-
富营养化水体大量繁殖的藻类分泌的嗅味物质长期以来一直是饮用水行业关注的主要问题[1-2]. 水体中嗅味物质的浓度通常在ng·L−1范围内,但通常因其嗅味阈值极低,嗅味物质直接影响饮用水的感官,以及公众对饮用水的信心和接受程度[3-4]. 土臭素(geosmin, GSM)和2-甲基异茨醇(2-methylisobornel, 2-MIB)是引发水体异嗅的主要物质,与大多数饮用水中土、霉味事件有关[5-6]. 我国生活饮用水卫生标准(GB 5749—2022)已经将GSM和2-MIB列入到出厂水和管网水需要检测的项目,并规定其含量不能超过10 ng·L−1[7].
目前传统处理工艺通常很难彻底去除在水体中赋存浓度极低的嗅味物质. 虽然基于颗粒活性炭和粉末活性炭等少数技术可以去除嗅味物质[8-9],但面临嗅味物质浓度升高而引起的去除效率低、达标困难和高成本等问题[10]. 随着对优质饮用水需求的不断增加,越来越多研究致力于发展更有效的嗅味物质替代处理工艺. 近年来,基于羟基自由基(hydroxyl radicals, ·OH)的高级氧化技术(advanced oxidation processes, AOPs),因具有选择性低和反应速度快等优点[11],成为了去除嗅味物质优先选择的技术[12]. 在众多AOPs中,紫外线(ultraviolet light, UV)和双氧水(H2O2)通过生成·OH去除嗅味物质,且具有工艺灵活便捷和无副产品等优势而受到广泛关注,但该方法也存在H2O2利用率低和残留等问题[13]. 近来研究表明,向UV/H2O2体系中加入低浓度O3可有效增加系统中的稳态自由基浓度,其与O3作用可促进水中有机物的降解[14-16]. 同时,UV/H2O2与生物活性炭(biological activated carbon, BAC)工艺联用(UV/H2O2-BAC)则可有效解决H2O2残留问题[17]. 目前基于UV/H2O2-BAC发展的嗅味物质去除工艺主要基于实验室小试装置或现场小规模试验进行,尽管能取得满意的去除效果,但其应用于大流量水的工艺条件少有优化,效果少有验证[18].
针对当前UV/H2O2-BAC工艺去除嗅味物质存在的难点,本研究利用增设的AOP中试装置,探究了UV/H2O2-BAC和UV/H2O2/O3-BAC两种耦合工艺对山东省某饮用水处理厂的砂滤池出水中GSM和2-MIB的去除效果,并考察了H2O2投加量、O3投加量、紫外线剂量及BAC对降解效率的影响,以期为发展绿色高效的嗅味物质去除技术提供数据和技术支撑.
-
自主研制的中试装置如图1所示,尺寸为3.6 m×1.2 m×2.6 m. 紫外复合催化反应器是中试装置的核心部件,其构型已从反应器内流态、光辐射和化学反应等方面实现优化,目的在于最大化利用紫外光能并提升·OH等活性物质的产率,从而促进嗅味物质的去除效率. 中试装置共有12支87 W的UV灯管,UV剂量通过控制UV灯开关调控,H2O2的投加量由蠕动泵控制,O3通过O3发生器制取并通过射流泵控制其投加量,同时分别在蠕动泵与射流泵前端配置监控流量计以确保H2O2和O3在AOPs体系中的流量和浓度稳定.
-
UV/H2O2-BAC和UV/H2O2/O3-BAC中试工艺设置在山东省潍坊市某自来水厂,其流程如图2所示. 该自来水水厂供水水源取自白浪河水库,水厂主体处理工艺为混凝-沉淀-砂滤-二氧化氯消毒. 研究增设AOP中试反应装置,其进水为自来水厂的砂滤池出水. 中试期间进水水质参数如表1所示,GSM和2-MIB的浓度分别在28.5—34.1 ng·L−1和83.2—94.1 ng·L−1之间. 为考察中试装置和工艺对大流量水的处理效果,进水流量设置为10 m3·h−1. 进水先通入混合器与投加试剂充分混合,随后进入AOP反应器去除嗅味物质(紫外光照时间为34 s),工艺末端为BAC罐,以去除反应器出水中残余的H2O2、O3和嗅味物质. H2O2和O3的投加量通过蠕动泵控制.
-
参数调整时,工艺稳定运行至少1 h后再取样. 水样采集点如图2所示,分别为中试进水,AOP反应器出水和BAC罐出水. 采集后的水样经过0.45 μm滤膜过滤后储存于棕色玻璃采样瓶,并于4 ℃条件下运送至实验室分析.
-
由于水源水中嗅味物质浓度较大波动,高浓度维持时间较短,中试工艺主要考察了H2O2投加量、O3投加量和UV剂量对GSM和2-MIB去除的影响. 中试装置对不同试验条件的操作如下:反应前使用滤池出水冲洗装置并排出,开启UV灯进行预热;分别调节H2O2和O3投加量与进水流量. 通过控制UV灯的开关、H2O2和O3的投加实现UV/H2O2、UV/H2O2/O3等多种AOP反应体系. 具体条件如下:首先在UV剂量为800 mJ·cm−2条件下,探究了H2O2投加量(0、1、2、3、5、10、20 mg·L−1),与O3投加量(0、0.5、1、2、3、5 mg·L−1)对UV/H2O2和UV/H2O2/O3体系的影响. 优化完成后,进一步探究了UV剂量(0、200、400、600、800 mJ·cm−2)对AOP反应体系的影响.
-
仪器:GSM和2-MIB提取和测定采用磁力搅拌水浴加热锅(SHJ-A6型,金坛区白塔安瑞实验仪器厂)和气相色谱-质谱联用仪(GC-MS, 6890N-5973C, Agilent).
试剂:GSM和2-MIB的标准品购自美国Sigma-Aldrich公司. 内标物(1, 2-二氯苯-D4)购自美国Accustandard公司. 氯化钠(NaCl)购自中国阿拉丁公司,使用前经450 ℃烘烤2 h然后保存于干燥器中备用.
-
GSM和2-MIB的测定参照GB/T 32470—2016《生活饮用水臭味物质 土臭素和2-甲基异莰醇检验方法》. GSM和2-MIB的前处理采用固相微萃取法[19]:在50 mL顶空瓶中加入30 mL水样、7.5 g离子强度调节剂NaCl和15 μL内标溶液1, 2-二氯苯-D4(浓度为20 μg·L−1),然后将顶空瓶置于磁力加热搅拌器搅拌加热(温度为60 ℃,搅拌速度为800 r·min−1),并采用萃取纤维(50/30 μm DVB/CAR/PDMS, Supelco)对水样中的嗅味物质进行萃取,萃取温度为60 ℃. 萃取30 min后,将萃取纤维头插入气相色谱进样口解吸5 min进行分析,解吸温度为250 ℃. GC-MS分析条件和升温程序如下:气相色谱柱为HP-5MS(30 m×0.25 mm×0.25 μm, Agilent),嗅味物质定量采用选择离子检测模式,进样口温度为250 ℃,离子源温度为230 ℃,压力为56.5 kPa,初始温度为60 ℃保持2.5 min,以8 ℃·min−1速率升至250 ℃保持5 min;质谱仪选用电子电离源,接口温度为280 ℃,离子化能量为70 eV. 降解产物分析采用全扫描模式.
-
图3(a, b)为H2O2投加量对UV/H2O2体系降解嗅味物质的影响. 结果表明,当不投加H2O2时,GSM和2-MIB的去除率分别为2.0%和1.2%,说明纯UV对嗅味物质去除效果有限. 投加H2O2后,嗅味物质的去除率显著上升,当H2O2浓度从1.0 mg·L−1增加至5.0 mg·L−1时,GSM和2-MIB的去除率分别从23.8%和25.9%显著提高至41.6%和45.8%. 在10 mg·L−1条件下进一步提高到48.2%和52.3%,表明UV/H2O2中生成的·OH在嗅味物质的去除中发挥了关键作用.
当H2O2投加量达到20 mg·L−1时,GSM和2-MIB的去除率相比10 mg·L−1发生了下降,这说明投加过高浓度的H2O2会抑制嗅味物质的去除. 当H2O2浓度低于10 mg·L−1时,增加H2O2浓度可以增加·OH的生成量,从而显著促进GSM和2-MIB的降解,但当H2O2达到20 mg·L−1时,过量的H2O2就会消耗·OH从而不利于GSM和2-MIB的去除. 相比实验室模拟条件,实际水体中嗅味物质在纯UV和UV/H2O2体系中的去除率有所降低[20],这与实际水体中的组分(例如有机质,CO32–、HCO3–)削减UV光强和消耗·OH有关.
图3(c, d)为H2O2投加量对UV/H2O2/O3体系降解嗅味物质的影响. 结果表明,纯O3浓度投加为3.0 mg·L−1时,无UV光照GSM和2-MIB的去除率分别为21.0%和21.6%,该数据明显低于常温条件下O3去除嗅味物质的效率[15,21-22],说明低温下O3的反应效率低,与先前文献报道一致[23],因此需引入UV和H2O2以促进基于O3的AOPs体系对嗅味物质的去除;有UV光照GSM和2-MIB的去除率为30.7%和30.1%,说明UV/O3能促进·OH的生成和嗅味物质的去除. 向UV/H2O2体系中投加O3能略微提升嗅味物质的去除率,H2O2投加浓度对UV/H2O2/O3体系去除嗅味物质的影响与UV/H2O2体系相似.
图4为H2O2投加量保持为5.0 mg·L−1时,O3投加量对UV/H2O2/O3体系去除嗅味物质的影响. 结果表明,当O3投加量从0.5 mg·L−1逐渐升至5.0 mg·L−1时,UV/H2O2/O3工艺对GSM和2-MIB的去除率维持在40%—50%之间,相较于UV/H2O2(GSM:41.6%和2-MIB:45.8%)促进效果并不明显. 投加O3浓度较低会导致嗅味物质去除率下降,这可能与O3的反应特性相关,O3会优先与有机质中的不饱和结构发生反应并导致有机质分子解聚变成小分子,从而竞争·OH引起嗅味物质去除率的下降[24]. 当O3投加到3.0 mg·L−1时,才会有余量O3促进·OH的产生和嗅味物质的降解. 在UV/O3体系中,GSM和2-MIB的去除率分别在19.7%—35.1%和25.4%—33.4%之间,说明H2O2的存在能显著促进·OH的产生和嗅味物质的去除. 综合成本和嗅味物质去除效果,确定UV/H2O2和UV/H2O2/O3工艺H2O2和O3的投加量分别为5.0 mg·L−1和3.0 mg·L−1.
图5为UV剂量对基于UV的AOPs工艺去除嗅味物质的影响. 结果表明,UV、UV/H2O2、UV/H2O2/O3工艺对GSM和2-MIB的去除率随UV剂量升高而增加.
当UV剂量从200 mJ·cm−2提高至800 mJ·cm−2时,对应工艺中GSM分别从3.0%、19.8%、23.4%提高至5.7%、40.1%和41.3%;2-MIB去除率分别从2.4%、20.8%、21.6%提高至9.5%、43.6%和45.6%. 而无UV时,单独投加H2O2对GSM和2-MIB的去除率仅有3.4%和6.8%,投加H2O2/O3对GSM和2-MIB的去除率略微提高至10.2%和7.5%,说明UV激发H2O2和O3产生的·OH对嗅味物质的去除具有重要的作用. 在相同的UV剂量下,UV/H2O2/O3对GSM和2-MIB的去除高于UV/H2O2,说明O3的投加促进了·OH的生成,并与O3联合作用去除水中的嗅味物质和其他有机物.
-
UV/H2O2和UV/H2O2/O3体系均存在H2O2、O3等残留问题,影响后续消毒等过程,需通过活性炭进一步去除. 同时与活性炭耦合后,也能有效发挥活性炭的吸附及生物降解作用,进一步提升嗅味物质的去除效果. 因此,工艺设计中将AOP与BAC耦合以去除残余H2O2,并利用BAC的吸附和微生物作用进一步去除嗅味物质. 如图6所示,耦合BAC显著提升了GSM和2-MIB的去除率,UV、UV/O3、UV/H2O2和UV/H2O2/O3对GSM的去除率从5.7%、30.7%、40.6%、45.8%分别提高至61.3%、70.8%、71.9%、90.1%,对2-MIB的去除率从9.5%、30.7%、43.6%、47.7%分别提高至58.0%、71.5%、73.0%、86.0%. BAC去除嗅味物质主要基于吸附和微生物降解作用:首先BAC通过物理化学吸附作用将嗅味物质吸附到活性炭表面和孔隙内,但吸附作用会在BAC吸附达到饱和后逐渐降低[25]. 随后,被吸附的嗅味物质会被BAC上的微生物降解,在BAC吸附饱和后,BAC对嗅味物质的吸附和降解引起的解吸达到平衡[26]. 尽管UV/H2O2/O3相比UV/H2O2对GSM和2-MIB提升不大,但经过BAC吸附降解处理后,前者与BAC耦合对嗅味物质去除率(GSM:90.1%,2-MIB:86.0%)显著高于后者与BAC耦合(GSM:71.9%,2-MIB:73.0%),这说明水体经UV/H2O2/O3处理后,嗅味物质在BAC上的吸附降解率高于UV/H2O2处理后的吸附量. 其可能原因在于UV/H2O2/O3更有利于有机质转化为小分子和矿化,进而更容易在BAC中被微生物降解,从而有更多的活性位点用于吸附并降解嗅味物质. 为证实该推测,对水样进行了TOC分析,如表2所示,发现UV/H2O2/O3处理出水的TOC明显低于其他工艺,耦合BAC后TOC更是降低至1.04 mg·L−1,远低于UV/H2O2和UV/O3耦合BAC出水的TOC含量. BAC出水TOC与嗅味物质浓度的下降和显著下降,表明BAC通过吸附和微生物降解等作用进一步去除了有机质、嗅味物质及其降解产物,这对于降低消毒副产物的生成和保障饮用水安全具有重要意义.
-
为探究GSM和2-MIB在UV/H2O2工艺中的降解途径和中间产物,利用气质联用仪对中试进水和UV/H2O2出水进行了全扫描分析,工艺参数为H2O2投加量10 mg·L−1,紫外线剂量800 mJ·cm−2,取样后加入0.1 mol·L−1 Na2S2O3猝灭残余H2O2. 检出的GSM和2-MIB降解产物如表3和表4所示,结合降解产物和文献调研[16,23-24],提出了UV/H2O2中GSM和2-MIB的降解途径,其降解主要由·OH引起,发生的主要反应包括脱甲基、脱水、加成、环开裂等[27-29].
GSM的降解途径如下:首先,·OH攻击GSM侧链的羟基和甲基进行氧化脱氢和去甲基反应,形成具有环状结构和双键的初级降解产物. 然后,初级降解产物与·OH发生加成反应,电子发生转移,断裂C—C键并打开双环结构,生成单环次级氧化降解产物. 最后,·OH与次级产物继续发生加成反应,使C—C和C=C键断裂,生成小分子醛酮等三级降解产物. 2-MIB的降解途径如下:首先,·OH攻击2-MIB侧链的羟基和甲基发生脱水和脱甲基反应,从而破坏2-MIB结构形成含有酮基的初级产物,樟脑是主要初级降解产物,这与先前文献报道一致[30-31]. 然后,·OH通过加成反应断开桥环结构并形成次级单环醛和酮基小分子. 随后,·OH与次级产物进行环加成反应并转移电子,使环状结构的化学键断裂,生成三级小分子醛、酮、酸等降解产物.
-
(1)实际运行条件下UV/H2O2和UV/H2O2/O3工艺对GSM和2-MIB均有良好的去除效果,其中后者的去除效果更好. 提高H2O2和O3投加量、以及紫外线剂量有利于AOPs工艺对GSM和2-MIB的去除,但过量H2O2会抑制嗅味物质的去除;
(2)UV/H2O2和UV/H2O2/O3耦合BAC进一步提高了GSM和2-MIB的去除率,后者更有利于嗅味物质和有机质的去除;
(3)利用GC-MS全扫描对UV/H2O2体系中GSM和2-MIB的降解产物进行了分析,其降解主要由·OH攻击侧链引起,发生的反应包括脱甲基、脱水、加成、环开裂等.
中试紫外高级氧化耦合生物活性炭工艺去除典型嗅味物质
Pilot test of removing typical odors by the coupled UV advanced oxidation and biological activated carbon processes
-
摘要: 利用增设的紫外高级氧化中试装置,研究了紫外/过氧化氢(UV/H2O2)和紫外/过氧化氢/臭氧(UV/H2O2/O3)与生物活性炭(BAC)耦合工艺对低温下大流量饮用水中较高含量土臭素(GSM)和2-甲基异莰醇(2-MIB)的去除效果. 重点考察了H2O2投加量、O3投加量、紫外线剂量及BAC对嗅味物质去除的影响,并分析了UV/H2O2过程中GSM和2-MIB的降解产物情况. 结果显示,相比UV/H2O2-BAC耦合工艺,UV/H2O2/O3-BAC耦合工艺对GSM(28.5—34.1 ng·L−1)和2-MIB(83.2—94.1 ng·L−1)具有更显著的去除效果,去除率分别可达90.1%和86.0%. 提高H2O2投加量、O3投加量和紫外线剂量促进了紫外高级氧化工艺段对GSM和2-MIB的去除,但H2O2投加量不可过高. 通过对降解产物进行分析,提出了UV/H2O2降解GSM和2-MIB的过程,其降解主要由羟基自由基引起,主要反应包括脱甲基、脱水、加成、环开裂等方式. 本研究可为发展绿色高效的嗅味物质去除技术提供技术指导和支撑.
-
关键词:
- 土臭素 /
- 2-甲基异莰醇 /
- 紫外/过氧化氢 /
- 紫外/过氧化氢/臭氧 /
- 生物活性炭.
Abstract: Using a pilot-scale UV advanced oxidation plant, this study aimed to investigate the efficacy of UV/H2O2 and UV/H2O2/O3 coupled with biological activated carbon (BAC) processes in removing high levels of geosmin (GSM) and 2-methylisoborneol (2-MIB) from drinking water. The effects of H2O2 dosage, O3 dosage, UV dose, and BAC on odour removal were investigated in detail, along with the degradation products of GSM and 2-MIB during the UV/H2O2 process. Results showed that the UV/H2O2/O3-BAC coupling process exhibited higher removal rates on GSM (28.5—34.1 ng·L−1) and 2-MIB (83.2—94.1 ng·L−1), with removal rates up to 90.1% and 86.0%, respectively, compared to the UV/H2O2-BAC coupling process. Increasing the H2O2, O3, and UV doses promoted the removal of GSM and 2-MIB in the UV advanced oxidation process unit; however, the H2O2 dosage should not be excessively high. Based on the degradation products, a proposed degradation process and mechanism of GSM and 2-MIB by UV/H2O2 suggested that hydroxyl radicals, including demethylation, dehydration, addition, and ring-opening, caused immediate reactions. This study provides fundamental data and technical support for developing green and efficient odour removal technologies.-
Key words:
- GSM /
- 2-MIB /
- UV/H2O2 /
- UV/H2O2/O3 /
- BAC
-

-
表 1 砂滤池的出水水质和嗅味物质浓度
Table 1. The quality and odors concentration of sand filter effluent
pH 温度/℃
Temperature溶解氧/(mg·L−1)
Dissolved oxygenTOC/
(mg·L−1)GSM浓度/(ng·L−1)
GSM concentration2-MIB浓度/(ng·L−1)
2-MIB concentration7.6—8.2 5.5—6.8 11.7—13.5 3.3—4.2 28.5—34.1 83.2—94.1 表 2 UV/H2O2-BAC和UV/H2O2/O3-BAC工艺的进出水TOC浓度
Table 2. TOC concentration of inlet and outlet water of UV/H2O2-BAC and UV/H2O2/O3-BAC
中试工艺
Pilot test process中试进水/(mg·L−1)
Pilot test influentAOP出水/(mg·L−1)
AOP effluentBAC出水/(mg·L−1)
BAC effluentUV+BAC 3.4±0.1 3.1±0.1 2.6±0.2 UV/H2O2+BAC 3.6±0.1 3.4±0.1 2.5±0.1 UV/O3+BAC 3.2±0.2 3.0±0.1 2.2±0.2 UV/H2O2/O3+BAC 3.3±0.2 3.1±0.3 1.0±0.2 表 3 UV/H2O2降解GSM的中间产物及其保留时间和质谱特征
Table 3. Retention time (tR) and mass spectral characteristics (M, m/z) of GMS degradation products in UV/H2O2
检出物质
Detected substancestR/min 分子量/Da
Molecular weight定性离子(m/z)
Qualitative ions结构式
StructureGSM 14.37 182.30 112, 125 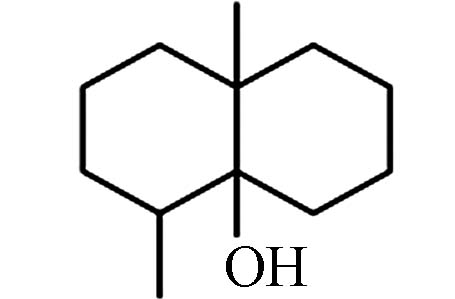
Trans-1,10-dimethyl-trans-9-decalinol 13.34 182.30 126, 112, 43 
4a-Methyl-4,4a,5,6,7,8-hexahydro-2(3H)-naphthalenone 12.44 164.24 164, 122, 107 
2-Ethyl-2-hexenal 11.96 126.20 126, 97, 55 
(1,2-Dimethylcyclopentyl)-methanol 11.83 128.21 97, 55 
Cyclohexanone 8.14 5.27 112, 98, 55 
Octanoic acid 7.98 144.21 101, 73, 60 
Pentanal 7.48 86.13 58, 44, 29 
表 4 UV/H2O2降解2-MIB的产物及其保留时间和质谱特征
Table 4. Retention time (tR) and mass spectral characteristics (M, m/z) of 2-MIB degradation products in UV/H2O2
检测物质
Detection of substancestR/min 分子量/Da
Molecular weight定性离子(m/z)
Qualitative ions结构式
Structure2-MIB 10.38 168.28 135, 95, 107 
(2,2,3-Trimethyl-cyclopent-3-enyl)-acetaldehyde 11.34 152.23 108, 95, 93 
(4Z)-4-chloro-6-ethoxy-2,6-dimethylhepta-2,4-diene 10.81 202.72 124, 109 
2,3,4,5-Tetramethyl-2-cyclopentenone 8.77 138.21 138, 123, 95 
2,6-Dimethyl-2,4-heptadiene 8.09 124.22 112, 69, 55 
D-camphor 7.80 152.23 95, 81, 69 
Bornane-2,5-dione 7.48 166.22 108, 93 
-
[1] ZHU J, STUETZ R M, HAMILTON L, et al. Management of biogenic taste and odour: From source water, through treatment processes and distribution systems, to consumers [J]. Journal of Environmental Management, 2022, 323: 116225. doi: 10.1016/j.jenvman.2022.116225 [2] 吕成旭, 石瑞洁, 季铭, 等. 高外源性藻类输入的城市河道嗅味物质分布特征及影响因素 [J]. 环境化学, 2022, 41(5): 1579-1590. doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2021010602 LV C X, SHI R J, JI M, et al. Distribution characteristics and influencing factors of odorants in urban rivers with high exogenous algae input [J]. Environmental Chemistry, 2022, 41(5): 1579-1590(in Chinese). doi: http://dx.doi.org/10.7524/j.issn.0254-6108.2021010602
[3] 李勇, 张晓健, 陈超. 我国饮用水中嗅味问题及其研究进展 [J]. 环境科学, 2009, 30(2): 583-588. LI Y, ZHANG X J, CHEN C. Review on the tastes and odors compounds in drinking water of China [J]. Environmental Science, 2009, 30(2): 583-588(in Chinese).
[4] KEHOE M J, CHUN K P, BAULCH H M. Who smells? Forecasting taste and odor in a drinking water reservoir [J]. Environmental Science & Technology, 2015, 49(18): 10984-10992. [5] WANG C M, GALLAGHER D L, DIETRICH A M, et al. Data analytics determines co-occurrence of odorants in raw water and evaluates drinking water treatment removal strategies [J]. Environmental Science & Technology, 2021, 55(24): 16770-16782. [6] ABD EL-HACK M E, EL-SAADONY M T, ELBESTAWY A R, et al. Undesirable odour substances (geosmin and 2-methylisoborneol) in water environment: Sources, impacts and removal strategies [J]. Marine Pollution Bulletin, 2022, 178: 113579. doi: 10.1016/j.marpolbul.2022.113579 [7] 李勇, 张晓健, 陈超. 水中嗅味评价与致嗅物质检测技术研究进展 [J]. 中国给水排水, 2008, 24(16): 1-6. LI Y, ZHANG X J, CHEN C. Research progress in evaluation of tastes and odors compounds in water and their detection technology [J]. China Water & Wastewater, 2008, 24(16): 1-6(in Chinese).
[8] 史嘉璐, 龙超, 李爱民. 饮用水源水中致嗅物质去除技术研究进展 [J]. 环境科学与技术, 2012, 35(3): 122-126. SHI J L, LONG C, LI A M. Progress in removal technology of taste and odor compounds-geosmin and 2-MIB in drinking water source [J]. Environmental Science & Technology, 2012, 35(3): 122-126(in Chinese).
[9] JUNG S W, BAEK K H, YU M J. Treatment of taste and odor material by oxidation and adsorption [J]. Water Science and Technology, 2004, 49(9): 289-295. doi: 10.2166/wst.2004.0588 [10] BERTONE E, CHANG C, THIEL P, et al. Analysis and modelling of powdered activated carbon dosing for taste and odour removal [J]. Water Research, 2018, 139: 321-328. doi: 10.1016/j.watres.2018.04.023 [11] LEE Y, GERRITY D, LEE M J, et al. Organic contaminant abatement in reclaimed water by UV/H2O2 and a combined process consisting of O3/H2O2 followed by UV/H2O2: Prediction of abatement efficiency, energy consumption, and byproduct formation [J]. Environmental Science & Technology, 2016, 50(7): 3809-3819. [12] HUANG X L, WANG S, WANG G X, et al. Kinetic and mechanistic investigation of geosmin and 2-methylisoborneol degradation using UV-assisted photoelectrochemical [J]. Chemosphere, 2022, 290: 133325. doi: 10.1016/j.chemosphere.2021.133325 [13] ZOSCHKE K, DIETRICH N, BÖRNICK H, et al. UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation [J]. Water Research, 2012, 46(16): 5365-5373. doi: 10.1016/j.watres.2012.07.012 [14] ARSLAN A, TOPKAYA E, ÖZBAY B, et al. Application of O3/UV/H2O2 oxidation and process optimization for treatment of potato chips manufacturing wastewater [J]. Water and Environment Journal, 2017, 31(1): 64-71. doi: 10.1111/wej.12227 [15] WANG X L, WANG X L, MI J R, et al. UV/H2O2/O3 removal efficiency and characterization of algae-derived organic matter and odorous substances [J]. Journal of Environmental Chemical Engineering, 2023, 11(1): 109128. doi: 10.1016/j.jece.2022.109128 [16] 刘超, 强志民, 张涛, 等. 臭氧和基于臭氧的高级氧化工艺降解农药的研究进展 [J]. 环境化学, 2011, 30(7): 1225-1235. LIU C, QIANG Z M, ZHANG T, et al. Research progress on degradation of pesticides by ozone and advanced oxidation process based on ozone [J]. Environmental Chemistry, 2011, 30(7): 1225-1235(in Chinese).
[17] PRADHAN S, FAN L H, RODDICK F A. Removing organic and nitrogen content from a highly saline municipal wastewater reverse osmosis concentrate by UV/H2O2–BAC treatment [J]. Chemosphere, 2015, 136: 198-203. doi: 10.1016/j.chemosphere.2015.05.028 [18] 王永磊, 刘杰, 王猛, 等. 紫外高级氧化工艺降解土臭素(GSM)和2-甲基异莰醇(2-MIB)的对比 [J]. 环境化学, 2022, 41(9): 3083-3093. doi: 10.7524/j.issn.0254-6108.2021050501 WANG Y L, LIU J, WANG M, et al. Comparison of degradation of geosmin (GSM) and 2- methyl isoborneol (2-MIB) by ultraviolet advanced oxidation process [J]. Environmental Chemistry, 2022, 41(9): 3083-3093(in Chinese). doi: 10.7524/j.issn.0254-6108.2021050501
[19] YOU YEAN-WOONG. 饮用水中2-甲基异莰醇(2-MIB)和土臭素的高灵敏检测 [J]. 环境化学, 2016, 35(8): 1733-1736. YOU Y W. Highly sensitive detection of 2- methyl isocamphene (2-MIB) and oxytocin in drinking water [J]. Environmental Chemistry, 2016, 35(8): 1733-1736(in Chinese).
[20] MENG T, SU X, SUN P Z. Degradation of geosmin and 2-methylisoborneol in UV-based AOPs for photoreactors with reflective inner surfaces: Kinetics and transformation products [J]. Chemosphere, 2022, 306: 135611. doi: 10.1016/j.chemosphere.2022.135611 [21] 米记茹. UV/H2O2/O3高级氧化去除藻源嗅味和有机物的效果及机理研究[D]. 济南: 山东建筑大学, 2021. MI J R. Study on the effect and mechanism of UV/H2O2/O3 advanced oxidation to remove odor and organic matter from algae[D]. Jinan: Shandong Jianzhu University, 2021 (in Chinese).
[22] BENIWAL D, TAYLOR-EDMONDS L, ARMOUR J, et al. Ozone/peroxide advanced oxidation in combination with biofiltration for taste and odour control and organics removal [J]. Chemosphere, 2018, 212: 272-281. doi: 10.1016/j.chemosphere.2018.08.015 [23] PARK G, YU M, KOO J Y, et al. Oxidation of geosmin and MIB in water using O3/H2O2: Kinetic evaluation [J]. Water Supply, 2006, 6(2): 63-69. doi: 10.2166/ws.2006.051 [24] SUN B, WANG Y, XIANG Y Y, et al. Influence of pre-ozonation of DOM on micropollutant abatement by UV-based advanced oxidation processes [J]. Journal of Hazardous Materials, 2020, 391: 122201. doi: 10.1016/j.jhazmat.2020.122201 [25] SCHOLZ M, MARTIN R J. Ecological equilibrium on biological activated carbon [J]. Water Research, 1997, 31(12): 2959-2968. doi: 10.1016/S0043-1354(97)00155-3 [26] ABROMAITIS V, RACYS V, van der MAREL P, et al. Effect of shear stress and carbon surface roughness on bioregeneration and performance of suspended versus attached biomass in metoprolol-loaded biological activated carbon systems [J]. Chemical Engineering Journal, 2017, 317: 503-511. doi: 10.1016/j.cej.2017.02.097 [27] ANTONOPOULOU M, EVGENIDOU E, LAMBROPOULOU D, et al. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media [J]. Water Research, 2014, 53: 215-234. doi: 10.1016/j.watres.2014.01.028 [28] JIANG Q Y, WANG Y L, TIAN L P, et al. Pilot-scale and mechanistic study of the degradation of typical odors and organic compounds in drinking water by a combined UV/H2O2-BAC process [J]. Chemosphere, 2022, 292: 133419. doi: 10.1016/j.chemosphere.2021.133419 [29] YUAN R F, WANG S N, LIU D, et al. Effect of the wavelength on the pathways of 2-MIB and geosmin photocatalytic oxidation in the presence of Fe-N co-doped TiO2 [J]. Chemical Engineering Journal, 2018, 353: 319-328. doi: 10.1016/j.cej.2018.07.123 [30] KIM T K, MOON B R, KIM T, et al. Degradation mechanisms of geosmin and 2-MIB during UV photolysis and UV/chlorine reactions [J]. Chemosphere, 2016, 162: 157-164. doi: 10.1016/j.chemosphere.2016.07.079 [31] MA L F, WANG C Y, LI H P, et al. Degradation of geosmin and 2-methylisoborneol in water with UV/chlorine: Influencing factors, reactive species, and possible pathways [J]. Chemosphere, 2018, 211: 1166-1175. doi: 10.1016/j.chemosphere.2018.08.029 -




 下载:
下载:





















