+淬灭的荧光.
根据NCDs荧光峰值处(Ex = 340 nm,Em = 440 nm)的变化情况,可通过下式计算F–存在下NCDs的荧光恢复率:
其中,F0为NCDs溶液的原始荧光强度,FAl和F分别为加入Al3+和F–后的NCDs荧光强度. 由图3c可以发现,NCDs荧光恢复率先随着F–浓度的升高而稳步上升,并在F–浓度超过300 μmol·L−1后逐渐平稳,说明已达NCDs荧光恢复的上限. 在20—300 μmol·L−1浓度范围内,NCDs的荧光恢复率与F–浓度具有很好的线性关系(R2 = 0.995,图3d),表明该NCDs/Al3+体系可用于定量检测水中的F–. 该方法对F–的检出限(LOD)为0.65 μmol·L−1(即0.012 mg L−1),远低于WHO和我国规定的饮用水F–浓度限值,也低于多数已报道F–荧光检测法的LOD值(见表1)[8, 10-12, 28-31],表明NCDs/Al3+方法具有良好的F–灵敏度.
-
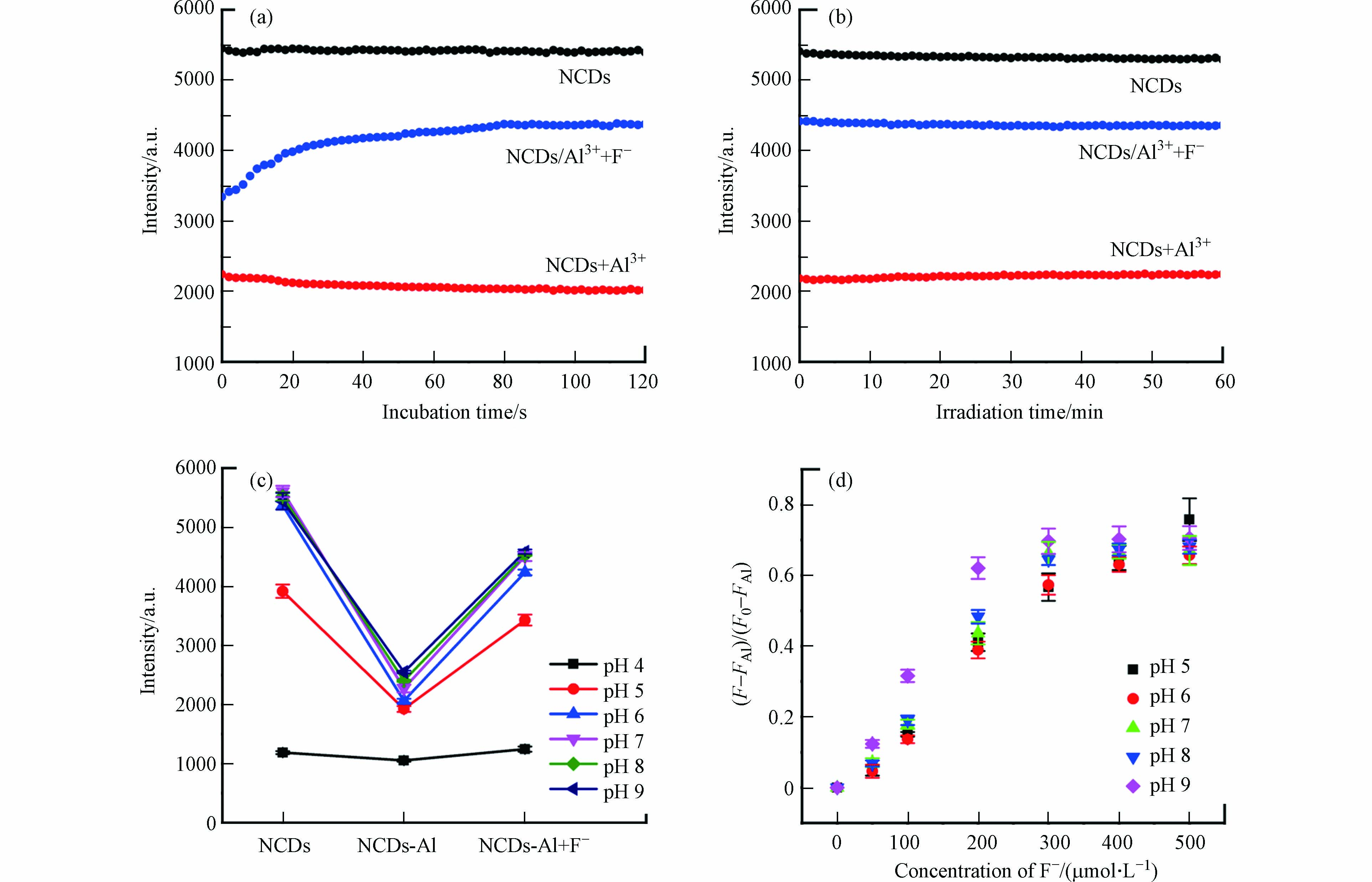
图4a显示了加入Al3+和F–后NCDs的荧光强度随时间的变化. 可以看出,Al3+淬灭NCDs荧光的速度极快,几乎在加入NCDs溶液的瞬时即达到淬灭平衡. F–恢复NCDs荧光的速度略慢于Al3+的淬灭速度,但也可在约1.0 min之内达到恢复平衡. 与文献报道的荧光方法相比(表1),NCDs/Al3+对F–的响应时间更短,具有更快的F–检测速度.
-
由于荧光探针是依靠荧光强度的改变来达到检测目的,因此探针在激发光下的荧光稳定性是其至关重要的性能指标. 如图4b所示,在激发光下连续照射1 h后,NCDs、NCDs/Al3+和NCDs/Al3+/F–溶液的荧光发射强度均未发生明显波动,表明该NCDs检测体系具有较强的荧光稳定性.
图4c和d为溶液pH值对NCDs检测F–性能的影响. 在溶液pH4.0时,NCDs表现出较弱的荧光发射,这可能是由于NCDs表面的含氧官能团在酸性条件下解离程度较低,NCDs疏水性较高,易因聚集产生荧光淬灭[34]. 当溶液pH由4.0升至9.0时,随着NCDs表面官能团解离程度的提高,NCDs的荧光强度逐渐上升,并在中性至弱碱性pH范围内(6.0—9.0)保持稳定(图4c). 在此pH范围内,Al3+和F–对NCDs荧光的淬灭和恢复程度也基本相同(图4d),表明NCDs有良好的pH适应性,在中性至弱碱性环境中均能保持稳定的荧光性能.
-
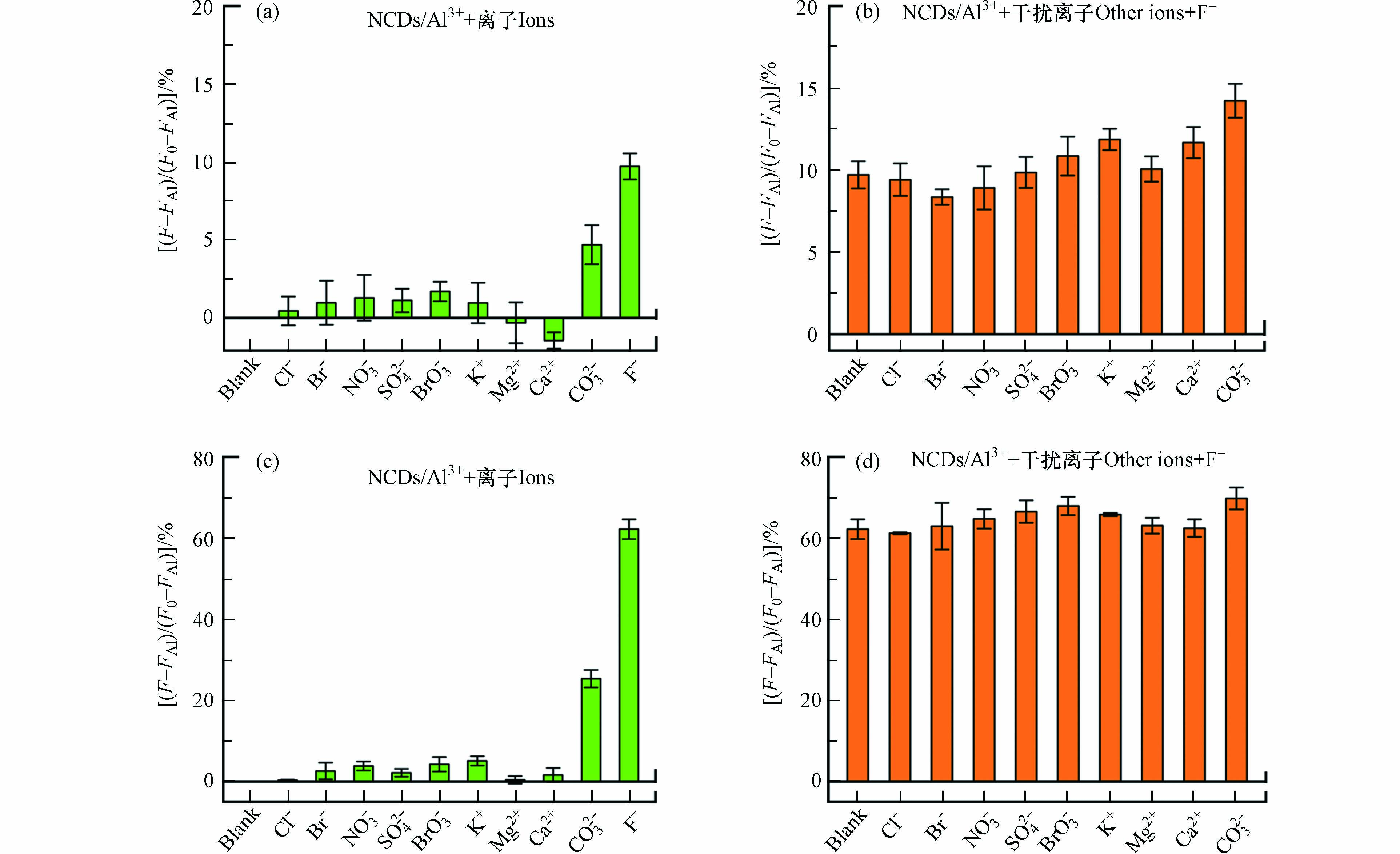
进一步考察NCDs/Al3+体系对F–的选择性,研究了水体常见阴离子(Cl–、Br–、NO3–、SO42–、BrO3–、CO32–)和阳离子(K+、Mg2+、Ca2+)对F–检测的影响. 如图5a和c所示,在浓度相同的情况下,F–恢复NCDs荧光的效率明显高于其它离子. 例如,低浓度(50 μmol·L−1)和高浓度(300 μmol·L−1)F–分别将NCDs的荧光恢复了10%和60%,而除CO32–外的其它离子的恢复率不足2%和5%.CO32–在两个浓度下的恢复效率分别为5%和25%,稍高于其它干扰离子,这可能是因为CO32–能够与Al3+在溶液中发生双水解反应. 尽管如此,CO32–的荧光恢复效率仍明显低于F–. 研究了F–与相同浓度干扰离子共存时,NCDs的荧光恢复效率(图5b和d). 在低浓度共存条件下,F–对NCDs的荧光恢复仅受CO32–微量影响(恢复率由10%增至14%);而当共存浓度较高时,所有测试离子均不影响NCDs检测F–. 结果表明, NCDs/Al3+体系对F–具有优秀的选择性. 该体系的F–选择性主要源于F–远高于其它离子的Al3+配位能力,F–的存在更容易使Al3+从NCDs上脱附.
-
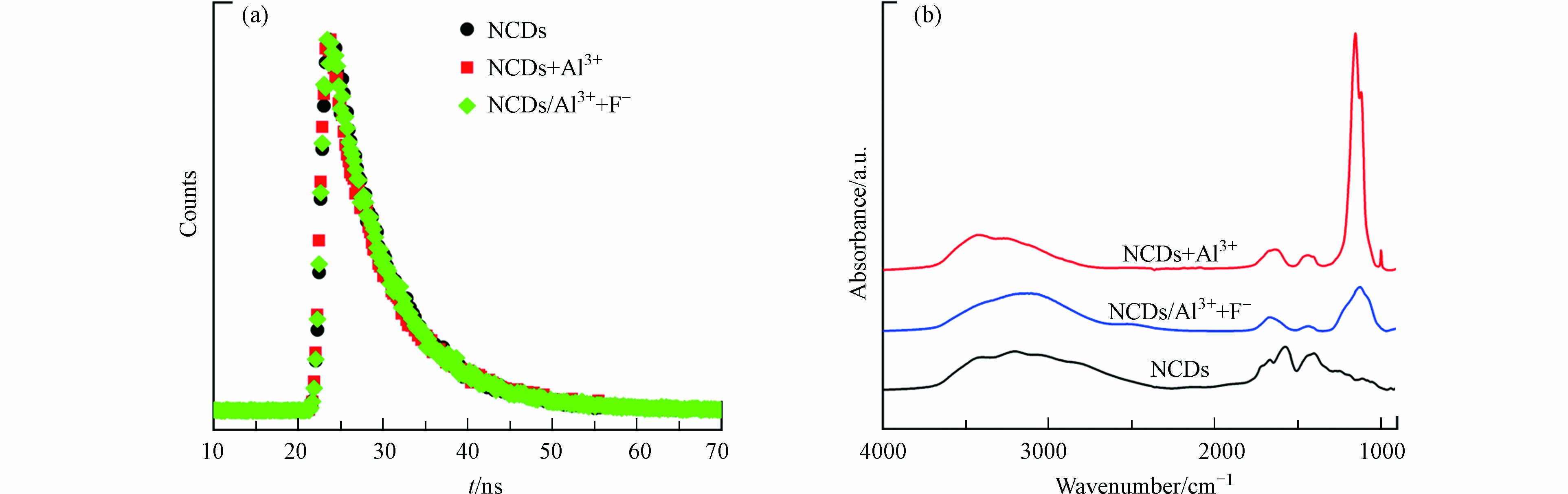
为探究NCDs对F–的检测机制,考察了Al3+和F–对NCDs荧光寿命的影响. 如图6a所示,NCDs、NCDs+Al3+、NCDs/Al3++F–的荧光衰减曲线几乎重叠,3个体系中NCDs的荧光寿命分别为7.01 ns、7.06 ns、7.02 ns,表明Al3+和F–对NCDs的荧光寿命无明显影响,二者不是通过能量转移过程影响NCDs荧光发光. 由此推测Al3+对NCDs荧光的淬灭机制为静态淬灭,即NCDs与Al3+发生相互作用,形成NCDs/Al3+静态复合物;当F–存在时,F–会与Al3+发生配位反应,使NCDs/Al3+复合物中的Al3+脱附,进而恢复NCDs荧光. 为了验证此假设,进一步分析了加入Al3+和F–后NCDs的表面电荷和FT-IR光谱变化. NCDs表面含有丰富含氧官能团,原始NCDs带负电,Zeta电位为-6.41 mV;加入Al3+后,由于Al3+与含氧官能团发生配位作用,因此NCDs的Zeta电位升至+1.40 mV;而加入F–后,F–与Al3+的配位导致NCDs表面的Al3+脱附,NCDs的Zeta电位又降至-3.97 mV. FT-IR光谱分析结果也印证了这一机制. 如图6b所示,与NCDs相比,NCDs/Al3+的FT-IR光谱在1120 cm−1处出现1个新强峰,对应于Al—OH的弯曲振动,证实了Al3+与NCDs的结合[35-36];加入F–后,Al—OH吸收峰的强度显著下降,表明F–的存在使Al3+从NCDs表面脱附.
-
为了评估NCDs/Al3+的可应用性,将其用于检测实际水体中的F–浓度. 所测试实际水样分别取自实验室自来水龙头和太湖,过0.45 μm滤膜后直接进行分析. 结果显示,两个水样中的F–浓度均低于方法的LOD值. 论文进一步开展了梯度加标回收实验(加标浓度为20、40、60、80、100 μmol·L−1,表2),测得F–回收率范围为88.2%—105.0%,与常规离子色谱法的检测回收率相当(91.4%—111.9%). 此外,NCDs/Al3+方法也具有较好的精密度,多次检测的相对标准偏差在2.67%以内(n=3),表明可利用NCDs检测实际水样中的F–.
-
本文利用简单的水热法成功地制备了具有优异荧光性能的掺氮碳量子点(NCDs). 该量子点能够以铝离子(Al3+)为介导,通过荧光“关闭”—“开启”模式选择性检测水体中的氟离子(F–). 与已报道的F–荧光检测方法相比,本论文基于NCDs的检测体系具有更低的F–检测限(0.65 μmol·L−1)和更快的检测速度(F–响应时间约1.0 min). 此外,NCDs还具有优异的荧光稳定性和F–选择性,在实际水样的F–分析中也显示了良好的应用性能. 本论文的研究结果可为水体F–的快速检测提供技术支持,也有助于拓展碳量子点材料在荧光检测中的应用.
基于氮掺杂碳量子点的水体氟离子选择性荧光开启检测
Rapid and selective “turn-on” fluorescent detection of fluoride ion in aqueous solution using nitrogen-doped carbon quantum dots
-
摘要: 本论文以柠檬酸为碳源、尿素为氮源,通过水热法制备了氮掺杂碳量子点(NCDs),将其作为荧光探针用于检测水体中的氟离子(F–). 利用透射电镜(TEM)、X射线光电子能谱(XPS)、红外光谱(FT-IR)、紫外-可见光谱(UV-vis)、荧光光谱等表征手段分析了NCDs的结构和光谱学性质. 考察了探针检测氟离子的灵敏度、稳定性和选择性,及其在天然水体样品中的适用性. 结果表明,NCDs可在紫外光激发下产生蓝色荧光,且具有较高的荧光量子产率(41%). NCDs富含羧基、羟基等含氧官能团,可与铝离子(Al3+)发生反应,这一过程会导致其荧光淬灭;而F–与Al3+的配位反应可置换出与NCDs结合的Al3+,使NCDs的荧光恢复,产生荧光“开启”效应.NCDs荧光恢复的程度与F–浓度线性正关系(R2 = 0.995),表明该方法可用于定量检测F–. 进一步研究显示,NCDs在检测F–时具有较快的响应时间(约1.0 min)、较宽的线性范围(20—300 μmol·L−1)、较低的检出限(0.65 μmol·L−1)和良好的选择性(水体常见阴阳离子对检测过程的影响低于5%). 此外,NCDs还具有良好的稳定性,在中性到弱碱性环境(pH 6.0—9.0)中均能有效检出F–. 在实际水体分析过程中,NCDs显示了良好的F–加标回收率(88.2%—105.0%)和检测精密度(相对标准偏差低于3.0%),表明其具有较好的应用潜能.Abstract: Nitrogen-doped carbon quantum dots (NCDs) were synthesized by a facile hydrothermal method using citric acid as the carbon source and urea as the nitrogen source, and were applied as a novel “turn-on” fluorescent probe for the detection of fluoride ions (F–) in water. The structural and spectroscopic properties of the NCDs were characterized by transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), infrared spectroscopy (FT-IR), ultraviolet-visible spectroscopy (UV-vis), and fluorescence spectroscopy. The sensitivity, stability and selectivity of the NCDs probe to detect F– as well as its applicability in natural water samples were investigated. NCDs showed blue fluorescence emission under ultraviolet light irradiation, and had a high fluorescence quantum yield of 41%. The NCDs can react with aluminium ions (Al3+) via the surface oxygen-containing groups, which would quench the fluorescence emission of NCDs. Due to the strong coordination affinity, F– can compete with NCDs for Al3+ and thus recover the fluorescence of NCDs. There existed a good linear relationship between the recovery ratio of NCDs fluorescence and F– concentration (R2 = 0.995), suggesting the possibility of NCDs in F– quantification. The NCDs-based fluorescence method for F– detection exhibited a short response time (approximately 1.0 min), wide linear range (20—300 μmol·L−1), low detection limit (0.65 μmol·L−1), good selectivity (influences of common ions below 5%), and satisfactory stability in environmentally relevant pH range (pH 6.0—9.0). Finally, the proposed method was successfully applied in the analysis of F– in real water samples with high recoveries (88.2%—105.0%) and precision (relative standard deviations lower than 3.0%).
-
Key words:
- nitrogen-doped carbon dots /
- fluoride ion /
- fluorescent detection /
- turn-on
-

-
图 3 (a)Al3+和(b)F–对NCDs溶液荧光光谱的影响(Ex= 340 nm);(c)不同浓度F–存在下NCDs在Ex = 340 nm/Em = 440 nm处的荧光恢复率;(d)NCDs在Ex = 340 nm/Em = 440 nm处的荧光恢复率与F–的相关关系
Figure 3. Fluorescence spectra of NCDs solution at Ex 340 nm upon the addition of (a) Al3+ and (b) F–; (c) fluorescence recovery efficiency of NCDs at Ex = 340 nm/Em = 440 nm as a function of F– concentration; (d) relationship between fluorescence recovery efficiency of NCDs at Ex = 340 nm/Em = 440 nm and F– concentration
图 4 NCDs的(a)荧光响应速度和(b)荧光稳定性随时间的变化;溶液pH值对NCDs的(c)荧光强度和(d)荧光恢复率的影响(Al3+浓度为100 μmol·L−1,F–浓度为300 μmol·L−1)
Figure 4. Fluorescence intensity of NCDs solution in the presence of Al3+ (100 μmol·L−1) and/or F– (300 μmol·L−1) as a function of (a) incubation time and (b) irradiation time; effect of solution pH on NCDs (c) fluorescence intensity and (d) fluorescence recovery efficiency by F–
图 5 (a)低浓度(50 μmol·L−1)和(c)高浓度(300 μmol·L−1)离子存在下NCDs的荧光恢复率;(b)低浓度(50 μmol·L−1)和(d)高浓度(300 μmol·L−1)干扰离子和F–共存时的NCDs荧光恢复率
Figure 5. Fluorescence recovery efficiency of NCDs in the presence of different ions at (a) low (50 μmol·L−1) and (c) high concentrations (300 μmol·L−1), and in the coexistence of F– and other ions at (b) low (50 μmol·L−1) and (d) high concentrations (300 μmol·L−1)
表 1 文献报道F–荧光检测法的分析性能
Table 1. Analytical performances of reported fluorescence methods for F– detection
探针
Probe检出限/(μmol·L−1)
LOD线性范围/(μmol·L−1)
Linear Range响应时间/min
Response time文献
References基于内部电荷转移的荧光探针 80 500—28000 25 [8] 基于Si—O键断裂的荧光探针 18 0—1000 45 [12] 含有多面体低聚硅氧烷的纳米粒子 10 10—100 1.67 [13] 基于硼酸的荧光碳点 110 0—26700 5.0 [14] 蒽基荧光受体 2.0 2—120 NAa [30] 基于1,1’-联萘基支架的荧光探针 1.86 5.0—45.0 200 [31] 基于壳聚糖凝胶的荧光碳点 6.6 6.6—50.6 2.0 [32] 基于羧酸桥联二铁络合物的
荧光探针6.7 0—30 NAa [33] NCDs 0.65 20—300 1.0 本论文 注:a “NA” 文中未提及. a “NA ” Not available. 表 2 基于NCDs的荧光法对实际水样中F–的检测结果(%, n=3)
Table 2. Analytical results for the determination of F– in real water samples
加标浓度/(μmol·L−1)
Spiked concentration自来水样加标回收率
Tap water recoveries太湖水样加标回收率
Taihu Lake water recoveries荧光法 离子色谱法 荧光法 离子色谱法 20 88.2 ± 2.24 111.7 ± 0.76 88.5 ± 2.66 111.9 ± 1.27 40 92.1 ± 2.63 98.5 ± 2.06 94.7 ± 2.01 107.2 ± 1.93 60 95.7 ± 2.67 94.0 ± 0.33 105.0 ± 1.97 99.9 ± 1.75 80 96.9 ± 1.90 92.0 ± 1.21 97.3 ± 2.20 94.7 ± 0.25 100 101.6 ± 1.58 91.4 ± 1.13 103.7 ± 2.01 91.5 ± 1.05 -
[1] AOBA T, FEJERSKOV O. Dental fluorosis: Chemistry and biology [J]. Critical Reviews in Oral Biology and Medicine:an Official Publication of the American Association of Oral Biologists, 2002, 13(2): 155-170. doi: 10.1177/154411130201300206 [2] AK S, BHATNAGAR M, VIG K, et al. Excess fluoride ingestion and thyroid hormone derangements in children living in Delhi, India [J]. Fluoride, 2005, 38(2): 98-108. [3] AYOOB S, GUPTA A K. Fluoride in drinking water: A review on the status and stress effects [J]. Critical Reviews in Environmental Science and Technology, 2006, 36(6): 433-487. doi: 10.1080/10643380600678112 [4] 刘东生, 陈庆沐, 余志成, 袁芷云. 我国地方性氟病的地球化学问题 [J]. 地球化学, 1980, 9(1): 13-22. doi: 10.3321/j.issn:0379-1726.1980.01.002 LIU D S, CHEN Q M, YU Z C, et al. Geochemical environment problems concerning the endemic fluorine disease in China [J]. Geochimica, 1980, 9(1): 13-22(in Chinese). doi: 10.3321/j.issn:0379-1726.1980.01.002
[5] DAY J K, BRESNER C, COOMBS N D, et al. Colorimetric fluoride ion sensing by polyborylated ferrocenes: Structural influences on thermodynamics and kinetics [J]. Inorganic Chemistry, 2008, 47(3): 793-804. doi: 10.1021/ic701494p [6] MORÉS S, MONTEIRO G C, SANTOS F D S, et al. Determination of fluorine in tea using high-resolution molecular absorption spectrometry with electrothermal vaporization of the calcium mono-fluoride CaF [J]. Talanta, 2011, 85(5): 2681-2685. doi: 10.1016/j.talanta.2011.08.044 [7] LI Y H, DUAN Y, ZHENG J, et al. Self-assembly of graphene oxide with a silyl-appended spiropyran dye for rapid and sensitive colorimetric detection of fluoride ions [J]. Analytical Chemistry, 2013, 85(23): 11456-11463. doi: 10.1021/ac402592c [8] ZHU B C, YUAN F, LI R X, et al. A highly selective colorimetric and ratiometric fluorescent chemodosimeter for imaging fluoride ions in living cells [J]. Chemical Communications, 2011, 47(25): 7098-7100. doi: 10.1039/c1cc11308a [9] ZHOU Y, ZHANG J F, YOON J. Fluorescence and colorimetric chemosensors for fluoride-ion detection [J]. Chemical Reviews, 2014, 114(10): 5511-5571. doi: 10.1021/cr400352m [10] LIU W, DAI X, BAI Z L, et al. Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal-organic framework equipped with abundant lewis basic sites: A combined batch, X-ray absorption spectroscopy, and first principles simulation investigation [J]. Environmental Science & Technology, 2017, 51(7): 3911-3921. [11] LIU W, DAI X, WANG Y, et al. Ratiometric monitoring of thorium contamination in natural water using a dual-emission luminescent europium organic framework [J]. Environmental science & technology, 2018, 53(1): 332-341. [12] CHENG X H, JIA H, FENG J, et al. “Reactive” probe for fluoride: “Turn-on” fluorescent sensing in aqueous solution and bioimaging in living cells [J]. Sensors and Actuators B:Chemical, 2014, 199: 54-61. doi: 10.1016/j.snb.2014.03.054 [13] DU F F, BAO Y Y, LIU B, et al. POSS-containing red fluorescent nanoparticles for rapid detection of aqueous fluoride ions [J]. Chemical Communications, 2013, 49(41): 4631-4633. doi: 10.1039/c3cc40810h [14] SHAMSIPUR M, SAFAVI A, MOHAMMADPOUR Z, et al. Fluorescent carbon nanodots for optical detection of fluoride ion in aqueous media [J]. Sensors and Actuators B:Chemical, 2015, 221: 1554-1560. doi: 10.1016/j.snb.2015.07.096 [15] LIU J B, YANG X H, WANG K M, et al. A switchable fluorescent quantum dot probe based on aggregation/disaggregation mechanism [J]. Chemical Communications, 2011, 47(3): 935-937. doi: 10.1039/C0CC03993D [16] SUN Y P, ZHOU B, LIN Y, et al. Quantum-sized carbon dots for bright and colorful photoluminescence [J]. Journal of the American Chemical Society, 2006, 128(24): 7756-7757. doi: 10.1021/ja062677d [17] ZHU S J, SONG Y B, ZHAO X H, et al. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective [J]. Nano Research, 2015, 8(2): 355-381. doi: 10.1007/s12274-014-0644-3 [18] ZHANG Y Q, MA D K, ZHUANG Y, et al. One-pot synthesis of N-doped carbon dots with tunable luminescence properties [J]. Journal of Materials Chemistry, 2012, 22(33): 16714-16718. doi: 10.1039/c2jm32973e [19] HU C, LI M Y, QIU J S, et al. Design and fabrication of carbon dots for energy conversion and storage [J]. Chemical Society Reviews, 2019, 48(8): 2315-2337. doi: 10.1039/C8CS00750K [20] 傅鹏, 周丽华, 唐连凤, 等. 碳量子点的制备及其在能源与环境领域应用进展 [J]. 应用化学, 2016, 33(7): 742-755. doi: 10.11944/j.issn.1000-0518.2016.07.150393 FU P, ZHOU L H, TANG L F, et al. Progress in preparation of carbon quantum dots and its application in the fields of energy and environment [J]. Chinese Journal of Applied Chemistry, 2016, 33(7): 742-755(in Chinese). doi: 10.11944/j.issn.1000-0518.2016.07.150393
[21] PENG D, ZHANG L, LIANG R P, et al. Rapid detection of mercury ions based on nitrogen-doped graphene quantum dots accelerating formation of Manganese porphyrin [J]. ACS Sensors, 2018, 3(5): 1040-1047. doi: 10.1021/acssensors.8b00203 [22] LIU H, LI R S, ZHOU J, et al. Branched polyethylenimine-functionalized carbon dots as sensitive and selective fluorescent probes for N-acetylcysteine via an off-on mechanism [J]. Analyst, 2017, 142(22): 4221-4227. doi: 10.1039/C7AN01136A [23] ZHENG M, XIE Z G, QU D, et al. On-off-on fluorescent carbon dot nanosensor for recognition of chromium(VI) and ascorbic acid based on the inner filter effect [J]. ACS Applied Materials & Interfaces, 2013, 5(24): 13242-13247. [24] CHEN B B, LIU M L, ZHAN L, et al. Terbium (III) modified fluorescent carbon dots for highly selective and sensitive ratiometry of stringent [J]. Analytical chemistry, 2018, 90(6): 4003-4009. doi: 10.1021/acs.analchem.7b05149 [25] ZHANG Z, ZHANG D, SHI C, et al. 3, 4-Hydroxypyridinone-modified carbon quantum dot as a highly sensitive and selective fluorescent probe for the rapid detection of uranyl ions [J]. Environmental Science:Nano, 2019, 6(5): 1457-1465. doi: 10.1039/C9EN00148D [26] TANG L, JI R, LI X, et al. Deep ultraviolet to near-infrared emission and photoresponse in layered N-doped graphene quantum dots [J]. ACS nano, 2014, 8(6): 6312-6320. doi: 10.1021/nn501796r [27] LI H, KONG W, LIU J, et al. Fluorescent N-doped carbon dots for both cellular imaging and highly-sensitive catechol detection [J]. Carbon, 2015, 91: 66-75. doi: 10.1016/j.carbon.2015.04.032 [28] QU D, ZHENG M, ZHANG L G, et al. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots [J]. Scientific Reports, 2014, 4: 5294. doi: 10.1038/srep05294 [29] LI L B, YU B, YOU T. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (Ⅱ) ions [J]. Biosensors and Bioelectronics, 2015, 74: 263-269. doi: 10.1016/j.bios.2015.06.050 [30] HUANG W W, LIN H, CAI Z, et al. A novel anthracene-based receptor: Highly sensitive fluorescent and colorimetric receptor for fluoride [J]. Talanta, 2010, 81(3): 967-971. doi: 10.1016/j.talanta.2010.01.045 [31] DONG M, PENG Y, DONG Y M, et al. A selective, colorimetric, and fluorescent chemodosimeter for relay recognition of fluoride and cyanide anions based on 1, 1'-binaphthyl scaffold [J]. Organic Letters, 2012, 14(1): 130-133. doi: 10.1021/ol202926e [32] BARUAH U, GOGOI N, MAJUMDAR G, et al. β-Cyclodextrin and calix[4]arene-25, 26, 27, 28-tetrol capped carbon dots for selective and sensitive detection of fluoride [J]. Carbohydrate Polymers, 2015, 117: 377-383. doi: 10.1016/j.carbpol.2014.09.083 [33] ZHOU Y H, DONG X L, ZHANG Y X, et al. Highly selective fluorescence sensors for the fluoride anion based on carboxylate-bridged diiron complexes [J]. Dalton Transactions, 2016, 45(16): 6839-6846. doi: 10.1039/C5DT03801D [34] WANG H, WANG Y, GUO J, et al. A new chemosensor for Ga3+ detection by fluorescent nitrogen-doped graphitic carbon dots [J]. RSC Advances, 2015, 5(17): 13036-13041. doi: 10.1039/C4RA15431B [35] SHI L H, WANG Q, ZHANG C, et al. Tricolor emission carbon dots for label-free ratiometric fluorescent and colorimetric recognition of Al3+ and pyrophosphate ion and cellular imaging [J]. Sensors and Actuators B:Chemical, 2021, 345: 130375. doi: 10.1016/j.snb.2021.130375 [36] LIU C S, SHIH K, GAO Y X, et al. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina [J]. Journal of Soils and Sediments, 2012, 12(5): 724-733. doi: 10.1007/s11368-012-0506-0 -



 下载:
下载: