-
矿物颗粒是大气颗粒物的重要组成部分,约占大气对流层颗粒物质量浓度的50%,据估算全球每年进入大气的矿物颗粒约有
1000 —3000 Tg[1 − 3]. 值得指出的是,在沙尘暴期间,北京大气中矿物颗粒的质量浓度在颗粒物中的占比可高达74%[4]. 矿物颗粒具有较长的大气寿命(数天到数周)[5],其在风力作用下在大气中能够迁移数千米. 在矿物颗粒迁移的过程中,由于其具有巨大的比表面积和较高的化学活性(表面活性位点、金属离子等),可以为大量的污染物提供丰富的吸附位点,因此,大量污染物可能吸附在矿物颗粒表面. 吸附在矿物颗粒表面的污染物的反应活性可能会与气相不同,导致其可能会存在与气相不同的反应机制,最终影响其大气归趋及影响[6 − 10]. 例如,Ji等[7]发现,相比甲醛和NO2的气相反应,吸附在二氧化硅矿物颗粒表面的甲醛与NO2反应生成HONO的能垒较低. Liu等[9]发现吸附在MgO颗粒表面的SO2,通过与NO形成不同于气相反应的独特[SO4−NO]络合物机制,促进NO向NO2转化. 因此,了解污染物在矿物颗粒表面的吸附行为对于评估其大气归趋及影响具有指导意义.芳香族污染物是大气中一类重要的污染物,其中芳香烃在城市大气中占总挥发性有机物浓度的20%—30%[11]. 它们通常来自含碳材料的不完全燃烧,石油裂解,森林火灾和火山喷发等[12 − 13],不但具有致癌和致畸作用,而且可能会在大气中经历一系列的转化过程生成比自身毒性更大的产物,多种芳香族污染物已被我国生态环境部和美国环保署列为优先控制污染物[14 − 15]. 芳香族污染物由于具有较高的持久性和长距离迁移性,因此很可能会吸附在矿物颗粒表面. 例如,在北京和天津地区的矿物颗粒表面上已检出芳香族污染物[16 − 17]. 然而,目前仅有个别研究考察了芳香族污染物(萘、蒽、菲、甲苯、苯酚、氟苯、氯苯、溴苯)在矿物颗粒表面的吸附行为[18 − 20],还有大量的芳香族污染物在矿物颗粒表面的吸附行为是未知的,严重阻碍了全面认识芳香族污染物的大气影响. 因此,亟需全面探究芳香族污染物在矿物颗粒表面的吸附行为.
吸附能(Eads)是描述污染物在矿物颗粒表面吸附行为的一个重要参数[21 − 22]. 鉴于大气中存在大量的芳香族污染物,逐一揭示芳香族污染物在矿物颗粒表面的Eads值,需要极大的工作量. 因此,亟需建立一种高通量的预测模型来预测芳香族污染物在矿物颗粒表面的Eads值. 定量结构-活性关系(QSAR)模型是目前最有效的模型之一,已被广泛应用于预测污染物在不同表面的Eads值[23 − 26]. 例如,Wang等[26]构建了预测水相和气相中有机污染物在石墨烯上Eads值的QSAR模型;Su等[24]建立了预测有机污染物在黑磷纳米颗粒上Eads值的多参数线性自由能关系(pp-LFERs)模型. 然而,由于目前缺乏芳香族污染物在矿物颗粒表面上Eads值的数据,未见报道用于预测芳香族污染物在矿物颗粒表面上Eads值的QSAR模型.
三水铝石(Al(OH)3)是地球表面最丰富且最常见的矿物颗粒之一[27 − 28],也是最稳定的一种氢氧化铝矿物形态[29]. 它是由OH—Al—OH配位八面体周期性排列而形成的薄的扁平六边形晶体,可以为大气中的芳香族污染物提供丰富的—OH吸附位点,进而影响芳香族污染物的环境行为. 本研究采取“吸附机制研究-QSAR模型构建”的两步策略,首先选取21个代表性芳香族污染物作为模型化合物,使用第一性原理的方法探究它们在三水铝石(001)面上的吸附行为,并计算相应的Eads值. 随后,基于计算得到的21个Eads值,采用多元线性回归(MLR)方法,建立预测芳香族污染物在三水铝石(001)面上Eads值的QSAR模型. 研究结果对于增强芳香族污染物在矿物颗粒表面上吸附行为的理解具有重要意义,并且为进一步揭示芳香族污染物在三水铝石(001)面上的大气转化奠定了基础.
-
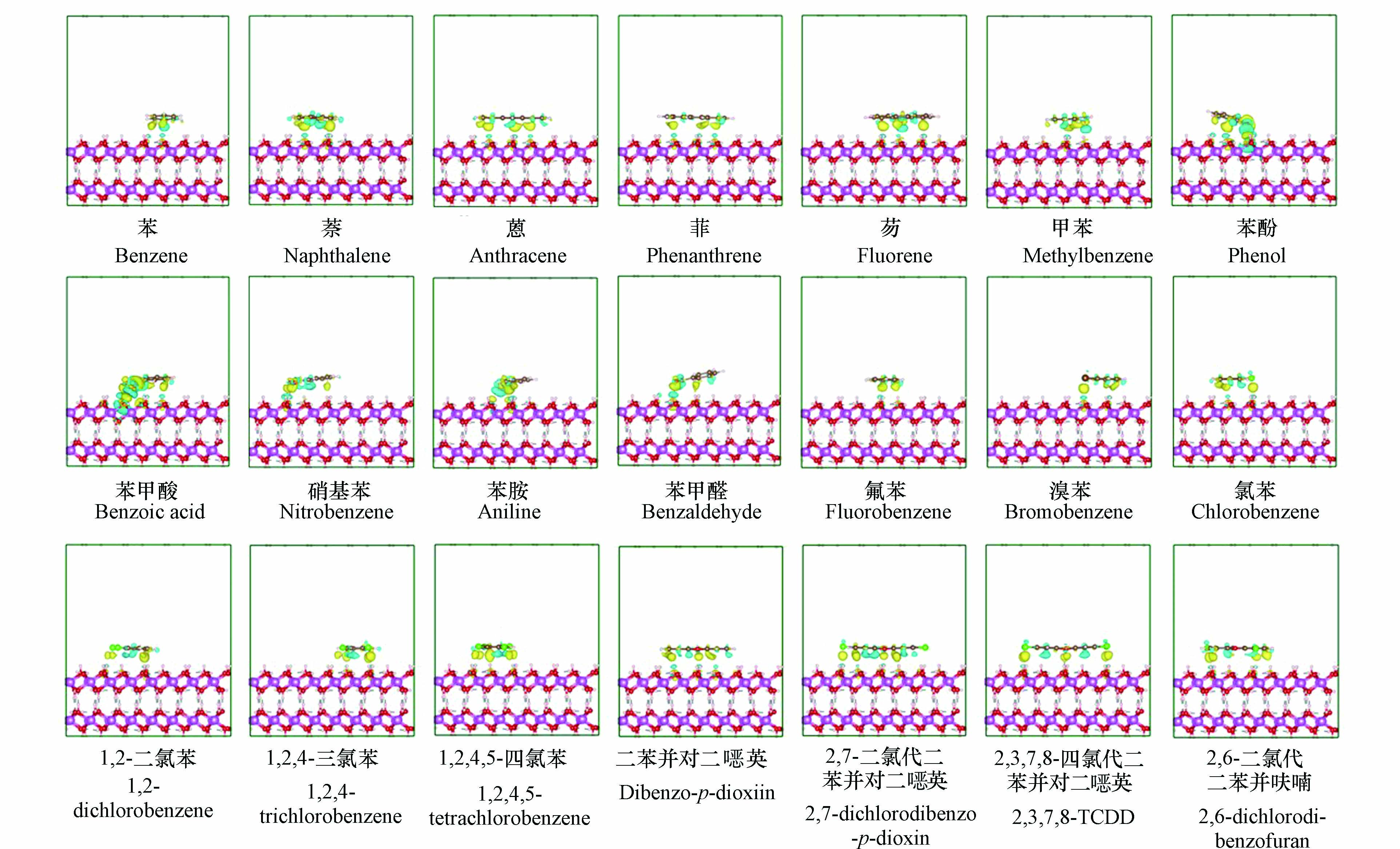
基于结构多样性原则,本研究选取了21个具有代表性的芳香族污染物作为模型化合物,包括苯、多环芳烃(PAHs)、取代苯(单\双\多)、二噁英,详见表1.
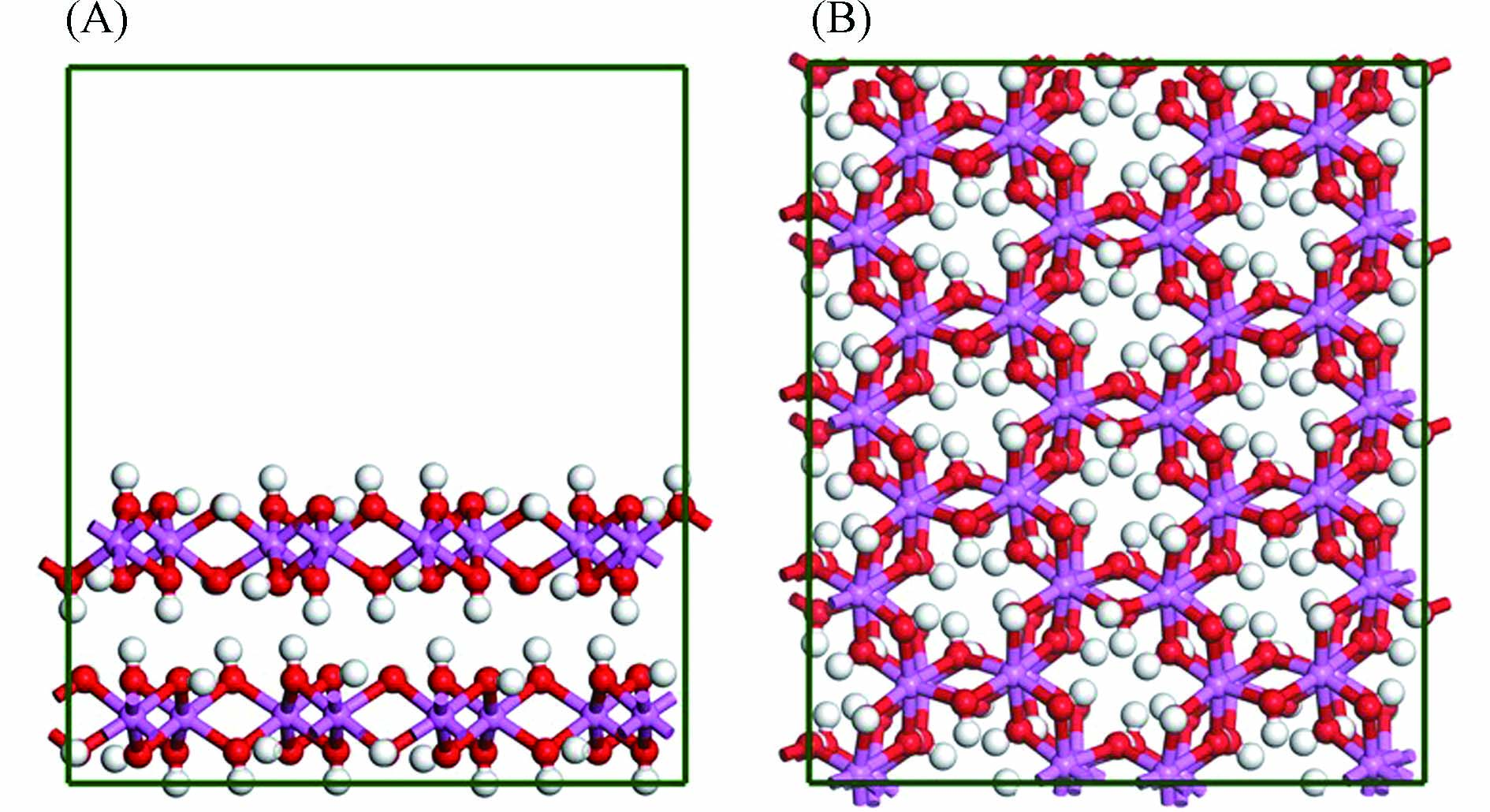
三水铝石的原子坐标取自Saalfeld等[30]报道的晶体结构,其晶胞参数为:a =
0.8684 nm,b =0.5078 nm,c =0.9736 nm. 为了更加接近自然界中三水铝石的真实体系,采用2 × 4 × 1的周期性超级晶胞结构. 由于三水铝石(001)面的表面能小,可稳定存在于自然界中[31 − 34],因此选取该表面来研究芳香族污染物在三水铝石矿物颗粒上的吸附行为. 此外,为了避免层与层之间的相互作用,沿z方向上设置了1.5 nm的真空层. 构建的三水铝石(001)面模型中共包含:64个Al原子、192个O原子和192个H原子,见图1. -
本文使用第一性原理计算软件VASP 5.4.4进行所有的密度泛函理论(DFT)计算[35]. 在进行结构优化时,使用Perdew等[36]提出的广义梯度近似(GGA)的Perdew-Burke-Ernzerhof (PBE)泛函描述交换关联势. 为了描述弱相互作用,采用Grimme等[37 − 39]提出的D3方法对范德华相互作用进行校正. 采用缀加平面波基组(PAW)描述离子与电子间相互作用[40 − 41],截断能为400 eV,布里渊区使用1 × 1 × 1的Γ点. 在结构优化时,所有原子是被允许放松的,能量和力的收敛标准分别设置为10−5 eV和0.4 eV·nm−1,该收敛标准已被成功应用于探究SO2在三水铝石矿物颗粒表面上的转化机制[42].
芳香族污染物在三水铝石(001)面上会存在不同的吸附构型. 通过研究者的化学直觉,设计了芳香族污染物在三水铝石(001)面上不同的吸附构型,并计算相应的Eads值. 最终选择每一个芳香族污染物在三水铝石(001)面上Eads值最低的吸附构型作为最稳定的吸附构型. Eads值的计算公式如下:
其中,EA−B为三水铝石(001)面和芳香族污染物复合体系的总能量,EA和EB分别为三水铝石(001)面和单个芳香族污染物的能量.
电子密度差(Δρ)已被成功地应用于分析吸附构型中存在的相互作用[43 − 45]. 因此,本研究计算了21个芳香族污染物在三水铝石(001)面上最稳定吸附构型的Δρ,分析它们与三水铝石(001)面之间的相互作用,并通过VESTA软件进行可视化[46]. Δρ的计算公式如下:
其中,ρA−B为三水铝石(001)面和芳香族污染物复合体系的电子密度,ρA和ρB分别为三水铝石(001)面和单个芳香族污染物的电子密度.
-
分子描述符的选择是构建QSAR模型的关键步骤. 本研究选择的分子描述符包括量子化学描述符、Dragon描述符和物理化学参数. 首先,本研究采用Gaussian 09软件包[47],在M06-2X/6-31+G(d,p)的理论水平下优化了21个芳香族污染物的结构,从优化得到的结果文件中提取量子化学描述符,包括偶极矩,极化率、最高占据分子轨道能、最低未占据分子轨道能. 另外,采用Dragon 6.0软件计算了21个芳香族污染物的Dragon描述符. 通过采用EPI suite 4.0软件和文献查找方式获得芳香族污染物的物理化学参数的实验值,包括正辛醇-空气分配系数(lgKOA)和亨利定律常数(lgKH). 若上述两种方式都找不到lgKOA和lgKH的实验值,则采用EPI suite 4.0软件进行预测. 最后,将计算得到的21个Eads值,按照4:1的比例随机分为训练集和验证集,采用MLR方法,建立预测芳香族污染物在三水铝石(001)面上Eads值的QSAR模型. 调整后的决定系数(R2adj),均方根误差(RMSE),去一法交叉验证系数(Q2LOO)和外部验证系数(Q2ext)用来评价QSAR模型的拟合能力、稳健性以及预测能力. 另外,基于计算标准残差(δ)和杠杆值(h)得到Williams图,表征模型的应用域[48].
-
图2列出了21个芳香族污染物在三水铝石(001)面上的最稳定吸附构型. 可以看出,除卤代苯之外,其他的取代苯(如苯酚、苯甲酸、硝基苯、苯胺以及苯甲醛)均倾斜地吸附在三水铝石(001)面上. 对于卤代苯、苯、多环芳烃以及二噁英,它们均平行吸附在三水铝石(001)面上. 通过仔细分析21个芳香族污染物在三水铝石(001)面上的吸附构型(图2)和电子密度差(图3),发现芳香族污染物与三水铝石(001)面之间的相互作用主要包括三类:O—H···π键、氢键和O—H···X (X = Cl/Br)相互作用. 其中,苯和多环芳烃的吸附主要是由O—H···π键驱动的,取代苯和二噁英主要是由这三类相互作用中的一类或两类或三类驱动的,如表2所示. 此外,本研究进一步计算了所有吸附体系的Bader电荷(表2),发现芳香族污染物与三水铝石(001)面之间的电荷转移量很小,在−0.039 e—0.057 e之间,两者之间的相互作用主要取决于它们之间的静电力[43],进一步表明O—H···π键、氢键和O—H···X (X = Cl/Br)相互作用是芳香族污染物在三水铝石(001)面上吸附的主要原因. 综合上述分析,可以推测芳香族污染物在三水铝石(001)面上的吸附属于物理吸附,与多环芳烃在石墨烯上的吸附类型相似[49].
表2列出了21个芳香族污染物在三水铝石(001)面上最稳定吸附构型的Eads值. 可以看出,芳香族污染物在三水铝石(001)面上的Eads值在−12 kcal·mol−1至−30 kcal·mol−1范围之间,在范德华(vdW)相互作用范围内[26]. 相比苯,多环芳烃在三水铝石(001)面上的Eads值更低,表明多环芳烃与三水铝石(001)面之间的相互作用更强. 此外,芳环的数量会影响芳香族污染物在三水铝石(001)面上的Eads值,如苯、萘和蒽在三水铝石(001)面上的Eads值的顺序为:苯 > 萘 > 蒽,表明芳环的数量越多,芳香族污染物在三水铝石(001)面上的Eads值越低,吸附能力越强,这与Dang等[20]发现的结果一致. 另外,取代基的存在会促进芳香族污染物在三水铝石(001)面上的吸附,例如,苯的Eads值大于取代苯,这与Yu等[50]的研究结果一致. 值得指出的是,不同类型的取代基对芳香族污染物在三水铝石(001)面上的吸附具有不同的促进效应,这主要是由两者之间所形成的相互作用类型不同导致的. 例如,甲苯由于其—CH3取代基不含有强电负性原子,导致其与三水铝石(001)面上的O—H键之间只能形成O—H···π键,不能形成氢键;而苯酚的—OH取代基和苯胺的—NH2取代基都含有强电负性原子,导致它们与三水铝石(001)面上的O—H键之间既可以形成O—H···π键,也可以形成O—H···O型氢键或N—H···O型氢键. 进一步对比不同氯取代个数的氯代苯在三水铝石(001)面上的吸附能力(Eads值:氯苯 > 1,2-二氯苯 > 1,2,4-三氯苯 > 1,2,4,5-四氯苯),发现取代基的个数越多,氯代苯的吸附能力越强. 此外,在不同氯取代个数的二苯并对二噁英体系中也出现了类似的现象(Eads值:二苯并对二噁英 > 2,7-二氯代二苯并对二噁英 > 2,3,7,8-四氯代二苯并对二噁英). 因此可以推测,相比单取代芳香族污染物,多取代芳香族污染物在三水铝石(001)面上的吸附能力更强.
-
基于计算得到的21个Eads值,建立了预测芳香族污染物在三水铝石(001)面上的Eads值的QSAR模型:
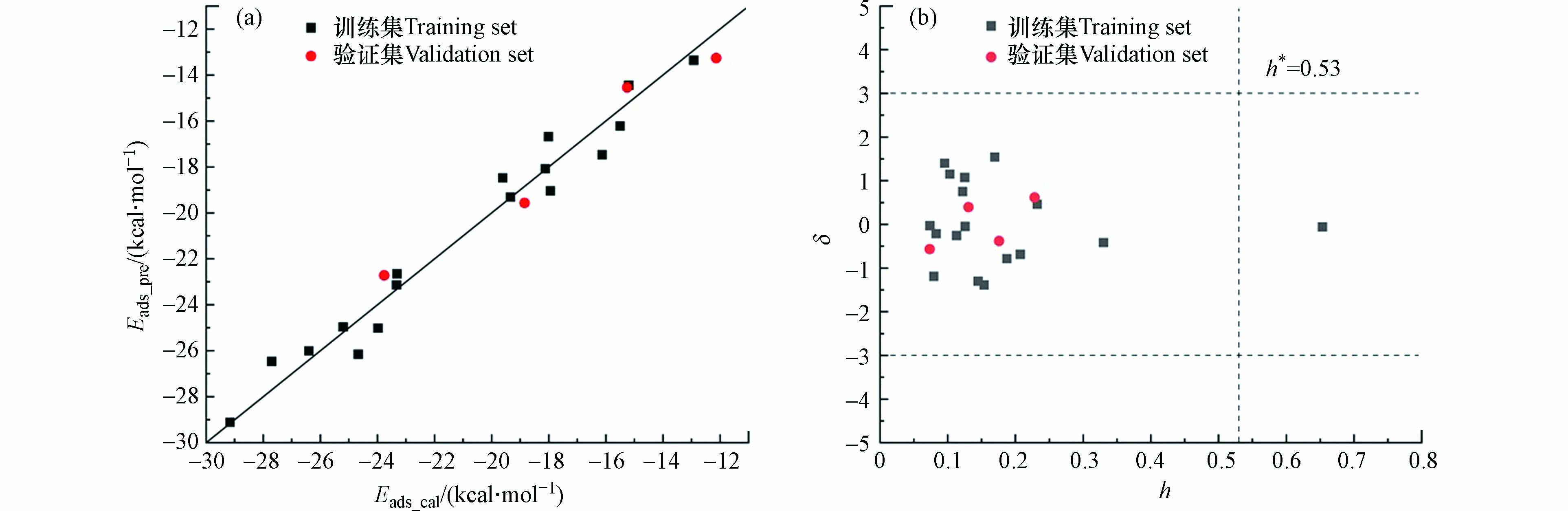
其中,Ntra和Next分别表示训练集和验证集中芳香族污染物的个数. 该模型包括两个分子描述符lgKOA和Am,其含义及相应数值分别见表3和表4. 从表3可知,两个描述符的方差膨胀因子(VIF)值均小于5,表明模型不存在多重共线性[51 − 52]. 模型的统计学评估参数R2tra = 0.961、Q2LOO = 0.966、RMSEt = 0.869、R2ext = 0.864、Q2ext = 0.955、RMSEe = 0.923,表明该预测模型具有很好的拟合优度、稳健性和预测能力[53]. 此外,模型中R2和Q2的差值小于0.3,表明模型没有过拟合[54].
从图4a可以看出,本研究中构建的QSAR模型预测的21个芳香族污染物在三水铝石(001)面上的Eads值与通过第一性原理计算的Eads值具有很好的一致性. 基于标准残差δ和杠杆值h的Williams图如图4b所示. 可以看出,所有芳香族污染物的标准残差均满足|δ|小于3,表明模型没有离群点. 此外,本研究发现除了2,3,7,8-四氯代二苯并对二噁英,模型中大部分芳香族污染物都在应用域内. 因此,该模型能够用来预测含有—CH3、—OH、—COOH、—NO2、—NH2、—CHO、—F、—Cl、—Br官能团的芳香族污染物. 对于2,3,7,8-四氯代二苯并对二噁英,虽然其h值大于警戒值h* = 0.53,但具有良好的拟合效果(|δ|<3),说明模型的训练集具有较好的代表性.
-
本研究构建的QSAR模型中共有两个描述符lgKOA和Am,其对应的P值和t检验值见表3. 可以看出,两个描述符的P值均小于0.04,表明它们与芳香族污染物在三水铝石(001)面上的Eads值具有相关性. 根据t检验值可以看出,物理化学参数lgKOA的t检验值的绝对值最大,是影响芳香族污染物在三水铝石(001)面上Eads值的最重要因素. lgKOA的t检验值和系数均为负数,表明其与Eads值具有负相关关系. 前人研究指出,lgKOA能够用来表征有机污染物在空气和颗粒相之间的分配行为[55 − 56],lgKOA越大的有机污染物,越容易存在于颗粒相中,这与本研究的结果一致. Dragon描述符Am反应了与芳香族污染物本身结构有关的信息,其与Eads值呈正相关,Am值越大,Eads值越大,芳香族污染物在三水铝石(001)面上的吸附能力越弱.
-
本研究采用第一性原理的方法,计算了21个芳香族污染物(如苯、PAHs、取代苯和二噁英)在三水铝石(001)面上的吸附能和吸附构型,揭示了氢键、O—H···π键以及O—H···X (X = Cl/Br)相互作用是导致芳香族污染物在三水铝石(001)面上吸附的主要原因. 本研究较为全面地考察了芳香族污染物在三水铝石(001)面上的吸附能,提高了目前对芳香族污染物在矿物颗粒表面吸附行为的理解. 此外,本研究构建了能够用来预测芳香族污染物在三水铝石(001)面上吸附能的QSAR模型,该模型可以用于评估其他芳香族污染物在三水铝石(001)面上的吸附能. 需要指出的是,本研究仅考察了芳香族污染物在三水铝石(001)面上的吸附行为,并未考察其在三水铝石(001)面上的转化行为. 因此,建议将来进一步探究芳香族污染物在三水铝石(001)面上的大气转化,进而更加全面的评价芳香族污染物的大气归趋和影响.
三水铝石矿物颗粒表面吸附芳香族污染物的计算模拟与预测
Computational simulation and prediction for adsorption of aromatic pollutants on gibbsite mineral particle surface
-
摘要: 了解芳香族污染物在矿物颗粒表面的吸附行为对于评估其大气归趋及影响具有重要意义. 吸附能(Eads)是描述芳香族污染物在矿物颗粒表面吸附行为的重要参数,然而,目前该数据十分匮乏. 考虑到大气中存在大量的芳香族污染物,逐个揭示芳香族污染物在矿物颗粒表面的Eads值需要巨大的计算资源和工作量. 因此,亟需建立一种能够快速预测芳香族污染物在矿物颗粒表面Eads值的预测模型. 本研究采取第一性原理的方法,探究了21个芳香族污染物在矿物颗粒三水铝石(001)面上的吸附机制. 结果发现,21个芳香族污染物与三水铝石(001)面主要形成氢键、O—H···π键以及O—H···X (X = Cl/Br)相互作用,其Eads值的范围在−12 kcal·mol−1至−30 kcal·mol−1之间. 基于计算得到的21个Eads值,采用多元线性回归分析方法,建立了预测芳香族污染物在三水铝石(001)面上Eads值的定量结构-活性关系模型. 该模型具有很好的拟合优度、稳健性和预测能力,能够用来预测含有—CH3、—OH、—COOH、—NO2、—NH2、—CHO、—F、—Cl、—Br官能团的芳香族污染物. 本研究结果对于提高目前对芳香族污染物在矿物颗粒表面上吸附行为的理解具有重要意义,并且为进一步揭示芳香族污染物在三水铝石(001)面上的大气转化奠定了基础.
-
关键词:
- 芳香族污染物 /
- 三水铝石 /
- 吸附能 /
- 第一性原理计算 /
- 定量结构-活性关系.
Abstract: Understanding the adsorption behavior of aromatic pollutants on the surface of mineral particles is significant for evaluating their atmospheric fate and impacts. The adsorption energy (Eads) is a crucial parameter to describe the adsorption behavior of aromatic pollutants on the surface of mineral particles. However, only limited aromatic pollutants (i.e., naphthalene, anthracene, toluene, phenol, et al.) have so far been studied. In view of the large amounts of aromatic pollutants in the atmosphere, it takes lots of work to evaluate the Eads values of aromatic pollutants on the surface of mineral particles one by one. Therefore, it is necessary to establish a high-throughput prediction model to predict the Eads values of aromatic pollutants on the surface of mineral particles. Herein, we employed first-principles calculations to investigate the adsorption mechanism of 21 aromatic pollutants on gibbsite (001) surface. Results indicated that three types of interactions including hydrogen bonds, O—H···π bonds and O—H···X (X = Cl/Br) are formed between 21 aromatic pollutants and gibbsite (001) surface. The calculated Eads values are in the range of −12—−30 kcal·mol−1. Based on the calculated 21 Eads values, a quantitative structure-activity relationship (QSAR) model for predicting the Eads values of aromatic pollutants on gibbsite (001) surface was established. The model has good goodness of fit, robustness and predictive ability, which can be used to predict the Eads values of aromatic pollutants containing —CH3, —OH, —COOH, —NO2, —NH2, —CHO, —F, —Cl and —Br functional groups. The results expand current understanding of the adsorption mechanism of aromatic pollutants on the surface of mineral particles, and provide a foundation for investigating the atmospheric transformation of aromatic pollutants on gibbsite (001) surface in the further. -

-
表 1 21个芳香族污染物的分类、名称、分子式和CAS号
Table 1. Classification, name, molecular formula and CAS number of the 21 aromatic pollutants
分类
Classification名称
Name分子式
Molecular formulaCAS 苯和PAHs 苯 C6H6 00071-43-2 萘 C10H8 00091-20-3 蒽 C14H10 00120-12-7 菲 C14H10 00085-01-8 芴 C13H10 00086-73-7 取代苯 甲苯 C7H8 00108-88-3 苯酚 C6H6O 00108-95-2 苯甲酸 C7H6O2 00065-85-0 硝基苯 C6H5NO2 00098-95-3 苯胺 C6H7N 00062-53-3 苯甲醛 C7H6O 00100-52-7 氟苯 C6H5F 00462-06-6 溴苯 C6H5Br 00108-86-1 氯苯 C6H5Cl 00108-90-7 1,2-二氯苯 C6H4Cl2 00095-50-1 1,2,4-三氯苯 C6H3Cl3 00120-82-1 1,2,4,5-四氯苯 C6H2Cl4 00095-94-3 二噁英 二苯并对二噁英 C12H8O2 00262-12-4 2,7-二氯代二苯并对二噁英 C12H6Cl2O2 33857 -26-02,3,7,8-四氯代二苯并对二噁英 C12H4Cl4O2 01746-01-6 2,6-二氯代二苯并呋喃 C12H6Cl2O 60390 -27-4表 2 21个芳香族污染物在三水铝石(001)面上的吸附能(Eads)、相互作用类型以及Bader电荷分析得到的各种芳香族污染物的电荷转移(Q)
Table 2. Adsorption energies (Eads), interaction types of 21 aromatic pollutants on gibbsite (001) surface and charge transfer (Q) of each aromatic pollutant obtained by Bader charge analysis
化合物
CompoundEads/
(kcal·mol−1)O—H···π键
O—H···π bond氢键
Hydrogen bondO—H···X
(X = Cl/Br)Q/e 苯 −12.13 ● ○ ○ 0.030 萘 −19.34 ● ○ ○ 0.057 蒽 −25.21 ● ○ ○ 0.056 菲 −24.83 ● ○ ○ 0.049 芴 −23.33 ● ○ ○ 0.042 甲苯 −15.25 ● ○ ○ 0.025 苯酚 −23.31 ● ● ○ −0.003 苯甲酸 −26.40 ● ● ○ −0.039 硝基苯 −19.61 ● ● ○ 0.015 苯胺 −17.94 ● ● ○ 0.005 苯甲醛 −16.13 ● ● ○ 0.015 氟苯 −12.92 ● ● ○ 0.021 溴苯 −15.50 ● ○ ● 0.052 氯苯 −15.20 ● ○ ● 0.037 1,2-二氯苯 −18.01 ● ○ ● 0.045 1,2,4-三氯苯 −18.12 ● ○ ● 0.050 1,2,4,5-四氯苯 −18.84 ● ○ ● 0.041 二苯并对二噁英 −23.76 ● ● ○ 0.031 2,7-二氯代二苯并对二噁英 −27.70 ● ● ● 0.037 2,3,7,8-四氯代二苯并对二噁英 −29.16 ○ ● ● 0.056 2,6-二氯代二苯并呋喃 −24.67 ● ● ● 0.056 注:正数Q表示芳香族污染物向三水铝石(001)面的电荷转移;●和○分别表示相互作用类型的有无.
Note: The positive number Q represents the charge transfer of each aromatic pollutant to gibbsite (001) surface; symbols ● and ○ indicate the presence and absence of interaction types, respectively.表 3 模型中涉及的分子描述符的含义及其方差膨胀因子(VIF)、t检验值和显著性水平P值
Table 3. Molecular descriptors involved in the model and their variable inflation factor (VIF), t-statistics, and significance-level P Values.
描述符
Descriptor含义
DefinitionVIF t P lgKOA 正辛醇-空气分配系数 2.600 −14.11 <0.001 Am 由质量测量的WHIM描述符 2.600 2.357 <0.040 表 4 QSAR模型中所涉及的描述符lgKOA和Am的值以及通过QSAR模型预测得到的吸附能(Eads_pre)
Table 4. Values of lgKOA and Am descriptors involved in the QSAR model and predicted adsorption energies (Eads_pre) by the QSAR model
化合物
CompoundlgKOA Am Eads_pre/(kcal·mol−1) 苯 2.7801) 1.289 −13.27 萘 5.1901) 4.034 −19.31 蒽 7.5501) 8.271 −25.01 菲 7.5701) 9.009 −24.96 芴 6.7901) 7.234 −23.13 甲苯 3.3101) 2.207 −14.55 苯酚 6.3262) 1.990 −22.66 苯甲酸 7.6782) 3.795 −26.00 硝基苯 4.8582) 3.736 −18.48 苯胺 4.9832) 2.021 −19.04 苯甲醛 4.4422) 2.902 −17.47 氟苯 2.8632) 2.114 −13.36 溴苯 3.9862) 3.238 −16.22 氯苯 3.3101) 2.921 −14.45 1,2-二氯苯 4.3601) 6.883 −16.69 1,2,4-三氯苯 4.9501) 8.160 −18.08 1,2,4,5-四氯苯 5.6301) 10.42 −19.57 二苯并对二噁英 6.6432) 7.322 −22.72 2,7-二氯代二苯并对二噁英 8.3601) 13.245 −26.46 2,3,7,8-四氯代二苯并对二噁英 10.0501) 26.200 −29.11 2,6-二氯代二苯并呋喃 8.3601) 15.394 −26.15 注:1)和2)分别表示EPI suite 4.0软件得到芳香族污染物的lgKOA的实验值和预测值.
Note: 1) and 2) represent the experimental and predicted lgKOA values of aromatic pollutants obtained by EPI suite 4.0, respectively. -
[1] ANDREAE M O, CHARLSON R J, BRUYNSEELS F, et al. Internal mixture of sea salt, silicates, and excess sulfate in marine aerosols[J]. Science, 1986, 232(4758): 1620-1623. doi: 10.1126/science.232.4758.1620 [2] DENTENER F J, CARMICHAEL G R, ZHANG Y, et al. Role of mineral aerosol as a reactive surface in the global troposphere[J]. Journal of Geophysical Research:Atmospheres, 1996, 101(D17): 22869-22889. doi: 10.1029/96JD01818 [3] HARRISON S P, KOHFELD K E, ROELANDT C, et al. The role of dust in climate changes today, at the last glacial maximum and in the future[J]. Earth-Science Reviews, 2001, 54(1/2/3): 43-80. [4] 韩力慧, 庄国顺, 孙业乐, 等. 北京大气颗粒物污染本地源与外来源的区分: 元素比值Mg/Al示踪法估算矿物气溶胶外来源的贡献[J]. 中国科学B辑, 2005, 35(3): 237-246. doi: 10.3321/j.issn:1006-9240.2005.03.010 HAN L H, ZHUANG G S, SUN Y (L /y), et al. Distinguishing between local sources and external sources of atmospheric particulate pollution in Beijing—Estimation of contribution of external sources of mineral aerosols by element ratio Mg/Al tracer method[J]. Science in China (Series B), 2005, 35(3): 237-246 (in Chinese). doi: 10.3321/j.issn:1006-9240.2005.03.010
[5] PROSPERO J M. Long-term measurements of the transport of African mineral dust to the southeastern United States: Implications for regional air quality[J]. Journal of Geophysical Research:Atmospheres, 1999, 104(D13): 15917-15927. doi: 10.1029/1999JD900072 [6] BAI F Y, DENG M S, CHEN M Y, et al. Atmospheric oxidation of fluoroalcohols initiated by ˙OH radicals in the presence of water and mineral dusts: Mechanism, kinetics, and risk assessment[J]. Physical Chemistry Chemical Physics, 2021, 23(23): 13115-13127. doi: 10.1039/D1CP01324F [7] JI Y M, WANG H H, LI G Y, et al. Theoretical investigation on the role of mineral dust aerosol in atmospheric reaction: A case of the heterogeneous reaction of formaldehyde with NO2 onto SiO2 dust surface[J]. Atmospheric Environment, 2015, 103: 207-214. doi: 10.1016/j.atmosenv.2014.12.044 [8] LANGHAMMER D, KULLGREN J, ÖSTERLUND L. Photoinduced adsorption and oxidation of SO2 on anatase TiO2(101)[J]. Journal of the American Chemical Society, 2020, 142(52): 21767-21774. doi: 10.1021/jacs.0c09683 [9] LIU C, WANG H H, MA Q X, et al. Efficient conversion of NO to NO2 on SO2-aged MgO under atmospheric conditions[J]. Environmental Science & Technology, 2020, 54(19): 11848-11856. [10] WANG W G, LIU M Y, WANG T T, et al. Sulfate formation is dominated by Manganese-catalyzed oxidation of SO2 on aerosol surfaces during haze events[J]. Nature Communications, 2021, 12: 1993. doi: 10.1038/s41467-021-22091-6 [11] LI M, ZHANG Q, ZHENG B, et al. Persistent growth of anthropogenic non-methane volatile organic compound (NMVOC) emissions in China during 1990–2017: Drivers, speciation and ozone formation potential[J]. Atmospheric Chemistry and Physics, 2019, 19(13): 8897-8913. doi: 10.5194/acp-19-8897-2019 [12] RAVINDRA K, SOKHI R, VAN GRIEKEN R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation[J]. Atmospheric Environment, 2008, 42(13): 2895-2921. doi: 10.1016/j.atmosenv.2007.12.010 [13] ZHANG Y X, TAO S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004[J]. Atmospheric Environment, 2009, 43(4): 812-819. doi: 10.1016/j.atmosenv.2008.10.050 [14] KEITH L, TELLIARD W. ES&T special report: Priority pollutants: I-a perspective view[J]. Environmental Science & Technology, 1979, 13(4): 416-423. [15] RAMÍREZ N, CUADRAS A, ROVIRA E, et al. Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites[J]. Environmental Health Perspectives, 2011, 119(8): 1110-1116. doi: 10.1289/ehp.1002855 [16] FU S, LI K, XIA X J, et al. Polycyclic aromatic hydrocarbons residues in sandstorm depositions in Beijing, China[J]. Bulletin of Environmental Contamination and Toxicology, 2009, 82(2): 162-166. doi: 10.1007/s00128-008-9537-z [17] WU S P, TAO S, XU F L, et al. Polycyclic aromatic hydrocarbons in dustfall in Tianjin, China[J]. Science of the Total Environment, 2005, 345(1/2/3): 115-126. [18] ABELARD J, WILMSMEYER A R, EDWARDS A C, et al. Adsorption of substituted benzene derivatives on silica: Effects of electron withdrawing and donating groups[J]. The Journal of Physical Chemistry C, 2016, 120(24): 13024-13031. doi: 10.1021/acs.jpcc.6b02028 [19] BERRO Y, GUEDDIDA S, LEBÈGUE S, et al. Atomistic description of phenol, CO and H2O adsorption over crystalline and amorphous silica surfaces for hydrodeoxygenation applications[J]. Applied Surface Science, 2019, 494: 721-730. doi: 10.1016/j.apsusc.2019.07.216 [20] DANG Y, LIU Y B, FENG X, et al. Effect of dispersion on the adsorption of polycyclic aromatic hydrocarbons over the γ-Al2O3 (110) surface[J]. Applied Surface Science, 2019, 486: 137-143. doi: 10.1016/j.apsusc.2019.05.020 [21] GLADICH I, CARIGNANO M A, FRANCISCO J S. Adsorption and isomerization of glyoxal and methylglyoxal at the air/hydroxylated silica surface[J]. The Journal of Chemical Physics, 2020, 152(16): 164702. doi: 10.1063/1.5143402 [22] WANG H M, LIU H Z, CHEN Z, et al. Interaction between SO2 and NO in their adsorption and photocatalytic conversion on TiO2[J]. Chemosphere, 2020, 249: 126136. doi: 10.1016/j.chemosphere.2020.126136 [23] MEHMETI V, SADIKU M. A comprehensive DFT investigation of the adsorption of polycyclic aromatic hydrocarbons onto graphene[J]. Computation, 2022, 10(5): 68. doi: 10.3390/computation10050068 [24] SU L H, WANG Y, WANG Z Y, et al. Simulating and predicting adsorption of organic pollutants onto black phosphorus nanomaterials[J]. Nanomaterials, 2022, 12(4): 590. doi: 10.3390/nano12040590 [25] WANG B Y, XU X Y, HAO D, et al. Quantitative structure-activity relationship of amine collector adsorption on quartz surface[J]. Journal of Northeastern University, 2020, 41: 131. [26] WANG Y, CHEN J W, WEI X X, et al. Unveiling adsorption mechanisms of organic pollutants onto carbon nanomaterials by density functional theory computations and linear free energy relationship modeling[J]. Environmental Science & Technology, 2017, 51(20): 11820-11828. [27] GALE J D, ROHL A L, MILMAN V, et al. An ab initio study of the structure and properties of aluminum hydroxide: gibbsite and bayerite[J]. The Journal of Physical Chemistry B, 2001, 105(42): 10236-10242. doi: 10.1021/jp011795e [28] LOUAER S, WANG Y, GUO L. Fast synthesis and size control of gibbsite nanoplatelets, their pseudomorphic dehydroxylation, and efficient dye adsorption[J]. ACS Applied Materials & Interfaces, 2013, 5(19): 9648-9655. [29] 杨晓芳, 王东升, 孙中溪. 三水铝石(γ-Al(OH)3)和α-Al2O3表面酸碱性质与磷酸根吸附研究[J]. 环境科学学报, 2007, 27(4): 637-642. doi: 10.3321/j.issn:0253-2468.2007.04.016 YANG X F, WANG D S, SUN Z X. Studies on the surface acid-base properties and phosphate adsorption behavior of gibbsite (γ-Al(OH)3) and α-Al2O3[J]. Acta Scientiae Circumstantiae, 2007, 27(4): 637-642 (in Chinese). doi: 10.3321/j.issn:0253-2468.2007.04.016
[30] SAALFELD H, WEDDE M. Refinement of the crystal structure of gibbsite, A1(OH)3[J]. Zeitschrift Für Kristallographie - Crystalline Materials, 1974, 139(1/2/3/4/5/6): 129-135. [31] FU W, VAUGHAN J, GILLESPIE A. in situ AFM investigation of gibbsite growth in high ionic strength, highly alkaline, aqueous media[J]. Hydrometallurgy, 2016, 161: 71-76. doi: 10.1016/j.hydromet.2016.01.030 [32] SHEN Z Z, ILTON E S, PRANGE M P, et al. Molecular dynamics simulations of the interfacial region between boehmite and gibbsite basal surfaces and high ionic strength aqueous solutions[J]. The Journal of Physical Chemistry C, 2017, 121(25): 13692-13700. doi: 10.1021/acs.jpcc.7b02463 [33] XU T Y, CATALANO J G. Impacts of surface site coordination on arsenate adsorption: Macroscopic uptake and binding mechanisms on aluminum hydroxide surfaces[J]. Langmuir, 2016, 32(49): 13261-13269. doi: 10.1021/acs.langmuir.6b03214 [34] YUN J N, ZHU C, WANG Q, et al. Strong affinity of mineral dusts for sulfur dioxide and catalytic mechanisms towards acid rain formation[J]. Catalysis Communications, 2018, 114: 79-83. doi: 10.1016/j.catcom.2018.06.011 [35] KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50. doi: 10.1016/0927-0256(96)00008-0 [36] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. doi: 10.1103/PhysRevLett.77.3865 [37] GRIMME S. Accurate description of van der Waals complexes by density functional theory including empirical corrections[J]. Journal of Computational Chemistry, 2004, 25(12): 1463-1473. doi: 10.1002/jcc.20078 [38] GRIMME S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction[J]. Journal of Computational Chemistry, 2006, 27(15): 1787-1799. doi: 10.1002/jcc.20495 [39] GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. doi: 10.1063/1.3382344 [40] BLÖCHL P E. Projector augmented-wave method[J]. Physical Review B, 1994, 50(24): 17953-17979. doi: 10.1103/PhysRevB.50.17953 [41] KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758-1775. doi: 10.1103/PhysRevB.59.1758 [42] YUN J N, ZHU C, WANG Q, et al. Catalytic conversions of atmospheric sulfur dioxide and formation of acid rain over mineral dusts: Molecular oxygen as the oxygen source[J]. Chemosphere, 2019, 217: 18-25. doi: 10.1016/j.chemosphere.2018.10.201 [43] HOUNFODJI J W, KANHOUNNON W G, KPOTIN G, et al. Molecular insights on the adsorption of some pharmaceutical residues from wastewater on kaolinite surfaces[J]. Chemical Engineering Journal, 2021, 407: 127176. doi: 10.1016/j.cej.2020.127176 [44] ZHAO N, JU F, SONG Q W, et al. A simple empirical model for phenanthrene adsorption on soil clay minerals[J]. Journal of Hazardous Materials, 2022, 429: 127849. doi: 10.1016/j.jhazmat.2021.127849 [45] LAINÉ J, FOUCAUD Y, BONILLA-PETRICIOLET A, et al. Molecular picture of the adsorption of phenol, toluene, carbon dioxide and water on kaolinite basal surfaces[J]. Applied Surface Science, 2022, 585: 152699. doi: 10.1016/j.apsusc.2022.152699 [46] MOMMA K, IZUMI F. VESTA: A three-dimensional visualization system for electronic and structural analysis[J]. Journal of Applied Crystallography, 2008, 41(3): 653-658. doi: 10.1107/S0021889808012016 [47] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09;Gaussian Inc. , 2009. [48] GRAMATICA P. Principles of QSAR models validation: Internal and external[J]. QSAR & Combinatorial Science, 2007, 26(5): 694-701. [49] LI B, OU P F, WEI Y L, et al. Polycyclic aromatic hydrocarbons adsorption onto graphene: A DFT and AIMD study[J]. Materials, 2018, 11(5): 726. doi: 10.3390/ma11050726 [50] YU S J, WANG X X, AI Y J, et al. Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides[J]. Journal of Materials Chemistry A, 2016, 4(15): 5654-5662. doi: 10.1039/C6TA00890A [51] O’BRIEN R M. A caution regarding rules of thumb for variance inflation factors[J]. Quality & Quantity, 2007, 41(5): 673-690. [52] TODESCHINI R, CONSONNI V, MAIOCCHI A. The K correlation index: Theory development and its application in chemometrics[J]. Chemometrics and Intelligent Laboratory Systems, 1999, 46(1): 13-29. doi: 10.1016/S0169-7439(98)00124-5 [53] GOLBRAIKH A, SHEN M, XIAO Z Y, et al. Rational selection of training and test sets for the development of validated QSAR models[J]. Journal of Computer-Aided Molecular Design, 2003, 17(2): 241-253. [54] ERIKSSON L, JAWORSKA J, WORTH A P, et al. Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs[J]. Environmental Health Perspectives, 2003, 111(10): 1361-1375. doi: 10.1289/ehp.5758 [55] LEE R G M, BURNETT V, HARNER T, et al. Short-term temperature-dependent air-surface exchange and atmospheric concentrations of polychlorinated naphthalenes and organochlorine pesticides[J]. Environmental Science & Technology, 2000, 34(3): 393-398. [56] LOHMANN R, HARNER T, THOMAS G O, et al. A comparative study of the gas-particle partitioning of PCDD/fs, PCBs, and PAHs[J]. Environmental Science & Technology, 2000, 34(23): 4943-4951. -



 下载:
下载: