-
黄钾铁矾类矿物作为一种典型次生矿物,在微生物湿法冶金、湿法炼锌及酸性矿山废水(Acid mine drainage, AMD)等环境广泛存在[1-3]。例如,在微生物湿法冶金领域,黄钾铁矾类矿物的生成不仅会消耗大量的三价铁浸出剂,导致生产成本增加;还会形成覆盖层,影响浸出矿物的后续再利用,导致大量浸出物废弃堆置[4-5]。而在湿法炼锌领域,黄钾铁矾类矿物为除铁的主要生成物,一方面生成量巨大,需要占用大量的土地进行堆置;另一方面,由于黄钾铁矾类矿物生成过程的共沉淀、吸附效应,会导致部分锌、铟等金属转移到黄钾铁矾类矿物废渣中,难以回收,造成资源浪费[6-8]。因此,探究绿色、经济可行的黄钾铁矾类矿物处理方式对实现黄钾铁矾类矿物减量化、资源化等有重要意义。
黄钾铁矾类矿物主要处理方法包括固定法、火法与湿法[9]。其中,固定法是将黄钾铁矾类矿物作为建筑级配材料,在与水泥、石膏等混合后固化实现减量化,但此法消纳量少,不能回收黄钾铁矾类矿物中的有价金属,因此运用不多[10]。火法是将黄钾铁矾类矿物进行单一或多元煅烧,能够回收黄钾铁矾类矿物中的有价金属,但是能耗高、渣量减量化效果不明显。湿法是采用强酸或微生物厌氧还原的方式将黄钾铁矾类矿物溶解,进行工艺组合能回收黄钾铁矾类矿物中的有价金属、硫酸盐、铁盐等,当进一步采用微生物厌氧还原处置时,其环境友好、能耗低,近年来受到广泛关注[7, 11]。在黄钾铁矾类矿物晶格中,含有Fe3+与SO42-。因此,通过还原其中的Fe3+(铁还原途径)与SO42-(硫酸盐还原途径),均能实现黄钾铁矾类矿物的溶解[12]。例如,采用硫酸盐还原细菌(Desulfovibrio vulgaris)将黄钾铁矾类矿物中的SO42-还原为S2-,能够实现黄钾铁矾类矿物的溶解(如式(1)所示)[13]。同样,采用希瓦氏菌(Shewanella putrefaciens)将黄钾铁矾类矿物中的Fe3+还原为Fe2+,亦能实现黄钾铁矾类矿物的溶解(如式(2)所示)[14]。从式(1)与式(2)可知,采用硫酸盐还原途径,每还原1 mol的黄钾铁矾类矿物,将消耗16 mol的电子;而还原同样的黄钾铁矾类矿物,采用铁还原途径,将仅需要3 mol电子,显然更具优势。
对微生物湿法冶金而言,好氧浸出多采样自养微生物如氧化亚铁硫杆菌、氧化硫硫杆菌等,导致有机底物存在往往会抑制自养微生物好氧浸出活性,因而,探究自养、铁还原微生物对黄钾铁矾类矿物处置有重要意义[15-16]。有研究发现,氧化亚铁硫杆菌在厌氧环境下,能够利用H2作为电子供体,将黄钾铁矾类矿物还原溶解,实现了通过单一菌株、控制“厌氧-好氧”环境变化,完成微生物湿法冶金中铁离子的循环利用[17-19]。由于H2在液相中溶解度较低,且H2易燃特性限制通过高压顶空方式供应氢气,因此,如何安全可靠地提高厌氧环境中氧化亚铁硫杆菌的H2可及性,是该工艺方法需要解决的关键问题。鉴于此,拟以2种典型中空纤维膜(无泡出气与微气泡出气)搭建中空纤维膜反应器(Hollow fiber membrane reactor, HfMR),探讨在强酸性环境下(pH在2左右)运用中空纤维膜向液相供应H2,并分析不同气体传质方式下氧化亚铁硫杆菌厌氧还原溶解黄钾铁矾类矿物特性,以为促进氧化亚铁硫杆菌厌氧还原溶解技术发展提供参考。
-
本研究所用的黄钾铁矾类矿物,由氧化亚铁硫杆菌[20](Acidithiobacillus ferrooxidans,At. ferrooxidans SW-02,GenBank序列号:KJ094412)在9K培养基[21]、好氧条件下,于空气恒温摇床(培养条件:30 ℃,170 r·min−1)中培养5 d后分离得到。
-
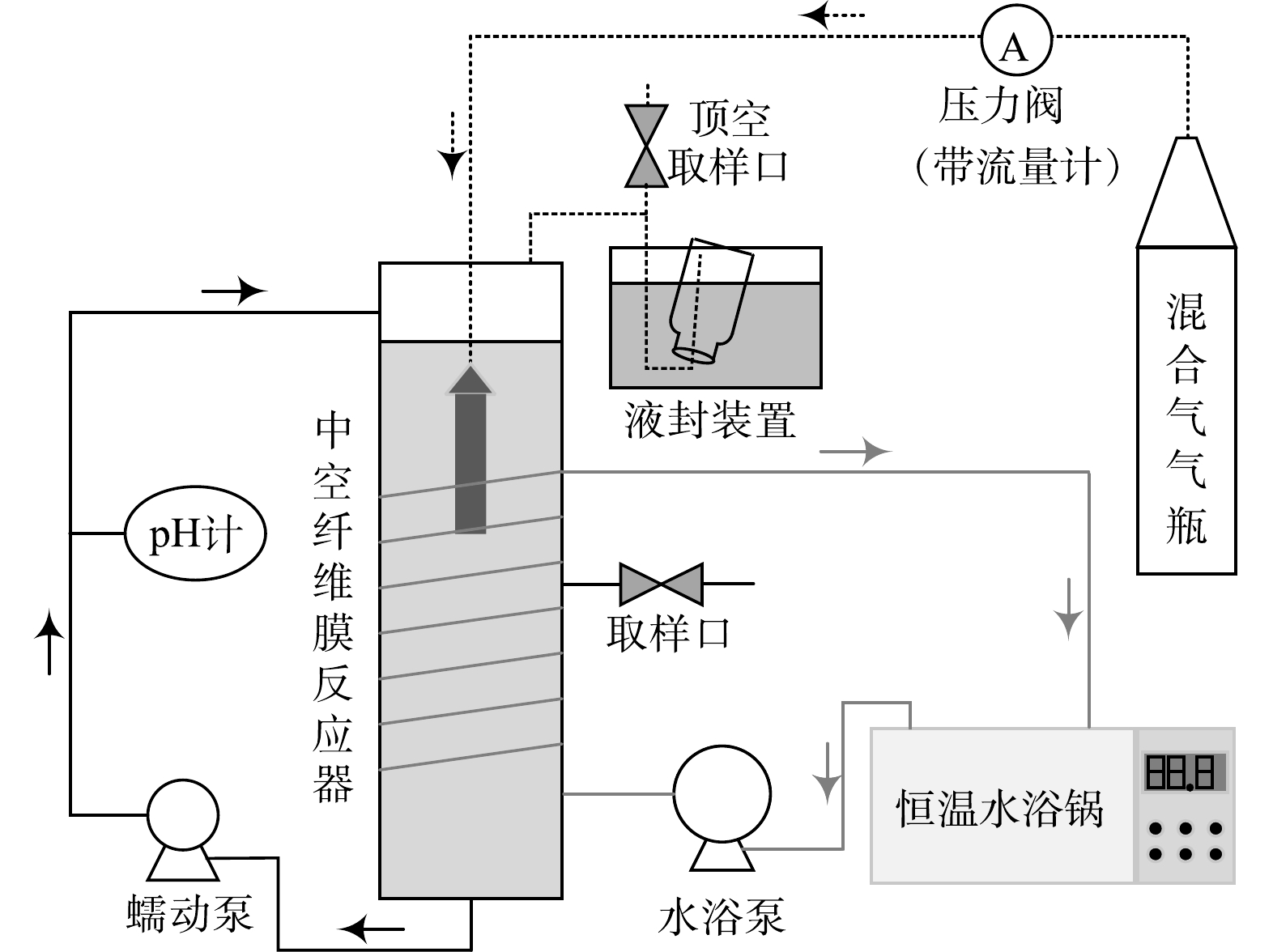
分别以中空纤维膜A(中国洁海瑞泉膜技术有限公司制造,膜丝的外径为1.3 mm,内径为0.7 mm[22])和中空纤维膜B(日本三菱集团制造,单根膜丝的外径为0.28 mm,内径为0.18 mm[23])搭建2个HfMR(编号分别为反应器 A与反应器 B,如图1所示),2个HfMR的工作体积和膜面积均为1.0 L和87.9 cm2。
-
1)细菌厌氧驯化。采用微量元素厌氧培养基[24],于120 mL血清瓶(上部顶空为70 mL的H2与CO2混合气(H2与CO2体积比为4∶1);下部工作液体为49 mL培养基与1 mL接种菌体)中厌氧驯化氧化亚铁硫杆菌(驯化条件为30 ℃、170 r·min−1),驯化电子受体采用黄钾铁矾类矿物(以Fe3+的浓度计,加入浓度为30 mmol·L−1)。不断用黄钾铁矾类矿物驯化培养细菌,待细菌在对数期后期数量稳定时(在本实验中,驯化稳定时,利用血球计数板测定的细菌对数期个数为2×107 cell·mL−1),取上清液作为接种体,用于中空纤维膜反应器黄钾铁矾类矿物还原。
2)黄钾铁矾类矿物还原溶解。分别向反应器 A与反应器 B中加入980 mL微量元素厌氧培养基(成分见表1)、20 mL厌氧细菌接种体、Fe2(SO4)3(以Fe3+的浓度计,加入浓度为12 mmol·L−1)与黄钾铁矾类矿物(以Fe3+的浓度计,加入浓度为30 mmol·L−1)。然后向反应器 A与反应器 B中通入H2与CO2混合气(H2与CO2体积比为4∶1)。其中,反应器 A的进气压力为20 kPa,反应器 B的进气压力为40 kPa(进气压力依据中空纤维膜各自耐压特性确定,采用高压气瓶供气,气体纯度为“高纯”)。还原溶解过程反应温度设为30 ℃,应器内循环流速由蠕动泵控制在200 mL·min−1。
-
1)溶解性Fe2+、Fe3+浓度分析。每天取样1 mL(经0.45 μm针头过滤器过滤)分析溶解性Fe2+、Fe3+浓度变化。其中,总溶解性铁离子(T-Fe)采用电感耦合等离子体发射光谱仪(Optima 8300美国PerkinElmer公司)测定;溶解性Fe2+采用邻菲啰啉分光光度法测定[25]。溶解性Fe3+浓度采用T-Fe浓度减去溶解性Fe2+浓度计算得到。
2) Zeta电位与矿物形貌(SEM)分析。每天取样1 mL用Zeta电位仪(Zetasizer Nano ZS90,英国Malvern公司)测定;矿物形貌变化采用场发射扫描电子显微镜(Ultra 55,德国ZEISS公司)进行分析。
3)反应器顶空气体成分分析。每天用0.5 mL气密针取反应器顶空气体用气相色谱仪(Trace 1300,美国Thermo Scientific公司)进行气体成分分析(电导检测器(ECD)。
4)细菌胞外聚合物三维荧光分析[26]。分析步骤为:先取10 mL混合液于15 mL无菌离心管中,先振荡30 s,然后在4 ℃、4 000 r·min−1液9 mL于另一只无菌离心管中,并在4 ℃、8 000 r·min−1下离心10 min,然后弃掉上清液;向离心管中加入5 mL已灭菌的厌氧培养基(pH=2.00),然后超声10 min(超声条件:200 W、40 KHz);最后,在4 ℃、11 000 r·min−1下离心10 min,取上清液采用三维荧光光谱仪(QuantaMaster-40,美国PTI公司)测定(狭缝宽度为5 nm,响应时间为0.1 s,重复扫描50次)。
-
如图2(a)所示,在前9 d中,反应器 B剩余的溶解性Fe3+的浓度低于反应器 A,且经过9 d的反应,反应器 A与反应器 B体系中加入的溶解性Fe3+均被全部还原。这说明,反应器 B还原溶解性Fe3+的速率快于反应器 A。进一步,如图2(b)所示,随着反应的持续进行,反应器 A与反应器 B的Fe2+浓度变化出现了较大的差异,反应器 B的Fe2+浓度明显高于反应器 A。由于Fe2+来源途径包括还原溶解性Fe3+与还原黄钾铁矾类矿物。考虑到2个HfMR在溶解性Fe3+还原途径差异不大(图2(a)),这说明反应器 A与反应器 B在还原溶解黄钾铁矾类矿物效率上存在不同。
为进一步分析反应器 A与反应器 B在还原溶解黄钾铁矾类矿物效率上的差异,对2个HfMR还原溶解黄钾铁矾类矿物产生的Fe2+浓度进行了估算(即总溶解性Fe2+浓度减去溶解性Fe3+(加入12 mmol·L−1)还原生成的Fe2+浓度),结果见图2(c)。可看出,反应器 B开始还原溶解黄钾铁矾类矿物的时间出现在第6 d,而反应器 A则在第9 d才出现黄钾铁矾类矿物的还原溶解。此外,反应器 B还原溶解黄钾铁矾类矿物的最大速率出现在第11 d,为5.82 mmol·(L·d)−1。与此相对应的是,反应器 A的最大速率出现在第10 d,为3.18 mmol·(L·d)−1。以上结果表明,反应器 B相比于反应器A,其还原溶解黄钾铁矾类矿物的启动时间更早,且最大还原速率亦占据优势。同时,图2(c)还展示了2个HfMR条件下氧化亚铁硫杆菌厌氧还原溶解黄钾铁矾类矿物的效率。在反应器 A中,经过16 d的反应,黄钾铁矾类矿物还原溶解的效率为53.8%,而反应器 B则达到了86.6%。
而在2个HfMR的pH变化中(图2(d)),pH变化呈现2个不同的阶段。在一阶段,即反应开始至3 d,反应器 B的pH低于反应器 A;而在第二阶段,及第4 d至反应结束,反应器 B的pH高于反应器 A。根据反应方程(式(3)与式(4))[18, 27],在反应的第一阶段,反应器 B的溶解性Fe3+还原更多,故通过还原溶解Fe3+释放的H+也越多,故其pH低于反应器 A。而在第二阶段,随着黄钾铁矾类矿物还原溶解反应的进行,反应器 B的H+消耗快于反应器 A,故其pH开始高于反应器 A。综上可知,对于还原溶解固相的黄钾铁矾类矿物,采用无泡出气传质方式的HfMR效果明显优于微气泡出气方式。
-
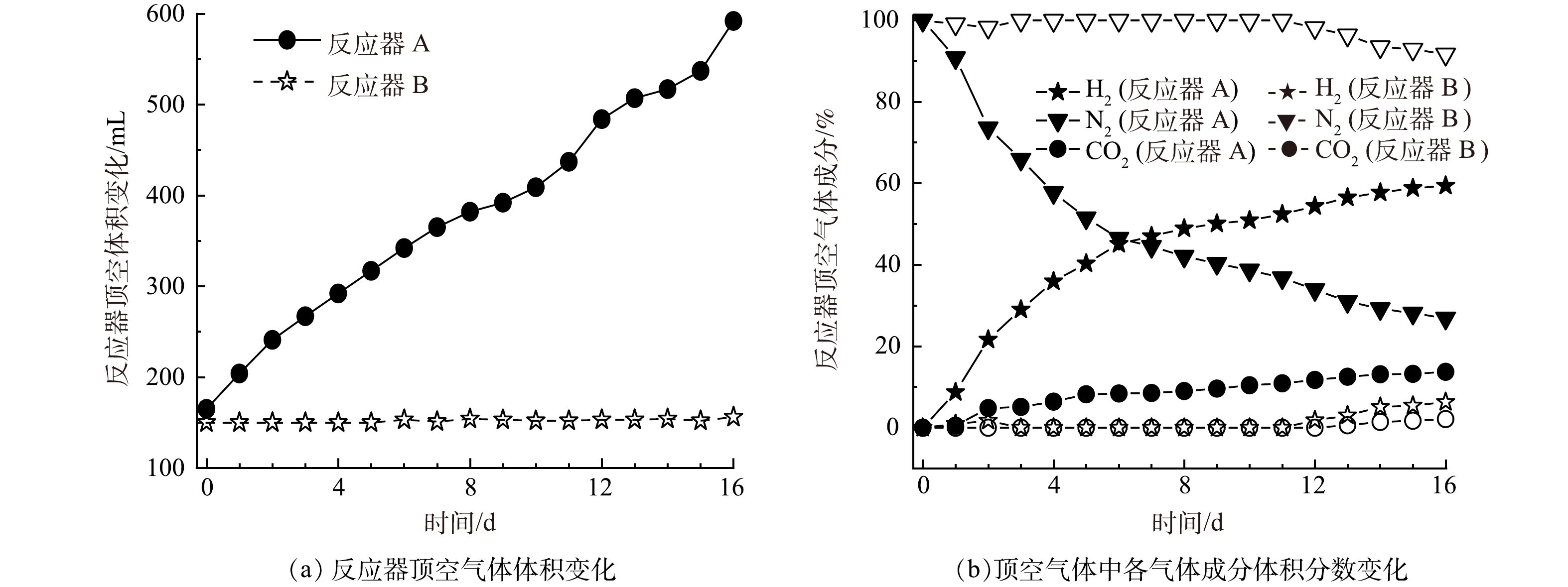
如图3(a)所示,反应器 A初始顶空气体体积约165 mL,而反应器 B初始顶空气体体积约150 mL。随着反应的进行,采用反应器 A(微气泡出气),其顶空气体明显增加,在反应结束时,顶空气体体积共约590 mL气体(不含顶空气体成分分析取样消耗的约10 mL气体);而采用反应器 B(无泡出气),其顶空气体无明显增加,在反应结束时,顶空气体体积约155 mL(不含顶空气体成分分析取样消耗的约10 mL气体)。进一步分析顶空气体成分变化(如图3(b)所示),在反应器 A中,初始顶空中的N2随着顶空气体的增加,浓度由接近100%降低至26.9%,呈现快速降低的趋势;而其H2和CO2浓度则随着反应的进行,在顶空中的浓度逐渐增加。与此相对的是,在反应器 B中,氮气浓度在前11 d无变化,而从第12 d开始才出现缓慢降低(后续检测发现膜丝存在轻微漏气)。这些结果说明,相比于反应器 A的微气泡出气,反应器 B的无泡出气能够大幅降低进入顶空的混合气量,从顶空气体难以回收再利用角度而言,反应器 B相比于反应器 A对减少顶空无效气体优势明显。
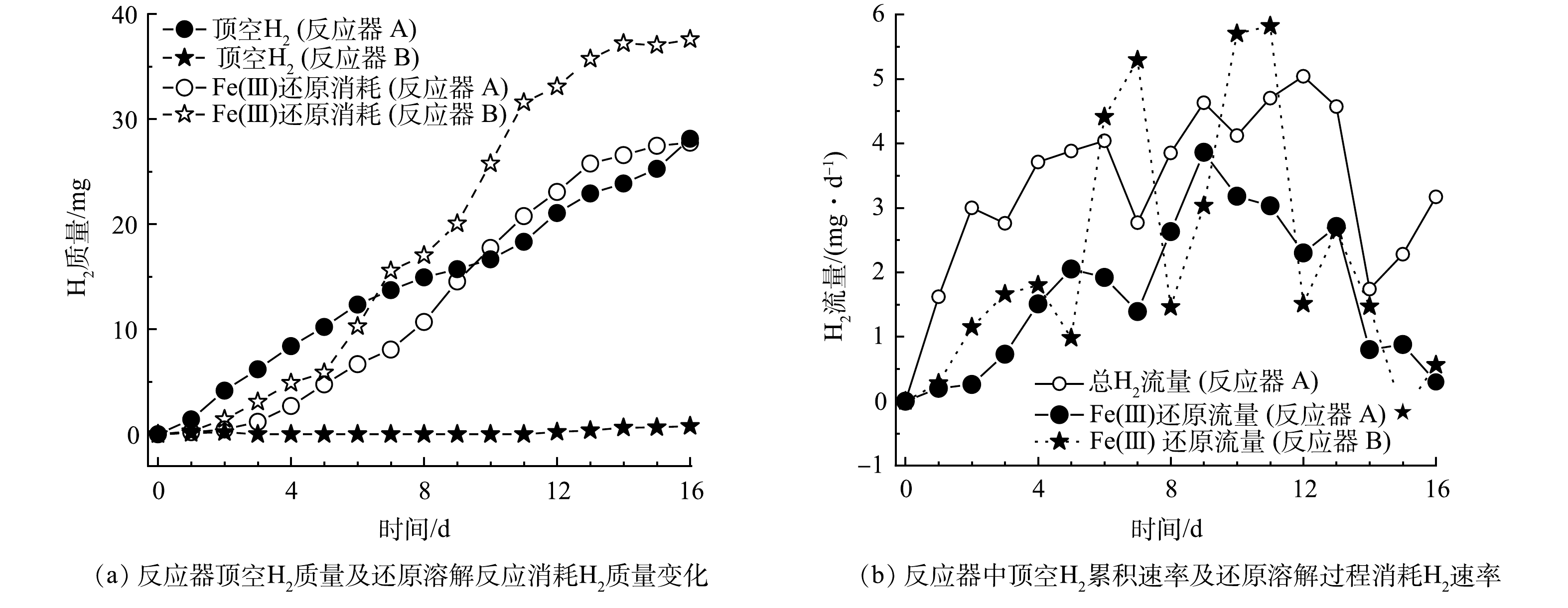
为进一步分析2种HfMR的H2传质流量,分别计算了去到顶空中的H2与作为电子供体参与还原过程消耗的H2量,结果见图4(a)。从结果上看,反应器 A条件下H2供应分为2个阶段,在前9 d,去到顶空中的H2量更多;而在后一阶段,即后7 d,参与还原反应的H2量更多。与此同时,在反应器 B中,参与还原反应的H2量明显高于去到顶空中的H2(顶空中的H2积累发生在第13 d)。
根据H2质量变化计算得到的H2流量如图4(b)所示。在反应器 A中,不管是其总的H2流量,还是用于还原反应的气体流量,变化幅度均小于反应器 B。这是因为,采用微气泡传质方式,其流量跟进气压力与膜表面的微孔尺寸有关,在本实验条件下,当进气压力一定时,进气流量相对较为稳定,且受制于微气泡在水相中停留时间短的问题,其最大H2传质流量受限,在体系H2消耗增加的情况下,其总的H2供应量并不能大幅提高。而在反应器 B中,其H2流量变化较为明显(由于反应器 B中H2几乎全部用于还原反应,故没有展示其总的H2流量)。当体系H2消耗量较低时,由于膜丝外表面水体中H2浓度较高,这时膜丝表面与水体间的传质扩散阻力较大,故H2流量较低,此时其流量低于反应器 A的H2流量;而当体系消耗H2量增加时,膜丝外表面水体中H2的浓度因消耗而大幅降低,这时膜丝表面与水体间的传质扩散阻力变小,H2流量开始明显增加,此时其H2流量又大于反应器 A的H2流量。由此可见,无泡出气的H2传质方式,相比于微气泡传质方式,或能更快响应还原溶解体系对H2的需求,从而加快黄钾铁矾类矿物的还原溶解效率,并大幅降低顶空无效H2的供应,提高H2利用率。
-
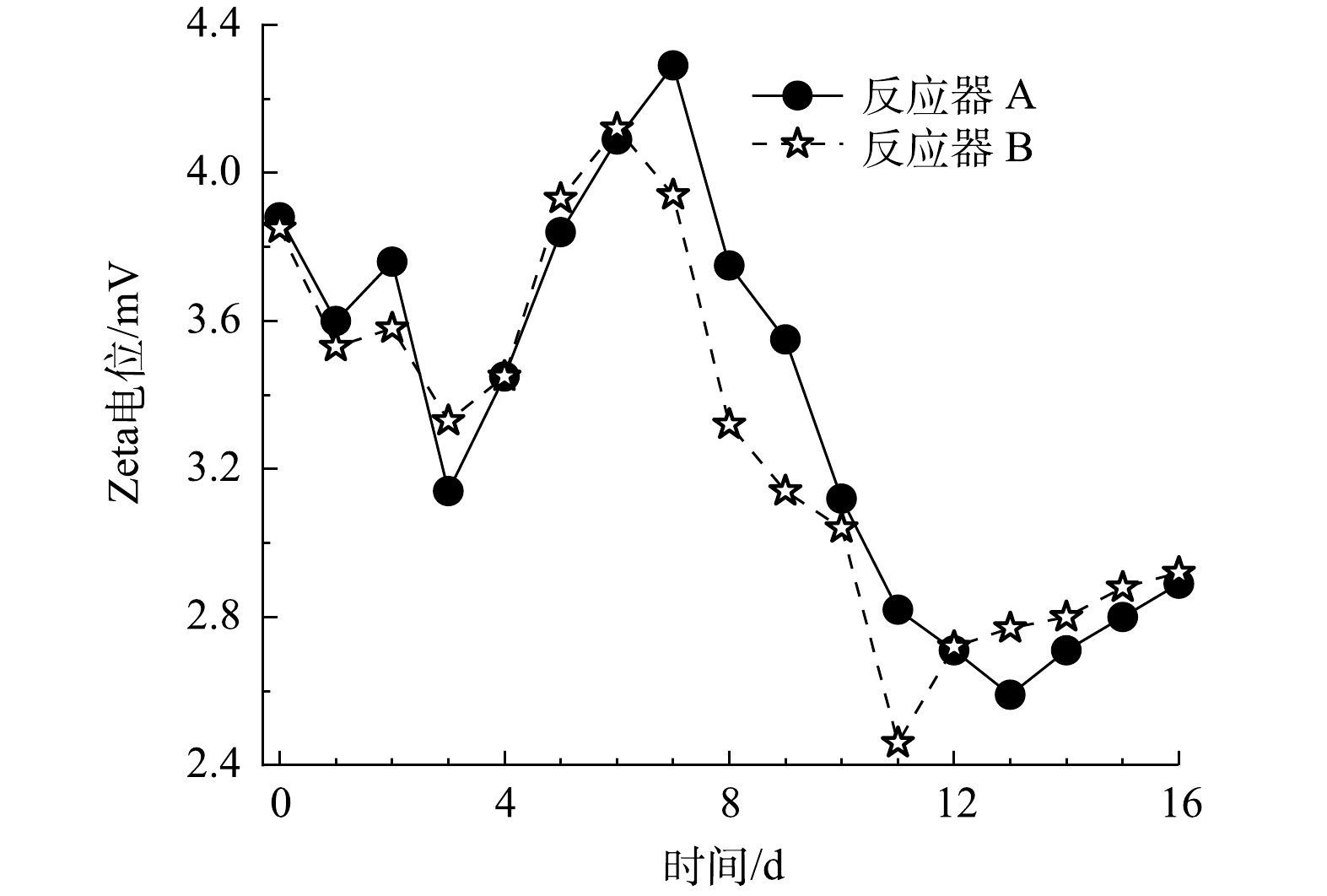
由2.1节的结果可知,无泡出气中空纤维膜加快了体系溶解性Fe3+的还原效率,而溶解性Fe3+对黄钾铁矾类矿物表面的双电层有压缩作用。因此,进一步分析HfMR条件下的Zeta电位变化,有助于深入了解黄钾铁矾类矿物的表面传质过程。2个HfMR的Zeta电位变化如图5所示。在加入溶解性Fe3+后,由于Fe3+对双电层的压缩作用,体系Zeta电位在前3 d出现持续降低;然后,紧接着在第4 d到第7 d,随着溶液中溶解性Fe3+被大量还原,体系Zeta电位开始快速升高。而在第8 d后,随着黄钾铁矾类矿物还原溶解,体系Fe2+浓度开始上升,由Fe2+产生的双电层压缩“效应”开始显现。在反应器 B中,由于其还原溶解黄钾铁矾类矿物的效率更快,Fe2+浓度更高,故其Zeta电位相比反应器 A在这一阶段降低更多。
-
2个HfMR条件下矿物形貌(SEM结果)如图6所示。在反应过程中,反应器 A中矿物表面形貌由最初的结晶状态,在第8 d变得光滑;然后,随着黄钾铁矾类矿物的还原溶解,最终变得粗糙(第16 d)。而在反应器 B中,其反应结束时的形貌与反应器 A没有明显差异。但是,在第8 d,其表面形貌与反应器 A存在明显差异。从反应器 B黄钾铁矾类矿物的形貌变化特征上看,其可能的原因或与不同供气方式改变了氧化亚铁硫杆菌生长代谢过程有关。
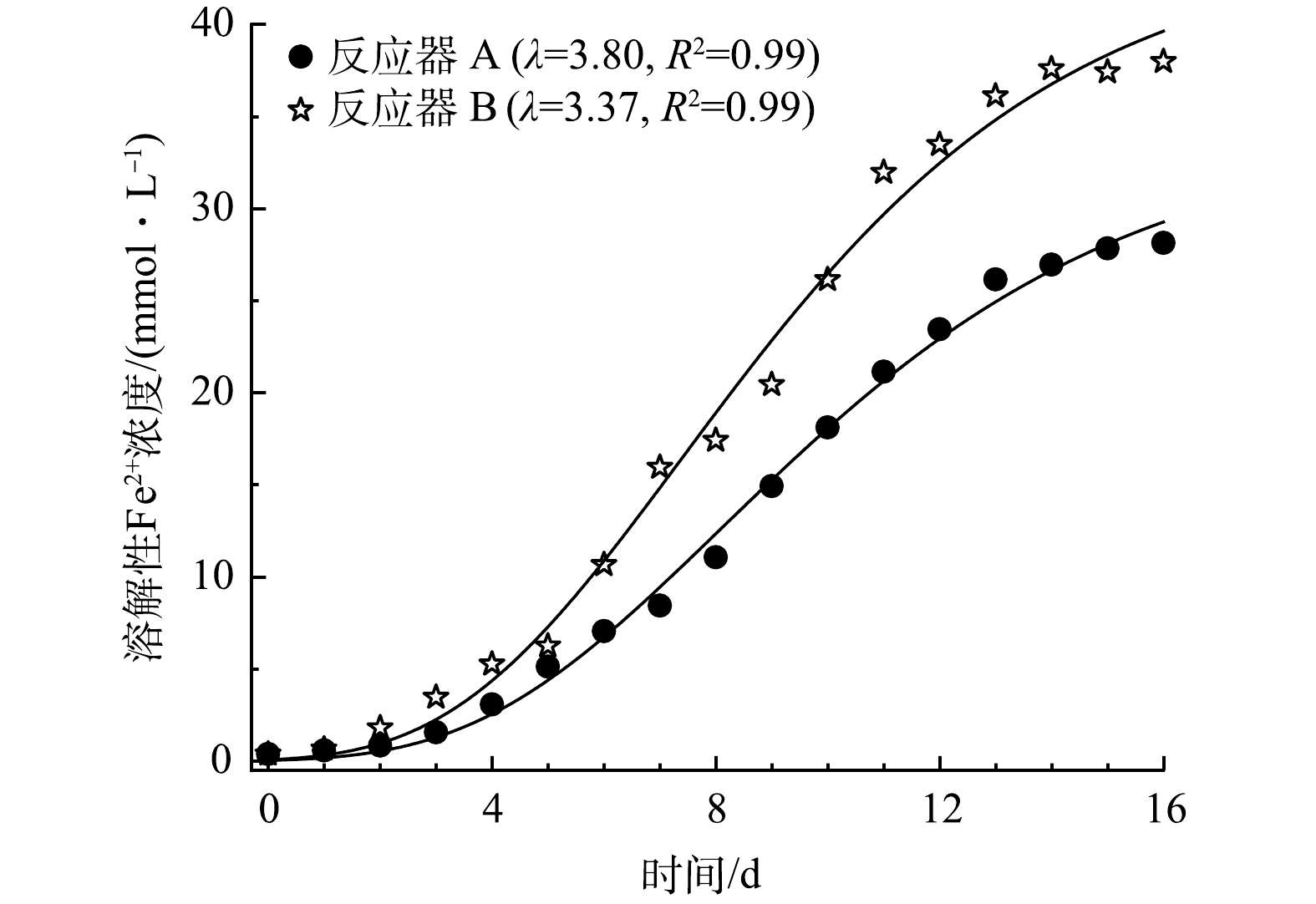
为评估HfMR条件下细菌的生长特征,采用修订的冈珀茨模型(式(5))对细菌代谢产生溶解性Fe2+的过程进行了拟合[28-29],结果如图7所示。从图上可以看出,采用反应器 B(无泡出气方式)供应H2,其生长迟滞时间最短,为3.37 d,高于反应器 B的3.8 d。这说明,采用无泡出气方式供应H2,能够降低细菌获取H2的难度,缩短其生长迟滞期,进而快速进入还原溶解过程。
式中:y代表Fe2+在时间t的浓度,mmol·L−1;A代表最高的Fe2+浓度,mmol·L−1;μm代表Fe2+最大的产生速率,mmol·(L·d)−1;λ代表迟滞时间,d;t代表反应时间,d。
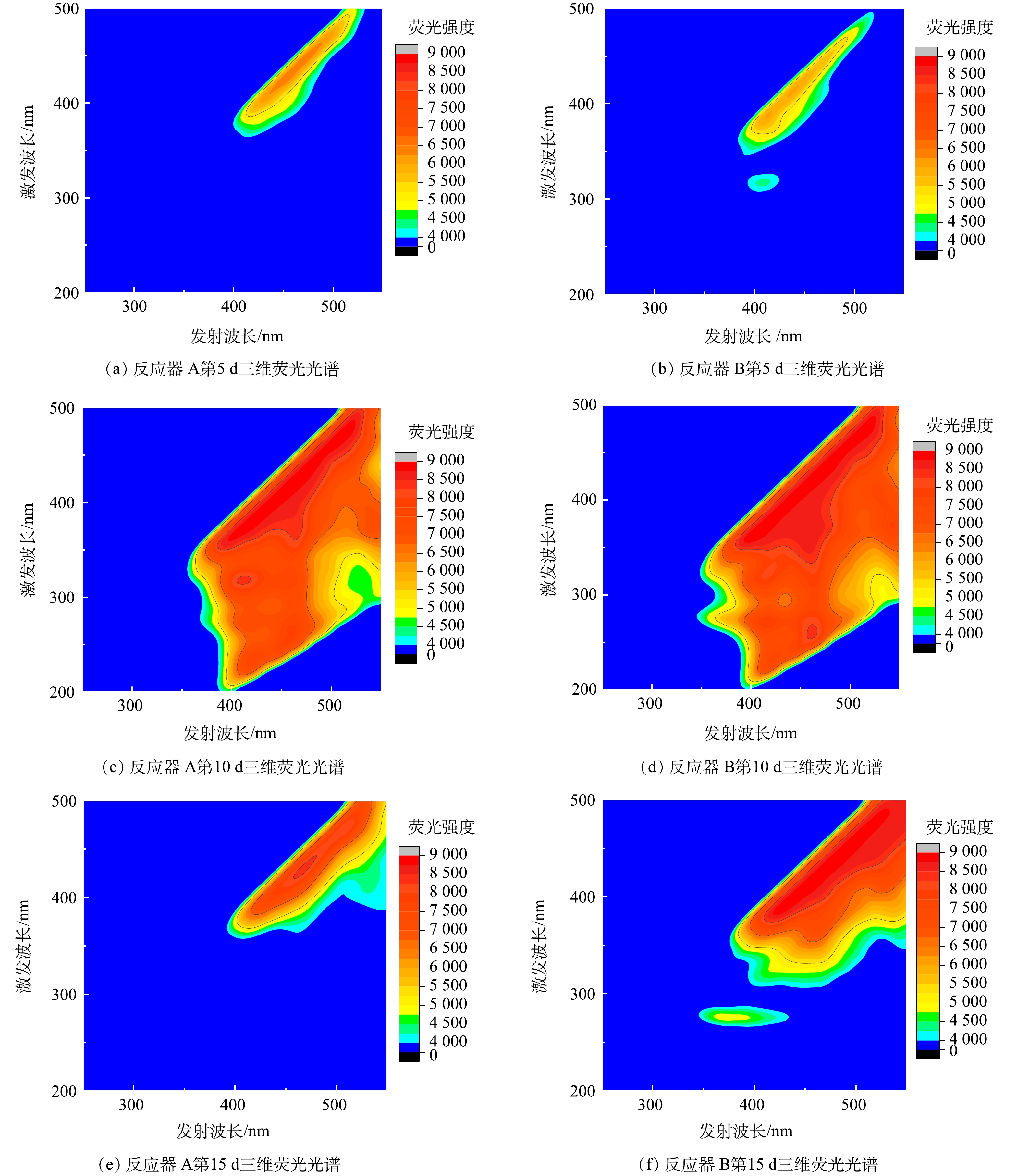
此外,对HfMR条件下氧化亚铁硫杆菌分泌的胞外聚合物的三维荧光光谱特性进行了分析,结果如图8所示。从胞外聚合物三维荧光光谱结果上看,相比于反应器 A供气模式,反应器 B条件下氧化亚铁硫杆菌胞外聚合物三维荧光光谱强度更强。此外,在反应趋近结束时,反应器 B条件下的胞外聚合物三维荧光光谱强度依然高于反应器 A。这一结果说明,采用反应器 B,即无泡出气供应H2,能让氧化亚铁硫杆菌分泌更多的胞外聚合物。
-
根据前述研究结果,在采用HfMR传质H2条件下,微气泡传质方式的H2传质速率受限于表面微孔与进气压力,供体速率较为稳定。此外,H2以微气泡的形式传质,在液相中停留时间较短,其气液交换量有限。在这些因素综合影响下,微气泡传质方式不能满足“还原溶解体系H2消耗速率快速变化”这一需求,进而在一定程度上“抑制”了细菌的厌氧代谢过程。由于溶解性Fe3+“易于还原”,这种“抑制”效应对细菌还原溶解性Fe3+的不利影响并不明显。但是,当还原相对难以还原溶解的黄钾铁矾类矿物时,这一“抑制”效用开始变得十分明显,故导致黄钾铁矾类矿物的还原溶解效率较低。
而采用无泡出气方式的中空纤维膜来传质H2,由于其能更快响应还原溶解体系对H2的需求,降低了细菌获取H2的难度,从而有利于细菌的厌氧代谢过程。故细菌在无泡出气传质方式下,其生长迟滞期缩短,并能分泌更多的胞外聚合物,而这些结果明显对黄钾铁矾类矿物的还原溶解过程有利。因此,其黄钾铁矾类矿物的溶解速率较高,相比于批次实验,能够大幅缩短反应需要的时间。此外,微气泡方式相比无泡出气方式,增加了混合气消耗,不利于成本节约且其顶空气累积的H2,亦增加了后期处理过程的安全隐患。
-
1)在2种类型的中空纤维膜中,微气泡出气方式H2在液相停留时间短,细菌还原过程中H2可及性较差,且H2易在顶空累积;而无泡出气方式能够提高细菌还原过程中H2可及性,避免顶空中过度累积H2。
2)采用无泡出气方式的中空纤维膜,能够降低细菌获取H2的难度,缩短厌氧代谢生长迟滞期,并让细菌分泌更多的胞外聚合物,改善黄钾铁矾类矿物的还原溶解过程,进而显著提高黄钾铁矾类矿物还原溶解效率,最大还原溶解速率达到5.82 mmol·(L·d)−1。
中空纤维膜传质H2对氧化亚铁硫杆菌还原溶解黄钾铁矾类矿物的影响
Effect of H2 mass transfer by hollow fiber membrane on anaerobic bio-dissolution of jarosites using Acidithiobacillus ferrooxidans
-
摘要: 为强化氧化亚铁硫杆菌厌氧还原溶解黄钾铁矾类矿物过程中的H2传质,以2种典型中空纤维膜(无泡出气与微气泡出气)搭建中空纤维膜反应器,研究中空纤维膜传质H2对氧化亚铁硫杆菌厌氧还原溶解黄钾铁矾类矿物的影响。结果表明,采用无泡出气中空纤维膜,黄钾铁矾类矿物最大还原溶解速率为5.82 mmol·(L·d)−1,16 d后黄钾铁矾类矿物还原溶解量达到86.6%;而采用微气泡中空纤维膜,黄钾铁矾类矿物最大还原溶解速率为3.18 mmol·(L·d)−1,16 d后黄钾铁矾类矿物还原溶解量仅达到53.9%。进一步地,无泡出气中空纤维膜相比微气泡出气中空纤维膜,在显著降低H2消耗的同时,能够让氧化亚铁硫杆菌更易获取H2,进而分泌更多胞外有机物,提高浸出效率。本研究结果可为无泡出气中空纤维膜在氧化亚铁硫杆菌还原溶解黄钾铁矾类矿物的应用提供参考。Abstract: To improve the H2 mass transfer during the jarosites anaerobic reduction dissolution process by Acidithiobacillus ferrooxidans, two typical hollow fiber membranes, including bubble free type and microbubble type as research objects, and the characteristics of anaerobic reduction dissolution of jarosites by Acidithiobacillus ferrooxidans in the hollow fiber membrane reactor were studied. The results showed that the maximum reduction dissolution rate of jarosites was 5.82 mmol·(L·d)−1 using the bubble free type of hollow fiber membrane, and the anaerobic reduction dissolution amount of jarosites reached 86.6% after 16 days. However, the maximum reduction dissolution rate of jarosites was 3.18 mmol·(L·d)−1 when using the microbubble type of hollow fiber membrane, and the anaerobic reduction dissolution amount of jarosites only reached 53.9%. Furthermore, compared with the microbubble type of hollow fiber membrane, the H2 consumption of reduction bio-dissolution process was reduced when using the bubble free type of hollow fiber membrane, and made it easier for Acidithiobacillus ferrooxidans to get H2 and secreted more extracellular organic matter. The results of this study can provide a reference for the application of bubble free type of hollow fiber membrane in the reduction and dissolution of jarosites by Acidithiobacillus ferrooxidans.
-

-
表 1 微量元素厌氧培养基组成
Table 1. Component content of anaerobic medium
mg·L−1 成分 质量浓度 (NH4)2SO4 132 K2HPO4 41 CuSO4·5H2O 2 CoCl2·6H2O 0.5 MgSO4·7H2O 490 CaCl2·2H2O 9 MnSO4·H2O 1 Na2SeO4·10H2O 1 KCl 52 ZnSO4·7H2O 1 NaMoO4·5H2O 0.5 NiCl2·6H2O 1 -
[1] HAN H, SUN W, HU Y, et al. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy[J]. Journal of Hazardous Materials, 2014, 278: 49-54. doi: 10.1016/j.jhazmat.2014.05.091 [2] DAS G, ACHARYA S, ANAND S, et al. Jarosites: a review[J]. Mineral Processing and Extractive Metullargy Review, 1996, 16(3): 185-210. doi: 10.1080/08827509708914135 [3] 周佳兴, 董燕, 刘奋武, 等. NaBH4对施氏矿物-黄铁矾生物化学合成的影响及矿物在催化降解甲基橙中的应用[J]. 环境工程学报, 2021, 15(4): 1242-1251. doi: 10.12030/j.cjee.202010103 [4] KAKSONEN A, MORRIS C, REA S, et al. Biohydrometallurgical iron oxidation and precipitation: Part II — Jarosite precipitate characterisation and acid recovery by conversion to hematite[J]. Hydrometallurgy, 2014, 147-148: 264-272. doi: 10.1016/j.hydromet.2014.04.015 [5] 辛靖靖, 刘金艳, 伍赠玲, 等. 黄铜矿生物浸出过程中的钝化作用研究进展[J]. 金属矿山, 2018(9): 15-21. doi: 10.19614/j.cnki.jsks.201809003 [6] MARTINEZ M, SOLAN A, HIDALGO A, et al. Characterization and mobilization of toxic metals from electrolytic zinc waste[J]. Chemosphere, 2019, 233: 414-421. [7] 刘鹏飞, 张亦飞, 游韶玮, 等. 热酸浸出回收黄钾铁矾渣中有价元素[J]. 过程工程学报, 2016, 16(4): 584-589. doi: 10.12034/j.issn.1009-606X.216125 [8] 薛佩毅, 巨少华, 张亦飞, 等. 焙烧-浸出黄钾铁矾渣中多种有价金属[J]. 过程工程学报, 2011, 11(1): 56-60. [9] ASOKAN P, SAXENA M, ASOLEKAR S. Hazardous jarosite use in developing non-hazardous product for engineering application[J]. Journal of Hazardous Materials, 2006, 137(3): 1589-1599. [10] 魏甲明, 杨斌 李若贵. 西北铅锌厂152 m2流态化焙烧炉与改良黄钾铁矾法炼锌项目的创新[J]. 中国有色冶金, 2018, 47(2): 11-13. doi: 10.3969/j.issn.1672-6103.2018.02.003 [11] OUYANG B, LU X, LIU H, et al. Reduction of jarosite by Shewanella oneidensis MR-1 and secondary mineralization[J]. Geochimica et Cosmochimica Acta, 2014, 124: 54-71. doi: 10.1016/j.gca.2013.09.020 [12] 王文静, 高坤, 叶翰, 等. 希瓦氏菌还原作用下黄钾铁矾的相转变特征及其负载铬的迁移转化规律[J]. 环境科学学报, 2021, 41(4): 1323-1332. [13] ZHOU C, ZHOU Y, RITTMANN B. Reductive precipitation of sulfate and soluble Fe(III) by Desulfovibrio vulgaris: Electron donor regulates intracellular electron flow and nano-FeS crystallization[J]. Water Research, 2017, 119: 91-101. doi: 10.1016/j.watres.2017.04.044 [14] CASTRO L, BLAZQUEZ M L, GONZALEZ F, et al. Anaerobic bioleaching of jarosites by Shewanella putrefaciens, influence of chelators and biofilm formation[J]. Hydrometallurgy, 2017, 168: 56-63. doi: 10.1016/j.hydromet.2016.08.002 [15] 赵尚明, 何环, 于忠琦, 等. 嗜酸氧化亚铁硫杆菌脱煤矸石中硫影响因素的筛选及条件优化[J]. 环境工程学报, 2015, 9(9): 4585-4590. doi: 10.12030/j.cjee.20150979 [16] 王莉莉, 孙秀云, 李桥, 等. 废弃印刷线路板中铜的两步浸出工艺优化[J]. 环境工程学报, 2018, 12(1): 250-258. doi: 10.12030/j.cjee.201705115 [17] YANG Y, CHEN S, YANG D, et al. Anaerobic reductive bio-dissolution of jarosites by Acidithiobacillus ferrooxidans using hydrogen as electron donor[J]. Science of The Total Environment, 2019, 686: 869-877. doi: 10.1016/j.scitotenv.2019.06.071 [18] YANG Y, CHEN S, WANG B, et al. Effect of ferric ions on the anaerobic bio-dissolution of jarosites by Acidithiobacillus ferrooxidans[J]. Science of The Total Environment, 2020, 710: 136334. doi: 10.1016/j.scitotenv.2019.136334 [19] 杨远坤, 谌书, 陈梦君, 等. 氧化亚铁硫杆菌浸提废旧线路板铜的浸出率与时间的关系[J]. 环境工程学报, 2013, 7(6): 2322-2326. [20] YANG Y, CHEN S, LI S, et al. Bioleaching waste printed circuit boards by Acidithiobacillus ferrooxidans and its kinetics aspect[J]. Journal of Biotechnology, 2014, 173: 24-30. doi: 10.1016/j.jbiotec.2014.01.008 [21] ZHU N, XIANG Y, ZHANG T, et al. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria[J]. Journal of Hazardous Materials, 2011, 192(2): 614-619. doi: 10.1016/j.jhazmat.2011.05.062 [22] WANG H, DAI K, WANG Y, et al. Mixed culture fermentation of synthesis gas in the microfiltration and ultrafiltration hollow-fiber membrane biofilm reactors[J]. Bioresource Technology, 2018, 267: 650-656. doi: 10.1016/j.biortech.2018.07.098 [23] TANG Y, ZHOU C, Van GINKEL S, et al. Hydrogen permeability of the hollow fibers used in H-2-based membrane biofilm reactors[J]. Journal of Membrane Science, 2012, 407: 176-183. [24] PRONK J T, de BRUYN J C, BOS P, et al. Anaerobic Growth of Thiobacillus ferrooxidans[J]. Applied and Environmental Microbiology, 1992, 58(7): 2227-2230. doi: 10.1128/aem.58.7.2227-2230.1992 [25] STOOKEY L L. Ferrozine--a new spectrophotometric reagent for iron[J]. Analytical Chemistry, 2002, 42(7): 779-781. [26] YU R, ZHONG D, MIAO L, et al. Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by Acidithiobacillus ferrooxidans[J]. Transactions of Nonferrous Metals Society Of China, 2011, 21(7): 1634-1640. doi: 10.1016/S1003-6326(11)60907-2 [27] AMEND J, SHOCK E. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and bacteria[J]. FEMS Microbiology Reviews, 2001, 25(2): 175-243. doi: 10.1111/j.1574-6976.2001.tb00576.x [28] ZWIETERING M, JONGENBURGER I, ROMBOUTS F, et al. Modeling of the bacterial growth curve[J]. Applied and Environmental Microbiology, 1990, 56(6): 1875-1881. doi: 10.1128/aem.56.6.1875-1881.1990 [29] PHUKOETPHIM N, SALAKKAM A, LAOPAIBOON P, et al. Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations: Logistic and modified Gompertz models[J]. Journal of Biotechnology, 2017, 243: 69-75. doi: 10.1016/j.jbiotec.2016.12.012 -



 下载:
下载: