-
地下水中砷的污染已在世界范围内严重威胁到人体健康,世界卫生组织和我国饮用水卫生标准(GB5749—2022)限值均为10 μg·L−1[1 − 2]. 含砷饮用水被人体长期摄入,会引起以皮肤病变为主的全身性慢性中毒疾病[3 − 4]. 中国大陆高砷地下水分布广泛,在我国高砷地下水主要分布于内蒙古[5 − 6]、湖北[7 − 8]、山西[9 − 11]、新疆[12 − 14]等多个地区. 受地质因素等影响,傍河型地下水是我国北方地区较为普遍的一种饮用水水源地类型,其水质水量受临近河道和地下径流补充双重影响. 海拉尔河沿线傍河型地下水源中砷浓度普遍较高,满洲里地区作为我国最大的口岸城市,长期采用傍河地下水的取水方式[15 − 16],地下水源中砷的健康风险状况广受关注.
地下水含水层中的砷通常会吸附在含氧金属矿物表面,通过微生物参与的氧化还原、竞争吸附和络合反应等特殊的地球化学过程,释放并富集在水体中[17 − 19]. 来自于地下水含水层本身和地表水输入的溶解性有机质(DOM),均可作为重要的能源物质和电子供体,参与地下水中砷的释放[20 − 22]. 据研究报道,DOM作为氧化还原反应中的主要能量提供物质[23],使氧化态的金属离子和砷接受DOM释放的电子,转化成还原态[17]. 而DOM中的有机酸,诸如富里酸和胡里酸均会与三价砷和五价砷竞争地下水矿物质的有效吸附点位,导致含铁矿物表面的砷解吸,从而释放到水体中,尤其在偏酸性环境中,砷的溶解性会增强[24 − 26]. 除此之外,DOM对金属离子和氧化物具有络合作用,可以和金属矿物表面的砷形成可溶解的络合物,促进砷的释放,其中铁、铝、锰等金属离子均可以充当DOM和五价砷的桥联作用物质[27 − 29]. 所以,在高砷地下水的形成过程中,DOM对微生物一系列反应均起到了显著的促进作用.
刘韩等对内蒙古河套平原砷的富集机制进行了较为全面的解析[5]. 满洲里市作为以傍河地下水为饮用水源的特殊地区,其近年来砷浓度升高的原因及其机制还鲜见报道. 本研究通过对满洲里地区地下水取水井及海拉尔河沿线地表水进行DOM三维荧光光谱的测量,基于平行因子分析法对其光谱图进行解译,以期解析地下水中溶解性有机物质的主要类型,探究傍河地下水水井中砷的富集机制及近年地下水砷浓度升高的原因,旨在对海拉尔河沿线地下水取水地区的饮用水安全保障策略的制定提供依据.
-
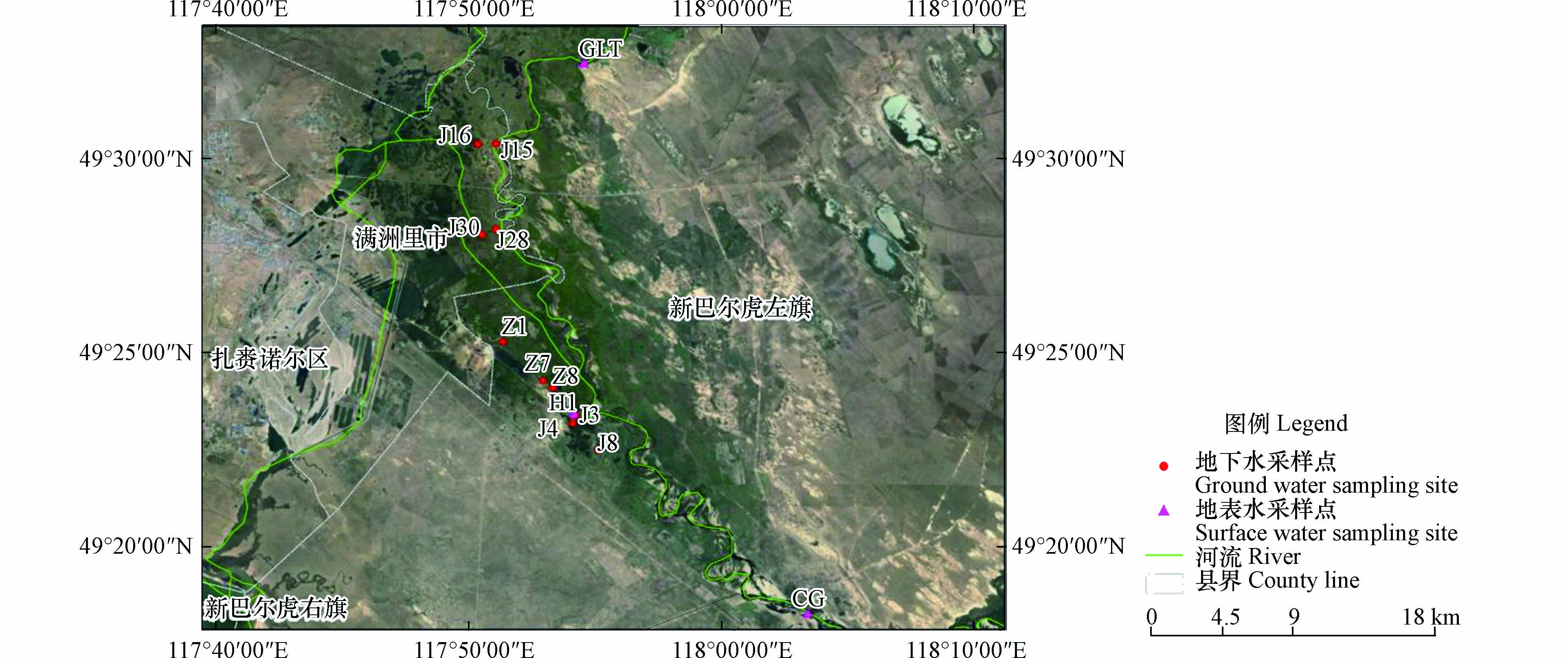
满洲里市位于内蒙古自治区呼伦贝尔市西部,东经117°12′—117°53′,北纬49°19′—49°41′,处于呼伦贝尔大草原和大兴安岭边缘过渡地带,主要地形为波状起伏的丘陵. 属中温带大陆性草原气候,年降水量在300 mm左右,主要集中在7月至8月. 海拉尔河在满洲里境内长度为12 km,面积约38.8 km2,占全市总面积5.34 %,年平均径流量30亿m3. 其干流区属于第四系冲积含水层分布的河滩地,岩性由粉砂、细砂和砂砾石组成,透水性良好. 其在满洲里市东湖区北部阿巴该图山以南分成两支,主流在阿巴该图山脚下转向东北,改称额尔古纳河[30].
满洲里市地下水水源地位于东经117°47′59′′—117°54′41′′,北纬49°26′55′′—49°30′44′′,所处大地构造位置为额尔古纳兴凯褶皱系与内蒙古—大兴安岭华力西褶皱系的交接部位,一级构造单元属兴安地槽褶皱系,二级构造单元属喜桂图旗中华力西期地槽褶皱带,三级构造单元属满洲里复、背斜. 满洲里市的地下水主要有第四纪孔隙潜水、基岩裂隙潜水和深层结构裂隙水,多分布在山间洼地、山丘底部及河谷滩地. 研究区地下水主要补给来源为大气降水入渗补给、邻区地下水侧向补给、地表水体补给和第四系冲积含水层垂直补给煤系地层风化裂隙含水层,含水层为孔隙潜水、介质为细砂,水位埋深30 m. 主要排泄于地面蒸发、海拉尔河、矿坑疏干排泄地下水及人工开采等[31].
-
为研究满洲里傍河地下水型饮用水水源地高砷成因,于2020年7月在研究区采集16组样品. 包括位于海拉尔河的地表水采样点4个(GLT、CG、H1、H2),地下水井12个(Z1、Z7、Z8、J3、J4、J8、J15、J16、J23、J24、J28、J30)(图1).
-
采集地表水样品时,润洗采水器及采样瓶3次后,采水器深入河水水面50 cm以下进行采集,装瓶保存. 采集地下水样品时,提前进行排放,以排除井管中长期滞留的地下水,保证抽取的地下水样品质量. 现场使用YSI多参数分析仪(YSI ProDss,USA)测试地表水和地下水温度、pH、溶解氧(DO)、总溶解性固体(TDS)、氧化还原点位(ORP)、电导率(EC)等现场指标.
现场用0.45 μm纤维滤膜过滤水样,用于实验室直接测试阴离子待用;经优先级纯硝酸酸化至pH< 2.0 保存,用于实验室测量阳离子和微量元素;经浓硫酸酸化后4 ℃冷藏,用于实验室测试溶解性有机碳(DOC)和DOM荧光分析.
利用滴定法测试HCO3−. As和Fe采用等离子体发射光谱仪(ICP﹣OES)(iCAP 7600,Thermo Fisher Scientific,美国)进行测定.
-
三维荧光光谱(excitation-emission-matrix spectra,EEM)采用分光光度计(DR6000,美国HACH)进行测试. 其中,激发波长(λEx)范围为200—450 nm,间隔为5 nm;发射波长(λEm)范围为250—550 nm,间隔为5 nm. 同时通过分别扣除 λEx < λEx+20 nm 和 λEx > 2 λEx−20 nm 区域,扣除一级和二级瑞利散射对荧光光谱的影响,被扣除部分均为零. 另外,为了纠正拉曼散射峰影响,利用R语言的DOMFlour扣除超纯水荧光信号,并使用对校正后的三维荧光光谱数据进行平行因子分析(PARAFAC).
对腐殖化指数(HIX)、生物指数(BIX)、荧光指数(FI)[32]指示参数进行计算. HIX是在254 nm激发波长,发射波长在435—480 nm与300—345 nm内峰面积的比值. HIX值越高则说明DOM的腐殖化程度越高[33]. 生物指数(BIX)是在310 nm激发波长下,发射波长分别为380 nm和430 nm的荧光强度值之比,表征DOM为微生物来源或外源. BIX大于0.8代表自生源特征明显,小于0.8为自生源特征不明显,受人为或陆源影响较大[34 − 35]. 荧光指数FI是在370 nm 激发波长下,发射波长分别为470 nm 和520 nm 的荧光强度的比值,可以表征溶解性有机物的来源. FI值较低(约1.3—1.4)表明是陆源有机物,而较高的FI值(约1.7—1.9)则代表了微生物代谢来源[32].
-
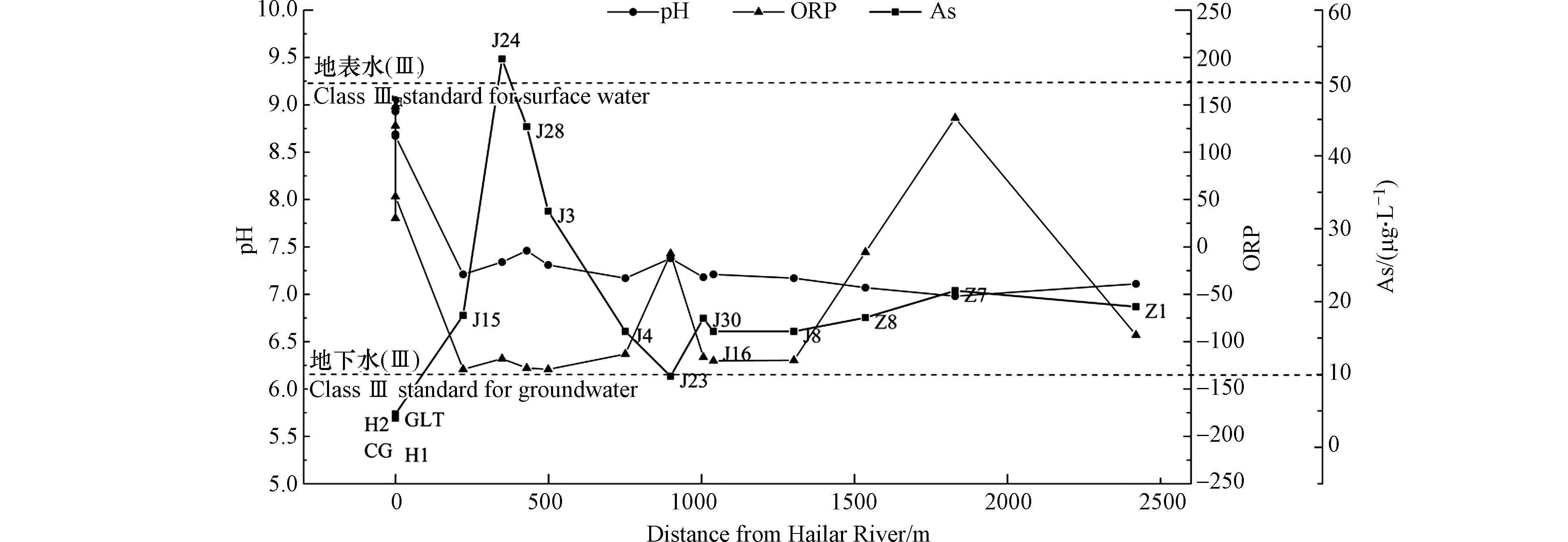
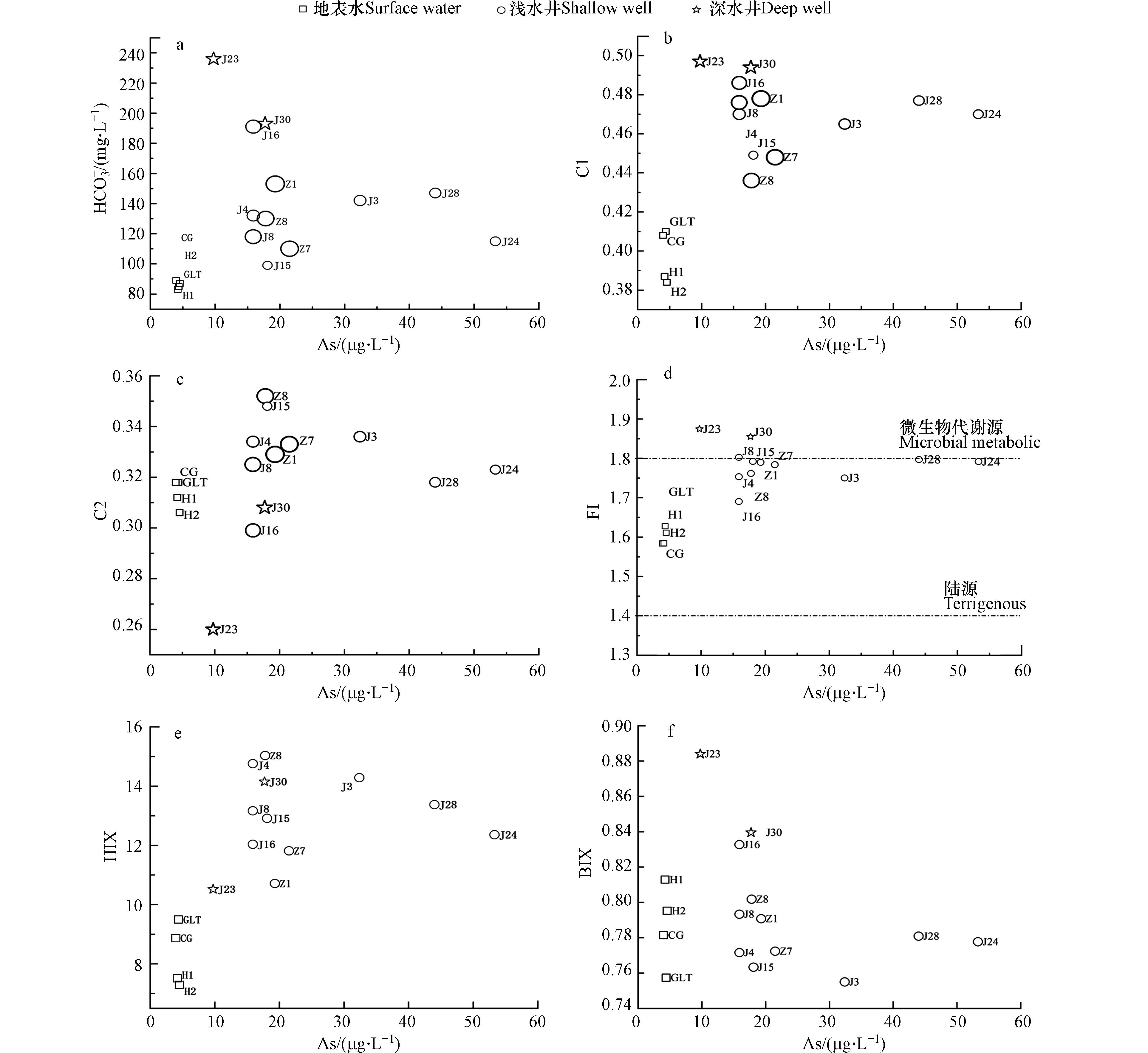
As的检出情况如表1所示,其中位于海拉尔河的地表水采样点4个(GLT、CG、H1、H2),地下水井12个(Z1、Z7、Z8、J3、J4、J8、J15、J16、J23、J24、J28、J30),所有样品As的总浓度范围介于 3.98—53.3 µg·L−1. 地表水As的浓度范围介于 3.98—4.57 µg·L−1,平均浓度为4.30 µg·L−1,海拉尔河自上游至下游4个采样点无明显差异,整体处于较低水平,均未超过Ⅲ类地表水限值(≤ 0.05 mg·L−1). 地下水As的浓度范围介于9.75—53.3 µg·L−1,平均浓度为23.46 µg·L−1,整体均处于较高水平,超标率为83.33 %. 其中仅J23的As浓度接近Ⅲ类地下水限值,为9.75 µg·L−1,其余监测点位均超过Ⅲ类地下水标准限值(≤ 0.01 mg·L−1),J24的As浓度为所有点位中最高,已超过Ⅴ类地下水水质标准(>0.05 mg·L−1). 从地下水井不同井深的As的浓度范围来看,浅水取水井(30—54 m)As的浓度范围介于15.9—53.3 µg·L−1,浅层承压水水井(136 m)As的浓度范围介于9.75—17.7 µg·L−1,浅水取水井As浓度较高于承压水井. 所有地下水井均处于还原条件(ORP ≤ −7),但J23还原环境较弱,其As的浓度较低,分析认为其受地表水的补给渗透影响较大. 距离海拉河较近的J24、J28的As浓度高于其他点位,浓度为53.3 µg·L−1和44 µg·L−1,两井距离较近,虽然两点的pH值和氧化还原电位与其他地下水井无明显差异,井深均处于35—40 m,但近河岸带易形成铁氧化物\氢氧化物聚集带,阻止地下水中的砷向地表水中排泄[36].
地下水中砷含量与pH值、ORP之间均呈现负相关关系(图2). pH越偏碱性,地下水中砷浓度降低,含水介质对砷的吸附能力会加强. 在水环境中,砷的存在形式主要为砷酸根和亚砷酸根,亚砷酸盐(H3AsO3)是一种具有很小解离系数的弱酸,随着pH的升高,H3AsO3逐渐解离生成大量H2AsO3−,H2AsO3−易被带正电荷的氧化物表面吸收,导致地下水中砷含量降低. As与 ORP 呈负相关,说明氧化还原环境对于As的活化起限制性作用,还原性越强,砷浓度越高. 在还原条件下,吸附砷的胶体变活跃,铁锰氧化物/氢氧化物更容易发生还原性溶解,导致吸附在矿物或晶格表面的砷释放到地下水中.
-
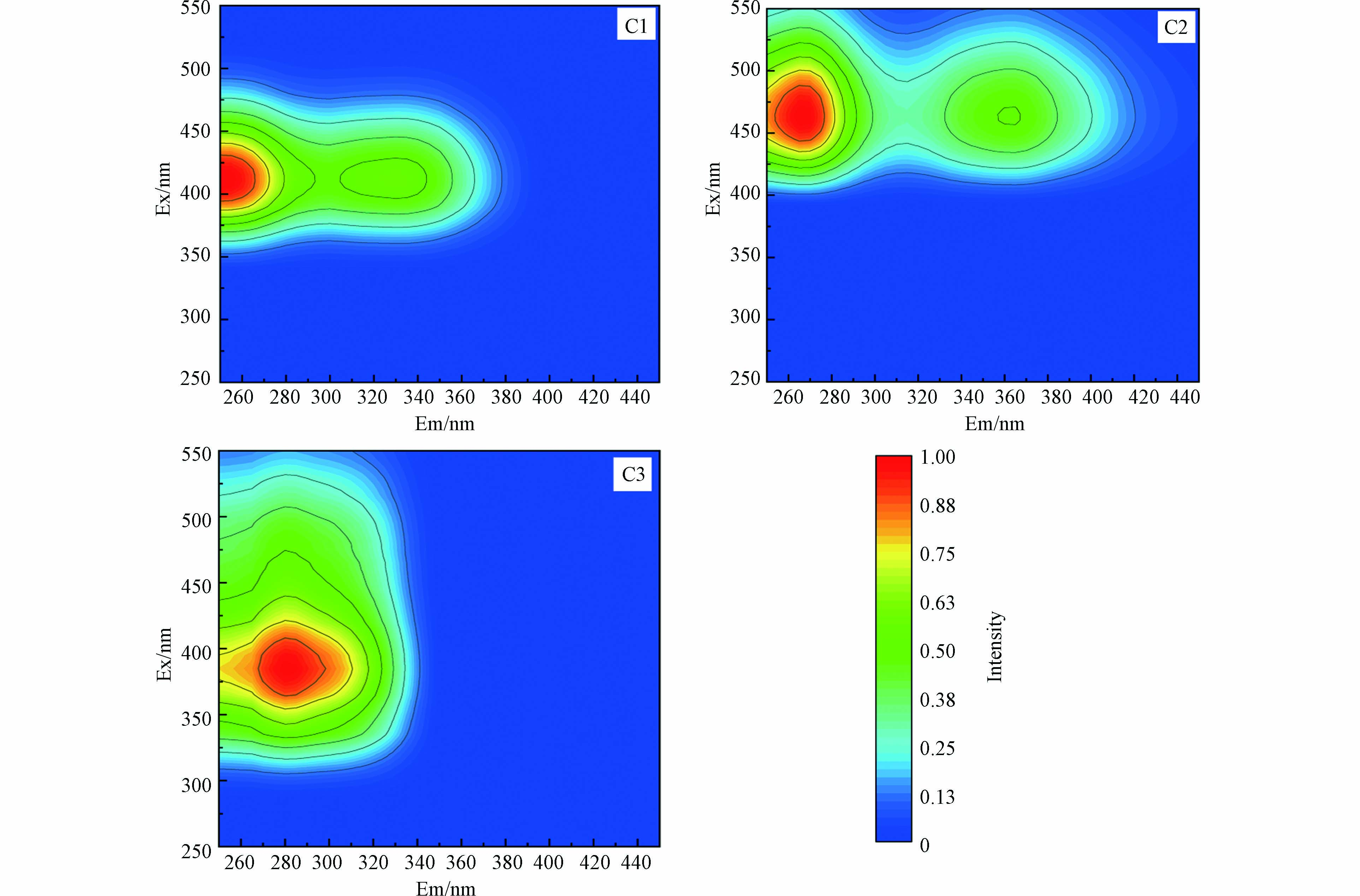
运用PAEAFAC模型,对16组水样进行了DOM三维荧光解析,所有样品均获取3个有效荧光组分(C1、C2、C3)(图3). 组分C1在250 nm/410 nm处具有单一激发峰和发射峰,C2在265(365) nm/465 nm具有两个激发峰和一个发射峰,C3在280 nm/385 nm具有单一激发峰和发射峰. C1为陆源类腐殖质,是天然水体中长期存在的组分,为传统A峰(λEx/λEm =237—260 nm/400—500 nm),该组分反映了紫外可见类腐殖质的荧光特性,容易与金属离子反应生成金属离子-有机物络合物,影响自然水环境的DOM荧光特征[37 − 38]. C2为类醌物质,是陆源水生环境中的微生物来源类腐殖质[39],C3为微生物来源腐殖质,接近于传统T峰(λEx/λEm =260—280 nm/320—360 nm),T峰表征该组分在水环境有机质中含有部分原位DOM,表明该组分为自源微生物活动的产物,与DOM中的芳环族氨基酸结构有关[40]. 3种组分的发射峰均大于380 nm,说明满洲里地区与多环芳烃类荧光团的联系较为密切[41].
根据地表水以及不同深度地下水样品中的最大荧光强度(Fmax)计算每个样品中各个组分的相对含量(图4). 在所有样品中,C1组分占比最高,为38.4%—49.7%,其中地表水相对含量小于地下水,分析认为由于在丰水期地表水的径流速度比地下水的渗透速度更快,并且陆源类腐殖质在深水井的含量高于浅水井,说明随着井深以及时间的增长,陆源有机物累积程度随之增大. C2组分在浅水井含量更高,微生物来源的类腐殖质组分更容易受到人为因素的影响,相比深水井,浅水井更容易被人为因素影响. C3组分随水位深度得降低而降低,但在深水井中有所回升.
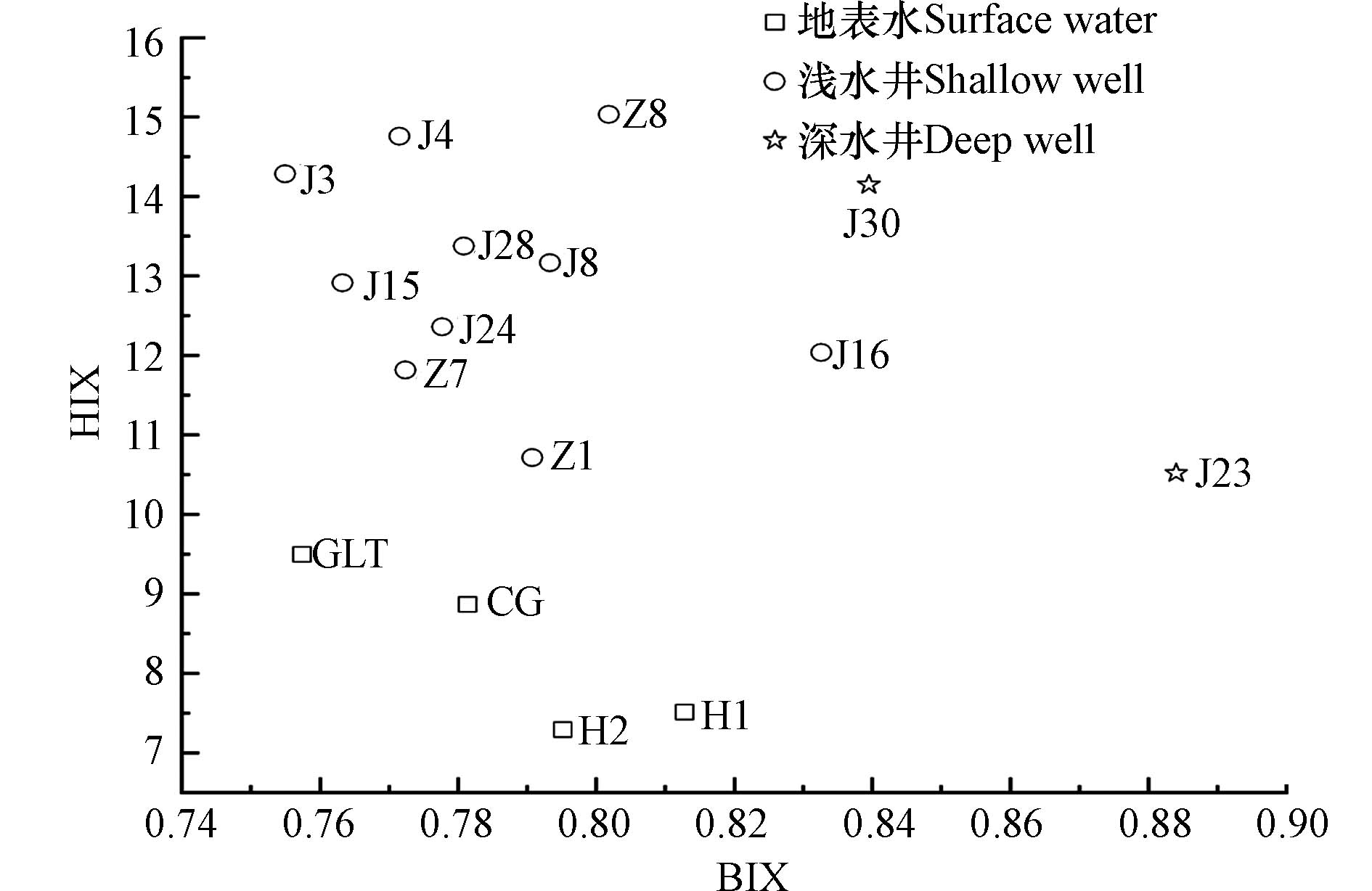
所有样品中腐殖化指数HIX均处于偏高水平(HIX>4),其中地表水腐化程度最低,HIX值在7.29—9.49(平均值为8.29),浅水井的HIX值在10.71—15.03(平均值为13.04),深水井的HIX值在10.52—14.15(平均值为12.33). 地下水井中的有机质腐化程度受其氧化还原环境的影响而发生波动[42],其中J23靠近于海拉尔河,虽然为承压水取水井,受到与地表水相互补给渗透的影响,有机质腐化程度偏低. 生物指数BIX值在0.75—0.88(平均值为0.79),值在0.8上下浮动,表明满洲里地区的有机质受自生源和外源两方面的同时影响. 且地表水与地下水BIX值无明显差异,但不同水体的HIX与BIX的关系具有明显的差异性(图5),说明傍河地下水井与地表水具有明显的交互作用.
地表水体中荧光指数FI值范围在1.58—1.64(平均值为1.60),地下水体FI范围在1.69—1.87(平均值为1.79),其中深水井FI值均达到1.8以上,说明满洲里地区DOM多为混合源,但地表水以陆源为主,陆源类有机物对地表水中As的迁移转化起到更为重要的作用,而地下水以微生物源为主,微生物源有机物在其代谢作用下参与了As的释放,起到更为重要的作用.
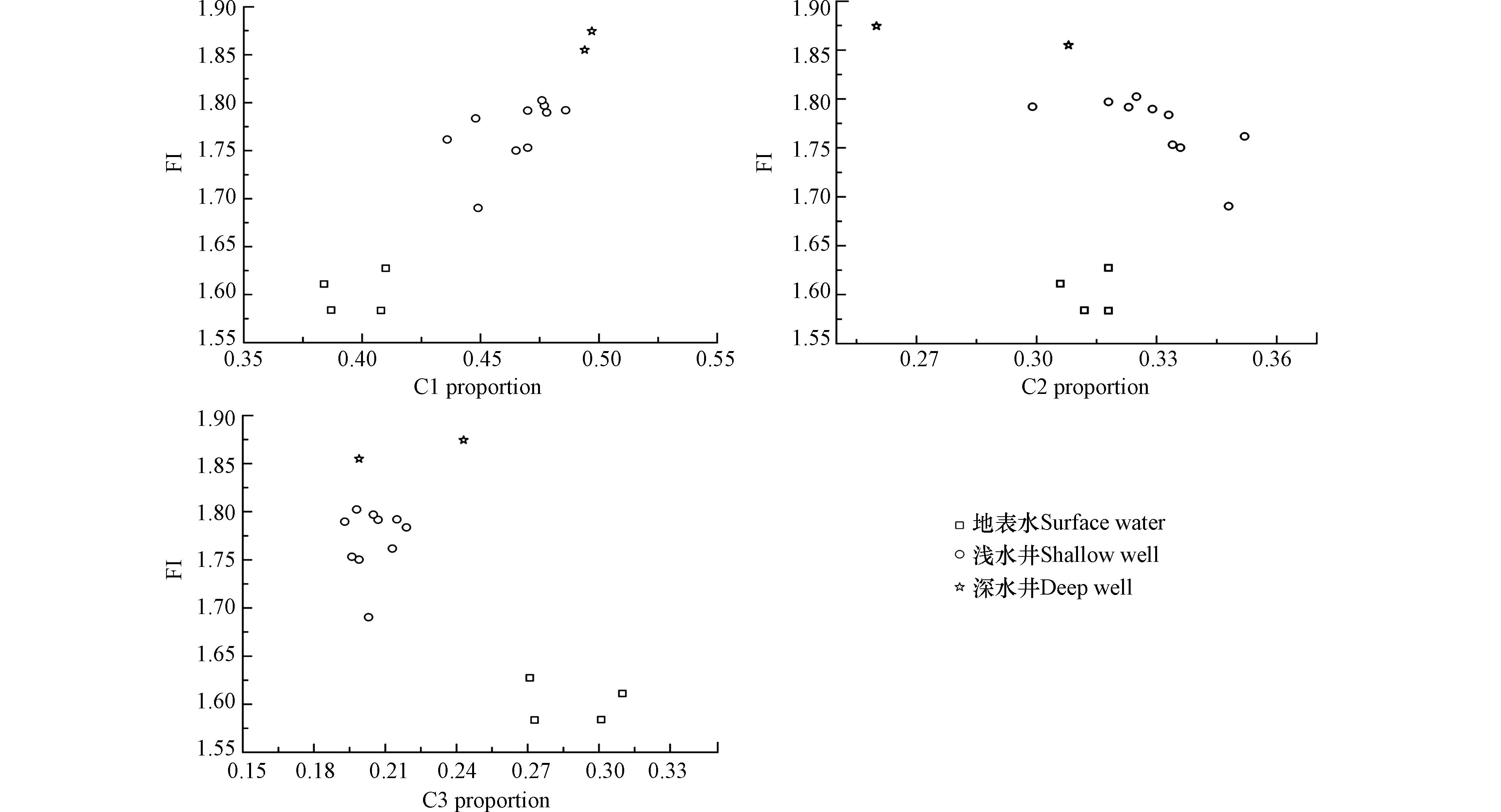
对不同组分所占比例与FI荧光指数之间的关系进行分析发现(图6),C1组分与FI之间随着水体的深度,呈现明显的正相关关系,C2组分未呈现明显的相关性,而C3组分与FI呈现负相关关系. 说明腐质化程度越高,地下水中陆源类腐殖质成分越多,越接近陆源,而微生物代谢成分越少.
-
地下水中的As在地壳表层循环过程中发生的一种环境水文地球化学现象,特定的地质、地理背景和环境条件均与As的迁移转化有关,地下水的水化学特征是影响地下水中砷的浓度的重要因素[43 − 44]. 满洲里地区地下水源As在碱性-还原的环境中,导致吸附在矿物表面或晶格里的砷更容易释放到地下水中. 在含水介质中,铁氧化物通常作为As的主要载体,有机物的存在会使铁氧化物溶解加快,同时有机质被氧化成重碳酸根[45 − 46]. 在本研究中,As与铁之间相关性均较弱,但在不同深度的水体中,HCO3−与As之间呈现不同关系(图7a),在深水井中HCO3− 浓度更高,As浓度越小,而在浅水井中,由于地下水井距离海拉尔河远近不一,其受HCO3−影响程度不同.
DOM是微生物介导下铁氧化物As释放的主要因素之一,本研究中,浅水井As的浓度水平随井深增加,C1荧光组分的浓度随As浓度升高而升高,3种荧光组分占比程度随井深的变化逐渐产生了差异性,(图7b),有研究表明溶解性有机物以多种形式促进砷在地下水中的迁移转化[47],C1主要通过络合作用提高As的溶解性. 类醌组分C2可以作为电子飞行物,穿梭于易分解的DOM、Fe和As之间,对微生物的氧化还原作用起传递作用(图7c). 不同深度下DOM的荧光特性不同,对砷的富集释放的影响也不同,从FI、HIX和BIX与As浓度间的关系图可以看出,不同深度的水体受到了陆源腐殖质不同程度的影响,在浅水井中As浓度虽然升高,但FI指数并未产生明显升高(图7d),说明地表水和浅层地下水中As的主要来源较为一致,均为混合来源,但深层地下水中的As则为陆源腐殖质来源更多. 在深水井中As与HIX呈现正相关性(图7e),而与BIX呈负相关性(图7f),反映了As的迁移转化起到重要作用的是腐殖化程度较高、难以被微生物利用的溶解性有机物. 主要组分为陆源类有机物,微生物利用内源碳降解有机质,促进地下水中砷的释放. 地下水中溶解性有机质为混合来源,微生物源有机质占据主导地位. 由于满洲里地区本身的岩层状况,地下水中微生物发生强烈的内源反应,加之外源环境变化影响,共同导致该地区地下水中砷的富集.
-
(1)满洲里地下水源中As的含量范围在9.75—53.3 µg·L−1,地下水均处于偏还原-弱碱性环境.
(2)该地区水体中DOM有3种组分:C1陆源类腐殖质、C2陆源水生环境中的微生物来源的类腐殖质类醌、C3微生物代谢产物. 其中地表水及地下水中均为C1组分占比最大,达到38.4%—48.7%.
(3)该地区地下水的HIX值偏高,腐殖化程度严重,陆源类腐殖质在深层井中更多,FI值则表明地表水多为陆源有机物,浅水井为混合源有机物,深水井为微生物源有机物.
(4)满洲里地区DOM通过多种方式促进砷在地下水中的富集,主要通过陆源类有机物和微生物利用内源有机碳共同作用,促使As在地下水中的富集和解吸.
海拉尔河典型傍河型地下水源砷的富集机制与溶解性有机物三维荧光特性解析
The enrichment mechanisms of arsenic and three-dimensional fluorescence spectroscopy characteristics of dissolved organic matters in typical riverside groundwater sources of the Hailar River
-
摘要: 傍河型地下水是我国北方地区较为普遍的一种饮用水水源地类型,其水质水量受临近河道和地下径流补充双重影响. 海拉尔河沿线傍河型地下水源中砷浓度较高,备受关注. 溶解性有机质(dissolved organic matter,DOM)是影响地下水中砷释放的主要因素,为此,本研究对海拉尔河和沿线地下水水源中砷(As)的含量和DOM进行调查监测,展开三维荧光光谱分析,利用平行因子法确定水体的有机物组分,对探究砷的富集机理具有重要意义. 结果表明,满洲里水体中DOM包括含陆源类腐殖质(C1)、类醌化合物(C2)和微生物来源的腐殖质(C3)的3种组分. 傍河地下水源的腐殖化程度高于地表河流,且浅水井多为陆源和微生物源混合来源有机物. 有机质通过多种方式影响地下水中砷的浓度,主要为C1通过络合方式促进溶解性砷浓度升高,C2则穿梭于易分解的DOM、Fe和As之间,对微生物的氧化还原作用起传递作用. 综上,在陆源类有机物和微生物利用内源有机碳的共同作用下,使As在地下水中的浓度逐年升高.Abstract: Riverside groundwater is a common type of drinking water source in northern China, and its water quality and quantity are affected by both neighboring river courses and groundwater runoff supplement. Arsenic concentration in riverside groundwater along the Hailar River is high, which has attracted much attention. Dissolved organic matter (DOM) is a key factor of arsenic release in groundwater. This study investigated and monitored the arsenic content and DOM of the Hailar River and groundwater sources along the line, carried out three-dimensional fluorescence spectrum analysis, determined the organic matter composition in water body by parallel factor method, which was of great significance to explore the enrichment mechanism of arsenic. The results showed that the DOM in the water of Manzhouli included three components, C1 was terrestrial humic-like; C2 was semiquinone-like; C3 was microbial humic. The degree of humification in groundwater was relatively higher, and most shallow wells were organic matters of mixed source from terrestrial and microbial sources. Organic matters affect the concentration of As in groundwater in a variety of ways. The main reason was that C1 promoted the increase of soluble arsenic concentration through complexation; and C2 shuttled to enhance microbial oxidation and reduction between DOM, Fe and As. In summary, the concentrations of As in groundwater increased year by year due to the simultaneous use of endogenous organic carbon by terrestrial organisms and microorganisms.
-

-
表 1 满洲里地表水和地下水主要理化指标和荧光指数
Table 1. Statistics of surface water and groundwater physical-chemistry in the Manzhouli area
样品名称
Sample类型
Water types井深/m
Well depth与海拉尔河距离/m
Distance from
Hailar RiverAs/
(µg·L−1)pH ORP/
mVMn/
(µg·L−1)Fe/
(µg·L−1)DOC/
(mg·L−1)FI HIX BIX GLT 地表水 0 0 4.40 8.69 127.9 120 64.5 3.02 1.63 9.49 0.76 CG 地表水 0 0 3.98 8.93 148.3 88 77.3 3.01 1.58 8.87 0.78 H1 地表水 0 0 4.23 9.05 30.3 45 77.3 3.35 1.58 7.51 0.81 H2 地表水 0 0 4.57 8.67 53.1 56 21.3 2.98 1.61 7.29 0.80 Z1 浅水井 30 2420 19.3 7.11 −93.1 352 30.3 4.12 1.79 15.03 0.80 Z7 浅水井 30 1829 21.5 6.98 −136.1 293 88.8 3.47 1.78 10.71 0.79 Z8 浅水井 30 1536 17.8 7.07 −5.5 464 164 2.97 1.76 11.82 0.77 J16 浅水井 30.3 1039 15.9 7.21 −120.2 711 32.2 5.31 1.79 12.04 0.83 J15 浅水井 31.5 221 18.1 7.21 −129.2 296 36.8 3.48 1.69 12.91 0.76 J24 浅水井 36.9 348 53.3 7.34 −118 204 18.44 3.35 1.79 12.36 0.78 J28 浅水井 39.8 429 44 7.46 −127.9 254 4.47 4.28 1.80 13.37 0.78 J3 浅水井 54 499 32.4 7.31 −129.3 324 14.1 3.82 1.75 14.28 0.75 J4 浅水井 54 751 15.9 7.17 −113.1 679 25.2 3.61 1.75 14.75 0.77 J8 浅水井 38.4 1302 15.9 7.17 −119.7 570 57.1 3.75 1.80 13.16 0.79 J23 深水井 136 899 9.75 7.38 −7.0 119 16.4 4.51 1.87 10.52 0.88 J30 深水井 136 1006 17.7 7.18 −116.4 606 36.7 5.17 1.85 14.15 0.84 -
[1] FENDORF S, MICHAEL H A, van GEEN A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia[J]. Science, 2010, 328(5982): 1123-1127. doi: 10.1126/science.1172974 [2] 国家市场监督管理总局, 国家标准化管理委员会. 生活饮用水卫生标准: GB 5749—2022[S]. 北京: 中国标准出版社, 2022. State Administration for Market Regulation, Standardization Administration of the People's Republic of China. Standards for drinking water quality: GB 5749—2022[S]. Beijing: Standards Press of China, 2022(in Chinese).
[3] 吴丰昌, 王立英, 黎文, 等. 天然有机质及其在地表环境中的重要性[J]. 湖泊科学, 2008, 20(1): 1-12. doi: 10.18307/2008.0101 WU F C, WANG L Y, LI W, et al. Natural organic matter and its significance in terrestrial surface environment[J]. Journal of Lake Sciences, 2008, 20(1): 1-12 (in Chinese). doi: 10.18307/2008.0101
[4] WANG Y X, PI K F, FENDORF S, et al. Sedimentogenesis and hydrobiogeochemistry of high arsenic Late Pleistocene-Holocene aquifer systems[J]. Earth-Science Reviews, 2019, 189: 79-98. doi: 10.1016/j.earscirev.2017.10.007 [5] 刘韩. 河套平原高砷地下水中异化铁还原菌对砷迁移转化的影响研究[D]. 武汉: 中国地质大学, 2021. LIU H. Arsenic mobilization affected by dissimilatory iron reducing bacteria from high arsenic groundwater in Hetao Basin[D]. Wuhan: China University of Geosciences, 2021 (in Chinese).
[6] 郭华明, 郭琦, 贾永锋, 等. 中国不同区域高砷地下水化学特征及形成过程[J]. 地球科学与环境学报, 2013, 35(3): 83-96. GUO H M, GUO Q, JIA Y F, et al. Chemical characteristics and geochemical processes of high arsenic groundwater in different regions of China[J]. Journal of Earth Sciences and Environment, 2013, 35(3): 83-96 (in Chinese).
[7] 鲁宗杰, 邓娅敏, 杜尧, 等. 江汉平原高砷地下水中DOM三维荧光特征及其指示意义[J]. 地球科学, 2017, 42(5): 771-782. LU Z J, DENG Y M, DU Y, et al. EEMs characteristics of dissolved organic matter and their implication in high arsenic groundwater of Jianghan plain[J]. Earth Science, 2017, 42(5): 771-782 (in Chinese).
[8] 韩莉, 甘义群, 于凯. 江汉平原高砷地下水中溶解性有机质来源的稳定碳同位素示踪研究[J]. 地质学报, 2015, 89(增刊1): 266-268. HAN L, GAN Y Q, YU K. Stable carbon isotope tracing study on the source of dissolved organic matter in high arsenic groundwater in Jianghan plain[J]. Acta Geologica Sinica, 2015, 89(Sup 1): 266-268 (in Chinese).
[9] 梁梦钰, 郭华明, 李晓萌, 等. 贵德盆地三河流域高砷地下水中溶解性有机物三维荧光特性及其指示意义[J]. 地学前缘, 2019, 26(3): 243-254. LIANG M Y, GUO H M, LI X M, et al. Excitation-emission matrix spectroscopic characteristics of dissolved organic matters and the significance in high arsenic groundwater research in the Guide Basin, China[J]. Earth Science Frontiers, 2019, 26(3): 243-254 (in Chinese).
[10] 关林瑞, 钱坤, 李俊霞, 等. 大同盆地地下水系统中碘迁移富集的生物标志物证据[J]. 地质科技情报, 2019, 38(1): 235-242. GUAN L R, QIAN K, LI J X, et al. Mobilization and enrichment of iodine in groundwater from the Datong Basin: Evidences from biomarker study[J]. Geological Science and Technology Information, 2019, 38(1): 235-242 (in Chinese).
[11] 张多, 谢先军, 刘文静, 等. 大同盆地地下水中溶解性有机质分子特征及其对砷迁移富集的影响[J]. 安全与环境工程, 2022, 29(5): 148-154. ZHANG D, XIE X J, LIU W J, et al. Molecular characteristics of dissolved organic matter and its impact on arsenic mobilization and enrichment in groundwater system in Datong Basin[J]. Safety and Environmental Engineering, 2022, 29(5): 148-154 (in Chinese).
[12] 王翔, 罗艳丽, 邓雯文, 等. 新疆奎屯地区高砷地下水DOM三维荧光特征[J]. 中国环境科学, 2020, 40(11): 4974-4981. WANG X, LUO Y L, DENG W W, et al. The 3D-EEM characteristics of DOM in high arsenic groundwater of Kuitun, Xinjiang [J]. China Environmental Science, 2020, 40(11): 4974-4981 (in Chinese).
[13] 王翔. 奎屯河下游区域地下水中砷的释放过程研究[D]. 乌鲁木齐: 新疆农业大学, 2021. WANG X. Mobilization processes of arsenic in groundwater of Kuitun River downsteam[D]. Urumqi: Xinjiang Agricultural University, 2021 (in Chinese).
[14] 袁翰卿, 李巧, 陶洪飞, 等. 新疆奎屯河流域地下水砷富集因素[J]. 环境化学, 2020, 39(2): 524-530. YUAN H Q, LI Q, TAO H F, et al. Groundwater arsenic enrichment factors of Kuitun River Basin, Xinjiang[J]. Environmental Chemistry, 2020, 39(2): 524-530 (in Chinese).
[15] 张坤锋, 昌盛, 付青, 等. 内蒙古东北部地区地下-地表饮用水源多环芳烃污染特征与风险[J]. 环境科学, 2022, 43(6): 3005-3015. ZHANG K F, CHANG S, FU Q, et al. Pollution characteristics and risks of polycyclic aromatic hydrocarbons in underground and surface drinking water sources in northeast Inner Mongolia[J]. Environmental Science, 2022, 43(6): 3005-3015 (in Chinese).
[16] 张坤锋, 赵少延, 孙兴滨, 等. 海拉尔河及傍河地下水饮用水源中挥发性有机物的污染特征与风险[J]. 河南师范大学学报(自然科学版), 2021, 49(5): 74-82. ZHANG K F, ZHAO S Y, SUN X B, et al. Pollution characteristics and risks of volatile organic compounds in drinking water sources of Hailar River and nearby rivers groundwater[J]. Journal of Henan Normal University (Natural Science Edition), 2021, 49(5): 74-82 (in Chinese).
[17] MLADENOV N, ZHENG Y, MILLER M P, et al. Dissolved organic matter sources and consequences for iron and arsenic mobilization in Bangladesh aquifers[J]. Environmental Science & Technology, 2010, 44(1): 123-128. [18] TUFANO K J, FENDORF S. Confounding impacts of iron reduction on arsenic retention[J]. Environmental Science & Technology, 2008, 42(13): 4777-4783. [19] 贾永锋, 郭华明. 高砷地下水研究的热点及发展趋势[J]. 地球科学进展, 2013, 28(1): 51-61. JIA Y F, GUO H M. Hot topics and trends in the study of high arsenic groundwater[J]. Advances in Earth Science, 2013, 28(1): 51-61 (in Chinese).
[20] HARVEY C F, SWARTZ C H, BADRUZZAMAN A B M, et al. Arsenic mobility and groundwater extraction in Bangladesh[J]. Science, 2002, 298(5598): 1602-1606. doi: 10.1126/science.1076978 [21] TUFANO K J, REYES C, SALTIKOV C W, et al. Reductive processes controlling arsenic retention: Revealing the relative importance of iron and arsenic reduction[J]. Environmental Science & Technology, 2008, 42(22): 8283-8289. [22] McARTHUR J M, RAVENSCROFT P, BANERJEE D M, et al. How paleosols influence groundwater flow and arsenic pollution: A model from the Bengal Basin and its worldwide implication[J]. Water Resources Research, 2008, 44(11): W11411. [23] HU X S, ZHOU Y Q, ZHOU L, et al. Urban and agricultural land use regulates the molecular composition and bio-lability of fluvial dissolved organic matter in human-impacted southeastern China[J]. Carbon Research, 2022, 1(1): 19. doi: 10.1007/s44246-022-00020-6 [24] SHARMA P, ROLLE M, KOCAR B, et al. Influence of natural organic matter on As transport and retention[J]. Environmental Science & Technology, 2011, 45(2): 546-553. [25] SAADA A, BREEZE D, CROUZET C, et al. Adsorption of arsenic (V) on kaolinite and on kaolinite-humic acid complexes. Role of humic acid nitrogen groups[J]. Chemosphere, 2003, 51(8): 757-763. doi: 10.1016/S0045-6535(03)00219-4 [26] BOWELL R J. Sorption of arsenic by iron oxides and oxyhydroxides in soils[J]. Applied Geochemistry, 1994, 9(3): 279-286. doi: 10.1016/0883-2927(94)90038-8 [27] REDMAN A D, MACALADY D L, AHMANN D. Natural organic matter affects arsenic speciation and sorption onto hematite[J]. Environmental Science & Technology, 2002, 36(13): 2889-2896. [28] LIN H T, WANG M C, LI G C. Complexation of arsenate with humic substance in water extract of compost[J]. Chemosphere, 2004, 56(11): 1105-1112. doi: 10.1016/j.chemosphere.2004.05.018 [29] 孟永霞, 程艳, 李琳, 等. 匹里青河夏季有色溶解性有机质(CDOM)分布特征及来源分析[J]. 环境化学, 2020, 39(11): 3213-3222. doi: 10.7524/j.issn.0254-6108.2019080902 MENG Y X, CHENG Y, LI L, et al. Distribution characteristics and source analysis of chromophoric dissolved organic matter(CDOM) in Piliqing River in summer[J]. Environmental Chemistry, 2020, 39(11): 3213-3222 (in Chinese). doi: 10.7524/j.issn.0254-6108.2019080902
[30] 严登华, 何岩, 邓伟, 等. 满洲里市水体中氟化物的环境特征[J]. 环境科学研究, 2001, 14(1): 24-26, 30. YAN D H, HE Y, DENG W, et al. The environmental characteristic of fluoride in the water body of Manzhouli city[J]. Research of Environmental Sciences, 2001, 14(1): 24-26, 30 (in Chinese).
[31] 张宝发. 满洲里市水文地质特征[J]. 吉林地质, 2013, 32(3): 77-79, 85. ZHANG B F. Hydrogeological characteristics of Manzhouli[J]. Jilin Geology, 2013, 32(3): 77-79, 85 (in Chinese).
[32] LAVONEN E E, KOTHAWALA D N, TRANVIK L J, et al. Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production[J]. Water Research, 2015, 85: 286-294. doi: 10.1016/j.watres.2015.08.024 [33] OHNO T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter[J]. Environmental Science & Technology, 2002, 36(4): 742-746. [34] 张紫薇, 周石磊, 张甜娜, 等. 岗南水库沉积物溶解性有机物光谱时空分布特征及环境意义[J]. 环境科学学报, 2021, 41(9): 3598-3611. ZHANG Z W, ZHOU S L, ZHANG T N, et al. Spatiotemporal evolution and environmental significance of dissolved organic matter (DOM) in sediments of Gangnan Reservoir[J]. Acta Scientiae Circumstantiae, 2021, 41(9): 3598-3611 (in Chinese).
[35] HUGUET A, VACHER L, RELEXANS S, et al. Properties of fluorescent dissolved organic matter in the Gironde Estuary[J]. Organic Geochemistry, 2009, 40(6): 706-719. doi: 10.1016/j.orggeochem.2009.03.002 [36] 鹿帅. 傍河开采驱动下地下水中砷的生物地球化学过程与模拟研究[D]. 长春: 吉林大学, 2018. LU S. Biogeochemical process of arsenic in groundwater and its simulation affected by groundwater exploitation in riverside[D]. Changchun: Jilin University, 2018 (in Chinese).
[37] CORY R M, McKNIGHT D M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter[J]. Environmental Science & Technology, 2005, 39(21): 8142-8149. [38] STEDMON C A, MARKAGER S, BRO R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy[J]. Marine Chemistry, 2003, 82(3/4): 239-254. [39] YAMASHITA Y, KLOEPPEL B D, KNOEPP J, et al. Effects of watershed history on dissolved organic matter characteristics in headwater streams[J]. Ecosystems, 2011, 14(7): 1110-1122. doi: 10.1007/s10021-011-9469-z [40] OSBURN C L, HANDSEL L T, MIKAN M P, et al. Fluorescence tracking of dissolved and particulate organic matter quality in a river-dominated estuary[J]. Environmental Science & Technology, 2012, 46(16): 8628-8636. [41] YAMASHITA Y, TANOUE E. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids[J]. Marine Chemistry, 2003, 82(3/4): 255-271. [42] 王振. 青海贵德盆地高砷地下水分布和成因探究[D]. 北京: 中国地质大学(北京), 2019. WANG Z. Distribution and genesis mechanism of high arsenic groundwater in the guide basin, Qinghai[D]. Beijing: China University of Geosciences, 2019 (in Chinese).
[43] ISLAM F S, GAULT A G, BOOTHMAN C, et al. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments[J]. Nature, 2004, 430(6995): 68-71. doi: 10.1038/nature02638 [44] 王焰新, 苏春利, 谢先军, 等. 大同盆地地下水砷异常及其成因研究[J]. 中国地质, 2010, 37(3): 771-780. WANG Y X, SU C L, XIE X J, et al. The genesis of high arsenic groundwater: A case study in Datong Basin[J]. Geology in China, 2010, 37(3): 771-780 (in Chinese).
[45] NICKSON R T, McARTHUR J M, RAVENSCROFT P, et al. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal[J]. Applied Geochemistry, 2000, 15(4): 403-413. doi: 10.1016/S0883-2927(99)00086-4 [46] AL LAWATI W M, RIZOULIS A, EICHE E, et al. Characterisation of organic matter and microbial communities in contrasting arsenic-rich Holocene and arsenic-poor Pleistocene aquifers, Red River Delta, Vietnam[J]. Applied Geochemistry, 2012, 27(1): 315-325. doi: 10.1016/j.apgeochem.2011.09.030 [47] POSTMA D, LARSEN F, MINH HUE N T, et al. Arsenic in groundwater of the Red River floodplain, Vietnam: Controlling geochemical processes and reactive transport modeling[J]. Geochimica et Cosmochimica Acta, 2007, 71(21): 5054-5071. doi: 10.1016/j.gca.2007.08.020 -



 下载:
下载: