-
有机磷酸酯(organophosphate esters,OPEs)作为阻燃剂或增塑剂被广泛应用于各类工业和民用产品,如塑料、涂料、橡胶、泡沫、纺织、电子、家具、建筑材料等[1]. 多溴二苯醚(polybrominated diphenyl ethers,PBDEs)等溴代阻燃剂在环境中具有持久性、远距离迁移性、生物累积性和高毒性[2],已经被逐渐淘汰和禁用[3 − 4]. 作为其替代品,OPEs的产量和消费量逐年增加,全球OPEs消费量从1992年的10万吨增加到2015年的68万吨,2018年增加到105万吨[5 − 7]. 由于OPEs通常以物理添加而非化学键合的方式添加到各种材料中,因此很容易通过挥发、浸出、磨损等方式释放到不同环境介质中[8 − 10]. OPEs已在世界各地的空气[11 − 13]、灰尘[11, 14 − 15]、水[16 − 18]、沉积物[16, 19]和土壤[20 − 22]等环境介质中广泛检出. 毒理学研究表明,OPEs对动物具有许多潜在毒性,如生殖毒性[23 − 24]、发育毒性[25 − 26]、神经毒性[27]等,长期接触OPEs可能会导致严重的健康问题.
OPEs的广泛使用和环境存在会不可避免导致人体暴露,人体会通过呼吸[14, 28]、皮肤接触[29 − 31]、饮食[32 − 33]等多种途径摄入OPEs. 目前已在人体尿液、血液、母乳、头发、指甲等多种生物基质中检出OPEs及其代谢物[34 − 37]. 进入人体内的大部分OPEs易经肝脏等代谢器官转化成二酯代谢物(di-OPE)和羟基化代谢物(OH-OPE),并通过尿液等方式排泄或在体内累积[38]. 尿液以非侵入性方式收集,较血液等基质更容易获取,可提供人体暴露OPEs的综合信息,涵盖所有类型的来源和暴露途径,因此尿液是人体OPEs暴露评估的最常用基质,尿液中di-OPEs是现阶段识别和量化人体暴露于OPEs的首选生物标志物[39 − 40].
大多数OPEs能在人体内被快速代谢,但也有部分OPEs的转化效率较低. 例如,体外研究发现,2-乙基己基二苯基磷酸酯(EHDPP)可稳定存在于人肝细胞内,48 h代谢率仅为6.12%[41];银鸥肝微粒将磷酸三苯酯(TPHP)转化为DPHP的转化率仅为15%±3%[42]. He等[43]在澳大利亚儿童尿液中检出了磷酸三(2-氯乙基)酯(TCEP)、磷酸三(1-氯-2-丙基)酯(TDCIPP)、磷酸三(2-乙基己基)酯(TEHP)、TPHP、EHDPP等多种OPEs. 人肝微粒的体外实验表明,OH-OPEs是许多OPEs的主要转化产物而非di-OPEs[44],一些研究也对尿液中OH-OPEs进行了分析[45 − 48]. 但鲜有研究同时对OPEs、di-OPEs和OH-OPEs进行定量分析,且目前关注的目标化合物种类较少,或要分别进行前处理操作,过程复杂,试剂用量大[49].
本研究优化了萃取和净化等前处理参数,建立了一种同时测定尿液样品中14种OPEs、7种di-OPEs和3种OH-OPEs的HPLC-MS/MS分析方法,用以评估人体OPEs暴露水平及代谢特征.
-
UltiMate
3000 高效液相色谱仪(美国Thermo Fisher公司);Triple QuadTM三重四极杆串联质谱检测系统(MS/MS,美国AB SCIEX公司);DP200D-1氮吹浓缩仪(东莞市普标仪器实验器材科技有限公司);STRATA-X-AW固相萃取小柱(60 mg,3 mL,美国Phenomenex);β-葡萄糖醛酸酶/芳基硫酸酯酶(2 mL,1.1 g·cm−3 (20 ℃),美国Sigma-Aldrich公司);甲醇(色谱纯,德国Merck公司);甲酸(色谱纯,美国Fluka公司);乙酸(优级纯,上海沪试化工有限公司);无水乙酸钠(99%,上海麦克林生化科技股份有限公司);氢氧化铵(优级纯,上海沪试化工有限公司);蒸馏水(4.5 L,屈臣氏);乙酸铵(97%,美国Alfa Aesar公司).14种OPEs及其替代内标的标准品:TEP、TMP、TCEP、TPHP、TMPP、TBOEP、EHDPP和CDPP购自德国Dr. Ehrenstorfer GmbH公司;TCEP-D12、TCIPP-D18购自加拿大Toronto Research Chemicals公司;TMP-D9、TEP-D15购自加拿大C/D/N Isotopes公司;V6、3IPPDPP、B3IPPPP、3tBPDPP、B3tBPPP、TnBP-D27和TPHP-D15购自美国Cambridge Isotope Laboratories公司. 7种di-OPEs及其替代内标的标准品:DnBP、DiBP、BBOEP、BCEP、BCIPP、BDCIPP、DPHP、DoCP、DpCP、DnBP-D27、BBOEP-D8、BCEP-D8、BCIPP-D12、BDCPP-D10、DPHP-D10、DoCP-D14和DpCP-D14均购自加拿大Toronto Research Chemicals公司. 3种OH-OPEs及其替代内标的标准品:BBOEHEP、4-OH-TPHP、5-OH-EHDPP、BBOEHEP-D4均购自加拿大Toronto Research Chemicals公司.
OPEs和OH-OPEs单标储备液及其内标储备液(
1000 mg·L−1)用乙腈稀释,配制成质量浓度为1 mg·L−1的混合标准储备液和混合内标储备液,di-OPEs单标储备液及其内标储备液(1000 mg·L−1)用甲醇稀释,配制成质量浓度为1 mg·L−1的混合标准储备液和混合内标储备液,于-4 ℃储存备用. -
尿样在室温解冻后取2 mL于10 mL玻璃离心管中,加10 μL内标混合溶液(1 mg·L−1)静置老化30 min以充分均匀,加入200 μL β-葡萄糖醛酸酶/芳基硫酸酯酶(
1000 单位/mL,pH=5,0.2 mol·L−1醋酸钠缓冲液),37 ℃水浴加热酶解6 h以上,加入800 μL 2%甲酸/水溶液涡旋混匀准备净化.STRATA-X-AW固相萃取柱依次用860 μL 2%甲酸/甲醇和860 μL 2%甲酸/水进行活化,前述准备好的酶解样品上样后依次用860 μL 2%甲酸/水和860 μL 2%甲酸/甲醇淋洗,最后用1.2 mL 2%氨水/甲醇洗脱. 分别收集2%甲酸/甲醇淋洗液和2%氨水/甲醇洗脱液,氮吹挥干后用1:1甲醇/水定容至250 μL,涡旋复溶后转移至放有250 μL玻璃内插管的进样小瓶中,进行HPLC-MS/MS检测.
-
样品分析采用配有双三元梯度泵、自动进样器和柱温箱的高效液相色谱(Ultimate
3000 ,Thermo Fisher Scientific)与配有电喷雾电离(ESI)源的三重四极杆质谱(API4500 )串联仪器.使用Acquity UPLC BEH C18色谱柱(2.1 mm × 100 mm, 1.7 µm,美国Waters)进行样品分离,柱温25 ℃,流动相A为5 mmol·L−1乙酸铵缓冲溶液,B为甲醇;流速为300 µL·min−1,OPEs和OH-OPEs的梯度洗脱程序为:0 min流动相B比例为10%,1 min内增加到40%,到4 min时增加到90%,4.1 min时设定为100% B流动相,在此状态下保持8.4 min,最后在0.1 min内恢复到10% B,并保持5.4 min,总分析时长为18 min. di-OPEs的梯度洗脱程序:初始流动相B比例为20%,保持0.5 min,1 min时增加到40%,到4 min时增加到100%,保持6 min,最后在0.1 min内恢复到20% B,保持6.9 min,总分析时长为17 min.
-
对于OPEs和OH-OPEs,采用电喷雾离子源正离子模式(ESI+)电离,多重反应监测模式(MRM)分析,气帘气压0.14 MPa,碰撞气压0.02 MPa,离子源喷雾电压
5000 V,温度600 ℃,雾化气0.34 MPa,辅助雾化气0.28 MPa. 对于di-OPEs,选择负离子模式(ESI-)电离MRM分析,气帘气压0.07 MPa,碰撞气压0.02 MPa,离子源喷雾电压-4500 V,温度500 ℃,雾化气0.34 MPa,辅助雾化气0.34 MPa. 其余质谱参数信息详见表1和表2. -
在整个采样和实验过程中,为保证样品检测结果的可靠性,尽量避免使用塑料和橡胶制品,所有玻璃器皿在每次使用前依次用超纯水和甲醇清洗3次. 样品前处理时,在每11个样品中加1个程序空白样品以监测前处理过程中可能引入的污染. 使用内标法对目标物质进行定量,仪器检测时,每15个样品加1针标准溶液(5 ng·mL−1)作为质控样,以监测仪器信号的稳定性,若质控样测定结果与其真实值的偏差大于±20%,则须重新绘制标准曲线.
-
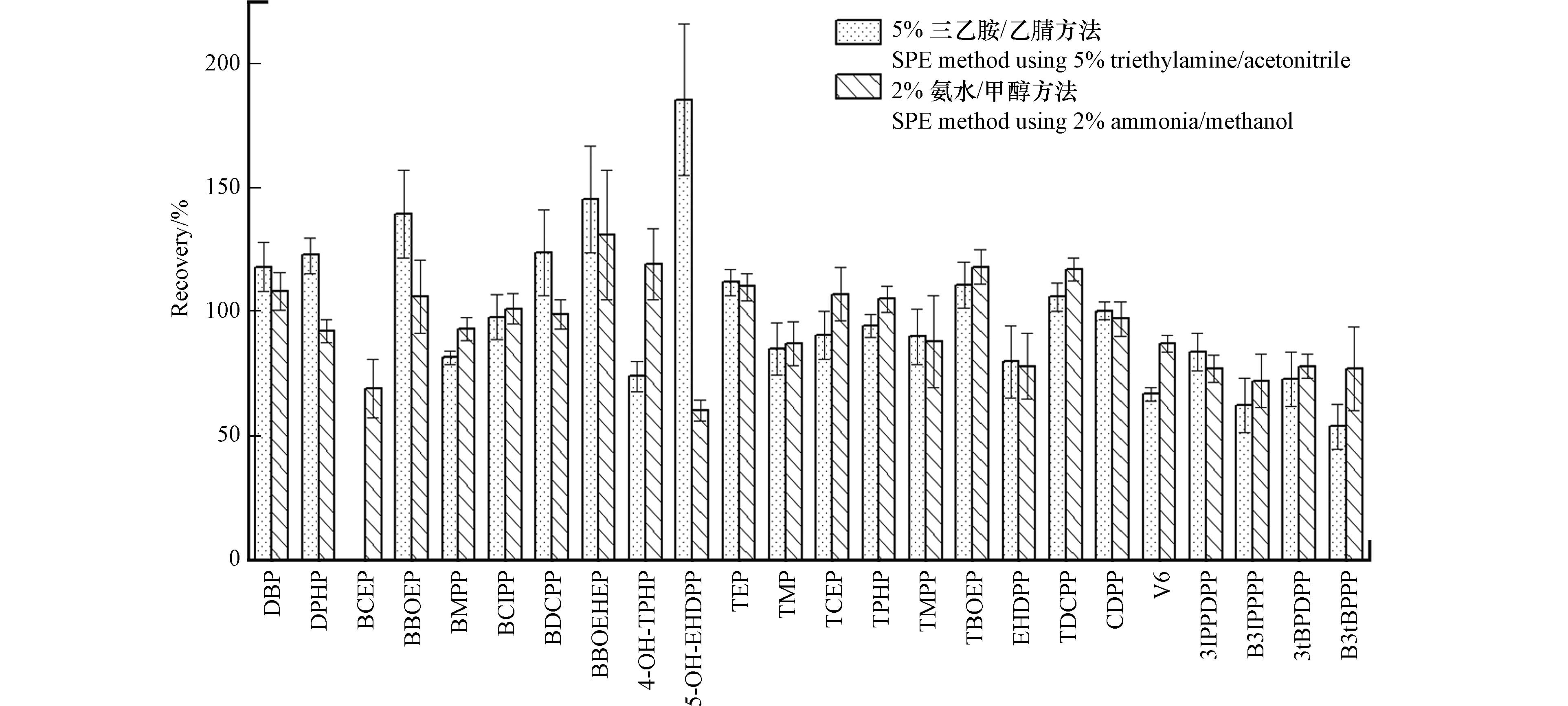
固相萃取小柱、淋洗与洗脱溶剂是决定样品富集净化效果的重要因素. 本研究选取尿液样本处理中最常用的STRATA-X-AW萃取小柱,考察了5%三乙胺/乙腈和2%氨水/甲醇两种溶剂对目标物加标回收结果的影响. 使用2%氨水/甲醇的固相萃取过程如“1.2”所述,使用另一种溶剂的净化过程为:用2 mL乙腈、2 mL水活化萃取小柱,上样后用2 mL水淋洗,最后用2 mL 5%三乙胺/乙腈洗脱目标物,将收集的洗脱液氮吹挥干后用1:1甲醇/水定容至1 mL.
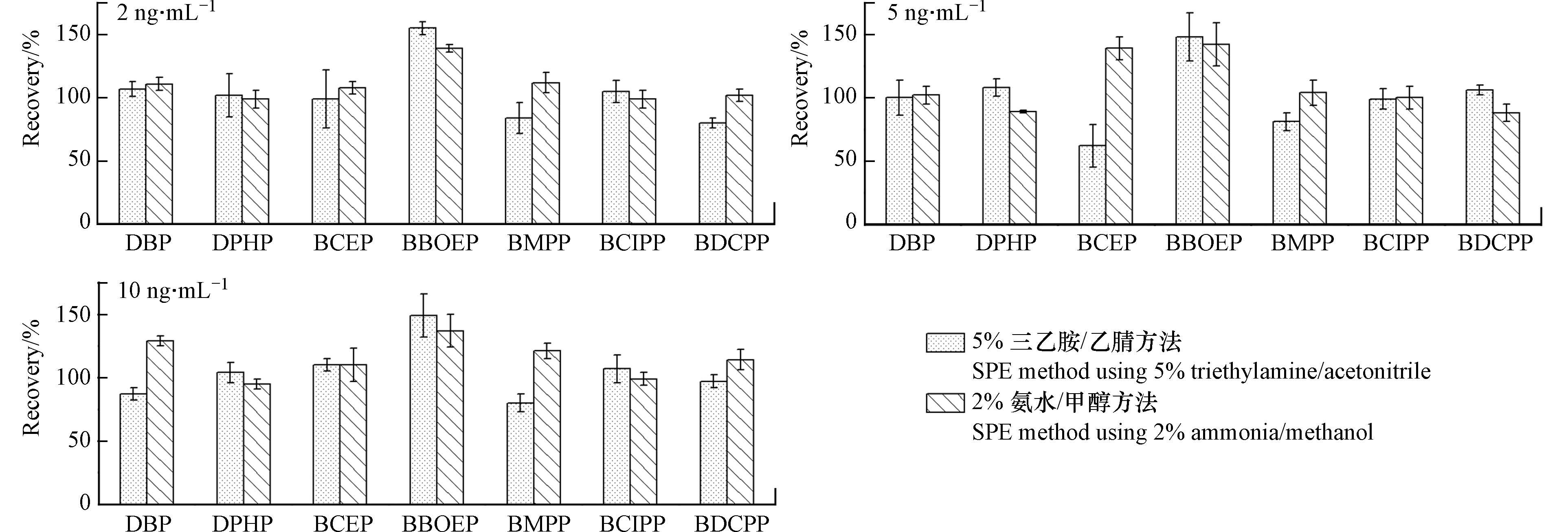
两种方法在2 ng·mL−1、5 ng·mL−1和10 ng·mL−1加标水平下对7种di-OPEs的加标回收率如图1所示,除BCEP(5 ng·mL−1加标水平)外,其余物质的回收率不存在明显差异. 5%三乙胺/乙腈洗脱方法中OPEs和OH-OPEs的加标回收率为60%—126%,RSD为2%—24%. 但2%氨水/甲醇洗脱液中多数OPEs和OH-OPEs未检出,检出物质的回收率低于1%,分析原因可能是该方法中使用的2%甲酸/甲醇酸性有机淋洗液即可将填料吸附的OPEs完全洗脱,因此收集前处理过程中2%甲酸/甲醇淋洗液氮吹浓缩后上机检测OPEs和OH-OPEs.
另外,考虑到实际尿液样本中OPEs及其代谢物浓度较低,本研究又在尿液样本中添加1 ng·mL−1的浓度水平下对比了两种方法对目标物的萃取回收结果(图2),2%氨水/甲醇洗脱方法所有物质加标回收率为60%—131%,5%三乙胺/乙腈洗脱方法中BCEP浓度小于检出限,其余目标物的加标回收率为54%—185%. 上述结果说明低浓度水平下,2%氨水/甲醇方法表现出更好的回收率,对目标物质的洗脱效率更高,且该方法浓缩倍数高,具有更低的方法检出限和更高的灵敏度,溶剂用量更少. 综合考虑,选择收集固相萃取过程中的2%甲酸/甲醇淋洗液测定OPEs及OH-OPEs,收集2%氨水/甲醇洗脱液测定di-OPEs.
-
本研究采用提取后添加法评估人体尿液样本的基质效应(matrix effect,ME)[50],取不添加任何目标物质以及内标的尿液样品,根据前处理过程进行萃取净化,将获得的萃取液混合得到基质空白. 在空白基质中加入标准物质和内标,浓度为2 ng·mL−1,设置4个平行,上机检测. 目标化合物的基质效应计算方法见公式(1):
其中,A0为混合基质空白中目标物的响应峰面积;A1为加标混合基质中目标物的响应峰面积;A2为纯溶剂中目标物的响应峰面积.
ME<100%,表示有基质抑制效应;ME>100%,表示有基质增强效应;ME=100%,表示无基质效应. 由表3可见,人体尿液中的24种目标物质的基质效应为24%—159%,大部分的di-OPEs和OH-OPEs呈现基质抑制效应,而大多数的OPEs呈现基质增强效应. 其中大部分物质的基质效应,如BBOEP(35%)、TBOEP(156%)等,可通过其相应的同位素内标(BBOEP-D8(35%)、TBOEP-D6(151%))进行消除,一些不具有对应同位素内标的化合物也可通过选择合适的内标定量抵消部分基质效应的影响,校正后OPEs及其代谢物的回收率为78%—125%,整体可达到微量分析的要求.
-
以1:1甲醇/水为溶剂,配制与样品浓度相对应的质量浓度范围为0.05—40 ng·mL−1的7种di-OPEs的混合标准溶液,以及3种OH-OPEs和15种OPEs的混合标准溶液,在“1.3”的仪器条件下按照标准溶液浓度从低到高的顺序进行检测,以目标化合物与对应内标物峰面积之比为纵坐标(y),以目标物质量浓度为横坐标(x)绘制标准曲线. 结果表明,所有目标化合物均在该范围内表现出良好的线性关系(相关系数r>0.99). 对于实验中存在过程空白的物质,以过程空白的3倍标准偏差作为方法检出限(MDL),不存在过程空白的物质,以3倍信噪比计算方法检出限,最终所得OPEs、OH-OPEs和di-OPEs的MDL分别为
0.0008 —0.32 ng·mL−1、0.0040 —0.0083 ng·mL−1和0.0072 —0.23 ng·mL−1(表4). -
通过尿液基质加标回收实验考察了方法的准确度和精密度. 将实际人体尿液样品作为实验基质,添加0.5 ng·mL−1和1 ng·mL−1水平的目标化合物混合标准溶液,每个浓度水平设置4个平行样本,进行前处理和分析. 结果如表5所示,7种di-OPEs加标回收率69%—121%,RSD为5%—19%;3种OH-OPEs和15种OPEs的加标回收率60%—131%,RSD为4%—22%,说明方法具有较好的准确度和精密度,能够满足检测的要求.
-
使用本方法对15个实际人体尿样中的OPEs及其代谢物(m-OPEs)进行分析,结果见表6. 7种di-OPEs和3种OH-OPEs的总含量为0.07—7.04 ng·mL−1,中位含量为 0.54 ng·mL−1. DPHP在15个样品中均被检出,DBP的检出率高于50%,这可能与两种di-OPEs具有直接的工业生产和应用有关,需对其来源进一步分析追溯. 14种OPEs的总含量为< LOD—0.68 ng·mL−1,中位含量为0.05 ng·mL−1,浓度和检出率较m-OPEs低,但一种新的有机磷酸酯阻燃剂3-异丙基苯基二苯基磷酸酯(3IPPDPP)的检出率为66.7%,需引起关注.
-
本研究建立了通过一次前处理即实现人体尿液中7种di-OPEs、3种OH-OPEs和14种OPEs的提取测定分析方法. 方法操作简便快捷,具有较低的检出限,对人体尿液中低浓度目标物有良好的回收率和重现性,可满足人体尿液中OPEs及其代谢物的分析要求.
固相萃取-液相色谱-串联质谱测定人体尿液中有机磷酸酯及其二酯和羟基代谢物
Determination of organophosphate esters and their diester and hydroxylated metabolites in human urine by high performance liquid chromatography-tandem mass spectrometry combined with solid phase extraction
-
摘要: 尿液中的有机磷酸酯二酯代谢物(di-OPEs)是现阶段识别和量化人体暴露于有机磷酸酯(OPEs)的首选生物标志物. 目前鲜有研究同时对OPEs及其二酯代谢物、羟基代谢物(OH-OPEs)进行分析测定,且关注的目标化合物种类较少. 本研究对尿液前处理过程中常用的净化浓缩方法进行了优化,建立了人体尿液样品中14种OPEs、7种di-OPEs和3种OH-OPEs的高效液相色谱-串联质谱分析方法(HPLC-MS/MS). 取2 mL样品经β-葡萄糖醛酸酶/芳基硫酸酯酶酶解6 h后,加入2% 甲酸/水调节pH,然后用STRATA-X-AW固相萃取柱进行净化,收集固相萃取过程中的2% 甲酸/甲醇淋洗液和2% 氨水/甲醇洗脱液氮吹浓缩后分别进行OPEs、OH-OPEs和di-OPEs的HPLC-MS/MS测定,OPEs和OH-OPEs的质谱检测选用电喷雾正离子模式电离,di-OPEs选用负离子模式,在多重反应监测模式(MRM)下测定. 尽管尿液样品中多数目标物质在检测时存在基质效应(均值24%—159%),但通过合适的同位素内标进行校正,可以抵消部分基质影响. 在优化的条件下,24种目标物质在0.05—40 ng·mL−1范围内线性关系良好(r>0.99),方法检出限(MDL)
0.0008 —0.32 ng·mL−1,加标回收率60%—131%,RSD为4%—22%. 采用本方法对实际人体尿液样本进行分析,7种di-OPEs和3种OH-OPEs的总含量为0.07—7.04 ng·mL−1,中位含量为 0.54 ng·mL−1,14种OPEs的总含量为<MDL—0.68 ng·mL−1,中位含量为0.05 ng·mL−1. 两种具有直接工业生产应用的di-OPEs(磷酸二苯基酯DPHP和磷酸二丁酯DBP)检出率高于60%,其来源值得追溯. 一种新型有机磷酸酯阻燃剂3-异丙基苯基二苯基磷酸酯(3IPPDPP)的检出率为66.7%,应该引起关注.-
关键词:
- 有机磷酸酯 /
- 代谢物 /
- 固相萃取 /
- 高效液相色谱-串联质谱 /
- 人体尿液
Abstract: Organophosphate diester (di-OPE) metabolites in urine are commonly used as biomarkers to identify and quantify human exposure to organophosphate esters (OPEs). Few studies have determined OPEs and their diester and hydroxyl metabolites (OH-OPEs) at the same time, and there were fewer types of substances of concern. In this study, purification and concentration parameters in the pretreatment process were optimized, and a high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method was established for the detection of 14 OPEs, 7 di-OPEs and 3 OH-OPEs in human urine samples. 2 mL of the sample was digested by β-glucuronidase/aryl sulfatase enzyme solution for 6 h. After adding 2% formic acid in water to adjust the pH value, the samples were cleaned up by a STRATA-X-AW solid phase extraction column, 2% formic acid/methanol rinse and 2% ammonia/methanol eluent were collected and concentrated for the detection of OPEs, OH-OPEs and di-OPEs by HPLC-MS/MS. OPEs and OH-OPEs were ionized in electrospray ionization positive mode, di-OPEs were ionized in electrospray ionization negative mode, then the analytes were detected in the multiple reaction monitoring (MRM) mode. Although most of the target analytes in urine samples have matrix effects (average in range of 24%—159%), the matrix effects can be partially cancelled out by choosing suitable isotopic internal standards for correction. Under the optimized condition, the linear relationships of all the analytes were good in the range of 0.05—40 ng·mL−1 (r>0.99). The method detection limits (MDL) ranged from0.0008 ng·mL−1 to 0.32 ng·mL−1. The average recoveries of 24 analytes in urine were in the range of 60%—131% and the RSDs were in the range of 4%—22%. The optimized method was applied to detect OPEs, di-OPEs and OH-OPEs in 15 human urine samples. The total concentrations of 7 di-OPEs and 3 OH-OPEs ranged from 0.07 ng·mL−1 to 7.04 ng·mL−1, with a median concentration of 0.54 ng·mL−1, and the total concentrations of 14 OPEs ranged from < MDL to 0.68 ng·mL−1, with a median concentration of 0.05 ng·mL−1. Diphenyl phosphate (DPHP) and dibutyl phosphate (DBP), which have direct industrial production and applications, were detected at a rate higher than 60%, so their sources need to be further studied. 3-isopropylphenyl diphenyl phosphate (3IPPDPP), a new organophosphate flame retardant, was detected at a rate of 66.7%, and it should be a cause for concern. -

-
表 1 7种di-OPEs的质谱参数
Table 1. MS parameters for 7 di-OPEs
化合物
Compounds缩写
Abbreviation母离子
Precursor ion(m/z)子离子
Product ion
(m/z)解簇电压/ V
DP入口电压/ V
EP碰撞出口
电压/ V
CXPDibutyl phosphate
(磷酸二丁酯)DBP 209 78.9*, 152.9 −70, −70 −10 −7, −8 Diphenyl phosphate
(磷酸二苯基酯)DPHP 248.9 92.9*, 155 −80, −80 −10 −6, −10 Bis(2-chloroethyl) phosphate
(磷酸二(2-氯乙基)酯)BCEP 221 35.1*, 36.9 −15, −15 −10 −10, −10 Bis(2-butoxyethy) phosphate
(磷酸二丁氧酯)BBOEP 297 78.8*, 197.0 −100, −100 −10 −6, −10 Di-o-tolyl-phosphate
(磷酸二甲苯酯)BMPP 277 107*, 169.0 −95, −95 −10 −8, −6 Bis(1-chloro-2-propyl) phosphate
(磷酸二(1-氯-2-丙基酯))BCIPP 248.8 34.9*, 37.0 −30, −30 −10 −9, −10 Bis(1,3-dichlo-ro-2-propyl) phosphate
(磷酸二(1,3-二氯-2-丙基)酯)BDCPP 316.9 35.0*, 37.0 −35, −35 −10 −5, −9 内标
ISDnBP-D18 227.1 78.9*, 163 −70, −20 −10 −7, −8 DPHP-D10 258.9 98.0*, 158.9 −90, −90 −10 −8, −8 BCEP-D8 229.0 35.0 −22 −10 −9 BCIPP-D12 260.6 35.0*, 37.0 −20, −20 −10 −9, −10 BBOEP-D8 305.0 78.9 −52 −10 −7 BMPP-D14 291 114.0*, 174.9 −100, −100 −10 −9, −12 BDCPP-D10 326.8 35 −40 −10 −9 注:*定量离子Quantitative ion;DBP包括DnBP (Di-n-butyl phosphate)和DiBP (Di-iso-butyl phosphate),BMPP包括DoCP (Di-o-tolyl-phosphate)和DpCP (Di-p-tolyl-phosphate). 表 2 14种OPEs和3种OH-OPEs的质谱参数
Table 2. MS parameters for 14 OPEs and 3 OH-OPEs
化合物
Compound缩写
Abbreviation母离子
Precursor
ion(m/z)子离子
Product ion
(m/z)解簇
电压/V
DP入口
电压/V
EP碰撞出口
电压/V
CXPTriethyl phosphate
(磷酸三乙酯)TEP 183.0 99.0*, 81.0 54, 60 10 7, 8 Trimethyl phosphate
(磷酸三甲酯)TMP 141.1 109.1*, 79.0 60, 60 10 10, 6 Tri(2-chloroethyl) phosphate
(磷酸三(2-氯乙基)酯)TCEP 285.0 63.0*, 99.2 80, 75 10 10, 10 Tri-phenyl phosphate
(磷酸三苯酯)TPHP 327.1 152.0*, 77.1 130, 130 10 11, 7 Trimethylphenyl phosphate
(磷酸三甲苯酯)TMPP 369.2 166.1*, 90.9 147, 147 10 11, 8 Tri(2-butoxyethyl) phosphate
(磷酸三丁氧酯)TBOEP 399.3 299.3*, 199.0 95, 95 10 10, 10 2-Ethylhexyl di-phenyl phosphate
2-乙基己基二苯基磷酸酯EHDPP 363.2 251.0*, 76.9 72,70 10 9,7 Tri(1,3-dichloro-2-propyl) phosphate
(磷酸三(1,3-二氯-2-丙基)酯)TDCPP 431.1 98.9*, 208.9 85, 84 10 9, 8 Cresyl diphenyl phosphate
(磷酸甲苯二苯酯)CDPP 341.1 152.1*, 165.1 135, 135 10 10, 10 2,2-bis(Chloromethyl) trimethylene
bis(bis(2-chloroethyl)phosphate)
2,2-双(氯甲基)三亚甲基双(双(2-氯乙基)磷酸酯)V6 582.9 361.1*, 234.7 111.6, 111.6 10 11, 16 3-isopropylphenyl diphenyl phosphate
3-异丙基苯基二苯基磷酸酯3IPPDPP 368.9 327.0*, 152.1 130, 130 10 11, 10 Bis(3-isopropylphenyl) phenyl phosphate
磷酸双(3-异丙基苯基)苯酯B3IPPPP 411.0 327.0*, 369.1 149, 149 10 12.5, 11 3-tert-Butylphenyl diphenyl phosphate
3-叔丁基苯基二苯基磷酸酯3tBPDPP 383.0 327.0*, 215.0 132, 132 10 12.5, 13 Bis(3-tert-butylphenyl) phenyl phosphate
磷酸双(3-叔丁基苯基)苯酯B3tBPPP 439.1 327.0*, 383.0 167, 167 10 16, 14 Bis(2-butoxyethyl) hydroxyethyl phosphate
双(2-丁氧基乙基)羟乙基磷酸酯BBOEHEP 343.2 243.1*, 101.1 72, 70 10 16, 10 4-hydroxyl triphenyl phosphate
4-羟基苯基二苯基磷酸酯4-OH-TPHP 343.1 141.1*, 215.1 119, 130 10 5, 8 2-ethyl-5-hydroxyhexyl diphenyl phosphate
2-乙基-5-羟基己基二苯基磷酸酯5-OH-EHDPP 379.1 251.0*, 153.1 70, 130 10 12, 7 内标
ISTEP-D15 198.0 101.9 65 10 8 TMP-D9 150.1 83.1 90 10 7 TCEP-D12 299.1 102.0 75 10 6 TBOEP-D18 426.2 208.2 85 10 15 TCIPP-D18 345.1 101.9 75 10 8 TDCPP-D15 445.8 101.9 85 10 8 TPHP-D15 342.3 160.0 135 10 10 BBOEHEP-D4 347.3 100.8 140 10 9 注:*定量离子Quantitative ion. 表 3 7种di-OPEs、3种OH-OPEs及14种OPEs在人体尿液中2 ng·mL−1 水平下的基质效应(n=4)
Table 3. Matrix effect of the 7 di-OPEs, 3 OH-OPEs and 14 OPEs in human urine at level of 2 ng·mL−1 (n=4)
化合物
Compound基质效应/%
ME精密度/%
RSD内标
Internal standard基质效应/%
ME精密度/%
RSDDBP 65 6 DnBP-D18 58 5 DPHP 59 8 DPHP-D10 59 0 BCEP 59 8 BCEP-D8 96 6 BBOEP 35 10 BBOEP-D8 35 5 BMPP 53 9 BMPP-D14 49 1 BCIPP 103 9 BCIPP-D12 88 3 BDCPP 52 8 BDCPP-D10 45 10 BBOEHEP 52 8 BBOEHEP-D4 29 6 4-OH-TPHP 34 13 TPHP-D15 22 7 5-OH-EHDPP 78 11 TBOEP-D6 141 4 TEP 84 2 TEP-D15 74 3 TMP 140 8 TMP-D9 136 6 TCEP 24 6 TCEP-D12 23 7 TPHP 86 9 TPHP-D15 89 3 TMPP 144 8 TPHP-D15 89 3 TBOEP 156 5 TBOEP-D6 151 4 EHDPP 139 20 TPHP-D15 89 3 TDCPP 73 10 TDCPP-D15 67 6 CDPP 112 5 TPHP-D15 89 3 V6 58 6 TCIPP-D18 68 4 3IPPDPP 147 6 TPHP-D15 89 3 B3IPPPP 155 6 TPHP-D15 89 3 3tBPDPP 147 5 TPHP-D15 89 3 B3tBPPP 159 6 TPHP-D15 89 3 表 4 7种di-OPEs、14种OPEs及3种OH-OPEs的线性范围、线性方程、相关系数和方法检出限
Table 4. Linear ranges, linear equations, correlation coefficients, detection limits and quantitation limits of 7 di-OPEs, 3 OH-OPEs and 14 OPEs
化合物Compound 内标
Internal Standard线性范围/(ng·mL−1)
Linear range线性方程
Linear equation相关系数
Correlation coefficient (r)方法检出限/(ng·mL−1)
MDLDBP DnBP-D18 0.1—40 y=0.156x+ 0.00739 0.9977 0.0076 DPHP DPHP-D10 0.05—20 y= 0.0397 x+0.00325 0.9979 0.015 BCEP BCEP-D8 0.5—20 y=0.144x+ 0.0057 0.9962 0.23 BBOEP BBOEP-D8 0.05—20 y= 0.0827 x-0.00404 0.9938 0.018 BMPP BMPP-D14 0.1—40 y=0.265x+ 0.00289 0.9969 0.0072 BCIPP BCIPP-D12 0.05—20 y=0.028x+ 0.00059 0.9983 0.020 BDCPP BDCPP-D10 0.05—20 y=0.146x+1.44×10-6 0.9992 0.028 BBOEHEP BBOEHEP-D4 0.05—20 y=1.55x+ 0.00241 0.9921 0.0056 4-OH-TPHP TPHP-D15 0.05—20 y=0.157x+ 0.00405 0.9974 0.0083 5-OH-EHDPP TBOEP-D6 0.05—20 y=0.113x+ 0.00154 0.9967 0.0040 TEP TEP-D15 0.05—20 y=0.122x+ 0.00335 0.9992 0.0081 TMP TMP-D9 0.5—20 y=0.147x- 0.00803 0.9986 0.32 TCEP TCEP-D12 0.05—20 y=0.155x+9.44×10-5 0.9996 0.014 TPHP TPHP-D15 0.05—20 y=0.161x+ 0.00159 0.9997 0.010 TMPP TPHP-D15 0.05—20 y=0.161x+ 0.00159 0.9997 0.0018 TBOEP TBOEP-D6 0.05—20 y= 0.0823 x+0.0118 0.9998 0.013 EHDPP TPHP-D15 0.05—20 y= 0.0782 x+0.00242 0.9992 0.011 TDCPP TDCPP-D15 0.05—20 y= 0.0342 x+0.00384 0.9987 0.0061 CDPP TPHP-D15 0.05—20 y=0.033x+ 0.000638 0.9990 0.036 V6 TCIPP-D18 0.05—20 y=0.22x+ 0.00543 0.9991 0.0036 3IPPDPP TPHP-D15 0.05—20 y=0.471x+ 0.0111 0.9982 0.0013 B3IPPPP TPHP-D15 0.05—20 y=0.506x+ 0.0125 0.9977 0.0008 3tBPDPP TPHP-D15 0.05—20 y=0.476x+ 0.00998 0.9983 0.0009 B3tBPPP TPHP-D15 0.05—20 y=0.36x+ 0.00378 0.9993 0.0008 表 5 7种di-OPEs、3种OH-OPEs及14种OPEs在人体尿液中低浓度水平下的加标回收率(n=4)
Table 5. Spiked recoveries and relative standard deviations of the 7 di-OPEs, 3 OH-OPEs and 14 OPEs in human urine at low concentration levels (n=4)
化合物
Compound加标浓度水平
Spiked concentration0.5 ng·mL−1 1 ng·mL−1 回收率/%
Recovery精密度/%
RSD回收率/%
Recovery精密度/%
RSDDBP 89 12 108 7 DPHP 90 9 92 5 BCEP 78 5 69 17 BBOEP 121 7 106 14 BMPP 114 19 93 5 BCIPP 108 5 101 6 BDCPP 87 6 99 6 BBOEHEP 125 16 131 20 4-OH-TPHP 95 15 119 12 5-OH-EHDPP 74 8 60 7 TEP 112 5 110 5 TMP 85 12 87 10 TCEP 90 11 107 10 TPHP 94 5 105 5 TMPP 90 13 88 21 TBOEP 111 8 118 6 EHDPP 80 18 78 17 TDCPP 106 5 117 4 CDPP 100 4 97 7 V6 67 4 87 4 3IPPDPP 84 9 77 7 B3IPPPP 62 18 72 15 3tBPDPP 73 15 78 6 B3tBPPP 64 17 77 22 表 6 15个人体尿样中m-OPEs和OPEs的浓度值(ng·mL−1)
Table 6. Concentrations of m-OPEs and OPEs in 15 samples (ng·mL−1)
化合物
Compound1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 DBP 0.38 — — — — 0.05 0.09 0.03 — 0.04 0.10 0.19 — 0.28 0.04 DPHP 0.56 0.41 0.62 0.07 0.13 0.10 0.08 0.08 0.20 0.10 0.36 1.82 4.4 0.49 0.08 BCEP — — — — 0.34 — — — 0.38 0.62 — — 0.66 — — BBOEP — — — — — — 0.07 0.05 — — — — — 0.22 — BMPP — — — — — — — — — — — — — — — BCIPP 0.15 — 0.76 — 0.07 — — — — — — — — — — BDCPP 0.18 0.06 5.65 — — — — — — — — — — — — BBOEHEP — — — — — — — — — — — — — — — 4-OH-TPHP — — — — — — — — — — — — — — — 5-OH-EHDPP — — — — — — — — — — — — — — — ∑m-OPEs 1.27 0.47 7.04 0.07 0.54 0.15 0.25 0.16 0.58 0.77 0.45 2.02 5.06 0.99 0.12 TEP — — 0.31 0.02 — 0.48 — 0.01 0.05 0.09 0.04 0.01 — 0.01 0.02 TMP — — — — — — — — — — — — — — — TCEP — — — — — — — — — — — — — — — TPHP — — — — — — — — — — — — — — — TMPP — — — — — — — — — — — — — — — TBOEP — — 0.02 — — — 0.03 — — — 0.04 — — 0.07 — EHDPP — — — — — — — — — — — — — — TDCPP — — 0.18 — — — — — — — — — — — — CDPP — — — — — — — — — — — — — — V6 — — — — — — — — — — — — — — 3IPPDPP 0.03 0.01 0.01 — 0.02 — 0.01 — — 0.08 0.02 0.05 — 0.08 0.05 B3IPPPP — — — — — — — — — — — — — — — 3tBPDPP — 0.00 0.16 — 0.03 — — — — 0.01 — 0.03 — 0.03 0.03 B3tBPPP — — — — — — — — — — — — — — — ∑OPEs 0.03 0.01 0.68 0.02 0.05 0.48 0.04 0.01 0.05 0.17 0.10 0.09 0 0.19 0.10 —:未检出. -
[1] WEI G L, LI D Q, ZHUO M N, et al. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure[J]. Environmental Pollution, 2015, 196: 29-46. doi: 10.1016/j.envpol.2014.09.012 [2] de WIT C A, HERZKE D, VORKAMP K. Brominated flame retardants in the Arctic environment: Trends and new candidates[J]. The Science of the Total Environment, 2010, 408(15): 2885-2918. doi: 10.1016/j.scitotenv.2009.08.037 [3] ABBASI G, LI L, BREIVIK K. Global historical stocks and emissions of PBDEs[J]. Environmental Science & Technology, 2019, 53(11): 6330-6340. [4] BETTS K. Does a key PBDE break down in the environment?[J]. Environmental Science & Technology, 2008, 42(18): 6780. [5] van der VEEN I, de BOER J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis[J]. Chemosphere, 2012, 88(10): 1119-1153. doi: 10.1016/j.chemosphere.2012.03.067 [6] WANG Y, SUN H W, ZHU H K, et al. Occurrence and distribution of organophosphate flame retardants (OPFRs) in soil and outdoor settled dust from a multi-waste recycling area in China[J]. The Science of the Total Environment, 2018, 625: 1056-1064. doi: 10.1016/j.scitotenv.2018.01.013 [7] LIN J N, ZHANG L T, GUO C S, et al. Inter-annual variation and comprehensive evaluation of organophosphate esters (OPEs) in the Yellow Sea, China[J]. Marine Pollution Bulletin, 2022, 176: 113440. doi: 10.1016/j.marpolbul.2022.113440 [8] CARLSSON H, NILSSON U, ÖSTMAN C. Video display units: an emission source of the contact allergenic flame retardant triphenyl phosphate in the indoor environment[J]. Environmental Science & Technology, 2000, 34(18): 3885-3889. [9] SUNDKVIST A M, OLOFSSON U, HAGLUND P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk[J]. Journal of Environmental Monitoring:JEM, 2010, 12(4): 943-951. doi: 10.1039/b921910b [10] SALTHAMMER T, FUHRMANN F, UHDE E. Flame retardants in the indoor environment: Part II: Release of VOCs (triethylphosphate and halogenated degradation products) from polyurethane[J]. Indoor Air, 2003, 13(1): 49-52. doi: 10.1034/j.1600-0668.2003.01150.x [11] HOU M M, SHI Y L, NA G S, et al. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure[J]. Environment International, 2021, 146: 106261. doi: 10.1016/j.envint.2020.106261 [12] CHEN S C, TAO F, LIU W B, et al. Emerging and traditional organophosphate esters in office air from Hangzhou, East China: Seasonal variations, influencing factors and human exposure assessment[J]. Environment International, 2023, 182: 108313. doi: 10.1016/j.envint.2023.108313 [13] LI R J, GAO H, HOU C, et al. Occurrence, source, and transfer fluxes of organophosphate esters in the South Pacific and Fildes Peninsula, Antarctic[J]. The Science of the Total Environment, 2023, 894: 164263. doi: 10.1016/j.scitotenv.2023.164263 [14] LIU B L, DING L J, LV L Y, et al. Organophosphate esters (OPEs) and novel brominated flame retardants (NBFRs) in indoor dust: A systematic review on concentration, spatial distribution, sources, and human exposure[J]. Chemosphere, 2023, 345: 140560. doi: 10.1016/j.chemosphere.2023.140560 [15] SHI Y M, ZHAO L C, ZHU H K, et al. Co-occurrence of phthalate and non-phthalate plasticizers in dust and hand wipes: A comparison of levels across various sources[J]. Journal of Hazardous Materials, 2023, 459: 132271. doi: 10.1016/j.jhazmat.2023.132271 [16] XIE C M, QIU N, XIE J L, et al. Organophosphate esters in seawater and sediments from the low-latitude tropical sea[J]. The Science of the Total Environment, 2024, 907: 167930. doi: 10.1016/j.scitotenv.2023.167930 [17] YANG Y, MENG Y, LIU S, et al. Insights into organophosphate esters (OPEs) in aquatic ecosystems: Occurrence, environmental behavior, and ecological risk[J]. Critical Reviews in Environmental Science and Technology, 2024, 54(8): 641-675. doi: 10.1080/10643389.2023.2266311 [18] LI X X, YAO Y M, ZHAO M S, et al. Nontarget identification of novel organophosphorus flame retardants and plasticizers in rainfall runoffs and agricultural soils around a plastic recycling industrial park[J]. Environmental Science & Technology, 2023, 57(34): 12794-12805. [19] FU J, FU K H, HU B Y, et al. Source identification of organophosphate esters through the profiles in proglacial and ocean sediments from ny-ålesund, the Arctic[J]. Environmental Science & Technology, 2023, 57(5): 1919-1929. [20] CHEN Y, XIAN H, ZHU C C, et al. The transport and distribution of novel brominated flame retardants (NBFRs) and organophosphate esters (OPEs) in soils and moss along mountain valleys in the Himalayas[J]. Journal of Hazardous Materials, 2024, 465: 133044. doi: 10.1016/j.jhazmat.2023.133044 [21] HAN B J, CHEN L Y, LI Y J, et al. Spatial distribution and risk assessment of 11 organophosphate flame retardants in soils from different regions of agricultural farmlands in mainland China[J]. The Science of the Total Environment, 2022, 842: 156806. doi: 10.1016/j.scitotenv.2022.156806 [22] TIAN Y X, WANG Y, CHEN H Y, et al. Organophosphate esters in soils of Beijing urban parks: Occurrence, potential sources, and probabilistic health risks[J]. The Science of the Total Environment, 2023, 879: 162855. doi: 10.1016/j.scitotenv.2023.162855 [23] LIU X S, JI K, JO A, et al. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio)[J]. Aquatic Toxicology, 2013, 134/135: 104-111. doi: 10.1016/j.aquatox.2013.03.013 [24] ZHU Y, ZHANG J Y, LIU Y X, et al. Environmentally relevant concentrations of the flame retardant tris(1, 3-dichloro-2-propyl) phosphate inhibit the growth and reproduction of earthworms in soil[J]. Environmental Science & Technology Letters, 2019, 6(5): 277-282. [25] DASGUPTA S, CHENG V, VLIET S M F, et al. Tris(1, 3-dichloro-2-propyl) phosphate exposure d ring the early-blastula stage alters the normal trajectory of zebrafish embryogenesis[J]. Environmental Science & Technology, 2018, 52(18): 10820-10828. [26] ZHANG Y K, YI X E, HUANG K, et al. Tris(1, 3-dichloro-2-propyl)phosphate Reduces Growth Hormone Expression via Binding to Growth Hormone Releasing Hormone Receptors and Inhibits the Growth of Crucian Carp[J]. Environmental Science & Technology, 2021, 55(12): 8108-8118. [27] HONG X S, CHEN R, HOU R, et al. Triphenyl phosphate (TPHP)-induced neurotoxicity in adult male Chinese rare minnows (Gobiocypris rarus)[J]. Environmental Science & Technology, 2018, 52(20): 11895-11903. [28] HOU M M, FANG J L, SHI Y L, et al. Exposure to organophosphate esters in elderly people: Relationships of OPE body burdens with indoor air and dust concentrations and food consumption[J]. Environment International, 2021, 157: 106803. doi: 10.1016/j.envint.2021.106803 [29] LIU X T, YU G, CAO Z G, et al. Occurrence of organophosphorus flame retardants on skin wipes: Insight into human exposure from dermal absorption[J]. Environment International, 2017, 98: 113-119. doi: 10.1016/j.envint.2016.10.021 [30] BELLO A, CARIGNAN C C, XUE Y L, et al. Exposure to organophosphate flame retardants in spray polyurethane foam applicators: Role of dermal exposure[J]. Environment International, 2018, 113: 55-65. doi: 10.1016/j.envint.2018.01.020 [31] MENDELSOHN E, HAGOPIAN A, HOFFMAN K, et al. Nail Polish as a source of exposure to triphenyl phosphate[J]. Environment International, 2016, 86: 45-51. doi: 10.1016/j.envint.2015.10.005 [32] HOU M M, SHI Y L, NA G S, et al. Increased human exposure to organophosphate esters via ingestion of drinking water from water dispensers: Sources, influencing factors, and exposure assessment[J]. Environmental Science & Technology Letters, 2021, 8(10): 884-889. [33] GBADAMOSI M R, ABDALLAH M A E, HARRAD S. A critical review of human exposure to organophosphate esters with a focus on dietary intake[J]. The Science of the Total Environment, 2021, 771: 144752. doi: 10.1016/j.scitotenv.2020.144752 [34] ALVES A, COVACI A, VOORSPOELS S. Method development for assessing the human exposure to organophosphate flame retardants in hair and nails[J]. Chemosphere, 2017, 168: 692-698. doi: 10.1016/j.chemosphere.2016.11.006 [35] ZHENG G M, SCHREDER E, DEMPSEY J C, et al. Organophosphate esters and their metabolites in breast milk from the United States: Breastfeeding is an important exposure pathway for infants[J]. Environmental Science & Technology Letters, 2021, 8(3): 224-230. [36] ZHAO F R, WAN Y, ZHAO H Q, et al. Levels of blood organophosphorus flame retardants and association with changes in human sphingolipid homeostasis[J]. Environmental Science & Technology, 2016, 50(16): 8896-8903. [37] SAILLENFAIT A M, NDAW S, ROBERT A, et al. Recent biomonitoring reports on phosphate ester flame retardants: A short review[J]. Archives of Toxicology, 2018, 92(9): 2749-2778. doi: 10.1007/s00204-018-2275-z [38] YANG Y, CHEN P, MA S T, et al. A critical review of human internal exposure and the health risks of organophosphate ester flame retardants and their metabolites[J]. Critical Reviews in Environmental Science and Technology, 2022, 52(9): 1528-1560. doi: 10.1080/10643389.2020.1859307 [39] CHEN Z F, TANG Y T, LIAO X L, et al. A QuEChERS-based UPLC-MS/MS method for rapid determination of organophosphate flame retardants and their metabolites in human urine[J]. Science of the Total Environment, 2022, 826: 153989. doi: 10.1016/j.scitotenv.2022.153989 [40] LU Q, LIN N, CHENG X M, et al. Simultaneous determination of 16 urinary metabolites of organophosphate flame retardants and organophosphate pesticides by solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry[J]. Chemosphere, 2022, 300: 134585. doi: 10.1016/j.chemosphere.2022.134585 [41] ZHU L F, HUANG X H, LI Z H, et al. Evaluation of hepatotoxicity induced by 2-ethylhexyldiphenyl phosphate based on transcriptomics and its potential metabolism pathway in human hepatocytes[J]. Journal of Hazardous Materials, 2021, 413: 125281. doi: 10.1016/j.jhazmat.2021.125281 [42] GREAVES A K, SU G Y, LETCHER R J. Environmentally relevant organophosphate triesters in herring gulls: in vitro biotransformation and kinetics and diester metabolite formation using a hepatic microsomal assay[J]. Toxicology and Applied Pharmacology, 2016, 308: 59-65. doi: 10.1016/j.taap.2016.08.007 [43] HE C, TOMS L M L, THAI P, et al. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children[J]. Environment International, 2018, 111: 124-130. doi: 10.1016/j.envint.2017.11.019 [44] PHILLIPS A L, HERKERT N J, ULRICH J C, et al. In vitro metabolism of isopropylated and tert-butylated triarylphosphate esters using human liver subcellular fractions[J]. Chemical Research in Toxicology, 2020, 33(6): 1428-1441. doi: 10.1021/acs.chemrestox.0c00002 [45] BASTIAENSEN M, MALARVANNAN G, BEEN F, et al. Metabolites of phosphate flame retardants and alternative plasticizers in urine from intensive care patients[J]. Chemosphere, 2019, 233: 590-596. doi: 10.1016/j.chemosphere.2019.05.280 [46] LI M Q, YAO Y M, WANG Y, et al. Organophosphate ester flame retardants and plasticizers in a Chinese population: Significance of hydroxylated metabolites and implication for human exposure[J]. Environmental Pollution, 2020, 257: 113633. doi: 10.1016/j.envpol.2019.113633 [47] ZHAO F R, KANG Q Y, ZHANG X H, et al. Urinary biomarkers for assessment of human exposure to monomeric aryl phosphate flame retardants[J]. Environment International, 2019, 124: 259-264. doi: 10.1016/j.envint.2019.01.022 [48] PHILLIPS A L, HAMMEL S C, HOFFMAN K, et al. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study[J]. Environment International, 2018, 116: 176-185. doi: 10.1016/j.envint.2018.04.013 [49] 张洛红, 秦瑞欣, 严骁, 等. 液液提取-固相萃取-LC-MS/MS测定人体尿液中有机磷系阻燃剂及其二酯类代谢物[J]. 环境化学, 2020, 39(1): 197-206. doi: 10.7524/j.issn.0254-6108.2019090201 ZHANG L H, QIN R X, YAN X, et al. Determination of organophosphate flame retardants and their diesters in urine by liquid-liquid extraction and solid-phase extraction coupled with LC-MS/MS[J]. Environmental Chemistry, 2020, 39(1): 197-206(in Chinese). doi: 10.7524/j.issn.0254-6108.2019090201
[50] 侯敏敏, 史亚利, 蔡亚岐. 液液提取-固相萃取-高效液相色谱-串联质谱测定人体血液中16种有机磷酸酯[J]. 色谱, 2021, 39(1): 69-76. doi: 10.3724/SP.J.1123.2020.07033 HOU M M, SHI Y L, CAI Y Q. Determination of 16 organophosphate esters in human blood by high performance liquid chromatography-tandem mass spectrometry combined with liquid-liquid extraction and solid phase extraction[J]. Chinese Journal of Chromatography, 2021, 39(1): 69-76(in Chinese). doi: 10.3724/SP.J.1123.2020.07033
-



 下载:
下载: