-
近几十年抗生素的滥用已造成了地表水和饮用水的污染[1-2].作为一种广谱抗菌剂—四环素(TC)在动物体内代谢不完全,其中60%—90%的母体化合物以原始或代谢形式排放到水生环境中,因其具有很好的化学稳定性,几乎不会自然分解[3].常规处理四环素的方法包括电解法[4]、光催化降解[5]、化学氧化[6]和生物过滤[7]这些方法因其流程复杂基础设施昂贵和可能二次污染而受到限制.因此开发一种高效、便捷和"绿色"去除TC的技术已成为一项重要而紧急的任务.
石墨烯是一种基于二维蜂窝状结构的纳米材料[8]。其比表面积理论值高达2630 m2·g−1,这为去除抗生素提供更多的吸附位点[9]。氧化石墨烯因其富含含氧官能团而具有优异的性能。活性较强的含氧官能团可与抗生素产生强相互作用,并具有很高的可修饰性[10−11]。早期人们侧重于石墨烯或氧化石墨烯吸附抗生素,但是由于石墨烯片层间的静电力使其具有疏水性以及石墨烯片层之间容易发生堆叠,这不利于抗生素的吸附[12−13]。因此,二维结构的石墨烯在应用中存在一定的问题。以石墨烯为基本单元的多孔三维石墨烯,可以克服二维石墨烯堆叠缺点,展现出更高的比表面积、较大的孔隙率,从而实现优异的吸附性能[14−15]。而且三维石墨烯作为吸附剂不仅避免了石墨烯吸附剂在水中造成的二次污染,还作为宏观体,更有利于固液分离[16]。
基于三维石墨烯多孔空间结构和超大比表面积,以及良好的吸附性能,采用环境友好的抗坏血酸还原氧化石墨烯,通过自组装形成具有三维网络结构的气凝胶三维石墨烯(3DG)。用扫描电镜(SEM)、透射电镜(TEM)和X射线衍射(XRD)等技术对3DG微观结构和化学组成进行了表征。将3DG作为吸附剂应用于去除水中四环素(TC),系统研究了TC的吸附机理和降解性能。
-
试剂:乙腈和甲酸为色谱纯,石墨粉、双氧水、高锰酸钾、硝酸钠、浓硫酸、乙醇、抗坏血酸、盐酸、氢氧化钠、四环素标准品均为分析纯,上海阿拉丁生物技术有限公司购买
仪器:Aglient 1260高效液相色谱仪:安捷伦科技有限公司;SB−5200DT超声波清洗机和Scientz−18ND真空冷冻干燥机:宁波新芝生物科技有限公司; UV−1800紫外可见分光光度计:上海美谱达仪器有限公司;PHS-3E pH 计:上海梅特勒−托利多仪器有限公司;HC−3018高速离心机:安徽中科中佳科学仪器有限公司
-
采用传统的 Hummers 方法制备 GO [17]。本研究在参考文献的基础上采用化学还原和冷冻干燥法制备三维石墨烯[18]。称取0.5 g GO置于250 mL 烧杯中,加入100 mL去离子水后超声溶解1 h ,可以得到5 mg·mL−1 GO溶液。取 10 mL GO溶液,放于圆柱形玻璃器皿中。向其中添加新配置的125 mg·mL−1的抗坏血酸溶液,在 65 ℃烘箱中还原3 h ,得到石墨烯水凝胶。将水凝胶置于低温下− 80 ℃ 冰箱中2 h以形成冰,随后冷冻干燥48 h,以获得成型的 3DG。
-
取一定浓度的50 mL的TC置于100 mL锥形瓶中,调节溶液的pH=2.0—12.0(1 mol·L−1的HCl或NaOH溶液调),加入一定质量的3DG,放置于恒温摇床中进行吸附。待吸附达到饱和后,取一定量的样品溶液,过0.45 μm的微孔滤膜后通过紫外分光光度计和HPLC系统进行检测。整个实验在恒温水浴摇床上进行,控制转速。TC的吸附过程保持在避光状态,避免抗生素发生光解。
高效液相色谱法(HPLC)。流动相:0.1 %甲酸水溶液和乙腈,柱温30 ℃ ,进样量10 μL,流速为1.0 mL·min−1,检测波长为360 nm(TC)
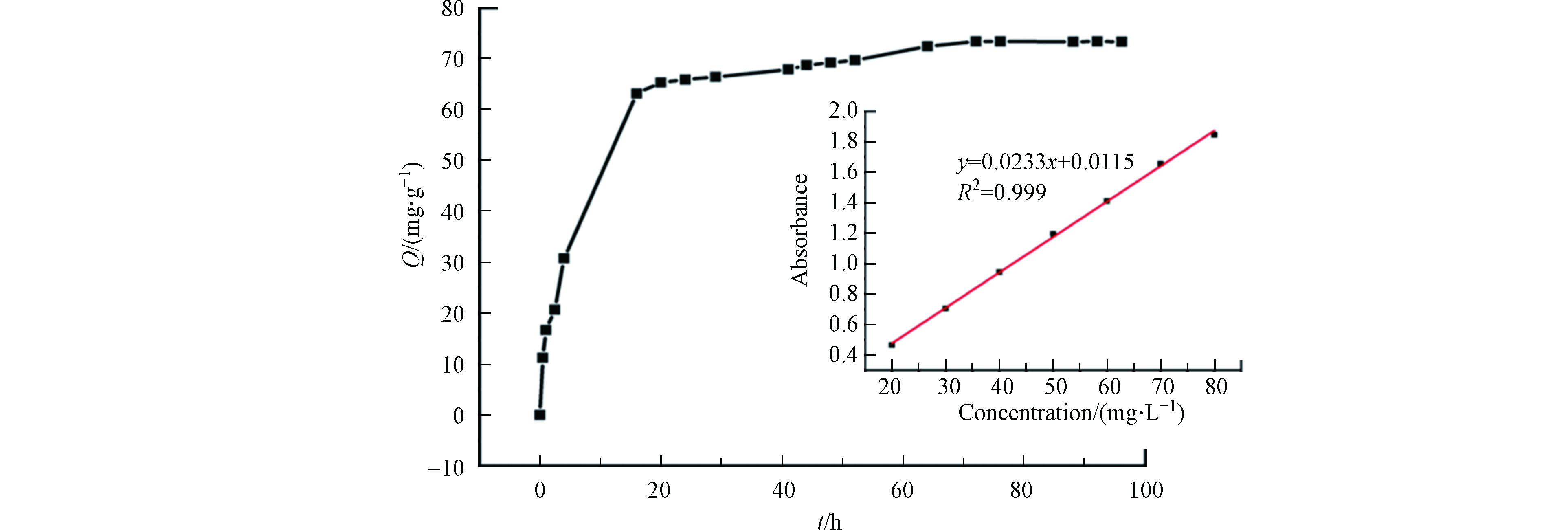
由 UV/ vis 测得 TC 最大吸收波长是360 nm,且在相同条件下对该实验中的样品做3次平行实验并求取平均值。其中通过公式 (1) 和 (2) 分别计算3DG的吸附量以及吸附效率
式中,Qe( mg·g−1 ):3DG平衡吸附量;C0( mg·L−1 ):TC初始浓度;Ce( mg·L−1 ):TC吸附平衡时的浓度;m( g ):3DG的质量;D:3DG的吸附效率
-
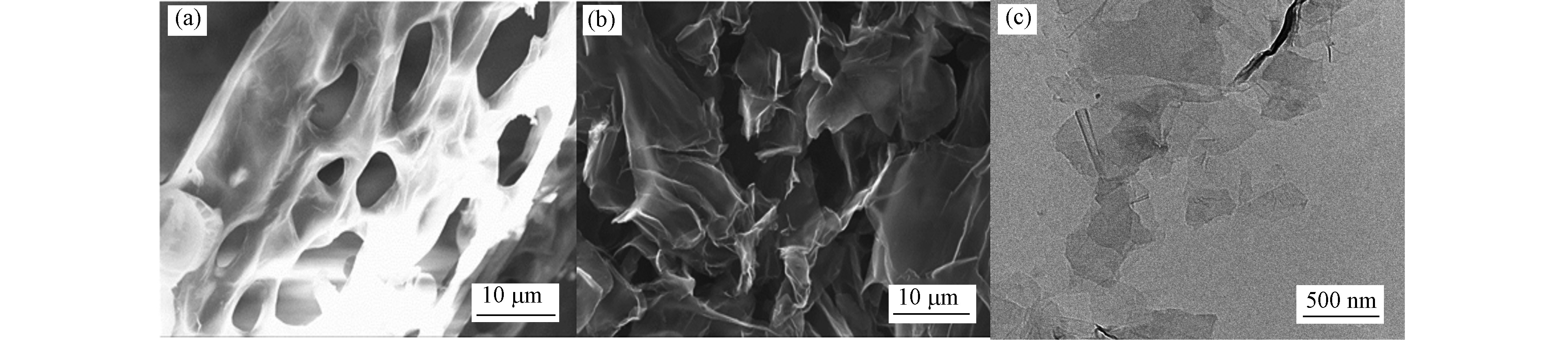
图1(a) 可以直观地看出 ,3DG 呈交联多孔结构,由疏松光滑的石墨烯片层堆叠而成,孔径约为5—20 μm。从图1(b) 中可观察到,3DG 内部呈交联卷曲且蓬松的薄纱状,含有很多褶皱。边缘卷曲折叠现象可能是由于热力学涨落效应,能够降低 3DG 表面能,使其更稳定地存在。1(c)是 3DG 的 TEM 图,很明显可以看出 3DG 的厚度不均匀,不同位置的颜色深浅不一,层数也不同。这是由于 3DG 在生长过程中液体分散不够均匀,致使在形成过程中不同部位的活性位点分布不太均匀,活性也不尽相同。
-
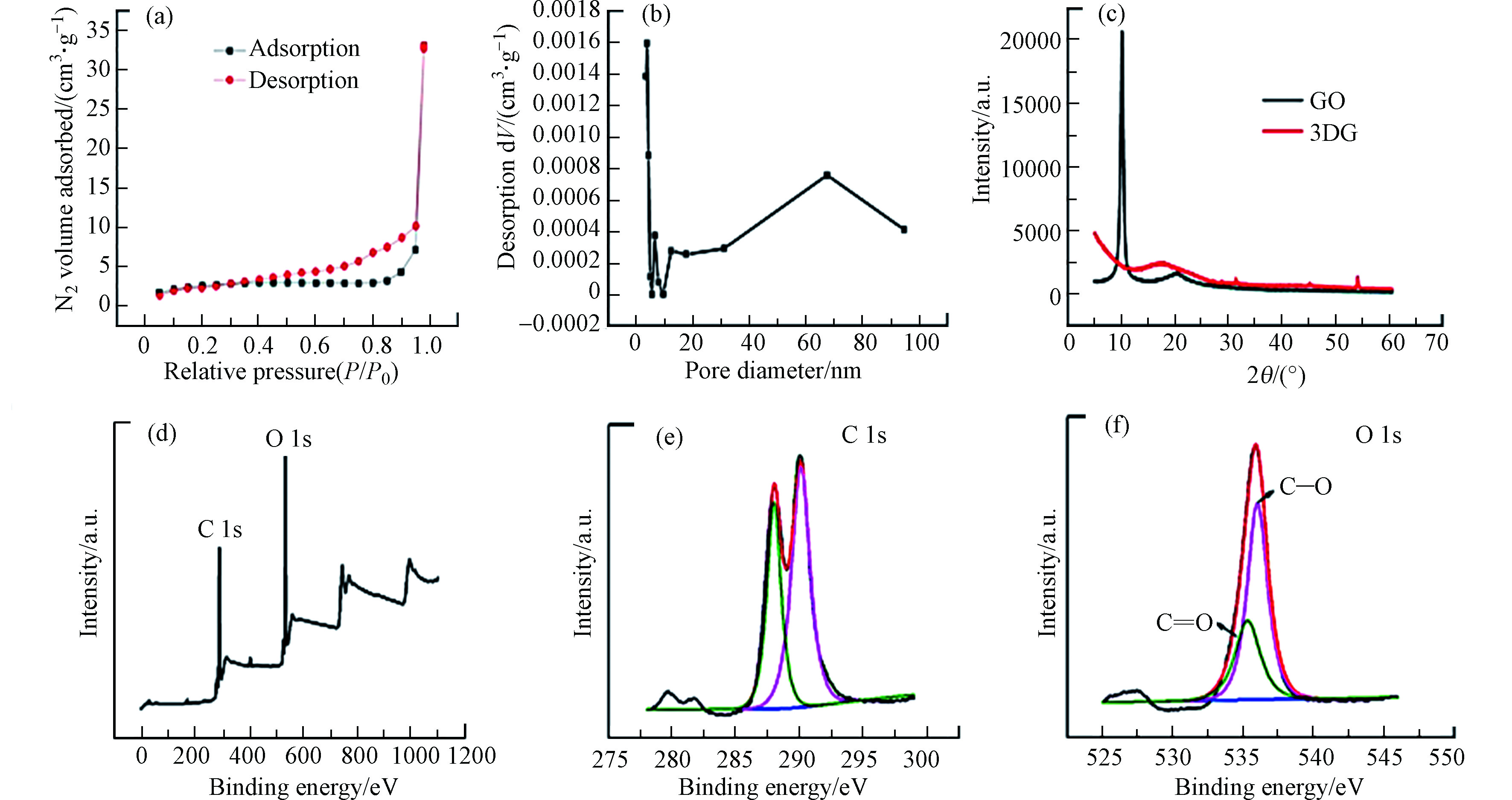
图2(a) 展示了 3DG 的吸脱附曲线。根据曲线特征判断此吸/脱附曲线属于IV型吸附等温线,此外在压力0.35—0.95之间有较明显的滞后环,可推出 3DG 的内部存在大量介孔且在吸附过程中出现了毛细凝聚的现象。图中的脱附曲线较陡峭,可能与介孔出现堵塞或者因空穴效应导致的挥发有关。根据N2吸脱附曲线计算3DG的比表面积是19.554 m2·g −1。结合孔径分布图(图2b)和使用BJH方程计算可知,3DG的孔以介孔为主,孔径为3.821 nm。石墨被充分氧化成功构建出GO。
图2(c)是GO 和3DG 的 XRD 图谱。由图2可以看到,在2θ=10.162°和17.985°分别出现了GO 和3DG 的特征衍射峰,用布拉格方程即2 dsinθ = λ 计算出GO 和3DG 内部片层间距是 0.87 nm和0.49 nm。GO 的峰窄且尖锐,但 3DG特征峰较宽,得出 GO结晶度较好,在其还原自组装 3DG的阶段,石墨烯纳米片厚度加大且层数增加。此外,在10.162 °中GO 的峰消失,可看出抗坏血酸已经完全还原了GO,形成 3DG。
图2 (d)展示了 GO 的 XPS 全谱图。其中,在288 eV、536 eV处的两个特征峰分别对应的是 C1s和O1s,图2(e)展示的是GO 中的 C1s 高分辨图谱,其中,两个不同的峰集中在288 eV和290.1 eV ,分别对应烷基或者芳香环中的C=C/C—C、环氧和烷氧基上的C—O或羟基。图2(f) 展示了 GO 中的 O 1s 高分辨图谱中,在535.3 eV和536 eV 处的峰对应羰基和酮基上的 C—O和C=O。可得出,GO 表面官能团以 C—O为主。
-
TC在3DG上的吸附量随时间的变化情况如图3所示。可以发现TC在3DG上吸附时间较长,初始阶段前18 h内吸附量迅速升高,而后逐渐变得缓慢,直至72 h时基本到达吸附−解吸的动态平衡。
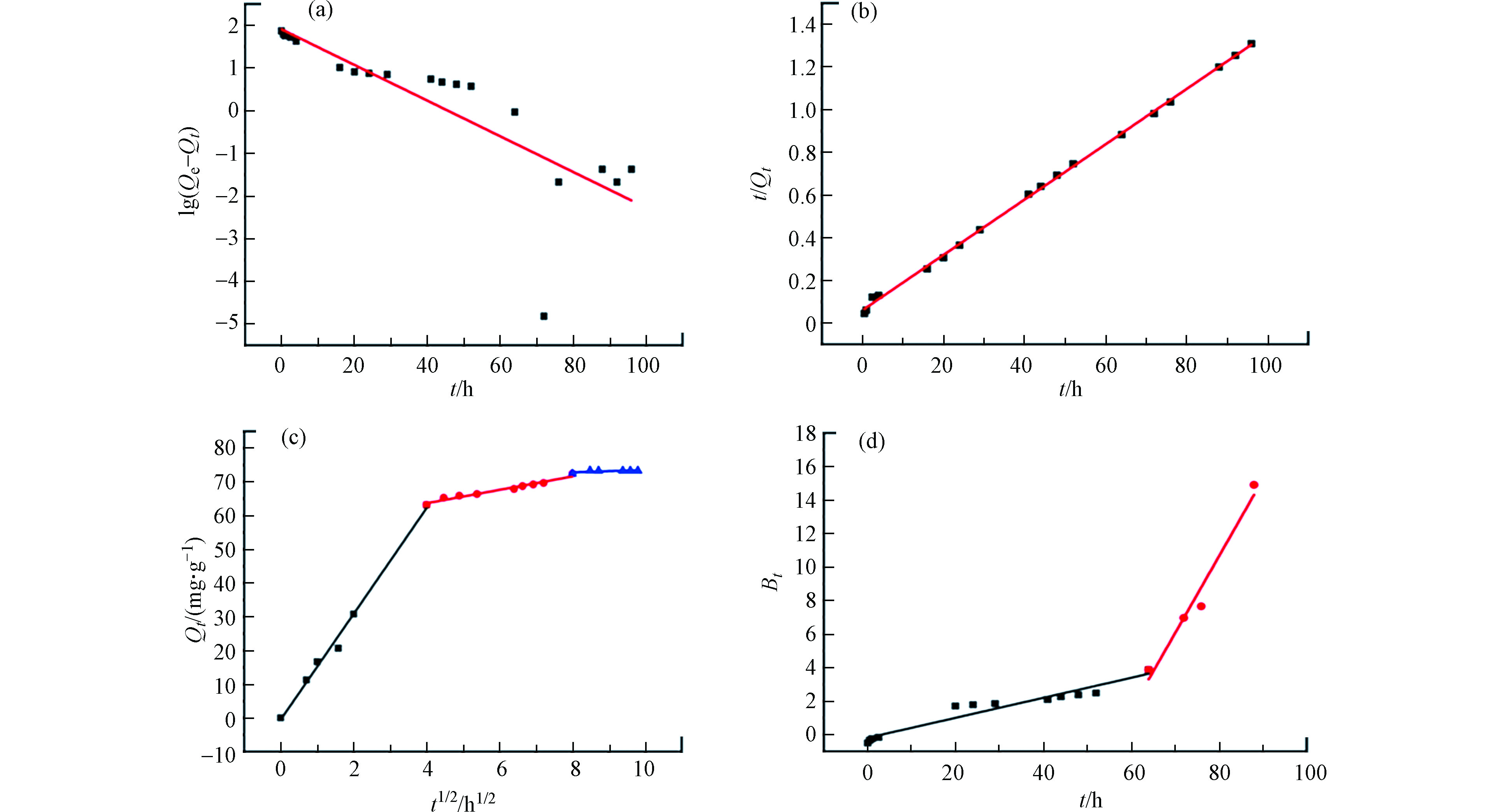
借助伪一级动力学、伪二级动力学、内扩散模型(The Weber and Morris Model)和外扩散控制模型(Boyd Model)探讨了3DG对TC的动力学。4种动力学模型的拟合曲线见图4,相关参数见表1。
准一级动力学方程如公式 (3) 所示:
准二级动力学方程如公式 (4) 所示:
内扩散模型方程如公式 (5) 所示:
外扩散模型方程如公式 (6) 所示:
其中,Qe1,e2( mg·g−1 )—平衡吸附量;Qt ( mg·g−1 )—在任一时间t的瞬时吸附量;k1—伪一级动力学的速率常数;k2—伪二级动力学的速率常数;Ci—涉及到厚度、边界层的常数;kip—内扩散速率常数;F(t)=Q/Qe;Bt—时间常数,与F(t)有关,Bt=0.4977−ln(1−F(t))。
拟合伪一级动力学曲线所得参数与线性拟合曲线发现R2非常小,仅有0.6686。此外,推算的Qe1与实际实验Qe极为各异,表明3DG对TC的吸附并非单纯的物理吸附。伪二级动力学参数R2高达0.9999,推算所得的Qe2与实际实验所得Qe的差距相对比较小,表明TC及3DG两种要素和其化学相互作用来决定吸附过程,3DG的最大吸附量与其反应活性位点个数成正比。内扩散模型的线性拟合曲线是一个三段式非线性图,表明3DG对于TC的吸附是连续的而且分为3个阶段。第一阶段为线性吸附,且和表层扩散有关,斜率的大小也说明了吸附速率的快慢。在这个阶段,由于水合作用,TC分子从溶液扩散到3DG表面且形成水膜。同时,TC分子克服液膜的阻力后,通过液膜至3DG外层。第二阶段是TC分子内扩散过程,相对缓慢,此阶段TC穿过3DG的外层进入到内层进行吸附位扩散。而在第三阶段中曲线的斜率最小,此时吸附达到了饱和。每个阶段的直线都不会经过原点,说明不仅只有内部扩散是此吸附中的速率控制因素。外扩散模型拟合结果是直线不通过原点。这说明液膜扩散过程是起始阶段中吸附速率的主要控制因素,后期可能还有其它影响因素。
-
为了探究3DG对于TC的热力学,该研究分别设置了288 、298、308、318、328 K的不同温度,探讨3DG对不同起始浓度(20—70 mg·L−1)的TC溶液的吸附效果。
在吸附热力学研究中, ∆G、∆S和∆H等热力学参数的公式如下:
其中,K−固液相吸附平衡常数;T−绝对温度;R−常数,8.314 J·mol−1·K−1。吸附热力学参数见表2。
可以看出,∆G均为负值。在起始浓度不变的前提下,随环境温度的升高,∆G反而减小,说明了3DG吸附TC的过程是自发而且可行,与此同时吸附性能随着环境温度的升高而增大。表2中的熵变化即 ∆S > 0,表明3DG对于TC的亲和力和增加了固体和液体界面的随机性;焓变化即 ∆H > 0时则说明此吸附过程应是吸热的。据经验可知,若∆H的值在2.17—20.9 kJ·mol−1之间,则以物理吸附为主,若在20.9—418.4 kJ·mol−1之间以化学吸附为主。本研究中∆H为10.755 kJ·mol−1,说明主要为物理吸附。
-
为了继续探究3DG对TC的吸附影响,本研究设置不同的温度分别为288、298、308、318 、328 K,以及3DG对不同初始浓度(20—70 mg·L−1)的TC溶液的吸附效果。选用Langmuir、Freundlich及Tempkin的3种等温吸附分析模型,对TC在3DG上的吸附特性和机理展开分析,3种分析模型和参数数值如图5和表3。
Langmuir模型:
式中,KL(L·mg−1)—Langmuir常数;Qmax—Langmuir最大吸附量。
Freundlich模型
式中,KF(L·mg−1)—Freundlich常数;n—Freundlich指数。
Tempkin等温吸附模型
式中,bT(J·mol−1)吸附常数,与吸附热有关;R常数,8.314 J·mol−1·K−1;KT(L·mg−1)Tempkin平衡结合常数。
Langmuir模型可用于化学吸附和物理吸附,图5(b) 由Ce和Ce/Qe做线性拟合得到,表3展示的是相应的参数。R2在0.9975—0.9996之间,这也说明了此吸附过程比较符合Langmuir模型,从而进一步说明了3DG均匀的吸附位点对TC发生了吸附反应。此外,随着温度的升高,Qmax和KL都会增加,这也证明了本吸附是吸热反应。
Freundlich模型不仅适用于单层吸附,还可适用于不均匀的表面吸附,可应用于物理吸附以及化学吸附。假定该模型的吸附过程发生在非均匀的表面上,相应参数如表3。R2的值在0.9491到0.9758之间,和Langmuir模型相比拟合较差。除此之外,在不同的环境温度下所有的1/n < 1,表明容易发生吸附。
Tempkin模型就是一种基于正负电荷之间相互作用的化学吸附模型,假设吸附剂具有非均匀性的表面,同时将表面分为许多均匀的吸附单元,每个单元吸附热为常数,表3记录了相应的参数。由表可知,在5种温度下,相关系数R2依次为0.9956、0.9942、0.9914、0.9969、0.997,该模型相对以上两种模型拟合程度较低。此外,在所有温度下的KT值都很小,说明TC与3DG之间的结合力较弱。
-
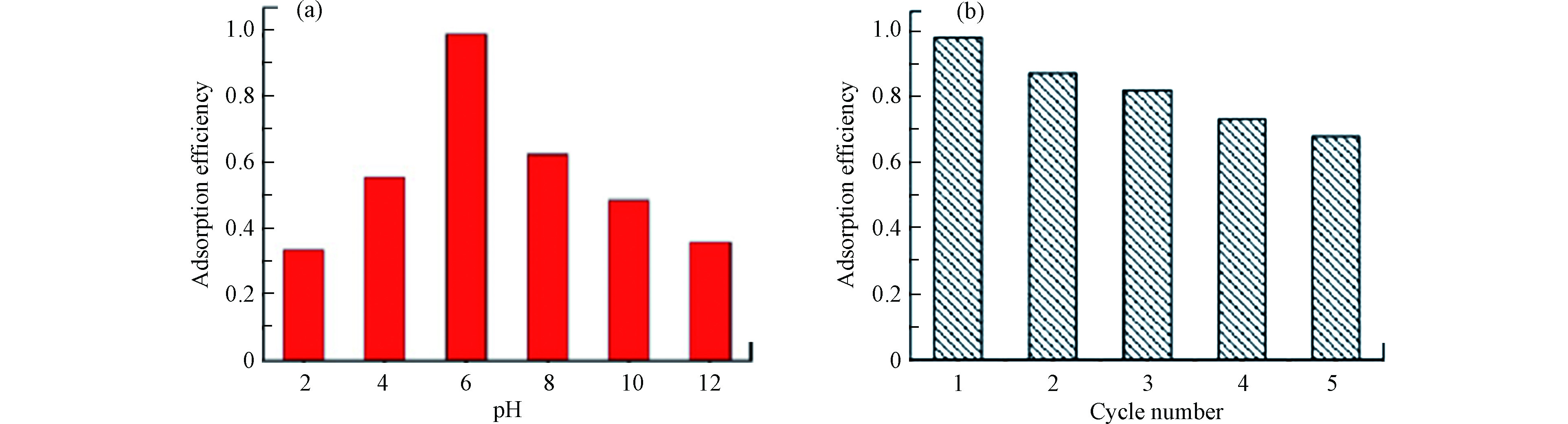
pH值可影响TC的存在形式、3DG的表面电荷甚至二者的结合位点等。因此,在本研究中我们设定了不同的pH值分别是2、4、6、8、10和12,探讨3DG对TC的吸附影响。从图6 (a)中可以看出,随着pH的加大,吸附效率先增加后减小,当pH= 6时吸附效率可以达到98.6 %。TC水溶液包括3个电离常数(pKa= 3.30、7.68和9.68),在pH < 3.3、3.4 < pH < 7.6、 7.7 < pH < 9.6和9.7 < pH < 12下,分别存在阳离子( TC+ )、两性离子( TC+−0 )和阴离子( TC−、 TC2− )的形式。在3DG的表面存在丰富的基团,如氨基、巯基和亚胺等,在偏酸或者偏碱环境下,这些活性基团分别发生质子化或者去质子化,导致3DG在不同的环境中带不同的电荷。因此,在偏酸的环境中,3DG带的正电荷和TC + 发生静电排斥,而在偏碱环境中,3DG带的负电荷和TC− 、TC2− 发生静电排斥,从而吸附效率极大降低。
在优化的最佳条件下,本研究的3DG对TC的吸附结果与相关研究进行了对比。从表4可以看出,本研究所制备的3DG对TC的最大吸附量要优于其他材料,说明该方法制备的3DG用于去除水中的TC性能较好。
-
进一步探究3DG的再生性能,对3DG进行多次重复吸附−解吸实验测定3DG的吸附性能。过滤分离吸附饱和后的3DG,在恒温摇床上用体积比50:50的乙醇水浸泡24 h后进行脱附,用超纯水洗涤多次随后烘干称重,重复吸附试验。如图6(b) 为经过5次循环后3DG的吸附效率。对一定质量的3DG 重复5次吸附-解吸后,吸附效率从98%降到了68%,说明3DG材料具有较好的再生性能。
-
(1)3DG对TC有良好的吸附效果,吸附时间在72 h后达到饱和,吸附过程较符合伪二级动力学模型,是吸热过程,温度升高有利于吸附的进行。
(2)与Freundlich和Tempkin模型相比, 3DG对TC的吸附行为更符合Langmuir模型。在328 K温度下,最佳pH值条件6,最大吸附量为322.58 mg·g−1。
(3)3DG材料具备良好再生性能,吸附-解吸重复试验进行5次后,材料仍具备良好的吸附性能,再次证明了3DG材料是一种优异的TC吸附剂。
基于三维石墨烯去除水体中四环素
The removal of tetracycline in water based on 3D grapheme
-
摘要: 三维石墨烯具有较大的比表面积和独特的空间孔结构,为捕获抗生素提供大量的活性位点,能够促进抗生素在多孔网络结构的运输。本研究采用化学还原自组装方法制备了有序多孔结构的三维石墨烯(3DG),并将其应用于去除水体中的四环素(TC)。研究结果显示, TC在3DG的吸附过程同时符合伪二级动力学和Langmuir方程。在最佳吸附pH=6时,3DG对TC最大饱和吸附量达到322.58 mg·g−1,并具备良好的再生性能,经过5次吸附-解吸重复试验后, 3DG对水体中TC的去除率仍可达68%。因此,3DG是一种在环境分析领域具有良好应用前景的吸附材料。Abstract: Three-dimensional graphene can provide abundant active sites for trapping the antibiotic and promoting the transportation of antibiotic in the porous network structure based on its large surface area and interconnected porous structure. In this study, porous three-dimensional graphene aerogel (3DG) was prepared by using chemical reduction and self-assembly method, and applied to remove tetracycline (TC) from water. The results revealed that 3DG exhibited good adsorption performance towards TC, with maximum adsorption amount being 322.58 mg·g−1 at pH=6, the adsorption process of TC on 3DG accords with pseudo-second-order kinetics and Langmuir model. 3DG has good regeneration performance. After 5 repeated adsorption-desorption experiments, the removal rate was kept 68%. Thus, 3DG is a promising adsorbent in field of environmental analysis.
-
Key words:
- three-dimensional graphene /
- tetracycline /
- removal /
- regeneration
-

-
表 1 3DG−TC的动力学模型参数
Table 1. Dynamic model parameters of 3DG−TC
C0/(mg·L−1) 伪一级动力学 伪二级动力学 K1/min−1 Qe1/(mg·g−1) R2 K2/(g·mg−1·min−1) Qe2/(mg·g−1) R2 50 0.0963 80.3526 0.6686 2.817×10−3 76.9231 0.9999 C0/(mg·L−1) The Weber and Morris Model K1p/
(g·mg−1·min−1/2)K2p/
(g·mg−1·min−1/2)K3p/
(g·mg−1·min−1/2)C1
(R2)C1
(R2)C.3
(R2)50 15.68 1.99 0.35 −0.52
(0.9912)55.67
(0.9591)70.04
(0.8583)C0/(mg·L−1) Boyd Model K1d K2d C1 C2 R12 R22 50 0.0596 0.4593 −0.1862 −26.0993 0.9274 0.9543 表 2 3DG吸附TC的热力学参数
Table 2. thermodynamic parameters on Adsorption of TC
熵变化(∆S)/
(J·mol−1)焓变化(∆H)/
(kJ·mol−1)吉布斯自由能变化(∆G)/(kJ·mol−1) 328 K 318 K 308 K 298 K 288 K 56.26 10.755 −2.938 −2.849 −2.759 −2.669 −2.580 表 3 3DG吸附TC的3种等温模型参数
Table 3. Parameters of three kinds of isotherm models on 3DG adsorption of TC
等温吸附模型 参数 288 K 298 K 308 K 318 K 328 K Langmuir Qmax/(mg·g−1) 277.78 294.12 303.03 312.5 322.58 KL/(L·mg−1) 0.0843 0.095 0.1162 0.2177 0.252 R2 0.9996 0.9993 0.9996 0.9975 0.9996 Freundlich 1/n 0.5626 0.5602 0.5573 0.557 0.5537 KF/(L·mg−1) 32.544 37.211 42.246 53.464 58.119 R2 0.9715 0.9491 0.9513 0.9758 0.9736 Tempkin KT/(L·mg−1) 0.6465 0.7533 0.8802 1.1909 1.3463 bT 34.924 34.519 34.399 33.064 33.713 R2 0.9956 0.9942 0.9914 0.9969 0.997 表 4 不同吸附剂对TC的最大吸附量
Table 4. Maximum adsorption capacity for TC using different adsorbents
-
[1] HE K, BLANEY L. Systematic optimization of an SPE with HPLC-FLD method for fluoroquinolone detection in wastewater [J]. Journal of Hazardous Materials, 2015, 282: 96-105. doi: 10.1016/j.jhazmat.2014.08.027 [2] LUO B, XU D, LI D, et al. Fabrication of a Ag/Bi3TaO7 plasmonic photocatalyst with enhanced photocatalytic activity for degradation of tetracycline [J]. ACS Applied Materials & Interfaces, 2015, 7(31): 17061-17069. [3] ZHU X, LIU Y, QIAN F, et al. Preparation of magnetic porous carbon from waste hydrochar bysimultaneous activation and magnetization for tetracycline removal [J]. Bioresource Technology, 2014, 154: 209-214. doi: 10.1016/j.biortech.2013.12.019 [4] DAI Q, ZHOU J, WENG M, et al. Electrochemical oxidation metronidazole with Co modified PbO2 electrode: Degradation and mechanism [J]. Separation and Purification Technology, 2016, 166: 109-116. doi: 10.1016/j.seppur.2016.04.028 [5] OBREGON S, HERNANDEZ-URESTI D B, VAZQUEZ A, et al. Electrophoretic deposition of PbMoO4 nanoparticles for photocatalytic degradation of tetracycline [J]. Applied Surface Science, 2018, 457: 501-507. doi: 10.1016/j.apsusc.2018.06.203 [6] REN X, ZENG G, TANG L, et al. Sorption, transport and biodegradation - An insight into bioavailability of persistent organic pollutants in soil [J]. Science of the Total Environment, 2018, 610: 1154-1163. [7] LIANG J, YANG Z, TANG L, et al. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost [J]. Chemosphere, 2017, 181: 281-288. doi: 10.1016/j.chemosphere.2017.04.081 [8] WU J, ZHAO H, CHEN R, et al. Adsorptive removal of trace sulfonamide antibiotics by water-dispersible magnetic reduced graphene oxide-ferrite hybrids from wastewater [J]. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 2016, 1029: 106-112. [9] LI X, WANG Z, LI Q, et al. Preparation, characterization, and application of mesoporous silica-grafted graphene oxide for highly selective lead adsorption [J]. Chemical Engineering Journal, 2015, 273: 630-637. doi: 10.1016/j.cej.2015.03.104 [10] ROSTAMIAN R, BEHNEJAD H. A comprehensive adsorption study and modeling of antibiotics as a pharmaceutical waste by graphene oxide nanosheets [J]. Ecotoxicology and Environmental Safety, 2018, 147: 117-123. doi: 10.1016/j.ecoenv.2017.08.019 [11] LAI K C, LEE L Y, HIEW B Y Z, et al. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms [J]. Journal of Environmental Sciences, 2019, 79: 174-199. doi: 10.1016/j.jes.2018.11.023 [12] FILIP J, ANDICSOVA-ECKSTEIN A, VIKARTOVSKA A, et al. Immobilization of bilirubin oxidase on graphene oxide flakes with different negative charge density for oxygen reduction. The effect of GO charge density on enzyme coverage, electron transfer rate and current density [J]. Biosensors & Bioelectronics, 2017, 89: 384-389. [13] HUANG G, SONG X, CHEN Y, et al. Study of the effect of chemical composition on the surface wettability of three-dimensional graphene foams [J]. Chinese Chemical Letters, 2020, 31(7): 1839-1842. doi: 10.1016/j.cclet.2020.02.053 [14] ZHOU G, YE Z, SHI W, et al. Applications of three dimensional graphene and its composite materials [J]. Progress in Chemistry, 2014, 26(6): 950-960. [15] ZHANG J, WEN C Y, LI Q, et al. Electro-enhanced solid-phase microextraction of bisphenol A from thermal papers using a three-dimensional graphene coated fiber [J]. Journal of Chromatography A, 2019, 1585: 27-33. doi: 10.1016/j.chroma.2018.11.063 [16] LOSURDO M, GIANGREGORIO M M, CAPEZZUTO P, et al. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure [J]. Physical Chemistry Chemical Physics, 2011, 13(46): 20836-20843. doi: 10.1039/c1cp22347j [17] MARCANO D C, KOSYNKIN D V, BERLIN J M, et al. Improved synthesis of graphene oxide [J]. ACS Nano, 2010, 4(8): 4806-4814. doi: 10.1021/nn1006368 [18] ZHANG X, SUI Z, XU B, et al. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources [J]. Journal of Materials Chemistry, 2011, 21(18): 6494-6497. doi: 10.1039/c1jm10239g [19] 张浩, 沈宇, 田丹碧, 等. 多壳层空心MOFs材料对水体中抗生素的高效吸附 [J]. 科学通报, 2019, 64(34): 3632-3639. ZHANG H, CHEN Y, TIAN D. B, et al. Multi-shelled hollow metal-organic frameworks for optimizing the adsorption performance of antibiotic [J]. Chinese Science Bulletin, 2019, 64(34): 3632-3639(in Chinese).
[20] ZHANG X, SHEN J, ZHUO N, et al. Interactions between antibiotics and graphene-based materials in water: A comparative experimental and theoretical investigation [J]. ACS Applied Materials & Interfaces, 2016, 8(36): 24273-24280. [21] 齐梦雨, 戴媛媛, 张莹, 等. 氧化石墨烯/适配体吸附剂对四环素类抗生素的吸附性能研究 [J]. 浙江师范大学学报(自然科学版), 2020, 43(02): 168-175. QI M Y, DAI Y Y, ZHANG Y, et al. Adsorption properties of graphene oxide/aptamer adsorbents for tetracycline antibiotics [J]. Journal of Zhejiang Normal University(Natural Sciences), 2020, 43(02): 168-175(in Chinese).
[22] ZHUANG Y, YU F, MA J, et al. Enhanced adsorption removal of antibiotics from aqueous solutions by modified alginate/graphene double network porous hydrogel [J]. Journal of Colloid and Interface Science, 2017, 507: 250-259. doi: 10.1016/j.jcis.2017.07.033 [23] 祝林, 许子牧. GO/TiO2复合纳米材料对四环素的吸附作用及其再生效果研究 [J]. 安徽农业大学学报, 2019, 46(6): 995-1002. ZHU L, XU Z M. Adsorption performance of tetracycline by GO/TiO2 composites and their regeneration capacity [J]. Journal of Anhui Agricultural University, 2019, 46(6): 995-1002(in Chinese).
[24] 连丽丽, 葛佳慧, 杨旭, 等. 多孔NiO的制备及其对水中四环素的吸附 [J]. 分析测试学报, 2020, 39(8): 1006-1011. doi: 10.3969/j.issn.1004-4957.2020.08.010 LIAN L L, GE J H, YANG X, et al. Preparation of Porous NiO and Its Adsorption for Tetracycline in Water [J]. Journal of Instrumental Analysis, 2020, 39(8): 1006-1011(in Chinese). doi: 10.3969/j.issn.1004-4957.2020.08.010
[25] NODEH H R, SERESHTI H. Synthesis of magnetic graphene oxide doped with strontium titanium trioxide nanoparticles as a nanocomposite for the removal of antibiotics from aqueous media [J]. RSCAdvances, 2016, 6(92): 89953-89965. doi: 10.1039/C6RA18341G [26] HUANG B, LIU Y, LI B, et al. Effect of Cu(Ⅱ) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite [J]. Carbohydrate Polymers, 2017, 157: 576-585. doi: 10.1016/j.carbpol.2016.10.025 -



 下载:
下载: