-
由于抗菌谱广、可以口服、吸收较迅速等优点,磺胺类抗生素(sulfonamides, SAs)被大量应用于治疗和预防疾病[1 − 3]. 但是由于早期的认知缺失,我国存在严重的SAs滥用情况,其对环境存在潜在的严重危害[4]. 以磺胺对甲氧嘧啶(sulfamethoxydiazine,SMD)为例,目前已监测到其在地表水和地下水中广泛存在[5 − 7]. 虽然SMD在环境中呈现出低浓度水平,但通过食物链的传播和积累,其潜在毒性不可忽视[8],极易产生致癌、致畸和致突变等系列危害. 目前,电化学高级氧化法(electrochemical advanced oxidation process, EAOPs)因其降解污染物彻底、处理无害、见效快以及温和的工艺条件等独特优势[9],成为降解磺胺类抗生素最有前景的技术,Tröster等[10]构建了以掺硼金刚石膜[11](boron doped diamond, BDD)电极为阳极的电化学氧化系统,研究结果表明BDD电极的化学惰性和独特的电化学性质对电化学水处理具有巨大潜力,EAOP适用于废水处理,且已经在实验室内获得成功. 从实验室研究到工业应用过程中,反应器放大所引起的尺度效应对于降解效率具有重要影响,因此反应器的尺度效应和尺寸的优化研究具有重要意义.
目前,已经有研究人员通过构建不同尺寸的反应器对污染物降解尺度效应做出探讨. 其中,Zhu等[12 − 13]构建了一个大型BDD阳极系统(阳极面积为2904 cm2)按比例放大后在批量处理模式下未发现效率明显降低,认为BDD阳极系统的电化学氧化反应器尺度放大具有可行性. 针对垃圾填埋场渗滤液电化学氧化实验,Urtiaga等[14 − 15]构建了BDD阳极面积为1.05 m2中试规模反应器,并对放大效果进行评估,结果表明,中试系统对铵离子去除率明显降低,反应器尺寸效应影响显著. 为了降低化学实验成本,计算流体力学软件(computational fluid dynamics,CFD)被引入到污染物降解模拟中,CFD模型在实验初和验证阶段可提供设计、优化和综合分析[16],并预测电化学反应器的性能[17]. van Walsem[18]等利用CFD模型计算新型多管反应器中吸附和解吸速率常数并利用实验确定的出口浓度分布,验证了该模型的模拟结果与实验数据具有良好的一致性,证实了CFD在化学实验研究中的可行性. Bagheri等[19]利用CFD模型对用于水处理的双波长紫外线(VUV/UV)光反应器进行了模拟,预测了VUV/UV反应器中污染物的降解速率. 由于CFD模型和化学实验分属不同的学科领域,利用CFD软件模拟反应器内的污染物降解机制,评估反应器放大效应对于降解效率的影响,目前尚缺乏深入的研究.
本研究以SMD为目标污染物,采用以BDD电极为阳极的电化学氧化系统处理模拟废水,建立循环系统中污染物降解的CFD模型,利用实验测试确定电极表面反应速率,结合反应器几何尺寸进行流场模拟研究,探究反应器放大倍数与降解效率的关系,为电化学降解有机污染物的工业化应用提供依据.
-
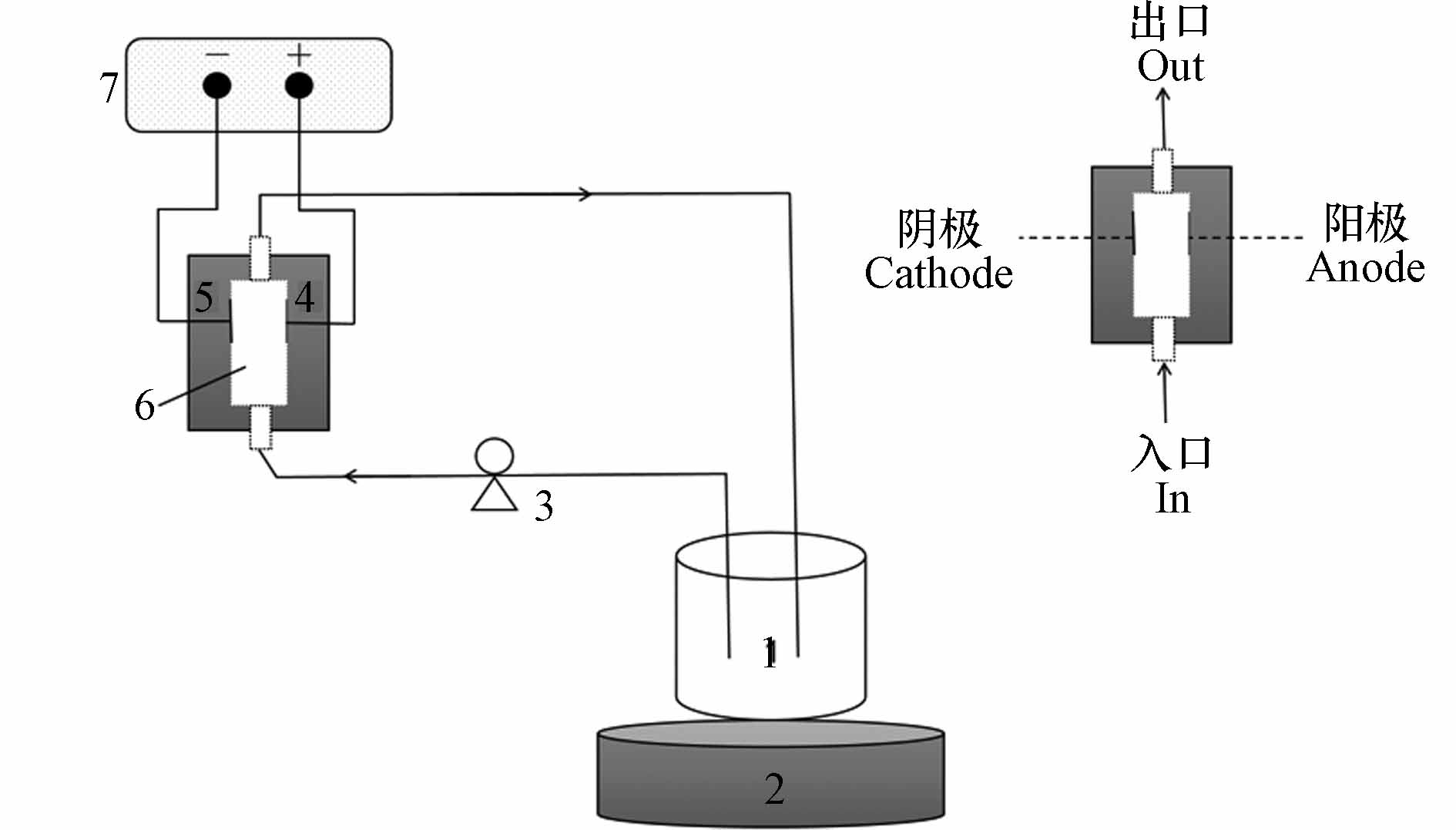
实验装置示意图如图1所示. 反应器为微分柱间歇式反应器(differential column batch reactor, DCBR)[20 − 21] 其中,BDD阳极板与电源正极相连,不锈钢板与负极相连,电极间距为14 mm. 可通过蠕动泵调节反应器进口流量,烧杯内含500 mL待降解溶液,溶液被蠕动泵从烧杯吸出,通过电化学反应器流动并回到烧杯中,实现污染物的循环降解.
实验条件及操作流程:在恒温、恒流(100 mL·min−1)条件下,将含0.1 mol·L−1 Na2SO4电解质的磺胺对甲氧嘧啶溶液(30 mg·L−1)作为模拟污水,采用BDD阳极体系进行连续的电化学氧化,每隔10 分钟从烧杯中抽取样品进行化学分析,循环持续1 h.
实验中使用的磺胺对甲氧嘧啶来自山东西亚化工有限公司;分析级 Na2SO4购自中国国药化学试剂北京有限公司;去离子水(电阻率 > 18.2 MΩ·cm)在室温下从Millipore纯水系统中获得;BDD阳极板来自德国厂家CONDIAS GmbH,尺寸为30 mm×80 mm×1 mm,相同尺寸的阴极不锈钢板来自北京丽都博益水处理科技有限公司.
样品中SMD的降解效率由日本津岛公司生产的LC-20A型高效液相色谱仪(HPLC)分析所得,使用的色谱柱型号为安捷伦SB-C18(4.6 mm×250 mm×5 μm). 其中,流动相甲醇:超纯水(0.1%甲酸和99.9%去离子水)为60:40,恒定流速为1.0 mL·min−1,在
$ \lambda $ =254 nm处进行检测(注射量为20 μL,柱温设定为25℃). -
基于有限体积法,网格采用非结构化网络划分如图2所示. 为计算方便,划分两个流体域作为反应区域与非反应区域. 其中反应区域是靠近壁表面的一层网格区域(图2中的绿色区域)尺寸为3 cm×8 cm×1.4 cm,其余部分为非反应区域,其中内部圆柱状衔接部分尺寸 0.0628 cm3(r = 0.2 cm,h = 0.5 cm). 其中,Y 正方向由反应器入口指向出口,Z 正方向指向阳极.
-
假设流体为不可压缩态且固壁无滑移,由于SMD浓度为30 mg·L−1,溶液的物理性质可近似认为与水相同,因此可用标准壁函数来预测近壁流动特性. 对于入口条件,半径r = 0.2 m,初始流量为0.1 L·min−1计算得流速为0.133 m·s−1并给定SMD的初始质量分数为3×10−5,出口则设置为压力出口边界条件,减少回流对流体流动的影响.
在基本控制方程中,反应速率以源相的形式出现在组分输运方程中. 因此反应速率和边界条件均采用用户自定义函数(user-defined functions,UDF)进行编译,使用C语言编程计算出反应器出口SMD的平均质量分数并将其赋给反应器的进口,从而实现液体的循环流动模拟,化学反应速率以质量组分源项的形式加入在阳极表面反应区域. 本文采用收敛残差的标准为10−4,各变量离散均采用二阶迎风格式,迭代方法为SIMPLE算法.
-
通过求解反应器中各组分的对流、扩散和反应的物质守恒方程,使用CFD软件Fluent 19.2,对SMD的传输和化学反应进行建模. 控制方程如下:
式中,ρ 为流体密度;
$ {u_i} $ 、$ {u_j} $ 为流体速度矢量;t为时间;P为流体微元体上的压力;$ {\tau _{ij}} $ 为黏性应力张量;C为浓度;S是源(汇)项;$ {f_i} $ 为单位质量力;$ {J_j} $ 为质量扩散通量,在湍流扩散流量中可以表示为$ {J}_{j}=\partial \dfrac{C}{\partial {x}_{j}}(\rho {D}_{i,m}+\dfrac{{u}_{t}}{S{c}_{t}}) $ ,其中Di,m是混合物中第i种物质的扩散系数,$ S{c_t} $ 是湍流施密特数并将默认值设置为0.7. RNG$ k-\varepsilon $ 模型包括湍流动能方程k和扩散方程$ \varepsilon $ 如下式:式中,ρ 为流体密度;
$ k $ 和$ \epsilon $ 分别为湍动动能和耗散率;$ {u_i} $ 和$ {x_j} $ 分别为速度分量和坐标分量;$ {u}_{\mathrm{e}\mathrm{f}\mathrm{f}} $ 为有效黏性系数;$ {G_K} $ 为湍动能产生项;$ {G_b} $ 是用于浮力引起的湍流动能;$ {Y}_{M} $ 为可压缩湍流中脉动膨胀对总耗散率的影响;$ {G}_{1\varepsilon } $ =1.42;$ {G}_{2\varepsilon } $ =1.68;$ {\alpha _k} $ 和$ {\alpha }_{\varepsilon } $ 分别为k和$ \varepsilon $ 的湍流Prandtl数$ {\alpha _k} $ =$ {\alpha }_{\varepsilon } $ =0.7179;$ {S}_{k} $ 和$ {S}_{\varepsilon } $ 为用户定义的源项;$ {S}_{\varepsilon } $ 为$ \varepsilon $ 方程中的附加源. -
化学实验中通过测量反应进出口污染物浓度差确定实验系统的降解速率,该值是电极板的表面降解能力和内部流动传质特性的共同作用结果. 反应器尺度变化后,内部流动传质特性随之变化,且进出口因此实验系统进出口测定的降解速率并不恒定. 与之不同,在BDD阳极体系中电极板对于污染物的降解取决于电极板的材料特性和电流特性,和实验系统的尺寸无关. 电极板的降解速率可以应用到不同尺度的反应器中.
假定电极板对于低浓度SMD电催化降解符合一级反应动力学方程:
式中,S是源(汇)项,k为电极板表面反应速率常数,
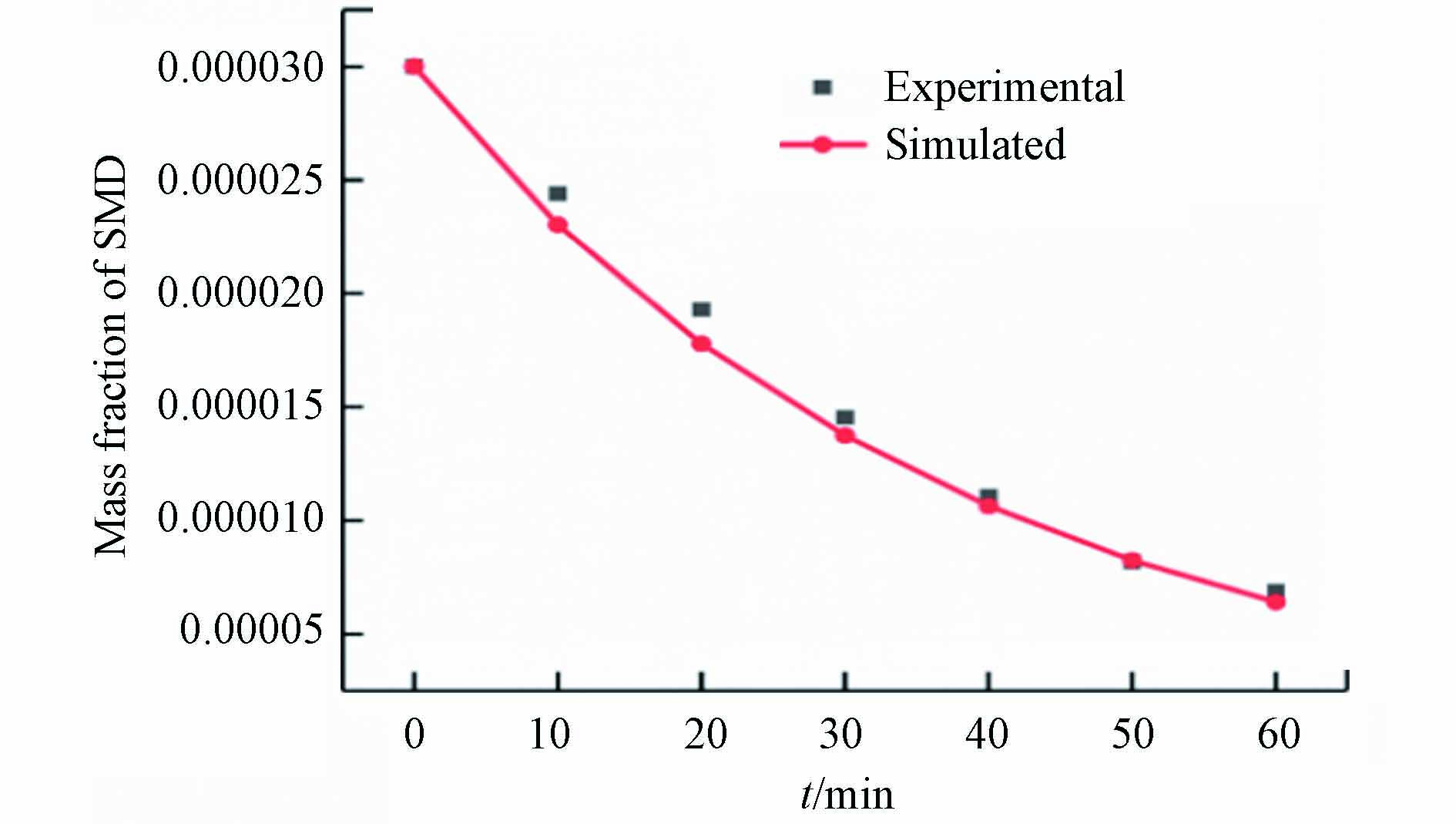
$ C $ 为$ t $ 时刻阳极表面污染物浓度.利用化学实验系统进出口污染物浓度差可以率定不同条件下的电极板表面反应速率. 数学模型显示,在20 mA·cm−2的电流密度下,电极板反应速率常数k的率定值为18. 图3给出了在上述电流密度的条件下,当流速为100 mL·min−1,电极间距为14 mm时,SMD质量分数随时间变化的实验值和模拟值. 结果表明,所得数据与实验结果吻合良好. 相关数据的对比如表1所示,模拟值与实验值的误差在合理的范围内,其中,反应速率的相对误差为0.4%,说明电极板的表面反应速率率定值具有较高的可靠性.
-
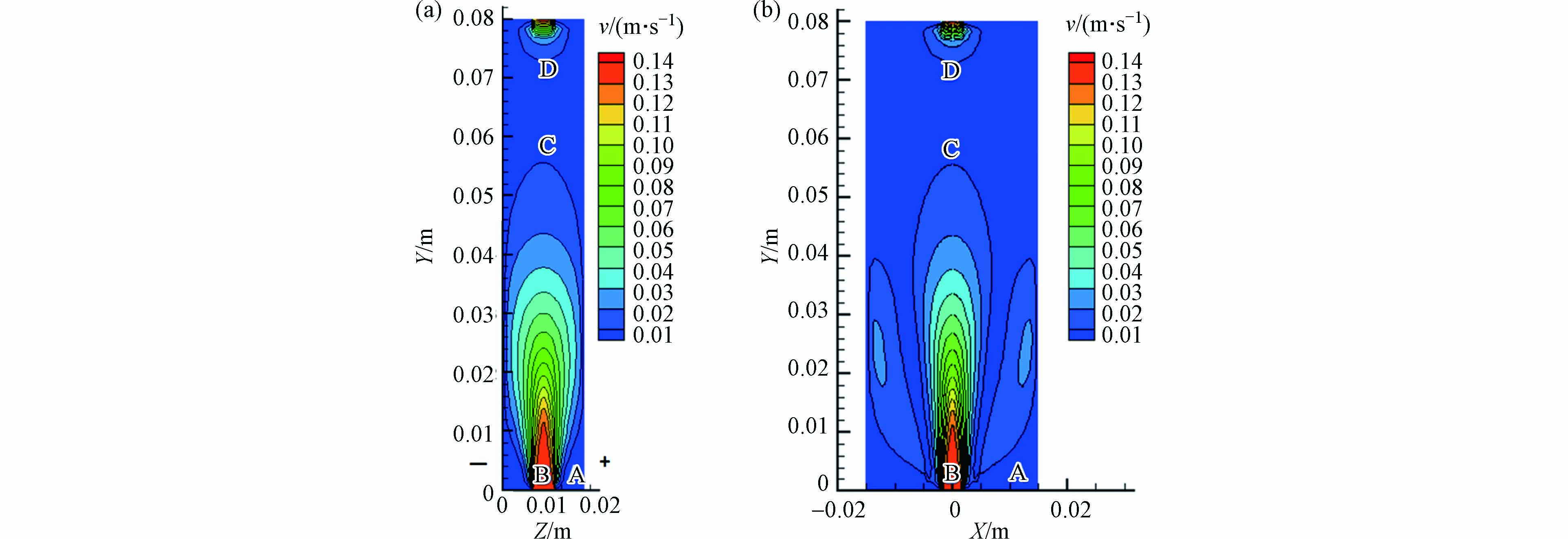
污染物的流动特征是影响反应器性能的重要因素. 流场处于稳定状态时,反应器中各处的速度分布如图4所示. 图4(a)显示了与电极板垂直中间面(X=半宽度)的流场分布,可大致分为4个区域A—D,各区域特点如下: A区域由于反应器入口和水段之间的差异导致入口处的速度较大,形成一定区域的流动死区,不利于污染物的降解;B区域为主流区,速度大小分布呈抛物线型,流体分布均匀对称;C区域的流体由于远离入口,速度均匀且较小;D区域是出口区域,受到能量守恒和收敛作用,流体速度增加.
图4(b)为与电极板平行中间面(Z=半厚度)的速度分布. 由于主流体具有强烈的夹带作用,从而驱动周围流体移动. 当液体靠近壁面时,受壁附近高剪应力的影响,会在壁附近出现逆流. 这部分流体通过主流夹带作用形成循环流. 根据射流理论,射流的半厚度遵循经典理论,其中x是距源点的距离,本研究中ε的计算值为0.126. 与Alberson等[22]的实验数据ε=0.154相比,相对偏差较小,表明结果可信.
-
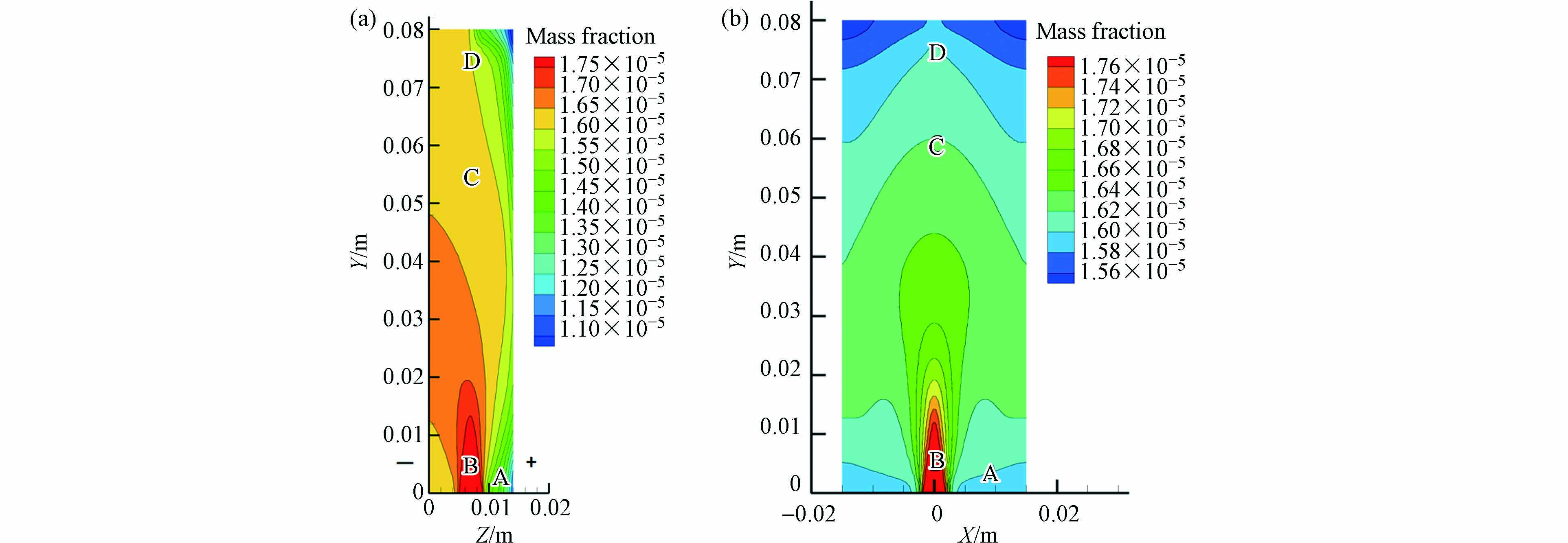
以20 mA·cm−2的电流密度为例,在20 min的循环降解时间内,反应器典型位置的污染物质量分数等值线如图5所示. Z的正方向指向阳极,即左面流体接触阴极,右面接触阳极. 由图5可知,从阴极到阳极,SMD质量分数逐渐减少. 如图5(a)所示,A区域靠近阳极且流速小,形成流动死区,与周围液体交换能力差,转移到阳极的污水较少,阳极反复降解部分污染物,虽然降解效果明显,但传质作用弱,导致B、C区域中的阳极部分未能充分发挥作用,D区由于接近出口处,流速增大使得传质效果加强,污染物降解效率得到提升.
图5(b)显示,在阳极的降解作用下,流体的质量分数由入口到出口不断减小. 在B区域,因为较大的流速加强了传质作用,使得SMD的质量分数变化范围小. 在C—D区域由于壁面附近速度较小,传质作用较弱,壁面附近区域污染物的的降解更为充分. 综上,传质在污染物降解反应中起着决定性的作用,反应器尺度变化导致的传质特性变化对污染物整体降解具有重要影响,其中,当反应器扩大后,进出水口处会加剧溶液扩散不均匀程度,这可能是放大后反应器效果变差的主要原因之一.
-
参考之前研究中的扩大尺寸:0.35 m2[23]、2800 cm2[24]、2904 cm2[13]、0.5 m2[10]和1.05 m2[14 − 15]等,本研究选择模拟2400 cm2(放大100倍)、5400 cm2(放大225倍)和9600 cm2(扩大400倍)的阳极面积,并参考实验室反应器面积(24 cm2)的降解效率以评估本研究中放大反应器的效果. 研究表明,增大电极间距不利于污染物降解且会升高电阻导致能耗增加[25],故扩大后的反应器保持与实验室反应器相同的电极间距1.4 cm,又由于电极板间距不变,故反应器进出口半径和尺寸均不变. 此外,为了维持单位面积阳极处理污染物的能力,在停留时间不变的情况下,通过增大流量来增加流速和模拟废水总体积以适应反应器放大规格,具体参数如表2所示.
对于3个放大系统,反应区域的划分和边界条件与实验室反应器大致相同. 反应器的网格精度和实验室反应器相同,网格数量按比例增加,保证了反应区域厚度和实验室反应器模拟的一致性(图6)。
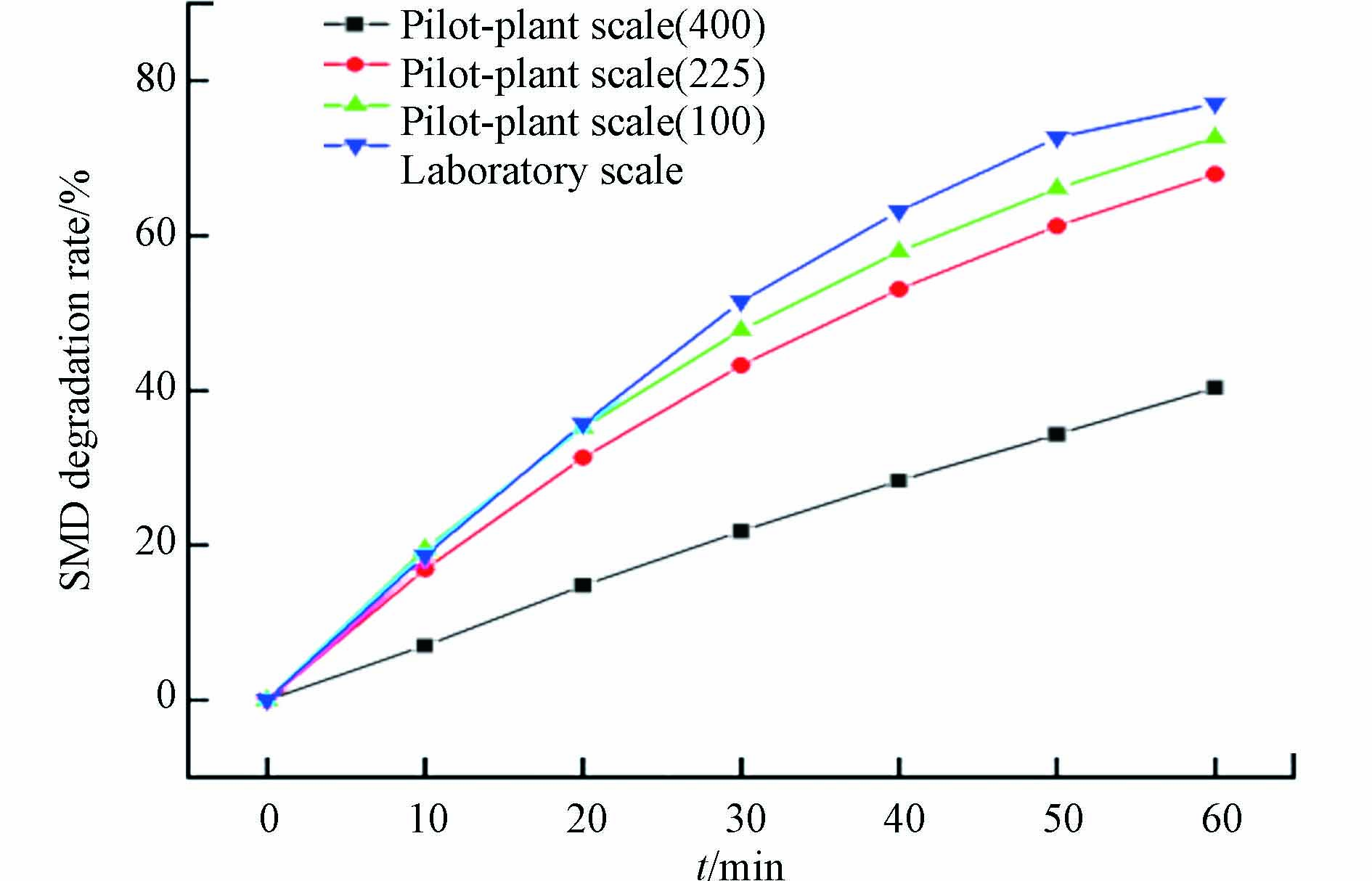
以20 mA·cm−2的电流密度为例,利用数学模型进行模拟计算. 上述各规模反应器中SMD降解率随时间变化如图7所示. 在60 min时,实验室反应器和3个扩大不同倍数反应器的降解率分别为77.1%、72.7%、68.0%和40.4%. 与实验室反应器相比,3个放大系统的降解能力分别下降了5.7%、11.8%和47.6%. 说明反应器放大对BDD系统降解有一定程度的影响.
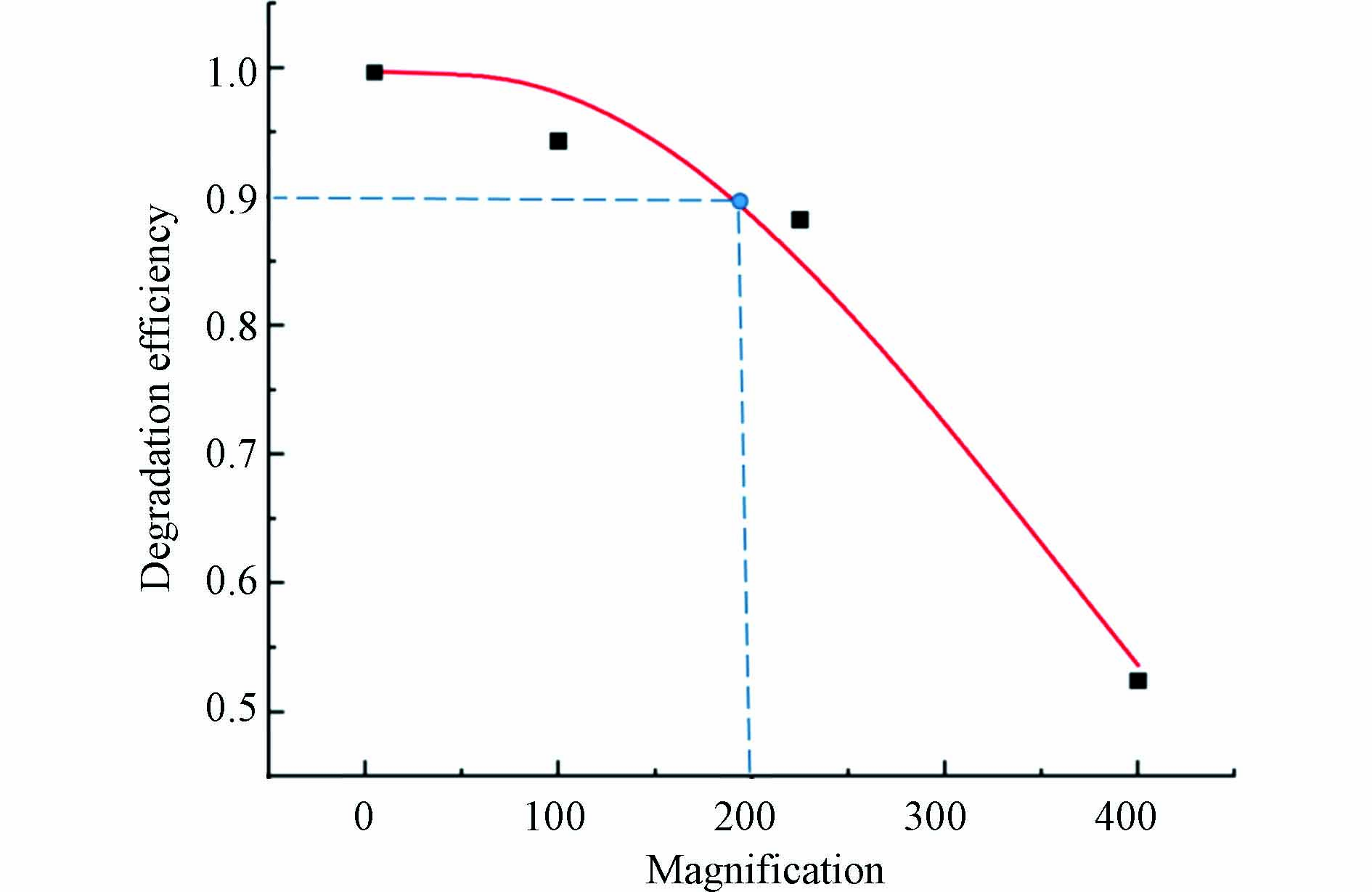
将上述模拟计算得出的SMD降解效率进行数据处理,以实验室降解效率为标准,将降解效率和对应的放大倍数作散点图并汇出拟合曲线如图8所示. 由图8可得,随着反应器放大倍数的增加,降解效率逐渐降低. 在反应器放大倍数为200倍时,降解效率为实验室降解效率的90%,如图中蓝色虚线所示. 综合考虑放大后的降解效率与实用性,建议放大倍数为200倍.
-
(1)利用CFD软件建立BDD阳极系统中SMD电化学氧化的数学模型. 通过对流场和质量分数场的研究表明,传质对反应器的降解有很大影响.
(2)基于化学实验结果参数和数学模型确定了污染物的降解速率. 在电流密度为20 mA·cm−2下的阳极板表面反应速率常数为18 min−1.
(3)在污染物降解系统中,反应器尺度效应明显,通过对反应器尺度效应的模拟发现在相同的电流密度、停留时间和废水特性下,3个放大的BDD阳极系统中的SMD降解率与实验室反应器相比分别下降了5.7%、11.8%和47.6%,并作图分析,建议尺度放大比例控制在200倍之内.
电化学氧化反应器尺度效应对于抗生素模拟废水降解的影响
Scale effect of electrochemical oxidation reactor on the degradation of antibiotic simulated wastewater
-
摘要: 反应器的尺寸对于污水处理效率具有显著的影响,是污水处理从实验室小型反应器到实际中大型反应器应用的关键. 本研究建立了尺寸为33.6 cm3 (3 cm×8 cm×1.4 cm)的电化学反应器,以掺硼金刚石膜(boron doped diamond,BDD)电极为阳极,对磺胺对甲氧嘧啶(sulfametoxydiazine,SMD)的氧化降解进行实验测试. 在实测数据的基础上,利用计算流体力学(Computational fluid dynamics,CFD)软件fluent建立电化学氧化反应器降解SMD的数学模型,率定不同电流密度下的电化学表面反应速率,分析反应器尺寸对污染物流动特征分布和浓度特征分布影响,利用CFD模拟,分别将BDD阳极面积放大100倍、225倍和400倍,探究3种工况下的尺度效应模拟. 结果表明,BDD体系降解反应器的尺度效应显著,在20 mA·cm−2的电流密度下持续60 min后,3种BDD阳极体系中SMD的降解率分别达到72.7%、68.0%和40.4%,相比实验室反应器降解能力分别下降5.7%、11.8% 和47.6%,建议反应器放大尺度小于200倍以免显著影响降解能力.Abstract: The size of reactor has a significant impact on the efficiency of wastewater treatment, which is the key to the application of wastewater treatment from laboratory small reactors to actual medium and large reactors. This study established a size of 33.6 cm3 (3 cm×8 cm×1.4 cm) electrochemical reactor, with BDD (Boron Doped Diamond) electrode as anode, to conduct experimental test on the oxidative degradation of sulfamethazine (SMD). On the basis of measured data, the mathematical model of electrochemical oxidation reactor degrading SMD was established by using Computational fluid dynamics (CFD) software fluent, the electrochemical surface reaction rate under different current densities was calibrated, and the influence of reactor size on the distribution of pollutant flow characteristics and concentration characteristics was analyzed. The BDD anode area was magnified by 100 times, 225 times and 400 times respectively by using CFD simulation, Explore the scale effect simulation under 3 working conditions. The results showed that the scale effect of the BDD system degradation reactor was significant. After 60 min at a current density of 20 mA·cm−2, the degradation rates of SMD in the three BDD anode systems were 72.7%, 68.0% and 40.4%, respectively, which decreased by 5.7%, 11.8% and 47.6% compared with the degradation capacity of the laboratory reactor. It was suggested that the reactor magnification should be less than 200 times to avoid significant impact on the degradation capacity.
-
Key words:
- mathematical model /
- scale effect /
- sulfamethoxine /
- electrochemical oxidation /
- BDD.
-

-
图 5 反应器不同表面SMD质量分数等值线:(a)
$ \mathit{X}= $ 半宽度,(b)$ \mathit{Z}= $ 半厚度Figure 5. Contours of SMD mass fraction in the various middle surfaces of the reactor:(a)
$ X=\mathrm{h}\mathrm{a}\mathrm{l}\mathrm{f}\mathrm{w}\mathrm{i}\mathrm{d}\mathrm{t}\mathrm{h} $ and (b)$ Z=\mathrm{h}\mathrm{a}\mathrm{l}\mathrm{f}\;\mathrm{t}\mathrm{h}\mathrm{i}\mathrm{c}\mathrm{k}\mathrm{n}\mathrm{e}\mathrm{s}\mathrm{s} $ 表 1 出口反应速率常数的实验值与模拟值的比较
Table 1. Comparison between experimental and simulated values of kinetic constants of first order reaction
电流密度/(mA·cm−2)
Current density数据来源
Data sources出口反应速率常数
Outlet reaction rate constant相对误差/%
Relative error20 实验值 0.0256 0.40 模拟值 0.0257 表 2 实验室反应器和中试反应器装置参数
Table 2. Parameter of the experimental set-ups at laboratory scale and pilot plant scale
运行参数
Operating parameters实验室反应器
Laboratory reactor扩大反应器
Expanded reactor扩大100倍
100 times扩大225倍
225 times扩大400倍
400 times流量/(L·min−1) 0.1 10 22.5 40 阳极面积/cm2 24 2400 5400 9600 电极间距/cm 1.4 1.4 1.4 1.4 处理的污水体积/L 0.5 50 112.5 200 -
[1] SAXENA S K, RANGASAMY R, KRISHNAN A A, et al. Simultaneous determination of multi-residue and multi-class antibiotics in aquaculture shrimps by UPLC-MS/MS[J]. Food Chemistry, 2018, 260: 336-343. doi: 10.1016/j.foodchem.2018.04.018 [2] MOUDGIL P, BEDI J S, AULAKH R S, et al. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk[J]. Food Analytical Methods, 2019, 12(2): 338-346. doi: 10.1007/s12161-018-1365-0 [3] HOFF R, PIZZOLATO T M, DIAZ-CRUZ M S, et al. Trends in sulfonamides and their by-products analysis in environmental samples using mass spectrometry techniques[J]. Trends in Environmental Analytical Chemistry, 2016, 9: 24-36. doi: 10.1016/j.teac.2016.02.002 [4] WANG J L, ZHOU A X, ZHANG Y L, et al. Research on the adsorption and migration of sulfa antibiotics in underground environment[J]. Environmental Earth Sciences, 2016, 75(18): 1-9. [5] QIN L T, PANG X R, ZENGH H, et al. Ecological and human health risk of sulfonamides in surface water and groundwater of Huixian Karst wetland in Guilin, China[J]. Science of the Total Environment, 2020, 708: 134552. doi: 10.1016/j.scitotenv.2019.134552 [6] FALEYE A C, ADEGOKE A A, RAMLUCKAN K, et al. Antibiotic Residue in the aquatic environment: Status in Africa[J]. Open Chemistry, 2018, 16: 890-903. doi: 10.1515/chem-2018-0099 [7] CARVALHO I T, SANTOS L. Antibiotics in the aquatic environments: A review of the European scenario[J]. Environment International, 2016, 94: 736-757. doi: 10.1016/j.envint.2016.06.025 [8] HE B S, YAN X H. Modifications of Au nanoparticle-functionalized graphene for sensitive detection of sulfanilamide[J]. Sensors (Basel, Switzerland), 2018, 18(3): 846. doi: 10.3390/s18030846 [9] AHMED M B, ZHOU J L, NGO H H, et al. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review[J]. Journal of Hazardous Materials, 2017, 323: 274-298. doi: 10.1016/j.jhazmat.2016.04.045 [10] TRÖSTER I, FRYDA M, HERRMANN D, et al. Electrochemical advanced oxidation process for water treatment using DiaChem® electrodes[J]. Diamond and Related Materials, 2002, 11(3/4/5/6): 640-645. [11] ZHU X P, NI J, LAI P. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using boron-doped diamond electrodes[J]. Water Research, 2009, 43(17): 4347-4355. doi: 10.1016/j.watres.2009.06.030 [12] ZHU X P, NI J R, WEI J J, et al. Scale-up of BDD anode system for electrochemical oxidation of phenol simulated wastewater in continuous mode[J]. Journal of Hazardous Materials, 2010, 184(1/2/3): 493-498. [13] ZHU X P, NI J R, WEI J J, et al. Scale-up of B-doped diamond anode system for electrochemical oxidation of phenol simulated wastewater in batch mode[J]. Electrochimica Acta, 2011, 56(25): 9439-9447. doi: 10.1016/j.electacta.2011.08.032 [14] URTIAGA, RUEDA A, ANGLADA A, et al. Integrated treatment of landfill leachates including electrooxidation at pilot plant scale[J]. Journal of Hazardous Materials, 2009, 166(2/3): 1530-1534. [15] ANGLADA Á, URTIAGA A M, ORTIZ I. Laboratory and pilot plant scale study on the electrochemical oxidation of landfill leachate[J]. Journal of Hazardous Materials, 2010, 181(1/2/3): 729-735. [16] TAGHIPOUR F, MOHSENI M. CFD simulation of UV photocatalytic reactors for air treatment[J]. AIChE Journal, 2005, 51(11): 3039-3047. doi: 10.1002/aic.10538 [17] KUMAR J, BANSAL A. Photocatalytic degradation in annular reactor: Modelization and optimization using computational fluid dynamics (CFD) and response surface methodology (RSM)[J]. Journal of Environmental Chemical Engineering, 2013, 1(3): 398-405. doi: 10.1016/j.jece.2013.06.002 [18] van WALSEM J, VERBRUGGEN S W, MODDE B, et al. CFD investigation of a multi-tube photocatalytic reactor in non-steady-state conditions[J]. Chemical Engineering Journal, 2016, 304: 808-816. doi: 10.1016/j.cej.2016.07.028 [19] BAGHERI M, MOHSENI M. Computational fluid dynamics (CFD) modeling of VUV/UV photoreactors for water treatment[J]. Chemical Engineering Journal, 2014, 256: 51-60. doi: 10.1016/j.cej.2014.06.068 [20] LI G C, ZHOU S Q, SHI Z, et al. Electrochemical degradation of ciprofloxacin on BDD anode using a differential column batch reactor: Mechanisms, kinetics and pathways[J]. Environmental Science and Pollution Research International, 2019, 26(17): 17740-17750. doi: 10.1007/s11356-019-04900-0 [21] XIE R Z, MENG X, SUN, P, et al. Electrochemical oxidation of ofloxacin using a TiO2-based SnO2-Sb/polytetrafluoroethylene resin-PbO2 electrode: Reaction kinetics and mass transfer impact[J]. Applied Catalysis B: Environmental, 2017, 203: 515-525. doi: 10.1016/j.apcatb.2016.10.057 [22] ALBERTSON M L, DAI Y B, JENSEN R A, et al. Diffusion of submerged jets[J]. Transactions of the American Society of Civil Engineers, 1950, 115(1): 639-664. doi: 10.1061/TACEAT.0006302 [23] MARTÍN de VIDALES M J, COTILLAS S, PEREZ-SERRANO J F, et al. Scale-up of electrolytic and photoelectrolytic processes for water reclaiming: A preliminary study[J]. Environmental Science and Pollution Research International, 2016, 23(19): 19713-19722. doi: 10.1007/s11356-016-7189-9 [24] URTIAGA A, GÓMEZ P, ARRUTI A, et al. Electrochemical removal of tetrahydrofuran from industrial wastewaters: Anode selection and process scale-up[J]. Journal of Chemical Technology & Biotechnology, 2014, 89(8): 1243-1250. [25] SINGH A, KAUSHIK A. Sustained energy production from wastewater in microbial fuel cell: Effect of inoculum sources, electrode spacing and working volume[J]. 3 Biotech, 2021, 11(7): 344. doi: 10.1007/s13205-021-02886-6 -



 下载:
下载: