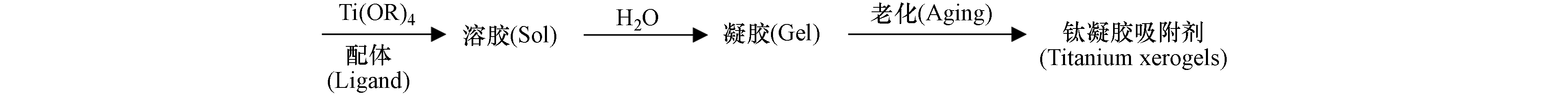-
重金属污染是威胁公众健康的世界性环境问题. 其中,砷(As)和锑(Sb)因毒性高、影响范围大而成为水体重金属污染研究的代表性元素,在水环境中常以三价或五价形式存在[1 − 4]. 长期接触含As水可能会引起神经系统症状,皮肤病变和癌症[5],而摄入过量Sb可能会伤害心脏和肝脏,甚至导致死亡[6]. 世界卫生组织将饮用水中As浓度限值规定为10 μg·L−1[7]. 中国、欧盟和日本对Sb的最高污染水平限定为5 μg·L−1[8]. 因此,开发水中As/Sb的深度去除技术是保障饮用水安全和生态环境健康的迫切需求.
目前,水环境中As、Sb污染的治理技术主要包括混凝技术、电化学技术、膜滤技术、离子交换技术、生物技术和吸附技术[9 − 10]. 由于操作简单、成本低等特点,吸附法一直是研究的热点[11 − 17]. 吸附法去除As、Sb的效果主要与所使用的吸附剂以及As、Sb的存在形态有关[12, 18 − 19]. 目前常见的重金属吸附剂主要是铁基、锰基等氧化物或氢氧化物纳米颗粒[20 − 24], 在应用过程中存在金属溶损率高、吸附容量低(< 150 mg·g−1)、受pH影响大、As(Ⅲ)去除效果差、纳米材料制备过程复杂等问题,限制了其大规模的应用[25 − 26]. 钛系吸附剂以稳定、无毒、抗腐蚀以及对As的高亲和力获得广泛关注[27]. 近期,一种通过溶胶凝胶法制备的新型钛凝胶吸附剂呈现As(Ⅲ)吸附容量大,受水环境(pH或共存离子等)变化影响小等特点. 由于材料表面具有较高的As(Ⅲ)亲和力,这种新型钛凝胶吸附剂对腐殖酸等共存有机质(50 mg·L−1)以及硫酸盐、碳酸盐等无机离子(10 mmol·L−1)具有较强的抗干扰能力[26]. 在固定床系统中可以有效的将As(Ⅲ)浓度从200 μg·L−1降至10 μg·L−1以下[26],表现出了良好的应用潜力.
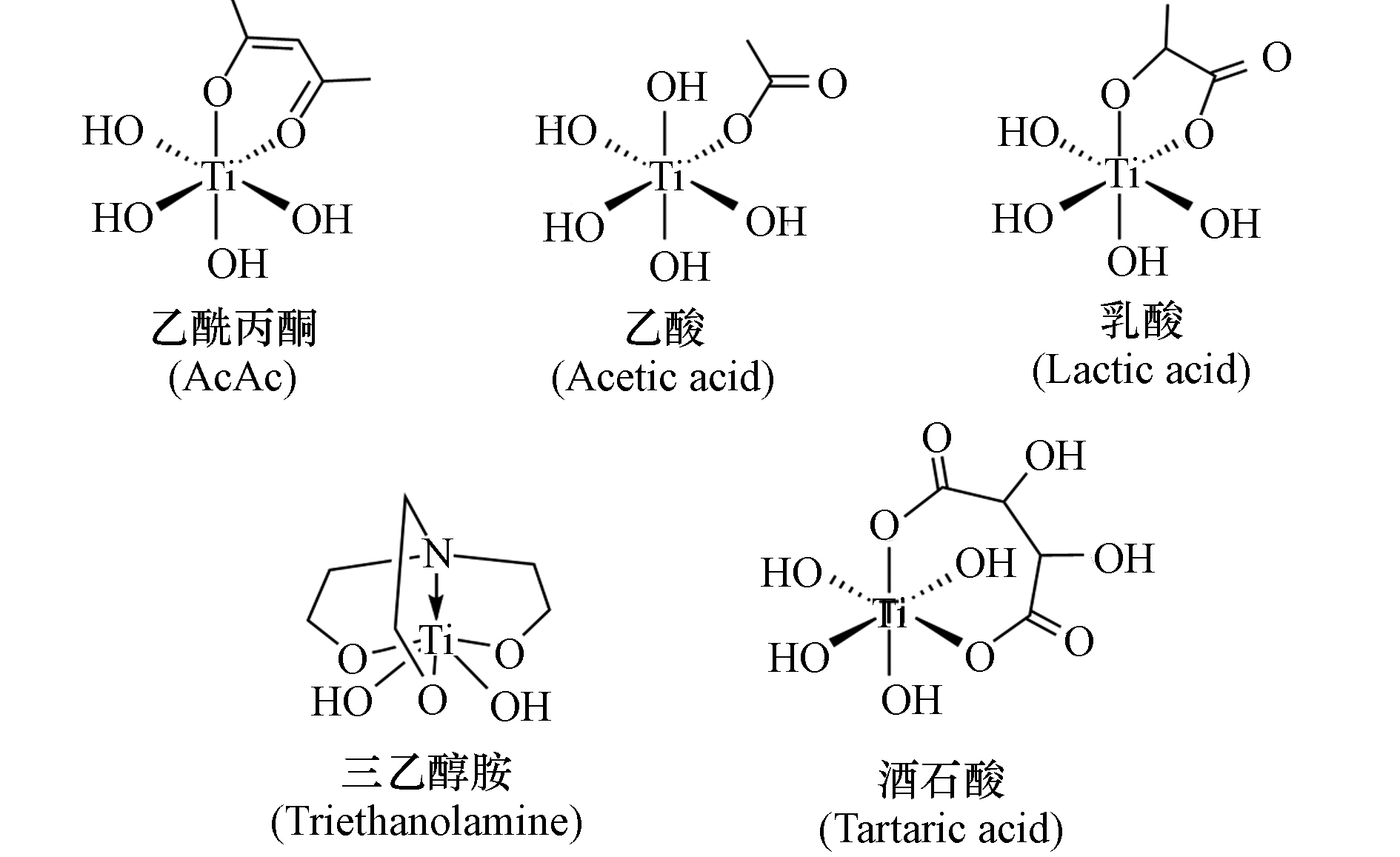
由于电负性低、极化能力强等特点,Ti4+极易在水中水解,形成不溶性氧化物而沉淀. 这对钛凝胶吸附剂的高效稳定制备带来挑战. 因此,选择合适的配体克服Ti4+不受控制的水解是溶胶凝胶法制备高性能钛凝胶吸附剂的关键. 先前所研发的钛凝胶吸附剂是以乙酰丙酮(AcAc)为桥联配体. 该配体在制备过程中通过络合作用提高了Ti4+离子的稳定性,限制了钛醇盐的水解反应,从而保护了Ti/O结构的生长. 然而,在溶胶凝胶法过程中加入其他种类配体是否可以表现出更佳的吸附性能尚未得到进一步的验证,配体结构与吸附剂性能之间的构效关系尚不明晰.
本文分别以11种有机配体(3种常见有机酸配体,1种醇胺类配体和7种乙酰丙酮结构衍生物)(图1)通过溶胶凝胶法合成了11种钛干凝胶,并与无添加钛凝胶材料进行了As、Sb吸附性能对比. 结合吸附动力学和吸附容量,初步考察了配体对钛干凝胶吸附 As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)和 Sb(Ⅴ)性能的影响,剖析了可能的影响机制,为高效钛系吸附剂的开发、适配体的筛选和设计提供理论指导.
-
除有特别说明,所有试剂均为分析纯,无需进一步纯化. 乙酰丙酮(AcAc)、酒石酸、冰乙酸、乳酸、三乙醇胺、3,5-庚二酮(97%)、钛酸四丁酯、焦锑酸钾均购自Aladdin. 3,3-二甲基-2,4-戊二酮(AcAc-(CH3)2, 97%)、亚砷酸钠、砷酸钠购自Sigma-Aldrich. 2,3-丁二酮、3-甲基-2,4-戊二酮(AcAc-CH3, 97%)分别购于上海化学试剂厂和日本TCI公司. 2-乙酰基环己酮(Cycle-diketone, 95%)、3,4-二氢-6-甲基-2氢-吡喃-5-甲基酮(Cycle-enol, 96%)均为南京大学化学化工学院合成. 除此以外,本研究使用的所有其他化学品均来自国药化学试剂有限公司. 所有溶液均用超纯水(18.25 MΩ·cm)配制.
-
加入不同配体,分别制备了12种钛凝胶吸附剂:4种常见有机酸、醇胺类配体(酒石酸、乙酸、乳酸、三乙醇胺)和7种AcAc及其衍生物(AcAc、丁二酮、3,5-庚二酮、AcAc-CH3、AcAc-(CH3)2、Cycle-diketone、Cycle-enol)以及无配体添加的对照组. 具体制备方法为:将0.75 mL配体与10 mL乙醇混合,添加10 mL钛酸四丁酯后,在室温下搅拌30 min,获得均一的溶胶,为A液. 将5 mL乙醇,3.3 mL水和0.2 mL 50%硝酸混合搅拌,为B液. 将B液缓慢滴入到A液中,不断搅拌后可以观察到混合液逐渐失去流动性,成为凝胶. 将所得凝胶收集到敞口的离心管中,并用冷冻干燥机干燥48 h,即可得到不同配体合成的钛凝胶吸附剂. 在上述配方中,钛酯与配体的摩尔比均为4:1,钛酯与水的摩尔比为4:25. 制备流程如图2所示.
-
本文旨在探究溶胶凝胶法合成钛凝胶吸附剂过程中桥联配体对其吸附性能的影响,为剥离其他因素影响,采用了相对简单的模拟金属离子体系. 配制100 mL含5 mg·L−1 As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)、Sb(Ⅴ)的样品溶液,用稀NaOH和HClO4溶液调节pH为7.0 ± 0.1. 将样品溶液加入摇瓶,准确称取0.02 g钛凝胶吸附剂加入摇瓶,在摇床上振荡(120 r·min−1,25 ℃),间隔一段时间取样. 所有实验组和对照组设置3个平行.
-
配置100 mL含50 mg·L−1 As/Sb的样品溶液,用稀NaOH和HClO4溶液调节pH为7.0 ± 0.1. 将样品溶液加入摇瓶,准确称取0.02 g钛凝胶吸附剂加入摇瓶,在摇床上振荡(120 r·min−1,25 ℃),48 h后取样. 所有实验组和对照组设置3个平行.
-
砷、锑和钛的质量浓度使用电感耦合等离子体发射光谱仪(iICP 7400 ICP-OES,美国)测定. 测定钛凝胶吸附剂的载钛量时,首先将材料用硝酸和高氯酸湿法消解,然后再进行质量浓度检测.
吸附剂的表面位点密度通过使用配备有组合玻璃电极(DGi115-SC,Mettler Toledo)的自动滴定系统(T50)的恒电位滴定法测定. 添加酸滴定剂为0.2 mol·L−1 HNO3,碱滴定剂为0.03 mol·L−1 NaOH,背景溶液为50 mL 0.01 mol·L−1 NaClO4,投加钛凝胶吸附剂的质量为20 mg.
-
材料的平衡吸附容量Qe(mg·g−1)根据公式(1)计算:
式中,C0、Ce(mg·L−1)分别为污染物初始质量浓度和平衡质量浓度,V(L)为反应体系的体积,m(g)为吸附剂的投加质量.
吸附速率常数(k2(g·(mg·h)−1)通过二级动力学[28](公式2)拟合:
式中,Qt(mg·g−1)为材料在t(h)时刻的吸附容量,Qe(mg·g−1)为平衡吸附容量.
-
利用原位格氏图(Gran plots)计算钛凝胶吸附剂的滴定平衡点和表面羟基位点密度[29 − 30]. 酸性端的格氏值(Ga)及碱性端的格氏值(Gb)计算如公式(3)和公式(4):
式中,V0为体系初始总体积(mL),Vat为酸滴定到体系pH为3.0时消耗的标准酸液的总体积(mL),Vb为碱滴定体系pH到11.0的过程中消耗的标准碱液的实时累计体积(mL).
表面羟基位点含量(Hs,mmol·g−1)的计算公式如式(5):
式中,m为材料质量(mg),Veb1与Veb2分别为格氏图中酸滴定曲线拟合线性回归方程与横坐标的交点(mL),sample和blank分别为含有吸附剂体系和空白体系,cb为所用滴定碱液浓度(mmol·L−1).
-
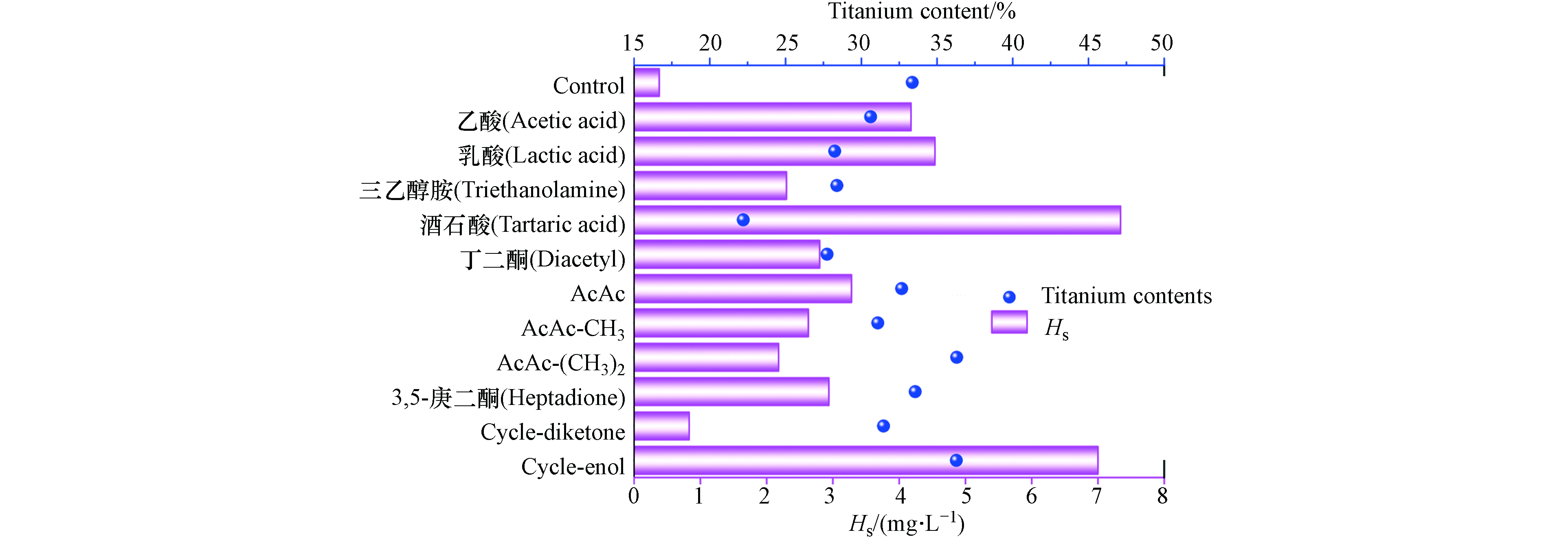
钛凝胶吸附剂中表面羟基基团含量是提升重金属吸附性能的重要因素[26]. Gran图解法常用于确定溶液中氧化物的表面活性位点密度. 加入各类配体后,合成的12种钛凝胶吸附剂的Gran曲线如图3所示. Veb1前段为加入的NaOH与水中的H+反应所用的量. Veb1和Veb2中间段为NaOH与材料表面的羟基(Ti—OH,活性位点)反应所用的碱量. Veb2后段为调节整个固液系统的pH所需碱量. 根据公式(5)计算得到材料表面的—OH位点的含量[29](图4). 结果表明,与不加入配体的对照组(Hs = 0.38 mmol·g−1)相比,加入有机配体均可以显著提高钛凝胶吸附剂的表面位点含量. 这些有机配体含有较多的羟基和羧基,可显著提高材料在水中缓冲氢离子的能力. 其中,加入Cycle-diketone配体后合成的钛凝胶材料表面羟基位点含量增加最少,Hs仅为0.83 mmol·g−1;而加入酒石酸和Cycle-enol后合成的钛凝胶材料表面羟基含量显著高于其他有机配体,Hs分别为7.34 mmol·g−1和7.0 mmol·g−1. 另外,有机羧酸类配体调控的钛凝胶材料Hs值普遍高于双酮类有机配体(除了Cycle-diketone外). 酒石酸和Cycle-enol的挥发性差,在常温下能以固体形态稳定存在,较高的Hs值可能是因为这些配体在材料中存在较多残留.
AcAc的中心碳上有α-H,能够发生烯醇和双酮式结构互变,其pKa值为8.9[30]. 烯醇式阴离子是一种常见的双齿配体,可与各类金属形成配合物[31]. 从图4中可以看出,随着中心碳上的α-H被甲基取代,烯醇式含量的减少,钛凝胶材料表面羟基位点含量逐渐降低. AcAc,AcAc-CH3,AcAc-(CH3)2的Hs值分别为3.28、2.6、2.18 mmol·g−1. 丁二酮不具有烯醇式结构,Cycle-diketone的α-H受到空间位阻的影响,电离难度增大. 因此,丁二酮和Cycle-diketone配体调控后的钛凝胶吸附剂Hs值均有所下降. 其中Cycle-diketone调控后的Hs值仅为 0.83 mmol·g−1,远低于AcAc. 以上结果表明,AcAc的烯醇式结构是影响材料表面位点含量的重要因素. 当烯醇式结构能够稳定且大量存在时,材料表面位点含量增加,反之则下降.
除了检测材料表面活性位点含量外,本文又通过ICP-OES测定了材料的钛含量(图4). 与无配体调控的钛凝胶材料相比,有机配体的加入并没有显著提升钛含量. AcAc及其衍生物合成的材料中钛含量在31.1%到36.3%之间浮动,而有机羧酸类配体以及不具有配位能力的丁二酮会降低材料中的钛含量至30%以下.
-
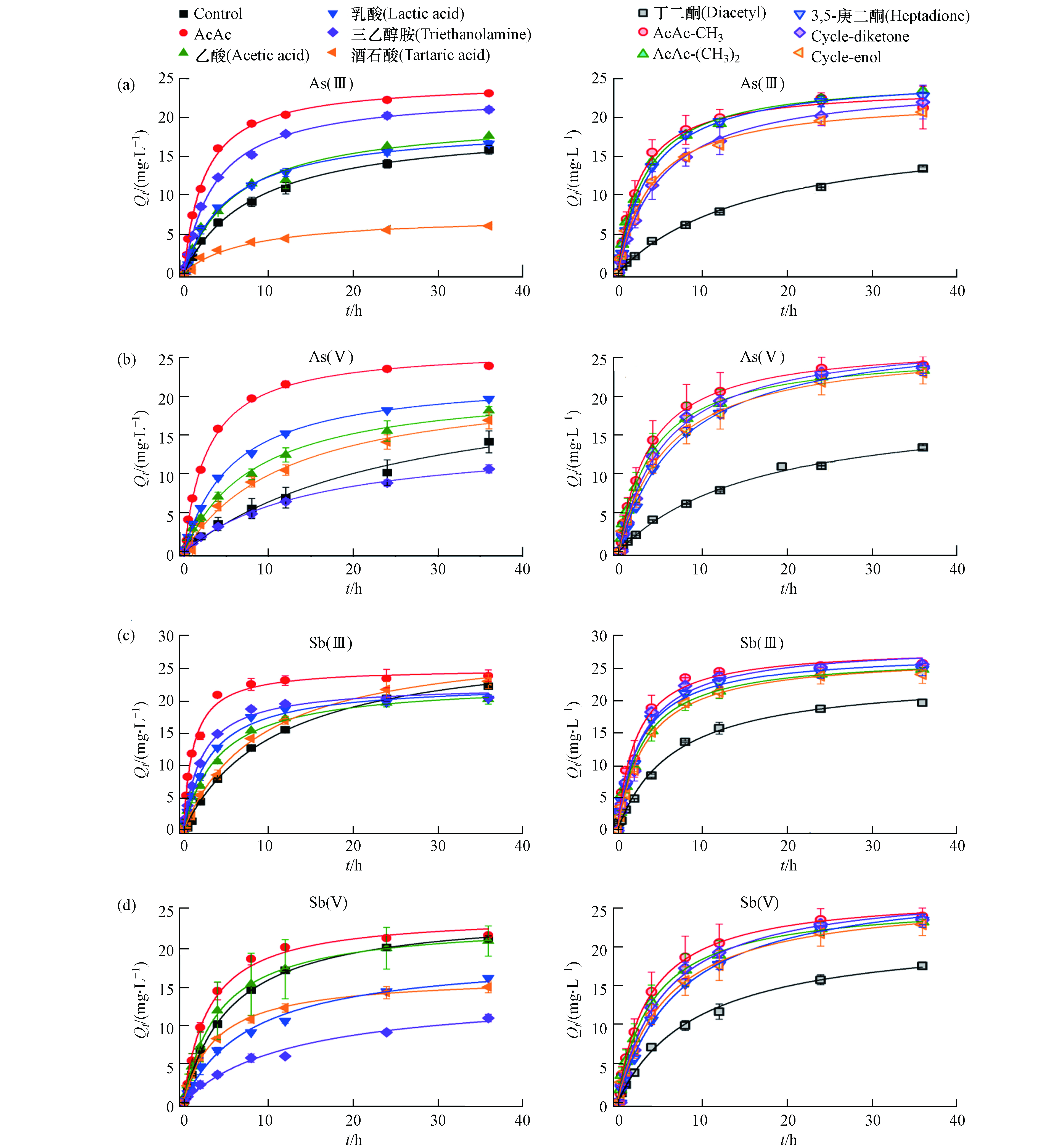
将12种钛凝胶吸附剂分别放置于含有As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)、Sb(Ⅴ)(初始浓度为5 mg·L−1)的溶液中,静态吸附35 h内对As\Sb的吸附动力学如图5所示. 加入有机配体合成的大多数钛凝胶混凝剂对As、Sb的吸附速率均快于对照组. 为了更准确地比较各类配体调控对钛凝胶材料吸附速率的影响,采用二级吸附动力学方程[28]对动力学数据进行拟合,得到表观吸附速率常数k2(图6). 11种桥联配体中AcAc对As\Sb的吸附速率提升效果最好,其对As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)、Sb(Ⅴ)的吸附速率分别是对照组的10.1、8.1、11.5、1.9倍. 丁二酮调控后对材料的吸附速率没有提升,甚至减慢了对As(Ⅲ)、Sb(Ⅴ)的吸附速率.
有机羧酸类配体的Hs都高于AcAc,却没有表现出更高的吸附速率. 这可能与较低的钛含量以及配体配位强度有关. 相比较五价的As/Sb,三价的As/Sb具有更高的生物毒性,而且在水中通常以电中性的形式存在,分离去除更加困难[6, 19, 26]. 从图6可以看出,除乙酸配体对As(Ⅲ)有显著吸附速率提升外,其他羧酸类配体对吸附速率提升效果有限,仅有1.28—1.85倍. 而对于Sb(Ⅲ)的吸附速率提升情况正好相反. 乙酸配体调控后几乎没有提升对Sb(Ⅲ)的吸附速率,其他羧酸类配体的提升效果显著(3.39—6.68倍).
对于β-双酮类配体而言,则呈现出较为明显的规律:随着AcAc中心碳上的α-H数量的减少,AcAc衍生物调控的钛凝胶材料对As/Sb的吸附速率逐渐降低. 这与材料表面活性位点含量变化一致. 3, 5-庚二酮,Cycle-diketone以及Cycle-enol对As/Sb吸附速率的提升效果普遍弱于AcAc中心碳取代的结构类似物,与有机酸类配体的提升效果相当.
-
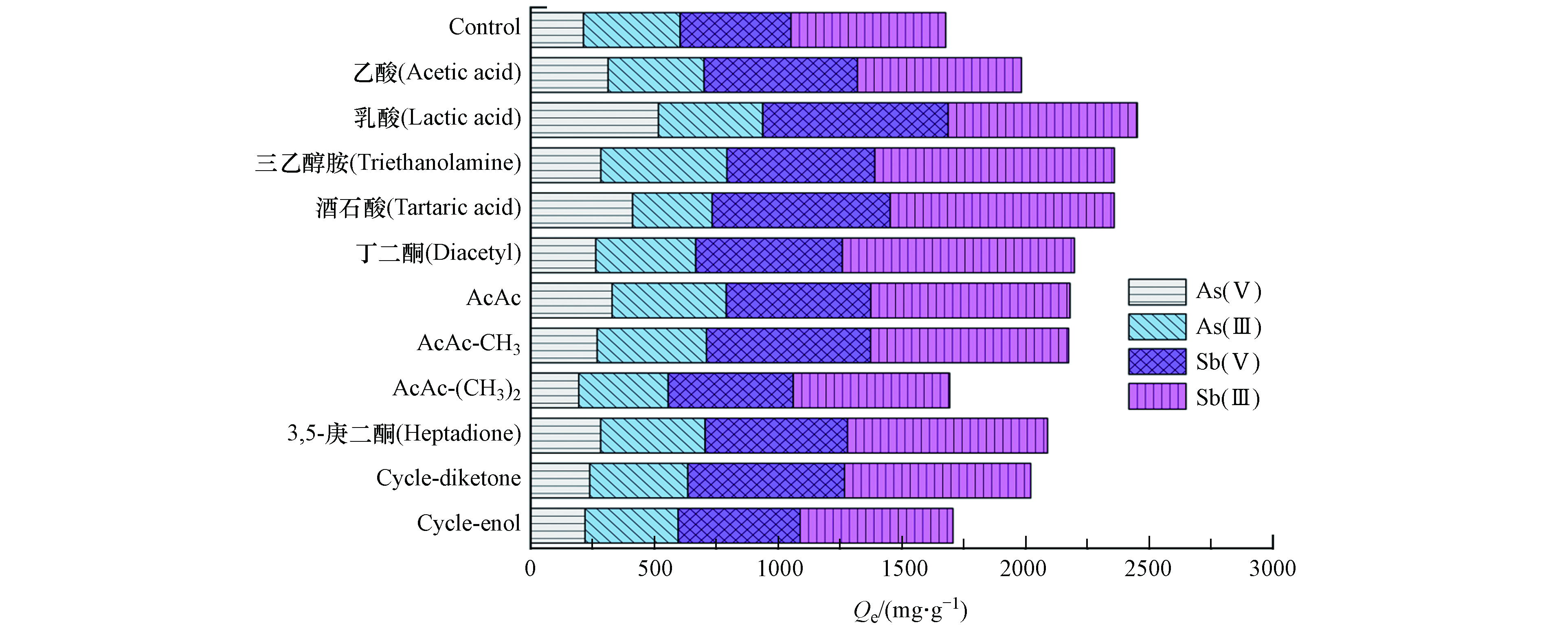
对12种钛凝胶吸附剂以单位质量Ti归一化后,分别计算了吸附初始浓度为50 mg·L−1的As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)、Sb(Ⅴ) 48 h后的吸附容量(图7).
加入各类配体后,大部分钛凝胶吸附剂的吸附容量均有所提升,对Sb的提升效果最为明显. AcAc调控后的钛凝胶材料对As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)、Sb(Ⅴ)的吸附容量分别达到了462、329、805、584 mg·g−1(以Ti计). 与无配体调控的对照组相比,分别提升了1.18、1.55、1.29、1.30倍. AcAc中心碳α-H全部取代的AcAc-(CH3)2以及环状双酮Cycle-enol则对吸附容量几乎没有提升效果. 在所有配体调控中,对As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)、Sb(Ⅴ)吸附容量提升最大的分别为三乙醇胺(510 mg·g−1)、乳酸(516 mg·g−1)、三乙醇胺(968 mg·g−1)以及酒石酸(750 mg·g−1).
在实际含As、Sb地下水处理过程中,吸附速率和吸附容量是决定工程设计和吸附效能的重要参数. 尽管有机酸类配体制得的钛凝胶对As/Sb的吸附容量略优于AcAc为配体的钛凝胶,但其它配体对吸附速率提升的贡献仅为AcAc的30%—60%. 综合来看,AcAc是制备高性能钛凝胶吸附剂的适宜配体. 此外,由于以AcAc为配体的钛凝胶吸附剂对As/Sb的结合较强,解析再生比较困难. 尽管如此,兼顾高吸附容量和快吸附速率的钛凝胶吸附剂与实际应急快速处理需求具有良好的适配性,未来可通过负载、复配等方式进一步提高吸附性能,对使用后的钛凝胶吸附剂通过煅烧等方式回收具有附加价值的二氧化钛,从而降低处理成本. 因此,以AcAc为配体的钛凝胶吸附剂具有良好的应用潜力.
-
配体主要从合成过程和吸附过程两个方面影响钛凝胶吸附剂的性能. 一方面,配体与钛的配位能力和配位方式可以有效控制水解和缩合步骤,进而影响聚合物凝胶的结构和吸附性能. 在溶胶-凝胶法制备过程中,钛酸四丁酯前体先进行水解和聚合反应形成溶胶,然后再缓慢聚合形成凝胶. 然而,钛烷氧基体系对水的敏感性很高,会快速地发生水解,并形成无定形水合氧化钛簇而沉淀,使得材料有序性变差,表面羟基活性位点变少(见对照组),从而影响吸附性能. 引入富含螯合电子的氧基配体可以有效提高对水解的抵抗力,阻止中钛水解-缩合反应位点,保护Ti/O结构的生长,从而增加了溶胶-凝胶过程中材料的有序性和表面羟基活性位点. 另一方面,在吸附过程中,配体与钛配位后,能否进一步水解形成新的吸附位点同样是影响钛凝胶材料对重金属吸附性能的重要因素. Zhang等研究发现,与无配体的对照组相比,AcAc调控的钛凝胶材料的物理结构(表面积和孔体积)和表面电荷(PZC)都不利于As的吸附,却呈现更高的吸附性能. 推测是因为在吸附过程中,钛凝胶表面发生原位水解,从而提供了新生的Ti—O—H和C=O/C—O—H位. 这些新位点通过具有强结合能的内球络合捕获As(Ⅲ)[26]. 另外,由于As\Sb含氧酸盐与配体的直接络合能力较弱,暴露在材料表面的未配位的自由配体较少,加之腐殖酸等共存配体的竞争干扰小[26],材料表面有机配体自身与As\Sb含氧酸盐的配位络合作用占比较小. 由此推测,理想的配体应当具有适中的钛配位能力. 配体配位能力太弱,在合成过程中则易生成无定形水合氧化钛簇,无法形成有序的凝胶结构. 配体配位能力太强,在吸附过程中难以快速水解释放吸附位点,导致较低的吸附速率. 该猜想与本文的配体影响相吻合.
11种配体与钛的常见配位模型如图8所示,丁二酮和AcAc-diketone与钛的配位能力最差,无法形成螯合结构,这些配体不利于生成结构有序的钛凝胶,具有较低的表面羟基活性位点密度以及较低的吸附速率. 除此以外,AcAc及其衍生物都能作为双齿配体与钛螯合形成较为稳定的共轭六元环. 羧酸类配体与Ti的配位能力相对较强,乙酸作为单齿配体与钛进行配位,乳酸和酒石酸可以作为双齿配体与钛螯合形成五元环和七元环,而三乙醇胺作为四齿配体可与钛形成强稳定性半包结配合物. 这些配体虽然具有较高的表面羟基活性位点密度和吸附容量,但强稳定的配位结构非常不利于吸附过程中吸附位点的水解释放,导致较低的吸附速率. 比较来看,AcAc是最为合适的桥联配体,其配位能力适中,既能有效控制Ti的快速水解,又能在吸附过程中迅速释放吸附位点,因而兼具吸附速率快和吸附容量高的优势. 另外,中心碳或端基碳的取代基效应会影响烯醇式AcAc的配位能力. 当中心碳上的α-H被推电子基团如甲基等取代后,其电离能力减弱,pKa变大,不利于向烯醇式的转化,配位能力减弱. 当中心碳上存在双甲基取代或在AcAc骨架上引入环状结构时,在吸附过程中可能会由于空间位阻效应,导致其无法作为一个锚点,降低了As/Sb的亲和力. 因此,AcAc-(CH3)2、Cycle-enol以及Cycle-diketone表现出较低的吸附速率和吸附容量.
-
本文系统研究了有机配体对溶胶凝胶法合成的钛吸附剂去除砷锑的性能影响. 总体而言,在合成中加入有机配体,均能显著提高钛凝胶表面羟基位点密度以及As、Sb吸附性能. 但配体的配位能力和配位结构对钛凝胶吸附性能的提升存在显著影响. 通过对比各类配体调控后的钛凝胶表面羟基位点密度、钛含量、吸附速率以及吸附容量等参数,发现AcAc是高性能钛凝胶吸附剂制备过程中的适宜配体.
配体主要从合成和吸附两个过程影响钛凝胶吸附剂的吸附性能. 在溶胶凝胶法制备过程中,配体能够通过配位作用抑制钛的过度水解,从而提高水解产物的有序性,得到多吸附位点的立体网状结构. 而在吸附过程中,钛配合物原位水解暴露吸附新位点的数量是钛凝胶有效捕获As(Ⅲ)、Sb(Ⅲ)的重要因素. AcAc的烯醇式结构易与Ti形成较为稳定的共轭六元环,其配位能力介于丁二酮、Cycle-enol等弱配体和酒石酸、三乙醇胺等强配体之间,兼顾了合成过程中抑制钛的过度水解作用和吸附过程中新活性位点的快速释放功能. 因而AcAc调控后的钛凝胶材料兼具吸附速率快和吸附容量大的优势. 当AcAc中心碳或端基碳被甲基取代后,会降低烯醇式AcAc的含量以及配位能力,进而减弱了材料吸附As、Sb的性能.
本文研究结果有望为高性能环境功能材料的设计提供新思路,但仍然存在不足之处. 后续可借助钛配位化学基本理论与现代仪器分析手段,明确钛氧簇凝胶合成过程中AcAc的作用机制及聚合网络的生成机制,为该方法的持续优化提供理论依据. 另外,还需要系统评价钛凝胶材料净污性能,考察实际水质条件下共存基质的影响及作用规律,探索该类材料规模化生产的技术要点,以推进该类材料的实用化进程.
桥联配体对钛凝胶吸附砷锑性能的影响及机制
Effects and mechanism of bridging ligands on the adsorption performance for arsenic and antimony removal by titanium xerogel
-
摘要: 针对钛凝胶吸附剂中桥联配体与吸附性能之间的关系不明问题,本文对比考察了由11种配体(有机羧酸类、醇胺类以及双酮类)合成的钛凝胶材料吸附砷(As)、锑(Sb)的性能. 乙酰丙酮(AcAc)作为桥联配体合成的钛凝胶材料表现出优异的As、Sb去除能力,其对As(Ⅲ)、As(Ⅴ)、Sb(Ⅲ)和Sb(Ⅴ)的吸附容量分别达到了329、461、584、805 mg·g−1(以钛计),吸附速率分别比无配体组提高了10.1、8.1、11.5、1.9倍. 各类配体对钛凝胶吸附容量的提升效果相当,但对吸附速率提升效果差异明显. 有机羧酸类配体调控后对As、Sb吸附速率仅为AcAc的30%—60%,而丁二酮等弱配体调控后几乎没有提升吸附速率. 与其他配体相比,AcAc的配位能力介于丁二酮和酒石酸、三乙醇胺等强配体之间,其适中的配位能力兼顾了制备过程中抑制钛快速水解和吸附过程中新活性位点的快速释放特性. 当AcAc中心碳或端基碳被甲基取代后,烯醇式AcAc的含量以及配位能力降低,进而减弱材料的砷锑吸附性能.Abstract: To clarify the relationship between the structure of bridging ligands and the adsorption performance, a series of titanium xerogels were synthesized with 11 ligands (organic carboxylic acids, alcohol-amines, and diketones) and their performance for arsenic and antimony removal was investigated. The titanium xerogel synthesized with acetylacetone (AcAc) as a bridging ligand showed an excellent adsorption performance: the adsorption capacities to As(Ⅲ), As(Ⅴ), Sb(Ⅲ), and Sb(Ⅴ) were 329, 461, 584 and 805 mg·g−1 (in titanium), respectively, and the adsorption rates were 10.1, 8.1, 11.5 and 1.9 times higher than that of the xerogel without organic ligand. All the tested ligands had comparable effects on the adsorption capacity, but led to significant differences in the adsorption rate. The xerogels with organic carboxylic acid ligands adsorbed As and Sb at rates of only 30%—60% of that with AcAc, while weak ligand, such as butanedione, had negligible effect on the adsorption rate. Compared with other ligands, AcAc had a moderate coordination capacity between butanedione and strong ligands (e.g., tartaric acid and triethanolamine), which balanced the inhibition of rapid hydrolysis of titanium during synthesis and the quick release of new active sites during adsorption. When the hydrogen at the central or terminal carbon of AcAc was substituted by methyl groups, the content of the enolic form and consequently the coordination ability were reduced, which in turn weakened the adsorption performance.
-
Key words:
- heavy metal pollution /
- titanium xerogel adsorbent /
- acetylacetone /
- ligands /
- sol-gel
-

-
-
[1] 柳凤娟, 张国平, 罗绪强, 等. Fe(Ⅱ)浓度对硫酸盐还原菌去除水体中砷和锑的影响[J]. 环境化学, 2021, 40(10): 3171-3179. doi: 10.7524/j.issn.0254-6108.2020060401 LIU F J, ZHANG G P, LUO X Q, et al. Effect of different contents of Fe(Ⅱ) on removal of arsenic and antimony from water by sulfate reducing bacteria[J]. Environmental Chemistry, 2021, 40(10): 3171-3179(in Chinese). doi: 10.7524/j.issn.0254-6108.2020060401
[2] 任杰, 刘晓文, 李杰, 等. 我国锑的暴露现状及其环境化学行为分析[J]. 环境化学, 2020, 39(12): 3436-3449. doi: 10.7524/j.issn.0254-6108.2019090701 REN J, LIU X W, LI J, et al. Analysis of exposure status quo and environmental chemical behaviors of antimony in China[J]. Environmental Chemistry, 2020, 39(12): 3436-3449(in Chinese). doi: 10.7524/j.issn.0254-6108.2019090701
[3] FANG Z Y, LI Z X, ZHANG X L, et al. Enhanced arsenite removal from silicate-containing water by using redox polymer-based Fe(III) oxides nanocomposite[J]. Water Research, 2021, 189: 116673. doi: 10.1016/j.watres.2020.116673 [4] YANG T, WU S S, LIU C P, et al. Efficient degradation of organoarsenic by UV/chlorine treatment: Kinetics, mechanism, enhanced arsenic removal, and cytotoxicity[J]. Environmental Science & Technology, 2021, 55(3): 2037-2047. [5] ARGOS M, KALRA T, RATHOUZ P J, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study[J]. The Lancet, 2010, 376(9737): 252-258. doi: 10.1016/S0140-6736(10)60481-3 [6] LI J Y, ZHENG B H, HE Y Z, et al. Antimony contamination, consequences and removal techniques: A review[J]. Ecotoxicology and Environmental Safety, 2018, 156: 125-134. doi: 10.1016/j.ecoenv.2018.03.024 [7] SMITH A H, LOPIPERO P A, BATES M N, et al. Public health. Arsenic epidemiology and drinking water standards[J]. Science, 2002, 296(5576): 2145-2146. doi: 10.1126/science.1072896 [8] KANG M, KAMEI T, MAGARA Y. Comparing polyaluminum chloride and ferric chloride for antimony removal[J]. Water Research, 2003, 37(17): 4171-4179. doi: 10.1016/S0043-1354(03)00351-8 [9] ZHANG X Y, XIE N Y, GOU Y, et al. Insights into adsorptive removal of antimony contaminants: Functional materials, evaluation and prospective[J]. Journal of Hazardous Materials, 2021, 418: 126345. doi: 10.1016/j.jhazmat.2021.126345 [10] SARKAR A, PAUL B. The global menace of arsenic and its conventional remediation - A critical review[J]. Chemosphere, 2016, 158: 37-49. doi: 10.1016/j.chemosphere.2016.05.043 [11] 曾辉平, 于亚萍, 吕赛赛, 等. 基于铁锰泥的除砷颗粒吸附剂制备及其比较[J]. 环境科学, 2019, 40(11): 5002-5008. ZENG H P, YU Y P, LÜ S S, et al. Preparation and comparison of arsenic removal granular adsorbent based on iron-manganese sludge[J]. Environmental Science, 2019, 40(11): 5002-5008(in Chinese).
[12] 李聪, 钟溢健, 解庆林, 等. 不同吸附材料处理水中砷的效应分析[J]. 现代化工, 2018, 38(7): 21-25. doi: 10.16606/j.cnki.issn0253-4320.2018.07.005 LI C, ZHONG Y J, XIE Q L, et al. Effect analysis on arsenic removal from water by different adsorption materials[J]. Modern Chemical Industry, 2018, 38(7): 21-25(in Chinese). doi: 10.16606/j.cnki.issn0253-4320.2018.07.005
[13] 许江城, 康得军, 杨天学, 等. 改性吸附材料处理水体中砷的研究进展[J]. 水处理技术, 2020, 46(10): 6-11. XU J C, KANG D J, YANG T X, et al. Research progress in the treatment of arsenic in water by modified adsorbent[J]. Technology of Water Treatment, 2020, 46(10): 6-11(in Chinese).
[14] CHI Z Y, XIE X J, PI K F, et al. Mineralogical controls on arsenite adsorption onto soils: Batch experiments and model-based quantification[J]. Science of the Total Environment, 2021, 767: 144920. doi: 10.1016/j.scitotenv.2020.144920 [15] CUONG D V, WU P C, CHEN L I, et al. Active MnO2/biochar composite for efficient As(Ⅲ) removal: Insight into the mechanisms of redox transformation and adsorption[J]. Water Research, 2021, 188: 116495. doi: 10.1016/j.watres.2020.116495 [16] UNGUREANU G, SANTOS S, BOAVENTURA R, et al. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption[J]. Journal of Environmental Management, 2015, 151: 326-342. [17] YU T C, WANG X H, LI C. Removal of antimony by FeCl3-modified granular-activated carbon in aqueous solution[J]. Journal of Environmental Engineering, 2014, 140(9): A4014001. doi: 10.1061/(ASCE)EE.1943-7870.0000736 [18] MATSUI Y, SHIRASAKI N, YAMAGUCHI T, et al. Characteristics and components of poly-aluminum chloride coagulants that enhance arsenate removal by coagulation: Detailed analysis of aluminum species[J]. Water Research, 2017, 118: 177-186. doi: 10.1016/j.watres.2017.04.037 [19] MOHAN D, PITTMAN C U Jr, Arsenic removal from water/wastewater using adsorbents—A critical review[J]. Journal of Hazardous Materials, 2007, 142(1/2): 1-53. [20] GUAN X H, DU J S, MENG X G, et al. Application of titanium dioxide in arsenic removal from water: A review[J]. Journal of Hazardous Materials, 2012, 215/216: 1-16. doi: 10.1016/j.jhazmat.2012.02.069 [21] KANEL S R, MANNING B, CHARLET L, et al. Removal of arsenic(Ⅲ) from groundwater by nanoscale zero-valent iron[J]. Environmental Science & Technology, 2005, 39(5): 1291-1298. [22] MISHRA P K, GAHLYAN P, KUMAR R, et al. Aero-gel based cerium doped iron oxide solid solution for ultrafast removal of arsenic[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 10668-10678. [23] SHARMA V K, ZBORIL R, VARMA R S. Ferrates: greener oxidants with multimodal action in water treatment technologies[J]. Accounts of Chemical Research, 2015, 48(2): 182-191. doi: 10.1021/ar5004219 [24] ZHANG T S, WANG J, ZHANG W T, et al. Amorphous Fe/Mn bimetal-organic frameworks: Outer and inner structural designs for efficient arsenic(Ⅲ) removal[J]. Journal of Materials Chemistry A, 2019, 7(6): 2845-2854. doi: 10.1039/C8TA10394A [25] 马文静, 阎莉, 张建锋. 二氧化钛对地下水中砷硅的吸附及再生回用[J]. 环境科学, 2018, 39(3): 1241-1247. doi: 10.13227/j.hjkx.201706112 MA W J, YAN L, ZHANG J F. Groundwater arsenic and silicate adsorption on TiO2 and the regeneration of TiO2[J]. Environmental Science, 2018, 39(3): 1241-1247(in Chinese). doi: 10.13227/j.hjkx.201706112
[26] ZHANG C, WU B D, PAN B C, et al. Deep removal of arsenite from water with no need for pre-oxidation or in-line oxidation[J]. Chemical Engineering Journal, 2020, 401: 126046. doi: 10.1016/j.cej.2020.126046 [27] NABI D, ASLAM I, QAZI I A. Evaluation of the adsorption potential of titanium dioxide nanoparticles for arsenic removal[J]. Journal of Environmental Sciences, 2009, 21(3): 402-408. doi: 10.1016/S1001-0742(08)62283-4 [28] GUO X, WANG J L. A general kinetic model for adsorption: Theoretical analysis and modeling[J]. Journal of Molecular Liquids, 2019, 288: 111100. doi: 10.1016/j.molliq.2019.111100 [29] GRAN G. Determination of the equivalence point in potentiometric titrations. part Ⅱ[J]. The Analyst, 1952, 77(920): 661-671. doi: 10.1039/an9527700661 [30] ANTONELLI D M, YING J Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified Sol-gel method[J]. Angewandte Chemie International Edition in English, 1995, 34(18): 2014-2017. doi: 10.1002/anie.199520141 [31] SCHUBERT U. Chemical modification of titanium alkoxides for Sol–gel processing[J]. Journal of Materials Chemistry, 2005, 15(35/36): 3701. -




 下载:
下载: