-
随着我国纺织印染行业的高速发展,纺织印染废水已经成为重要的工业废水排放来源. 据统计,我国染料年产量达到77万—79万t,约占全世界总产量的2/3[1]. 印染纺织废水具有成分复杂、色度高、可生化性差等特性,成为难处理工业废水之一. 活性红3BS是一种典型偶氮染料被广泛的应用于纺织印染行业中,对水体中的各类生物具有强烈的毒害作用,经过食物链富集进入人体后可以引起人类恶性肿瘤病变,引发各种恶性疾病[2].
染料的降解工艺主要包括生物法、物理法和化学法. 生物法具有成本低、环境友好等优点,但微生物容易被染料的毒性抑制,对高浓度染料废水去除效果并不理想;物理法主要是利用吸附剂的高比表面积将液相中的污染物吸附至固体表面或内部孔道,对多种类型的染料具备处理能力,但没有实现从根本上消除污染物,吸附剂的再生和处置可能会造成二次污染等问题[3]. 因此,基于硫酸根盐自由基(SO4·−)的高级氧化技术因其能高效、快速降解水体各类高浓度染料而受到广泛关注与研究[4]. 一般而言,激活过一硫酸盐(PMS)产生SO4·−的方法包括超声、光辐射、紫外光等,耗能较大且对设备要求较高[5];而在均相反应中引入过渡金属虽然能够提高催化效果,但又易造成金属离子溶出形成污染[6]. 因此,制备碳基载铁改性材料作为非均相催化剂既可有效激活PMS增强降解效率[7],又能避免金属离子的二次污染. 除此之外,磁性生物炭的磁响应特征可以提高催化材料的可回收率[8],具有较高的应用研究价值.
本文通过生物沥滤将离子态铁负载到生物炭上,通过对铁负载材料进行二次热解改性制备出具有磁性的铁基生物炭(Fe3O4@BC)用于高浓度染料废水脱色研究. 生物沥滤驱动制备的磁性生物炭相较于传统方法简洁高效,为磁性生物炭的制备提供了新的的方法与路径.
-
氧化亚铁硫杆菌(Acidithiobacillus ferrooxidans,简称A.f 319)保存于武汉纺织大学微生物实验室. 使用改良后的9K培养基配方:KCL:0.1 g·L−1, (NH4)2SO4 :3 g·L−1 ,K2HPO4:0.5 g·L−1 ,MgSO4·7H2O :0.5 g·L−1,FeSO4·7H2O:40.0 g·L−1. 初始pH调至3.0. 实验所使用的活性红3BS、过一硫酸盐(PMS)、等试剂购于国药集团化学试剂有限公司. 生物炭材料来自湖北省通山县农业秸秆和木材气化副产物.
-
将活化后的A.f 319添加入生物炭材料的9 K培养基中富集培养,60 h后抽滤分离,60 ℃烘干备用. 该生物炭材料命名为BBC. 将烘干后的BBC放入管式炉中热解,升温至700 ℃,全程氮气气氛保护,升温速率5 ℃·min−1,保温1 h. 温度降至室温后取出,使用去离子水反复清洗烘干备用,该材料标注为PBC.
-
本实验使用扫描电子显微镜(Zeiss Gemini 300 ,德国Zeiss)分析材料的形貌特征;X射线衍射仪(Bruke D8 Advance ,德国Bruker )和傅立叶红外光谱仪(Thermo Scientific Nicolet 6700,美国)分析材料晶体结构和表面官能团;X射线光电子能谱技术(Thermo Scientific K-Alpha,美国 Thermo Scientific)分析材料表面元素及价态变化.
-
染料浓度设置为200 mg·L−1,考察BC、BBC、PBC不同材料(添加量固定为1 g·L−1)对活性红3BS吸附实验;染料浓度设置为200 mg·L−1,考察BC、BBC、PBC不同材料(添加量固定为1 g·L−1)PMS(添加量固定为0.5 g·L−1)对活性红3BS的催化降解实验.
活性氧化物种猝灭实验是先将猝灭剂加入200 mg·L−1的活性红3BS的溶液中再进行催化降解实验. 选取甲醇(MeOH)、叔丁醇、对苯醌、L-组氨酸对·OH和SO4·−、·OH、·O2、1O2进行淬灭.
催化剂重复使用性能实验是在反应后进行固液分离,对分离出的催化剂使用超纯水多次冲洗,放入真空干燥箱干燥,循环5次.
-
图1(a)、(b)所示为黄钾铁矾负载于生物炭上的扫描电镜图. 结晶状的黄钾铁矾负载于生物炭上,结晶颗粒呈现不规则的块状,粒径大小从0.5—1 μm不等,经ICP测试载铁含量达到197.6 mg·g−1 . 生物沥滤将离子态铁负载到了生物炭上,可能的反应见式(1—3)[9]:
图1(c)、(d)为热解过后的生物炭,SEM可观察到黄钾铁矾的晶体形态发生了变化,晶体粒径变小,金属颗粒均匀的分散在生物炭上,以更加规整的圆形或椭圆形附着,无明显的团聚.
-
用N2-BET对BC、BBC、PBC的比表面积和孔容孔径进行分析. 结果表明,PBC的比表面积(147.621 m2·g−1)相比BBC(52.743 m2·g−1)和BC(41.090 m2·g−1)显著提高(P<0.05),证明了Fe3O4负载于生物炭上可以提高材料的比表面积,增加有效的吸附点位,促进污染物在材料表面的吸附.
-
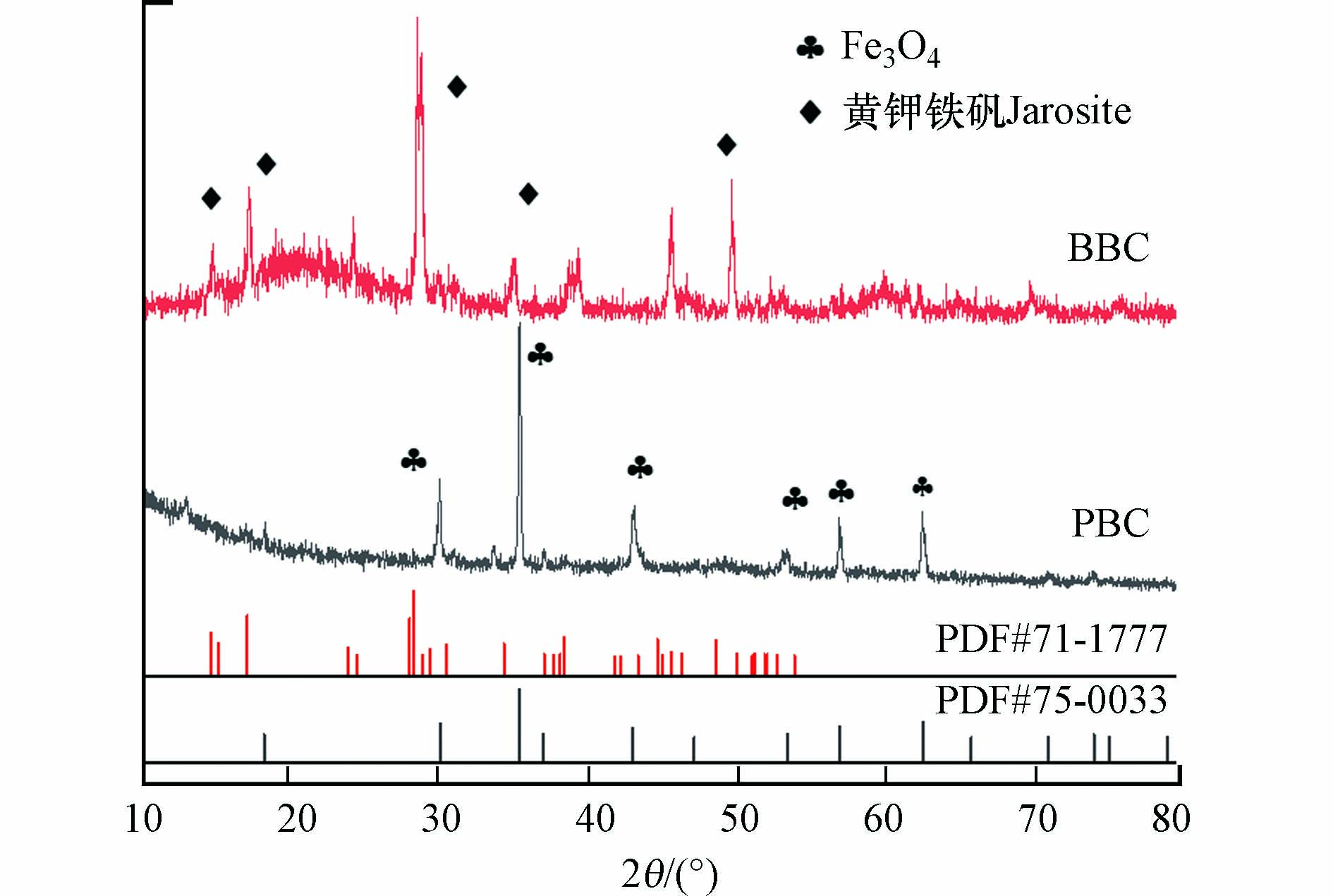
图2为负载黄钾铁矾的生物炭(BBC)与热解后的磁性生物炭(PBC)XRD图谱. BBC的主衍射峰与黄钾铁矾标准卡片PDF#71-1777中的17.4°、28.6°、28.9°、46.8°、49.9°高度吻合,表明黄钾铁矾被成功的负载于生物炭上. Fe3O4的衍射峰在29.9°、35.3°、43.1°、53.4°、57.2°和62.5°对应纯立方尖晶结构的(220)、(311)、(400)、(422)、(511)和(440)晶面,这些结果对应标准卡片PDF#75-0033,可以证明该材料为Fe3O4. 结合SEM图可以看出Fe3O4的比表面积明显大于热解前,暗示着在激活PMS产生自由基的催化反应中具有更好的活性位点[10].
-
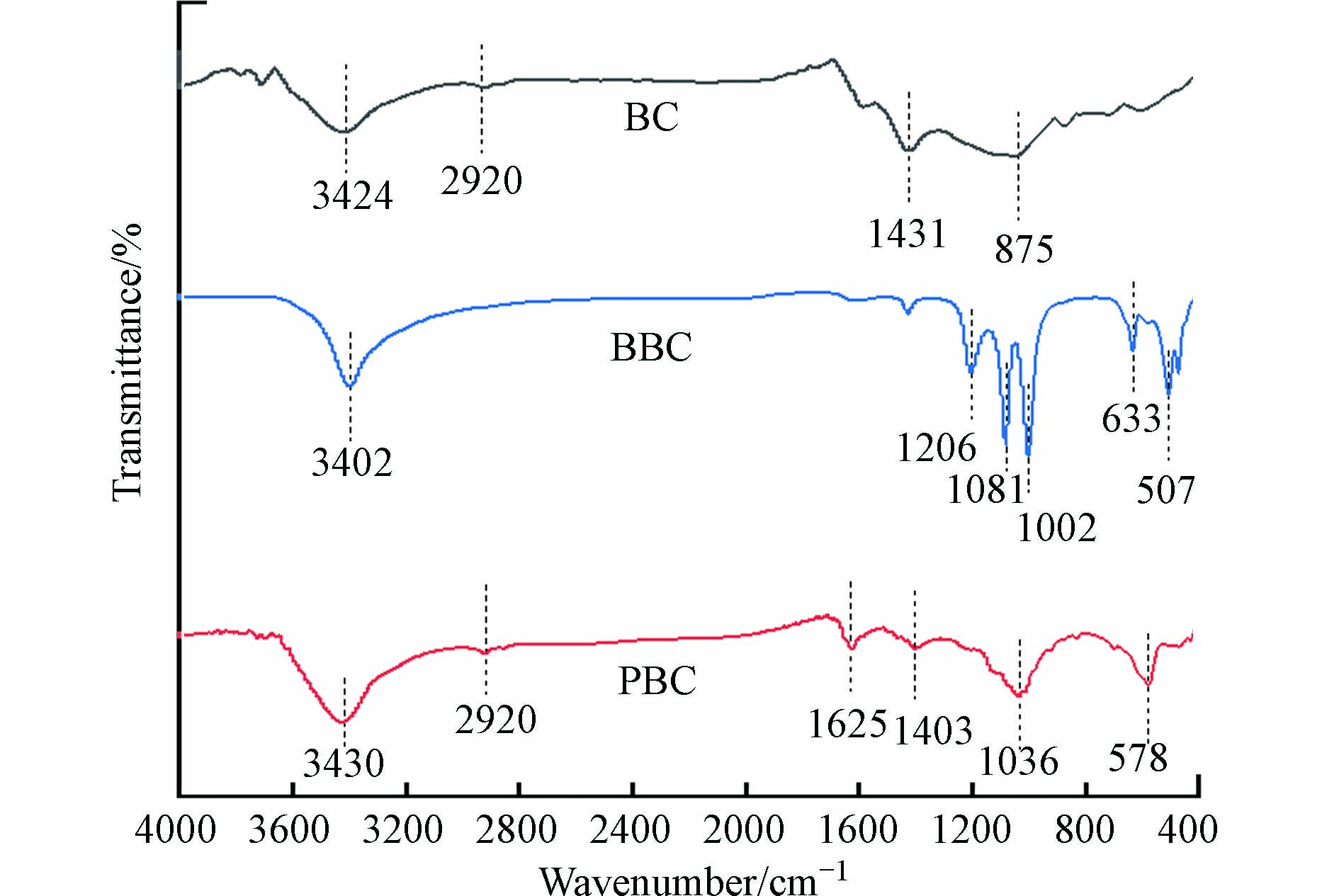
傅里叶红外光谱(FTIR)分析结果表明: BC、BBC和PBC的主要官能团有O—H、C—H、C=O和C—O组成(图3). 其中在3424 cm−1处的特征峰归证实存在O—H的伸缩振动,2920 cm−1处的特征峰归属于C—H的弯曲振动峰[11],1625 cm−1处的特征峰是C=O的伸缩振动,1036 cm−1处是C—O的伸缩振动特征峰[12]. BBC在1000 cm−1到1500 cm−1产生了大量的C—O组,507 cm−1处和633 cm−1处特征峰应归属于Fe—O特征峰. PBC的FTIR谱图显示在578 cm−1出现了属于Fe—O的典型特征峰[13].
-
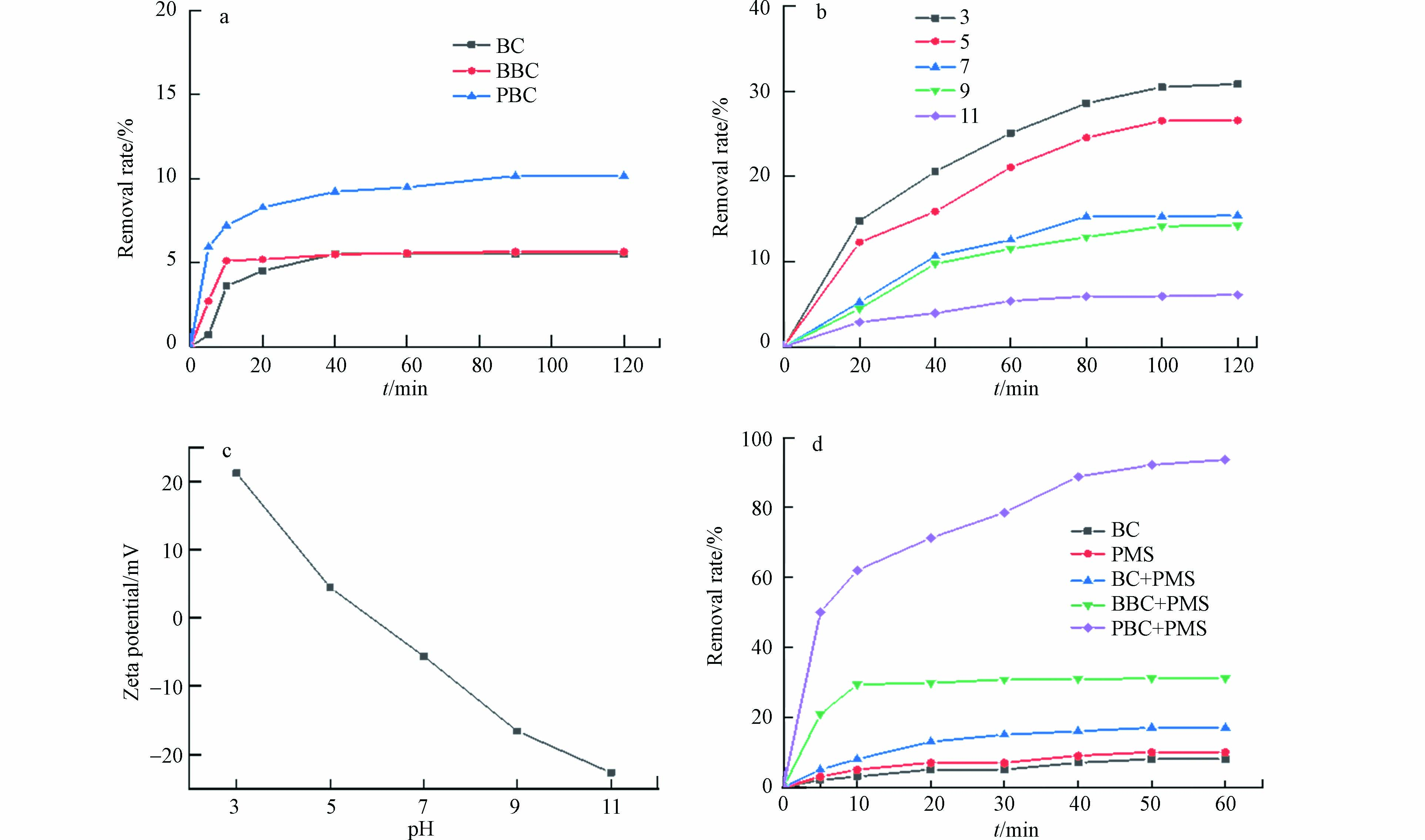
与BC和BBC相比,PBC显著提高了对活性红3BS(200 mg·L−1)的吸附效果,吸附能力分别提高了84.1%和79.5%(见图4a),Fe3O4的载入改善了生物炭的孔隙结构,提高材料的比表面积(表1),增强PBC对染料的吸附性能. 探讨了PBC在pH 3—11的范围内对染料的吸附作用,结果表明pH的增加会导致吸附效果逐渐下降(图4b). 结合Zeta电位结果(图4c),pH较低时碳材料表面带有强烈的正电荷,更容易吸附阴离子染料;随着pH的升高,碳材料表面去质子化带负电,与阴离子染料产生静电排斥从而降低了染料的去除效率[14].
PBC激活PMS对染料的催化降解能力明显优于BC、BBC以及单一的PMS,该反应体系在60 min内对200 mg·L−1染料去除率可达93.8 %(图4d),降解效率优于部分文献报道[15]. Fe3O4负载于生物炭表面不仅增强了对染料的吸附性能,而且能够通过Fe3O4中Fe2+和Fe3+电子穿梭进一步激活PMS产生更多活性物种,提高复合材料的协同降解能力. 在反应60 min后,活性红3BS的TOC去除率达到了67.1 %,这表明仍有部分物质没有被完全矿化[16].
-
pH的变化会对Fe3O4@BC/PMS降解活性红3BS产生显著影响. 由图5(a)可知,在弱酸性条件下Fe3O4@BC对PMS的激活效果较好,pH为5时60 min内的降解率达到了84.69 %. 碱性条件下,随着pH的升高降解效率显著下降,推测是pH偏高时Fe3O4@BC表面的活性位点发生钝化,促进铁离子形成氢氧化铁沉淀,减弱了PBC对染料的吸附能力,同时降低了PMS产生自由基的能力.
PMS投加量的增加可以提高活性红3BS的去除效率. 当PMS的投加量增加到0.75 g·L−1时,对染料的去除率达到93.8 %. 然而,当PMS的投加量继续提高,污染物的降解效率却不增反降. 分析原因,可能是过量的PMS会在短时间内产生大量的·OH和SO4·−与PMS自身产生淬灭反应,生成了氧化能力较弱的SO5·−,导致了SO4·−的利用率降低[17].
-
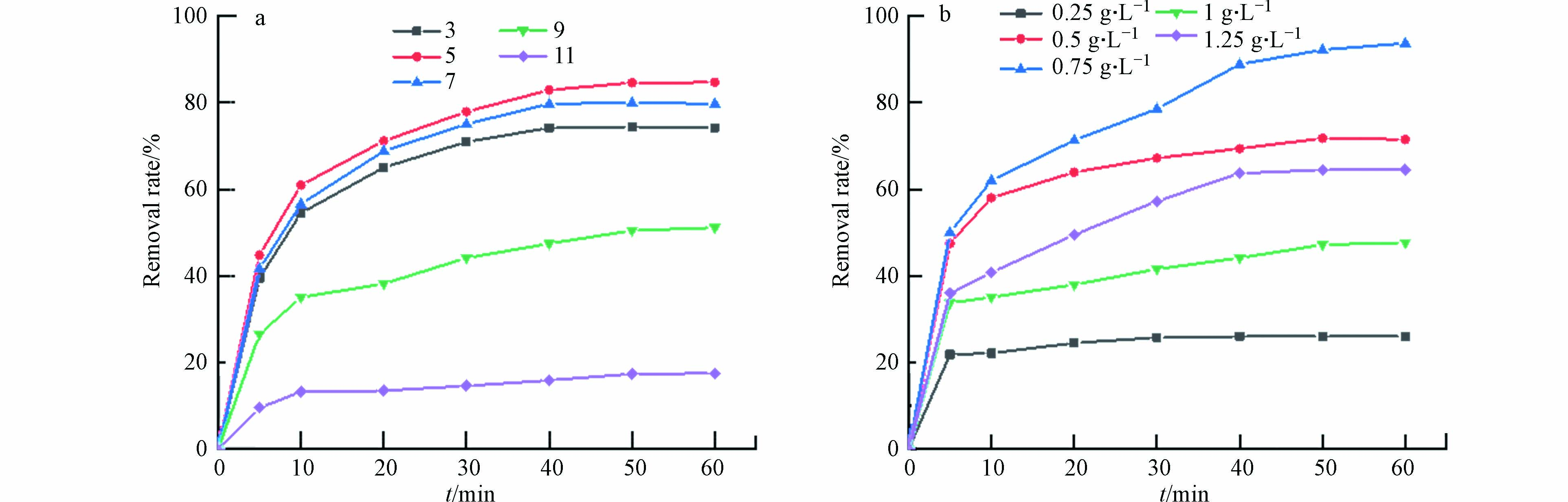
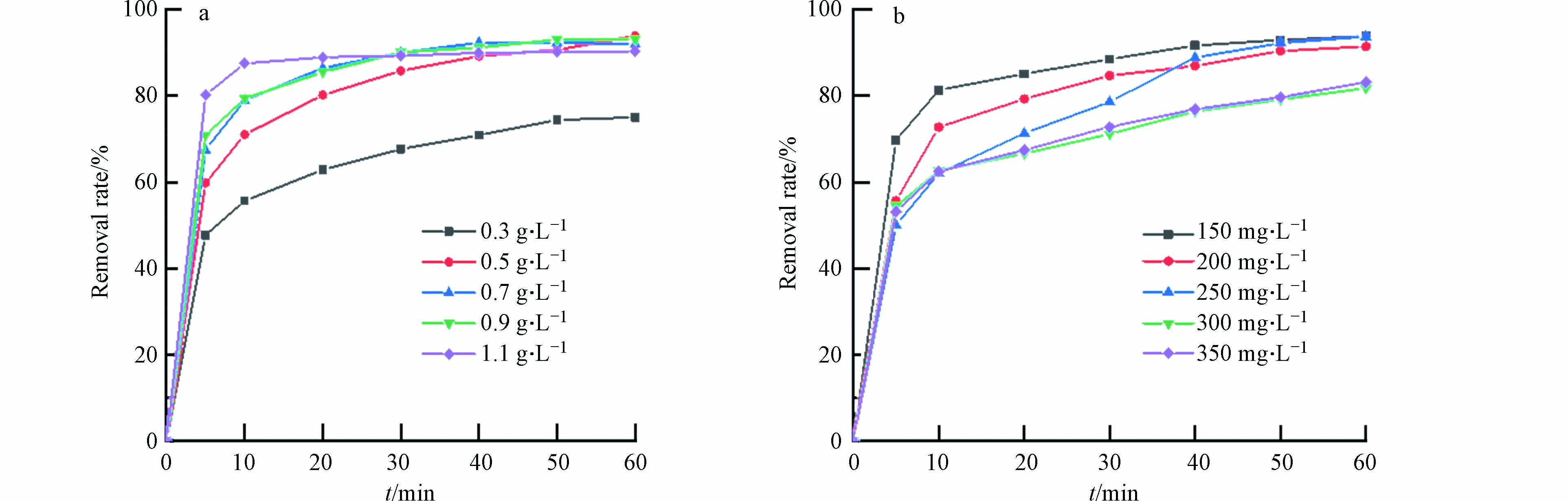
随着催化剂用量的增加,材料对活性红3BS的去除率也会逐步提升. 如图6(a)所示,当催化剂用量为0.5 g·L−1时,去除率达到了93.8 %. 继续增加催化剂的投加量,发现去除效果并不明显,这可能是该体系下PMS不足导致.
污染物的初始浓度升高对降解率影响并不显著. 如图6(b)所示,当污染物的初始浓度由150 mg·L−1增加到350 mg·L−1,60 min内污染物的降解率由95 %只下降到了81.8 %,可见Fe3O4@BC对高浓度染料废水也有较好的催化降解效果.
-
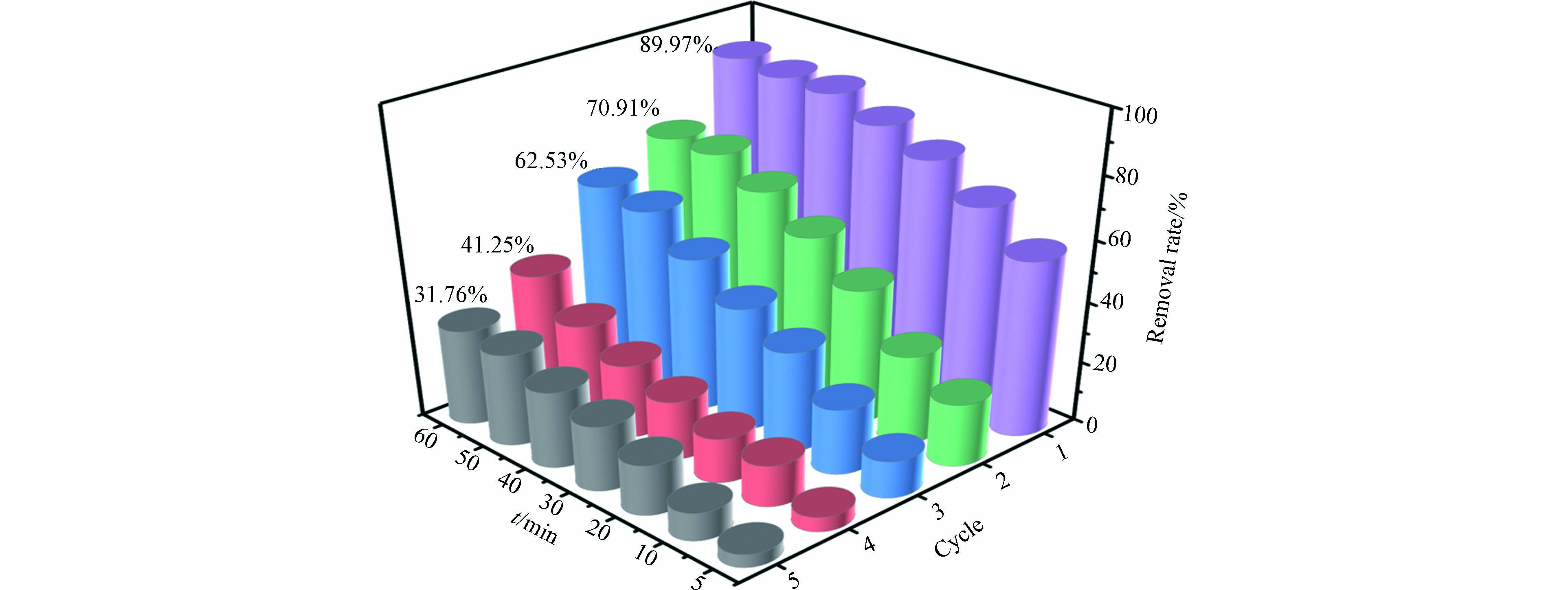
Fe3O4@BC在5次循环利用之后对活性红3BS仍然有一定的催化降解效果. 如图7所示,第一次实验活性红3BS降解率最高,污染物可以被大部分去除;第二次循环使用后,去除率达到70.91%;在随后的第3、4、5次循环中,去除率逐步下降,原因是反应过程中生物炭上的Fe3+和Fe2+循环参与对PMS的活化,造成铁离子溶出,导致了催化活性降低. 5次循环利用之后对污染物的降解率依然能够达到31.76 %,说明该材料是一种具有一定重复利用性的磁性催化剂.
-
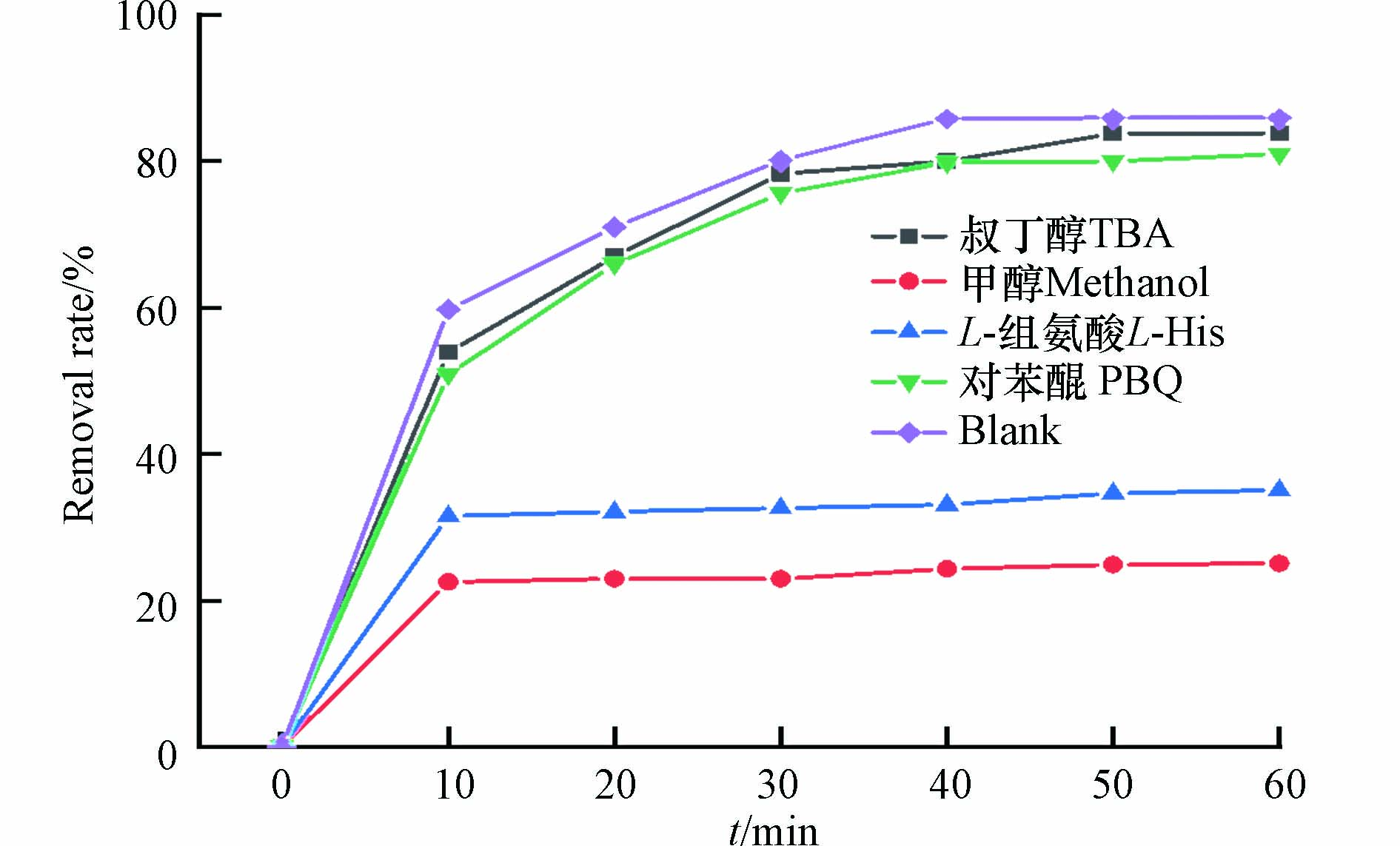
自由基淬灭实验确定磁性催化剂激活PMS起主要作用的自由基活性组分. 淬灭剂选用甲醇(MeOH)、叔丁醇(TBA)、对苯醌(PBQ)、L-组氨酸进行活性氧化物种猝灭实验. 甲醇是一种常见的·OH和SO4·−的自由基猝灭剂,二级反应速率常数k[MeOH,·OH]=9.7×108 mol·L−1·s−1,k[MeOH, SO4−·]=3.2×106 mol·L−1·s−1;叔丁醇是常用的·OH猝灭剂,反应常数k[·OH,TBA]=(3.8—7.6×108 mol·L−1·s−1);对苯醌可以抑制超氧自由基(·O2−), 反应速率常数为k[·O2−,PBA]=(1.0×109 mol·L−1·s−1);L-组氨酸捕获(1O2)的反应速率常数为1.6×106 mol·L−1·s−1[18].
图8展示了不同自由基淬灭剂对磁性生物炭活化PMS降解染料的影响. 投加叔丁醇和对苯醌对染料的去除并没有较大影响;投加甲醇会使染料的去除率下降60.79 %;L-组氨酸的加入会使染料的去除率下降50.82 %. 表明Fe3O4@BC/PMS体系中可能主要产生SO4−·和1O2而非·OH和·O2−[19]. 结合XPS分析反应前后 PBC 的化学价态变化(图9),PBC 拟合出的709.6 eV(Fe2+)和712.9 eV(Fe3+)两组峰,Fe2+和Fe3+的含量分别为66.1 %和33.9 %,反应后Fe2+的含量降低到了37.4 %,说明Fe3O4上颗粒的Fe3+和Fe2+存在电子转移机制,转化过程中的电子转移加速了污染物的降解[20]. 除此之外,生物炭表面的缺陷点位和部分官能团也可以作为活性点位活化PMS.
根据上述分析推测可能的降解机制. Fe3O4@BC活化PMS降解染料的机制主要包括自由基途径(SO4−·)和非自由基途径(1O2). 可能的反应过程见式(4—7):
-
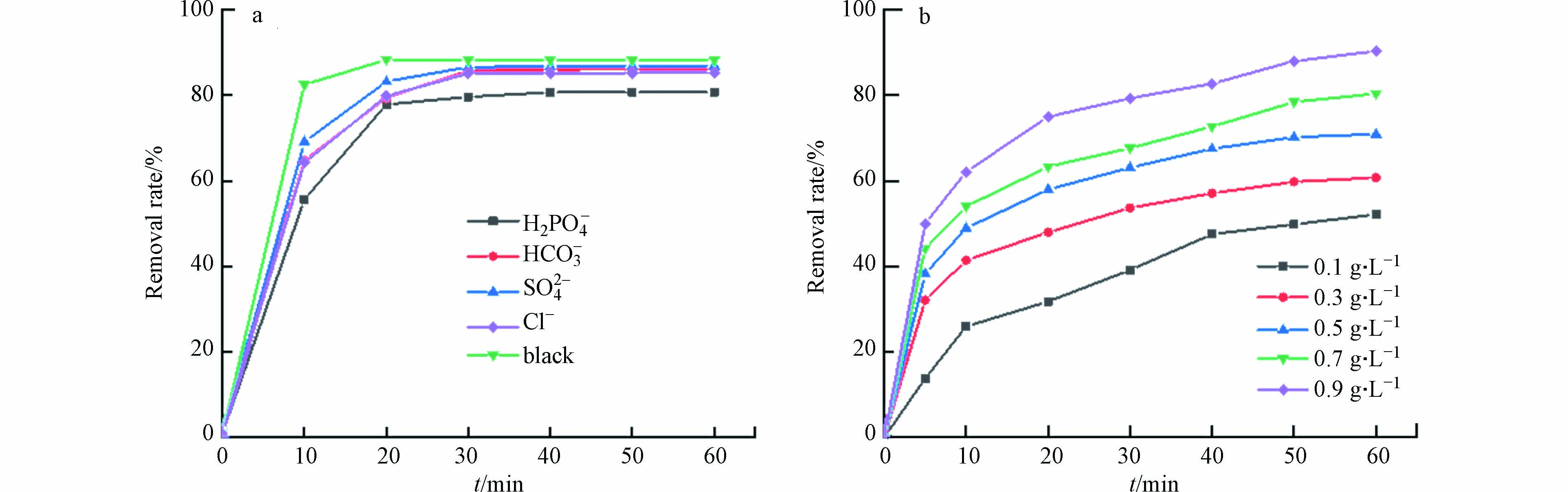
无机阴离子普遍存在于染料废水中,并且会对染料的降解效果产生影响[21]. 本实验选取了Cl−、HCO3−、SO42-和H2PO4−作为干扰离子,探究这些离子对活性红3BS催化降解的影响. 实验结果如图10(a)所示:干扰离子的添加对降解效率产生影响,尤其是H2PO4−的存在会使降解效率从88.2%下降到80.7%. 其原因可能是:(1)这些无机离子的引入会与染料竞争Fe3O4@BC上的活性位点;(2)无机离子可以清除体系中的自由基.
为考察Fe3O4@BC/PMS体系对其他类型染料降解能力,选取难降解蒽醌类染料亚甲基蓝作为目标污染物. 结果显示Fe3O4@BC/PMS体系对100 mg·L−1的亚甲基蓝具有良好的去除效果,催化剂投加量为0.9 g·L−1时,60 min内对亚甲基蓝的降解效率可以达到90.3%.
-
本文利用生物沥滤将离子态铁负载到生物炭上,对铁负载生物炭二次热解改性,成功制备出具备磁性效应的铁基生物炭. Fe3O4@BC/PMS体系相较于原始生物炭的催化降解能力显著提升,对几种类型染料具有催化降解能力,多次循环利用后仍然保持较高的降解效率. 自由基猝灭实验探讨了复合材料对染料的降解机制,Fe3O4@BC/PMS体系中SO4−·和1O2主导了对染料的降解过程. 结果表明Fe3O4@BC材料在处理难降解的染料废水方面具有较高的应用前景,本研究为磁性生物炭改性材料处理染料废水方面提供了新方法.
生物沥滤法制备Fe3O4@BC协同PMS对活性红3BS染料的降解
Study on the decolorization effect of bioleaching-driven Fe3O4@BC-activated PMS on reactive red 3BS
-
摘要: 磁性生物炭的制备方法包括物理法和化学法,制备要求较高. 本文通过生物沥滤将离子态铁负载到生物炭上,对铁负载生物炭二次热解改性,运用SEM、XRD、XPS、FTIR等手段对材料进行表征,制备出具有磁性的铁基生物炭(Fe3O4@BC). 研究材料改性前后及协同PMS对活性红3BS染料的降解过程及机理,探讨了环境因素对染料降解效果的影响,在最佳反应条件下,协同作用对200 mg·L−1的活性红3BS染料废水脱色效果达到93.8 %. 自由基猝灭实验证实反应机理是Fe3O4@BC/PMS体系中自由基和非自由基途径生成的SO4·−和1O2参与了对染料的协同降解. 五次循环利用后催化体系对染料的降解效率仍能达到31.2 %. 本研究为磁性生物炭在高浓度染料废水处理方向提供了新的方法和路径.Abstract: The preparation methods of magnetic biochar include physical and chemical methods with high preparation requirements. In this paper, iron-based biochar (Fe3O4@BC) with magnetic properties was prepared by loading ionic iron onto biochar through bioleaching, modifying iron-loaded biochar by secondary pyrolysis, and characterizing the material by using SEM, XRD, XPS, and FTIR. The degradation process and mechanism of reactive red 3BS dyestuff before and after material modification and synergistic PMS were investigated, and the influence of environmental factors on the degradation effect of dyestuff was discussed. Under the optimal reaction conditions, the synergistic effect on decolorization of 200 mg·L−1 reactive red 3BS dyestuff wastewater reached 93.8%. The free radical burst experiments confirmed the reaction mechanism that SO4·− and 1O2 generated by free radical and non-free radical pathways in the Fe3O4@BC/PMS system were involved in the synergistic degradation of the dye. The degradation efficiency of the catalytic system for the dye could still reach 31.2% after five cycles of recycling. This study provides a new method and pathway for magnetic biochar in the direction of high concentration dye wastewater treatment.
-
Key words:
- bioleaching /
- magnetic biochar /
- PMS /
- Azo dyes /
- decolorization
-

-
表 1 BC、BBC、PBC的比表面积和孔隙结构
Table 1. Specific surface area and pore structure of BC
样品
Sample比表面积/(m2·g−1)
Surface area孔容/(cm3·g−1)
Pore volume平均孔径/ nm
Average pore diameterBC 41.090 0.022 3.439 BBC 52.743 0.057 4.834 PBC 147.621 4.725 2.586 -
[1] 沈彧彧. 纺织行业节能减排现状与主要废水处理技术综述[J]. 中国资源综合利用, 2018, 36(1): 93-96. SHEN Y Y. Current status of energy saving and emission reduction in textile lndustry and summary of main wastewater treatment technologies[J]. China Resources Comprehensive Utilization, 2018, 36(1): 93-96(in Chinese).
[2] 王启明, 石旭, 朱茜茜, 等. 外加电场作用下希瓦氏菌对活性红3BS脱色效果研究[J]. 江西化工, 2019(1): 155-157. doi: 10.3969/j.issn.1008-3103.2019.01.038 WANG Q M, SHI X, ZHU X X, et al. Decolorization of reactive red 3BS by Shewanellaoneidensis applied electric field[J]. Jiangxi Chemical Industry, 2019(1): 155-157(in Chinese). doi: 10.3969/j.issn.1008-3103.2019.01.038
[3] OTHMAN N H, ALIAS N H, SHAHRUDDIN M Z, et al. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 2803-2811. doi: 10.1016/j.jece.2018.04.024 [4] XIAO R Y, LUO Z H, WEI Z S, et al. Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies[J]. Current Opinion in Chemical Engineering, 2018, 19: 51-58. doi: 10.1016/j.coche.2017.12.005 [5] SHAD A CHEN J, QU R J, et al. Degradation of sulfadimethoxine in phosphate buffer solution by UV alone, UV/PMS and UV/H2O2: Kinetics, degradation products, and reaction pathways[J]. Chemical Engineering Journal, 2020, 398: 125357. doi: 10.1016/j.cej.2020.125357 [6] 胡昊, 潘顺龙, 聂溪, 等. CoFe2O4的制备及其对有机膦酸的去除性能研究[J]. 环境科学学报, 2022, 42(8): 156-165. HU H, PAN S L, NIE X, et al. Preparation of CoFe2O4 as catalyst for the removal of phosphonates[J]. Acta Scientiae Circumstantiae, 2022, 42(8): 156-165(in Chinese).
[7] ZHU S S, HUANG X C, MA F, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms[J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. [8] HOSLETT J, GHAZAL H, KATSOU E, et al. The removal of tetracycline from water using biochar produced from agricultural discarded material[J]. The Science of the Total Environment, 2021, 751: 141755. doi: 10.1016/j.scitotenv.2020.141755 [9] 钟萍丽, 伍赠玲, 季常青, 等. 酸性矿山废水生物矿化源头控制技术研究进展[J]. 湿法冶金, 2022, 41(4): 289-294. ZHONG P L, WU Z L, JI C Q, et al. Research progress on source control technologies of biological mineralization for acid mine drainage[J]. Hydrometallurgy of China, 2022, 41(4): 289-294 (in Chinese).
[10] SUN J, ZHOU S B, HOU P, et al. Synthesis and characterization of biocompatible Fe3O4 nanoparticles[J]. Journal of Biomedical Materials Research Part A, 2007, 80A(2): 333-341. doi: 10.1002/jbm.a.30909 [11] WANG Y, YU L, WANG R T, et al. Microwave catalytic activities of supported perovskite catalysts MOx/LaCo0.5Cu0.5O3@CM (M = Mg, Al) for salicylic acid degradation[J]. Journal of Colloid and Interface Science, 2020, 564: 392-405. doi: 10.1016/j.jcis.2019.12.130 [12] HE X D, LI P Y. Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): Occurrence, sources and health risks[J]. Exposure and Health, 2020, 12(3): 385-401. doi: 10.1007/s12403-020-00344-x [13] HUANG H X, GUO T, WANG K, et al. Efficient activation of persulfate by a magnetic recyclable rape straw biochar catalyst for the degradation of tetracycline hydrochloride in water[J]. Science of the Total Environment, 2021, 758: 143957. doi: 10.1016/j.scitotenv.2020.143957 [14] CELIK S, DUMAN N, SAYIN F, et al. Microbial cells immobilized on natural biomatrix as a new potential ecofriendly biosorbent for the biotreatment of reactive dye contamination[J]. Journal of Water Process Engineering, 2021, 39: 101731. doi: 10.1016/j.jwpe.2020.101731 [15] ARSLAN H, BOUCHAREB R, ARIKAN E B, et al. Iron-loaded leonardite powder for Fenton oxidation of Reactive Red 180 dye removal[J]. Environmental Science and Pollution Research International, 2022, 29(51): 77071-77080. doi: 10.1007/s11356-022-21306-7 [16] LIU J, CUI J N, ZHAO T Y, et al. Fe3O4-CeO2 loaded on modified activated carbon as efficient heterogeneous catalyst[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 565: 59-69. [17] 方志勇, 郭枭杰, 周鑫, 等. Fe3O4/MoS2强化过氧化单硫酸盐活化去除2, 4-二氯苯氧乙酸[J]. 环境化学, 2022, 41(4): 1435-1443. doi: 10.7524/j.issn.0254-6108.2020122107 FANG Z Y, GUO X J, ZHOU X, et al. Removal of 2, 4-dichlorophenoxyacetic acid by Fe3O4/MoS2 enhanced PMS activation[J]. Environmental Chemistry, 2022, 41(4): 1435-1443(in Chinese). doi: 10.7524/j.issn.0254-6108.2020122107
[18] DU W Y, ZHANG Q Z, SHANG Y N, et al. Sulfate saturated biosorbent-derived nanoarchitecture as an efficient catalyst for peroxymonosulfate activation[J]. Applied Catalysis B:Environmental, 2020, 262: 118302. doi: 10.1016/j.apcatb.2019.118302 [19] YANG Y, BANERJEE G, BRUDVIG G W, et al. Oxidation of organic compounds in water by unactivated peroxymonosulfate[J]. Environmental Science & Technology, 2018, 52(10): 5911-5919. [20] WANG Q R, SHI Y X, LV S Y, et al. Peroxymonosulfate activation by tea residue biochar loaded with Fe3O4 for the degradation of tetracycline hydrochloride: Performance and reaction mechanism[J]. RSC Advances, 2021, 11(30): 18525-18538. doi: 10.1039/D1RA01640G [21] LI W Q, LI S Q, TANG Y, et al. Highly efficient activation of peroxymonosulfate by cobalt sulfide hollow nanospheres for fast ciprofloxacin degradation[J]. Journal of Hazardous Materials, 2020, 389: 121856. doi: 10.1016/j.jhazmat.2019.121856 -




 下载:
下载: