-
由于在河流和湖泊等水系统中检测到的浓度越来越高,抗生素污染已成为一个全球性问题,对环境和人类健康造成潜在风险[1-2]。在众多抗生素中,氯霉素(chloramphenicol, CAP)是一种广谱抗生素,用于治疗由革兰氏阳性和革兰氏阴性菌引起的感染。我国规定不得在动物源性食品中检出氯霉素,但由于其易于获得、价格低廉、抗菌效果稳定、抑菌效果好,仍有人在动物源性食品生产中违规添加[3]。氯霉素在水体中的存在会对生态系统造成危害,对人类健康造成风险。
自然水体当中抗生素的去除方法主要有吸附法[4-5]、生物降解法[6]、光催化法[7]和化学氧化法[8]等。吸附法由于其低能量成本、吸附能力高以及环境友好性,是一种有效的去除抗生素方法。生物炭是一种优异的吸附剂,由生物质(如农林废弃物)在缺氧条件下热解形成[9],具有比表面积高、孔容大、介电常数高等优越特性,能够有效地实现物理吸附和化学吸附,从而达到降低水体中有机污染物浓度的目的[10]。山竹是藤本植物科山竹属的一种热带季节性植物,在亚洲国家有大量种植,每公斤山竹大约会产生0.6 kg果壳[11],由于尚未被发现开发利用的价值,造成每年有大量的山竹壳被当作农业废物丢弃,对环境造成极大的负担,利用热解方式回收废弃山竹壳制备生物炭是一种很有前景的方法。山竹壳主要成分是纤维素、半纤维素、木质素和果胶等,经热解和表面改性后,其比表面积、孔结构和表面官能团均发生了变化[12-15]。较大的比表面积和多孔结构以及丰富的表面官能团均有利于对Cr(Ⅵ)、Ni(Ⅱ)、Pb(Ⅱ)等重金属的吸附[14],也为利用山竹壳炭去除废水中抗生素尤其是氯霉素提供了可能。
直接热解得到的生物炭通常难以达到足够高的比表面积和孔容,而通过化学活化的方法可以显著改善生物炭的孔隙结构。常用的化学活化剂为强碱(KOH、NaOH)、盐(Na2CO3、K2CO3和K2C2O4)和强酸(H3PO4和H2SO4)等[16]。其中,K2C2O4因价格低廉、腐蚀性弱且活化性能优异受到关注。CUI等[17]对比了K2C2O4、KOH和K2CO3对辣椒秸秆的活化效果及对Hg的吸附能力影响,发现K2C2O4活化得到的辣椒秸秆炭比表面积和孔容最大,吸附效果最佳。
生物炭吸附性能优越且价格低廉,但在实际应用中,却因传统的过滤分离方法易造成活性炭的流失以及筛网的堵塞,分离回收困难。为此,将磁性介质铁、钴和镍等引入到生物炭上,制备具有一定磁性的生物炭,从而可利用磁性辅助分离技术对生物炭进行分离、回收逐渐成为研究的热点[18]。
因此,本研究以废弃山竹壳为原料,通过控制赋磁剂硝酸铁(Fe(NO3)3)和活化剂草酸钾(K2C2O4)的用量,对制得的磁性山竹壳炭理化性能进行研究,并进一步考察山竹壳炭对废水中氯霉素的吸附行为,以期揭示氯霉素吸附的微观机制,为废弃山竹壳的资源化利用及废水中氯霉素的去除提供参考。
-
所用山竹壳购于安徽省芜湖市某超市;氯霉素USP级,购买于上海蓝季生物;其余药品均为分析纯,购买于麦克林生化科技公司。
-
将新鲜的山竹壳掰成约5 mm×5 mm碎片,用蒸馏水冲洗杂质后干燥、破碎、过80目筛待用。准确称取2.0 g K2C2O4和1.5 g Fe(NO3)3制成30 mL混合溶液,加入5 g山竹粉,静置6 h后80 ℃烘干多余水分。干燥后样品采用管式炉850 ℃炭化2 h,升温速率5 ℃·min−1。炭化后山竹壳经80 ℃水浴6 h后用蒸馏水洗涤至中性,烘干后命名为PGC-4-850。按表1改变制备条件,得样品PGC-4-800、PGC-4-900、PGC-3-850、PGC-5-850、PC-850、GC-850和C-850。
-
比表面积和孔径采用全自动比表面积分析仪(NOVA4200E, Quantachrome, USA)在77 K下测量; 样品表面形貌和元素分布采用扫描电子显微镜(S-3400N, Hitachi, Japan)进行显微观察; 表面官能团采用傅里叶变换红外光谱仪(Nicolet iS 50, Thermo Fisher, USA)分析;磁滞回归线采用振动样品磁强计(Lake Shore 7404, Lake Shore Cryotronics, Inc, USA)分析;物相分析由X射线衍射仪(Bruker D8 ADVANCE, Germany)分析;采用X射线光电子能谱(Perkin-Elmer PHI 550 ESCA/SAM, USA)对活性炭样品表面元素的化学形态进行表征;样品Zeta电位由Zeta电位及粒度分析仪(NanoBrook 90PlusPALS, USA)分析,测量固液比为1 g·L−1。
-
分别称取不同条件下制备的山竹壳炭0.03 g,放置到100 mL初始质量浓度为125 mg·L−1的氯霉素溶液中, 30 ℃恒温水浴、300 r·min−1条件下进行吸附,依次间隔5、10、15、25、30、60、60、60、60、60、60、120 min取上层清液1 mL过0.45 μm滤膜,滤液用紫外-可见分光光度计于278 nm处测量吸光度。通过式(1)计算氯霉素的吸附量。分别采用拟一级动力学方程(式(2))、拟二级动力学方程(式(3))和Bangham方程(式(4))对吸附过程进行拟合。
式中:
$ {C}_{0} $ 为吸附前氯霉素溶液的质量浓度,mg·L−1;$ {C}_{\mathrm{e}} $ 为吸附后氯霉素溶液的质量浓度,mg·L−1;$ V $ 为氯霉素溶液的体积,L;$ m $ 为生物炭的用量,g。式中:
$ {q}_{\mathrm{e}} $ 为平衡时的吸附量,mg·g−1;$ {q}_{t} $ 为时间t的吸附量,mg·g−1; t为吸附时间,min;$ {k}_{1} $ 为拟一级动力学吸附速率常数,min−1;$ {k}_{2} $ 为拟二级动力学吸附速率常数,g·(mg·min)−1;m为物性参数,$ k $ 3为速率常数。 -
称取30 mg磁性山竹壳炭PGC-3-850于50 mL离心管中,分别加入初始质量浓度 25、50、75、100、125、150 mg·L−1的50 mL氯霉素溶液中,用恒温金属摇匀仪在300 r·min−1、20 ℃下振荡24 h后,取上层清液1 mL过0.45 μm滤膜,滤液用紫外-可见分光光度计于278 nm处测量吸光度,计算得到吸附值。改变恒温金属摇匀仪的温度得到30 ℃和40 ℃下等温吸附数据。分别用Langmuir方程(式(5))、Freundlich方程(式(6))、D-R方程(式(7))和Temkin方程(式(8))拟合实验数据。根据式(9)~式(11)计算吸附过程热力参数。
式中,
$ {Q}_{\mathrm{m}\mathrm{a}\mathrm{x}} $ 为理论最终大吸附容量,mg·g−1;$ {K}_{\mathrm{L}} $ 为Langmuir方程平衡常数;$ {K}_{\mathrm{F}} $ 为Freundlich 常数;n为经验常数;$ {R}_{\mathrm{L}} $ 为分离因子;$ {Q}_{\mathrm{m}} $ 是饱和吸附容量,mg·g−1;B和ε是D-R方程中的吸附能参数和能量参数;$ {a}_{t} $ 和$ {b}_{t} $ 分别是Temkin方程的模型参数,L·g−1和J·mol−1。式中,
$ {K}_{\mathrm{c}} $ 为平衡分配系数常数;T为绝对温度,K;R为理想气体常数,为8.314 J·(mol·K)−1。 -
以PGC-3-850为吸附剂,以125 mg·L−1氯霉素溶液为吸附质,用稀NaOH和盐酸溶液调节溶液pH=3~11,其余条件同1.4,考察溶液pH对山竹壳炭吸附氯霉素的影响。
-
溶剂再生:分别以50%乙醇溶液和水为脱附剂解离吸附在PGC-3-850上的氯霉素,循环进行 5 次吸附-解吸实验,以评估PGC-3-850的溶剂再生性能;热法再生:吸附后的PGC-3-850置于300 ℃烘箱,恒温2 h以解吸氯霉素,循环进行5次吸附-解吸实验,以评估PGC-3-850的热法再生性能。
-
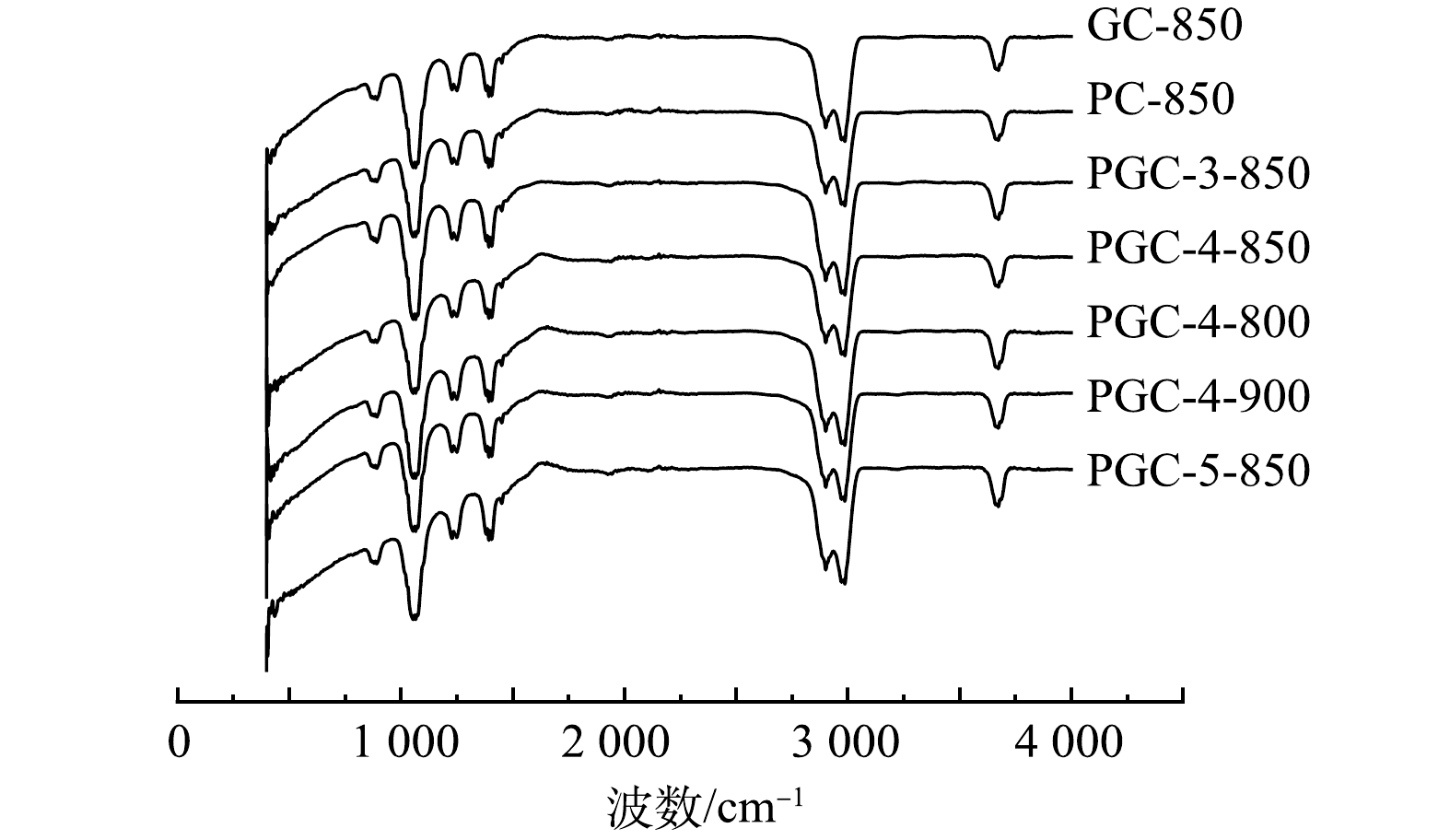
采用FTIR方法定性检测不同制备条件下山竹壳炭表面官能团的相似性和差异性,图1为各样品的透射图谱。可见,各样品FTIR谱图相似,吸收峰主要为3 670 cm−1对应的羟基O—H伸缩振动峰、2 987 cm−1和2 901 cm−1对应的C—H(—CH3和—CH2—)的伸缩振动峰,1 280~1 410 cm−1为O—H的吸收峰,1 050 cm−1和1 230 cm−1分别为伯醇和酚的C—O吸收峰,而900 cm−1左右的特征峰被认为与芳香结构有关。在 500~600 cm−1没有出现 Fe3O4中的Fe—O特征峰,可能是因为样品中的铁氧化物含量较低且分散[19]。总的来说,无论是否添加Fe(NO3)3或K2C2O4,山竹壳炭中均含有丰富的羟基表面官能团和芳香结构,能够为污染物的吸附提供大量的吸附位点。
-
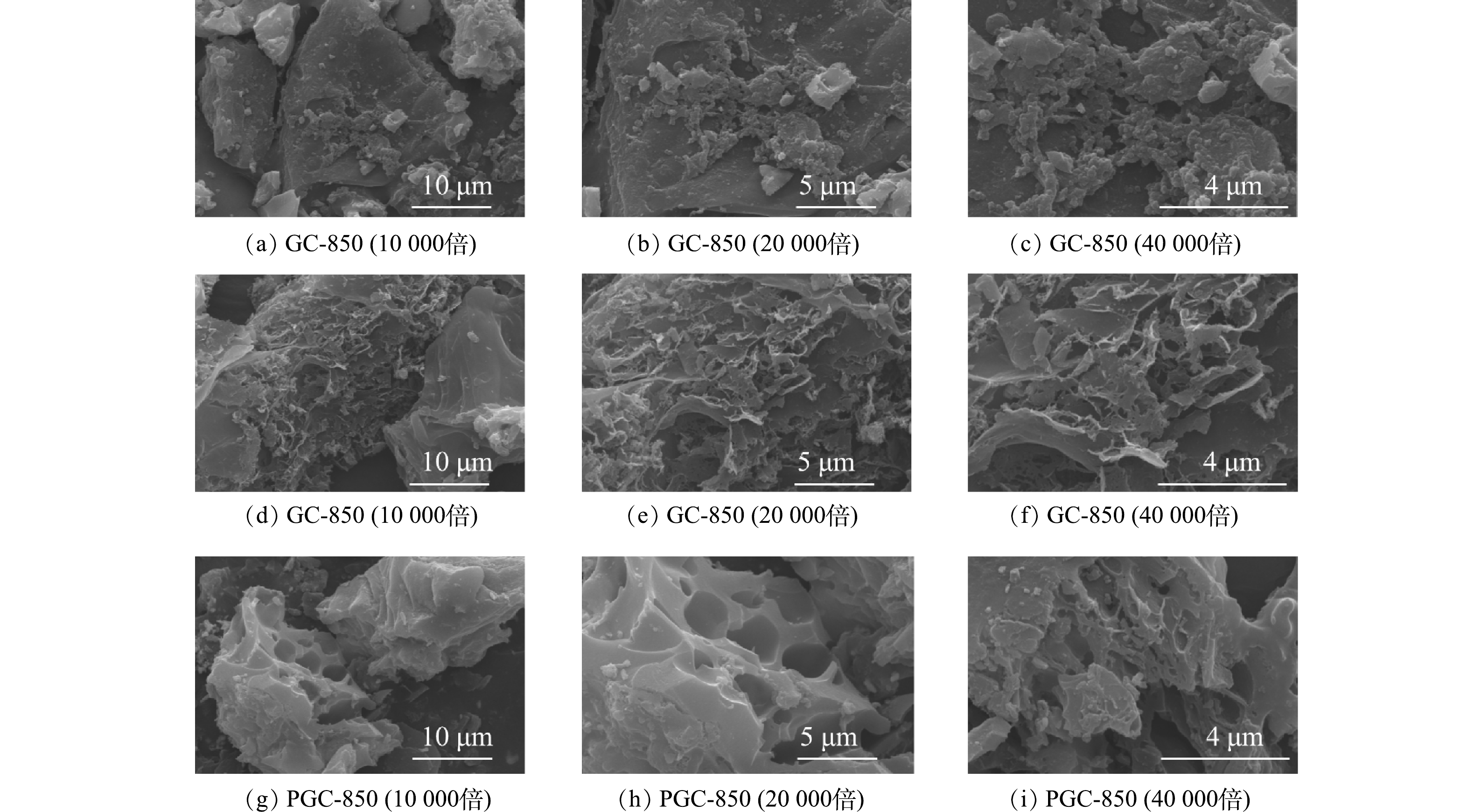
通过扫描电子显微镜可以观察出添加K2C2O4和Fe(NO3)3对山竹壳炭材料微观形貌和结构的影响。如图2(a)所示,当仅添加Fe(NO3)3时,虽然Fe(NO3)3分解过程中会产生气体,但得到的样品PC-850并没有明显的孔隙结构。图2(b)为仅添加K2C2O4的样品,可以看出K2C2O4活化造孔效果明显,得到的GC-850蓬松多孔。当同时添加K2C2O4和Fe(NO3)3时,PGC-3-850山竹壳炭表面同样遭到严重破坏,有明显的孔结构存在,孔隙结构发达,有利于吸附性能的提高。
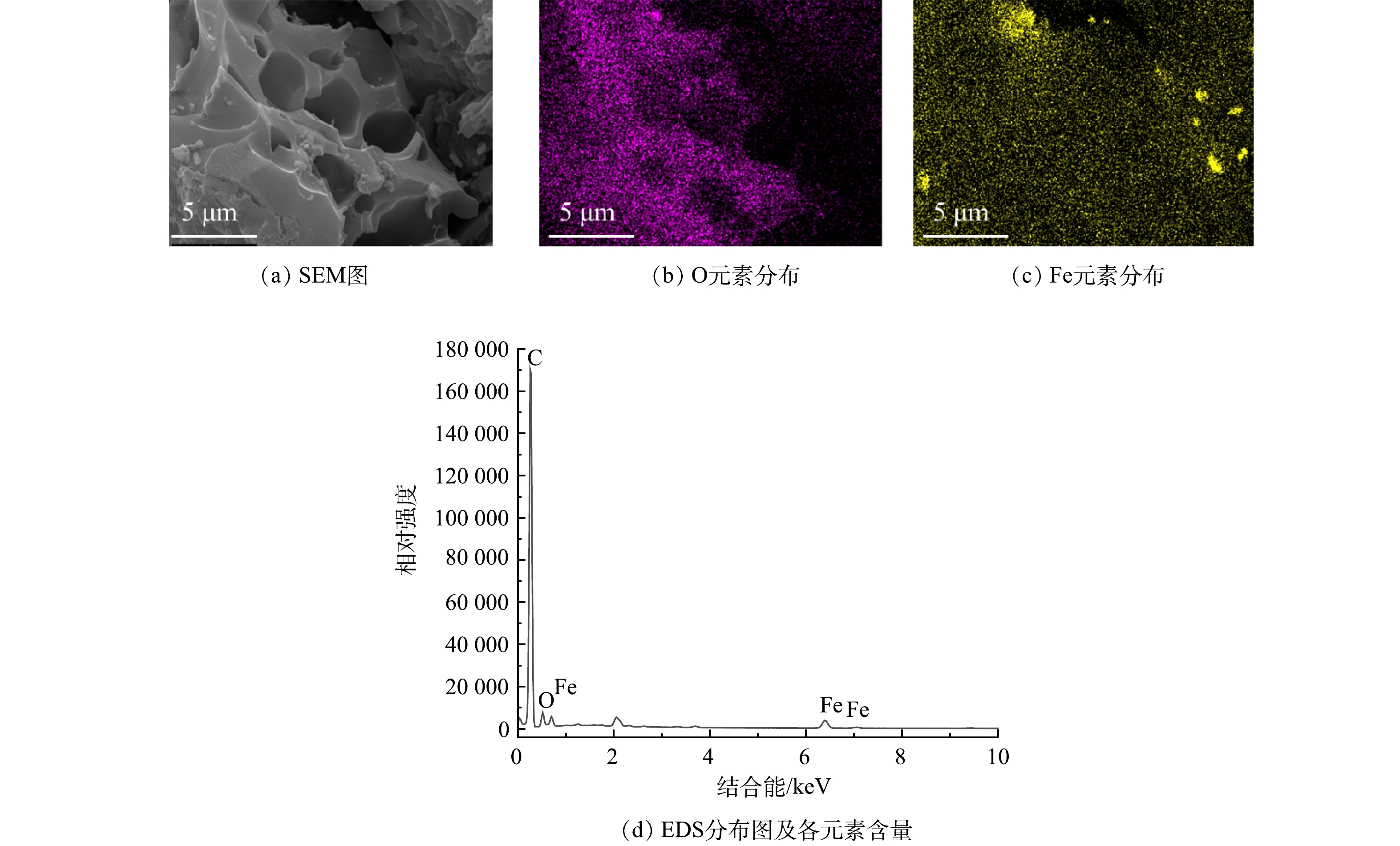
由图3中Mapping 照片和EDS表征结果可以看出,PGC-3-850主要含有C、O、Fe元素,其中C、Fe、O元素的质量占比分别为63.92%、9.15%和17.91%。由此可见,该吸附材料是由吸附前体C元素及磁性材料前体Fe元素组成,水浴洗涤后依然保留大量的Fe元素,同时O元素也较为丰富,这和山竹壳炭本身含有较多的酚醇羟基有关。由Mapping图还可以看出,Fe元素并未均匀分布在山竹壳炭中,少部分Fe元素以颗粒状富集在山竹壳炭表面。对比Fe元素和O元素的分布图,发现O元素分布并未同Fe元素呈现相应的富集一致。这是因为PGC-3-850表面的颗粒状Fe元素并非以氧化物的形式存在,而是主要以单质Fe的形式存在。
-
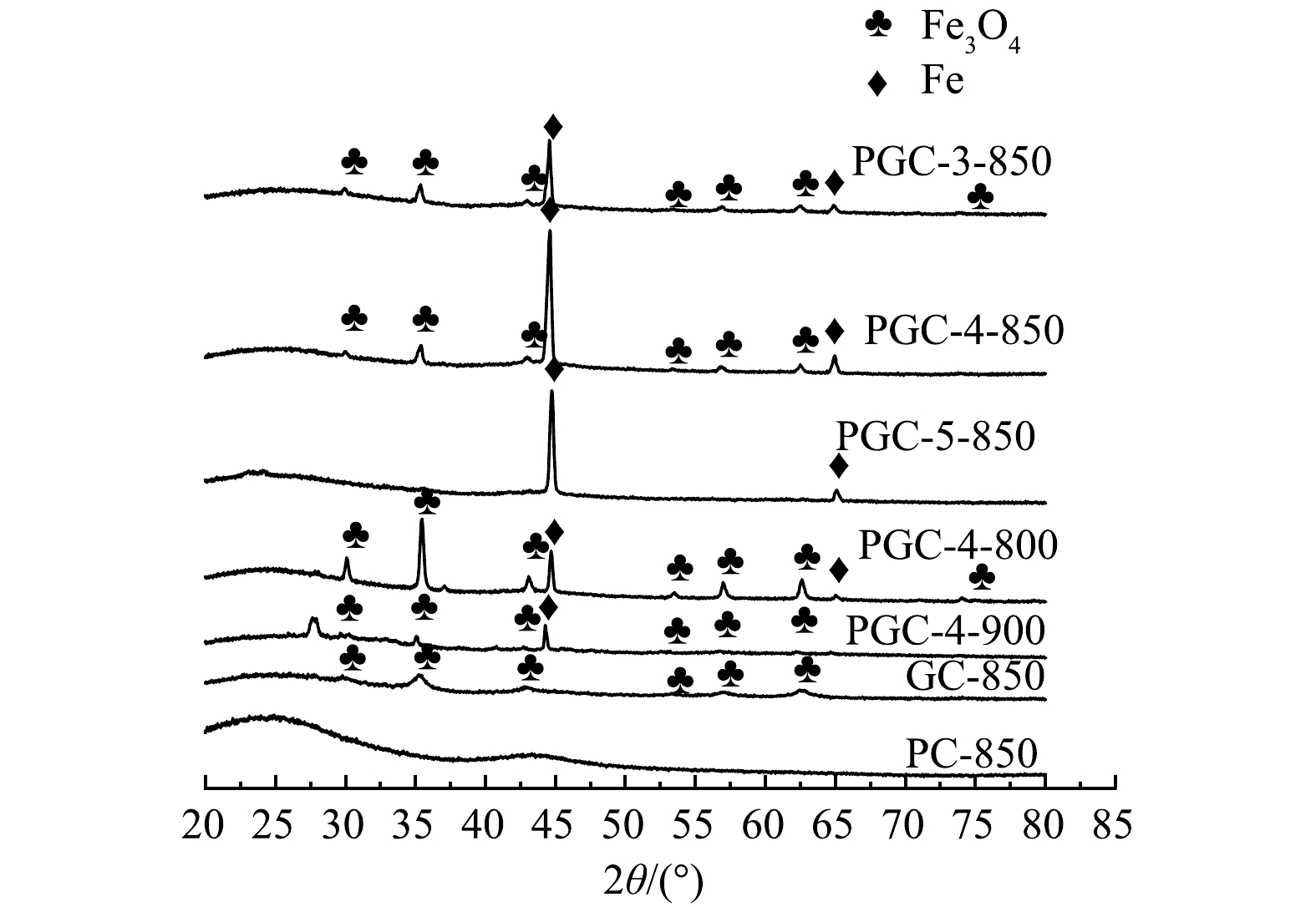
由图4可以看出,添加Fe(NO3)3的各样品在衍射角30.1°、35.4°、43.1°、56.9°、62.5°处有明显的磁铁矿Fe3O4的衍射特征峰。这是因为Fe(NO3)3高温下分解产生的Fe2O3会被炭化过程产生的CO逐步还原成Fe3O4。添加K2C2O4后,由于K2C2O4可分解成K2CO3和CO,CO将与Fe3O4继续反应生成FeO。继续增加K2C2O4用量,Fe元素将全部被还成单质Fe[20]。在衍射角为44.7°和65.0°处出现的单质Fe特征峰也证明了这一点。然而,在各样品的XRD图谱中均未发现FeO的吸收峰。
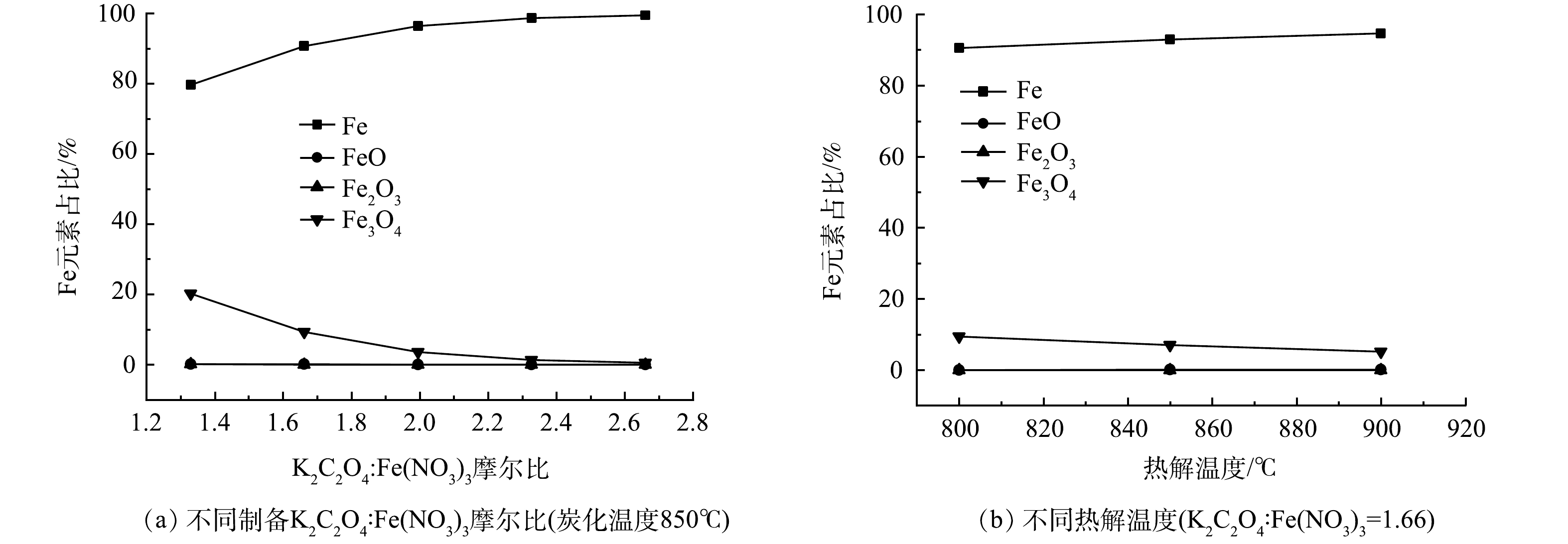
为了分析不同K2C2O4/ Fe(NO3)3摩尔比下Fe元素的相态演变,利用Aspen plus 软件,采用Rstoic反应器模拟K2C2O4与C生成CO和K的反应,以及Fe(NO3)3热分解反应,采用Gibbs反应器模拟各产物在热力学平衡时,以Fe、FeO、Fe2O3或Fe3O4形式存在的铁元素占总铁元素的质量比,模拟结果见图5。由图5(a)可以看出,在实验范围内,铁元素主要以单质Fe和Fe3O4的形式存在,单质Fe的质量含量远大于以Fe3O4形式存在的铁含量。随着K2C2O4含量的增加,Fe3O4逐渐被还原成单质Fe,当K2C2O4与Fe(NO3)3的摩尔比接近2.7时,Fe3O4接近消失。由图5(b)可以看出,随着碳化温度升高,Fe3O4的质量占比逐渐降低,单质Fe质量占比逐渐升高。上述结果表明,PGC-3-850中Fe元素主要以单质Fe和Fe3O4形式存在。
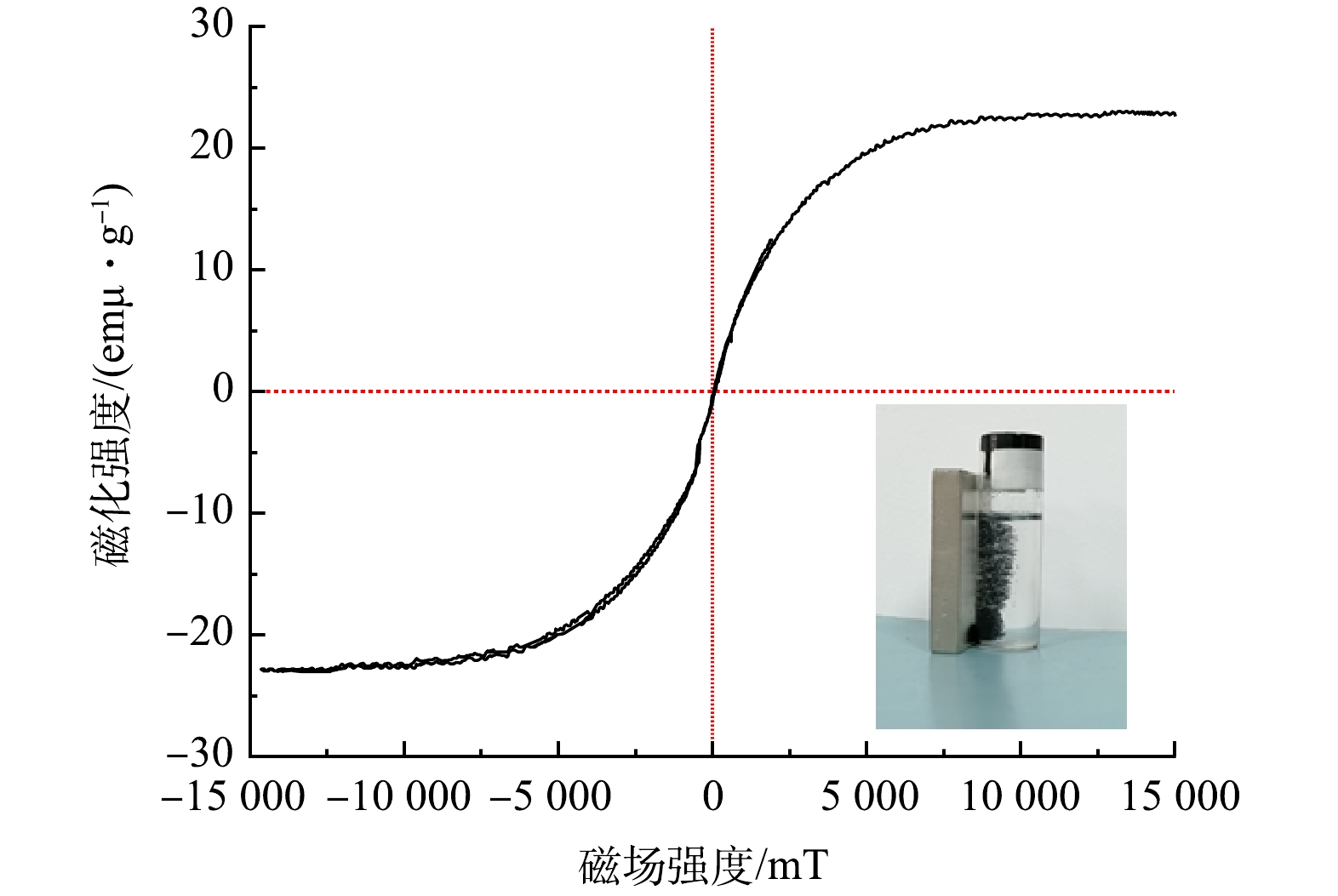
图6为室温(25°C)下获得的PGC-3-850磁滞回线。可以看出,PGC-3-850的饱和磁化强度约为22 emu·g−1,且矫顽力较小,磁化滞后可忽略。这可以防止样品被永久磁化,有利于样品在没有外部磁场的情况下再分散[21]。由图6可以看出,在外加磁场的作用下,吸附剂聚集到磁场的一侧,表明PGC-3-850有明显的磁响应特性,该特性使得吸附剂在使用后方便回收。因此,可以证明Fe(NO3)3在活化过程中明显转化为铁氧体磁性材料。虽然饱和磁化强度值小于文献中报到的纳米Fe3O4颗粒的89.0 emu·g−1,但依然能够实现废水与山竹壳炭的分离。
-
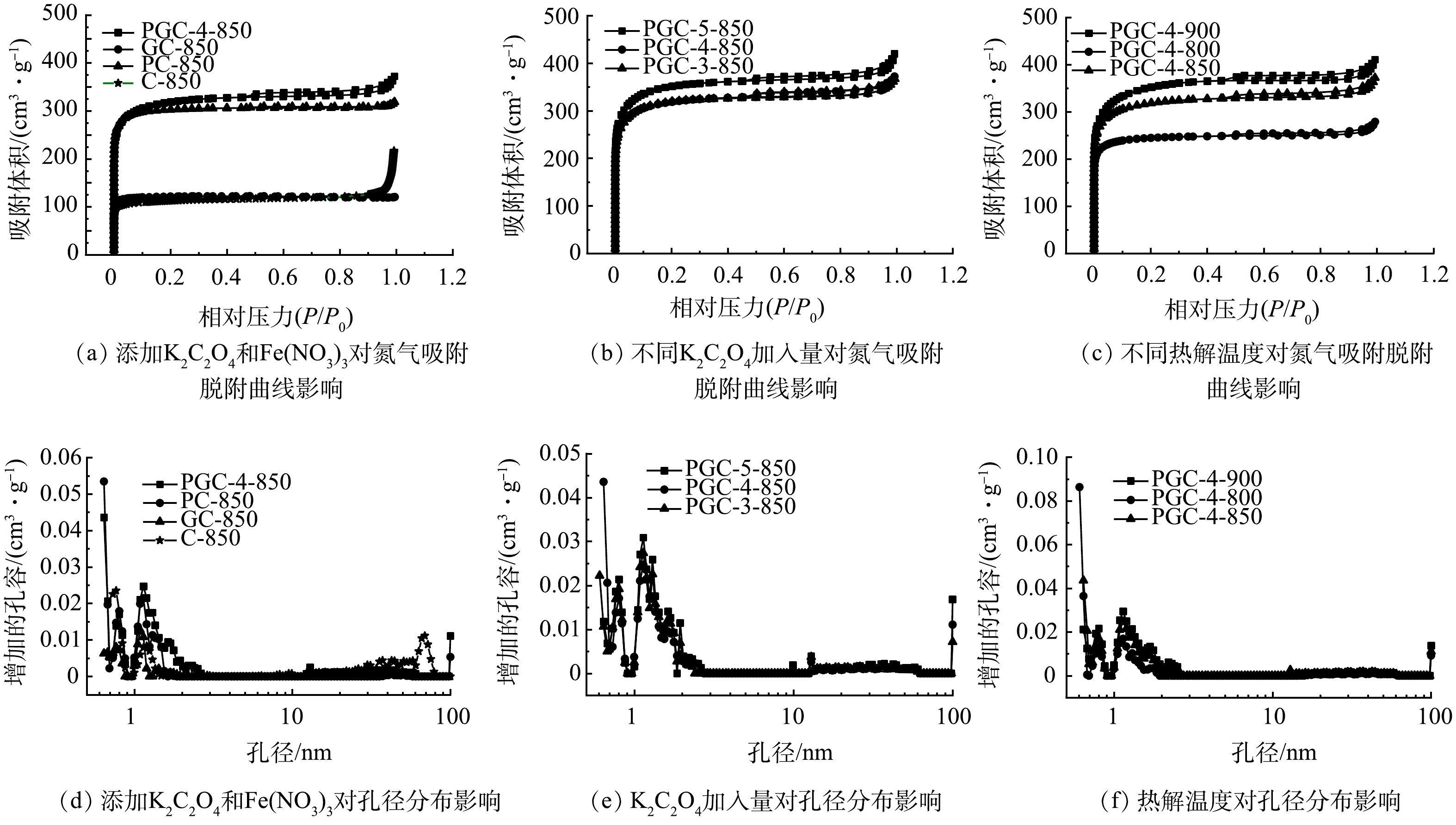
采用氮气吸附/脱附测试对各样品的比表面积和孔径分布进行分析。如图7所示,各样品的氮气吸附/脱附等温线均属于具有 H4 滞后环的 I 型曲线。I型的等温线通常认为是微孔吸附的标志。H4型滞后环的出现归因于具有小窄的狭缝状孔吸附。结合孔径分布可以看出绝大部分为微孔和介孔,孔径在0.8~10 nm,平均孔径为4~6 nm。由图7(b)和图7(c)可以看出,在炭化温度为850 ˚C下,磁性山竹壳炭的氮气吸附量随着K2C2O4用量的增加而增加;碳化温度也是影响氮气吸附量的另一个重要因素,当碳化温度由800 ˚C升到900 ˚C,氮气吸附量明显增加。
表2给出了各样品的孔性参数。空白组C-850的比表面为550.95 m2·g−1,单独添加Fe(NO3)3的样品GC-850,比表面积略有降低,为493.94 m2·g−1,而单独添加K2C2O4后得到PC-850样品,比表面积达到1 208.88 m2·g−1。显而易见,K2C2O4是一种优异造微孔剂,随着K2C2O4用量增加,比表面积和孔容较空白组均显著提升,是空白组的2倍以上。同时添加K2C2O4和Fe(NO3)3的样品PGC-4-850与PC-850相比,比表面积相同,但总孔容增加了47.7%,其中以介孔为主。结合Fe的元素分布情况,可能是因为Fe的加入形成了一种硬支撑,阻止了活化过程介孔的塌缩。同时,由图7(f)可知,提高煅烧温度微孔和总孔体积均显著增加,炭化温度900 ˚C时,微孔孔容最高,可达到0.483 m3·g−1。
-
各样品对初始质量浓度为125 mg·L−1的氯霉素溶液的吸附情况见图8。在吸附的前20 min,吸附速率快,山竹壳炭表面大量的活性吸附位点几乎完全暴露,山竹壳炭表面浓度与氯霉素溶液中的浓度差异很大,大量的氯霉素分子从水溶液中转移到山竹壳炭表面,随着吸附时间的增加,氯霉素占据生物炭表面大部分活性中心,吸附剂逐渐达到饱和状态,活性位点减少,吸附速率逐渐减小[22]。由图8还可以看出,C-850和GC-850具有最低的氯霉素吸附效率,PC-850和PGC-4-800具有相近的吸附能力,均远小于其他样品,吸附速率也低于其他样品。PGC-4-900具有最高的吸附容量,可达316.3 mg·g−1。与水稻秸秆炭[22](127.56 mg·g−1)、玉米秸秆炭[23](32.3 mg·g−1)、葡萄浆废料[24](3.104 mg·g−1)、稻草生物炭[25](60.2 mg·g−1)、香蒲炭[26](137 mg·g−1)和花生壳炭[4](423.7 mg·g−1)等农林废弃物相比,磁性山竹壳炭具有较好的吸附能力,是一种理想的吸附材料。
拟一级动力学方程、拟二级动力学方程和Bangham方程拟合后的参数见表3。可知,各样品的吸附速率曲线更符合拟二级吸附速率方程(R2>0.98),且由模型拟合出的平衡吸附量与实验值更相近。这说明本研究制得的磁性山竹壳炭对氯霉素的吸附过程为物理吸附与化学吸附的复合过程,且受化学吸附控制。Bangham 孔道扩散模型的可决系数R2较低,表明颗粒内孔道扩散不是吸附过程的控制步骤。
-
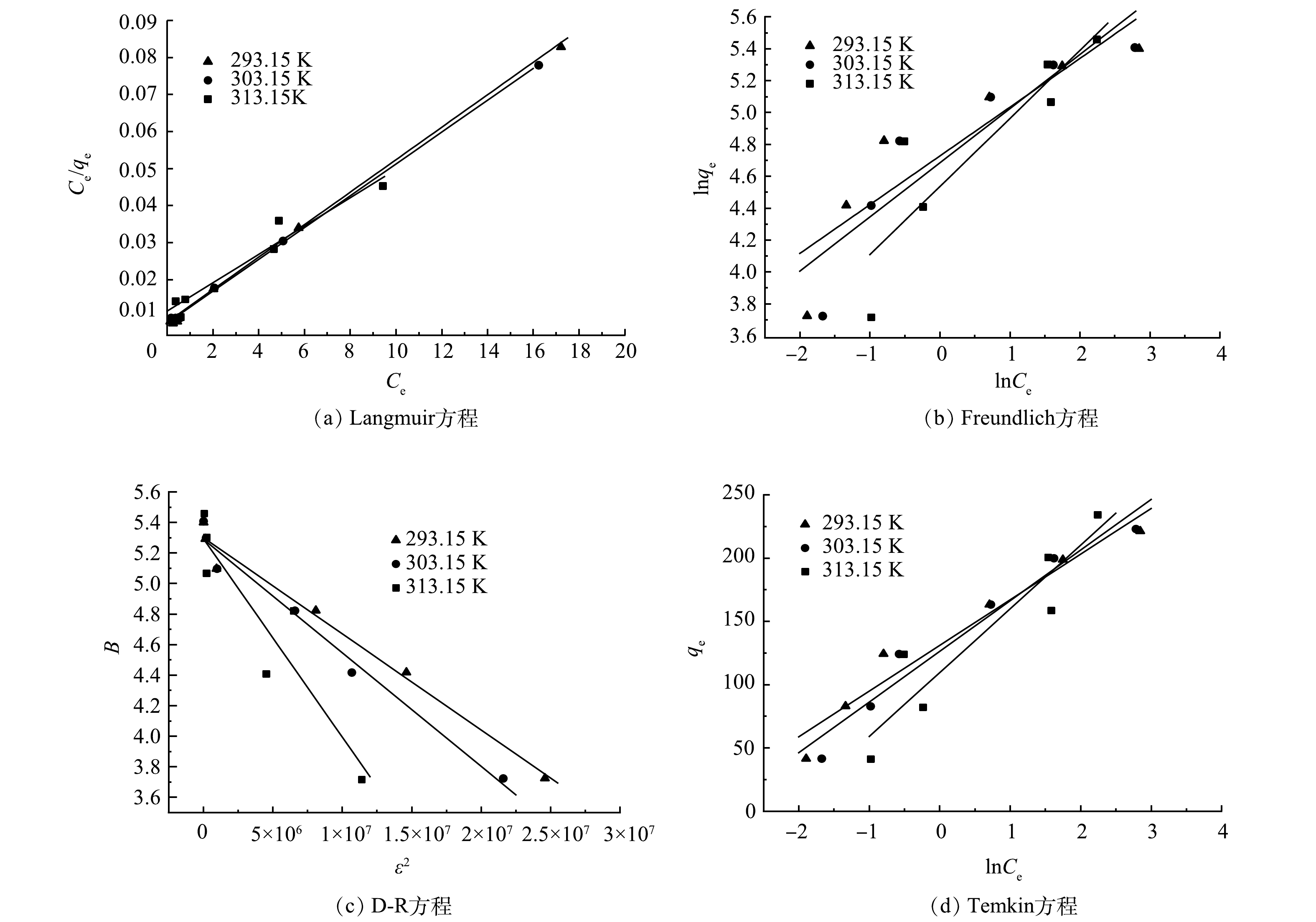
分别利用4种等温线方程对所测得的实验数据进行拟合,得到图9的吸附等温线和表4的吸附等温线拟合参数。由表4可以看出,利用Langmuir方程所拟合得到的可决系数R2大于0.98,高于其他3种模型。因此,PGC-3-850对氯霉素的吸附过程更符合Langmuir模型,即吸附为单分子层吸附[27]。Qmax和
$ {K}_{\mathrm{L}} $ 是吸附剂性能的特征参数,Qmax越大,则表明在同一条件下吸附剂的吸附容量越大,$ {K}_{\mathrm{L}} $ 越大表示吸附速率越快。可以看出在实验范围内,随着吸附温度的升高,吸附容量逐渐变大。通过研究吸附热力学参数来研究吸附过程所能达到的程度,吸附过程的热力学参数主要包括自由能(
$ \Delta G° $ )、焓($ \Delta H° $ )和熵($ \Delta S° $ )等,可由式(9)~式(11)计算得到。计算结果如表5所示,$ \Delta G° $ <0,表明PGC-3-850吸附氯霉素的过程是自发进行;$ \Delta S° $ >0,表明吸附过程中,体系熵值增大,即吸附过程中,整个水溶液体系的混合度增加;$ \Delta H° $ >0,吸附过程吸热,升高温度有利于吸附过程,与吸附等温线数据相吻合。 -
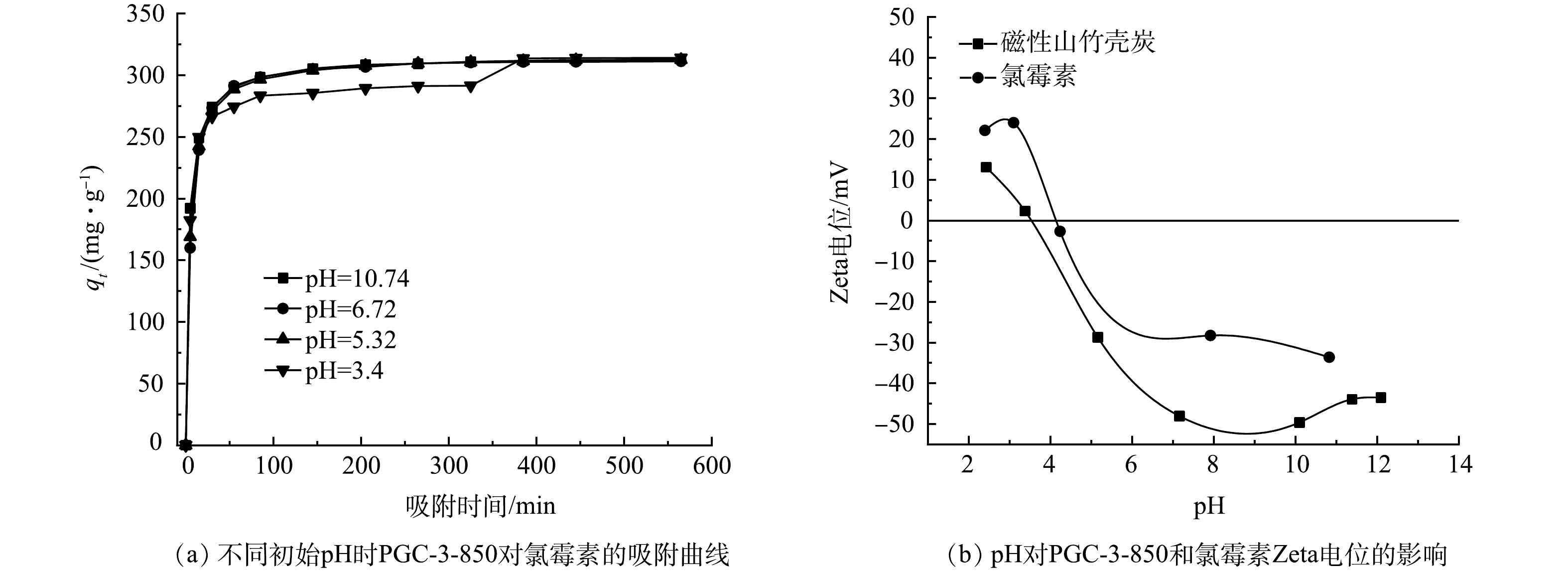
初始pH是影响生物炭吸附性能的最重要参数之一,它既能影响吸附物在溶液中的电离程度,又能够影响生物炭表面官能团的质子化-去质子转变,从而影响生物炭的吸附性能。由图10(a)可以看出,本研究中PGC-3-850对氯霉素的吸附容量受pH影响并不明显。由图10(b) PGC-3-850的ζ电位与pH关系可知,磁性山竹壳活性炭PGC-3-850的pHzpc=3.5。当溶液pH<3.5时,样品表面带正电;当溶液pH>3.5时,样品表面带负电。而当125 mg·L−1氯霉素溶液的pH<4.1时,溶液带正电,当溶液pH>4.1时,溶液带负电。本实验条件下,氯霉素溶液与PGC-3-850均带同种电荷,二者之间为静电排斥,并不能促进PGC-3-850对氯霉素的吸附。这说明静电作用非磁性山竹壳炭对氯霉素吸附的主要机理,本研究制备的磁性山竹壳炭在进行氯霉素吸附时具有宽泛的pH适应性,可用于不同酸碱度条件下含氯霉素污水的修复与治理。
-
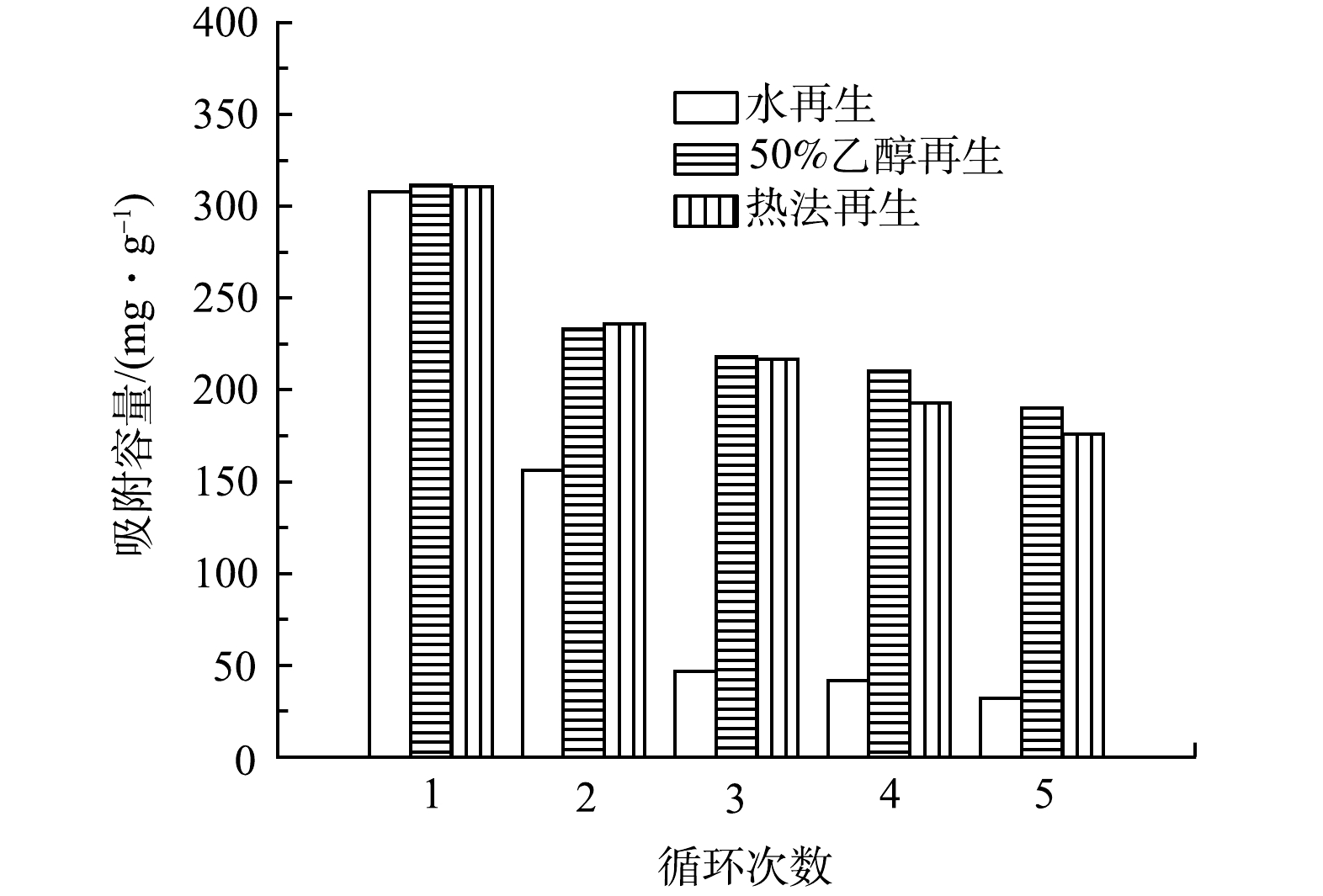
由图11可见,随着循环次数的增加,经3种方法再生的PGC-3-850对氯霉素的吸附效率均呈下降趋势。其原因可能是比表面积和孔容积的减少以及表面官能团的消耗[28]。第1次再生后水作为脱附剂的PGC-3-850对氯霉素的吸附量有较大的下降,由311.3 mg·g−1降到156.3 mg·g−1,5次循环后吸附效率仅为最初的20%。50%乙醇和热法再生的数据相当,一次再生后吸附容量分别为233.2 mg·g−1和236.1 mg·g−1。经过5次循环后,50%乙醇和热法再生仍能保持约60%和55%的去除率。虽然50%乙醇再生效果略好于热法再生,但再生后的再生液产生量很大,难以处理。而热再生法是目前国内外应用最广泛的活性炭再生方法,目前氯霉素的脱附机理、再生产物及其变化规律并不清楚,再生温度和时间也远没有达到最优,将是下一步研究的重点。
-
磁性山竹壳炭对氯霉素的吸附符合Langmuir等温方程和拟二级动力学方程,吸附过程不是简单的扩散,而是物理吸附和化学吸附的共同作用,受化学吸附控制的单分子层吸附。对比吸附前后PGC-3-850的吸附等温线(图12)可以发现,吸附后的PGC-3-850中0.55~1.5 nm的微孔已经完全被氯霉素填充。理论上,当孔径比吸附剂分子大1.7~3倍时,吸附剂具有最佳的吸附性能[29]。从数值上来说,氯霉素分子的尺寸为0.46 nm[4],被填充的孔径小于1.5 nm,更接近氯霉素分子的1.7~3倍,吸附过程中存在微孔填充效应。
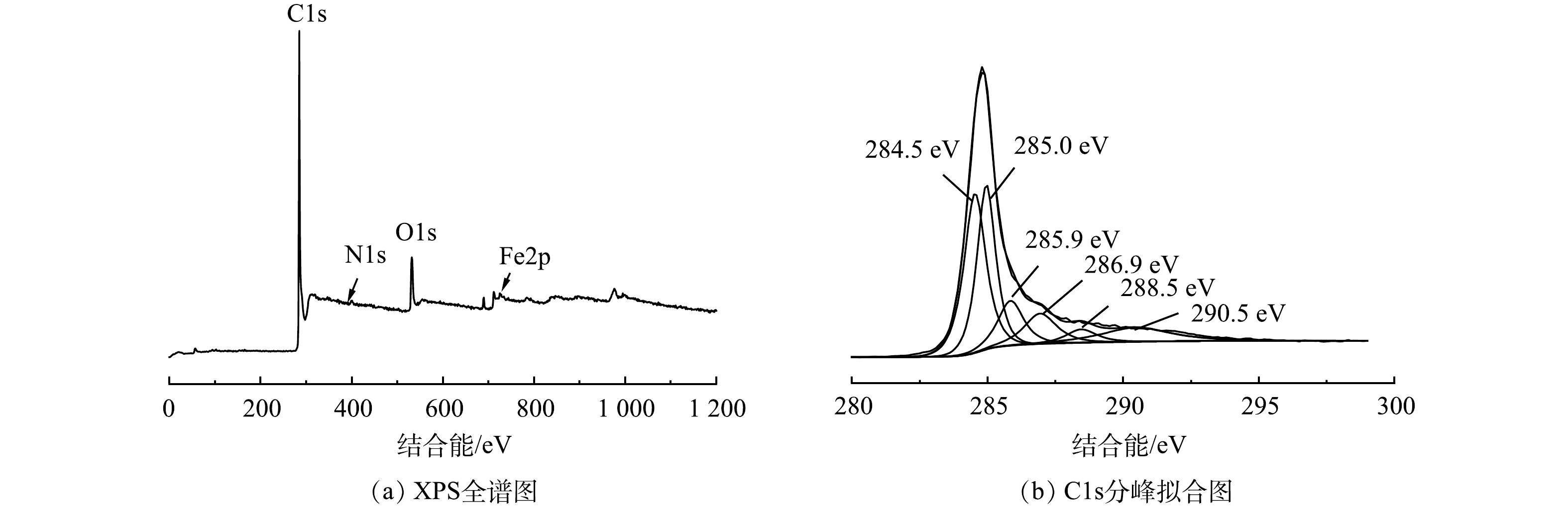
化学吸附主要与吸附剂和吸附质表面的化学基团种类、数量有关,其主要因素有静电作用、π-π键和氢键作用等。溶液 pH 会直接影响生物炭表面的电荷性质,从而促进生物炭与吸附质之间的静电作用,由前文可知,磁性山竹壳炭与氯霉素之间存在静电斥力,静电作用并不会促进对氯霉素的吸附。由图13可知,磁性山竹壳炭PGC-3-850表面有大量的C、O、Fe、N元素。对C1s进行分峰,分别对应石墨碳(C—C,284.5 eV(34.7%)),脂肪面碳 (C—C,284.9 eV(30.3%)) ,醇中的碳(C—O,285.9 eV(11.4%)),羰基(C=O,286.9 eV(9.8%)),酯基(C=O,288.5 eV(4%))和π—π键(290.5 eV(9.7%))。石墨碳是碳质元素结晶矿物,其结晶格架为六边形层状结构。FTIR表征也证实磁性山竹壳炭中存在芳香结构。这些结构能与氯霉素的苯环结构之间产生π—π共轭,在反应过程中充当π-电子受体[30],有利于吸附剂吸附目标污染物。除此之外,氯霉素中的N—H、—OH与磁性山竹壳炭中的含氧官能团能形成氢键,利于山竹壳炭吸附氯霉素[31]。
-
1)利用Fe(NO3)3和K2C2O4活化法制备了具有微孔结构的磁性山竹壳炭,随着K2C2O4用量的增加和炭化温度的升高,磁性山竹壳炭的比表面积和孔容有所增加,但Fe3O4逐渐被还原为单质Fe。样品PGC-4-900对氯霉素吸附量最高,为316.3 mg·g−1。
2)磁性山竹壳炭吸附氯霉素的过程是以化学吸附为主的单分子层吸附,拟二级动力学和 Langmuir 模型可以较好地拟合吸附过程。吸附为自发,吸热和无序度增加的过程。
3)磁性山竹壳炭对氯霉素溶液pH不敏感,可用于不同酸碱度环境中含氯霉素污水的修复与治理。循环利用性能良好,经5次热再生循环后对氯霉素的去除率仍可维持55%以上。
4)磁性山竹壳炭对氯霉素吸附主要机理主要有主孔隙填充、π—π作用和氢键作用。
磁性山竹壳炭的制备及对氯霉素吸附性能
Preparation of biochar based on magnetic mangosteen shell and its adsorption towards chloramphenicol
-
摘要: 以山竹壳为原料,K2C2O4为活化剂,Fe(NO3)3为赋磁剂制备了磁性山竹壳炭。考察了制备条件对山竹壳炭理化性质的影响,并探究其对水体中氯霉素的吸附性能。结果表明,随着K2C2O4用量的增加和炭化温度的升高,磁性山竹壳炭的比表面积和孔容增加,但Fe3O4逐渐被还原为单质Fe。当磁性山竹壳炭PGC-4-900投加量为0.3 g·L−1,溶液质量浓度为125 mg·L−1时,对氯霉素吸附容量最大可达316.3 mg∙g−1。吸附过程为自发、吸热和无序度增加的过程。吸附动力学符合拟二级动力学模型,等温模型可用Langmuir方程描述。磁性山竹壳炭在吸附氯霉素方面具有宽泛的pH适应性,静电作用非磁性山竹壳炭对氯霉素吸附主要机理,孔隙填充和π—π作用在氯霉素吸附过程中起主导作用。Abstract: Mangosteen shell were chosen as raw material for preparing magnetic biochar via K2C2O4 activation and Fe(NO3)3 magnetization. The influences of preparation conditions on the physical and chemical properties of biochar were investigated, and its adsorption performance towards chloramphenicol(CAP) was also studied. Results showed that the specific surface area and pore volume of magnetic activated biochar increased with the increase of Carbonization temperature and dosage of K2C2O4, but Fe3O4 was gradually reduced to elemental Fe. When the dosage of PGC-4-900 was 0.3 g·L−1, and the CAP solution concentration was 125 mg·L−1, the adsorption capacity towards CAP could reach 316.3 mg∙g−1. The CAP adsorption process by the biochar was a spontaneous, endothermic, and randomness increase one. CAP adsorption process on the biochar could be well fitted with pseudo-second order kinetics and Langmuir models. Magnetic mangosteen shell biochar had a wide pH adaptability during CAP adsorption. Pore filling and π-π interaction were the main mechanisms for CAP removal by magnetic biochar of mangosteen shell, while electrostatic effect could be ignored.
-

-
表 1 山竹壳炭样品制备条件
Table 1. Preparing condition of mangosteen shell biochar samples
样品编号 K2C2O4/g Fe(NO3)3/g 炭化温度/ ℃ C-850 0 0 850 PC-850 2 0 850 GC-850 0 1.5 850 PGC-3-850 1.5 1.5 850 PGC-4-850 2 1.5 850 PGC-5-850 2.5 1.5 850 PGC-4-800 2 1.5 800 PGC-4-900 2 1.5 900 表 2 各样品的孔性特征对比
Table 2. Textural properties of the prepared samples
样品编号 BET比表面积/(m2·g−1) 微孔表面积/(m2·g−1) 微孔孔容/(cm3·g−1) 总孔容/(cm3·g−1) 微孔/总孔容/% 平均孔径/nm C-850 550.95 546.85 0.191 0.204 93.6 1.48 GC-850 493.94 469.92 0.161 0.186 86.6 10.06 PC-850 1 208.88 1 128.70 0.381 0.493 77.9 4.15 PGC-4-900 1 321.41 961.20 0.483 0.636 75.9 3.89 PGC-4-800 969.81 859.68 0.284 0.431 65.9 5.95 PGC-4-850 1 219.08 999.27 0.309 0.576 53.6 4.55 PGC-5-850 1 333.52 998.11 0.295 0.649 45.4 4.93 PGC-3-850 1 218.61 916.96 0.274 0.566 48.1 4.09 表 3 各样品吸附氯霉素动力学参数
Table 3. Kinetics parameters for CAP adsorption on mangosteen shell biochars
样品 吸附容量/
(mg·g−1)拟一级动力学方程 拟二级动力学方程 Bangham方程 k1 qe R2 k2 qe R2 k3 m R2 GC-850 26.95 0.021 75 22.34 0.738 7 0.1175 26.2 0.815 6 3.371 3.119 0.487 1 PC-850 241.14 0.032 2 214.8 0.896 8 420.7 228.7 0.983 4 55.59 4.172 0.983 0 PGC-4-900 316.34 0.222 9 306.5 0.971 1 142.3 315.5 0.999 8 213.1 14.3 0.847 8 PGC-4-800 219.33 0.055 987 197.5 0.883 9 -0.222 1 223.1 0.998 2 74.69 5.633 0.982 3 PGC-4-850 307.94 0.178 2 292.7 0.950 7 0.413 8 310.5 1 182.9 11.09 0.909 6 PGC-5-850 313.08 0.300 4 305.1 0.981 3 135.9 312.5 0.999 9 236.7 20.21 0.859 0 PGC-3-850 311.08 0.123 1 303.1 0.983 5 107.9 310.1 0.999 7 165.4 8.824 0.756 2 表 4 PGC-3-850吸附等温线拟合参数
Table 4. Isotherm parameters for CAP adsorption onto PGC-3-850
温度/K Langmuir方程 Freundlich方程 qmax/(mg·g−1) KL/(L·mg−1) R2 n KF/(mg·g−1) R2 293.15 228 1.26 0.999 2 3.27 112.96 0.815 8 303.15 232.6 1.35 0.999 5 2.943 108.2 0.826 0 313.15 271 0.666 7 0.981 6 2.343 93.22 9.784 3 温度/K D-R方程 Temkin方程 Qm/(mg·g−1) B R2 at/(L·mg−1) Bt/(J·mol−1) R2 293.15 200.36 6.30E-08 0.982 8 37.63 67.52 0.951 6 303.15 198.34 7.44E-08 0.981 3 23.34 62.89 0.953 4 313.15 199.14 1.30E-07 0.853 6 8.77 51.65 0.867 2 表 5 PGC-3-850吸附氯霉素的热力学参数
Table 5. Thermodynamic parameters for CAP adsorption onto PGC-3-850
初始溶液质量
浓度/(mg·L−1)ΔH˚/
(kJ·mol−1)ΔS˚/
(J·(mol·K)−1)ΔG˚/(kJ·mol−1) 293.15 K 303.15 K 313.15 K 100 3.68 44.56 −9.38 −9.83 −10.27 125 8.38 53.84 −7.40 −7.94 −8.48 150 24.89 101.17 −4.77 −5.78 −6.79 -
[1] SINGH R, SINGH A P, KUMAR S, et al. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies[J]. Journal of Cleaner Production, 2019, 234: 1484-1505. doi: 10.1016/j.jclepro.2019.06.243 [2] BÖGER B, SUREK M, VILHENA R D O, et al. Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil[J]. Journal of Hazardous Materials, 2021, 402: 123448. doi: 10.1016/j.jhazmat.2020.123448 [3] HANEKAMP J C, BAST A. Antibiotics exposure and health risks: Chloramphenicol[J]. Environmental Toxicology and Pharmacology, 2015, 39(1): 213-220. doi: 10.1016/j.etap.2014.11.016 [4] YANG J, JI G, GAO Y, et al. High-yield and high-performance porous biochar produced from pyrolysis of peanut shell with low-dose ammonium polyphosphate for chloramphenicol adsorption[J]. Journal of Cleaner Production, 2020, 264: 121516. doi: 10.1016/j.jclepro.2020.121516 [5] 李一冉. 香蒲活性炭的制备、原位改性及对抗生素和重金属的吸附机理研究[D]. 济南: 山东大学, 2018. [6] HOLANDA F H E, BIROLLI W G, MORAIS E D S, et al. Study of biodegradation of chloramphenicol by endophytic fungi isolated from Bertholletia excelsa (Brazil nuts)[J]. Biocatalysis and Agricultural Biotechnology, 2019, 20: 101200. doi: 10.1016/j.bcab.2019.101200 [7] CHATZITAKIS A, BERBERIDOU C, PASPALTSIS I, et al. Photocatalytic degradation and drug activity reduction of Chloramphenicol[J]. Water Research, 2008, 42(1/2): 386-394. [8] Li Y B, WANG C P, ZHENG M Y, et al. Optimum and efficiency of chloramphenicol degradation by UV/H2O2 process[J]. Advanced Materials Research, 2014, 838-841: 2677-2680. [9] CHEN W, MENG J, HAN X, et al. Past, present, and future of biochar[J]. Biochar, 2019, 1(1): 75-87. doi: 10.1007/s42773-019-00008-3 [10] ZHOU Y, LIU X, XIANG Y, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245: 266-73. doi: 10.1016/j.biortech.2017.08.178 [11] LI Y, WANG X, CAO M. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors[J]. Journal of CO2 Utilization, 2018, 27: 204-216. doi: 10.1016/j.jcou.2018.07.019 [12] NASRULLAH A, SAAD B, BHAT A H, et al. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal[J]. Journal of Cleaner Production, 2019, 211: 1190-1200. doi: 10.1016/j.jclepro.2018.11.094 [13] ZEIN R, SUHAILI R, EARNESTLY F, et al. Removal of Pb(II), Cd(II) and Co(II) from aqueous solution using Garcinia mangostana L. fruit shell[J]. Journal of Hazardous Materials, 2010, 181(1): 52-56. [14] 单锐, 谈莉, 陈凤鸣, 等. 改性山竹壳炭对废水中Ni(Ⅱ)的吸附效果与机理研究[J]. 安全与环境学报, 2022, 22(6): 3473-3483. doi: 10.13637/j.issn.1009-6094.2021.1619 [15] 黄晓东, 刘明华, 林春香. 氨基硫脲改性山竹壳对Cd2+、Pb2+的吸附[J]. 环境科学与技术, 2012, 35(5): 24-27. [16] MA P, WANG S, WANG T, et al. Effect of bifunctional acid on the porosity improvement of biomass-derived activated carbon for methylene blue adsorption[J]. Environmental Science and Pollution Research, 2019, 26(29): 30119-30129. doi: 10.1007/s11356-019-06177-9 [17] CUI Y, HUO Q, CHEN H, et al. Biomass carbon magnetic adsorbent constructed by one-step activation method for the removal of Hg0 in flue gas[J]. ACS Omega, 2022, 7(11): 9244-9253. doi: 10.1021/acsomega.1c05857 [18] 王芳君, 桑倩倩, 邓颖, 等. 磁性铁基改性生物炭去除水中氨氮[J]. 环境科学, 2021, 42(04): 1913-1922. doi: 10.13227/j.hjkx.202009047 [19] YANG K, PENG H, WEN Y, et al. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles[J]. Applied Surface Science, 2010, 256(10): 3093-3097. doi: 10.1016/j.apsusc.2009.11.079 [20] 张晓华. 生物质基炭材料的结构调控及其电化学性能研究[D]. 太原: 太原理工大学, 2019. [21] YANG W, CHEN H, HAN X, et al. Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal[J]. Journal of Hazardous Materials, 2020, 381: 120981. doi: 10.1016/j.jhazmat.2019.120981 [22] 刘庆玉, 王永超, 梅自力, 等. 氯化锌浸渍水稻秸秆制备生物炭吸附氯霉素的机理研究[J]. 沈阳农业大学学报, 2021, 52(05): 557-567. [23] CHENG X X, ZHENG C L, LU Q, et al. Adsorption of furazolidone, D-cycloserine, and chloramphenicol on granular activated carbon made from corn stover[J]. Journal of Environmental Engineering, 2019, 145(7): 04019038. doi: 10.1061/(ASCE)EE.1943-7870.0001546 [24] RUMBIDZAI, CHITONGO, BEATRICE, et al. Abatement of amoxicillin, ampicillin, and chloramphenicol from aqueous solutions using activated carbon prepared from grape slurry[J]. Clean – Soil Air Water, 2019, 47(2): e1800077. doi: 10.1002/clen.201800077 [25] YANG F, ZHANG Q, JIAN H, et al. Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol[J]. Journal of Hazardous Materials, 2020, 396: 122598. doi: 10.1016/j.jhazmat.2020.122598 [26] LI Y R, ZHANG J, LIU H. Removal of chloramphenicol from aqueous solution using low-cost activated carbon prepared from typha orientalis[J]. Water, 2018, 10(4): 351. doi: 10.3390/w10040351 [27] LIU H, XU C, WEI X, et al. 3D hierarchical porous activated carbon derived from bamboo and its application for textile dye removal: Kinetics, isotherms, and thermodynamic studies[J]. Water, Air, & Soil Pollution, 2020, 231(10): 504. [28] MIRZAEIAN M, ABBAS Q, HUNT M R C, et al. Pseudocapacitive effect of carbons doped with different functional groups as electrode materials for electrochemical capacitors[J]. Energies, 2020, 13(21): 5577. doi: 10.3390/en13215577 [29] TANG L, YU J, PANG Y, et al. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal[J]. Chemical Engineering Journal, 2018, 336: 160-169. doi: 10.1016/j.cej.2017.11.048 [30] YAN L, LIU Y, ZHANG Y, et al. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline[J]. Bioresource Technology, 2019, 297: 122381. [31] 柳慧. 碳基复合材料对两类常用抗生素的去除及机理研究[D]. 长沙: 湖南大学, 2019. -



 下载:
下载: