-
塑料制品因其成本低廉、耐腐蚀和持久性等特点在世界范围内得到广泛应用,同时塑料制品的低回收率和难降解等问题使之在环境中大量积累. 环境中塑料经物理、化学和生物形式降解为直径小于5 mm的塑料颗粒被称为微塑料(microplastics, MPs). 目前,人类已经在海洋[1]、河流[2 − 3]、湖泊[4 − 5]、生物[6 − 7]甚至人体[8 − 9]中检测到微塑料的存在. 微塑料具有尺寸小、孔隙率高、比表面积大、疏水性强等特点,使之能有效吸附环境中如有机污染物、重金属、农药、病原体等污染物. 鱼类作为水生环境中最常见的生物,经呼吸和摄食作用两种途径摄入微塑料[10]:呼吸作用将水体中微塑料沾附于鳃组织上;当食物和微塑料混在一起时,产生一种“味觉陷阱”,致使大多数随食物一同被吞食,进而在消化系统中积累. 微塑料对鱼类等水产品的污染已成为全球粮食安全的新问题[11],通过水产品摄入微塑料被认为是人类摄入化学添加剂和持续性有毒污染物的潜在途径[6]. 鱼类等水产品中微塑料的高效提取和准确定量是确定其污染程度的首要前提.
密度分离法利用微塑料与杂质的密度不同,通过添加不同密度盐溶液将目标微塑料从样品中分离出来. 经典的直接密度分离法[12 − 13]是在烧杯或锥形瓶中与盐溶液直接混合,静置后倾倒上清液,该方法简单方便、成本低,但在上清液转移过程中微塑料总不可避免地黏附于瓶壁上而无法收集,从而造成微塑料的损失. 目前,已有很多研究开发出许多密度分离装置,尽管其具有较高的回收率[14 − 16],但这些装置普遍存在结构繁琐、操作复杂、难以推广等问题. 本文借鉴分液漏斗分液的原理,将样品和浮选盐溶液转移进常见的分液漏斗中,在静置结束后将废弃液从下口排出,上清液从上口转移,瓶壁可多次冲洗,以避免黏附于瓶壁上微塑料的损失.
NR是一种低成本、无毒、疏水、光化学稳定的染料,相比于其他染料,NR具有对塑料吸附性高、荧光强度高、培育时间短及对多种聚合物亲和力好等优点[17]. 基于NR的微塑料荧光检测技术具有快速、灵敏度高、检测限低(已被应用于>3 μm微塑料检测)[18 − 20]、可实现自动化计数等优点,大大提高了目视检测的准确率,可能具有与FTIR/Roman等光谱工具同等的效力[17,21],该技术已被应用于水体[19 − 20]、生物[6,18,21] 、食品[22 − 24]等样本中微塑料检测. 但在生物样本中,NR同样能够染色未完全消解的有机物,如有机物去除过程中的脂肪、肥皂等,严重阻碍微塑料的鉴定[23,25].
某些富含大量脂质的生物组织在碱性溶液中发生皂化反应,消解过程中钾基盐形成凝胶状的软肥皂;浮选溶液如氯化钠或碘化钠浮选溶液的加入,产生钠基盐形成硬肥皂[26]. 生成的肥皂不仅会堵塞过滤器,而且会捕获部分微塑料,从而降低过滤效率和微塑料回收率[27]. 因此,有必要确定适当的方法以去除皂化反应生成的肥皂.
本文为建立富含脂质的生物样本中微塑料检测方法,对传统的KOH消解生物样本进行优化:采用分液漏斗双密度分离法以提高对不同密度和不同粒径微塑料的回收率;探寻皂化反应生成的肥皂的去除方法,以减少其对NR染色观察的影响;利用NR染色以提高微塑料识别的准确率. 建立鱼体中微塑料提取的实验流程,并应用到实际样本中,以期为鱼类等生物样本中微塑料的高效提取理论依据和技术支持.
-
尼罗红(HPLC级,上海麦克林公司);正己烷、氢氧化钾、氯化钠、乙醇(分析纯,广东西陇科学股份有限公司);碘化钠(分析纯,上海阿拉丁生化科技公司);粒径为10—100 μm、100—500 μm、500—1000 μm和1—5 mm的PA(聚酰胺)、PE(聚乙烯)、PP(聚丙烯)、PS(聚苯乙烯)、PET(聚对苯二甲酸乙二醇酯)、PVC(聚氯乙烯)塑料(纯度99%,湖南瑞祥塑料有限公司);BSA(纯度≥96%,安徽兰杰柯科技有限公司);玻璃纤维滤膜(直径50 mm,孔径0.45 μm ,上海兴亚净化材料厂)
FA2004B型电子天平(精确度0.0001 g,上海佑科仪器仪表有限公司);自然对流烘箱(UN-30,德国Memmert公司);体视显微镜(SZ61,日本Olympus公司);傅里叶变换红外光谱(Spectrum two,美国Perkinelmer公司);显微傅里叶变换红外光谱仪(Carry660+610+600,美国Aglient公司);相差荧光显微镜(IX71,日本Olympus公司);水浴振荡摇床(8WB20D,澳大利亚Ratek公司).
-
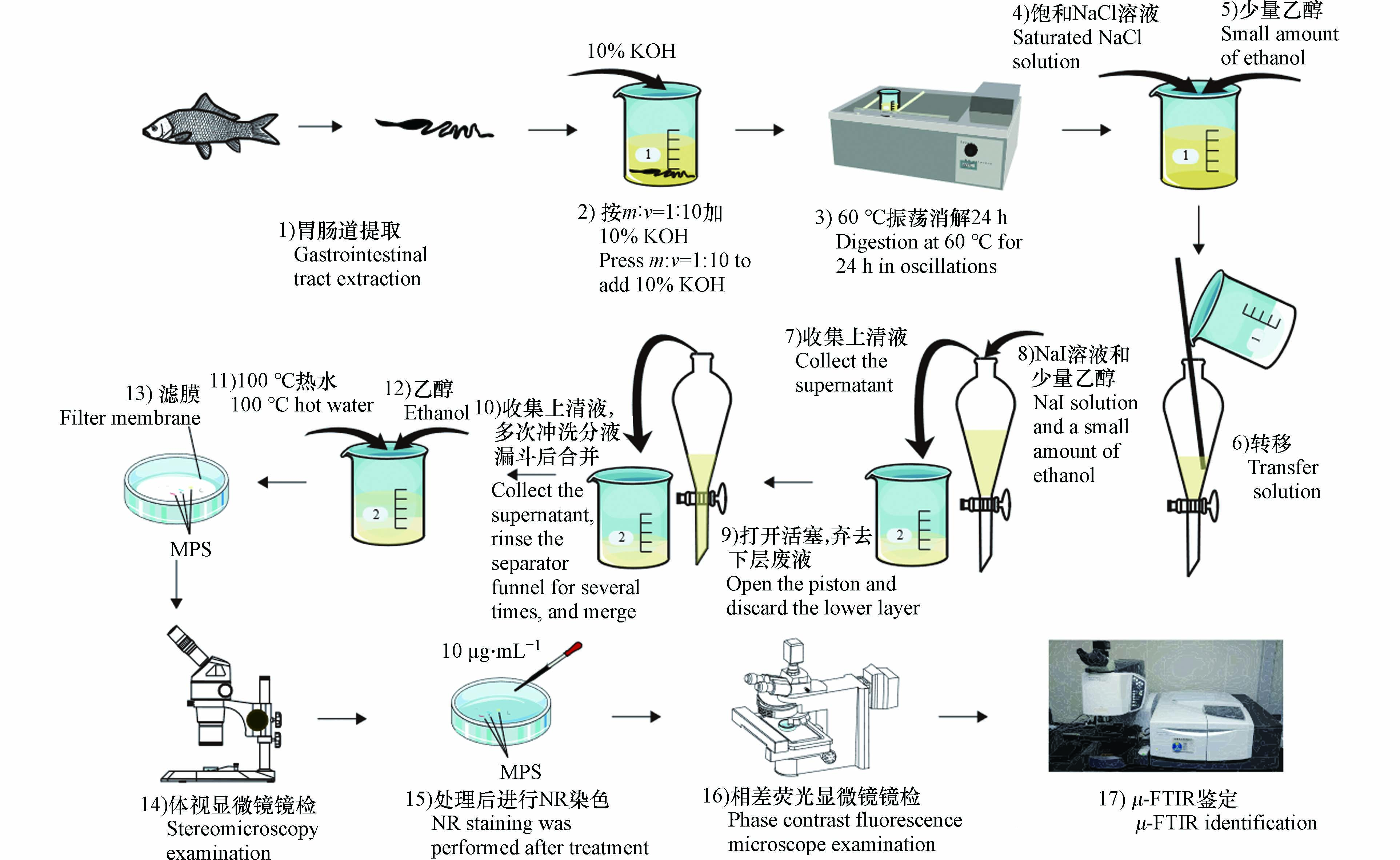
选取草鱼为方法建立所需样本. 市售草鱼体型普遍较大,胃肠道弯曲盘绕且表面富含丰富的脂质. 从当地市场购草鱼若干,直接带回实验室. 在超净工作台中,使用金属解刨剪从鱼肛门开始沿着腹侧一直到腮盖,从而暴露内部器官,取出其胃肠道(包括食道、幽门盲肠和肠道)[28]. 夹紧胃肠道两端,超纯水冲洗胃肠道外表面,称重,并使用金属剪刀将其剪碎.
-
KOH消解法已被证明是生物样品中微塑料提取的常用方法,该方法消解效果好,回收率高,而且对微塑料表面形貌没有明显影响[25,29]. 按质量体积比(W:V=1:10)向胃肠道中加10% KOH,铝箔封口,60 ℃水浴振荡摇床24 h,至溶液澄清,消解液底部无生物样本组织. 若未完全消解,继续加10% KOH,相同条件下继续培养.
-
将消解液转移至250 mL分液漏斗,随后使用50 mL饱和NaCl(ρ=1.2 g·cm−3)溶液多次冲洗烧杯,将消解液完全转移至分液漏斗中,防止微塑料损失. 充分振荡,静置过夜. 取上清液,向分液漏斗中添加50 mL NaI(ρ=1.8 g·cm−3)溶液,充分振荡,静置12 h. 打开分液漏斗活塞,移去下层溶液,转移上清液,并仔细冲洗分液漏斗,将两次密度分离的上清液和冲洗液合并. 使用称重法计算浮选溶液密度:
式中,ρ为溶液密度(g·mL−1),Wa为溶液和量筒的质量(g),Wb为量筒质量(g),V为溶液体积(mL).
NaI溶液浓度高,回收效果好,但价格昂贵[30 − 32]. 参考Kedzierski等[31]研究对使用过的NaI溶液进行重复利用,具体过程为:使用20 μm的尼龙滤膜对回收的NaI溶液进行过滤,以除去较大杂质,后通过加热蒸发浓缩溶液体积或添加碘化钠固体,提高溶液密度,并使用0.45 μm的玻璃纤维滤膜多次过滤以确保无外部微塑料污染,置于棕色瓶中,4 ℃避光保存. 每次使用前,测定NaI溶液密度.
-
使用10% KOH在60 ℃下消解24 h后,样品溶液整体呈透明澄清,但加入氯化钠和碘化钠浮选溶液后,溶液变得浑浊,出现大量絮状较软的肥皂,静置一段时间后,这些软肥皂汇集在液体表面形成硬肥皂层. 将硬肥皂单独分离,称重,等分成若干份,分别探讨不同温度热水(60 ℃、80 ℃、100 ℃)和不同有机溶剂(甲醇、乙醇[27]、丙酮[25,33])及联用对肥皂的去除效率,重复3次. 使用公式(2)评价肥皂的去除效率.
肥皂单独分离再去除的预实验显示出热水处理和有机溶剂处理各自的优缺点,因此将热水和有机溶剂联用以优化实验流程:每次加入浮选盐溶液后添加20 mL有机溶剂,向合并上清液中添加100 ℃热水和有机溶剂以去除肥皂,随后抽滤至0.45 μm玻璃纤维滤膜上. 共设计3组实验:A:热水+甲醇;B:热水+乙醇;C:热水+丙酮,重复3次. 使用公式(3)评价整个实验流程包括KOH消解和肥皂去除对生物样本的消解效率.
式中,E1为肥皂去除效率,E2为生物样本的消解效率,W1为肥皂质量(g),W2为胃肠道质量(g),Wc为过滤后滤膜的干重(g),Wd为过滤前滤膜的干重(g).
-
向过滤后的滤膜中添加10 mL乙醇,超声振荡处理10 min,乙醇完全冲洗滤膜. 烘干. 100 mg NR溶于100 mL丙酮溶液制成1 mg·mL−1 NR母液,正己烷稀释成10 μg·mL−1的NR工作液[17,20]. 滴加几滴10 μg·mL−1 NR染液,避光条件下培养30 min,转移至相差荧光显微镜. 分别使用常用于识别染色微生物的3种滤光片(蓝色、绿色和紫色),以确定被染色合成聚合物合适的观测条件.
-
为探究该方法对不同密度(高密度:PET和PVC[30],ρ>1.3 g·cm−3、低密度:PP、PE、PS和PA[34],ρ<1.15 g·cm−3)和不同粒径(10—100 μm、100—500 μm、500—1000 μm和1—5 mm)微塑料的影响,设计不同组回收率验证实验(表1),使用公式(4)评价回收率. 实验所需的微塑料均在质量浓度为2%牛血清白蛋白(BSA)中培养24 h,以减少微塑料颗粒间的静电荷,防止结块[20].
式中,R为微塑料回收率,Wm为添加微塑料质量(g),Wn为处理后微塑料质量(g).
-
CI是指羰基的吸光度与不同聚合物参考峰吸光度之比[35],可用于表征该实验流程对实验前后微塑料老化降解的影响,CI增加的越高表明微塑料老化程度越高. 使用FTIR的衰变全反射模式(ATR-FTIR)对实验前后标准微塑料的红外光谱进行测定,扫描次数为32次,范围为450—4000 cm−1. CI使用公式(5)表示:
式中,A1指羰基在1715 cm−1的吸光度[36];A2是每种聚合物在不同波长下参考峰的吸光度. PE:1462 cm−1、PP:1454 cm−1、PS:1450 cm−1、PA:1461 cm−1、PET:1504 cm−1、PVC:1428 cm−1[25].
-
昌江,长江鄱阳湖流域饶河支流,发源于安徽祁门县,过倒湖进入江西境内. 于2022年6月22日至24日,从昌江的5个采样点收集不同种类的鱼类样品. 铝箔包裹密封带回实验室,−20 ℃冷冻保存. 使用时室温下解冻,测量并称重,按图1流程进行实验. 其中,体视显微镜镜检过程按照如下规则对微塑料进行计数: 1)没有可见的细胞或有机结构;2)纤维质地均匀;3)颗粒呈均匀的颜色,排除假阳性的可能;4)薄膜边缘光滑或有棱角;5)发泡在压力下易变形[34].
在相差荧光显微镜下对疑似微塑料荧光图像进行拍照(曝光时间:1—30 ms),使用image J计数和测量. 计数完成后,首先在相差荧光显微镜下挑选具有荧光的疑似微塑料,后在体视显微镜下挑选相同数量的疑似微塑料,分别转移至μ-FTIR进行红外光谱测定(参数:扫描次数为64次,范围650—4000 cm−1,分辨率2 cm−1,ATR模式),并与Know It-ALLTM软件库中标准图谱进行匹配,匹配度>70即被认定为该聚合物成分.
-
使用Origin 2021对肥皂去除和生物样本消解的效率进行分析,对回收率和实验前后微塑料的CI进行统计. 使用image J对微塑料图像进行计数和测量. 使用SPSS 27.0对体视法和NR法的微塑料检出率进行配对样本T检验,*P<0.05表示差异显著.
-
实验人员身着棉质实验服,带丁腈手套;实验尽可能在通风橱中进行,使用无水乙醇进行擦拭;实验过程中避免使用塑料材质设备,无刻度的玻璃容器在使用前放置到马弗炉中500 ℃加热2 h[3],其他玻璃容器在使用前用超纯水冲洗3次;配置的试剂过滤后使用;未使用的样品或试剂及时用滤膜覆盖表面,避免其与空气接触;野外样品处理时,使用程序空白以评估空气等环境的污染,该空白使用与样品相同的处理步骤,样本中最终微塑料数量扣除空白组.
-
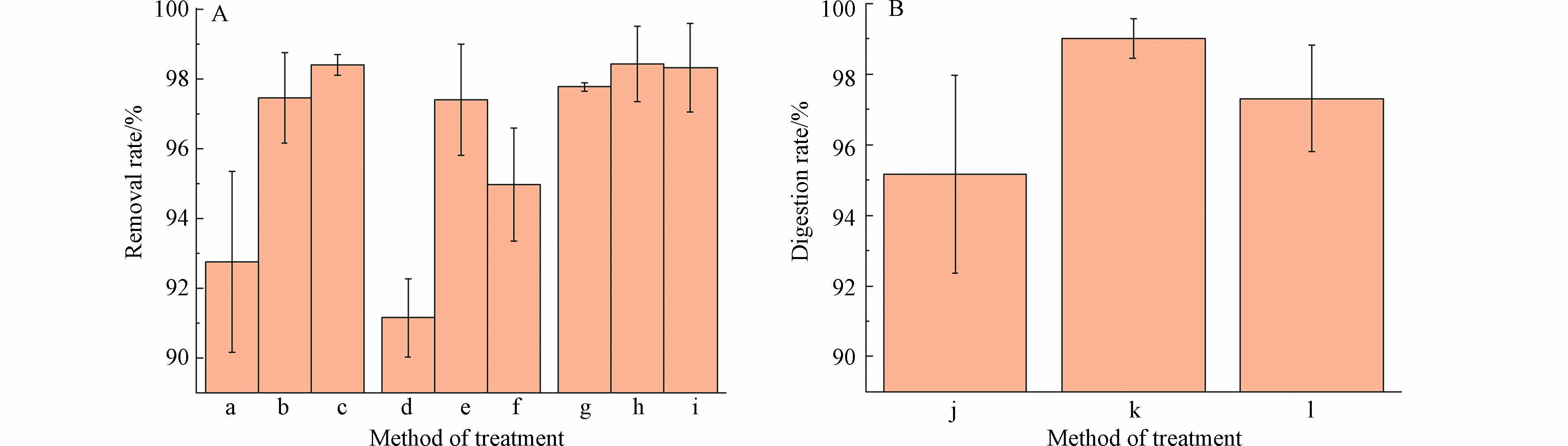
分液漏斗密度分离法对不同密度和不同粒径微塑料的回收率如表1所示,即使是对10—100 μm的微塑料,其回收率也有91%,且随着微塑料粒径越大回收率越高,最高回收率达100%,平均回收率在96%以上,远高于传统密度分离法39.8%的回收率[13]. 同时实验结果表明,分液漏斗密度分离法对于相同粒径不同密度的微塑料的回收率无明显差异. 这取决于NaI浮选溶液提供较高的密度(1.8 g·cm−3),使之能够分离出密度较大的微塑料,提高微塑料的回收率,同时NaI溶液的重复利用已被证明是可行的[31].
分液漏斗密度分离法结构简单,操作方便,对微塑料的回收率较高(91%以上),且NaI溶液的多次回收能够显著降低成本,减低对环境的危害. 但该装置仍存在一些问题:对于较复杂的样本,如较大粒径泥沙颗粒、未完全消解的鱼骨等不能通过下口排出,适用性较差.
-
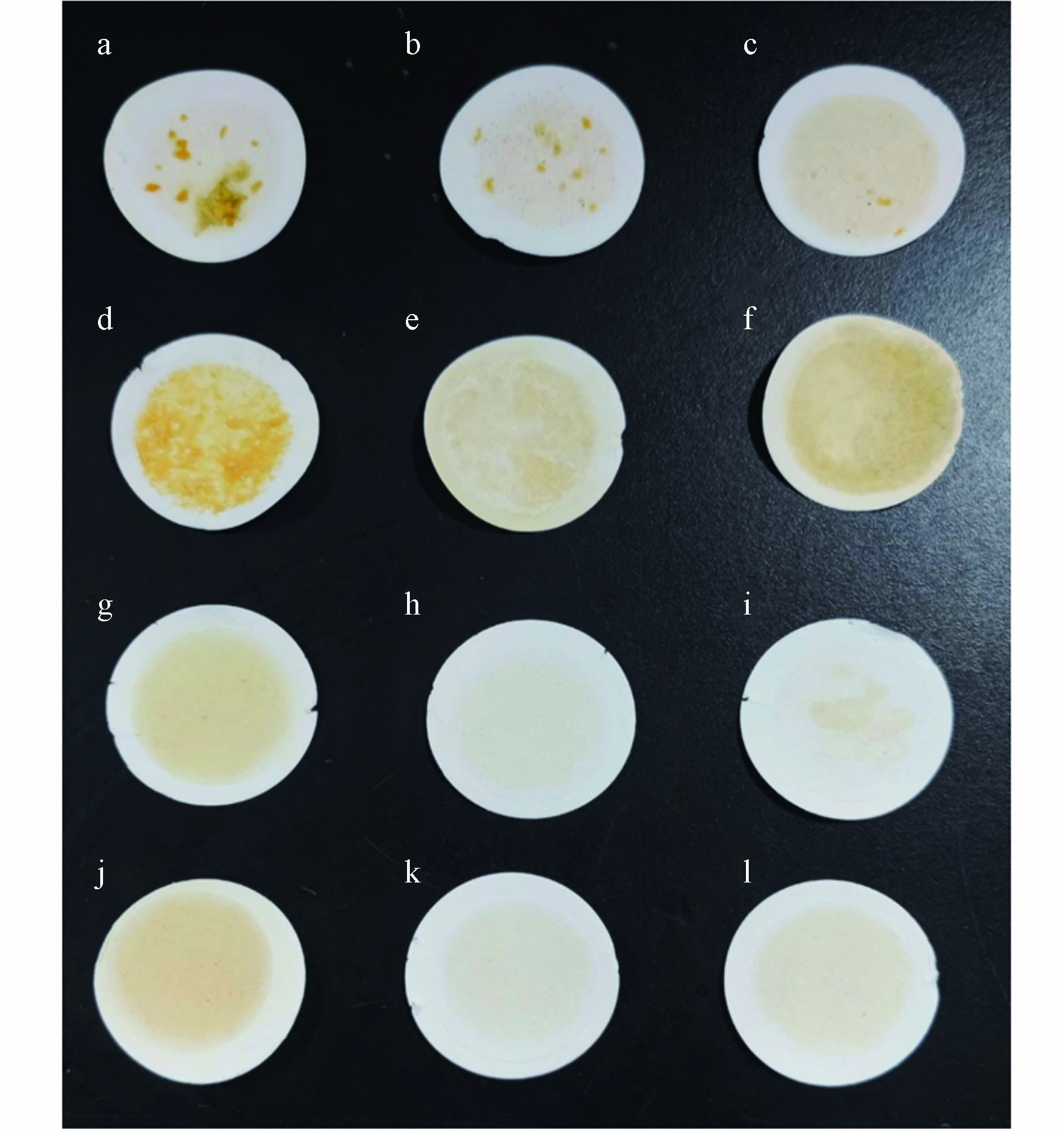
随着热水温度的升高,肥皂的去除效率逐渐升高,去除效率最高达98.77%±0.58%(图2),这说明溶液温度的升高显著提高了肥皂的溶解度,大块的凝胶状肥皂重新悬浮成细小的肥皂颗粒(如图3a—3c),但分散后的溶液依然浑浊. 尽管绝大多数细小肥皂颗粒可以穿过滤膜,但在滤膜上仍观察到少量肥皂块和较黄的背景,且前期预实验表明温度降低部分肥皂会重新出现. 甲醇、乙醇和丙酮等有机溶剂的单独使用均能去除大部分肥皂,在使用相同量的有机溶剂时,肥皂去除效率:乙醇>丙酮>甲醇. 甲醇的去除效率最低且滤膜上能明显看到未去除的物质(图3d);乙醇和丙酮处理后溶液整体透明澄清,但过滤后滤膜表面存在一层较薄的油脂层(图3e和3f). 上述结果表明,热水对肥皂的去除效率高,但分散后的溶液浑浊且温度降低肥皂会重新出现;有机溶剂处理后溶液整体透明澄清,但仍存在部分油脂,这可能是有机溶剂添加量较少的缘故. 因此综合二者的优劣,将热水和有机溶剂联合使用以除去肥皂,结果表明100 ℃热水+甲醇、100 ℃热水+乙醇和100 ℃热水+丙酮处理对肥皂的去除效率分别为97%±0.12%、98%±1.08%和98%±1.98%. 虽相对于热水的单独使用,其去除效率相差不大,但除甲醇处理组(图3g)外,乙醇和丙酮处理组(图3h和3i)滤膜表面无明显黄色背景.
肥皂单独分离后再去除操作复杂,而且转移过程可能会造成微塑料的损失. 因此根据上述结果,将热水和有机溶剂的联合使用引入原有的实验流程,以评价整个实验流程对生物样本的消解效率:在每次加入浮选盐溶液后,加少量有机溶剂去除部分肥皂,使皂化反应所生成的肥皂只集中于分液漏斗上层,而不至于充满整个分液漏斗,这避免了弃去下层液过程中肥皂吸附某些微塑料而造成的损失,同时已分散的肥皂不会再次凝聚;向合并的上清液中添加热水和有机溶剂,100 ℃热水能显著提高肥皂的溶解度,凝胶状肥皂重新悬浮成细小的肥皂颗粒,少量的有机溶剂溶解细小的肥皂颗粒使溶液整体透明澄清. 但实验过程中发现,在添加相同量的有机溶剂后,甲醇对肥皂的去除效果不明显,肥皂仍充满整个分液漏斗,且最终热水+甲醇对肥皂的去除效率低于另外两种有机溶剂(图2). 100 ℃热水+乙醇和100 ℃热水+丙酮联用均能高效去除凝胶状的肥皂,生物样本的消解率分别为99%±0.56%和97%±1.61%. 乙醇作为常见的多功能有机溶剂,能够溶解许多极性和非极性物质,且已被证明对微塑料影响较小[27]. 丙酮易挥发、有刺激性气味和一定的毒性,且丙酮会使PS发生表面变化、CI值升高[37],最终选用100℃热水+乙醇处理作为生物样本消解的最佳方案.
实验过程中,注意到不同种类的鱼胃肠道等组织表面富含脂质的量各有不同,如草鱼作为常见的食草性鱼类,体型较大,胃肠道较长且弯曲盘旋,表面附着有丰富的脂质. 而鲫鱼等杂食性鱼类,体型较小,其胃肠道较短且呈一条直线,表面脂质较少. 目前,绝大多数研究的是鱼胃肠道中的微塑料丰度,其表面的脂质并不是我们所需要的,因此在消解前我们可以夹紧胃肠道两端,小心去除胃肠道表面脂质.
-
如表2所示,PE在蓝色滤光片下显示出明显的黄绿色荧光;PS、PP在蓝色滤光片下显示出黄色荧光;PET在蓝色滤光片下显示出较弱的黄色荧光,在绿色滤光片下显示出红色荧光,在紫色滤光片下显示出独特的紫色荧光;多数聚合物在绿色滤光片下显示出较强的红色荧光,但背景的染色一定程度上阻碍了塑料颗粒的清晰识别. 同时不同聚合物在不同滤光片下荧光颜色的不同,使之能在一定程度上作为判断微塑料种类的辅助手段.
-
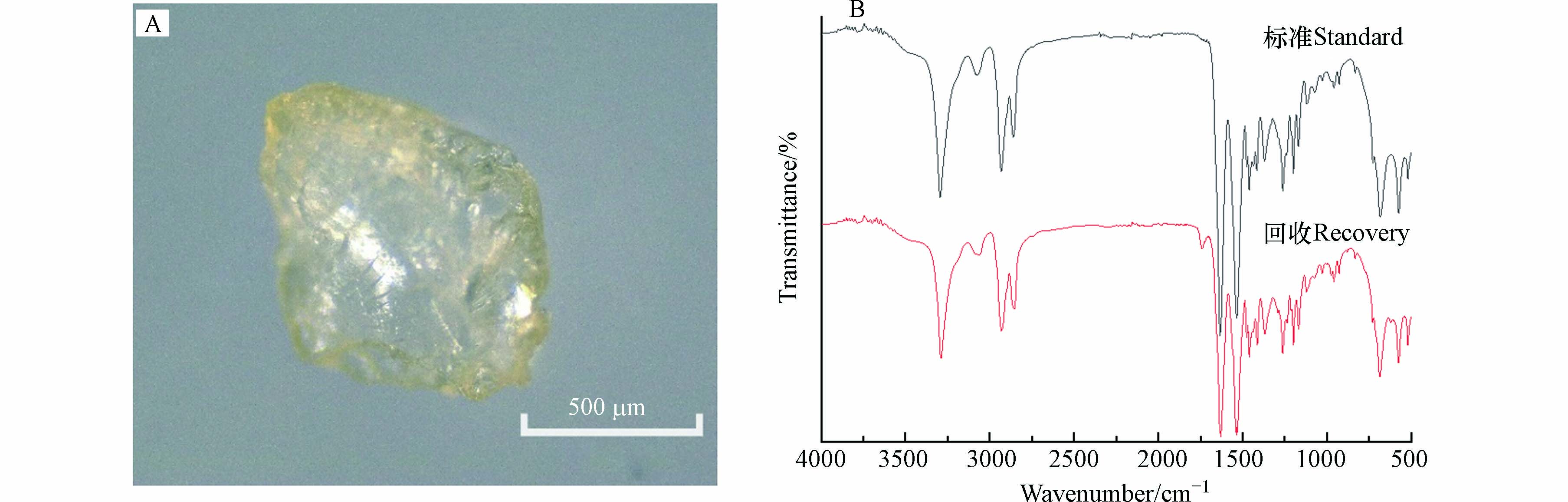
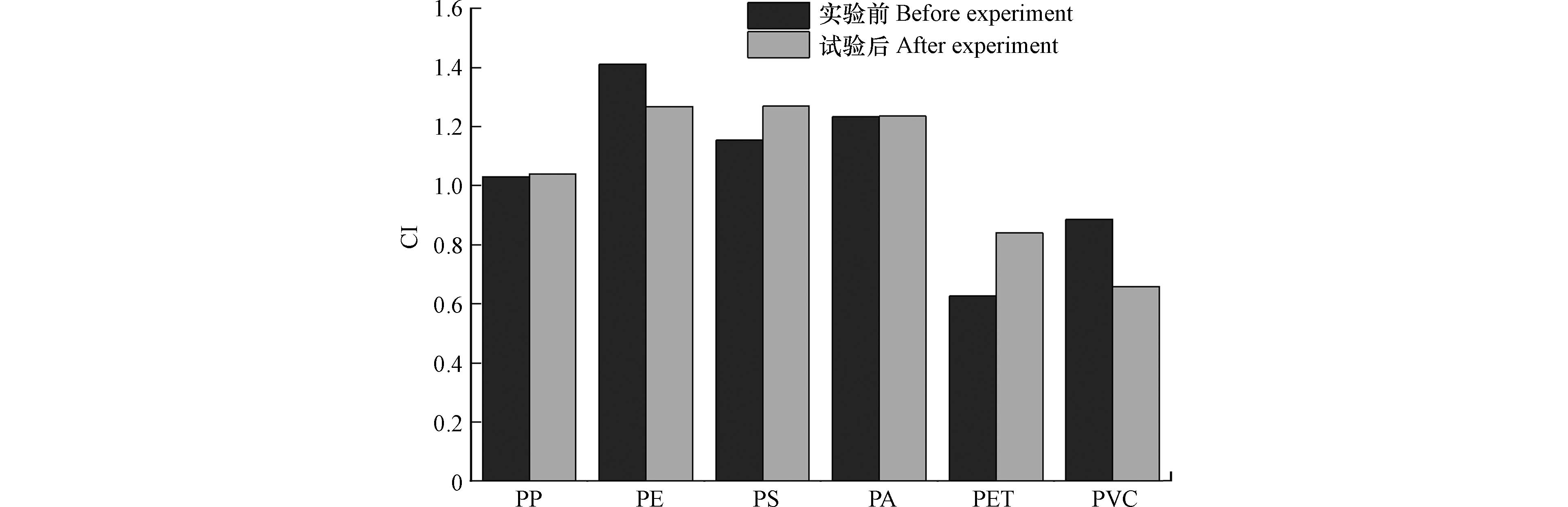
本文所添加的标准塑料颗粒呈白色或透明色,因此无法直观的观察到KOH碱溶液是否对某些其他颜色的微塑料产生漂白作用,尽管某些研究发现KOH消解法会漂白某些具有颜色微塑料[27]. 实验中发现部分PA微塑料变黄(图4A),但其与标准微塑料的红外光谱无明显差异(图4B),CI值相近(图5),表明该方法不会对PA红外测定产生干扰.
实验前后羰基(C=O,1715 cm−1)吸光度值变化较小(图5),这表明大多数微塑料几乎不发生降解,该方法对微塑料红外光谱影响很小. 同时,热水与乙醇相结合消除肥皂的方法并未出现应用丙酮导致PS的CI值显著增加的情况[25].
-
体视显微镜镜检法是基于较低倍率下对样品表面形貌的目视检查,根据样品形态、颜色等信息筛选潜在微塑料,该方法操作简单、成本低,是鉴定微塑料的常用方法[38]. 然而,该方法耗时耗力、可靠性差、误检率高. 镜检复杂环境样品时,有些自然材料如硅藻壳、泥沙和盐晶体等可能被误认为微塑料[39],同时由于体视显微镜较低的倍率可能会造成粒径较小微塑料的漏检.
通过对昌江不同河段的多种鱼胃肠道内微塑料进行检测,结果表明,所建立的方法对多种野生鱼类的消解效率达97%—100%,表明该方法的普遍适用性. 同时发现经NR染色结合荧光显微镜观察到的微塑料数量基本高于直接在体视显微镜镜检,NR法检测到微塑料的平均数量是体视法的1.2倍(表3). 为比较两种方法对微塑料识别的准确性,分别在体视显微镜和荧光显微镜下随意挑选相同数量的微塑料,转移至μ-FTIR进行红外光谱测定. 结果表明,体视法对微塑料的平均检出率仅有54.64%,而NR法平均检出率为84.70%,在某些样品中最高达100%. 对体视法和NR法的微塑料检出率进行配对样本T检验,P<0.05,表明NR法对微塑料识别的准确性显著高于体视法.
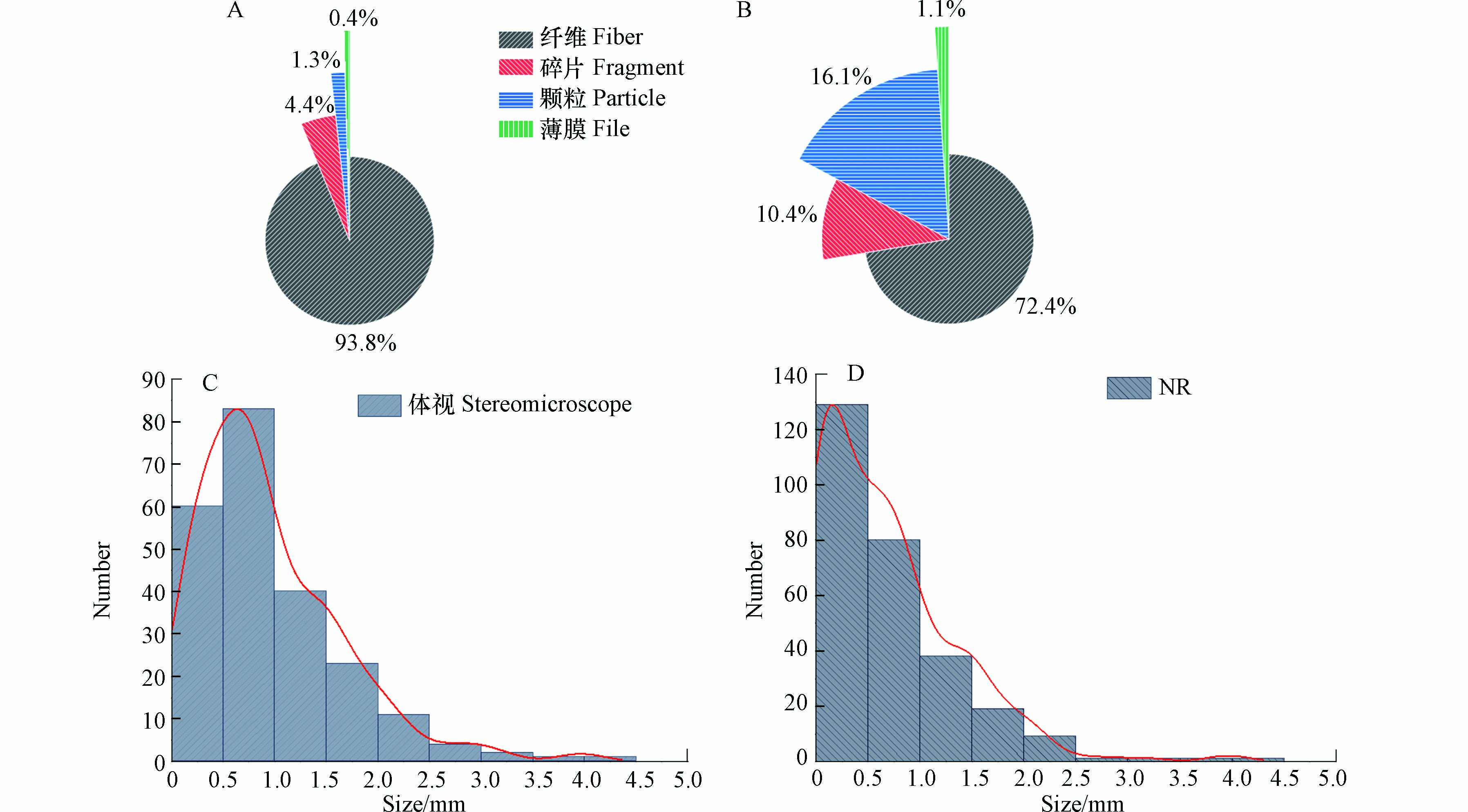
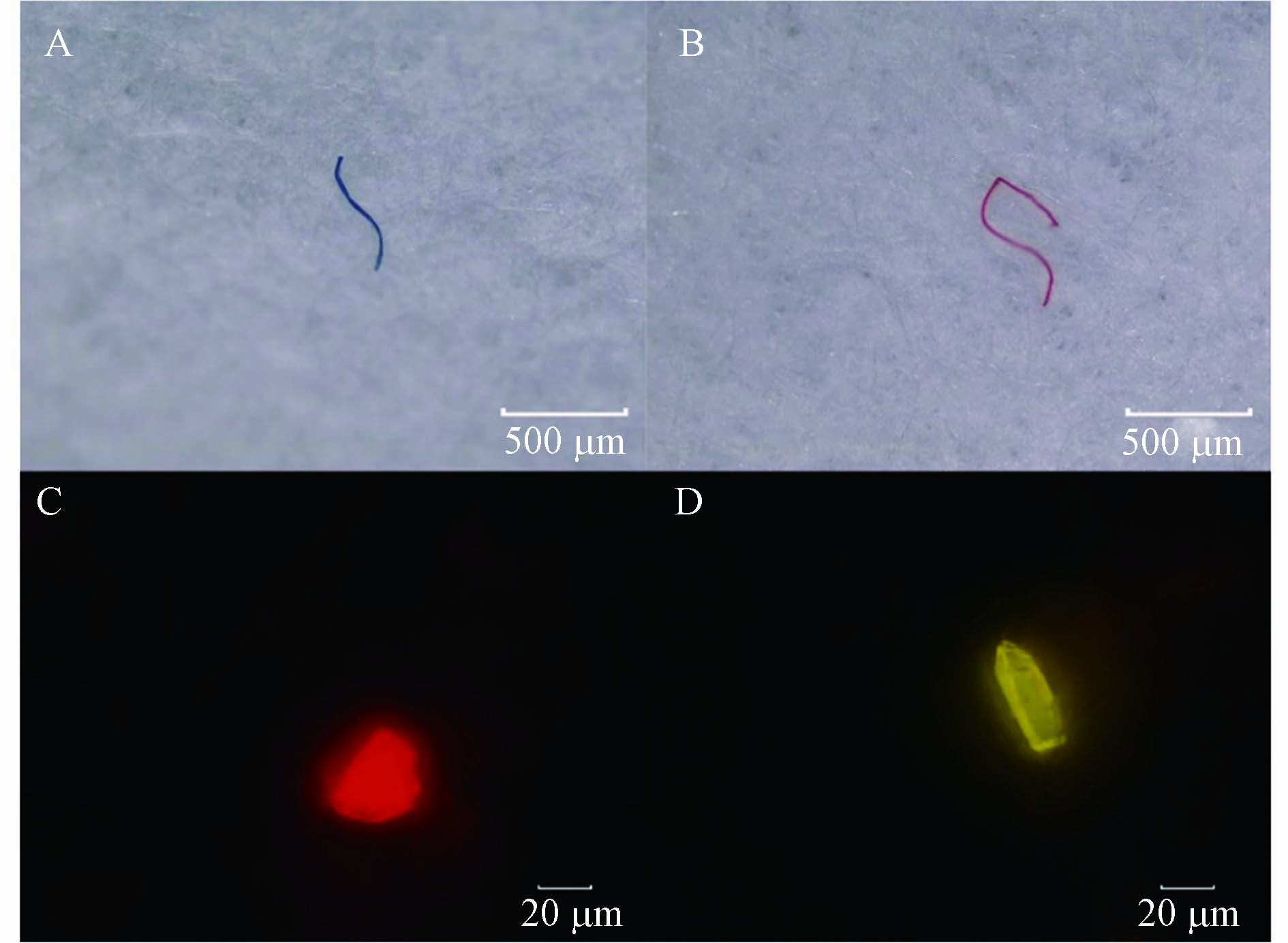
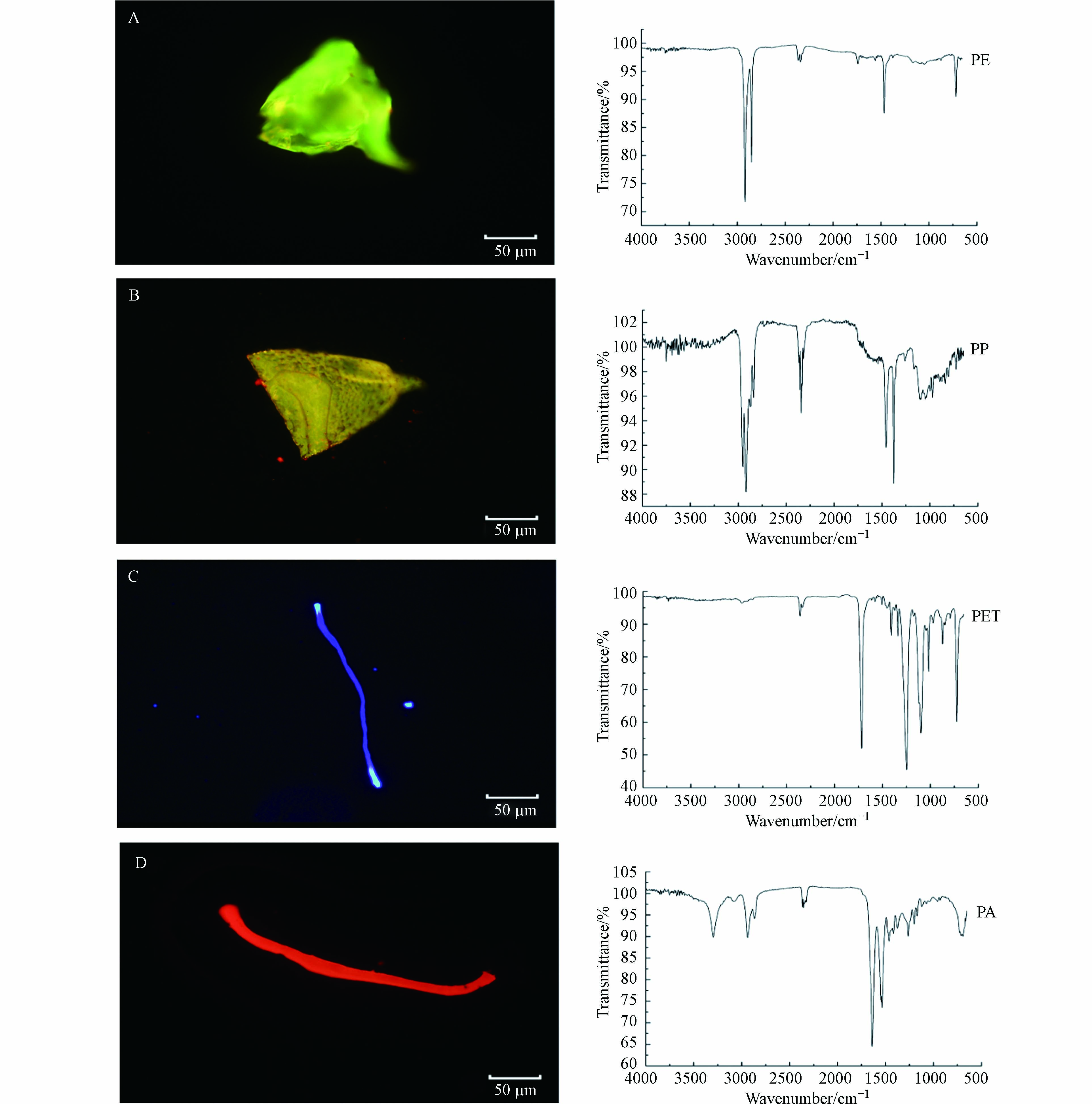
对体视法和NR法所得到的微塑料不同形状和粒径进行统计得到图6,体视法中纤维微塑料占比达93.8%,碎片4.4%,颗粒1.3%,薄膜0.4%(图6A),且微塑料粒径分布主要集中于0.5—1 mm(图6C). 这可能是因为纤维具有较长且带明显颜色(如红色、蓝色等)的特性,使之在体视显微镜下更容易分辨出来(图7A和7B). 尽管部分碎片、颗粒等粒径较小,在体视显微镜下难以分辨,但经NR染色后在荧光显微镜下能观察到明显的荧光(图7C和7D). 因此,NR法观察到颗粒、碎片分别占16.1%和10.4%(图6B),且微塑料主要集中于0—0.5 mm更小的粒径范围内(图6D). 同时,在不同滤光片下微塑料表现出不同颜色的荧光,这有助于对聚合物种类进行初步判断. 对野生鱼体内微塑料NR染色并进行红外光谱分析,结果发现,在蓝色滤光片下PE、PP分别显示出明显的绿色荧光(图8A)和黄色荧光(图8B),在紫色滤光片下PET显示出明显的紫色荧光,纤维两端有其他颜色荧光(图8C),PA纤维在绿色滤光片下显示出强烈的红色荧光(图8D).
-
(1)分液漏斗双密度浮选法操作简便、成本低,能减少微塑料转移中的损失和泥沙等高密度物质的干扰,对不同粒径、不同密度的6种常见微塑料回收率达91%—100%. NaI的重复利用显著降低成本,减少对环境的危害.
(2)热水与乙醇结合能高效去除富含脂质组织与碱液皂化反应生成的肥皂(去除率98%),除PA塑料外,对PS、PP、PE、PET、PVC的红外光谱测定及CI值无明显影响. 同时,肥皂的去除降低了NR染色中有机物共染的问题.
(3)NR染色后,不同滤光片下不同聚合物显示出不同颜色的荧光,有助于对微塑料种类进行逐步判断.
(4)该方法对不同河段、不同种类的野生鱼类样品的消解率达97%—100%,减少了小粒径微塑料的漏检,对微塑料的检出率(84.7%)显著高于体视显微镜镜检法(54.64%).
鱼富脂胃肠道中微塑料检测
Detection of microplastics in gastrointestinal tract of lipid-rich fish
-
摘要: 为建立富含脂质的生物样本中微塑料的检测方法,以鱼胃肠道为研究对象,选取不同粒径(10—100 μm、100—500 μm、500—1000 μm和1—5 mm)、不同密度(ρ<1.15 g·cm−3、ρ>1.3 g·cm−3)的6种微塑料为验证对象,对传统KOH消解法中回收率低、皂化反应干扰、计数不准确等问题进行优化. 结果表明,分液漏斗双密度浮选法能有效减少微塑料损失和降低泥沙等干扰,回收率达91%—100%;100 ℃热水和乙醇联用对皂化反应产生的肥皂的去除效率达98%±1.08%,生物样品的消解效率达99%±0.56%;尼罗红(Nile Red,NR)染色后,不同类型微塑料在不同滤光片下能观察到明显的荧光;除部分聚酰胺(PA)微塑料部分颜色变黄,该方法对PA、聚乙烯(PE)、聚丙烯(PP)、聚苯乙烯(PS)、聚对苯二甲酸乙二醇酯(PET)和聚氯乙烯(PVC)的红外光谱和羰基指数(carbonyl index,CI)无明显影响;实际样本应用表明,该方法对不同河段、不同种类的野生鱼类样本的消解率达97%—100%,对微塑料的检出率(84.70%)显著高于传统的体视显微镜镜检法(54.64%).Abstract: In order to establish the detection method of microplastics in lipid-rich biological samples, taking the gastrointestinal tract of fish as the research object, 6 types of microplastics with different particle sizes (10—100 μm, 100—500 μm, 500—1000 μm and 1—5 mm) and different density (ρ<1.15 g·cm−3, ρ>1.3 g·cm−3) were selected as the verification object, and the problems of low recovery, saponification reaction interference and inaccurate counting in the traditional KOH digestion method were optimized. The results showed that the separation funnel double-density flotation method can effectively reduce the loss of microplastics and the interference of sediment, and the recovery rate was 91%—100%. The removal efficiency of soap induced by saponification reaction occurred by the combination of 100 ℃ hot water and ethanol was 98%±1.08%, and the digestion efficiency of biological samples was 99%±0.56%. We find different types of microplastic stained with Nile Red present distinguishable fluorescence under different light filters. Except that some polyamide (PA) microplastics turn yellow, this method has no obvious effect on the infrared spectrum and carbonyl index of PA, polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET) and polyvinyl chloride (PVC). The actual sample application showed that the digestion rate of this method for different reaches and different kinds of wild fish samples was 97%—100%, and the detection rate of microplastics (84.70%) was significantly higher than that of traditional stereomicroscope (54.64%).
-
Key words:
- microplastics /
- lipid /
- density flotation /
- saponification reaction /
- Nile Red.
-

-
图 6 (A)和(B)分别为体视法和NR法检测得到不同形状微塑料的占比,(C)和(D)分别为体视法和NR法检测得到微塑料的粒径分布频数直方图及曲线拟合
Figure 6. (A)and(B)show the proportions of microplastics with different shapes detected by the stereomicroscope method and the NR method respectively, (C) and (D) obtained histogram and curve fitting of particle size distribution frequency of microplastics detected by stereomicroscope method and NR method respectively
图 8 (A)PE颗粒在蓝色滤光片下的荧光图像及其红外光谱图,(B)PP薄膜在蓝色滤光片下的荧光图像及其红外光谱图,(C)PET纤维在紫色滤光片下的荧光图像及其红外光谱图,(D)PA纤维在绿色滤光片下的荧光图像及其红外光谱图
Figure 8. (A)Fluorescence image and infrared spectrum of PE particle under blue filter,(B)Fluorescence image and infrared spectrum of PP film under blue filter,(C)Fluorescence image and infrared spectrum of PET fiber under purple filter and(D)Fluorescence image and infrared spectrum of PA fiber under green filter
表 1 不同组回收率验证实验的微塑料添加量及回收率
Table 1. The amount and recovery of microplastics in different recovery verification experiments
分组
Group粒径
Size种类及量
Type and quantity回收率/%
Recovery rate1 10—100 μm PA、PS、PE、PP各0.1 g 91.97±1.71 2 100—500 μm PA、PS、PE、PP各0.1 g 90.55±1.69 3 500—1000 μm PA、PS、PE、PP各0.1 g 100.67±1.53 4 1—5 mm PA、PS、PE、PP各0.1 g 100.4±6.41 5 10—100 μm PET、PVC各0.1 g 95±5.93 6 100—500 μm PET、PVC各0.1 g 93±4.05 7 500—1000 μm PET、PVC各0.1 g 96.8±0.8 8 1—5 mm PET、PVC各0.1 g 99.87±0.23 表 2 不同类型的聚合物在不同滤光片下的荧光图像(20×)
Table 2. Fluorescence images of different types of polymers under different filters (20×)

表 3 野外环境中不同鱼类的消解率,体视法和NR法测定微塑料的丰度及检出率
Table 3. The digestion rate of different fish in the wild environment, the abundance and detection rate of microplastics determined by the stereomicroscope method and the NR method
种类
Type经度
Longitude纬度
Latitude鱼长/cm
Length of fish鱼重/g
Weight of fish消解率/%
Digestion rate微塑料丰度/(个·条−1)
Abundance of microplastics微塑料检出率/%
Detection rate of microplastics体视
StereomicroscopeNR 体视
StereomicroscopeNR 鲫鱼 117.197783 29.263332 14.6 23.7 98.64 32 35 60 80 117.381327 29.675387 14.5 18.92 99.38 11 15 60 100 116.505457 29.029374 17.2 20.43 100 25 31 50 80 草鱼 117.510502 29.704057 18.7 38.5 99.73 11 12 40 80 116.505457 29.029374 19.4 30.73 98.32 14 16 60 100 117.510502 29.704057 15.6 22.78 98.07 16 19 62.5 75 黄花鱼 117.197783 29.263332 18.6 49.79 97.74 17 17 66.67 83.33 117.510502 29.704057 13.6 36.68 97.90 13 15 60 100 翘嘴鲌 117.381327 29.675387 13.3 12.62 99.65 16 20 62.5 87.5 117.381327 29.675387 11.3 7.84 99.49 10 15 60 80 117.776792 29.898412 9.3 5.27 100 9 15 33.33 66.67 鳊鱼 117.776792 29.898412 7.8 5.96 100 26 38 40 70 117.510502 29.704057 14.7 16.35 98.79 14 14 60 100 117.510502 29.704057 13.8 12.53 99.14 11 17 50 83.33 平均 99.06 16.07 19.93 54.64 84.70 -
[1] PENG X, CHEN M, CHEN S, et al. Microplastics contaminate the deepest part of the world’s ocean[J]. Geochemical Perspectives Letters, 2018: 1-5. [2] HAN M, NIU X R, TANG M, et al. Distribution of microplastics in surface water of the lower Yellow River near estuary[J]. Science of the Total Environment, 2020, 707: 135601. doi: 10.1016/j.scitotenv.2019.135601 [3] TREILLES R, GASPERI J, TRAMOY R, et al. Microplastic and microfiber fluxes in the Seine River: Flood events versus dry periods[J]. Science of the Total Environment, 2022, 805: 150123. doi: 10.1016/j.scitotenv.2021.150123 [4] WANG W F, YUAN W K, CHEN Y L, et al. Microplastics in surface waters of Dongting Lake and Hong Lake, China[J]. Science of the Total Environment, 2018, 633: 539-545. doi: 10.1016/j.scitotenv.2018.03.211 [5] ZHANG Q J, LIU T, LIU L, et al. Distribution and sedimentation of microplastics in Taihu Lake[J]. Science of the Total Environment, 2021, 795: 148745. doi: 10.1016/j.scitotenv.2021.148745 [6] IMASHA H U E, BABEL S. Microplastics contamination in commercial green mussels from selected wet markets in Thailand[J]. Archives of Environmental Contamination and Toxicology, 2021, 81(3): 449-459. doi: 10.1007/s00244-021-00886-4 [7] PARVIN F, JANNAT S, TAREQ S M. Abundance, characteristics and variation of microplastics in different freshwater fish species from Bangladesh[J]. Science of the Total Environment, 2021, 784: 147137. doi: 10.1016/j.scitotenv.2021.147137 [8] LESLIE H A, van VELZEN M J M, BRANDSMA S H, et al. Discovery and quantification of plastic particle pollution in human blood[J]. Environment International, 2022, 163: 107199. doi: 10.1016/j.envint.2022.107199 [9] YAN Z H, LIU Y F, ZHANG T, et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status[J]. Environmental Science & Technology, 2022, 56(1): 414-421. [10] 韩旭. 微塑料在斑马鱼不同组织中的生物累积[D]. 大连: 大连海事大学, 2020. HAN X. Bioaccumulation of microplastics in different tissues of zebrafish[D]. Dalian: Dalian Maritime University, 2020 (in Chinese).
[11] MISTRI M, SFRISO A A, CASONI E, et al. Microplastic accumulation in commercial fish from the Adriatic Sea[J]. Marine Pollution Bulletin, 2022, 174: 113279. doi: 10.1016/j.marpolbul.2021.113279 [12] LIANG T, LEI Z Y, FUAD M T I, et al. Distribution and potential sources of microplastics in sediments in remote lakes of Tibet, China[J]. Science of the Total Environment, 2022, 806: 150526. doi: 10.1016/j.scitotenv.2021.150526 [13] THOMPSON R C, OLSEN Y, MITCHELL R P, et al. Lost at sea: Where is all the plastic?[J]. Science, 2004, 304(5672): 838. doi: 10.1126/science.1094559 [14] 徐舟影, 陈奥飞, 赵胤祺, 等. 武汉城市污水中微塑料的分离、鉴定及其微观特征分析[J]. 环境科学研究, 2021, 34(3): 637-645. doi: 10.13198/j.issn.1001-6929.2020.07.17 XU Z Y, CHEN A F, ZHAO Y Q, et al. Separation, identification and microscopic characteristics analysis of microplastics in Wuhan municipal sewage[J]. Research of Environmental Sciences, 2021, 34(3): 637-645 (in Chinese). doi: 10.13198/j.issn.1001-6929.2020.07.17
[15] CLAESSENS M, van CAUWENBERGHE L, VANDEGEHUCHTE M B, et al. New techniques for the detection of microplastics in sediments and field collected organisms[J]. Marine Pollution Bulletin, 2013, 70(1/2): 227-233. [16] IMHOF H K, SCHMID J, NIESSNER R, et al. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments[J]. Limnology and Oceanography: Methods, 2012, 10(7): 524-537. doi: 10.4319/lom.2012.10.524 [17] SHRUTI V C, PÉREZ-GUEVARA F, ROY P D, et al. Analyzing microplastics with Nile red: Emerging trends, challenges, and prospects[J]. Journal of Hazardous Materials, 2022, 423(Pt B): 127171. [18] IANNILLI V, PASQUALI V, SETINI A, et al. First evidence of microplastics ingestion in benthic amphipods from Svalbard[J]. Environmental Research, 2019, 179: 108811. doi: 10.1016/j.envres.2019.108811 [19] TONG H Y, JIANG Q Y, HU X S, et al. Occurrence and identification of microplastics in tap water from China[J]. Chemosphere, 2020, 252: 126493. doi: 10.1016/j.chemosphere.2020.126493 [20] WIGGIN K J, HOLLAND E B. Validation and application of cost and time effective methods for the detection of 3–500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California[J]. Marine Pollution Bulletin, 2019, 143: 152-162. doi: 10.1016/j.marpolbul.2019.03.060 [21] DOWARAH K, PATCHAIYAPPAN A, THIRUNAVUKKARASU C, et al. Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet[J]. Marine Pollution Bulletin, 2020, 153: 110982. doi: 10.1016/j.marpolbul.2020.110982 [22] MASON S A, WELCH V G, NERATKO J. Synthetic polymer contamination in bottled water[J]. Frontiers in Chemistry, 2018, 6: 407. doi: 10.3389/fchem.2018.00407 [23] SHIM W J, SONG Y K, HONG S H, et al. Identification and quantification of microplastics using Nile Red staining[J]. Marine Pollution Bulletin, 2016, 113(1/2): 469-476. [24] YARANAL N A, SUBBIAH S, MOHANTY K. Identification, extraction of microplastics from edible salts and its removal from contaminated seawater[J]. Environmental Technology & Innovation, 2021, 21: 101253. [25] PRATA J C, SEQUEIRA I F, MONTEIRO S S, et al. Preparation of biological samples for microplastic identification by Nile Red[J]. Science of the Total Environment, 2021, 783: 147065. doi: 10.1016/j.scitotenv.2021.147065 [26] KONKOL K L, RASMUSSEN S C. An ancient cleanser: Soap production and use in antiquity[M]//ACS Symposium Series. Washington, DC: American Chemical Society, 2015: 245-266. [27] DAWSON A L, MOTTI C A, KROON F J. Solving a sticky situation: Microplastic analysis of lipid-rich tissue[J]. Frontiers in Environmental Science, 2020, 8: 563565. doi: 10.3389/fenvs.2020.563565 [28] NELMS S E, GALLOWAY T S, GODLEY B J, et al. Investigating microplastic trophic transfer in marine top predators[J]. Environmental Pollution, 2018, 238: 999-1007. doi: 10.1016/j.envpol.2018.02.016 [29] 邹亚丹, 徐擎擎, 张哿, 等. 6种消解方法对荧光测定生物体内聚苯乙烯微塑料的影响[J]. 环境科学, 2019, 40(1): 496-503. doi: 10.13227/j.hjkx.201804072 ZOU Y D, XU Q Q, ZHANG G, et al. Influence of six digestion methods on the determination of polystyrene microplastics in organisms using the fluorescence intensity[J]. Environmental Science, 2019, 40(1): 496-503 (in Chinese). doi: 10.13227/j.hjkx.201804072
[30] GOHLA J, BRAČUN S, GRETSCHEL G, et al. Potassium carbonate (K2CO3) - A cheap, non-toxic and high-density floating solution for microplastic isolation from beach sediments[J]. Marine Pollution Bulletin, 2021, 170: 112618. doi: 10.1016/j.marpolbul.2021.112618 [31] KEDZIERSKI M, Le TILLY V, CÉSAR G, et al. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling[J]. Marine Pollution Bulletin, 2017, 115(1/2): 120-129. [32] 宋小卫, 吴晓凤, 宋小平, 等. 微塑料的提取分离方法研究进展[J]. 环境化学, 2022, 41(3): 793-800. doi: 10.7524/j.issn.0254-6108.2020112401 SONG X W, WU X F, SONG X P, et al. Research progress on the extraction and separation methods of microplastics[J]. Environmental Chemistry, 2022, 41(3): 793-800 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020112401
[33] PRATA J C, SILVA A L P, Da COSTA J P, et al. Microplastics in internal tissues of companion animals from urban environments[J]. Animals, 2022, 12(15): 1979. doi: 10.3390/ani12151979 [34] KERSHAW P J, TURRA A, GALGANI F, et al. Guidelines for the Monitoring and assessment of plastic litter and microplastics in the ocean[R]. London, UK: GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection, 2019. [35] PRATA J C, REIS V, PAÇO A, et al. Effects of spatial and seasonal factors on the characteristics and carbonyl index of (micro)plastics in a sandy beach in Aveiro, Portugal[J]. Science of the Total Environment, 2020, 709: 135892. doi: 10.1016/j.scitotenv.2019.135892 [36] RODRIGUES M O, ABRANTES N, GONÇALVES F J M, et al. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal)[J]. Science of the Total Environment, 2018, 633: 1549-1559. doi: 10.1016/j.scitotenv.2018.03.233 [37] KAPUKOTUWA R W M G K, JAYASENA N, WEERAKOON K C, et al. High levels of microplastics in commercial salt and industrial salterns in Sri Lanka[J]. Marine Pollution Bulletin, 2022, 174: 113239. doi: 10.1016/j.marpolbul.2021.113239 [38] 王昆, 林坤德, 袁东星. 环境样品中微塑料的分析方法研究进展[J]. 环境化学, 2017, 36(1): 27-36. doi: 10.7524/j.issn.0254-6108.2017.01.2016051704 WANG K, LIN K D, YUAN D X. Research progress on the analysis of microplastics in the environment[J]. Environmental Chemistry, 2017, 36(1): 27-36 (in Chinese). doi: 10.7524/j.issn.0254-6108.2017.01.2016051704
[39] LÖDER M G J, IMHOF H K, LADEHOFF M, et al. Enzymatic purification of microplastics in environmental samples[J]. Environmental Science & Technology, 2017, 51(24): 14283-14292. -



 下载:
下载: