-
近年来,汞(Hg)作为最危险的有毒重金属之一,已引起了人们的广泛关注[1]。汞离子(Hg2+)具有生物聚集性,在自然界中,Hg2+能通过放大作用,在食物链之间不断传递,自下而上积累,营养级越高,Hg2+的浓度就越大。Hg2+还具有强毒性,进入人体以后,会破环肾脏,影响人体的呼吸系统和神经系统[2]。因此,急需开发一种有效的Hg2+选择性检测技术,并将其应用于生物科学和环境科学。
光学检测(通过荧光变化或比色变化)是最方便的测量技术。因此,近年来荧光探针因其操作简单、灵敏度高、对生物系统无损伤等特点而备受关注[3-4]。在过去的几十年里,大多数测定Hg2+的荧光探针都是基于螯合反应的荧光猝灭型探针,Hg2+充当荧光猝灭剂,然而它们往往会受到其他具有相似配位性能的金属离子的影响[5-6]。相比之下,基于Hg2+促进脱硫反应释放荧光团从而增强荧光强度的方法,就具有更好的专一性。目前,科研工作者开发了大量荧光增强型探针,并将它们用于Hg2+的选择性检测[7-12]。然而,它们有些激发/发射波长较短,有些则需要大量有机试剂作为助溶剂。因此,亟待发展发射波长长、水溶性好、灵敏度和选择性高的新型荧光增强型探针,并将其用于环境中Hg2+浓度的特异性检测。
半花菁席夫碱染料具有良好的光物理和光化学性质,如理想的激发/发射波长,大的摩尔吸收系数,其在水溶液中存在席夫碱(SB)和质子化席夫碱(PSB)两种形式,SB形式的吸收波长较短,没有荧光,而PSB形式的吸收波长较长,并且有荧光,SB形式转换为PSB形式之后,会造成体系荧光信号的增加,这非常有利于构建以半花菁席夫碱染料为荧光团的荧光探针[13-14]。基于此,本文报道了一种新型的以半花菁席夫碱染料为荧光团,与Hg2+有较高的亲和力的硫代碳酸酯基团为识别受体的荧光探针(MC-Hg)。由于硫代碳酸酯基团强的吸电子能力,探针MC-Hg的pKa值较低,在弱酸和中性条件下,主要以SB形式存在,没有荧光,当与Hg2+反应后,硫代碳酸酯离去,释放出半花菁荧光团MCF,pKa值升高,在弱酸和中性条件下,主要以PSB形式存在,有荧光,从而实现对Hg2+的选择性检测(机理示意图如图1所示)。
-
日立F-4600荧光分光光度计(日本,日立公司);岛津UV-2600紫外分光光度计(日本,岛津公司);布鲁克micrOTOF-QⅡ型质谱仪(德国,布鲁克公司)。
硫代氯甲酯苯酯、N, N-二异丙基乙胺(DIPEA)、硝酸汞均购于上海阿拉丁生化技术有限公司,其它化学试剂均为分析纯试剂。整个实验中所用水均为二次蒸馏水。
-
将探针(5.7 mg,0.01 mmol)溶于10 mL丙酮中,得到储备溶液(1.0 × 10−3 mol·L−1),密封避光保存。金属阳离子Na+、K+、Cu2+、Zn2+、Mn2+、Ni2+、Cd2+、Pb2+、Co2+、Hg2+、Mg2+、Ca2+、Al3+、Fe2+,阴离子Cl−、CO32−分别溶解在蒸馏水中以制备浓度为10−2 mol·L−1的溶液。
在10 mL比色管中依次加入25 μL探针储备液、500 μL乙醇、500 μL不同pH的磷酸氢二钠-柠檬酸缓冲液(0.2 mol·L−1),蒸馏水定容至5 mL,摇匀。移取3 mL到1 cm × 1 cm的比色皿中,分别测试荧光光谱图和紫外-可见吸收光谱图,激发波长为580 nm,激发和发射狭缝均为5 nm,扫描速度为2400 nm·min−1。
-
实验之前,将自来水和汉江水(汉中段)用微量滤膜过滤,再用磷酸氢二钠-柠檬酸缓冲液(2.0 ×10−2 mol·L−1,pH 6.4)调节实际水样的pH值。随后,在实际水样中加入不同浓度的Hg2+和探针MC-Hg,摇匀,在室温下反应3 min后,检测645 nm处的荧光强度。
-
化合物1的合成:见参考文献[15]。
化合物2的合成(图2):向25 mL的圆底烧瓶中加入30 mL二氯甲烷、硫代氯甲酸苯酯(0.4665 g,2.7 mmol)、化合物1(0.5643 g,2.7 mmol)和N, N-二异丙基乙胺(DIPEA)(0.3705 g,2.80 mmol),混合后在室温下搅拌2 h,至反应完全后,用柱色谱法纯化(体积比为石油醚:乙酸乙酯 = 10:1)。分离后旋干,得到白色固体为化合物2(0.80 g,产率85%)。1H NMR (600 MHz, CDCl3) 化学位移(δ): 7.468—7.441 (m, 4H), 7.32 (t, J = 7.4 Hz, 1H), 7.217—7.202 (m,2H), 7.155—7.146 (m,2H), 6.53 (s,1H), 1.521 (s,9H). 13C NMR (150 MHz, CDCl3) δ: 195.23, 153.72, 152.75, 148.94, 137.09, 129.80, 126.95, 122.41, 121.95, 119.48, 116.29, 80.99, 28.44. HRMS (ESI, m/z) calculated for [C18H19NO4S+H]+: 346.1113,found: 346.1575。
化合物3的合成(如图2):向25 mL的圆底烧瓶加入2 mL三氟乙酸、8 mL二氯甲烷和化合物2(0.1794 g,0.52 mmol),混合后在常温下搅拌反应2.5 h,等反应完成后,旋干。用柱色谱法纯化,得到白色的固体产物为化合物3(0.12 g,产率98%)。1H NMR (600 MHz, DMSO-d6) δ: 7.50 (t, J = 7.9 Hz, 2H), 7.36 (t, J = 7.4 Hz, 1H), 7.31 (d, J = 7.8 Hz, 2H), 7.20 (d, J = 8.8 Hz, 2H), 7.04 (d, J = 8.7 Hz, 2H). 13C NMR (150 MHz, DMSO) δ: 195.11, 153.66, 148.32, 130.35, 127.39, 124.78, 123.03, 122.23, 119.69, 116.60. HRMS (ESI, m/z) calculated for [C13H11NO2S+H]+: 246.0589,found: 246.0510。
化合物4的合成(图2):见参考文献[16]。1H NMR (400 MHz, CDCl3), δ: 10.28 (s, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.26—7.24 (m, 1H), 6.99 (t, J = 8.0 Hz, 1H), 6.77 (d, J = 4.0 Hz, 1H), 5.56 (d, J = 6.0 Hz, 1H), 3.81 (q, J = 8.0 Hz, 2H), 2.63 (t, J = 4.0 Hz, 2H), 2.53 (t, J = 4.0 Hz, 2H), 1.81 (t, J = 8.0 Hz, 2H), 1.68 (s, 6H), 1.34(t, J = 8.0 Hz, 3H). 13C NMR (100 MHz, CDCl3), δ: 191.19, 162.14, 149.21, 144.05, 139.76, 131.84, 128.89, 128.34, 123.42, 122.30, 121.33, 107.22, 92.85, 47.04, 30.14, 28.77, 27.12, 25.01, 24.72, 21.37, 11.67. HRMS (ESI): m/z calcd for C21H25ClNO [M+H]+: 342.1625; found: 342.1613。
探针MC-Hg的合成(如图2):向50 mL圆底烧瓶中加入15 mL乙醇、化合物4(0.1671 g,0.49 mmol)、化合物3(0.1200 g,0.49 mmol)和醋酸钠(0.1805 g,2.20 mmol),在80 ℃的条件下搅拌并回流45 min。再用柱色谱法纯化(二氯甲烷:甲醇 = 50:1),得到蓝色固体(MC-Hg)为探针MC-Hg(0.25 g,产率90%)。1H NMR (600 MHz, DMSO-d6), δ: 8.54 (s, 1 H), 7.47—7.44 (m, 3H), 7.34—7.32 (m, 4H), 7.23—7.20 (m, 4H), 6.82—6.62 (m, 3H), 5.57 (d, J = 6.0 Hz, 1H), 4.12 (q, J = 8.0 Hz, 2H), 2.83 (t, J = 4.0 Hz, 2H), 2.63 (t, J = 4.0 Hz, 2H), 1.79 (t, J = 8.0 Hz, 2H), 1.69 (s, 6H), 1.36(t, J = 8.0 Hz, 3H). 13C NMR (150 MHz, DMSO-d6), δ: 189.19, 172.11, 162.16, 153.32, 151.22, 148.21, 146.02, 144.05, 139.76, 132.21, 131.84, 127.86, 127.14, 126.23, 123.42, 122.30, 121.33, 117.51, 116.32, 107.22, 104.36, 47.04, 30.14, 38.87, 28.12, 25.21, 23.62, 21.27, 13.67. HRMS (ESI, m/z) calculated for [C34H33ClN2O2S]: 568.1951,found: 568.3020。
-
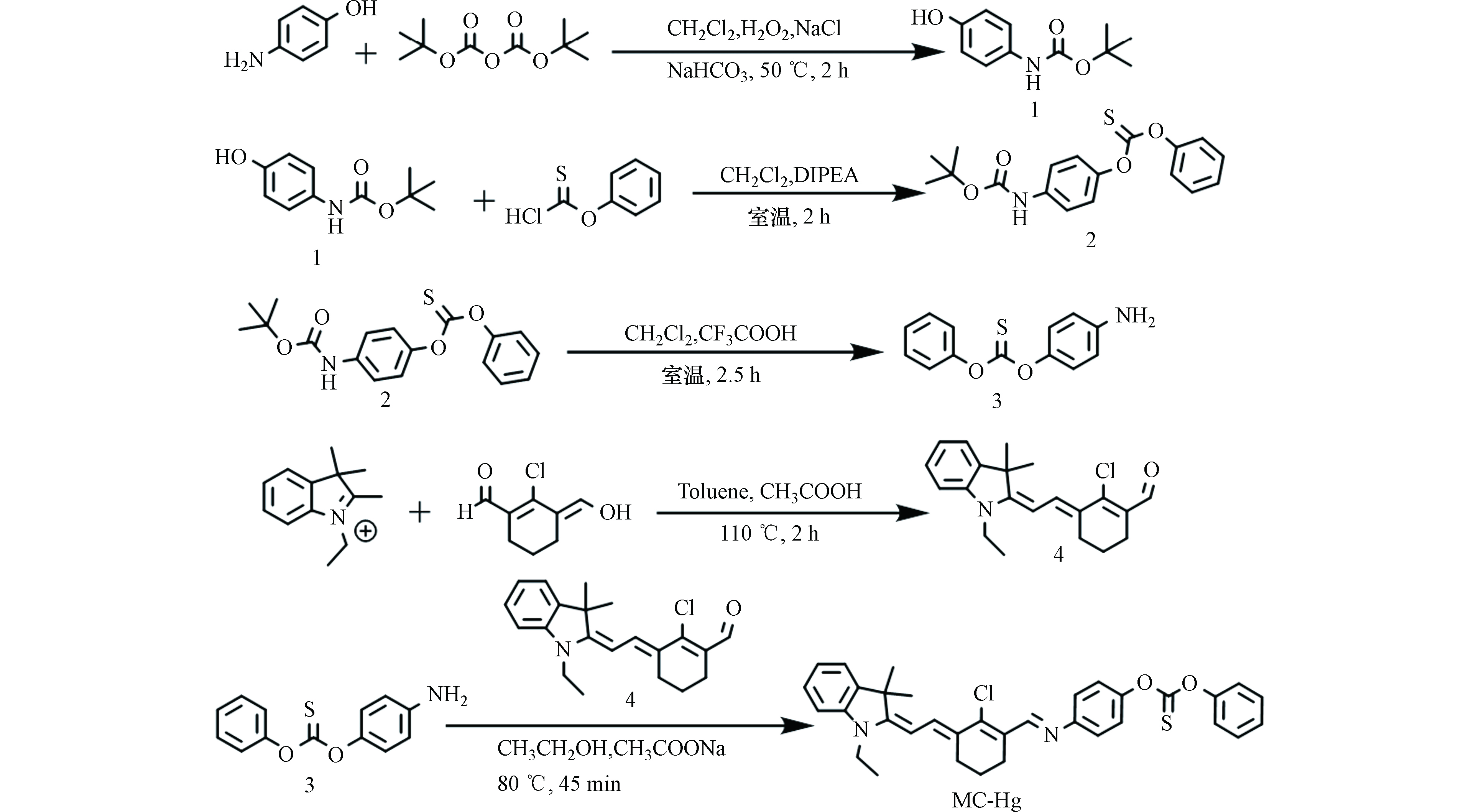
首先,本工作研究了MC-Hg在不同pH值溶液中吸收光谱的变化,结果如图3a所示。当pH ≥ 5.0时,MC-Hg主要以没有荧光的SB形式存在,最大吸收峰在467 nm处。随着溶液H+浓度的增加,MC-Hg的最大吸收峰红移至612 nm处。这一现象说明探针MC-Hg在酸性溶液中主要以具有荧光的PSB形式存在。图3b为MC-Hg在612 nm处吸收强度随H+浓度变化的趋势,可以得出MC-Hg的pKa值为4.5[17]。
研究了MC-Hg荧光光谱随pH值变化的趋势图(图4)。当pH从8.0减少到3.0时,MC-Hg溶液的荧光强度增加了183倍,这一现象表明随着溶液pH值的降低,探针从没有荧光的SB形式转变为具有荧光的PSB形式。图4b为645 nm处MC-Hg荧光强度随H+浓度变化的趋势,可以得出探针的pKa值为4.7,这与上述紫外-可见吸收光谱数据得到的pKa值基本一致。
-
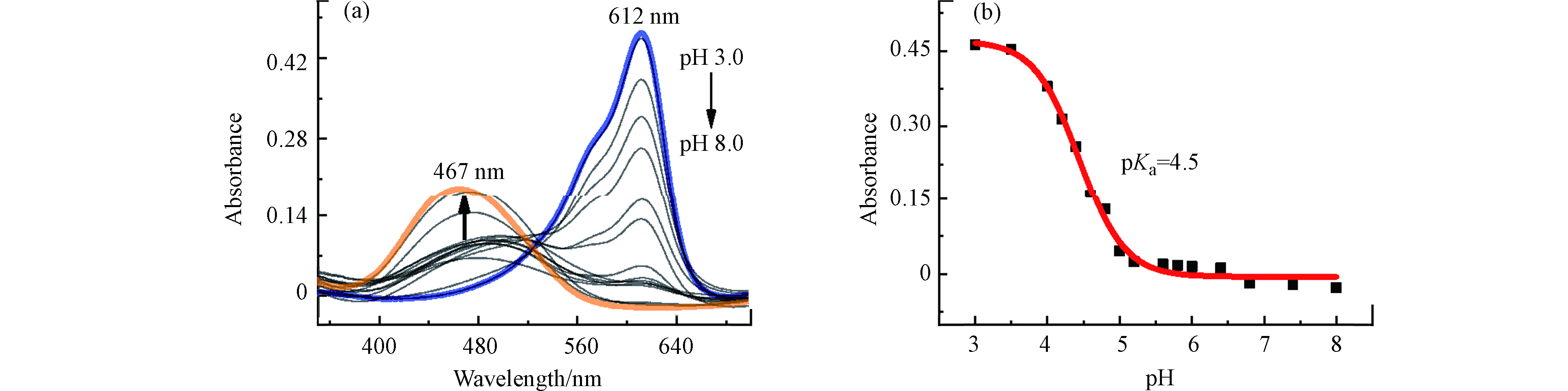
研究了溶液pH对MC-Hg与Hg2+反应的影响。如图5a所示,随着溶液pH值的增加,MC-Hg与Hg2+反应体系荧光强度的变化值先增加后减小,在pH = 6.4时达到最大值。因此,MC-Hg与Hg2+反应的最佳pH值为6.4。
研究了加入Hg2+前后,探针MC-Hg溶液的荧光强度随时间的变化。如图5b所示,探针MC-Hg的荧光强度不随时间的变化而变化,表明探针在该检测条件下具有较好的稳定性。此外,加入Hg2+后,探针MC-Hg的荧光强度迅速增加,在3 min内基本完成反应,相比之下,响应速度优于很多已经报道的荧光探针[18-21]。因此,所有测量的检测时间设置为3 min。
为了研究探针在酸性条件下的稳定性,测试了pH 6.4下,探针MC-Hg溶液的紫外吸收和荧光光谱随时间的变化。如图6所示,在80 min内,探针MC-Hg溶液的紫外-可见吸收和荧光光谱没有明显的变化,表明探针在pH 6.4条件下具有很好的稳定性。
-
考察了MC-Hg对不同浓度Hg2+的荧光响应。如图7a所示,在pH = 6.4时,MC-Hg主要以没有荧光的SB形式存在,体系的荧光很弱。随着Hg2+的加入,MC-Hg的荧光强度逐渐增大。当Hg2+的浓度高于12 × 10−6 mol·L−1时,荧光强度达到最大。此外,MC-Hg在645 nm处的荧光信号与Hg2+的浓度呈现良好的线性关系,线性范围为0.0—7.0 × 10−6 mol·L−1(如图7b所示)。
检出限(LOD)为2.7 × 10−7 mol·L−1(S/N=3)。另外我们用等摩尔法连续变化法测定了探针MC-Hg与Hg2+的化学计量关系。如图8所示,从图中可以看出反应的摩尔分数为0.5左右,这可以证明探针MC-Hg与Hg2+的反应是按化学计量关系为1: 1反应的。
-
根据文献报道,Hg2+和硫化物具有高亲和力且吸电子基团可以显著降低半花菁席夫碱染料中亚胺基团的pKa值[22]。因此,半花菁探针MC-Hg以具有强吸电子能力且可选择性识别Hg2+的碳酸酯基团为识别受体。由于碳酸酯基团的强吸电子能力,MC-Hg的pKa值较低(如图3和图4所示),与Hg2+反应后,碳酸酯基团断裂,释放出pKa值较高的荧光团,经二氯甲烷萃取、柱色谱分离后,对所得产物进行表征:1H NMR (600 MHz, DMSO-d6), δ: 9.46 (s, 1H), 8.74 (s, 1H), 7.52 (d, J = 12.7 Hz, 1H), 7.29 (d, J = 7.3 Hz, 1H), 7.18 (t, J = 7.8 Hz, 1H), 7.07 (d, J = 8.4 Hz, 2H), 6.89—6.83 (m, 2H), 6.77 (d, J = 8.5 Hz, 2H), 6.64 (dd, J = 28.0, 9.9 Hz, 1H), 5.57 (d, J = 12.7 Hz, 1H), 3.78 (q, J = 7.1 Hz, 2H), 2.67 (t, J = 6.1 Hz, 2H), 2.60 (t, J = 6.3 Hz, 2H), 1.75 (t, J = 6.2 Hz, 2H), 1.57 (s, 6H), 1.15 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, DMSO), δ: 159.21, 156.55, 155.79, 144.01, 140.08, 139.13, 129.22, 128.37, 127.47, 123.71, 122.77, 122.26, 120.56, 116.29, 115.61, 107.30, 92.78, 46.08, 36.75, 28.31, 26.88, 26.67, 21.43, 14.56. HRMS (ESI): m/z calcd for C27H29ClN2O [M+H]+: 433.2047; found: 433.2040. 实验结果表明所生成产物为MCF(该反应机理已有文献报道[8]),从而导致检测体系的吸收波长红移,荧光强度显著升高(如图1和图7所示)。
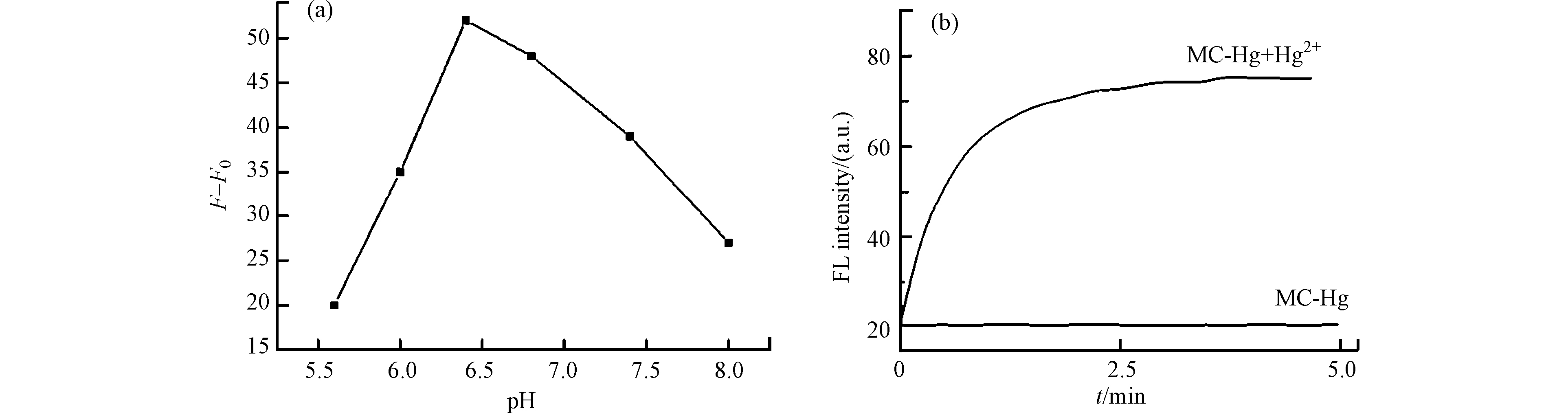
为了进一步探究MC-Hg与Hg2+的反应机理,考察了MC-Hg和Hg2+在磷酸氢二钠-柠檬酸缓冲液中(pH 6.4,2.0 × 10−2 mol·L−1)反应后,紫外-可见吸收光谱的变化。如图9所示,探针在短波长处有一个主要的吸收峰,表明它在此检测条件下主要以SB形式存在。
然而,随着一系列不同浓度的Hg2+加入,MC-Hg在612 nm处的吸收强度逐渐增加。紫外-可见吸收光谱的变化表明水溶液中PSB形式的增加,也就是说MC-Hg与Hg2+反应后,所生成荧光团MCF的pKa值升高,主要以PSB形式存在。这些结果证明MC-Hg与Hg2+高选择性反应前后,pKa值产生移动(由低到高),造成感应体系中荧光信号的增强。
-
在上述条件下,考察了MC-Hg对Hg2+的选择性(如图10所示)。
通过监测探针MC-Hg在加入其他离子后荧光发射信号的变化,研究其对Hg2+的选择性。结果表明,只有Hg2+会导致检测体系荧光强度的明显升高,其他常见离子,如Na+、K+、Cu2+、Zn2+、Mn2+、Ni2+、Cd2+、Pb2+、Co2+、Mg2+、Ca2+、Al3+、Fe2+、Cl−、CO32−,均没有明显的变化。为了进一步验证这些竞争离子对Hg2+的荧光检测没有影响,在其他离子同时存在的情况下,研究了探针MC-Hg对Hg2+的反应。从图10中可以看出,探针MC-Hg在645 nm处的荧光强度响应值只有轻微的变化。所有实验结果表明,其他竞争离子对Hg2+的检测没有或影响不大,因此,探针MC-Hg对Hg2+表现出较高的选择性。
-
利用标准加入法研究了探针MC-Hg检测实际水样中Hg2+浓度的能力[8, 23-24]。将实验室自来水和从汉江汉中段取得的水样过滤,pH值调节到6.4。将3种Hg2+浓度(1.0×10−6、2.0×10−6、4.0×10−6 mol·L−1)分别加入到实际水样中,然后加入MC-Hg,反应3 min后进行检测。结果如表1所示,用上述方法检测水样中的Hg2+浓度时,回收率均不低于96%,表明该探针MC-Hg可实现水样中Hg2+的定量检测,回收率良好.
最后,将MC-Hg检测Hg2+的特性与已报道的Hg2+荧光探针相比较。结果表明,半花菁荧光探针MC-Hg对Hg2+的检测具有响应速度快、水溶性好的特点(表2)[25-29],但该方法的检出限还需在后续的工作中继续改进。
-
结果表明,本文成功制备了一种合成步骤简单、成本低的新型荧光探针MC-Hg,其对Hg2+的检测具有较高的灵敏度和选择性。相关竞争离子对Hg2+检测的干扰很小或不存在,说明硫代碳酸酯基团可以选择性地识别Hg2+。此外,在实际应用中,探针MC-Hg对环境水样中Hg2+的测定具有很好的回收率。以上这些显著的优点表明MC-Hg在环境样品Hg2+的检测方面具有良好的应用前景。
荧光恢复型半花菁荧光探针的构建及其在水样中汞离子检测中的应用
Construction of a merocyanine-based turn-on fluorescent probe and its application in the detection of mercury ion in water sample
-
摘要: 汞离子(Hg2+)对人体有很大的危害,对Hg2+的检测具有重要意义。本文构建了一种荧光恢复型半花菁荧光探针MC-Hg,该探针以半花菁席夫碱类染料为荧光团,硫代碳酸酯为识别基团,可实现水溶液中的Hg2+的测定。含有硫代碳酸酯基团的MC-Hg具有较低的pKa值,在弱酸和中性条件下,主要以席夫碱(SB)形式存在,没有荧光。然而,当与Hg2+反应后,硫代碳酸酯离去后,释放出半花菁荧光团,pKa值升高,在弱酸和中性条件下,主要以质子化席夫碱(PSB)形式存在,荧光增强,从而实现对Hg2+的检测。实验研究了探针对Hg2+的响应性能,考察了pH和干扰离子对探针MC-Hg与Hg2+响应性能的影响。结果表明,探针MC-Hg具有良好的稳定性和水溶性,对Hg2+的检测具有响应速度快、选择性高的特点。在pH值为6.4时,荧光探针MC-Hg对Hg2+的响应效果最佳。随着Hg2+的加入,检测体系在645 nm处的荧光强度逐渐增强,与Hg2+浓度呈良好的线性关系,线性范围为0.0—7.0×10−6 mol·L−1,且检测体系在612 nm处形成新的吸收峰,并逐渐增强。此外,探针MC-Hg具有潜在的应用价值,已成功应用于实际水样中Hg2+的测定。Abstract: Mercury ion (Hg2+) is very harmful to human health, so it is of great significance to detect Hg2+. In this paper, a new merocyanine-based fluorescent turn-on probe MC-Hg was developed, which used merocyanine Schiff base dye as fluorophore and thiocarbonate as recognition group for the determination of Hg2+ in aqueous solution. MC-Hg with thiocarbonate has a low pKa value, and it mainly exists in the form of Schiff base (SB) without fluorescence under weak acid and neutral conditions. After reacting with Hg2+, its thiocarbonate group was removed to afford a merocyanine dye as the final product, whose pKa value increased, and was present mainly as the fluorescent protonated Schiff base (PSB) form under weak acid and neutral conditions, which can be utilized for the detection of Hg2+. The response of the probe to Hg2+ was studied, and the effects of pH and interference ions on the response of the probe MC-Hg to mercury ions were investigated. The results showed that the probe MC-Hg had good stability and water solubility, and show fast response and high selectivity toward Hg2+. Furthermore, the fluorescence probe MC-Hg showed the best response to mercury ions at pH 6.4. With the addition of Hg2+, the fluorescence intensity of the detection system at 645 nm gradually increased linearly with Hg2+ concentration in the range of 0.0—7.0× 10−6 mol·L−1, and the UV absorption value formed a new peak at 612 nm, and also gradually increased. In addition, the probe MC-Hg has potential application and has been successfully applied to the determination of Hg2+ in real water samples.
-
Key words:
- mercury ion /
- fluorescent probe /
- merocyanine /
- high selectivity /
- water samples
-

-
图 5 加入Hg2+前后的MC-Hg溶液荧光强度的差值随pH值的变化图(a);探针MC-Hg和加入Hg2+的MC-Hg溶液的荧光强度随时间的变化图(b);
Figure 5. (a) Fluorescence intensity of MC-Hg solution added with Hg2+ varied with pH value where F0 and F are the fluorescence intensity of the probe before and after adding Hg2+; (b) Time-coursed fluorescence responses of probe MC-Hg to Hg2+;
表 1 实际水样中Hg2+浓度的检测
Table 1. Analysis of Hg2+ concentrations in real water samples
水样
Water samples加入Hg2+(×10−6)/(mol·L−1)
Hg2+ spikeda回收Hg2+(×10−6)/(mol·L−1)
Hg2+ recovered回收率/%
Recovery汉江水
Hanjiang River0.0 未检出 1.0 1.02 102 2.0 1.96 98.0 4.0 3.93 98.3 自来水
Tap water0.0 未检出 1.0 0.96 96.0 2.0 2.01 101 4.0 3.97 99.3 a3次测量结果的平均值. aMean of three determinations. 表 2 检测Hg2+的荧光分析方法对比
Table 2. A comparison of fluorescent methods for detection of Hg2+
材料
Materials线性范围/
(μmol·L−1)
Linear range检出限/
(nmol·L−1)
Detection limit响应时间/
min
Response time激发/发射
波长/nm
λex/λem溶剂
Solvent文献
References基于花菁染料的荧光探针 1.5—7.5 7.3 5.0 600/790 乙醇/水
(5/95,V/V)[25] 基于半花菁染料的荧光探针 2.0—10.0 320 5.0 630/710 乙腈/水
(1/99,V/V)[26] 基于半花菁染料的荧光探针 3.0—5.5 180 5.0 587/708 二甲基亚砜/水(2/8,V/V) [27] 基于罗丹明B染料的荧光探针 1.0—20.0 330 15.0 530/583 乙醇/水
(2/8,V/V)[28] 基于苯并噻唑染料的荧光探针 5.0—100.0 310 30.0 405/525 乙腈/水
(1/1,V/V)[29] 基于半花菁染料的荧光探针 0.0—7.0 270 3 580/645 丙酮/水
(1/99,V/V)本工作 -
[1] GHARAMI S, AICH K, PATRA L, et al. Detection and discrimination of Zn2+ and Hg2+ using a single molecular fluorescent probe [J]. New Journal of Chemistry, 2018, 42(11): 8646-8652. doi: 10.1039/C8NJ01212A [2] 张云苹. 水中重金属离子的检测及其应用研究[D]. 济南: 济南大学, 2016. ZHANG Y P. Research on detection and application of heavy metal ions in the water[D]. Jinan: University of Jinan, 2016(in Chinese).
[3] 陈丽娟, 刘仁勇, 赵丹, 等. 聚乙烯亚胺修饰的碳点荧光探针用于检测汞离子 [J]. 分析化学, 2020, 48(8): 1067-1074. doi: 10.19756/j.issn.0253-3820.191528 CHEN L J, LIU R Y, ZHAO D, et al. A novel fluorescent probe for mercury ion detection based on polyethyleneimine modified carbon dots [J]. Chinese Journal of Analytical Chemistry, 2020, 48(8): 1067-1074(in Chinese). doi: 10.19756/j.issn.0253-3820.191528
[4] ZHANG B X, ZHANG H J, ZHONG M, et al. A novel off-on fluorescent probe for specific detection and imaging of cysteine in live cells and in vivo [J]. Chinese Chemical Letters, 2020, 31(1): 133-135. doi: 10.1016/j.cclet.2019.05.061 [5] 张磊, 许森, 赵雅梦, 等. 稀土配位聚合物荧光探针的制备及其对痕量汞离子的检测 [J]. 分析测试学报, 2020, 39(12): 1527-1532. doi: 10.3969/j.issn.1004-4957.2020.12.014 ZHANG L, XU S, ZHAO Y M, et al. Preparation of a fluorescent probe based on terbium coordination polymers and its detection on trace mercury ions [J]. Journal of Instrumental Analysis, 2020, 39(12): 1527-1532(in Chinese). doi: 10.3969/j.issn.1004-4957.2020.12.014
[6] ZHU X J, FU S T, WONG W K, et al. A near-infrared-fluorescent chemodosimeter for mercuric ion based on an expanded porphyrin [J]. Angewandte Chemie, 2006, 45(19): 3150-3154. doi: 10.1002/anie.200600248 [7] 管怡晗, 黎广进, 刘盛华, 等. 汞离子比色型荧光探针的合成与性质 [J]. 环境化学, 2021, 40(8): 2544-2550. doi: 10.7524/j.issn.0254-6108.2020041201 GUAN Y H, LI G J, LIU S H, et al. Synthesis and properties of colorimetric fluorescent probe for mercury ions [J]. Environmental Chemistry, 2021, 40(8): 2544-2550(in Chinese). doi: 10.7524/j.issn.0254-6108.2020041201
[8] DUAN Q X, LV X Y, LIU C Y, et al. Dichlororesorufin-based colorimetric and fluorescent probe for ultrasensitive detection of mercury ions in living cells and zebrafish [J]. Industrial & Engineering Chemistry Research, 2018, 58(1): 11-17. [9] 张勇, 王强, 李伟, 等. 基于硫磺素T的可视化检测汞离子的荧光探针 [J]. 有机化学, 2014, 34(2): 403-408. doi: 10.6023/cjoc201309001 ZHANG Y, WANG Q, LI W, et al. Thioflavin T-based fluorescent probe for visual detection of Hg2+ [J]. Chinese Journal of Organic Chemistry, 2014, 34(2): 403-408(in Chinese). doi: 10.6023/cjoc201309001
[10] WANG Y, ZHANG L, HAN X Y, et al. Fluorescent probe for mercury ion imaging analysis: Strategies and applications [J]. Chemical Engineering Journal, 2021, 406: 127166. doi: 10.1016/j.cej.2020.127166 [11] 秦思瑶. 新型汞离子荧光探针的设计、合成及应用[D]. 杭州: 浙江理工大学, 2019. QIN S Y. The design, synthesis, and application of fluorescent probes for the detection of mercury ions[D]. Hangzhou: Zhejiang Sci-Tech University, 2019(in Chinese).
[12] WANG Z L, ZHANG Y, YIN J, et al. A novel camphor-based “turn-on” fluorescent probe with high specificity and sensitivity for sensing mercury(Ⅱ) in aqueous medium and its bioimaging application [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(33): 12348-12359. [13] ZHANG S R, WANG Q, LIU X W, et al. Sensitive and selective fluorescent probe for selenol in living cells designed via a pK a shift strategy [J]. Analytical Chemistry, 2018, 90(6): 4119-4125. doi: 10.1021/acs.analchem.8b00066 [14] CAO J F, SUN W, FAN J L. Insights into bishemicyanines with long emission wavelengths and high sensitivity in viscous environments [J]. Chinese Chemical Letters, 2020, 31(6): 1402-1405. doi: 10.1016/j.cclet.2019.10.006 [15] SERVINIS L, HENDERSON L C, ANDRIGHETTO L M, et al. A novel approach to functionalise pristine unsized carbon fibre using in situ generated diazonium species to enhance interfacial shear strength [J]. Journal of Materials Chemistry A, 2015, 3(7): 3360-3371. doi: 10.1039/C4TA04798B [16] 张晟瑞. 半花菁类荧光探针的构建及应用研究[D]. 西安: 西北大学, 2018. ZHANG S R. Development of fluorescent probes based on hemicyanines and their applications[D]. Xi'an: Northwest University, 2018(in Chinese).
[17] VALEUR B, BERBERAN-SANTOS M N . Molecular fluorescence: Principles and applications[M]. Weinheim: Wiley-VCH, [2013] [18] LI G J, WANG J L, LI D Y, et al. A Hg(Ⅱ)-specific probe for imaging application in living systems and quantitative analysis in environmental/food samples [J]. Chinese Chemical Letters, 2021, 32(4): 1527-1531. doi: 10.1016/j.cclet.2020.09.040 [19] YANG Y, SHEN R, WANG Y Z, et al. A selective turn-on fluorescent sensor for Hg (II) in living cells and tissues [J]. Sensors and Actuators B:Chemical, 2018, 255: 3479-3487. doi: 10.1016/j.snb.2017.09.180 [20] GU B, HUANG L Y, SU W, et al. A benzothiazole-based fluorescent probe for distinguishing and bioimaging of Hg2+ and Cu2+ [J]. Analytica Chimica Acta, 2017, 954: 97-104. doi: 10.1016/j.aca.2016.11.044 [21] YUAN Z H, YANG Y S, LV P C, et al. Recent progress in small-molecule fluorescent probes for detecting mercury ions [J]. Critical Reviews in Analytical Chemistry, 2022, 52(2): 250-274. doi: 10.1080/10408347.2020.1797466 [22] PUYOL M, ENCINAS C, RIVERA L, et al. Characterisation of new norcyanine dyes and their application as pH chromoionophores in optical sensors [J]. Dyes and Pigments, 2007, 73(3): 383-389. doi: 10.1016/j.dyepig.2006.01.006 [23] ZHANG D X, XU N, LI H D, et al. Probing thiophenol pollutant in solutions and cells with BODIPY-based fluorescent probe [J]. Industrial & Engineering Chemistry Research, 2017, 56(33): 9303-9309. [24] 孙雪花, 张锦婷, 赵李艳, 等. 基于氮掺杂碳量子点的制备及其对Hg2+的响应 [J]. 环境化学, 2021, 40(1): 321-326. doi: 10.7524/j.issn.0254-6108.2020061504 SUN X H, ZHANG J T, ZHAO L Y, et al. Preparation of nitrogen-doped carbon quantum dots and its response to Hg2+ [J]. Environmental Chemistry, 2021, 40(1): 321-326(in Chinese). doi: 10.7524/j.issn.0254-6108.2020061504
[25] LI G J, GUAN Y H, YE F Y, et al. Cyanine-based fluorescent indicator for mercury ion and bioimaging application in living cells [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2020, 239: 118465. doi: 10.1016/j.saa.2020.118465 [26] WANG Y, HOU X F, LI Z S, et al. A novel hemicyanine-based near-infrared fluorescent probe for Hg2+ ions detection and its application in living cells imaging [J]. Dyes and Pigments, 2020, 173: 107951. doi: 10.1016/j.dyepig.2019.107951 [27] YUAN G, LV H H, LIU H, et al. Hemicyanine-based colorimetric and near-infrared fluorescent off-on probe for Hg2+ detection and imaging in living cells and zebrafish [J]. Dyes and Pigments, 2020, 183: 108674. doi: 10.1016/j.dyepig.2020.108674 [28] ZHANG W T, YU C W, YANG M, et al. Characterization of a Hg2+-selective fluorescent probe based on rhodamine B and its imaging in living cells [J]. Molecules (Basel, Switzerland), 2021, 26(11): 3385. doi: 10.3390/molecules26113385 [29] WANG L H, CHEN H, ZHANG N N, et al. Reaction-based two novel fluorescent probes for Hg2+ detection using benzothiazole derivatives via ESIPT mechanism in aqueous solution and serum [J]. Tetrahedron Letters, 2021, 64: 152735. doi: 10.1016/j.tetlet.2020.152735 -




 下载:
下载: