-
四环素类抗生素(TCs,表1)是由放线菌产生,以氢化并四苯为基本骨架的一类广谱抗生素,广泛应用于人类医疗、畜牧业和水产养殖业. 据报道,TCs的生产和使用量在我国位居第一[1]、世界第二[2]. TCs主要包括四环素(TC)、土霉素(OTC)、金霉素(CTC)和多西霉素(DXC),使用后大部分会以原型或代谢产物形式随尿液和粪便排出并通过各种途径进入环境,对生态系统和公共健康产生严重影响[3 − 5].
以往的研究报道TCs在河流、湖泊和土壤中被广泛检出[6 − 9],但是主要集中于上述几种原型抗生素,缺乏对其转化产物的监测及潜在风险研究. 一方面,已有研究表明TCs由于水解和光解等作用容易在C4位可逆地形成差向异构产物(如ETC和ECTC)、在C6位形成脱水产物及其差向异构产物(如ATC和EATC)、在b环发生开环反应生成内酯型异构体(如α-apo-OTC和β-apo-OTC)、在c环发生开环生成异构体(如ICIC)[10 − 12]. 另一方面,尽管一些TCs转化产物的抗菌活性不如其原型化合物,但毒性却大大增强,如含有EATC和ETC降解产物的变质TC制剂能引起范康尼综合征[13];Halling-Sorensen等发现EATC和ATC对环境中的一些耐药菌和土壤细菌的杀灭作用比原型化合物更强[14]. 因此,环境中TCs转化产物存在引起的风险不容忽视.
目前,相关研究还主要停留在实验室条件下TCs转化产物的结构鉴定、形成规律及影响因素等方面[9,11],关于它们在实际环境中的污染水平和分布研究还非常少,且已有的检测方法主要针对几种原型化合物,需要进一步研究环境水样萃取条件对转化产物富集浓缩效果的影响、TCs易于差向异构导致同分异构体普遍存在对特异检测的影响等. 本研究针对7种TCs和13种转化产物(包括7组15个同分异构体),应用固相萃取结合超高效液相色谱串联质谱仪(UPLC-MS/MS)建立高灵敏同时分析方法,并应用于永定河(北京段)水体样品,初步调查TCs及转化产物的浓度水平、组成及分布特征,以期为后续的环境行为和风险评估研究奠定基础.
-
超高效液相色谱串联质谱仪(ACQUITY UPLC/Xevo TQ-XS,Waters,美国);固相萃取仪(Supelco,美国);Oasis HLB和Oasis MAX固相萃取柱(500 mg/6 cc,Waters).
甲醇、乙腈(HPLC级,Fisher,美国);二氯甲烷(HPLC 级,ROE Science,美国);甲酸(分析级,北京);Na2EDTA(分析级,北京),超纯水(Milli-Q 超纯水系统,Merk,美国). 实验用标准品(表1)均购自百灵威科技有限公司,纯度均大于97.0%.
-
2022年1月,对从北向南流经北京的永定河(115.71°—116.26° E,39.58°—40.1° N)进行GPS定位采样. 水样在水面以下0.5 m处进行采集,保存于预先润洗过的棕色玻璃瓶中,共计27个水样. 样品采集后在2 h 内运回实验室,并立即进行样品前处理.
-
水样经1.2 µm玻璃纤维滤膜过滤后量取水样1 L,添加10 ng同位素过程内标(DXC-d3,TC-d6,CTC-13C-d3),再加入Na2EDTA(5 g·L−1)充分溶解后,使用Oasis HLB固相萃取柱进行富集浓缩. Oasis HLB小柱首先依次使用6 mL二氯甲烷、6 mL甲醇以及12 mL超纯水活化,然后水样以5—10 mL·min−1的流速上样,经12 mL超纯水淋洗后负压下抽去柱内残留水分,最后用6 mL含1%甲酸的甲醇溶液洗脱. 洗脱液棕色玻璃瓶收集、氮气吹干后100 µL甲醇定容,过滤膜后待测. 水样处理过程中避光,尽量避免光降解的影响.
-
采用超高效液相色谱串联质谱联用仪进行分析处理.色谱柱为Waters BEH C18柱(100 mm×2.1 mm, 1.7 µm),柱温保持40 ℃. 流动相为0.1%甲酸(A)和甲醇(B),采用梯度洗脱,具体程序为:B在1.5 min内由10%升至16%,继续在7.5 min内升至100%,恢复初始状态. 流速设为 0. 3 mL·min−1,进样体积为2 μL.
串联质谱采用ESI正离子模式,毛细管电压为0.5 kV,离子源温度为150 ℃,脱溶剂气温度为500 ℃,锥孔气流量50 L·h−1,脱溶剂气流量为150 L·h−1;扫描方式为多反应监测模式(MRM).
-
对于7种原型TCs和13种转化产物,ESI-MS/MS在正离子模式响应高于负离子,所有目标化合物的母离子均为质子化分子离子([M+H]+). 通过直接将标准品引入质谱对每个目标化合物的MS/MS参数进行优化、获得响应最高的两个碎片离子. 如表1所示,所有目标化合物均以氢化并四苯为基本骨架,质谱碎片离子经常展现相同的碎裂模式. 母离子质荷比(m/z)发生17 Da和35 Da的中性丢失、结构上分别对应氨基([M+H−NH3]+)和随后水([M+H−NH3−H2O]+)的损失是大多数TCs响应最高的两个碎片离子. 从结构上损失水、二甲基氨基或酰胺基侧链产生[M+H−H2O−N(CH3)2]+(如12-AMINO)、[M+H−H2O−(CH3)2N−NH2CO]+(如MINO)、[M+H−2H2O−(CH3)2N−NH2CO]+(如DXC,6-EDXC),a环发生断裂生成m/z 154 Da(如ATC,EATC,CTC和ICTC)等也是质谱响应最高的碎片离子之一.
比较有趣的是,TCs与它们的差向异构体尽管碎裂模式相同,但是碎片离子生成的难易程度和相对强度关系却不尽相同. 图1显示的是ATC和EATC的MS/MS质谱图. 如图1所示,m/z 410([M+H−NH3]+)、321([M+H−H2O−(CH3)2N−NH2CO]+)、m/z 269和154是主要的碎片离子,m/z 269和154是母离子a环发生碎裂对应产生的两个碎片离子[15 − 16]. 在相同的碰撞能量下(26 eV),ATC小分子碎片离子的丰度比例明显高于EATC(图1,a和c);进一步结合碰撞能量增加碎片离子相对强度变化的情况看(图1,b和d),ATC和EATC两个差向异构体的化学结构在质谱电离过程中稳定性明显不同,ATC更易于断裂,EATC的结构更加稳定. 这可能是由于在分子空间构象中a环与b、c和d环不在同一平面,ATC结构中a环C4位二甲氨基远离b, c和d环平面,而EATC朝向平面、相对难于被攻击断裂[15].
-
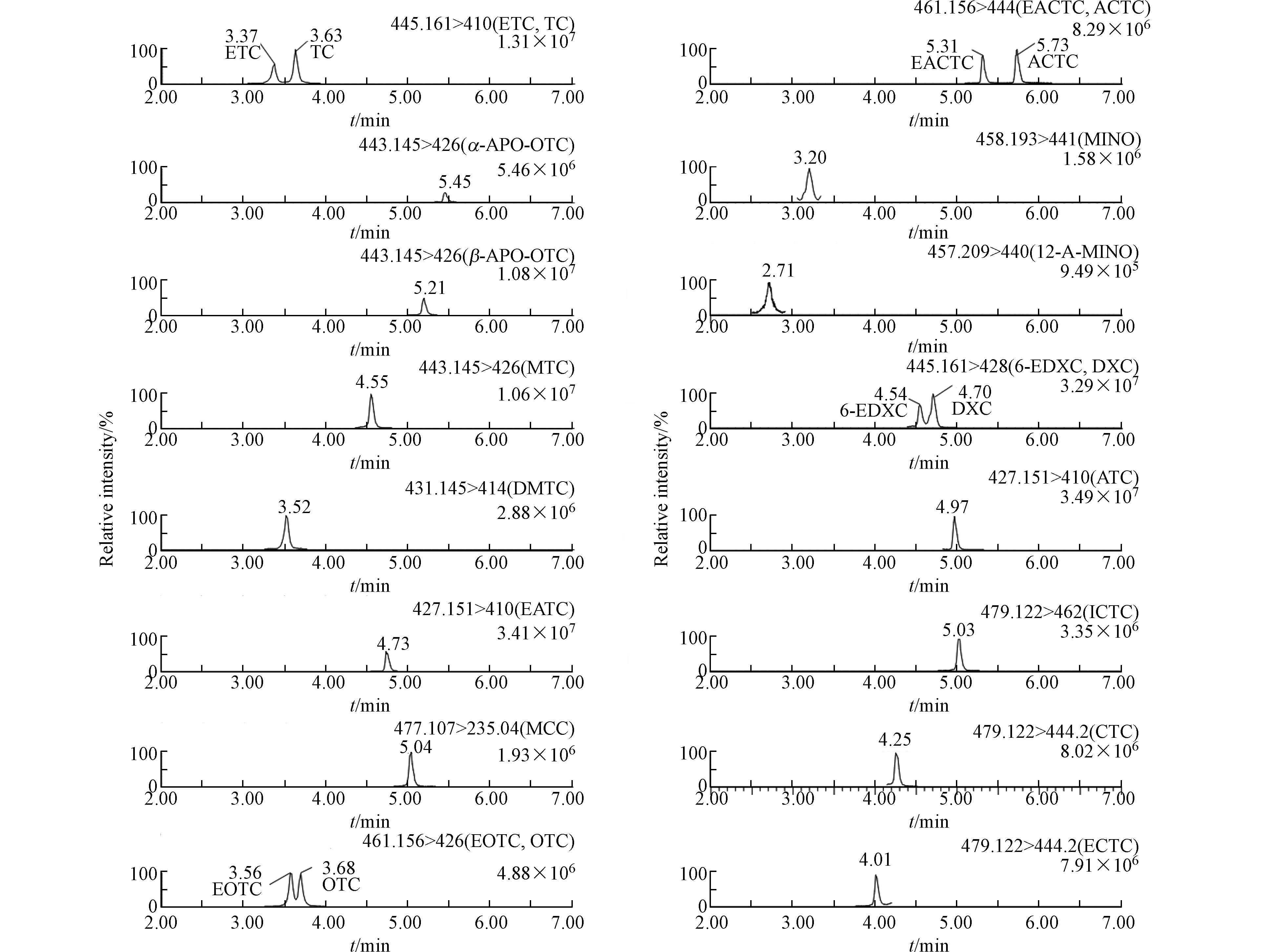
色谱分离是LC-MS/MS特异检测环境样品中TCs及其转化产物面临的主要问题. 在色谱分离过程中,TCs分子中的β-二酮(C10—C12位)和羧酰胺(C2位)结构能够与残留的金属离子形成螯合物,进一步吸附于固定相上的硅烷醇基团,造成TCs化合物易于出现峰拖尾现象. 因此,本研究选择低硅烷醇活性的C18反相色谱柱、并添加甲酸进一步抑制硅烷醇活性,结合UPLC采用1.7 µm小颗粒填充柱使色谱峰更加尖锐和对称,显著提高色谱分离效能. 图2为优化梯度条件后20种TCs及其转化产物的UPLC-MS/MS MRM色谱图,典型色谱峰峰宽小于0.15 min,所有目标化合物在9 min以内完成分析.
由于结构相近,TCs及其转化产物的色谱保留行为也表现出典型的特征和规律(表1和图2). 对于7种原型TCs,出峰先后顺序是MINO、TC、OTC、CTC、MTC、DXC和MCC. 它们的结构差异主要在于C5、C6和C7位取代基的不同. 相比TC,除MINO在C7位氢被二甲氨基取代增大极性保留时间提前,其余5种化学结构中氢被甲基取代(OTC,C5位)、氢被氯取代(CTC和MCC,C7位)、脱水成烯(MTC和MCC,C6位)和脱羟基(DXC,C6位),极性减弱、色谱保留行为增强、保留时间增长. 对于转化产物,所有差向异构体的色谱保留均弱于原型,这可能还是由于分子空间构象中差向异构体C4位二甲氨基相比于原型不易被固定相保留所导致;形成的脱水和内酯型产物极性均减弱,保留时间增长.
-
本研究应用目标化合物绝对回收率结果考察了Na2EDTA添加量、固相萃取柱、洗脱溶剂3个因素对固相萃取的影响.
-
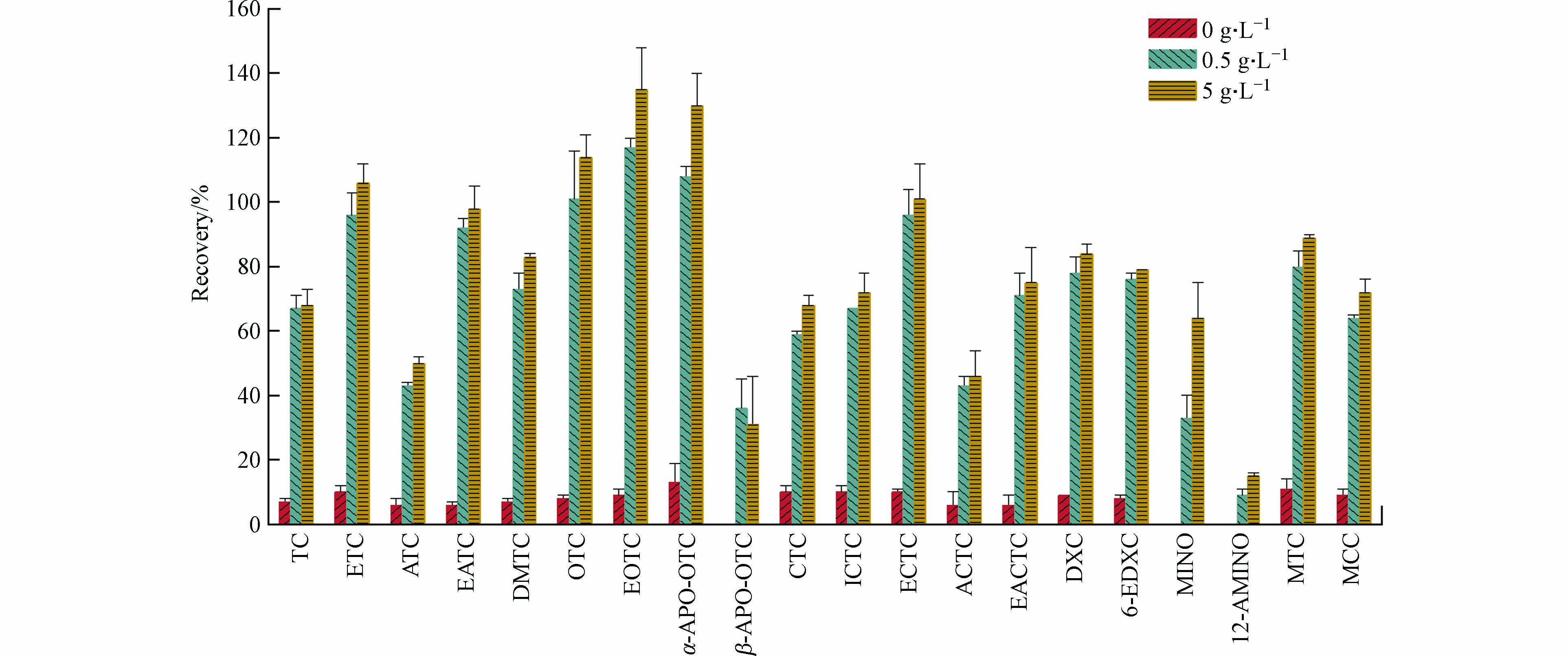
TCs类污染物易于与金属离子形成螯合物,为了准确检测水样中TCs,需要在萃取之前加入Na2EDTA等与水样中的金属阳离子进行螯合、释放出与金属离子结合的部分[17 − 19]. 以往研究Na2EDTA添加水平经常为0.5 g·L−1或者更低,本研究设置0、0.5、5 g·L−1的3个Na2EDTA添加水平,研究Na2EDTA添加量对20种TCs及其转化产物萃取效果的影响. 如图3所示,水样在萃取前加入Na2EDTA显著提高TCs类化合物的萃取效率,且5 g·L−1高Na2EDTA添加量还是高于较低的0.5 g·L−1水平. 值得注意的是,在0.5 g·L−1和5 g·L−1两个Na2EDTA添加水平下,差向异构体的萃取效率均显著高于原型(P < 0.05),而脱水产物的萃取效率均显著低于原型(P < 0.05). TCs类污染物具有多个可电离官能团,具有非常复杂的酸碱化学,这导致其分子在不同pH条件下的行为发生变化. 以往的研究报道TCs类污染物在pH 3—5范围内萃取效率最好,0.5 g·L−1和5 g·L−1 Na2EDTA添加水平下水样的pH分别是5.0和4.5. 因此,本研究在水样萃取之前选择Na2EDTA的添加水平为5 g·L−1.
-
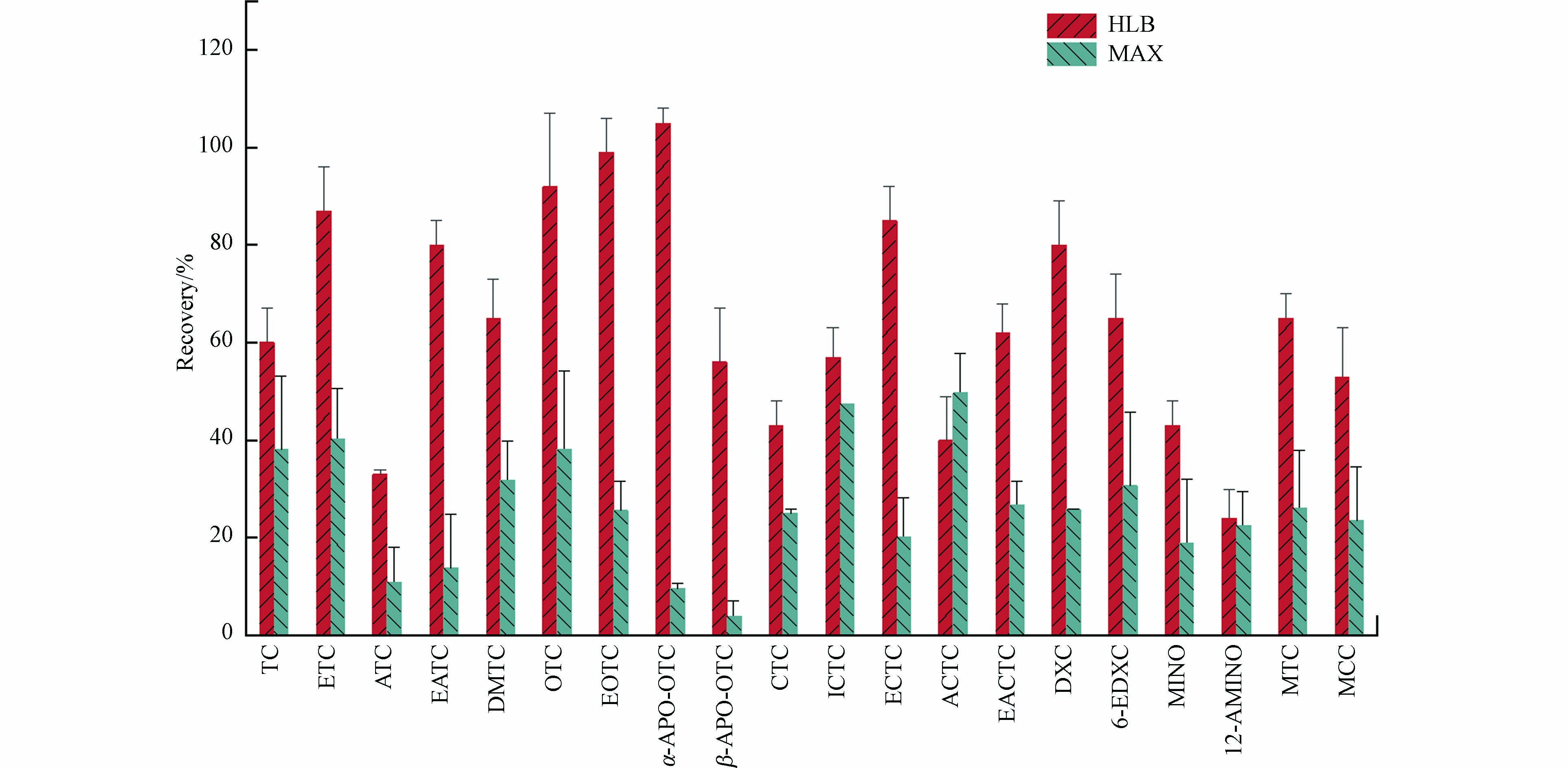
已经有不少研究表明,与C18等常用的硅胶基质固相萃取柱相比,Oasis HLB柱pH值稳定性更好、适用溶剂更广泛,更加适合TCs类极性化合物的保留[20 − 22]. 近年来有研究报道混合型阴离子交换柱Oasis MAX柱也能够有效萃取一些TCs,特别对于土壤等复杂基质具有较好的净化效果[23 − 25]. 根据文献报道的Oasis MAX柱萃取条件(pH 2.8),对比两种萃取柱的萃取效果,结果如图4所示,Oasis HLB柱的萃取效率总体来讲好于Oasis MAX柱. 值得注意的是,本研究中水样上样体积较大(1 L),而Oasis MAX柱因为一些TCs化合物极性大、水样体积达到1 L时易于被穿透而保留性能大大减弱(TC和OTC纯水回收率低至30%)[26]. 鉴于以往基于COD、NH3-N等水质指标研究报道2019年以后永定河水质较好[27],可以通过增大水样浓缩倍数的方式增大TCs化合物检出的可能性,因此,本研究选取Oasis HLB柱进行水样中TCs及其转化产物的富集萃取.
-
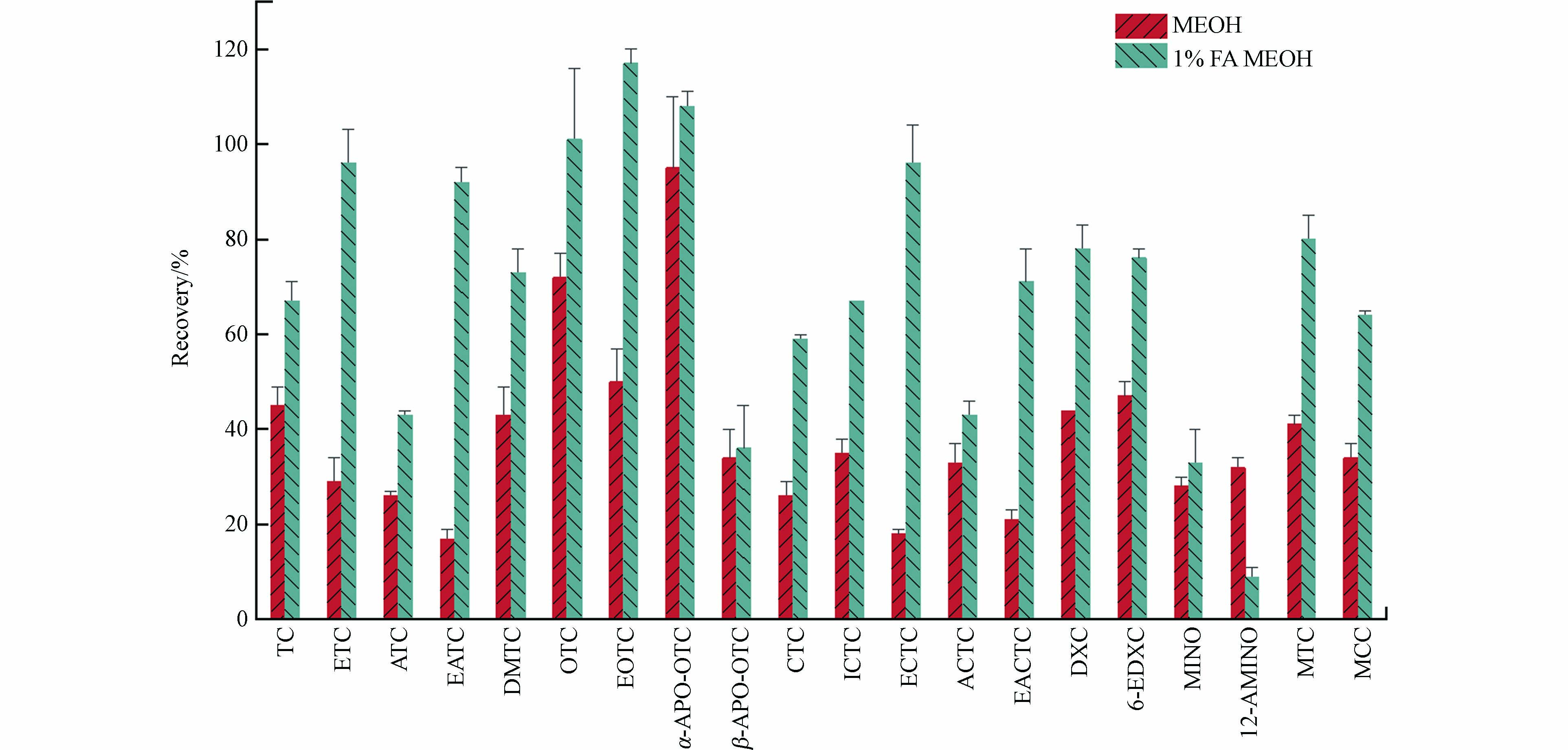
TCs类污染物和固相萃取柱吸附剂的性质,以及固相萃取柱洗脱液的组成共同决定了洗脱效果. 由于TCs类污染物是强极性化合物,甲醇经常被用于洗脱该类污染物[22,28 − 29]. 本研究也获得同样的结果,与乙腈等溶剂相比,甲醇更适合. 但是,针对本研究的20种TCs及其转化产物,绝大多数回收率都低于60%,特别是差向异构转化产物结果更低. 为了提高固相萃取回收率,进一步用甲酸酸化的甲醇进行洗脱. 结果如图5所示,大多数目标化合物回收率明显提高,说明甲酸酸化的甲醇用于Oasis HLB柱上TCs类污染物、特别是转化产物的洗脱效果更好.
-
采用全过程内标校正法定量分析,定量标准曲线包括0.1、0.5、1.0、5.0 、10.0、50.0、100.0 μg·L−1 的7个浓度水平. 在此范围内,所有目标化合物均具有良好的线性,相关系数均大于0.99. 实际采样过程中设置空白样品与平行样品(10%样品点位,3个),并在上游和下游各选取1个点位进行加标回收率实验(n=3). 其中空白样品中未检出任何目标抗生素,平行样品的相对标准偏差均在10%以内,20种加标样品的回收率除β-APO-OTC(46.8%)和12-AMINO(42.2%)外,其他均在60%—130%之间,相对标准偏差(RSD)小于15%. 方法检出限参照《环境监测分析方法标准制定技术导则》(HJ 168—2020),取永定河水1 L添加最后浓度为3 ng·L−1的20种目标化合物(n=7),经固相萃取浓缩后检测分析;计算7个添加样品目标化合物浓度的标准偏差,根据式(1)计算方法检出限,结果为24.5—927 pg·L−1 (表2),与以往报道的方法相比,略低于在线固相萃取-LC-MS/MS太湖水样中的分析方法(5 mL水样体积;TC,OTC,DXC和CTC检出限为500—1500 pg·L−1 [17])和离线固相萃取-UPLC-MS/MS南明河水样中的分析方法(1000 mL浓缩到1 mL;TC和OTC检出限为210—550 pg·L−1 [30]).
式中,t(n-1,0.99)当置信水平为99%且自由度为n-1时,双边分布的值. t(6,0.99)=3.413. Kf为样品的富集率.
-
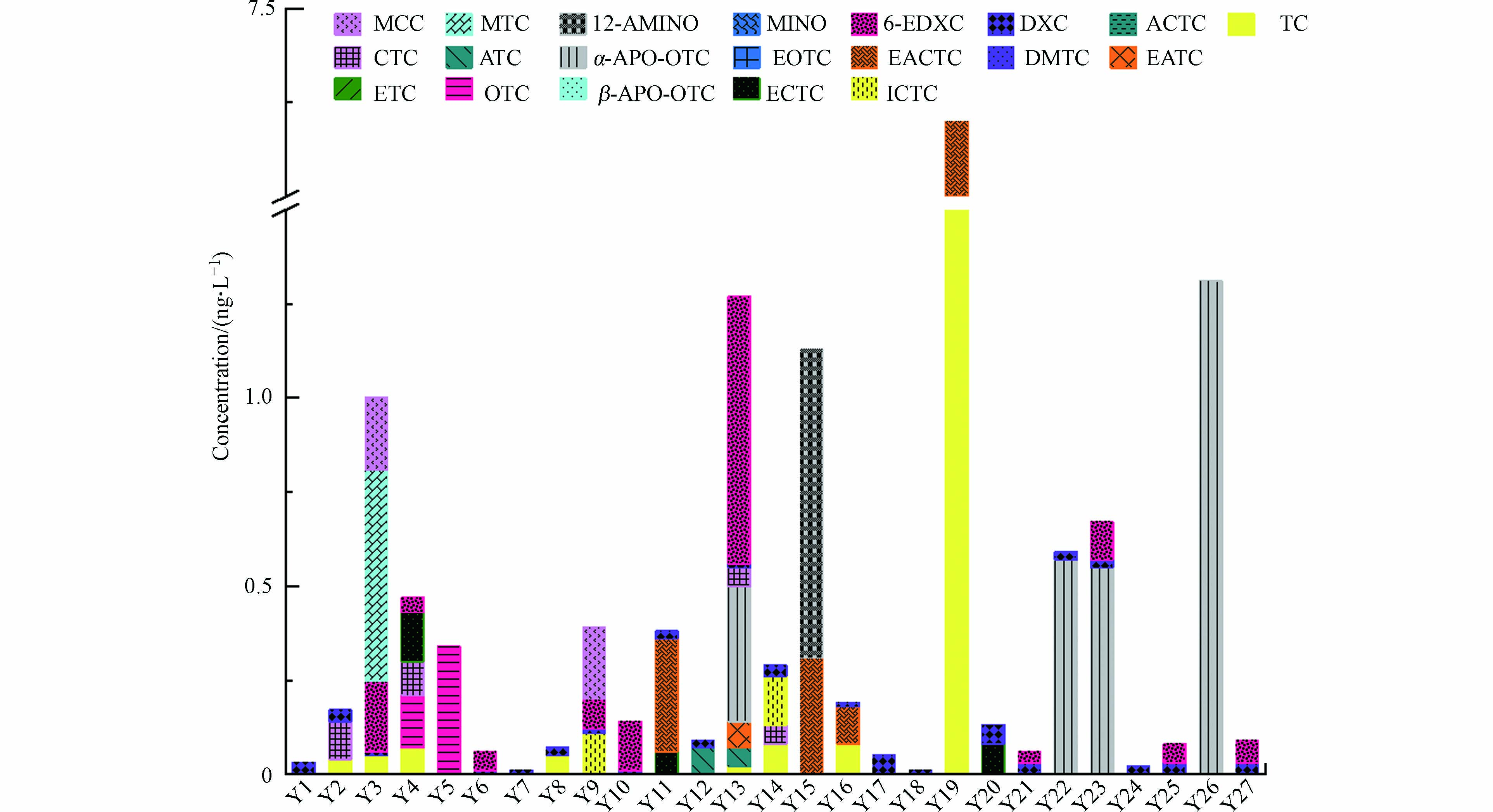
基于上述优化方法,对永定河水中四环素类及降解转化产物的赋存种类和含量水平进行了分析(表3和图6). 针对20种目标物质,检出了6种原型物质,它们的平均浓度和检出率分别是TC(520 pg·L−1, 14.8%), OTC(240 pg·L−1, 7.4%), CTC(100 pg·L−1, 7.4%), DXC(350 pg·L−1, 29.6%), MTC(560 pg·L−1, 3.7%)和MCC(190 pg·L−1, 7.4%). 可以看出,永定河水中四环素抗生素含量水平普遍处于较低的污染水平,浓度普遍低于1 ng·L−1. 本研究采集的永定河水样主要是官厅水库下游北京段,从2019年开始的官厅水库和引黄工程生态补水可能大大降低了污染物的含量水平[27]. 除了这些原型物质,8种降解转化产物也同时检出,包括差向异构产物ETC(平均浓度:1980 pg·L−1, 检出率:3.7%)和ECTC(80.0 pg·L−1, 14.8%),脱水产物及其差向异构产物ATC(70.0 pg·L−1, 3.7%), EATC(90.0 pg·L−1, 7.4%)和EACTC(1280 pg·L−1, 14.8%). 永定河水中CTC的差向异构产物相对于TC比例和频率更高. 这可能是由于C7位氯原子的空间排斥效应,CTC在C4位比TC更有可能发生差向异构转化所致. 另外,本研究没有检出OTC的差向异构和脱水产物. 其中,OTC在C5位的羟基与C-4位的二甲氨基相互作用可能一定程度上解释OTC比CTC和TC更难发生差向异构的现象[10]. 据报道,OTC的脱水产物相比于TC和CTC环境稳定性差、c环易于破裂生成内酯化产物[31]. 本研究的检出结果与之相符,OTC内酯化产物α-apo-OTC的含量水平和检出率(700 pg·L−1, 14.8%)均高于OTC. 本研究还检出了DXC在c环C-6位甲基差向异构物质6-EDXC,检出浓度(140 pg·L−1, 37.0%)甚至经常高于DXC.
尽管一些实验室研究报道四环素类抗生素在酸性条件下易于发生差向异构,在碱性条件下易于发生分子内脱水. 最新的研究表明,四环素类抗生素能够在河水基质(pH值)中发生差向异构和脱水反应[12],但是缺少真实水环境中的数据验证. 本研究在针对20种四环素类及降解产物建立同时分析检测技术的基础上,揭示了永定河水中四环素类抗生素降解产物广泛存在、且含量水平与本体在同一水平上. 有研究表明,四环素及其降解转化产物更易于吸附于颗粒物[32 − 33],说明沉积物中可能浓度更高. 由于四环素类降解产物可能产生毒性更强的效应,因此,应进行进一步调查评估、更加重视四环素类转化产物、及其与本体化合物复合暴露造成的环境和健康风险.
-
(1)基于固相萃取技术结合超高效液相色谱串联质谱建立了同时检测地表水体中7种TCs及13种转化产物的高灵敏分析方法,能有效识别和定量分析地表水体中的痕量污染.
(2)永定河北京段水体中TCs及其转化产物处于较低的浓度水平(基本低于1 ng·L−1).
(3)TCs的差向异构和脱水转化产物能够与其原型化合物能够同时存在于环境水体中,且浓度水平和检出频率可能不低,应重视TCs转化产物引起的环境风险.
地表水中20种四环素类抗生素及其转化产物的同时检测方法研究和应用
Research and application of simultaneous detection method for 20 tetracyclines and transformation products in surface water
-
摘要: 四环素类抗生素(TCs)由于水解和光解等作用容易生成多种转化产物、且有些转化产物具有更强的毒性效应,因此,TCs转化产物的环境存在引起的风险不容忽视. 本研究建立了超高效液相色谱串联三重四极杆质谱(UPLC-MS/MS)同时检测地表水体中7种TCs及13种转化产物的分析方法,细致研究了TCs及其转化产物的质谱碎裂和液相色谱分离规律. 水样添加5 g·L−1 Na2EDTA后经Oasis HLB固相萃取柱富集净化、1%甲酸甲醇洗脱后用UPLC-MS/MS测定. 流动相为甲醇和0.1%甲酸水溶液,在Waters BEH C18柱上采用梯度洗脱,实现了20种TCs及其转化产物的准确鉴定. 实际水样中绝大多数目标污染物的加标回收率为60%—130%,方法检出限在24.5—927 pg·L−1之间. 应用此方法于永定河(北京段)27个样品,检测出6种原型TCs(平均浓度70.0—560 pg·L−1;检出频率3.7%—29.6%)和9种转化产物(70.0—1980 pg·L−1;3.7%—37%),转化产物与原型物质的检出浓度和检出频率在同一水平上、甚至稍高,应该重视与TCs同时存在的转化产物所引起的环境和健康风险.Abstract: Due to the hydrolysis and photolysis of tetracycline antibiotics (TCs), they are easy to generate a variety of transformation products, and some of them even have stronger toxicities. Therefore, the risks caused by the environmental occurrences of TC transformation products could not be ignored. A method of ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) combined with solid-phase extraction (SPE) has been developed for simultaneous analysis of 7 TCs and 13 transformation products in surface water. It was studied in detail about their mass spectrometric fragmentation pattern and chromatographic separation. After adding 5 g·L−1 Na2EDTA, the water samples were enriched through Oasis HLB cartridges, and eluted with 1% formic acid in methanol for UPLC-MS/MS detection. The detection used gradient elution process on Waters BEH C18 column with methanol and 0.1% formic acid in water as the mobile phase to achieve accurate identification of these 20 analytes. The method detection limits were 24.5—927 pg·L−1 and spiking recoveries of 60%—130% for most target analytes in surface sample. Application of this method for 27 water samples collected from the Yongding River in Beijing area showed that 6 TCs and 9 transformation products were detected with the average concentrations of 70.0—560 pg·L−1 (detection frequencies of 3.7%—29.6%) and 70.0—1980 pg·L−1(3.7%—37%), respectively. The detection concentrations and detection frequencies of transformation products are close to those of TCs, thus more attention should be paid to the environmental and health risks caused by the coexisting TC transformation products.
-
Key words:
- emerging contaminants /
- antibiotics /
- tetracyclines /
- transformation products /
- UPLC-MS/MS /
- surface water.
-

-
表 1 20种TCs及其转化产物的结构信息和质谱参数
Table 1. Structure information and MS parameters of 20 tetracyclines and transformation products
结构
Structure中文名称
Chinese
Name英文名称
Compounds简称
Abbreviation结构
StructureR1 R2 R3 R4 R5 R6 R7 R8 R9 MRM CE/eV Cone/V 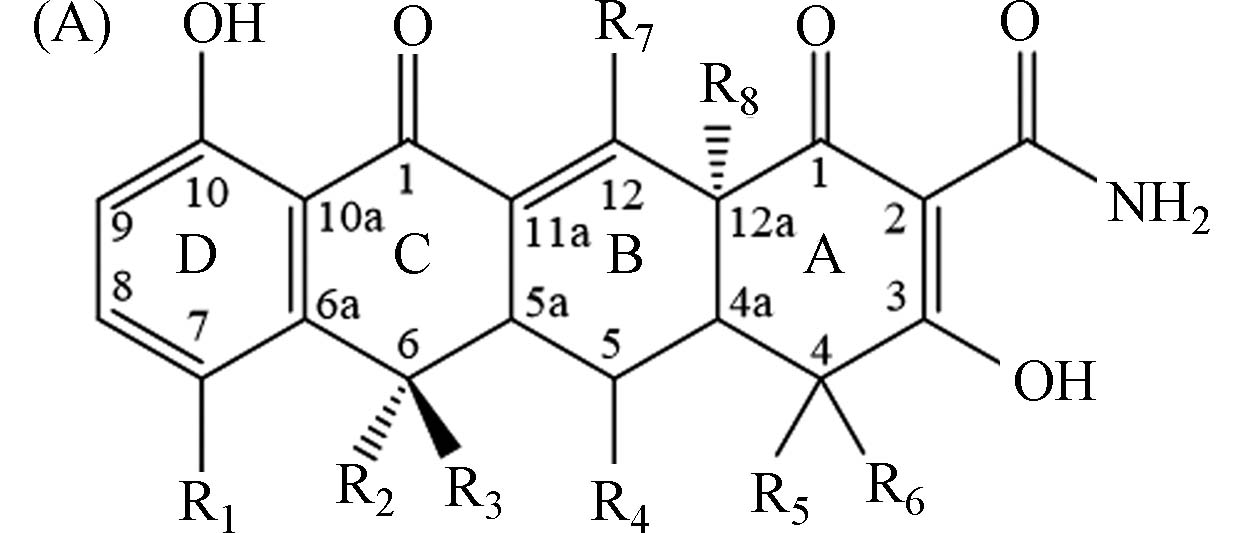
四环素 tetracycline TC A H OH CH3 H H N(CH3)2 OH OH — 445>410
445>42820
1630
304-差向四环素 4-epi-tetracycline ETC A H OH CH3 H N(CH3)2 H OH OH — 445>410
445>42820
1630
30脱水四环素 anhydrotetracycline ATC A H CH3 H H H N(CH3)2 OH OH — 427>410
427>15413
3030
404-差向脱水四环素 4-epi-anhydrotetracy-cline EATC A H CH3 H H N(CH3)2 H OH OH — 427>410
427>15413
3030
40去甲环素 demethyltetracy-cline DMTC A H OH H H H N(CH3)2 OH OH — 431>414
431>15413
2230
34土霉素 oxytetracycline OTC A H OH CH3 CH3 H N(CH3)2 OH OH — 461>426
461>20116
3040
404-差向土霉素 4-epi-oxyltracycline EOTC A H OH CH3 CH3 N(CH3)2 H OH OH — 461>426
461>44416
1040
40金霉素 chlortetracycline CTC A Cl OH CH3 H H N(CH3)2 OH OH — 479>444
479>15420
2610
144-差向金霉素 4-epi-chlortetracyeline ECTC A Cl OH CH3 H N(CH3)2 H OH OH — 479>444
479>15420
2610
14脱水四环霉素 anhydrochlortetracy-cline ACTC A Cl CH3 H H H N(CH3)2 OH OH — 461>444
461>42610
1640
404-差向脱水四环霉素 4-epi-anhydrochlortetracy-cline EACTC A Cl CH3 H H N(CH3)2 H OH OH — 461>444
461>42610
1640
40强力霉素 doxycycline DXC A H H CH3 OH H N(CH3)2 OH OH — 445>428
445>32116
2630
30β-多西环素 6-epi doxycycline 6-EDXC A H CH3 H OH H N(CH3)2 OH OH — 445>428
445>32116
2630
30米诺环素 minocycline MINO A N(CH3)2 H H H H N(CH3)2 OH OH — 458>441
458>35216
2030
30δ-亚氨基米诺环素 12-amino minocycline 12-A MINO A N(CH3)2 H H H H N(CH3)2 NH2 OH — 457>440
457>39516
2620
20美他环素 methacycline MTC A H =CH2 OH H N(CH3)2 OH OH — 443>426
443>20120
3610
24甲氯环素 meclocycline MCC A Cl =CH2 OH H N(CH3)2 OH OH — 477>235
477>9742
2614
14四环素-D6 tetracycline-D6 TC- D6 A H OH CH3 H H N(CD3)2 OH OH — 451>433
451>41616
2030
30金霉素-13C-D3 chlortetracycline-13C-D3 CTC-13C-D3 A Cl OH CH3 H H N213CC D3H3 OH OH — 485>450
485>15820
2614
14强力霉素-D3 doxycycline-D3 DXC-D3 A H D CH2D OH D N(CH3)2 OH OH — 448>431 16 20 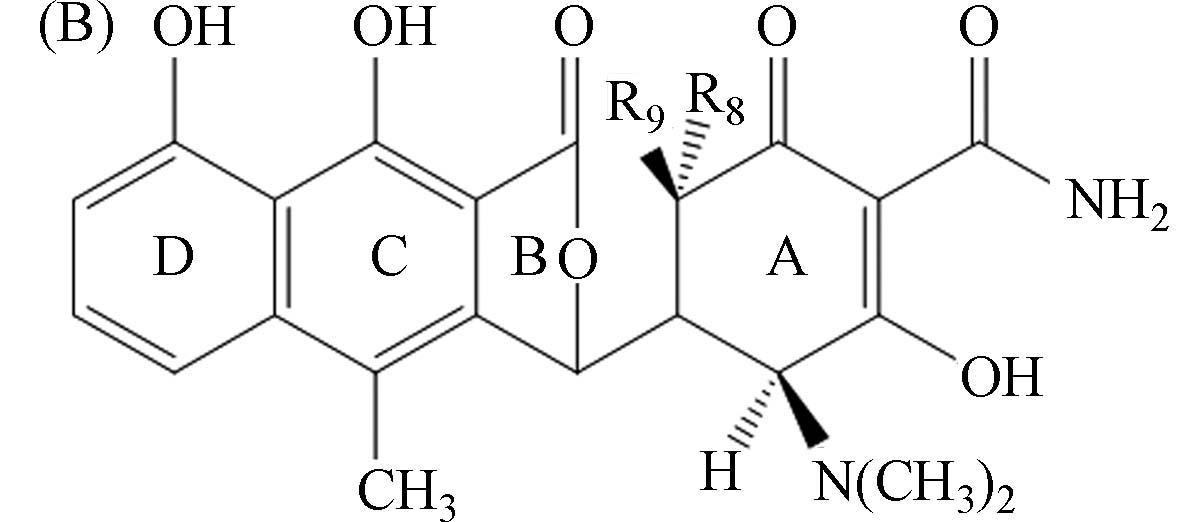
α-载脂蛋白-土霉素 α-apo-oxytetracycline α-apo-OTC B H OH — — — — — OH H 443>426
443>40820
2310
30β-载脂蛋白-土霉素 β-apo-oxytetracycline β-apo-OTC B OH H — — — — — H OH 443>426
443>40820
2310
30
异氯四环素 isochlortetracycline ICTC C — — — — — — — — — 479>462
479>15423
2610
14注:MRM(Multiple reaction monitoring);质谱多反应监测;CE(Collision energy):碰撞能量;Cone(Cone voltage):锥孔电压. 表 2 20种TCs及其转化产物的方法检出限和加标回收率
Table 2. Method detection limit and recovery of 20 TCs and transformation products
抗生素
Antibiotics线性回归方程
Regression equationR2 方法检出限/(pg·L−1)
Method detection limit回收率/%
RecoveryRSD/% 内标
Internal standardTC y=2.13x-1.79 0.993 67.0 74.8 2.10 TC-D6 ETC y=0.496x+0.0857 0.992 55.0 100.0 13.8 TC-D6 ATC y=5.99x-5.34 0.990 64.4 72.7 8.50 CTC-13C-D3 EATC y=2.60x-4.13 0.992 53.0 85.9 7.40 CTC-13C-D3 DMTC y=0.548x-0.486 0.994 103.0 84.2 5.20 TC-D6 OTC y=1.06x-1.76 0.990 14.7 124.8 2.00 TC-D6 EOTC y=0.206x-0.00990 0.999 127.0 86.3 8.60 TC-D6 α-APO-OTC y=1.71x-1.77 0.990 128.0 80.4 2.10 CTC-13C-D3 β-APO-OTC y=1.53x-0.0564 0.996 118.0 46.8 3.80 CTC-13C-D3 CTC y=1.43x-1.07 0.994 85.0 99.8 1.30 CTC-13C-D3 ICTC y=0.622x-0.942 0.991 53.0 60.0 2.40 CTC-13C-D3 ECTC y=0.388x-0.673 0.990 40.5 103.9 5.90 CTC-13C-D3 ACTC y=1.15x-0.667 0.991 927.0 82.8 6.70 CTC-13C-D3 EACTC y=0.621x-0.588 0.990 125.0 76.9 3.40 CTC-13C-D3 DXC y=3.29x-3.35 0.992 36.0 74.7 4.70 DXC-D3 6-EDXC y=4.76x-2.19 0.993 24.5 91.2 5.20 CTC-13C-D3 MINO y=0.798x-0.740 0.991 88.7 129.8 6.90 TC-D6 12-AMINO y=0.623x-0.300 0.996 63.8 42.2 8.50 TC-D6 MTC y=0.668x+0.171 0.990 98.4 83.3 3.10 TC-D6 MCC y=0.373x-0.287 0.991 32.2 61.3 4.20 CTC-13C-D3 表 3 永定河(北京段)水样中TCs及其转化产物含量和检出率
Table 3. Concentrations and detection frequencies of TCs and transformation products in water samples from the Yongding River (Beijing)
抗生素
Antibiotics含量范围/(pg·L−1)
Concentration range平均值/(pg·L−1)
Average concentration检出率/%
Detection ratioTC 70.0—1830 520 14.8 ETC 1980 1980 3.70 ATC 70.0 70.0 3.70 EATC 70.0—110 90.0 7.40 OTC 140—340 240 7.40 α-APO-OTC 360—1310 700 14.8 CTC 90.0—100 100 7.40 ICTC 110—130 120 7.40 ECTC 50.0—130 80.0 14.8 EACTC 300—3230 1280 14.8 DXC 30.0—50.0 350 29.6 6-EDXC 30.0—710 140 37.0 12-AMINO 820 820 3.70 MTC 560 560 3.70 MCC 190 190 7.40 -
[1] SHEN Q B, WANG Z Y, YU Q, et al. Removal of tetracycline from an aqueous solution using Manganese dioxide modified biochar derived from Chinese herbal medicine residues[J]. Environmental Research, 2020, 183: 109195. doi: 10.1016/j.envres.2020.109195 [2] PAN S F, ZHU M P, CHEN J P, et al. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane–Implications for antibiotics recovery[J]. Separation and Purification Technology, 2015, 153: 76-83. doi: 10.1016/j.seppur.2015.08.034 [3] ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance[J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. [4] WANG H L, CHEN T H, CHEN D, et al. Sulfurized oolitic hematite as a heterogeneous Fenton-like catalyst for tetracycline antibiotic degradation[J]. Applied Catalysis B:Environmental, 2020, 260: 118203. doi: 10.1016/j.apcatb.2019.118203 [5] 贾世杰, 高会旺, 祁建华. 海洋环境中抗生素抗性基因研究进展[J]. 环境化学, 2023, 42(3): 792-804. doi: 10.7524/j.issn.0254-6108.2022103101 JIA S J, GAO H W, QI J H. Antibiotic resistance genes in marine environment–a review[J]. Environmental Chemistry, 2023, 42(3): 792-804 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022103101
[6] CHEN Y L, JIANG C X, WANG Y L, et al. Sources, environmental fate, and ecological risks of antibiotics in sediments of Asia’s longest river: A whole-basin investigation[J]. Environmental Science & Technology, 2022, 56(20): 14439-14451. [7] WANG C, ZHAO Y P, LIU S, et al. Contamination, distribution, and risk assessment of antibiotics in the urban surface water of the Pearl River in Guangzhou, South China[J]. Environmental Monitoring and Assessment, 2021, 193(2): 98. doi: 10.1007/s10661-021-08887-5 [8] GU J Y, CHEN C Y, HUANG X Y, et al. Occurrence and risk assessment of tetracycline antibiotics in soils and vegetables from vegetable fields in Pearl River Delta, South China[J]. Science of the Total Environment, 2021, 776: 145959. doi: 10.1016/j.scitotenv.2021.145959 [9] 张晶晶, 陈娟, 王沛芳, 等. 中国典型湖泊四大类抗生素污染特征[J]. 中国环境科学, 2021, 41(9): 4271-4283 doi: 10.19674/j.cnki.issn1000-6923.20210510.007 ZHANG J J, CHEN J, WANG P F, et al. Pollution characteristics of four-type antibiotics in typical lakes in China[J]. China Environmental Science, 2021, 41(9): 4271-4283(in Chinese) doi: 10.19674/j.cnki.issn1000-6923.20210510.007
[10] ZHONG S F, YANG B, XIONG Q, et al. Hydrolytic transformation mechanism of tetracycline antibiotics: Reaction kinetics, products identification and determination in WWTPs[J]. Ecotoxicology and Environmental Safety, 2022, 229: 113063. doi: 10.1016/j.ecoenv.2021.113063 [11] LEICHTWEIS J, VIEIRA Y, WELTER N, et al. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic[J]. Process Safety and Environmental Protection, 2022, 160: 25-40. doi: 10.1016/j.psep.2022.01.085 [12] DZOMBA P, ZARANYIKA M F. Degradation of tetracycline in tropical river ecosystems: Generation and dissipation of metabolites;kinetic and thermodynamic parameters[J]. Reaction Kinetics, Mechanisms and Catalysis, 2022, 135(4): 2115-2136. doi: 10.1007/s11144-022-02249-z [13] 张业旺, 谭强, 刘瑞江, 等. 青霉素酰化酶制备6-APA的研究进展[J]. 中国抗生素杂志, 2008, 33(7): 385-391. doi: 10.13461/j.cnki.cja.004238 ZHANG Y W, TAN Q, LIU R J, et al. Progress in enzymatic production of 6-aminopenicillanic acid with penicillin acylase[J]. Chinese Journal of Antibiotics, 2008, 33(7): 385-391 (in Chinese). doi: 10.13461/j.cnki.cja.004238
[14] HALLING-SØRENSEN B, SENGELØV G, TJØRNELUND J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria[J]. Archives of Environmental Contamination and Toxicology, 2002, 42(3): 263-271. doi: 10.1007/s00244-001-0017-2 [15] ZHU P X, ZHOU L X, JIANG K Z, et al. Diastereomer recognition of three pairs of tetracyclines by electrospray ionization mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2022, 36(2): e9221. doi: 10.1002/rcm.9221 [16] ŠALA M, KOČAR D, LUKEŽIČ T, et al. Rapid identification of atypical tetracyclines using tandem mass spectrometric fragmentation patterns[J]. Rapid Communications in Mass Spectrometry, 2015, 29(17): 1556-1562. doi: 10.1002/rcm.7252 [17] SHEN F, XU Y J, WANG Y, et al. Rapid and ultra-trace levels analysis of 33 antibiotics in water by on-line solid-phase extraction with ultra-performance liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography. A, 2022, 1677: 463304. doi: 10.1016/j.chroma.2022.463304 [18] ŁUKASZEWICZ P, BIAŁK-BIELIŃSKA A, DOŁŻONEK J, et al. A new approach for the extraction of tetracyclines from soil matrices: Application of the microwave-extraction technique[J]. Analytical and Bioanalytical Chemistry, 2018, 410(6): 1697-1707. doi: 10.1007/s00216-017-0815-7 [19] VIDAL J L M, AGUILERA-LUIZ M D, ROMERO-GONZÁLEZ R, et al. Multiclass analysis of antibiotic residues in honey by ultraperformance liquid chromatography-tandem mass spectrometry[J]. Journal of Agricultural and Food Chemistry, 2009, 57(5): 1760-1767. doi: 10.1021/jf8034572 [20] ANDRADE-EIROA A, CANLE M, LEROY-CANCELLIERI V, et al. Solid-phase extraction of organic compounds: A critical review (Part Ⅰ)[J]. TrAC Trends in Analytical Chemistry, 2016, 80: 641-654. doi: 10.1016/j.trac.2015.08.015 [21] ANDRADE-EIROA A, CANLE M, LEROY-CANCELLIERI V, et al. Solid-phase extraction of organic compounds: A critical review (Part Ⅱ)[J]. TrAC Trends in Analytical Chemistry, 2016, 80: 655-667. doi: 10.1016/j.trac.2015.08.014 [22] 廖杰, 李青松. 测定13种抗生素的固相萃取-高效液相色谱串联质谱法优化与应用[J]. 环境化学, 2022, 41(5): 1538-1547. doi: 10.7524/j.issn.0254-6108.2022021302 LIAO J, LI Q S. Optimization and application of solid phase extraction-high performance liquid chromatography-tandem mass spectrometry for determination of 13 antibiotics[J]. Environmental Chemistry, 2022, 41(5): 1538-1547 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022021302
[23] JIA A, XIAO Y, HU J Y, et al. Simultaneous determination of tetracyclines and their degradation products in environmental waters by liquid chromatography–electrospray tandem mass spectrometry[J]. Journal of Chromatography A, 2009, 1216(22): 4655-4662. doi: 10.1016/j.chroma.2009.03.073 [24] ZHENG W L, ZHANG L F, ZHANG K Y, et al. Determination of tetracyclines and their epimers in agricultural soil fertilized with swine manure by ultra-high-performance liquid chromatography tandem mass spectrometry[J]. Journal of Integrative Agriculture, 2012, 11(7): 1189-1198. doi: 10.1016/S2095-3119(12)60114-2 [25] ZHOU J L, MASKAOUI K, LUFADEJU A. Optimization of antibiotic analysis in water by solid-phase extraction and high performance liquid chromatography–mass spectrometry/mass spectrometry[J]. Analytica Chimica Acta, 2012, 731: 32-39. doi: 10.1016/j.aca.2012.04.021 [26] de ZAN M M, GIL GARCÍA M D, CULZONI M J, et al. Solving matrix-effects exploiting the second order advantage in the resolution and determination of eight tetracycline antibiotics in effluent wastewater by modelling liquid chromatography data with multivariate curve resolution-alternating least squares and unfolded-partial least squares followed by residual bilinearization algorithms[J]. Journal of Chromatography A, 2008, 1179(2): 106-114. doi: 10.1016/j.chroma.2007.11.091 [27] 杨艳红, 王欣, 白璇. 引黄生态补水对官厅水库水质时空分布影响及污染源解析[J]. 水资源开发与管理, 2022, 8(3): 61-69. doi: 10.16616/j.cnki.10-1326/TV.2022.03.12 Yang Y H, Wang X, Bai X. Influence of ecological water supplement of the Yellow River Diversion on time and space distribution of Guanting Reservoir water quality and analysis of pollution sources[J]. Water Resources Development and Management, 2022, 8(3): 61-69 (in Chinese). doi: 10.16616/j.cnki.10-1326/TV.2022.03.12
[28] ZHAO W J, ZUO H Y, GUO Y, et al. Porous covalent triazine-terphenyl polymer as hydrophilic–lipophilic balanced sorbent for solid phase extraction of tetracyclines in animal derived foods[J]. Talanta, 2019, 201: 426-432. doi: 10.1016/j.talanta.2019.04.010 [29] SHAO B, JIA X F, WU Y N, et al. Multi-class confirmatory method for analyzing trace levels of tetracyline and quinolone antibiotics in pig tissues by ultra-performance liquid chromatography coupled with tandem mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2007, 21(21): 3487-3496. doi: 10.1002/rcm.3236 [30] LINGHU K L, WU Q X, ZHANG J, et al. Occurrence, distribution and ecological risk assessment of antibiotics in Nanming River: Contribution from wastewater treatment plant and implications of urban river syndrome[J]. Process Safety and Environmental Protection, 2023, 169: 428-436. doi: 10.1016/j.psep.2022.11.025 [31] WU X F, WEI Y S, ZHENG J X, et al. The behavior of tetracyclines and their degradation products during swine manure composting[J]. Bioresource Technology, 2011, 102(10): 5924-5931. doi: 10.1016/j.biortech.2011.03.007 [32] JI L L, WAN Y Q, ZHENG S R, et al. Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: Implication for the relative importance of black carbon to soil sorption[J]. Environmental Science & Technology, 2011, 45(13): 5580-5586. [33] 徐龙凤, 魏群山, 吕强, 等. 水体模拟颗粒物对四环素的吸附特性及基本规律[J]. 环境科学, 2018, 39(4): 1668-1676. doi: 10.13227/j.hjkx.201707225 XU L F, WEI Q S, LÜ Q, et al. Adsorption of tetracycline on simulated suspended particles in water[J]. Environmental Science, 2018, 39(4): 1668-1676 (in Chinese). doi: 10.13227/j.hjkx.201707225
-




 下载:
下载: