-
全氟烷基羧酸(perfluoroalkyl carboxylates,PFCAs)是一类合成有机物,被广泛应用于防水织物、生物材料及泡沫灭火剂等[1]. PFCAs在环境介质中被广泛检出,如地表水[2]、地下水[3]、雨水[4]、土壤[5]、大气[6]、沉积物[7]等. 由于具有持久性、生物累积性和高毒性,PFCAs的污染问题已引起广泛关注:3M公司于2000年开始逐步停止使用碳原子数为8的全氟化合物;2006年美国环境保护局将全氟辛烷羧酸(perfluorooctanoic acid,PFOA)列为“可能”或“疑似”致癌物;2019年PFOA及其相关盐类被列入《关于持久性有机污染物的斯德哥尔摩公约》附件A.
随着全氟化合物逐渐被禁用,其他全氟和多氟化合物正在逐渐替代传统的PFOA,如短链PFCAs、氟调醇(fluorotelomer alcohols,FTOHs),全氟醚羧酸(perfluoroether carboxylic acids,PFECAs),氟调酸(fluorotelomer carboxylic acids,FTCAs)等. 其中,FTCAs是一类重要的多氟化合物,也是一类工业品[8]. 研究表明,FTCAs中的6:2 FTCA可作为PFOA的替代加工助剂[9]. 此外,研究发现另一类重要的含氟表面活性剂原料——FTOHs,在微生物作用下[10]及大气环境中[11]均可能转化为FTCAs,我们的前期工作已经发现FTOHs在城市污水处理厂污水污泥中普遍存在[12]. FTCAs被发现在雨水(0.12 ng·L−1)[13]、灰尘(n.d.(未检出)—0.85 ng·m−3,<0.1—26 ng·g−1)[14 − 15]和地表水(n.d.—35.2 ng·L−1)及沉积物(n.d.—23.6 ng·g−1)[16 − 17]等环境介质中存在. 近年来研究发现FTCAs对水蚤[18]、摇蚊幼虫[19]、端足虫[20]等水生生物具有急性毒性效应. 此外FTCAs能够转化为全氟烷基羧酸[21],也可能带来潜在的环境风险.
污水处理厂受纳生活污水及工业废水,部分出水排放进入地表水,是环境中污染物的源和汇. Gallen等[22]调研了2010—2020年澳大利亚某污水处理厂中6:2 FTCA和5:3 FTCA的浓度分布,进水中的平均浓度分别为<LOD(检出限)—100 ng·L−1和3.4—436 ng·L−1;Gremmel等[23]在德国某工业污水处理厂进水中检出FTCAs的浓度为0.98 μg·L−1—4.68 μg·L−1,出水中仍有1.37 μg·L−1—8.92 μg·L−1残留;Eriksson等[24]检测了2015年瑞典3座污水处理厂中5种FTCAs的浓度,进水及二沉池出水中的总浓度分别为0.5—3.5 ng·L−1和<LOD—0.4 ng·L−1. 中国是全氟化合物重要的生产和使用国,但目前FTCAs在中国城市污水处理厂中的分布及排放特征的研究和报道仍非常有限.
本研究调研了中国5个城市9座城市污水处理厂的进水、二沉池出水、三级处理出水及污泥中6种FTCAs和12种PFCAs的浓度分布、去除及排放特征,并利用风险商初步评估了出水中的潜在环境风险. 本研究将为我国城市污水处理厂中微量新污染物的管理提供数据基础.
-
实验试剂:6种FTCAs包括5:3 饱和氟调酸(5:3 fluorotelomer carboxylic acid,5:3 FTCA)、6:2 不饱和氟调酸(6:2 fluorotelomer unsaturated carboxylic acid,6:2 FTUCA)、6:2 饱和氟调酸(6:2 fluorotelomer carboxylic acid,6:2 FTCA)、7:3 饱和氟调酸(7:3 fluorotelomer carboxylic acid,7:3 FTCA)、8:2 不饱和氟调酸(8:2 fluorotelomer unsaturated carboxylic acid,8:2 FTUCA)和8:2 饱和氟调酸(8:2 fluorotelomer carboxylic acid,8:2 FTCA),12种PFCAs包括全氟丁烷羧酸(perfluorobutyric acid,PFBA)、全氟戊烷羧酸(perfluoropentanoic acid,PFPeA)、全氟己烷羧酸(perfluorohexanoic acid,PFHxA)、全氟庚烷羧酸(perfluoroheptanoic acid,PFHpA)、PFOA、全氟壬烷羧酸(perfluorononanoic acid,PFNA)、全氟癸烷羧酸(perfluorodecanoic acid,PFDA)、全氟十一烷羧酸(perfluoroundecanoic acid,PFUnDA)、全氟十二烷羧酸(perfluorododecanoic acid,PFDoDA)、全氟十三烷羧酸(perfluorotridecanoic acid,PFTriDA)、全氟十四烷羧酸(perfluorotetradecanoic acid,PFTeDA)、全氟十六烷羧酸(perfluorohexadecanoic acid,PFHxDA)以及11种同位素内标(13C4-PFBA、13C4-PFHxA、13C4-PFOA、13C4-PFNA、13C4-PFDA、13C4-PFUnDA、13C2-PFDoDA、13C2-6:2 FTCA、13C2-8:2 FTCA、13C2-6:2 FTUCA、13C2-8:2 FTUCA),纯度均>98%,购自加拿大Wellington Laboratories Inc.公司. 其他试剂包括:甲醇(色谱纯)、乙酸(优级纯)、氨水(HPLC级)、乙酸铵(HPLC级),超纯水(电阻率>18.2 MΩ·cm).
实验耗材:WAX固相萃取柱(6 cc,Waters公司)、有机相针式过滤器(13 mm,0.22 μm,CNW公司)、液相分析柱Acquity UPLC·BEH C18(2.1 mm×100 mm,1.7 μm,Waters公司)、C18预柱Waters ACUITY UPLC BEH(2.1 mm × 50 mm,1.7 μm,Waters公司).
实验仪器:Waters ACQUITY UPLCTM超高效液相色谱与Waters XEVOTM串联四极杆质谱联用仪(Waters公司).
-
2018—2022年对中国不同地区5个城市(北京、天津、青岛、开封、无锡)采用不同处理工艺的9座城市污水处理厂进行了调研,采集了进水、二沉池出水、三级处理出水和污泥样品. 利用SD900采样器(HACH公司)采集24 h混合样各5 L并置于聚丙烯瓶中,避光、干冰条件下运输,24 h内完成前处理,所有样品设置3个平行样. 选取的9座城市污水处理厂的生物处理工艺主要有:厌氧-缺氧-好氧(anaerobic-anoxic-oxic,A/A/O)、膜生物反应器(membrane bioreactor,MBR)及氧化沟(oxidation ditch,OD)工艺. 三级处理工艺主要为混凝沉淀过滤消毒、反渗透和臭氧工艺. 9座城市污水处理厂的详细信息及参数见表1.
-
FTCAs及PFCAs的污水污泥样品前处理方法参考文献[25]. 取500 mL过滤后水样加入25 μL的0.2 mg·L−1混合内标. 将样品以10 mL·min−1的流速通过活化后的WAX固相萃取柱,氮气吹干;用4 mL的0.5%氨水甲醇洗脱,氮气吹至0.5 mL,过滤后进行超高效液相色谱-三重四极杆串联质谱分析. 污泥样品冷冻干燥,研磨过筛后取0.1 g至50 mL聚丙烯离心管中,加入25 μL的0.2 mg·L−1混合内标,加入7.5 mL的1%乙酸水,60 ℃下超声20 min,3500 r·min−1离心10 min,收集上清液;再加入1.7 mL的90:10的甲醇:1%乙酸(体积分数)溶液,超声并离心后收集上清液;以上两个步骤重复两次,上清液合并,加入7.5 mL的1%乙酸水,过WAX固相萃取柱(同污水前处理步骤).
-
样品采用超高效液相色谱-三重四极杆串联质谱仪进行检测分析. 色谱流动相为甲醇(A)和5 mmol·L−1乙酸铵(B),流速0.2 mL·min−1,采用梯度分离. 初始流动相比例为甲醇10%,在6 min内升至65%,然后在7 min内升至75%,在11 min内升到100%,保持2 min后回到初始比例,平衡色谱柱4 min. 进样体积5 μL,柱温40 ℃,样品温度10 ℃. 质谱采用电喷雾离子源,采用负离子模式,毛细管电压为2 kV,脱溶剂气流量为600 L·h−1,锥孔气流量为50 L·h−1,源温度为150 ℃,脱溶剂气温度为350 ℃. 具体质谱参数见表2.
-
实验中所采用的器皿均为聚丙烯材质,使用前用甲醇润洗,实验采用内标法定量,目标化合物及内标对应关系见表2. 对采集的污水及污泥进行了加标回收率实验,FTCAs和PFCAs的加标量为5 ng. 目标物的检出限(limit of detection,LOD)定义为信噪比大于3的浓度,定量限(limit of quantitation,LOQ)定义为信噪比大于10的浓度. 6种FTCAs和12种PFCAs的回收率为75%—119%. 污水中LOD为0.01—0.11 ng·L−1,LOQ为0.01—0.38 ng·L−1;污泥中检测限为0.01—0.10 ng·g−1,定量限为0.02—0.35 ng·g−1.
-
城市污水处理厂三级处理出水及污泥中FTCAs及PFCAs排放量的计算见式(1):
式中,M(g·d−1)为污水处理厂三级处理出水及污泥中FTCAs或PFCAs的排放量;Cwater(ng·L−1)为污水处理厂三级处理出水中的FTCAs或PFCAs浓度;Qwater(m3·d−1)为污水处理厂日处理污水量;Csludge(ng·g−1 dw)为污水处理厂污泥中的FTCAs或PFCAs浓度;Qsludge(kg·d−1)为污水处理厂日处理干污泥量.
采用风险商(risk quotient)评估城市污水处理厂出水中FTCAs及PFCAs对鱼类的潜在环境风险,如式(2)所示:
式中,RQ为风险商;MEC为检测环境浓度(measured enrivonment concentration,MEC);PNEC为预测无效应浓度(predicted no effect concentration,PNEC),用50%效应浓度(EC50)、50%致死浓度(LC50)或最低观察效应浓度(LOEC)与评价因子(AF)的比值计算,此处AF值取1000. 本研究中的毒性数据来自美国EPA ECOTOX数据库(https://cfpub.epa.gov/ecotox/). 当RQ < 0.1时,为低风险;当0.1 ≤ RQ < 1时,为中风险;当RQ ≥ 1时,为高风险.
-
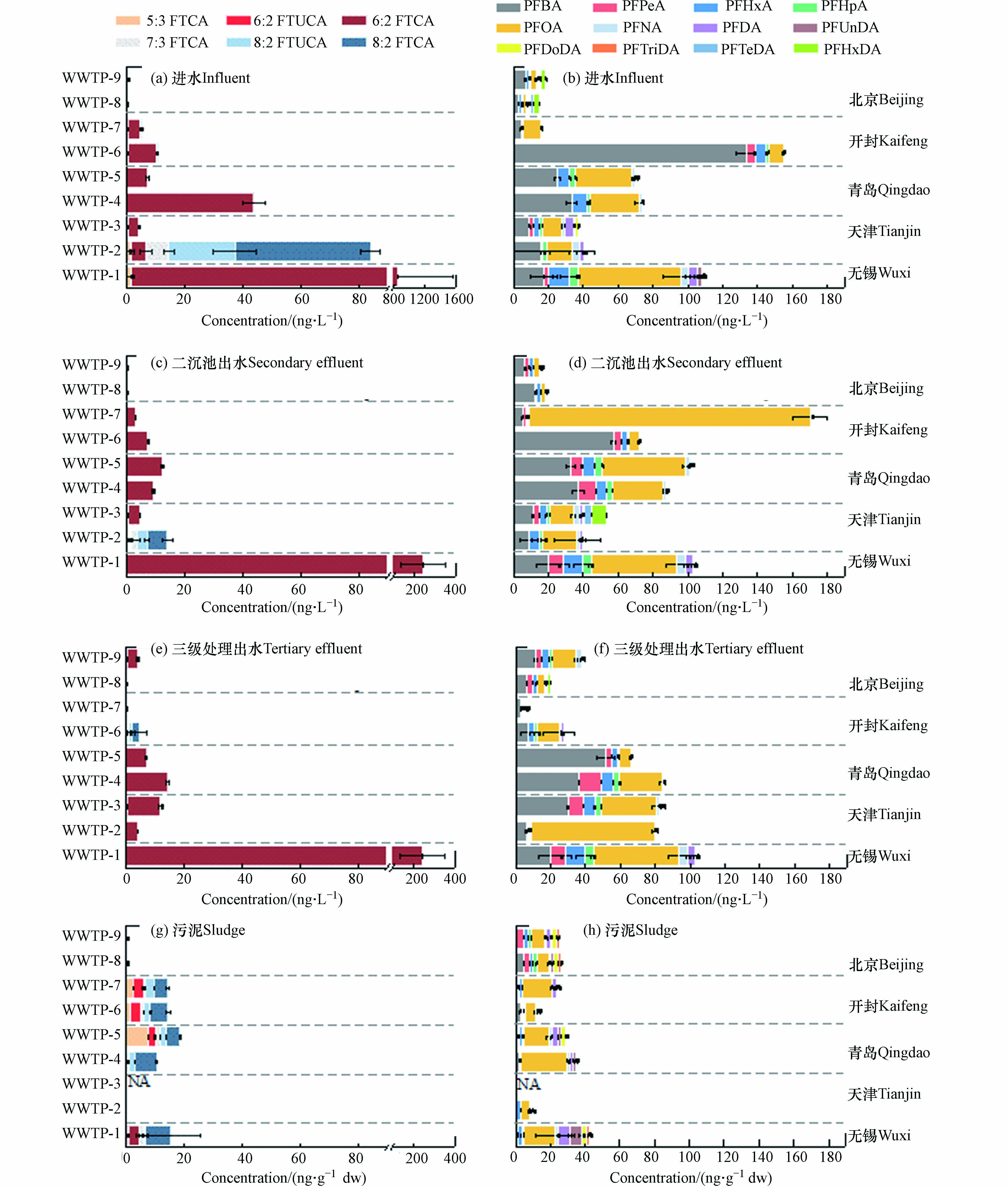
FTCAs及PFCAs在污水处理厂中被广泛检出,浓度分布见表3. 在污水样品中6种FTCAs及12种PFCAs均有检出. 进水中两类物质的总浓度分别为0.52—2.00×103 ng·L−1和12.4—156 ng·L−1;在二沉池出水中总浓度分别为0.23—404 ng·L−1和14.1—172 ng·L−1;三级处理出水中两类物质的总浓度分别为0.17—404 ng·L−1和7.25—126 ng·L−1. 6种FTCAs及12种PFCAs在污泥样品中同样均被检出,浓度分别为<LOD—33.1 ng·g−1 dw和11.2—53.9 ng·g−1 dw.
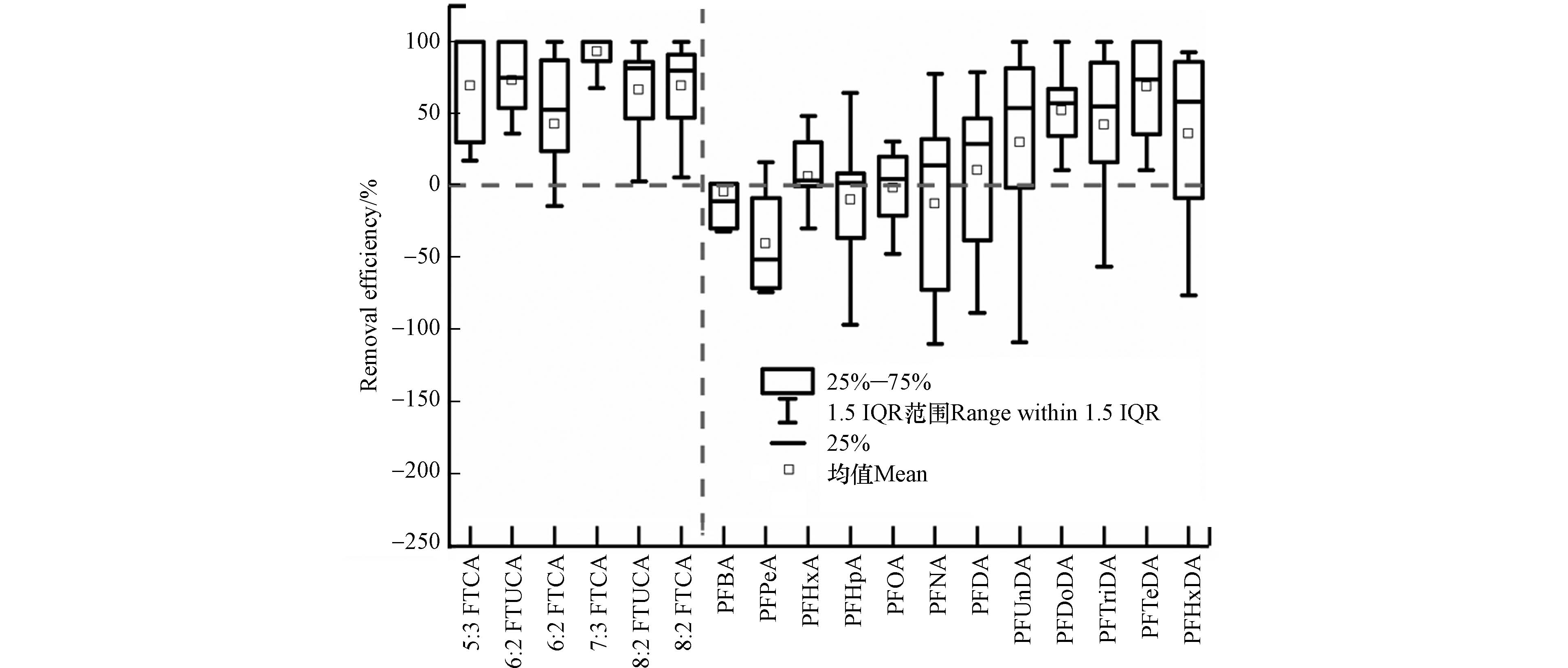
9座污水处理厂污水及污泥样品中FTCAs及PFCAs的浓度组成如图1所示. WWTP-1和WWTP-2的进水中收纳了部分工业废水,进水中FTCAs总浓度(67.9—2.00×103 ng·L−1)高于其他5座主要受纳生活污水的污水处理厂(0.52—44.0 ng·L−1),表明工业废水的排入会影响城市污水处理厂中FTCAs的浓度水平. 6:2 FTCA和8:2 FTCA是主要的组成物质,在9座污水处理厂中的浓度分别为<LOD—2.00×103 ng·L−1和<LOD—50.3 ng·L−1. 位于无锡的WWTP-1中6:2 FTCA为主要的组成物质,而位于天津的WWTP-2中8:2 FTCA为主,组成的差异可能是由于两座污水处理厂受纳的工业废水类型不同. 澳大利亚某污水处理厂未受纳工业废水时进水中6:2 FTCA及5:3 FTCA的浓度(平均浓度分别为<LOD—5.3 ng·L−1和6.4—8.7 ng·L−1)显著低于间歇性受纳工业废水时进水中的浓度(浓度分别为<LOD—100 ng·L−1和3.4—436 ng·L−1)[22],表明工业废水的排放可能导致污水处理厂进水中全氟类物质浓度的升高.
在二沉池出水中,WWTP-1厂中仍有高浓度FTCAs残留,总浓度为139—404 ng·L−1,同进水组成一致,6:2 FTCA为主要的组成物质(139—403 ng·L−1);其他污水厂二沉池出水中FTCAs总浓度为0.23—15.3 ng·L−1. 其他的研究报道同样发现二沉池出水中仍有FTCAs残留,在德国某工业污水处理厂进水中FTCAs的浓度为0.98 μg·L−1(6:2 FTUCA)—4.68 μg·L−1(5:3 FTCA),出水中仍有1.37 μg·L−1(8:2 FTUCA)—8.92 μg·L−1(6:2 FTUCA)[23];瑞典3座污水处理厂进水中5种FTCAs的总浓度为0.5—3.5 ng·L−1,二沉池出水仍有<LOD—0.4 ng·L−1残留[24]. 以上结果表明生物处理工艺无法完全去除FTCAs. 在三级处理出水中FTCAs仍有残留,总浓度为0.17—404 ng·L−1,6:2 FTCA和8:2 FTCA是主要的组成物质. 在污泥样品中,FTCAs总浓度为<LOD—24.9 ng·g−1 dw,8:2 FTCA为主要组成物质. Eriksson等[24]报道了瑞典污水处理厂污泥中FTCAs的浓度,其中5:3 FTCA和7:3 FTCA为主要的物质,浓度分别为0.02—68.0 ng·g−1 dw和0.02—17.3 ng·g−1 dw.
对于PFCAs,在进水、二沉池出水和三级处理出水中的总浓度分别为12.4—156 ng·L−1、14.1—172 ng·L−1和7.25—126 ng·L−1,其中PFBA和PFOA为主要的组成物质,在污水中的浓度分别为< 0.10—133 ng·L−1和1.40—162 ng·L−1,分别占污水中PFCAs总浓度的0%—86%和5%—94%. 其中WWTP-7二沉池出水中PFCAs总浓度高达172 ng·L−1,可能是由于进水中存在PFCAs其他前体物,如多氟烷基磷酸酯(polyfluoroalkyl phosphate esters,PAPs)、氟调聚磺酸盐(fluorotelomer sulfonates,FTSAs)等物质,也可能通过生物转化生成PFCAs[26]. 在污泥样品中PFCAs的总浓度为11.2—53.9 ng·g−1 dw,其中PFOA为主要的组成物质,浓度为4.85—30.3 ng·g−1 dw,占污泥中PFCAs总浓度的15%—27%.
-
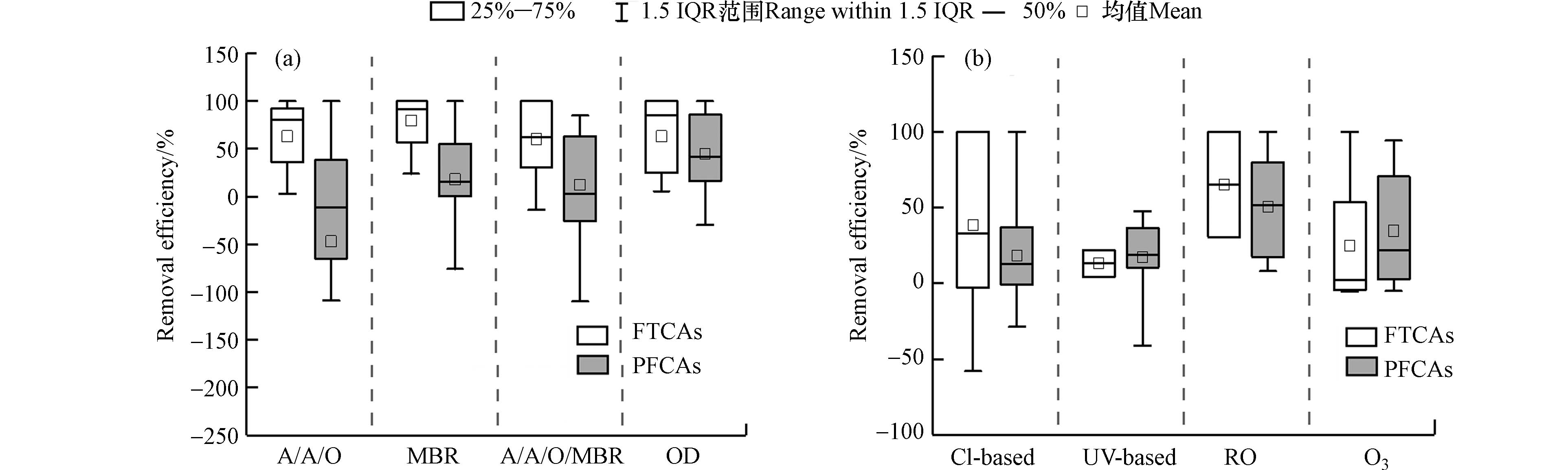
计算了FTCAs和PFCAs在生物处理工艺中的去除率,如图2所示. 生物处理工艺能够去除部分FTCAs,5:3 FTCA、6:2 FTUCA、6:2 FTCA、7:3 FTCA、8:2 FTUCA和8:2 FTCA的平均去除率分别为43%、70%、73%、70%、93%和66%. 12种PFCAs的平均去除率为−40%—69%,PFBA、PFPeA、PFHpA、PFOA和PFNA均存在负去除,平均去除率分别为−4%、−40%、−9%、−2%和13%,表明存在FTCAs等前体物向PFCAs的转化. 此外,部分FTCAs在生物处理工艺中存在负去除,可能存在FTCAs的前体物的转化导致FTCAs浓度升高. 有研究通过实验室模拟实验发现,在纯菌[27]、混合细菌[28 − 29]、消化污泥[30]、活性污泥[31 − 34]等条件下,8:2 FTOHs和6:2 FTOH能够转化为FTCAs和FTUCAs,而FTCAs和FTUCAs可以经过多个步骤生成PFCAs[35]. 进一步分析了不同生物处理工艺对FTCAs和PFCAs的去除率,如图3所示. A/A/O、MBR、A/A/O/MBR及OD对FTCAs的平均去除率分别为63%、79%、60%和63%,无显著差异(P > 0.05). 四种工艺对PFCAs的去除率分别为30%、18%、12%和44%,同样均无显著差异(P > 0.05). 此外PFCAs在不同生物处理工艺中均存在负去除,表明FTCAs在不同工艺中均可能存在向PFCAs的转化.
在三级处理工艺中,6种FTCAs的去除率为-58%—100%,表明三级处理工艺对FTCAs仍不能实现完全去除,并且可能存在FTCAs前体物的转化导致FTCAs浓度升高. 12种PFCAs在三级处理工艺中的去除率为−66%—100%. 对比不同三级深度处理工艺对FTCAs和PFCAs的去除情况(图3),除RO外,PFCAs均存在负去除,课题组前期工作在对全国11座城市污水处理厂的研究中同样发现过滤、紫外、氯、臭氧等三级处理技术均无法有效去除PFCAs,甚至存在负去除现象[36].
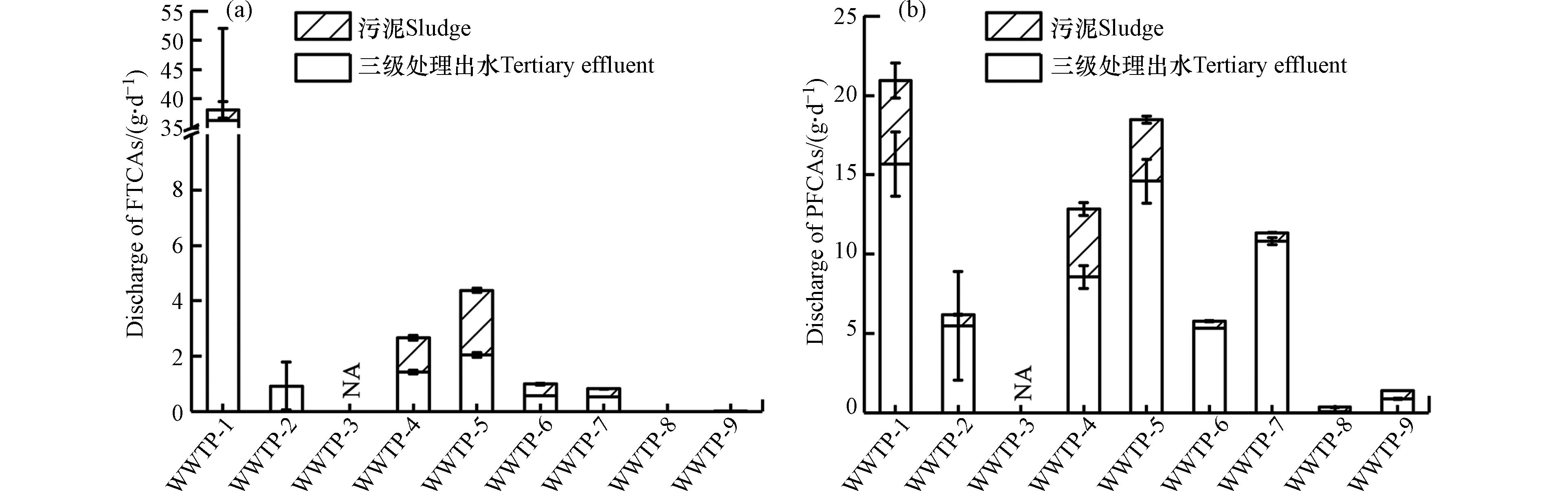
污水处理厂是环境中污染物的重要排放源,因此计算了8座污水处理厂三级处理出水及污泥中FTCAs和PFCAs的排放量(WWTP-3未采集到污泥样品,故此处仅计算8座污水处理厂的排放量),如图4所示. 三级处理出水中的∑FTCAs排放量平均值为12.6 g·d−1,污泥中的排放量平均值为0.98 g·d−1,污水污泥中总排放量为13.6 g·d−1. 其中6:2 FTCA是主要的物质,总排放量为12.6 g·d−1,其次为8:2 FTCA,总排放量为0.60 g·d−1. ∑PFCAs在三级处理出水及污泥中的平均排放量分别为9.50 g·d−1和2.69 g·d−1,总排放量为12.2 g·d−1. PFOA和PFBA为主要的组成物质,短链PFCAs主要由污水排放,长链PFCAs则主要通过污泥排放,这是由于随着碳链的增长,PFCAs更易吸附于污泥中. 之前的研究探究了中国11座污水处理厂深度处理出水中PFCAs的排放量,PFBA和PFOA为主要的物质,排放量分别为0.18—9.81 g·d−1和0.08—23.10 g·d−1 [36].
-
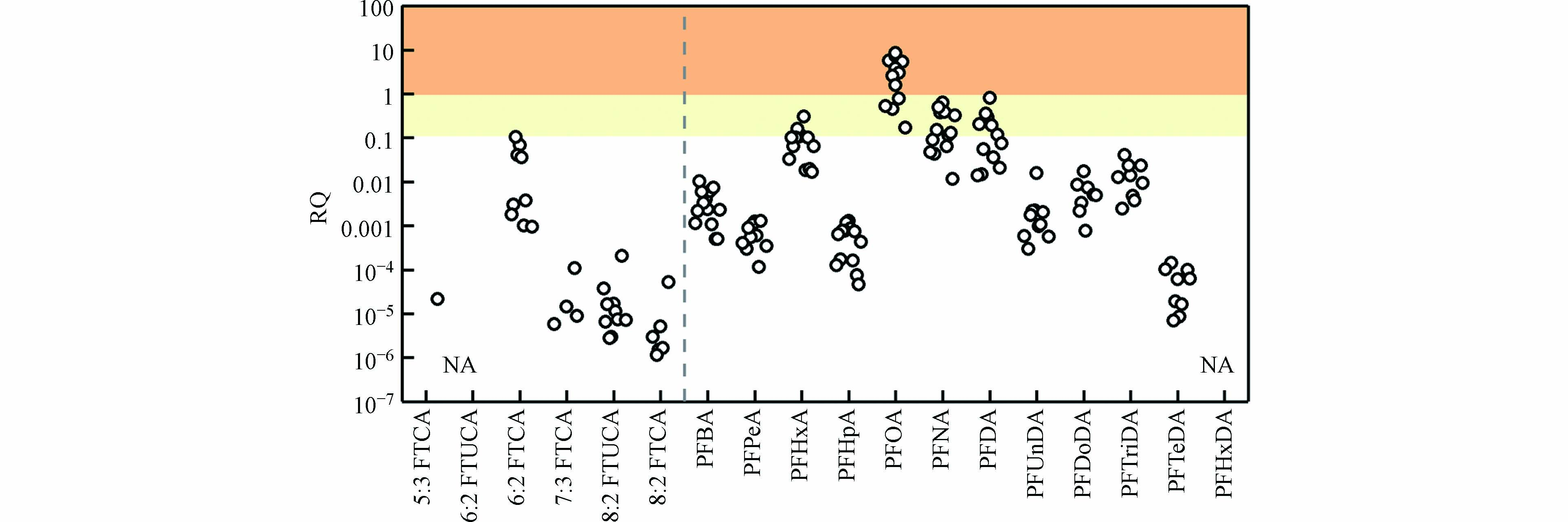
分别计算了9座污水处理厂三级处理出水中FTCAs及PFCAs对鱼类的风险商(RQ),如图5所示. 5:3 FTCA、7:3 FTCA、8:2 FTUCA和8:2 FTCA的RQ值均<0.1,表明对水生环境具有较低的环境生态风险. 6:2 FTCA的RQ值为0.00—0.10,可能会带来中风险. 对于PFCAs,其中7种PFCAs对鱼类的RQ值<0.1,表明对水生生物的影响较小. PFHxA、PFNA和PFDA的RQ值分别为0.02—0.30、0.01—0.63和0.00—0.82,对水生生物具有中风险. PFOA对鱼类的RQ值为0.17—8.58,表明具有较高的潜在风险. 对于PFCAs,Mu等[37]调研了中国148座城市污水处理厂出水中PFASs的浓度,并应用AF及SSD方法计算了PFASs的生态风险,结果表明PFASs对水生生物的风险较低. Zhou等[38]用污水中污染物平均浓度和EC50、LC50或LOEC比值计算了PFOA的相对环境风险,RQ值<1为低风险. 此外有研究报道了天津市某水源地周边不同介质中PFCAs的RQ值均<0.1,不会对水生生态系统产生不利影响[39]. FTCAs对水环境的潜在风险的报道仍然有限,本研究表明城市污水处理系统三级处理出水中仍含有较高浓度的FTCAs残留,并对水环境存在中风险,应引起关注.
-
1)FTCAs在城市污水处理厂中广泛存在,进水浓度为0.52—2.00×103 ng·L−1,6:2 FTCA和8:2 FTCA是主要的物质;受纳工业废水的城市污水处理厂(WWTP-1和WWTP-2)进水中FTCAs的浓度高于其他主要受纳生活污水的城市污水处理厂,表明工业废水的排入会影响城市污水处理厂中FTCAs的浓度水平.
2)生物处理工艺对FTCAs的平均去除率为43%—93%,对PFCAs的去除率为-40%—69%,表明可能存在FTCAs等前体物向PFCAs的转化. 三级处理工艺无法完全去除FTCAs和PFCAs,并存在负去除,三级处理出水中仍含有0.17—404 ng·L−1和7.25—126 ng·L−1的FTCAs和PFCAs残留. ∑FTCAs在三级处理出水及污泥中的总排放量为13.6 g·d−1,6:2 FTCA和8:2 FTCA是主要的组成物质.
3)三级处理出水中6:2 FTCA、PFHxA、PFNA和PFDA对鱼类的RQ值为0.00—0.82,为中风险;PFOA的RQ值为0.17—8.58,具有较高的潜在风险.
氟调酸在我国典型城市污水处理厂中的浓度分布及排放
Occurrence and discharge of fluorotelomer carboxylic acids (FTCAs) in typical municipal WWTPs in China
-
摘要: 氟调酸(fluorotelomer carboxylic acids,FTCAs)是全氟烷基羧酸(perfluoroalkyl carboxylates,PFCAs)的前体物,同时也是替代加工助剂. 本研究应用固相萃取-液相色谱-三重四极杆质谱仪联用的检测分析方法,探究了中国5个城市9座城市污水处理厂进水、二沉池出水、三级处理出水和污泥中6种FTCAs和12种PFCAs的分布及排放特征. FTCAs在城市污水处理厂进水中的浓度为0.52—2.00×103 ng·L−1,受纳工业废水的城市污水处理厂进水中FTCAs的浓度高于其他主要受纳生活污水的城市污水处理厂,表明工业废水的排放会影响城市污水处理厂中FTCAs的浓度水平. 生物处理工艺对FTCAs和PFCAs的去除率分别为43%—93%和−40%—69%. ∑FTCAs在三级处理出水及污泥中的总排放量为13.6 g·d−1,6 : 2 FTCA和8 : 2 FTCA为主要的组成物质. 三级处理出水中FTCAs及PFCAs的风险商分析表明三级处理出水中6 : 2 FTCA、PFHxA、PFOA、PFNA和PFDA可能对鱼类存在潜在风险. 本研究为我国城市污水处理厂中新污染物的管控提供了数据基础.Abstract: Fluorotelomer carboxylic acids (FTCAs) are the precursors and substitutes of perfluoroalkyl carboxylates (PFCAs). In this study, the distribution and discharge characteristics of six FTCAs and twelve PFCAs in influent, secondary effluent, tertiary effluent and sludge samples from nine municipal wastewater treatment plants (WWTPs) in five cities in China were investigated by using the detection and analysis method of solid-phase extraction and liquid chromatography coupled with triple quadrupole mass spectrometry. The total concentrations of FTCAs in the influent from nine municipal WWTPs were 0.52—2.00×103 ng·L−1. The concentrations of FTCAs in the influent from municipal WWTPs receiving industrial wastewater were higher than those from other WWTPs mainly receiving domestic sewage, suggesting the influence of industrial wastewater on the concentration level of FTCAs. The removal efficiencies of FTCAs and PFCAs during biological treatment were 43%—93% and −40%—69%, respectively. The total discharge of FTCAs in the tertiary effluent and sludge was 13.6 g·d−1, 6:2 FTCA and 8:2 FTCA as the predominant components. The risk quotient analysis of FTCAs and PFCAs in the tertiary effluent suggested that 6:2 FTCA, PFHxA, PFOA, PFNA and PFDA in the tertiary effluent may pose potential risks to fish in aquatic environment. This study provides the data basis for the management and control of emerging contaminants in municipal WWTPs in China.
-

-
表 1 9座城市污水处理厂的运行参数
Table 1. Operational parameters of nine municipal WWTPs
污水处理厂
WWTPs城市
Cities生物处理
工艺
Biological
treatment三级处
理工艺
Tertiary
treatment水力停
留时间/h
HRT污泥停
留时间/d
SRT设计流量/
(m3·d−1)
Treatment capacity服务人口
(×104)
Service
population污泥产量/
(kg·d−1)
Sludge
yield进水组成
Influent
composition采样时间
Sampling timeWWTP-1 无锡 MBR 消毒 20 15 150000 50 120000 生活污水和
30%工业废水2020年8月和12月2021年3月和12月 WWTP-2 天津 多级A/O 混凝沉淀过滤消毒 18 15 200000 NA 60000 生活污水和
部分工业废水2018年6月和11月 WWTP-3 天津 OD 反渗透 NA NA 70000—
80000NA NA 生活污水 2022年3月 WWTP-4 青岛 A/A/O 混凝沉淀过滤紫外 21 13 100000 26 118000 生活污水 2021年3月 WWTP-5 青岛 A/A/O 混凝沉淀过滤消毒 21 19 170000 80 126000 生活污水 2021年3月 WWTP-6 开封 OD 磁混凝消毒 12 12 80000 NA 29000 生活污水 2021年5月 WWTP-7 开封 A/A/O 混凝沉淀过滤消毒 13 17 133000 NA 20000 生活污水 2021年5月 WWTP-8 北京 A/A/O/MBR 反渗透 19 NA 10000 NA 10000 生活污水 2022年6月 WWTP-9 北京 A/A/O/MBR 臭氧 15 NA 45000 25—30 20000 生活污水 2022年6月 NA,数据不可用,data was not available;MBR,膜生物反应器,membrane bioreactor;A/A/O,厌氧-缺氧-好氧工艺,anaerobic-anoxic-oxic;OD,氧化沟工艺,oxidation ditch 表 2 三重四极杆质谱中FTCAs和PFCAs的质谱参数
Table 2. Mass spectrometry parameters of FTCAs and PFCAs in triple quadrupole instrument
物质类别
Compounds物质
Acronym母离子
Parent ion子离子
Daughter ion锥孔电压/V
Cone voltage碰撞能/eV
Collision energy内标
Surrogate standardFTCAs 5:3 FTCA 341 217 15 25 13C2-6:2 FTUCA 237 15 6:2 FTUCA 357 243 12 36 13C2-6:2 FTUCA 293 18 6:2 FTCA 377 243 15 30 13C2-6:2 FTCA 293 20 7:3 FTCA 441 317 25 15 13C2-8:2 FTUCA 337 10 8:2 FTUCA 457 343 10 36 13C2-8:2 FTUCA 393 15 8:2 FTCA 477 343 15 40 13C2-8:2 FTCA 393 25 PFCAs PFBA 213 119 15 17 13C4-PFBA 169 8 PFPeA 263 119 11 17 13C2-PFHxA 219 5 PFHxA 313 119 11 17 13C2-PFHxA 269 7 PFHpA 363 169 12 16 13C4-PFOA 319 6 PFOA 413 169 11 15 13C4-PFOA 369 7 PFNA 463 219 12 14 13C5-PFNA 419 8 PFDA 513 269 14 14 13C2-PFDA 469 8 PFUnDA 563 219 14 16 13C2-PFUnDA 519 10 PFDoDA 613 169 16 10 13C2-PFDoDA 569 18 PFTriDA 663 269 10 20 13C2-PFDoDA 619 10 PFTeDA 713 219 10 20 13C2-PFDoDA 669 10 PFHxDA 813 169 20 30 13C2-PFDoDA 769 15 表 3 9座城市污水处理厂进水、二沉池出水、三级处理出水和污泥中FTCAs及PFCAs的浓度
Table 3. Concentration of PFCAs and FTCAs in influent, secondary effluent, tertiary effluent and sludge samples from nine WWTP municipals in China.
物质
Compounds进水/(ng·L−1, n=13)
Influent二沉池出水/(ng·L−1, n=13)
Secondary effluent范围
Range平均值
Mean中位值
Median检出率/%
Freq范围
Range平均值
Mean中位值
Median检出率/%
Freq5:3 FTCA <0.02—2.63 0.60 <0.02 38 <0.01—1.25 0.13 <0.01 23 6:2 FTUCA <0.01—1.76 0.28 <0.01 46 <0.01—0.82 0.10 0.04 54 6:2 FTCA <0.11—2.00×103 270 7.11 85 <0.07—403 77.0 7.31 77 7:3 FTCA <0.08—9.72 1.83 0.56 92 <0.07—3.17 0.32 <0.07 38 8:2 FTUCA <0.01—30.3 3.66 0.18 77 <0.01—4.32 0.62 0.08 85 8:2 FTCA <0.11—50.3 7.42 0.29 54 <0.07—8.84 1.17 0.11 54 ∑FTCAs 0.52—2.00×103 284 44.0 100 0.23—404 79.4 12.4 100 PFBA <0.10—133 24.1 12.8 92 2.94—57.4 19.8 13.8 100 PFPeA <0.07—6.39 1.41 <0.07 31 <0.06—12.7 5.07 4.52 85 PFHxA <0.01—19.6 6.23 6.37 92 0.77— 18.9 6.27 6.36 100 PFHpA 0.55—5.93 2.66 1.65 100 0.34—6.40 2.88 3.19 100 PFOA 1.42—74.8 27.8 27.3 100 2.65—162 38.6 33.0 100 PFNA 0.35—6.46 2.57 1.91 100 0.28—6.28 2.47 1.84 100 PFDA 0.25—6.89 2.54 1.59 100 0.13—8.18 1.84 1.16 100 PFUnDA <0.02—7.01 1.17 0.31 92 <0.02—3.45 0.61 0.33 85 PFDoDA <0.07—2.47 0.44 0.14 77 <0.04—1.02 0.20 0.16 69 PFTriDA <0.02—0.59 0.17 0.09 62 <0.01—0.67 0.16 0.08 69 PFTeDA <0.03—2.30 0.36 0.13 62 <0.02—4.02 0.41 0.03 54 PFHxDA <0.04—3.81 0.65 0.13 69 <0.02—8.45 0.79 0.06 77 ∑PFCAs 12.4—156 70.1 71.7 100 14.1—172 79.0 89.1 100 物质
Compounds三级处理出水/(ng·L−1, n=13)
Tertiary effluent污泥/(ng·g−1dw, n=12)
Sludge范围
Range平均值
Mean中位值
Median检出率/%
Freq范围
Range平均值
Mean中位值
Median检出率/%
Freq5:3 FTCA <0.01—0.36 0.03 <0.01 8 <0.03—7.72 1.07 <0.03 33 6:2 FTUCA <0.01—0.55 0.09 0.04 54 <0.02—3.54 0.96 0.40 67 6:2 FTCA <0.06—403 77.4 6.87 69 <0.09—4.93 1.26 <0.09 33 7:3 FTCA <0.05—1.08 0.11 <0.05 31 <0.08—3.51 0.89 0.56 83 8:2 FTUCA <0.01—2.03 0.24 0.07 77 <0.01—3.04 1.06 0.55 50 8:2 FTCA <0.06—5.26 0.50 <0.06 46 <0.01—24.9 4.88 2.36 50 ∑FTCAs 0.17—404 78.3 8.92 100 <LOD—33.1 10.1 8.10 83 PFBA 2.48—51.6 18.1 11.8 100 <0.10—4.44 1.14 0.96 58 PFPeA 0—13.08 5.36 4.14 77 <0.07—4.29 0.92 0.42 58 PFHxA 1.05—18.9 5.78 6.29 100 0.83—3.52 2.25 2.25 100 PFHpA 0.23—6.40 2.78 3.17 100 <0.02—2.14 0.63 0.41 75 PFOA 1.40—71.1 28.8 24.6 100 4.85—30.3 13.8 9.06 100 PFNA 0.11—6.28 2.20 1.28 100 <0.05—2.47 1.26 1.19 92 PFDA <0.01—8.18 1.69 0.74 92 0.88—7.75 3.60 2.15 100 PFUnDA <0.01—3.45 0.46 0.21 77 0.49—7.88 2.79 1.10 100 PFDoDA <0.04—1.02 0.22 0.13 62 <0.07—4.25 1.86 2.00 92 PFTriDA <0.01—0.64 0.17 0.08 69 <0.04—2.40 1.18 1.15 83 PFTeDA <0.02—0.72 0.20 0.08 69 <0.07—1.23 0.31 0.17 67 PFHxDA <0.01—1.32 0.28 0.06 77 <0.07—0.83 0.21 0.11 75 ∑PFCAs 7.25—126 66.1 81.4 100 11.2—53.9 29.9 29.1 100 -
[1] LEHMLER H J. Synthesis of environmentally relevant fluorinated surfactants—a review[J]. Chemosphere, 2005, 58(11): 1471-1496. doi: 10.1016/j.chemosphere.2004.11.078 [2] WANG S Q, DING G H, LIU Y H, et al. Legacy and emerging persistent organic pollutants in the marginal seas of China: Occurrence and phase partitioning[J]. The Science of the Total Environment, 2022, 827: 154274. doi: 10.1016/j.scitotenv.2022.154274 [3] ZHOU J, LI S J, LIANG X X, et al. First report on the sources, vertical distribution and human health risks of legacy and novel per- and polyfluoroalkyl substances in groundwater from the Loess Plateau, China[J]. Journal of Hazardous Materials, 2021, 404: 124134. doi: 10.1016/j.jhazmat.2020.124134 [4] HAN T Z, GAO L Y, CHEN J H, et al. Spatiotemporal variations, sources and health risk assessment of perfluoroalkyl substances in a temperate bay adjacent to metropolis, North China[J]. Environmental Pollution, 2020, 265(Pt A): 115011. [5] MA D H, ZHONG H F, LV J T, et al. Levels, distributions, and sources of legacy and novel per- and perfluoroalkyl substances (PFAS) in the topsoil of Tianjin, China[J]. Journal of Environmental Sciences, 2022, 112: 71-81. doi: 10.1016/j.jes.2021.04.029 [6] WANG S Q, LIN X P, LI Q, et al. Neutral and ionizable per-and polyfluoroalkyl substances in the urban atmosphere: Occurrence, sources and transport[J]. Science of the Total Environment, 2022, 823: 153794. doi: 10.1016/j.scitotenv.2022.153794 [7] LI F S, SUN H W, HAO Z N, et al. Perfluorinated compounds in Haihe River and Dagu drainage canal in Tianjin, China[J]. Chemosphere, 2011, 84(2): 265-271. doi: 10.1016/j.chemosphere.2011.03.060 [8] Krafft M P, Riess J G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability—part one [J]. Chemosphere, 2015, 129: 4-19. doi: 10.1016/j.chemosphere.2014.08.039 [9] SHI G H, CUI Q Q, PAN Y T, et al. 6: 2 fluorotelomer carboxylic acid (6: 2 FTCA) exposure induces developmental toxicity and inhibits the formation of erythrocytes during zebrafish embryogenesis[J]. Aquatic Toxicology, 2017, 190: 53-61. doi: 10.1016/j.aquatox.2017.06.023 [10] WANG N, SZOSTEK B, BUCK R C, et al. Fluorotelomer alcohol biodegradation-direct evidence that perfluorinated carbon chains breakdown[J]. Environmental Science & Technology, 2005, 39(19): 7516-7528. [11] ELLIS D A, MARTIN J W, de SILVA A O, et al. Degradation of fluorotelomer alcohols: A likely atmospheric source of perfluorinated carboxylic acids[J]. Environmental Science & Technology, 2004, 38(12): 3316-3321. [12] CHEN H R, PENG H, YANG M, et al. Detection, occurrence, and fate of fluorotelomer alcohols in municipal wastewater treatment plants[J]. Environmental Science & Technology, 2017, 51(16): 8953-8961. [13] LOEWEN M, HALLDORSON T, WANG F Y, et al. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada[J]. Environmental Science & Technology, 2005, 39(9): 2944-2951. [14] ERIKSSON U, KÄRRMAN A. World-wide indoor exposure to polyfluoroalkyl phosphate esters (PAPs) and other PFASs in household dust[J]. Environmental Science & Technology, 2015, 49(24): 14503-14511. [15] ZHAO Z, YUE L X, QIAO H Q, et al. Perfluoroalkyl acids in dust on residential indoor/outdoor window glass in Chinese Cities: Occurrence, composition, and toddler exposure[J]. Environmental Science and Pollution Research, 2022, 29(10): 13881-13892. doi: 10.1007/s11356-021-16653-w [16] ZHAO Z, CHENG X H, HUA X, et al. Emerging and legacy per- and polyfluoroalkyl substances in water, sediment, and air of the Bohai Sea and its surrounding rivers[J]. Environmental Pollution, 2020, 263(Pt A): 114391. [17] 李琦路, 程相会, 赵祯, 等. 黄河中游(渭南—郑州段)全/多氟烷基化合物的分布及通量[J]. 环境科学, 2019, 40(1): 228-238. doi: 10.13227/j.hjkx.201805240 LI Q L, CHENG X H, ZHAO Z, et al. Distribution and fluxes of perfluoroalkyl and polyfluoroalkyl substances in the middle reaches of the Yellow River(Weinan-Zhengzhou section)[J]. Environmental Science, 2019, 40(1): 228-238 (in Chinese). doi: 10.13227/j.hjkx.201805240
[18] PHILLIPS M M, DINGLASAN-PANLILIO M J A, MABURY S A, et al. Fluorotelomer acids are more toxic than perfluorinated acids[J]. Environmental Science & Technology, 2007, 41(20): 7159-7163. [19] PHILLIPS M M, DINGLASAN-PANLILIO M J A, MABURY S A, et al. Chronic toxicity of fluorotelomer acids to Daphnia magna and Chironomus dilutus[J]. Environmental Toxicology and Chemistry, 2010, 29(5): 1123-1131. [20] MITCHELL R J, MYERS A L, MABURY S A, et al. Toxicity of fluorotelomer carboxylic acids to the algae Pseudokirchneriella subcapitata and Chlorella vulgaris, and the amphipod Hyalella azteca[J]. Ecotoxicology and Environmental Safety, 2011, 74(8): 2260-2267. doi: 10.1016/j.ecoenv.2011.07.034 [21] LEE H, D'EON J, MABURY S A. Biodegradation of polyfluoroalkyl phosphates as a source of perfluorinated acids to the environment[J]. Environmental Science & Technology, 2010, 44(9): 3305-3310. [22] GALLEN C, BIGNERT A, TAUCARE G, et al. Temporal trends of perfluoroalkyl substances in an Australian wastewater treatment plant: A ten-year retrospective investigation[J]. Science of the Total Environment, 2022, 804: 150211. doi: 10.1016/j.scitotenv.2021.150211 [23] GREMMEL C, FRÖMEL T, KNEPPER T P. HPLC–MS/MS methods for the determination of 52 perfluoroalkyl and polyfluoroalkyl substances in aqueous samples[J]. Analytical and Bioanalytical Chemistry, 2017, 409(6): 1643-1655. doi: 10.1007/s00216-016-0110-z [24] ERIKSSON U, HAGLUND P, KÄRRMAN A. Contribution of precursor compounds to the release of per- and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs)[J]. Journal of Environmental Sciences, 2017, 61: 80-90. doi: 10.1016/j.jes.2017.05.004 [25] MA C M, PENG H, CHEN H R, et al. Long-term trends of fluorotelomer alcohols in a wastewater treatment plant impacted by textile manufacturing industry[J]. Chemosphere, 2022, 299: 134442. doi: 10.1016/j.chemosphere.2022.134442 [26] 杨琳, 李敬光. 全氟化合物前体物质生物转化与毒性研究进展[J]. 环境化学, 2015, 34(4): 649-655. YANG L, LI J G. Perfluorinated compound precursors: Biotransformation and toxicity[J]. Environmental Chemistry, 2015, 34(4): 649-655 (in Chinese).
[27] KIM M H, WANG N, McDONALD T, et al. Biodefluorination and biotransformation of fluorotelomer alcohols by two alkane-degrading Pseudomonas strains[J]. Biotechnology and Bioengineering, 2012, 109(12): 3041-3048. doi: 10.1002/bit.24561 [28] KIM M H, WANG N, CHU K H. 6: 2 Fluorotelomer alcohol (6: 2 FTOH) biodegradation by multiple microbial species under different physiological conditions[J]. Applied Microbiology and Biotechnology, 2014, 98(4): 1831-1840. doi: 10.1007/s00253-013-5131-3 [29] DINGLASAN M J A, YE Y, EDWARDS E A, et al. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids[J]. Environmental Science & Technology, 2004, 38(10): 2857-2864. [30] ZHANG S, SZOSTEK B, McCAUSLAND P K, et al. 6: 2 and 8: 2 fluorotelomer alcohol anaerobic biotransformation in digester sludge from a WWTP under methanogenic conditions[J]. Environmental Science & Technology, 2013, 47(9): 4227-4235. [31] YU X L, TAKABE Y, YAMAMOTO K, et al. Biodegradation property of 8: 2 fluorotelomer alcohol (8: 2 FTOH) under aerobic/anoxic/anaerobic conditions[J]. Journal of Water and Environment Technology, 2016, 14(3): 177-190. doi: 10.2965/jwet.15-056 [32] YU X L, NISHIMURA F, HIDAKA T. Effects of microbial activity on perfluorinated carboxylic acids (PFCAs) generation during aerobic biotransformation of fluorotelomer alcohols in activated sludge[J]. Science of the Total Environment, 2018, 610/611: 776-785. doi: 10.1016/j.scitotenv.2017.08.075 [33] WANG N, SZOSTEK B, FOLSOM P W, et al. Aerobic biotransformation of 14C-labeled 8-2 telomer B alcohol by activated sludge from a domestic sewage treatment plant[J]. Environmental Science & Technology, 2005, 39(2): 531-538. [34] LI F, SU Q F, ZHOU Z M, et al. Anaerobic biodegradation of 8: 2 fluorotelomer alcohol in anaerobic activated sludge: Metabolic products and pathways[J]. Chemosphere, 2018, 200: 124-132. doi: 10.1016/j.chemosphere.2018.02.065 [35] 陈红瑞, 张昱, 杨敏. 全氟化合物前体物氟调醇的检测方法、环境分布及转化研究进展[J]. 环境化学, 2015, 34(12): 2170-2178. doi: 10.7524/j.issn.0254-6108.2015.12.2015052602 CHEN H R, ZHANG Y, YANG M. Research progress on the detection methods, environmental distribution and transformation of perfluorinated compounds’ precursors fluorotelomer alcohols[J]. Environmental Chemistry, 2015, 34(12): 2170-2178 (in Chinese). doi: 10.7524/j.issn.0254-6108.2015.12.2015052602
[36] 马春萌, 陈红瑞, 马洁, 等. 短链全氟烷酸替代物在城市污水深度处理工艺中的分布和排放[J]. 生态毒理学报, 2020, 15(5): 147-157. MA C M, CHEN H R, MA J, et al. Occurrence and discharge of short-chain perfluoroalkyl acids(PFAAs)substitutes during advanced treatment process in municipal wastewater treatment plants[J]. Asian Journal of Ecotoxicology, 2020, 15(5): 147-157 (in Chinese).
[37] MU H X, LI J H, CHEN L, et al. Distribution, source and ecological risk of per- and polyfluoroalkyl substances in Chinese municipal wastewater treatment plants[J]. Environment International, 2022, 167: 107447. doi: 10.1016/j.envint.2022.107447 [38] ZHOU Y Q, MENG J, ZHANG M, et al. Which type of pollutants need to be controlled with priority in wastewater treatment plants: Traditional or emerging pollutants?[J]. Environment International, 2019, 131: 104982. doi: 10.1016/j.envint.2019.104982 [39] CAO X H, WANG C C, LU Y L, et al. Occurrence, sources and health risk of polyfluoroalkyl substances (PFASs) in soil, water and sediment from a drinking water source area[J]. Ecotoxicology and Environmental Safety, 2019, 174: 208-217. doi: 10.1016/j.ecoenv.2019.02.058 -



 下载:
下载: