-
药物和个人护理用品(pharmaceutical and personal care products, PPCPs)作为水中新兴污染物受到人们的广泛关注。美托洛尔(MTP)是目前临床上使用最多的3种β受体阻滞剂之一,也是一种典型的PPCPs,被广泛应用于治疗高血压、冠心病、心力衰竭等多种心血管疾病。近年来,随着人口老龄化不断地加剧,这类药物被大量生产和使用,并且MTP及其代谢产物可以通过人体排泄的方式进入市政排水管网,随后汇集到污水处理厂。由于污水处理厂现有工艺难以有效去除此类药物,因此相当一部此类药物会随着污水厂的出水进入到天然水体中,进而会污染饮用水水源。相关报道表明,MTP在水环境中被频繁检出,其浓度水平达到ng·L−1至μg·L−1级别[1]。此外,MTP对水生生物存在一定的毒性危害[2-3]。因此亟需寻求一种高效的去除方法,以减轻或消除该类污染物的环境负面效应。
高级氧化工艺(AOP)是一种降解水中PPCPs的有效方法。其中,基于紫外的高级氧化工艺由于具有操作简便,处理成本低,去除效率高,二次污染较小等优点,受到研究者的关注[4]。氯是一种强氧化剂,由于价格便宜,具有较强的杀菌能力,被广泛应用于水处理中的消毒工艺。而且,紫外线照射也是一种常见的消毒手段,常用于二沉池出水的消毒。在紫外光照射下,氯可以分解产生羟基自由基(·OH)和氯自由基(如Cl·、ClO·、Cl2·−等),其中,·OH是一种非选择性的活性基团,能够较快地与大多数有机物进行反应,而氯自由基是一种选择性的活性基团,更倾向于与含有苯环等富电子基团有机物进行反应。紫外光和氯组合工艺(UV/Cl2)已被证实能够有效地降解水体中的微量有机污染物,如布诺芬、卡马西平、对乙酰氨基酚、萘普生等[5-8]。因而,UV/Cl2工艺作为一种新型高级氧化工艺在水处理中具有潜在的应用前景。
本研究采用UV/Cl2工艺降解水中的MTP,主要考察不同体系(单独UV、避光氯化、UV/Cl2工艺)、溶液pH值、氯投加量、阴离子(HCO3−、Cl−)以及天然有机物对MTP降解效果的影响。并且利用LC/MS/MS鉴定了MTP降解的主要中间产物,阐明了降解机理。研究结果可为UV/Cl2工艺控制水中此类污染物的提供理论基础。
-
美托洛尔(MTP,纯度为98%)、次氯酸钠(NaClO,有效氯含量6%—14%)购于阿拉丁(上海),活性氯的浓度采用N,N-二乙基对苯二胺分光光度法测定。硝基苯(NB,纯度为98%)和腐殖酸(Humic acid, HA)购于Sigma-Aldrich公司(美国)。其他药剂NaH2PO4·2H2O、Na2HPO4·12H2O、Na2SO4、NaHCO3、NaCl、Na2S2O3·5H2O等购于国药集团化学试剂有限公司,均为分析纯。实验用水均采用超纯水(电导率18.3 MΩ·cm)。
-
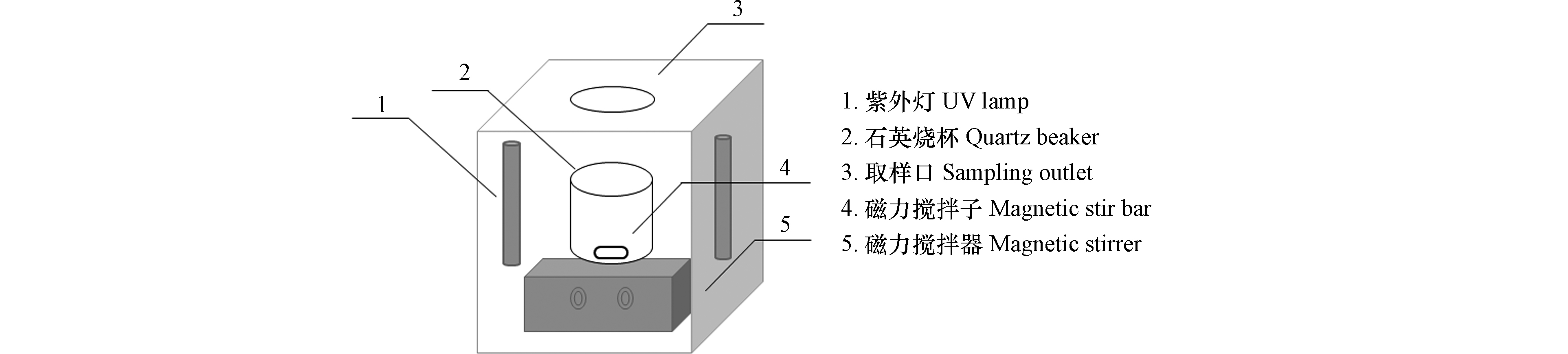
试验所用的反应装置如图1所示,紫外光源采用波长为254 nm的低压汞灯(4W,飞利浦,荷兰).
实验时,将装有100 mL的MTP溶液的石英烧杯置于反应装置中,利用磁力搅拌子保证反应时溶液能够混合均匀,当加入一定量的氯后,立即打开紫外灯开始降解反应。同时,分别进行单独紫外光光解和避光氯化降解MTP的对照实验。溶液的pH值采用磷酸盐缓冲溶液调节。通过分别向MTP溶液中加入既定量的NaHCO3、NaCl和HA,来考察阴离子和天然有机物对MTP降解效果的影响。在特定的反应时刻取样,并立即用过量的Na2S2O3终止反应,随后用HPLC或LC/MS/MS进行测定。每组实验进行3次平行实验,实验结果取平均值。
-
MTP和NB的浓度采用HPLC(Waters e2695,USA)进行测定;色谱柱为Waters C18柱(4.6 mm × 250 mm,5 μm);MTP的流动相为乙腈和10 mmol·L−1醋酸铵(体积比40∶60);NB的流动相为乙腈和水(体积比60∶40);采用紫外检测器,MTP和NB的检测波长分别为222 nm和262 nm;流速为0.8 mL·min−1;进样体积为10 μL。
MTP降解中间产物采用LC/MS/MS(Thermo TSQ quantum Access Max, USA)进行测定,色谱条件:Thermo Hypersil Gold C18柱(2.1 mm × 150 mm,5 μm);流动相A和B分别为10 mmol·L−1醋酸铵和乙腈,流速为0.25 mL· min−1,采用梯度洗脱程序:0—5 min,1%的B;5—15 min,B从1%均匀升高到50%;15—25 min,恢复到1%的B,进样体积为10 μL;质谱条件:全扫描(m/z = 50—350)和子离子扫描模式,阳离子模式。
生态风险采用ECOSAR软件计算出的鱼类96 h半数致死浓度(96 h-LC50)、水蚤48 h半数致死浓度(48 h-LC50)和绿藻96 h半数效应浓度(96 h-LC50)数值进行预测与评估。
-
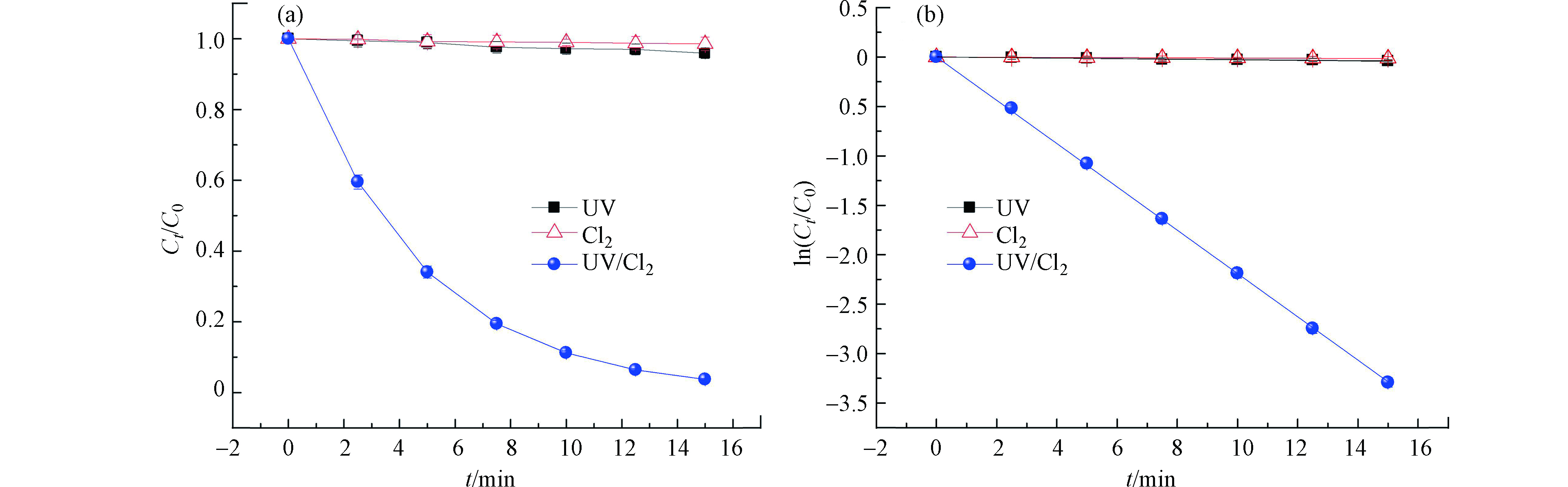
在MTP初始浓度为25 μmol·L−1,氯投加量为250 μmol·L−1,溶液pH值为7条件下,分别考察单独紫外光照射,避光氯化和UV/Cl2工艺对MTP的降解效果,结果如图2所示。结果表明,避光氯氧化对MTP降解的几乎没有作用,15 min时去除率仅为1.4%。单独紫外光对MTP的降解效率同样较低,15 min时去除率为4.1%,表明紫外光解和单独氯氧化都不能有效降解MTP。而紫外联合氯工艺(UV/Cl2)在15 min时对MTP的去除率高达96.2%。对MTP降解过程进行拟合后发现,不同体系中,MTP的降解都遵循拟一级反应动力学模型,且降解速率常数可根据式(1)得出。
式中, [MTP]t为t时刻MTP的浓度,μmol·L−1;[MTP]0为MTP反应初始浓度,μmol·L−1;t为反应时间,min;kobs为拟一级速率常数,min−1。
单独紫外光光解,氯氧化以及UV/Cl2工艺过程中,MTP的降解速率分别为0.00272、0.00103、0.219 min−1。一般而言,某种有机物的光解效率与其摩尔吸光系数及量子产率有关,据报道,由于MTP的摩尔吸光系数为560 L·(mol·cm)−1,光量子产率为0.005 mol·E−1,二者均不高,因此,单独紫外光光解MTP的效率并不高[9]。据报道,在中性条件下,MTP与氯的反应速率常数仅为(0.123 ± 0.011) L·(mol·s)−1,所以避光氯化降解MTP的效率也很低[10]。与单独紫外光光解和氯氧化相比,UV/Cl2工艺降解MTP的效率大大提升。这是因为氯在水溶液中会以HOCl和OCl−的形式存在(式(2)),而二者均会在紫外光照射下分解产生羟基自由基(·OH)和氯自由基(Cl·)(式(3)),进而促进了MTP的氧化降解[5]。
-
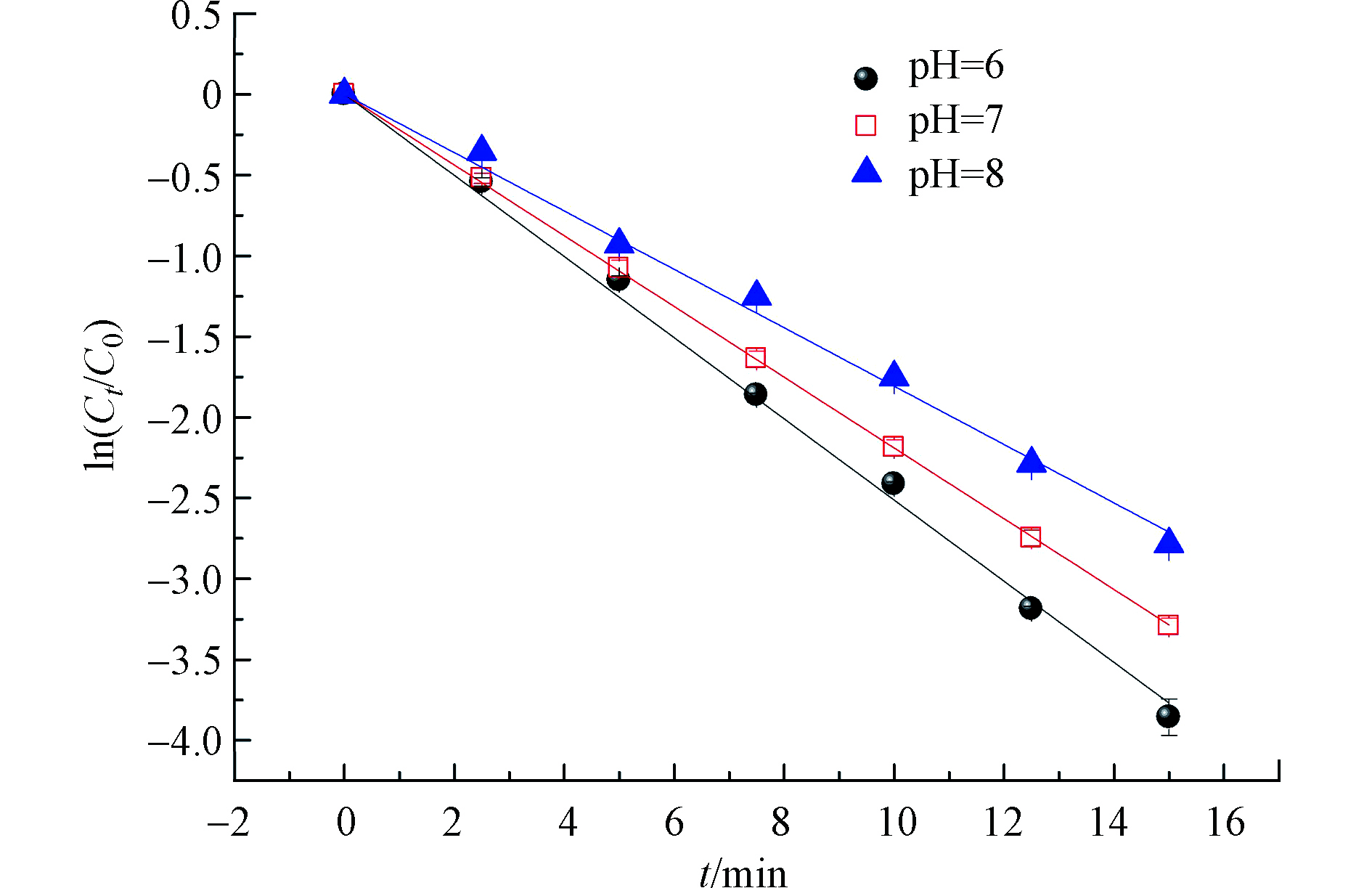
在MTP初始浓度为25 μmol·L−1,氯投加量为250 μmol·L−1时,考察不同溶液pH值对MTP降解的影响,结果如图3所示。结果表明,不同pH值条件下,MTP的降解速率常数满足以下顺序:pH = 6 (0.251 min−1) > pH = 7 (0.219 min−1) > pH = 8 (0.181 min−1)。很明显,酸性条件更有利于MTP的降解。这是因为,水溶液中HOCl与OCl−的比例与溶液的pH值有关(pKa,HOCl = 7.5)。当pH = 6时,HOCl和OCl−的比例分别96.9%和3.1%,当溶液的pH值的升高时,溶液中OCl−组分的比例逐渐增大(当pH = 8时,HOCl和OCl−的比例分别24.0%和76.0%)。虽然HOCl和OCl−均能够在紫外照射下产生·OH和RCS,但由于OCl−在254 nm处的光量子产率低于HOCl的光量子产率,因此,当溶液pH值逐渐升高时,体系中OCl−升高,导致·OH和RCS的生成量减小,进而导致MTP的降解速率下降[11]。
为了进一步探究不同活性组分对MTP降解的贡献,采用动力学模型对MTP的降解进行分析[12]。UV/Cl2体系中,MTP的氧化降解可以用式(4)来表示:
式中,kUV、kRCS分别为UV和RCS降解MTP的一级反应速率常数,min−1;
$ {k_{{\text{gOH/MTP}}}} $ 为·OH与MTP的二级反应速率,为8.1 × 109 L·(mol·s)−1[13],[·OH]为UV/Cl2体系中·OH的浓度,mol·L−1。其中,[·OH]可通过式(5)间接测得。式中,
$ {k_{ \cdot {\text{OH/NB}}}} $ 为·OH与NB的二级反应速率,为3.9 × 109 L·(mol·s)−1[11]。将式(4)和式(5)进行积分,得到式(6)和式(7):式中,[NB]0和[NB]t为NB在t时刻和0时刻的浓度。因此,在UV/Cl2体系中,单独UV、·OH和RCS对MTP去除率的贡献可由式(8)来表示:
式中,R为t时间内MTP的总去除率,
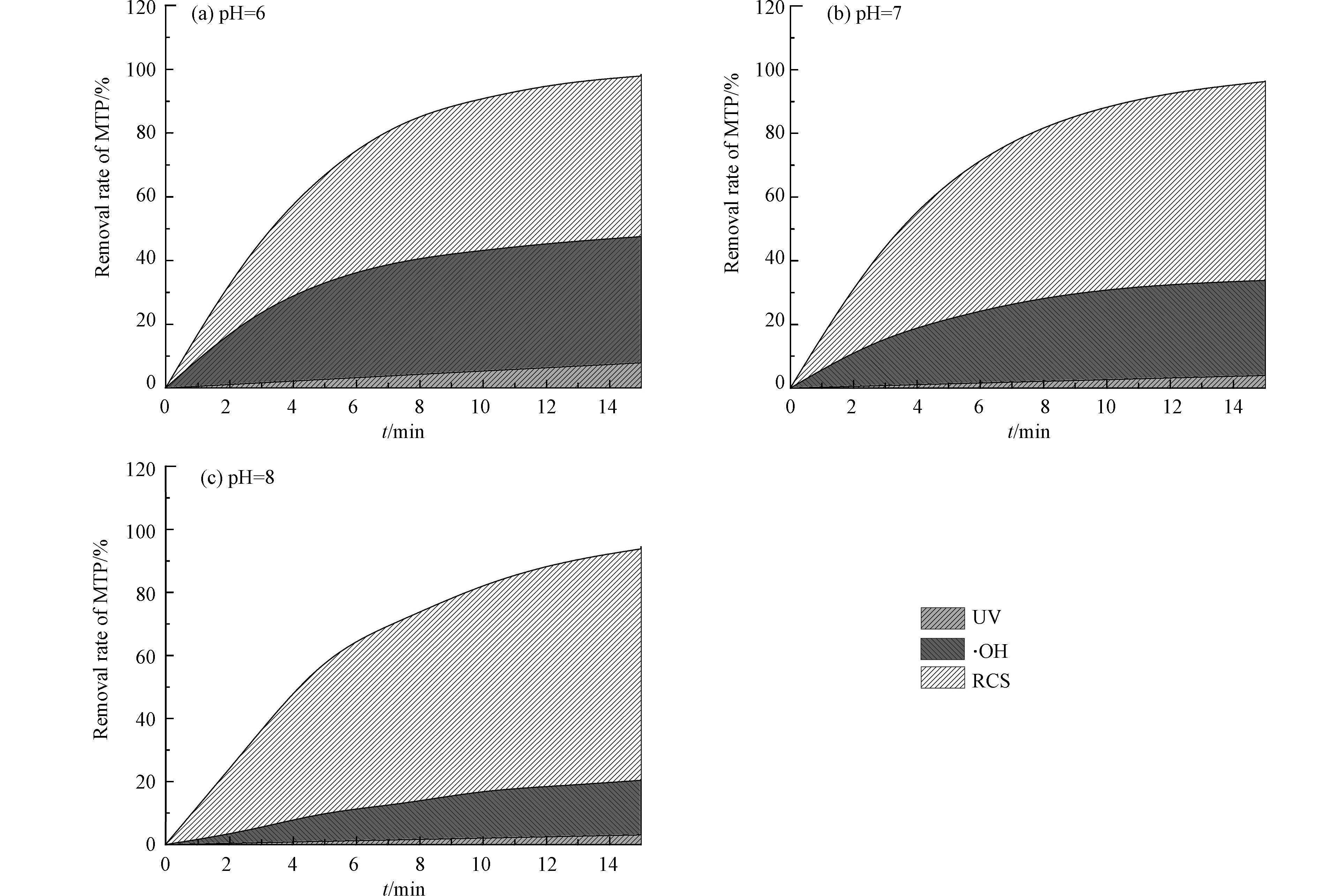
$ {R_{{\text{UV}}}} $ ,$ {R_{ \cdot {\text{OH}}}} $ 和$ {R_{{\text{RCS}}}} $ 分别为t时间内UV,·OH和RCS对MTP的去除率的贡献。实验采用NB作为探针化合物,将初始浓度为5 μmol·L−1的NB加入到25 μmol·L−1的MTP溶液中,氯投加量为250 μmol·L−1,考察UV,·OH和RCS在不同溶液pH值对MTP的去除率的贡献,结果如图4所示。结果表明,不同活性组分对MTP的降解的贡献与溶液的pH值息息相关。在酸性条件下,·OH对MTP的降解占主导作用,而在中性和碱性条件下,RCS对MTP的降解占主导作用。同时,RCS对MTP降解的贡献随着溶液pH值的升高而增大。在15 min时,当溶液pH值从6增加到8时,RUV和R·OH分别从8.1%和48.5%降低到3.3%和21.7%,而RRCS从43.4%增加到75.0%。一方面,随着溶液pH值的升高,溶液中OCl−逐渐增多,故·OH的生成量减小;另一方面,和HOCl相比,OCl−消耗·OH的速率较快,由此造成体系中·OH浓度进一步降低[11]。此外,据报道,·OH的氧化能力随着溶液pH值升高而降低[14]。所以,当溶液pH值升高时,·OH对MTP去除率的贡献减小,而RCS对MTP降解的贡献增加。
-
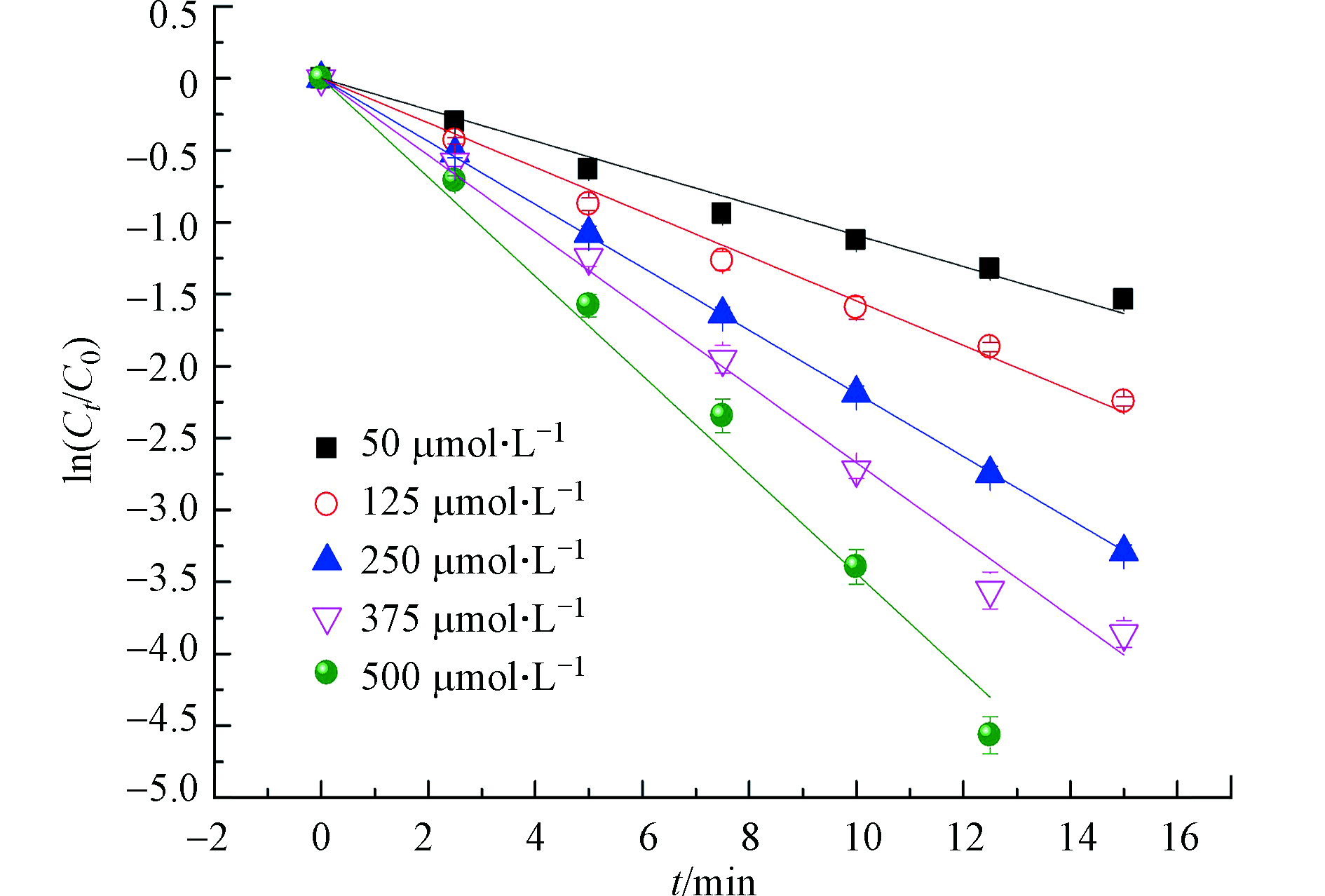
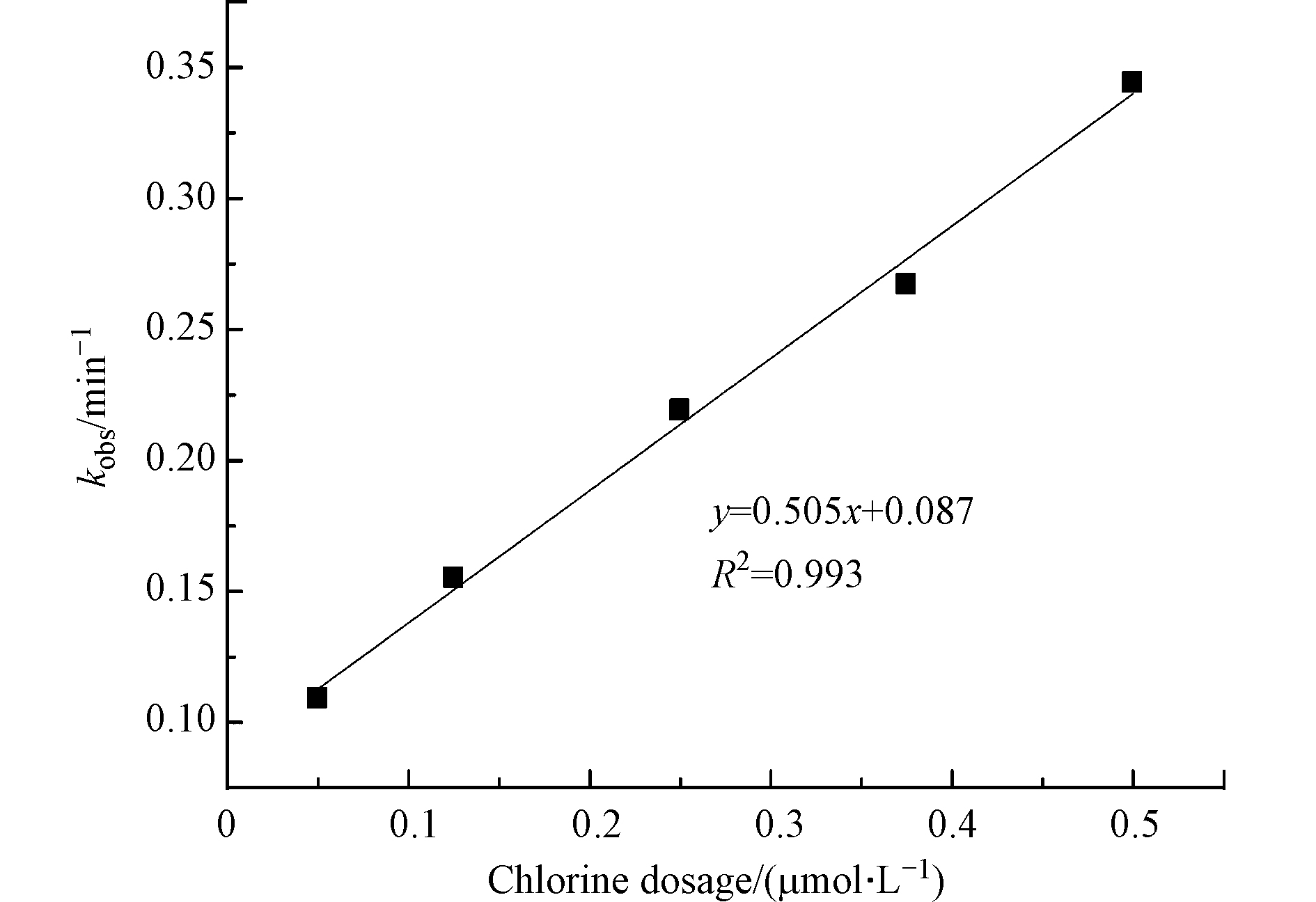
在MTP初始浓度为25 μmol·L−1,溶液pH值为7条件下,考察不同氯投加量对MTP降解的影响,结果如图5所示。结果表明,MTP的降解速率与氯投加量正相关,当氯投加量从50 μmol·L−1增加到500 μmol·L−1时,MTP的降解速率从0.109 min−1增加为0.344 min−1。同时,氯投加量与MTP降解速率常数之间的线性关系良好(R2 = 0.993)。这是因为,在UV/Cl2体系中,氯是产生·OH和RCS的主要来源,因此自由基的浓度会随着氯投加量的增加而增加,这将会强化MTP的降解。也有研究表明,随着氯投加量的持续增加,过量的HOCl和OCl−会淬灭生成的·OH和RCS,产生活性较低的ClO·,如式(9)—(12)所示,由此表现出降解速率增幅变缓的现象[15],而在本实验中并未出现降解速率随着氯投加量持续增加而增幅变缓的现象。
-
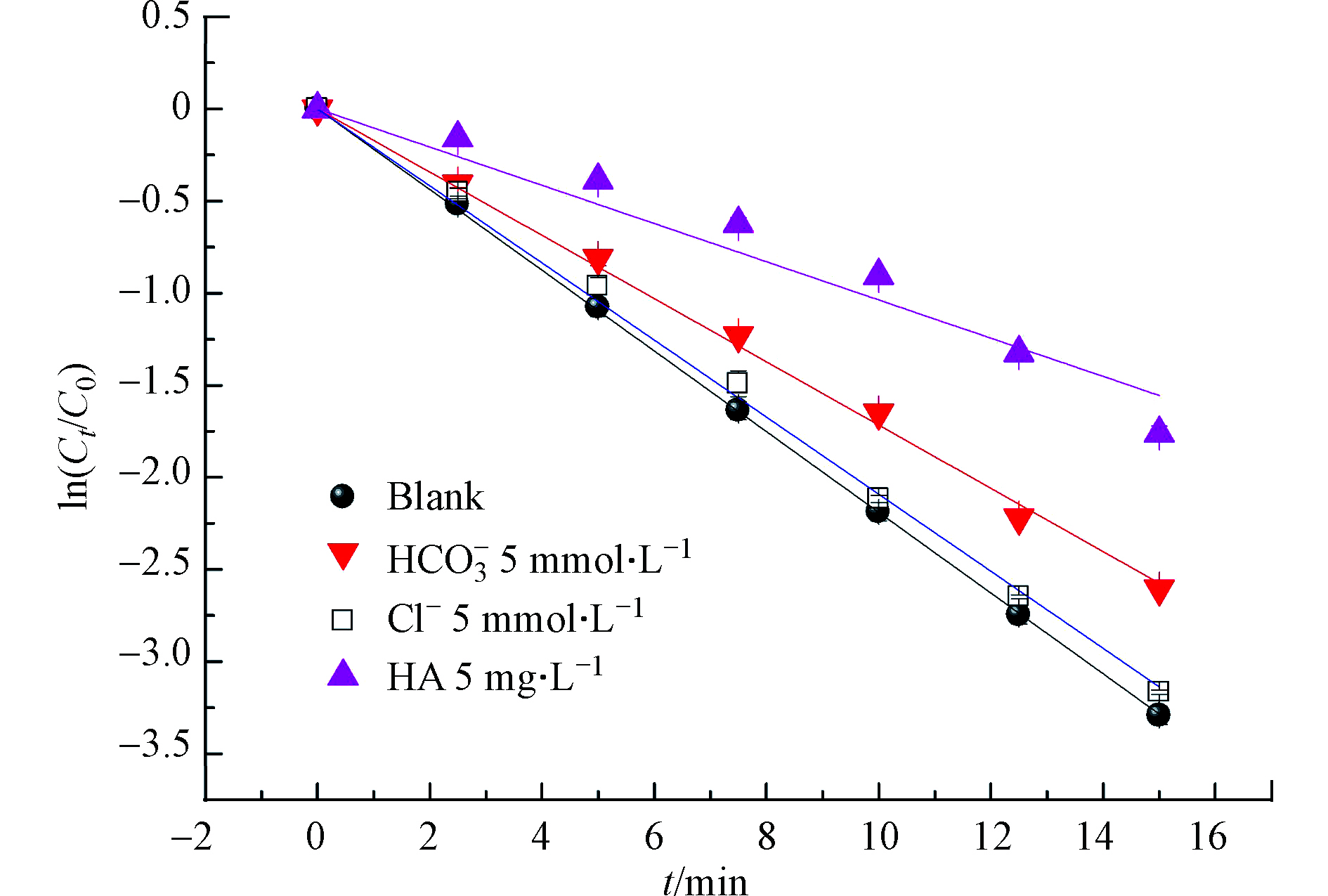
实际水体中广泛存一些阴离子和溶解性有机物会影响高级氧化反应的效果。因此,在MTP初始浓度为25 μmol·L−1,氯投加量为250 μmol·L−1,溶液pH值为7条件下,向体系中投加定量的NaHCO3,NaCl和腐殖酸,来考察HCO3−、Cl−及天然有机物对MTP降解的影响,结果如图7所示。
结果表明,HCO3−和HA明显抑制了MTP的降解,而Cl−的影响不大。和对照组相比,当投加5 mmol·L−1的HCO3−于反应溶液后,MTP的降解速率从0.219 min−1降低至0.171 min−1。这是因为HCO3−是典型的自由基清除剂,它能够与·OH和Cl·进行较快的反应,生成活性较弱的CO3·–,如式(11)和(12)所示[16],从而抑制了MTP的降解;与HCO3−相比,Cl−的抑制程度较低,与对照组相比,当投加5 mmol·L−1的HCO3−于反应溶液后,MTP的降解速率仅降低了4.5%,其原因在于Cl−能够与·OH和Cl·反应生成活性较低的ClOH·–和Cl2·–,但另一方面,ClOH·–和Cl2·–也会以较快的速率分解为·OH和Cl·,如式(13)和(14)所示,故Cl−对反应速率的影响较小[17]。HA显著抑制MTP的降解,和对照组相比,当投加5 mg·L−1后,MTP的降解速率从0.219 min−1降低至0.104 min−1。这是因为腐殖酸能够过滤紫外线,进而与氯竞争光子的吸收,降低自由基产量;同时,HA作为自由基清除剂,也能够消耗体系中的生成的·OH和RCS,从而抑制了MTP的降解[11]。
-
采用LC/MS/MS对UV/Cl2工艺降解MTP的中间产物进行鉴定,结合二级质谱碎片和相关文献,共检测出9种降解中间产物,结果见表1所示。
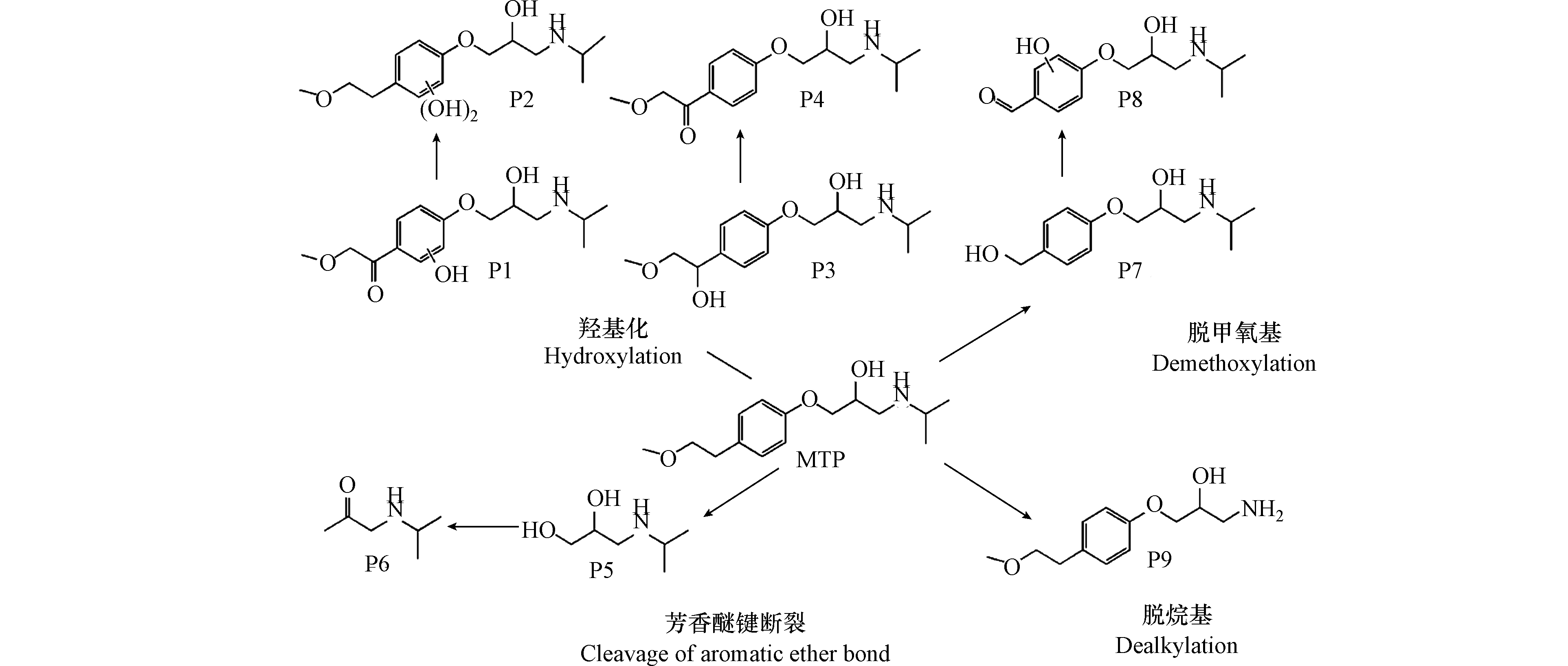
根据鉴定到的中间产物,图8给出了MTP在UV/Cl2体系中降解的可能路径。由于单独紫外光和避光氯化对MTP的降解效果有限,因此,·OH和RCS对MTP的降解占主导作用。首先,苯环是富电子基团,·OH亲电子作用于MTP苯环形成羟基化产物,如产物P1和P2,值得注意的是,ClO●与苯环发生电子转移作用也会生成MTP羟基化产物[12]。与此同时,MTP甲氧乙基侧链上的β-碳原子也容易受到·OH的攻击,形成产物P3。接着,P3的羟基进一步氧化为酮基,形成产物P4[18]。其次,β受体阻滞剂的芳香醚键易受到自由基的攻击而发生断裂,因此,MTP的芳香醚键由于受到·OH的攻击而发生断裂,生成产物3-异丙氨基-1,2-丙二醇(产物P5)。Romero等[19]利用光芬顿工艺降解MTP时,也同样检测到了3-异丙氨基-1,2-丙二醇。Tay等[20]利用臭氧氧化MTP的过程中也检测到了3-异丙氨基-1,2-丙二醇,这表明芳香醚键的断裂是MTP降解的主要途径之一。随着3-异丙氨基-1,2-丙二醇的进一步氧化,生成产物P6。MTP甲氧乙基侧链上的β-碳原子受到自由基的攻击,导致MTP脱去甲氧基,形成产物P7,接着P7经过脱氢、羟基化作用形成产物P8。此外,受到自由基的攻击,MTP侧链上氮原子和α-碳原子发生电子转移,导致N-脱烷基形成产物P9[21]。总体来说,UV/Cl2体系降解MTP主要包括四种机理,即羟基化反应、芳香醚键的断裂、脱甲氧基反应、脱烷基作用等。
-
对降解中间产物的进行毒性评价对UV/Cl2工艺应用于实际水处理具有重要的意义。因此,本实验采用ECOSAR模型对MTP与其中间降解产物进行生态毒性评价[22],结果如表2所示。
从表2中可以看出,MTP的LC50(鱼96 h)值为81.6 mg·L−1,而中间产物P8的LC50(鱼96 h)值为28.7 mg·L−1,因此,与MTP相比,P8对鱼具有较高的毒性。然而其余的中间产物的LC50(鱼96 h)值均高于MTP,说明这些中间产物的急性毒性与MTP相比均有所降低。研究表明,可根据预测值来判定化合物的生态毒性风险等级:低风险,LC50(EC50) > 100 mg·L−1;中等风险,10 mg·L−1 < LC50(EC50) < 100 mg·L−1;高风险,1 mg·L−1 < LC50(EC50) < 10 mg·L−1;极高风险,LC50(EC50) < 1 mg·L−1[23]。因而,尽管与MTP相比,中间产物对水蚤、绿藻的急性毒性均有所降低,但这些中间产物仍存在一定的生态风险,在降解过程中需要进一步去除。
-
(1)与单独紫外光光解和避光氯氧化相比,UV/Cl2工艺极大地提高了MTP的降解效率,氯在紫外光辐照下,产生的·OH和RCS是MTP降解的主要活性物种。
(2)不同工况下UV/Cl2工艺对MTP的降解都遵循拟一级反应动力学模型,酸性条件下有利于MTP的降解,且RCS对MTP降解的贡献随着pH值的升高而增大,经过计算,当溶液pH值从6增加到8时,UV和·OH对MTP降解的贡献分别从8.1%和48.5%降低到3.3%和21.7%,而RCS对MTP降解的贡献从43.4%增加到75.0%。MTP的降解速率随着氯投加量的增大而线性增加;HCO3−和HA抑制了MTP在UV/Cl2体系中的降解,而Cl−对MTP的降解效果的影响不大。
(3)LC/MS/MS结果表明,UV/Cl2工艺降解MTP过程中主要产生了9种中间产物,其中主要的反应途径包括羟基化反应、芳香醚键的断裂、脱甲氧基反应、脱烷基作用等机制。此外,UV/Cl2工艺在一定程度上能够降解MTP的生态环境风险。
紫外光-氯工艺降解美托洛尔的影响因素与机理
Influencing factors and mechanism of metoprolol degradation by UV/chlorine process
-
摘要: 利用紫外光和氯组合工艺(UV/Cl2)降解水中β受体阻滞剂美托洛尔(MTP)。考察了不同体系(单独UV、避光氯化、UV/Cl2)、溶液pH值、氯投加量、HCO3−、Cl−以及天然有机物对MTP降解的影响,并通过液相色谱质谱联用检测了降解中间产物,提出了可能的降解路径,并评估了生态风险。结果表明,单独的紫外光或避光氯几乎不能够降解MTP,而UV/Cl2工艺降解MTP的效率大大提高,15 min时MTP的去除率高达96.2%,且UV/Cl2工艺对MTP的降解遵循拟一级反应动力学模型。酸性条件下有利于MTP的降解,且氯自由基(RCS)对MTP降解的贡献随着溶液pH值的增加而增加。HCO3−和天然有机物均不同程度地抑制了MTP的降解,而Cl−的影响可忽略不计。通过中间产物分析可知,MTP的降解的主要反应途径包括羟基化、芳香醚键的断裂、脱甲氧基、脱烷基等机制。ECOSAR模型预测结果表明,UV/Cl2工艺能够降低MTP的生态风险。Abstract: UV/chlorine process was adopted to degrade metoprolol (MTP), a β-blockers in water. The effects of different systems (UV alone, dark chlorination, UV/chlorine), solution pH value, chlorine dosage, HCO3−, Cl− and natural organic matter on the MTP degradation were investigated. The degradation intermediate products were identified by LC/MS/MS, and the possible degradation pathways were proposed. The ecological risk was also evaluated. The results indicated that UV alone or dark chlorination can hardly degrade MTP, while the degradation efficiency is greatly improved by the UV/chlorine process, and the removal rate can reach to 96.2% within 15 minutes. The degradation of MTP also well followed with the pseudo-first-order kinetic model. Acidic condition favored MTP degradation, and the contribution of reactive chlorine specie (RCS) towards MTP degradation increased as the solution pH value increased. HCO3− and natural organic matter inhibited MTP degradation to different extents, while the effect of Cl− was negligible. Through the analysis of intermediate products, the degradation mechanism of MTP may involve hydroxylation, the cleavage of aromatic ether bond, demethoxylation and dealkylation, etc. The predicted results of ECOSAR model showed that the UV/chlorine process can reduce the ecological risk of MTP.
-
Key words:
- UV/chlorine process /
- metoprolol /
- influencing factors /
- degradation mechanism /
- toxicity evaluation
-

-
表 1 MTP及其降解中间产物质谱参数
Table 1. Mass spectrum parameters of MTP and its intermediates
化合物
Compounds分子量
Molecular
weight保留时间
Retention
time/min质荷比
Mass-to-charge
ratio(m/z)MS/MS碎片
MS/MS
fragmentation分子结构
Molecular structureMTP 267 14.88 268 56,74,92,116,133,159 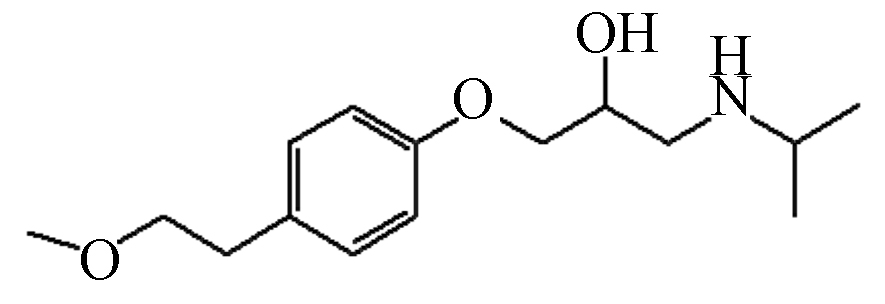
P1 283 14.03 284 116,175,234,252 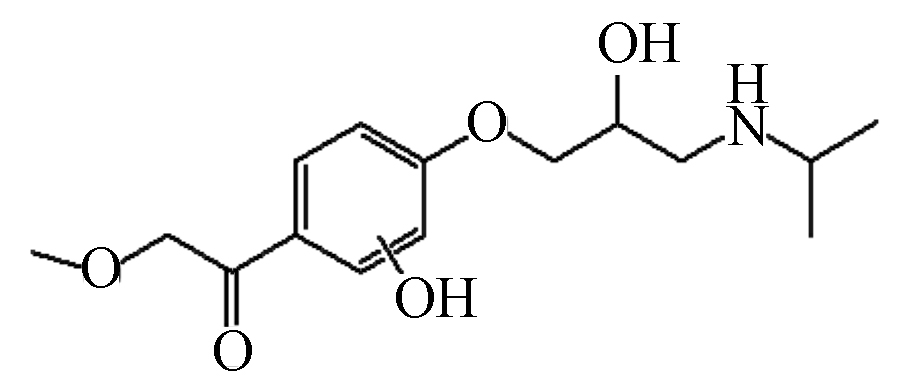
P2 299 12.56 300 238,282 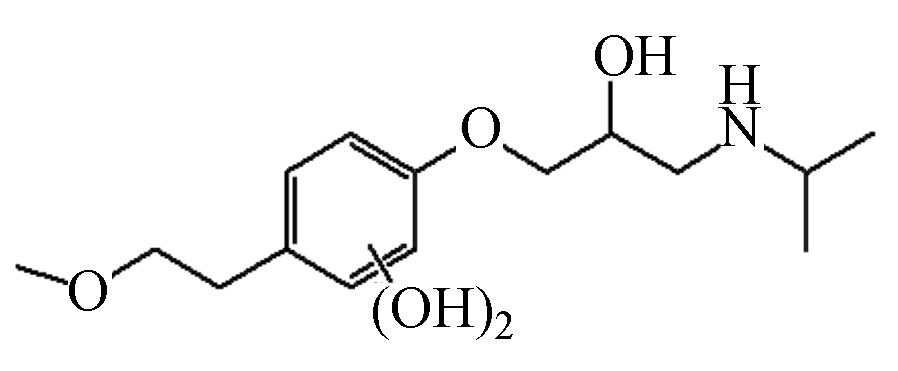
P3 283 2.46 284 116,175,224,248 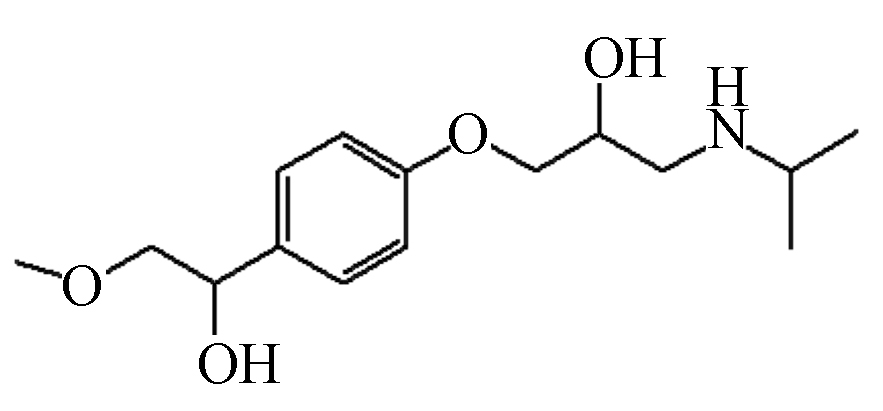
P4 281 14.74 282 98,218,240 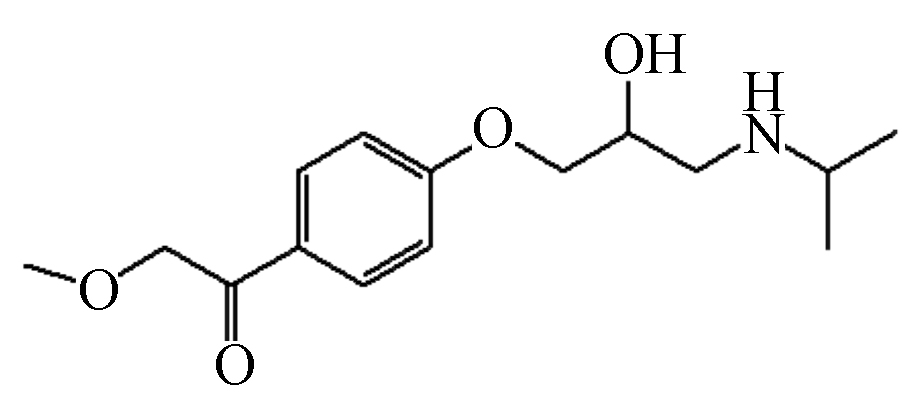
P5 133 1.84 134 56,74,92,116 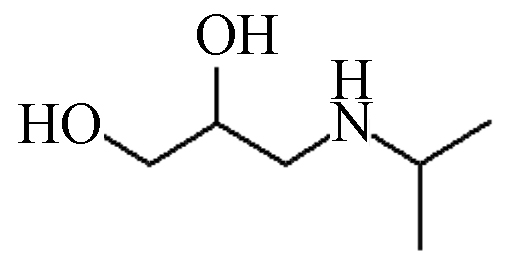
P6 115 14.96 116 56,74,98 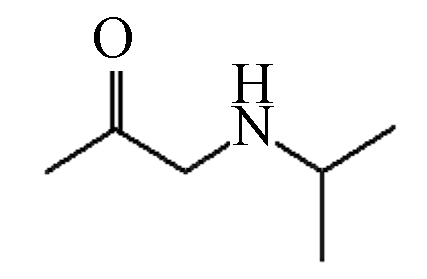
P7 239 2.22 240 74,163,198 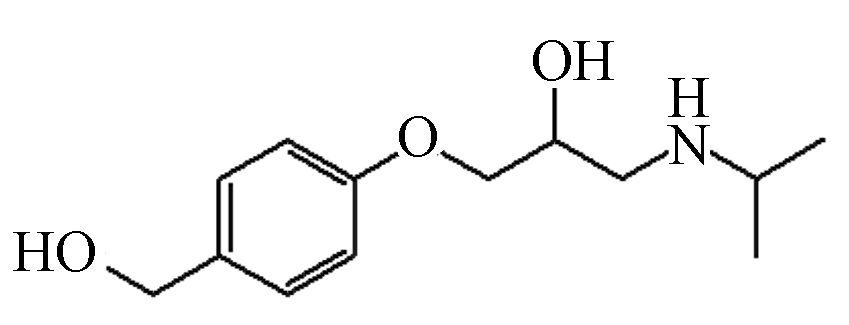
P8 253 12.02 254 133,159,236 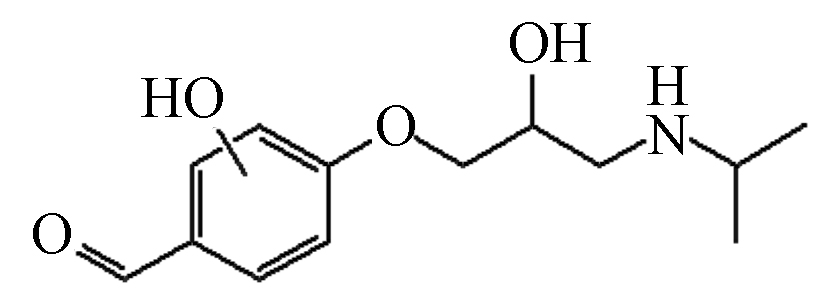
P9 225 14.91 226 56,74,133,191 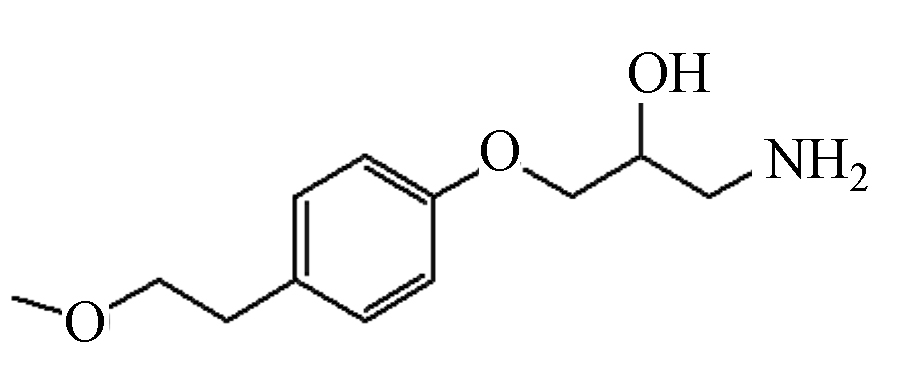
表 2 MTP及其降解中间产物急性毒性
Table 2. Acute toxicity of MTP and its intermediates
化合物
Compounds鱼Fish
96 h-LC50/(mg·L−1)水蚤Daphnid
48 h-LC50/( mg·L−1)绿藻Green algae
96 h-EC50/( mg·L−1)MTP 81.6 9.38 8.31 P1 263 27.8 29.2 P2 390 40.2 44.3 P3 479 48.3 55.5 P4 669 65.8 79.6 P5 1990 170 272 P6 238 23.6 28 P7 239 25.1 26.6 P8 28.7 27.6 15.3 P9 550 53.9 65.5 -
[1] HUGGETT D B, KHAN I A, FORAN C M, et al. Determination of beta-adrenergic receptor blocking pharmaceuticals in United States wastewater effluent [J]. Environmental Pollution, 2003, 121(2): 199-205. doi: 10.1016/S0269-7491(02)00226-9 [2] CLEUVERS M. Initial risk assessment for three beta-blockers found in the aquatic environment [J]. Chemosphere, 2005, 59(2): 199-205. doi: 10.1016/j.chemosphere.2004.11.090 [3] 彭祖华, 池剑, 孙立伟, 等. β-肾上腺受体阻滞剂的水生态毒理学研究进展 [J]. 生态毒理学报, 2013, 8(4): 494-503. doi: 10.7524/AJE.1673-5897.20120608001 PENG Z H, CHI J, SUN L W, et al. Current progress in the aquatic ecotoxicology of β-adrenergic receptor blockers [J]. Asian Journal of Ecotoxicology, 2013, 8(4): 494-503(in Chinese). doi: 10.7524/AJE.1673-5897.20120608001
[4] CANONICA S, MEUNIER L, VON GUNTEN U. Phototransformation of selected pharmaceuticals during UV treatment of drinking water [J]. Water Research, 2008, 42(1/2): 121-128. [5] XIANG Y Y, FANG J Y, SHANG C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process [J]. Water Research, 2016, 90: 301-308. doi: 10.1016/j.watres.2015.11.069 [6] WANG W L, WU Q Y, HUANG N, et al. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species [J]. Water Research, 2016, 98: 190-198. doi: 10.1016/j.watres.2016.04.015 [7] LI J Q, ZHOU S Q, LI M, et al. Mechanism insight of acetaminophen degradation by the UV/chlorine process: Kinetics, intermediates, and toxicity assessment [J]. Environmental Science and Pollution Research, 2019, 26(24): 25012-25025. doi: 10.1007/s11356-019-05747-1 [8] 伊学农, 方佳男, 高玉琼, 等. 紫外线-氯联合高级氧化体系降解水中的萘普生 [J]. 环境工程学报, 2019, 13(5): 1030-1037. doi: 10.12030/j.cjee.201811102 YI X N, FANG J N, GAO Y Q, et al. Degradation of naproxen in water by UV/chlorine advanced oxidation process [J]. Chinese Journal of Environmental Engineering, 2019, 13(5): 1030-1037(in Chinese). doi: 10.12030/j.cjee.201811102
[9] RIVAS F J, GIMENO O, BORRALHO, et al. UV-C radiation based methods for aqueous metoprolol elimination [J]. Journal of Hazardous Materials, 2010, 179(1-3): 357-362. doi: 10.1016/j.jhazmat.2010.03.013 [10] ACERO J L, BENITEZ F J, REAL F J, et al. Kinetics of aqueous chlorination of some pharmaceuticals and their elimination from water matrices [J]. Water Research, 2010, 44(14): 4158-4170. doi: 10.1016/j.watres.2010.05.012 [11] FANG J Y, FU Y, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system [J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. [12] WU Z H, FANG J Y, XIANG Y Y, et al. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways [J]. Water Research, 2016, 104: 272-282. doi: 10.1016/j.watres.2016.08.011 [13] WOLS B A, HARMSEN D J H, BEERENDONK E F, et al. Predicting pharmaceutical degradation by UV (LP)/H2O2 processes: A kinetic model [J]. Chemical Engineering Journal, 2014, 255: 334-343. doi: 10.1016/j.cej.2014.05.088 [14] ZHANG Y Q, ZHANG J F, XIAO Y J, et al. Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate [J]. Chemical Engineering Journal, 2016, 302: 526-534. doi: 10.1016/j.cej.2016.05.085 [15] 赵刘柱, 吴敏, 朱延平, 等. 紫外—氯降解非那西丁影响因素及机理研究 [J]. 水处理技术, 2019, 45(3): 69-73,78. ZHAO L Z, WU M, ZHU Y P, et al. Influencing factors and mechanism of ultraviolet/chlorine degradation on phenacetin [J]. Technology of Water Treatment, 2019, 45(3): 69-73,78(in Chinese).
[16] DENG J, WU G X, YUAN S J, et al. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, mechanisms and degradation pathways [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2019, 371: 151-158. doi: 10.1016/j.jphotochem.2018.10.043 [17] JIA X R, JIN J, GAO R, et al. Degradation of benzophenone-4 in a UV/chlorine disinfection process: Mechanism and toxicity evaluation [J]. Chemosphere, 2019, 222: 494-502. doi: 10.1016/j.chemosphere.2019.01.186 [18] RADJENOVIC J, ESCHER B I, BABAEY K. Electrochemical degradation of the β-blocker metoprolol by Ti/Ru0.7Ir0.3O2 and Ti/SnO2-Sb electrodes [J]. Water Research, 2011, 45: 3205-3214. doi: 10.1016/j.watres.2011.03.040 [19] ROMERO V, ACEVEDO S, MARCO P, et al. Enhancement of Fenton and photo-Fenton processes at initial circumneutral pH for the degradation of the β-blocker metoprolol [J]. Water Research, 2016, 88: 449-457. [20] TAY K S, RAHMAN N A, ABAS M R B. Ozonation of metoprolol in aqueous solution: Ozonation by-products and mechanisms of degradation [J]. Environmental Science and Pollution Research, 2013, 20(5): 3115-3121. doi: 10.1007/s11356-012-1223-3 [21] WILDE M L, MAHMOUD W M M, KUMMERER K, et al. Oxidation-coagulation of β-blockers by K2FeVIO4 in hospital wastewater: Assessment of degradation products and biodegradability [J]. Science of The Total Environment, 2013, 452-453: 137-147. doi: 10.1016/j.scitotenv.2013.01.059 [22] YANG Y, LU X L, JIANG J, et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate [J]. Water Research, 2017, 118: 196-207. doi: 10.1016/j.watres.2017.03.054 [23] WANG J B, ZHI D, ZHOU H, et al. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode [J]. Water Research, 2018, 137: 324-334. doi: 10.1016/j.watres.2018.03.030 -



 下载:
下载: