-
2022年5月,国务院发布《新污染治理行动方案》,吹响了新污染物全面管控的号角. 氯代有机污染物(chlorinated organic pollutants,COPs)是持久性有机污染物中的一种,属于新污染物管控范畴. 因C—Cl键的存在,COPs分子结构稳定,很难自然降解;另外,氯代有机污染物脂溶性较强,生物易富集,在痕量水平具有“三致效应”,环境风险高. COPs污染的处理,目前有氧化法和还原法两种. 氧化法主要通过焚烧、光催化氧化[1]和芬顿(Fenton)氧化等技术实现,可将COPs转化为无毒无害的CO2,H2O和氯离子;还原法则主要利用零价铁(Fe0)[2]和H2[3]等具有还原性化学试剂,以Fe0作为电子供体,COPs通过Fe0表面的氧化还原反应得到电子实现脱氯. 近年来,电催化加氢脱氯(electrocatalytic hydrodechlorination,EHDC)技术是还原法的一种,只是它通过电源驱动电子的转移,无需添加化学药剂,因此逐渐受到研究者的关注,它的主要优势在于:(1)反应条件温和,多在常温常压下进行;(2)过程及产物可控,降低有毒副产物产生的风险;(3)设备简便、无需额外化学试剂;(4)低二次污染风险.
目前,EHDC领域主要集中在高效、稳定催化剂的研发和脱氯机理的解析. 催化剂是EHDC技术的核心,对电极/污染物界面电子转移速率、活性物种产生速率、污染物活化、污染物吸脱附等过程有决定性影响. 设计高活性和高稳定性的电催化材料处理水体和废水中的COPs仍是研究热点和长期目标. 过渡金属被发现拥有较高的EHDC活性,通过形貌尺寸、电子结构、晶格应变、界面效应等策略进一步调控,可提高催化剂活性、选择性和耐久性. 本文针对EHDC领域中金属基催化剂的类型、性能以及Pd基催化剂调控策略等领域的研究进展进行了综述,并总结电催化技术工业化应用现存的壁垒,提出了该技术可能的发展前景.
-
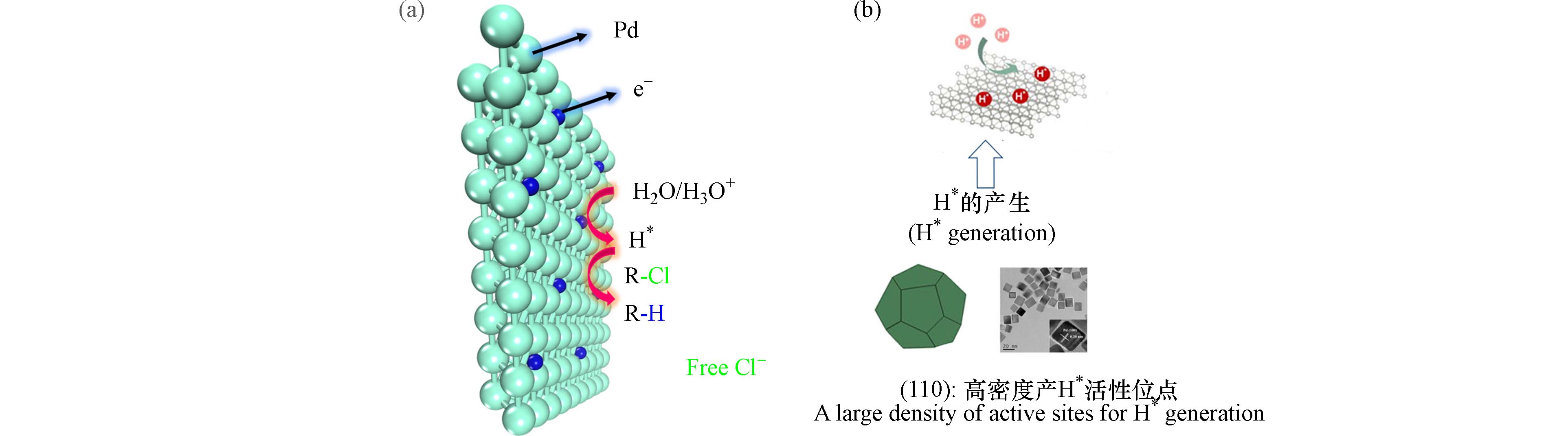
金属钯(Pd)在EHDC反应中展现出的优异性能,使其逐渐成为研究者们的首选催化剂[4]. 通过Pd基催化剂表面原位电解水形成的活性氢(H*)物种攻击裂解C—Cl键,使氯原子脱离,以降低COPs分子的毒性和稳定性. Pd基催化剂表面的EHDC反应过程按照方程(1—4)进行:
Pd基优异的催化脱氯性能主要来源于两个方面. 首先,Pd独特的电子轨道能较好地吸附COPs并活化C—Cl键[5 − 7]. 其次,H*介导的Pd基催化剂间接还原途径中(图1),H*的产量与产生速率是控制EHDC反应效率的关键[8]. Pd在H*产生上具有两个显著优势:(1)Pd表面产生H*过电位较低;(2)Pd-H*结合能适中,能抑制析氢反应(HER),减少H*以H2形式逸出的损失,增加H*的利用率. Jiang等的研究表明,Pd表面上产生H*的活性位点密度是关键结构特征,具有高密度活性位点的Pd(110)是促进H*生成的理想晶面[9]. Zhao等研究表明了缺电子和富电子的Pd物种分别主导C—Cl键的活化和H*的产生[7]. 此外,Lou等还提出在Pd高折射率晶面的台阶位点上可以产生更多H*,是脱氯反应的高活性位点[10].
-
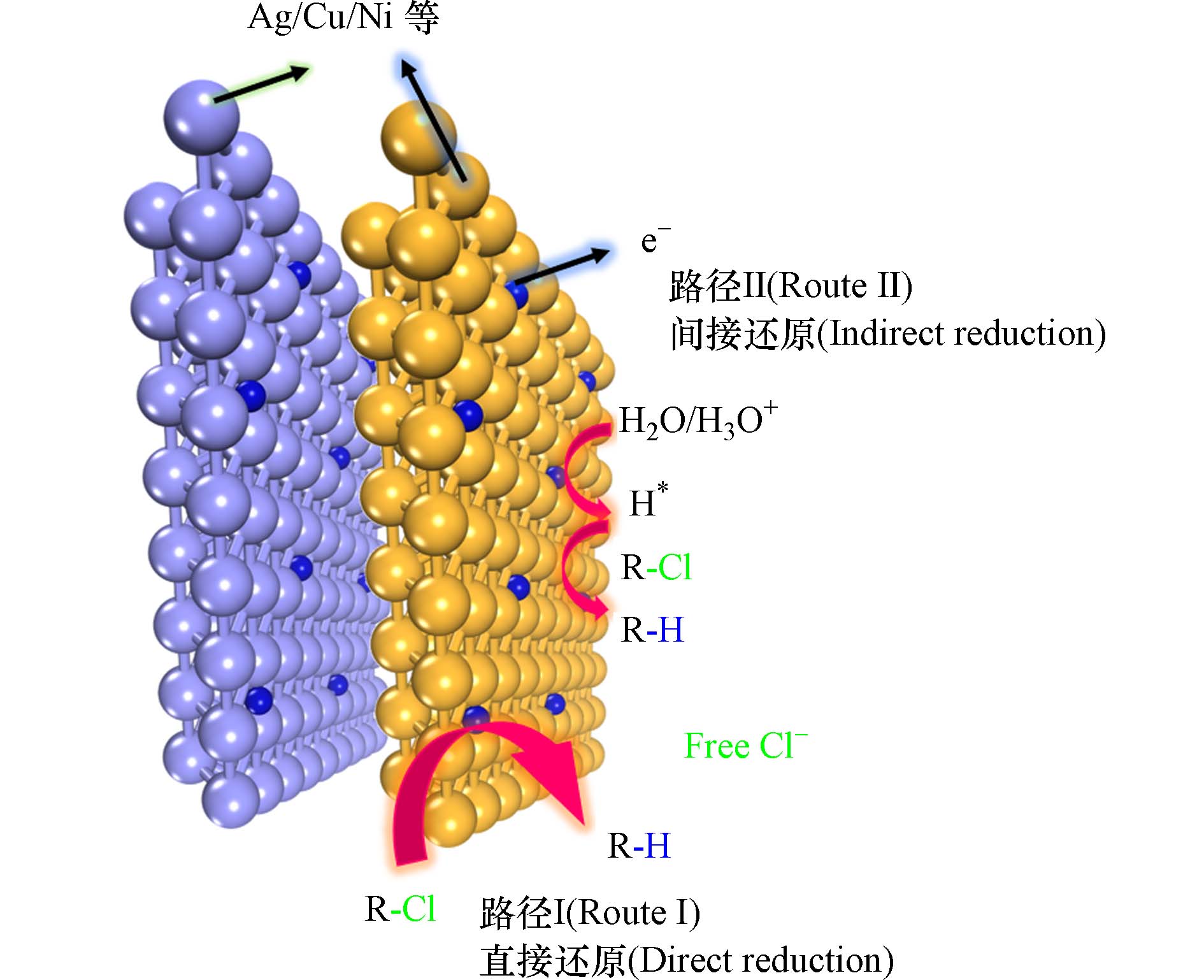
除Pd基催化剂外,银(Ag)、铜(Cu)等金属也展现出一定的EHDC活性. 与Pd不同,Ag、Cu等金属表面电子(e−)可以直接穿过亥姆荷茨溶液层,直接电催化还原裂解C—Cl. 同时,部分COPs也通过H*的间接还原实现脱氯(图2). Huang等提出Ag的催化特性主要源于较低的内在能垒而非COPs的还原活化自由能,从而导致需要克服的热力学驱动力降低,COPs在Ag表面的脱氯还原电位发生正移,脱氯反应更容易发生[11]. 此外,Ag对卤素有较好的亲和力,COPs在Ag表面的吸附发挥着关键作用. Ma等制备的Ag基催化剂在碱性条件下可实现3,4,5,6-四氯吡啶甲酸中4位或5位氯原子的脱氯并保留完整的羧基(—COOH)[12]. Lou等制备的高Ag负载量(13.7 mg·cm−3)、高比表面积(26 m2·g−1)和低密度的Ag基催化剂,在甲草胺的脱氯中展现出优异的脱氯性能,甲草胺的转化率高达96%—99%,电流效率达33%[13]. 纳米枝晶形态的Ag基催化剂也对COPs具有良好脱氯性能,Liu等报道了对氯乙酸展现出良好脱氯性能的Ag纳米枝晶,其形态主要由施加电位所控制,在优化后的制备条件下所制备的枝晶数量多、均匀型好、具有开孔结构[14]. 然而,因为Ag容易与脱氯产物即Cl−络合形成AgCl而失活,因此在实际应用中活性较低.
Cu基催化剂,价格低廉,无毒性,因此也被广泛应用于EHDC领域. 与商业Cu泡沫电极对比,Mao等使用化学气相沉积法制备的3D石墨烯Cu泡沫(GR-Cu)电极对三氯乙酸表现出了更优异的脱氯性能. 在Cu泡沫表面通过电子的直接还原,20 min内可消除97.33%的三氯乙烯酸(500 μg·L−1)[15]. Gan等的研究表明,具有丰富Cu-N4配位位点的单原子Cu基催化剂,在催化1,2-二氯乙烯脱氯产乙烯的反应中有99%的选择性,其法拉第电流效率高达64%,远优于Ni、Ag和Ru等金属的催化效果[16]. Liu等则使用Cu-Ni双金属阴极催化剂来应对低渗透区三氯乙烯(TCE)修复的挑战,在1—4 V·cm−1电压区间内,Cu-Ni双金属电极都达到了98%的脱氯活性,这主要得益于Ni对于Cu电子结构的调控[17].
金属镍(Ni)具有较强的产氢能力,金属钴(Co)具有适当的H原子结合能,有利于H*的产生和释放[18]. 催化剂中Ni和Co的掺杂可以有效提升催化剂的脱氯性能. Yin等报道了一种Cu-Ni双金属催化剂,Ni表面产生H*并溢流到临近Cu粒子表面参与脱氯[19]. Liu等报道了一种单原子钴和Cu纳米团簇复合催化剂,Cu纳米团簇同时充当导电桥和吸附位点,从而加速了与2,4-二氯苯酚脱氯反应相关的直接电子转移[20]. Ni和Co也被单独作为催化剂进行研究,Xu等比较了固定在氮化石墨烯上的原子级Ni、Fe和Co催化剂,其中单原子Ni催化剂对氯乙酸展现出最优的催化性能[21]. Li等将水热组装制备的单原子Co催化剂(Co-SG)用作双效催化剂电催化还原-氧化2,4-二氯苯甲酸,阴极表面催化H*产生,阳极催化H2O2电化学合成中均表现出优异性能[22]. Wang等设计合成的棒状Cu-Co纳米尖晶石,富含氧空位和吸附活性位点,表现出较高的电催化脱氯活性和法拉第电流效率(21.61%)[23]. Wang等制备的自支撑3D分层异质结Ni-WC电极通过增强界面电荷转移,实现了高效的脱氯反应,3 h内4-氯酚的EHDC效率为100%,表现优于同等条件的Pd/C[24].
-
截止目前,Pd基催化剂脱氯性能调控的方式主要包括尺寸形貌调控、电子结构调控及表面有机配体修饰几种方式(图3)。
-
Pd纳米颗粒的合成方式简单、可控,是EHDC反应的主流催化剂形貌. 通常有化学还原和电还原两种合成方式. 化学还原一般采用硼氢化钠或H2等还原剂来还原[PdCl4]2-物种. EHDC效率受Pd纳米颗粒尺寸影响明显,Shu等合成的均匀分散在多壁碳纳米管(MWCNTs)上的Pd颗粒(粒径6.4—13.1 nm)对于4-氯酚的催化活性遵循Pd(100%,6.4 nm)>Pd(60%,9.5 nm)>Pd(29%,13.1 nm). 小颗粒的大比表面积提高了电极面积和溶液体积之比(A/V比)为加氢脱氯提供更多的反应位点[25]. Yang等通过Ni与超细Pd(粒径为(8.91±0.23)nm)形成足够的微界面,也可以提高催化脱氯性能,120 min内可完成99.5% 氟苯尼考(20 mg·L−1)的脱氯[26]. Zhao等使用聚多巴胺(PDA)作为有机物包覆层调控Pd纳米点的粒径,证实了PDA层可使得纳米Pd均匀分布在TiO2纳米表面,Pd的粒径约为1.5—2 nm. 在1.5 mA·cm−2和1 mmol 4-CP的条件下,TiO2@PDA/Pd电极表现出较高的活性,达到了23.96 min−1·g−1的质量活性. 除纳米颗粒外,Liu等还制备了一种富含缺陷的Pd纳米线. 相比于Pd/C,Pd纳米线比表面积更大,且富含缺陷位点,增加了对反应物的吸附和催化活性;同时吸附、产生、保留和释放H*的能力更强,这是Pd纳米线EHDC性能提高的重要来源[8].
-
将孤立的Pd原子稳定在合适的载体上是减少Pd用量,提高原子利用率的可行策略[27 − 28],代表了催化材料领域的前沿进展. 纳米颗粒的团簇形式会导致大部分活性位点的掩埋,而金属位点的原子分散使每个原子都参与目标反应,产生100%的理论原子利用率. 此外,不饱和的金属配位环境和均匀的几何及电子结构使得催化剂表面具有更多的吸附位点,可以更有效地选择特定的吸附模式和反应途径,从而提高催化活性[29]. Chu等将Pd分散在SiC表面,发现邻近的单Pd原子催化剂在保留传统单Pd原子催化剂高选择性的基础上,邻近原子之间的协同作用极大地增强了碳-卤素键的氢化活性[30]. 相邻的Pd单原子协同作用降低了4-氯酚亚稳态反应的初始脱附和最终加氢产物苯酚的脱附这两个关键亚稳态反应步骤的能量,同时不改变有机卤素中的其它键的结构. Huang等将Pdnano缩小为Pd1锚定在rGO上,可显著提升Pd的原子效率高达14倍,并且能够抑制氯离子引起的催化剂中毒现象,通过Pd-O配位和独特的单原子结构增强界面电子转移并抑制Pd1上的H2生成来提高催化效率[31]. Mao等利用氮掺杂碳锚定Pd制备的原子级分散的Pd基电催化剂(A-Pd-NC),对4-氯酚转化为苯酚的脱氯活性高达98.9 mmol·g−1·h−1,选择性约100%[32].
-
Pd电子结构可通过界面或表面应力效应等进行调控,具体可通过引入载体、构建异质结[33 − 34]、金属或非金属掺杂等手段达成. 载体对于设计和制备性能优异的负载型Pd基催化剂尤为重要,除了作为“骨架”,在一定程度上还能起到共催化或助催化的作用. 负载型Pd基催化剂具有较高的催化活性和选择性,同时拥有耐高温、抗氧化和耐腐蚀等优良性能,常见的选择有碳材料(C)、金属、金属氧化物(Al2O3、TiO2、MnO2)、金属氮化物(TiN)、金属碳化物(TiC)[35]等. 碳材料稳定的物理化学性质和发达的孔隙结构,可支撑和分散Pd颗粒,如炭黑(CB)、多壁碳纳米管(MWCNTs)和颗粒活性炭(GAC)等[36 − 37]. 此外,氮(N)掺杂C还用于制备原子级分散Pd基催化剂,比商业Pd/C的催化脱氯活性整整高出41.2倍[32]. 泡沫Ni具有三维立体均匀网状结构,孔隙度高达96%—98%,并保持了良好的物理、化学性能. Mao等制备的超细Pd@Ni-foam脱氯反应速率常数分别是纯Ni-foam和商业Pd/C的44倍和4.4倍,其优异性能主要源于镍泡沫框架和Pd-Ni原子微界面导致的Pd-Ni原子间强协同效应以及高产量的H*[26]. 但是,泡沫Ni上的Pd易脱落,不利于反应长期稳定地进行且存在水质二次污染风险. 金属氧化物作为中间层可有效避免活性物质的脱落并加速电子转移,比如直接生长在Ni泡沫上的MnO2可分别与Pd端和Ni端形成的强黏附和电子相互作用. MnO2一方面作为载体利用还原形成的空穴捕获Pd2+,另一方面作为电子供体利用过剩电子将Pd2+还原为Pd0[38 − 39]. Li等提出以NiCo-MOF作为中间层可辅助更小Pd颗粒的形成和H*的吸附,显著提高催化活性,在40 min内对氯霉素的去除率达到了95%以上[40]. 肖特基异质结诱导的金属载体相互作用,可优化Pd电子结构并且平衡Pd上物质的解/吸附行为,半导体金属Pd-TiO2(电子传递方向:TiO2→Pd)肖特基异质结催化剂的质量活性、EHDC效率和能量选择性明显优于传统的Pd/C催化剂[41]. Lou等利用Pd和Co-MNSs导电夹层的协同作用及界面耦合产生的异质结构形成电子富集的Pd表面,在Ni泡沫上针对氯霉素去除的表观速率常数是Pd/Ni的3.5倍,商用Pd/C涂层Ni泡沫电极的6.8倍[42]. Li等发现Pd与Ni2P协同可明显提升4-氯酚的EHDC效率,NiP代替Pd0产生H*并促进Pd2+的产生[43].
通过将Pd与Cu[44]、Ag[45]、In[46,47]和Pt[48]等金属合金化调节表面晶格应力来提升其催化活性是一种较为成熟的策略,其中Pd与其他金属成功合金化及恰当的金属比例是关键. Peng等发现Ag-Pd合金的组成为Ag32Pd68时具有最高的EHDC活性,恰当的Ag比例削弱催化剂对于产物苯酚的吸附能力[45],苯酚的吸附也被认为是抑制EHDC反应速率的决定步骤之一[49]. Chen等则提出Pd-Au合金中Pd7Au3合金纳米粒子具有最高的EHDC效率,富电子的Pd作为高效电化学活性点位,质量活性为纯Pd的7.83倍[50]. 非金属掺杂剂(硼、磷)可以改变Pd的电子结构从而提高其电催化性能. 准金属硼(B)原子比Pd原子小得多,可掺杂在Pd的晶格空间中,而非取代Pd原子[51]. 磷(P)的掺杂也促使Pd-P催化性能比纯金属Pd显著提高[52]. B、P共掺杂协同调控Pd纳米棒的电子结构,其电催化活性远优于商业Pd/C、Pd/B、Pd/P和纯Pd纳米棒.
-
催化剂表面有机配体功能化是近年来一种较为新兴的调控策略,通过配体中的N[53]、O、S和P等元素键合向金属表面泵送电子,从而形成富电子催化表面[54]. 值得关注的是,有机配体功能化不仅限于催化剂本身的调控,还能通过调控原子H*、污染物和产物等的吸脱附能力间接促进EHDC反应. 十六烷基溴化铵(CTAB)修饰的Pd纳米颗粒,明显提高催化剂附近2,4-二氯苯甲酸的浓度并加快其降解速率[55]. 此外,四乙基氯化铵在还原电位下转化为分子胺,Pd表面配体环境中强的N-Pd配合作用可以明显提升Pd纳米颗粒的EHDC性能. 机理研究表明,胺配体会引入3种效应:(1)H+泵效应——增加局部H+浓度;(2)电子效应;(3)空间效应——缓解苯酚的强吸附,释放吸附位点[56]. Fan等利用亲水性聚合物聚乙二醇(PEG),增强材料表面的亲水性,从而改善Pd与废水之间微环境的质量传递,并提高Pd/C在2,4-二氯苯酚的电化学脱卤反应中的活性和法拉第效率约4—5倍[57].
除小分子有机配体外,还有一类大分子有机配体——导电聚合物(conducting polymer,CP)值得关注[58]. CP是典型的π-共轭聚合物,可以传输电子,常见的导电聚合物有聚乙烯亚胺(PEI)、聚丙烯酸(PAA)、聚苯胺(PAN)等. 将CP应用于电催化剂的功能化修饰,极大改变了催化剂的形貌、电子结构、表面环境和形成机制,还有助于多相催化中独特的空间效应,是一种高效的界面工程策略[59 − 61]. 催化活性和有机物比例呈典型的火山型趋势,过高的有机物比例会增强析氢副反应(HER)反应的发生机率,对于中间产物或毒副产物的吸附也将增强. 目前,在EHDC领域,此类电催化剂较为稀少,但该类调控策略在提升催化剂的催化活性、稳定性、抗毒性等方面都十分具有应用前景.
-
催化剂及相应电极的制备是电化学技术在环境领域应用的关键步骤. 目前EHDC的主流催化剂大都为粉末,需通过聚合物黏结剂将其涂覆于导电基板上. 常用的聚合物黏结剂包括全氟磺酸(Nafion)、聚四氟乙烯(PTFE)、酚醛树脂(PF)和聚偏二氟乙烯(PVDF)等. 黏结剂不导电,它的使用不可避免地会掩盖部分活性位点,阻断电子传输通道,降低催化活性. 因此,工业上更需要的是一体化的自支撑电极[62 − 63],可克服粉末催化剂在电极制备过程中的问题. 此外,实验室催化剂制备工艺往往不足以支撑工业上高达几千小时的连续运转,且不能承载大于500 mA·cm−2的电流密度[64],极大限制电催化技术成果向工业化应用的转化. 因此,设计合成可大规模使用的高稳定、高活性、低成本的工业型催化剂是势在必行的研究趋势.
-
COPs的直接氧化降解工艺反应能垒高,产物难以控制,有产生短链脂肪氯代化合物的风险. 与其它脱氯工艺相比,EHDC过程沿可控途径开展,如2,4-二氯苯酚(2,4-DCP)在Pd表面的EHDC路径为:2,4-DCP→氯酚(4-CP/2-CP)→苯酚(P),最终得到完整的脱氯产物苯酚,从而降低分子的毒性和稳定性. 含酚废水的直接排放也不被允许,联用阳极氧化可实现苯酚的进一步降解[65],通过投加Cl−可增强阳极的反应活性,但不排除Cl2及新COPs产生的风险,因此降毒性和矿化两段联用工艺的设计十分迫切,如已见报道的光-电、磁-电和生物-电等技术的联用. 此外,为响应国家“双碳”号召,资源化回收利用是首选,已有报道可进一步电催化苯酚还原,转化为高经济价值的环己醇[62 − 63,66 − 67],此类研究具有十分可观的利益,但目前研究尚浅,还待进一步探索.
-
地表水、自来水等天然水体中电解质含量较少,难以满足电催化反应的需求. 为提高水体导电性,实验室一般添加额外电解质,常用的有硫酸盐溶液、磷酸盐缓冲溶液、KOH溶液等,成本投入较高还存在高盐、高磷、高碱度废水排放的问题. 此外,医药、燃料、化工等工业废水往往具有高盐、高COD、排放量大等问题,Hu等已证明高盐度废水中的EHDC过程明显受到结垢和Cl−毒化的双重掣肘,严重影响其在污染治理中的应用[68]. Mao等也发现自来水中天然的Ca2+、Mg2+等离子也存在结垢问题,削弱催化性能并缩减电极寿命[69]. pH也是限制电催化工业应用的重要因素,Jiang等表明弱酸性是EHDC过程的理想条件[70]. 在中性自然水体(pH—7左右)和偏酸或偏碱的水体中均不能发挥出稳定的催化活性[71],而在强酸强碱中催化剂几乎不能发挥作用.
-
电催化技术处理水和废水中的COPs是最有前景的策略之一,通过其高选择性和催化性能实现高效率的污染物脱毒. 目前,对于EHDC的直接和间接还原机理研究已经比较明朗,而对于催化剂的研究更是百花齐放. Pd基催化剂在电催化加氢脱氯方向一骑绝尘,然而减少其使用量或寻找可替代金属仍然是一个热点的问题. 催化剂性能的提升除了尺寸电子结构等调控外,表面有机配体功能化也是一种十分可取的调控策略,不仅可以调控催化剂本身的形貌、电子结构、界面结构,还可以调控反应物及产物的选择性和吸脱附,这种双边调控的能力,达到了1+1>2的效果. 但是,电催化技术在工业应用方面还存在着巨大的阻碍,第一步就是要克服实验室和自然水体或工业废水的水质差异性. 除了调控催化剂的耐受性外,投加具有辅助效果的药剂也是一种选择. 其次,克服粉末催化剂的缺点也是一个亟待解决的问题,优异的催化剂是催化反应稳定、高效进行的先决条件. 同时,为响应国家“双碳”目标,资源化回收是首选. 因此,电催化领域未来的研究方向应以提高在实际工业应用中的适用性为目标,突破反应条件、处理量等的限制.
电催化氢解脱氯反应研究进展及应用可行性分析
Review on the progress and evaluation on the application feasibility of electrocatalytic hydrodechlorination technology in detoxificiation of chlorinated organic pollutants
-
摘要: 氯代有机物是一类重要的化工原料和中间体,广泛应用于医药、农药、染料等领域,而大量的使用致使其环境暴露量增加,造成污染. 因碳-氯键(C—Cl)的存在,氯代有机污染物(chlorinated organic pollutants,COPs)分子结构稳定,难自然降解,易生物累积且具三致效应,环境风险极高. 电催化氢解脱氯(electrocatalytic hydrodechlorination,EHDC)是目前处理水体COPs的热点技术,其通过在催化剂表面原位电解水形成活性氢(H*)攻击C—Cl键,使氯原子脱落转化为Cl−,C—Cl键转化为C—H键,从而大大减小COPs分子毒性和稳定性,增加废水可生化性. 相比以Fe0或H2驱动的氢解脱氯技术,EHDC技术主要优势在于:(1)反应条件温和,过程可控;(2)无需额外添加化学试剂;(3)反应选择性高,毒副产物少. 金属钯(Pd)具有独特的电子轨道,吸附及活化C—Cl键能力强,同时在产H*方面具有显著优势,因此被广泛用于EHDC. 本文重点综述了EHDC领域研究者在催化剂筛选、电子转移路径、表界面反应机制及Pd基催化剂性能调控策略等方面的研究进展,总结了电催化技术推广应用现存的壁垒,提出了该技术可能的发展前景.Abstract: The massive use of the chlorinated organic compounds in the pharmaceutical, pesticide and dye industries has led to their overexposure in environment. Due to the bearing carbon-chlorine (C-Cl) bonds, chlorinated organic pollutants (COPs) are generally chemically stable and highly resistant to natural degradation. They are also highly toxic, carcinogenic and bio-accumulative, thus delivering significant environmental risks. Electrocatalytic hydrodechlorination (EHDC) represents a promising technology for COPs treatment. It proceeds by the in-situ generation of atomic hydrogen (H*) on the catalyst surface via water dissociation. These H* are highly active for the hydrodechlorination of C—Cl bonds, which enable the conversion of the C—Cl bond and Cl atom to the C—H bond and Cl−, respectively. Overall, the EHDC process can significantly reduce the molecular toxicity and chemical stability of COPs, and improve the biodegradability of the wastewater. Compared to the hydrodechlorination technology driven by Fe0 or H2, EHDC technology is superior in the aspects of (1) mild reaction conditions and controllable process, (2) low chemical input and (3) high reaction selectivity and few yield of toxic by-products. Palladium metal (Pd) has been preferably developed as EHDC catalyst, owing to its robust performance in H* generation, adsorption and activation of C—Cl bonds. This work reviews the research progress achieved in the EHDC field, including the rules to design active catalysts, the insight into the electron transfer path and the interfacial reaction mechanism as well as the strategies to tune the performance of Pd-based catalysts. We also summarize the remaining challenges to the scale application of EHDC technology in practical environmental pollution abatement, and put forward the possible development prospects for this technology.
-
Key words:
- COPs /
- Pd-based catalysts /
- catalyst modulation /
- application feasibility analysis.
-

-
-
[1] XIONG L Q, TANG J W. Strategies and challenges on selectivity of photocatalytic oxidation of organic substances[J]. Advanced Energy Materials, 2021, 11(8): 2003216. doi: 10.1002/aenm.202003216 [2] MENG F X, XU J, DAI H W, et al. Even incorporation of nitrogen into Fe0 nanoparticles as crystalline Fe4N for efficient and selective trichloroethylene degradation[J]. Environmental Science & Technology, 2022, 56(7): 4489-4497. [3] WEIDLICH T, KAMENICKÁ B, MELÁNOVÁ K, et al. Hydrodechlorination of different chloroaromatic compounds at room temperature and ambient pressure—Differences in reactivity of Cu- and Ni-based Al alloys in an alkaline aqueous solution[J]. Catalysts, 2020, 10(9): 994. doi: 10.3390/catal10090994 [4] CHAPLIN B P, REINHARD M, SCHNEIDER W F, et al. Critical review of Pd-based catalytic treatment of priority contaminants in water[J]. Environmental Science & Technology, 2012, 46(7): 3655-3670. [5] XU J, LIU X, LOWRY G V, et al. Dechlorination mechanism of 2, 4-dichlorophenol by magnetic MWCNTs supported Pd/Fe nanohybrids: Rapid adsorption, gradual dechlorination, and desorption of phenol[J]. ACS Applied Materials & Interfaces, 2016, 8(11): 7333-7342. [6] XU J, CAO Z, LIU X, et al. Preparation of functionalized Pd/Fe-Fe3O4@MWCNTs nanomaterials for aqueous 2, 4-dichlorophenol removal: Interactions, influence factors, and kinetics[J]. Journal of Hazardous Materials, 2016, 317: 656-666. doi: 10.1016/j.jhazmat.2016.04.063 [7] ZHAO Z F, YU L, ZHENG L X, et al. TiO2@PDA inorganic-organic core-shell skeleton supported Pd nanodots for enhanced electrocatalytic hydrodechlorination[J]. Journal of Hazardous Materials, 2022, 435: 128998. doi: 10.1016/j.jhazmat.2022.128998 [8] LIU R, ZHAO H C, ZHAO X Y, et al. Defect sites in ultrathin Pd nanowires facilitate the highly efficient electrochemical hydrodechlorination of pollutants by H*ads[J]. Environmental Science & Technology, 2018, 52(17): 9992-10002. [9] JIANG G M, LI X J, SHEN Y, et al. Mechanistic insight into the electrocatalytic hydrodechlorination reaction on palladium by a facet effect study[J]. Journal of Catalysis, 2020, 391: 414-423. doi: 10.1016/j.jcat.2020.09.008 [10] LOU Y Y, XIAO C, FANG J Y, et al. High activity of step sites on Pd nanocatalysts in electrocatalytic dechlorination[J]. Physical Chemistry Chemical Physics:PCCP, 2022, 24(6): 3896-3904. doi: 10.1039/D1CP04975E [11] HUANG B B, ZHU Y Y, LI J, et al. Uncovering the intrinsic relationship of electrocatalysis and molecular electrochemistry for dissociative electron transfer to polychloroethanes at silver cathode[J]. Electrochimica Acta, 2017, 231: 590-600. doi: 10.1016/j.electacta.2017.02.055 [12] MA C A, LI M C, LIU Y N, et al. in situ FTIR studies on the electrochemical hydrodechlorination of 3, 4, 5, 6-tetrachloropicolinic acid on Ag cathode[J]. Electrochimica Acta, 2010, 55(9): 3171-3174. doi: 10.1016/j.electacta.2009.12.086 [13] LOU Y Y, HE W Y, VERLATO E, et al. Ni-coated graphite felt modified with Ag nanoparticles: A new electrode material for electro-reductive dechlorination[J]. Journal of Electroanalytical Chemistry, 2019, 849: 113357. doi: 10.1016/j.jelechem.2019.113357 [14] LIU B Z, DING C, XIAO B, et al. Electrocatalytic dechlorination of chloroacetic acids on silver nanodendrites electrode[J]. Materials Science and Engineering:C, 2014, 37: 108-112. doi: 10.1016/j.msec.2014.01.015 [15] MAO R, LI N, LAN H C, et al. Dechlorination of trichloroacetic acid using a noble metal-free graphene-Cu foam electrode via direct cathodic reduction and atomic H[J]. Environmental Science & Technology, 2016, 50(7): 3829-3837. [16] GAN G Q, ZHANG X Q, BU S Y, et al. Metal-nitrogen-carbon single-atom aerogels as self-supporting electrodes for dechlorination of 1, 2-dichloroethane[J]. Advanced Functional Materials, 2022, 32(48): 2206263. doi: 10.1002/adfm.202206263 [17] LIU B, LI G H, MUMFORD K G, et al. Low permeability zone remediation of trichloroethene via coupling electrokinetic migration with in situ electrochemical hydrodechlorination[J]. Chemosphere, 2020, 250: 126209. doi: 10.1016/j.chemosphere.2020.126209 [18] BARANTON S, COUTANCEAU C. Nickel cobalt hydroxide nanoflakes as catalysts for the hydrogen evolution reaction[J]. Applied Catalysis B:Environmental, 2013, 136/137: 1-8. doi: 10.1016/j.apcatb.2013.01.051 [19] YIN L F, DAI Y R, NIU J F, et al. Rapid dechlorination of chlorophenols in aqueous solution by[Ni| Cu]microcell[J]. Journal of Hazardous Materials, 2012, 209/210: 414-420. doi: 10.1016/j.jhazmat.2012.01.044 [20] LIU L, CHEN Y R, LI S L, et al. Enhanced electrocatalytic cathodic degradation of 2, 4-dichlorophenoxyacetic acid based on a synergistic effect obtained from Co single atoms and Cu nanoclusters[J]. Applied Catalysis B:Environmental, 2023, 332: 122748. doi: 10.1016/j.apcatb.2023.122748 [21] XU Y H, YAO Z Q, MAO Z C, et al. Single-Ni-atom catalyzes aqueous phase electrochemical reductive dechlorination reaction[J]. Applied Catalysis B:Environmental, 2020, 277: 119057. doi: 10.1016/j.apcatb.2020.119057 [22] LI N, SONG X Z, WANG L, et al. Single-atom cobalt catalysts for electrocatalytic hydrodechlorination and oxygen reduction reaction for the degradation of chlorinated organic compounds[J]. ACS Applied Materials & Interfaces, 2020, 12(21): 24019-24029. [23] WANG J, FAN S Y, LI X Y, et al. Rod-like nanostructured Cu-co spinel with rich oxygen vacancies for efficient electrocatalytic dechlorination[J]. ACS Applied Materials & Interfaces, 2023, 15(10): 12915-12923. [24] WANG Q, DU J, MA Y Y, et al. Noble-metal-free 3D hierarchical Ni-WC heterostructure with enhanced interfacial charge transfer for efficient electrocatalytic hydrodechlorination[J]. Chemical Engineering Journal, 2023, 451: 139107. doi: 10.1016/j.cej.2022.139107 [25] SHU X Y, YANG Q, YAO F B, et al. Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes[J]. Chemical Engineering Journal, 2019, 358: 903-911. doi: 10.1016/j.cej.2018.10.095 [26] YANG L M, CHEN Z L, CUI D, et al. Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol[J]. Chemical Engineering Journal, 2019, 359: 894-901. doi: 10.1016/j.cej.2018.11.099 [27] ZHANG Z Q, CHEN Y G, ZHOU L Q, et al. The simplest construction of single-site catalysts by the synergism of micropore trapping and nitrogen anchoring[J]. Nature Communications, 2019, 10: 1657. doi: 10.1038/s41467-019-09596-x [28] YANG K X, KONG Y J, HUANG L Z, et al. Catalytic elimination of chlorinated organic pollutants by emerging single-atom catalysts[J]. Chemical Engineering Journal, 2022, 450: 138467. doi: 10.1016/j.cej.2022.138467 [29] LI J C, LI M, LI J, et al. Hydrodechlorination and deep hydrogenation on single-palladium-atom-based heterogeneous catalysts[J]. Applied Catalysis B:Environmental, 2021, 282: 119518. doi: 10.1016/j.apcatb.2020.119518 [30] CHU C H, HUANG D H, GUPTA S, et al. Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis[J]. Nature Communications, 2021, 12: 5179. doi: 10.1038/s41467-021-25526-2 [31] HUANG D H, KIM D J, RIGBY K, et al. Elucidating the role of single-atom Pd for electrocatalytic hydrodechlorination[J]. Environmental Science & Technology, 2021, 55(19): 13306-13316. [32] MAO Z C, LIU L H, YANG H B, et al. Atomically dispersed Pd electrocatalyst for efficient aqueous phase dechlorination reaction[J]. Electrochimica Acta, 2021, 391: 138886. doi: 10.1016/j.electacta.2021.138886 [33] CHEN M, SHU S, LI J X, et al. Activating palladium nanoparticles via a Mott-Schottky heterojunction in electrocatalytic hydrodechlorination reaction[J]. Journal of Hazardous Materials, 2020, 389: 121876. doi: 10.1016/j.jhazmat.2019.121876 [34] JIANG K X, SHI X L, CHEN M, et al. Optimizing the metal-support interactions at the Pd-polymer carbon nitride Mott-Schottky heterojunction interface for an enhanced electrocatalytic hydrodechlorination reaction[J]. Journal of Hazardous Materials, 2021, 411: 125119. doi: 10.1016/j.jhazmat.2021.125119 [35] LOU Z M, LI Y Z, ZHOU J S, et al. TiC doped palladium/nickel foam cathode for electrocatalytic hydrodechlorination of 2, 4-DCBA: Enhanced electrical conductivity and reactive activity[J]. Journal of Hazardous Materials, 2019, 362: 148-159. doi: 10.1016/j.jhazmat.2018.08.066 [36] ZHOU J S, LOU Z M, XU J, et al. Enhanced electrocatalytic dechlorination by dispersed and moveable activated carbon supported palladium catalyst[J]. Chemical Engineering Journal, 2019, 358: 1176-1185. doi: 10.1016/j.cej.2018.10.098 [37] ZHOU J S, LOU Z M, YANG K L, et al. Electrocatalytic dechlorination of 2, 4-dichlorobenzoic acid using different carbon-supported palladium moveable catalysts: Adsorption and dechlorination activity[J]. Applied Catalysis B:Environmental, 2019, 244: 215-224. doi: 10.1016/j.apcatb.2018.11.052 [38] LI J X, PENG Y Y, ZHANG W D, et al. Hierarchical Pd/MnO2 nanosheet array supported on Ni foam: An advanced electrode for electrocatalytic hydrodechlorination reaction[J]. Applied Surface Science, 2020, 509: 145369. doi: 10.1016/j.apsusc.2020.145369 [39] LOU Z M, ZHOU J S, SUN M, et al. MnO2 enhances electrocatalytic hydrodechlorination by Pd/Ni foam electrodes and reduces Pd needs[J]. Chemical Engineering Journal, 2018, 352: 549-557. doi: 10.1016/j.cej.2018.07.057 [40] LI J J, WANG Y, ZHAO B, et al. Unraveling kinetics and mechanism of electrocatalytic hydrodechlorination of chlorinated PPCPs by nickel-cobalt metal organic framework supported palladium composite electrode[J]. Applied Catalysis B:Environmental, 2023, 332: 122754. doi: 10.1016/j.apcatb.2023.122754 [41] WANG K F, SHU S, CHEN M, et al. Pd-TiO2 Schottky heterojunction catalyst boost the electrocatalytic hydrodechlorination reaction[J]. Chemical Engineering Journal, 2020, 381: 122673. doi: 10.1016/j.cej.2019.122673 [42] LOU Z M, YU C C, WEN X F, et al. Construction of Pd nanoparticles/two-dimensional Co-MOF nanosheets heterojunction for enhanced electrocatalytic hydrodechlorination[J]. Applied Catalysis B:Environmental, 2022, 317: 121730. doi: 10.1016/j.apcatb.2022.121730 [43] LI J X, CHEN Y J, BAI R Y, et al. Construction of Pd/Ni2P-Ni foam nanosheet array electrode by in-situ phosphatization-electrodeposition strategy for synergistic electrocatalytic hydrodechlorination[J]. Chemical Engineering Journal, 2022, 435: 134932. doi: 10.1016/j.cej.2022.134932 [44] FU C H, SHU S, HU L, et al. Electrocatalytic nitrate reduction on bimetallic Palladium-Copper Nanowires: Key surface structure for selective dinitrogen formation[J]. Chemical Engineering Journal, 2022, 435: 134969. doi: 10.1016/j.cej.2022.134969 [45] PENG Y Y, CUI M Y, ZHANG Z Y, et al. Bimetallic composition-promoted electrocatalytic hydrodechlorination reaction on silver–palladium alloy nanoparticles[J]. ACS Catalysis, 2019, 9(12): 10803-10811. doi: 10.1021/acscatal.9b02282 [46] ZHOU Y J, ZHANG G, JI Q H, et al. Enhanced stabilization and effective utilization of atomic hydrogen on Pd-In nanoparticles in a flow-through electrode[J]. Environmental Science & Technology, 2019, 53(19): 11383-11390. [47] LEE J H, KATTEL S, JIANG Z, et al. Tuning the activity and selectivity of electroreduction of CO2 to synthesis gas using bimetallic catalysts[J]. Nature Communications, 2019, 10(1): 3724. doi: 10.1038/s41467-019-11352-0 [48] ZHANG J, HU L, QIAN Y, et al. Synthesis of intermetallic FePtPd nanoparticles and their enhanced catalysis for electro-oxidation of methanol[J]. Surfaces and Interfaces, 2022, 35: 102485. doi: 10.1016/j.surfin.2022.102485 [49] FU W Y, SHU S, LI J X, et al. Identifying the rate-determining step of the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. Nanoscale, 2019, 11(34): 15892-15899. doi: 10.1039/C9NR04634H [50] CHEN Y J, FENG C, WANG W H, et al. Electronic structure engineering of bimetallic Pd-Au alloy nanocatalysts for improving electrocatalytic hydrodechlorination performance[J]. Separation and Purification Technology, 2022, 289: 120731. doi: 10.1016/j.seppur.2022.120731 [51] LI J, CHEN J X, WANG Q A, et al. Controllable increase of boron content in B-Pd interstitial nanoalloy to boost the oxygen reduction activity of palladium[J]. Chemistry of Materials, 2017, 29(23): 10060-10067. doi: 10.1021/acs.chemmater.7b03732 [52] LUO F, ZHANG Q, YU X X, et al. Palladium phosphide as a stable and efficient electrocatalyst for overall water splitting[J]. Angewandte Chemie, 2018, 130(45): 15078-15083. doi: 10.1002/ange.201810102 [53] WANG P, SHI X L, FU C H, et al. Strong pyrrolic-N-Pd interactions boost the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. Nanoscale, 2020, 12(2): 843-850. doi: 10.1039/C9NR07528C [54] MA L, WANG C M, GONG M, et al. Control over the branched structures of platinum nanocrystals for electrocatalytic applications[J]. ACS Nano, 2012, 6(11): 9797-9806. doi: 10.1021/nn304237u [55] ZHOU J S, LOU Z M, WANG Z N, et al. Electrocatalytic dechlorination of 2, 4-DCBA using CTAB functionalized Pd/GAC movable granular catalyst: Role of adsorption in catalysis[J]. Chemical Engineering Journal, 2021, 414: 128758. doi: 10.1016/j.cej.2021.128758 [56] JIANG G M, SHI X L, CUI M Y, et al. Surface ligand environment boosts the electrocatalytic hydrodechlorination reaction on palladium nanoparticles[J]. ACS Applied Materials & Interfaces, 2021, 13(3): 4072-4083. [57] FAN Z M, ZHAO H C, WANG K F, et al. Enhancing electrocatalytic hydrodechlorination through interfacial microenvironment modulation[J]. Environmental Science & Technology, 2023, 57(3): 1499-1509. [58] GUO X G, FACCHETTI A. The journey of conducting polymers from discovery to application[J]. Nature Materials, 2020, 19(9): 922-928. doi: 10.1038/s41563-020-0778-5 [59] XU G R, LIU F Y, LIU Z H, et al. Ethanol-tolerant polyethyleneimine functionalized palladium nanowires in alkaline media: The “molecular window gauze” induced the selectivity for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2015, 3(42): 21083-21089. doi: 10.1039/C5TA06644A [60] XU G R, BAI J A, YAO L, et al. Polyallylamine-functionalized platinum tripods: Enhancement of hydrogen evolution reaction by proton carriers[J]. ACS Catalysis, 2017, 7(1): 452-458. doi: 10.1021/acscatal.6b03049 [61] XU G R, BAI J, JIANG J X, et al. Polyethyleneimine functionalized platinum superstructures: Enhancing hydrogen evolution performance by morphological and interfacial control[J]. Chemical Science, 2017, 8(12): 8411-8418. doi: 10.1039/C7SC04109H [62] GAO M T, TAN H, ZHU P Q, et al. Why phenol is selectively hydrogenated to cyclohexanol on Ru (0001): An experimental and theoretical study[J]. Applied Surface Science, 2021, 558: 149880. doi: 10.1016/j.apsusc.2021.149880 [63] GU Z N, ZHANG Z Y, NI N, et al. Simultaneous phenol removal and resource recovery from phenolic wastewater by electrocatalytic hydrogenation[J]. Environmental Science & Technology, 2022, 56(7): 4356-4366. [64] MORALES-GUIO C G, STERN L A, HU X L. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution[J]. Chemical Society Reviews, 2014, 43(18): 6555-6569. doi: 10.1039/C3CS60468C [65] YANG K C, ZHAO Y X, ZHOU X, et al. “Self-degradation” of 2-chlorophenol in a sequential cathode-anode cascade mode bioelectrochemical system[J]. Water Research, 2021, 206: 117740. doi: 10.1016/j.watres.2021.117740 [66] SONG Y, GUTIÉRREZ O Y, HERRANZ J, et al. Aqueous phase electrocatalysis and thermal catalysis for the hydrogenation of phenol at mild conditions[J]. Applied Catalysis B:Environmental, 2016, 182: 236-246. doi: 10.1016/j.apcatb.2015.09.027 [67] WANG S, ZHU T H, JIANG N, et al. Hydrogenation of phenol to cyclohexanol using carbon encapsulated Ni–Co alloy nanoparticles[J]. Reaction Chemistry & Engineering, 2022, 7(2): 429-441. [68] HU L, SHI L, SHEN F, et al. Electrocatalytic hydrodechlorination system with antiscaling and anti-chlorine poisoning features for salt-laden wastewater treatment[J]. Water Research, 2022, 225: 119210. doi: 10.1016/j.watres.2022.119210 [69] MAO R, ZHAO X, LAN H C, et al. Graphene-modified Pd/C cathode and Pd/GAC particles for enhanced electrocatalytic removal of bromate in a continuous three-dimensional electrochemical reactor[J]. Water Research, 2015, 77: 1-12. doi: 10.1016/j.watres.2015.03.002 [70] JIANG G M, WANG K F, LI J Y, et al. Electrocatalytic hydrodechlorination of 2, 4-dichlorophenol over palladium nanoparticles and its pH-mediated tug-of-war with hydrogen evolution[J]. Chemical Engineering Journal, 2018, 348: 26-34. doi: 10.1016/j.cej.2018.04.173 [71] GUO J X, ZHANG X Q, SUN Y F, et al. NiMoS3 nanorods as pH-tolerant electrocatalyst for efficient hydrogen evolution[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 9006-9013. -



 下载:
下载: