-
目前,有研究[1]表明,已经有超过5 000种药物在使用后被释放到环境中,其中由于抗生素类药物及其代谢物在低浓度下仍具有一定的生物活性,未经代谢就被排出体外,长期残留在环境中会促使抗生素耐药菌和抗生素耐药基因的形成,对人体健康和生态环境有潜在影响[2-4]。许多研究[5-7]已经证实在地表水、地下水和废水等各类水体中普遍存在抗生素。同时,通过常规污水处理厂去除水中抗生素的处理效果不理想[8-10]。因此,亟需开发出更加绿色经济、稳定高效的抗生素废水处理工艺[11]。目前,电化学高级氧化工艺(electrochemical advanced oxidation process, EAOPs)被认为能够有效去除水中的抗生素[12-15]。在电芬顿(electro-Fenton, EF)体系中,气体扩散电极(gas diffusion electrode, GDE)的气-液-固三相界面结构可以促使空气迅速与溶液接触并发生还原反应,驱动体系实现H2O2的高效产生[16]。ZHANG等[17]用生物质衍生碳对GDE电极进行改性,表现出优秀的H2O2生产能力,电流效率在30 min内可达到95%。但GDE电极还原Fe3+能力较差,因此基于GDE阴极的EF体系的催化效率取决于Fe3+/Fe2+间还原循环速率[18-21]。
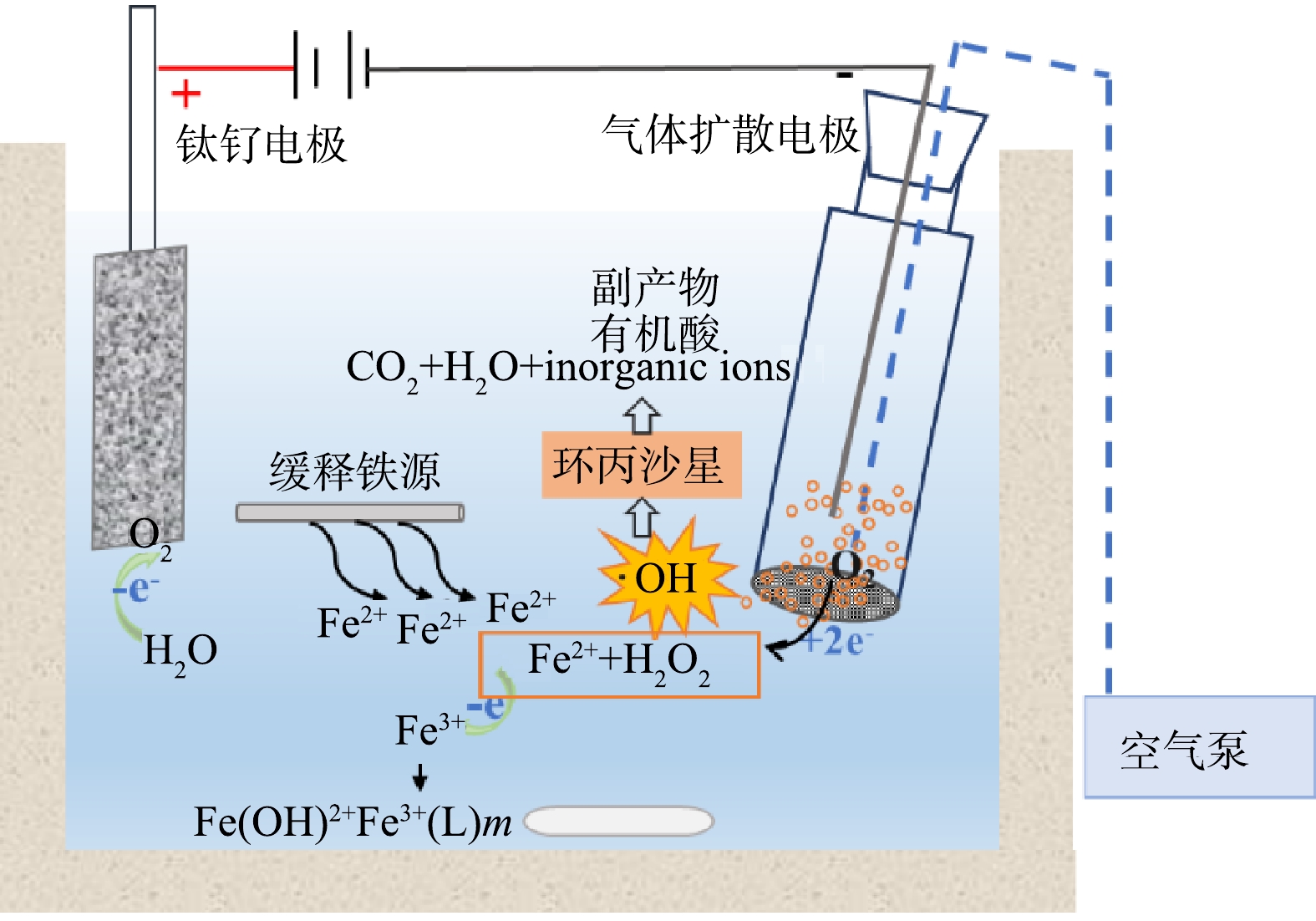
基于此,本研究提出通过引入柱状铁棒作为缓释铁源,优化铁离子投加方式,构建了缓释铁源EF体系(slow-release iron source, SRIS-EF),以实现高效同步生成芬顿反应所需Fe2+和H2O2,从而提高体系氧化能力。本研究系统考察了100 mg·L−1 CIP在电化学生成H2O2(electrochemical oxidation-H2O2, EO-H2O2)、EF和SRIS-EF 3种体系中的降解效能,对比了柱状铁棒面积、电流密度和初始pH对SRIS-EF体系降解效能的影响,解析了体系中H2O2、Fe2+和·OH的变化,结合液相色谱及离子色谱等手段,探讨了缓释铁源强化GDE电极的SRIS-EF体系催化降解环丙沙星(ciprofloxacin, CIP)的反应机理。
-
盐酸环丙沙星(C17H18O3FN3-HCl, MW 367.8)纯度为97.5%,购自上海生工生物。七水合硫酸亚铁(FeSO4·7H2O,99%)、无水硫酸钠(Na2SO4,99%)、硫酸(H2SO4,96%),氢氧化钠(NaOH,98%)和二甲基亚砜(DMSO)购自国药化学试剂公司,2,4-二硝基苯肼(DNPH)购自Aladdin。不同尺寸柱状铁棒为市购,含铁量99%,使用前用砂纸打磨去除表面氧化层。所用溶液用Millipore Milli-Q Integral 5系统制得的超纯水(18.2 MΩ·cm)配制。
-
实验装置示意图如图1所示。3种体系的实验均在单层石英玻璃反应器中进行,反应体积为150 mL,阳极为RuO2/Ti板,阴极为GDE电极,阴阳极面积均为3.0 cm2,极间距为1.0 cm。空气泵以36 L·h−1将空气通入GDE电极。EO-H2O2体系中无外加铁源,用于表征其基于电化学还原O2原位生成H2O2的氧化能力,同时可以与EF体系的降解能力进行对比。EF体系是通过外加1.0 mmol·L−1 Fe2+作为催化剂降解污染物。SRIS-EF体系中,采用柱状铁棒作为铁源持续析出Fe2+。反应体系均采用0.2 mol·L−1 H2SO4或NaOH调节溶液初始pH,反应过程中溶液利用磁力搅拌器持续搅拌(550 r·min−1)。在设定时间进行取样后使用0.22 μm PES过滤器进行过滤。
-
使用岛津U-2550紫外可见分光光度计,通过1,10-邻菲罗啉法测定体系中的Fe2+和总铁浓度,检测波长为510 nm[22]。H2O2浓度通过硫酸氧钛(TiOSO4)法测定:TiOSO4中Ti(IV)与H2O2反应形成黄色化合物,测定其在408 nm处吸光度[23]。生成H2O2的电流效率(current efficiency,CE)通过式(1)进行计算[24]。
式中:ηCE为电流效率,%;n为生成H2O2所转移的电子数;
${C}_{{\text{H}}_{\text{2}}{\text{O}}_{\text{2}}} $ 为H2O2的浓度,mol·L−1;V为溶液体积,L;I为外部施加电流,A;t为时间,s。通过DMSO-DNPH法测定体系中产生·OH的浓度[25-26]:·OH和DMSO反应后定量生成甲醛(HCHO),HCHO可与DNPH反应生成HCHO-DNPH,通过岛津Prominence LC-20AT液相色谱测定HCHO-DNPH浓度,使用Waters Xbridge Shield RP18 C-18色谱柱(5 μm,250 mm×4.6 mm),检测波长为355 nm。
利用上述的液相色谱检测CIP浓度,色谱柱相同,检测波长为278 nm。降解产生的有机酸浓度也采用液相色谱进行测定,色谱柱为BioRad Aminex HPX 87H色谱柱(300 mm×7.8 mm (I.D)),检测波长为210 nm。CIP降解过程中生成的无机离子(NO3−和F−)采用配备DS5电导检测器的DIONEX ICS-600离子色谱仪(Dionex Co., USA)测定,采用IonPac® AS23阴离子交换柱(4 mm×250 mm)和IonPac® AG23保护柱(4 mm×50 mm)。体系中生成的氨氮(NH4+-N)浓度采用苯酚钠光度法测定,检测波长为620 nm[27]。溶液中总有机碳(total organic carbon, TOC)浓度利用岛津TOC-LCSN TOC分析仪在NPOC模式下进行分析。体系的矿化电流效率(mineralization current efficiency,MCE)通过式(2)计算。
式中:ηMCE为矿化电流效率,%;F为法拉第常数,96 487 C·mol−1;Vs为溶液体积,L;ΔCTOC为TOC浓度变化,mg·L−1;4.32×107为单位转换常数;m为污染物分子中碳原子数目;I为电流强度,A;t为反应时间,h;n为电子转移数目,取78(式(3))。
-
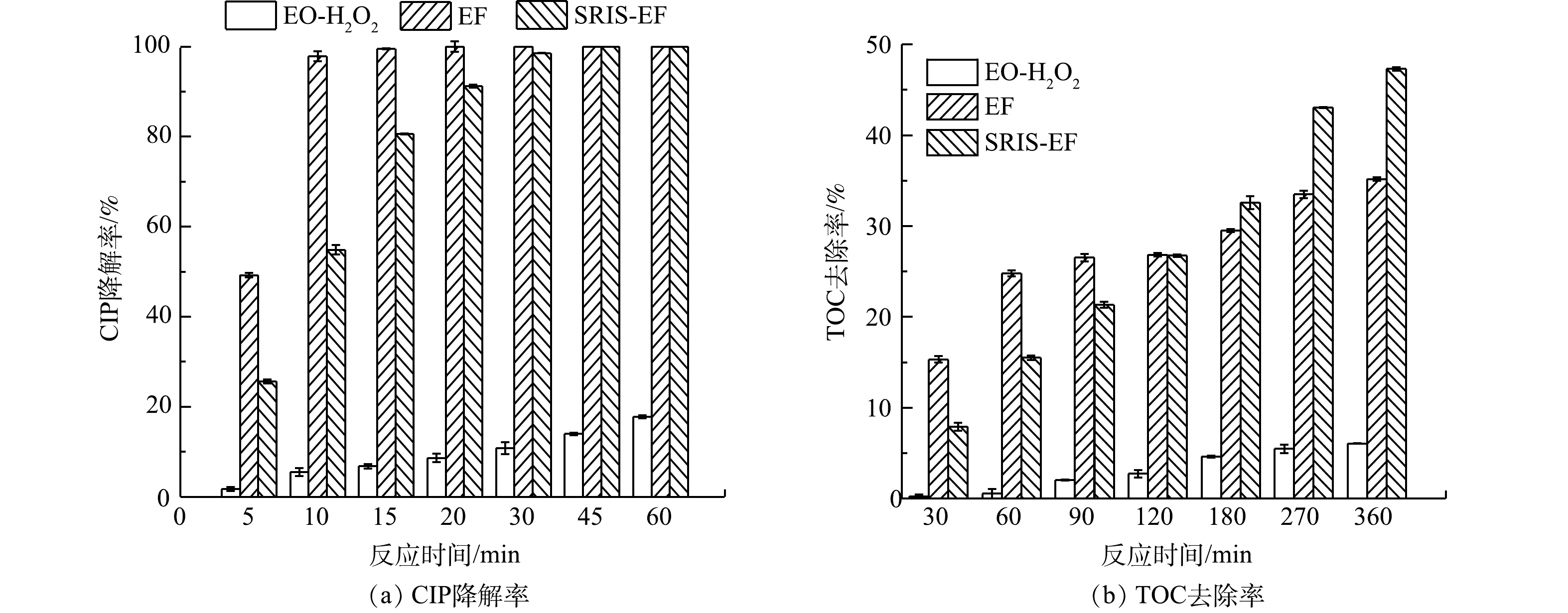
首先对比了EO-H2O2、EF和SRIS-EF对CIP降解效能的影响,结果如图2所示。对这3个体系的动力学表观速率常数进行线性拟合后发现,其降解过程均符合一级动力学,计算结果如表1所示。由于反应初期迅速产生的高浓度·OH具有极强的氧化能力,EF和SRIS-EF均能在30 min内将CIP完全降解。而在EO-H2O2体系中,反应120 min后 CIP去除率仅29%。这一现象与体系对CIP矿化能力相吻合。反应360 min后,EO-H2O2、EF和SRIS-EF体系的矿化率分别为6.1%,35.2%和47.3%。在EO-H2O2中,CIP降解率和矿化率均很低,这是由于该过程的基本没有·OH生成,体系中的H2O2氧化能力较弱,对CIP分子结构破坏能力有限。SRIS-EF体系的矿化率远高于EF体系。是由于EF体系外加Fe2+在反应初期就与电化学产生的H2O2迅速反应,而GDE阴极对Fe3+还原效果较差,抑制了芬顿反应持续发生,而在SRIS-EF体系中缓释铁源可持续析出Fe2+,确保了芬顿反应所需的Fe2+和H2O2高效产生,体系中可持续生成大量·OH,实现高效矿化CIP。
-
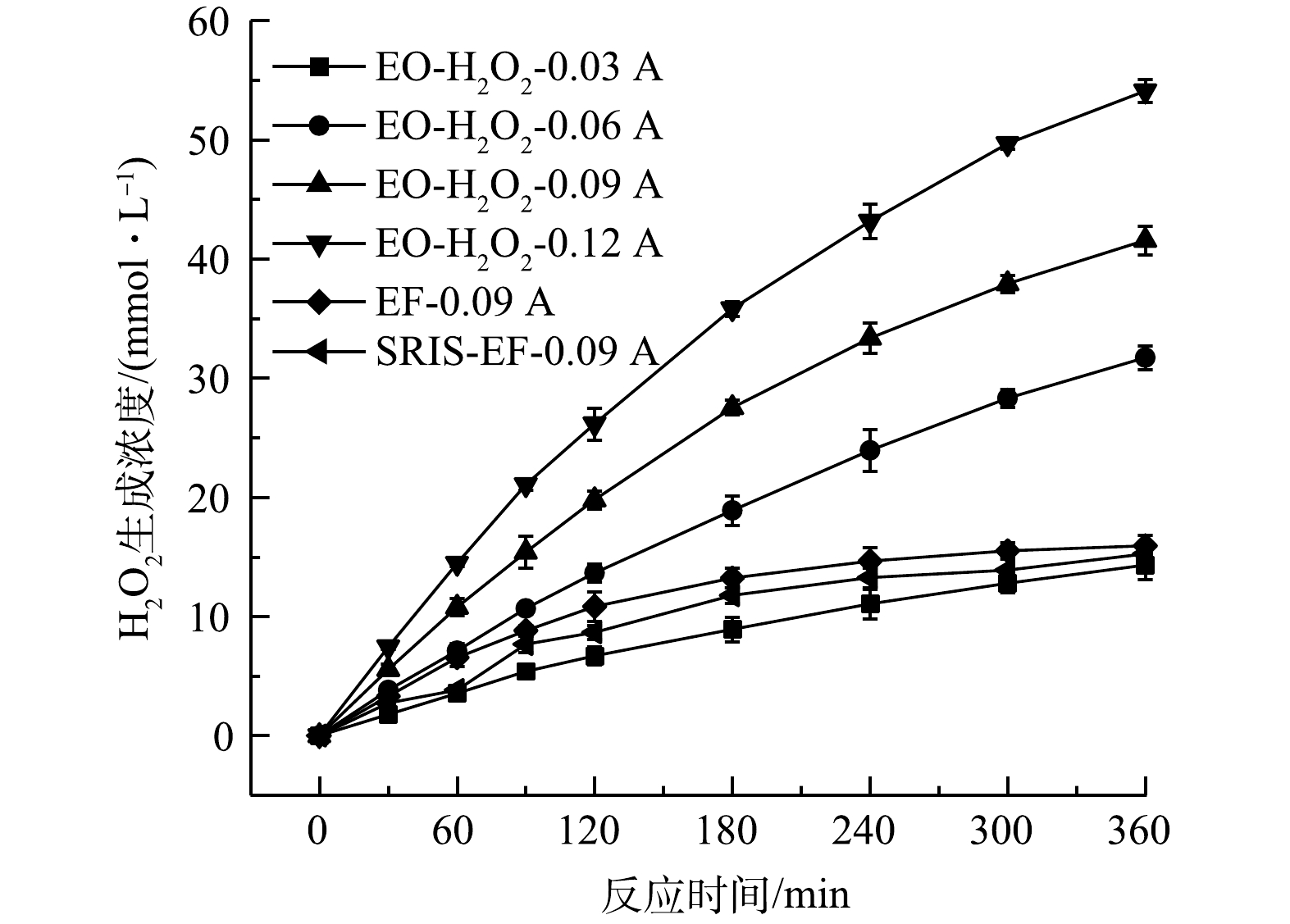
1) GDE电极H2O2生成性能。测定了不同电流条件下EO-H2O2、EF和SRIS-EF体系中H2O2的生成情况。由图3可以看出,反应360 min后,0.03、0.06、0.09和0.12 A·电流对应的H2O2产量可达14.31、31.71、41.55和54.08 mmol·L−1,对应的电流效率分别为63.93%、70.82%、61.86%和60.39%。这表明GDE阴极能有效催化2e−-ORR发生。H2O2浓度在反应初期呈线性增加,180 min后积累速率稍微减慢,这是由于电极上发生副反应会消耗部分H2O2(式(4))。当向体系中投加Fe2+或利用缓释铁棒原位产生Fe2+时,产生的H2O2会与Fe2+发生芬顿反应,H2O2浓度迅速降低。由于SRIS-EF体系中Fe2+持续析出,芬顿反应速率高于常规EF体系,SRIS-EF体系内残留H2O2浓度略低于EF体系,反应240 min后,SRIS-EF和EF体系内残留H2O2浓度趋于稳定,此时H2O2生成和分解达到相对平衡。
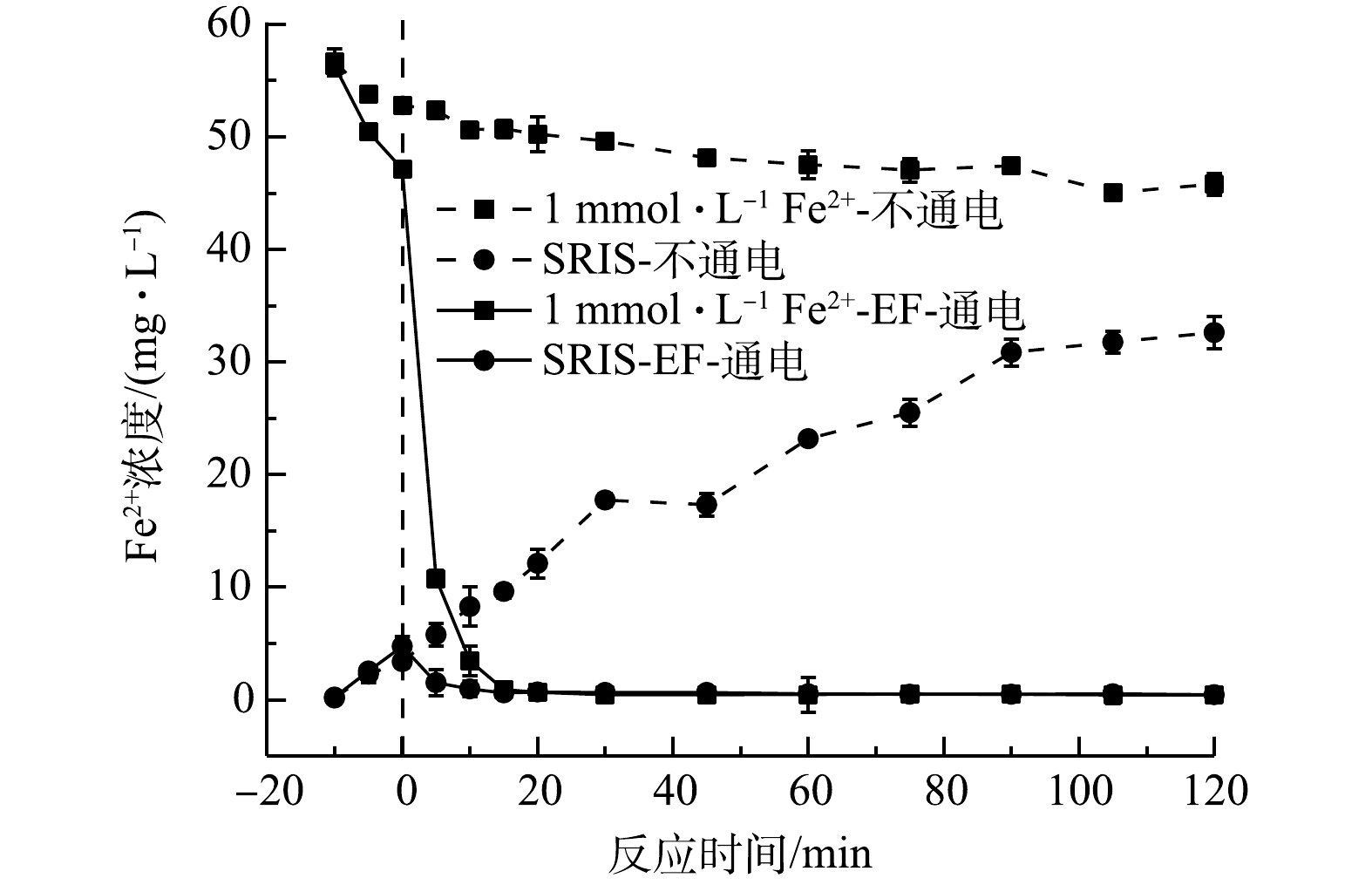
2)体系中Fe2+浓度变化。由图4可看出,在不通电时,SRIS-EF体系主要以Fe2+形式持续溶出,反应进行到120 min时,溶出的Fe2+含量为32.59 mg·L−1。在通电时,Fe2+会被快速消耗,20 min后Fe2+浓度趋于零。但SRIS-EF过程中,Fe2+溶出与消耗是一个动态过程,SRIS-EF体系中Fe2+会高效利用,持续产生·OH。而EF体系中,通电后Fe2+浓度随电解时间迅速降低,这是因为Fe2+会与电化学产生的高浓度H2O2迅速反应,Fe2+迅速转化为Fe3+,20 min后Fe2+浓度也降低为零,但这时由于GDE电极较差的Fe3+还原能力,这时芬顿反应基本停止,不再持续产生·OH。
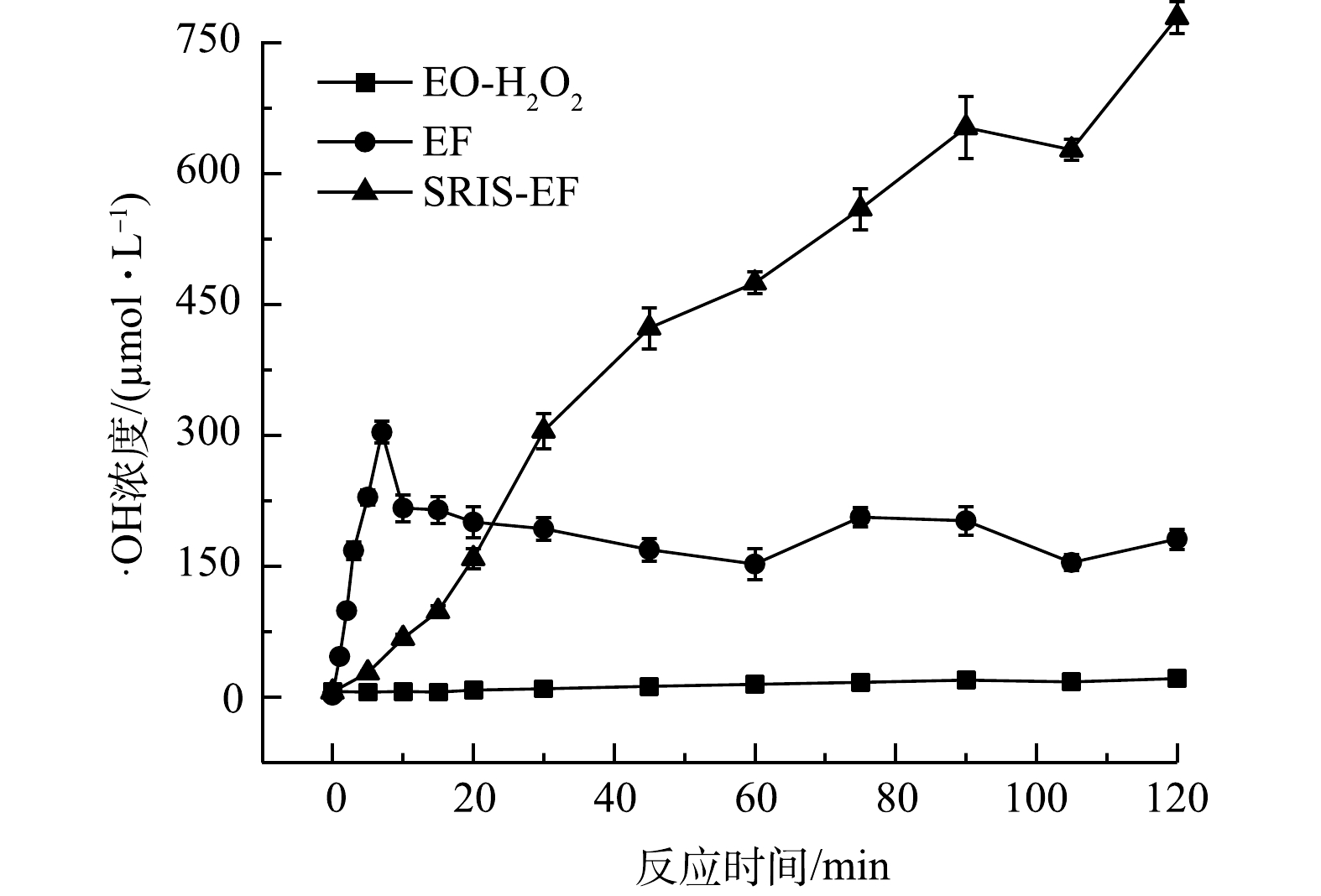
3)不同体系中·OH生成情况。体系中生成的·OH浓度决定了其氧化能力大小,也可反映不同体系之间降解效能的区别。由图5可知,EO-H2O2体系仅有少量·OH生成(21.2 μmol·L−1),表明该体系中H2O2不能有效生成·OH,氧化能力很弱。在常规EF体系,反应初期特别是前5 min内,体系中初始投加的Fe2+与电化学生成H2O2迅速反应,·OH生成速率很快。之后·OH浓度几乎不再继续增长,逐渐趋于稳定,说明Fe2+被消耗完后,Fe2+/Fe3+还原转化效率较低,不能持续发生芬顿反应产生·OH。
在SRIS-EF体系中,Fe2+可逐渐产生,持续与电化学生成的H2O2发生芬顿反应,从而保证·OH的持续生成,120 min后生成·OH浓度可达778.7 μmol·L−1,是常规EF体系生成·OH浓度的2.56倍。这是由于·OH生成速率与H2O2和Fe2+的含量成正比(式(5))[28]。本研究构建的体系通过缓释铁源提供较为稳定的亚铁离子,同时通过GDE电极可稳定生成H2O2,有助于产生高浓度的·OH。根据·OH生成情况得出各体系氧化能力顺序为EO-H2O2<<EF<SRIS-EF。
式中:C•OH是·OH浓度,μmol·L−1;k是芬顿反应的二阶速率常数,(μmol·min)−1·L;λ是·OH的平均寿命,min;CH2O2和CFe2+分别是H2O2和Fe2+浓度,μmol·L−1。
-
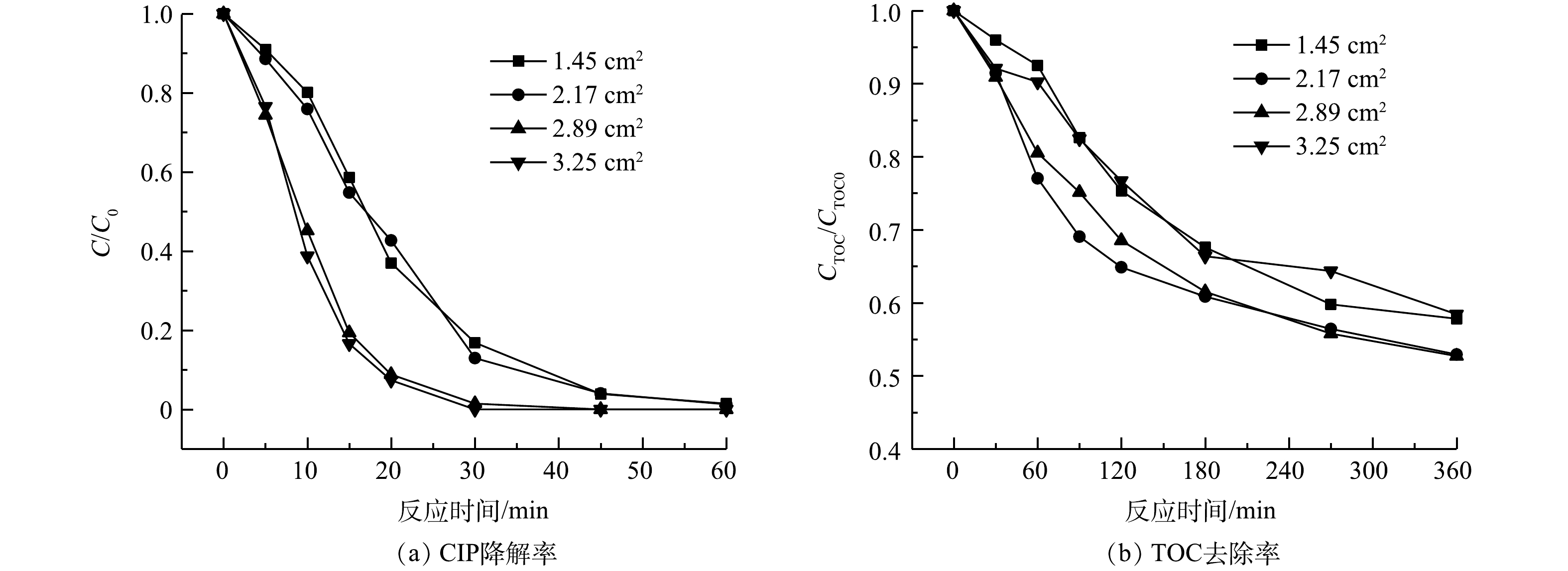
1)柱状铁棒面积。由表2可见,随着铁棒面积的增加,亚铁生成速率和H2O2利用速率逐渐提高,可加速·OH的生成。图6中的数据也证实了铁棒表面积越大,SRIS-EF体系对CIP的降解速率越高。在处理360 min后,铁棒面积分别为1.45、2.17和2.89 cm2的SRIS-EF体系中的TOC去除率分别为42.2%、47.1%、47.3%。但当铁棒面积超过2.89 cm2时,体系对CIP矿化能力受到抑制,360 min后TOC去除率为41.6%。这是由于体系中溶出过多Fe2+会消耗·OH,从而降低CIP矿化效率(式(6))[29-30]。
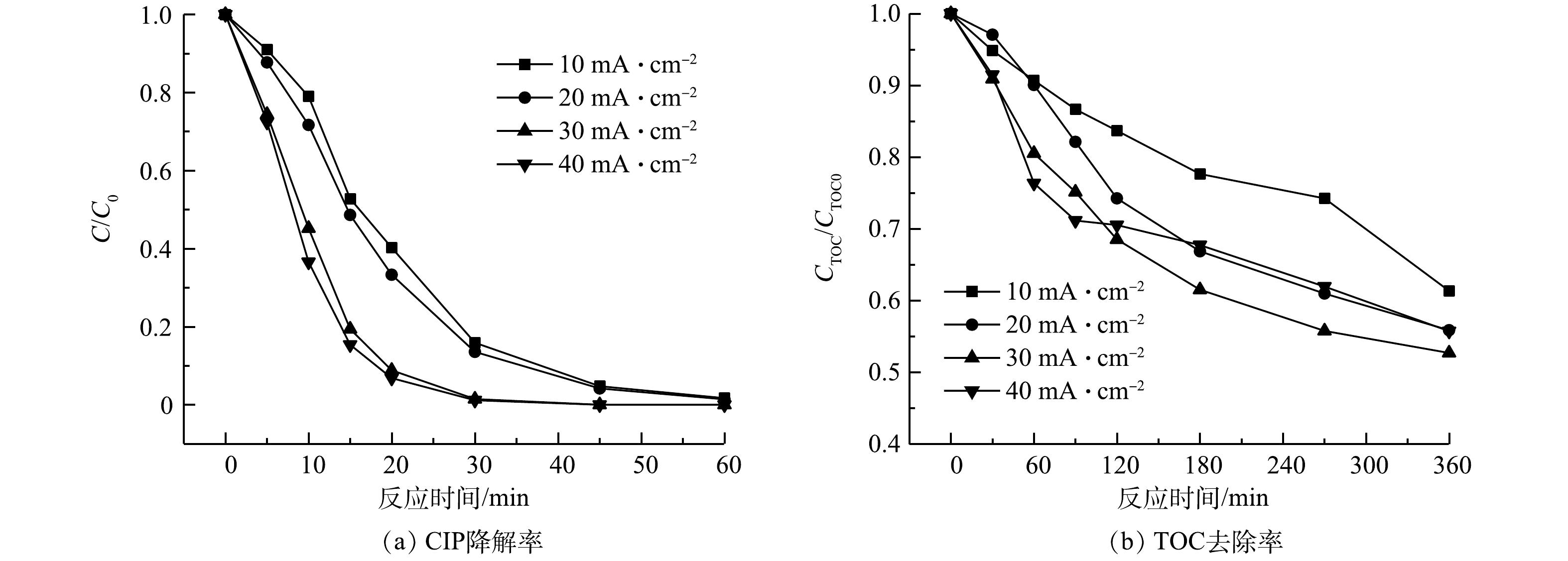
2)电流密度。图7反映了电流密度影响CIP在SRIS-EF体系中降解与矿化的情况。当电流密度从10 mA·cm−2提升到30 mA·cm−2时,H2O2产量提升为原来的2.90倍,同时体系TOC的去除率也由38.7%提高到47.3%。电流密度超过30 mA·cm−2时,电极极化反应加剧,消耗了体系内H2O2和·OH,体系生成H2O2的电流利用效率也仅为60.39%。析氢析氧等副反应增多也影响了CIP降解效果。反应360 min后不同电流密度条件下SRIS-EF体系的TOC去除率分别为38.7%、44.1%、47.3%和44.3%,表明电流密度为30 mA·cm−2时SRIS-EF的处理效果最好。
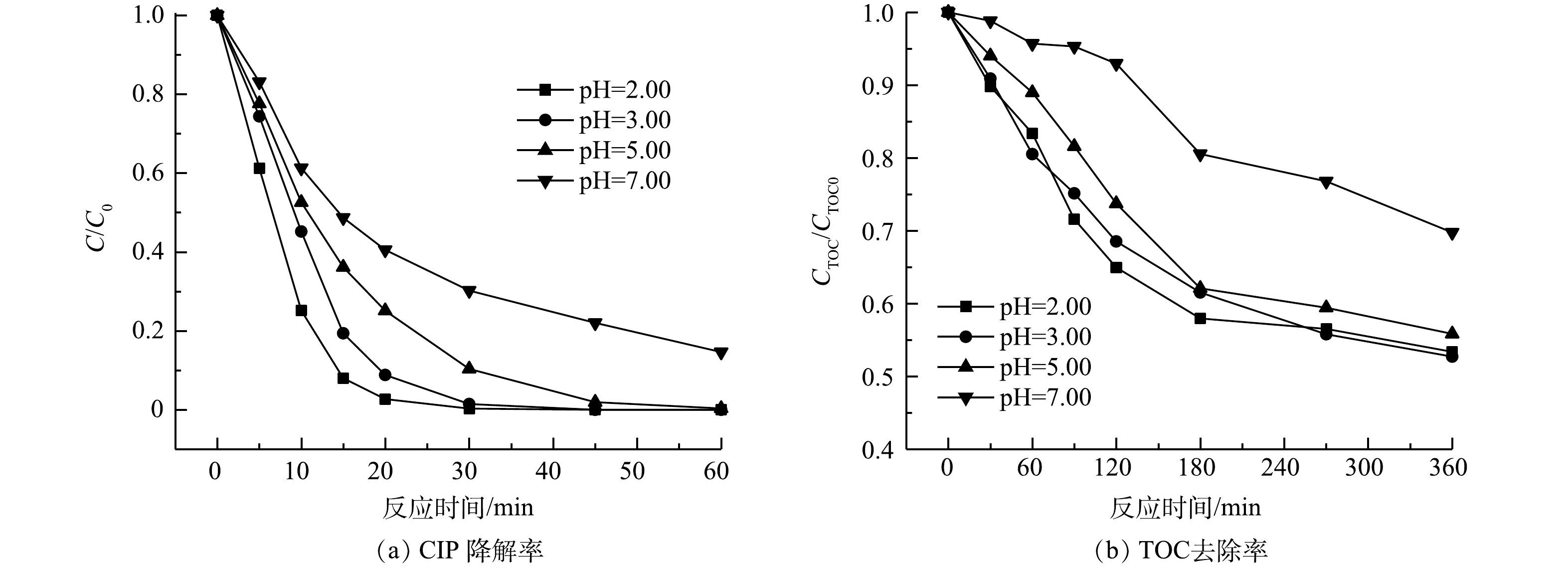
3)初始pH。由图8可见,在酸性条件下CIP降解效果更好,中性条件下延长反应时间也可实现CIP完全降解,SRIS-EF体系的pH适用范围较广。当初始pH为3.00时,TOC去除率为47.3%,此时体系的氧化能力最强。pH=2.00时体系矿化效果稍差,TOC去除率为46.2%。这是由于强酸环境中H+浓度过高,会使Fe3+的还原反应受阻,同时过多的H+会与H2O2结合形成稳定的过氧离子(H3O2+),会抑制·OH生成(式(7))[31]。当pH=7.00时,处理360 min后TOC去除率仅为30.2%,因为较高pH条件下Fe2+不易从铁棒溶出,且易生成沉淀,同时H2O2还会分解成H2O和O2,降低·OH的产生,造成CIP降解效率下降[32-34]。
-
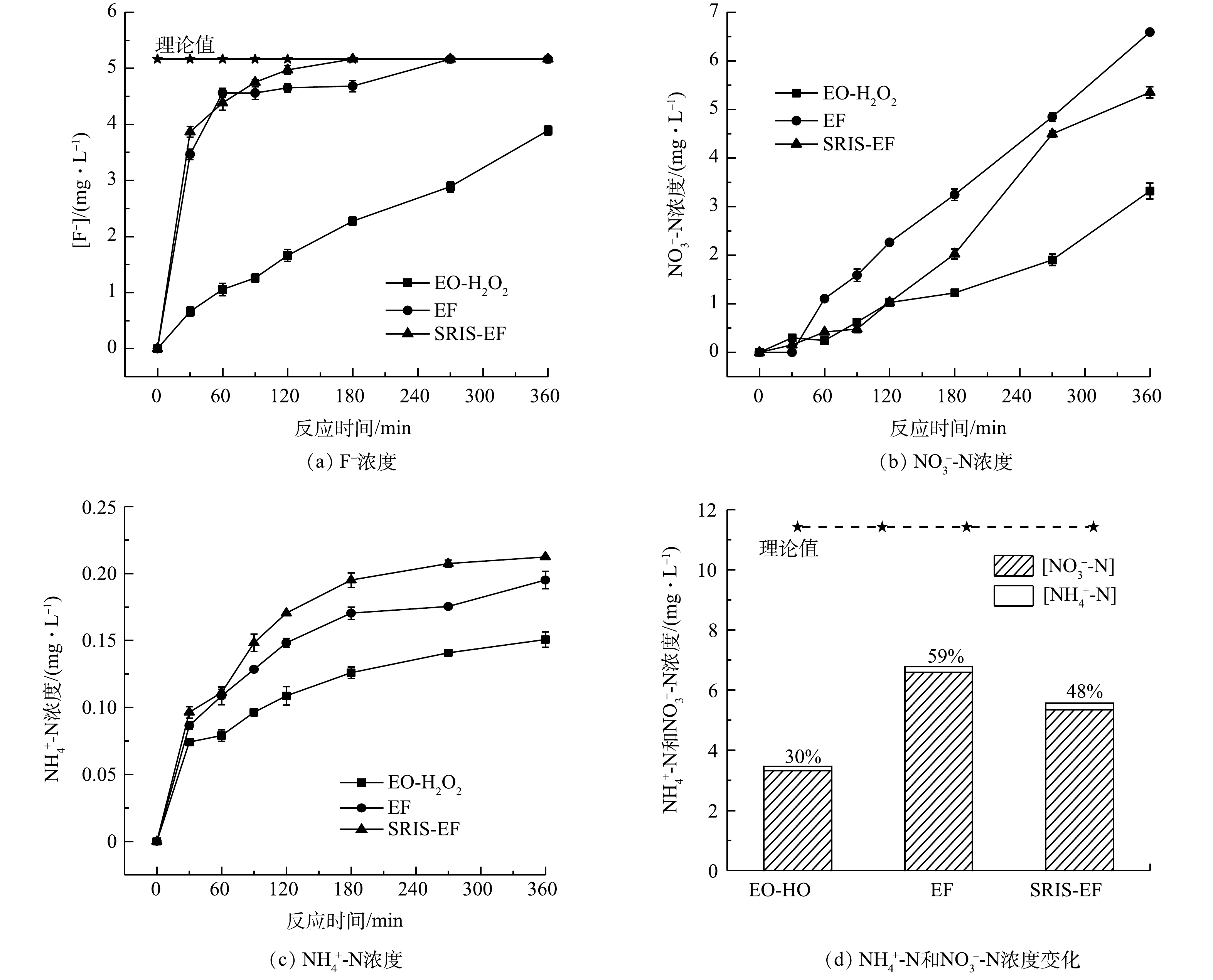
CIP分子中杂原子转化可反映其化学键的断裂情况。由图9(a)可见,生成F−的浓度随着电解时间延长而增加。在EF和SRIS-EF电解360 min后,F−释放量接近达到理论值的100%。这表明CIP分子中的C-F在·OH攻击下极易被破坏发生断裂[35-36]。由图9(d)可见,CIP降解过程中N元素主要以NO3−-N释放到溶液中,反应360 min后NO3−-N和NH4+-N质量浓度分别为5.35 mg·L−1和0.21 mg·L−1。考虑到CIP分子中哌嗪环不稳定,易受到·OH进攻裂解,可推测NO3−-N是来自哌嗪环断裂后N原子的氧化[37]。
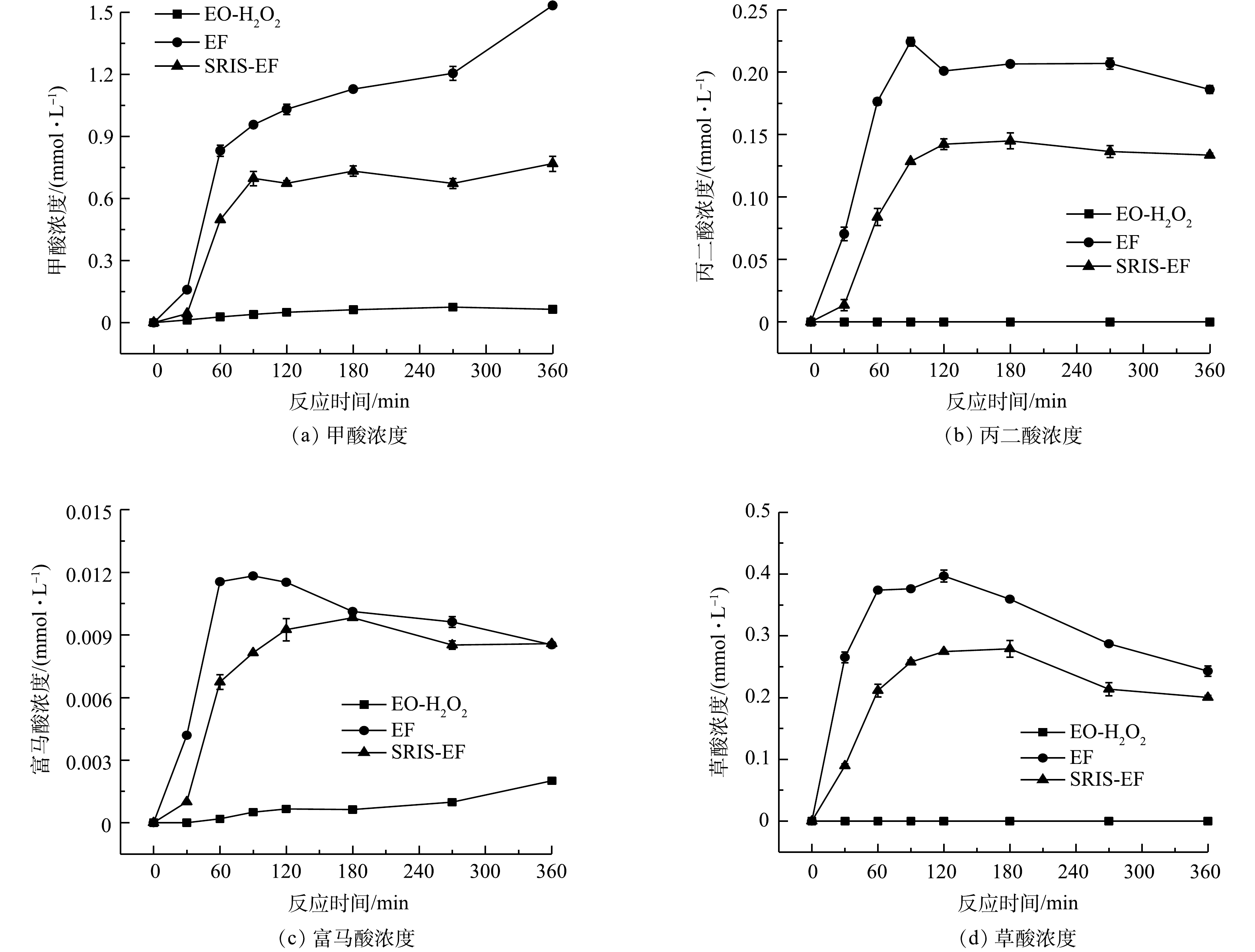
降解过程中短链羧酸生成情况也可反映体系对CIP分子的破环程度[36]。图10反映了不同体系降解CIP过程中短链羧酸的生成情况,发现在各体系中均生成了包括草酸、甲酸、富马酸和丙二酸4种有机酸。其中甲酸浓度随着电解时间延长而持续升高,反应360 min后在EO-H2O2、EF和SRIS-EF体系中其浓度分别达到0.064、1.53和0.77 mmol·L−1。还检测到少量的富马酸和草酸,其浓度呈现先升高后保持稳定或略微下降的趋势。
-
1)与常规EF体系相比较,SRIS-EF反应360 min后TOC矿化率由35.2%提高到47.3%,说明缓释铁源耦合GDE电极能有效强化体系的矿化性能。SRIS-EF体系处理效果受铁棒面积、初始pH、电流密度等因素影响。通过多条件优化实验确定了当铁棒面积为2.89 cm2、初始pH为3.00、电流密度为30 mA·cm−2时体系处理效果最好。
2)在30 mA·cm−2时GDE电极可生成41.55 mmol·L−1 H2O2 。Fe2+浓度变化结果验证了SRIS-EF体系能持续产生Fe2+,以确保芬顿反应的持续发生。SRIS-EF产生的·OH浓度可达778.7 μmol·L−1,说明优化铁离子投加方式可有效提高体系氧化能力。
3) CIP结构中的C-F键易受到攻击后断裂,CIP氧化后释放到溶液中主要以NO3−-N形式存在,推测是CIP分子的哌嗪环断裂后N原子被氧化后形成的。在EF和SRIS-EF体系中检测到的有机酸种类和含量均高于EO-H2O2体系。
缓释铁源耦合气体扩散电极强化电芬顿降解环丙沙星
Enhanced degradation of the antibiotic ciprofloxacin by electro-Fenton using a sustained-release iron source coupled with a gas diffusion cathode
-
摘要: 气体扩散电极(GDE)气液固三相界面可高效产生H2O2,但对铁离子还原能力较弱。针对这一问题,本文构建了一种基于缓释铁源的电芬顿体系(SRIS-EF),通过引入柱状铁棒与GDE电极协同作用加强体系的氧化能力。该体系旨在改进Fe2+投加方式,通过电场与溶液酸碱度协同作用持续生成Fe2+,提高羟基自由基(·OH)的生成和利用率,加速污染物的降解与矿化。以100 mg·L−1环丙沙星(CIP)为目标物,通过定量分析体系内生成的主要活性氧化物质(ROS)表征其氧化能力,结果表明SRIS-EF能够持续生成更高浓度的·OH。对比了不同体系对CIP的矿化能力,发现SRIS-EF的TOC矿化率与常规EF相比提高了12.1%。此外,考察了SRIS-EF体系中柱状铁棒面积、初始pH、电流密度等因素对降解效能的影响,得出了SRIS-EF体系的最佳条件为:铁棒面积2.89 cm2,初始pH 3.00,电流密度30 mA·cm−2,在此条件下反应60 min即可被完全去除CIP,处理360 min后TOC去除率可达47.3%。还对体系中无机离子和小分子有机酸的生成情况进行了测定,检测出CIP内的N和F主要以NH4+、NO3−和F−形式释放,降解过程中生成了草酸、甲酸、富马酸和丙二酸等四种短链羧酸,其中甲酸浓度较高。研究证实了SRIS-EF体系在降解水中难降解有机污染物方面优异的氧化性能。Abstract: The electro-Fenton (EF) using the gas diffusion electrode (GDE) can produce H2O2 efficiently at the gas-liquid-solid three-phase interface, but its reduction ability for Fe3+ is very weak. A novel EF process of SRIS-EF based on slow-release iron source (SRIS) was constructed, in which the oxidation capacity was enhanced by the synergistic effect of the columnar iron rod and GDE electrode. In SRIS-EF, SRIS can continuously generate Fe2+ by the coeffect of the electric field and the acidity of solution, the generation and utilization of hydroxyl radicals (·OH) are enhanced, the degradation and mineralization of pollutants were accelerated accordingly. The oxidative capacity was characterized by quantifying the major reactive oxygen species (ROS) generated during treating 100 mg·L−1 ciprofloxacin (CIP). The results showed that SRIS-EF could consistently generate more ·OH than EF alone. The mineralization ability of different processes was also compared, the TOC removal rate of SRIS-EF process was 12.1% higher than that of the conventional EF process. In addition, the effects of iron rod area, initial pH and current density of SRIS-EF were studied. The optimal conditions were determined as follows: the area of iron rod was 2.89 cm2, the initial pH was 3.00, and the current density was 30 mA·cm−2. Under the optimal condition, CIP was completely degraded within 60 min, and 47.3% TOC was removed after 360 min treatment. The generations of inorganic ions and organic acids in different processes were measured. The N and F atoms of CIP were mainly released as NH4+, NO3− and F−. Four short-chain carboxylic acids: oxalic acid, formic acid, fumaric acid and malonic acid were detected, the higher concentration of formic acid was generated in SRIS-EF process. The study verified the excellent oxidation performance of SRIS-EF process on the degradation of refractory organic pollutants in water.
-

-
表 1 在不同体系中降解过程的一级动力学表观速率常数及电流矿化效率
Table 1. Apparent pseudo first-order rate constants and mineralization current efficiency in different systems
降解体系 k1/min−1 R2 MCE/% EO-H2O2 0.003 0.989 0.03 EF 0.379 0.963 2.53 SRIS-EF 0.144 0.968 6.57 表 2 不同柱状铁棒面积下反应体系中Fe2+浓度、H2O2浓度和TOC去除率的变化
Table 2. Evolution of Fe2+, H2O2 and TOC removal rates in SRIS-EF with different columnar iron rod areas
铁钉面积/
cm2Fe2+浓度/
(mg·L−1)H2O2累积浓度/
(mmol·L−1)TOC
去除率/%0 — 41.55 6.1 1.45 16.42 17.79 38.7 2.17 25.34 14.31 44.1 2.89 32.59 12.26 47.3 3.25 36.70 7.78 44.3 -
[1] DORIVAL‐GARCíA N, ZAFRA‐GóMEZ A, CANTARERO S, et al. Simultaneous determination of 13 quinolone antibiotic derivatives in wastewater samples using solid‐phase extraction and ultra performance liquid chromatography–tandem mass spectrometry[J]. Microchemical Journal, 2013, 106: 323-333. doi: 10.1016/j.microc.2012.09.002 [2] VAN DOORSLAER X, DEWULF J, VAN LANGENHOVE H, et al. Fluoroquinolone antibiotics: an emerging class of environmental micropollutants[J]. Science of the Total Environment, 2014, 500: 250-269. [3] DORIVAL-GARCIA N, ZAFRA-GOMEZ A, NAVALON A, et al. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions[J]. Journal of Environment Management, 2013, 120: 75-83. [4] LI W, SHI Y, GAO L, et al. Occurrence and removal of antibiotics in a municipal wastewater reclamation plant in Beijing, China[J]. Chemosphere, 2013, 92(4): 435-444. doi: 10.1016/j.chemosphere.2013.01.040 [5] CAO S S, DUAN Y P, TU Y J, et al. Pharmaceuticals and personal care products in a drinking water resource of Yangtze River Delta Ecology and Greenery Integration Development Demonstration Zone in China: Occurrence and human health risk assessment[J]. Science of the Total Environment, 2020, 721: 137624. doi: 10.1016/j.scitotenv.2020.137624 [6] BLAIR B D, CRAGO J P, HEDMAN C J, et al. Evaluation of a model for the removal of pharmaceuticals, personal care products, and hormones from wastewater[J]. Science of the Total Environment, 2013, 444: 515-521. doi: 10.1016/j.scitotenv.2012.11.103 [7] KOSMA C I, LAMBROPOULOU D A, ALBANIS T A. Comprehensive study of the antidiabetic drug metformin and its transformation product guanylurea in Greek wastewaters[J]. Water Research, 2015, 70: 436-448. doi: 10.1016/j.watres.2014.12.010 [8] ENIOLA J O, KUMAR R, BARAKAT M A, et al. A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment[J]. Journal of Cleaner Production, 2022, 356: 131826. doi: 10.1016/j.jclepro.2022.131826 [9] MACKUĽAK T, ČERNANSKý S, FEHéR M, et al. Pharmaceuticals, drugs, and resistant microorganisms - environmental impact on population health[J]. Current Opinion in Environmental Science & Health, 2019, 9: 40-48. [10] 徐维海, 张干, 邹世春, 等. 典型抗生素类药物在城市污水处理厂中的含量水平及其行为特征[J]. 环境科学, 2007, 28(8): 1779-1783. doi: 10.3321/j.issn:0250-3301.2007.08.023 [11] CAI Y, SUN T, LI G, et al. Traditional and emerging water disinfection technologies challenging the control of antibiotic-resistant bacteria and antibiotic resistance genes[J]. ACS ES& T Engineering, 2021, 1(7): 1046-1064. [12] KLAVARIOTI M, MANTZAVINOS D, KASSINOS D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes[J]. Environment International, 2009, 35(2): 402-417. doi: 10.1016/j.envint.2008.07.009 [13] MONDAL S K, SAHA A K, SINHA A. Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization[J]. Journal of Cleaner Production, 2018, 171: 1203-1214. doi: 10.1016/j.jclepro.2017.10.091 [14] BRILLAS E, SIRéS I. Electrochemical removal of pharmaceuticals from water streams: Reactivity elucidation by mass spectrometry[J]. TRAC Trends in Analytical Chemistry, 2015, 70: 112-121. doi: 10.1016/j.trac.2015.01.013 [15] TOPOLOVEC B, ŠKORO N, PUАČ N, et al. Pathways of organic micropollutants degradation in atmospheric pressure plasma processing: A review[J]. Chemosphere, 2022, 294: 133606. doi: 10.1016/j.chemosphere.2022.133606 [16] BRILLAS E, SIRéS I, OTURAN M A. Electro-Fenton process and related electrochemical technologies based on fenton’s reaction chemistry[J]. Chemical Reviews, 2009, 109(12): 6570-6631. doi: 10.1021/cr900136g [17] ZHANG Y, DANIEL G, LANZALACO S, et al. H2O2 production at gas-diffusion cathodes made from agarose-derived carbons with different textural properties for acebutolol degradation in chloride media[J]. Journal of Hazardous materials, 2022, 423: 127005. doi: 10.1016/j.jhazmat.2021.127005 [18] SIRéS I, OTURAN N, OTURAN M A, et al. Electro-Fenton degradation of antimicrobials triclosan and triclocarban[J]. Electrochimica Acta, 2007, 52(17): 5493-5503. doi: 10.1016/j.electacta.2007.03.011 [19] ISARAIN-CHáVEZ E, ARIAS C, CABOT P L, et al. Mineralization of the drug β-blocker atenolol by electro-Fenton and photoelectro-Fenton using an air-diffusion cathode for H2O2 electrogeneration combined with a carbon-felt cathode for Fe2+ regeneration[J]. Applied Catalysis B:Environmental, 2010, 96(3-4): 361-369. doi: 10.1016/j.apcatb.2010.02.033 [20] SIRéS I, GARRIDO J A, RODRíGUEZ R M, et al. Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene[J]. Applied Catalysis B:Environmental, 2007, 72(3-4): 382-394. doi: 10.1016/j.apcatb.2006.11.016 [21] YANG Z, SHAN C, PAN B, et al. The Fenton reaction in water assisted by picolinic acid: accelerated iron cycling and co-generation of a selective Fe-based oxidant[J]. Environmental Science and Technology, 2021, 55(12): 8299-8308. doi: 10.1021/acs.est.1c00230 [22] 中国国家环境保护总局. 水和废水监测分析方法(第四版)[J]. 北京:中国环境科学出版社, 2002: 368-370. [23] WELCHER F J. Standard methods of chemical analysis[M]. New York Krieger Publishing Company, 1975. [24] YU F, ZHOU M, YU X. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration[J]. Electrochimica Acta, 2015, 163: 182-189. doi: 10.1016/j.electacta.2015.02.166 [25] TAI C, PENG J F, LIU J F, et al. Determination of hydroxyl radicals in advanced oxidation processes with dimethyl sulfoxide trapping and liquid chromatography[J]. Analytica Chimica Acta, 2004, 527(1): 73-80. doi: 10.1016/j.aca.2004.08.019 [26] ABOU DALLE A, DOMERGUE L, FOURCADE F, et al. Efficiency of DMSO as hydroxyl radical probe in an electrochemical advanced oxidation process: reactive oxygen species monitoring and impact of the current density[J]. Electrochimica Acta, 2017, 246: 1-8. doi: 10.1016/j.electacta.2017.06.024 [27] HORN D B, SQUIRE C R. The estimation of ammonia using the indophenol blue reaction[J]. Clinica Chimica Acta, 1966, 14(2): 185-194. doi: 10.1016/0009-8981(66)90085-4 [28] WANG A, LI Y-Y, RU J. The mechanism and application of the electro-Fenton process for azo dye Acid Red 14 degradation using an activated carbon fibre felt cathode[J]. Journal of Chemical Technology & Biotechnology, 2010, 85(11): 1463-1470. [29] SKOUMAL M, RODRíGUEZ R M, CABOT P L, et al. Electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton degradation of the drug ibuprofen in acid aqueous medium using platinum and boron-doped diamond anodes[J]. Electrochimica Acta, 2009, 54(7): 2077-2085. doi: 10.1016/j.electacta.2008.07.014 [30] 王爱民, 曲久辉, 史红星, 等. 活性碳纤维阴极电芬顿反应降解微囊藻毒素研究[J]. 高等学校化学学报, 2005, 26(9): 1669-1672. doi: 10.3321/j.issn:0251-0790.2005.09.013 [31] WEN Z, WANG A, ZHANG Y, et al. Mineralization of cefoperazone in acid medium by the microwave discharge electrodeless lamp irradiated photoelectro-Fenton using a RuO2/Ti or boron-doped diamond anode[J]. Journal of Hazardous materials, 2019, 374: 186-194. doi: 10.1016/j.jhazmat.2019.03.124 [32] RADWAN M, GAR ALALM M, ELETRIBY H. Optimization and modeling of electro-Fenton process for treatment of phenolic wastewater using nickel and sacrificial stainless steel anodes[J]. Journal of Water Process Engineering, 2018, 22: 155-162. doi: 10.1016/j.jwpe.2018.02.003 [33] 高迎新, 张昱, 杨敏, 等. Fe3+或Fe2+均相催化H2O2生成羟基自由基的规律[J]. 环境科学, 2006, 27(2): 305-309. doi: 10.3321/j.issn:0250-3301.2006.02.021 [34] 龚月湘, 兰华春, 李久义, 等. 光电芬顿矿化草甘膦有机废水[J]. 环境工程学报, 2016, 10(8): 3999-4003. doi: 10.12030/j.cjee.201503080 [35] SOPAJ F, OTURAN N, PINSON J, et al. Effect of the anode materials on the efficiency of the electro-Fenton process for the mineralization of the antibiotic sulfamethazine[J]. Applied Catalysis B:Environmental, 2016, 199: 331-341. doi: 10.1016/j.apcatb.2016.06.035 [36] ANTONIN V S, SANTOS M C, GARCIA-SEGURA S, et al. Electrochemical incineration of the antibiotic ciprofloxacin in sulfate medium and synthetic urine matrix[J]. Water Research, 2015, 83: 31-41. doi: 10.1016/j.watres.2015.05.066 [37] 李张丽, 杨健, 贾漫珂, 等. 非水溶性铁Schiff碱配合物活化H2O2光催化降解环丙沙星[J]. 武汉大学学报(理学版), 2021, 67(4): 367-374. -



 下载:
下载: