-
双酚A(bisphenol A,简称BPA),分子式为C15H16O2,由一个丙烷骨架和两个苯环组成,每个苯环上含有一个羟基. BPA难溶于水,21.5℃下在水中的溶解度为1 g·L–1,挥发性较弱,生物降解性差. BPA是制造塑料的前体物质,由苯酚和丙酮在酸性介质中缩合制得[1],常用于生产聚碳酸酯塑料、环氧树脂[2] 、阻燃和涂料等产品[3],是重要的有机合成中间体,同时也是最常用的塑料添加剂之一[4] . BPA在食品和各种消费品中广泛应用,是世界上生产量最大的化学品之一[5],2016年全球年产量约为800万t[6],到2022年可达1060万吨[7]. 亚洲是BPA使用量最多的地区[8],而中国的BPA消耗量约占全球的一半[9]. 由于BPA应用广泛,普遍存在于人们的生活中,其一般通过生活污水、农业径流和工业废水进入环境[10],同时由于其难生物降解,会持久性存在于环境中[11],严重威胁人类和动物健康[12-13]. 因此,如何有效的处理BPA废水成为众多研究者的关注重点. BPA废水处理方法通常有三大类:物理法、生物法和化学法[14-15]. 本文综述了上述几种方法在处理BPA过程中的作用机理、优缺点及研究进展,可为进一步推动实际BPA废水的处理提供理论依据.
-
BPA能够破坏生物体的激素组成,引起内分泌紊乱[16],是一种典型的内分泌干扰物(EDCs)[17]. 大量研究表明BPA具有潜在的生物毒性[18-19],接触BPA会导致糖尿病、癌症、肿瘤、心血管疾病[20],甚至器官中毒[21]. 实验室动物的研究表明,即使在低剂量下(2 ng·g−1)也会对健康产生不利影响,早期接触BPA会导致荷尔蒙的改变,从而影响免疫系统和身体功能[22]. 对此,欧洲食品安全局(EFSA)审查了BPA暴露和毒性问题,降低了BPA可耐受每日摄入量(TDI),由此前50 μg·kg−1·bw−1·d−1降到4 μg·kg−1·bw−1·d−1[23],以此引起对BPA的重视.
近年来,随着BPA产品的广泛使用,其在各流域水体、土壤、沉积物、空气、城市垃圾、饮用水和食品中均已被检出[24-27] ,最终通过各种途径进入环境水体. 表1列出了我国不同地区环境水体中BPA的浓度数据,可以看出BPA检出率较高,已成为我国环境水体中不可忽视的污染物. 此外,BPA在地下水和沉积物中可稳定保持达70 d, 现已成为环境中能检测到的最微量污染物之一[28-29] . 面对当前我国对水生态安全和水质量健康方面的需求,急需开发高效经济的BPA废水处理方法.
-
吸附法是深度处理BPA废水的重要方法之一[38],该方法通过吸附材料把BPA富集,再利用其他方法将BPA回收. 常用的吸附材料有活性炭、高分子材料(树脂)、硅质材料(黏土、沸石)、矿化垃圾、生物材料(农业固废)等[39].
活性炭因较大的比表面积和孔容,对BPA具有较好的吸附能力[40]. Norah等[41]利用废烟蒂,通过水热炭化工艺制备了氮掺杂碳气凝胶(NDC)用于吸附BPA. 实验表明,BPA在NDC上的吸附符合拟二级动力学模型和Langmuir等温吸附模型;热力学结果表明该吸附过程是自发吸热的. NDC可再生,经7次再生循环后NDC对BPA去除率保持在92.47%. Sun等[42]采用直接热解法合成氮掺杂碳(PDA-C)来吸附水中BPA,实验表明制备的PDA-C可以快速吸附BPA(t < 5 min),且具有高吸附量( qm = 1351 mg·g–1),吸附效率稳定. 吸附机理表明,sp2 C和杂原子N的存在改善了PDA-C对π-π电子给体-受体的吸附作用及与BPA之间的疏水效应,是优越去除性能的主要源头. Hugo等[43]通过水热炭化松果壳合成活化氢炭,用于BPA吸附去除,实验表明在pH为7.0时吸附容量最大达708 mg·g–1,吸附主要通过单层形成,是一个吸热自发过程.
吸附法具有安全、操作方便、流程简单等优点,即使在复杂水环境中也能保持高效性能[44],但无法用于修复流域/区域污染,另外因污染物没有被彻底降解,吸附后的材料存在二次污染风险.
在这种情况下,将吸附技术与其他技术,如光催化氧化,联用将是较好的补强方式[45]. Zhou等[46]采用化学法制备了分子印迹的硫掺杂二氧化钛光催化剂(MICST),并通过选择性光降解的方法去除BPA,实验表明,MICST对BPA有较高的吸附能力(Qmax = 191 mg·g–1),BPA在MICST上是单层吸附,且符合Langmuir等温模型,BPA的光催化与吸附具有协同作用.
-
膜技术以其占地面积小、操作简单、分离性好[47]、能耗低且在处理过程中不产生代谢产物而广受关注[48-49]. 膜技术去除BPA的重要原理是尺寸排斥和静电排斥[50],根据膜滤径大小可分为微滤、超滤、纳滤、反渗透、深吸、电渗析和渗透蒸发等. 其中,纳滤和反渗透[51]在废水处理中应用较广,对BPA有较高的截留率.
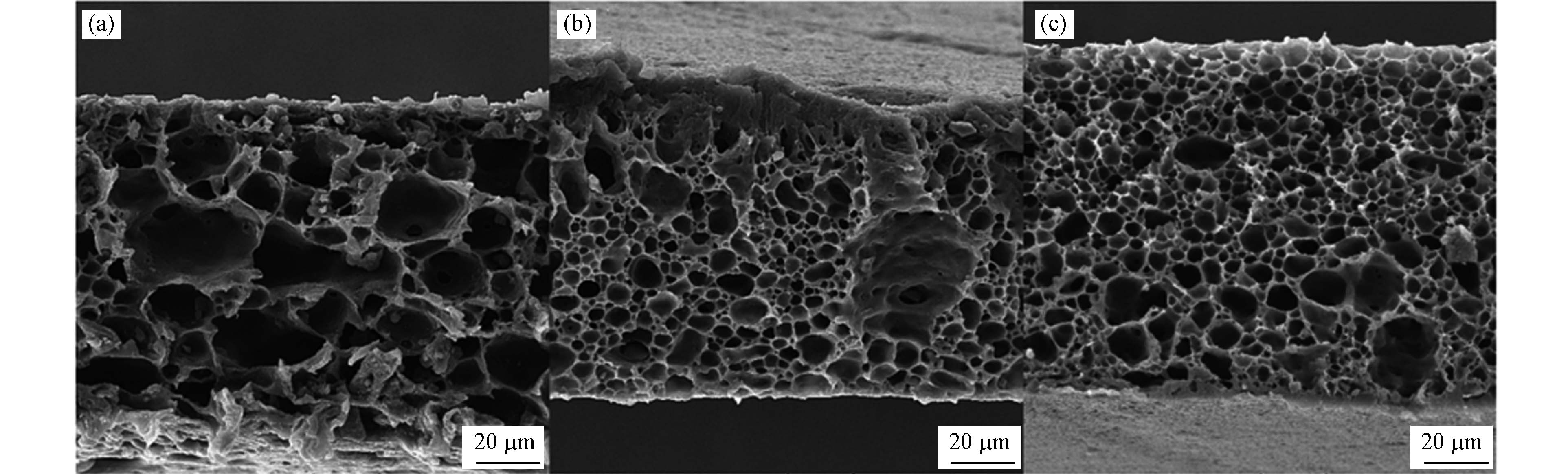
Azizul等[52]采用静电纺丝法制备的PVDF-PVP-MnO2纳米复合膜用于去除BPA,纳米纤维直径为105.01 nm,拉伸强度为2.93 MPa,表面接触角为102.10°,孔隙率为97.88%. 实验表明PVDF-PVP-MnO2纳米复合膜对BPA去除效率较好,且可重复使用,适用于饮用水中低浓度BPA去除. Hou等[53]采用改性双极射流静电纺丝技术制备了新型双功能纳米纤维膜(APAN/CS/PVA-NM),用于过滤水中BPA. 其对BPA的去除主要是通过分子间力和氢键作用,BPA的过滤突破曲线符合Thomas模型. 实验表明,经过10次循环,APAN/CS/PVA-NM对BPA仍能保持较高的去除率. Simin等[54]采用相转化法合成了聚砜/氧化石墨烯纳米复合膜(PSF/GO,图1为横截面FESEM图像),其中GO含量为0.4%的PSF/GO膜对PBA去除效率最佳,对7.5 mg·L–1BPA的去除率可达93%,相应输入压力、操作时间、BPA初始浓度和pH分别为102 kPa、10.6 min和5.5. 与单一PSF膜相比,PSF/GO膜在孔隙率、zeta电位和纯水通量方面表现出更好的特性.
高操作压力、低渗透性及膜污染是当前膜分离技术在水处理过程中尚存的问题[55]. 目前研究者多将膜技术与其他技术联用来改善膜处理中的技术难题,如将膜技术和光催化技术联合,可提高污染物去除效率,减轻膜污染,延长膜的使用寿命. Wang等[56]通过相转化法制备光催化Fe掺杂TiO2/PSF复合超滤膜,并采用水热法合成光催化剂. 实验表明,合成催化剂的最佳条件是Fe/TiO2掺杂比例为1:200,反应温度为160℃,反应时间为12 h,制备的Fe-TiO2质量比为0.20的复合膜在180 min内对BPA的去除率达到90.78%,光催化剂的加入使复合膜具有可见光光催化和超滤的双重特性. 反应机理如下:由于PSF的静电吸附,BPA分子迅速聚集在膜表面和孔内,复合膜内的Fe-TiO2颗粒在可见光照射产生光电子(e−)和空穴(h+),分别与O2和H2O反应生成自由基,这些强氧化性自由基可将BPA分解为小分子、CO2和H2O.
-
微生物法是利用微生物对外来污染物进行生物转化/吸收以实现污染物去除的一种高效[57]和环境友好的方法[58-60]. BPA进入环境后可被微生物利用并将其从环境中去除[61-62]. Hou等[63]分离出一种具有BPA降解能力的奇异变形杆菌SQ-2. 实验表明,SQ-2在98 h内可降解77.72%的BPA,且在河水中表现出与在最小盐介质中相同的降解性能. Sarma等[64]研究了3种细菌菌株(HAWD1,HAWD2和HAWD3)和直接从河流沉积物中分离的细菌联合体(BCC1)对BPA的生物降解作用. 研究表明,BCC1对BPA的去除率最快,72 h内可将BPA完全转化为代谢物. 在实验条件下,所有的生物降解都遵循零阶反应. Gloria等[65]测试了5种潜在的益生菌,即乳酸乳球菌、枯草芽孢杆菌、植物乳杆菌、粪肠球菌和酿酒酵母对BPA的毒性耐受性及其去除BPA的能力. 实验表明,BPA毒性在所有微生物身上都很明显,五种研究菌株显示出相当高的BPA去除效率,其中枯草芽孢杆菌对BPA的去除率最高,为51.9%. 细菌和酵母对BPA的去除可能是通过静电力或化学亲和力或生物降解在细菌表面吸附的结果.
生物法操作周期较长、对高浓度样品的处理效果较差、生物降解后雌激素活性增加等限制了绿色生物方法的应用. 将化学法(作为前处理或后处理)与生物法联合使用可达到比单个方法更好的处理效果. Wu等[66]制备了一种新型的光子-酶级联催化体系HRP-CN/Cu3(PO4)2杂化纳米花,对BPA进行无害化处理. 实验表明,HRP-CN/Cu3(PO4)2不仅表现出良好的固定化酶的酶学性质,而且具有更好的耐酸、耐碱和耐高温性能. HRP-CN/Cu3(PO4)2在60 min内对BPA的去除率可达72.98%,催化过程既不会产生不必要的有害环境的物质,又能充分利用太阳能.
-
臭氧氧化法利用催化材料表面的活性位点加速臭氧分解形成O·和OH·[67],将水中有机物转化为无毒小分子物质,因其氧化能力强(pH=7时氧化电位为2.07V)[68]、无二次污染[69]、效率高而成为高效降解BPA的方法之一[70]. Mu等[71]构建了三维(3D)氧化铈(CeOx)/SBA-16纳米复合材料,该催化剂可提高臭氧利用效率,同时促进主要活性氧物种·OH的形成. CeOx/SBA-16/O3在90 min内可矿化60.9%的BPA. 缪倩倩等[72]利用Cu(NO3)2·3H2O对螯合树脂D851沉淀改性,改性后的树脂可去除水体中86.71%的BPA(初始浓度为10 mg·L–1),且重复使用5次后稳定性依然良好. Li等[73]采用微波辅助法制备了双金属共掺杂的生物炭复合材料 (CoFe2O4@BC),在室温下8 min内可催化降解95.8%的BPA (100 mg·L–1).
但臭氧氧化也存在传质效率差、臭氧利用率低[74]、催化剂制备复杂、成本高[75]等问题. 在这种情况下,将臭氧氧化与吸附法联合使用或有更好的效果. Zhang等[76]制备了Fe3O4-MnO2磁性复合材料,并将其作为催化剂用于BPA在水溶液中的催化臭氧化. 实验表明,Fe3O4-MnO2磁性复合材料对BPA降解具有较高的催化活性、稳定性和可循环利用性. 在最佳实验条件下,BPA在Fe3O4-MnO2/O3工艺中的去除效率达到97.0%. 机理如下:Fe3O4-MnO2表面吸附的H2O分解成OH−和H+,形成表面羟基(等式(1)). 水性臭氧吸附在Fe3O4-MnO2表面,并与表面羟基相互作用生成HO˙,进而氧化污染物. 此外,O3分子与M-O2˙ ̄反应产生一个HO3˙自由基,HO3˙自由基可以释放到溶液中,并迅速还原生成HO˙(等式(2—4)).
-
光催化氧化法利用可再生的太阳能或人工光源激活光催化剂产生强氧化性自由基(如·OH),将有机物矿化为CO2和H2O. 该技术无需外加化学物质[77],能耗较低,是一种绿色[78]、经济[79]的有机污染物去除技术[80]. 与传统处理方法不同,光催化氧化法较适用于低浓度污染物去除[81]. 吴瞳等[82]利用甲醇辅助溶剂热方法制备了新型氮化碳同质结光催化剂(x% MeCN-CN ,其中x为甲醇预处理尿素产物与尿素的质量百分比),其中30% MeCN-CN具有最佳光催化性能,4 h内对BPA去除率可达98.9%,经过5次光催化循环使用后,依旧具有良好光催化活性. Chukwuka等[83]以邻苯二甲酸锰为原料,采用水热釜法合成了ZnWO4MnPc,其在365 nm紫外照射下对BPA去除率达60%. 在H2O2浓度为5 mmol·L−1的条件下,辐照4 h后去除率可达80%左右. 在相同浓度的H2O2存在下,在450 nm可见光照射30 min后,去除率几乎达到100%. Hunge等[84]采用水基沉淀法合成了TiO2@ND复合光催化剂,其在中性和酸性条件均可降解BPA. 在紫外照射下,8 mg TiO2@ND复合材料在pH为5.1时可实现BPA的完全降解. TiO2@ND优异的光催化活性归因于其对光的强吸收和其内部有效的光生载流子分离.
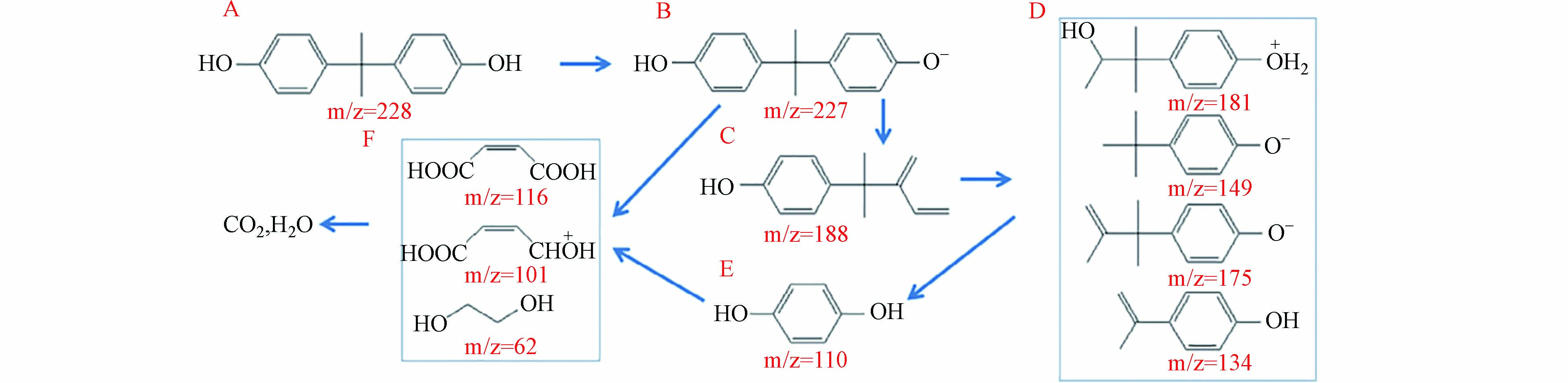
然而,目前的光催化氧化处理工艺由于量子产率低、光生活性氧(ROS)利用率低、高压过滤回收悬浮光催化剂的能量要求高等原因,存在能量效率低的问题[85]. 在这种情况下,联合生物酶处理或能解决这些问题. Zhang等[86]将辣根过氧化物酶(HRP)固定在介孔石墨氮化碳(MCN)表面,制备了光催化剂酶异质结(PEH)用于去除BPA. 实验表明,MCN/HRP PEH在50 min内对BPA的去除率可达85.7%,具有较高的降解活性和循环稳定性. MCN/HRP PEH降解性能的提高主要是由于载流子可见光捕获能力和分离效率的提高,以及光酶协同催化作用,在MCN/HRP样品上,BPA分子在降解反应过程中可能的中间体和途径如图2所示.
-
电催化氧化法可通过阳极直接氧化实现BPA去除[87-88],改法因无需额外添加氧化剂,且操作简便、效率高、产毒性低[89]、化学稳定性高[90]等特点,被认为是一种很有前途[91]的有机污染物降解技术[92]. 阳极材料是电催化氧化方法的关键,决定了电极对有机污染物的去除效率. 符远航等[93]采用电沉积-热分解法制备了负载多壁碳纳米管(MWCNTs)的多孔Ti/SnO2-Sb-Ni电极,该电极可在60 min内去除99.76%的BPA(50 mg·L–1). Mohammad等[94]采用阳极沉积法制备了石墨/β-PbO2阳极,在三维电化学反应器(3DER)中研究了石墨/β-PbO2阳极和颗粒活性炭(GAC)颗粒电极在降解BPA时的电催化协同作用. 实验表明,石墨/β-PbO2阳极和GAC颗粒电极的3DER氧化体系可以高效、低能耗地降解和矿化BPA. 在最佳条件下,优化后的3DER体系对BPA的去除率可达98.8%. Chen等[95]通过一步水热法制备了稀土Er掺杂CdWO4纳米材料 (Er-CdWO4),用来去除BPA. 实验表明,质量分数为0.5 % Er-CdWO4是测试样品中最好的电催化剂,有效克服了电子转移性能受限和对BPA催化活性弱的缺陷,表现出最快的电子转移性能和最强的BPA电催化能力.
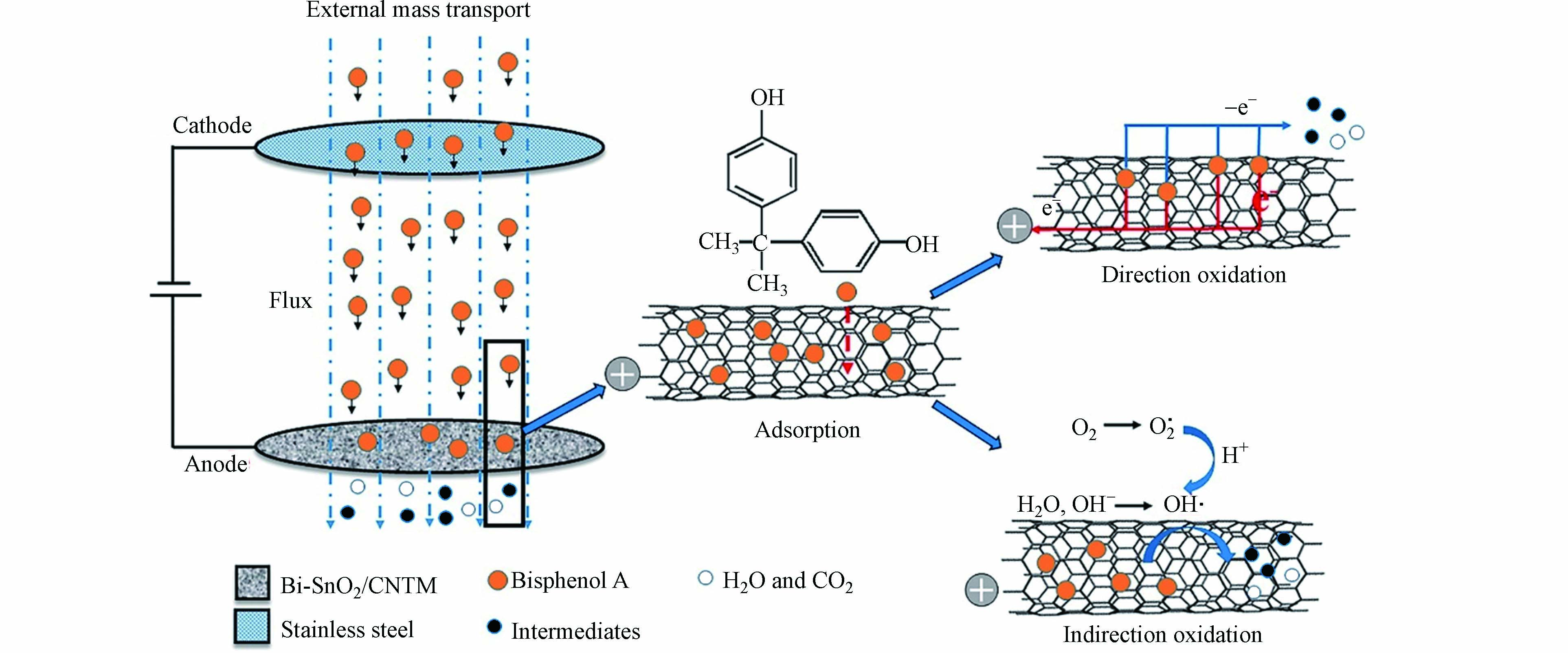
尽管如此,当前的阳极大多为平板电极,针对浓度不高、扩散性较差的有机污染物降解效率和能耗受传质限制以及电极极化的问题,可联合膜技术将污染物吸附后再进行电催化氧化. Zhao等[96]通过简单的真空过滤-电吸附-水热工艺,设计并制备了Bi-SnO2包覆的三维多孔碳纳米管电催化膜(Bi-SnO2/CNTM)来去除BPA. 实验表明,Bi-SnO2/CNTM具有良好的电催化活性和稳定性,比纯CNTM具有更好的BPA去除性能,Bi-SnO2/CNTM在30 mg·L–1BPA溶液中连续处理6 h,BPA去除率可达65.9%,图3为电催化降解BPA的机理,包括4个步骤:有机分子向膜表面的传质、物理吸附、电催化降解反应和降解产物的脱附. 由图3可见,电催化降解机制可归因于两个过程,一方面,吸附在膜表面的BPA分子通过高浓度的孔直接被氧化. 另一方面,BPA分子通过产生大量的活性自由基间接氧化.
电催化与膜技术联合使用,通过改变絮状物颗粒与膜表面之间的附着力来减少膜污染和水中污染物的浓度. 基于膜的水处理系统中的天然有机物和抗生素会引起严重的膜污染,导致滤饼或凝胶层的形成以及孔隙堵塞等问题,从而降低过滤和纯化效率. 可将电催化氧化技术用作预处理,把大分子有机物转化为小的中等有机分子并聚集无机离子,催化剂可被膜收集,从而达到去除膜污染和净化废水的目的. 目前电催化氧化与膜技术联合使用存在催化剂负载困难、颗粒难以控制、催化剂容易流失等问题,如何解决这些问题是决定电催化氧化与膜技术联合使用未来发展的关键.
-
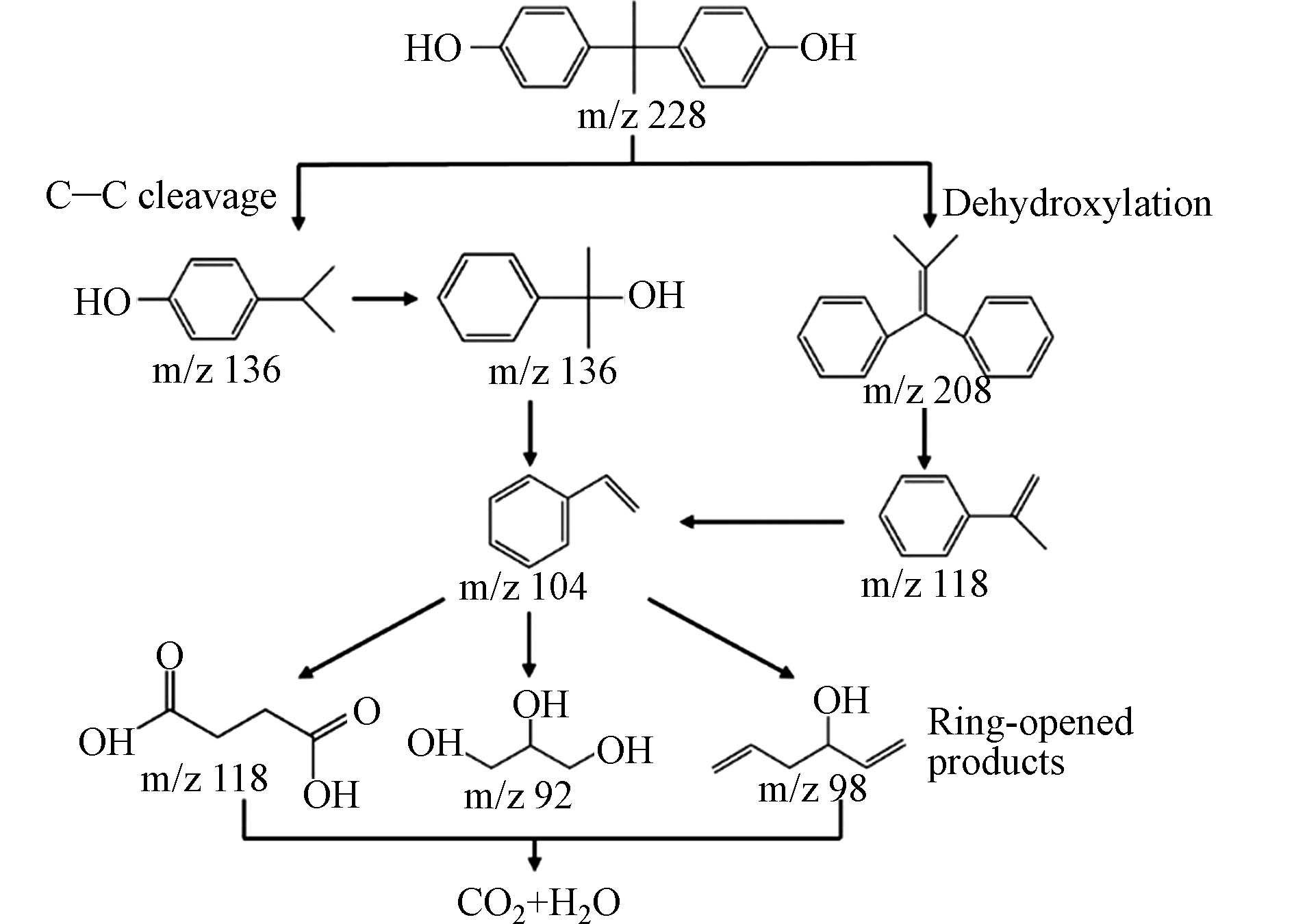
过硫酸盐氧化法通常采用光热刺激或过渡金属活化过硫酸盐产生氧化性强的硫酸根自由基(SO4·−)[97]以矿化降解有机分子的技术[98],因反应条件温和、反应速度快[99]、选择性高而受到广泛关注. 常见的过硫酸盐有过一硫酸盐(PMS)和过二硫酸盐(PDS),其中,PMS具有较高的氧化还原电位,可被催化剂活化生成SO4·−和OH·[100-102]. Chen等[103]通过驱动双前驱体咪唑酸沸石骨架(ZIF-67)和羊角草构建了碳包覆Co3O4和生物炭(BC)的复合材料(Co3O4/C-BC). Co3O4/C-BC内部丰富的多孔结构有利于PMS的扩散和传质,在30 min内可100%去除20 mg·L–1的BPA. 机理研究表明BPA和PMS分子可以吸附在Co3O4/C-BC表面,PMS被Co3O4活性位点迅速激活,随后吸附的BPA分子与相邻的自由基发生反应然后形成中间体,最后被矿化为CO2和H2O. 刘畅等[104]采用水热法合成具有高催化活性的CuMnFe三元金属氢氧化物(LDHs)来去除BPA. 实验表明,在Cu与Mn摩尔比为1:1时,Cu/Mn-Fe LDHs具有良好的稳定性和重复利用性,在太阳光/1:1CMF/PMS反应体系中,在PMS最佳浓度为0.4 mmol·L−1和催化剂最佳投加量为0.6 g·L–1时,BPA的去除率在15 min内达到93.5%,该降解反应的过程符合一级反应动力学. Hu等[105]采用水热耦合热解法制备表面含丰富氧官能团的碳催化剂,通过激活PMS降解BPA. 实验表明,水热处理使氧元素含量增加,尤其是酮基含量增加,有利于PMS催化氧化降解有机污染物. 在最佳反应条件下,20 min内BPA的去除率为100%,HT190-SC600/PMS系统上BPA可能降解途径如图4所示.
过渡金属已被公认为活化过硫酸盐的高效催化剂,一些金属如Cu,Mn,Co,Fe和Mo表现出很高的催化活性[106],但在实际应用中工作pH值范围窄,金属离子易溶出造成二次污染,且粉末催化剂需要后处理,不易回收再利用. 膜技术可有效解决上述问题. Liu等[107]采用共沉淀和微波处理法成功合成了具有优异催化性能的CuO@CuS/PVDF膜活化过硫酸盐(PS)降解四环素. 实验表明,CuO@CuS具有优异的活化活化性能,可活化过硫酸盐产生羟基自由基(·OH)、硫酸根自由基(SO4·−)和单线态氧(1O2),CuO@CuS/PVDF-PS体系可在60 min内去除83.4%的四环素,且CuO@CuS/PVDF膜具有良好的稳定性和可重复使用性.
-
在外加电场的作用下,介质放电产生包括电子、各种离子、原子和自由基在内的混合体,等离子体技术就是利用这些混合体,使污染物在短时间内发生分解,以达到降解污染物的目的. 等离子体技术是一种绿色高效[108]、无需额外添加氧化剂[109]和催化剂的有机污染物降解技术[110],有脉冲放电等离子体(PDP)和介质阻挡放电(DBD)的两种模式. 等离子体技术具有操作方便、氧化效率高等优点[111],但也存在能量利用率低的问题[112],限制了其商业应用[113]. Yang等[114]采用介质阻挡放电(DBD)低温等离子体技术用于BPA去除. 实验表明,DBD体系产生的·OH是BPA降解的主要活性氧,当pH为6.0时,在30 min内可100%去除50 mg·L–1的BPA(初始浓度).
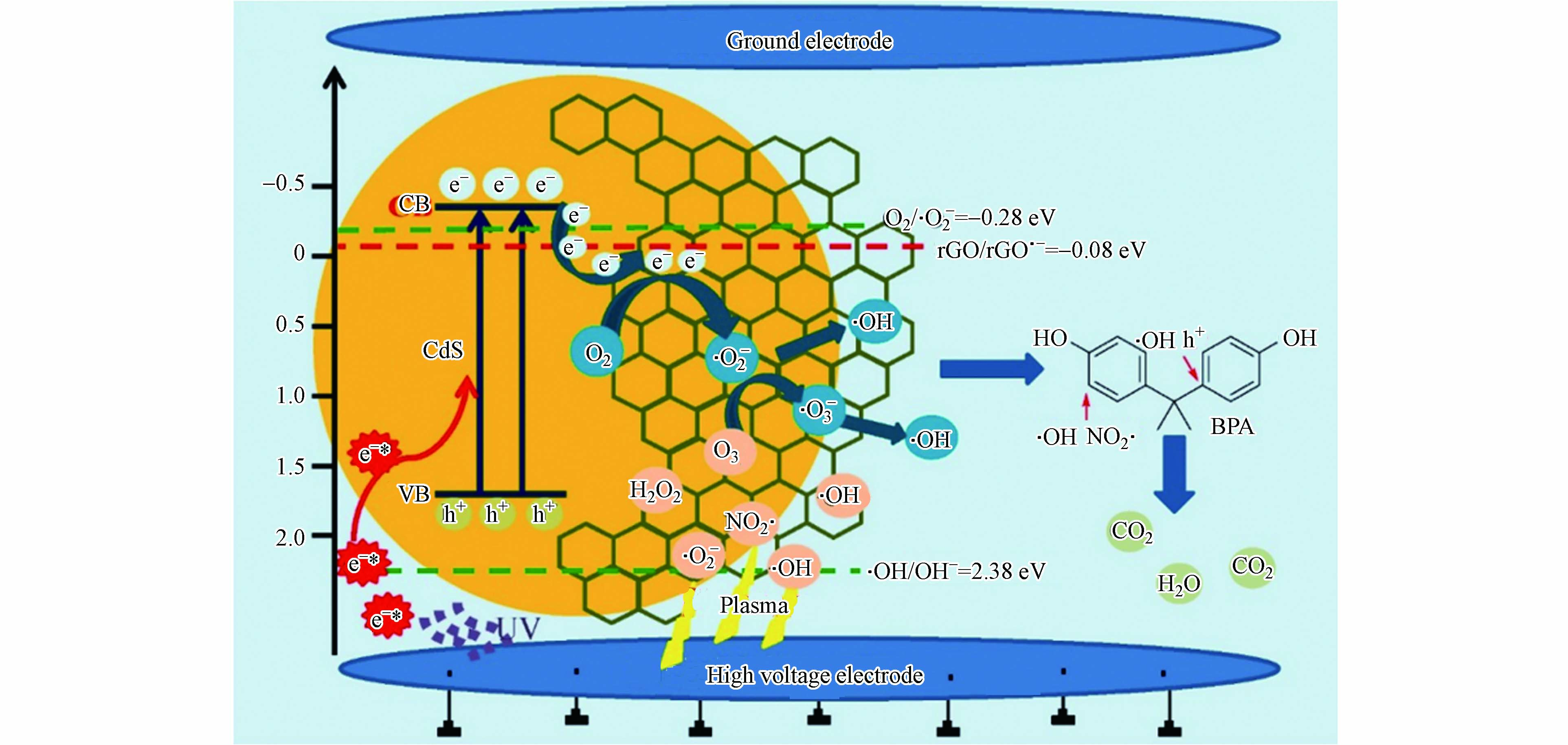
等离子体也可作为协同技术与光催化联合使用,Jiang等[115]采用水热法制备一系列石墨烯-CdS(rGO(x)/CdS)纳米复合材料,并将其引入脉冲气液混合放电(PHD)体系中,用于BPA的降解. 实验表明,PHD等离子体与rGO(x)/CdS纳米复合材料的结合对BPA的降解有明显的协同增强作用,rGO(5)/CdS等离子体辅助催化降解60 min后,最高去除率为87.58%. rGO对CdS具有良好的保护作用,减少了rGO(5)/CdS纳米复合材料的光腐蚀,提高了稳定性,图5为rGO(5)/CdS纳米复合材料等离子体辅助催化过程中BPA降解的反应机理. 由图5可见,rGO(5)/CdS纳米复合材料很容易被激活并导致光生电子和空穴分离,光生电子迅速转移到rGO表面,与吸附的O2和O3反应,反应产物能有效攻击BPA的苯环,通过破环反应使其分解,最终矿化为CO2和H2O.
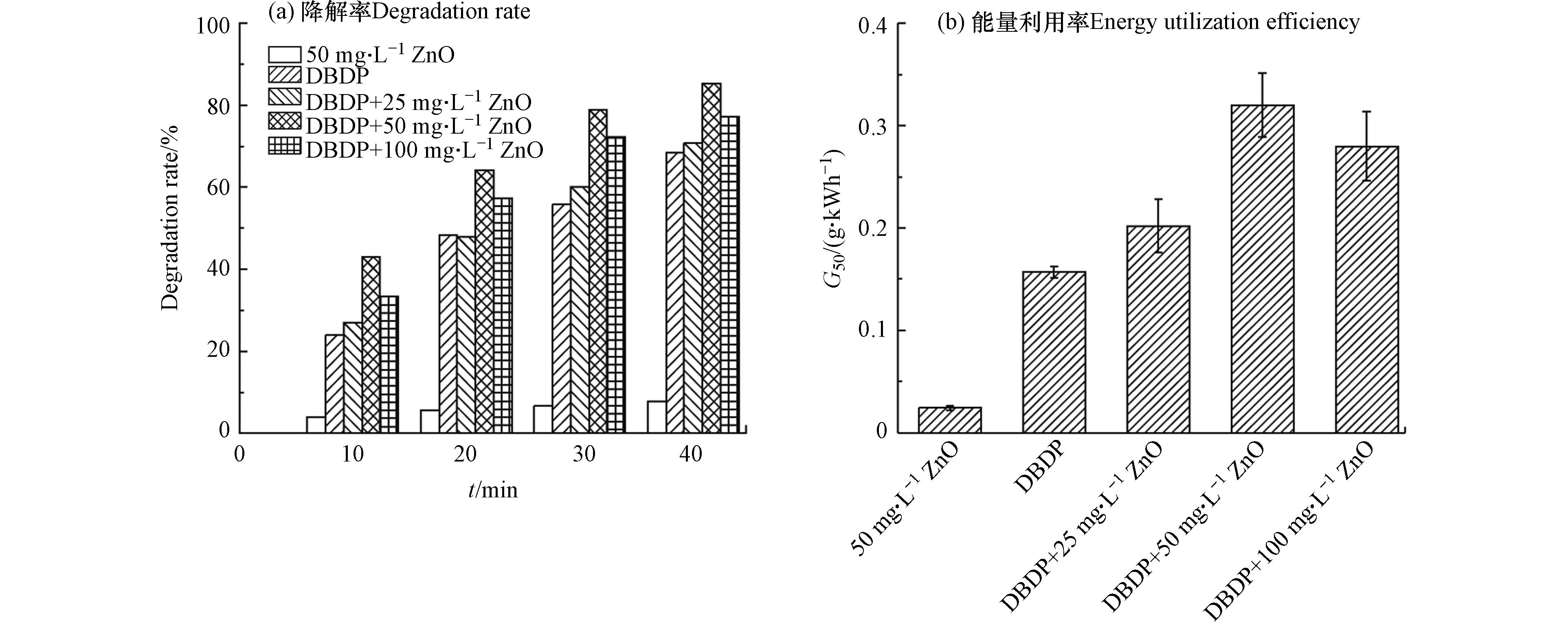
等离子体降解操作方便,效果好,但在实验过程中发现,等离子体降解过程中会产生臭氧等副产物,且能量效率并不高. 近年来,等离子体降解法和催化剂的联合技术被认为是最具有发展前景的技术之一,颇受研究者的关注. 在等离子体反应体系中加入适当的催化剂,利用等离子体可在常温常压下进行反应以及催化剂具有高选择性的特点,将两者的优势结合起来,使反应更加具有选择性,可有效地抑制副产物的产生,提高选择性. Wang等[116]采用介质阻挡放电等离子体(DBDP)技术和纳米WO3催化技术,研究在DBDP/WO3协同体系中对BPA的降解. 实验表明,WO3的最佳添加量为175 mg·L–1,中性溶液条件和氧气鼓泡有利于BPA在反应体系中的降解. 在DBDP/WO3体系中,0.5 L·min–1O2为载气,反应30 min后BPA的去除率可达100%,所制备的WO3经过四次循环使用后仍具有良好的稳定性. 闫欣等[117]建立了介质阻挡放电等离子体(DBDP)和纳米氧化锌(ZnO)相协同的难降解有机物降解体系,以BPA作为目标污染物. 实验表明,DBDP和ZnO的结合对BPA的降解有协同作用,在最佳实验条件下,DBDP/ZnO协同体系反应40 min后,BPA的去除率为85.4%,不同催化剂浓度下BPA的降解如图6所示.
-
面对复杂多样的废水环境,单一处理技术的处理效果已不能满足实际要求. 如物理法处理速度快,但二次污染风险大;生物法经济性高,但抗干扰弱,处理周期长;化学法降解效果显著,但成本高. 鉴于此,建议通过多种技术联合使用来处理BPA废水.
1) 吸附法和光催化降解联合. 通过吸附材料富集污染物,再利用光催化降解污染物. 吸附富集可提高催化剂表面污染物浓度,强化光催化降解效率,而污染物的降解又可解决单一吸附法存在二次污染风险的问题.
2) 高级氧化和生物法联合. 将高级氧化技术作为预处理,提高废水的可生化性,再利用微生物降解污染物. 高级氧化反应提供的羟基自由基可将有机物转化为可生物降解的中间产物,为微生物代谢提供适宜的底物,而生物法又可降低单一氧化法成本高的问题.
3) 电催化降解与膜技术联合. 通过膜技术分离富集污染物,再利用电催化降解污染物. 膜分离不仅可以提高污染物浓度,而且膜可以收集催化剂,解决催化剂后分离的问题,而催化剂又可以减轻膜污染,达到循环使用的目的.
不过技术的联合应用,也要提前分析不同技术的适用性,探究联合技术对污染物去除转化的协同机理. 除此之外,还要关注联合技术处理污染物过程中的毒性变化,建议使用QSAR模型预测污染物毒性,提前预测反应过程中的风险.
典型内分泌干扰物双酚A废水处理研究进展
Progress in the treatment technologies toward endocrine disrupter bisphenol A
-
摘要: 双酚A是一种典型的内分泌干扰物,环境暴露量逐渐升高;其分子结构稳定,不易降解,且具有一定的毒性,对生态环境安全和哺乳动物健康造成威胁,因此发展高效、绿色且经济的双酚A处理技术已成为一个研究重点. 本文简单介绍了环境中双酚A的来源与危害,综述了近期国内外处理双酚A废水的技术方法,着重论述了吸附法、膜技术、生物法、臭氧氧化法、光催化氧化法、电催化氧化法及过硫酸盐氧化法、等离子体降解法在双酚A废水处理方面的反应机制和技术优缺点,分析了相关技术目前所取得的研究进展. 总体而言,目前这些单一的处理技术在矿化效率、催化剂合成、二次污染控制和处理成本等问题上难以兼顾,因此我们建议可采用多种技术联合实施的处理方案,以满足不同应用场景下对含双酚A废水的高效、安全降解.Abstract: Bisphenol A (BPA), a typical endocrine disruptor chemical, features the high environmental exposure, the strong persistence to natural degradation and the large health risk to the human and mammals. How to remove BPA from water in an efficient and economical way has, therefore, been receiving increasing research interests. This paper briefly introduces the harmfulness and origin of BPA, and then summarizes the recent progress achieved in the development of the technologies for treatment of BPA-contaminated wastewater, including adsorption, membrane filtration, biological, photo-/electrocatalytic oxidation, persulfate-mediated oxidation and plasma-triggered degradation. Specifically, the removal mechanism as well as the merits/demerits of each technology are discussed. Finally, we propose a joint implementation of the above technologies for practical application, considering that the performance of the single technology is unsatisfied in the aspect of mineralization efficiency, catalyst synthesis, secondary pollution control or treatment cost.
-
Key words:
- bisphenol A /
- endocrine disruptors /
- wastewater treatment /
- catalysts
-

-
图 1 合成膜横截面的扫描电镜图像
Figure 1. Scanning electron microscope image of the cross-section of the as-synthesized membranes [54]
表 1 我国不同地区环境水体中BPA浓度
Table 1. BPA concentrations in different areas of China
地区
Areas采样时间
Sampling time范围/(ng·L–1)
Range均值/(ng·L–1)
Mean value检出率/%
Detection ratio参考文献
References松花江 2006 22—49 [30] 辽河 2013 5.9—141.0 47 100 [31] 海河 2003 nd—106 41 93 [32] 黄河 2008 13—172 40 100 [33] 长江 2015 nd—50.1 26.6 92.9 [34] 珠江 2007—2008 43—639 232 100 [35] 太湖 2015 28—560 97 100 [36] 东湖 2013-2014 nd—37 3.2 [37] 注:nd 表示未检出/低于检出限,not detected. -
[1] 严燕, 黄蔷, 牟敬锋, 等. 深圳居民日常饮用水中双酚A的污染状况调查及暴露评估 [J]. 中国卫生检验杂志, 2016, 26(22): 3310-3312,3316. YAN Y, HUANG Q, MU J F, et al. Investigation and exposure assessment of bisphenol A for drinking water in Shenzhen [J]. Chinese Journal of Health Laboratory Technology, 2016, 26(22): 3310-3312,3316(in Chinese).
[2] ANDALURI G, MANICKAVACHAGAM M, SURI R. Plastic toys as a source of exposure to bisphenol-A and phthalates at childcare facilities [J]. Environmental Monitoring and Assessment, 2018, 190(2): 65. doi: 10.1007/s10661-017-6438-9 [3] VASILJEVIC T, HARNER T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels [J]. The Science of the Total Environment, 2021, 789: 148013. doi: 10.1016/j.scitotenv.2021.148013 [4] FERRER-POLONIO E, ALVIM C B, FERNÁNDEZ-NAVARRO J, et al. Influence of bisphenol A occurrence in wastewaters on biomass characteristics and activated sludge process performance [J]. The Science of the Total Environment, 2021, 778: 146355. doi: 10.1016/j.scitotenv.2021.146355 [5] BHATNAGAR A, ANASTOPOULOS I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review [J]. Chemosphere, 2017, 168: 885-902. doi: 10.1016/j.chemosphere.2016.10.121 [6] WU L H, ZHANG X M, WANG F, et al. Occurrence of bisphenol S in the environment and implications for human exposure: A short review [J]. Science of the Total Environment, 2018, 615: 87-98. doi: 10.1016/j.scitotenv.2017.09.194 [7] YANG Y, OK Y S, KIM K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review [J]. Science of the Total Environment, 2017, 596/597: 303-320. doi: 10.1016/j.scitotenv.2017.04.102 [8] JALAL N, SURENDRANATH A R, PATHAK J L, et al. Bisphenol A (BPA) the mighty and the mutagenic [J]. Toxicology Reports, 2018, 5: 76-84. doi: 10.1016/j.toxrep.2017.12.013 [9] MARTÍN-LARA M A, CALERO M, RONDA A, et al. Adsorptive behavior of an activated carbon for bisphenol A removal in single and binary (bisphenol A—Heavy metal) solutions [J]. Water, 2020, 12(8): 2150. doi: 10.3390/w12082150 [10] ANA K M S, ESPINO M P. Occurrence and distribution of hormones and bisphenol A in Laguna Lake, Philippines [J]. Chemosphere, 2020, 256: 127122. doi: 10.1016/j.chemosphere.2020.127122 [11] WANG Y C, CHEN D Z, YU Y W, et al. Magnetic porous carbon nanopolyhedron modified rGO composites as recyclable sorbent for effective removal of bisphenol A from water [J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 105911. doi: 10.1016/j.jece.2021.105911 [12] HU Y, ZHU Q Q, YAN X T, et al. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review [J]. Environmental Research, 2019, 178: 108732. doi: 10.1016/j.envres.2019.108732 [13] WANG L, YUN J, ZHANG H X, et al. Degradation of Bisphenol A by ozonation in rotating packed bed: Effects of operational parameters and co-existing chemicals [J]. Chemosphere, 2021, 274: 129769. doi: 10.1016/j.chemosphere.2021.129769 [14] HAYAT K, MENHAS S, BUNDSCHUH J, et al. Microbial biotechnology as an emerging industrial wastewater treatment process for arsenic mitigation: A critical review [J]. Journal of Cleaner Production, 2017, 151: 427-438. doi: 10.1016/j.jclepro.2017.03.084 [15] ZHANG X Y, ZHANG H X, XIANG Y Y, et al. Synthesis of silver phosphate/graphene oxide composite and its enhanced visible light photocatalytic mechanism and degradation pathways of tetrabromobisphenol A [J]. Journal of Hazardous Materials, 2018, 342: 353-363. doi: 10.1016/j.jhazmat.2017.08.048 [16] SHAFEI A, RAMZY M M, HEGAZY A I, et al. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer [J]. Gene, 2018, 647: 235-243. doi: 10.1016/j.gene.2018.01.016 [17] WANG J Q, ZHENG M, DENG Y, et al. Generality and diversity on the kinetics, toxicity and DFT studies of sulfate radical-induced transformation of BPA and its analogues [J]. Water Research, 2022, 219: 118506. doi: 10.1016/j.watres.2022.118506 [18] FAROOQ M U, JALEES M I, Qurat-ul-Ain, et al. Health risk assessment of endocrine disruptor bisphenol A leaching from plastic bottles of milk and soft drinks [J]. Environmental Science and Pollution Research, 2021, 28(40): 57090-57098. doi: 10.1007/s11356-021-14653-4 [19] DREOLIN N, AZNAR M, MORET S, et al. Development and validation of a LC-MS/MS method for the analysis of bisphenol a in polyethylene terephthalate [J]. Food Chemistry, 2019, 274: 246-253. doi: 10.1016/j.foodchem.2018.08.109 [20] BILAL M, IQBAL H M N, BARCELÓ D. Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues - A review [J]. Science of the Total Environment, 2019, 689: 160-177. doi: 10.1016/j.scitotenv.2019.06.403 [21] MA Y, LIU H H, WU J X, et al. The adverse health effects of bisphenol A and related toxicity mechanisms [J]. Environmental Research, 2019, 176: 108575. doi: 10.1016/j.envres.2019.108575 [22] ABRAHAM A, CHAKRABORTY P. A review on sources and health impacts of bisphenol A [J]. Reviews on Environmental Health, 2020, 35(2): 201-210. doi: 10.1515/reveh-2019-0034 [23] RUSSO G, BARBATO F, MITA D G, et al. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe [J]. Food and Chemical Toxicology, 2019, 131: 110575. doi: 10.1016/j.fct.2019.110575 [24] CUI F P, YANG P, LIU C, et al. Urinary bisphenol A and its alternatives among pregnant women: Predictors and risk assessment [J]. Science of the Total Environment, 2021, 784: 147184. doi: 10.1016/j.scitotenv.2021.147184 [25] WANG H, LIU Z H, ZHANG J, et al. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants [J]. Science of the Total Environment, 2019, 692: 107-116. doi: 10.1016/j.scitotenv.2019.07.134 [26] LIU X M, SHI H H, XIE B, et al. Microplastics as both a sink and a source of bisphenol A in the marine environment [J]. Environmental Science & Technology, 2019, 53(17): 10188-10196. [27] WANG J P, ZHANG M. Adsorption characteristics and mechanism of bisphenol A by magnetic biochar [J]. International Journal of Environmental Research and Public Health, 2020, 17(3): 1075. doi: 10.3390/ijerph17031075 [28] MPATANI F M, HAN R P, ARYEE A A, et al. Adsorption performance of modified agricultural waste materials for removal of emerging micro-contaminant bisphenol A: A comprehensive review [J]. Science of the Total Environment, 2021, 780: 146629. doi: 10.1016/j.scitotenv.2021.146629 [29] ABOOTALEBI JAHROMI F, MOORE F, KESHAVARZI B, et al. Bisphenol A (BPA) and polycyclic aromatic hydrocarbons (PAHs) in the surface sediment and bivalves from Hormozgan Province coastline in the Northern Persian Gulf: A focus on source apportionment [J]. Marine Pollution Bulletin, 2020, 152: 110941. doi: 10.1016/j.marpolbul.2020.110941 [30] 邵晓玲, 马军. 松花江水中13种内分泌干扰物的初步调查 [J]. 环境科学学报, 2008, 28(9): 1910-1915. doi: 10.13671/j.hjkxxb.2008.09.010 SHAO X L, MA J. Preliminary investigation on 13 endocrine disrupting chemicals in the Songhua River [J]. Acta Scientiae Circumstantiae, 2008, 28(9): 1910-1915(in Chinese). doi: 10.13671/j.hjkxxb.2008.09.010
[31] JIN H B, ZHU L Y. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China [J]. Water Research, 2016, 103: 343-351. doi: 10.1016/j.watres.2016.07.059 [32] JIN X L, JIANG G B, HUANG G L, et al. Determination of 4-tert-octylphenol, 4-nonylphenol and bisphenol A in surface waters from the Haihe River in Tianjin by gas chromatography-mass spectrometry with selected ion monitoring [J]. Chemosphere, 2004, 56(11): 1113-1119. doi: 10.1016/j.chemosphere.2004.04.052 [33] WANG L, YING G G, CHEN F, et al. Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools [J]. Environmental Pollution, 2012, 165: 241-249. doi: 10.1016/j.envpol.2011.10.005 [34] WANG W F, NDUNGU A W, WANG J. Monitoring of endocrine-disrupting compounds in surface water and sediments of the Three Gorges Reservoir region, China [J]. Archives of Environmental Contamination and Toxicology, 2016, 71(4): 509-517. doi: 10.1007/s00244-016-0319-z [35] GONG J, RAN Y, CHEN D Y, et al. Occurrence and environmental risk of endocrine-disrupting chemicals in surface waters of the Pearl River, South China [J]. Environmental Monitoring and Assessment, 2009, 156(1): 199-210. [36] YAN Z Y, LIU Y H, YAN K, et al. Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: Occurrence, distribution, source apportionment, and ecological and human health risk [J]. Chemosphere, 2017, 184: 318-328. doi: 10.1016/j.chemosphere.2017.06.010 [37] WU C X, HUANG X L, LIN J, et al. Occurrence and fate of selected endocrine-disrupting chemicals in water and sediment from an urban lake [J]. Archives of Environmental Contamination and Toxicology, 2015, 68(2): 225-236. doi: 10.1007/s00244-014-0087-6 [38] WU Z X, ZHAO D Y. Ordered mesoporous materials as adsorbents [J]. Chemical Communications, 2011, 47(12): 3332-3338. doi: 10.1039/c0cc04909c [39] 方梦祥, 姚鹏, 岑建孟, 等. 活性炭吸附处理含酚废水的研究进展 [J]. 化工进展, 2018, 37(2): 744-751. FANG M X, YAO P, CEN J M, et al. Adsorption treatment of phenolic wastewater by activated carbon: A review [J]. Chemical Industry and Engineering Progress, 2018, 37(2): 744-751(in Chinese).
[40] 蒋博龙, 史顺杰, 蒋海林, 等. 金属有机框架材料吸附处理苯酚污水机理研究进展 [J]. 化工进展, 2021, 40(8): 4525-4539. doi: 10.16085/j.issn.1000-6613.2020-1811 JIANG B L, SHI S J, JIANG H L, et al. Research progress in phenol adsorption mechanism over metal-organic framework from wastewater [J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4525-4539(in Chinese). doi: 10.16085/j.issn.1000-6613.2020-1811
[41] ALHOKBANY N S, NAUSHAD M, KUMAR V, et al. Self-nitrogen doped carbons aerogel derived from waste cigarette butts (cellulose acetate) for the adsorption of BPA: Kinetics and adsorption mechanisms [J]. Journal of King Saud University - Science, 2020, 32(8): 3351-3358. doi: 10.1016/j.jksus.2020.09.021 [42] SUN Z Q, ZHAO L, LIU C H, et al. Fast adsorption of BPA with high capacity based on π-π electron donor-acceptor and hydrophobicity mechanism using an in-situ sp2 C dominant N-doped carbon [J]. Chemical Engineering Journal, 2020, 381: 122510. doi: 10.1016/j.cej.2019.122510 [43] de LIMA H H C, LLOP M E G, dos SANTOS MANIEZZO R, et al. Enhanced removal of bisphenol A using pine-fruit shell-derived hydrochars: Adsorption mechanisms and reusability [J]. Journal of Hazardous Materials, 2021, 416: 126167. doi: 10.1016/j.jhazmat.2021.126167 [44] 孙刘鑫, 王培茗, 杨俊浩, 等. 离子强度对吸附有机污染物影响的研究进展 [J]. 化工进展, 2021, 40(6): 3239-3257. doi: 10.16085/j.issn.1000-6613.2020-1477 SUN L X, WANG P M, YANG J H, et al. Research progress on the effect of ionic strength on the removal of organic pollutants from wastewater by adsorbents [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3239-3257(in Chinese). doi: 10.16085/j.issn.1000-6613.2020-1477
[45] MENESES I P, NOVAES S D, DEZOTTI R S, et al. CTAB-modified carboxymethyl cellulose/bagasse cryogels for the efficient removal of bisphenol A, methylene blue and Cr(VI) ions: Batch and column adsorption studies [J]. Journal of Hazardous Materials, 2022, 421: 126804. doi: 10.1016/j.jhazmat.2021.126804 [46] ZHOU G Z, CAO Y Y, JIN Y Q, et al. Novel selective adsorption and photodegradation of BPA by molecularly imprinted sulfur doped nano-titanium dioxide [J]. Journal of Cleaner Production, 2020, 274: 122929. doi: 10.1016/j.jclepro.2020.122929 [47] MODI A, BELLARE J. Copper sulfide nanoparticles/carboxylated graphene oxide nanosheets blended polyethersulfone hollow fiber membranes: Development and characterization for efficient separation of oxybenzone and bisphenol A from water [J]. Polymer, 2019, 163: 57-67. doi: 10.1016/j.polymer.2018.12.040 [48] PAN Z L, YU F P, LI L, et al. Electrochemical microfiltration treatment of bisphenol A wastewater using coal-based carbon membrane [J]. Separation and Purification Technology, 2019, 227: 115695. doi: 10.1016/j.seppur.2019.115695 [49] CHEN Z H, LIU Z, HU J Q, et al. β-Cyclodextrin-modified graphene oxide membranes with large adsorption capacity and high flux for efficient removal of bisphenol A from water [J]. Journal of Membrane Science, 2020, 595: 117510. doi: 10.1016/j.memsci.2019.117510 [50] S E, G A, DAS D B. Embedding low-cost 1D and 2D iron pillared nanoclay to enhance the stability of polyethersulfone membranes for the removal of bisphenol A from water [J]. Separation and Purification Technology, 2021, 266: 118560. doi: 10.1016/j.seppur.2021.118560 [51] MOREIRA C G, MOREIRA M H, SILVA V M O C, et al. Treatment of Bisphenol A (BPA) in water using UV/H2O2 and reverse osmosis (RO) membranes: Assessment of estrogenic activity and membrane adsorption [J]. Water Science and Technology, 2019, 80(11): 2169-2178. doi: 10.2166/wst.2020.024 [52] ZAHARI A M, SHUO C W, SATHISHKUMAR P, et al. A reusable electrospun PVDF-PVP-MnO2 nanocomposite membrane for bisphenol A removal from drinking water [J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 5801-5811. doi: 10.1016/j.jece.2018.08.073 [53] HOU Z A, WEN Z B, WANG D D, et al. Bipolar jet electrospinning bi-functional nanofibrous membrane for simultaneous and sequential filtration of Cd2+ and BPA from water: Competition and synergistic effect [J]. Chemical Engineering Journal, 2018, 332: 118-130. doi: 10.1016/j.cej.2017.09.064 [54] NASSERI S, EBRAHIMI S, ABTAHI M, et al. Synthesis and characterization of polysulfone/graphene oxide nano-composite membranes for removal of bisphenol A from water [J]. Journal of Environmental Management, 2018, 205: 174-182. [55] ZHAO Z Y, MUYLAERT K, SZYMCZYK A, et al. Harvesting microalgal biomass using negatively charged polysulfone patterned membranes: Influence of pattern shapes and mechanism of fouling mitigation [J]. Water Research, 2021, 188: 116530. doi: 10.1016/j.watres.2020.116530 [56] WANG Q, YANG C Y, ZHANG G S, et al. Photocatalytic Fe-doped TiO2/PSF composite UF membranes: Characterization and performance on BPA removal under visible-light irradiation [J]. Chemical Engineering Journal, 2017, 319: 39-47. doi: 10.1016/j.cej.2017.02.145 [57] MOUSSAVI G, ABBASZADEH HADDAD F. Bacterial peroxidase-mediated enhanced biodegradation and mineralization of bisphenol A in a batch bioreactor [J]. Chemosphere, 2019, 222: 549-555. doi: 10.1016/j.chemosphere.2019.01.190 [58] YUE W L, YIN C F, SUN L M, et al. Biodegradation of bisphenol-a polycarbonate plastic by Pseudoxanthomonas sp. strain NyZ600 [J]. Journal of Hazardous Materials, 2021, 416: 125775. doi: 10.1016/j.jhazmat.2021.125775 [59] TAGHIZADEH T, TALEBIAN-KIAKALAIEH A, JAHANDAR H, et al. Biodegradation of bisphenol A by the immobilized laccase on some synthesized and modified forms of zeolite Y [J]. Journal of Hazardous Materials, 2020, 386: 121950. doi: 10.1016/j.jhazmat.2019.121950 [60] RAMPINELLI J R, de MELO M P, ARBIGAUS A, et al. Production of Pleurotus sajor-caju crude enzyme broth and its applicability for the removal of bisphenol A [J]. Anais Da Academia Brasileira De Ciências, 2021, 93(1): e20191153. [61] CYDZIK-KWIATKOWSKA A, ZIELIŃSKA M. Microbial composition of biofilm treating wastewater rich in bisphenol A [J]. Journal of Environmental Science and Health, Part A, 2018, 53(4): 385-392. doi: 10.1080/10934529.2017.1404326 [62] FERRO OROZCO A M, MORALES URREA D A, CONTRERAS E M, et al. Loss of bisphenol A removal ability of activated sludge in semi-continuous reactors (SCR) [J]. Journal of Environmental Chemical Engineering, 2020, 8(3): 103778. doi: 10.1016/j.jece.2020.103778 [63] HOU S Y, YANG P. BPA biodegradation driven by isolated strain SQ-2 and its metabolism mechanism elucidation [J]. Biochemical Engineering Journal, 2022, 185: 108540. doi: 10.1016/j.bej.2022.108540 [64] SARMA H, NAVA A R, MANRIQUEZ A M E, et al. Biodegradation of bisphenol A by bacterial consortia isolated directly from river sediments [J]. Environmental Technology & Innovation, 2019, 14: 100314. [65] KYRILA G, KATSOULAS A, SCHORETSANITI V, et al. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties [J]. Journal of Hazardous Materials, 2021, 413: 125363. doi: 10.1016/j.jhazmat.2021.125363 [66] WU J C, MA X N, LI C M, et al. A novel photon-enzyme cascade catalysis system based on hybrid HRP-CN/Cu3(PO4)2 nanoflowers for degradation of BPA in water [J]. Chemical Engineering Journal, 2022, 427: 131808. doi: 10.1016/j.cej.2021.131808 [67] 张瑞阳, 王姝焱, 黎邦鑫, 等. 气相臭氧分解催化材料的研究进展 [J]. 材料导报, 2021, 35(21): 21037-21049. ZHANG R Y, WANG S Y, LI B X, et al. Research progress of gaseous ozone decomposition catalysts [J]. Materials Reports, 2021, 35(21): 21037-21049(in Chinese).
[68] TIAN J, LI B B, QU R J, et al. Influence of anions on ozonation of bisphenol AF: Kinetics, reaction pathways, and toxicity assessment [J]. Chemosphere, 2022, 286: 131864. doi: 10.1016/j.chemosphere.2021.131864 [69] 钱媛媛, 王永杰, 杨雪晶. 臭氧相关水处理工艺及其传质特征研究进展[J]. 化工进展, 2021, 40(增刊1): 411-425. QIAN Y Y, WANG Y J, YANG X J. Application of ozone for water treatment and implication of mass transfer characteristics[J]. Chemical Industry and Engineering Progress, 2021, 40(Sup 1): 411-425 (in Chinese).
[70] HUANG Y X, YANG T T, LIANG M L, et al. Ni-Fe layered double hydroxides catalized ozonation of synthetic wastewater containing Bisphenol A and municipal secondary effluent [J]. Chemosphere, 2019, 235: 143-152. doi: 10.1016/j.chemosphere.2019.06.162 [71] MU J X, LI S Y, WANG J, et al. Efficient catalytic ozonation of bisphenol A by three-dimensional mesoporous CeOx-loaded SBA-16 [J]. Chemosphere, 2021, 278: 130412. doi: 10.1016/j.chemosphere.2021.130412 [72] 缪倩倩, 孟冠华, 刘宝河, 等. 铜氧化物/D851树脂催化臭氧氧化降解双酚A [J]. 环境工程学报, 2019, 13(7): 1557-1564. MIAO Q Q, MENG G H, LIU B H, et al. Degradation of bisphenol A through catalytic ozonation process with copper oxide/D851 resin [J]. Chinese Journal of Environmental Engineering, 2019, 13(7): 1557-1564(in Chinese).
[73] LI S, WU Y N, ZHENG Y J, et al. Free-radical and surface electron transfer dominated bisphenol A degradation in system of ozone and peroxydisulfate co-activated by CoFe2O4-biochar [J]. Applied Surface Science, 2021, 541: 147887. doi: 10.1016/j.apsusc.2020.147887 [74] 杜明辉, 王勇, 高群丽, 等. 臭氧微气泡处理有机废水的效果与机制 [J]. 化工进展, 2021, 40(12): 6907-6915. DU M H, WANG Y, GAO Q L, et al. Mechanism and efficiency of ozone microbubble treatment of organic wastewater [J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6907-6915(in Chinese).
[75] JABESA A, GHOSH P. Oxidation of bisphenol-a by ozone microbubbles: Effects of operational parameters and kinetics study [J]. Environmental Technology & Innovation, 2022, 26: 102271. [76] ZHANG H, HE Y L, LAI L D, et al. Catalytic ozonation of Bisphenol A in aqueous solution by Fe3O4-MnO2 magnetic composites: Performance, transformation pathways and mechanism [J]. Separation and Purification Technology, 2020, 245: 116449. doi: 10.1016/j.seppur.2019.116449 [77] CHEN Y H, WANG B, HOU W C. Graphitic carbon nitride embedded with graphene materials towards photocatalysis of bisphenol A: The role of graphene and mediation of superoxide and singlet oxygen [J]. Chemosphere, 2021, 278: 130334. doi: 10.1016/j.chemosphere.2021.130334 [78] WANG Q, LV G H, CAO Y T, et al. Rational design of 2D ultrathin BiO(HCOO)xI1-x composite nanosheets: The synergistic effect of ultrathin structure and hybridization in the effective elimination of BPA under visible light irradiation [J]. Separation and Purification Technology, 2022, 282: 120153. doi: 10.1016/j.seppur.2021.120153 [79] 王燚凡, 佘少桦, 孙传智, 等. 超薄硫掺杂石墨相氮化碳纳米片光催化降解双酚A [J]. 环境科学研究, 2021, 34(12): 2859-2866. WANG Y F, SHE S H, SUN C Z, et al. Photocatalytic degradation of bisphenol A using ultrathin S-doped graphitic carbon nitride nanosheets [J]. Research of Environmental Sciences, 2021, 34(12): 2859-2866(in Chinese).
[80] TANG Y, LI X L, ZHANG H, et al. Cobalt-based ZIF coordinated hybrids with defective TiO2-x for boosting visible light-driven photo-Fenton-like degradation of bisphenol A [J]. Chemosphere, 2020, 259: 127431. doi: 10.1016/j.chemosphere.2020.127431 [81] HE X, WU M, AO Z M, et al. Metal-organic frameworks derived C/TiO2 for visible light photocatalysis: Simple synthesis and contribution of carbon species [J]. Journal of Hazardous Materials, 2021, 403: 124048. doi: 10.1016/j.jhazmat.2020.124048 [82] 吴瞳, 顾佳玉, 彭晨, 等. 石墨相氮化碳同质结光催化处理水中双酚A [J]. 中国环境科学, 2021, 41(7): 3255-3265. WU T, GU J Y, PENG C, et al. Study on photocatalytic degradation of bisphenol A in water by graphite phase carbon nitride homojunction [J]. China Environmental Science, 2021, 41(7): 3255-3265(in Chinese).
[83] ANUCHA C B, ALTIN I, BIYIKLIOGLU Z, et al. Synthesis, characterization, and photocatalytic evaluation of manganese (III) phthalocyanine sensitized ZnWO4 (ZnWO4MnPc) for bisphenol A degradation under UV irradiation [J]. Nanomaterials, 2020, 10(11): 2139. doi: 10.3390/nano10112139 [84] HUNGE Y M, YADAV A A, KHAN S, et al. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination [J]. Journal of Colloid and Interface Science, 2021, 582: 1058-1066. doi: 10.1016/j.jcis.2020.08.102 [85] GARCÍA-DÍAZ E, ZHANG D N, LI Y L, et al. TiO2 microspheres with cross-linked cyclodextrin coating exhibit improved stability and sustained photocatalytic degradation of bisphenol A in secondary effluent [J]. Water Research, 2020, 183: 116095. doi: 10.1016/j.watres.2020.116095 [86] ZHANG H B, WU J C, HAN J, et al. Photocatalyst/enzyme heterojunction fabricated for high-efficiency photoenzyme synergic catalytic degrading Bisphenol A in water [J]. Chemical Engineering Journal, 2020, 385: 123764. doi: 10.1016/j.cej.2019.123764 [87] PELEYEJU M G, VILJOEN E L. WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water - A review [J]. Journal of Water Process Engineering, 2021, 40: 101930. doi: 10.1016/j.jwpe.2021.101930 [88] LI S P, LIU C L, LIU H J, et al. Effective stabilization of atomic hydrogen by Pd nanoparticles for rapid hexavalent chromium reduction and synchronous bisphenol A oxidation during the photoelectrocatalytic process [J]. Journal of Hazardous Materials, 2022, 422: 126974. doi: 10.1016/j.jhazmat.2021.126974 [89] GOULART L A, ALVES S A, MASCARO L H. Photoelectrochemical degradation of bisphenol A using Cu doped WO3 electrodes [J]. Journal of Electroanalytical Chemistry, 2019, 839: 123-133. doi: 10.1016/j.jelechem.2019.03.027 [90] ZHOU Q X, WANG M Y, TONG Y Y, et al. Improved photoelectrocatalytic degradation of tetrabromobisphenol A with silver and reduced graphene oxide-modified TiO2 nanotube arrays under simulated sunlight [J]. Ecotoxicology and Environmental Safety, 2019, 182: 109472. doi: 10.1016/j.ecoenv.2019.109472 [91] WANG W K, ZHU W Z, MAO L, et al. Two-dimensional TiO2-g-C3N4 with both TiN and CO bridges with excellent conductivity for synergistic photoelectrocatalytic degradation of bisphenol A [J]. Journal of Colloid and Interface Science, 2019, 557: 227-235. doi: 10.1016/j.jcis.2019.08.088 [92] SHAO H X, WANG Y B, ZENG H B, et al. Enhanced photoelectrocatalytic degradation of bisphenol a by BiVO4 photoanode coupling with peroxymonosulfate [J]. Journal of Hazardous Materials, 2020, 394: 121105. doi: 10.1016/j.jhazmat.2019.121105 [93] 符远航, 刘安迪, 黄纬斌, 等. 负载多壁碳纳米管的多孔Ti/SnO2-Sb-Ni电极电催化氧化双酚A [J]. 环境科学, 2022, 43(5): 2640-2649. FU Y H, LIU A D, HUANG W B, et al. Electrocatalytic oxidation of bisphenol A by porous Ti/SnO2-Sb-Ni electrode loaded with multi-wall carbon nanotubes [J]. Environmental Science, 2022, 43(5): 2640-2649(in Chinese).
[94] SAMARGHANDI M R, ANSARI A, DARGAHI A, et al. Enhanced electrocatalytic degradation of bisphenol A by graphite/β-PbO2 anode in a three-dimensional electrochemical reactor [J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106072. doi: 10.1016/j.jece.2021.106072 [95] CHEN S Y, LIU P, LI Y, et al. Engineering the doping amount of rare earth element erbium in CdWO4: Influence on the electrochemical performance and the application to the electrochemical detection of bisphenol A [J]. Journal of Electroanalytical Chemistry, 2022, 904: 115867. doi: 10.1016/j.jelechem.2021.115867 [96] ZHAO L, ZHANG X Q, LIU Z M, et al. Carbon nanotube-based electrocatalytic filtration membrane for continuous degradation of flow-through Bisphenol A [J]. Separation and Purification Technology, 2021, 265: 118503. doi: 10.1016/j.seppur.2021.118503 [97] GUO R N, WANG Y Y, LI J J, et al. Sulfamethoxazole degradation by visible light assisted peroxymonosulfate process based on nanohybrid manganese dioxide incorporating ferric oxide [J]. Applied Catalysis B:Environmental, 2020, 278: 119297. doi: 10.1016/j.apcatb.2020.119297 [98] 李广英, 杜敏洁, 谈成英, 等. 锰铁氧体活化PMS降解双酚A的过程机制 [J]. 环境工程学报, 2021, 15(9): 2952-2962. LI G Y, DU M J, TAN C Y, et al. Mechanism of BPA degradation in a system of peroxymonosulfate activated by a Mn/Fe bimetallic oxide catalysts [J]. Chinese Journal of Environmental Engineering, 2021, 15(9): 2952-2962(in Chinese).
[99] YOU Y, ZHAO Z J, SONG Y R, et al. Synthesis of magnetized nitrogen-doped biochar and its high efficiency for elimination of ciprofloxacin hydrochloride by activation of peroxymonosulfate [J]. Separation and Purification Technology, 2021, 258: 117977. doi: 10.1016/j.seppur.2020.117977 [100] XU H D, JIANG N, WANG D, et al. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways [J]. Applied Catalysis B:Environmental, 2020, 263: 118350. doi: 10.1016/j.apcatb.2019.118350 [101] ZHENG W T, YOU S J, YAO Y, et al. Development of atomic hydrogen-mediated electrocatalytic filtration system for peroxymonosulfate activation towards ultrafast degradation of emerging organic contaminants [J]. Applied Catalysis B:Environmental, 2021, 298: 120593. doi: 10.1016/j.apcatb.2021.120593 [102] HUANG Y, NENGZI L C, ZHANG X Y, et al. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism [J]. Chemical Engineering Journal, 2020, 388: 124274. doi: 10.1016/j.cej.2020.124274 [103] CHEN X L, LI F, ZHANG M Y, et al. Highly dispersed and stabilized Co3O4/C anchored on porous biochar for bisphenol A degradation by sulfate radical advanced oxidation process [J]. Science of the Total Environment, 2021, 777: 145794. doi: 10.1016/j.scitotenv.2021.145794 [104] 刘畅, 王宇寒, 胡清, 等. 太阳光/CuMnFe LDHs催化剂/过一硫酸盐体系降解双酚A [J]. 环境工程学报, 2021, 15(11): 3545-3560. LIU C, WANG Y H, HU Q, et al. Degradation of bisphenol A using CuMnFe LDHs catalyst and peroxymonosulfate under solar light [J]. Chinese Journal of Environmental Engineering, 2021, 15(11): 3545-3560(in Chinese).
[105] HU W R, TONG W H, LI Y L, et al. Hydrothermal route-enabled synthesis of sludge-derived carbon with oxygen functional groups for bisphenol A degradation through activation of peroxymonosulfate [J]. Journal of Hazardous Materials, 2020, 388: 121801. doi: 10.1016/j.jhazmat.2019.121801 [106] WANG A W, NI J X, WANG W, et al. MOF Derived Co−Fe nitrogen doped graphite[email protected]magnetic chitosan Micro−nanoreactor for environmental applications: Synergy enhancement effect of adsorption−PMS activation [J]. Applied Catalysis B:Environmental, 2022, 319: 121926. doi: 10.1016/j.apcatb.2022.121926 [107] LIU Y, GUO R N, SHEN G H, et al. Construction of CuO@CuS/PVDF composite membrane and its superiority for degradation of antibiotics by activation of persulfate [J]. Chemical Engineering Journal, 2021, 405: 126990. doi: 10.1016/j.cej.2020.126990 [108] MAO D N, YAN X, WANG H J, et al. Catalysis of rGO-WO3 nanocomposite for aqueous bisphenol A degradation in dielectric barrier discharge plasma oxidation process [J]. Chemosphere, 2021, 262: 128073. doi: 10.1016/j.chemosphere.2020.128073 [109] ABDEL-FATTAH E. Atmospheric pressure helium plasma jet and its applications to methylene blue degradation [J]. Journal of Electrostatics, 2019, 101: 103360. doi: 10.1016/j.elstat.2019.103360 [110] 王小平, 梅洁. 泡膜式介质阻挡放电等离子体去除模拟生产废水中的四溴双酚S [J]. 环境工程学报, 2021, 15(7): 2305-2313. WANG X P, MEI J. Removal of TBBPS from simulated production wastewater by bubble film dielectric barrier discharge plasma [J]. Chinese Journal of Environmental Engineering, 2021, 15(7): 2305-2313(in Chinese).
[111] ZHOU W, GUAN Z, ZHAO M G, et al. Characteristics and mechanism of toluene removal from gas by novelty array double dielectric barrier discharge combined with TiO2/Al2O3 catalyst [J]. Chemosphere, 2019, 226: 766-773. doi: 10.1016/j.chemosphere.2019.04.005 [112] GUO H, JIANG N, WANG H J, et al. Degradation of flumequine in water by pulsed discharge plasma coupled with reduced graphene oxide/TiO2 nanocomposites [J]. Separation and Purification Technology, 2019, 218: 206-216. doi: 10.1016/j.seppur.2019.03.001 [113] DENG R Y, HE Q, YANG D X, et al. Enhanced synergistic performance of nano-Fe0-CeO2 composites for the degradation of diclofenac in DBD plasma [J]. Chemical Engineering Journal, 2021, 406: 126884. doi: 10.1016/j.cej.2020.126884 [114] YANG J R, ZENG D Q, HASSAN M, et al. Efficient degradation of Bisphenol A by dielectric barrier discharge non-thermal plasma: Performance, degradation pathways and mechanistic consideration [J]. Chemosphere, 2022, 286: 131627. doi: 10.1016/j.chemosphere.2021.131627 [115] JIANG N, LI X C, GUO H, et al. Plasma-assisted catalysis decomposition of BPA over graphene-CdS nanocomposites in pulsed gas-liquid hybrid discharge: Photocorrosion inhibition and synergistic mechanism analysis [J]. Chemical Engineering Journal, 2021, 412: 128627. doi: 10.1016/j.cej.2021.128627 [116] WANG H J, SHEN Z, YAN X, et al. Dielectric barrier discharge plasma coupled with WO3 for bisphenol A degradation [J]. Chemosphere, 2021, 274: 129722. doi: 10.1016/j.chemosphere.2021.129722 [117] 闫欣, 依成武, 毛丹妮, 等. 纳米氧化锌协同介质阻挡放电等离子体降解双酚A [J]. 环境工程学报, 2021, 15(1): 152-161. YAN X, YI C W, MAO D N, et al. Degradation of bisphenol A by dielectric barrier discharge plasma combined with nano-ZnO [J]. Chinese Journal of Environmental Engineering, 2021, 15(1): 152-161(in Chinese).
-



 下载:
下载: