-
工业废水的种类繁多,难降解污染物含量高,经常规物化及生化工艺处理后,一般难以达标排放. 深度处理是工业废水达标排放的必要工艺,主要有芬顿技术[1-2]、臭氧催化[3]、电化学高级氧化[4],但这些技术存在药剂加量大、反应时间长、投资成本高等缺陷[5]. 近一二十年,兴起了一种叫做“COD去除剂”的药剂,其主要成分是氯酸钠. 工程应用发现,投加该药剂可显著降低水中COD,使得工业废水无需经过深度处理即可实现“达标”排放. 投加“COD去除剂”工艺操作简单,试剂成本较低,无需复杂的硬件设施,因此应用较为普遍[6].
经实践证明,投加氯酸盐并不能真实地去除水中COD. 更为重要的是氯酸盐是一种迁移性强、潜在毒性大、暴露途径多的污染物,对食品安全和人体健康构成巨大威胁[7-8]. 因此,投加氯酸盐已被环境保护部门判定为违法行为. 例如,2020年9—11月,陕西省生态环境厅查清神木市污水处理厂累计投加131余吨“COD去除剂”处理污水和不正常运行水污染防治设施等环境违法行为,地方生态环境局对该污水处理厂使用“COD去除剂”构成“通过篡改、伪造监测数据逃避监管的方式违法排放污染物”和不正常运行水污染防治设施等违法行为进行立案处罚,处以20万元和40万元罚款,并责令立即停止违法行为,同时将该污水处理厂涉嫌环境违法的问题移送公安部门[9].
目前,人们已经意识到氯酸盐对于COD测定存在掩蔽作用,但并没有提出避免氯酸盐干扰COD测定的方法. 要消除氯酸盐对COD测定的影响,最直接的方法是去除氯酸盐,但前提条件是不影响水中COD的数值. 目前,已有许多技术用来去除水中氯酸盐,包括生物电化学还原[10]、电还原[11]、活性炭[12]、和生物降解等方法,但是这些方法操作复杂,反应条件苛刻,同时存在改变原始COD含量的可能性. 对此,本文提出了一种以亚硫酸盐为还原剂的COD测定的预处理工艺,即通过投加相应的亚硫酸氢钠完全还原去除氯酸盐,进而测出真实的COD值;同时考察了温度和[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比对于该方法的影响,确定了最佳温度和[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比;根据ESR分析推测出该方法的反应路径,并通过TOC和三维荧光分析验证了该方法的切实可靠,为完善COD测定方法及理论提供参考. -
试验水样采用的是来自济宁某化工企业的工业废水:COD为120 mg·L−1,TOC为75 mg·L−1,
${\rm{SO}}_4^{2-} $ 为356 mg·L−1,Cl−为151 mg·L−1,${\rm{NO}}_3^{-} $ -N为76.6 mg·L−1,${\rm{NH}}_4^{+} $ -N为4.6 mg·L−1,pH为8.7. -
集热式恒温加热磁力搅拌器,巩义市英峪高科仪器厂. PTFE 微滤膜(0.45 μm),长沙斯普林公司. 重铬酸钾(分析纯),浓硫酸(分析纯),硫酸汞(分析纯),硫酸银(分析纯),氯酸钠(分析纯);亚硫酸氢钠(分析纯),无水乙醇(分析纯),十二水磷酸氢二钠(分析纯),二水磷酸二氢钠(分析纯),国药集团化学试剂有限公司. 乙二胺四乙酸二钠(分析纯),上海麦克林生化科技有限公司. 2-硝基苯甲酸(分析纯),上海阿拉丁生化科技股份有限公司. 所有溶液均采用超纯水(电阻率≤18.2 MΩ·cm)制备.
-
氯酸盐的投加量对COD掩蔽作用的影响. 分别取100 mL工业废水加入8个烧杯中,分别加入不同投加量的氯酸钠(0、0.5、1、2、3、5 、8、10 mmol·L−1),室温下用磁力搅拌器搅拌3 min,然后静置30 min,取上清液测定水样的COD和TOC值. 消除氯酸盐对COD测定的影响:在确定能够将水样COD值降到综合排放标准(100 mg·L−1)以下的氯酸钠量后,首先将水样pH调至4.0左右,按照一定的[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比投加亚硫酸氢钠去除氯酸盐,使COD恢复到初始值.温度对消除氯酸盐掩蔽作用的影响. 首先将水样pH调至4.0左右,在250 mL的锥形瓶中加入100 mL的水样,按照[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为5先后投加氯酸钠和亚硫酸氢钠,将锥形瓶放置在集热式恒温加热磁力搅拌器中分别在20 、40 、60 、80 °C,保持转速为20 r·min−1,考察温度对氯酸钠去除的影响.[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比对消除氯酸盐掩蔽作用的影响. 首先将水样pH调至4.0左右,在250 mL的锥形瓶中加入100 mL的水样,放置在集热式恒温加热磁力搅拌器中保持恒温60 °C,保持转速为20 r·min−1,分别按照[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为3、4、5、6、7先后投加氯酸钠和亚硫酸氢钠,考察[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比对氯酸钠去除的影响. -
COD采用快速消解分光光度法测定,检测范围为15—250 mg·L−1,允许测定误差为±3.0%(HJ/T 399-2007:水质 化学需氧量测定 快速消解分光光度法)[13];TOC 采用总有机碳分析仪测定;
${\rm{ClO}}_3^- $ 采用离子色谱法测定,测定条件:流量为 1.33 mL·min−1、抑制电流为100 mA、分析时间为9 min;亚硫酸根采用紫外分光光度计测定;${\rm{SO}}_3^{ {\rm{·}}-}$ 、${\rm{SO}}_4^{ {\rm{·}}-}$ 检测采用电子自旋共振(ESR),5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为捕获剂. -
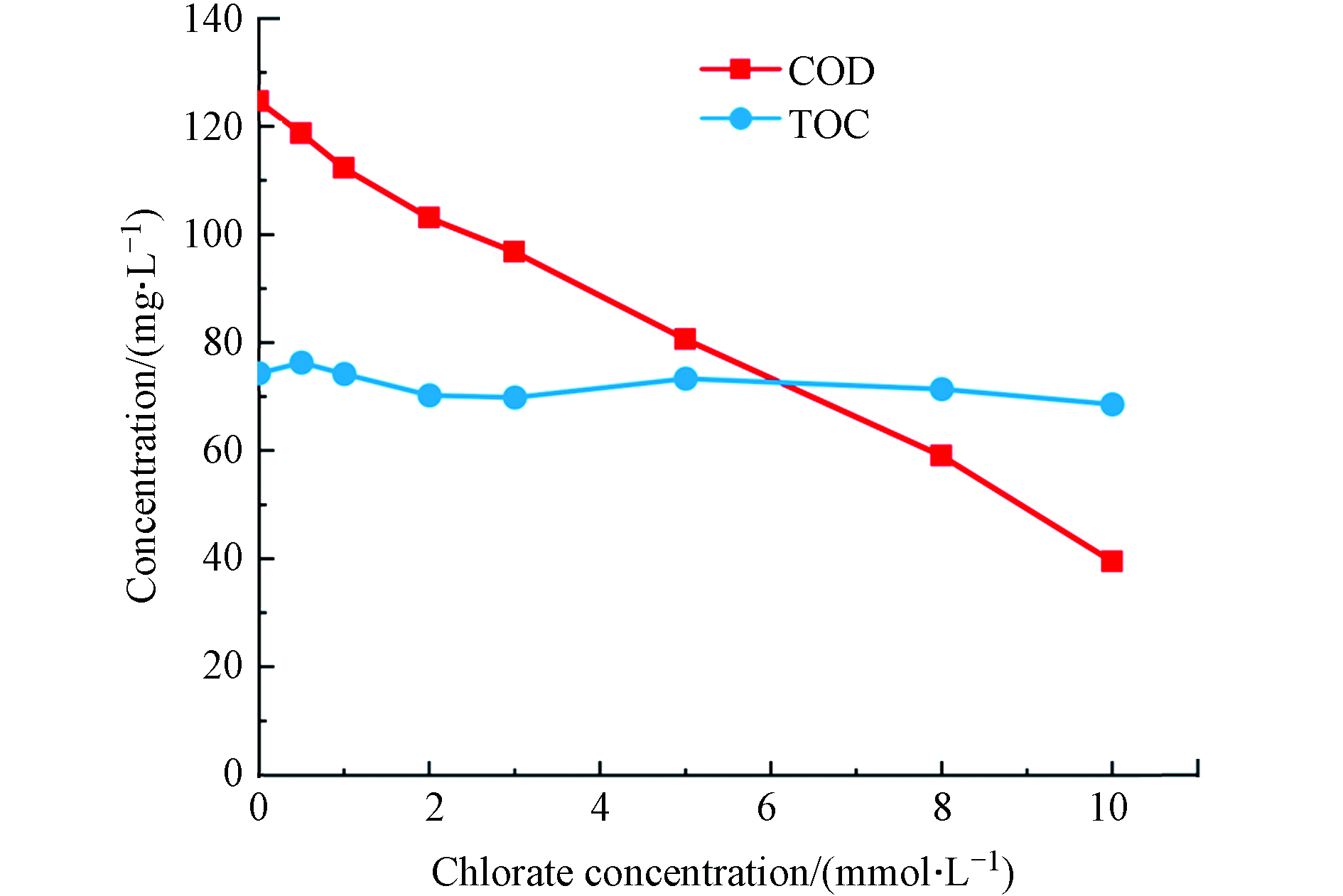
氯酸盐投加量是影响其对水体COD掩蔽作用的关键因素. 如图1所示,当氯酸盐投加量为0.5、1、2、3 、5、8、10 mmol·L−1时,COD浓度由初始的124 mg·L−1分别降至118、112、103、97、81、59、39 mg·L−1. 一般而言,COD与TOC呈现一定的正相关性[14];但是,在以上反应条件下,TOC保持在69—76 mg·L−1范围内,并未发生显著变化.
并且COD的降低与氯酸盐反应时间、反应pH和反应温度无关,仅与氯酸盐用量有关,氯酸盐用量越高,COD越低[15];其原因是,在酸性高温消解条件下,水样中的
${\rm{ClO}}_3^ - $ 可与有机物发生氧化还原反应,减少${\rm{Cr}}_2 {\rm{O}}_7^{2-} $ 的消耗量,同时大部分${\rm{ClO}}_3^ - $ 被转化为Cl−,并被硫酸汞络合稳定下来,从而导致COD检测值偏低[6,15-16].根据中国《污水综合排放标准》(中国环境保护部,1996年),工业废水COD一级排放标准为100 mg·L−1[17]. 从图1可以看出3 mmol·L−1氯酸盐可使COD降到100 mg·L−1以下,因此在下文中根据3 mmol·L−1的氯酸盐按照不同的[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比投加亚硫酸氢钠来消除氯酸盐对COD测定的影响. -
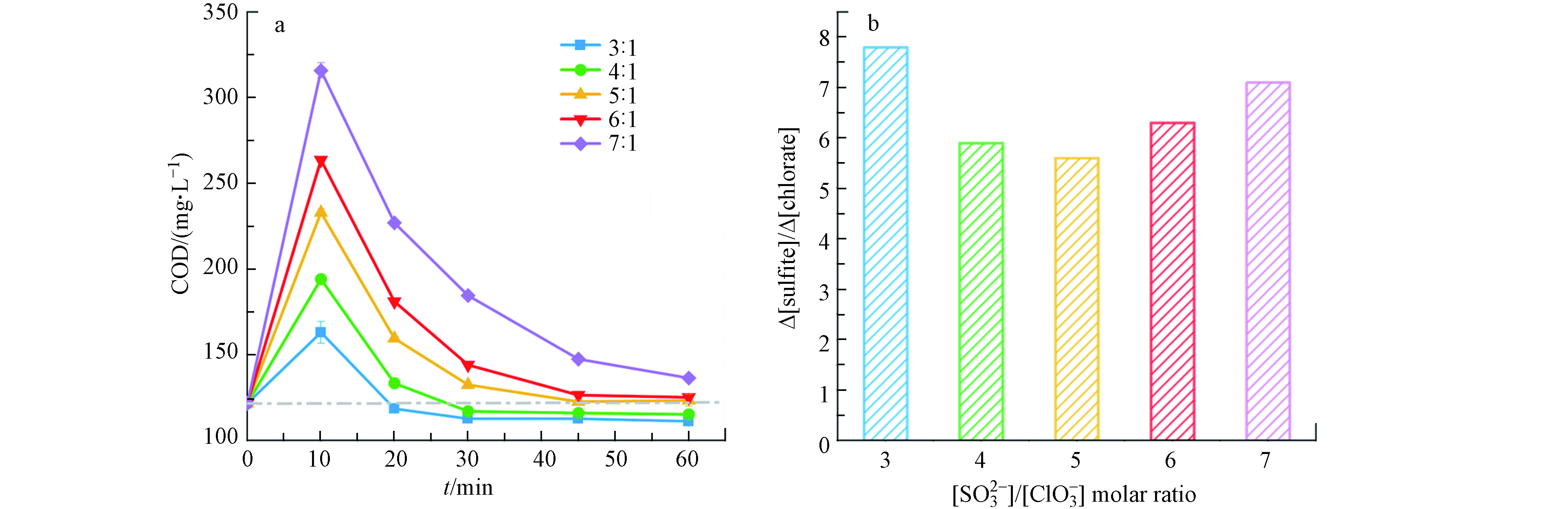
温度是影响消除氯酸盐掩蔽作用的关键参数. 图2考察了[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为5时,温度对消除氯酸盐掩蔽作用的影响. 如图2(a)所示,在每个温度下COD值都会呈现先上升后下降的趋势;反应10 min时,20、40、60、80 °C的COD值分别从初始浓度上升到328、294、233、165 mg·L−1,原因是投加的亚硫酸盐具有较强的还原性,在测定COD的过程中亚硫酸根与重铬酸钾反应,消耗了更多的重铬酸钾,使得测定的COD数值偏高;随着反应进行,亚硫酸根不断被消耗,COD开始呈下降趋势,且随着氯酸根的完全还原,COD最终恢复到初始值,并保持不变.从图2(a)还可以看出,经过1 h的反应,20 °C时COD未恢复到初始值,此时还有1.4 mmol·L−1氯酸根和8.7 mmol·L−1亚硫酸根未反应完全,且反应进行至1.5 h时COD也未能恢复到初始值,这是由于温度为20 °C时,亚硫酸根和氯酸根之间的氧化还原反应较慢;尽管40 °C 条件下COD恢复到初始值,但仍检测出有0.8 mmol·L−1氯酸根和1.7 mmol·L−1亚硫酸根未反应完全,原因是氯酸根的氧化作用和亚硫酸根的还原作用,使得测定的COD表观上恢复到初始值;当温度为80 °C时,经过30 min的反应氯酸根和亚硫酸根消耗完全,但COD未能恢复到初始值,这可能是因为温度较高,存在溶剂蒸发,导致COD上升,因此该温度并不适用于此反应;而60 °C时经45 min COD便恢复到初始值,且亚硫酸根已经反应完全,剩余的氯酸根也不会对COD造成影响. 与其他温度相比,60 °C下反应迅速,
${\rm{SO}}_3^{2-} $ 和${\rm{ClO}}_3^ - $ 同步耗尽,COD很快恢复到初始水平,因此60 °C为最佳反应温度. -
[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比是影响消除氯酸盐掩蔽作用的决定因素. 图3考察了温度为60 °C时,[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比对消除氯酸盐掩蔽作用的影响,图3(a)显示,与温度的影响趋势一致,COD数值先上升后下降,且随着摩尔比的升高COD上升的幅度越大. 当[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为3和4时,COD值最终分别降至110 mg·L−1和114 mg·L−1,且保持不变,未恢复到初始值,原因是投加的亚硫酸根不足以完全去除氯酸根;[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为5和6时,亚硫酸根完全耗尽,COD恢复到初始值,且剩余的氯酸根对COD的影响可忽略不计,实际[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比分别为5.5和6.2(图3 b);尽管当[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为7时氯酸根被完全还原,但是COD无法恢复到初始值,这是因为有剩余的亚硫酸根存在,使得COD测定数值偏高. 因此,可以得出结论当[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比在5—6时为最佳的投加计量,能够使COD快速有效的恢复到初始值.为了验证该方法对于不同浓度氯酸盐的适用性,还考察了当水样中氯酸盐浓度为3 mmol·L−1以上和3 mmol·L−1以下时对COD掩蔽作用的消除情况. 如图4所示,在[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为5—6、温度为60 °C条件下,当氯酸盐浓度小于3 mmol·L−1时,45 min内COD恢复良好. 而氯酸盐浓度高于3 mmol·L−1时,COD未能恢复到初始值,这可能是因为投加的亚硫酸盐过多或产生了还原性物质导致COD未能恢复. 因此当废水中氯酸盐浓度高于3 mmol·L−1时,可先将氯酸盐浓度稀释至3 mmol·L−1以下,再投加相应的亚硫酸盐. -
根据前面分析得到结论,当[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为5—6、温度为60 °C时,可以使COD快速有效的恢复到初始值,且${\rm{SO}}_3^{2-} $ 和${\rm{ClO}}_3^ - $ 同步耗尽. 其中温度是关键因素,根据阿伦尼乌斯定律,亚硫酸盐还原${\rm{ClO}}_3^ - $ 过程的速率常数与反应温度正相关,在较高的反应温度下,大部分反应物分子有足够的能量越过活化能垒,也就意味着温度升高,两种物质反应越快,COD也越快恢复到初始值. 此外,在较高的反应温度下,${\rm{ClO}}_3^ - $ 与亚硫酸盐的快速反应有利于${\rm{HCO}}_3^{-} $ (在pH为3.0 —7.0时亚硫酸盐的主要存在形式)中H+的释放,从而加速${\rm{ClO}}_3^ - $ 被亚硫酸盐还原. 但当温度升高至80 °C时,存在溶剂蒸发导致COD未能恢复到初始值.理论上氯酸根与亚硫酸氢根在1:3时可以反应完全,但经过实验得出需要的亚硫酸盐的量始终高于方程式1计算的理论值,过量的亚硫酸盐消耗主要归因于溶解氧(DO)的参与(方程式2)和
${\rm{ClO}}_3^ - $ 与亚硫酸盐反应过程中反应中间体(自由基)的生成(方程式3)[18]. 将反应装置密封,通过持续输入氮气来创造无氧环境,在60 °C条件下按照[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为3先后投加氯酸盐和亚硫酸盐,如图5(a)所示,经过1 h的反应COD恢复到初始值且保持不变,实际[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为3.3(图5 b);当[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为4时,COD未能恢复到初始值,这是因为投加的亚硫酸盐过量,导致COD偏高. 而在空气中[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比达到5—6时才能有效消除氯酸盐对COD的掩蔽作用,因此溶解氧的存在是亚硫酸盐过度消耗的重要原因.根据上述讨论,在
${\rm{ClO}}_{3}^{-} $ -亚硫酸盐体系中,反应中间体(自由基)的生成也可以解释亚硫酸盐的过度消耗. 为了对中间体的生成提供直接证据,利用DMPO作为捕获剂收集了电子自旋共振谱图(ESR):在温度为60 °C、初始pH为4.0时,按照1:5的比例先后加入氯酸钠和亚硫酸氢钠,且在反应初始阶段加入了100 mmol·L−1的DMPO,得到的结果与之前报道的 DMPO-${\rm{SO}}_3^{ {\rm{·}}-}$ 加合物的结果一致,意味着${\rm{SO}}_3^{ {\rm{·}}-}$ 的产生(图6 a)[19].因为
${\rm{SO}}_3^{·-} $ 与溶解氧会以扩散控制的速率 (2.5×109 mol·L−1·s−1) 快速反应,生成活性自由基${\rm{SO}}_5^{ {\rm{·}}-}$ ,并进一步转化为${\rm{SO}}_4^{ {\rm{·}}-}$ [20],因此在相同条件下,当反应进行10 min后加入100 mmol·L−1的DMPO(在实验一开始加入过量的DMPO会捕获生成的${\rm{SO}}_3^{ {\rm{·}}-}$ ,阻断了后面的反应,进而检测不到${\rm{SO}}_4^{ {\rm{·}}-}$ 的生成),在图6(b)中可以观察到典型的 DMPO-${\rm{SO}}_4^{ {\rm{·}}-}$ 和 DMPO-·OH信号;然而,${\rm{SO}}_4^{·-} $ 加合物可以通过亲核取代反应与H2O/OH-反应生成相应的·OH加合物,反应速度相当快[21-22],因此DMPO-·OH信号可能是由DMPO-${\rm{SO}}_4^{ {\rm{·}}-}$ 经亲核取代反应产生的. 通过ESR分析可得出在ClO3--亚硫酸盐体系中的确形成了反应中间体(自由基),其中${\rm{SO}}_4^{ {\rm{·}}-}$ 为主要的中间体.在
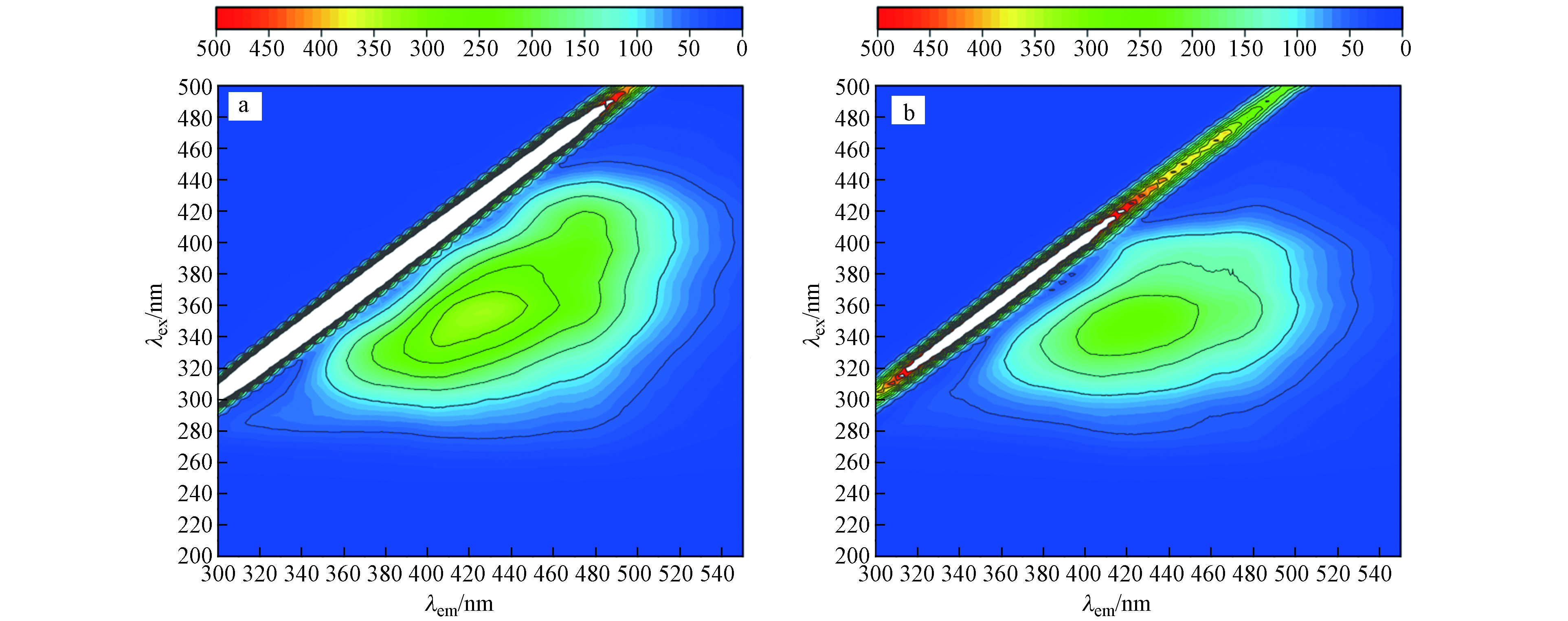
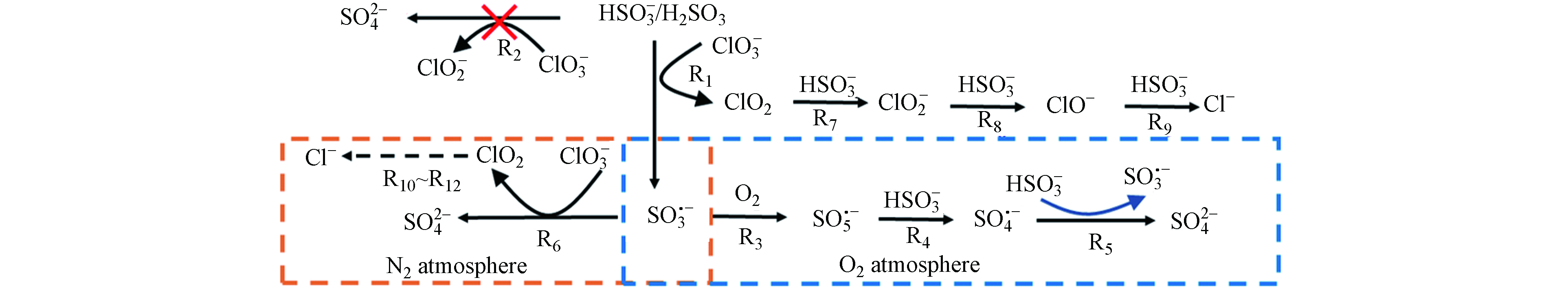
${\rm{ClO}}_3^ - $ -亚硫酸盐体系中形成的高氧化${\rm{SO}}_4^{ {\rm{·}}-} $ 具有通过降解水样中有机物来降低COD的潜力,因此可能影响亚硫酸盐还原法消除ClO3-干扰测定COD的可行性. 通过三维荧光光谱图可以看出,该废水中主要存在的物质为腐殖酸,反应前后相对比,图7(b)荧光强度要比图7(a)的低,是因为在该体系中产生了自由基,导致部分物质被氧化,但反应前后TOC值保持不变,并未实现有效矿化,且COD恢复到初始值,原因是过量的亚硫酸盐能够快速捕获${\rm{SO}}_4^{ {\rm{·}}-} $ . 因此该方法能够有效去除${\rm{ClO}}_3^ - $ ,且不改变原始COD.${\rm{ClO}}_3^ - $ 被亚硫酸盐还原的途径如图8所示,${\rm{ClO}}_3^ - $ 与亚硫酸盐的反应可能通过直接单电子转移(R1)或直接氧转移反应(R2)进行,但是通过密度泛函理论(DFT)计算R1和R2的吉布斯自由能变化(ΔG)分别为−3.4×105 J·mol−1和1.4×106 J·mol−1,因此,与R2相比R1有更高的热力学可能性引发亚硫酸盐还原${\rm{ClO}}_3^ - $ ,生成${\rm{SO}}_3^{ {\rm{·}}-} $ 和ClO2,这与ESR分析中检测到DMPO-${\rm{SO}}_3^{ {\rm{·}}-} $ 加合物的结果是一致的(图6a);而ClO2则会被亚硫酸盐通过氧转移反应最终还原为Cl−(R7→R9)[23]. 当${\rm{ClO}}_3^ - $ -亚硫酸盐体系暴露于空气中时,${\rm{SO}}_3^{ {\rm{·}}-} $ 容易与O2发生反应,并通过R3→R4反应生成${\rm{SO}}_4^{ {\rm{·}}-} $ (图6b),${\rm{SO}}_4^{ {\rm{·}}-} $ 最终氧化亚硫酸盐分别生成${\rm{SO}}_4^{2-} $ 和${\rm{SO}}_3^{ {\rm{·}}-} $ (R5),这导致了亚硫酸盐的过度消耗. 而在氮气氛围下,ESR分析并没有检测到${\rm{SO}}_4^{ {\rm{·}}-} $ 的信号,所以产生的${\rm{SO}}_3^{ {\rm{·}}-} $ ($ E^0_{{\rm{SO}}_3^{ {\rm{·}}-}/ {\rm{SO}}_3^{2-}} $ =0.72 VNHE)可能主要被${\rm{ClO}}_3^ - $ ($E^0_{{{\rm{ClO}}_3^ - }/{{\rm{Cl}}^-}}$ =1.45 VNHE)所捕获生成${\rm{SO}}_4^{2-} $ (R6),这也解释了在无氧条件下[${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为3时即可使COD恢复到初始值(图5),且${\rm{ClO}}_3^ - $ 最终被还原为Cl−(R10→R12). -
本文中提出的以亚硫酸盐为还原剂的预处理工艺不但可以去除废水中存在的氯酸盐,而且有效消除了氯酸盐对于COD测定的掩蔽作用. 除了废水中固定存在的或人为投加的氯酸盐以外,在电化学高级氧化过程(EAOPs)中,废水中普遍存在的Cl-也可通过多步氧化反应生成
${\rm{ClO}}_3^ - $ ,因此电化学实际去除的COD量可能会低于测定的COD去除量;如表1所示,在Ti/RuOx-IrOx电极上,含105 mmol·L−1 Cl−的尿液废水经8 h处理后可产生26 mmol·L−1的${\rm{ClO}}_3^ - $ [24];BDD作为阳极处理含35 mmol·L−1 Cl-的尿液废水时,最大可产生20 mmol·L−1的${\rm{ClO}}_3^ - $ ,且COD从初始的1710降至100 mg·L–1[25]. 在各种EAOPs中,毒性${\rm{ClO}}_3^ - $ 的形成一直是人们关注的焦点,但其对COD测定的干扰却被完全忽略了,这可能导致对电化学COD去除性能的评价过高,造成EAOPs处理后出水COD达标的假象,同时经电化学处理后表观COD达标的废水排放到自然环境中,可能对水体安全构成潜在威胁. 因此,提出一种消除氯酸盐对COD掩蔽作用的方法,对于正确评价电化学COD去除性能具有重要意义.为了进一步验证目前改进的COD测定方法的有效性,研究了该策略在“真实场景”中的实施情况. 在测定COD值之前,一个不能忽视的问题是在大多数情况下,水样中的
${\rm{ClO}}_3^ - $ 含量可能是未知的. 因此,首先开发了一种快速试纸用于定性检测水样中的${\rm{ClO}}_3^ - $ (图9a),该方法基于${\rm{ClO}}_3^ - $ 将I-转化为I2,具体而言,首先将制备的试纸浸入用浓硫酸调节的含${\rm{ClO}}_3^ - $ 水样中,然后将试纸与比色卡进行对比获得${\rm{ClO}}_3^ - $ 的大致浓度范围. 然后,采用基于碘的分光光度法来定量测量${\rm{ClO}}_3^ - $ (图9b). 不可否认的是,其他的一些氧化剂,比如铁、高锰酸盐离子、氯、臭氧等会干扰分光光度法对于${\rm{ClO}}_3^ - $ 的测定,导致测定数值与水样中${\rm{ClO}}_3^ - $ 的实际浓度有偏差. 但是事实上,这些强氧化物质在生物处理后几乎不存在于工业废水中[27-28],并且通过向水样中投加5 mmol·L−1${\rm{ClO}}_3^ - $ 来验证该方法的有效性,测定数值为4.8 mmol·L−1,因此上述方法可以测定出水样中${\rm{ClO}}_3^ - $ 的含量. 然而,对于电解体系中${\rm{ClO}}_3^ - $ 的测定,由于电化学过程中产生的其他氯氧化物(ClO−,${\rm{ClO}}_2^ - $ ,${\rm{ClO}}_4^ - $ )对显色反应的潜在干扰,离子色谱仪的应用可能更有利. -
向废水中投加氯酸盐会对COD的测定存在掩蔽作用,且随着氯酸盐投加量越大掩蔽作用越强,但TOC基本保持不变. 为了消除氯酸盐的影响本文提出了一种以亚硫酸盐为还原剂的预处理工艺. 根据水样中氯酸盐的含量,投加相应的亚硫酸氢钠去除,进而得到真实的COD;并且在温度为60 °C、[
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比为5—6时,能够使COD快速有效的恢复到初始值. 但是当废水中氯酸盐浓度高于3 mmol·L−1时,可先将氯酸盐浓度稀释至3 mmol·L−1以下,再投加相应的亚硫酸盐. 通过ESR分析揭示了${\rm{SO}}_3^{ {\rm{·}}-} $ 和${\rm{SO}}_4^{ {\rm{·}}-} $ 的产生,提出亚硫酸盐与氯酸盐首先通过单电子转移反应生成${\rm{SO}}_3^{ {\rm{·}}-} $ ,溶解氧氧化${\rm{SO}}_3^{ {\rm{·}}-} $ 进一步生成${\rm{SO}}_4^{ {\rm{·}}-} $ ,${\rm{ClO}}_3^ - $ 的还原中间体如ClO2、${\rm{ClO}}_2^ - $ 和ClO−通过氧转移反应被亚硫酸盐还原,最终产物为Cl−. 反应前后的TOC保持不变,基本不会改变原水的水质,且COD恢复到初始值. 在电化学处理含氯废水的过程中会产生大量的${\rm{ClO}}_3^ -$ ,这可能会导致过高评价电化学COD去除性能,同时排放经电化学处理后的达标废水还会带入新的污染物质(氯酸盐),对生态环境造成潜在的危害.
氯酸盐屏蔽废水COD测定的消除策略及反应机制
Elimination strategy and reaction mechanism for the interference of chlorate on COD
-
摘要: 向废水中投加氯酸盐,即所谓的“COD(化学需氧量)去除剂”,可降低COD检测值,呈现出水符合COD排放标准的假象. 从本质上而言,氯酸盐的添加并未实际去除COD,而仅仅是作为氧化剂减少了在高温消解过程中重铬酸钾的消耗量,导致测定的COD数值偏小,因此氯酸盐只是起到了掩蔽作用. 为消除氯酸盐对COD的掩蔽作用,提出了一种以亚硫酸盐为还原剂的预处理工艺:根据氯酸盐含量投加相应的亚硫酸氢钠进行去除,进而测定出真实的COD. 结果表明,当[
${\rm{SO}}_3^{2-} $ ]/[ClO3−]摩尔比为5—6、温度为60 °C时,经过1 h的反应可以有效的消除氯酸盐对COD测定的影响,此时${\rm{SO}}_3^{2-} $ 和${\rm{ClO}}_3^- $ 同步耗尽. 基于ESR(电子自旋共振)分析,可知氯酸盐与亚硫酸盐首先通过单电子转移反应生成${\rm{SO}}_3^{\cdot-} $ ,生成的${\rm{SO}}_3^{\cdot−} $ 与溶解氧进一步发生反应生成${\rm{SO}}_4^{\cdot-} $ ,而${\rm{ClO}}_3^- $ 的还原中间体(ClO2、${\rm{ClO}}_2^- $ 和ClO−)可与亚硫酸根继续通过氧转移反应而被还原,最终产物为Cl−;通过反应前后的三维荧光光谱图发现生成的自由基虽然可以氧化部分有机物,但是并未发生矿化反应,因此经处理后所测定的COD值即为真实值. 总而言之,本研究所提出的亚硫酸盐消除氯酸盐对COD测定干扰的方法切实可行,为完善COD测定方法及理论提供参考.Abstract: Deliberate addition of mildly oxidative chlorate (${\rm{ClO}}_3^- $ ), so-called “COD (chemical oxygen demand) degrader”, into wastewater induces the false COD reduction, which would bring about false appearance of effluents meeting the COD discharge standards. In essence, chlorate doesn’t really remove COD but just masks the COD determination process, where it acts as an alternative oxidant to dichromate and therefore the consumption of dichromate is reduced. Herein, a pretreatment process using sulfite as reducing agent was proposed to reduce chlorate and therefore eliminate the masking effect of chlorate on COD determination. It was demonstrated that the COD value could be restored well after 1 h reduction of${\rm{ClO}}_3^- $ by sulfite at 60 °C with the [sulfite]/[chlorate] molar ratio value of 5—6, accompanying the synchronously complete depletion of these two reactants. Based on ESR (electron spin-resonance spectroscopy) analysis, the reaction between${\rm{ClO}}_3^- $ and sulfite mainly proceeded via one-electron transfer process with the generation of${\rm{SO}}_3^{\cdot-} $ , the subsequent formation of${\rm{SO}}_4^{\cdot-} $ derived from the oxidation of${\rm{SO}}_3^{\cdot-} $ by the dissolved O2. The${\rm{ClO}}_3^- $ reduction intermediates, such as ClO2,${\rm{ClO}}_2^- $ and ClO−, could be further reduced by sulfite via oxygen transfer process with Cl− as the final product. Although the three-dimensional fluorescence spectra indicating that the generated free radicals could oxidize part of the organic matter, no mineralization reaction occurred, so the COD value measured after the treatment is the true value. In general, this study pioneered an effective pretreatment method using sulfite as reducing agent to eliminate the interference of chlorate on COD determination and provide reference for improving COD determination method and theory.-
Key words:
- chlorate /
- COD /
- masking effect /
- sulfites /
- free radicals
-

-
图 3 [
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ]摩尔比对ClO3−-亚硫酸盐体系中COD(a)和Δ[${\rm{SO}}_3^{2-} $ ]/Δ [${\rm{ClO}}_3^ - $ ](b)的影响.Figure 3. Effect of [
${\rm{SO}}_3^{2-} $ ]/[${\rm{ClO}}_3^ - $ ] molar ratio on COD (a) and Δ[${\rm{SO}}_3^{2-} $ ]/Δ [${\rm{ClO}}_3^ - $ ] (b) in the${\rm{ClO}}_3^ - $ -sulfite system.表 1 氯酸盐电化学生成的研究汇总
Table 1. Summary of Electrochemical Chlorate Formation in Previous Studies
水样
Water treated阳极
Anode电流密度/(mA·cm−2)
Current density[COD]0/[COD]t/
(mg·L−1)处理时间/h
t[Cl−]0
/
(mmol·L−1)[ClO3−]max/ (mmol·L−1) 参考文献
References尿液
UrineTi/RuOx-IrOx 100 — 8 105 26 [24] TDIROF 20 1710/600 25 35 25 [25] BDD 20 1710/100 17.5 35 20 [25] 厕所废水
LatrineTiO2/IrO2 15 500/(<30) 12 100 73 [26] BDD 15 500/(<30) 2 30 12 [26] TDIROF,热分解氧化铱膜. TDIROF,Thermally decomposed iridium oxide film. -
[1] 郭庆英, 刘晓茜, 李晶. 芬顿高级氧化用于工业污水厂深度处理提标改造 [J]. 中国给水排水, 2019, 35(10): 64-67. doi: 10.19853/j.zgjsps.1000-4602.2019.10.012 GUO Q Y, LIU X Q, LI J. Application of Fenton advanced oxidation process for upgrading and reconstruction project of an industrial wastewater treatment plant [J]. China Water & Wastewater, 2019, 35(10): 64-67(in Chinese). doi: 10.19853/j.zgjsps.1000-4602.2019.10.012
[2] SU T, WANG Z K, ZHOU K, et al. Advanced treatment of secondary effluent organic matters (EfOM) from an industrial park wastewater treatment plant by Fenton oxidation combining with biological aerated filter [J]. Science of the Total Environment, 2021, 784: 147204. doi: 10.1016/j.scitotenv.2021.147204 [3] IKHLAQ A, BROWN D R, KASPRZYK-HORDERN B. Mechanisms of catalytic ozonation: An investigation into superoxide ion radical and hydrogen peroxide formation during catalytic ozonation on alumina and zeolites in water [J]. Applied Catalysis B:Environmental, 2013, 129: 437-449. doi: 10.1016/j.apcatb.2012.09.038 [4] GANZOURY M A, GHASEMIAN S, ZHANG N, et al. Mixed metal oxide anodes used for the electrochemical degradation of a real mixed industrial wastewater [J]. Chemosphere, 2022, 286: 131600. doi: 10.1016/j.chemosphere.2021.131600 [5] 赵传勋, 李德辉, 余黄杰, 等. 污水COD达标深度处理技术研究进展 [J]. 北京石油化工学院学报, 2021, 29(3): 61-66. doi: 10.19770/j.cnki.issn.1008-2565.2021.02.0012 ZHAO C X, LI D H, YU H J, et al. Research progress of the wastewater COD advanced treatment technology for meeting the standard requirements [J]. Journal of Beijing Institute of Petrochemical Technology, 2021, 29(3): 61-66(in Chinese). doi: 10.19770/j.cnki.issn.1008-2565.2021.02.0012
[6] MENG X S, KHOSO S A, LYU F, et al. Study on the influence and mechanism of sodium chlorate on COD reduction of minerals processing wastewater [J]. Minerals Engineering, 2019, 134: 1-6. doi: 10.1016/j.mineng.2019.01.009 [7] 方齐乐, 陈宝梁. 新型环境污染物高氯酸盐的环境化学行为、食品安全及健康风险 [J]. 科学通报, 2013, 58(26): 2626-2642. doi: 10.1360/972013-195 FANG Q L, CHEN B L. Environmental transport behaviors of perchlorate as an emerging pollutant and their effects on food safety and health risk [J]. Chinese Science Bulletin, 2013, 58(26): 2626-2642(in Chinese). doi: 10.1360/972013-195
[8] 张小磊, 苍岩, 宋伟, 等. 二氧化氯预氧化含藻水过程中副产物的生成规律 [J]. 环境化学, 2019, 38(2): 306-316. doi: 10.7524/j.issn.0254-6108.2018040203 ZHANG X L, CANG Y, SONG W, et al. By-product formation in algae-containing water pre-oxidized by chlorine dioxide [J]. Environmental Chemistry, 2019, 38(2): 306-316(in Chinese). doi: 10.7524/j.issn.0254-6108.2018040203
[9] 仲和. 污水处理厂使用“COD去除剂”被认定为数据造假[J]. 中国环境监察, 2021(S1): 69. ZHONG H. The use of "COD removers" in sewage treatment plants was found to be data fraud [J]. China Environment Supervision, 2021(Sup 1): 69(in Chinese).
[10] TORRES-ROJAS F, MUÑOZ D, TAPIA N, et al. Bioelectrochemical chlorate reduction by Dechloromonas agitata CKB [J]. Bioresource Technology, 2020, 315: 123818. doi: 10.1016/j.biortech.2020.123818 [11] LAKHIAN V, DICKSON-ANDERSON S E. Reduction of bromate and chlorate contaminants in water using aqueous phase Corona discharge [J]. Chemosphere, 2020, 255: 126864. doi: 10.1016/j.chemosphere.2020.126864 [12] GONCE N, VOUDRIAS E A. Removal of chlorite and chlorate ions from water using granular activated carbon [J]. Water Research, 1994, 28(5): 1059-1069. doi: 10.1016/0043-1354(94)90191-0 [13] 中华人民共和国环境保护部. 水质 化学需氧量的测定 快速消解分光光度法: HJ/T 399—2007[S]. 北京: 中国环境科学出版社, 2008. Ministry of Environmental Protection of the People's Republic of China. Water quality-Determination of the chemical oxygen demand-Fast digestion-Spectrophotometric method: HJ/T 399—2007[S]. Beijing: China Environment Science Press, 2008(in Chinese).
[14] 王德明. 水体TOC与CODCr、BOD5、CODMn相关性研究 [J]. 化学分析计量, 2010, 19(3): 61-64. doi: 10.3969/j.issn.1008-6145.2010.03.050 WANG D M. Study on the correlation of toc with codcr, bod5 and codmn in water [J]. Chemical Analysis and Meterage, 2010, 19(3): 61-64(in Chinese). doi: 10.3969/j.issn.1008-6145.2010.03.050
[15] 缪佳, 陈开榜, 朱佳, 等. 氯酸盐对电镀废水COD检测的掩蔽机理初步分析 [J]. 中国给水排水, 2018, 34(23): 80-84. doi: 10.19853/j.zgjsps.1000-4602.2018.23.016 MIAO J, CHEN K B, ZHU J, et al. Preliminary analysis of masking mechanism of chlorate on COD detection in electroplating wastewater [J]. China Water & Wastewater, 2018, 34(23): 80-84(in Chinese). doi: 10.19853/j.zgjsps.1000-4602.2018.23.016
[16] 陈开榜. 氯酸盐对电镀废水COD检测掩蔽机理分析[D]. 杭州: 浙江工业大学, 2018: 44-45. CHEN K B. Study on the masking mechanism of chlorate on electroplating wastewater COD(cr) detection[D]. Hangzhou: Zhejiang University of Technology, 2018: 44-45. (in Chinese)[知网硕士中文][知网硕士英文] [17] 国家环境保护总局. 污水综合排放标准: GB 8978—1996[S]. 北京: 中国标准出版社, 1998. State Environmental Protection Administration of the People's Republic of China. Integrated wastewater discharge standard: GB 8978—1996[S]. Beijing: Standards Press of China, 1998(in Chinese).
[18] QIAO J L, FENG L Y, DONG H Y, et al. Overlooked role of sulfur-centered radicals during bromate reduction by sulfite [J]. Environmental Science & Technology, 2019, 53(17): 10320-10328. [19] ZAMORA P L, VILLAMENA F A. Theoretical and experimental studies of the spin trapping of inorganic radicals by 5, 5-dimethyl-1-pyrroline N-oxide (DMPO). 3. Sulfur dioxide, sulfite, and sulfate radical anions [J]. The Journal of Physical Chemistry. A, 2012, 116(26): 7210-7218. doi: 10.1021/jp3039169 [20] BRANDT C, van ELDIK R. Transition metal-catalyzed oxidation of sulfur(IV) oxides. atmospheric-relevant processes and mechanisms [J]. Chemical Reviews, 1995, 95(1): 119-190. doi: 10.1021/cr00033a006 [21] TIMMINS G S, LIU K J, BECHARA E J H, et al. Trapping of free radicals with direct in vivo EPR detection: A comparison of 5, 5-dimethyl-1-pyrroline-N-oxide and 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide as spin traps for HO and SO4•− [J]. Free Radical Biology and Medicine, 1999, 27(3/4): 329-333. [22] DAVIES M J, GILBERT B C, STELL J K, et al. Nucleophilic substitution reactions of spin adducts. Implications for the correct identification of reaction intermediates by EPR/spin trapping [J]. Journal of the Chemical Society, Perkin Transactions 2, 1992(3): 333. doi: 10.1039/p29920000333 [23] HALPERIN J, TAUBE H. The transfer of oxygen atoms in oxidation—reduction reactions. III. the reaction of halogenates with sulfite in aqueous solution [J]. Journal of the American Chemical Society, 1952, 74(2): 375-380. doi: 10.1021/ja01122a026 [24] 王飞, 刘峻峰, 张杰, 等. 电化学氧化对尿液处理过程中消毒副产物的生成控制和去除 [J]. 环境工程学报, 2021, 15(9): 2973-2984, 2846. doi: 10.12030/j.cjee.202104054 WANG F, LIU J F, ZHANG J, et al. Control and removal of disinfection by-products(DBPs) during electrochemical oxidation of urine [J]. Chinese Journal of Environmental Engineering, 2021, 15(9): 2973-2984, 2846(in Chinese). doi: 10.12030/j.cjee.202104054
[25] ZÖLLIG H, REMMELE A, FRITZSCHE C, et al. Formation of chlorination byproducts and their emission pathways in chlorine mediated electro-oxidation of urine on active and nonactive type anodes [J]. Environmental Science & Technology, 2015, 49(18): 11062-11069. [26] JASPER J T, YANG Y, HOFFMANN M R. Toxic byproduct formation during electrochemical treatment of latrine wastewater [J]. Environmental Science & Technology, 2017, 51(12): 7111-7119. [27] RITTMANN B E. Aerobic biological treatment. Water treatment processes [J]. Environmental Science & Technology, 1987, 21(2): 128-136. [28] WANG X, DAIGGER G, LEE D J, et al. Evolving wastewater infrastructure paradigm to enhance harmony with nature [J]. Science Advances, 2018, 4(8): eaaq0210. doi: 10.1126/sciadv.aaq0210 -



















 下载:
下载: